Abstract
Background
Acute enteropathy is a trigger of chronic gastrointestinal (GI) disease in humans.
Objective
To report the prevalence of and explore possible risk factors for signs of chronic GI disease in dogs after an episode of acute hemorrhagic diarrhea (AHD).
Animals
One hundred and fifty‐one dogs, 80 dogs with a historical diagnosis of AHD, 71 control dogs with no history of AHD.
Methods
In this retrospective longitudinal study, data were collected from dogs with a historical diagnosis of AHD and healthy controls matched by breed, age and sex, aged between 1 year and 15 years of age, for which a follow‐up of at least 12 months after enrolment was available. Dog owners responded to a questionnaire to determine the history of signs of chronic GI disease.
Results
There was a higher prevalence of signs of chronic GI disease in the dogs with a previous episode of AHD compared to control dogs (AHD 28%; controls 13%; P = .03; odds ratio = 2.57; confidence interval [CI] 95% 1.12‐6.31) over a similar observation time (median 4 years; range, 1‐12 years).
Conclusions and Clinical Importance
Severe intestinal mucosal damage and associated barrier dysfunction might trigger chronic GI disease later in life.
Keywords: AHDS, canine, enteropathy, HGE, IBD, intestinal
Abbreviations
- AHD
acute hemorrhagic diarrhea
- AHDS
acute hemorrhagic diarrhea syndrome
- CADS‐Index
Canine Acute Diarrhea Severity‐Index
- CE
chronic enteropathy
- CI
confidence interval
- CIBDAI
Canine Inflammatory Bowel Disease Activity Index
- CPE
Clostridium perfringens enterotoxin gene
- CPV
canine parvovirus
- GI
gastrointestinal
- HGE
hemorrhagic gastroenteritis
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- PI
postinfectious
- qPCR
quantitative polymerase chain reaction
- rRNA
ribosomal ribonucleic acid
- TRegs
regulatory T cells
- WBC
white blood cells
1. INTRODUCTION
Acute enteropathy is a risk factor for development of chronic gastrointestinal (GI) disease in humans. Acute enteropathy could lead to an increased risk for chronic intestinal and extraintestinal disorders such as postinfectious (PI) irritable bowel syndrome (IBS), 1 inflammatory bowel disease (IBD) 2 and reactive arthritis. 3 Data about long‐term consequences of acute GI disorders in dogs are sparse. Puppies surviving canine parvovirus (CPV) enteritis have an increased risk for developing chronic enteropathy (CE) later in life. 4 A loss of tolerance to food components and altered intestinal microbiota after severe intestinal injury is a potential mechanism for development of CE. 4 It is postulated that barrier dysfunction and dysbiosis during the acute phase of disease are factors responsible for sensitization of the immune system. 5 , 6 During infancy and early childhood the immune system as well as the composition of the intestinal microbiome are immature. 7 , 8 , 9 Therefore, young age might represent an additional risk factor for developing chronic disease.
An example for acute GI damage in adult dogs is acute hemorrhagic diarrhea syndrome (AHDS), which results in intestinal barrier dysfunction and dysbiosis. Characteristic histopathological findings in dogs with AHDS include severe lesions with destruction of intestinal villi and epithelial necrosis in the entire small and large intestine. 10 According to recent findings, it seems likely that infection by Clostridium perfringens type A strains associated with release of NetF toxins, possibly in concert with other toxins such as NetE, NetG and C. perfringens enterotoxin gene (CPE), is responsible for the necrotic enterocolitis in many dogs with AHDS. 11 Moreover, dogs with AHDS have alterations in their microbiome. 12 Fecal samples analyzed by 16S rRNA gene sequencing and qPCR revealed increases in proportions for C. perfringens‐like sequences as well as decreases in proportions for Blautia and Turicibacter spp. 12
Although further studies are needed, we suspect that similar intestinal damage is present in other acute diseases leading to hemorrhagic diarrhea. Therefore, to assess the long‐term impact of acute hemorrhagic diarrhea (AHD), longitudinal studies are warranted. This may allow future research on epidemiology, mechanisms, and possibly treatment to prevent development of signs of chronic GI disease. Therefore, the aim of this study was to investigate the prevalence of signs of chronic GI disease in dogs after an episode of AHD compared to that of a control group and to identify possible risk factors for a progression to chronicity.
2. MATERIALS AND METHODS
2.1. Study design
The study was designed as a retrospective longitudinal study. Data were collected from dogs that were presented with AHD. Control dogs, which had no reported previous signs consistent with AHD according to the owner, were matched by breed, sex, age and time of presentation. Dogs of both groups with a follow‐up period of at least 1 year were identified by reviewing medical records from the Clinic of Small Animal Internal Medicine of the LMU Munich, Germany as well as of the Clinic of Small Animal Medicine Ismaning, Germany and the AniCura Clinic of Small Animal Medicine Haar, Germany from May 2006 to December 2018. Owners of dogs suitable for the study were contacted by telephone, and after giving consent to participate in the survey, a questionnaire was mailed. This study was approved by the ethics committee of the Centre of Veterinary Medicine, LMU Munich, Germany (approval number: 215‐14‐05‐2020).
2.1.1. AHD group
We searched our databases for dogs with the diagnosis “acute hemorrhagic diarrhea syndrome.” Dogs presenting with severe AHD were included in this group when no causes were identified and a provisional diagnosis of AHDS was made by the clinician. To detect underlying disorders for AHD, a CBC (79/80), biochemistry profile (70/80), fecal examination (flotation, Giardia ELISA; 52/80), abdominal sonography (44/80), abdominal x‐ray (17/80), basal cortisol (7/80), coagulation times (24/80), and cPLi SNAP test (2/80) were performed. Dogs that received drugs potentially harmful to the intestinal mucosa (eg, NSAIDs, glucocorticoids) and with signs of chronic GI disease before the acute episode with hemorrhagic diarrhea were excluded. AHDS is still a diagnosis of exclusion and not every diagnostic test necessary to rule out other causes of AHD were performed consistently in every dog, we defined the included study sample as dogs with AHD.
2.1.2. Control group
Dogs of the same sex and breed, with a similar age (±1 year) presented for routine preventative health examinations, vaccinations, elective surgery or minor diseases in the same year (±1 year) as the corresponding dogs with AHD were enrolled in the control group. These dogs were selected randomly but were not included if they had a history of AHD or signs of chronic GI disease before the matched time of presentation. Dogs receiving immunosuppressive drugs, including steroids, or antibiotics at the time of presentation were not included. For a few dogs (eg, rare breeds—see Supporting Information), no matched control dogs could be recruited.
2.1.3. Questionnaire
The questionnaire comprised general questions such as age of the dog, origin of the animal, diet and wellness routines (vaccinations, deworming, etc) and specific questions regarding chronic GI disorders (see Supporting Information). The GI‐related questions referred to the period with the most severe clinical signs of the chronic disease and were assessed by the Canine Inflammatory Bowel Disease Activity Index (CIBDAI) according to Jergens and others. 13 Chronic disease was defined as episodes of signs of GI disease that lasted longer than 3 weeks, or recurrent episodes after AHD which lasted longer than 3 days. An owner observation time of at least 1 year was required to identify the development of signs of chronic GI disease after an acute episode of AHD. The questionnaire was internally validated by testing comprehensibility in randomly chosen dog owners without a medical background and had been successfully used in a previous study. 4
2.2. Statistical analysis
Power analysis showed that a minimum of 60 dogs in each group were required to detect a clinically significant difference of 30% in the prevalence of chronic disorders later in life with P < .05 and a power of >90%. In order to assure the quality of the match between AHD and control groups, several variables were compared between these groups univariantly, namely, the breed using Fishers' exact test, sex and prevention of endoparasites using Pearson's chi‐squared test, and age and time span of observation using Wilcoxon rank sum test. The confidence intervals (CIs) for the prevalence of having CE in AHD and control groups were calculated with the Wald method. The comparison of frequency of having CE between AHD and control groups (“groups” being 1 of 2 predictors) and sex (“sex” been the second predictor) was assessed with a multiple logistic regression. The difference in severity of the disease, expressed as a number (CIBDAI) on ordinal scale, between these 2 groups was studied by the Wilcoxon rank sum test. The influence of 5 variables (administration of antibiotics, duration of hospitalization, sex, age, leukocytes, albumin) on the risk of developing a chronic disease after an episode after AHD only in dogs with AHD (ie, only in AHD group, no control dogs in these models were present) was studied with multiple logistic regression. A manual backwards selection was applied to reduce the number of variables in the final model and to potentially improve the model fit. The models were compared using 3 performance quality indicators: Akaike's Information Criterion (AIC), Bayesian Information Criterion (BIC), and Tjur's R2. Because of the absence of any significant improvement in model quality after backwards selection, the results of all 5 variables are presented in this paper. A potential collinearity among predictors was studied with variation inflation factor (VIF). A P value <.05 was considered significant. Power analysis was performed in Prism6, GraphPad, San Diego, all other statistical analyses by using R.
3. RESULTS
3.1. Comparison of baseline data between AHD and control group
One hundred and fifty‐one completed questionnaires were available for final analysis, 80 questionnaires from the AHD group and 71 from the control group, respectively. The AHD group consisted of 65 purebred dogs, and the most represented breeds were Dachshund (n = 7), Chihuahua (n = 5), and Yorkshire Terrier (n = 4). Fifteen dogs of the AHD group were mixed breed dogs. Median age at presentation was 5 years (range, 1‐15), median time span of observation was 4 years (1‐12). At the time of data acquisition 63 dogs were still alive. All 80 AHD dogs had presented with an acute onset of watery‐bloody diarrhea and 74 dogs additionally had vomiting. In 54/80 dogs with AHD, treatment included administration of antibiotics: 44 with 1 single antibiotic of these 33 amoxicillin/clavulanic acid, 9 metronidazole, 1 amoxicillin, 1 tylosin. Seven dogs were treated with 2, 2 with 3 and 1 dog with 5 antibiotics.
Fifty‐eight dogs of the control group were purebred, most represented breeds were Dachshund (n = 7) and Yorkshire Terrier (n = 4). Thirteen dogs were of mixed breed. Median age at presentation was 5 years (range, 1‐15) and median observation time was 4 years (range, 1‐12). The main reasons for presentation were orthopedic problems or elective surgery, for example, spaying (n = 26), routine preventative health examinations including vaccinations (n = 18), ophthalmologic problems (n = 8), or acute diseases excluding AHD (n = 19).
No significant differences with respect to sex, breed, median age at presentation, or median time span of observation were identified between the 2 groups (Tables 1 and 2).
TABLE 1.
Comparison of baseline data between dogs with AHD and control group
| Variable | AHD group (n = 80) | Control group (n = 71) | P value |
|---|---|---|---|
| Age at presentation (years) | Median 5 (range, 1‐15) | Median 5 (1‐15) | .55 |
| Time span of observation (years) | Median 4 (1‐12) | Median 4 (1‐12) | .52 |
| Sex | .44 | ||
| Female | 50% (n = 40) | 56% (n = 40) | |
| Male | 50% (n = 40) | 44% (n = 31) | |
| Prevention of endoparasites | .37 | ||
| Regularly | 80% (n = 64) | 87% (n = 62) | |
| Not regularly | 18% (n = 14) | 13% (n = 9) | |
| Unknown | 2% unknown (n = 2) | 0% unknown (n = 0) |
Abbreviation: AHD, acute hemorrhagic diarrhea.
TABLE 2.
Breeds
| Group | ||||
|---|---|---|---|---|
| Characteristic | AHD | Control | Total | P‐value a |
| Breed | >.9 | |||
| Australian Shepherd | 1 (50%) | 1 (50%) | 2 (100%) | |
| Cairn Terrier | 1 (50%) | 1 (50%) | 2 (100%) | |
| Cavalier King Charles Spaniel | 2 (50%) | 2 (50%) | 4 (100%) | |
| Chihuahua | 5 (62%) | 3 (38%) | 8 (100%) | |
| Collie | 1 (50%) | 1 (50%) | 2 (100%) | |
| Dachshund | 7 (50%) | 7 (50%) | 14 (100%) | |
| Dalmatian | 1 (50%) | 1 (50%) | 2 (100%) | |
| Deutsch Drahthaar | 1 (50%) | 1 (50%) | 2 (100%) | |
| French Bulldog | 1 (50%) | 1 (50%) | 2 (100%) | |
| German Shorthaired Pointer | 1 (50%) | 1 (50%) | 2 (100%) | |
| Giant Schnauzer | 2 (50%) | 2 (50%) | 4 (100%) | |
| Golden Retriever | 1 (50%) | 1 (50%) | 2 (100%) | |
| Griffon | 1 (50%) | 1 (50%) | 2 (100%) | |
| Havanese | 2 (40%) | 3 (60%) | 5 (100%) | |
| Hovavart | 1 (100%) | 0 (0%) | 1 (100%) | |
| Irish Setter | 1 (100%) | 0 (0%) | 1 (100%) | |
| Italian Greyhound | 1 (50%) | 1 (50%) | 2 (100%) | |
| Jack Russel Terrier | 2 (50%) | 2 (50%) | 4 (100%) | |
| Labrador | 2 (50%) | 2 (50%) | 4 (100%) | |
| Maltese | 3 (60%) | 2 (40%) | 5 (100%) | |
| Mini Pinscher | 1 (33%) | 2 (67%) | 3 (100%) | |
| Mini Schnauzer | 2 (50%) | 2 (50%) | 4 (100%) | |
| Mixed breed | 15 (54%) | 13 (46%) | 28 (100%) | |
| Parson Russel Terrier | 3 (60%) | 2 (40%) | 5 (100%) | |
| Pekingese | 2 (50%) | 2 (50%) | 4 (100%) | |
| Pinscher | 1 (100%) | 0 (0%) | 1 (100%) | |
| Pomeranian | 1 (50%) | 1 (50%) | 2 (100%) | |
| Poodle | 2 (67%) | 1 (33%) | 3 (100%) | |
| Prague Rattler | 2 (50%) | 2 (50%) | 4 (100%) | |
| Pug | 1 (50%) | 1 (50%) | 2 (100%) | |
| Pumi | 1 (50%) | 1 (50%) | 2 (100%) | |
| Rhodesian Ridgeback | 1 (50%) | 1 (50%) | 2 (100%) | |
| Rottweiler | 1 (50%) | 1 (50%) | 2 (100%) | |
| Seguigo Italiano | 1 (100%) | 0 (0%) | 1 (100%) | |
| Shepherd | 2 (50%) | 2 (50%) | 4 (100%) | |
| Shih Tzu | 1 (50%) | 1 (50%) | 2 (100%) | |
| Springer Spaniel | 1 (50%) | 1 (50%) | 2 (100%) | |
| Tibet Spaniel | 1 (50%) | 1 (50%) | 2 (100%) | |
| Yorkshire Terrier | 4 (50%) | 4 (50%) | 8 (100%) | |
| Total | 80 (53%) | 71 (47%) | 151 (100%) | |
Abbreviation: AHD, acute hemorrhagic diarrhea.
Fisher's exact test.
3.2. Comparison between the AHD and control groups
Twenty‐two of 80 (28%; CI 95% 17.7‐37.3) dog owners from the AHD group and 9/71 (13%; CI 95% 4.9‐20.4) from the control group reported chronic or chronic recurrent signs of GI disease later in life, indicating a higher prevalence of chronic GI disorders in dogs after an episode of AHD (P = .03; odds ratio = 2.57; CI 95% 1.12‐6.31). With specific reference to the severity of chronic disease, quantified by the CIBDAI, dogs with AHD had a similar disease activity (median 3; range, 1‐12) compared to the control group (median 4; range, 1‐10; P = .93; Figure 1). Nine of 22 dog owners from the AHD group reported an improvement of signs of GI disease with a change of diet, whereas 3 dogs were nonresponsive to a diet change. In 11 dogs, no elimination diet trial was performed. In the control group 2 of 9 dog owners reported an improvement of signs of GI disease with a change of diet, whereas 1 dog was nonresponsive to a diet change and in 6 dogs no elimination diet trial was performed.
FIGURE 1.
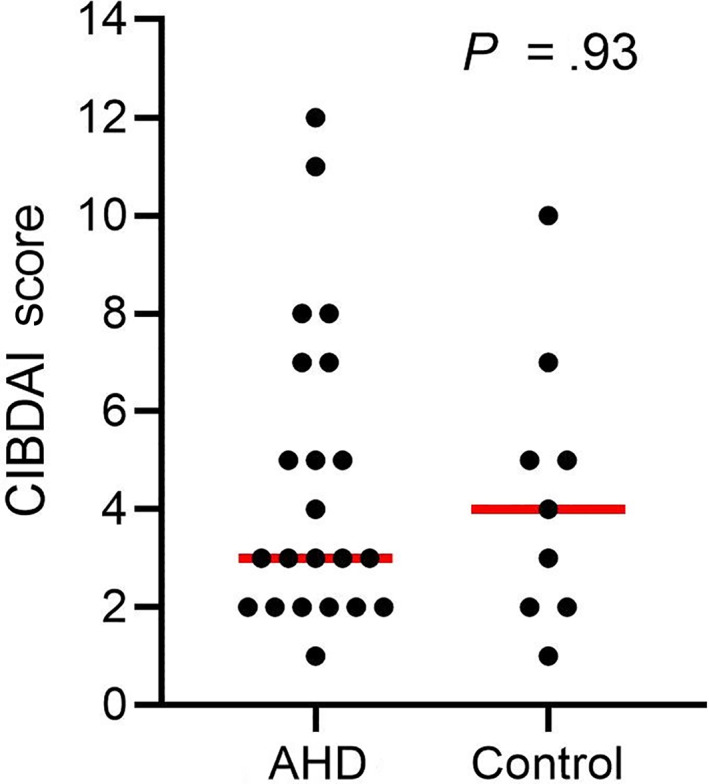
Comparison of the Canine Inflammatory Bowel Disease Activity Index (CIBDAI) between dogs with AHD and control dogs. Red lines indicate the median. CIBDAI score was evaluated for the time point with the most severe clinical signs. AHD, acute hemorrhagic diarrhea
3.3. Risk factors for development of chronic diarrhea in dogs with AHD
No significant difference in the age at presentation for AHD between dogs with (median 5.5 years) and without development of signs of chronic GI disease (median 5 years) was observed (P = .96; Figure 2). In addition, in the group of dogs with AHD no association between the following investigated variables and an increased prevalence of signs of chronic GI disease later in life was identified: time of hospitalization (P = .06); administration of antibiotics during the episode of AHD (P = .44) and selected laboratory variables reflecting the severity of mucosal damage and systemic inflammatory response (number of white blood cell [WBC] P = .65, serum albumin level P = .13) recorded during hospitalization.
FIGURE 2.
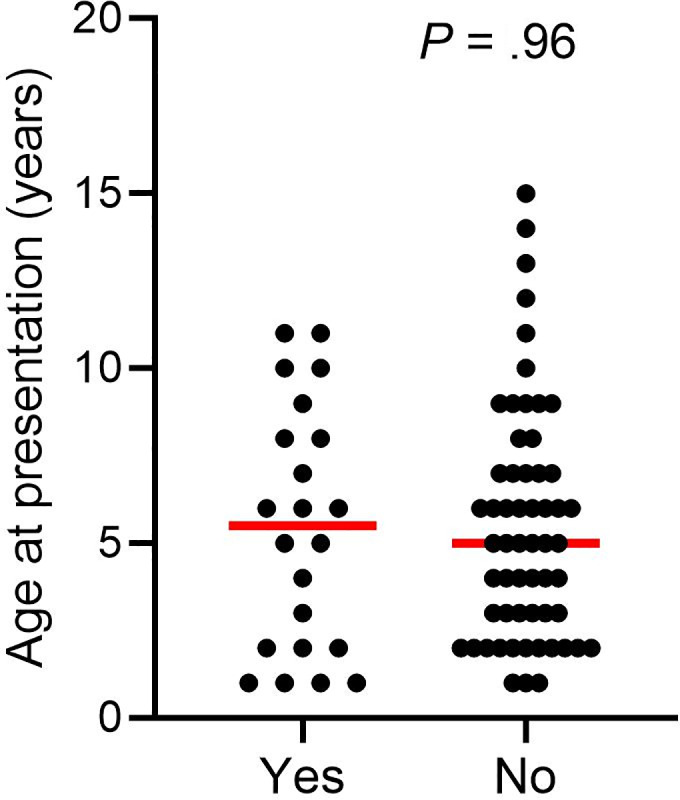
Age at the time of presentation for AHD in dogs with (yes) and without (no) development of signs of chronic GI disease later in life. Red lines indicate the median age. AHD, acute hemorrhagic diarrhea; GI, gastrointestinal
4. DISCUSSION
The present study shows that dogs with a previous episode of AHD have an increased prevalence of signs of chronic GI disease later in life. This is in accordance with puppies surviving CPV infection, in which there is an increased risk for chronic GI disease. 4
One major difference between dogs presented for CPV infection and dogs in our AHD group is the age at onset of the acute intestinal disease (median age: CPV 12 weeks vs AHD 5 years). Early life changes in the intestinal microbiota composition and diversity represent 1 mechanism that drives allergic diseases. In experimental studies using gnotobiotic mice, selective colonization of the intestinal tract has been demonstrated to protect against food allergen sensitization. In these same mice, antibiotic treatment enhances food allergen sensitization. 14 Especially in early life a critical level of microbial diversity is required to induce an immunoregulatory network that protects from allergic disorders. 15 In human medicine, there is a correlation between altered infant gut microbiome and childhood atopy or asthma, 16 and there is an increased risk for allergies and autoimmune diseases after an early‐life exposure to antibiotics. 15 , 17 , 18 It is assumed that the human gut microbiome remains vulnerable until approximately 3 years of age, creating a so‐called early life “window‐of‐opportunity.” 7
While in a cohort of dogs with CPV infection the young age at the onset of an acute intestinal disorder might represent 1 factor increasing the risk for chronic diarrhea later in life, our study shows that the time point of an insult is not the only or main predisposing factor for chronic disorders later in life. Therefore, other mechanisms such as severity of intestinal barrier dysfunction, changes in the composition and function of the commensal microbiota, and the type of the infectious agent inciting acute enteropathy might also play a role.
In humans, IBS represents a frequently observed PI long‐term sequelae. IBS is characterized by increased visceral hypersensitivity leading to chronic abdominal pain and altered intestinal motility resulting in either diarrhea or constipation without evidence of an underlying disorder (eg, lactose intolerance, coeliac disease) and without evidence of damage or inflammation of the intestinal mucosa. 19 The risk for IBS is higher in individuals exposed to infectious agents and moreover depends on the type of the causative agent. For example, in 1252 people with verified giardiasis the prevalence of PI‐IBS is 47.9% (339/707) in the infected and only 14.3% (149/1042) in the control group. 20 This prevalence of long‐term PI consequences after an acute giardiasis is much higher than the pooled prevalence of PI‐IBS with 10.1%. In a meta‐analysis comprising 21 421 humans, 42% develop IBS after diarrhea incited by protozoa or parasites and 14% by bacterial infection. 1 There is strong evidence that clostridial overgrowth and toxin release is responsible for the AHD in our dogs. 21
Dogs with AHDS characteristically have mucosal necrosis over the entire length of the small and large intestine. 21 Such severe destruction of the GI barrier, which is also suspected in our study sample, could enable the transmucosal translocation of bacteria and food antigens. It is hypothesized that allergens crossing the epithelial barrier trigger a breakdown of tolerance and this represents 1 main risk factor for developing food allergy. 5 , 6 Nine of the 22 dogs in the AHD group, which developed chronic diarrhea, had a complete resolution of clinical signs on an elimination diet. This might indeed imply that barrier dysfunction in dogs with AHD represents a risk factor for developing food allergy in a subset of dogs. Unfortunately, the exact number of dogs with chronic diarrhea responding to a diet is unknown, because in half of the dogs with chronic diarrhea no diet trials were performed. It is also unknown if the dogs which responded to an elimination diet had a true hypersensitivity to food components or a nonimmunological condition. 22
Commensal bacteria are important to protect against food allergen sensitization. 14 Bacterial products are taken up by colonic dendritic cells that induce differentiation of bacterial‐specific regulatory T cells (TRegs). These TRegs migrate to all areas of the intestine, where they release Interleukin 10 to maintain a tolerogenic immune environment; which means tolerance to harmless bacteria and food components. 23 A relevant number of dogs with AHD have a dysbiosis mainly caused by the overgrowth of C. perfringens. 10 , 12 Moreover, a large number of dogs with AHD are treated with broad‐spectrum antibiotics, which additionally induces major shifts in the intestinal microbiota. 24 Dysbiosis associated with functional or immunological alterations (eg, reduced immunomodulatory secondary bile acids, short‐chain fatty acids, indoles, and other immunomodulatory metabolites) could potentiate an allergic response. 25 No significant correlation between the use of antibiotics and the development of signs of chronic GI disease was detected in our study. However, the impact of alterations on the microbiome due to antibiotics depends on several factors including antibiotic spectrum, dosage, duration and administration route, the pharmacokinetics, and pharmacodynamic properties. 26
Children with food allergy have intestinal permeability that correlates positively with severity of symptoms. 27 We correlated variables potentially reflecting the severity of AHD and mucosal damage (eg, time of hospitalization, number of WBC, serum albumin concentration) with the prevalence of signs of chronic GI disease later in life but could not identify any additional risk factor.
This study has some limitations. First, AHDS is still a diagnosis of exclusion and the diagnostic tests were not performed identically in a standardized study design. Therefore, the term AHDS was not used for our sample group, although it was specifically searched for dogs with the diagnosis AHDS. However, the clinical sign “hemorrhagic diarrhea,” reflecting severe intestinal mucosal damage, was present in every dog. Second, the limited number of dogs in specific subcategories (eg, dogs not administered antibiotics, dogs without abnormally high WBC) might be the reason that additional risk factors for progression to chronicity could not be identified. Third, the most important component (eg, severity of barrier dysfunction, intestinal dysbiosis, type of infectious agent) responsible for driving chronicity after an acute intestinal disease cannot be determined based on the design of our study. Nevertheless, the authors believe it can be concluded that dogs with AHD have an increased risk for signs of chronic GI disease later in life. A large number of dogs with chronic diarrhea after an episode with AHD will respond to elimination diets, which is essential information to convey to the owner in such cases. It is important to have data about long‐term prognosis in dogs of AHD and this information should serve as the basis for further research to identify specific risk factors for progression to chronicity and to identify new treatment strategies.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the ethics committee of the Centre of Veterinary Medicine, Ludwig‐Maximilians‐University Munich, Germany (approval number: 215‐14‐05‐2020).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1: Supporting information.
ACKNOWLEDGMENT
No funding was received for this study. Presented at the 29th Annual ECVIM‐CA Congress, September 19‐21, 2019, Milano, Italy.
Skotnitzki E, Suchodolski JS, Busch K, et al. Frequency of signs of chronic gastrointestinal disease in dogs after an episode of acute hemorrhagic diarrhea. J Vet Intern Med. 2022;36(1):59-65. doi: 10.1111/jvim.16312
REFERENCES
- 1. Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta‐analysis. Gastroenterology. 2017;152:1042‐1054. e1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia Rodriguez LA, Ruigomez A, Panes J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology. 2006;130:1588‐1594. [DOI] [PubMed] [Google Scholar]
- 3. Ajene AN, Walker CLF, Black RE. Enteric pathogens and reactive arthritis: a systematic review of Campylobacter, Salmonella and Shigella‐associated reactive arthritis. J Health Popul Nutr. 2013;31:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kilian E, Suchodolski JS, Hartmann K, Mueller RS, Wess G, Unterer S. Long‐term effects of canine parvovirus infection in dogs. PLoS One. 2018;13:e0192198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol. 2005;115:3‐12. [DOI] [PubMed] [Google Scholar]
- 6. Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol. 2012;5:232‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson AMF, DePaolo RW. Window‐of‐opportunity: neonatal gut microbiota and atopy. Hepatobiliary Surg Nutr. 2017;6:190‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nylund L, Satokari R, Salminen S, de Vos WM. Intestinal microbiota during early life—impact on health and disease. Proc Nutr Soc. 2014;73:457‐469. [DOI] [PubMed] [Google Scholar]
- 9. Blake AB, Cigarroa A, Klein HL, et al. Developmental stages in microbiota, bile acids, and clostridial species in healthy puppies. J Vet Intern Med. 2020;34:2345‐2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Unterer S, Busch K, Leipig M, et al. Endoscopically visualized lesions, histologic findings, and bacterial invasion in the gastrointestinal mucosa of dogs with acute hemorrhagic diarrhea syndrome. J Vet Intern Med. 2014;28:52‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sindern N, Suchodolski JS, Leutenegger CM, et al. Prevalence of Clostridium perfringens netE and netF toxin genes in the feces of dogs with acute hemorrhagic diarrhea syndrome. J Vet Intern Med. 2019;33:100‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suchodolski JS, Markel ME, Garcia‐Mazcorro JF, et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS One. 2012;7:e51907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17:291‐297. [DOI] [PubMed] [Google Scholar]
- 14. Stefka AT, Feehley T, Tripathi P, et al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci. 2014;111:13145‐13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cahenzli J, Koller Y, Wyss M, et al. Intestinal microbial diversity during early‐life colonization shapes long‐term IgE levels. Cell Host Microbe. 2013;14:559‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujimura KE, Sitarik AR, Havstad S, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22:1187‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hirsch AG, Pollak J, Glass TA, et al. Early‐life antibiotic use and subsequent diagnosis of food allergy and allergic diseases. Clin Exp Allergy. 2017;47:236‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roubaud‐Baudron C, Ruiz VE, Swan AM Jr, et al. Long‐term effects of early‐life antibiotic exposure on resistance to subsequent bacterial infection. mBio. 2019;10(6):1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949‐958. [DOI] [PubMed] [Google Scholar]
- 20. Wensaas KA, Hanevik K, Hausken T, et al. Postinfectious and sporadic functional gastrointestinal disorders have different prevalences and rates of overlap: results from a controlled cohort study 3 years after acute giardiasis. Neurogastroenterol Motil. 2016;28:1561‐1569. [DOI] [PubMed] [Google Scholar]
- 21. Leipig‐Rudolph M, Busch K, Prescott JF, et al. Intestinal lesions in dogs with acute hemorrhagic diarrhea syndrome associated with netF‐positive Clostridium perfringens type A. J Vet Diagn Invest. 2018;30:495‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mueller R, Unterer S. Adverse food reactions: pathogenesis, clinical signs, diagnosis and alternatives to elimination diets. Vet J. 2018;236:89‐95. [DOI] [PubMed] [Google Scholar]
- 23. Plunkett CH, Nagler CR. The influence of the microbiome on allergic sensitization to food. J Immunol. 2017;198:581‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ortiz V, Klein L, Channell S, et al. Evaluating the effect of metronidazole plus amoxicillin‐clavulanate versus amoxicillin‐clavulanate alone in canine haemorrhagic diarrhoea: a randomised controlled trial in primary care practice. J Small Anim Pract. 2018;59:398‐403. [DOI] [PubMed] [Google Scholar]
- 25. Suchodolski JS. Diagnosis and interpretation of intestinal dysbiosis in dogs and cats. Vet J. 2016;215:30‐37. [DOI] [PubMed] [Google Scholar]
- 26. Jernberg C, Lofmark S, Edlund C, et al. Long‐term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology (Reading). 2010;156:3216‐3223. [DOI] [PubMed] [Google Scholar]
- 27. Järvinen KM, Konstantinou GN, Pilapil M, et al. Intestinal permeability in children with food allergy on specific elimination diets. Pediatr Allergy Immunol. 2013;24:589‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information.


