Graphical abstract
Keywords: Ultrasound/chlorine process, Synergy, Reactive chlorine species (RCS), Mineral anions, Natural organic matter
Highlights
-
•
Ultrasound/chlorine combination produced synergistic effect toward Allura Red AC degradation.
-
•
Mineral anions and humic acid decreased the degradation efficiency of the sono-hybrid process.
-
•
Ultrasound/chlorine process is efficient even in real natural mineral water.
Abstract
In this work, after exploring the first report on the synergism of combining ultrasound (US: 600 kHz) and chlorine toward the degradation of Allura Red AC (ARAC) textile dye, as a contaminant model, the impact of various mineral water constituents (Cl−, SO42−, NO3−, HCO3− and NO2−) and natural organic matter, i.e., humic acid (HA), on the performance of the US/chlorine sono-hybrid process was assessed for the first time. Additionally, the process effectiveness was evaluated in a real natural mineral water (NMW) of a known composition. Firstly, it was found that the combination of ultrasound and chlorine (0.25 mM) at pH 5.5 in cylindrical standing wave ultrasonic reactor (f = 600 kHz and Pe = 120 W, equivalent to PA ∼ 2.3 atm) enhanced in a drastic manner the degradation rate of ARAC; the removal rate being 320% much higher than the arithmetic sum of the two separated processes. The source of the synergistic effect was attributed to the effective implication of reactive chlorine species (RCS: Cl•, ClO• and Cl2•−) in the degradation process. Radical probe technique using nitrobenzene (NB) as a specific quencher of the acoustically generated hydroxyl radical confirmed the dominant implication of RCS in the overall degradation rate of ARAC by US/chlorine system. Overall, the presence of humic acid and mineral anions decreased the efficiency of the sono-hybrid process; however, the inhibition degrees depend on the type and the concentration of the selected additives. The reaction of these additives with the generated RCS is presumably the reason for the finding results. The inhibiting effect of Cl−, SO42−, NO3− and NO2− was more pronounced in US/chlorine process as compared to US alone, whereas the inverse scenario was remarked for the effect of HA. These outcomes were associated to the difference in the reactivity of HA and mineral anions toward RCS and •OH oxidizing species, in addition to the more selective character of RCS than hydroxyl radical. The displacement of the reaction zone with increasing the additive concentration may also be another influencing factor that favors competition reactions, which subsequently reduce the available reactive species in the reacting medium. The NMW exerted reductions of 43% and 10% in the process efficiency at pH 5.5 and 8, respectively, thereby confirming the RCS-quenching mechanism by the water matrix constituents. Hence, this work provided a precise understanding of the overall mechanism of chlorine activation by ultrasound to promote organic compounds degradation in water.
1. Introduction
Azo dyes are broadly used in dying, weaving, tanning and paper industries. This class of dyes represent over 70% of the total production of synthetic dyes [1]. They are aromatic compounds with large structural diversity that always provide a high degree of photolytic, biological and chemical stability; in simply words, they resist to degradation [2]. A numerous of these substances are toxic, carcinogens and mutagenic, making them potential risk for human health and aquatic environments [3], [4]. Accordingly, industrial wastewaters containing azo dyes cannot be treated with conventional methods and, consequently, an exigent challenge is devoted in evolving more appropriate technologies for the complete removal of these persistent chemicals before discharging their effluents in environmental water basins.
Advanced oxidation processes (AOPs) based on hydroxyl or sulfate radicals have often gained recognition as a promising approach towards removing recalcitrant textile dyes from aqueous effluent in water [5]. UV/H2O2 (or S2O82−), Fe(II)/H2O2 (or S2O82−), UV/O3, photocatalysis, sonolysis and other innovative AOPs like UV/IO4− and H2O2/IO4− have shown efficient degradation of textile dyes, even in very complex matrices [6], [7], [8], [9], [10], [11]. Thanks to the high oxidation potentials of •OH and SO4•− (E0 = 2.8 and 2.6 V/SHE, respectively), the degradation of micropollutants may be forced for up to mineralization [5].
The basic event of sonication in aqueous solution is the acoustic cavitation phenomenon, which is defined as the formation, growth and collapse of short-lived tiny bubbles in water [12]. The quasi-adiabatic collapse of these bubbles compresses intensively the gas and water vapor trapped therein up to achieving dramatic thermodynamic conditions, i.e. temperature and pressure of the order of thousand kelvin and hundred atmospheres [13]. Hydroxyl and hydrogen radicals have been then generated from the homolytic cleavage of water vapor molecules (H2O → •OH + H•), and with other gases present, i.e. O2 and air, further reactive species like HO2• and atomic oxygen (O) can be formed during the radical-driven chain reactions inside the cavity at the collapse [14], [15], [16], [17]. Of all, •OH radical is the most recognized oxidizing agent, as identified by EPR-spin trapping [18], [19], chemical dosimetries [20], [21] and chemical probe-techniques [22], [23]. •OH can drive oxidation reaction in the gas phase for volatile substrates, at the bubble/solution interface for hydrophobic compounds and in the bulk solution for hydrophilic substances [24]. However, the highest concentration of these transient species is located at the bubble/solution interface, and only about 10% of this quantity can achieve the bulk of the solution [25].
Chlorine is generally the most used chemical agent for drinking water disinfection [26]. Despite its low activity on microorganisms in biofilms, chlorine can lead to a significant removal of the majority of planktonic bacteria [26]. Lately, ultraviolet (UV) combined with chlorine (i.e., the UV/chlorine process) is being one promising AOP for the degradation of persistent micropollutants [27], [28]. The synergy resulted from applying UV/chlorine process for the degradation of diverse organic pollutants was attributed to the implication of •OH and reactive chlorine species RCS (Cl•, ClO• and Cl2•–) in the degradation process [29], [30], [31], [32], [33]. Primarily •OH and chlorine (Cl•) radicals and secondary ClO• and Cl2•− are formed in the system through UV photolysis of HClO/ClO− and their subsequent chain-reaction [28]. A similar reaction mechanism has also been reported for the catalytic activation of chlorine with iron (II and III), which has been successfully applied for fastly degrading of textile dyes [34], [35]. Redox potentials of RCS are of 2.43 V/SHE for Cl•, 2.13 V/SHE for Cl2•− and 1.5–1.8 V/SHE for ClO•, and they react with organic matter with second order rate constants varies from ∼ 108 –1011 M−1s−1 for Cl• to ∼ 107 –109 M−1s−1 for ClO• and 102 –106 M−1s−1 for Cl2•− [28], [36]. In such multiple-free radical systems, •OH and RCS work together to facilitated the degradation of micropollutants. While •OH is non-selective oxidant, RCS are selective and preferentially react with contaminants containing electron rich moieties [37], [38], with similar reaction mechanisms as for •OH (i.e. electron transfer, H-atom abstraction and addition in unsaturated band). In UV/chlorine system, RCS were found to be the key species implicated in the destruction of trimethoprim [39] and benzoic acid [40], whereas •OH dominates for the degradation of ronidazole [41] and ibuprofen [42].
The present work will primarily provide the first report on chlorine activation by high frequency ultrasound (US: 600 kHz) at near neutral pH. The US/chlorine process has been applied for the degradation of Allura Red AC (ARAC) synthetic dye, as a contaminant model. ARAC is a very persistent textile dye of established carcinogenic and toxic effects [43], [44], [45] and, therefore, any presence of this compound could has a detrimental effect in aquatic life. After exploring the synergism of combining US and chlorine toward the degradation of ARAC, the impact of various mineral water constituents (Cl−, SO42−, NO3−, HCO3− and NO2−) and natural organic matter, i.e., humic acid (HA), on the performance of the US/chlorine sono-hybrid process has been assessed for the first time. Additionally, degradation experiments have been conducted in a natural mineral water to check the limit of applicability of this new sono-hybrid process for water treatment.
2. Materials and methods
Throughout the study, ultrapure water was used for solutions and samples preparation. Sodium hypochlorite solution (∼16% available active chlorine basis) and Allura Red AC (abbreviation: ARAC; CAS number: 25956–17-6; chemical formula: C18H14N2Na2O8S2; molecular weight: 496,42 g mol−1) were supplied by Sigma-Aldrich. The molecular structure of ARAC is given in Fig. S1 (supplementary data). All other reagents (NaOH, H2SO4, KI, ((NH4)6Mo7⋅4H2O, nitrobenzene, humic acid, Na2SO4, NaCl, NaHCO3, NaNO3 and NaNO2) were commercial products of the purest grade available (Sigma-Aldrich).
Sonolytic runs were conducted using 150 mL of open to air-solution in the cylindrical water-jacketed glass reactor presented in Fig. S2 (supplementary data). The sonication system operates at a single frequency (600 kHz) and electric power (120 W) and emits irradiation waves in continuous mode from a piezoelectric disc (diameter of 4 cm) pasted on stainless steel plate (diameter 5 cm) fixed at the bottom of the reactor. The temperature of the irradiating liquid was controlled through the cooling jacket and displayed by a thermocouple (Hanna Instruments) immersed in the solution. The acoustic energy dissipated in the solution (∼23 W, corresponding to an acoustic intensity In = 1.83 W/cm2) was estimated by the calorimetric method.
Stock solutions of chlorine (100 mM, pH 5) and ARAC (500 mg L−1, pH ∼ 5.5) were prepared and stored in the dark at 4 °C. Experiments were carried out under different conditions at pH 5.5 and ambient temperature (25 ± 1 °C). The pH of the solution was adjusted using NaOH or H2SO4 (0.1 M). Quantitative analysis of the dye concentration was performed using a Biochrom WPA Lightwave II UV–vis spectrophotometer at λmax = 504 nm. Hydrogen peroxide concentrations were quantified according to the iodometric method. To ensure reproducibility of the results, all runs were performed in triplicate and results were presented as averages. Error bars, ported in relevant data, represent the deviation of means.
In tests of chlorination alone (without sonication), runs were conducted in a cylindrical water-jacketed glass cell of 200 mL under a fixed magnetic stirring (300 rpm). When US is applied, no mechanical stirring is applied; the stirring is efficiently ensured by sonication itself. Sonication through the acoustic cavitation is known to create an efficient micro-stirring of the reaction system (please see [46], [47], for example, where the advancing role of stirring by ultrasound is shown on the extraction of arsenic(V) and copper (II) ions for the aqueous solution by an ELM ‘Emulsion Liquid Membrane’ system).
3. Results and discussion
3.1. Characteristics of ARAC chlorination
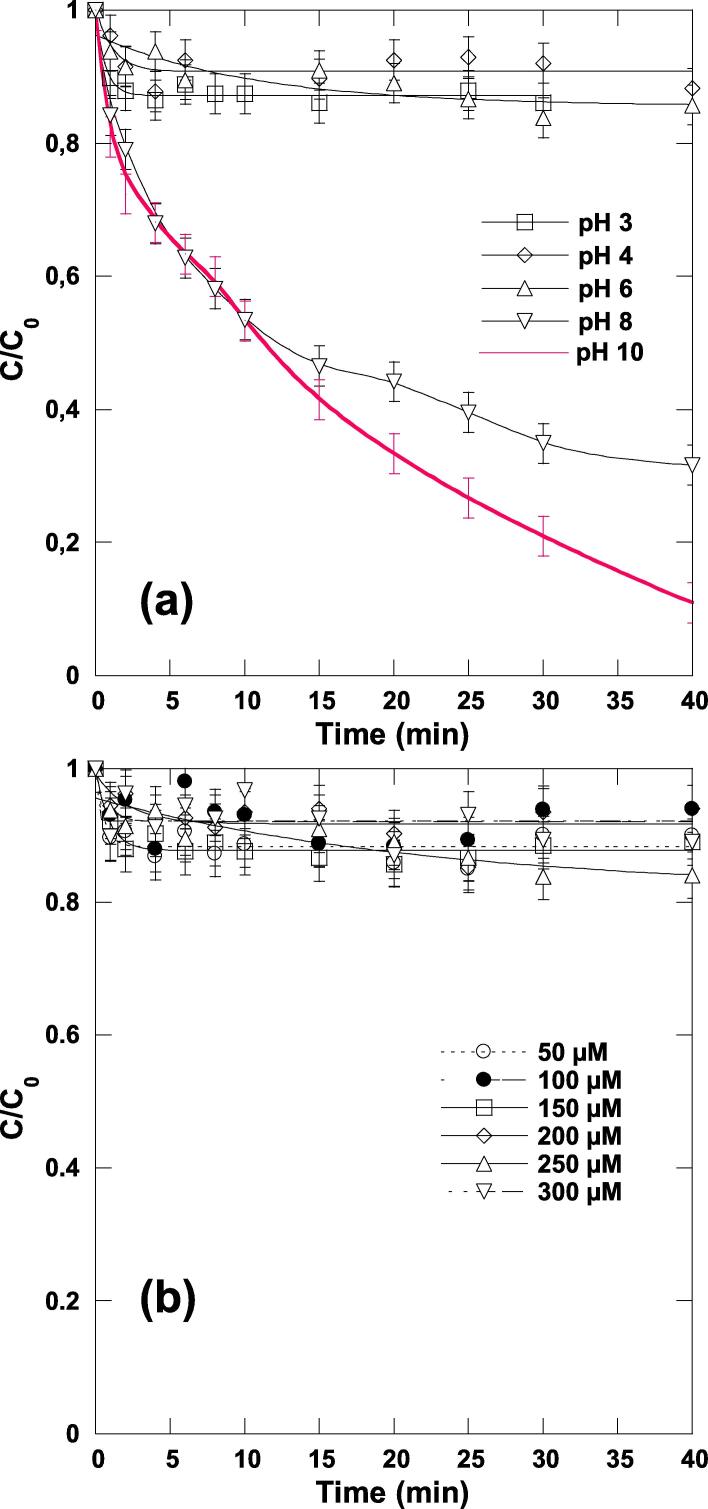
The effect of pH in the range of 3 to 10 and chlorine dosage (0.05–0.3 mM) on direct chlorination of ARAC (C0 = 5 mg L−1) at 25 °C is shown in Fig. 1(a) and (b). In all these runs, magnetic agitation is performed at 300 rpm. After a long contact time, i.e., 40 min, low removals of ∼ 10–15% were obtained at pH 3–6, whereas a relatively faster rate was observed at pH 8 and 10, where ∼ 69% and 90% of ARAC was eliminated after 40 min of treatment, respectively (Fig. 1(a)).
Fig. 1.
ARAC chlorination kinetics for different solution pH (a) and diverse chlorine dosages (b) (conditions: C0 = 5 mg L−1 (10 µM), [chlorine]0 = 0.25 mM for (a) and 0.05–0.3 mM for (b), pH 3–10 for (a) and pH 5.5 for (b), V = 150 mL, temperature: 25 ± 1 °C, stirring speed: 300 rpm).
In aqueous solution, the main free chlorine species are Cl2, HOCl and OCl− [28], [36]. At 25 °C, the calculated distribution of chlorine species vs. solution pH is shown in Fig. S3 for a chlorine concentration of 0.5 mM. Cl2 is only present at low pH values (pH < 3). HOCl is the predominant free chlorine species at pH < 7.5, and ClO− at pH > 7.5. >98% of free chlorine is present as HOCl in the pH range of 2.5–6, and as ClO− at pH > 9. At pH 8, chlorine exists as mixture of 26% HOCl and 74% of OCl−. Therefore, under typical water treatment conditions in the pH range 6–9, hypochlorous acid and hypochlorite are the main chlorine species. It is important to note that in addition to these major chlorine species, other chlorine intermediates such as Cl3− and Cl2O, can also be formed [36], but their concentrations are very low to be considered in chlorination processes [36]. In addition, given that the pKa value of ARAC is 11.4 [48], the dye could not change its structure between pH 3–8. Therefore, ARAC reacts with OCl− (pH 8 and 10), whereas the dye molecules showed a strong persistence toward the reaction with HOCl (pH 3–6), even at varying chlorine dosages as stated in Fig. 1(b).
As a recapitulation, at pH 8–10, the breakdown of ARAC by chlorination alone is ensured via its reaction with OCl− present in a large amount (Fig. S3 of the Supplementary Data) whereas, in the pH’s range 3–6, the reaction of ARAC with HOCl (present in a large quantity in this range of pH according to Fig. S3) is very slow even at varying chlorine dosages as stated in Fig. 1(b).
3.2. Degradation synergism upon US/chlorine sono-hybrid treatment
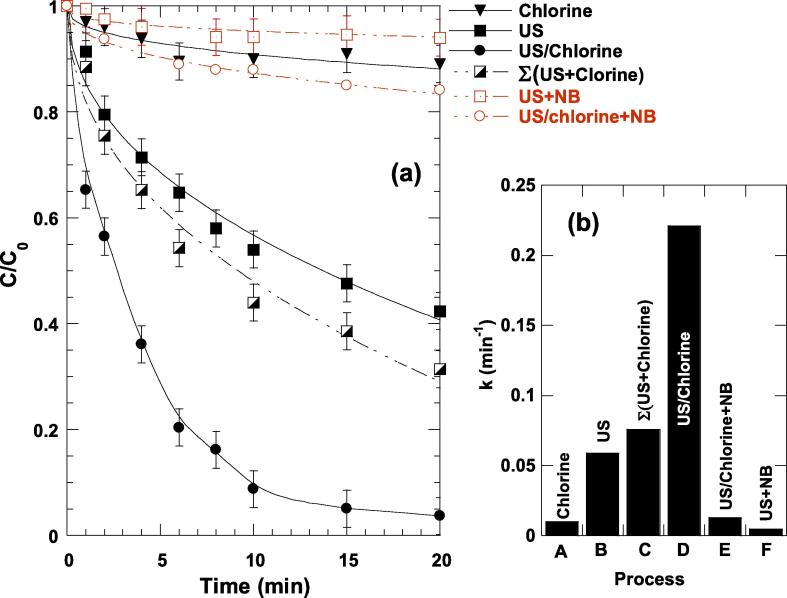
Degradation experiments were carried out at pH 5.5 for different reaction systems including chlorination, sonication (US: 600 kHz, 120 W) as well as US/chlorine combination for C0 = 5 mg L−1 (10 µM) and [chlorine]0 = 0.25 mM. As seen from Fig. 2(a), after 20 min of treatment, 96.2% of ARAC was eliminated by US/chlorine against 10% for chlorine sole, 57% for sonication alone and 68.5% for the sum of two separated processes, i.e., Σ(US + Chlorine). Besides, the dye concentration decays exponentially with time following a pseudo-first order kinetics, which can be described as -d[ARAC]/dt = k[ARAC]. Values of 0.01, 0.059 and 0.221 min−1 were recorded, respectively, for chlorination, sonication and US/chlorine combination (Fig. 2(b)), giving a strong synergy index SI equal to 3.2 at pH 5.5. The synergy index is calculated as SI = kUS+Chlorine/(kUS + kChlorine). Therefore, the sono-chlorination process ensures 320% increase in the degradation rate as compared to the sum of the two separated processes. This higher synergism was maintained for pH 4–6 where the sole chlorination did not affect significantly the degradation of the dye (Fig. 1(a)). At pH 8–10, ARAC chlorination happened at appreciable initial rates of 0.41 mg L−1 min−1 (Fig. 1(a)). Besides, Fig. S4(a) of the Supplementary Material showed that the sonolytic degradation of ARAC in basic medium (pH 8–10) is as higher as that ensured by the sole chlorination (0.35 mg L−1 min−1). Consequently, at pH 8 and 10, the effect of applying US/chlorine is additive as the synergistic index is equal to 1 (i.e., r0,US/Chlorine ∼ 0.8 mg L−1 min−1, r0,US ∼ 0.35 mg L−1 min−1, r0,Cl ∼ 0.41 mg L−1 min−1). Therefore, the synergism of applying US/Chlorine treatment was could be only obtained at pH 4–6 where HOCl is the sole chlorine species (Fig. S3).
Fig. 2.
ARAC degradation kinetics (a), and corresponding pseudo-first order rate constant (b), obtained under different oxidation systems, i.e., chlorine, ultrasound (US), US/chlorine, US/NB, and US/chlorine/NB (conditions: frequency: 600 kHz, power: 120 W, C0 = 5 mg L−1 (10 µM), [chlorine]0 = 0.25 mM, [NB]0 = 1 mM, 25 ± 1 °C, pH 5.5). NB: nitrobenzene.
For the purpose of process improvement, the ARAC degradation by the US/chlorine system has been evaluated through assisting the sonicated system by a mechanical agitation. The obtained results are reported in Fig. S5 of the Supplementary Material. As seen, solution stirring by 100 to 300 rpm has practically no effect on the degradation performance of ARAC. This confirms the performing action of sonication as a perfect stirring tool. Above 500 rpm, the process performance is reduced, mainly to the negative impact of stirring on the wave propagation, as a great part of the acoustic energy dissipated into the solution could be lost by the wave diffraction at the surface of the propeller, in addition to the fact that higher stirring speed could accelerate the coalescence of bubbles which could reduce the number of active bubbles, and then the radicals generation rate in the sonicated solution. Therefore, the conclusion was that there is no need to assist the sonochemical treatment by mechanical stirring; the strategy which has been adopted in all following sections. Also, pH adjustment with acids other than H2SO4 can affect the process performance; however, this scenario is not posed as the substitution of H2SO4 with HCl did not show any difference (Fig. S6 of the Supplementary Material).
Although it is impossible to make an exact comparison with other advanced oxidation process (AOPs) due to the disparity in the experimental conditions and the substrate pollutant, we have tried to conduct a qualitative comparison with three chlorine-based AOPs (i.e., UV/chlorine, Fe(II)/chlorine and Fe(III)/chlorine) where the degradation process is similar to that of US/chlorine (i.e., •OH and RCS pathways). The comparison is only based on the removal yield of the different pollutants at given degradation times. The comparison data was added in Table S1 of the Supplementary Data. As shown in this Table, the US/chlorine provides degradation performance as higher as other ones, particularly after a long irradiation time (10 min in this case). At the initial stage, the UV/chlorine provided them most advancement; however, this is because of the lower power supply of our ultrasonic device. If higher power is applied, the process performance of the US/chlorine will be much preferment, as you can see in [49]. Besides, our discovered system is more efficient than the Fe(III)/chlorine process either at the initial or at the final stage of the treatment. On the other hand, despite the fact that the US/chlorine started slowly than the Fe(II)/chlorine system (35% for the former against 80% for the latter), the sono-chlorination overlapped the Fe(II)/chlorine at the last stage of reaction (10 min), where 92% of removal was achieved against 84% for the Fe(II)/chlorine. Furthermore, the performance of the US/chlorine is incomparable with those of US/persulfate and US/periodate. These lasts induced a rather limited enhancement in the degradation of dyes [50], [51] as compared to the drastic improvement caused by the US/chlorine process (of this study). This is in addition to the fact that we do not need to remove the residual chlorine after the treatment, while this operation is necessary in the case of persulfate and periodate. Besides, all iron-based AOPs require supplementary steps for removing iron; the problem which is not posed by the developed sono-chlorination system.
3.3. Source of the synergism
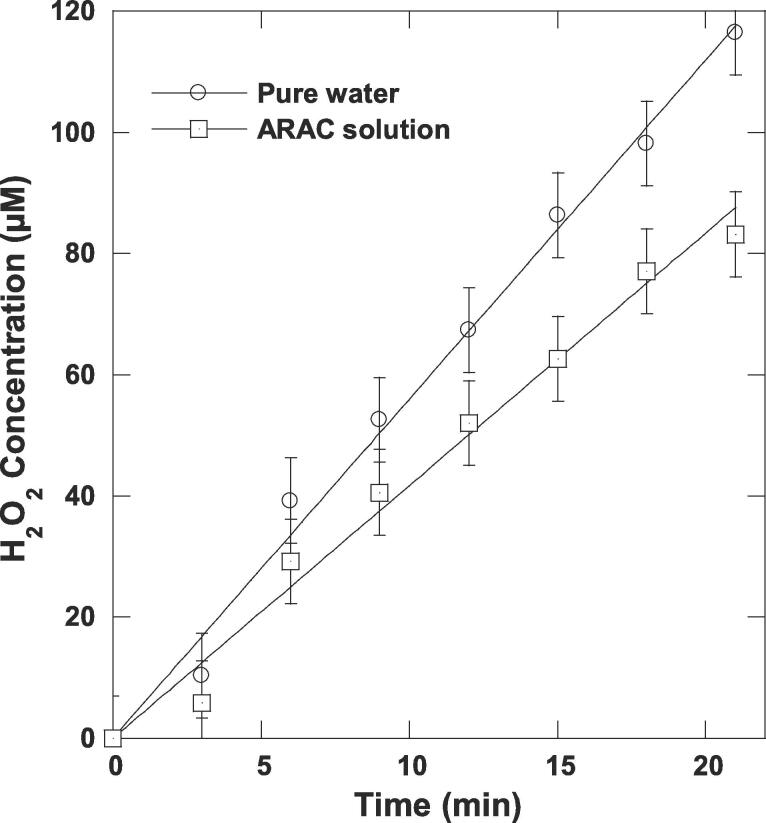
ARAC is a highly water soluble solute (solubility: 225 g L−1, log Kow = − 0.55 [52]) of negligible volatility. This compound cannot enter the bubbles to be pyrolyzed but it could be oxidized outside the bubble by •OH radical ejected from the bubbles inside at the collapse. We confirmed this mechanism by adding nitrobenzene (NB, a nonvolatile substrate) as a specific scavenger of •OH (kNB,•OH = 3.9 × 109 [36]). NB at 1 mM inhibited the sonolytic degradation of ARAC by >91% as stated in Fig. 1(a) and (b), i.e., the k value decreased from 0.059 to 0.005 min−1 due NB addition. It seems that the ARAC degradation occurred preferentially at the interfacial region, as it was confirmed by H2O2 analysis (Fig. 3). H2O2 could mainly be formed at the bubble/solution interface via 2•OH → H2O2 (k = 5.5 × 109 M−1 s−1) [20], [53]. The rate of hydrogen peroxide formation decreased from 5.6 µM min−1 in pure water to 4.17 µM min−1 in ARAC aqueous solution (5 mg L−1), meaning that ARAC molecules scavenge an appreciable portion of hydroxyl radicals located at the reactive interfacial region. Consequently, the sono-degradation of ARAC mainly takes place at the bubble/solution interface via •OH reaction pathway, but some non-negligible degradation reactions can take place in the bulk solution as NB did not quench completely the dye removal (∼90% of ARAC stayed not degraded after a long irradiation time in the presence of NB).
Fig. 3.
H2O2 evolution during the sonolysis of pure water and 5 mg L−1 of ARAC solution (conditions: V = 150 mL, temperature: 25 ± 1 °C, frequency: 600 kHz, power: 120 W). Accumulation rates: 5.6 µM min−1 in pure water and 4.17 µM min−1 in ARAC solution (ARAC induced reduction of in the accumulation rate of H2O2, confirming the •OH pathway at the bubble/solution interface for the sonolytic degradation of ARAC).
On the other hand, at pH 5.5, hypochlorous acid (HOCl) is the main species of chlorine in aqueous solution (Fig. S3). This species is highly reactive toward the acoustically generated reactive species (•OH, H•, HO2• and H2O2) and the susceptible reactions between them can generate several reactive chlorine species (RCS: Cl•, ClO•, HOCl•−and Cl2•−) as can be summarized in the following reaction scheme [2], [28], [36], [54], [55]:
| •OH + HOCl → ClO• + H2O k1 = 2 × 109 M−1 s−1[28] (1) |
| •H + HClO → •OH + HCl k2 = 5 × 108 M−1 s−1[56] (2) |
| •H + HClO → Cl• + H2O k3 = 5 × 108 M−1 s−1[56] (3) |
| •H + HClO → ClO• + H2 k4 = 5 × 108 M−1 s−1[56] (4) |
| HO2•/O2•− + HClO → Cl• + O2 + H2O/OH− k5 = 7.5 × 106 M−1 s−1[54] (5) |
| H2O2 + HClO → HCl + O2 + H2O k6 = 1.1 × 104 M−1 s−1[28] (6) |
| HCl ⇌ H+ + Cl− p Ka = − 6.3 (7) |
| Cl• + Cl− ⇌ Cl2•− k8 = 8.5 × 109 M−1 s−1[57] (8) |
| •OH + Cl− ⇌ HClO•−k9 = 4.3 × 109 M−1 s−1[57] (9) |
| Cl• + HClO → ClO• + H+ + Cl− k10 = 3 × 109 M−1 s−1[40] (10) |
| Cl• + H2O → HClO•− + H+ k11 = 2.5 × 105 s−1[28] (11) |
| Cl2•− + H2O → Cl− + HClO•− + H+ k12 = 1 × 105 M−1 s−1[36] (12) |
| HClO•− ⇌ •OH + Cl− k13 = 6.9 × 109 M−1 s−1[36] (13) |
| HClO•− + H+ → Cl• + H2O k14 = 2.1 × 1010 M−1 s−1[36] (14) |
| HClO•− + Cl− → Cl2•− + OH− k15 = 1 × 105 M−1 s−1[36] (15) |
| Cl2•− + •OH → HClO + Cl− k16 = 1 × 109 M−1 s−1[28] (16) |
| Cl2•− + H• → 2Cl− + H+ k17 = 8 × 109 M−1 s−1[58] (17) |
| Cl2•− + HO2• → 2Cl− + H+ + O2 k18 = 3 × 109 M−1 s−1[58] (18) |
| Cl2•− + Cl• → Cl2 + Cl− k19 = 2.1 × 109 M−1 s−1[28] (19) |
| •OH + •OH → H2O2 k20 = 5.5 × 109 M−1 s−1[59] (20) |
| Cl• + Cl• → Cl2 k21 = 8.8 × 107 M−1 s−1[28] (21) |
| Cl2•− + Cl2•− → Cl2 + 2Cl− k22 = 6.3 × 108 M−1 s−1[28] (22) |
| ClO• + ClO• → Cl2O2 k23 = 7.5 × 109 M−1 s−1[28] (23) |
The synergism resulted from the application of US/Chlorine process may therefore be attributed to the sonochemical activation of chlorine (Eqs. 1–15), presumably at the bubble/solution interface due to the high chlorine concentration, 0.25 mM. The generated RCS in the sonicating reaction medium can create further reaction pathway, which makes the degradation of micropollutants very fast. To confirm this statement, ARAC degradation run by US/chlorine was performed in the presence of NB. NB react efficiently with •OH (kNB,OH = 3.9 × 109 [36]), while its reaction with RCS (Cl•, Cl2•− and ClO•) and chlorine is negligible [34]. With adding 1 mM of NB into the reacting medium, the dye removal after 20 min drops from 99% without NB to 15% (Fig. 2(a)), recording about 85% of loss in the removal yield and ∼ 94% of reduction in the pseudo-first order rate constant (kUS/chlorine/NB = 0.013 min−1). Therefore, the acoustically generated •OH is the main activator of chlorine, although the other ones (H• and HO2•) may participate but with a minor portion. This means that •OH radicals act principally on HOCl (in addition to ARAC) rather than reacting only with ARAC present in solution. Therefore, a set of 23 chemical reactions has been proposed for the activation of chlorine (HOCl is the main form of chlorine at pH 5.5) via hydroxyl radicals. Through this mechanism, we will attempt to increase the amount of RCS (Cl•, Cl2•− and ClO•) by the action of •OH radicals (produced within the bubble) on HOCl.
3.4. Effect of NOM on the performance of the US/chlorine sono-hybrid process
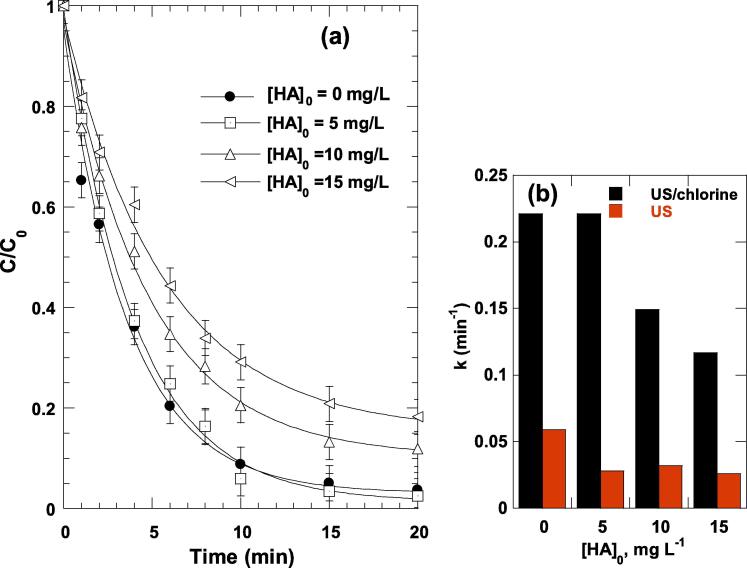
Natural organic matter (NOM) is comprised of humic and fulvic acids, found in almost all surface waters, and are due to breakdown of vegetation [60]. Surface waters contain NOM in the concentration range of 0.1–20 mg L−1 [60]. Humic acids (HA) have molecular weights ranging from several hundred to several thousand and are comprised primarily of aromatic compounds (i.e., with benzene-rings in various forms) [60]. NOM has a negative charge, i.e., hydrophilic compounds, which stay relatively far from the reactive interfacial zone of the cavitation bubbles and being hardly degraded by ultrasound [60]. To look at the effect of HA on the performance of US/chlorine sono-hybrid process, ARAC (5 mg L−1) degradation runs were conducted in the presence of 5, 10 and 15 mg L−1 of HA at pH 5.5. The results for US/chlorine process are shown in Fig. 4(a) and the corresponding effect of HA concentration on the pseudo-first order rate constant (k) for US and US/chlorine treatment of ARAC are presented in Fig. 4(b).
Fig. 4.
Effect of HA concentration on ARAC degradation kinetics (a), and corresponding effect of [HA] on the pseudo-first order rate constant (k) for US and US/chlorine treatment of ARAC (b) (conditions: frequency: 600 kHz, power: 120 W, C0 = 5 mg L−1 (10 µM), [chlorine]0 = 0.25 mM, [HA]0 = 0–15 mg L−1, 25 ± 1 °C, pH 5.5). HA: Humic acid.
For the sono-chlorination process, HA at 5 mg L−1 did not affect the degradation rate whereas 8% and 15% of reduction in the dye removal at 20 min were recorded for 10 and 15 mg L−1 of HA, respectively (Fig. 4(a)). The corresponding decrease in the rate constant (k) were 32% and 47% for [HA]0 = 10 and 15 mg L−1, respectively (Fig. 4(b)). Conversely, a nearly constant reduction of about 17 ± 2% in the dye elimination at 20 min and ∼ 50% in the rate constant k were obtained for the three HA concentrations upon the sole sonication of ARAC (Fig. 4(b)). This latter observation confirms that HA can compete for hydroxyl radical reaction (i.e., kHA-●OH = 2.5 × 104 M−1s−1 [61]) in the bulk solution only since no further reductions in the removal efficiency was obtained for [HA]0 > 5 mg L−1. Hamdaoui and Merouani [23] showed an insignificant effect of HA (5–40 mg L−1) on the sonochemical degradation of acid orange 7 (20 mg L−1, 57.09 µM) at 600 kHz and 120 W. Similar outcome has also been reported by Neppolian et al. [62] for the oxidation of Arsenic(III) to Arsenic(V) using an initial concentration of 10.10 µM (0.7567 mg L−1) by 20 kHz sonication. The difference between our findings and those of Hamdaoui and Merouani [23] and Neppolian et al. [62] resides in the difference of initial pollutant concentration (C0). For lower C0, like in our case, degradation may occur in both the bubble/solution interface and the bulk of the solution whereas, for higher C0, the bubbler/solution interface is the preferable reaction zone for pollutant oxidation [63]. Thus, addition of HA for cases where higher C0 is used could not affect the degradation rate as the pollutant could be degraded at the interfacial reactive zone where the availability of HA is unsuspected due to its too high hydrophilic nature [23]. However, a competition could take place between the contaminant and HA to react with hydroxyl radical if the degradation reaction happens also in the bulk solution, i.e., when lower C0 is used.
For the system, US/chlorine, the appreciable decrease in the rate constant (k) when [HA]0 increased from 10 to 15 mg L−1, i.e., 32% and 47%, respectively, was attributed to competition between ARAC molecules and HA to react with both •OH and RCS (i.e., kHA-Cl● =1.3 × 104 M−1 s−1, kHA-ClO● = 4.5 × 104 M−1 s−1, [2], [64]); whose able to drive reaction in the bulk solution due to their higher lifetime than •OH (i.e., 5 µs for Cl• [65] and fractions of milliseconds for Cl2•− [65], against ∼ 1 ns for •OH). HA may also react directly with HOCl (k = 0.7–5 M−1s−1) [66] leading to an instant consumption of available chlorine [67]. Fang et al. [40] have showed that the k value for benzoic acid degradation in UV/chlorine process decreased by about the half when the concentration of NOM increased from 0 to 10 mg/L. Recently, Dong et al. [68] have showed that the removal yield of ciprofloxacin in UV/chlorine system decreased from 98.5% to 62.2% in the presence of only 0.4 mg L−1 of HA. With the continuous increase of HA to 4 mg L−1, the degradation efficiency further decreased to 43.2% [68]. In their detailed study on the degradation of 34 pharmaceuticals and personal care products (PPCPs), Guo et al. [37] have recorded a significant reduction in the degradation rate of some PPCPs, while the degradation of some others where less affected by NOM. The calculated •OH-degradation rate constant (k’•OH) decreased by ∼ 10%, while that of RCS (k’RCS) decreased by 19.3% to 90% [37]. Moreover, the steady-state concentration of •OH, Cl• and Cl2•− decreased by < 20% [37]. The authors concluded that RCS is more sensitive toward NOM than •OH [37].
Besides, the molecular weight of humic acid (Sigma-product) ranges of 2,000–500,000 g/mol, against only 496.42 g/mol for ARAC. Thus, if we consider only 2000 g/mol for HA, the 5 mg/L of HA corresponds to 2.5 µM whereas the 5 mg/L of ARAC corresponds to 10 µM. Thus, for 5 mg/L for each one (ARAC and HA), the molar concentration of HA in the solution is much lower than that of ARAC, by about 4-fold (10/2.5 = 4). This justifier the non-significance of HA addition at 5 mg/L on the removal rate of ARAC (5 mg/L) by the US/chlorine process, as demonstrated in Fig. 4(a). However, as the concentration of HA increases to 10 and 15 mg/L (i.e., 5 and 7.5 µM), this could create a competition with the ARAC (5 mg/L or 10 µM) because the molar concentrations of both substrates are quite similar. It is also seen that even with the use of 15 mg/L of HA, there is no much decrease in the degradation rate of ARAC either in the US or in the US/chlorine systems (Fig. 4(b)). This is because the competition between HA and ARAC to react with free radicals did not depend on the substrates concentration only, but also to the second order rate constants [ri = ki-OHCOHCi , where ri: degradation rate and i = ARAC or HA]. If we consider only the reactivity with hydroxyl radicals, the ki-OH values for HA and ARAC (with •OH) are 2.5 × 104 and ∼ 109 M−1 s−1, respectively. Thus, for [HA] = 15 mg/L (7.5 µM for WHA = 2000 g/mol) and [ARAC] = 5 mg/L (10 µM), we have:
Therefore, a lower competition is always maintained as the reaction rate of •OH with ARAC is much higher than that of •OH with HA, this if we assume that all reactions take place in the same reaction region. However, because the degradation of ARAC occurs mainly at the bubble interface (as demonstrated in section 3.3) and HA is more hydrophilic compound (stays in the bulk solution), there is certainly a difference in the reaction zone, which means that the contribution of HA in consuming free radicals is lower, even with the use of higher HA concentration compared to that of ARAC (also, because the concentration of •OH in the bulk solution is about 10% of that at the bubbles interface). Finally, it should be noted that we cannot increase further the HA concentration (>15 mg/L) to not exceed its typical concentration in wastewater (between 5 and 20 mg/L in most cases).
3.5. Effect of water mineral anions on the performance of the US/chlorine sono-hybrid process
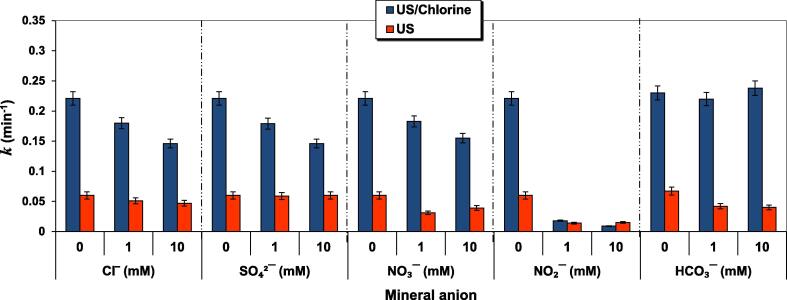
Inorganic ions constitute a major part of wastewater. The effect of various mineral water constitutes, i.e., SO42−, Cl−, NO3−, HCO3− and NO2− at 1 and 10 mM on the degradation rate constant (k) of ARAC (5 mg L−1) upon US and US/chlorine sono-hybrid system is shown in Fig. 5 for [chlorine]0 = 0.25 mM and pH 5.5, except for HCO3− where the initial pH was 8 to ensure maximum quantity of bicarbonate in the reacting medium [69]. Practically, all mineral anions act as radical scavengers, thereby decreasing the degradation rate of the dye in both US and US/chlorine system. However, the degree of reduction differs from one anion species to another and it sometimes may dependent of the applied process.
Fig. 5.
Effect of mineral anions on ARAC degradation rate constant (k) for US and US/chlorine treatments (conditions: frequency: 600 kHz, power: 120 W, C0 = 5 mg L−1 (10 µM), [chlorine]0 = 0.25 mM, [anions]0 = 0–10 mM, 25 ± 1 °C, pH 5.5, except for HCO3− (pH 8)).
The degradation rate of the dye upon the sole sonication was decreased by ∼ 15% and 20% with adding 1 and 10 mM of Cl−, respectively, whereas more inhibiting effect of 18% and 34% was recorded for the sono-hybrid system for 1 and 10 mM of chloride, respectively. In the US system, Cl− can react with •OH (Eq. 9), thereby decreasing the degradation rate through the generation of the less reactive radical anion HClO•− [70]. The competition between ARAC and Cl− to react with ●OH may take place in the bulk solution at low Cl− dosage, but it was also probable at the bubble/solution interface in the presence of a high quantity of chloride, i.e., 10 mM. The increase in competition with increasing Cl− concentration could result in lowering the degradation rate of the dye. However, even though HClO•− can simultaneously be decomposed via several routes to generate Cl•, Cl2•− and also •OH (Eqs. 13–15), no enhancing-degradation rate was observed, which is may be attributed to the fact that the consequent created radicals from Eqs. 13–15 could also react with Cl− itself which is still in excess as compared to the dye molecules (10 µM), thereby decreasing the overall yield of radicals in the solution. The same tendency of chloride effect was observed for several available sonochemical treatments [22], [71], [72].
In the light of the foregoing, the detrimental effect exerted by Cl− in the US/chlorine system, as compared to the sole sonolysis, can be associated to (i) the scavenging of the acoustically generated •OH by the excess of Cl− like in the case of sonolysis alone and (ii) the consumption of HClO by chloride ions according to the reaction (24), which reduced the available concentration of chlorine in the solution (i.e., the yield of RCS could then be lower than that in the absence of Cl−).
| HOCl + Cl− → Cl2OH− k24 = 1.5 × 104 M−1 s−1[54] (24) |
On the other hand, no significant effect was observed for sulfate ions on the sonolytic degradation of ARAC, while remarkable losses of 19% and 34% in the degradation rate constant (k) were obtained for the US/chlorine treatment in the presence of 1 and 10 mM of sulfate, respectively. Sulfate is generally inert toward •OH-based AOPs [8], [71], [73]. Also, no effect of sulfate ions was reported for the degradation of reactive green 12 (RG12) dye by UV/chlorine process, where •OH was determined as the most reactive species in the medium [2]. Conversely, the addition of SO42− at 50 mM significantly reduced the degradation of RG12 by Fe(II)/chlorine and Fe(III)/chlorine processes (i.e., by 37% and 20%, respectively), in which Cl2•− was established to be the key oxidizing agent of the dye [34], [35]. Meghraoui et al. [34], [35] have suggested that SO42− could react with Cl2•− but only at high sulfate concentration (i.e., no second-order rate constant of the reaction was provided). Therefore, the reducing effect of SO42− on the degradation rate of ARAC by the US/chlorine process may be due to the quench of reactive chlorine species by sulfate ions.
Nitrite drastically inhibited the degradation rate of the dye by both US and US/chlorine processes. For the US/chlorine system, the ARAC degradation rate constant (k) drops from 0.221 min−1 to 0.018 and 0.009 min−1, respectively, for 1 and 10 mM of NO2− (i.e., 92% and 96% of drops, respectively). The corresponding recorded loss for the sonolytic system was 75% for both 1 and 10 mM of nitrite. In the US system, NO2− can react efficiently with •OH with a second-order rate constant of 1 × 1010 M−1 s−1 (Eq. 25) [2], [35]. Therefore, the dye removal could be quenched even at a low concentration of NO2−. In the US/chlorine system, NO2− not only quenches •OH but also consume RCS as well as free chlorine (HOCl) (Eqs. 26 and 27) [2], resulting in a more inhibiting effect of the degradation rate as compared to the sonication in the absence of HOCl (Fig. 5).
| NO2− + •OH → OH− + NO2•k25 = 1 × 1010 M−1s−1 (25) |
| NO2− + Cl2•− → 2Cl− + NO2•k26 = 2.5 × 108 M−1s−1 (26) |
| NO2− + HOCl → NO3− + HCl k27 = 1.386 × 106 M−1s−1 (27) |
Contrarily to nitrite, NO3− moderately inhibited the degradation of ARAC by US and US/chlorine processes (Fig. 5). Losses of 17% and 30% in the k value were calculated for ARAC degradation in US/chlorine system in the presence of 1 and 10 mM of nitrite, respectively. Accordingly, 35% and 48% of losses in the k value were recorded in the US reaction system. It is therefore noted that the decelerating effect of NO3− vis-à-vis ARAC degradation was more pronounced for US than US/chlorine system. In the US system, NO3− can react rapidly with the acoustically generated •OH and H• according to reactions (28) and (29), thereby producing the lesser reactive NO2• and NO3• radicals [74]. For the system, US/chlorine, RCS are the main driver of the pollutant degradation. Based on the obtained results, it seems that NO3− is less reactive toward RCS as for •OH and H•. Unfortunately, there is no available data of second-order rate constants to confirm this suggestion. The one found reaction is that of NO3− with Cl•, given in reaction (30).
| NO3− + •OH → OH− + NO3•k28 = 1 × 106 M−1s−1 (28) |
| NO3− + H• → NO2• + OH−k29 = 4.4 × 106 M−1s−1 (29) |
| NO3− + Cl• → NO3• + Cl −k30 = 1 × 108 M−1s−1 (30) |
Bicarbonate can react at high second-order rate constants with •OH, Cl• and Cl2•− (Eqs. 31–33), while ClO• is not reactive toward HCO3− ions (k < 600 M−1 s−1) [64], [69].
| HCO3− + •OH → CO3•− + H2O k31 = 8.5 × 106 M−1s−1[64] (31) |
| HCO3− + Cl• → CO3•− + Cl− + H+k32 = 2.2 × 108 M−1s−1[64] (32) |
| HCO3− + Cl2•− → CO3•− + 2Cl− + H+k33 = 8 × 107 M−1s−1[64] (33) |
As a result, the concentration of •OH, Cl• and Cl2•− could be decreased in the presence of HCO3−, while that of CO3•− increased. The oxidation potential of carbonate radical (1.78 and 1.59 V/SHE at pH 7 and pH 12.5, respectively [69]) is less than those of Cl• (2.43 V/SHE) and Cl2•− (2.13 V/SHE) [28], [34]. This radical, even less reactive than •OH and RCS, has shown a rate enhancement of the sonochemical degradation of several water contaminants when the pollutant concentration is low and the HCO3− loading is relatively high [69], [72], [75], [76]. The mechanism of enhancement was usually attributed to the selectivity and the long lifetime of CO3•− as compared to •OH. However, this scenario was valid only where the CO3•− radical is reactive toward the target pollutant [2]. In fact, the production of carbonate radical in the UV/chlorine oxidation system has decreased the degradation of some pollutants like naproxen, nalidixic acid, metronidazole, ibuprofen, diethyltoluamide, Propranolol, flumequine, N,N-diethyl-m-toluamide, and caffeine [37], [38], [77]. However, the degradation of other ones were practically not affected (e.g. erythromycin, azithromycin, roxithromycin, venlafaxine, and Salbutamol) or even increased like the case of Ractopamine and Clenbuterol [37]. In our case, bicarbonate addition decreased the sonolytic degradation of ARAC by about 40% (Fig. 5), indicating that ARAC is presumably not reactive with CO3•−. Thus, the substitution of a certain amount of •OH by CO3•− could reduce the degradation via decreasing the available concentration of hydroxyl radical. Accordingly, Fig. 5 showed that bicarbonate has an insignificant effect on ARAC degradation by US/chlorine process, perhaps for the same reason as reported early for the US system. However, the huge generation of RCS may offset the remarkable reducing effect of bicarbonate on the sonolytic reaction system.
3.6. Process efficiency in natural mineral water
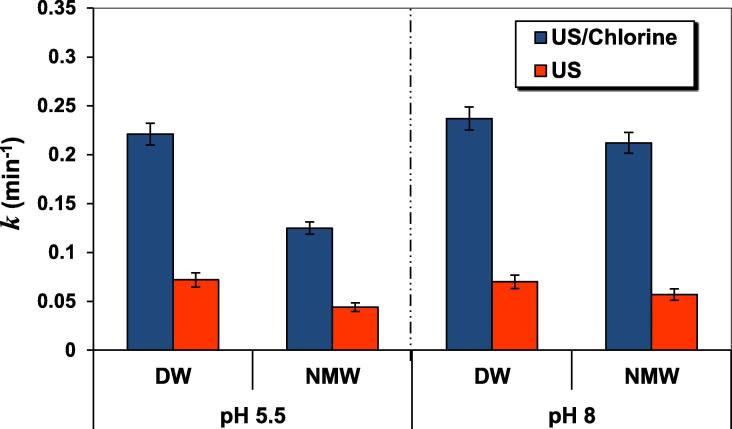
Complementary degradation experiments using US/chlorine process for ARAC degradation were performed in natural mineral water (NMW) whose the characteristics are: Ca2+ = 81 mg L−1, Mg2+ = 24 mg/L, Na+ = 15.8 mg L−1, Cl− = 72 mg L−1 (2 mM), SO42− = 53 mg L−1 (0.55 mM), HCO3− = 265 mg L−1 (4.34 mM). The achieved results are presented in Fig. 6 for pH 5.5 and 8. For the US/chlorine system, the degradation rate constant in the NMW at pH 5.5 was 0.125 min−1, compared to 0.221 min−1 in deionized water, thus resulting in 43% of decrease in the value of k. A comparable decrease of 39% was provoked by the NMW for the US system (Fig. 6). The reason of these decreases is the quenching effect of mineral anions toward RCS than •OH, as discussed in the previous section. The indicated inhibition of 43% in the NMW at pH 5.5 compared to DW is clearly explained by the scavenging effect (on HOCl) of the different ionic species present in solution, where this was detailed in section 3.5. It should be noted that at pH 5.5, HOCl is the predominant form of chlorine (>98%, Fig. S3 of the Supplementary Data); therefore, hypochlorous acid activation is relatively reduced by the different anions present in solution (see section 3.5). At a pH 8, chlorine is principally present as ClO− (∼74%) and HOCl (∼26%) (Fig. S3 of the Supplementary Data). Consequently, at this pH, the lower inhibition (10%) of the US/chlorine performance in the NMW compared to the DW matrix is probably owing to the fact that the inhibition of HOCl (through the different anions) is offset by the action of ClO− on ARAC. However, as it was indicated previously, the efficacy of US/chlorine process is clearly shown in the pH’s range 5.5–8 (Fig. 4). However, the decrease in the k values at pH 8 due to NMW is much lower for US/chlorine against US alone (i.e., 10% against 18%, respectively), meaning that the process is very effective at the near neutral pH. Thus, the US/chlorine process is a promising technique for ARAC removal in real natural mineral waters.
Fig. 6.
Effect of water matrices on ARAC degradation rate constant (k) for US and US/chlorine treatments (conditions: frequency: 600 kHz, power: 120 W, C0 = 5 mg L−1 (10 µM), [chlorine]0 = 0.25 mM, 25 ± 1 °C, pH 5.5 and 8). DW: deionized water, NMW: natural mineral water.
3.7. Energetic cost of US/chlorine process
It should be noted that the efficiency of US process is certainly improved by the addition of chlorine to the medium as an oxidation enhancer. This is corroborated through the different findings obtained in Fig. 2, Fig. 4, Fig. 5, Fig. 6. The technological cost of our system (US/chlorine process) may be determined through the energy consumed (kWh) per 1 g (or mol) of ARAC converted (under the action of •OH radicals and RCS), in addition to the amount of chlorine added to the sonoreactor. Considering the maximal elimination of ARAC (Fig. 2) where 96.2% (5 mg/L) is decomposed in 20 min, this gives a conversion of 4.008 × 10−6 g L−1 s−1. On the other hand, the power delivered to the sonicated medium (150 mL) is equal to 120 W, which gives a power density of 800 W/L. As a result, our energetical consumption for ARAC conversion (5 mg/L) is equal to 14.43 g/kWh (2.907 × 10−2 mol/kWh) at an ultrasound frequency of 600 kHz. Regarding the consumption of chlorine (cheap compound), if we suppose that the total chlorine (0.25 mM) is activated (via US) during the conversion of ARAC (10 µM), a chlorine consumption of 2.083 × 10−7 mol L−1 s−1 is obtained for the same acoustic conditions (600 kHz and 120 W). Regarding the challenges of the US application in the proposed process (US/chorine AOP), this issue is resumed in the following points:
-
(i)
Reduction of energetic consumption of sonoreactor through the optimization of ultrasound frequency, acoustic intensity and liquid temperature in accordance with the amount of chlorine added to the sonicated solution.
-
(ii)
Amelioration of sonoreactor design and efficiency through the improvement of its form, construction materials, and transducer placement around the reactor wall.
-
(iii)
Constructing continuous-flow reactors.
-
(iv)
Extending the US/chlorine process to the industrial application (large-scale operation).
4. Conclusion
US/chlorine is being discovered as one promising advanced oxidation process which can alternate for US/O3 toward the degradation of persistent organic contaminants. Chlorine activation with ultrasound can promote the degradation of pollutants in similar manner as for UV/chlorine AOP, in which both •OH and RCS may participate in the oxidation process. The process was found efficiently synergistic toward the degradation of ARAC. Overall, the presence of NOM and mineral anions decreased the efficiency of the sono-hybrid process; however, the degree of inhibition depends on the type and the concentration of the selected additives.
This study could open effective perspectives for the application of US/chlorine process as alternative AOP for water treatment. Nevertheless, the viability of the process in treating real wastewater treatment is still depending on by-products analysis and evaluating the TOC abatement and the toxicity of the residual effluent. Even though the objective of the present work was to provide initial results on the effect of HA and mineral anion in term of pollutant removal, further complementally in-depth studies are under realization to check the effective applicability of the US/chlorine process, by which degradation products, TOC evolution and toxicity of the residual effluent will be analyzed.
Finally, with simple comparison to US/O3 process, the most examined hybrid treatment, the US/chlorine process may present several initial advantages, like:
-
(i)
US/chlorine is facile to handling with more safety as chlorine is less harmful as ozone. Additionally, the liquid phase of chlorine facilitates its employment instead of O3 which is used in a gas phase (toxic element).
-
(ii)
Chlorine is available and less expensive than ozone, i.e., the latter requires in situ generation via a specific expensive apparatus. Thus, the cost of US/chlorine treatment could be lower than that of US/O3 for the same experimental operation.
-
(iii)
The synergy resulted from US/chlorine is more important than that reported for US/O3 process. Therefore, the US/chlorine system allows achieving higher removal yield in a short treatment time than US/O3.
-
(iv)
The US/chlorine process does not require removal of the residual chlorine as it is originally used as a disinfectant, whereas the elimination of residual O3 after the sono-ozonation is obligatory.
CRediT authorship contribution statement
Oualid Hamdaoui: Investigation, Conceptualization, Methodology, Formal analysis, Project administration, Supervision, Data curation, Funding acquisition, ResourcesVisualization, Writing – review & editing. Slimane Merouani: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Meriem Ait Idir: Investigation, Visualization, Writing – review & editing. Hadjer C. Benmahmoud: Investigation, Visualization, Writing – review & editing. Aissa Dehane: Validation, Visualization, Writing – review & editing. Abdulaziz Alghyamah: Validation, Visualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.105918.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Carmen Z., Daniela S. Org. Pollut. Ten Years after Stock. Conv. - Environ. Anal. Updat. 2012. Textile organic dyes – characteristics , polluting effects and separation / elimination procedures from industrial effluents – A critical overview; pp. 55–86. [Google Scholar]
- 2.Belghit A., Merouani S., Hamdaoui O., Bouhelassa M., Al-Zahrani S. The multiple role of inorganic and organic additives in the degradation of reactive green 12 by UV/chlorine advanced oxidation process. Environ. Technol. 2020:1–27. doi: 10.1080/09593330.2020.1807609. [DOI] [PubMed] [Google Scholar]
- 3.Brown M.A., De Vito S.C. Predicting azo dye toxicity. Crit. Rev. Env. Sci. Technol. 1993;23(3):249–324. doi: 10.1080/10643389309388453. [DOI] [Google Scholar]
- 4.Bendjama H., Merouani S., Hamdaoui O., Bouhelassa M. Efficient degradation method of emerging organic pollutants in marine environment using UV/periodate process: Case of chlorazol black. Mar. Pollut. Bull. 2018;126:557–564. doi: 10.1016/j.marpolbul.2017.09.059. [DOI] [PubMed] [Google Scholar]
- 5.M.I. Stefan, Advanced oxidation processes for water treatment: Fundamentals and applications, IWA Publishing, London, UK, 2017.
- 6.Ghodbane H., Hamdaoui O. Decolorization of antraquinonic dye, C.I. Acid Blue 25, in aqueous solution by direct UV irradiation, UV/H2O2 and UV/Fe(II) processes. Chem. Eng. J. 2010;160:226–231. doi: 10.1016/j.cej.2010.03.049. [DOI] [Google Scholar]
- 7.Ghodbane H., Hamdaoui O., Merouani S. Degradation of C.I. acid blue 25 in water using UV/K2S2O8 process: Effect of salts and environmental matrix. Desalin. Water Treat. 2017;74:395–401. doi: 10.5004/dwt.2017.20612. [DOI] [Google Scholar]
- 8.Bekkouche S., Merouani S., Hamdaoui O., Bouhelassa M. Efficient photocatalytic degradation of Safranin O by integrating solar-UV/TiO2/persulfate treatment: Implication of sulfate radical in the oxidation process and effect of various water matrix components. J. Photochem. Photobiol. A Chem. 2017;345:80–91. doi: 10.1016/j.jphotochem.2017.05.028. [DOI] [Google Scholar]
- 9.Modirshahla N., Behnajady M.A., Ghanbary F. Decolorization and mineralization of C.I. Acid Yellow 23 by Fenton and photo-Fenton processes. Dye. Pigment. 2007;73(3):305–310. doi: 10.1016/j.dyepig.2006.01.002. [DOI] [Google Scholar]
- 10.Hsing H.-J., Chiang P.-C., Chang E.-E., Chen M.-Y. The decolorization and mineralization of Acid Orange 6 azo dye in aqueous solution by advanced oxidation processes: A comparative study. J. Hazard. Mater. 2007;141(1):8–16. doi: 10.1016/j.jhazmat.2006.05.122. [DOI] [PubMed] [Google Scholar]
- 11.Lee C., Yoon J. Application of photoactivated periodate to the decolorization of reactive dye: Reaction parameters and mechanism. J. Photochem. Photobiol. A Chem. 2004;165(1-3):35–41. doi: 10.1016/j.jphotochem.2004.02.018. [DOI] [Google Scholar]
- 12.Bhangu S.K., Ashokkumar M. Theory of sonochemistry. Top. Curr. Chem. 2016;374 doi: 10.1007/s41061-016-0054-y. [DOI] [PubMed] [Google Scholar]
- 13.Yasui K. In: Theor. Exp. Sonochemistry Involv. Inorg. Syst. PankajAshokkumar M., editor. Springer ScienceþBusiness Media; New York: 2011. Fundamentals of acoustic cavitation and sonochemistry; pp. 1–29. [Google Scholar]
- 14.BlakePerutz J.R., Suslick K.S., Didenko Y., Fang M.M., Hyeon T., Kolbeck K.J., McNamara W.B., Mdleleni M.M., Wong M. Acoustic cavitation and its chemical consequences. Philos. Trans. R. Soc. 1999;357(1751):335–353. doi: 10.1098/rsta.1999.0330. [DOI] [Google Scholar]
- 15.Suslick K.S., Flannigan D.J. Inside a collapsing bubble: sonoluminescence and the conditions during cavitation. Annu. Rev. Phys. Chem. 2008;59(1):659–683. doi: 10.1146/annurev.physchem.59.032607.093739. [DOI] [PubMed] [Google Scholar]
- 16.Makino K., Mossoba M.M., Riesz P. Chemical effects of ultrasound on aqueous solutions. Evidence for •OH an •H by spin trapping. J. Am. Chem. Soc. 1982;104:3537–3539. doi: 10.1021/ja00376a064. [DOI] [Google Scholar]
- 17.Merouani S., Hamdaoui O., Rezgui Y., Guemini M. Sensitivity of free radicals production in acoustically driven bubble to the ultrasonic frequency and nature of dissolved gases. Ultrason. Sonochem. 2014;22:41–50. doi: 10.1016/j.ultsonch.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Makino K., Mossoba M.M., Riesz P. Chemical effects of ultrasound on aqueous solutions. Formation of hydroxyl radicals and hydrogen atoms. J. Phys. Chem. 1983;87(8):1369–1377. doi: 10.1021/j100231a020. [DOI] [Google Scholar]
- 19.Wei Z., Villamena F.A., Weavers L.K. Kinetics and Mechanism of Ultrasonic Activation of Persulfate: An in Situ EPR Spin Trapping Study. Environ. Sci. Technol. 2017;51:3410–3417. doi: 10.1021/acs.est.6b05392. [DOI] [PubMed] [Google Scholar]
- 20.Merouani S., Hamdaoui O., Saoudi F., Chiha M. Influence of experimental parameters on sonochemistry dosimetries: KI oxidation, Fricke reaction and H2O2 production. J. Hazard. Mater. 2010;178:1007–1014. doi: 10.1016/j.jhazmat.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 21.Mark G., Tauber A., Laupert R., Schuchmann H.P., Schulz D., Mues A., von Sonntag C., Dorothea Schulz A.M., Von Sonntag C., Schulz D., Mues A., Von Sonntag C. OH-radical formation by ultrasound in aqueous solution-Part II: Terephthalate and Fricke dosimetry and the influence of various conditions on the sonolytic yield. Ultrason. Sonochem. 1998;5:41–52. doi: 10.1016/S1350-4177(98)00012-1. [DOI] [PubMed] [Google Scholar]
- 22.Taamallah A., Merouani S., Hamdaoui O. Sonochemical degradation of basic fuchsin in water. Desalin. Water Treat. 2016;57(56):27314–27330. doi: 10.1080/19443994.2016.1168320. [DOI] [Google Scholar]
- 23.Hamdaoui O., Merouani S. Ultrasonic destruction of acid Orange 7: Effect of humic acid, surfactants and complex matrices. Water Environ. Reasearch. 2017;89(3):250–259. doi: 10.2175/106143016X14798353399539. [DOI] [PubMed] [Google Scholar]
- 24.Adewuyi Y.G. Sonochemistry: environmental science and engineering applications. Ind. Eng. Chem. Res. 2001;40:4681–4715. doi: 10.1021/ie010096l. [DOI] [Google Scholar]
- 25.Tauber A., Mark G., Schuchmann H.-P., von Sonntag C. Sonolysis of tert-butyl alcohol in aqueous solution. Sonolysis of tert -butyl alcohol in aqueous solution. 1999;(6):1129–1136. doi: 10.1039/a901085h. [DOI] [Google Scholar]
- 26.Deborde M., Von Gunten U. Reactions of chlorine with inorganic and organic compounds during water treatment — Kinetics and mechanisms : A critical review. Water Res. 2008;42:13–51. doi: 10.1016/j.watres.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Dong H., Qiang Z., Hu J., Qu J. Degradation of chloramphenicol by UV/chlorine treatment: Kinetics, mechanism and enhanced formation of halonitromethanes. Water Res. 2017;121:178–185. doi: 10.1016/j.watres.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 28.De Laat J., Stefan M. In: Adv. Stefan M.I., editor. IWA Publishing, London, UK; Oxid. Process. Water Treat.: 2017. UV/chlorine process; pp. 383–428. [Google Scholar]
- 29.Wang W.L., Wu Q.Y., Li Z.M., Lu Y., Du Y., Wang T., Huang N., Hu H.Y. Light-emitting diodes as an emerging UV source for UV/chlorine oxidation: Carbamazepine degradation and toxicity changes. Chem. Eng. J. 2017;310:148–156. doi: 10.1016/j.cej.2016.10.097. [DOI] [Google Scholar]
- 30.Huang N., Wang T., Wang W., Wu Q., Li A., Hu H. UV/chlorine as an advanced oxidation process for the degradation of benzalkonium chloride: Synergistic effect, transformation products and toxicity evaluation. Water Res. 2017;114:246–253. doi: 10.1016/j.watres.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Li D., Chen D., Yao Y., Lin J., Gong F., Wang L., Luo L., Huang Z., Zhang L. Strong enhancement of dye removal through addition of sulfite to persulfate activated by a supported ferric citrate catalyst. Chem. Eng. J. 2016;288:806–812. doi: 10.1016/j.cej.2015.12.008. [DOI] [Google Scholar]
- 32.Gao Z.-C., Lin Y.-L., Xu B., Pan Y., Xia S.-J., Gao N.-Y., Zhang T.-Y., Chen M. Degradation of acrylamide by the UV/chlorine advanced oxidation process. Chemosphere. 2017;187:268–276. doi: 10.1016/j.chemosphere.2017.08.085. [DOI] [PubMed] [Google Scholar]
- 33.Javier Benitez F., Real F.J., Acero J.L., Casas F. Assessment of the UV/Cl2 advanced oxidation process for the degradation of the emerging contaminants amitriptyline hydrochloride, methyl salicylate and 2-phenoxyethanol in water systems. Environ. Technol. (United Kingdom). 2017;38:2508–2516. doi: 10.1080/09593330.2016.1269836. [DOI] [PubMed] [Google Scholar]
- 34.Meghlaoui F.Z., Merouani S., Hamdaoui O., Bouhelassa M., Ashokkumar M. Rapid catalytic degradation of refractory textile dyes in Fe (II)/chlorine system at near neutral pH: Radical mechanism involving chlorine radical anion (Cl2●−)-mediated transformation pathways and impact of environmental matrices. Sep. Purif. Technol. 2019;227 doi: 10.1016/j.seppur.2019.115685. [DOI] [Google Scholar]
- 35.Meghlaoui F.Z., Merouani S., Hamdaoui O., Alghyamah A., Bouhelassa M., Ashokkumar M. Fe(III)-catalyzed degradation of persistent textile dyes by chlorine at slightly acidic conditions: the crucial role of Cl2●− radical in the degradation process and impacts of mineral and organic competitors, Asia-Pacific. J Chem. Eng. 2020:1–12. doi: 10.1002/apj.2553. [DOI] [Google Scholar]
- 36.Remucal C.K., Manley D. Emerging investigators series: The efficacy of chlorine photolysis as an advanced oxidation process for drinking water treatment. Environ. Sci. Water Res. Technol. 2016;2:565–579. doi: 10.1039/c6ew00029k. [DOI] [Google Scholar]
- 37.Guo K., Wu Z., Shang C., Yao B.o., Hou S., Yang X., Song W., Fang J. Radical Chemistry and Structural Relationships of PPCP Degradation by UV/Chlorine Treatment in Simulated Drinking Water. Environ. Sci. Technol. 2017;51(18):10431–10439. doi: 10.1021/acs.est.7b0205910.1021/acs.est.7b02059.s001. [DOI] [PubMed] [Google Scholar]
- 38.Wu Z., Guo K., Fang J., Yang X.X., Xiao H., Hou S., Kong X., Shang C., Yang X.X., Meng F., Chen L. Factors affecting the roles of reactive species in the degradation of micropollutants by the UV/chlorine process. Water Res. 2017;126:351–360. doi: 10.1016/j.watres.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 39.Wu Z., Fang J., Xiang Y., Shang C., Li X., Meng F., Yang X. Roles of reactive chlorine species in trimethoprim degradation in the UV/chlorine process: Kinetics and transformation pathways. Water Res. 2016;104:272–282. doi: 10.1016/j.watres.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Fang J., Fu Y., Shang C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ. Sci. Technol. 2014;48(3):1859–1868. doi: 10.1021/es4036094. [DOI] [PubMed] [Google Scholar]
- 41.Qin L., Lin Y.L., Xu B., Hu C.Y., Tian F.X., Zhang T.Y., Zhu W.Q., Huang H., Gao N.Y. Kinetic models and pathways of ronidazole degradation by chlorination, UV irradiation and UV/chlorine processes. Water Res. 2014;65:271–281. doi: 10.1016/j.watres.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 42.Xiang Y., Fang J., Shang C. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process. Water Res. 2016;90:301–308. doi: 10.1016/j.watres.2015.11.069. [DOI] [PubMed] [Google Scholar]
- 43.J.F. Borzelleca, J.W. Olson, F.E. Reno, Lifetime toxicity/carcinogenicity study of FD & C Red No. 40 (Allura Red) in Sprague-Dawley rats, Food Chem. Toxicol. 27 (1989) 701–705. 10.1016/0278-6915(89)90074-4. [DOI] [PubMed]
- 44.Garole V.J., Choudhary B.C., Tetgure S.R., Garole D.J., Borse A.U. Detoxification of toxic dyes using biosynthesized iron nanoparticles by photo-Fenton processes. Int. J. Environ. Sci. Technol. 2018;15(8):1649–1656. doi: 10.1007/s13762-017-1510-0. [DOI] [Google Scholar]
- 45.C. V. Vorhees, R.E. Butcher, R.L. Brunner, V. Wootten, T.J. Sobotka, Development toxicity and psychotoxicity of FD and C red dye no. 40 (Allura red AC) in rates, Toxicology. 28 (1983) 207–217. [DOI] [PubMed]
- 46.Kiani S., Mousavi S.M. Ultrasound assisted preparation of water in oil emulsions and their application in arsenic (V) removal from water in an emulsion liquid membrane process. Ultrason. Sonochem. 2013;20(1):373–377. doi: 10.1016/j.ultsonch.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Chiha M., Hamdaoui O., Ahmedchekkat F., Pétrier C. Study on ultrasonically assisted emulsification and recovery of copper(II) from wastewater using an emulsion liquid membrane process. Ultrason. Sonochem. 2010;17(2):318–325. doi: 10.1016/j.ultsonch.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Sun Q., Yang L., Yang J., Liu S., Hu X. Study on the interaction between Rhodamine dyes and Allura Red based on fluorescence spectra and its analytical application in soft Drinks. Anal. Sci. 2017;33(10):1181–1187. doi: 10.2116/analsci.33.1181. [DOI] [PubMed] [Google Scholar]
- 49.Boutamine Z., Hamdaoui O., Merouani S. Sonochemical and photosonochemical degradation of endocrine disruptor 2-phenoxyethanol in aqueous media. Sep. Purif. Technol. 2018;206:356–364. doi: 10.1016/j.seppur.2018.06.010. [DOI] [Google Scholar]
- 50.Ferkous H., Merouani S., Hamdaoui O., Pétrier C. Persulfate-enhanced sonochemical degradation of naphthol blue black in water: Evidence of sulfate radical formation. Ultrason. Sonochem. 2017;34:580–587. doi: 10.1016/j.ultsonch.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 51.Hamdaoui O., Merouani S. Improvement of sonochemical degradation of brilliant blue R in water using periodate ions: Implication of iodine radicals in the oxidation process. Ultrason. Sonochem. 2017;37:344–350. doi: 10.1016/j.ultsonch.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 52.Allura Red AC, PubChem. (2020). https://pubchem.ncbi.nlm.nih.gov/compound/Allura-Red-AC (last visit 7/9/2020).
- 53.Pétrier C., Francony A. Ultrasonic waste-water treatment: incidence of ultrasonic frequency on the rate of phenol and carbon tetrachloride degradation. Ultrason. Sonochem. 1997;4:295–300. doi: 10.1016/S1350-4177(97)00036-9. [DOI] [PubMed] [Google Scholar]
- 54.Bulman D.M., Mezyk S.P., Remucal C.K. The Impact of pH and Irradiation Wavelength on the Production of Reactive Oxidants during Chlorine Photolysis. Environ. Sci. Technol. 2019;53(8):4450–4459. doi: 10.1021/acs.est.8b0722510.1021/acs.est.8b07225.s00110.1021/acs.est.8b07225.s002. [DOI] [PubMed] [Google Scholar]
- 55.A.A. Belghit, S. Merouani, O. Hamdaoui, M. Bouhelassa, A. Alghyamah, M. Bouhelassa, Influence of processing conditions on the synergism between UV irradiation and chlorine toward the degradation of refractory organic pollutants in UV/chlorine advanced oxidation system, Sci. Total Environ. 736 (2020) 139623_1-139623_10. 10.1016/j.scitotenv.2020.139623. [DOI] [PubMed]
- 56.Vogt R., Schindler R.N. Product channels in the photolysis of HOCl. J. Photochem. Photobiol. A Chem. 1992;66(2):133–140. [Google Scholar]
- 57.Buxton G.V., Bydder M., Arthur Salmon G. Reactivity of chlorine atoms in aqueous solution Part 1The equilibrium Cl• + Cl- → Cl2•-, J. Chem. Soc. Faraday Trans. 1998;94:653–657. doi: 10.1039/a707377a. [DOI] [Google Scholar]
- 58.Neta P., Huie R.E., Ross A.B. Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data. 1988;17(3):1027–1284. [Google Scholar]
- 59.Buxton G.V., Greenstock C.L., Helman W.P., Ross A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O − in Aqueous Solution. J. Phys. Chem. Ref. Data. 1988;17(2):513–886. [Google Scholar]
- 60.Hendricks D. Fundamentals of water treatment unit processes: Physical, chemical, and biological, IWA Publishing, London. UK. 2011 doi: 10.1142/p063. [DOI] [Google Scholar]
- 61.Kong X., Wu Z., Ren Z., Guo K., Hou S., Hua Z., Li X., Fang J. Degradation of lipid regulators by the UV/chlorine process: Radical mechanisms, chlorine oxide radical (ClO•)-mediated transformation pathways and toxicity changes. Water Res. 2018;137:242–250. doi: 10.1016/j.watres.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 62.B. Neppolian, A. Doronila, F. Grieser, Simple and Efficient Sonochemical Method for the Oxidation of Arsenic (III) to Arsenic (V), 43 (2009) 6793–6798. [DOI] [PubMed]
- 63.Chadi N.E., Merouani S., Hamdaoui O., Bouhelassa M. New aspect of the effect of liquid temperature on sonochemical degradation of nonvolatile organic pollutants in aqueous media. Sep. Purif. Technol. 2018;200:68–74. doi: 10.1016/j.seppur.2018.01.047. [DOI] [Google Scholar]
- 64.K. Guo, Z. Wu, J. Fang, UV-based advanced oxidation process for the treatment of pharmaceuticals and personal care products, in: Arturo J. Hernández-Maldonado, Lee Blaney (Eds.), Contam. Emerg. Concern Water Wastewater, Elsevier Inc., 2020: pp. 367–408. 10.1016/B978-0-12-813561-7.00010-9.
- 65.Alegre M.L., Geronés M., Rosso J.A., Bertolotti S.G., Braun A.M., Mártire D.O., Gonzalez M.C. Kinetic Study of the Reactions of Chlorine Atoms and Cl2•- Radical Anions in Aqueous Solutions. 1. Reaction with Benzene. J. Phys. Chem. A. 2000;104(14):3117–3125. doi: 10.1021/jp9929768. [DOI] [Google Scholar]
- 66.Chuang Y.-H., Chen S., Chinn C.J., Mitch W.A. Comparing the UV/Monochloramine and UV/Free Chlorine Advanced Oxidation Processes (AOPs) to the UV/Hydrogen Peroxide AOP under Scenarios Relevant to Potable Reuse. Environ. Sci. Technol. 2017;51(23):13859–13868. doi: 10.1021/acs.est.7b0357010.1021/acs.est.7b03570.s001. [DOI] [PubMed] [Google Scholar]
- 67.Westerhoff P., Chao P., Mash H. Reactivity of natural organic matter with aqueuos chlorine and bromine. Water Res. 2004;38:1502–1513. doi: 10.1016/j.watres.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 68.Deng J., Wu G., Yuan S., Zhan X., Wang W., Hu Z.H. Ciprofloxacin degradation in UV/chlorine advanced oxidation process: Influencing factors, mechanisms and degradation pathways. J. Photochem. Photobiol. A Chem. 2019;371:151–158. doi: 10.1016/j.jphotochem.2018.10.043. [DOI] [Google Scholar]
- 69.Merouani S., Hamdaoui O., Saoudi F., Chiha M., Pétrier C. Influence of bicarbonate and carbonate ions on sonochemical degradation of Rhodamine B in aqueous phase. J. Hazard. Mater. 2010;175(1-3):593–599. doi: 10.1016/j.jhazmat.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 70.Yuan R., Wang Z., Hu Y., Wang B., Gao S. Probing the radical chemistry in UV/persulfate-based saline wastewater treatment: Kinetics modeling and byproducts identification. Chemosphere. 2014;109:106–112. doi: 10.1016/j.chemosphere.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 71.Merouani S., Hamdaoui O., Saoudi F., Chiha M. Sonochemical degradation of Rhodamine B in aqueous phase: Effects of additives. Chem. Eng. J. 2010;158:550–557. doi: 10.1016/j.cej.2010.01.048. [DOI] [PubMed] [Google Scholar]
- 72.Pétrier C., Torres-Palma R., Combet E., Sarantakos G., Baup S., Pulgarin C. Enhanced sonochemical degradation of bisphenol-A by bicarbonate ions. Ultrason. Sonochem. 2010;17(1):111–115. doi: 10.1016/j.ultsonch.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 73.Chadi N.E., Merouani S., Hamdaoui O., Bouhelassa M., Ashokkumar M. Influence of mineral water constituents, organic matter and water matrices on the performance of the H2O2/IO4–advanced oxidation process. Environ. Sci. Water Res. Technol. 2019;5(11):1985–1992. [Google Scholar]
- 74.L. Venault, De l'influence des ultrasons sur la reactivite de l'uranium (U(IV)/U(VI)) et du plutonium (PU(III)/PU(IV)) en solution aqueuse nitrique, Ph.D. Thesis, Paris 11 University, 1997.
- 75.Minero C., Pellizzari P., Maurino V., Pelizzetti E., Vione D. Enhancement of dye sonochemical degradation by some inorganic anions present in natural waters. Appl. Catal. B Environ. 2008;77(3-4):308–316. doi: 10.1016/j.apcatb.2007.08.001. [DOI] [Google Scholar]
- 76.Villegas-Guzman P., Silva-Agredo J., Giraldo-Aguirre A.L., Florez-Acosta O., Petrier C., Torres-Palma R.A., Florez-Acosta O., Petrier C., Torres-Palma R.A. Enhancement and inhibition effects of water matrices during the sonochemical degradation of the antibiotic dicloxacillin. Ultrason. Sonochem. 2015;22:211–219. doi: 10.1016/j.ultsonch.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Pan M., Wu Z., Tang C., Guo K., Cao Y., Fang J. Comparative study of naproxen degradation by the UV/chlorine and the UV/H2O2 advanced oxidation processes. Environ. Sci. Water Res. Technol. 2018;4:1219–1230. doi: 10.1039/c8ew00105g. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.