Abstract
Background
Orthostatic tremor (OT) is a rare movement disorder characterized by high‐frequency (>12 Hz) involuntary, rhythmic, sinusoidal movements affecting predominantly the limbs while standing.
Objective
To describe the signalment, presenting complaints, phenotype, diagnostic findings, treatment, and outcome of a large sample of dogs with OT.
Animals
Sixty dogs diagnosed with OT based on conscious electromyography.
Methods
Multicenter retrospective case series study. Dogs were included if they had a conscious electromyography consistent with muscle discharge frequency >12 Hz while standing.
Results
Fifty‐three cases were diagnosed with primary OT (POT). Giant breed dogs represented most cases (83%; 44/53). Most dogs (79%; 42/53) were younger than 2 years of age at onset of signs, except for Retrievers which were all older than 3.5 years of age. The most common presenting complaints were pelvic limb tremors while standing (85%; 45/53) and difficulty when rising or sitting down (45%; 24/53). Improvement of clinical signs occurred in most dogs (85%; 45/53) treated medically with phenobarbital, primidone, gabapentin, pregabalin or clonazepam, but it was mostly partial rather than complete. Orthostatic tremor‐plus was seen in 7 dogs that had concurrent neurological diseases.
Conclusions and Clinical Importance
Primary OT is a progressive disease of young, purebred, giant/large‐breed dogs, which appears to begin later in life in Retrievers. Primary OT apparently responds partially to medications. Orthostatic tremor‐plus exists in dogs and can be concomitant or associated with other neurological diseases.
Keywords: canine, paroxysmal movement disorders, postural tremor, shaky legs syndrome, tremor syndromes
Abbreviations
- BAER
brainstem auditory evoked responses
- CI
confidence interval
- CSF
cerebrospinal fluid analysis
- CSM
cervical spondylomyelopathy
- CT
computed tomography
- EEG
electroencephalography
- EMG
electromyography
- IQR
interquartile range
- IVDP
intervertebral disc protrusion—Hansen type II
- MNCV
motor nerve conduction velocities
- MRI
magnetic resonance imaging
- OT
orthostatic tremor
- OT‐Plus
orthostatic tremor‐plus
- POT
primary orthostatic tremor
- QoL
quality of life
- SOT
secondary orthostatic tremor
- WEBLT
weight‐bearing lifting test
1. INTRODUCTION
Orthostatic tremor (OT) is a rare movement disorder characterized by pathognomonic high‐frequency (>12 Hz) involuntary, rhythmic, sinusoidal movements affecting predominantly limbs, but also trunk or head, which are triggered exclusively while standing and relieved when sitting, lying, or walking. 1 , 2 , 3 OT is classified as an action‐related postural tremor as it occurs during voluntary movement of the muscle in its attempt to maintain a posture against gravity. 1 , 4 , 5 In humans, OT is a clinical sign, which could manifest as a sole sign usually of idiopathic etiology (primary OT [POT]), or concomitant with neurological, electrophysiological, or imaging evidence of other neurological diseases (OT‐Plus). When there is an association between OT‐Plus and concomitant disease, then OT is secondary or symptomatic (SOT). 6 In humans, it has been hypothesized that a central oscillator, located in brainstem or cerebellum, generates OT. 2 Primary OT has rarely been described in dogs, with few case reports, mainly in giant breed dogs: Great Dane, 7 , 8 , 9 Scottish Deerhound, 10 Weimaraner 11 and Mastiff, 8 while OT‐Plus has been observed in Great Danes 4 and Jack Russell terriers 12 but the concomitant disease was not described.
Diagnosis of OT is based on presence of typical clinical features, confirmed by conscious electromyography (EMG) on a weight‐bearing posture (eg, while standing), including (a) high frequency visible or palpable limb tremor with or without head or trunk involvement while standing, (b) absence of tremors during nonweight‐bearing postures or gait, (c) presence of “helicopter sign,” and (d) reduction/discontinuation of tremors on weight‐bearing lifting test (WEBLT). 7 , 10 , 13 The “helicopter sign” is defined as the sound resembling a distant helicopter during auscultation with a stethoscope over the tremoring limb, which corresponds to the sound generated during EMG of affected muscles (Video S1), and it is considered a unique clinical feature of OT in humans. 14 WEBLT is a clinical test where OT decreases or discontinues after lifting and keeping the affected limbs air‐floating (Video S2). Conscious standing EMG confirms the clinical suspicion of OT revealing the characteristic high frequency tremors (>12 Hz). 2 , 3
The aim of the present study is to expand the knowledge of OT in dogs by describing phenotype, results of diagnostic tests, treatment, and outcome in this case‐series.
2. MATERIALS AND METHODS
This is a retrospective, multicenter, case‐series study conducted by 4 veterinary teaching hospitals and 5 private referrals (Belgium, Italy, United Kingdom, United States). Ethical approval was granted by the local Research Ethics Committee (EA15/20).
Cases were recruited using an online veterinary forum (Veterinary Information Network, American College of Veterinary Internal Medicine / European College of Veterinary Neurology Neurologists ListServe) asking for veterinary neurologists to participate if they had worked‐up cases of OT in dogs.
Inclusion criteria consisted of (a) complete medical records, (b) clinical features of OT as mentioned before, and (c) muscle discharges >12 Hz frequency on conscious standing EMG (or under light sedation and application of external weight‐bearing on the examined limb).
Classification of breeds based on body weight was adopted based on previous publications as follows: (a) small (<10 kg), (b) medium (10‐25 kg), (c) large (26‐45 kg), and giant breeds (>45 kg). 15
Dogs were divided into 2 groups based on results of investigations: (a) POT: if no other neurological disease was identified; and (b) OT‐Plus: if concomitant neurological, electrophysiological, or imaging evidence of another neurological disease was present. 6 Characterization of OT as secondary/symptomatic was not feasible as not all of the cases underwent full investigations and therefore correlations between concomitant diseases and OT were subjective.
Complete medical records consisted of a minimum database of history/signalment/presenting complaints, physical and neurological examinations, conscious (or light sedation) EMG, treatment, and outcome. Auscultation findings of affected muscles or WEBLT results were recorded where available. Any video recordings were reviewed by 2 board‐certified neurologists for further study and analysis of OT features. Other diagnostic modalities recorded where available included: complete blood count, serum biochemistry including creatine kinase (CK), thyroid profile, urinalysis, magnetic resonance imaging (MRI) of the brain with or without spinal cord, cerebrospinal fluid (CSF) analysis, infectious diseases tests, muscle/nerve biopsy, genetic tests, electroencephalography (EEG), brainstem auditory evoked response (BAER) or motor nerve conduction studies. MRI devices used included high‐field or low‐field magnets. MRI scans included a minimum of T2‐weighted, fluid attenuated inversion recovery (FLAIR), T1‐weighted before and after contrast sequences. EMG was performed mainly with concentric needles using 8 different electrodiagnostic device models (Supporting Information S1).
Progression of the disease, characterized by intensification or/and generalization of tremors, was based exclusively on clinical history provided by owners at the time of initial presentation. Follow‐up was achieved by reexaminations, video recording evaluations or by contacting the referring veterinarians. An online questionnaire was distributed to the owners (Supporting Information S2) of dogs diagnosed with OT that were still alive, to avoid recall bias and for ethical reasons. Questions were closed‐ended, multiple‐choice, or open‐ended. The aim of the questionnaire was accessory: to obtain a long‐term follow‐up from the owners' perspective, to help us understand further the features of the disease obtained on clinical history, current quality of life of the dog and owners' perspective on treatment efficiency.
2.1. Statistical analysis
Numerical variables were presented as median, interquartile range (IQR) and range. Categorical variables were summarized as counts and percentages. The 95% confidence intervals (CI 95%) for percentages were calculated using the Wilson score method. The groups were compared by the Mann‐Whitney U test (2 groups) or Kruskal‐Wallis H test (>2 groups) in the case of numerical variables, and by the maximum likelihood G test or Fisher's exact test (if the expected count in any cell of the contingency table <5) in case of categorical variables. Correlations between 2 numerical or ordinal variables were determined using the Spearman's rank correlation coefficient (R s). Agreement beyond chance between clinical localization of tremors and EMG discharges was assessed using Cohen's kappa coefficient and classed according to Landis and Koch. 16 Relationship between demographic and clinical characteristics of dogs and severity of OT as well as the occurrence and extent of remission were first tested in the univariable analysis and only variables whose P‐value was <.1 were entered into the multivariable analysis. The latter was performed using the multivariable logistic regression according to the backward stepwise procedure. The strength of relationship estimated in the univariable and multivariable analysis was expressed as crude (ORc) and adjusted (ORadj) odds ratio, respectively. A significance level (α) was set at .05 and all statistical tests were 2‐tailed. Statistical analysis was performed in TIBCO Statistica 13.3 (TIBCO Software Inc, Palo Alto, California) and IBM SPSS Statistics 26 (IBM Corporation, Armonk, New York).
3. RESULTS
Sixty dogs met the inclusion criteria from 4 countries: United States (n = 43), United Kingdom (n = 9), Belgium (n = 4), and Italy (n = 4). Fifty‐three of them (88%) were diagnosed with POT, and 7 (12%) with OT‐Plus.
3.1. Primary orthostatic tremor
3.1.1. Animals
Twenty‐four dogs were males (16 neutered) and 29 females (25 neutered). Median age at presentation was 20 months (IQR, 14‐25 months; range, 8 months‐8.8 years). Most dogs were purebred (51/53; 96%) and most of them (52/53; 98%) belonged to giant (n = 44) or large (n = 8) breeds while 1 was medium size (Labrador‐cross, 15 kg). The most represented breeds were Great Dane (n = 21), Newfoundland (n = 9), Retriever (n = 7), Irish Wolfhound (n = 6), and Mastiff (n = 6). Other breeds included Scottish Deerhound, Kuvasz, and Border Collie (Table 1). Retrievers included Labrador (n = 4), Labrador‐cross (n = 2) and Golden Retriever (n = 1). Median body weight at presentation was 55 kg (IQR, 49‐64 kg; range, 15‐101 kg).
TABLE 1.
Signalment features of dogs with primary orthostatic tremor and orthostatic tremor‐plus
| Breed | n (%) | Sex | Body weight (kg) | Breed size | |
|---|---|---|---|---|---|
| Male (neutered) | Female (neutered) | Median, IQR (range) | |||
| Primary OT (n = 53) | |||||
| Great Dane | 21 (39.6) | 9 (5) | 12 (10) | 62, 56‐66 (48‐97) | Giant breed |
| Newfoundland | 9 (17) | 4 (3) | 5 (4) | 52, 51‐57(46‐62) | Giant breed |
| Irish Wolfhound | 6 (11.3) | 1 (1) | 5 (5) | 52, 50‐53 (49‐66) | Giant breed |
| Mastiff | 6 (11.3) | 3 (2) | 3 (3) | 77, 69‐91 (66‐101) | Giant breed |
| Labrador retriever (including 2 Labrador‐cross) | 6 (11.8) | 4 (3) | 2 (1) | 32, 31‐35 (15‐41) | Large breed |
| Scottish Deerhound | 2 (3.8) | 1 (1) | 1 (1) | 44‐48 | Giant breed |
| Kuvasz | 1 (1.9) | 1 (0) | 0 | 49 | Giant breed |
| Border Collie | 1 (1.9) | 1 (1) | 0 | 29 | Large breed |
| Golden Retriever | 1 (1.9) | 0 | 1 (1) | 26 | Large breed |
| Breed | n | Sex | Body weight (kg) | Breed size | |
|---|---|---|---|---|---|
| Male (neutered) | Female (neutered) | Median, IQR (range) | |||
| OT‐Plus (n = 7) | |||||
| Great Dane | 3 | 2 (2) | 1 (1) | 65, 64‐79 (64‐93) | Giant breed |
| Bloodhound | 1 | 1 (1) | 0 | 51 | Giant breed |
| Labrador retriever | 1 | 1 (0) | 0 | 37 | Large breed |
| Jack Russell terrier | 2 | 0 | 1 (1) | 6.5‐7 | Small breed |
3.1.2. Onset of clinical signs
Median age of dogs at onset of signs was 12 months (IQR, 9‐15 months; range, 4 months‐7 years). Most dogs (80%) were 2‐year‐old or younger, while half of dogs were 1‐year‐old or younger. Median duration of clinical signs before diagnosis was 6 months (IQR, 4‐9 months; range, 1‐58 months). Duration of clinical signs before diagnosis was positively correlated with age at onset of signs (R s = 0.40; P = .003). No significant relationship between sex (P = .7), neutering status (P = .28), breed (P = .39) or body weight (P = .97) and the onset of clinical signs was observed.
3.1.3. Presenting signs
Most dogs (52/53; 98%) were reported by the owners to have limb tremors while standing (28/53; 53%), difficulty rising/sitting (7/53; 13%) or both (17/53; 32%). One dog was referred for none of these signs but due to a “dancing sign.” Other complaints were difficulty in maintaining posture while eating (5/53; 9%), reluctance to exercise (4/53; 8%), suspected pain (2/53; 4%), wide‐based stance (2/58; 4%), “dancing sign” (2/53; 4%), head tremors accompanying limb tremors (2/53; 4%), limb weakness (2/53; 4%) and 1 (1/53; 2%) of each: difficulty maintaining posture during defecation, exacerbation of tremors when stressed, collapse when jumping, limb incoordination and anxiety.
Progression of clinical signs from onset to diagnosis was reported by owners at presentation in 33/53 dogs (62%; CI 95%: 49%, 74%); however, the time which elapsed from onset of signs to presentation did not differ between dogs whose signs progressed or not (P = .16). No relationship was revealed between progression of clinical signs and sex (P = .55), neutering status (P = .72), breed (P = .7), body weight (P = .35), nor age at which signs started (P = .75).
3.1.4. Physical and neurological examination findings
Physical examination abnormalities were found in 7 dogs (13%) and all of them were unrelated and nonspecific (eg, heart murmur, obesity, joint crepitus/pain). No abnormalities were found on neurological examination of dogs with POT other than OT and associated features (Figure 1).
FIGURE 1.
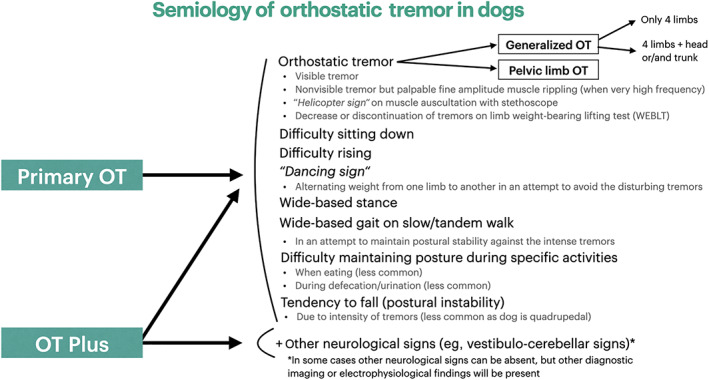
Flowchart of semiology of orthostatic tremor in dogs
3.1.5. Tremor characteristics
Clinical localization of tremors at the time of presentation included all limbs (43/53; 81%; equal tremors [n = 20], pelvic more intense than thoracic limbs [n = 14], thoracic more intense than pelvic limbs [n = 2]), isolated pelvic limbs (10/53; 19%), all limbs and head (6/53; 11%) or all limbs and trunk (1/53; 2%; Video S3). Tail involvement was reported at least in 1 of the generalized OT cases, but it was not systematically recorded. The older the dog was at the onset of signs the less generalized tremors it developed (R s = −0.55; P < .001). However, no relationship between generalization of tremors and sex (P = .28), neutering status (P = .41), breed (P = .06), or body weight (P = .36) were found.
Occurrence of tremors was evaluated during neurological examination and revealed that tremors occurred while standing (51/53; 96%), rising (45/53; 85%), and sitting (23/53; 43%). Tremors while walking slowly for the initial few steps (tandem gait) occurred only in 2 dogs (2/53; 4%). Only 1 dog (1/53; 2%) tended to fall due to tremor intensity. In 10 dogs (19%), tremors were exacerbated by anxiety. No dog manifested tremors when trotting, running or in sternal or lateral recumbency. There were 2 dogs that did not have obvious tremors while standing; in these dogs, tremors were palpable, and they had difficulty transitioning to a sitting posture. The number of activities where tremors were evident differed between breeds (P < .001). In all 9 Newfoundland dogs, tremors accompanied all postures and activities. Tremors when rising were absent in 3 Great Danes, 2 Labrador retrievers, 1 Golden retriever, 1 Border Collie and 1 Kuvasz. Large breeds (7 Retrievers, 1 Border Collie) manifested tremors when rising less often (4/8; 50%) than other breeds (42/45; 93%; P = .007). Dogs without tremors when rising were older at onset of signs (n = 8; median, 42 months; IQR, 22‐59 months; range, 8‐76 months) than dogs with tremors when rising (n = 45; median, 11; IQR, 9‐15 months; range, 4‐89 months; P = .03).
Other clinical features that were occasionally observed included wide‐based stance when rising/sitting (3/53; 6%), wide‐based stance on tandem gait that was resolving on running/trotting (1/53; 2%) and “dancing sign” represented by shifting weight from 1 limb to another (2/53; 4%; Supporting Information Video S3).
Auscultation of affected limbs with a stethoscope was performed in 12/53 cases and was compatible with “helicopter sign.” WEBLT was performed in 40/53 dogs revealing a decrease (31/40; 78%) or complete resolution of tremors (9/40; 23%).
3.1.6. Electromyography
EMG was performed mainly in conscious dogs while standing (48/53; 91%); however, there was a small number of dogs who did not tolerate conscious EMG and therefore they were examined under light sedation in lateral recumbency with application of external resistance at the examined limb resembling weight‐bearing. Tremor frequency varied from 13 to 21 Hz (Figure 2). It was stable in 38 dogs (72%), while it fluctuated in 15 dogs (28%). In 3 dogs, tremor frequency reached or exceeded 19 Hz. Control EMG was performed in 5 dogs (9%) under general anesthesia and in 10 conscious dogs (19%) in lateral recumbency revealing muscle silence. EMG discharges were observed on pelvic limbs (24/53; 45%), all limbs (22/53; 42%), all limbs and head (4/53; 8%) or all limbs and trunk (3/53; 6%); however, not all parts of the body were examined thoroughly in all cases especially if tremors were not visually observed in this region.
FIGURE 2.
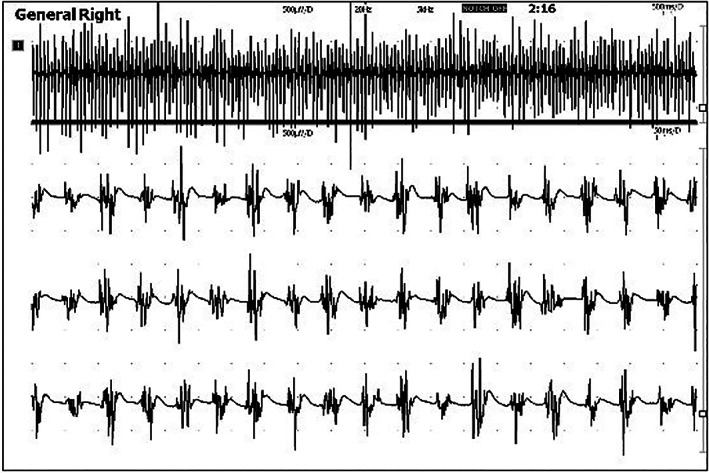
Typical conscious electromyography findings of a dog with primary orthostatic tremor, demonstrating high‐frequency muscle discharges (18 Hz)
Agreement between clinical evaluation of tremors and EMG was moderate (Cohen's kappa = 0.45; CI 95%: 0.37, 0.53). When there was clinical evidence of pelvic limb tremors, usually EMG discharges from pelvic limbs were evident (90%). When there was clinical evidence of all limb tremors, usually EMG discharges from all limbs (58%) or only pelvic limbs (36%) were evident. Unfortunately, not all parts of the body were consistently examined electromyographically in all cases especially if tremors were not visually observed in that region, and thus this represents of a major limitation.
3.1.7. Other diagnostic tests
Serum CK activity was measured in 42/53 dogs, and was slightly increased (based on variability in reference intervals from different labs) in 20 dogs (48%) with a median value of 242 U/L (IQR, 202‐305 U/L; range, 155‐510 U/L). Other diagnostic test findings were nonspecific (Supporting Information S3).
3.1.8. Medical treatment and outcome
Many dogs (28/53; 53%) were treated with gabapentin (median dose, 20 mg/kg PO q8‐12h; IQR, 10‐27 mg/kg; range, 10‐40 mg/kg), in 2 of which it was later replaced by phenobarbital and for 1 dog phenobarbital was added to existing therapy. Fourteen (26%) dogs were treated with phenobarbital (median dose, 2.5 mg/kg PO q12h; IQR, 2.5‐3.0 mg/kg; range, 2.0‐3.2 mg/kg), in 1 of which it was replaced by gabapentin. The remaining dogs were treated with clonazepam (6/53; 11%; median dose, 0.4 mg/kg PO q8h; range, 0.35‐3.5 mg/kg), pregabalin (3/53; 6%; dose, 3.5 mg/kg PO q8‐12h), primidone (2/53; 4%; dose, 30 mg/kg PO q12h), valproic acid (2/53; 4%; dose, 45 mg/kg PO q8h), or B‐complex vitamins (1/53; 2%). All dogs received at least a single medication.
Given that primidone is metabolized to phenobarbital and pregabalin is similar to gabapentin, categories regarding effectiveness of medications in bringing partial or complete remission were simplified to phenobarbital or primidone (n = 15/15; effectiveness of 100%; CI 95%: 80%, 100%), gabapentinoids (n = 25/29; effectiveness of 86%; CI 95%: 69%, 95%), clonazepam (n = 5/6; effectiveness of 83%; CI 95%: 44%, 97%), and others (valproic acid, vitamin B‐complex; n = 0; 0%; CI 95%: 0%, effectiveness of 56%). Statistical comparison of the effectiveness of the first 3 groups did not reveal any significant difference between them (P = .15). Treatment with phenobarbital or primidone, gabapentinoids, or clonazepam was associated with higher probability of remission than other treatments (ORc = 57.9; CI 95%: 2.6, 1275; P = .002).
Improvement in tremors after treatment occurred in 45/53 (85%) of dogs, while it did not improve or even worsened in 8 dogs (15%). Degree of improvement was known for 38/45 dogs (84.4%) and was more commonly partial (n = 27/38; 71%; CI 95%: 55%, 83%), than complete (11/38; 29%; CI 95%: 17%, 45%; Figure 3). Median follow‐up time was 5 months (IQR, 0‐8 months; range, 0‐12 months) after diagnosis.
FIGURE 3.
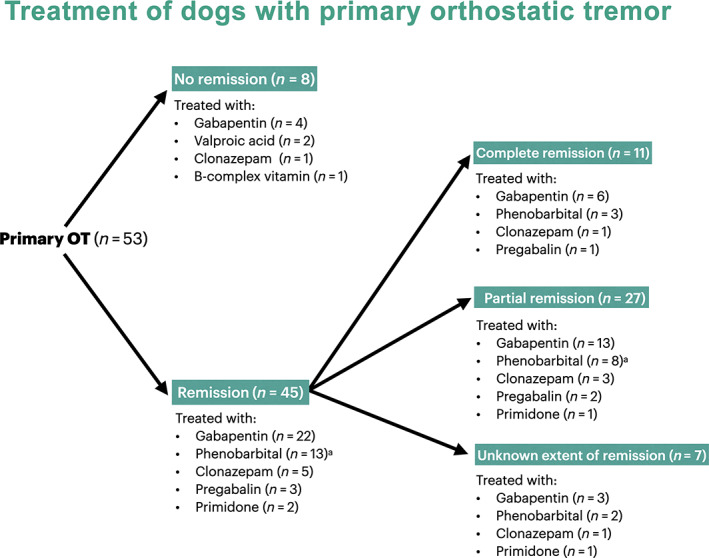
Flowchart of treatment and outcome. aOne dog treated first with gabapentin (ineffective) and then phenobarbital added
Univariable analyses of potential variables associated with treatment response are presented in Supporting Information S4. The only variable that remained significant in the multivariable analysis was that Retrievers were less likely to show improvement with medical treatment (ORadj = 0.07; CI 95%: 0.01, 0.44; P = .004; Supporting Information S5).
However, as Retriever breeds had the lightest bodyweight and only 7 representatives of this group were enrolled in the study, multivariable analysis was rerun without the variable Retriever breeds to investigate the role of the body weight adjusted by other variables significant in univariable analysis (Supporting Information S6). Body weight proved not to be linked to the probability of responding to medical treatment (ORadj = 0.09; CI 95%: 0.02, 0.49; P = .005), while the only variable significant was age at onset of clinical signs and therefore dogs older than 24 months at onset of signs were less likely to show improvement with medical treatment (P = .007).
Univariable analysis investigating factors contributing to the extent of remission is presented in Supporting Information S7. The only variable that remained significant in the multivariable analysis was that Newfoundland dogs were more likely to show complete remission with medical treatment (ORadj = 6.67; CI 95%: 1.23, 36.1; P = .03; Supporting Information S8).
3.1.9. Retrievers
Retriever breeds dogs were a subpopulation of dogs with POT which warrants more careful investigation because they were (a) older at onset of clinical signs (median, 76 months; IQR, 42‐84 months) compared to general population (median, 11 months; IQR, 9‐15 months; P < .001), (b) lighter (median, 32 kg; IQR, 26‐35 kg) compared to general population (median, 59 kg; IQR, 51‐66 kg; P < .001), and (c) less likely to respond to medical treatment: only 3/7 retrievers (43%) showed improvement compared to 42/46 (93%; P = .007).
3.2. Orthostatic tremor‐plus
Seven cases were diagnosed with OT‐Plus represented by Great Dane (n = 3), Jack Russel Terrier (n = 2), Labrador Retriever (n = 1) and Bloodhound (n = 1; Supporting Information S9). Four of them were diagnosed with compressive myelopathy such as osseous‐ (n = 2) or disc‐associated (n = 1) cervical spondylomyelopathy (CSM), and lumbosacral intervertebral disc protrusion (IVDP; n = 1). In 2 of them, CSF analysis revealed albuminocytological dissociation presumptively related to compressive myelopathy. Improvement of OT occurred in all of them on medical treatment; the dog treated with levetiracetam had a partial improvement for 4 months and then deteriorated. One dog that was operated for CSM continued manifesting OT after surgery.
Two elderly Jack Russell terriers manifested vestibulo‐cerebellar signs and simultaneous generalized OT‐Plus confirmed by EMG. WEBLT was decreased in 1 dog and without change in the other dog. Tremors were evident on sternal or lateral recumbency and during tandem gait, but not when running/trotting. MRI revealed diffuse brain atrophy and thalamic infarct in 1 dog, while diffuse brain microbleeds on MRI (Figure 4) and albuminocytological dissociation on CSF analysis were evident in both dogs. Both dogs were diagnosed with vascular encephalopathy, 1 of which had chronic kidney disease, and they were treated with gabapentin (11‐16 mg/kg PO q8h) showing partial improvement for a follow‐up period of 2 and 5 months.
FIGURE 4.

Magnetic resonance imaging (MRI) of a 15‐year‐old female neutered Jack Russell terrier diagnosed with vascular encephalopathy and secondary orthostatic tremor. A small (height, 0.3 cm) interthalamic adhesion is evident at the T2‐weighted sagittal sequence of the brain (B) which along with the widened cerebral sulci of the T2‐weighted transverse sequence of the brain are consistent with brain atrophy involving both cerebrum and cerebellum and possibly related with cognitive dysfunction syndrome. There are also several small (1 mm) round focal T2 iso‐ and hypointense lesions throughout all lobes of the cerebrum in the T2‐weighted sequences (A, B) that correspond to slightly larger (<3 mm) areas of signal void on the gradient echo sequence (C), consistent with cerebral microbleeds
The last case of OT‐Plus was a 7.5‐year‐old male entire Labrador Retriever with a 2‐year history of limb tremors while standing, which the owner reported that they possibly improved on prednisolone in the past. Aside from tremors, neurological examination was unremarkable. WEBLT revealed marked reduction of tremors. Generalized OT was confirmed on EMG. Motor nerve conduction velocities (MNCV) were normal in sciatic, tibial, and ulnar nerves; however, polyphasia and temporal dispersion of the compound muscle action potential were detected, leading to a diagnosis of a presumptive subclinical neuropathy and OT‐Plus. Serology for infectious diseases was negative. The dog was treated initially with prednisolone (1 mg/kg PO q24h), where only a questionable response was evident which prompted tapering of the treatment. When gabapentin (10 mg/kg PO q8h) was added in the scheme, there was partial improvement of tremors at 1‐month clinical and a 4‐year questionnaire‐based follow‐up.
Whether the concomitant diseases of these dogs could be responsible for OT‐Plus is unknown. We suspect that the Jack Russell Terriers with OT‐Plus might resemble SOT as OT manifested concurrently and simultaneously with the vestibulo‐cerebellar signs; however, this conclusion can be only presumptive.
Compared to dogs with POT, dogs with OT‐Plus were older at onset of signs (P = .04), comprised small breeds (2 Jack Russell terriers), usually manifested other neurological signs, had more often tremors when recumbent (3 dogs with OT‐Plus, 0 dogs with POT; P = .001) and walking slowly (3 dogs with OT‐Plus, 1 dog with POT; P = .004) which were almost never observed in POT. A negative WEBLT was represented only from a single case from the OT‐Plus group. Tremors on recumbency and tremors when walking slowly in tandem gait occurred more often in dogs with OT‐Plus (P = .02). Their tremors always involved at least all limbs (so tremors were more generalized).
3.3. Questionnaires
Of the questionnaires sent (n = 17), 15 owners responded (response rate, 88%), 13 of them had dogs diagnosed with POT and two with OT‐Plus due to presumptive subclinical neuropathy (n = 1) and lumbosacral IVDP (n = 1). Overall, 62% of the owners of dogs with POT treated with medications considered that none of the medications helped their dog regarding reducing tremor satisfactorily, while at the same time most of them (12/13) considered that tremors were not affecting markedly QoL of their dogs with a median grade of 7/10 (IQR, 6‐8; range, 3‐10). The owners of the single dog diagnosed with OT‐Plus due to presumptive subclinical neuropathy considered that their dog's QoL was very good (9/10), while the owners of the dog with lumbosacral IVDP and degenerative joint disease scored their dog's QoL as poor (1/10; Supporting Information S10).
4. DISCUSSION
This study revealed that POT in dogs is, within the dogs available for this study, mainly a disease of purebred, giant and large breed dogs, neither with any sex predisposition nor related to the neutering status. Initial signs are from when the dog is at least 4‐month‐old and usually between 9‐months and 2‐years‐old, except for Retriever breeds. Apparent overrepresentation of some breeds (eg, Great Dane) that manifest the disease at a particularly young age suggests a genetic predisposition. However, a possible bias might exist as particularly Great Dane was the first breed where OT was described and therefore the likelihood of recognition of the disease was more likely compared to other breeds. In humans, POT is usually manifested around 60 years of age (range, 13‐85 years), females seem to be overrepresented, and familial cases have been reported. 2
Although the exact pathophysiology is currently unknown, it has been hypothesized that a central oscillator, located in the brainstem or the cerebellum, generates OT. 2 , 17 However, as tremor intensity is modulated dependently on the gait cycle, being most prominent while standing (in the presence of muscle contractions under load) and most absent during the swing phase (when limb lifted off‐the‐floor), it appears that the central oscillatory pattern only manifests peripherally in muscles being contracted under load (eg, during activation of Golgi tendon organ afferents that directly project to Ib interneurons). 18 Therefore, it is suggested that the peripheral manifestation of OT does not occur via a direct projection of central oscillatory sources to spinal motor neurons but is rather mediated via spinal interneurons that signal the loading state of respective muscles. 18 This hypothesis explains why OT is evident while standing, and not when walking, trotting or running although all of these situations include weight‐bearing.
Primary OT in dogs seems to be a progressive disease, similarly to humans. 2 Based on that, a neurodegenerative process could be suspected for canine POT. 2 Tremors in POT were mostly observed in all limbs with or without head or trunk involvement and less frequently isolated on pelvic limbs, while standing, when rising and less often when sitting and can often be accompanied by a specific semiology (Figure 1). Tremors disappeared when recumbent, trotting or running in all dogs. Additionally, there was no dog without either partial or complete response to WEBLT. Therefore, clinical features of OT appeared to characterize well the OT phenotype in dogs and as a result, they should be systematically used in evaluation of limb tremors in dogs to support clinical suspicion of OT. In general, OT in dogs appeared to be visible and severe. In only 2 dogs, obvious tremors were not easily detected visually but rather palpable and audible with a stethoscope. These dogs' semiology included a “dancing sign.” In contrast, in humans OT is often not visible and might only be apparent on palpation, auscultation with a stethoscope or EMG, while its diagnosis is suspected by subjective description of feeling unsteady while standing and relief when sitting. 2 This dance is defined as shifting weight from 1 limb to another. Similarly, humans with OT tend to avoid situations in which they have to stand still for long period (eg, waiting in line), and in an attempt to stop the disturbing tremors they usually try to alternate weight from 1 leg to the other, walk in place or lean on an object. 2 This dance looks like chorea (which in Greek means dance being an involuntary, abrupt, unsustained contraction of different muscle groups which represents a clinical sign of paroxysmal dyskinesias), 4 but the fact this might be voluntary would suggest a different pathophysiology. In dogs, differential diagnoses for POT include (a) tremors due to paresis (eg, myelopathy, neuromuscular disease), 19 , 20 (b) tremors due to painful conditions (eg, orthopedic disease especially if bilateral), 20 , 21 (c) physiological tremors (eg, shivering thermogenesis during hypothermia), 22 , 23 (d) cerebellar tremors, 21 (e) tremors due to hypomyelination, 20 (f) essential (senile) tremors, 4 , 24 , 25 or (g) twitches associated with intoxications (eg, metaldehyde/mycotoxins) or metabolic disease (eg, hypoglycemia/hypocalcemia). 4 Other differential diagnoses from human medicine, such as orthostatic myoclonus (which has a myoclonic phenotype) and pseudo‐orthostatic tremor (phenotype of OT but with low frequencies) 2 have not been reported in animals, while parkinsonian‐like tremor is extremely rare in dogs. 26 , 27 Fortunately, most tremors have either specific characteristics (eg, intention tremor) or are accompanied by other neurological signs (eg, cerebellar signs) or clinical findings (eg, femoral pulse deficits) that help differentiating them from OT, as well as lack of OT's clinical features (eg, WEBLT response). Most pathologic tremors have a frequency range of 4 to 8 Hz and usually this varies among affected body parts, 5 therefore EMG and measurement of the frequency of muscle discharges (>12 Hz) in a conscious dog is needed to distinguish reliably OT from other types of tremors. Where EMG is not available, alternative techniques using smartphone applications or electrocardiography have been used for identification of tremor frequency in humans. 28 , 29 , 30
This study showed that more than 3 quarters of dogs on medical treatment exhibited improvement of OT, but this was 2 times more likely to be partial than complete. Regardless of the extent of improvement, it appeared that phenobarbital, primidone, gabapentin, pregabalin or clonazepam achieved some degree of response; however, there were no untreated dogs for comparison. In humans, clonazepam includes the first‐line medication, while gabapentin, primidone, sodium valproate, carbamazepine, and phenobarbital comprise second‐line medications for OT. 2 However, response to medical treatment is often minimal, transient or not effective in humans and deep brain stimulation has been used successfully. 2 , 31 , 32 , 33 , 34 , 35 The high rate of improvement of OT in dogs compared to humans might be a result of the limited follow‐up time obtained in dogs or the fact that improvement was mainly partial, which seemed to be acceptable for pet owners but not in human patients. 36
Based on the owners' questionnaire, it seems that QoL of dogs was not severely affected by OT. In contrast, in humans it is considered that QoL is severely affected in POT, with “fear of falling” identified as 1 of the major factors. 36 This discrepancy could be explained due to difficulty and subjectivity of assessing QoL in dogs by their owners or the fact that dogs are quadrupedal and less affected by tremor‐related postural instability.
Retriever breeds with POT seemed to have different features compared to the remaining population. Retrievers manifest OT later in life, in a less severe form and that appears to be refractory to treatment. Whether it is the Retriever breed or the age at onset of signs which really contributes to the probability of remission warrants further investigation. Interestingly, in OT‐Plus dogs there was 1 Labrador Retriever with presumptive subclinical neuropathy. This case must be cautiously interpreted as it was a single case and although some temporal dispersion and polyphasia were detected in sciatic/tibial/ulnar nerves, MNCV and EMG at rest were completely normal and no clinical deficits ascribable to polyneuropathy were detected. In this case some degree of response to prednisolone was reported by owners. Interestingly, SOT has been observed in humans with chronic relapsing polyradiculoneuropathy and can be responsive to steroids. 37 MCNV study was performed in only 2 cases with POT and not in any Retriever with POT, so a direct comparison of the findings is not possible. Therefore, primary etiology of OT in Retrievers in this study might be presumptive and a possible etiology of age‐related degenerative neuropathy cannot be ruled out and therefore further studies should be conducted to study OT in these particular breeds.
Two Jack Russell terriers with concurrent OT and vestibulo‐cerebellar signs were also diagnosed with OT‐Plus and an MRI showed cerebral microbleeds and brain atrophy. Another 2 Jack Russell terriers had been previously reported with cerebellar signs and a presumptive diagnosis of OT‐Plus; however, none of them had an MRI. 12 In humans, brain atrophy, leukoaraiosis, small vessel disease (including cerebral amyloid angiopathy) and cerebral infarctions have been found in MRI or computed tomography (CT) of the brain in SOT cases. 31 , 38 It is important to distinguish OT from essential (senile) tremors. Essential tremors have been reported in elderly dogs especially terriers; however, they have not been extensively studied in veterinary medicine. 4 Although they share similar phenotypic features with OT as they are both postural tremors, senile tremors have a lower frequency on conscious standing EMG (4‐12 Hz). 2
Limitations of this study include (a) its retrospective nature, (b) the limited number of cases, especially for large breed dogs affected and cases with OT‐Plus given the rarity of the disease, which affects the statistical comparison, (c) the inconsistent therapeutic protocols with a variety of different medications, (d) the lack of long‐term follow‐up, (e) the limited number of questionnaire responses, (f) recall bias for the questionnaire responses as they were distributed not immediately after diagnosis, and (g) the diagnosis of POT was presumptive as not all dogs underwent all the necessary investigations in order to rule out primary diseases for potential OT‐Plus.
In conclusion, this retrospective study of OT in dogs revealed that POT is a rare paroxysmal movement disorder of large and giant breed dogs with specific semiology based on almost pathognomonic clinical (tremor while standing—absent on gait, WEBLT response, “helicopter sign”) and electrophysiological features (EMG frequency >12 Hz). Improvement could be associated with medical treatment but is more often partial than complete (at the ratio of 7 : 3). Retrievers seem to manifest a late‐onset less pharmacoresponsive POT. Orthostatic tremor‐plus exists in dogs and further investigations to detect an underlying disease are mandatory in these cases to clarify if they are of primary or secondary etiology.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the local Research Ethics Committee (EA15/20).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1. Supporting Information S1‐S10.
Video S1. Video of conscious electromyography of a 5.5‐year‐old Labrador Retriever with primary orthostatic tremor demonstrating muscle discharges of high frequency (17 Hz) and the so‐called electromyographic “helicopter sign”.
Video S2. Video demonstration of a positive limb weight bearing test (WEBLT) in a 7.5‐year‐old Labrador Retriever with primary orthostatic tremor. Note that the tremors almost cease when the pelvic limbs are lifted up.
Video S3. Video compilation of phenotypic features of dogs diagnosed with primary orthostatic tremor.
ACKNOWLEDGMENT
No funding was received for this study.
Liatis T, Gutierrez‐Quintana R, Mari L, et al. Primary orthostatic tremor and orthostatic tremor‐plus in dogs: 60 cases (2003‐2020). J Vet Intern Med. 2022;36(1):179-189. doi: 10.1111/jvim.16328
REFERENCES
- 1. Abdo WF, van de Warrenburg BPC, Burn DK, et al. The clinical approach to movement disorders. Nat Rev Neurol. 2010;6:29‐37. [DOI] [PubMed] [Google Scholar]
- 2. Benito‐Leon J, Domingo‐Santos A. Orthostatic tremor: an update on a rare entity. Tremor Other Hyperkinet Mov. 2016;6:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhatia KP, Bain P, Bajaj N, et al. Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33:75‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lowrie M, Garosi L. Classification of involuntary movements in dogs: tremors and twitches. Vet J. 2016;214:109‐116. [DOI] [PubMed] [Google Scholar]
- 5. Elble R. Tremor. In: Tousi B, Cummings J, eds. Neuro‐Geriatrics. USA: Springer; 2017:311‐326. [Google Scholar]
- 6. Erro R, Bhatia KP, Cordivari C. Shaking on standing: a critical review. Mov Disord Clin Pract. 2014;1:173‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garosi LS, Rossmeisl JH, de Lahunta A, et al. Primary orthostatic tremor in Great Danes. J Vet Med Intern. 2005;19:606‐609. [DOI] [PubMed] [Google Scholar]
- 8. Garosi L, Rossmeisl J. Primary orthostatic tremor in giant breeds. J Small Anim Pract. 2006;47:229. [DOI] [PubMed] [Google Scholar]
- 9. Giza EG, Nicpon JE, Wrzosek MA. Clinical and electrodiagnostic findings in a cohort of 61 dogs with peripheral nervous system diseases—a retrospective study. Pakistan Vet J. 2014;34:149‐154. [Google Scholar]
- 10. Platt SR, De Stefani A, Wieczorek L. Primary orthostatic tremor in a Scottish deerhound. Vet Rec. 2006;159:495‐497. [DOI] [PubMed] [Google Scholar]
- 11. Montoliu P, De Risio L, Beltran E, et al. Orthostatic tremor in a Weimaraner. J Vet Med Intern. 2009;23:396‐416. [Google Scholar]
- 12. Santifort K. Orthostatic tremor plus in two geriatric Jack Russel terriers. In: Proceedings 31st ESVN‐ECVN Symposium 2018, Copenhagen. J Vet Intern Med. 2020;34:488‐530. [Google Scholar]
- 13. Deuschl G, Bain P, Brin M, et al. Consensus statement of the movement disorder society on tremor. Mov Disord. 1998;13:2‐23. [DOI] [PubMed] [Google Scholar]
- 14. DeOrchis VS, Geyer HL, Herskovitz S. Teaching video neuroimages: the helicopter sign. Neurology. 2013;80:e161. [DOI] [PubMed] [Google Scholar]
- 15. Mugnier A, Mila H, Guiraud F, et al. Birth weight as a risk factor for neonatal mortality: breed‐specific approach to identify at‐risk puppies. Prev Vet Med. 2019;171:104746. [DOI] [PubMed] [Google Scholar]
- 16. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159‐174. [PubMed] [Google Scholar]
- 17. Schoberl F, Feil K, Xiong G, et al. Pathological ponto‐cerebello‐thalamo‐cortical activations in primary orthostatic tremor during lying and stance. Brain. 2017;140:83‐97. [DOI] [PubMed] [Google Scholar]
- 18. Wuehr M, Schlick C, Möhwald K, Schniepp R. Walking in orthostatic tremor modulates tremor features and is characterized by impaired gait stability. Sci Rep. 2018;8:14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bagley RS. Tremor syndromes in dogs: diagnosis and treatment. J Small Anim Pract. 1991;33:485‐490. [Google Scholar]
- 20. de Lahunta A, Glass E, Kent M. Lower motor neuron: spinal nerve, general somatic efferent system. In: de Lahunta A, Glass E, Kent M, eds. Veterinary Neuroanatomy and Clinical Neurology. 4th ed. Missouri: Elsevier Saunders; 2015:102‐161. [Google Scholar]
- 21. Bagley RS, Platt S. Tremors, involuntary movements and paroxysmal disorders. In: Platt S, Olby N, eds. BSAVA Manual of Canine and Feline Neurology. 4th ed. Gloucester, UK: British Small Animal Veterinary Association; 2014:232‐251. [Google Scholar]
- 22. Oncken AK, Kirby R, Rudloff E. Hypothermia in critically ill dogs and cats. Compend Contin Educ Pract Vet N Am Ed. 2001;23:506‐520. [Google Scholar]
- 23. McAllen RM, Tanaka M, Ootsuka Y, et al. Multiple thermoregulatory effectors with independent central controls. Eur J Appl Physiol. 2010;109:27‐33. [DOI] [PubMed] [Google Scholar]
- 24. Fenner WR. Neurology of the geriatric patient. Vet Clin N Am Small Anim Pract. 1988;18:711‐724. [DOI] [PubMed] [Google Scholar]
- 25. De Lahunta A, Glass EN, Kent M. Classifying involuntary muscle contractions. Compend Contin Educ Pract Vet N Am Ed. 2006;28:516‐529. [Google Scholar]
- 26. De Lahunta A, Averill DR. Hereditary cerebellar cortical and extrapyramidal nuclear abiotrophy in Kerry Blue Terriers. J Am Vet Med Assoc. 1976;168:1119‐1124. [PubMed] [Google Scholar]
- 27. O'Brien DP, Johnson GS, Schnabel RD, et al. Genetic mapping of canine multiple system degeneration and ectodermal dysplasia loci. J Hered. 2005;96:727‐734. [DOI] [PubMed] [Google Scholar]
- 28. Bhatti D, Thompson R, Hellman A, Penke C, Bertoni JM, Torres‐Russotto D. Smartphone apps provide a simple, accurate bedside screening tool for orthostatic tremor. Mov Disord Clin Pract. 2017;4:852‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chien JH, Torres‐Russotto D, Wang Z, Gui C, Whitney D, Siu KC. The use of smartphone in measuring stance and gait patterns in patients with orthostatic tremor. PLoS ONE. 2019;14:e0220012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Littmann L. Fact or artifact? The electrocardiographic diagnosis of orthostatic tremor. J Electrocardiol. 2010;43:270‐273. [DOI] [PubMed] [Google Scholar]
- 31. Hassan A, Ahlskog JE, Matsumoto JY, Milber JM, Bower JH, Wilkinson JR. Orthostatic tremor: clinical, electrophysiologic, and treatment findings in 184 patients. Neurology. 2016;86:458‐464. [DOI] [PubMed] [Google Scholar]
- 32. Lehn AC, O'Gorman C, Olson S, et al. Thalamic ventral intermediate nucleus deep brain stimulation for orthostatic tremor. Tremor Other Hyperkinet Mov. 2017;7:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Merola A, Fasano A, Hassan A, et al. Thalamic deep brain stimulation for orthostatic tremor: a multicenter international registry. Mov Disord. 2017;32:1240‐1244. [DOI] [PubMed] [Google Scholar]
- 34. Evidente VGH, Baker ZJ, Evidente MH, Garrett R, Lambert M, Ponce FA. Orthostatic tremor is responsive to bilateral thalamic deep brain stimulation: report of two cases performed asleep. Tremor Other Hyperkinet Mov. 2018;8:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gilmore G, Murgai A, Nzer A, et al. Zona incerta deep‐brain stimulation in orthostatic tremor: efficacy and mechanism of improvement. J Neurol. 2019;266:2829‐2837. [DOI] [PubMed] [Google Scholar]
- 36. Maugest L, McGovern EM, Mazalovic K, et al. Health‐related quality of life is severely affected in primary orthostatic tremor. Front Neurol. 2017;8:747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gabellini AS, Martinelli P, Gulli MR, et al. Orthostatic tremor: essential and symptomatic cases. Acta Neurol Scand. 1990;81:113‐117. [DOI] [PubMed] [Google Scholar]
- 38. Gerschlager W, Brown P. Orthostatic tremor—a review. In: Weiner WJ, Tolosa E, eds. Handbook of Clinical Neurology. Vol 100. 3rd ed. Amsterdam, The Netherlands: Elsevier; 2011:457‐462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information S1‐S10.
Video S1. Video of conscious electromyography of a 5.5‐year‐old Labrador Retriever with primary orthostatic tremor demonstrating muscle discharges of high frequency (17 Hz) and the so‐called electromyographic “helicopter sign”.
Video S2. Video demonstration of a positive limb weight bearing test (WEBLT) in a 7.5‐year‐old Labrador Retriever with primary orthostatic tremor. Note that the tremors almost cease when the pelvic limbs are lifted up.
Video S3. Video compilation of phenotypic features of dogs diagnosed with primary orthostatic tremor.


