Abstract
This study aimed to investigate the subclinical symptom and histological lesions of 21-day-old and 42-day-old broilers exposure to low concentration aflatoxin B1 (AFB1), and the preventive effect with adsorbent (Toxo-MX) supplementation. A total of 576 one-day-old Arbor Acres broilers were randomly allotted into 6 treatments 8 replicates and 12 birds per cage, fed with 0 ppb, 60 ppb and 120 ppb AFB1 contamination diet with or without Toxo-MX supplementation. Results showed both 60 ppb and 120 ppb AFB1 contamination significantly reduced growth performance in 21-day-old broilers (P < 0.05), but not in 42-day-old broilers (P > 0.05), however, AFB1 contamination in diet caused a higher feed to gain ratio (P < 0.05). Broilers of 21-day-old exposure to 60 ppb and 120 ppb AFB1 increased mRNA expression of hepatic inflammatory cytokines, and superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) activity (P < 0.05), 42-day-old broilers showed a same change in 120 ppb but not in 60 ppb of AFB1 contamination (P < 0.05). mRNA expressions of clauding-1, Zonula occludens-1 (ZO-1), and occludin decreased, but Bax, Bcl-2, and caspase-3 increased in 21-day-old broilers exposure to 60 ppb and 120 ppb AFB1 (P < 0.05), broilers of 42-day-old resisted on intestinal aflatoxicosis impairment against 60 ppb AFB1 contamination (P < 0.05), but not in 120 ppb (P < 0.05). Toxo-MX supplementation significantly reversed the detrimental effects on growth performance in both age broilers and reduced the accelerated feed to gain ratio caused by AFB1 (P < 0.05). Intestinal mRNA expression of tight junction and apoptotic genes in both age broilers were recovered by Toxo-MX supplementation (P < 0.05). However, Toxo-MX did not restore the accelerated expression of hepatic inflammation cytokines and SOD, GSH-Px in 120ppb AFB1 group (P < 0.05). The data demonstrated that diet supplementation with Toxo-MX reversed the detrimental effect on growth performance and intestine in broilers exposure to 60 ppb and 120 ppb AFB1. However, did not completely recovered hepatic inflammation induced by AFB1.
Key words: aflatoxin B1, broiler, immunity, hepatic inflammation, intestinal barrier function
INTRODUCTION
Aflatoxins are carcinogens and hepatotoxins which as the secondary metabolites produced by the fungal genus of Aspergillus are frequently contaminated in poultry feeds and associated with increased risk of disease outbreak and growth performance impairment (Dersjant-Li et al., 2003; Pankaj et al., 2018). The predominant aflatoxin is aflatoxin B1 (AFB1) (Rawal et al., 2010). Worldwide economic losses caused by AFB1 contamination could reach billions annually (Wu, 2015; Mitchell et al., 2016). However, unlike those relative high dosage used in experiment setting (Gowda et al., 2009; Yarru et al., 2009), natural occurrence of AFB1 in feed ingredients were usually between 0 and 1 mg/kg (Dersjant-Li et al., 2003). Nations in the world established aflatoxin contamination regulations which are aimed to protect human health and reduce residues in animal products: US Food and Drug Administration (FDA) set AFB1 contamination action levels in mature poultry feeds are less than 300 μg/kg in cottonseed meal, 100 μg/kg in corn, and 20 μg/kg in other feed ingredients. Moreover, for immature poultry are set at less than 20 μg/kg (Cai et al., 2020). The EU legal limit for AFB1 in processed cereal is 20 µg/kg (Agriopoulou et al., 2020), China set at less than 50 μg/kg (Ma et al., 2018). Furthermore, contaminated ingredients could be diluted before use, therefore, the toxic effect and prevention strategy of low concentration AFB1 contamination should be emphasis in experiment design.
Whenever mycotoxins are produced in crops, they directly create negative impact on animal health and directly or indirectly produce serious health issues (Cao et al., 2020). Recently study reported the positive rate of AFB1 contamination in feed ingredients could reach as high as 80.8% (Wu et al., 2016), discard the contaminated ingredient is not always practical, the treatment of AFB1 contaminated feeds have significant economic implications for animal producer. Thus, decontamination procedures are important for both safety and economic efficiency. AFB1 decontamination methods focused on degrading, inactivating, or removing AFB1 by physical, chemical, and biological methods (Rawal et al., 2010; Adebo et al., 2016; Rushing and Selim, 2019). A cost-effective to the problem is to use non-nutritive and inert adsorbents in the diet to bind AFB1 and reduced their absorption, however, the efficiency of those adsorbents is highly related to the concentration of AFB1 because of the rapid absorption rate of AFB1 (LI et al., 2010; Vila-Donat et al., 2018). Therefore, evaluation of the adsorbent efficiency in a natural occurrence low dosage of AFB1 is necessary in practical production.
Liver is the principal target organ of aflatoxicosis, hepatocytes exposure to AFB1 could activate hepatic inflammation responses, release inflammatory cytokines, and change antioxidant enzyme activity for detoxification reaction (Wang et al., 2019). The phase Ⅰ of AFB1 detoxification in poultry appears to convert the original molecules into hydrophilic compound by hydrolytic or oxidant-reduction enzymes, phase Ⅱ is characterized by conjugation of original molecules or its metabolites principal with endogenous glutathione (GSH), a reaction catalyzed by glutathione S-transferases (GSTs) (Dohnal et al., 2014). Likewise, aflatoxicosis caused impairment metabolism of liver, resulted in reduction of nutrition metabolism and feed efficiency (Iqbal et al., 2005; Saleemi et al., 2020). Besides, age is another important factor that affects the resistance of poultry to AFB1 contamination. Younger poultry is more sensitive to AFB1 than older ones, and the resistance is associated with development of hepatic microsomal enzyme system and GSH activity, and also the age-related increasing in the expression of GST isoforms (Dohnal et al., 2014; Wang et al., 2018). After intake of AFB1 contamination diet, AFB1 could directly contact to intestinal epithelial cell and altered intestinal morphology and digestibility (Yunus et al., 2010, 2011; Jiang et al., 2015), inhibition cellular 20S proteasome and increase caspase-3 activity to activate apoptotic pathway (Towner et al., 2003; Amici et al., 2007). However, these changes were varied with AFB1 dosage, length of exposure, age of poultry, species, and diet nutritional statues (Yunus et al., 2010, 2011).
Based on above information, the purpose of this study was to examine histological changes, aflatoxicosis symptoms and molecular mechanism in liver and intestine of 21-day-old and 42-day-old broilers exposure to 60 ppb or 120 ppb AFB1, evaluate the preventive effect of dietary supplementation with a commercial smectite clay minerals adsorbent (TOXO-MX).
MATERIALS AND METHODS
Sample Preparation
AFB1 were purchased from Pribiolab (Purity >99%, Qingdao, China), The adsorbent TOXO-MX were obtained from Trouw Nutrition, Amersfoort, The Netherlands. TOXO-MX consists of a high content of smectite clay which contained 94% dioctahedral smectite, 3% sodium feldspar, 1% each of illite and maghemite, and traces of quartz and calcite, as assessed by an X-ray diffraction study.
Diet Formulation
Diet formulation was based on NRC 1994 (Nutrient Requirements of Poultry, 1994), diet composition and nutrition level were presented in Table 1, AFB1 were added into the premix before mixture with feed ingredients, and the final concentration of AFB1 in the feed were determined by ELISA (Enzyme-linked Biotechnology Co., Ltd, Shanghai, China). The measured final concentration of AFB1 is presented in Table 2. Toxo-MX (2.5 kg/T) supplementation was performed according to the products’ instructions.
Table 1.
1Diet composition and nutrition level.
| Ingredients | Proportion1 (%) |
Nutrition |
|||
|---|---|---|---|---|---|
| 0–21 d | 22–42 d | Item | 0–21 d | 22–42 d | |
| Corn | 56.51 | 62.21 | ME (kCal/kg) | 3,039.94 | 3,111.17 |
| Soybean | 35.30 | 29.20 | CP (%) | 21.00 | 18.28 |
| Soybean oil | 4.50 | 4.90 | Ca (%) | 0.90 | 0.91 |
| CaCO3 | 1.52 | 1.60 | P (%) | 0.29 | 0.29 |
| CaHPO4 | 1.00 | 1.00 | Lys (%) | 1.27 | 1.09 |
| L-Lys | 0.35 | 0.30 | Met (%) | 0.47 | 0.41 |
| L-Met | 0.16 | 0.13 | Thr (%) | 0.83 | 0.75 |
| L-Thr | 0.06 | 0.06 | |||
| NaCl | 0.30 | 0.30 | |||
| Premix1 | 0.30 | 0.30 | |||
| Total | 100 | 100 | |||
Provided per kilogram of diet: vitamin A, 9500 IU; vitamin D3, 62.5 g; vitamin K3, 2.65 mg; vitamin B1, 2 mg; vitamin B2, 6 mg; vitamin B12, 0.025 mg; vitamin E, 30 IU; biotin, 0.0325mg; folic acid, 1.25 mg; pantothenic, 12 mg; niacin, 50 mg; Cu, 8 mg; Zn, 75 mg; Fe, 80 mg; Mn, 100 mg; Se, 0.15 mg; I, 0.35 mg.
Table 2.
The measured concentration of aflatoxin B1 (AFB1) in the diet of broilers.
| Item | 0 ppb AFB1 | SD | 60 ppb AFB1 | SD | 120 ppb AFB1 | SD |
|---|---|---|---|---|---|---|
| 0–21 d (ppb) | 0.51 | 0.44 | 57.10 | 2.39 | 117.84 | 8.74 |
| 22–42 d (ppb) | 0.09 | 0.17 | 62.00 | 4.11 | 126.54 | 10.06 |
The data represent mean of 5 replicates, tested sample were collected by using stratified sampling method, and AFB1 in the feed were determined by ELISA (Enzyme-linked Biotechnology Co., Ltd, Shanghai, China).
Animal Management
Hunan Agricultural University Animal Ethics Committee (Changsha, China) reviewed and approved all experimental protocols. A total of 576 one-day-old Arbor Acres broilers were housed in an environmentally controlled room, and temperature was set at 34°C at first day, and gradually decreased to reach 20°C from d 28 of age and for the rest of the experiment, the average air humidity was set at 65%. Broilers with equivalent gender were completely randomized allotted to a total of 6 treatments 8 replicates and 12 birds per cage of 0 ppb, 60 ppb and 120 ppb AFB1 with or without Toxo-MX supplementation. Broilers were free access to water and feed, experiment period was 42 d, include brood period of 1 d to 21 d and growth period of 22 d to 42 d. ADFI was measured by subtracting the amount of feed that was rejected from the birds that was offered, bodyweight (BW) were determined every 3 d, average daily gain (ADG), average daily feed intake (ADFI), and feed efficiency (G/F) were calculated. Four birds from each cage were sacrificed at 21 d and 42 d, respectively. Thymus, spleens, and bursa of fabricius were weighted. A lobe of liver, duodenum, jejunum and ileum of broilers from each cage were preserved in 4% buffered paraformaldehyde (4% Formaldehyde Solution in PBS, adjusted pH to 7.4, Sinopharm, Beijing, China) for histological analysis, the remaining hepatic and enteric parts were washed by 0.90% w/v of NaCl solution, freeze in the liquid nitrogen and then stored in −80°C for further analysis.
Serum Biochemical Assay and Serum Immunoglobulin, Acute-phase Protein Quantitation
Blood was collected from brachial vein of broiler, and serum were obtained in a blood collection tube (Separable microtubes, FUCHIGAMI, 170720, Kyoto, Japan) by centrifuging at 1,500 × g for 10 min and stored at −20°C until use. The levels of serum aspartate transaminase (AST), alanine transaminase (ALT), Serum total protein (TP), albumin (ALB), alkaline phosphatase (ALP), and total bilirubin (T-Bil) were measured with an automated analyzer for clinical chemistry analysis (Mindray BS-200 Chemistry Analyzer, Shenzhen, China), Serum Gamma-Glutamyl transferase (GGT) were tested by a GGT instrument Specific kit (Mindray, Shenzhen, China). Serum globulin (GLO) is calculated using the formula: . Urea nitrogen (BUN) was measured by using a BUN Colorimetric Detection Kit (Thermo, Shanghai, China) according to the manufacturer's instructions.
Serum immunoglobulin (IgG, IgA, and IgM) were quantitated by using chicken immunoglobulin ELISA kit according to the manufacturer's instructions (Cusabio, Wuhan, China). Serum acute-phase protein including serum amyloid A (SAA) and alpha-1-acid glycoprotein (α1-AGP) were measured by using chicken SAA ELISA kit and chicken alpha 1 acid glycoprotein ELISA kit according to the manufacturer's instructions (Abcam, Shanghai, China).
Hepatic and Enteric Histological Lesion
Lobes of liver, duodenum, jejunum, and ileum were preserved 24 h in 4% buffered paraformaldehyde, then were dewatered gradually with 10 to 30% sucrose water, changing every 12 h. After dewatering, the liver, duodenum, jejunum, and ileum from each treatment were embedded by paraffin wax. Paraffin blocks were cross sectioned at 5 μm by a microtome system machine (Yamato, Saitama, Japan). Sliced sections of liver, duodenum, jejunum, and ileum from each treatment mounted on poly-L-lysine-coated glass slides and stained with hematoxylin-eosin, observed under a microscope (Keyence, Tokyo, Japan) and were taken at 40 × and 100 × magnification for morphological analysis. Ten villi and 10 crypts of intestines from each segment of each bird were quantified by the software in microscopic imaging system (Keyence). The villus height and crypt depth per villus were measured in each segment. TUNEL assay was performed by using a TUNEL Assay Kit (Abcam) according to manufacturer's instructions. The number of TUNEL-positive cells were counted from 10 random fields from each intestinal cross-section by the software in microscopic imaging system (Keyencen), TUNEL positive rate were calculated by average the number of TUNEL-positive cells to total number of nuclei ratio per area and normalized to TUNEL signal of negative control group.
Real-Time Quantitative PCR
Jejunal total RNA was extracted by a Total RNA II kit (Omega Bio-tek, Guangzhou, China) according to the manufacturer's manual. PrimeScript RT Reagent Kit (Takara, Tokyo, Japan) were used to synthesize the cDNA. Expressions of claudin-1, occluding, Zonula occludens-1 (ZO-1), B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), Caspase-3, and β-actin were measured by real-time quantitative PCR (qPCR) using One-step PrimeScript RT-PCR kit (Takara). The reaction condition consisted 12.5 μL SYBR Premix Ex Taq Ⅱ (Applied Biosystems, Foster city, CA), 2 μl cDNA, 1 μL of 10 μM 1:1 forward and reverse target primer mixture and 8.5 μL of nuclease-free water. Primers for claudin-1, occluding, ZO-1, Bcl-2, Bax, Caspase-3, and β-actin are listed in Table 3. The thermal cycling conditions were composed of 50°C for 2 min followed by an initial denaturation step at 95°C for 10 min, 45 cycles at 95°C for 30 s, 60°C for 30 s and 72°C for 30 s. Real-time PCR was carried out using an ABI PRISM 7500 Sequence Detection System (Applied Biosystems). Reactions were run in triplicate in 3 independent experiments. The geometric mean of housekeeping gene β-actin was used as an internal control to normalize the variability in expression levels. Expression data were normalized to the geometric mean of housekeeping gene GAPDH to control the variability in expression levels and were analyzed using the 2-ΔΔCT method described by previous report (Livak and Schmittgen, 2001).
Table 3.
Primer sequences used for real-time quantitative PCR (5′ to 3′).
| Genes | Primer sequence (5’-3’)1 | Accession number |
|---|---|---|
| β-Actin | F: GAGAAATTGTGCGTGACATCA | NM_205518.1 |
| R: CCTGAACCTCTCATTGCCA | ||
| Bax | F: TCCTCATCGCCATGCTCAT | XM_422067 |
| R: CCTTGGTCTGGAAGCAGAAGA | ||
| Bcl-2 | F: GATGACCGAGTACCTGAACC | NM_205339 |
| R: CAGGAGAAATCGAACAAAGGC | ||
| Caspase-3 | F: TGGCCCTCTTGAACTGAAAG | NM_204725 |
| R: TCCACTGTCTGCTTCAATACC | ||
| ZO-1 | F: GCGCCTCCCTATGAGGAGCA | XM_413773.4 |
| R: CAAATCGGGGTTGTGCCGGA | ||
| Occluding | F: CCGTAACCCCGAGTTGGAT | NM_205128.1 |
| R: ATTGAGGCGGTCGTTGATG | ||
| Claudin-1 | F: GCAGATCCAGTGCAAGGTGTA | NM_001012611.2 |
| R: CACTTCATGCCCGTCACAG | ||
| IFN-γ | F: ATCATACTGAGCCAGATTGTTTCG | AJ634956.1 |
| R: ACGCCATCAGGAAGGTTGTT | ||
| IL-6 | F: AAATCCCTCCTCGCCAATCT | NM_204628.1 |
| R: CTCACGGTCTTCTCCATAAACG | ||
| TGF-β1 | F: CGGGACGGATGAGAAGAACTG | JQ423909.1 |
| R: CAGGCACGGACCACCATATTG | ||
| TNF-α | F: GGAATGAACCCTCCGCAGTA | AY765397.1 |
| R: CAGAGCATCAACGCAAAAGG | ||
| TLR-4 | F: TTTGAAAATGTAAGGCGGCTC | NM_001030693.1 |
| R: ATTTCCAGGGCTGAGTCCGA | ||
| IL-1β | F: ACTGGGCATCAAGGGCTA | NM_204524 |
| R: GGTAGAAGATGAAGCGGGTC |
Statistical Analysis
Results were expressed as mean ± SEM. The significant differences between treatments were analyzed by the One-Way ANOVA followed Tukey's multiple comparison post-test, and by using the GLM procedure of SPSS 19.0 (SPSS Inc., Chicago, IL). ANOVA annotation uses letters (a, b, c, etc.), where treatments not sharing the same letter indicates P < 0.05.
RESULTS
Growth Performance
Aflatoxicosis of AFB1 in poultry industry is characterized by reduction of growth performance and feed efficiency. In the present study, 60 ppb and 120 ppb AFB1 contaminated diet significantly decreased BW and ADG in 21-day-old broilers (Table 4, P < 0.05), F:G ratio were higher compared to control group (P < 0.05). Diet supplementation with Toxo-MX reversed the reduction of growth performance and feed efficiency (P < 0.05). The 42-day-old broilers fed AFB1 contaminated diet have no significant difference in BW (P > 0.05), however, an increased ADFI and F:G ratio has been showed (P < 0.05). Broilers fed both 60 ppb and 120 ppb AFB1 contaminated diet increased F:G ratio during the whole experiment period (P < 0.05), ADG of broilers fed with 120 ppb AFB1 contaminated diet significantly lower than control group (P < 0.05). Both 60 ppb and 120 ppb AFB1 contaminated diet supplement with Toxo-MX ameliorated F:G ratio and ADG (P < 0.05), and had no significant differences compared with control group (P > 0.05).
Table 4.
Growth performance of broilers fed dietary contaminated aflatoxin B1 (AFB1) with or without Toxo-MX.
| AFB1 |
0 ppb AFB1 |
60 ppb AFB1 |
120 ppb AFB1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | Toxo-MX | - | + | - | + | - | + | SEM | P | |
| 1–21 d | Initial BW (g) | 49.11 | 48.47 | 49.11 | 48.28 | 48.63 | 48.19 | 0.17 | 0.677 | |
| Final BW (g) | 655.36aW | 662.42a | 635.15b | 645.96ab | 611.82c | 641.78ab | 10.95 | 0.014 | ||
| ADG (g) | 28.87a | 29.24a | 27.91b | 28.46a | 26.82b | 28.27a | 0.52 | 0.018 | ||
| ADFI (g) | 44.54 | 44.74 | 44.92 | 43.80 | 44.48 | 43.99 | 0.51 | 0.462 | ||
| F:G | 1.54b | 1.53b | 1.61a | 1.54b | 1.66a | 1.56b | 0.03 | 0.021 | ||
| 22–42 d | Final BW (g) | 2,459.31 | 2,463.22 | 2,406.06 | 2,460.83 | 2,389.86 | 2,456.71 | 32.44 | 0.104 | |
| ADG (g) | 85.90 | 85.75 | 84.33 | 86.42 | 84.67 | 86.43 | 2.31 | 0.126 | ||
| ADFI (g) | 147.87c | 149.22c | 156.18ab | 149.73c | 160.03a | 153.56b | 3.96 | 0.002 | ||
| F:G | 1.72b | 1.74b | 1.85a | 1.73b | 1.89a | 1.78ab | 0.06 | 0.028 | ||
| 1–42 d | ADG (g) | 57.39a | 57.49a | 56.12ab | 57.44a | 55.74b | 57.35a | 1.25 | 0.012 | |
| ADFI (g) | 96.21 | 96.98 | 100.55 | 96.77 | 102.26 | 98.78 | 2.04 | 0.077 | ||
| F:G | 1.68b | 1.69b | 1.79a | 1.68b | 1.83a | 1.72b | 0.04 | 0.004 | ||
The data represent mean of 8 replicates, and different letters (abc) in the same column indicate significant differences (P < 0.05).
Serum Parameters
There are a number of serum biomarkers have been validated to evaluate the toxic effect of AFB1 in poultry. In the present study, a higher UA were shown in 21-day-old broilers fed AFB1 contaminated diet (Table 5, P < 0.05), Toxo-MX supplementation in 60ppb AFB1 contaminated diet recovered the UA levels (P < 0.05), but did not in 120 ppb AFB1 contaminated diet (P < 0.05). Forty two-day-old broilers exposure to both 60 ppb and 120 ppb AFB1 still have a higher serum UA (P < 0.05), but with Toxo-MX addition in AFB1 contaminated diet, broilers have no significant difference in serum UA compared with control group (P > 0.05). BUN had no significant changes among all the treatments in both age broilers (P > 0.05). Serum ALP was significantly increased in broilers exposure to both concentration of AFB1 in both age, Toxo-MX supplementation reversed the changes in 42-day-old broilers (P > 0.05), but not in 21-day-old birds (P < 0.05). In addition, AST, ALT, and GGT significantly increased in both 21-day-old and 42-day-old broilers fed AFB1 contaminated diet (P < 0.05), Toxo-MX supplementation ameliorated the higher AST and ALT in the birds exposure to AFB1 (P < 0.05), but the levels were still higher than control group (P < 0.05). Serum T-Bil and TP have no significant difference among the treatments in both age broilers (P > 0.05), however, ALB significantly decreased while GLB significantly increased in the 21-day-old broilers fed with AFB1 contaminated diet than control group (P < 0.05), in 42-day-old broilers exposure with 120 ppb AFB1 group, serum ALB and GLB was still higher (P < 0.05), but 60 ppb AFB1 exposure group have no significant difference (P > 0.05). A total of 120 ppb and 60 pb AFB1 contaminated diet add Toxo-MX have no significant differences of serum ALB and GLB in 21-day-old broilers (P > 0.05), but have lower ALB:GLB ratio (P < 0.05). In 42-day-old broilers, Toxo-MX supplementation reversed the changes in 60 ppb AFB1 exposure (P > 0.05), but not in 120 ppb exposure (P < 0.05).
Table 5.
Serum parameters of broilers fed dietary contaminated aflatoxin B1 (AFB1) with or without Toxo-MX.
| AFB1 |
0 ppb AFB1 |
60 ppb AFB1 |
120 ppb AFB1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | Toxo-MX | - | + | - | + | - | + | SEM | P | |
| 21 d | UA (mg/dL) | 7.34c | 7.45c | 8.89b | 7.79c | 10.12a | 9.11a | 0.09 | <0.001 | |
| BUN (mg/dL) | 3.42 | 3.30 | 3.21 | 3.54 | 3.45 | 3.24 | 0.15 | 0.724 | ||
| ALP (U/L) | 126.16b | 131.54b | 179.36a | 169.93a | 174.86a | 183.78a | 9.27 | 0.014 | ||
| AST (U/L) | 109.18d | 105.73d | 175.08b | 146.27c | 220.24a | 144.56c | 6.34 | <0.001 | ||
| ALT (U/L) | 3.23d | 3.44d | 44.37a | 13.27c | 42.58a | 23.02b | 2.32 | <0.001 | ||
| GGT (U/L) | 7.52b | 7.69b | 15.21a | 11.65b | 14.76a | 10.44b | 1.67 | <0.001 | ||
| T-Bil (μmol/L) | 35.92 | 34.85 | 35.60 | 33.11 | 36.11 | 35.26 | 1.18 | 0.237 | ||
| TP (g/L) | 58.96 | 57.64 | 58.52 | 59.40 | 59.62 | 58.08 | 0.13 | 0.536 | ||
| ALB (g/L) | 27.61a | 26.25a | 22.39b | 24.99ab | 21.65b | 24.92ab | 0.75 | 0.003 | ||
| GLB (g/L) | 31.35b | 31.39b | 36.13a | 34.41ab | 37.97a | 33.16ab | 0.41 | 0.016 | ||
| ALB:GLB | 0.88a | 0.84a | 0.62c | 0.73b | 0.57c | 0.75b | 0.01 | <0.001 | ||
| 42 d | UA (mg/dL) | 7.02d | 7.03d | 8.16b | 7.14d | 9.49a | 7.54cd | 0.10 | <0.001 | |
| BUN (mg/dL) | 3.64 | 3.55 | 3.41 | 3.67 | 3.41 | 3.39 | 0.21 | 0.609 | ||
| ALP (U/L) | 139.92b | 137.39b | 152.96a | 133.64b | 154.37a | 129.66b | 7.69 | 0.037 | ||
| AST (U/L) | 134.86b | 140.42b | 218.80a | 154.65b | 222.56a | 164.20b | 11.66 | <0.001 | ||
| ALT (U/L) | 3.51c | 3.96c | 13.05a | 6.22b | 13.26a | 8.43b | 0.77 | <0.001 | ||
| GGT (U/L) | 10.11c | 9.69c | 26.64a | 15.42b | 27.72b | 18.56a | 2.01 | <0.001 | ||
| T-Bil (μmol/L) | 20.59 | 21.09 | 21.57 | 19.83 | 21.73 | 20.46 | 0.87 | 0.501 | ||
| TP (g/L) | 58.32 | 59.84 | 60.06 | 60.50 | 61.82 | 60.72 | 0.07 | 0.249 | ||
| ALB (g/L) | 26.18a | 26.45a | 25.61a | 26.69a | 23.04b | 24.71b | 0.36 | 0.018 | ||
| GLB (g/L) | 32.44c | 33.39c | 34.45c | 33.81c | 38.78a | 36.01b | 0.54 | 0.048 | ||
| ALB:GLB | 0.80a | 0.79a | 0.74a | 0.79a | 0.59b | 0.69b | 0.01 | <0.001 | ||
The data represent mean of 8 replicates, and different letters (abc) in the same column indicate significant differences (P < 0.05).
Abbreviations: ALB, albumin; ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate transaminase; BUN, Urea nitrogen; GGT, Serum Gamma-Glutamyl transferase; T-Bil, total bilirubin; TP, serum total protein.
Serum Immunoglobulin and Acute-Phase Protein
AFB1 has been demonstrated that involved in outbreak of infectious disease due to immunosuppression (Tessari et al., 2006), thus, we investigated serum immunoglobulin and acute-phase protein levels in both age broilers. As shown in Table 6, serum IgA and IgG concentration significantly reduced in 21-day-old broilers exposure to 120 ppb AFB1 (P < 0.05), IgM has no significant difference (P > 0.05), AFB1 contaminated diet with Toxo-MX addition restored the decreasing of serum IgA, IgG concentration (P < 0.05), but IgG concentration was still less than control group (P < 0.05). Forty two-day-old broilers fed with 120 ppb AFB1 showed a less serum IgG concentration than control group (P < 0.05). Toxo-MX supplementation reversed the negative effect (P < 0.05), serum IgA and IgM concentration have no significant differences among all the treatments in 42-day-old broilers (P > 0.05). Moreover, both 60 ppb and 120 ppb AFB1 exposure showed no significant negative effect to the concentration of acute-phase proteins including SAA, α1-AGP, and α1-AT in both age birds (P > 0.05).
Table 6.
Serum levels of immunoglobulin and acute-phase protein of broilers fed dietary contaminated aflatoxin B1 (AFB1) with or without Toxo-MX.
| AFB1 |
0 ppb AFB1 |
60 ppb AFB1 |
120 ppb AFB1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | Toxo-MX | - | + | - | + | - | + | SEM | P | |
| 21d | IgA (ng/mL) | 530.53a | 552.67a | 577.24a | 548.64a | 291.32b | 464.85a | 23.45 | <0.001 | |
| IgG (ng/mL) | 1,604.65a | 1,556.23a | 1,448.66a | 1,546.9a | 945.18c | 1,238.34b | 62.36 | <0.001 | ||
| IgM (ng/mL) | 145.1 | 142.47 | 146.63 | 156.47 | 137.35 | 147.31 | 7.75 | 0.361 | ||
| SAA (ng/mL) | 237.73 | 226.45 | 233.11 | 223.19 | 241.42 | 220.27 | 18.73 | 0.842 | ||
| α1-AGP (ng/mL) | 146.8 | 146.1 | 133.87 | 133.94 | 132.54 | 140.51 | 19.48 | 0.224 | ||
| 42d | IgA (ng/mL) | 586.07 | 601.66 | 596.57 | 609.1 | 622.82 | 607.85 | 49.92 | 0.701 | |
| IgG (ng/mL) | 1,418.24ab | 1,391.94ab | 1,350.66b | 1,514.23a | 1,122.44c | 1,546.61a | 121.13 | <0.001 | ||
| IgM (ng/mL) | 276.45 | 281.17 | 264.44 | 273.21 | 278.11 | 269.59 | 23.53 | 0.383 | ||
| SAA (ng/mL) | 255.39 | 274.87 | 276.32 | 279.26 | 283.01 | 267.45 | 19.79 | 0.238 | ||
| α1-AGP (ug/mL) | 168.55 | 170.73 | 176.08 | 174.96 | 178.71 | 167.68 | 14.26 | 0.524 | ||
The data represent mean of 8 replicates, and different letters (ab) in the same column indicate significant differences (P < 0.05).
Relative Immune Organ Weight
Relative immune organ weights of the broilers in this study were presented in Table 7. Relative thymus weight significantly increased in 21-day-old broilers receiving diets with 120 ppb AFB1 (P < 0.05), meanwhile, relative weight of bursa of Fabricius was significantly reduced (P < 0.05), and there was no significant difference in the relative weight of spleen (P > 0.05). In the 42-day-old broilers, no significant difference was seen in the relative immune organ weight among the treatments (P > 0.05).
Table 7.
Relative immune organ weights (organ weight/body weight × 100) of broilers fed dietary contaminated aflatoxin B1 (AFB1) with or without Toxo-MX.
| AFB1 |
0 ppb AFB1 |
60 ppb AFB1 |
120 ppb AFB1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | Toxo-MX | - | + | - | + | - | + | SEM | P | |
| 21 d | Thymus | 0.45b | 0.43b | 0.49ab | 0.44b | 0.57a | 0.47b | 0.019 | 0.017 | |
| Spleen | 0.14 | 0.13 | 0.16 | 0.14 | 0.14 | 0.15 | 0.007 | 0.522 | ||
| Bursa of Fabricius | 0.32a | 0.34a | 0.26b | 0.34a | 0.24b | 0.32a | 0.011 | 0.021 | ||
| 42 d | Thymus | 0.32 | 0.31 | 0.33 | 0.32 | 0.34 | 0.31 | 0.015 | 0.741 | |
| Spleen | 0.30 | 0.31 | 0.29 | 0.30 | 0.34 | 0.31 | 0.010 | 0.124 | ||
| Bursa of Fabricius | 0.22 | 0.21 | 0.23 | 0.22 | 0.24 | 0.23 | 0.009 | 0.635 | ||
The data represent mean of 8 replicates, and different letters (ab) in the same column indicate significant differences (P < 0.05).
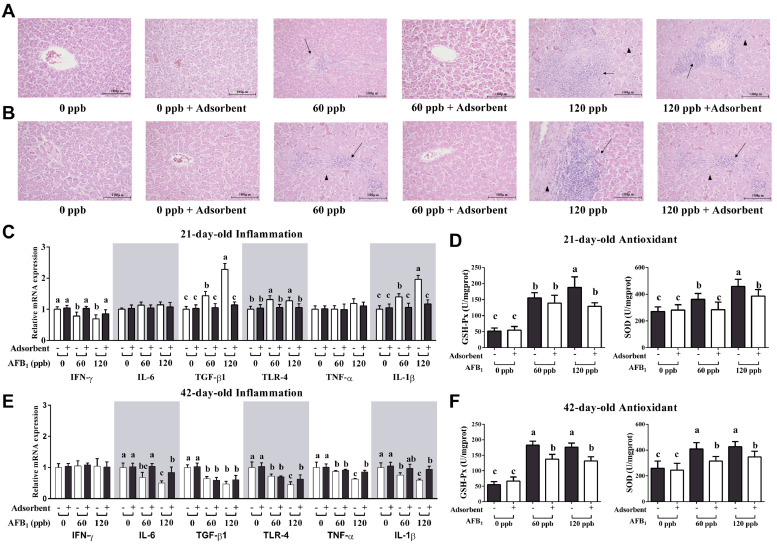
Hepatic Histological Assay, mRNA Expression of Inflammation Genes, and Levels of Antioxidant Enzymes
Leukocyte infiltration were showed in the livers of both 21-day-old and 42-day-old broilers fed AFB1 contaminated diet and the combination of 120ppb AFB1 and Toxo-MX diet (Figures 1A and 1B). Relative mRNA expression of hepatic inflammation cytokines of 21-day-old broilers were shown in Figure 1C, TGFβ-1, TLR-4, and IL-1β were significantly increased in the liver of broilers fed AFB1 contaminated diet (P < 0.05), however, a relative decreased of IFN-γ expression were showed in the birds fed AFB1 contaminated diet (P < 0.05), and these changes were reversed when supplemented with Toxo-MX (P < 0.05). Moreover, hepatic antioxidant enzyme including GSH-Px and SOD were significantly increased in the broilers exposure to AFB1 contamination in 21-day-age (Figure 1D, P < 0.05), Toxo-MX supplementation partly decreased the antioxidant enzyme increasing induced by AFB1 exposure (P < 0.05), but still higher than control group in the 120 ppb AFB1 contamination group (P < 0.05).
Figure 1.
The mRNA expression of hepatic inflammation and antioxidant enzyme genes of broilers fed dietary contaminated AFB1 with or without adsorbent. (A) Sliced hepatic tissue of 21-day-old broilers from each group was stained by hematoxylin–eosin (H&E) stain and was observed under a microscope (Keyence, Tokyo, Japan) with 200 × original magnification. (B) Sliced hepatic tissue of 42-day-old broilers. (C) Hepatic tissue mRNA expression of IFN-γ, IL-6, TGF-β1, TLR-4, TNF-α, IL-1β in 21-day-old broilers. (D) Hepatic GSH-Px and SOD levels in 21-day-old broilers. (E) Hepatic tissue mRNA expression of IFN-γ, IL-6, TGF-β1, TLR-4, TNF-α, IL-1β in 42-day-old broilers. (F) Hepatic GSH-Px and SOD levels in 42-day-old broilers. The data represent mean ± SD of 8 replicates, and different letters in the same column indicate significant differences (P < 0.05).
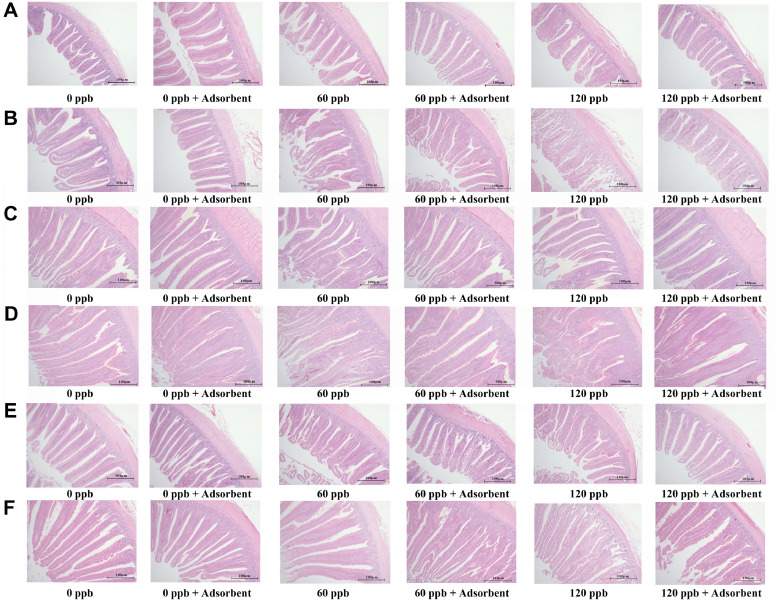
Figure 2.
Representative histopathology section of intestines. (A) Sliced duodenal tissue of 21-day-old broilers from each group was stained by H&E staining and was observed under a microscope (Keyence, Tokyo, Japan) with 400 × original magnification. (B) Sliced duodenal tissue of 42-day-old broilers. (C) Sliced jejunal tissue of 21-day-old broilers. (D) Sliced jejunal tissue of 42-day-old broilers. (E) Sliced ileal tissue of 21-day-old broilers. (F) Sliced ileal tissue of 42-day-old broilers.
In the 42-day-old broilers, hepatic IL-6, TGFβ-1, TLR-4, TNF-α, and IL-1β mRNA expression were significantly reduced in the AFB1 treatment groups (Figure 1E, P < 0.05), and these changes have not reversed in AFB1 plus Toxo-MX diet compared to control group (P < 0.05). Furthermore, hepatic GSH-Px and SOD were higher in both age broilers fed AFB1 contaminated diet (Figure 1F, P < 0.05), Toxo-MX addition reduced the accelerated GSH-Px and SOD but still higher than control group (P < 0.05).
Intestinal Villus Height and Crypt Depth
AFB1 intake could directly contact with the intestinal villus of broilers and impaired their immune defense and absorption function. As shown in Figure 2 and Table 8, in the 21-day-old broilers with 60 ppb and 120 ppb AFB1 exposure, jejunal and ileal villus height were significantly decreased (P < 0.05), and jejunal crypt depth increased (P < 0.05), which resulted in a significant lower VH:CD ratio (P < 0.05). Toxo-MX supplementation reversed the adverse effect in 21-day-old broilers fed AFB1 contamination diet (P < 0.05). Aflatoxicosis at intestinal barrier remained in 42-day-old broilers, the data showed a significant shorter villus height in the jejunum and ileum (P < 0.05), and a significant less VH:CD ratio (P < 0.05), Toxo-MX also recovered the decreased villus height in 42-day-old broilers (P < 0.05). Interestingly, villus height and crypt depth of duodenum has no significant difference among all the treatments in both age broilers (P > 0.05).
Table 8.
Intestinal villus height and crypt depth of broilers fed dietary contaminated aflatoxin B1 (AFB1) with or without Toxo-MX.
| Item | AFB1 |
0 ppb AFB1 |
60 ppb AFB1 |
120 ppb AFB1 |
SEM | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Toxo-MX | - | + | - | + | - | + | |||||
| 21 d | Duodenum | VH | 311.38 | 304.40 | 307.75 | 315.37 | 313.83 | 306.68 | 27.46 | 0.559 | |
| CD | 46.79 | 48.48 | 44.38 | 47.99 | 47.45 | 44.46 | 3.40 | 0.302 | |||
| VH:CD | 6.65 | 6.28 | 6.93 | 6.57 | 6.61 | 6.90 | 0.27 | 0.219 | |||
| Jejunum | VH | 692.94a | 688.87a | 561.83b | 685.04a | 574.09b | 660.97a | 37.40 | <0.001 | ||
| CD | 76.12b | 73.44b | 91.27a | 75.50b | 102.85a | 76.07b | 15.37 | 0.007 | |||
| VH:CD | 9.10a | 9.38a | 6.16b | 9.07a | 5.58b | 8.69ab | 1.43 | <0.001 | |||
| Ileum | VH | 268.69a | 271.26a | 188.17b | 258.07a | 183.11b | 264.36a | 22.98 | 0.023 | ||
| CD | 33.33 | 31.84 | 34.85 | 32.74 | 38.52 | 36.66 | 3.39 | 0.124 | |||
| VH:CD | 8.06a | 8.52a | 5.40c | 7.88a | 4.75c | 7.21ab | 1.19 | 0.008 | |||
| 42 d | Duodenum | VH | 336.79 | 329.00 | 315.72 | 321.41 | 315.04 | 336.11 | 26.96 | 0.755 | |
| CD | 48.45 | 47.08 | 49.38 | 51.09 | 51.07 | 49.97 | 5.33 | 0.347 | |||
| VH:CD | 6.95 | 6.99 | 6.39 | 6.29 | 6.17 | 6.73 | 0.80 | 0.819 | |||
| Jejunum | VH | 779.32a | 756.22a | 556.83b | 713.65a | 495.47b | 727.29a | 41.29 | <0.001 | ||
| CD | 79.30b | 78.57b | 98.57a | 75.33b | 99.60a | 79.54b | 12.01 | <0.001 | |||
| VH:CD | 9.83a | 9.62a | 5.65b | 9.47a | 4.97b | 9.14a | 1.46 | <0.001 | |||
| Ileum | VH | 274.88a | 295.44a | 250.97ab | 269.28a | 213.46b | 275.50a | 33.24 | 0.008 | ||
| CD | 44.70 | 47.38 | 49.04 | 46.64 | 48.42 | 47.65 | 4.38 | 0.217 | |||
| VH:CD | 6.15a | 6.24a | 5.12b | 5.77ab | 4.41c | 5.78ab | 1.21 | 0.014 | |||
The data represent mean of 8 replicates, and different letters (abc) in the same column indicate significant differences (P < 0.05).
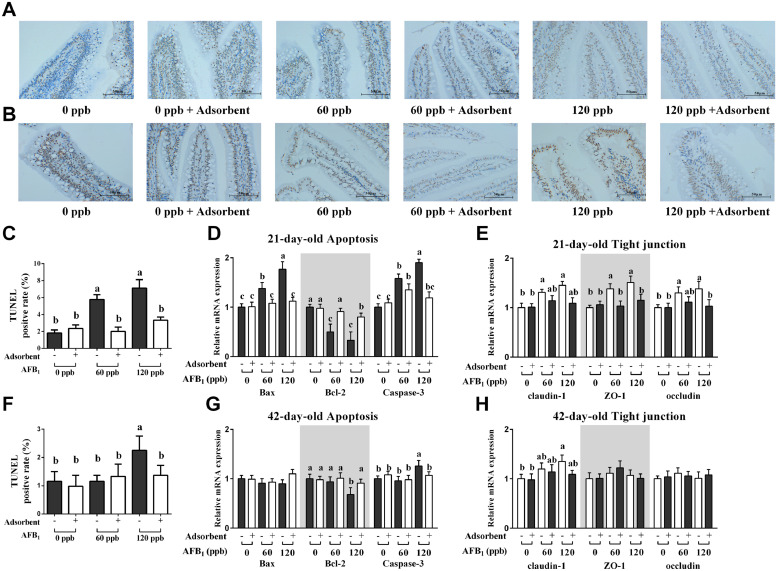
Jejunal TUNEL Assay, mRNA Expression of Epithelial Cells Apoptosis, and Tight Junction Protein Genes
Jejunal TUNEL assay were presented in Figure 3A, and TUNEL positive rate were normalized by the nuclei per area quantitated TUNEL signal of control group (Figures 3C and 3F). As shown in Figure 3C, TUNEL positive rate were significant higher in 21-day-old broilers fed both 60 ppb and 120 ppb AFB1 contaminated diet (P < 0.05), and diet Toxo-MX addition significantly reversed the increasing (P < 0.05). Bcl-2 mRNA expression of jejunal epithelial cells was significantly reduced, and Bax, caspase-3 significantly increased in 21-d broilers with both 60 ppb and 120 ppb AFB1 exposure (Figure 3D, P < 0.05). Twenty one-day-old broilers with Toxo-MX supplementation significantly restored the overexpression of Bax and caspase-3 (P < 0.05), the decreased mRNA expression of Bcl-2 recovered in 60 ppb AFB1 plus Toxo-MX (P < 0.05), but had not completely recovered in 120 ppb AFB1 plus Toxo-MX (P < 0.05). Tight junction protein of claudin-1, ZO-1 and occluding were increased in 21-day-old broilers fed both 60 ppb and 120 ppb AFB1 contaminated diet (Figure 3E, P < 0.05), tight junction protein of AFB1 plus Toxo-MX groups have no significant differences when compared with control group (P > 0.05).
Figure 3.
Jejunal TUNEL assay and mRNA expression of apoptosis and tight junction protein genes. (A) Sliced jejunal tissue of 21-day-old broilers from each group was labeled by TUNEL signal and was observed under a microscope (Keyence, Tokyo, Japan) with 400 × original magnification. (B) Sliced jejunal tissue of 42-day-old broilers. (C) TUNEL positive of jejunal tissue in 21-day-old broilers. (D) Jejunal tissue mRNA expression of Bax, Bcl-2, Casepase-3 in 21-day-old broilers. (E) Jejunal tissue mRNA expression of claudin-1, ZO-1, and occludin in 21-day-old broilers. (F) TUNEL positive of jejunal tissue in 42-day-old broilers. (D) Jejunal tissue mRNA expression of Bax, Bcl-2, Casepase-3 in 42-day-old broilers. (E) Jejunal tissue mRNA expression of claudin-1, ZO-1 and occludin in 42-day-old broilers. The data represent mean ± SD of 8 replicates, and different letters in the same column indicate significant differences (P < 0.05).
In 42-day-old broilers, jejunal TUNEL positive rate were only higher in 120 ppb AFB1 treatment group compared to control group (Figure 3F, P < 0.05), and only in 120ppb exposure group showed a lower Bcl-2 and higher caspase-3 mRNA expression mRNA expression when compared to control group (Figure 3G, P < 0.05), moreover, in tight junction protein mRNA expression, the claudin-1 was still higher in 120 ppb AFB1 exposure group (Figure 3H, P < 0.05), but ZO-1 and occluding have no significant difference among all the groups (P > 0.05). And all these changes induced by 120 ppb AFB1 were recovered with Toxo-MX supplementation (P < 0.05).
DISCUSSION
The toxic effect of AFB1 in poultry varies with dose, length of exposure, age, species, and diet nutritional statues. Generally, young broilers are more susceptible than older ones to the toxic effect (Wang et al., 2018). In the present study, 21-day-old broilers fed both 60 ppb and 120 ppb AFB1 contaminated diet showed a less BW and feed efficiency, however, 42-day-old broilers only showed less feed efficiency but had no numerical difference in BW. AFB1 induced significant changes in serum ALP, ALT, AST concentration, and these changes in serum biochemical parameters became less severe with the age increasing of broilers. According to previous study (Hsieh, 1994), broilers prolonged intake of low concentration AFB1 seemed to occur adaptive responses (Dersjant-Li et al., 2003; Yunus et al., 2011), in diet with a constant concentration of AFB1 contamination, the absorption of AFB1 equaled to rate of elimination from the body, growth performance may numerically showed no significant differences, particularly in mature animals. Thus, the less effect on growth performance of older broilers to aflatoxicosis may due to the immunity system development and the adaptive responses to long-term chronic exposure.
Previous study has showed aflatoxin is rapidly absorbed from the small intestine into the mesenteric venous blood, and accumulated in the liver of broilers, where the AFB1 and its metabolites bind with nucleic acid and proteins. Kidney has taken part in detoxification and is also among the organs where most of the aflatoxin residues are detected (Sawhney et al., 1973). Eliminated of aflatoxin appears slowly and primarily with urine and bile (Mabee et al., 1973; Sawhney et al., 1973). In this study, serum UA, hepatic SOD, and GSH-Px activity were significantly higher in both age broilers exposure to AFB1 contaminated diet. Consistent with present study, hepatic SOD, GSH-Px increased in broilers exposure to AFB1 had also been reported (Yang et al., 2012), and the increasing were not dose dependent, but related to hepatic protein synthesis function of broilers. Under the stimulation of low AFB1 levels, broilers met the requirement of detoxification by upregulate hepatic antioxidant enzymes, however, when large amount AFB1 accumulated in hepatic and inhibited protein synthesis or impaired hepatocytes, which turned into antioxidant enzymes expression impairment. Thus the detoxification responses in liver may vary with length of exposure and dose of AFB1 (Yang et al., 2012; Bhatti et al., 2021). Toxo-MX supplementation in this study had not completely reversed the negative effect in liver and serum parameters, especially to the increased GSH-Px and SOD. This may because of the rapid absorption rate of AFB1, while adsorbent showed less efficient binding rate with increasing AFB1 concentration, or the binding of high concentration AFB1 is not sufficient, the unbound AFB1 were absorbed into circulation, thus broilers presented the detoxification response with higher serum UA and hepatic SOD, GSH-Px, and may resulted in growth performance depletion under high concentration AFB1 exposure.
Thymus and bursa of Fabricius of broilers is the central immune organ, spleen is where immune responses toward systemically administered antigens are located. In the present study, the relative weight of thymus and bursa of Fabricius significantly decreased in broilers fed with 120 ppb of AFB1 contaminated diet at the age of 21 d but have no significant differences in 42-day-old broilers, indicated that aflatoxicosis may retard the immune organ development in young broilers. Moreover, It is reported that AFB1 exposure has detrimental effect on T and B lymphocytes proliferation and diversification (Chen et al., 2013; Rajput et al., 2017), and therefore, inhibit antibody production against aflatoxicosis (Qureshi et al., 1998; Ortatatli et al., 2005; Tessari et al., 2006). Furthermore, The chronic aflatoxicosis reduced protein utilization and synthesis which also link to serum globulin production (Kubena et al., 1993; Ledoux et al., 1999), the impaired protein synthesis function could be observed in serum ALB and GLB levels before major symptom occurred (Ortatatli et al., 2005). Our study showed a significantly decreased in serum ALB concentration of broilers exposure to AFB1, furthermore, serum IgG and IgA also reduced in 21-day-old broilers exposure to both concentration of AFB1, and the decreasing serum IgG were remain in 42-day-old birds' exposure to 120 ppb AFB1. This reduction in serum immunoglobulin was consistent with the other reports (Qureshi et al., 1998; Ortatatli et al., 2005; Chen et al., 2014). In addition, leukocyte infiltration was observed in the livers of both age broilers fed AFB1 contaminated diet and 120 ppb AFB1 with Toxo-MX diet, inflammation cytokines mRNA expressions, such as, TGFβ-1, TLR-4, and IL-1β were increased in 21-day-old broilers, however, decreased in 42-day-old broilers. The hepatic lesions results consistent with previous study that administration 50 μg/kg AFB1 contaminated diet to broilers (Magnoli et al., 2011), another study administrated a hydrated sodium calcium aluminosilicate (HSCAS) reported that bioavailability of aflatoxin and its residues decreased in liver, but not prevented hepatic lesions associated with aflatoxicosis (Neeff et al., 2013). These data indicated that dietary adsorbent supplementation could not reverse the hepatic and serum inflammation induced by AFB1. Hepatic immunity defense may firstly increase to against chronic aflatoxicosis and finally inhibit during long-term exposure, suggested broilers may be increasingly susceptible to disease because of suppression of immunity after long-term chronic aflatoxicosis.
Intestinal mRNA expression of tight junction protein increase is a response of recovery of impaired tight junction protein (Chen et al., 2016). Tight junction proteins are the crucial constituent for gut barrier function, therefore, any damage to tight junction proteins may resulted in increase of permeability and gut inflammation during AFB1 contamination (González-Mariscal et al., 2003; Saleemi et al., 2020). This study showed an increasing of tight junction protein mRNA expression in the broilers fed AFB1 contamination diet. In addition, aflatoxicosis induced elevation of the apoptotic gene expression in intestinal epithelial cells of 21-day-old broilers, although the elevated expression of apoptotic genes reduced with the age of broiler increasing, but still resulted in a lower villus height to crypt depth rate, suggested that aflatoxicosis impaired digestion ability of intestinal is not reversible with broiler age increasing.
The histological changes of small intestine in broilers associated with the increasing of endogenous nitrogen loss, and decreased energy and amino acid digestibility (Denli et al., 2009; Chen et al., 2016; Liu et al., 2018). Increased gut permeability may also facilitate the absorption of other antigens through the paracellular route that are normally could not be absorbed, leading to synergistic diseases outbreak (Girgis et al., 2010; Antonissen et al., 2014). These data explained that broilers exposure to AFB1 presented higher F:G ratio compared with control group in present study, indicated Toxo-MX supplementation may as economical solution for aflatoxicosis problem, making poultry production more cost-effective. Furthermore, Long term exposure to AFB1 suppressed hepatic inflammatory cytokines expression in 42-day-old broiler, which may also link to intestinal impairment and increased the disease-associated antigens intake. Although Toxo-MX supplementation significantly recovered the villus height and crypt depth and decreased the overexpression of apoptotic gene in both age broilers, however, showed less effect on hepatic inflammation. Another study reported a clay adsorbent against AFB1 contamination on intestine of young ducklings also showed ameliorated on villus height and villus/crypt ratio of the duodenum and jejunum, but did not totally protect the ducklings from the toxic effects on serum IgG and IgM of AFB1 concentrations above 100 ppb (Wan et al., 2013). With molecular or nano-clay binders in turkey against AFB1 aflatoxicosis showed improved on intestinal goblet cell hyperplasia, villous atrophy and diffuse lymphocytic enteritis, but with concentration above 110 ppb of AFB1 suggested perivascular inflammatory insult and the sequela of fibroplasia (Lala et al., 2016). These accumulated data demonstrated that protection effect of adsorbents decreased with the concentration of AFB1 increased, and absorbents are commonly more effective against the aflatoxicosis in gastrointestinal tract, but incapable for the aflatoxins that entered into circulation.
In summary, the data demonstrated that diet supplementation with Toxo-MX reversed the detrimental effect on growth performance and F:G ratio in broilers exposure to 60 ppb and 120 ppb AFB1, protected intestinal tight junction and restored increased apoptotic gene expression. However, did not completely recovered hepatic inflammation induced by AFB1, particularly in 120 ppb group. Suggested that Toxo-MX prevented gastrointestinal aflatoxicosis under low concentration of AFB1 contamination, but had less effect on hepatic aflatoxicosis, particularly in young broilers.
ACKNOWLEDGMENTS
This research was supported by National key R&D Program of intergovernmental Key Projects of China (2018YFE0101700), Support project for Scientific and technical talents in Hunan Province (2020TJ-Qo2). Double first-class construction project of Hunan Agricultural University (SYL201802015)
Author contributions: Zehe Song and Xi He: Conceptualization, Methodology. Kun Xie: Data curation, Writing- Original draft preparation. Guili Hu: Formal analysis, Investigation. Haihan Zhang and Yuguang Chen: Validation. De-Xing Hou: Writing- Review & Editing. All authors approved the final version of the manuscript to be published.
DISCLOSURES
The authors declare no competing interests linked to this manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101634.
Appendix. Supplementary materials
REFERENCE
- Adebo O.A., Njobeh P.B., Mavumengwana V. Degradation and detoxification of AFB1 by Staphylocococcus warneri, Sporosarcina sp. and Lysinibacillus fusiformis. Food Control. 2016;68:92–96. [Google Scholar]
- Agriopoulou S., Stamatelopoulou E., Varzakas T. Advances in occurrence, importance, and mycotoxin control strategies: prevention and detoxification in foods. Foods. 2020;9:137. doi: 10.3390/foods9020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici M., Cecarini V., Pettinari A., Bonfili L., Angeletti M., Barocci S., Biagetti M., Fioretti E., Maria Eleuteri A. Binding of aflatoxins to the 20S proteasome: effects on enzyme functionality and implications for oxidative stress and apoptosis. Biol. Chem. 2007;388:107–117. doi: 10.1515/BC.2007.012. [DOI] [PubMed] [Google Scholar]
- Antonissen G., Martel A., Pasmans F., Ducatelle R., Verbrugghe E., Vandenbroucke V., Li S., Haesebrouck F., Van Immerseel F., Croubels S. The impact of Fusarium Mycotoxins on human and animal host susceptibility to infectious diseases. Toxins (Basel) 2014;6:430–452. doi: 10.3390/toxins6020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti S.A., Khan M.Z., Saleemi M.K., Hassan Z.U. Combating immunotoxicity of aflatoxin B1 by dietary carbon supplementation in broiler chickens. Environ. Sci. Pollut. Res. 2021;28:49089–49101. doi: 10.1007/s11356-021-14048-5. [DOI] [PubMed] [Google Scholar]
- Cai Y.T., McLaughlin M., Zhang K. Advancing the FDA/office of regulatory affairs mycotoxin program: new analytical method approaches to addressing needs and challenges. J. AOAC Int. 2020;103:705–709. doi: 10.1093/jaocint/qsz007. [DOI] [PubMed] [Google Scholar]
- Cao S., Wan S., Chen Z., Saleemi M.K., Wang N., Naseem M.N., Munawar J. Mycotoxins - a global one health concern: a review. Agrobiol. Rec. 2020;2:1–16. [Google Scholar]
- Chen K., Fang J., Peng X., Cui H., Chen J., Wang F., Chen Z., Zuo Z., Deng J., Lai W., Zhou Y. Effect of selenium supplementation on aflatoxin B1-induced histopathological lesions and apoptosis in bursa of Fabricius in broilers. Food Chem. Toxicol. 2014;74:91–97. doi: 10.1016/j.fct.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Chen X., Naehrer K., Applegate T.J. Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks. Poult. Sci. 2016;95:1312–1325. doi: 10.3382/ps/pew022. [DOI] [PubMed] [Google Scholar]
- Chen K., Yuan S., Chen J., Peng X., Wang F., Cui H., Fang J. Effects of sodium selenite on the decreased percentage of T cell subsets, contents of serum IL-2 and IFN-γ induced by aflatoxin B1 in broilers. Res. Vet. Sci. 2013;95:143–145. doi: 10.1016/j.rvsc.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Denli M., Blandon J.C., Guynot M.E., Salado S., Perez J.F. Effects of dietary AflaDetox on performance, serum biochemistry, histopathological changes, and aflatoxin residues in broilers exposed to aflatoxin B1. Poult. Sci. 2009;88:1444–1451. doi: 10.3382/ps.2008-00341. [DOI] [PubMed] [Google Scholar]
- Dersjant-Li Y., Verstegen M.W.A., Gerrits W.J.J. The impact of low concentrations of aflatoxin, deoxynivalenol or fumonisin in diets on growing pigs and poultry. Nutr. Res. Rev. 2003;16:223–239. doi: 10.1079/NRR200368. [DOI] [PubMed] [Google Scholar]
- Dohnal V., Wu Q., Kuča K. Metabolism of aflatoxins: key enzymes and interindividual as well as interspecies differences. Arch. Toxicol. 2014;88:1635–1644. doi: 10.1007/s00204-014-1312-9. [DOI] [PubMed] [Google Scholar]
- Girgis G.N., Barta J.R., Brash M., Smith T.K. Morphologic changes in the intestine of broiler breeder pullets fed diets naturally contaminated with Fusarium mycotoxins with or without coccidial challenge. Avian Dis. 2010;54:67–73. doi: 10.1637/8945-052809-Reg.1. [DOI] [PubMed] [Google Scholar]
- González-Mariscal L., Betanzos A., Nava P., Jaramillo B.E. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Gowda N.K.S., Ledoux D.R., Rottinghaus G.E., Bermudez A.J., Chen Y.C. Antioxidant efficacy of curcuminoids from turmeric (Curcuma longa L.) powder in broiler chickens fed diets containing aflatoxin B1. Br. J. Nutr. 2009;102:1629–1634. doi: 10.1017/S0007114509990869. [DOI] [PubMed] [Google Scholar]
- Hsieh D.P.H. Acad. Press; Washington, DC: 1994. Pharmacokinetics and excretion of aflatoxins. The Toxicology of Aflatoxins; pp. 73–88. [Google Scholar]
- Iqbal M., Pumford N.R., Tang Z.X., Lassiter K., Ojano-Dirain C., Wing T., Cooper M., Bottje W. Compromised liver mitochondrial function and complex activity in low feed efficient broilers are associated with higher oxidative stress and differential protein expression. Poult. Sci. 2005;84:933–941. doi: 10.1093/ps/84.6.933. [DOI] [PubMed] [Google Scholar]
- Jiang M., Peng X., Fang J., Cui H., Yu Z., Chen Z. Effects of aflatoxin B1 on t-cell subsets and mRNA expression of cytokines in the intestine of broilers. Int. J. Mol. Sci. 2015;16:6945–6959. doi: 10.3390/ijms16046945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubena L.F., Harvey R.B., Huff W.E., Elissalde M.H., Yersin A.G., Phillips T.D., Rottinghaus G.E. Efficacy of a hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and diacetoxyscirpenol. Poult. Sci. 1993;72:51–59. doi: 10.3382/ps.0720051. [DOI] [PubMed] [Google Scholar]
- Lala A.O., Ajayi O.L., Oso A.O., Ajao M.O., Oni O.O., Okwelum N., Idowu O.M.O. Effect of dietary supplementation with clay-based binders on biochemical and histopathological changes in organs of turkey fed with aflatoxin-contaminated diets. J. Anim. Physiol. Anim. Nutr. (Berl). 2016;100:1191–1202. doi: 10.1111/jpn.12421. [DOI] [PubMed] [Google Scholar]
- Ledoux D.R., Rottinghaus G.E., Bermudez A.J., Alonso-Debolt M. Efficacy of a hydrated sodium calcium aluminosilicate to ameliorate the toxic effects of aflatoxin in broiler chicks. Poult. Sci. 1999;78:204–210. doi: 10.1093/ps/78.2.204. [DOI] [PubMed] [Google Scholar]
- LI J.juan, SUO D.cheng, SU X.ou. Binding capacity for aflatoxin B1 by different adsorbents. Agric. Sci. China. 2010;9:449–456. [Google Scholar]
- Liu N., Wang J., Deng Q., Gu K., Wang J. Detoxification of aflatoxin B1 by lactic acid bacteria and hydrated sodium calcium aluminosilicate in broiler chickens. Livest. Sci. 2018;208:28–32. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma R., Zhang L., Liu M., Su Y.T., Xie W.M., Zhang N.Y., Dai J.F., Wang Y., Rajput S.A., Qi D.S., Karrow N.A., Sun L.H. Individual and combined occurrence of mycotoxins in feed ingredients and complete feeds in china. Toxins (Basel) 2018;10:113. doi: 10.3390/toxins10030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabee M.S., Chipley J.R., Applegate K.L. Preparation of labeled aflatoxin B1 from acetate-1, 2-14C. J. Labelled Compd. 1973;9:277–279. [Google Scholar]
- Magnoli A.P., Monge M.P., Miazzo R.D., Cavaglieri L.R., Magnoli C.E., Merkis C.I., Cristofolini A.L., Dalcero A.M., Chiacchiera S.M. Effect of low levels of aflatoxin b1 on performance, biochemical parameters, and aflatoxin b1 in broiler liver tissues in the presence of monensin and sodium bentonite. Poult. Sci. 2011;90:48–58. doi: 10.3382/ps.2010-00971. [DOI] [PubMed] [Google Scholar]
- Mitchell N.J., Bowers E., Hurburgh C., Wu F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. - Part A Chem. Anal. Control. Expo. Risk Assess. 2016;33:540–550. doi: 10.1080/19440049.2016.1138545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeff D.V., Ledoux D.R., Rottinghaus G.E., Bermudez A.J., Dakovic A., Murarolli R.A., Oliveira C.A.F. In vitro and in vivo efficacy of a hydrated sodium calcium aluminosilicate to bind and reduce aflatoxin residues in tissues of broiler chicks fed aflatoxin B1. Poult. Sci. 2013;92:131–137. doi: 10.3382/ps.2012-02510. [DOI] [PubMed] [Google Scholar]
- Nutrient Requirements of Poultry . The National Academies Press; Washington, DC: 1994. Ninth Revised Edition. [Google Scholar]
- Ortatatli M., Oǧuz H., Hatipoǧlu F., Karaman M. Evaluation of pathological changes in broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Res. Vet. Sci. 2005;78:61–68. doi: 10.1016/j.rvsc.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Pankaj S.K., Shi H., Keener K.M. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci. Technol. 2018;71:73–83. [Google Scholar]
- Qureshi M.A., Brake J., Hamilton P.B., Hagler W.M., Nesheim S. Dietary exposure of broiler breeders to aflatoxin results in immune dysfunction in progeny chicks. Poult. Sci. 1998;77:812–819. doi: 10.1093/ps/77.6.812. [DOI] [PubMed] [Google Scholar]
- Rajput S.A., Sun L., Zhang N., Khalil M.M., Gao X., Ling Z., Zhu L., Khan F.A., Zhang J., Qi D. Ameliorative effects of grape seed proanthocyanidin extract on growth performance, immune function, antioxidant capacity, biochemical constituents, liver histopathology and aflatoxin residues in broilers exposed to aflatoxin b1. Toxins (Basel) 2017;9:371. doi: 10.3390/toxins9110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal S., Kim J.E., Coulombe R. Aflatoxin B1 in poultry: toxicology, metabolism and prevention. Res. Vet. Sci. 2010;89:325–331. doi: 10.1016/j.rvsc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Rushing B.R., Selim M.I. Ameliorative effects of grape seed proanthocyanidin extract on growth performance, immune function, antioxidant capacity, biochemical constituents, liver histopathology and aflatoxin residues in broilers exposed to aflatoxin b1. Food Chem. Toxicol. 2019;124:81–100. [Google Scholar]
- Saleemi M.K., Ashraf K., Gul S.T., Naseem M.N., Sajid M.S., Mohsin M., He C., Zubair M., Khan A. Toxicopathological effects of feeding aflatoxins B1 in broilers and its ameliosration with indigenous mycotoxin binder. Ecotoxicol. Environ. Saf. 2020;187:109712. doi: 10.1016/j.ecoenv.2019.109712. [DOI] [PubMed] [Google Scholar]
- Sawhney D.S., Vadehra D.V., Baker R.C. The metabolism of 14C aflatoxins in laying hens. Poult. Sci. 1973;52:1302–1309. doi: 10.3382/ps.0521302. [DOI] [PubMed] [Google Scholar]
- Tessari E.N.C., Oliveira C.A.F., Cardoso A.L.S.P., Ledoux D.R., Rottinghaus G.E. Effects of aflatoxin B1 and fumonisin B1 on body weight, antibody titres and histology of broiler chicks. Br. Poult. Sci. 2006;47:357–364. doi: 10.1080/00071660600756071. [DOI] [PubMed] [Google Scholar]
- Towner R.A., Qian S.Y., Kadiiska M.B., Mason R.P. In vivo identification of aflatoxin-induced free radicals in rat bile. Free Radic. Biol. Med. 2003;35:1330–1340. doi: 10.1016/j.freeradbiomed.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Vila-Donat P., Marín S., Sanchis V., Ramos A.J. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018;114:246–259. doi: 10.1016/j.fct.2018.02.044. [DOI] [PubMed] [Google Scholar]
- Wan X.L., Yang Z.B., Yang W.R., Jiang S.Z., Zhang G.G., Johnston S.L., Chi F. Toxicity of increasing aflatoxin B1 concentrations from contaminated corn with or without clay adsorbent supplementation in ducklings. Poult. Sci. 2013;92:1244–1253. doi: 10.3382/ps.2012-02748. [DOI] [PubMed] [Google Scholar]
- Wang H., Li W., Muhammad I., Sun X., Cui X., Cheng P., Qayum A., Zhang X. Biochemical basis for the age-related sensitivity of broilers to aflatoxin B1. Toxicol. Mech. Methods. 2018;28:361–368. doi: 10.1080/15376516.2018.1428258. [DOI] [PubMed] [Google Scholar]
- Wang X.H., Li W., Wang X.H., Han M.Y., Muhammad I., Zhang X.Y., Sun X.Q., Cui X.X. Water-soluble substances of wheat: a potential preventer of aflatoxin B1-induced liver damage in broilers. Poult. Sci. 2019;98:136–149. doi: 10.3382/ps/pey358. [DOI] [PubMed] [Google Scholar]
- Wu F. Global impacts of aflatoxin in maize: trade and human health. World Mycotoxin J. 2015;8:137–142. [Google Scholar]
- Wu L., Li J., Li Y., Li T., He Q., Tang Y., Liu H., Su Y., Yin Y., Liao P. Aflatoxin B1, zearalenone and deoxynivalenol in feed ingredients and complete feed from different Province in China. J. Anim. Sci. Biotechnol. 2016;7:63. doi: 10.1186/s40104-016-0122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Bai F., Zhang K., Bai S., Peng X., Ding X., Li Y., Zhang J., Zhao L. Effects of feeding corn naturally contaminated with aflatoxin B1 and B2 on hepatic functions of broilers. Poult. Sci. 2012;91:2792–2801. doi: 10.3382/ps.2012-02544. [DOI] [PubMed] [Google Scholar]
- Yarru L.P., Settivari R.S., Gowda N.K.S., Antoniou E., Ledoux D.R., Rottinghaus G.E. Effects of turmeric (curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poult. Sci. 2009;88:2620–2627. doi: 10.3382/ps.2009-00204. [DOI] [PubMed] [Google Scholar]
- Yunus A.W., Awad W.A., Kröger S., Zentek J., Böhm J. In vitro aflatoxin B1 exposure decreases response to carbamylcholine in the jejunal epithelium of broilers. Poult. Sci. 2010;89:1372–1378. doi: 10.3382/ps.2009-00617. [DOI] [PubMed] [Google Scholar]
- Yunus A.W., Ghareeb K., Abd-El-Fattah A.A.M., Twaruzek M., Böhm J. Gross intestinal adaptations in relation to broiler performance during chronic aflatoxin exposure. Poult. Sci. 2011;90:1683–1689. doi: 10.3382/ps.2011-01448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.