Abstract
Introduction
Early identification of etiology is very important for initiating appropriate therapy promptly in patients with meningoencephalitis (ME). BioFire FilmArray® meningitis/encephalitis (FA-ME) panel is a fully automated multiplex polymerase chain reaction (PCR) that detects 14 pathogens simultaneously in an hour. There is a dearth of studies highlighting its usefulness in ME syndrome in Indian patients.
Materials and methods
We performed a retrospective analysis of patients, admitted to the Kerala Institute of Medical Sciences Hospital, Thiruvananthapuram, Kerala, South India, with meningitis/encephalitis syndrome who underwent the multiplex PCR test on cerebrospinal fluid (CSF) over a period of 2 years from 2016 to 2018. Patients presenting with clinical diagnosis of acute meningitis, encephalitis, or ME who underwent CSF FA-ME panel were studied. The performance of the FA-ME panel was compared to CSF bacterial culture.
Results
Two-hundred and fifty-nine patients between December 2016 and December 2018 underwent the FA-ME test in CSF. FA-ME test detected pathogens in 61 (23.6%) out of 259 patients with ME syndrome. Among the pathogens detected by FA-ME panel, enterovirus was the commonest accounting for 29 cases (47.5%), followed by varicella in 11 patients (18%) and pneumococci in 9 (14.8%). CSF bacterial culture yield was low, positive only in 8 (3%) out of 259 cases, and matched with FA-ME panel in only one sample that grew Streptococcus pneumoniae. Bacterial culture yielded seven pathogens in those whose FA-ME panels were negative.
Conclusion
FA-ME panel improves diagnostic yield as compared to bacterial culture (26.3 vs 3%). FA-ME test helps in the early initiation of targeted antibiotic therapy and greater antibiotic de-escalation.
How to cite this article
Chandran S, Arjun R, Sasidharan A, Niyas VKM, Chandran S. Clinical Performance of FilmArray Meningitis/Encephalitis Multiplex Polymerase Chain Reaction Panel in Central Nervous System Infections. Indian J Crit Care Med 2022;26(1):67–70.
Keywords: Acute meningitis, Encephalopathy, Polymerase chain reaction
Introduction
Acute central nervous system (CNS) infections are among the most serious clinical conditions in medicine. Bacterial meningitis is associated with morbidity due to neurological complications and also mortality in both children and adults. It has an annual incidence of 4–6 cases per 100,000 population.1 In meningitis and encephalitis, rapid and accurate detection of etiology is extremely important in providing appropriate therapy to patients and also in avoiding unnecessary use of broad-spectrum antibiotics, thus preventing the development of drug resistance. Etiological surveillance is also crucial to identify targets for immunization, chart preventive strategies and to help formulate rational empirical therapy. Streptococcus pneumoniae and Neisseria meningitidis are responsible for 80% of the cases of bacterial meningitis globally.1 Enteroviruses are currently the leading recognizable cause of aseptic meningitis syndrome accounting for 85–95% of all pathogens identified.2
Novel and rapid molecular techniques are critical in identifying etiology and initiating appropriate therapy quickly in patients with meningoencephalitis (ME). Polymerase chain reaction (PCR) assays to diagnose CNS infection still remain underutilized. A fully automated multiplex PCR, the BioFire FilmArray® meningitis/encephalitis (FA-ME) panel, detects 14 pathogens simultaneously in an hour.3 There are studies highlighting its usefulness in ME syndrome. As there is not much data from India, we undertook this study to understand its performance.
Materials and Methods
To understand the performance of the FA-ME panel (BioFire Diagnostics, USA), we performed a retrospective analysis of patients, both adult and pediatric population, admitted with meningitis/encephalitis syndrome and who underwent the multiplex PCR test on CSF. Patients presenting with clinical diagnosis of acute meningitis, encephalitis, or ME with any of the following symptoms and signs of fever, headache, vomiting, photophobia, neck stiffness, focal neurological deficit, and who underwent CSF FA-ME panel were studied. This study was done in the Kerala Institute of Medical Sciences Hospital, Trivandrum, Kerala, South India, from December 2016 to December 2018. The performance of the FA-ME panel was compared to that of routine tests done on CSF, namely cell count, biochemistry, Gram's stain, bacterial culture, and other relevant tests.
Patient details including age, sex, address, month of admission, history, and clinical examination findings were noted. Basic blood parameters and other relevant investigations like blood culture, serologies for relevant infections including tropical fever like leptospirosis, scrub typhus, dengue virus, infectious virology, and brain imaging results were also noted. CSF parameters like total count, differential count, protein, sugar, Gram's stain, other special stains (acid-fast bacteria (AFB) stain, India ink, KOH stain), CSF culture, and sensitivity for bacterial, fungal, and mycobacterial were looked into. The CSF FA-ME panel (BioFire Diagnostics) results were noted and compared with standard microbiological methods for the diagnostic yield of the FA-ME panel as compared to routine tests and its utility in a tropical country like India.
Results
There were 259 patients between December 2016 and December 2018 who underwent this test. Turnaround time for the FA-ME panel was 2 hours. The age group ranged from 3 months to 86 years. The FA-ME panel detected pathogens in 61 (23.6%) out of 259 patients with ME syndrome. Among these 61 patients, 18 belonged to the pediatric age group. Among patients who had a positive result in FA-ME panel, 53 had >5 white cell counts, while 8 had <5 cells in the CSF. Viruses were commoner accounting for one-third (70.4%) of the pathogens, bacteria were found in 24.5%, and two patients had two organisms in a single sample (enterovirus and Haemophilus influenzae on both occasions). Among the pathogens detected by FA-ME panel, enterovirus was the commonest accounting for 29 cases (47.5%), followed by varicella in 11 patients (18%) and pneumococci in 9 (14.8%). Human herpesvirus 6 (HHV 6) was detected in three cases, H. influenzae, Streptococcus agalactiae and Listeria were detected in two cases each (Fig. 1). Meningococci, E. coli, Cryptococcus, and other viruses were not noted in this study.
Fig. 1.
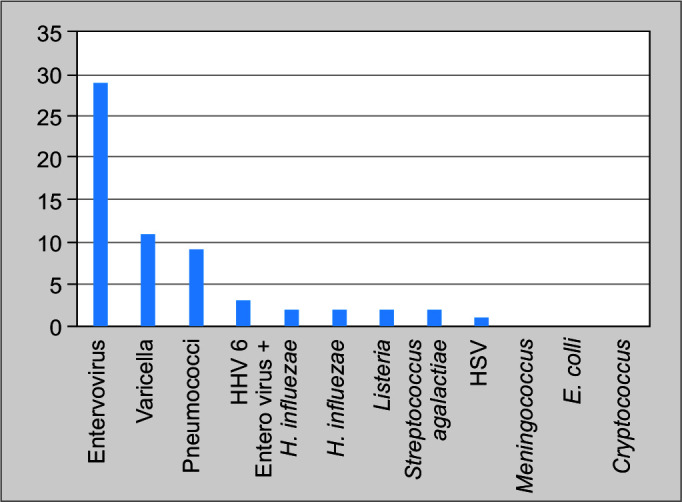
Distributions of pathogens (in numbers) identified by FA-ME panel
CSF bacterial culture yield was low, positive only in 8 (3%) out of 259 cases, and matched with FA-ME panel in only one sample which grew S. pneumoniae. Bacterial culture of CSF yielded seven other pathogens in those whose FA-ME panels were negative. The organism that grew in blood culture and the clinical diagnosis noted are as follows—Aerococcus viridans (aseptic meningitis), Streptococcal species (pyogenic meningitis), Micrococcus luteus (meningitis with epidural abscess), Aeromonas salmonicida (autoimmune encephalitis), Pseudomonas species (septic cavernous sinus thrombophlebitis with meningitis), and Klebsiella pneumoniae in two samples (both post-traumatic and post-op meningitis) (Table 1). FA-ME panel tests for six bacteria commonly causing community-acquired and neonatal meningitis and the above organisms are not included in the panel.
Table 1.
Pathogens identified in CSF culture whose FA-ME panel was negative
| Clinical diagnosis | CSF bacterial culture |
|---|---|
| Aseptic meningitis | Aerococcus viridans |
| Pyogenic meningitis | Streptococcus species |
| Meningitis/epidural abscess | Micrococcus luteus |
| Auto-immune encephalitis | Aeromonas salmonicida |
| Septic cerebral venous thrombosis, meningitis | Pseudomonas species |
| Traumatic brain injury | Klebsiella pneumoniae |
| Traumatic brain injury | K. pneumoniae |
One hundred and ninety-one patients (73.7%) were treated as CNS infection although FA-ME panel results were negative. CNS tuberculosis (TB) was diagnosed in 6 of these 259 patients, in one case the cartridge-based nucleic acid amplification test (CB-NAAT by Xpert MTB RIF) was positive, and in the rest, it was clinical TB based on clinical, CSF, and image findings. Seven patients had aseptic meningitis due to tropical infections, diagnosed based on serology of which five were dengue fever and two were scrub typhus. The rest of the patients (26.3%) had alternative diagnosis like autoimmune, malignancy, metabolic, and vascular etiology.
Enterovirus meningitis occurred almost equally in pediatric and adult age groups. It showed seasonal prevalence and was found commonly during the period October until December. Among the 29 cases, 24 enteroviral meningitis cases occurred during this period (Fig. 2).
Fig. 2.
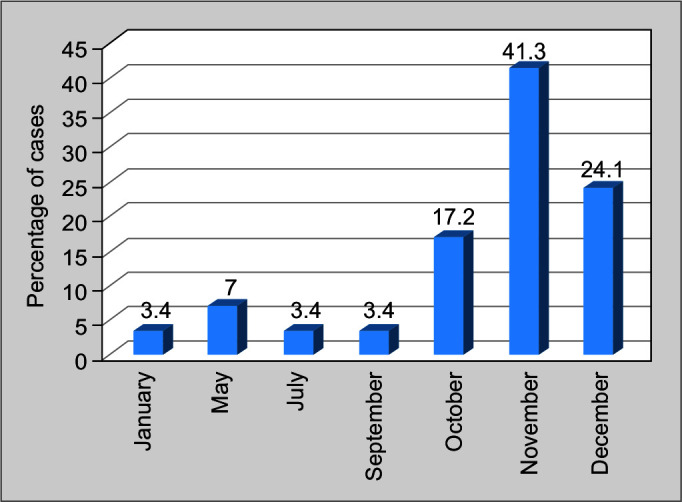
Month-wise distribution of enteroviral meningitis occurrence
Among the FA-ME panel positive group, antibiotic de-escalation was noted in 49%, whereas in the negative group, de-escalation happened in only 32% and the difference was statistically significant (p = 0.042).
Discussion
In the majority of the cases, diagnosis of ME is challenging as diagnostic yield with the tests commonly done is low. Moreover, CNS infections are associated with morbidity and mortality and a test with a quick turnaround time is important to clear the diagnostic dilemma. PCR-based assays are becoming popular for syndromic diagnosis like respiratory, gastrointestinal, and sepsis.4–6 Multiplex PCR panel to diagnose ME is more often used and becoming the standard of care in many countries. BioFire FA-ME panel is a Food and Drug Administration (FDA)-approved multiplex PCR test for CNS infections, simultaneously detecting 14 pathogens with minimal CSF volume and rapid turnaround time.
Infectious Diseases Society of America (IDSA) guidelines on the management of encephalitis released in 2008 include molecular diagnostics in the evaluation of encephalitis syndrome.7 There are quite a few studies looking at the performance of PCR-based studies and have shown better yield as compared to routine microbiological testing. An earlier study by Tzanakaki et al., wherein multiplex PCR was used to detect the top three pathogens causing meningitis, namely S. pneumoniae, H. influenzae type b, and N. meningitidis, was done. It was a single-tube assay where specific targets for the above pathogens were detected with good positive and negative predictive values.8 In a large prospective observational study, Nesher et al., compare non-PCR era (1999–2008) with a later period (2008–2013) when PCR was commonly used. Among the 323 patients enrolled, PCR provided the highest diagnostic yield (24·2%) but was ordered only for 39.6% of the patients. The yield of blood cultures was (10.3%) and that of CSF cultures was 4%. Even with PCR-based testing, in a good number of patients, diagnosis remained unknown, but with PCR, the diagnostic yield was better (38–47%); this change was attributed to diagnosing more viral pathogens, 8.3 and 26.3%, respectively.9
Only a limited number of studies are available in India on multiplex PCR for CNS infection. An Indian study conducted in 2017 demonstrated that multiplex PCR Syndrome Evaluation System (SES, Xcyton Diagnostics Limited) had a detection rate of 42.18% and clinical specificity of 100% which are higher than the results of our study.10 SES results elicited changes in therapy and superior patient outcome was observed. S. pneumoniae and mycobacterium TB were the most common bacterial pathogens. Varicella-zoster virus (VZV) was the most often detected viral pathogens by multiplex PCR. Another retrospective study conducted by Ramalingam et al. showed that SES (SES, Xcyton Diagnostics Limited) had a clinical sensitivity of 57.4% and specificity of 95.6%.11 S. pneumoniae and Pseudomonas aeruginosa were the top two bacterial pathogens isolated, and herpes simplex was the most common viral pathogen isolated. SES result helped in changing empiric to targeted therapy in 30% of the cases.
In our study the yield of FA-ME panel was 23.6% as compared to 3% by the standard microbiological methods. Majority were viruses contributing to better diagnostic yield by FA-ME panel. In previous studies as well, viruses accounted for the majority.9 Bacterial pathogens were also picked up better by FA-ME panel (1.6% by culture vs 24.5% by FA-ME panel). The overall low yield of the FA-ME panel was probably due to prior antibiotic therapy as the majority come after initial empiric antibiotic exposure. It is to be noted that similar results were found in CSF FA-ME when studied in negative Gram's stain cases by Wootton et al.3 Also it is to be noted that 26.3% had a noninfectious cause for their symptoms which would have contributed to low yield.
The commonest organism was enteroviruses, closely followed by varicella and pneumococci. Meningococcus and cryptococcus were not noted and none in the study had HIV. A seasonal prevalence of enterovirus infection was also noted in the study with clustering of cases in the period from October to December. Previous data reveal that though enteroviral meningitis can occur round the year, it usually peaks during the summer.12 Only further study will help to understand seasonality in this part of the world and avoid unnecessary antimicrobials.
FA-ME panel has some inadequacies as it can fail to detect nosocomial pathogens in a setting of post-trauma or post-op meningitis as these are usually caused by gram-negative bacteria like K. pneumoniae, Acinetobacter, Pseudomonas, and methicillin-resistant Staphylococcus aureus (MRSA). This may explain the pathogens that were detected by conventional culture but were missed in the FA-ME panel (Table 1). Certain community onset pathogens that can present as acute meningitis syndrome like leptospirosis, scrub typhus, dengue fever, and common subacute to chronic meningitis like TB can be missed by using this panel as a stand alone test. This is similar to a study by Wootton et al., where West Nile virus and histoplasmosis which are the important causes of ME in their geographic area were not detected by the panel.3 Hence in appropriate clinical situations, serology for the above tropical infections as well as CB-NAAT for TB should be additionally done.
FA-ME panel can help in antimicrobial stewardship if prompt revision in treatment is made based on the PCR reports. Among the FA-ME panel positive group, antibiotic de-escalation was noted in 49%, whereas in the negative group, de-escalation happened in only 32%, the difference being statistically significant. In his study by MacVane looking at the FilmArray blood culture identification (FA-BCID) panel, when it was linked to antimicrobial stewardship program (ASP) intervention, antibiotic de-escalation happened more effectively than without.13
Conclusion
FA-ME panel improves diagnostic yield as compared to bacterial culture (26.3 vs 3%). Viruses were the comonest and the majority were enteroviruses which is the likely reason for improved yield. Enteroviruses showed seasonal prevalence and occurred commonly between October and December. Those bacteria that grew in CSF culture but not identified by FA-ME panel were not in the panel, were nosocomial pathogens or unusual pathogens and the gap in diagnosis need to be understood. Also, if ME syndrome is considered secondary to TB, tropical infections, or zoonotic neurotropic viruses, additional tests are needed for diagnosis. The group that had positive results by this panel resulted in greater antibiotic de-escalation.
Research Quality and Ethics Statement
The authors of this manuscript declare that this scientific work complies with reporting quality, formatting, and reproducibility guidelines set forth by the EQUATOR Network. The authors also attest that this clinical investigation was determined not to require the Institutional Review Board/Ethics Committee review, and the corresponding protocol/approval number is not applicable. We also certify that we have not plagiarizedthe contents in this submission and have done a plagiarism check.
Acknowledgment
The authors would like to thank the team members of the Kerala Institute of Medical Sciences for their support for the clinical research.
Orcid
Sarath Chandran https://orcid.org/0000-0002-8452-9360
Rajalakshmi Arjun https://orcid.org/0000-0002-4838-183X
Aswathy Sasidharan https://orcid.org/0000-0002-8153-4236
Vettakkara KM Niyas https://orcid.org/0000-0002-7255-6257
Suresh Chandran https://orcid.org/0000-0001-8721-1107
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.World Health Organization. Geneva: World Health Organization; 2006. Neurological disorders: public health challenges. pp. 97–102. p. [Google Scholar]
- 2.Bennet JE, Blaser MJ, Dolin R, editors. 8th ed. Canada: Saunders; 2015. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. pp. 1097–1163. p. [Google Scholar]
- 3.Wootton SH, Aguilera E, Salazar L, Hemmert AC, Hasbun R. Enhancing pathogen identification in patients with meningitis and a negative Gram's stain using the BioFire FilmArray® Meningitis/Encephalitis panel. Ann Clin Microbiol Antimicrob. 2016;15(1):26. doi: 10.1186/s12941-016-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poritz MA, Blaschke AJ, Byington CL, Meyers L, Nilsson K, Jones DE, et al. FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: development and application to respiratory tract infection. PLoS One. 2011;6(10):e26047. doi: 10.1371/journal.pone.0026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buss SN, Leber A, Chapin K, Fey PD, Bankowski MJ, Jones MK, et al. Multicenter evaluation of the BioFire FilmArray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. J Clin Microbiol. 2015;53(3):915–925. doi: 10.1128/JCM.02674-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaschke AJ, Heyrend C, Byington CL, Fisher MA, Barker E, Garrone NF, et al. Rapid identification of pathogens from positive blood cultures by multiplex polymerase chain reaction using the FilmArray system. Diagn Microbiol Infect Dis. 2012;74(4):349–355. doi: 10.1016/j.diagmicrobio.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ, Marra CM, Roos KL, et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47(3):303–327. doi: 10.1086/589747. [DOI] [PubMed] [Google Scholar]
- 8.Tzanakaki G, Tsopanomichalou M, Kesanopoulos K, Matzourani R, Sioumala M, Tabaki A, et al. Simultaneous single-tube PCR assay for the detection of Neisseria meningitidis, Haemophilus influenzae type b and Streptococcus pneumoniae. Clin Microbiol Infect. 2005;11(5):386–390. doi: 10.1111/j.1469-0691.2005.01109.x. [DOI] [PubMed] [Google Scholar]
- 9.Nesher L, Hadi CM, Salazar L, Wootton SH, Garey KW, Lasco T, et al. Epidemiology of meningitis with a negative CSF Gram's stain: under-utilization of available diagnostic tests. Epidemiol Infect. 2016;144(1):189–197. doi: 10.1017/S0950268815000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Javali M, Acharya P, Mehta A, John AA, Mahale R, Srinivasa R. Use of multiplex PCR based molecular diagnostics in diagnosis of suspected CNS infections in tertiary care setting—a retrospective study. Clin Neurol Neurosurg. 2017;161:110–116. doi: 10.1016/j.clineuro.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Ramalingam RK, Chakraborty D. Retrospective analysis of multiplex polymerase chain reaction-based molecular diagnostics (SES) in 70 patients with suspected central nervous system infections: a single-center study. Ann Indian Acad Neurol. 2016;19(4):482. doi: 10.4103/0972-2327.192483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pons-Salort M, Oberste MS, Pallansch MA, Abedi GR, Takahashi S, Grenfell BT, et al. The seasonality of nonpolio enteroviruses in the United States: patterns and drivers. Proc Natl Acad Sci USA. 2018;115(12):3078–3083. doi: 10.1073/pnas.1721159115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacVane SH, Nolte FS. Benefits of adding a rapid PCR-based blood culture identification panel to an established antimicrobial stewardship program. J Clin Microbiol. 2016;54(10):2455–2463. doi: 10.1128/JCM.00996-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


