Abstract
Background
Metabolic syndrome is associated with formation of calcium oxalate (CaOx) uroliths in humans.
Objectives
To investigate the association between obesity and hyperlipidemia with CaOx lower urinary tract uroliths in client‐owned dogs.
Animals
Dogs with (n = 55, U [uroliths]‐dogs) and without (n = 39, UF [uroliths‐free]‐dogs) CaOx lower urinary tract uroliths.
Methods
Case‐control study. U‐dogs were retrospectively enrolled and compared to UF‐dogs. Body condition score (BCS; 1‐9 scoring scale), serum triglyceride (TG) and total cholesterol (CH) concentrations and glycemia (after >12‐hour food withholding) were recorded in both groups.
Results
On univariate logistic regression, when excluding Miniature Schnauzers, odds of having uroliths increased by a factor of 3.32 (95% CI 1.38‐11.12) for each mmol/L of TG (P = .027), of 39 (95% CI 9.27‐293.22) for each mmol/L of glycemia (P < .0001), and of 2.43 (95% CI 1.45‐4.45) per unit of BCS (P = .002). In multivariable models, the effect of TG was retained when all breeds were included for analysis and odds of having uroliths increased by a factor of 4.34 per mmol/L of TG (95% CI 1.45‐19.99; P = .02).
Conclusions and Clinical Importance
Serum lipid screening in dogs diagnosed with CaOx uroliths might be recommended to improve their medical staging and management.
Keywords: body condition score, canine, cholesterol, glycemia, obesity, obesity‐related metabolic dysfunction, triglycerides, urolithiasis, uroliths
Abbreviations
- BCS
body condition score
- BMI
body mass index
- CaOx
calcium oxalate
- CH
serum total cholesterol
- HCH
hypercholesterolemia
- HTG
hypertriglyceridemia
- LUT
lower urinary tract
- MetS
metabolic syndrome
- ORMD
obesity‐related metabolic dysfunction
- SBP
systolic blood pressure
- TG
serum triglycerides
- USG
urine specific gravity
1. INTRODUCTION
Based on global data from the Minnesota Urolith Center, calcium oxalate (CaOx) uroliths represent 36% of urolith submissions in dogs. 1 The increasing prevalence of CaOx uroliths relative to other urinary tract urolith types over the last 2 decades is of concern. 2 , 3 , 4 Overall, the pathophysiology of CaOx urinary tract urolith formation is complex, and much still remains to be understood. The 2 major factors involved in crystal formation are supersaturation of urine with calcium and oxalate and the imbalance between substances that promote and those that inhibit CaOx formation. 5
In humans, the prevalence and incidence of CaOx uroliths have markedly increased over the past several decades, especially in developed countries in which certain predisposing factors, such as obesity, have been identified. 6 Obesity represents a major and increasing healthcare concern in both human and veterinary medicine. 7 , 8 , 9 The population prevalence of overweight or obese dogs is estimated at 20 to 60% across various countries. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Overweight or obese people have an increased risk of CaOx and uric acid urolith formation and recurrence. 19 , 20 , 21 Especially, hyperlipidemia—defined as hypertriglyceridemia (HTG), hypercholesterolemia (HCH), or both—is related to an increased risk of nephrolithiasis in people. 22 , 23 , 24 , 25 , 26
Human metabolic syndrome (MetS) defined as concurrent obesity, hypertension, dyslipidemia and insulin resistance, 27 , 28 is associated with alterations in urine mineral content leading to an increased risk of both CaOx and uric acid nephrolithiasis formation and recurrence. 24 , 29 , 30 , 31 , 32 , 33 , 34 As dogs do not experience many of the complications associated with MetS identified in humans, such as atherosclerosis, coronary heart disease or stroke and type 2 diabetes mellitus, defining MetS for dogs has been questioned, 35 and the MetS criteria have therefore been adapted for this species defining obesity‐related metabolic dysfunction (ORMD). 36 The diagnosis of ORMD in dogs relies on 2 criteria: (a) body condition score (BCS) of 7‐9/9; AND (b) any 2 of the following: (1) serum TG > 2.3 mmol/L; (2) serum CH > 7.8 mmol/L; (3) systolic blood pressure > 160 mm Hg; (4) plasma glucose > 5.6 mmol/L, or previously diagnosed diabetes mellitus. 36 When compared with healthy dogs, dogs with ORMD have higher serum concentrations of CH, TG and glucose. 37 Approximately 20% of dogs with naturally occurring obesity have concurrent ORMD. 36
The aim of this study was to investigate the association between obesity and hyperlipidemia (CH, TG) with CaOx lower urinary tract uroliths in dogs. Hypotheses are that obesity and hyperlipidemia are associated with CaOx lower urinary tract uroliths in dogs.
2. MATERIALS AND METHODS
The study protocol defined and compared 2 groups: dogs with (U [uroliths]‐dogs) and without (UF [uroliths‐free]‐group) CaOx lower urinary tract uroliths. For U‐dogs, medical records between January 2013 and January 2020 were reviewed for dogs having concurrent CaOx lower urinary tract uroliths and serum TG measurement after >12‐hour food withholding. Since 2013, all dogs that presented to the Internal Medicine Department at the Veterinary Teaching Hospital, University of Montreal, for further assessment of lower urinary tract uroliths, underwent a systematic screening for hyperlipidemia after recent evidence that hyperlipidemia might be related to an increased risk of lithiasis in people. 22 , 23 , 24 , 25 , 26 CaOx monohydrate or dihydrate lower urinary tract uroliths were defined as having a composition of ≥70% of these minerals on chemical analysis. 38 All uroliths were analyzed by the Canadian Veterinary Urolith Centre (CVUC), University of Guelph, Laboratory Services Division, Guelph, ON, N1H 8J7, Canada. UF‐dogs enrolled in the study were apparently healthy staff pets from the Veterinary Teaching Hospital, University of Montreal, and were recruited between November and December 2019. Dogs with comorbidities or receiving concurrent pharmaceuticals that could influence lipid metabolism, glucose metabolism or urine composition were excluded from both U‐ and UF‐groups. The study was approved by the ethics committee of the University of Montreal (19‐Rech‐2049). Informed owner consents were obtained before enrollment of UF‐dogs.
The following data were recorded: signalment, previous medical history (medical background, current treatments, and diet), physical examination findings with BCS (1 to 9 points scale), 39 complete blood cell count (CBC) and biochemistry profile including electrolytes (ionized calcium if needed), CH and TG (within 3 months of CaOx lower urinary tract urolith removal), and urinalysis (free‐catch or cystocentesis). Samples were collected after food was withheld for >12 hours. Dogs in the control group (UF‐dogs) were prospectively recruited based on absence of lower urinary tract uroliths confirmed by the absence of visible uroliths on ultrasound and on 2 abdominal radiographs (orthogonal views and a lateral view with the legs extended to evaluate the course of the urethra in male dogs). UF‐dogs also underwent a complete physical examination including evaluation of BCS, CBC, biochemistry profile including TG, CH, and serum glucose after >12‐hour food withholding, and urinalysis (37 cystocentesis and 2 free‐catch samples) and only systemically healthy dogs were included. All blood and urine samples from U‐ and UF‐dogs were processed by the same laboratory at the Veterinary Teaching Hospital, University of Montreal. The laboratory analyzers and technique for evaluating biochemical profiles including serum TG, CH and glucose were the same from 2013 through to 2020.
A single investigator (MVP) recorded BCS of UF‐dogs whereas the BCS of U‐dogs was evaluated by the attending veterinarian. Overweight was defined as a BCS ≥6 and obesity by a BCS ≥7. 39 , 40 Systemic hypertension was present if the mean of the systolic blood pressure (SBP) measurements was above 160 mm Hg measured by a Doppler device. 41 Cholestasis was suspected with a 2‐fold increase in alkaline phosphatase (≥226 U/L) and concomitant ultrasonographic evidence of 1—mobile gallbladder sludge with a dilated gallbladder lumen; or 2—calculi with a dilated gallbladder lumen; or 3—characteristics of a gall bladder mucocele. 42 , 43 The diagnosis of ORMD in dogs relied on 2 criteria: (a) body condition score (BCS) of 7‐9/9; AND (b) any 2 of the following: (1) serum TG >2.3 mmol/L (>203.7 mg/dL); (2) serum CH >7.8 mmol/L (>301.6 mg/dL); (3) SBP >160 mm Hg; (4) plasma glucose >5.6 mmol/L (>100.8 mg/dL), or previously diagnosed diabetes mellitus. 36
Statistical analyses and graphs were produced using GraphPad PRISM 9 software version 9.2.0 (San Diego, CA). Qualitative data were presented as percentages and analyzed with a Fisher's exact test. Quantitative data were presented as medians and ranges and were analyzed with a Mann‐Whitney test. A P‐value of <.05 was considered significant. Serum lipid concentrations were analyzed as dichotomous (abnormal vs normal) and as continuous variables to evaluate their relationship with CaOx lower urinary tract uroliths. The cutoffs used were 7.8 mmol/L (301.6 mg/dL) for HCH and 2.3 mmol/L (203.7 mg/dL) for HTG. 36
Univariate logistic regressions were used to examine the effect of each risk factor on the odds of having uroliths. The effect of these risk factors was further studied in multivariable logistic regressions controlling for confounding variables. The logic was to include variables that were significant in the univariate models. Body condition scores were not available in many dogs with CaOx uroliths, and the logistic regressions were therefore performed with and without BCS. Odds ratios (OR) associated with serum glucose concentration produced unreliable estimates as evidenced by the large confidence intervals in the univariate model. Hence, we did not consider this variable in the multivariate models. Apart from glycemia, the multivariable models included the same cofactors: in 1 model, age and sex were included; in another model, age, sex, BCS and urine specific gravity were included. Analyses were repeated including or not breeds predisposed to primary hyperlipidemia, which only referred to Miniature Schnauzers in the current study.
3. RESULTS
The review of medical records between January 2013 and January 2020 for dogs having concurrent CaOx lower urinary tract uroliths (≥ 70% of CaOx monohydrate or dihydrate on chemical analysis) and >12‐hour food withholding serum TG measurement identified 66 dogs. Eight dogs with CaOx lower urinary tract uroliths presented with comorbidities that could influence lipid metabolism, glucose metabolism or urine composition and were excluded (2 dogs with hypothyroidism, 2 dogs with pituitary‐dependent hyperadrenocorticism, and 4 dogs with suspected cholestasis). Three dogs with CaOx lower urinary tract uroliths had received corticosteroids or phenobarbital within 3 months before presentation and were excluded.
Finally, 55 dogs were retrospectively recruited in the U‐group, including Miniature Schnauzer (22%, 12/55), Shih Tzu (18%, 10/55), Yorkshire Terrier (16%, 9/55), Poodle (15%, 8/55), Bichon (3 Maltese and 1 Frise; 6%, 4/55) and 10 other breeds (3 Dachshund, 2 Chihuahua, 2 Lhasa Apso, 1 Miniature Pinscher, 1 Portuguese Water Dog, 1 Coton de Tulear, 1 Pug, 1 Boston Terrier). Epidemiological and clinical findings in U‐dogs are detailed in Table 1.
TABLE 1.
Epidemiological and clinical findings in dogs with and without lower urinary tract CaOx uroliths
| U‐dogs (n = 55) | UF‐dogs (n = 39) | P | |
|---|---|---|---|
| Sex | |||
| Male | 72% (40/55) | 33% (13/39) | <.001 |
| Neutered males | 67% (37/55) | 33% (13/39) | |
| Intact males | 5% (3/55) | 0% (0/39) | |
| Female | |||
| Spayed females | 27% (15/55) | 59% (23/39) | |
| Intact females | 0% (0/55) | 8% (3/39) | |
| Age (median, range), months | 100 [24‐155] | 78 [12‐149] | .008 |
| Weight (median, range), kg | 7.2 [1.6‐28.7] | 9 [1.77‐23.7] | .07 |
| Clinical signs on presentation | |||
| Hematuria | 60% (33/55) | 0 | |
| Pollakiuria | 53% (29/55) | 0 | |
| Dysuria | 22% (12/55) | 0 | |
| Stranguria | 11% (6/55) | 0 | |
| Periuria | 7% (4/55) | 0 | |
| Nocturia | 4% (2/55) | 0 | |
| Oliguria or anuria | 2% (1/55) | 0 | |
| Discomfort on caudal abdominal palpation | 16% (9/55) | 0 | |
| Urolith prevention diet fed for >3 months before presentation (dry or wet not known) | 44% (24/55) | 0 |
Serum TG, CH and glucose values were available for 55, 32 and 42 U‐dogs respectively. Abdominal radiographs and urinary tract ultrasound were performed in 98% (54/55) and 84% (46/55) of U‐dogs respectively. Among U‐dogs, 36% (20/55) had only bladder uroliths, 2% (1/55) had only urethral uroliths and 62% (34/55) had both bladder and urethral uroliths. Minimally invasive procedures (percutaneous cystolithotomy, cystoscopic basket removal, urohydropulsion, and laser lithotripsy) were performed in 98% (54/55) of U‐dogs and open surgical cystotomy was performed in 1 dog (2%). Among the 55 U‐dogs, 47 dogs (85%) had CaOx monohydrate lower urinary tract uroliths and 8 dogs (15%) had CaOx dihydrate lower urinary tract uroliths.
Forty dogs were prospectively recruited based on absence of lower urinary tract uroliths. Thirty‐nine dogs were deemed systemically healthy and were enrolled in UF‐group. The last dog was excluded after being diagnosed with hypothyroidism. The 39 remaining UF‐dogs included mixed breeds (28%, 11/39), Yorkshire Terrier (13%, 5/39), and 13 other breeds (3 Cavalier King Charles Spaniel, 1 American Cocker Spaniel, 1 Pembroke Welsh Corgi, 1 Shiba Inu, 3 Boston Terrier, 2 Shih Tzu, 1 Maltese, 1 Bichon Frise, 1 Jack Russell Terrier, 3 Chihuahua, 2 Australian Shepherd, 1 Pug, 2 Pit Bull). Serum TG, CH and glucose measurements were available for all 39 UF‐dogs. Epidemiological and clinical findings in UF‐dogs are detailed in Table 1.
Serum TG concentrations were significantly higher in U‐dogs than UF‐dogs, with (0.93 mmol/L, n = 55, range: 0.21‐11.96; 0.49 mmol/L, n = 39, range: 0.16‐1.96; P = .0002) or without Miniature Schnauzers (0.87 mmol/L, n = 43, range: 0.21‐11.96; 0.49 mmol/L, n = 39, range: 0.16‐1.96; P = .005; Table 2, Figure 1). HTG was significantly more frequent in U‐dogs than UF‐dogs, with (24%, 13/55; 0%, 0/39; P = .0006) or without Miniature Schnauzers (19%, 8/43; 0%, 0/39; P = .01; Table 2). Serum CH concentrations and hypercholesterolemia did not differ significantly between the 2 groups (P = .17 and P = .51, respectively; Table 2). The effect of TG was maintained when excluding dogs receiving urinary diets in both U‐ and UF‐groups, whether Miniature Schnauzers were included: median TG 1.29 mmol/L [0.41‐9.6] in U‐dogs (n = 31) vs 0.49 mmol/L [0.16‐1.96] in UF‐dogs (n = 39), P < .0001; or excluded: 0.97 mmol/L [0.41‐9.6] in U‐dogs (n = 23) vs 0.49 mmol/L [0.16‐1.96] in UF‐dogs (n = 39), P < .0001.
TABLE 2.
Hyperlipidemia in dogs with and without CaOx lower urinary tract uroliths
| U‐dogs | UF‐dogs | P | |
|---|---|---|---|
| TG (mmol/L; median, range) | 0.93 (0.21‐11.96), n = 55 | 0.49 (0.16‐1.96), n = 39 | .0002* |
| TG without Miniature Schnauzers (mmol/L; median, range) | 0.87 (0.21‐11.96), n = 43 | 0.49 (0.16‐1.96), n = 39 | .005* |
| HTG (%) | 24%, n = 55 | 0%, n = 39 | .0006* |
| HTG without Miniature Schnauzers (%) | 19%, n = 43 | 0%, n = 39 | .01* |
| CH (mmol/L; median, range) | 6.47 (3.08‐9.58), n = 32 | 5.65 (2.94‐12.6), n = 39 | .17 |
| HCH (%) | 21%, n = 29 | 13%, n = 39 | .51 |
Abbreviations: CH, serum total cholesterolemia; HCH, hypercholesterolemia; HTG, hypertriglyceridemia; TG, serum triglyceridemia.
Indicates significant differences.
FIGURE 1.
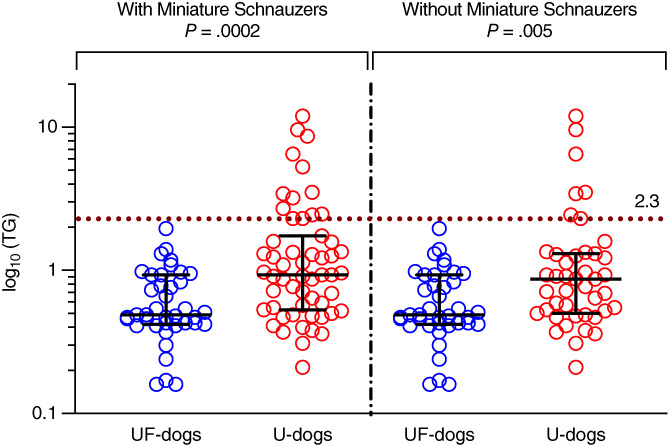
Triglyceridemia (TG) in dogs with (55 U‐dogs) and without (39 UF‐dogs) CaOx lower urinary tract uroliths. Medians with interquartile ranges are presented
Body condition scores were significantly higher in U‐dogs (median 5, n = 43, range: 4‐9) than UF‐dogs (median 5, n = 39, range: 4‐7; P = .04; Table 3, Figure 2). Obesity (BCS ≥7) was significantly more frequent in U‐dogs (23%, 10/43) than UF‐dogs (3%, 1/39; P = .008) but a similar proportion of the subjects were overweight in the 2 groups (P = .11; Table 3). Median glycemia was significantly higher in U‐dogs (5.8 mmol/L, n = 42, range: 4.3‐7.0) than UF‐dogs (4.7 mmol/L, n = 39, range: 3.0‐6.2; P < .0001; Table 3, Figure 3). Systolic blood pressures were reported in 31 U‐dogs (mean 156, n = 31, range: 110‐211; Table 3). Given the retrospective recruitment of U‐dogs, all ORMD criteria (as defined by Tvarijonaviciute et al., 2012) 36 were available for 12 U‐dogs (22%), from which 1 (2%) was diagnosed with ORMD. The ORMD could not be assessed in UF‐dogs since the study design did not allow to measure SBP.
TABLE 3.
Other components of obesity‐related metabolic dysfunction (ORMD) in dogs with and without CaOx lower urinary tract uroliths
| U‐dogs | UF‐dogs | P | |
|---|---|---|---|
| BCS (median, range) | 5 (4‐9), n = 43 | 5 (4‐7), n = 39 | .04* |
| Overweight (BCS ≥6; %) | 46%, n = 43 | 28%, n = 39 | .11 |
| Obese (BCS ≥7; %) | 23%, n = 43 | 3%, n = 39 | .008* |
| Glycemia (mmol/L; median, range) | 5.8 (4.3‐7.0), n = 42 | 4.7 (3.0‐6.2), n = 39 | <.0001* |
| SBP (mm Hg; mean, range) | 156 (110, 211), n = 31 |
Abbreviations: BCS, body condition score; SBP, systolic blood pressure.
Indicates significant results.
FIGURE 2.
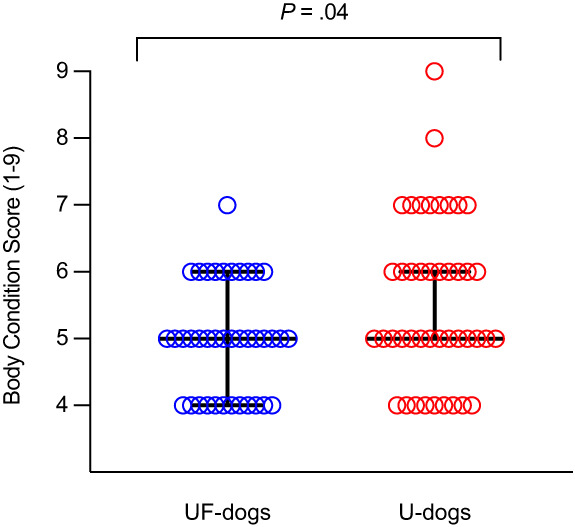
Body condition score (BCS) in dogs with (43 U‐dogs) and without (39 UF‐dogs) CaOx lower urinary tract uroliths. Medians with interquartile ranges are presented
FIGURE 3.
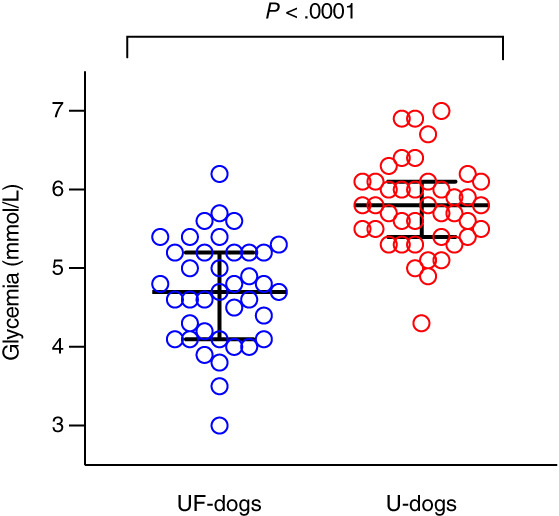
Glycemia in dogs with (42 U‐dogs) and without (39 UF‐dogs) CaOx lower urinary tract uroliths. Medians with interquartile ranges are presented
Univariate OR for having uroliths are presented in Table 4. No dogs in the UF‐group had HTG therefore this categorical variable could not be tested. Odds of having uroliths, when excluding Miniature Schnauzers, significantly increased per unit of serum TG (OR = 3.32, 95% CI 1.38‐11.12, P = .027), BCS (OR = 2.43, 95% CI 1.45‐4.45, P = .002), glycemia (OR = 39.37, 95% CI 9.27‐293.22, P < .0001), additional month of age (OR = 1.017, 95% CI 1.0038‐1.031, P = 0.01), and in males vs females (OR = 8.75, 95% CI 3.29‐25.51, P < .0001) and decreased per unit of USG (OR = 0.95, 95% CI 0.91‐0.99, P = .02; Table 4). Cholesterol and urinary pH were not significantly associated with CaOx lower urinary tract uroliths. Similar effects were observed when including Schnauzers as well.
TABLE 4.
Univariate odds ratio (OR) of some components of ORMD in dogs with and without CaOx lower urinary tract uroliths, including or excluding Miniature Schnauzers
| Variables | OR with Miniature Schnauzers [95% CI] (P‐value) | OR without Miniature Schnauzers [95% CI] (P‐value) |
|---|---|---|
| TG (mmol/L) | 4.52 [1.87, 14.55] (.004*) | 3.32 [1.38, 11.12] (.027*) |
| CH (mmol/L) | 1.15 [0.88, 1.54] (.32) | 1.13 [0.84, 1.54] (.42) |
| pH | 1.34 [0.92, 2.00] (.13) | 1.33 [0.89, 2.047] (.17) |
| Age (months) | 1.018 [1.0057, 1.031] (.005*) | 1.017 [1.0038, 1.031] (.01*) |
| Urine specific gravity | 0.94 [0.90, 0.98] (.006*) | 0.95 [0.91, 0.99] (.02*) |
| BCS | 1.69 [1.097, 2.74] (.02*) | 2.43 [1.45, 4.45] (.002*) |
| Glycemia (mmol/L) | 23.47 [7.09, 117.25] (<.0001*) | 39.37 [9.27, 293.22] (<.0001*) |
| Sex (males vs females) | 5.33 [2.23, 13.40] (.0002*) | 8.75 [3.29, 25.51] (<.0001*) |
Abbreviations: BCS, body condition score; CH, serum total cholesterolemia; TG, serum triglyceridemia.
Indicates significant results.
In all multivariable models, a significant effect of TG only occurred when including Schnauzers adjusting for age and sex or adjusting for age, sex, BCS and USG (Table 5). When adjusted for age and sex, odds of having uroliths increased by a factor of 3.23 per unit of TG when all dog breeds were included (95% CI 1.33‐12.08; P = .04; Table 5). When adjusted for age, sex, BCS, and urine specific gravity, odds of having uroliths increased by a factor of 4.34 per mmol/L of TG (95% CI 1.45‐19.99; P = .02) when all breeds are included. In all multivariable models, CH did not have a statistically significant association with uroliths.
TABLE 5.
Multivariate odds ratio (OR) of TG and CH in dogs with and without CaOx lower urinary tract uroliths, including and excluding Miniature Schnauzers
| Variables | OR with Miniature Schnauzers [95% CI] (P‐value) | OR without Miniature Schnauzers [95% CI] (P‐value) |
|---|---|---|
| Adjusted for age and sex | ||
| TG (mmol/L) |
3.23 [1.33, 12.08] (.04*) (55 U‐dogs and 39 UF‐dogs) |
1.70 (0.87, 7.25; .37) (43 U‐dogs and 39 UF‐dogs) |
| Age (months) | 1.013 [0.99, 1.03] (.095) | 1.018 [1.0016, 1.037] (.04*) |
| Sex (males vs females) | 6.37 [2.34, 19.24] (.0005*) | 9.91 [3.30, 34.76] (.0001*) |
| CH (mmol/L) | 1.11 [0.79, 1.52] (.53) | 1.12 (0.74, 1.67; .57) |
| Age (months) | 1.018 [1.0025, 1.035] (.03*) | 1.023 [1.0043, 1.044] (.02*) |
| Sex (males vs females) | 6.98 [2.39, 29.96] (.0007*) | 20.18 [4.96, 118.01] (.0001*) |
| Adjusted for age, sex, BCS and USG | ||
| TG (mmol/L) |
4.34 [1.45, 19.99] (.02*) (33 U‐dogs and 39 UF‐dogs) |
2.0049 [0.83, 12.76] (.39) (27 U‐dogs and 39 UF‐dogs) |
| Age (months) | 1.011 [0.99, 1.031] (.25) | 1.019 [0.99, 1.044] (.10) |
| Sex (males vs females) | 5.97 [1.70, 25.21] (.008*) | 13.71 [3.05, 90.03] (.002*) |
| BCS | 1.39 [0.77, 2.69] (.30) | 1.91 [0.95, 4.33] (.09) |
| USG | 0.93 [0.87, 0.98] (.008*) | 0.92 [0.85, 0.98] (.01*) |
| CH (mmol/L) | 0.90 [0.57, 1.37] (.64) | 0.95 [0.52, 1.64] (.86) |
| Age (months) | 1.022 [1.0031, 1.044] (.03*) | 1.032 [1.0075, 1.066] (.02*) |
| Sex (males vs females) | 9.24 [2.30, 48.23] (.004*) | 39.27 [5.51, 571.5] (.001*) |
| BCS | 0.93 [0.44, 1.91] (.85) | 1.61 [0.65, 4.27] (.31) |
| USG | 0.93 [0.88, 0.99] (.02*) | 0.92 [0.83, 0.99] (.04*) |
Abbreviations: BCS, body condition score; CH, serum total cholesterolemia; TG, serum triglyceridemia; USG, urine specific gravity.
Indicates significant results.
In U‐dogs, median BCS was 5 (n = 37) and 6 (n = 6) in dogs with monohydrate and dihydrate uroliths, respectively. Median SBP was 155 mm Hg in obese (BCS ≥7) and 160 mm Hg in nonobese (BCS < 7) U‐dogs (P = .83), and 156 and 158 mm Hg in overweight and nonoverweight U‐dogs, respectively (P = .44). When U‐ and UF‐dogs were pooled together, median BCS was 5 for both male and female dogs (P = .09). Proportion of obese males and obese females was not significantly different in U‐group (23% and 25%, respectively, P = 1). Median urine pH was 6.25 in U‐dogs (n = 42) and 6 in UF‐dogs (n = 39; P = .13). Median USG was significantly lower in U‐dogs (1.031, n = 41) than in UF‐dogs (1.039, n = 39; P = .001).
4. DISCUSSION
On univariate logistic regression when excluding Miniature Schnauzers, odds of having uroliths increased per unit of TG, glycemia and BCS. In multivariable models, the effect of TG was retained when all breeds were included for analysis and odds of having uroliths increased by a factor of 4.34 per mmol/L of TG (95% CI 1.45‐19.99; P = .02).
Dyslipidemia, characterized by the elevation of serum CH, TG, and a low high‐density lipoprotein, is associated with upper urinary tract urolith risk, formation and recurrence in people. 24 , 26 Hypercalciuria, hyperuricosuria, hyperoxalaturia and hypocitraturia are documented in these patients and these anomalies predispose to urolith formation. 25 Hypercalciuria and hyperoxaluria occur in rat models with HTG and HCH. 44
In the present study, Miniature Schnauzer was the only breed predisposed to primary hyperlipidemia in U‐group. 45 , 46 None of the UF‐breeds have been reported with primary hyperlipidemia. 45 , 46 Serum TG concentrations were significantly higher in U‐dogs than UF‐dogs, with and without Miniature Schnauzers, which suggests an association between HTG and CaOx lower urinary tract urolith formation in dogs. The significant effect of TG was maintained in the univariate model. However, in multivariable models, the significant effect of TG occurred only when including Miniature Schnauzers. The decrease in OR when excluding Miniature Schnauzers suggests that inclusion of this breed was responsible for the strong association between CaOx uroliths and TG. A larger sample of non‐Miniature Schnauzer dogs is needed to better discern the association between TG concentrations and CaOx uroliths. Univariate and multivariate analyzes captured age and sex distribution when TG concentrations were assessed (similar weights were reported in U‐ and UF‐groups, P = .07). Besides, serum TG does not seem to be influenced by age and sex. 47 There is statistical difference between male and female Miniature Schnauzers with regards to the prevalence of hypertriglyceridemia. 48 Although trends in commercial dog food formulation can vary over time, TG concentrations were measured after >12‐hour food withholding, and several diets do not influence triglyceridemia after food was withheld overnight. 47 The effect of TG was maintained when excluding dogs receiving urinary diets, whether Miniature Schnauzer were included or excluded. In humans, serum TG concentrations are significantly higher in patients with nephrolithiasis 22 , 49 and especially with CaOx 22 , 24 , 49 and uric acid nephrolithiasis. 24 , 49 High TG concentrations are the most important trait of MetS associating with human nephrolithiasis. 50 Urolith‐free humans with HTG are significantly more likely to have recurrence of uroliths with shorter urolith‐free intervals. 49 , 51 Urolith recurrence was not been evaluated in the present study.
Serum CH concentrations were not significantly different between U‐dogs and UF‐dogs. In humans, the association between high CH and uroliths is unclear: both increased 22 and decreased 26 , 49 serum CH concentrations are associated with all types of nephroliths. Serum CH concentrations are significantly lower in patients with CaOx nephrolithiasis and a negative association is identified between high CH and CaOx nephrolithiasis. 49 In these studies, it has been suggested that the association between HCH and CaOx nephrolithiasis might differ based on ethnicity, geography, genetic background, and lifestyle.
The proportion of obese dogs (BCS ≥7) was significantly higher for U‐dogs than UF‐dogs, as previously reported, 40 but a similar proportion of the subjects were overweight in the 2 groups. The significant effect of BCS was retained on univariate models. However, the effect of BCS was not retained in multivariable models, suggesting that age, sex, or both, explained the significantly higher proportion of obese dogs in U‐group than UF‐dogs. The Minnesota Urolith Center reported that overweight dogs were 2.2 times as likely to have CaOx uroliths, compared with dogs with an ideal body weight. 52 Conversely, another study evaluating 452 dogs with CaOx uroliths at general care veterinary hospitals did not show any significant difference between proportion of thin, normal or overweight dogs. 4 Since UF‐dogs enrolled in the study were apparently healthy staff pets, the lower incidence of obesity in this group could reflect a selection bias. Staff members of a veterinary hospital might monitor their pets' weight more closely. Similarly, staff members might not consider their obese dogs to be healthy and thus not volunteer them for study inclusion. However, when staff members shared their concerns regarding their pets' weight at the time of the inclusion, the authors encouraged them to enroll their dogs regardless of their body condition scores. In small companion animals, because of species, breed and age variation, measurement and subsequent diagnosis of obesity is complex. 7 The subjective and semiquantitative BCS (1 to 9 points scale) used in the study involved visual and tactile assessment characteristics correlating with abdominal and subcutaneous fat. 8 , 17 BCS scale has a good correlation with more accurate methods (chemical analysis, dual‐energy X‐ray absorptiometry, total body water using D2O, bioelectrical impedance), 53 , 54 , 55 and agreement among experienced and inexperienced operators is excellent when evaluating BCS using a 9 point scoring scale, suggesting that the method is reliable even when used without prior training. 8 Besides, BCS charts were available in each examination room, and all medical records were reassessed by an ACVIM board‐certified veterinarian. Although BCS measurement by several attending veterinarians can be a source of bias, the latter is considered negligeable. In large human epidemiologic studies, a larger body size is associated with an increased occurrence 21 , 49 , 56 , 57 and risk 30 , 58 , 59 of kidney uroliths, mainly composed of CaOx and uric acid. 34 , 60 , 61 In our study, the proportion of obese males and females were similar in U‐group, which contrasts with a previous study 13 , 15 , 62 in which obese females were significantly more likely to be affected than obese males. 9 , 16 , 18 In people, although studies report that overweight women had an increased risk for urolith formation 63 and number of episodes of urolith formation, 57 other studies revealed that overweight and obese men were more prone to urolith formation. 19 In our study, the median BCS was higher but not significantly in U‐dogs with CaOx dihydrate than monohydrate lower urinary tract uroliths. This correlates with human data where the significantly higher BMI in people who form uroliths is more prominent with uric acid and CaOx dihydrate uroliths than with monohydrate uroliths. 22
Although there is some evidence that obesity and dyslipidemia increase the risk of uroliths, the exact pathogenesis underlying the association between dyslipidemia and nephrolithiasis remains unclear. 49 In humans, obesity has been shown to significantly increase urine excretion of oxalate, 63 calcium, 34 , 63 , 64 uric acid, 34 , 63 , 64 sodium, 64 potassium, 64 magnesium, 64 creatinine, 64 calcium phosphate, 64 increase 64 or decrease 34 , 63 citrate excretion, and decrease fluid intake. 51 There is also a strong negative correlation between urine pH and body weight, 65 , 66 BMI, 65 , 66 or MetS 34 in patients with nephrolithiasis. Obesity and MetS are also associated with insulin resistance, 67 , 68 , 69 that decreased the production and transport of ammonia resulting in a low urine pH. 22 , 70 , 71 A low urine pH caused by an increase in the concentration of soluble nondissociated uric acid precipitates to favor uric acid urolith formation. 50 , 65 , 72 , 73 CaOx uroliths could form from uric acid‐induced crystallization of calcium salts by the process of heterogeneous nucleation. 19 , 63 , 72 , 74 , 75 , 76 , 77 However, the latter highlights a potential relevant species difference between humans and dogs. Whereas hyperuricosuria is a risk factor for CaOx uroliths in humans, 78 this mechanism seems unlikely in dogs since uric acid is usually further metabolized to allantoin (a more soluble product) by hepatic uricase. 79 Insulin resistance is the most important factor of MetS and kidney urolith formation in humans. 70 Dogs with ORMD had a significantly higher serum calcium concentration in comparison with dogs without ORMD, which could promote CaOx urolith formation. 37 Given that only 1 U‐dogs were diagnosed with ORMD in the current study, comparison between serum calcium concentration in dogs with and without ORMD was not possible.
In the light of the promising univariate OR of BCS and TG with and without CaOx lower urinary tract uroliths, further studies should be conducted to examine the effect of dietary and lifestyle interventions as preventive modalities on recurrent urolith formation in hyperlipidemic dogs, as it is recommended in humans in which a high consumption of simple carbohydrates or fat and a sedentary lifestyle contribute to HTG. 80 In dogs, obesity is associated with a higher number of meals, 81 high fat diets, 17 snacks fed, 14 , 81 feeding of kitchen/table scraps, 81 fresh meat, 81 and canine commercial treats. 81 The impact of weight loss is significant to the extent that it markedly improves all aspects of MetS in overweight humans. 82 Lifestyle changes such as exercise and decrease in stressful events also play an important role in decreasing HTG. 7 Exercise programs were recommended by the American Association of Clinical Endocrinologists for overweight humans. 83 , 84 , 85 For dogs, reduced daily exercise has been associated with obesity. 11 , 13 , 14 , 81 It has been postulated that exercise could potentially improve insulin sensitivity, thus, increasing urine pH and minimizing the risk of CaOx and uric acid urolith formation. 24 , 34 The combination of diet and exercise have been found to be significantly more effective than diet or exercise alone in the prevention of kidney uroliths in people with MetS. 86 A potential relationship between HTG and CaOx lower urinary tract uroliths in dogs might lead to the evaluation of statin drugs to prevent formation and recurrence of CaOx uroliths. In humans, besides diet restriction, statins are recommended as first‐line drug therapy for HTG. 23 Individuals taking statin medications have significantly less urolith formation compared to those not taking statins. 23
Glycemia was significantly higher in dogs with CaOx lower urinary tract uroliths and odds of having uroliths significantly increased per unit of glucose in the univariate model. Unfortunately, ORs associated with glycemia produced unreliable estimates as evidenced by the large confidence intervals in the univariate model, and glycemia could therefore not be considered in multivariate models. An association between high blood glucose and nephrolithiasis exists in people. 30 , 50 , 58 , 59
Urine pH is not lower in overweight dogs. 40 Evaluation of dogs with a wider range of BCSs than that in the current study sample might reveal an association between BCS and urine pH. Urine pH was also not associated with the presence of CaOx lower urinary tract uroliths as previously reported. 40 In humans, acidic urine is a risk factor for CaOx crystalluria, and the risk for the development of CaOx crystalluria is greatest when the urine pH is 4.5‐5.5. 87 In dogs, normal urine pH ranges from 5.0 to 8.0, and the risk of CaOx urolith formation might be less within that pH range as compared to lower pH values. 88 All U‐dogs in our study had a urine pH > 5.0, that is not significantly different from UF‐dogs. Our hypothesis is that as 44% of U‐dogs in our study were fed a urolith prevention diet at least 3 months before presentation, this might explain the higher urine pH. Specific gravity was significantly higher in UF‐dogs and odds of having uroliths significantly decreased per unit of USG. There is a negative association between USG and the presence of CaOx uroliths. 40 Increased water consumption was commonly recommended by the referring veterinarian when uroliths were diagnosed and urolith prevention diets also increase water consumption, this might account for the significantly lower USG in U‐dogs.
On univariate logistic regression, our study suggests a link between BCS, TG and glucose when excluding Miniature Schnauzers. Odds ratios associated with glucose produced unreliable estimates, and glycemia could therefore not be considered in the multivariate model. In multivariable models, the effect of BCS was not retained and the effect of TG was retained when all breeds were included for analysis. The decrease in OR when excluding Miniature Schnauzers suggests that inclusion of this breed was responsible for the strong association between uroliths and TG. A larger sample of non‐Miniature Schnauzer dogs is needed to better discern the association between TG concentrations and CaOx lower urinary tract uroliths. Serum lipid screening in dogs diagnosed with CaOx lower urinary tract uroliths might be recommended to improve their medical staging and management.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the University of Montreal, Canada, approval 19‐Rech 2049.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Funding provided by Hill's Pet Nutrition Canada.
Paulin MV, Dunn M, Vachon C, Beauchamp G, Conversy B. Association between hyperlipidemia and calcium oxalate lower urinary tract uroliths in dogs. J Vet Intern Med. 2022;36(1):146-155. doi: 10.1111/jvim.16324
REFERENCES
- 1. 2019 Minnesota Urolith Center Global Data Generated by Minnesota Urolith Center; March 2020. https://www.vetmed.umn.edu/sites/vetmed.umn.edu/files/globaldata.pdf. Accessed Feb 27 2021.
- 2. Osborne CA, Lulich JP, Kruger JM, et al. Analysis of 451,891 canine uroliths, feline uroliths, and feline urethral plugs from 1981 to 2007: perspectives from the Minnesota Urolith Center. Vet Clin North Am Small Anim Pract. 2009;39:183‐197. [DOI] [PubMed] [Google Scholar]
- 3. Low WW, Uhl JM, Kass PH, et al. Evaluation of trends in urolith composition and characteristics of dogs with urolithiasis: 25,499 cases (1985‐2006). J Am Vet Med Assoc. 2010;236:193‐200. [DOI] [PubMed] [Google Scholar]
- 4. Okafor CC, Lefebvre SL, Pearl DL, et al. Risk factors associated with calcium oxalate urolithiasis in dogs evaluated at general care veterinary hospitals in the United States. Prev Vet Med. 2014;115:217‐228. [DOI] [PubMed] [Google Scholar]
- 5. Nolan BG, Labato MA. Calcium oxalate urolithiasis. In: Bonagura JD, Twedt DC, eds. Kirk's Current Veterinary Therapy. XV ed. St Louis, MO: Elsevier; 2014:897‐901. [Google Scholar]
- 6. Obligado SH, Goldfarb DS. The association of nephrolithiasis with hypertension and obesity: a review. Am J Hypertens. 2008;21:257‐264. [DOI] [PubMed] [Google Scholar]
- 7. Chandler M, Cunningham S, Lund EM, et al. Obesity and associated comorbidities in people and companion animals: a one health perspective. J Comp Pathol. 2017;156:296‐309. [DOI] [PubMed] [Google Scholar]
- 8. German AJ. The growing problem of obesity in dogs and cats. J Nutr. 2006;136:1940S‐1946S. [DOI] [PubMed] [Google Scholar]
- 9. Fuchs T, Loureiro MP, Macedo LE, et al. Animal models in metabolic syndrome. Rev Col Bras Cir. 2018;45:e1975. [DOI] [PubMed] [Google Scholar]
- 10. McGreevy PD, Thomson PC, Pride C, et al. Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Vet Rec. 2005;156:695‐702. [DOI] [PubMed] [Google Scholar]
- 11. Courcier EA, Thomson RM, Mellor DJ, et al. An epidemiological study of environmental factors associated with canine obesity. J Small Anim Pract. 2010;51:362‐367. [DOI] [PubMed] [Google Scholar]
- 12. Corbee RJ. Obesity in show dogs. J Anim Physiol Anim Nutr (Berl). 2013;97:904‐910. [DOI] [PubMed] [Google Scholar]
- 13. Mao J, Xia Z, Chen J, et al. Prevalence and risk factors for canine obesity surveyed in veterinary practices in Beijing, China. Prev Vet Med. 2013;112:438‐442. [DOI] [PubMed] [Google Scholar]
- 14. Robertson ID. The association of exercise, diet and other factors with owner‐perceived obesity in privately owned dogs from metropolitan Perth, WA. Prev Vet Med. 2003;58:75‐83. [DOI] [PubMed] [Google Scholar]
- 15. Colliard L, Ancel J, Benet JJ, et al. Risk factors for obesity in dogs in France. J Nutr. 2006;136:1951S‐1954S. [DOI] [PubMed] [Google Scholar]
- 16. Gossellin J, McKelvie J, Sherington J, et al. An evaluation of dirlotapide to reduce body weight of client‐owned dogs in two placebo‐controlled clinical studies in Europe. J Vet Pharmacol Ther. 2007;30(suppl 1):73‐80. [DOI] [PubMed] [Google Scholar]
- 17. Laflamme DP. Understanding and managing obesity in dogs and cats. Vet Clin North Am Small Anim Pract. 2006;36:1283‐1295. [DOI] [PubMed] [Google Scholar]
- 18. Loftus JP, Wakshlag JJ. Canine and feline obesity: a review of pathophysiology, epidemiology, and clinical management. Vet Med (Auckl). 2015;6:49‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siener R, Glatz S, Nicolay C, et al. The role of overweight and obesity in calcium oxalate stone formation. Obes Res. 2004;12:106‐113. [DOI] [PubMed] [Google Scholar]
- 20. Curhan GC, Willett WC, Rimm EB, et al. Body size and risk of kidney stones. J Am Soc Nephrol. 1998;9:1645‐1652. [DOI] [PubMed] [Google Scholar]
- 21. Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455‐462. [DOI] [PubMed] [Google Scholar]
- 22. Inci M, Demirtas A, Sarli B, et al. Association between body mass index, lipid profiles, and types of urinary stones. Ren Fail. 2012;34:1140‐1143. [DOI] [PubMed] [Google Scholar]
- 23. Sur RL, Masterson JH, Palazzi KL, et al. Impact of statins on nephrolithiasis in hyperlipidemic patients: a 10‐year review of an equal access health care system. Clin Nephrol. 2013;79:351‐355. [DOI] [PubMed] [Google Scholar]
- 24. Torricelli FC, De SK, Gebreselassie S, et al. Dyslipidemia and kidney stone risk. J Urol. 2014;191:667‐672. [DOI] [PubMed] [Google Scholar]
- 25. Kang HW, Seo SP, Kim WT, et al. Hypertriglyceridemia is associated with increased risk for stone recurrence in patients with urolithiasis. Urology. 2014;84:766‐771. [DOI] [PubMed] [Google Scholar]
- 26. Kang HW, Lee SK, Kim WT, et al. Hypertriglyceridemia and low high‐density lipoprotein cholesterolemia are associated with increased hazard for urolithiasis. J Endourol. 2014;28:1001‐1005. [DOI] [PubMed] [Google Scholar]
- 27. American Heart Association , National Heart, Lung, and Blood Institue , Grundy SM, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13:322‐327. [PubMed] [Google Scholar]
- 28. Wong Y, Cook P, Roderick P, et al. Metabolic syndrome and kidney stone disease: a systematic review of literature. J Endourol. 2016;30:246‐253. [DOI] [PubMed] [Google Scholar]
- 29. Sakhaee K, Maalouf NM. Metabolic syndrome and uric acid nephrolithiasis. Semin Nephrol. 2008;28:174‐180. [DOI] [PubMed] [Google Scholar]
- 30. Jeong IG, Kang T, Bang JK, et al. Association between metabolic syndrome and the presence of kidney stones in a screened population. Am J Kidney Dis. 2011;58:383‐388. [DOI] [PubMed] [Google Scholar]
- 31. Sakhaee K, Capolongo G, Maalouf NM, et al. Metabolic syndrome and the risk of calcium stones. Nephrol Dial Transplant. 2012;27:3201‐3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hess B. Metabolic syndrome, obesity and kidney stones. Arab J Urol. 2012;10:258‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kohjimoto Y, Sasaki Y, Iguchi M, et al. Association of metabolic syndrome traits and severity of kidney stones: results from a nationwide survey on urolithiasis in Japan. Am J Kidney Dis. 2013;61:923‐929. [DOI] [PubMed] [Google Scholar]
- 34. Iba A, Kohjimoto Y, Mori T, et al. Insulin resistance increases the risk of urinary stone formation in a rat model of metabolic syndrome. BJU Int. 2010;106:1550‐1554. [DOI] [PubMed] [Google Scholar]
- 35. Verkest KR. Is the metabolic syndrome a useful clinical concept in dogs? A review of the evidence. Vet J. 2014;199:24‐30. [DOI] [PubMed] [Google Scholar]
- 36. Tvarijonaviciute A, Ceron JJ, Holden SL, et al. Obesity‐related metabolic dysfunction in dogs: a comparison with human metabolic syndrome. BMC Vet Res. 2012;8:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tvarijonaviciute A, Baric‐Rafaj R, Horvatic A, et al. Identification of changes in serum analytes and possible metabolic pathways associated with canine obesity‐related metabolic dysfunction. Vet J. 2019;244:51‐59. [DOI] [PubMed] [Google Scholar]
- 38. Ulrich LK, Bird KA, Koehler LA, et al. Urolith analysis. Submission, methods, and interpretation. Vet Clin North Am Small Anim Pract. 1996;26:393‐400. [DOI] [PubMed] [Google Scholar]
- 39. Laflamme D. Development and validation of a body condition score system for dogs. Canine Pract. 1997;22:10‐15. [Google Scholar]
- 40. Kennedy SM, Lulich JP, Ritt MG, et al. Comparison of body condition score and urinalysis variables between dogs with and without calcium oxalate uroliths. J Am Vet Med Assoc. 2016;249:1274‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Acierno MJ, Brown S, Coleman AE, et al. ACVIM consensus statement: guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med. 2018;32:1803‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsukagoshi T, Ohno K, Tsukamoto A, et al. Decreased gallbladder emptying in dogs with biliary sludge or gallbladder mucocele. Vet Radiol Ultrasound. 2012;53:84‐91. [DOI] [PubMed] [Google Scholar]
- 43. Center SA. Diseases of the gallbladder and biliary tree. Vet Clin North Am Small Anim Pract. 2009;39:543‐598. [DOI] [PubMed] [Google Scholar]
- 44. Schmiedl A, Schwille PO, Bonucci E, et al. Nephrocalcinosis and hyperlipidemia in rats fed a cholesterol‐ and fat‐rich diet: association with hyperoxaluria, altered kidney and bone minerals, and renal tissue phospholipid‐calcium interaction. Urol Res. 2000;28:404‐415. [DOI] [PubMed] [Google Scholar]
- 45. Xenoulis PG, Steiner JM. Lipid metabolism and hyperlipidemia in dogs. Vet J. 2010;183:12‐21. [DOI] [PubMed] [Google Scholar]
- 46. Xenoulis PG. Cholesterol, triglycerides. In: Ettinger SJ, Feldman EC, Côté E, eds. Textbook of Veterinary Internal Medicine. Vol 1. 8th ed. St Louis, MO: Elsevier; 2017:252‐256. [Google Scholar]
- 47. Pasquini A, Luchetti E, Cardini G. Plasma lipoprotein concentrations in the dog: the effects of gender, age, breed and diet. J Anim Physiol Anim Nutr (Berl). 2008;92:718‐722. [DOI] [PubMed] [Google Scholar]
- 48. Xenoulis PG, Suchodolski JS, Levinski MD, et al. Investigation of hypertriglyceridemia in healthy Miniature Schnauzers. J Vet Intern Med. 2007;21:1224‐1230. [DOI] [PubMed] [Google Scholar]
- 49. Ding Q, Ouyang J, Fan B, et al. Association between dyslipidemia and nephrolithiasis risk in a Chinese population. Urol Int. 2019;103:156‐165. [DOI] [PubMed] [Google Scholar]
- 50. Cho ST, Jung SI, Myung SC, et al. Correlation of metabolic syndrome with urinary stone composition. Int J Urol. 2013;20:208‐213. [DOI] [PubMed] [Google Scholar]
- 51. Ahmed MH, Ahmed HT, Khalil AA. Renal stone disease and obesity: what is important for urologists and nephrologists? Ren Fail. 2012;34:1348‐1354. [DOI] [PubMed] [Google Scholar]
- 52. Lekcharoensuk C, Lulich JP, Osborne CA, et al. Patient and environmental factors associated with calcium oxalate urolithiasis in dogs. J Am Vet Med Assoc. 2000;217:515‐519. [DOI] [PubMed] [Google Scholar]
- 53. German AJ, Holden SL, Morris PJ, et al. Comparison of a bioimpedance monitor with dual‐energy x‐ray absorptiometry for noninvasive estimation of percentage body fat in dogs. Am J Vet Res. 2010;71:393‐398. [DOI] [PubMed] [Google Scholar]
- 54. Mawby DI, Bartges JW, d'Avignon A, et al. Comparison of various methods for estimating body fat in dogs. J Am Anim Hosp Assoc. 2004;40:109‐114. [DOI] [PubMed] [Google Scholar]
- 55. Jeusette I, Greco D, Aquino F, et al. Effect of breed on body composition and comparison between various methods to estimate body composition in dogs. Res Vet Sci. 2010;88:227‐232. [DOI] [PubMed] [Google Scholar]
- 56. Hall WD, Pettinger M, Oberman A, et al. Risk factors for kidney stones in older women in the southern United States. Am J Med Sci. 2001;322:12‐18. [DOI] [PubMed] [Google Scholar]
- 57. Curhan GC, Willett WC, Speizer FE, et al. Twenty‐four‐hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001;59:2290‐2298. [DOI] [PubMed] [Google Scholar]
- 58. Jung HS, Chang IH, Kim KD, et al. Possible relationship between metabolic syndrome traits and nephrolithiasis: incidence for 15 years according to gender. Korean J Urol. 2011;52:548‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim YJ, Kim CH, Sung EJ, et al. Association of nephrolithiasis with metabolic syndrome and its components. Metabolism. 2013;62:808‐813. [DOI] [PubMed] [Google Scholar]
- 60. Mosli HA, Mosli HH, Kamal WK. Kidney stone composition in overweight and obese patients: a preliminary report. Res Rep Urol. 2013;5:11‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chou YH, Su CM, Li CC, et al. Difference in urinary stone components between obese and non‐obese patients. Urol Res. 2011;39:283‐287. [DOI] [PubMed] [Google Scholar]
- 62. Perez‐Sanchez AP, Del‐Angel‐Caraza J, Quijano‐Hernandez IA, et al. Obesity‐hypertension and its relation to other diseases in dogs. Vet Res Commun. 2015;39:45‐51. [DOI] [PubMed] [Google Scholar]
- 63. Sarica K, Altay B, Erturhan S. Effect of being overweight on stone‐forming risk factors. Urology. 2008;71:771‐774. [DOI] [PubMed] [Google Scholar]
- 64. Al‐Hayek S, Schwen ZR, Jackman SV, et al. The impact of obesity on urine composition and nephrolithiasis management. J Endourol. 2013;27:379‐383. [DOI] [PubMed] [Google Scholar]
- 65. Maalouf NM, Sakhaee K, Parks JH, et al. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422‐1425. [DOI] [PubMed] [Google Scholar]
- 66. Najeeb Q, Masood I, Bhaskar N, et al. Effect of BMI and urinary pH on urolithiasis and its composition. Saudi J Kidney Dis Transpl. 2013;24:60‐66. [DOI] [PubMed] [Google Scholar]
- 67. Ferrannini E, Natali A, Bell P, et al. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR). J Clin Invest. 1997;100:1166‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta‐cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456‐1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta‐cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663‐1672. [DOI] [PubMed] [Google Scholar]
- 70. Strohmaier WL, Wrobel BM, Schubert G. Overweight, insulin resistance and blood pressure (parameters of the metabolic syndrome) in uric acid urolithiasis. Urol Res. 2012;40:171‐175. [DOI] [PubMed] [Google Scholar]
- 71. Domingos F, Serra A. Metabolic syndrome: a multifaceted risk factor for kidney stones. Scand J Urol. 2014;48:414‐419. [DOI] [PubMed] [Google Scholar]
- 72. Sakhaee K, Adams‐Huet B, Moe OW, et al. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971‐979. [DOI] [PubMed] [Google Scholar]
- 73. Wagner CA, Mohebbi N. Urinary pH and stone formation. J Nephrol. 2010;23(suppl 16):S165‐S169. [PubMed] [Google Scholar]
- 74. Ratkalkar VN, Kleinman JG. Mechanisms of stone formation. Clin Rev Bone Miner Metab. 2011;9:187‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Coe FL, Strauss AL, Tembe V, et al. Uric acid saturation in calcium nephrolithiasis. Kidney Int. 1980;17:662‐668. [DOI] [PubMed] [Google Scholar]
- 76. Coe FL, Kavalach AG. Hypercalciuria and hyperuricosuria in patients with calcium nephrolithiasis. N Engl J Med. 1974;291:1344‐1350. [DOI] [PubMed] [Google Scholar]
- 77. Coe FL, Moran E, Kavalich AG. The contribution of dietary purine over‐consumption to hyperpuricosuria in calcium oxalate stone formers. J Chronic Dis. 1976;29:793‐800. [DOI] [PubMed] [Google Scholar]
- 78. Moe OW, Xu LHR. Hyperuricosuric calcium urolithiasis. J Nephrol. 2018;31:189‐196. [DOI] [PubMed] [Google Scholar]
- 79. Kerl ME. Renal tubular disease. In: Ettinger SJ, Feldman EC, Côté E, eds. Textbook of Veterinary Internal Medicine. Vol 2. 8th ed. St Louis, MO: Elsevier; 2017:1972‐1977. [Google Scholar]
- 80. Aguilar‐Salinas CA, Gomez‐Perez FJ, Rull J, et al. Prevalence of dyslipidemias in the Mexican National Health and Nutrition Survey 2006. Salud Publica Mex. 2010;52(suppl 1):S44‐S53. [DOI] [PubMed] [Google Scholar]
- 81. Bland IM, Guthrie‐Jones A, Taylor RD, et al. Dog obesity: owner attitudes and behaviour. Prev Vet Med. 2009;92:333‐340. [DOI] [PubMed] [Google Scholar]
- 82. Case CC, Jones PH, Nelson K, et al. Impact of weight loss on the metabolic syndrome. Diabetes Obes Metab. 2002;4:407‐414. [DOI] [PubMed] [Google Scholar]
- 83. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23:1‐87. [DOI] [PubMed] [Google Scholar]
- 84. Meschi T, Nouvenne A, Borghi L. Lifestyle recommendations to reduce the risk of kidney stones. Urol Clin North Am. 2011;38:313‐320. [DOI] [PubMed] [Google Scholar]
- 85. Sorensen MD, Chi T, Shara NM, et al. Activity, energy intake, obesity, and the risk of incident kidney stones in postmenopausal women: a report from the Women's Health Initiative. J Am Soc Nephrol. 2014;25:362‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Anderssen SA, Carroll S, Urdal P, et al. Combined diet and exercise intervention reverses the metabolic syndrome in middle‐aged males: results from the Oslo Diet and Exercise Study. Scand J Med Sci Sports. 2007;17:687‐695. [DOI] [PubMed] [Google Scholar]
- 87. Berg C, Tiselius HG. The effect of pH on the risk of calcium oxalate crystallization in urine. Eur Urol. 1986;12:59‐61. [DOI] [PubMed] [Google Scholar]
- 88. Johnson KY, Lulich JP, Osborne CA. Evaluation of the reproducibility and accuracy of pH‐determining devices used to measure urine pH in dogs. J Am Vet Med Assoc. 2007;230:364‐369. [DOI] [PubMed] [Google Scholar]


