Abstract
Objective:
Evaluate the effect of age on opioid consumption after traumatic injury.
Summary Background Data:
Older trauma patients receive fewer opioids due to decreased metabolism and increased complications, but adequacy of pain control is unknown. We hypothesized that older trauma patients require fewer opioids to achieve adequate pain control.
Methods:
A secondary analysis of the MAST Trial evaluating the effectiveness of two multi-modal pain regimens in 1,561 trauma patients aged 16 to 96 was performed. Older patients (≥55 years) were compared to younger patients. Median daily oral morphine milligram equivalents (MME) consumption, average numeric rating scale (NRS) pain scores, complications, and death were assessed. Multivariable analyses were performed.
Results:
Older patients (n=562) had a median age of 68 years (IQR 61–78) compared to 33 (24–43) in younger patients. Older patients had lower injury severity scores (13 [9–20] vs 14 [9–22], p=0.004), lower average pain scores (NRS 3 [1–4] vs 4 [2–5], p<0.001), and consumed fewer MME/day (22 [10–45] vs 52 [28–78], p<0.001). The MAST MMPR was effective at reducing opioid consumption at all ages. Additionally, on multivariable analysis including pain score adjustment, each decade age increase after 55 years was associated with a 23% reduction in MME/day consumed.
Conclusion:
Older trauma patients required fewer opioids than younger patients with similar characteristics and pain scores. Opioid dosing for post-traumatic pain should consider age. A 20 to 25% dose reduction per decade after age 55 may reduce opioid exposure without altering pain control.
Mini-Abstract:
This secondary analysis of the MAST randomized, clinical trial evaluated the effect of age on opioid consumption after traumatic injury. Older trauma patients required fewer opioids and fewer opioid prescriptions than younger patients with similar injuries and pain scores. Consideration of age in opioid dosing may decrease opioid exposure while maintaining adequate pain control.
Introduction
The proportion of older patients presenting to a hospital with an acute injury is rapidly increasing. From 2003 to 2009, the proportion of older (>65 years) injured patients presenting to trauma centers participating in the National Trauma Data Bank increased from 23% to 30% and some regions face more rapid growth of the older trauma patient population. 1 Numerous challenges exist in the treatment of an aging trauma population, who suffer higher morbidity and mortality than similarly injured young patients. 2
Optimal management of acute pain associated with traumatic injuries is necessary to reduce respiratory and other complications and to facilitate physical therapy and rehabilitation. 3,4 Increasingly, more focus is being placed on achieving adequate pain control while also minimizing opioids. Among older trauma patients, reducing opioid prescribing may be even more important given the physiologic changes and increasing prevalence of comorbidities with aging. While older patients have similar opioid absorption and distribution of opioid medications as younger patients, decreased hepatic metabolism and decreased renal elimination result in increased free serum drug levels and prolonged half-lives of opioid medications. 5–7 Furthermore, there is evidence that older adults experience enhanced pharmacodynamic sensitivity, where a medication has a stronger effect at a given concentration. 8 These issues may contribute to physicians’ hesitancy to administer opioids to older trauma patients.
While avoidance of opioid medications for the treatment of post-traumatic pain is preferable, it is unknown if an opioid-minimizing strategy is effective among older patients. There are multiple reports that older patients’ pain is undertreated, resulting in uncontrolled pain and additional adverse effects. 1,9 Thus, the optimal strategy for striking a fine balance between adequate pain control and avoidance of analgesic complications warrants further investigation among this important and growing trauma population. 10 We hypothesized that an opioid-minimizing multi-modal pain regimen (MMPR) would be effective in older trauma patients. Our secondary hypothesis was that older trauma patients require fewer opioids to achieve adequate pain control.
Methods
A secondary analysis of the multimodal analgesic strategy for trauma (MAST) trial (NCT03472469) was performed. The MAST trial was a pragmatic, randomized, comparative effectiveness trial comparing the original MMPR with the MAST MMPR. The original MMPR was implemented at the Red Duke Trauma Institute at Memorial Hermann Hospital-Texas Medical Center in 2013 and included 5 medication classes administered on a fixed dosing and timing schedule (Table 1). The medication classes included central prostaglandin inhibitors, non-steroidal anti-inflammatory drugs (NSAID), gabapentinoids, weak mu-opioid agonists, and a local anesthetic. Opioids were then applied as needed. The MAST MMPR used cheaper, generic alternatives and avoided scheduled tramadol. Opioid medications were administered on an as-needed basis for breakthrough pain in both groups. Modifications of the MMPR were done at the discretion of the physician, in keeping with the pragmatic trial design. Examples of modifications frequently made at the study institution include decreasing gabapentinoid dose in cases of acute or chronic renal insufficiency, withholding NSAIDs in cases of renal insufficiency, cirrhosis, or congestive heart failure, and re-starting home medications for patients previously undergoing treatment for chronic pain. It was not routine to withhold NSAIDs for orthopedic injuries, intracranial hemorrhage, or operative intervention. The trial was approved by the McGovern Medical School at UTHealth institutional reviewed board and was conducted between April 2, 2018 and March 31, 2019.
Table 1:
Pain Regimens
| Original MMPR | MAST MMPR |
|---|---|
| IV Acetaminophen 1g q6h x24h -> PO Acetaminophen 1g q6h | PO Acetaminophen 1g q6h |
| Ketorolac 30mg x1 dose | Ketorolac 30mg x1 dose |
| Celecoxib 200 mg PO q6h x48h -> Naproxen 500 mg PO q12h | Naproxen 500 mg PO q12h |
| Pregabalin 100 mg PO q8h x48h -> Gabapentin 300mg PO q8h | Gabapentin 300mg PO q8h |
| Lidocaine Patch q12h | Lidocaine Patch q12h |
| Tramadol 100mg PO q6h | Tramadol as needed |
| Opioids as needed | Opioids as needed |
The MAST trial protocol and results have previously been published. 11,12 Adults (≥16 years) admitted to the trauma service were randomized to receive the original MMPR or the MAST opioid-minimizing MMPR in a 1:1 allocation ratio. The level of care (floor, intermediate care unit, or intensive care unit [ICU]) was used as the stratification variable. The allocated MMPR was initiated on hospital admission. Written, informed consent was obtained from all study participants or their legally authorized representative.
The primary outcome was opioid exposure, estimated by oral morphine milligram equivalents (MMEs) per day using a standardized conversion factors, divided by length of hospital stay. Secondary outcomes included total MMEs and hospital discharge with an opioid prescription. Complications and mortality rates were recorded according to the National Trauma Databank Dictionary. 13 Adapted Clavien-Dindo in Trauma (ACDiT), which grades complications from 0 (no complication) to V (death), was used to score the severity of complications suffered by each patient. 14 Routine pain scores measured by nursing assessment utilized an 11-point scale with either the patient-stated numeric rating scale (NRS) or the nursing-assigned behavioral pain scale (BPS). 15,16 Pain was assessed at the time of vital sign evaluation at pre-determined minimum by unit of admission. Floor level patients were assessed for pain at least every 4 hours, intermediate unit level patients were assessed at least every 2 hours, and ICU level patients were assessed at least hourly. The NRS pain scale was administered verbally by the nurses and patient responses were recorded. In the event that a patient had impaired communication, the BPS was utilized as an alternative. 17 Prior opioid use was defined as either historical or current use of opioid. Urine drug screen was considered positive if cocaine, barbiturate, cannabis, or amphetamine were present in the urine.
Comparisons were made between two groups of patients: older and younger. Older was defined as greater than age 55 as numerous prior studies of trauma have demonstrated worsened outcomes after trauma above 55. 18,19 Demographics and outcomes with continuous variables were presented as medians and interquartile ranges (IQRs). Chi-square and Wilcoxon rank-sum tests were utilized to compare categorical and continuous demographic data and outcomes. Multivariable regression models were utilized to assess the relationship between outcomes and age as a continuous variable. Best model fit was assessed by minimization of Akaike information criterion Age was modelled using natural cubic splines to account for possible non-linear association with MME/day. 20 All associations were reported as risk ratios or absolute differences with 95% confidence intervals. Covariables known or suspected to be confounders between age and MME consumption were chosen a priori: factors including sex, number of rib fractures, presence of long bone fractures, presence of pelvic fractures, injury severity score, thoracotomy required, laparotomy required, amputation required, MMPR regimen allocation, history of prior opioid use, admission unit, and average pain score category. Average pain scores were converted into categories high, medium, or low, according to average pain score tertile. For patients who had both an average NRS and an average BPS pain score, the NRS was used to assign the patient to a pain score category. To evaluate our hypothesis regarding the effectiveness of the MAST MMPR at decreasing MME/day regardless of age, another multivariable model was built including randomization group, age (using a natural cubic spline), interaction term between group and age, and unit of admission as covariables. All data analyses were completed using R version 3.53 (R Core Team. 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
Results
Of 1,561 total patients included in the MAST trial, 562 (36%) were older. (Table 2) Older patients were more frequently female and injured by blunt mechanism. Older patients were less severely injured than younger patients. Older patients more frequently had a history of opioid use but less frequently arrived with a positive urine drug screen or with alcohol intoxication. Older patients were less frequently smokers.
Table 2:
Demographics, Younger and Older
| All Patients (n=1,561) | Younger (n=999) | Older (n=562) | P | |
|---|---|---|---|---|
| Age, years | 45 (29–63) | 33 (24–43) | 68 (61–78) | <0.001 |
|
| ||||
| Male Sex | 1061 (68%) | 736 (74%) | 325 (58%) | <0.001 |
|
| ||||
| Race/Ethnicity | ||||
| White | 726 (47%) | 371 (37%) | 355 (63%) | |
| Black | 307 (20%) | 233 (23%) | 74 (13%) | <0.001 |
| Hispanic | 487 (31%) | 374 (37%) | 113 (20%) | |
| Other | 41 (3%) | 21 (2%) | 20 (4%) | |
|
| ||||
| Blunt Mechanism of Injury | 1288 (83%) | 760 (76%) | 528 (94%) | <0.001 |
|
| ||||
| Injury Severity Score | 14 (9–22) | 14 (9–22) | 13 (9–20) | 0.004 |
|
| ||||
| Unit of Admission | ||||
| Floor | 585 (38%) | 408 (41%) | 177 (32%) | |
| Intensive Monitoring Unit | 380 (24%) | 195 (20%) | 185 (33%) | <0.001 |
| Intensive Care Unit | 552 (35%) | 369 (37%) | 183 (33%) | |
| Other | 44 (3%) | 27 (3%) | 17 (3%) | |
|
| ||||
| Number of Rib Fractures | 0 (0–4) | 0 (0–3) | 2 (0–5) | <0.001 |
|
| ||||
| Long Bone Fracture | 501 (32%) | 325 (33%) | 176 (31%) | 0.66 |
|
| ||||
| Pelvis Fracture | 285 (18%) | 193 (19%) | 92 (16%) | 0.17 |
|
| ||||
| Traumatic Brain Injury | 305 (20%) | 182 (18%) | 123 (22%) | 0.09 |
|
| ||||
| Abbreviated Injury Scale | ||||
| Head | 0 (0–2) | 0 (0–2) | 0 (0–0) | 0.31 |
| Face | 0 (0–0) | 0 (0–0) | 0 (0–0) | <0.001 |
| Chest | 2 (0–3) | 2 (0–3) | 2 (0–3) | 0.003 |
| Abdomen | 0 (0–2) | 0 (0–2) | 0 (0–2) | <0.001 |
| External | 1 (0–1) | 1 (0–1) | 1 (0–1) | <0.001 |
| Extremity | 2 (0–2) | 2 (0–3) | 2 (0–2) | 0.005 |
|
| ||||
| History of Opioid Use | 117 (8%) | 50 (5%) | 67 (12%) | <0.001 |
|
| ||||
| Positive Urine Drug Screen | 377 (24%) | 327 (33%) | 50 (9%) | <0.001 |
|
| ||||
| Positive EtOH on Arrival | 248 (16%) | 208 (21%) | 40 (7%) | <0.001 |
|
| ||||
| History of Smoking | 460 (30%) | 344 (34%) | 116 (21%) | <0.001 |
|
| ||||
| Underwent Laparotomy | 182 (12%) | 149 (15%) | 33 (6%) | <0.001 |
|
| ||||
| Underwent Thoracotomy | 64 (4%) | 41 (4%) | 23 (4%) | 1 |
|
| ||||
| Underwent Amputation | 21 (1%) | 19 (2%) | 2 (0.4%) | 0.02 |
|
| ||||
| MAST MMPR Allocation | 774 (50%) | 506 (51%) | 268 (48%) | 0.28 |
Continuous data presented as: median (IQR)
EtOH – Alcohol; MAST – Multimodal Analgesic Strategies in Trauma; MMPR – Multi-modal Pain Regimen
Older patients utilized fewer total MMEs and fewer MME/day than younger patients.(Table 3) Without adjustment, older patients utilized approximately half the total MMEs and MME/day than younger patients. Older patients were less frequently sent home with an opioid prescription. Older patients reported lower NRS pain scores and were assigned similar BPS scores as younger patients. There were 54 patients (27 in each age group) who had neither NRS nor BPS pain score assessments. Older patients more frequently suffered complications and with a higher ACDiT score than their younger counterparts. They had longer lengths of hospitalization, longer ventilator duration, and similar ICU lengths of stay. Older patients less frequently were discharged to home and suffered a higher mortality than younger patients.
Table 3:
Outcomes, Younger and Older
| Outcome | All Patients (n=1,561) | Younger (n=999) | Older (n=562) | P |
|---|---|---|---|---|
| Average NRS | 3 (2–5) | 4 (2–5) | 3 (1–4) | <0.001 |
|
| ||||
| Average BPS | 2 (2–3) | 2 (2–3) | 3 (2–3) | 0.06 |
|
| ||||
| Opioid Rx | 1003 (64%) | 714 (71%) | 289 (51%) | <0.001 |
|
| ||||
| Death | 65 (4%) | 21 (2%) | 44 (8%) | <0.001 |
|
| ||||
| Cardiac Arrest | 31 (2%) | 12 (1%) | 19 (3%) | 0.006 |
|
| ||||
| Unplanned ICU Admission | 61 (4%) | 25 (3%) | 36 (6%) | <0.001 |
|
| ||||
| Unplanned Intubation | 32 (2%) | 9 (1%) | 23 (4%) | <0.001 |
|
| ||||
| Acute Renal Failure | 43 (3%) | 11 (1%) | 32 (6%) | <0.001 |
|
| ||||
| ACDiT | <0.001 | |||
| 0 | 753 (48%) | 534 (54%) | 219 (39%) | |
| 1 | 192 (12%) | 119 (12%) | 73 (13%) | |
| 2 | 295 (19%) | 116 (17%) | 129 (23%) | |
| 3a | 48 (3%) | 29 (3%) | 19 (3%) | |
| 3b | 65 (4%) | 51 (5%) | 14 (3%) | |
| 4a | 109 (7%) | 64 (6%) | 45 (8%) | |
| 4b | 34 (2%) | 15 (2%) | 19 (3%) | |
| 5a | 19 (1%) | 5 (1%) | 14 (3%) | |
| 5b | 46 (3%) | 16 (2%) | 30 (5%) | |
|
| ||||
| Deep Vein Thrombosis | 9 (1%) | 5 (0.3%) | 4 (1%) | 0.9 |
|
| ||||
| Pulmonary Embolus | 20 (1%) | 14 (1%) | 6 (1%) | 0.7 |
|
| ||||
| Cerebral Vascular Accident | 12 (1%) | 4 (0.3%) | 8 (1%) | 0.05 |
|
| ||||
| Sepsis | 16 (1%) | 7 (0.4%) | 9 (2%) | 0.2 |
|
| ||||
| Length of Stay, Days | 5 (3–11) | 5 (3–11) | 6 (3–11) | 0.008 |
|
| ||||
| ICU Days | 0 (0–3) | 0 (0–3) | 0 (0–3) | 0.7 |
|
| ||||
| Patients on Ventilator | 352 (23%) | 245 (24%) | 107 (19%) | 0.02 |
| Ventilator Days* | 2 (1–6.3) | 2 (1–5) | 3 (1–11) | 0.02 |
|
| ||||
| Milligram Morphine Equivalents, total | 198 (77–473) | 246 (100–584) | 124 (42–300) | <0.001 |
|
| ||||
| Average Milligram Morphine Equivalents per Day | 41 (18–68) | 52 (28–78) | 21 (10–45) | <0.001 |
|
| ||||
| Discharge Disposition | <0.001 | |||
| Home | 1188 (76%) | 861 (86%) | 327 (58%) | |
| Rehabilitation | 157 (10%) | 32 (3%) | 125 (22%) | |
| Long-Term Care | 38 (2%) | 19 (2%) | 19 (3%) | |
| Skilled Nursing Fac. | 113 (7%) | 66 (7%) | 47 (8%) | |
| Death | 65 (4%) | 21 (2%) | 44 (8%) | |
Continuous data presented as: median (IQR)
Limited to patients requiring ventilation
NRS – Numeric rating scale; BPS – Behavioral Pain Score; Rx – Prescription; ICU – Intensive Care Unit; ACDiT – Adapted Clavien Dindo in Trauma
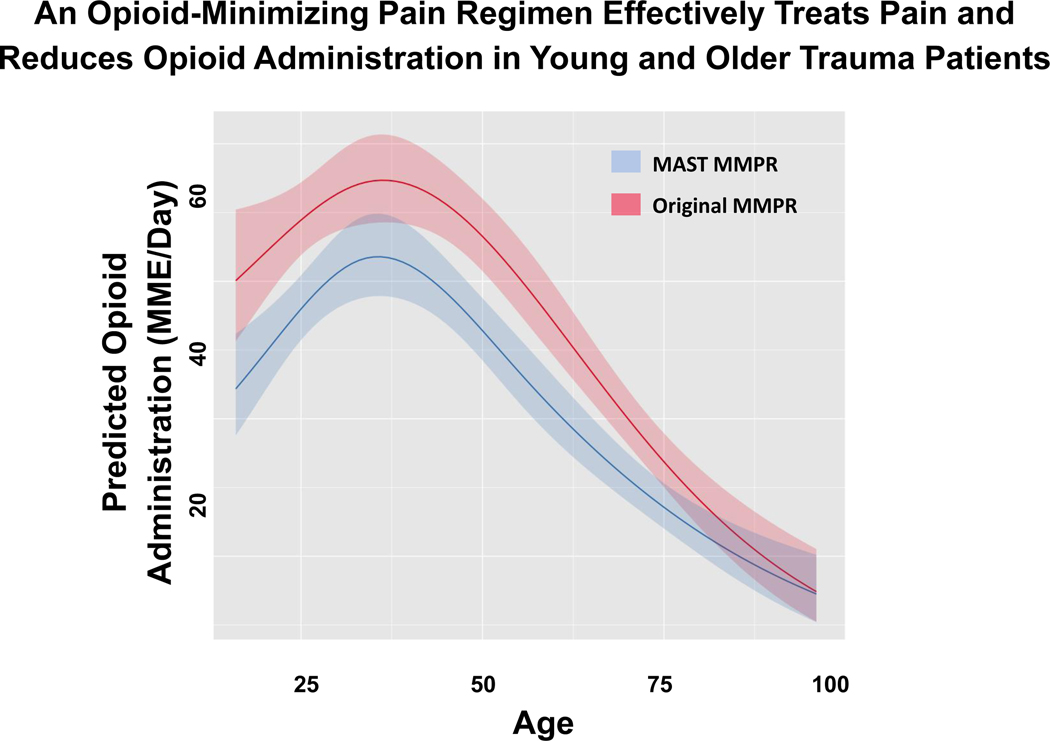
The MAST MMPR was effective at decreasing MME/day across all ages. (Figure 1) After adjustment for age, the MAST MMPR was associated with a 20% (IQR 6% to 23%) relative reduction in MME/day consumed. There was no interaction between the MAST MMPR allocation and age. Finally, the MAST MMPR was not associated with increased complications (RR 1.02, IQR 0.69–1.51).
FIGURE 1:
An opioid-minimizing pain regimen effectively treats pain and reduces opioid administration in young and older trauma patients.
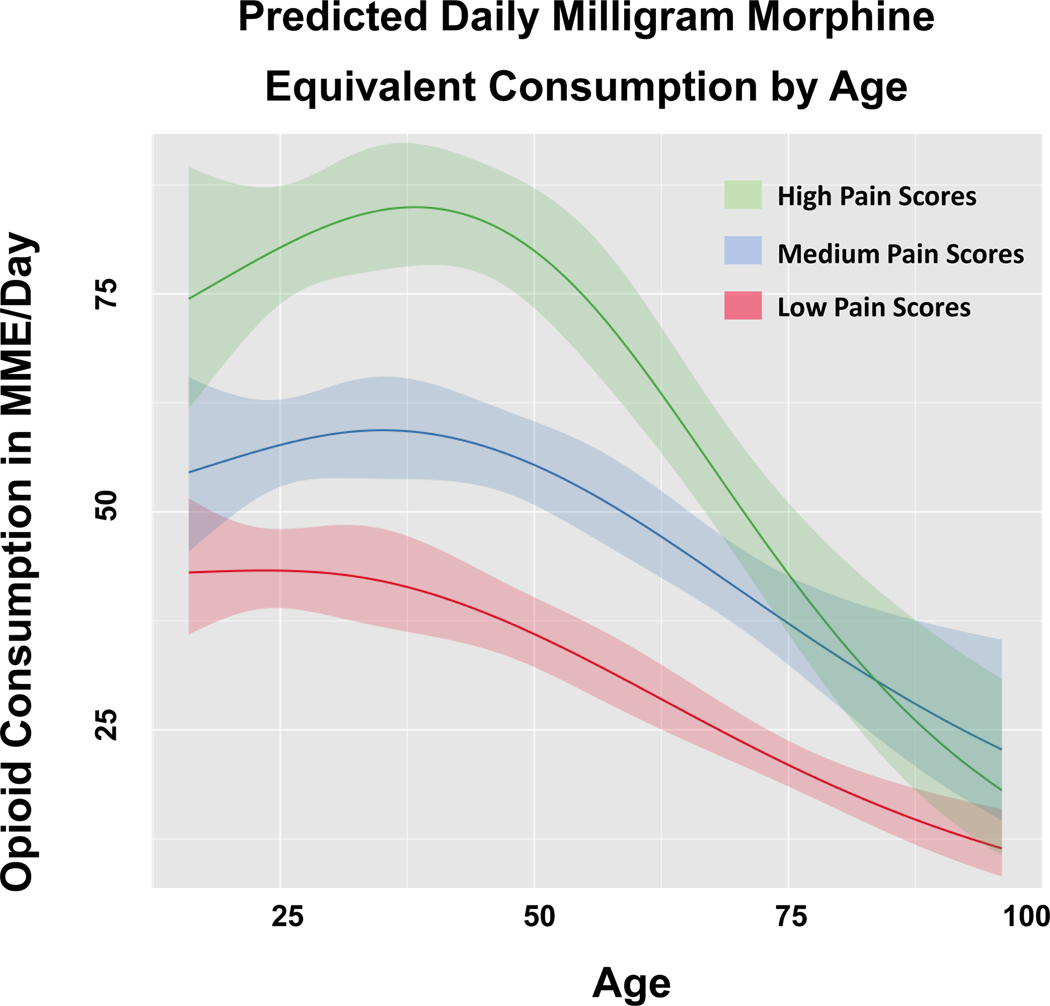
There was a curvilinear relationship between age and MME/day. After adjustment, patients with high pain scores received more daily opioids than patients with medium and low pain scores until age 80, at which time patients with high pain scores consumed similar amounts of MME/day as those with medium pain scores. (Figure 2). Patients in the 30 to 40 year-old range with high pain scores consumed the most MME/day, while increasing age thereafter resulted in a steady decrease in MME/day consumed. Across pain scores, the amount of MME/day consumed decreased slightly for patients younger than 55 with an average 6% relative reduction for each ten-year increase in age. However, above age 55 there was a rapid decline in MME/day with an average of 23% per ten-year age increase.
FIGURE 2:
Predicted daily milligram morphine equivalent consumption by age.
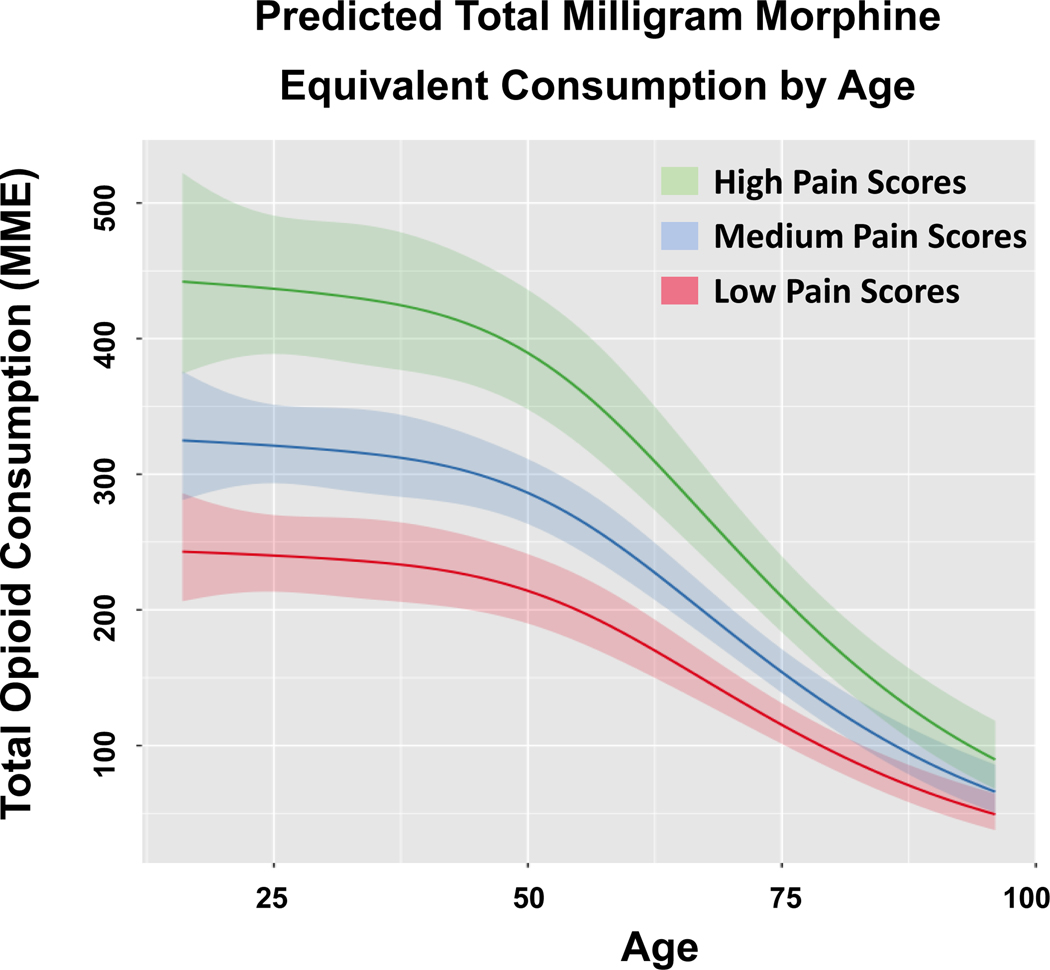
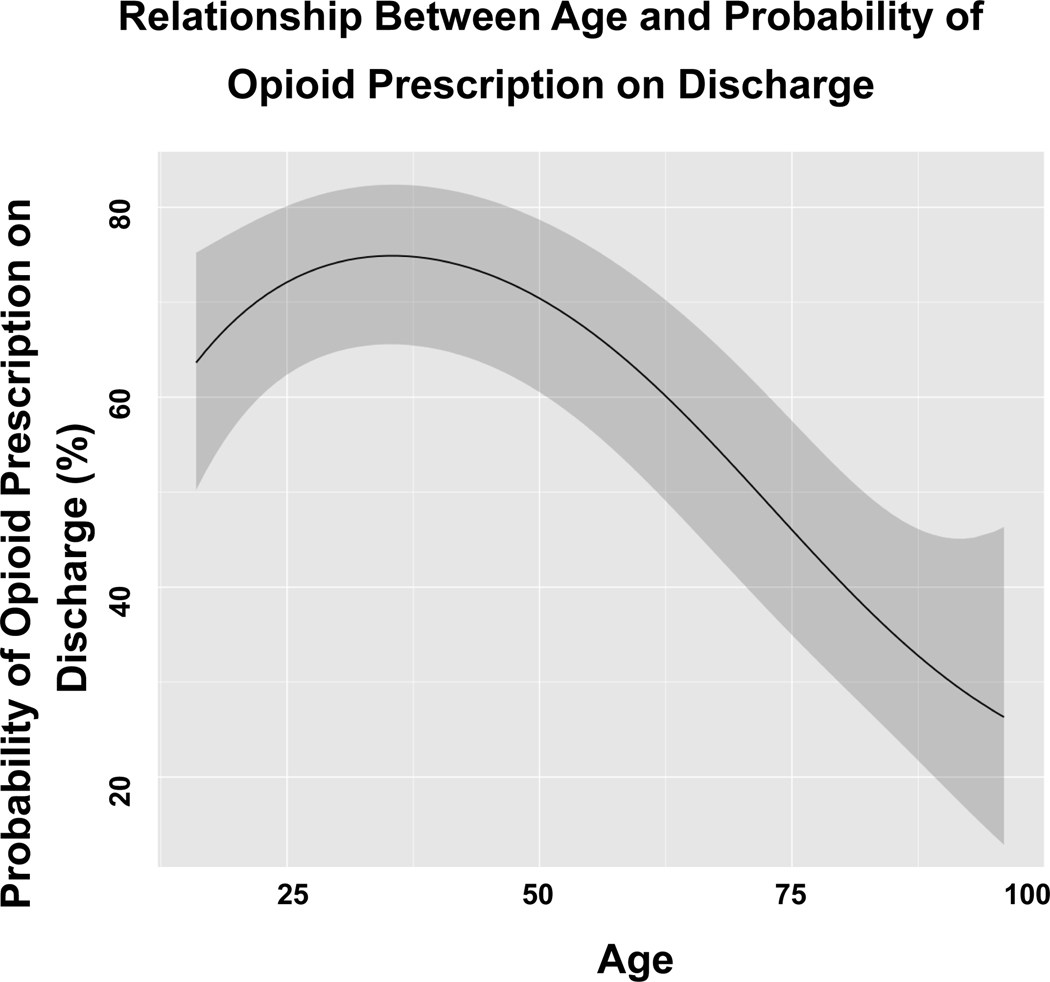
Similarly, among patients aged 55 years and younger, total MME consumed decreased by an average of 11% for each ten-year increase in age. (Figure 3) Total MME consumed decreased by 29% per ten-year age increase after the age of 55. Finally, probability of being discharged with an opioid prescription was similar (0% average change per ten-year age increase) for patients 55 years old and younger. (Figure 4) However, after age 55 years, there was an average 19% decline in the probability of being discharged with an opioid prescription per ten-year age increase.
FIGURE 3:
Predicted total milligram morphine equivalent consumption by age.
FIGURE 4:
Relationship between age and probability of opioid prescription on discharge.
Discussion
In this secondary analysis of the MAST clinical trial, older patients consumed fewer opioids to achieve similar pain scores as younger patients. This finding was consistent with our hypothesis. Advancing age was an independent predictor of decreased total opioids consumed and decreased odds of being discharged with an opioid prescription. The findings of this study provide quantitative evidence that supports a potential 6% decrease in opioid dose per additional decade in younger trauma patients and a 23% decrease in opioid dose per additional decade in older trauma patients. Furthermore, the MAST MMPR was found to be effective for opioid reduction without adversely affecting pain control across the age continuum.
Increasing evidence suggests that opioid-minimizing or opioid-sparing pain management regimens are feasible and result in similar pain management and patient-reported outcomes. 21–23 In a study of an opioid-sparing post-operative pathway with opioids only for breakthrough pain, patients reported high satisfaction and pain control. Patients requiring opioids for breakthrough pain were older (59 versus 52 years). 23 Reduction of in-hospital opioid use is likely to result in decreased post-discharge opioid demand and development of an opioid use disorder. 24. Furthermore, opioid use remains widely variable among and within individual trauma centers 25. Implementation of opioid-minimizing MMPRs may decrease the adverse effects of opioids on both for the individual and the community. 26
Inadequate analgesia disproportionately affects older adults. 27 This may partially be the result of a scarcity of evidence regarding effective, opioid-minimizing pain regimens for older trauma patients. Use of opioids in the elderly is associated with increased cognitive impairment and increased risk of delirium. 28 However, undertreatment of severe pain in older adults is also a risk factor for delirium, prolonged hospitalization, shortened or missed physical therapy sessions, and delays to ambulation. 28–31. Opioid minimization is optimal to both avoid patient-level side effects of opioid administration and community-level adverse effects by increasing opioid diversion. 32 The MAST clinical trial showed that a widely available multimodal pain regimen is effective at decreasing opioid use after trauma, and this present study confirmed effectiveness of this regimen across the age spectrum. Additionally, this study supports that clinicians can minimize opioid exposure in older trauma patients while simultaneously ensuring adequate analgesia.
Older trauma patients are at an elevated risk of having suboptimal analgesia due to reluctance to administer opioids and more frequent presence of contraindications to non-opioid analgesics. The American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons advocates for initiating opioid treatment at a dose lower than usually required and slowly titrating upward as needed. 33 Other authors advocate for a dose reduction by one-half to account for the alterations in opioid metabolism with aging. 34 A prior population-based study of postoperative, non-trauma patients suggested that each year increase in age was associated with a decrease of 0.77 oral morphine equivalents. 35 The present study provides evidence to support the intuitive practice of decreasing opioid dose in older patients. Additionally, the findings of the present study support a nuanced approach to opioid dosing, with modification of opioid dosing adjustment based on the patient’s decade of life. The present study also supports the use of opioid-minimizing MMPRs at all ages. These regimens remain a challenge in older patients because drug interactions and altered metabolism are significant in non-opioid analgesics as well. The prevalence of chronic kidney disease, peptic ulcer disease, cirrhosis, and coronary artery disease in older patients frequently makes prescribing NSAIDs potentially hazardous. 36,37 Gabapentinoids must be adjusted in cases of chronic kidney disease, but have otherwise been shown to be relatively safe in older adults and with few drug-drug interactions. 38 Finally, there may be hesitancy to prescribe acetaminophen in patients with liver dysfunction. As the study was a pragmatic clinical trial, guidelines were not provided regarding when a medication, such as NSAIDs or gabapentinoids should be withheld and physician discretion was utilized for these modifications.
Additional work is needed to continue to address this challenge for older trauma patients. The optimal starting dose of opioid analgesia remains unknown. The present study suggests that age is an important factor in opioid consumption; however, a starting opioid dose remains an individualized choice. Adoption of a standardized analgesic protocol for injured patients, with individualization based on pain severity and age, may minimize opioid administration without adversely affecting pain control. Impact on opioid-related adverse effects, length of stay, and discharge destination should be measured. Finally, the optimal strategies for escalation and de-escalation have yet to be elucidated.
This work has several limitations. First, conclusions cannot be made about the effectiveness of individual medications used in the MMPR. In addition, many patients received multiple sources of opioids, such as tramadol, oxycodone, and morphine, which were converted to MMEs. Second, the measurement of acute pain is challenging. This study simplified the complex and dynamic pain experience into an average pain score from all collected pain scores obtained while hospitalized. Third, it was assumed that pain severity reporting was equivalent in younger and older patients. However, impaired communication may lead to patients with severe cognitive deficiency having difficulty using the NRS tool. (2, 12) In the present study, patients who were unable to communicate and use the NRS tool had their pain assessed using the BPS. A minority of patients had pain reported exclusively with the BPS but it is notable that there was no difference in pain scores reported this way between older and younger age groups. Usual practice at the study institution is to choose between the NRS or the BPS in pain assessment. However, the BPS tool may not be an appropriate tool for those with chronic cognitive disability as it was originally designed for intubated and sedated patients. There is no current consensus on which tool should be used for elderly trauma patients. 39,40
Older trauma patients required fewer opioids than younger patients with similar characteristics and pain scores. Opioid dosing for post-traumatic pain should consider age. A 20 to 25% dose reduction per decade after age 55 may reduce opioid exposure without altering pain control. Furthermore, the MAST MMPR was effective at decreasing daily opioid consumption across the age continuum.
DISCUSSANT: Dr. Rosemary Kozar
I would like to congratulate the authors for their continues investigations into the reduction of opioids after trauma and especially congratulate Dr. Hatton for her excellent presentation. The current study is a secondary analysis of the MAST trial but focusing here on age-related opioid exposure after trauma.
I have a few questions:
If I understand correctly, the authors combined results of the original MMPR with the MAST MMPR. Can you comment if there were notable differences in the opioid use in the elderly between the 2 protocols? I was curious if perhaps the elderly were more reluctant to accept IV narcotics in the first protocol but more willing to accept an oral agent in the second?
How can the authors explain the discrepancy between the increased history of opioid use but less frequent UDS + screens? As a follow up, how do you handle chronic opioid users in terms of dosing. In seems that in Baltimore we have a high percentage of our elderly trauma patients that are on chronic opioids or using illicit narcotics.
The authors nicely discuss the issues of pain control and elderly in the manuscript with “too much” and “too little” both being risk factors for delirium. If indeed you have achieved a “just right” combination, can you at least speculate on your incidence of delirium since instituting this protocol? It seems like this would be an important endpoint to track in future studies.
I would like again to thank Dr Hatton on her excellent work and thank the American Surgical for the privilege of discussing this excellent paper.
Dr. Hatton Response:
To address your first question, yes, the main results were gathered after combining the two original study groups. Within the elderly cohort itself, there were no differences in administration of intravenous opioids and no difference that we saw in application of an opioid infusion. The primary difference in opioid use between the MMPR groups came from differences in oral opioid consumption. The original protocol used scheduled tramadol while the masked MMPR gave tramadol as needed, resulting in lower opioid consumption in the masked MMPR.
Second, a history of opioid use was common in our older cohort as you’ve pointed out. This variable was not specific for current opioid use, and many older patients may have previously used opioids for an acute event but were no longer actively taking those medications. Additionally, the urine drug screen was more frequently negative for older patients, also as pointed out. We did not consider a drug screen positive if it reflected systemic opioids or benzodiazepines as these are frequently given by our EMS services. Our data indicated a urine drug screen as positive only if cocaine, cannabis, amphetamines, or barbiturates were present in the system on arrival. It would be interesting to evaluate the individual components of the drug screen; however, we don’t have the data currently that reflects systemic opioid presence at the time of injury.
Regarding chronic opioid use, our general practice is to restart a patient’s home dose of medication and to add the multimodal pain regimen in addition to a home regimen to address their additional acute posttraumatic pain. These regimens are then titrated and adjusted as needed. Additionally, adjuncts such as intravenous ketamine or intravenous lidocaine are used if pain is difficult to control.
Finally, the study unfortunately did not assess delirium as a prospectively collected outcome. However, the surgical literature cites a wide range of delirium among the elderly with an incidence ranging between 20% to 70%, which is closely associated with opioid use. Without a standardized delirium assessment protocol in place at our institution, I can only presume our incidence of delirium is similar to these reports. Regarding the findings of the present study, we may hypothesize that delirium is decreased with decreased opioid consumption as long as analgesia is also adequate. We agree that the effect of opioid-minimizing multimodal pain regimens on delirium needs to be assessed in future studies to come to this conclusion.
Acknowledgments
Funding: JAH was supported by the Center for Clinical and Translational Sciences, which is funded by NIH Clinical and Translational Award UL1 TR000371 and KL2 TR000370 from the National Center for Advancing Translational Sciences. This work was also supported in part by the NMOU Core Resource, funded by NIH Clinical and Translational Science Award UL1TR003167. GEH and HRK are supported by the National Institute of General Medical Sciences of the National Institutes of Health [5T32GM008792].
Disclosures: CEW receives funding from the William Stamps Farish Fund, the Howell Family Foundation, the James H. “Red” Duke Professorship. GEH and HRK are supported by the National Institute of General Medical Sciences of the National Institutes of Health [5T32GM008792]. JAH was supported by the Center for Clinical and Translational Sciences, which is funded by NIH Clinical and Translational Award UL1 TR000371 and KL2 TR000370 from the National Center for Advancing Translational Sciences. He is also supported by Center for Clinical and Translational Sciences 1UL1TR003167. GEH is supported by the National Institute of General Medical Sciences of the National Institutes of Health [5T32GM008792].
Footnotes
Conflicts of Interest: All authors have no conflicts of interest to report.
All others have no disclosures to report.
References
- 1.Kozar RA, Arbabi S, Stein DM, et al. Injury in the aged: Geriatric trauma care at the crossroads. J Trauma Acute Care Surg. 2015;78(6):1197–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams SD, Cotton BA, McGuire MF, et al. Unique pattern of complications in elderly trauma patients at a Level I trauma center. J Trauma Acute Care Surg. 2012;72(1):112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baratta JL, Schwenk ES, Viscusi ER. Clinical consequences of inadequate pain relief: barriers to optimal pain management. Plast Reconstr Surg. 2014;134(4 Suppl 2):15S–21S. [DOI] [PubMed] [Google Scholar]
- 4.Jones J Jr., Southerland W, Catalani B. The Importance of Optimizing Acute Pain in the Orthopedic Trauma Patient. Orthop Clin North Am. 2017;48(4):445–465. [DOI] [PubMed] [Google Scholar]
- 5.McKeown JL. Pain Management Issues for the Geriatric Surgical Patient. Anesthesiol Clin. 2015;33(3):563–576. [DOI] [PubMed] [Google Scholar]
- 6.Baillie SP, Bateman DN, Coates PE, Woodhouse KW. Age and the pharmacokinetics of morphine. Age Ageing. 1989;18(4):258–262. [DOI] [PubMed] [Google Scholar]
- 7.Liukas A, Kuusniemi K, Aantaa R, et al. Plasma concentrations of oral oxycodone are greatly increased in the elderly. Clin Pharmacol Ther. 2008;84(4):462–467. [DOI] [PubMed] [Google Scholar]
- 8.McLachlan AJ, Bath S, Naganathan V, et al. Clinical pharmacology of analgesic medicines in older people: impact of frailty and cognitive impairment. Br J Clin Pharmacol. 2011;71(3):351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fallon WF Jr., Rader E, Zyzanski S, et al. Geriatric outcomes are improved by a geriatric trauma consultation service. J Trauma. 2006;61(5):1040–1046. [DOI] [PubMed] [Google Scholar]
- 10.Mangram AJ, Mitchell CD, Shifflette VK, et al. Geriatric trauma service: a one-year experience. J Trauma Acute Care Surg. 2012;72(1):119–122. [DOI] [PubMed] [Google Scholar]
- 11.Harvin JA, Albarado R, Truong VTT, et al. Multi-Modal Analgesic Strategy for Trauma: A Pragmatic Randomized Clinical Trial. J Am Coll Surg. 2021;232(3):241–251 e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvin JA, Green CE, Vincent LE, et al. Multi-modal Analgesic Strategies for Trauma (MAST): protocol for a pragmatic randomized trial. Trauma Surg Acute Care Open. 2018;3(1):e000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trauma TCo. National Trauma Data Standard Data Dictionary: 2019 Admissions. https://www.facs.org/~/media/files/quality%20programs/trauma/ntdb/ntds/data%20dictionaries/ntdb_data_dictionary_2019_revision.ashx. Published 2018. Accessed 2021.
- 14.Naumann DN, Vincent LE, Pearson N, et al. An adapted Clavien-Dindo scoring system in trauma as a clinically meaningful nonmortality endpoint. J Trauma Acute Care Surg. 2017;83(2):241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008;101(1):17–24. [DOI] [PubMed] [Google Scholar]
- 16.Payen JF, Bru O, Bosson JL, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Critical care medicine. 2001;29(12):2258–2263. [DOI] [PubMed] [Google Scholar]
- 17.Schuster J, Hoyer C, Ebert A, Alonso A. Use of analgesics in acute stroke patients with inability to self-report pain: a retrospective cohort study. BMC neurology. 2020;20(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatton GE, McNutt MK, Cotton BA, Hudson JA, Wade CE, Kao LS. Age-Dependent Association of Occult Hypoperfusion and Outcomes in Trauma. J Am Coll Surg. 2020;230(4):417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fakhry SM, Morse JL, Garland JM, et al. Redefining geriatric trauma: 55 is the new 65. J Trauma Acute Care Surg. 2021;90(4):738–743. [DOI] [PubMed] [Google Scholar]
- 20.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in medicine. 1989;8(5):551–561. [DOI] [PubMed] [Google Scholar]
- 21.Padilla JA, Gabor JA, Schwarzkopf R, Davidovitch RI. A Novel Opioid-Sparing Pain Management Protocol Following Total Hip Arthroplasty: Effects on Opioid Consumption, Pain Severity, and Patient-Reported Outcomes. J Arthroplasty. 2019;34(11):2669–2675. [DOI] [PubMed] [Google Scholar]
- 22.Anderson M, Hallway A, Brummett C, Waljee J, Englesbe M, Howard R. Patient-Reported Outcomes After Opioid-Sparing Surgery Compared With Standard of Care. JAMA Surg. 2021;156(3):286–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallway A, Vu J, Lee J, et al. Patient Satisfaction and Pain Control Using an Opioid-Sparing Postoperative Pathway. J Am Coll Surg. 2019;229(3):316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon JH, Suchting R, Kessler D, et al. Utility of a brief assessment of opioid demand among post-discharge trauma care patients. Exp Clin Psychopharmacol. 2020. [DOI] [PubMed] [Google Scholar]
- 25.Harvin JA, Truong VTT, Green CE, et al. Opioid exposure after injury in United States trauma centers: A prospective, multicenter observational study. J Trauma Acute Care Surg. 2020;88(6):816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei S, Green C, Truong VTT, et al. Implementation of a multi-modal pain regimen to decrease inpatient opioid exposure after injury. Am J Surg. 2019;218(6):1122–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platts-Mills TF, Esserman DA, Brown DL, Bortsov AV, Sloane PD, McLean SA. Older US emergency department patients are less likely to receive pain medication than younger patients: results from a national survey. Ann Emerg Med. 2012;60(2):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58(1):76–81. [DOI] [PubMed] [Google Scholar]
- 30.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12(1):50–55. [DOI] [PubMed] [Google Scholar]
- 31.Hwang U, Platts-Mills TF. Acute pain management in older adults in the emergency department. Clin Geriatr Med. 2013;29(1):151–164. [DOI] [PubMed] [Google Scholar]
- 32.Inciardi JA, Surratt HL, Lugo Y, Cicero TJ. The Diversion of Prescription Opioid Analgesics. Law Enforc Exec Forum. 2007;7(7):127–141. [PMC free article] [PubMed] [Google Scholar]
- 33.American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older P. Pharmacological management of persistent pain in older persons. Pain Med. 2009;10(6):1062–1083. [DOI] [PubMed] [Google Scholar]
- 34.Guerriero F. Guidance on opioids prescribing for the management of persistent non-cancer pain in older adults. World J Clin Cases. 2017;5(3):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howard R, Fry B, Gunaseelan V, et al. Association of Opioid Prescribing With Opioid Consumption After Surgery in Michigan. JAMA Surg. 2019;154(1):e184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page J, Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an underrecognized public health problem. Arch Intern Med. 2000;160(6):777–784. [DOI] [PubMed] [Google Scholar]
- 37.Hatton GE, Bell C, Wei S, Wade CE, Kao LS, Harvin JA. Do early non-steroidal anti-inflammatory drugs for analgesia worsen acute kidney injury in critically ill trauma patients? An inverse probability of treatment weighted analysis. J Trauma Acute Care Surg. 2020;89(4):673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed GF, Bathena SP, Brundage RC, et al. Pharmacokinetics and Saturable Absorption of Gabapentin in Nursing Home Elderly Patients. AAPS J. 2017;19(2):551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lichtner V, Dowding D, Esterhuizen P, et al. Pain assessment for people with dementia: a systematic review of systematic reviews of pain assessment tools. BMC Geriatr. 2014;14:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malec M, Shega JW. Pain management in the elderly. Med Clin North Am. 2015;99(2):337–350. [DOI] [PubMed] [Google Scholar]