Abstract
Major depressive disorder is a debilitating disorder affecting millions of people each year. Brain-derived neurotrophic factor (BDNF) and inflammation are two prominent biologic risk factors in the pathogenesis of depression that have received considerable attention. Many clinical and animal studies have highlighted associations between low levels of BDNF or high levels of inflammatory markers and the development of behavioral symptoms of depression. However, less is known about potential interaction between BDNF and inflammation, particularly within the central nervous system. Emerging evidence suggests that there is bidirectional regulation between these factors with important implications for the development of depressive symptoms and anti-depressant response. Elevated levels of inflammatory mediators have been shown to reduce expression of BDNF, and BDNF may play an important negative regulatory role on inflammation within the brain. Understanding this interaction more fully within the context of neuropsychiatric disease is important for both developing a fuller understanding of biological pathogenesis of depression and for identifying novel therapeutic opportunities. Here we review these two prominent risk factors for depression with a particular focus on pathogenic implications of their interaction.
Keywords: Brain-derived neurotrophic factor, Microglia, Neuroinflammation, Growth factors, Depression
Core Tip: Low levels of brain-derived neurotrophic factor (BDNF) and high inflammation have both been implicated as risk factors in the pathogenesis of major depressive disorder. Here we review the role BDNF and inflammation play in the etiology of depression and the interaction between them. Recent evidence suggests a bidirectional connection between these two risk factors: inflammation reduces BDNF expression, and BDNF may have a negative regulatory role in resolving neuroinflammation. Understanding of this interaction in the context of neuropsychiatric disease is important for a fuller understanding of biological pathogenesis of depression and for identifying novel therapeutic opportunities.
INTRODUCTION
Research has made important advances in recent decades towards the understanding and treatment of major depressive disorder, a debilitating disorder with a heterogeneous range of symptoms. Despite these advancements, depression remains a leading cause of disability with an estimated 264 million individuals worldwide affected by the disorder[1]. In the United States, the economic burden of major depressive disorder is an estimated 210.5 billion dollar[2] with substantial lost productivity and diminished quality of life for affected patients and their families. Recent interest has turned to biomarker and genetic analysis to predict those who may be vulnerable to developing depression and to understand the etiology of patients’ existing diagnosis in order to better prevent and treat this debilitating disorder. Two notable biological risk factors for depression are of particular interest: A deficiency in brain-derived neurotrophic factor (BDNF) and inflammation. In this review, we will highlight the mechanisms by which these factors are known to contribute to the development of depression and summarize emerging evidence suggesting that interactions between these two factors within the brain are important in the pathogenesis of depression.
BRAIN DERIVED NEUROTROPHIC FACTOR
BDNF, a member of the neurotrophin family of growth factors, has been well-studied for its role in the pathogenesis of major depressive disorder and antidepressant efficacy. BDNF is a small protein expressed by the bdnf gene on chromosome 11 in humans[3]. Transcription of the bdnf gene is controlled by nine distinct promoters. The bdnf gene contains up to 11 exons; exons II, III, IV, and VII are brain-specific[4]. BDNF is first synthesized as the precursor pre-proBDNF in the endoplasmic reticulum. The pre- domain is cleaved off and proBDNF is transported to the Golgi apparatus. ProBDNF may be secreted in the precursor form or proteolytically cleaved intracellularly or extracellularly to form mature BDNF (mBDNF)[5,6]. Both pro- and mature forms of the BDNF protein are neuroactive, though the activity of proBDNF and mBDNF have largely opposite effects. ProBDNF binds and activates the pan-neurotrophin receptor p75NTR, a member of the tumor necrosis factor receptor family, promoting apoptosis[7]. mBDNF binds with high affinity to the tyrosine kinase receptor tropomycin receptor kinase B (TrkB).
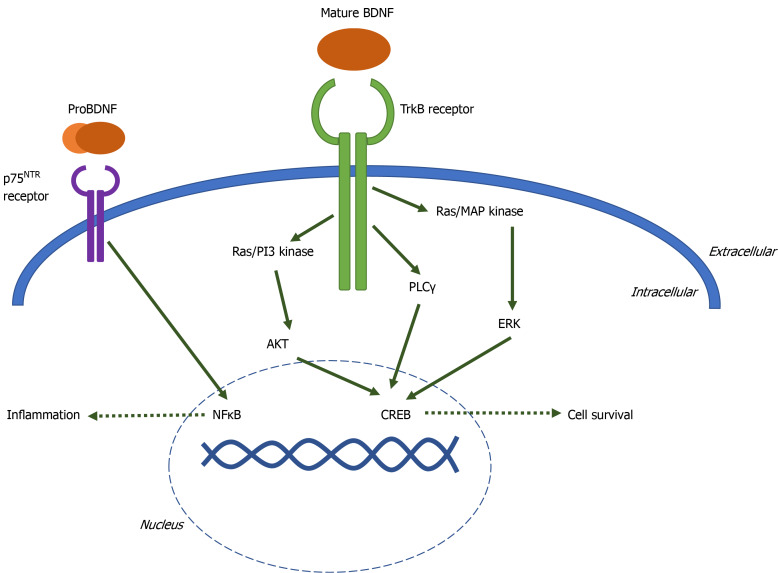
When mature BDNF, or neurotrophins with lesser affinity for TrkB including neurotrophin-4 and neurotrophin-3, bind to the extracellular domain of TrkB, the intracellular domains of the receptor dimerize and autophosphorylate one of three tyrosine residues. Phosphorylation at each residue initiates a distinct signaling cascade: Ras-PI3K-Akt, Ras-MAP kinase-Erk, or phospholipase Cγ[8]. These signaling cascades activate transcription factors such as CREB, resulting in cell proliferation, cell survival, synaptogenesis, and memory formation (Figure 1).
Figure 1.
Brain-derived neurotrophic factor signaling cascade diagram. When TrkB is activated by binding mature brain-derived neurotrophic factor (BDNF), the intracellular domains of the receptor dimerize and autophosphorylate one of three tyrosine residues. Phosphorylation at each residue initiates a distinct signaling cascade: Ras-PI3K-Akt, Ras-MAP kinase-Erk, or phospholipase Cγ. These signaling cascades activate transcription factor CREB, resulting in cell proliferation, cell survival, synaptogenesis, and memory formation. ProBDNF binds to pan-neurotrophin receptor P75NTR. P75NTR signaling activates transcription factor NFκB, leading to inflammation and apoptosis. BDNF: Brain-derived neurotrophic factor.
BDNF and TrkB are expressed both peripherally and within the central nervous system. In the periphery, BDNF has been detected in the heart and spleen[9], expressed by myoblasts[10], dorsal root ganglion cells[11], vascular endothelial cells[12], leukocytes[13] and is stored in platelets[14]. In the brain, BDNF is expressed by neurons, astrocytes[15], and microglia[16]. BDNF is highly expressed in the hippocampus and is found in lower concentrations in the cerebral cortex and brainstem[17]. TrkB is expressed in neurons, microglia, and astrocytes throughout the brain[18,19].
A number of factors may modulate BDNF expression or function. Prenatal, early life, social, and unpredictable stress are all associated with reduced BDNF expression or protein levels[20]. Exercise increases BDNF expression[21] and environmental enrichment protects against the effects of stress and early life inflammation on BDNF expression[22,23]. BDNF levels may also decline with age[24,25] and low BDNF levels are associated with age-related neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease[26,27]. However, some studies suggest BDNF expression does not change with age[28,29].
While a number of genetic factors may contribute to a reduction of BDNF expression or function[30-33], the val66met mutation has garnered considerable attention due to its relevance in psychiatric conditions like bi-polar disorder and suicidality[34,35]. The single nucleotide polymorphism (SNP; rs6265) at nucleotide 196 (G/A) occurs on the 5’ pro-BDNF sequence, producing a valine to methionine substitution within codon 66. This SNP does not appear to alter BDNF expression or biological activity, but impairs translocation and activity-dependent secretion[36], thus reducing BDNF- TrkB signaling. The val66met SNP is also associated with reduced serum BDNF protein levels in the periphery[37].
BDNF IN DEPRESSION
The negative correlation between BDNF levels and symptoms of depression have been well established; researchers have been interested in BDNF as a biomarker for depression for decades[38-40]. Clinical data has often demonstrated that patients suffering from major depression disorder are more likely to have alterations in their BDNF-TrkB signaling activity. Numerous studies have found that depressed or suicidal patients have lower BDNF levels than healthy controls[41-48]. Keller et al[30] found that suicide victims were more likely to have DNA methylation in the BDNF promotor/exon IV compared to control subjects, suggesting a link between epigenetic down-regulation of BDNF and suicidal behavior. Further, psychosocial stress, a known precursor to depression and anxiety, reduces BDNF levels[20].
Genetic analysis reveals several polymorphisms that are associated with susceptibility to developing depression or suicide, such as rs12273363, rs7124442, rs10767664, rs962369, rs908867[31,33]. Of these polymorphisms, the rs6265 SNP known as val66met has been most extensively studied in psychiatric conditions. Some studies suggest that individuals carrying the val66met polymorphism are more vulnerable to developing depression[37,49-52], suicidality[53,54], or to be nonresponsive to antidepressant treatment[55]. However, others dispute this association[55-60]. The val66met polymorphism has been linked to depression in breast cancer patients/survivors[61,62], but also appears to be protective against chemotherapy-associated cognitive impairments in breast cancer patients[63]. The mixed findings pertaining to association between the val66met SNP and psychiatric disorders suggest that the mutation alone is likely not sufficient to cause pathology. Rather it is a risk factor that interacts with other genetic or environmental factors to contribute to pathogenesis of depression or depressive symptoms.
Clinical studies investigating BDNF have been limited to measuring BDNF in the blood or cerebral spinal fluid, direct measurement of mRNA or protein in the brain being only available in post-mortem tissue samples. However, BDNF does cross the blood-brain barrier (BBB)[64], and Karege et al[65] found that brain and serum levels of BDNF are positively correlated in rats. For this reason, measuring peripheral BDNF levels are a feasible indicator of central BDNF expression. Moreover, there is a negative correlation between serum BDNF stored in platelets and depression in humans[66]. BDNF release from platelets may be impaired in depressed patients[67] while antidepressants increase BDNF release from platelets[68], suggesting platelet-derived BDNF is a contributing factor to the interaction between peripheral BDNF levels and depression.
Recent preclinical studies revealed that mice heterozygous for the BDNF allele, which reduces BDNF levels within the brain by about half[69], are susceptible to depressive-like phenotypes after a challenge such as mild stress or acute inflammation[70,71,201]. Direct infusion of BDNF into the rodent brain[72,73] and periphery[74] is protective against the behavioral consequences of stress in the forced swim test and learned helplessness models of depressive-like despair behavior. Further, manipulation of the BDNF-TrkB signaling activity through TrkB agonist 7,8-dihydroxyflavone (DHF)[75] reduces depressive-like behavioral changes induced by social defeat stress[76] and acute inflammation[77]. Many antidepressant treatments increase levels of circulating BDNF[46,68,78-84]. In the brain, anti-depressant treatment induces BDNF mRNA expression in neurons[85], astrocytes[86-88], and microglia[88]. Up-regulation of BDNF may be necessary for the anti-depressant response[89-93].
INFLAMMATION IN THE PERIPHERY AND THE BRAIN
As suggested above, dysregulation in the BDNF-TrkB system may not be a pathological factor that acts alone, rather perturbation within this neurotrophic factor expression/signaling may engender a foundation of vulnerability to subsequent insults to increase the risk of depression or lead to pathology (Figure 2). Inflammation is one risk factor that may well fit this profile.
Figure 2.
Hypothesis diagram. Brain-derived neurotrophic factor (BDNF) deficiency and elevated or chronic neuroinflammation independently confer vulnerability to development of major depressive disorder. BDNF plays a negative regulatory role in resolving neuroinflammation, and high inflammation reduces BDNF expression. BDNF: Brain-derived neurotrophic factor. BDNF: Brain-derived neurotrophic factor.
The ancient Roman encyclopedist Celcus defined inflammation by the presence of “rubor, calor, dolor, tumor”, or redness, heat, pain, and swelling. Modern scientists have a deeper understanding of inflammation as a consequence of the innate immune system’s activation in response to an irritant or loss of homeostatic control due to factors such as stress, obesity, and aging. Acute inflammation occurs when a tissue injury, pathogen, or noxious stimuli is detected. Leukocytes travel to the impacted region to remove the stimuli and repair damage. Chronic inflammation is a persistent and maladaptive response that can be caused by many factors, such as chronic somatic diseases, advancing age, obesity, smoking, and high fat diets. In addition to contributing directly to risk of depression, chronic inflammation may lead to chronic illnesses such as allergies, arthritis, and autoimmune disease that also have high comorbidity with depression.
Invading pathogens or signals released by damaged cells are detected by toll-like receptors (TLR) in the plasma membrane of innate immune cells. TLRs are classified as pattern recognition receptors (PRRs). PRRs recognize and bind pathogen-associated molecular patterns (PAMPs)[94], such as lipopolysaccharide (LPS) on the gram-negative bacterial cell wall, or damage-associated molecular patterns (DAMPs) in a pathogen-independent process known as “sterile inflammation”. Activation of TLRs initiate an intracellular signaling cascade, activating the transcription factor NFκB, causing up-regulation of pro-inflammatory mediators including cytokines, chemokines, cellular adhesion molecules[95], and downstream induction of reactive oxygen species[96]. Of these mediators, macrophage-derived TNFα, IL-1β, IL-6, and IL-10 have received extensive attention due their roles in regulating the immune system and their effects on the body[97].
Inflammation as a function of the immune response is necessary to protect the life of the organism. Recently, intentional induction of inflammation has been wielded as a promising tool against cancer as immunotherapy[98]. However, numerous studies have shown prolonged and elevated immune activation has significant impacts on physiological, metabolic, and neural/behavioral processes. The effects of peripheral inflammation or immune challenge do not remain in the periphery; inflammatory conditions impact the CNS through several possible mechanisms. The BBB created by the tight junctions of brain endothelium restricts diffusion of pathogens and non-select solutes from the blood into the brain. Peripheral inflammation may disrupt this boundary, increasing the permeability of the BBB and allowing infiltration by circulating monocytes, cytokines, and other substances[99,100]. Cytokines and monocytes attracted by the expression of chemokines such as monocyte chemoattractant protein 1 will travel to the brain and enter through leaky regions of the BBB or through active transport systems. Peripheral cytokines, PAMPs, and DAMPs can also impact brain homeostasis by signaling through the vagus nerve[101] or by signaling through PRRs on the BBB endothelial cells[102,103]. These inflammatory signaling pathways across the BBB initiate the neuroinflammatory response within the brain.
Numerous animal studies have demonstrated that microglia, the resident immune cell in the brain, adopt an “activated” phenotype following peripheral inflammation induced by LPS and live or heat-killed pathogens[100]. In their resting state, microglia are “ramified” with small somas and long highly branched processes. Once microglia detect an immune challenge, their morphology shifts toward an “amoeboid” shape with enlarged soma and shorter, thicker processes. Microglia are the primary source for brain-borne cytokines and other inflammatory mediators.
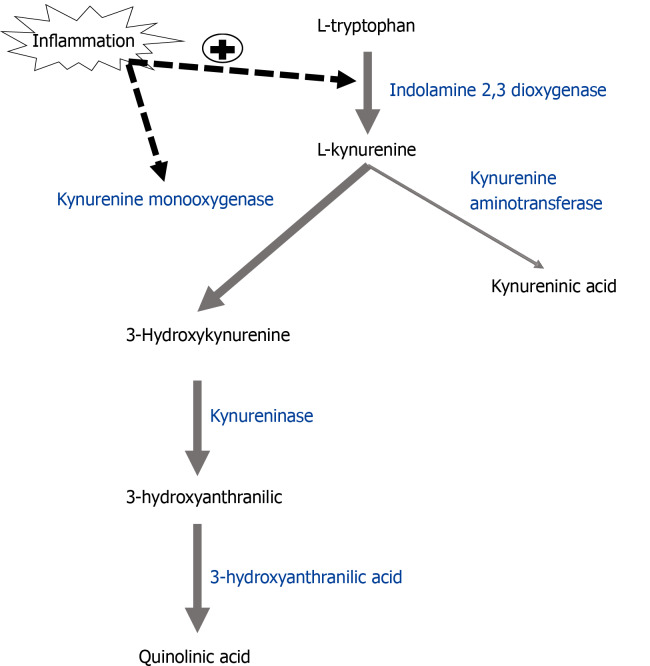
In addition to producing cytokines, inflammatory microglia also synthesize metabolites of the tryptophan-kynurenine pathway associated with oxidative stress. Tryptophan is converted to kynurenine by the enzyme indolamine-2,3 dioxygenase. Kynurenine metabolism then splits into distinct branches: Kyurenic acid, a metabolite with NMDA receptor antagonist activity, is produced in astrocytes by the enzyme kynurenine aminotransferase, while the enzyme kynurenine monooxygenase (KMO) produces 3-hydroxykynurenine (3-HK) in microglia (Figure 3). 3-HK is further metabolized by the enzyme 3-hydroxyanthranilate 3,4-dioxygenase (HAAO) into the neuroactive NMDA receptor agonist quinolinic acid (QA). 3-HK and QA are also free radical inducers and are necessary for the development of inflammation-induced development of depressive-like phenotypes[104], described below.
Figure 3.
Inflammation shifts kynurenine metabolism pathway towards oxidative stress-associated metabolites. The enzyme indolamine-2,3-dioxygenase (IDO) converts tryptophan to kynurenine. Kynurenine aminotransferase converts kynurenine to kynureninic acid in astrocytes while kynurenine monooxygenase (KMO) metabolizes kynurenine to 3-hyroxykynurenine (3-HK) in microglia. 3-HK is metabolized by kynureninase to 3-hydroxyanthranilic acid and 3-hydroxyanthranilic acid dioxygenase to quinolinic acid. Inflammation up-regulates the enzymes IDO and KMO, resulting in increased levels of KMO-dependent metabolites associated with oxidative stress and depression. IDO: Indolamine-2,3-dioxygenase; KAT: Kynurenine aminotransferase; KMO: Kynurenine monooxygenase; 3-HK: 3-hyroxykynurenine; KYNU: Kynureninase; HAAO: 3-hydroxyanthranilic acid dioxygenase; QA: Quinolinic acid.
NEUROINFLAMMATION IN DEPRESSION
Symptoms of typical “sickness behaviors” which cease upon recovery – fatigue, loss of appetite, pain sensitivity, anhedonia, cognitive deficits, social withdrawal – have significant overlap with symptoms of major depressive disorder[105]. In fact, a subset of patients with chronic inflammatory diseases will suffer from longer-lasting symptoms of depression[106]. Individuals suffering from depression but who are otherwise medically healthy often have higher baseline levels of circulating pro-inflammatory mediators, particularly TNFα and IL-6[82,107-110]. Some anti-depressant treatments may reduce neuroinflammation[111,112], but most studies suggest that conventional antidepressants have reduced efficacy in depressed patients who have high inflammation. Conversely, while direct TNFα inhibition was ineffective as an anti-depressant in treatment resistant depression patients with low-moderate CRP levels, it was quite effective in treatment resistant patients with high inflammation[113]. This finding underscores the notion that anti-depressant treatment decisions and efficacy may be improved by integrating understanding of a patient’s inflammatory status. At the cellular/molecular level, post-mortem studies indicate that microglia density in the dorsolateral prefrontal cortex, anterior cingulate cortex, and mediodorsal thalamus[114,115], expression levels of IL-1β, IL-6, and TNFα in the prefrontal cortex[116,117] and blood[118], and production of QA in the ACC[119] is significantly higher in suicide victims compared to non-suicide controls. Further, anti-depressants with secondary anti-inflammatory properties are more effective in treatment-resistant patients with high baseline levels of inflammatory markers IL-6 and C-reactive protein[120]. These studies suggest a strong association between inflammation and the development of depression.
Inflammation on its own may be sufficient to promote the development of depressive symptoms. Subsets of patients report feelings of depression following cytokine treatment for hepatitis or cancer[121,122]. Experimental treatment with endotoxin[123] or Salmonella typhi vaccine in healthy subjects similarly induced symptoms of depression and anxiety alongside acute inflammation[124] and increased kynurenine pathway metabolism. Numerous rodent studies have likewise demonstrated that inflammation can induce depressive-like phenotypes[125].
Inflammation can arise from multiple sources and events. In humans and rodents, acute and chronic stress is known to promote activation of the innate immune system[126,127] and induce microglial activation[115,128]. Psychological stress, a frequent trigger for depression and suicidality in humans, is commonly modeled in rodents using acute or chronic stressors such as social defeat, restraint, or home cage disruption. You et al[129] found that rats exposed to chronic mild stress have elevated central and peripheral pro-inflammatory cytokines, reduced neurogenesis in the hippocampus, and display anhedonia-like behavior as measured by the sucrose preference test. Hodes et al[109] found that mice with higher baseline levels of circulating IL-6 are more susceptible to developing depressive-like behavioral phenotypes after chronic social stress; IL-6-/- mice were resilient to the effects of social stress. Aging similarly increases vulnerability to neuroinflammation and subsequent depressive-like behaviors. Peripheral LPS treatment promotes a more robust inflammatory responses and sickness behavior in aged mice compared to young adults[130,131]. Culley et al[132] found that LPS increases pro-inflammatory cytokine expression in the prefrontal cortex and impairments in prefrontal cortex-dependent cognition in aged rats. Inflammation associated with obesity[133] and alcohol consumption[134] have similarly been shown to induce depressive symptoms and behaviors in humans and animals.
Researchers have extensively studied depressive-like behavioral changes induced by peripheral immune challenge in rodents[125]. The viral mimetic Poly:IC, attenuated bacterial strain Bacillus Calmette-Guerin (BCG), and LPS are common models used to induce chronic or acute innate immune activation in animal models. Poly:IC increases expression of IL-1β, TNFα, and CD11b and elevates kynurenine levels in the rat brain, followed by a reduction in saccharin preference up to 72 h after treatment[135]. BCG inoculation induces chronic inflammation, up-regulates TNFα, INFy, and the tryptophan-kynurenine enzymes IDO and HAAO, and drives despair-like behavior measured by immobility in forced swim test and tail suspension test one week after infection[136,137]. LPS treatment models acute inflammation: Pro-inflammatory cytokine up-regulation and sickness behaviors resolve within 24 h after administration. Once motor activity and food intake is restored at 24 h, mice continue to display anhedonia-like, despair-like, and anxiety-like behavior[131,138,139]. Anti-inflammatory compounds ameliorate the depressive-like behaviors after LPS[138,140-144]. Moreover, many of these effects appear to be dependent on neurotoxic kynurenine metabolism. Inhibition of, or targeted deletion of, the gene for the rate-limiting enzyme IDO prevents development of LPS- and BCG-induced depressive-like behaviors, despite the elevation of pro-inflammatory cytokines[136,145,146]. KMO-/- and HAAO-/- mice are likewise are protected against many of the depressive-like behavioral effects of LPS, while direct administration of 3-HK provokes immobility in the tail suspension test and hippocampal-dependent cognitive impairment in the y-maze without an increase in pro-inflammatory cytokines[147], suggesting a causative role of downstream metabolites 3-HK and QA.
However, of the total human population that is exposed to high levels of inflammation, only a relatively small subset goes on to develop symptoms of major depressive disorder. For this reason, researchers have lately turned to investigating the environmental and genetic risk factors that contribute to a patient’s vulnerability to developing depression. Recent research has revealed a role for BDNF in modulating the effects of neuroinflammation in a psychiatric context. A deficiency in BDNF may prime the system to develop neuropsychiatric symptoms in a maladaptive response to neuroinflammation-induced sickness behavior (Figure 2).
PATHOGENIC TUG OF WAR?
Mounting evidence has revealed negative correlations between BDNF and neuroinflammation, particularly in psychiatric populations[148,149]. Depression is frequently comorbid with chronic inflammatory conditions, and BDNF deficiency has been identified as a risk factor. Breast cancer survivors are more likely to suffer from inflammation-associated depression if they carry the Met allele in the val66met SNP[62]. BDNF expression is reduced in animals models and patients with rheumatoid arthritis, a disease characterized by chronic inflammation, and associated with major depressive disorders[150]. In Hepatitis C patients undergoing IFNα therapy, elevated cytokine levels are predictive of lower BDNF levels, and both BDNF and cytokine expression are associated with depressive symptoms[151]. Uint et al[152] found that elevated levels of both IL-1β and BDNF were predictive of treatment-resistant depression, but posited that this relationship may be due to the patients’ long-term use of anti-depressant medications buoying their BDNF levels. Treating rats with viral mimetic Poly:IC increases expression of IL-1β, TNFα, IL-6, and CD11b and decreases BDNF and TrkB in the frontal cortex and hippocampus and reduces saccharin preference (anhedonia-like behavior)[135].
Numerous anti-inflammatory treatments have shown promising effects in alleviating depressive-like symptoms and increasing BDNF. Clinically, zinc monotherapy decreases depressive symptoms and increases BDNF in obese subjects[153]. In pre-clinical studies, insulin-like growth factor-1[81] and drugs such as resveratrol[140,154], imipramine[89,144], doxycycline[144], fluoxetine[155], etazolate[156], chaihu-shugan-san[157], dihydromyricetin[158], minocycline[159], ketamine[160], and caffeine[161] all inhibit inflammation, increase BDNF, and improve depressive-like behavioral phenotypes.
BDNF activity likewise appears to impact stress or inflammation-induced depression. Mice with genetically reduced baseline levels of BDNF (BDNF+/- mice) develop an exaggerated neuroinflammatory and anhedonia-like response to peripheral LPS challenge compared to wild-type controls[201] and increased despair-like behavior in the forced swim test after acute mild stress[71]. Both the TrkB agonist DHF and the TrkB antagonist ANA-12 are anti-depressant in mice treated with LPS, likely due to opposing effects of BDNF-TrkB activity between the hippocampus and nucleus accumbens[77]. INFα therapy patients with the Val66Met polymorphism display symptoms of suicidal ideation and depression compared to those with the Val allele[162]. Mice with the humanized val66met polymorphism (Val/Met mice) are more sensitive to LPS-induced depressive-like behaviors than Val/Val mice and exhibit microglia with an already primed morphology (unpublished data).
Additionally, investigating the interaction between BDNF-TrkB system and inflammation may be relevant for addressing the sex differences in the presentation of depression. Women report experiencing depression at up to twice the rate of men. BDNF is expressed differentially in various regions of the CNS between males and females and environmental conditions modulate BDNF expression differentially between males and females, although circulating levels of peripheral BDNF appear consistent between sexes[163]. Female BDNF conditional KO mice display more depressive-like behaviors and attenuated anti-depressant response than male BDNF conditional KO mice[164]. Women may also be more vulnerable to developing inflammation-induced depression. Females tend to have higher baseline levels of inflammation than males[165] and have a larger pro-inflammatory and depressive response to endotoxin exposure[166]. In the brain, while male microglia appear to be more reactive early in life than female microglia, female microglia may be reactive and inflammatory later in life, when neuropsychiatric disorders tend to manifest[167]. Estrogen may also play a role: Rodent models of estrogen deficiency results in increased depressive-like behaviors, pro-inflammatory cytokine expression, and increased levels of kynurenine pathway enzyme IDO in the hippocampus[168]. There is also evidence that estrogen regulates expression of BDNF and that the estrogen receptor may be necessary for the protective effects of TrkB activation[163]. These findings suggest the relationship between BDNF, inflammation, and sex warrants further investigation.
BDNF AND NEUROINFLAMMATION: BI-DIRECTIONAL MODULATION
Mounting evidence suggests that the connection between BDNF expression and neuroinflammation regulation is bi-directional in nature (Figure 2). Interestingly, Gomes et al[169] found in vitro that microglia acutely increase extracellular secretion of BDNF in response to LPS, leading to reduced intracellular levels of BDNF. Cultured human monocyte cells constitutively secrete BDNF, and BDNF secretion is increased when monocytes are stimulated by TNFα or IL-6, although no change in BDNF mRNA was detected[170]. Astrocytes likewise express BDNF when stimulated by TNFα[15] and increase expression of BDNF, TNFα, and IL-6 after LPS treatment[171]. BDNF regulates proliferation and survival of microglia[172]. This acutely elevated BDNF secretion may be necessary for microglia proliferation and activation after immune challenge[173].
Alternatively, increased BDNF secretion may be a means of inhibitory feedback, as BDNF dampens microglial activation. In spinal cord injury, locally applied BDNF reduces microglial density and inhibits free radical production around injury site[174]. Exogenous BDNF infusion dampens microglial activation by LPS in the substantia nigra in aged mice[175]. In a mouse model of Type I diabetes, overexpressing BDNF in the hippocampus suppressed microglial activation and expression of TNFα and IL-6 induced by hyperglycemia[176]. Further, hypermethylation of BDNF is associated with higher levels of serum IL-6 in patients with acute coronary syndrome[177]. Exogenous BDNF administration significantly decreases TNFα and increases expression of the anti-inflammatory cytokine IL-10 in rodent models of stroke, multiple sclerosis, and pneumococcal meningitis[178-181]. Along this line, BDNF+/- mice have reduced expression of IL-10 and kynurenic acid levels while 3-HK is increased in the brain compared to wild-type controls following chronic mild stress[182]. After LPS treatment, BDNF+/- mice have increased expression of pro-inflammatory cytokines IL-1β and TNFα and elevated levels of kynurenine and QA[201]. Reduced BDNF after viral mimetic poly:IC treatment is likewise accompanied by a shift in the tryptophan/kynurenine ratio[135]. In vitro studies in BV2 microglia by Park et al[183] have demonstrated that TrkB activation by the agonist DHF inhibits production of nitric oxide, TNFα, and IL-1β, and translocation and transcriptional activity of NFκB. These data suggest a role for BDNF-TrkB activity in modulating and resolving the neuroinflammatory response to immune challenge with implications for the development of the depressive-like behavioral phenotypes (Figure 4).
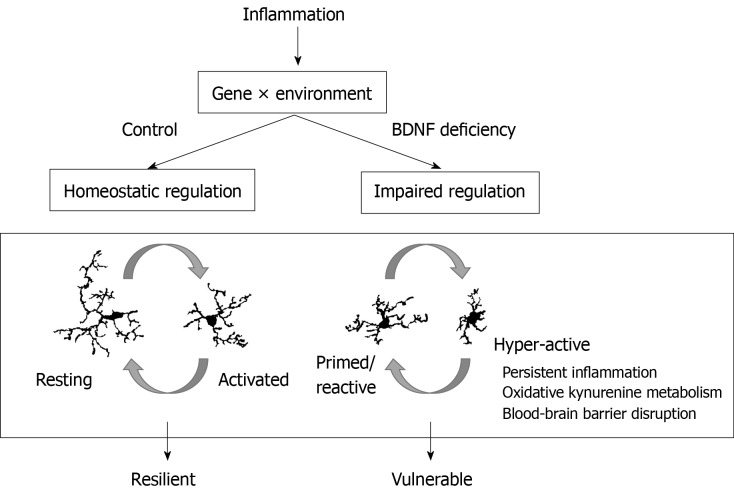
Figure 4.
Brain-derived neurotrophic factor deficiency impairs resolution of microglial inflammatory phenotypes. Brain-derived neurotrophic factor (BDNF) levels are altered by genetics and environmental circumstances. Reduced levels of BDNF impair microglia regulation after inflammation. Hyper-active microglia contribute to blood-brain barrier disruption and express higher levels of pro-inflammatory cytokines and kynurenine metabolism pathway enzymes. Individuals with hyper-active microglia are vulnerable to developing symptoms of depression after inflammatory challenge. BDNF: Brain-derived neurotrophic factor.
While BDNF secretion may be acutely increased after immune challenge, long-term BDNF expression is hindered in an inflammatory environment. Patients undergoing INFα treatment have significantly reduced BDNF levels[151,162], and Lotrich et al[162] found this effect was largest in those with the Val66Met genotype. In rodents, BDNF mRNA is significantly reduced after peripheral injection of LPS in the hippocampus[184,185], substantia nigra[186], and in the whole brain[161]. Similarly, poly I:C also reduces BDNF expression in the brain[135] and E. coli treatment down-regulated BDNF and reduced levels of phosphorylated TrkB receptors in the hippocampus of aged animals[187].
Down-regulation of BDNF may be driven by the pro-inflammatory cytokines IL-1β. In vitro experiments have shown that IL-1β treatment inhibits the neuroprotective effects of BDNF through the PI3-K and MAPK pathways and activity of the CREB transcription factor[188]. In rodents, exogenous IL-1β treatment blocks BDNF expression in the hippocampus[189-191], and while BDNF expression was not directly measured, chronic inflammation induced by BCG reduces neurogenesis[192] which is a BDNF-TrkB dependent process and correlate of anti-depressant efficacy.
THERAPEUTIC IMPLICATIONS
BDNF as a treatment target for inflammation-associated depression has its challenges. While BDNF and TrkB ligands do cross the b BBB[64,193], BDNF has opposing effects in different cell types and brain regions. For example, LPS treatment down-regulates BDNF in the hippocampus but up-regulates BDNF in the nucleus accumbens[77]. BDNF and TrkB agonists have anti-depressant-like effects in the hippocampus, but pro-depressive-like effects in the nucleus accumbens; inhibiting TrkB activation is anti-depressant in the nucleus accumbens[77]. Further, peripheral infusion of BDNF induces hyperalgesia[194] which, together with differential regionally-distinct CNS effects, precludes the therapeutic utility of systemic BDNF infusion and flooding the CNS with BDNF or TrkB ligands. However, intranasal ketamine, which was recently approved for anti-depressant use, activates BDNF-TrkB signaling directly in the brain, suggesting that therapeutic strategies that deliver BDNF-TrkB modulators directly to target regions within the CNS could prove efficacious.
Peripheral levels of BDNF and inflammatory markers may be useful as biomarkers for treatment-resistant depression, although this approach also is not without challenge. An ideal biomarker of risk or diagnosis should be reliably sensitive to predicting the disease in an asymptomatic individual and be specific to the disorder in question with little to no overlap with other diseases[195]. While low BDNF and high inflammation markers are frequently measured together in depressed individuals, there are many individuals who meet criteria but report no symptoms of depression, or become depressed without diverging from average serum levels of each marker. Additionally, low peripheral BDNF and elevated inflammatory markers are reported in other neurodegenerative or neuropsychiatric disorders, including Parkinson’s disease, bi-polar disorder, and schizophrenia[196-198]. Epigenetic patterns that disrupt inflammatory homeostasis or functional immunoreactivity of circulating immune cells may provide better prognostic value in predicting vulnerability.
Despite the obstacles, the association between BDNF and inflammation may have utility in deciding treatment options for depressed patients. Patients with inflammation and dysregulation of their BDNF-TrkB system may respond better to anti-depressant drugs with known anti-inflammatory properties, or anti-inflammatory drugs that incidentally have anti-depressant actions, and are able to elevate BDNF levels. Further mechanistic investigations of the interaction between BDNF expression and secretion and pro-inflammatory microglial responses may illuminate potentials targets for novel anti-depressant medication. One emerging approach that has yielded positive results in neurodegenerative disease is to use genetically modified hematopoietic stem cells that express growth factor and traffic specifically to the areas of the brain where pathology occurs[199,200]. While this approach has not yet been tested, it could be viable in cases of severe treatment-resistant depression.
CONCLUSION
Researchers have long recognized BDNF and neuroinflammation as key players in the development of neuropsychiatric conditions, notably major depressive disorder. Recent research has uncovered bi-directional modulation between these two risk factors in the development of depression with promising implications for predicting vulnerability to and treatment of depression. Future studies exploring the mechanisms of BDNF modulation by inflammatory signals, and the anti-inflammatory effects of BDNF in the brain, will provide greater insight into the complex pathogenesis of depression and other neuropsychiatric disorders.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Society for Neuroscience
Peer-review started: April 28, 2021
First decision: July 14, 2021
Article in press: December 2, 2021
Specialty type: Neurosciences
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bagheri-Mohammadi S, Han J S-Editor: Liu M L-Editor: A P-Editor: Liu M
Contributor Information
Grace A Porter, Department of Pharmacology, UT Health San Antonio, San Antonio, TX 78229, United States.
Jason C O’Connor, Department of Pharmacology, University of Texas Health San Antonio, San Antonio, TX 78229, United States; Audie L. Murphy VA Hospital, South Texas Veterans Health System, San Antonio, TX 78229, United States. oconnorj@uthscsa.edu.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) J Clin Psychiatry. 2015;76:155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- 3.Maisonpierre PC, Le Beau MM, Espinosa R 3rd, Ip NY, Belluscio L, de la Monte SM, Squinto S, Furth ME, Yancopoulos GD. Human and rat brain-derived neurotrophic factor and neurotrophin-3: gene structures, distributions, and chromosomal localizations. Genomics. 1991;10:558–568. doi: 10.1016/0888-7543(91)90436-i. [DOI] [PubMed] [Google Scholar]
- 4.Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90:397–406. doi: 10.1016/j.ygeno.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- 6.Lessmann V, Brigadski T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci Res. 2009;65:11–22. doi: 10.1016/j.neures.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta VK, You Y, Gupta VB, Klistorner A, Graham SL. TrkB receptor signalling: implications in neurodegenerative, psychiatric and proliferative disorders. Int J Mol Sci. 2013;14:10122–10142. doi: 10.3390/ijms140510122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto M, Sobue G, Yamamoto K, Terao S, Mitsuma T. Expression of mRNAs for neurotrophic factors (NGF, BDNF, NT-3, and GDNF) and their receptors (p75NGFR, trkA, trkB, and trkC) in the adult human peripheral nervous system and nonneural tissues. Neurochem Res. 1996;21:929–938. doi: 10.1007/BF02532343. [DOI] [PubMed] [Google Scholar]
- 10.Mousavi K, Jasmin BJ. BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J Neurosci. 2006;26:5739–5749. doi: 10.1523/JNEUROSCI.5398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho HJ, Kim SY, Park MJ, Kim DS, Kim JK, Chu MY. Expression of mRNA for brain-derived neurotrophic factor in the dorsal root ganglion following peripheral inflammation. Brain Res. 1997;749:358–362. doi: 10.1016/S0006-8993(97)00048-6. [DOI] [PubMed] [Google Scholar]
- 12.Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- 13.Noga O, Englmann C, Hanf G, Grützkau A, Seybold J, Kunkel G. The production, storage and release of the neurotrophins nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 by human peripheral eosinophils in allergics and non-allergics. Clin Exp Allergy. 2003;33:649–654. doi: 10.1046/j.1365-2222.2003.01586.x. [DOI] [PubMed] [Google Scholar]
- 14.Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87:728–734. [PubMed] [Google Scholar]
- 15.Saha RN, Liu X, Pahan K. Up-regulation of BDNF in astrocytes by TNF-alpha: a case for the neuroprotective role of cytokine. J Neuroimmune Pharmacol. 2006;1:212–222. doi: 10.1007/s11481-006-9020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 17.Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- 18.Frisén J, Verge VM, Fried K, Risling M, Persson H, Trotter J, Hökfelt T, Lindholm D. Characterization of glial trkB receptors: differential response to injury in the central and peripheral nervous systems. Proc Natl Acad Sci U S A. 1993;90:4971–4975. doi: 10.1073/pnas.90.11.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakajima K, Kikuchi Y, Ikoma E, Honda S, Ishikawa M, Liu Y, Kohsaka S. Neurotrophins regulate the function of cultured microglia. Glia. 1998;24:272–289. doi: 10.1002/(sici)1098-1136(199811)24:3<272::aid-glia2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Bath KG, Schilit A, Lee FS. Stress effects on BDNF expression: effects of age, sex, and form of stress. Neuroscience. 2013;239:149–156. doi: 10.1016/j.neuroscience.2013.01.074. [DOI] [PubMed] [Google Scholar]
- 21.Bechara RG, Kelly ÁM. Exercise improves object recognition memory and induces BDNF expression and cell proliferation in cognitively enriched rats. Behav Brain Res. 2013;245:96–100. doi: 10.1016/j.bbr.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Dandi Ε, Kalamari A, Touloumi O, Lagoudaki R, Nousiopoulou E, Simeonidou C, Spandou E, Tata DA. Beneficial effects of environmental enrichment on behavior, stress reactivity and synaptophysin/BDNF expression in hippocampus following early life stress. Int J Dev Neurosci. 2018;67:19–32. doi: 10.1016/j.ijdevneu.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Kentner AC, Khoury A, Lima Queiroz E, MacRae M. Environmental enrichment rescues the effects of early life inflammation on markers of synaptic transmission and plasticity. Brain Behav Immun. 2016;57:151–160. doi: 10.1016/j.bbi.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, McAuley E, Kramer AF. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palasz E, Wysocka A, Gasiorowska A, Chalimoniuk M, Niewiadomski W, Niewiadomska G. BDNF as a Promising Therapeutic Agent in Parkinson's Disease. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21031170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanila H. The role of BDNF in Alzheimer's disease. Neurobiol Dis. 2017;97:114–118. doi: 10.1016/j.nbd.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Ziegenhorn AA, Schulte-Herbrüggen O, Danker-Hopfe H, Malbranc M, Hartung HD, Anders D, Lang UE, Steinhagen-Thiessen E, Schaub RT, Hellweg R. Serum neurotrophins--a study on the time course and influencing factors in a large old age sample. Neurobiol Aging. 2007;28:1436–1445. doi: 10.1016/j.neurobiolaging.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Lapchak PA, Araujo DM, Beck KD, Finch CE, Johnson SA, Hefti F. BDNF and trkB mRNA expression in the hippocampal formation of aging rats. Neurobiol Aging. 1993;14:121–126. doi: 10.1016/0197-4580(93)90087-r. [DOI] [PubMed] [Google Scholar]
- 30.Keller S, Sarchiapone M, Zarrilli F, Videtic A, Ferraro A, Carli V, Sacchetti S, Lembo F, Angiolillo A, Jovanovic N, Pisanti F, Tomaiuolo R, Monticelli A, Balazic J, Roy A, Marusic A, Cocozza S, Fusco A, Bruni CB, Castaldo G, Chiariotti L. Increased BDNF promoter methylation in the Wernicke area of suicide subjects. Arch Gen Psychiatry. 2010;67:258–267. doi: 10.1001/archgenpsychiatry.2010.9. [DOI] [PubMed] [Google Scholar]
- 31.Ropret S, Zupanc T, Komel R, Videtič Paska A. Single nucleotide polymorphisms in the BDNF gene and suicide in the Slovenian sample. Neurosci Lett. 2015;602:12–16. doi: 10.1016/j.neulet.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 32.Hing B, Sathyaputri L, Potash JB. A comprehensive review of genetic and epigenetic mechanisms that regulate BDNF expression and function with relevance to major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. 2018;177:143–167. doi: 10.1002/ajmg.b.32616. [DOI] [PubMed] [Google Scholar]
- 33.Hing B, Davidson S, Lear M, Breen G, Quinn J, McGuffin P, MacKenzie A. A polymorphism associated with depressive disorders differentially regulates brain derived neurotrophic factor promoter IV activity. Biol Psychiatry. 2012;71:618–626. doi: 10.1016/j.biopsych.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarchiapone M, Carli V, Roy A, Iacoviello L, Cuomo C, Latella MC, di Giannantonio M, Janiri L, de Gaetano M, Janal MN. Association of polymorphism (Val66Met) of brain-derived neurotrophic factor with suicide attempts in depressed patients. Neuropsychobiology. 2008;57:139–145. doi: 10.1159/000142361. [DOI] [PubMed] [Google Scholar]
- 35.Pregelj P, Nedic G, Paska AV, Zupanc T, Nikolac M, Balažic J, Tomori M, Komel R, Seler DM, Pivac N. The association between brain-derived neurotrophic factor polymorphism (BDNF Val66Met) and suicide. J Affect Disord. 2011;128:287–290. doi: 10.1016/j.jad.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 37.Ozan E, Okur H, Eker C, Eker OD, Gönül AS, Akarsu N. The effect of depression, BDNF gene val66met polymorphism and gender on serum BDNF levels. Brain Res Bull. 2010;81:61–65. doi: 10.1016/j.brainresbull.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin Neurosci. 2010;64:341–357. doi: 10.1111/j.1440-1819.2010.02113.x. [DOI] [PubMed] [Google Scholar]
- 39.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Chakrapani S, Eskander N, De Los Santos LA, Omisore BA, Mostafa JA. Neuroplasticity and the Biological Role of Brain Derived Neurotrophic Factor in the Pathophysiology and Management of Depression. Cureus. 2020;12:e11396. doi: 10.7759/cureus.11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 42.Pandey GN, Ren X, Rizavi HS, Conley RR, Roberts RC, Dwivedi Y. Brain-derived neurotrophic factor and tyrosine kinase B receptor signalling in post-mortem brain of teenage suicide victims. Int J Neuropsychopharmacol. 2008;11:1047–1061. doi: 10.1017/S1461145708009000. [DOI] [PubMed] [Google Scholar]
- 43.Chauhan VS, Khan SA, Kulhari K. Correlation of brain-derived neurotrophic factor with severity of depression and treatment response. Med J Armed Forces India. 2020 doi: 10.1016/j.mjafi.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 46.Aydemir C, Yalcin ES, Aksaray S, Kisa C, Yildirim SG, Uzbay T, Goka E. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1256–1260. doi: 10.1016/j.pnpbp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 47.Cattaneo A, Bocchio-Chiavetto L, Zanardini R, Milanesi E, Placentino A, Gennarelli M. Reduced peripheral brain-derived neurotrophic factor mRNA levels are normalized by antidepressant treatment. Int J Neuropsychopharmacol. 2010;13:103–108. doi: 10.1017/S1461145709990812. [DOI] [PubMed] [Google Scholar]
- 48.Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Zhang H, Pavuluri MN. Brain-derived neurotrophic factor gene and protein expression in pediatric and adult depressed subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:645–651. doi: 10.1016/j.pnpbp.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Bukh JD, Bock C, Vinberg M, Werge T, Gether U, Vedel Kessing L. Interaction between genetic polymorphisms and stressful life events in first episode depression. J Affect Disord. 2009;119:107–115. doi: 10.1016/j.jad.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 50.Hwang JP, Tsai SJ, Hong CJ, Yang CH, Lirng JF, Yang YM. The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiol Aging. 2006;27:1834–1837. doi: 10.1016/j.neurobiolaging.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 51.Zhao M, Chen L, Yang J, Han D, Fang D, Qiu X, Yang X, Qiao Z, Ma J, Wang L, Jiang S, Song X, Zhou J, Zhang J, Chen M, Qi D, Yang Y, Pan H. BDNF Val66Met polymorphism, life stress and depression: A meta-analysis of gene-environment interaction. J Affect Disord. 2018;227:226–235. doi: 10.1016/j.jad.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 52.Lin E, Hong CJ, Hwang JP, Liou YJ, Yang CH, Cheng D, Tsai SJ. Gene-gene interactions of the brain-derived neurotrophic-factor and neurotrophic tyrosine kinase receptor 2 genes in geriatric depression. Rejuvenation Res. 2009;12:387–393. doi: 10.1089/rej.2009.0871. [DOI] [PubMed] [Google Scholar]
- 53.Schenkel LC, Segal J, Becker JA, Manfro GG, Bianchin MM, Leistner-Segal S. The BDNF Val66Met polymorphism is an independent risk factor for high lethality in suicide attempts of depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:940–944. doi: 10.1016/j.pnpbp.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 54.Kim YK, Lee HP, Won SD, Park EY, Lee HY, Lee BH, Lee SW, Yoon D, Han C, Kim DJ, Choi SH. Low plasma BDNF is associated with suicidal behavior in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:78–85. doi: 10.1016/j.pnpbp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 55.Kocabas NA, Antonijevic I, Faghel C, Forray C, Kasper S, Lecrubier Y, Linotte S, Massat I, Mendlewicz J, Noro M, Montgomery S, Oswald P, Snyder L, Zohar J, Souery D. Brain-derived neurotrophic factor gene polymorphisms: influence on treatment response phenotypes of major depressive disorder. Int Clin Psychopharmacol. 2011;26:1–10. doi: 10.1097/yic.0b013e32833d18f8. [DOI] [PubMed] [Google Scholar]
- 56.Gyekis JP, Yu W, Dong S, Wang H, Qian J, Kota P, Yang J. No association of genetic variants in BDNF with major depression: a meta- and gene-based analysis. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:61–70. doi: 10.1002/ajmg.b.32122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herbert J, Ban M, Brown GW, Harris TO, Ogilvie A, Uher R, Craig TK. Interaction between the BDNF gene Val/66/Met polymorphism and morning cortisol levels as a predictor of depression in adult women. Br J Psychiatry. 2012;201:313–319. doi: 10.1192/bjp.bp.111.107037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skibinska M, Groszewska A, Kapelski P, Rajewska-Rager A, Pawlak J, Dmitrzak-Weglarz M, Szczepankiewicz A, Twarowska-Hauser J. Val66Met functional polymorphism and serum protein level of brain-derived neurotrophic factor (BDNF) in acute episode of schizophrenia and depression. Pharmacol Rep. 2018;70:55–59. doi: 10.1016/j.pharep.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 59.Ryan KM, Dunne R, McLoughlin DM. BDNF plasma levels and genotype in depression and the response to electroconvulsive therapy. Brain Stimul. 2018;11:1123–1131. doi: 10.1016/j.brs.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 60.Froud A, Murphy J, Cribb L, Ng CH, Sarris J. The relationship between dietary quality, serum brain-derived neurotrophic factor (BDNF) level, and the Val66met polymorphism in predicting depression. Nutr Neurosci. 2019;22:513–521. doi: 10.1080/1028415X.2017.1415281. [DOI] [PubMed] [Google Scholar]
- 61.Kim JM, Jang JE, Stewart R, Kim SY, Kim SW, Kang HJ, Shin IS, Park MH, Yoon JH, Yoon JS. Determinants of suicidal ideation in patients with breast cancer. Psychooncology. 2013;22:2848–2856. doi: 10.1002/pon.3367. [DOI] [PubMed] [Google Scholar]
- 62.Dooley LN, Ganz PA, Cole SW, Crespi CM, Bower JE. Val66Met BDNF polymorphism as a vulnerability factor for inflammation-associated depressive symptoms in women with breast cancer. J Affect Disord. 2016;197:43–50. doi: 10.1016/j.jad.2016.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ng T, Teo SM, Yeo HL, Shwe M, Gan YX, Cheung YT, Foo KM, Cham MT, Lee JA, Tan YP, Fan G, Yong WS, Preetha M, Loh WJ, Koo SL, Jain A, Lee GE, Wong M, Dent R, Yap YS, Ng R, Khor CC, Ho HK, Chan A. Brain-derived neurotrophic factor genetic polymorphism (rs6265) is protective against chemotherapy-associated cognitive impairment in patients with early-stage breast cancer. Neuro Oncol. 2016;18:244–251. doi: 10.1093/neuonc/nov162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 65.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- 66.Lee BH, Kim YK. Reduced platelet BDNF level in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:849–853. doi: 10.1016/j.pnpbp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 2005;57:1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe K, Hashimoto E, Ukai W, Ishii T, Yoshinaga T, Ono T, Tateno M, Watanabe I, Shirasaka T, Saito S, Saito T. Effect of antidepressants on brain-derived neurotrophic factor (BDNF) release from platelets in the rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1450–1454. doi: 10.1016/j.pnpbp.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 69.Ibarguen-Vargas Y, Surget A, Vourc'h P, Leman S, Andres CR, Gardier AM, Belzung C. Deficit in BDNF does not increase vulnerability to stress but dampens antidepressant-like effects in the unpredictable chronic mild stress. Behav Brain Res. 2009;202:245–251. doi: 10.1016/j.bbr.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 70.Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 71.Advani T, Koek W, Hensler JG. Gender differences in the enhanced vulnerability of BDNF+/- mice to mild stress. Int J Neuropsychopharmacol. 2009;12:583–588. doi: 10.1017/S1461145709000248. [DOI] [PubMed] [Google Scholar]
- 72.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35:2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, Han M, Hashimoto K. Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 2015;232:4325–4335. doi: 10.1007/s00213-015-4062-3. [DOI] [PubMed] [Google Scholar]
- 77.Zhang JC, Wu J, Fujita Y, Yao W, Ren Q, Yang C, Li SX, Shirayama Y, Hashimoto K. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- 80.Haile CN, Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Foulkes A, Iqbal S, Mahoney JJ 3rd, De La Garza R 2nd, Charney DS, Newton TF, Mathew SJ. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol. 2014;17:331–336. doi: 10.1017/S1461145713001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park SE, Dantzer R, Kelley KW, McCusker RH. Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice. J Neuroinflammation. 2011;8:12. doi: 10.1186/1742-2094-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, Craig IW, Anacker C, Zunsztain PA, McGuffin P, Pariante CM. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline 'predictors' and longitudinal 'targets'. Neuropsychopharmacology. 2013;38:377–385. doi: 10.1038/npp.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ping G, Qian W, Song G, Zhaochun S. Valsartan reverses depressive/anxiety-like behavior and induces hippocampal neurogenesis and expression of BDNF protein in unpredictable chronic mild stress mice. Pharmacol Biochem Behav. 2014;124:5–12. doi: 10.1016/j.pbb.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 84.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 85.Lepack AE, Bang E, Lee B, Dwyer JM, Duman RS. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology. 2016;111:242–252. doi: 10.1016/j.neuropharm.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takano K, Yamasaki H, Kawabe K, Moriyama M, Nakamura Y. Imipramine induces brain-derived neurotrophic factor mRNA expression in cultured astrocytes. J Pharmacol Sci. 2012;120:176–186. doi: 10.1254/jphs.12039fp. [DOI] [PubMed] [Google Scholar]
- 87.Allaman I, Fiumelli H, Magistretti PJ, Martin JL. Fluoxetine regulates the expression of neurotrophic/growth factors and glucose metabolism in astrocytes. Psychopharmacology (Berl) 2011;216:75–84. doi: 10.1007/s00213-011-2190-y. [DOI] [PubMed] [Google Scholar]
- 88.Hisaoka-Nakashima K, Kajitani N, Kaneko M, Shigetou T, Kasai M, Matsumoto C, Yokoe T, Azuma H, Takebayashi M, Morioka N, Nakata Y. Amitriptyline induces brain-derived neurotrophic factor (BDNF) mRNA expression through ERK-dependent modulation of multiple BDNF mRNA variants in primary cultured rat cortical astrocytes and microglia. Brain Res. 2016;1634:57–67. doi: 10.1016/j.brainres.2015.12.057. [DOI] [PubMed] [Google Scholar]
- 89.Peng CH, Chiou SH, Chen SJ, Chou YC, Ku HH, Cheng CK, Yen CJ, Tsai TH, Chang YL, Kao CL. Neuroprotection by Imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur Neuropsychopharmacol. 2008;18:128–140. doi: 10.1016/j.euroneuro.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 90.Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry. 2008;63:642–649. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adachi M, Autry AE, Mahgoub M, Suzuki K, Monteggia LM. TrkB Signaling in Dorsal Raphe Nucleus is Essential for Antidepressant Efficacy and Normal Aggression Behavior. Neuropsychopharmacology. 2017;42:886–894. doi: 10.1038/npp.2016.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castrén E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiménez-Dalmaroni MJ, Gerswhin ME, Adamopoulos IE. The critical role of toll-like receptors--From microbial recognition to autoimmunity: A comprehensive review. Autoimmun Rev. 2016;15:1–8. doi: 10.1016/j.autrev.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shih RH, Wang CY, Yang CM. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front Mol Neurosci. 2015;8:77. doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lingappan K. NF-κB in Oxidative Stress. Curr Opin Toxicol. 2018;7:81–86. doi: 10.1016/j.cotox.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abdulkhaleq LA, Assi MA, Abdullah R, Zamri-Saad M, Taufiq-Yap YH, Hezmee MNM. The crucial roles of inflammatory mediators in inflammation: A review. Vet World. 2018;11:627–635. doi: 10.14202/vetworld.2018.627-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zanos TP, Silverman HA, Levy T, Tsaava T, Battinelli E, Lorraine PW, Ashe JM, Chavan SS, Tracey KJ, Bouton CE. Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity. Proc Natl Acad Sci U S A. 2018;115:E4843–E4852. doi: 10.1073/pnas.1719083115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Engblom D, Ek M, Saha S, Ericsson-Dahlstrand A, Jakobsson PJ, Blomqvist A. Prostaglandins as inflammatory messengers across the blood-brain barrier. J Mol Med (Berl) 2002;80:5–15. doi: 10.1007/s00109-001-0289-z. [DOI] [PubMed] [Google Scholar]
- 103.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Parrott JM, O'Connor JC. Kynurenine 3-Monooxygenase: An Influential Mediator of Neuropathology. Front Psychiatry. 2015;6:116. doi: 10.3389/fpsyt.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O'Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 107.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 108.Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctôt KL, Carvalho AF. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- 109.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Lebonté B, Horn S, Lapidus KA, Stelzhammer V, Wong EH, Bahn S, Krishnan V, Bolaños-Guzman CA, Murrough JW, Merad M, Russo SJ. Individual differences in the peripheral immune system promote resilience vs susceptibility to social stress. Proc Natl Acad Sci U S A. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dahl J, Ormstad H, Aass HC, Malt UF, Bendz LT, Sandvik L, Brundin L, Andreassen OA. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. 2014;45:77–86. doi: 10.1016/j.psyneuen.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 111.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ohgi Y, Futamura T, Kikuchi T, Hashimoto K. Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav. 2013;103:853–859. doi: 10.1016/j.pbb.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 113.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 115.Suzuki H, Ohgidani M, Kuwano N, Chrétien F, Lorin de la Grandmaison G, Onaya M, Tominaga I, Setoyama D, Kang D, Mimura M, Kanba S, Kato TA. Suicide and Microglia: Recent Findings and Future Perspectives Based on Human Studies. Front Cell Neurosci. 2019;13:31. doi: 10.3389/fncel.2019.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, Conley RR, Dwivedi Y. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res. 2012;46:57–63. doi: 10.1016/j.jpsychires.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pandey GN, Rizavi HS, Zhang H, Bhaumik R, Ren X. Abnormal protein and mRNA expression of inflammatory cytokines in the prefrontal cortex of depressed individuals who died by suicide J Psychiatry Neurosci 2018; 43: 376-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Black C, Miller BJ. Meta-Analysis of Cytokines and Chemokines in Suicidality: Distinguishing Suicidal Versus Nonsuicidal Patients. Biol Psychiatry. 2015;78:28–37. doi: 10.1016/j.biopsych.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 119.Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, Mawrin C, Brisch R, Bielau H, Meyer zu Schwabedissen L, Bogerts B, Myint AM. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang C, Wardenaar KJ, Bosker FJ, Li J, Schoevers RA. Inflammatory markers and treatment outcome in treatment resistant depression: A systematic review. J Affect Disord. 2019;257:640–649. doi: 10.1016/j.jad.2019.07.045. [DOI] [PubMed] [Google Scholar]
- 121.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 122.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 123.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 124.Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 125.Remus JL, Dantzer R. Inflammation Models of Depression in Rodents: Relevance to Psychotropic Drug Discovery. Int J Neuropsychopharmacol. 2016;19 doi: 10.1093/ijnp/pyw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 127.Stepanichev M, Dygalo NN, Grigoryan G, Shishkina GT, Gulyaeva N. Rodent models of depression: neurotrophic and neuroinflammatory biomarkers. Biomed Res Int. 2014;2014:932757. doi: 10.1155/2014/932757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sugama S, Fujita M, Hashimoto M, Conti B. Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neuroscience. 2007;146:1388–1399. doi: 10.1016/j.neuroscience.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 129.You Z, Luo C, Zhang W, Chen Y, He J, Zhao Q, Zuo R, Wu Y. Pro- and anti-inflammatory cytokines expression in rat's brain and spleen exposed to chronic mild stress: involvement in depression. Behav Brain Res. 2011;225:135–141. doi: 10.1016/j.bbr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 130.Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 131.Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Culley DJ, Snayd M, Baxter MG, Xie Z, Lee IH, Rudolph J, Inouye SK, Marcantonio ER, Crosby G. Systemic inflammation impairs attention and cognitive flexibility but not associative learning in aged rats: possible implications for delirium. Front Aging Neurosci. 2014;6:107. doi: 10.3389/fnagi.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Capuron L, Lasselin J, Castanon N. Role of Adiposity-Driven Inflammation in Depressive Morbidity. Neuropsychopharmacology. 2017;42:115–128. doi: 10.1038/npp.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kelley KW, Dantzer R. Alcoholism and inflammation: neuroimmunology of behavioral and mood disorders. Brain Behav Immun. 2011;25 Suppl 1:S13–S20. doi: 10.1016/j.bbi.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gibney SM, McGuinness B, Prendergast C, Harkin A, Connor TJ. Poly I:C-induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain Behav Immun. 2013;28:170–181. doi: 10.1016/j.bbi.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 136.O'Connor JC, Lawson MA, André C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW. Induction of IDO by bacille Calmette-Guérin is responsible for development of murine depressive-like behavior. J Immunol. 2009;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.O'Connor JC, André C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O'Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Frenois F, Moreau M, O'Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ge L, Liu L, Liu H, Liu S, Xue H, Wang X, Yuan L, Wang Z, Liu D. Resveratrol abrogates lipopolysaccharide-induced depressive-like behavior, neuroinflammatory response, and CREB/BDNF signaling in mice. Eur J Pharmacol. 2015;768:49–57. doi: 10.1016/j.ejphar.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 141.Sulakhiya K, Keshavlal GP, Bezbaruah BB, Dwivedi S, Gurjar SS, Munde N, Jangra A, Lahkar M, Gogoi R. Lipopolysaccharide induced anxiety- and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin. Neurosci Lett. 2016;611:106–111. doi: 10.1016/j.neulet.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 142.Ji WW, Wang SY, Ma ZQ, Li RP, Li SS, Xue JS, Li W, Niu XX, Yan L, Zhang X, Fu Q, Qu R, Ma SP. Effects of perillaldehyde on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav. 2014;116:1–8. doi: 10.1016/j.pbb.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 143.Yao W, Zhang JC, Dong C, Zhuang C, Hirota S, Inanaga K, Hashimoto K. Effects of amycenone on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav. 2015;136:7–12. doi: 10.1016/j.pbb.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 144.Mello BS, Monte AS, McIntyre RS, Soczynska JK, Custódio CS, Cordeiro RC, Chaves JH, Vasconcelos SM, Nobre HV Jr, Florenço de Sousa FC, Hyphantis TN, Carvalho AF, Macêdo DS. Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J Psychiatr Res. 2013;47:1521–1529. doi: 10.1016/j.jpsychires.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 145.Salazar A, Gonzalez-Rivera BL, Redus L, Parrott JM, O'Connor JC. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm Behav. 2012;62:202–209. doi: 10.1016/j.yhbeh.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heisler JM, O'Connor JC. Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory. Brain Behav Immun. 2015;50:115–124. doi: 10.1016/j.bbi.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O'Connor JC. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry. 2016;6:e918. doi: 10.1038/tp.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang JC, Yao W, Hashimoto K. Brain-derived Neurotrophic Factor (BDNF)-TrkB Signaling in Inflammation-related Depression and Potential Therapeutic Targets. Curr Neuropharmacol. 2016;14:721–731. doi: 10.2174/1570159X14666160119094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carniel BP, da Rocha NS. Brain-derived neurotrophic factor (BDNF) and inflammatory markers: Perspectives for the management of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2021;108:110151. doi: 10.1016/j.pnpbp.2020.110151. [DOI] [PubMed] [Google Scholar]
- 150.Pedard M, Quirié A, Tessier A, Garnier P, Totoson P, Demougeot C, Marie C. A reconciling hypothesis centred on brain-derived neurotrophic factor to explain neuropsychiatric manifestations in rheumatoid arthritis. Rheumatology (Oxford) 2021;60:1608–1619. doi: 10.1093/rheumatology/keaa849. [DOI] [PubMed] [Google Scholar]
- 151.Kenis G, Prickaerts J, van Os J, Koek GH, Robaeys G, Steinbusch HW, Wichers M. Depressive symptoms following interferon-α therapy: mediated by immune-induced reductions in brain-derived neurotrophic factor? Int J Neuropsychopharmacol. 2011;14:247–253. doi: 10.1017/S1461145710000830. [DOI] [PubMed] [Google Scholar]
- 152.Uint L, Bastos GM, Thurow HS, Borges JB, Hirata TDC, França JID, Hirata MH, Sousa AGMR. Increased levels of plasma IL-1b and BDNF can predict resistant depression patients. Rev Assoc Med Bras (1992) 2019;65:361–369. doi: 10.1590/1806-9282.65.3.361. [DOI] [PubMed] [Google Scholar]
- 153.Solati Z, Jazayeri S, Tehrani-Doost M, Mahmoodianfard S, Gohari MR. Zinc monotherapy increases serum brain-derived neurotrophic factor (BDNF) levels and decreases depressive symptoms in overweight or obese subjects: a double-blind, randomized, placebo-controlled trial. Nutr Neurosci. 2015;18:162–168. doi: 10.1179/1476830513Y.0000000105. [DOI] [PubMed] [Google Scholar]
- 154.Yang XH, Song SQ, Xu Y. Resveratrol ameliorates chronic unpredictable mild stress-induced depression-like behavior: involvement of the HPA axis, inflammatory markers, BDNF, and Wnt/β-catenin pathway in rats. Neuropsychiatr Dis Treat. 2017;13:2727–2736. doi: 10.2147/NDT.S150028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tan X, Du X, Jiang Y, Botchway BOA, Hu Z, Fang M. Inhibition of Autophagy in Microglia Alters Depressive-Like Behavior via BDNF Pathway in Postpartum Depression. Front Psychiatry. 2018;9:434. doi: 10.3389/fpsyt.2018.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guo J, Lin P, Zhao X, Zhang J, Wei X, Wang Q, Wang C. Etazolate abrogates the lipopolysaccharide (LPS)-induced downregulation of the cAMP/pCREB/BDNF signaling, neuroinflammatory response and depressive-like behavior in mice. Neuroscience. 2014;263:1–14. doi: 10.1016/j.neuroscience.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 157.Li L, Yu AL, Wang ZL, Chen K, Zheng W, Zhou JJ, Xie Q, Yan HB, Ren P, Huang X. Chaihu-Shugan-San and absorbed meranzin hydrate induce anti-atherosclerosis and behavioral improvements in high-fat diet ApoE-/- mice via anti-inflammatory and BDNF-TrkB pathway. Biomed Pharmacother. 2019;115:108893. doi: 10.1016/j.biopha.2019.108893. [DOI] [PubMed] [Google Scholar]
- 158.Ren Z, Yan P, Zhu L, Yang H, Zhao Y, Kirby BP, Waddington JL, Zhen X. Dihydromyricetin exerts a rapid antidepressant-like effect in association with enhancement of BDNF expression and inhibition of neuroinflammation. Psychopharmacology (Berl) 2018;235:233–244. doi: 10.1007/s00213-017-4761-z. [DOI] [PubMed] [Google Scholar]
- 159.Miao H, Li R, Han C, Lu X, Zhang H. Minocycline promotes posthemorrhagic neurogenesis via M2 microglia polarization via upregulation of the TrkB/BDNF pathway in rats. J Neurophysiol. 2018;120:1307–1317. doi: 10.1152/jn.00234.2018. [DOI] [PubMed] [Google Scholar]
- 160.Yang Y, Song Y, Zhang X, Zhao W, Ma T, Liu Y, Ma P, Zhao Y, Zhang H. Ketamine relieves depression-like behaviors induced by chronic postsurgical pain in rats through anti-inflammatory, anti-oxidant effects and regulating BDNF expression. Psychopharmacology (Berl) 2020;237:1657–1669. doi: 10.1007/s00213-020-05490-3. [DOI] [PubMed] [Google Scholar]
- 161.Basu Mallik S, Mudgal J, Hall S, Kinra M, Grant GD, Nampoothiri M, Anoopkumar-Dukie S, Arora D. Remedial effects of caffeine against depressive-like behaviour in mice by modulation of neuroinflammation and BDNF. Nutr Neurosci. 2021:1–9. doi: 10.1080/1028415X.2021.1906393. [DOI] [PubMed] [Google Scholar]
- 162.Lotrich FE, Albusaysi S, Ferrell RE. Brain-derived neurotrophic factor serum levels and genotype: association with depression during interferon-α treatment. Neuropsychopharmacology. 2013;38:985–995. doi: 10.1038/npp.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chan CB, Ye K. Sex differences in brain-derived neurotrophic factor signaling and functions. J Neurosci Res. 2017;95:328–335. doi: 10.1002/jnr.23863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 165.Derry HM, Padin AC, Kuo JL, Hughes S, Kiecolt-Glaser JK. Sex Differences in Depression: Does Inflammation Play a Role? Curr Psychiatry Rep. 2015;17:78. doi: 10.1007/s11920-015-0618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology. 2015;40:1709–1716. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Han J, Fan Y, Zhou K, Blomgren K, Harris RA. Uncovering sex differences of rodent microglia. J Neuroinflammation. 2021;18:74. doi: 10.1186/s12974-021-02124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu Y, Sheng H, Tang Z, Lu J, Ni X. Inflammation and increased IDO in hippocampus contribute to depression-like behavior induced by estrogen deficiency. Behav Brain Res. 2015;288:71–78. doi: 10.1016/j.bbr.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 169.Gomes C, Ferreira R, George J, Sanches R, Rodrigues DI, Gonçalves N, Cunha RA. Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J Neuroinflammation. 2013;10:16. doi: 10.1186/1742-2094-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schulte-Herbrüggen O, Nassenstein C, Lommatzsch M, Quarcoo D, Renz H, Braun A. Tumor necrosis factor-alpha and interleukin-6 regulate secretion of brain-derived neurotrophic factor in human monocytes. J Neuroimmunol. 2005;160:204–209. doi: 10.1016/j.jneuroim.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 171.Tu Z, Li Y, Dai Y, Li L, Lv G, Chen I, Wang B. MiR-140/BDNF axis regulates normal human astrocyte proliferation and LPS-induced IL-6 and TNF-α secretion. Biomed Pharmacother. 2017;91:899–905. doi: 10.1016/j.biopha.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 172.Zhang J, Geula C, Lu C, Koziel H, Hatcher LM, Roisen FJ. Neurotrophins regulate proliferation and survival of two microglial cell lines in vitro. Exp Neurol. 2003;183:469–481. doi: 10.1016/s0014-4886(03)00222-x. [DOI] [PubMed] [Google Scholar]
- 173.Zhang X, Zeng L, Yu T, Xu Y, Pu S, Du D, Jiang W. Positive feedback loop of autocrine BDNF from microglia causes prolonged microglia activation. Cell Physiol Biochem. 2014;34:715–723. doi: 10.1159/000363036. [DOI] [PubMed] [Google Scholar]
- 174.Joosten EA, Houweling DA. Local acute application of BDNF in the lesioned spinal cord anti-inflammatory and anti-oxidant effects. Neuroreport. 2004;15:1163–1166. doi: 10.1097/00001756-200405190-00016. [DOI] [PubMed] [Google Scholar]
- 175.Wu SY, Pan BS, Tsai SF, Chiang YT, Huang BM, Mo FE, Kuo YM. BDNF reverses aging-related microglial activation. J Neuroinflammation. 2020;17:210. doi: 10.1186/s12974-020-01887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Han R, Liu Z, Sun N, Liu S, Li L, Shen Y, Xiu J, Xu Q. BDNF Alleviates Neuroinflammation in the Hippocampus of Type 1 Diabetic Mice via Blocking the Aberrant HMGB1/RAGE/NF-κB Pathway. Aging Dis. 2019;10:611–625. doi: 10.14336/AD.2018.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim JM, Stewart R, Kim JW, Kang HJ, Lee JY, Kim SY, Kim SW, Shin IS, Hong YJ, Ahn Y, Jeong MH, Yoon JS. Modifying effects of depression on the association between BDNF methylation and prognosis of acute coronary syndrome. Brain Behav Immun. 2019;81:422–429. doi: 10.1016/j.bbi.2019.06.038. [DOI] [PubMed] [Google Scholar]
- 178.Jiang Y, Wei N, Zhu J, Lu T, Chen Z, Xu G, Liu X. Effects of brain-derived neurotrophic factor on local inflammation in experimental stroke of rat. Mediators Inflamm. 2010;2010:372423. doi: 10.1155/2010/372423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Makar TK, Bever CT, Singh IS, Royal W, Sahu SN, Sura TP, Sultana S, Sura KT, Patel N, Dhib-Jalbut S, Trisler D. Brain-derived neurotrophic factor gene delivery in an animal model of multiple sclerosis using bone marrow stem cells as a vehicle. J Neuroimmunol. 2009;210:40–51. doi: 10.1016/j.jneuroim.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 180.Jiang Y, Wei N, Lu T, Zhu J, Xu G, Liu X. Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience. 2011;172:398–405. doi: 10.1016/j.neuroscience.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 181.Xu D, Lian D, Wu J, Liu Y, Zhu M, Sun J, He D, Li L. Brain-derived neurotrophic factor reduces inflammation and hippocampal apoptosis in experimental Streptococcus pneumoniae meningitis. J Neuroinflammation. 2017;14:156. doi: 10.1186/s12974-017-0930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dugan AM, Parrott JM, Redus L, Hensler JG, O'Connor JC. Low-Level Stress Induces Production of Neuroprotective Factors in Wild-Type but Not BDNF+/- Mice: Interleukin-10 and Kynurenic Acid. Int J Neuropsychopharmacol. 2015;19:pyv089. doi: 10.1093/ijnp/pyv089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Park HY, Park C, Hwang HJ, Kim BW, Kim GY, Kim CM, Kim ND, Choi YH. 7,8-Dihydroxyflavone attenuates the release of pro-inflammatory mediators and cytokines in lipopolysaccharide-stimulated BV2 microglial cells through the suppression of the NF-κB and MAPK signaling pathways. Int J Mol Med. 2014;33:1027–1034. doi: 10.3892/ijmm.2014.1652. [DOI] [PubMed] [Google Scholar]
- 184.Guan Z, Fang J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun. 2006;20:64–71. doi: 10.1016/j.bbi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 185.Golia MT, Poggini S, Alboni S, Garofalo S, Ciano Albanese N, Viglione A, Ajmone-Cat MA, St-Pierre A, Brunello N, Limatola C, Branchi I, Maggi L. Interplay between inflammation and neural plasticity: Both immune activation and suppression impair LTP and BDNF expression. Brain Behav Immun. 2019;81:484–494. doi: 10.1016/j.bbi.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 186.Wu SY, Wang TF, Yu L, Jen CJ, Chuang JI, Wu FS, Wu CW, Kuo YM. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun. 2011;25:135–146. doi: 10.1016/j.bbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 187.Cortese GP, Barrientos RM, Maier SF, Patterson SL. Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLCgamma1, and ERK in hippocampal synaptoneurosomes. J Neurosci. 2011;31:4274–4279. doi: 10.1523/JNEUROSCI.5818-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tong L, Balazs R, Soiampornkul R, Thangnipon W, Cotman CW. Interleukin-1 beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol Aging. 2008;29:1380–1393. doi: 10.1016/j.neurobiolaging.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lapchak PA, Araujo DM, Hefti F. Systemic interleukin-1 beta decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience. 1993;53:297–301. doi: 10.1016/0306-4522(93)90196-m. [DOI] [PubMed] [Google Scholar]
- 190.Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 191.Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 192.Solano Fonseca R, Mahesula S, Apple DM, Raghunathan R, Dugan A, Cardona A, O'Connor J, Kokovay E. Neurogenic Niche Microglia Undergo Positional Remodeling and Progressive Activation Contributing to Age-Associated Reductions in Neurogenesis. Stem Cells Dev. 2016;25:542–555. doi: 10.1089/scd.2015.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu C, Chan CB, Ye K. 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Transl Neurodegener. 2016;5:2. doi: 10.1186/s40035-015-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Groth R, Aanonsen L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain. 2002;100:171–181. doi: 10.1016/s0304-3959(02)00264-6. [DOI] [PubMed] [Google Scholar]
- 195.Davis J, Maes M, Andreazza A, McGrath JJ, Tye SJ, Berk M. Towards a classification of biomarkers of neuropsychiatric disease: from encompass to compass. Mol Psychiatry. 2015;20:152–153. doi: 10.1038/mp.2014.139. [DOI] [PubMed] [Google Scholar]
- 196.Kauer-Sant'Anna M, Kapczinski F, Andreazza AC, Bond DJ, Lam RW, Young LT, Yatham LN. Brain-derived neurotrophic factor and inflammatory markers in patients with early- vs. late-stage bipolar disorder. Int J Neuropsychopharmacol. 2009;12:447–458. doi: 10.1017/S1461145708009310. [DOI] [PubMed] [Google Scholar]
- 197.Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson's disease. J Neural Transm Suppl. 2000:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- 198.Zhang XY, Tan YL, Chen DC, Tan SP, Yang FD, Wu HE, Zunta-Soares GB, Huang XF, Kosten TR, Soares JC. Interaction of BDNF with cytokines in chronic schizophrenia. Brain Behav Immun. 2016;51:169–175. doi: 10.1016/j.bbi.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 199.Chen C, Li X, Ge G, Liu J, Biju KC, Laing SD, Qian Y, Ballard C, He Z, Masliah E, Clark RA, O'Connor JC, Li S. GDNF-expressing macrophages mitigate loss of dopamine neurons and improve Parkinsonian symptoms in MitoPark mice. Sci Rep 2018; 8: 5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chen C, Guderyon MJ, Li Y, Ge G, Bhattacharjee A, Ballard C, He Z, Masliah E, Clark RA, O'Connor JC, Li S. Non-toxic HSC Transplantation-Based Macrophage/Microglia-Mediated GDNF Delivery for Parkinson's Disease. Mol Ther Methods Clin Dev. 2020;17:83–98. doi: 10.1016/j.omtm.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Parrott JM, Porter GA, Redus L, O'Connor JC. Brain derived neurotrophic factor deficiency exacerbates inflammation-induced anhedonia in mice. Psychoneuroendocrinology. 2021;134:105404. doi: 10.1016/j.psyneuen.2021.105404. [DOI] [PMC free article] [PubMed] [Google Scholar]