Abstract
Background
Endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography (EDGE) has emerged as a viable completely endoscopic method for performing pancreaticobiliary interventions in patients with Roux-en-Y gastric bypass anatomy. The aims of this systematic review were: (1) to describe the indications, outcomes, and complications of EDGE; and (2) to identify deficiencies in our knowledge of important technical approaches and clinical outcomes.
Methods
A systematic review was conducted via comprehensive searches of Medline, Scopus, CINAHL, and Cochrane to identify studies focusing on EDGE outcomes. Simple descriptive statistics were derived from case series only. Case reports were only included to qualitatively describe additional indications, techniques, and adverse events.
Results
The initial search identified 2143 abstracts. Nine case series and eight case reports were included. In the nine case series, 169 patients underwent EDGE. The technical success rate was 99% (168/169) for gastrogastrostomy/jejunogastrostomy creation and 98% (166/169) for subsequent ERCP. Minor adverse events specifically related to EDGE occurred in 18% (31/169) and included intraprocedural stent migration/malposition (n = 27) and abdominal pain (n = 4). Moderate adverse events specific to EDGE occurred in 5% (9/169): including bleeding (2 %), persistent fistula (1%), and perforation (1 %). Severe adverse events occurred in one patient with a perforation requiring surgery. Deficiency in reporting on the clinical significance of adverse events was identified.
Conclusion
Based on limited observational data, in expert hands, EDGE has a high rate of technical success and an acceptable rate of adverse events. As a novel procedure, many knowledge gaps need to be addressed to inform the design of meaningful comparative studies and guide informed consent.
Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) is challenging in patients with Roux-en-Y gastric bypass (RYGB) owing to their altered anatomy. The limitations of the currently available techniques for performing pancreaticobiliary interventions in this population has led to the emergence of endoscopic ultrasound (EUS)-directed transgastric ERCP (EDGE) as a viable completely endoscopic alternative that has been adopted by many ERCP providers in academic medical centers [1–3].
The EDGE procedure involves the creation of a fistula tract between the gastric pouch or proximal jejunum and the excluded stomach using a lumen-apposing metal stent (LAMS) placed under EUS guidance (Fig. 1). ERCP can subsequently be performed through the LAMS. The main advantage and likely appeal to many endoscopists is that EDGE is a completely endoscopic technique that allows the endoscopist to perform ERCP using a traditional duodenoscope and standard accessories [1, 2]. Case series have shown this procedure, in expert hands, has a high rate of technical and clinical success, with adverse event rates at low levels [4, 5]. Owing to the novelty of this procedure, it is challenging for endoscopists to fully inform patients undergoing EDGE of its appropriate indications, rates of technical success, clinical success, and adverse events, and the required management of any complications that might occur. Additionally, data to inform the design of studies comparing EDGE to other established approaches, such as laparoscopic- or enteroscopy-assisted ERCP, remain limited.
Fig. 1.
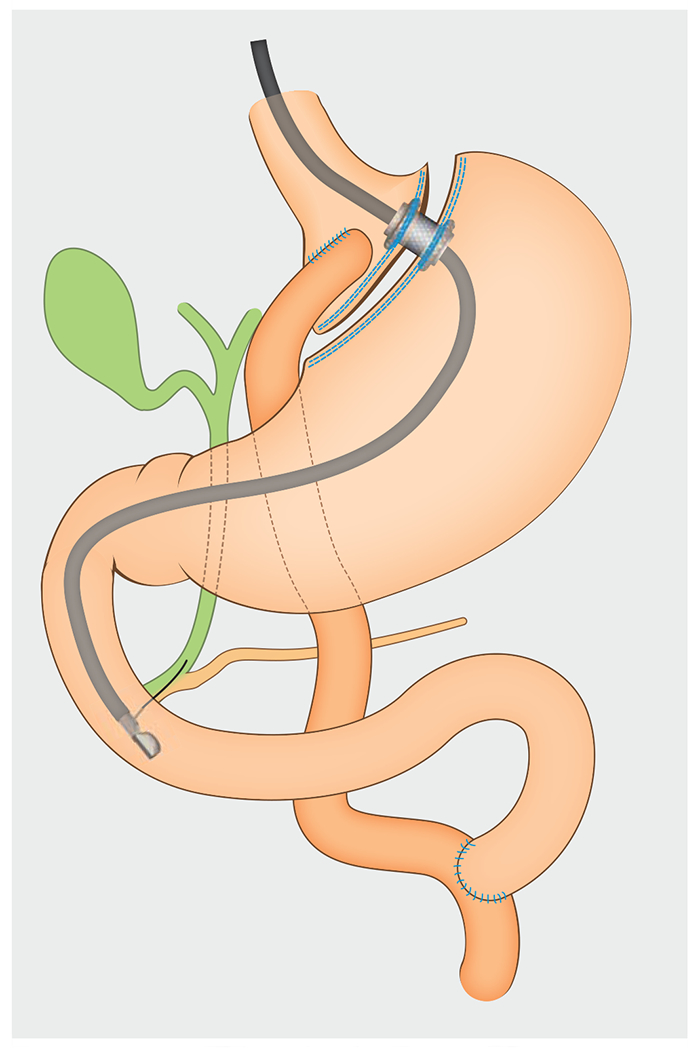
Schematic showing the use of endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography (EDGE) to facilitate endoscopic retrograde cholangiopancreatography in a patient with Roux-en-Y anatomy. In this example, a lumen-apposing metal stent (LAMS) is used to create a gastro-gastrostomy and access the excluded stomach.
The aims of this systematic review were to: (1) describe the indications, outcomes, and complications of EDGE; and (2) identify deficiencies in our knowledge of the important technical approaches and clinical outcomes.
Methods
This systematic review was conducted in accordance with the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines [6]. We performed a comprehensive search of Medline, Scopus, CINAHL and Cochrane from 1 January 2011 to 1 September 2020. The search was limited to start in 2011 as this corresponded to the first published description of a lumen-apposing metal stent (LAMS) [7]. As per protocol, all abstracts identified in the search were screened independently by two authors (R.M., S.P.) to determine their eligibility for full-text review. Manuscripts selected for full-text review were reviewed independently by the same two authors (R.M., S.P.) to assess their eligibility for inclusion in this study. Conflicts for both the abstract review and full-text review were resolved by consensus between the two reviewing authors (R.M., S.P.).
The inclusion criteria were as follows: (1) case series, cohort studies, or case reports; (2) manuscripts in the English language only; (3) studies containing human subjects only; (4) studies restricted to patients who had previously undergone an RYGB; (5) studies that examined the EDGE procedure, with the critical component of EDGE being defined as placement of a LAMS under EUS guidance from the gastric pouch or jejunum to the excluded stomach with a subsequent ERCP performed through the LAMS; and (6) manuscripts that described the indications, success rates, techniques, and/or adverse events of EDGE. Narrative reviews that described previously published literature were excluded.
Owing to the risk of duplicate data, if a study included data from a single center that was later published in a larger single or multicenter study, we preferentially included the larger or multicenter study. For studies with overlapping time periods, we contacted the study authors to determine if there were duplicate patients. If duplicate patients were present, we did not include the study. Additionally, if the same data were published both as an abstract and then later as a full-text manuscript, only the full-text manuscript was included.
The Medline search was: (((“Gastric Bypass”[MeSH] OR bypass OR transgastric OR roux-en-y OR gastrogastrostomy OR gastrojejunostomy* OR “surgically altered”) AND (“Cholangiopancreatography, Endoscopic Retrograde”[MeSH] OR endoscopic retrograde cholangiopancreatograph* OR ERCP))) OR ((“Endosonography”[MeSH] OR endoscopic ultrasound OR EUS) AND transgastric). Search terms used for Scopus, CINAHL, and Cochrane are available in Appendix 1s, see online-only Supplementary material.
Adverse events and their severity were defined based on the American Society of Gastrointestinal Endoscopy (ASGE) guidelines [8].
Statistical analysis
The included case series and/or cohort studies were used to create simple descriptive statistics for EDGE. Descriptive statistics used for this study included mean and standard deviation. SPSS version 25 was used to calculate the mean and standard deviation for the study. The pooled rate and confidence intervals (CIs) were calculated using Comprehensive Meta-Analysis, version 3.
Case reports were not used to generate descriptive statistics because of the lack of a denominator, but were reviewed to identify indications, techniques, or adverse events that were not reported in cohort studies.
Results
Study identification
A total of 2143 abstracts were identified from the initial search of the three databases during the target time period, with 17 articles selected for the final study [9–25]. A detailed report of the abstract and full-text reviews is shown in Fig. 2. The included studies were retrospective cohort studies (n = 9) and case reports (n = 8).
Fig. 2.
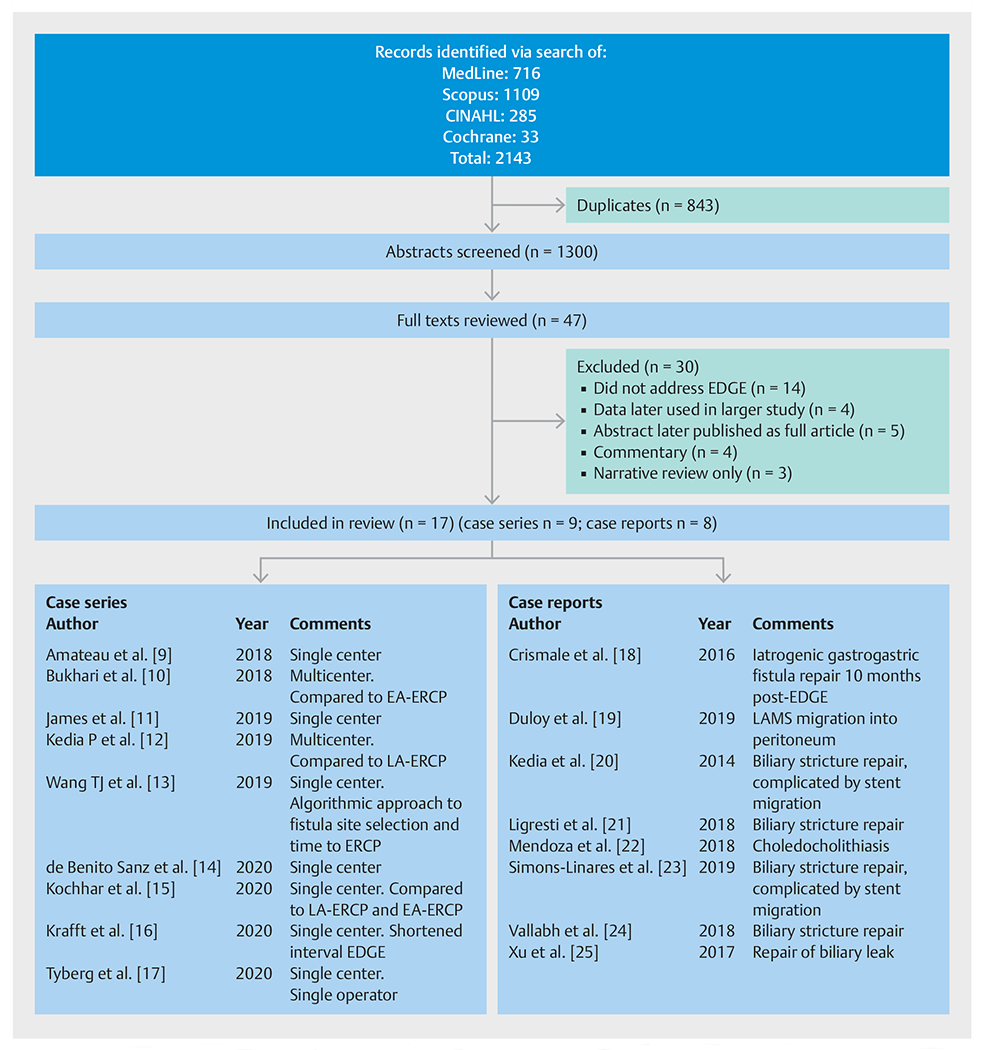
PRISMA diagram of the abstract and full-text review. EDGE, endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography; EA-ERCP, enteroscopy-assisted endoscopic retrograde cholangiopancreatography; LA-ERCP, laparoscopy-assisted ERCP; LAMS, lumen-apposing metal stent.
Demographics and indications
The nine selected case series, which included 169 patients, were used to generate the following descriptive statistics. Patients were predominantly female (80.9%; 115/142), with a mean age of 56.4 years (Table 1). The indication for EDGE was available for 135 patients in the cohort studies. The most common indication was choledocholithiasis (44%; 60/135), followed by biliary obstruction (12%; 16/135), pancreatitis (14%; 19/135), cholangitis (10%; 14/135), abnormal laboratory results (5.2%; 7/135), symptomatic biliary dilation (4.4%; 6/135), bile leak (4.4%; 6/135), biliary sludge (2.2%; 3/135), pancreatic duct dilation (n = 2), Sphincter of Oddi dysfunction (n = 1), and papillary stenosis (n = 1) [9–11, 13–17]
Table 1.
Indications, success rates, and adverse events for endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography (EDGE).
| Author (year) | Patients, n | Sex, female, n(%); age, mean (range), years | Indication* | Technical success rates | Adverse events | Adverse events specific to ERCP | |||
|---|---|---|---|---|---|---|---|---|---|
| Accessing excluded stomach | Performing ERCP | Minor | Moderate | Severe | |||||
| Amateau et al. (2018) | 3 | 2 (66%); 59 (50–67) | 2 biliary 1 pancreatic | 3/3 (100%) | 3/3 (100 %) | 1 stent migration | – | – | – |
| Bukhari et al. (2018) | 30 | 27 (90%); 52.5 (NA) | 26 biliary 4 pancreatic | 30/30 (100 %) | 30/30 (100%) | 2 stent migrations | 1 bleed 1 persistent fistula | – | – |
| James et al. (2019) | 19 | 15 (79%); 56 (39–71) | 13 biliary 6 pancreatic | 19/19 (100 %) | 19/19 (100%) | 6 stent malpositions | 1 persistent fistula | – | – |
| Kedia et al. (2019) | 29 | 25 (86%); 56 (35–82) | 23 biliary 6 pancreatic | 28/29 (97%) | 28/29 (97%) | 3 stent migrations | 1 bleed | 1 perforation | 2 pancreatitis |
| Wang et al. (2019) | 9 | NA; NA | 9 biliary | 9/9 (100%) | 9/9 (100 %) | 2 stent migrations | 1 bleed | – | – |
| de Benito Sanz et al. (2020) | 14 | 12 (86%); 56 (31–67) | 11 biliary 2 pancreatic 1 other | 14/14 (100 %) | 13/14 (93 %) | 4 stent migrations 2 abdominal pain | 1 perforation | – | 1 bleed |
| Kochhar et al. (2020) | 26 | 20 (77%); 60.8 (NA) | 22 biliary 4 pancreatic | 26/26 (100%) | 26/26 (100%) | 1 stent migration | – | – | 2 bleeds |
| Krafft et al. (2020) | 21 | 14 (67%); 57.6 (NA) | 20 biliary 1 pancreatic | 21/21 (100 %) | 20/21 (95 %) | 6 stent migrations 2 abdominal pain | – | – | – |
| Tyberg et al. (2020) | 18 | NA; NA | 11 biliary 3 pancreatic 4 other | 18/18 (100 %) | 17/18 (94 %) | 2 stent malpositions | 2 bleeds 1 perforation | – | 1 pancreatitis |
| Total | 169 | 115/142 (81 %); 56.4 | 137 biliary; 27 pancreatic; 5 other | 168/169 (99%) | 166/169 (98%) | 31/169 (18.3%) | 9/169 (5.3 %) | 1/169 (0.6 %) | 6/169 (3.5 %) |
ERCP, endoscopic retrograde cholangiopancreatography; NA, not available.
Specific indications – biliary: biliary choledocholithiasis (n = 60); biliary obstruction (n = 16); cholangitis (n = 14); abnormal laboratory results (n = 7); symptomatic biliary dilation (n = 6); bile leak (n = 6); biliary sludge (n = 3); Sphincter of Oddi dysfunction (n = 1); papillary stenosis (n = 1); not specified (n = 23); – pancreatic: pancreatitis (n = 19), pancreatic duct dilation (n = 2), not specified (n = 6);– other (n = 5).
Technical success and clinical outcomes
Overall, the technical success rate for creation of a gastrogastrostomy or jejunogastrostomy with placement of a LAMS was 99% (168/169), and the success rate for ERCP was 98% (166/169) (Table 1) [9–17]. The pooled success rate for ERCP was 96.8% (95%CI 92.6%–98.7%). Only one study commented on technical failure in creating a gastrogastric or jejunogastric fistula. This technical failure was due to malfunction of the LAMS during deployment, causing perforation that required immediate surgical intervention [12]. The remainder of the retrospective studies did not comment on any technical failures for the creation of the gastrogastric or jejunogastric fistula.
Technical approach
Patients had creation of either a gastrogastric fistula (61%; 74/122) or a jejunogastric fistula (39 %; 48/122) to facilitate EDGE [9–11, 13–16]. The 15-mm LAMS (n = 132) was used more commonly than the 20-mm LAMS (n = 37). A cautery-assisted LAMS was more commonly used (77%; 94/122) than a noncautery-assisted LAMS (23%; 28/122) [9–11, 13–16]. Most patients (62.1%; 105/169) underwent delayed ERCP after the initial creation of the gastrogastric or jejunogastric fistula [9–17]. For patients who underwent delayed ERCP, the weighted mean time from LAMS placement to ERCP was 24 days (n = 92), varying across studies from a mean of 2 days up to 48 days [10–14, 16,17].
The methods for fistula closure, when reported (n = 137), varied across studies and included: over-the-scope (OTS) clip and/or endoscopic suturing (n = 67), spontaneous (n = 39), double-pigtail stent placement to chaperone closure (n = 15), argon plasma coagulation (n = 13), and surgery (n = 3) (Table 2) [10–15, 17]. Fistula closure after completion of EDGE was assessed in four studies (n = 68) [10, 11, 13, 15]. The methods to assess closure included upper gastrointestinal (GI) series (49%; 33/68), esophagogastroduodenoscopy (16%; 11/68), weight change (28%; 19/68), computed tomography (CT) scan (4%; n = 3/68), or a combination of these strategies (3%; 2/68).
Table 2.
Variation in techniques for endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography (EDGE).
| Author (year) | Patients, n | Procedure time, minutes | Access type | Method for stomach insufflation | Size of LAMS, mm | Cautery/non-cautery | Single/delayed | Time to ERCP, days | Hospital stay, days | Fistula closure |
|---|---|---|---|---|---|---|---|---|---|---|
| GG/JG | ||||||||||
| Amateau et al. (2018) | 3 | Single session 53±22; delayed NA | 2/1 | Not reported | 15 | 0/3 | 3/0 | 0 | 1 | NA |
| Bukhari et al. (2018) | 30 | Single session 49±26; delayed NA | 17/13 | 5–10 mL contrast & 120–300 mL sterile water | 15 | 14/16 | 8/22 | 21.5 | 1 | 7 OTS clips, 8 OTS suturing device, 15 spontaneous |
| James et al. (2019) | 19 | All patients 116±88 | 8/11 | Saline + contrast solution | 15 | 14/5 | 4/15 | 48 | NA | 6 spontaneous, 13 APC |
| Kedia et al. (2019) | 29 | All patients 73 (24–230) | NA | 120 mL water + contrast | 15 | NA | 3/26 | 21–28 | 0.8 | OTS suturing |
| Wang et al. (2019) | 9 | All patients 142±37 | 3/6 | > 200mL contrast 50:50 dilution | 15 | 9/0 | 7/2 | 14–21 | NA | Double-pigtail stent placement |
| de Benito Sanz et al. (2020) | 14 | NA | 9/5 | >100mL of water-soluble contrast + sterile water | 20 | 10/4 | 10/4 | 22 | NA | 5 double-pigtail, 1 OTS clip, 1 OTS clip & pigtail |
| Kochhar et al. (2020) | 26 | All patients 79±31 | 22/4 | Mixture of contrast + saline | 15 (×24) 20 (× 2) | 26/0 | 13/13 | NA | 1.6 | 18 spontaneous, 3 surgically, 3 endoscopic clips |
| Krafft et al. (2020) | 21 | NA | 13/8 | >100mL of contrast + sterile water | 20 | 21/0 | 11/10 | 2 | NA | NA |
| Tyberg et al. (2020) | 18 | All patients 54.5 (31–88)* | NA | Contrast + 120 mL water | 15 | NA | 5/13 | 14–21 | NA | OTS clip and/or endoscopic sutures |
GG, gastrogastrostomy; JG, jejunogastrostomy; LAMS, lumen-apposing metal stent; ERCP, endoscopic retrograde cholangiopancreatography; OTS, over the scope; APC, argon plasma coagulation; NA, not available.
Includes one patient who only underwent endoscopic ultrasound.
Four studies reported the total procedure time based on single-session vs. multisession EDGE [9–11, 15]. The mean total procedure time was 94.2 minutes for same-session EDGE (n = 22) and 110.2 minutes for delayed EDGE (n = 17). Four studies reported a combined mean procedure time of 92 minutes (n = 83) [11–13, 15]. Two studies did not report the procedure time [14, 16]. One study had a mean procedure time of 54.5 minutes but included one patient who did not undergo ERCP [17]. Three studies reported that the mean length of hospitalization after EDGE or LAMS was 1 day or less [9, 10, 12]; one study reported a mean hospitalization of 1.6 days [15]; hospital stay was not reported in five studies [11, 13, 14, 16, 17].
Adverse events
Adverse events occurred in 27.8% of EDGE procedures (47/169). Of these, 41 adverse events were specifically related to the EDGE procedure and six were recognized complications of ERCP (Table 1). Minor adverse events specific to EDGE, as defined according to the ASGE lexicon, occurred in 18% of patients (31/169) and were due to intraprocedural stent migration (n = 19), stent malposition during deployment (n = 8), and post-procedure abdominal pain (n = 4). Stent migration was reported as occurring during attempted ERCP when the LAMS was traversed [9, 10, 12–16]. In all reported cases, stent migration was managed endoscopically with replacement or manipulation of the LAMS, or placement of an esophageal stent. It was not possible to determine if this adverse event represented a level A or B minor adverse event as no data were reported regarding the need for further post-procedural management (such as antibiotics or hospitalization) related to the stent migration.
Moderate adverse events specific to EDGE and defined according to the ASGE lexicon occurred in 5.3% of patients (9/169). These included bleeding (n = 5), persistent fistula (n = 2), and perforation (n = 2). Significant bleeding was reported in five patients [10, 12, 13, 17]. The causes of bleeding were fistula creation (n = 1), LAMS exchange for a double-pigtail stent (n = 1), or unknown/not reported (n = 3). Two patients developed a persistent fistula (one gastrogastric and one jejunogastric) after LAMS removal [9, 11]. These fistulas were identified at 6 weeks with an upper GI series in one patient and at 7 months with an upper GI series that was performed because of significant weight gain in another. There was one reported severe adverse event in which a patient developed a perforation owing to LAMS delivery system malfunction and required emergent surgery [12]. Table 3 describes the management of the various complications described in the retrospective studies and case reports.
Table 3.
Complications of endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography (EDGE).
| Complication | Author (year) | Number of patients | Details regarding complication |
|---|---|---|---|
| Bleeding | Bukhari et al. (2018) | 1 | During gastrogastrostomy creation, managed with transfusion only |
| Wang et al. (2019) | 1 | Following LAMS exchange for a double-pigtail stent, treated with epinephrine and LAMS to tamponade bleeding | |
| Kedia et al. (2019) | 1 | No further clarification | |
| de Benito Sanz et al. (2020) | 1 | Post-sphincterotomy bleeding, managed endoscopically | |
| Kochhar et al. (2020) | 2 | Sphincterotomy-related bleeding, managed with balloon tamponade | |
| Tyberg et al. (2020) | 2 | One case managed with endoscopic hemoclips, the other with a bridging stent to tamponade the bleed | |
| Perforation | Kedia et al. (2019) | 1 | Due to malfunction of the catheter delivery system during LAMS deployment process, managed with immediate surgical repair |
| de Benito Sanz et al. (2020) | 1 | Duodenal perforation closed by OTS clips | |
| Tyberg et al. (2020) | 1 | Jejunal perforation during duodenoscope advancement, managed endoscopically | |
| Persistent fistula | Bukhari et al. (2018) | 1 | Persistent leak identified on upper GI series 6 weeks after closure was attempted with endoscopic suture, managed with OTS clip closure and confirmed on follow-up upper GI series |
| Crismale et al. (2016) | 1 | EDGE, initially performed for cholangitis, was complicated by LAMS dislodgement requiring esophageal stent placement; presented 10 months post-procedure with 20 lb weight gain; stent removed using grasping forceps, APC to denude gastrogastric fistula before an endoscopic suturing device used to place a running suture, then oversewn with an interrupted stitch | |
| Stent malposition | Tyberg et al. (2020) | 2 | Flange deployed outside target lesion, managed with bridging esophageal FCSEMS |
| James et al. (2020) | 6 | Rescue procedures with placement of esophageal FCSEMS in four patients | |
| Stent migration (intraprocedural) | Bukhari et al. (2018) | 2 | During ERCP stage of EDGE, stent repositioned across fistula in one patient and FCSEMS placed in the other |
| Duloy et al. (2019) | 1 | Stent migration during scope withdrawal, resulting in stent migration into peritoneum, managed with placement of a second larger LAMS to connect the gastric pouch and remnant stomach | |
| Kedia et al. (2019) | 3 | Managed with bridging esophageal FCSEMS, mineral oil used to lubricate the shaft of the duodenoscope to prevent further occurrences | |
| Wang et al. (2019) | 2 | During ERC Pstage of single-session EDGE, endoscopically corrected | |
| de Benito Sanz et al. (2020) | 4 | Two occurred during single-session EDGE and two occurred during deferred ERCP; three cases were managed with LAMS relocation and one by OTS clip closure | |
| Krafft et al. (2020) | 6 | Five occurred during single-session EDGE and one during shortened-interval EDGE; incomplete LAMS dislodgement in four patients (managed with bridging esophageal FCSEMS deployed OTW) and complete LAMS dislodgement in two patients (managed with bridging esophageal FCSEMS, one deployed via NOTES and one OTW) | |
| Stent migration (postprocedural) | Simmons-Linares et al. (2018) | 1 | LAMS migration into the excluded stomach while waiting for maturation, managed by passing a wire through the fistula to the excluded stomach and TTS placement of an 18-mm × 6-cm FCSEMS, with ERCP then successfully performed |
| Kochhar et al. (2020) | 1 | LAMS dislodgement after ERCP, managed by removing the original LAMS and stenting the fistula with a new LAMS | |
| Abdominal pain | Krafft et al. (2020) | 2 | Post-procedural abdominal pain requiring prolongation of hospitalization, occurring in one patient with complete LAMS dislodgement requiring NOTES and in another with aborted ERCP because of duo denoscope angulation |
LAMS, lumen apposing metal stent; OTS, over the scope; GI, gastrointestinal; APC, argon plasma coagulation; FCSEMS, fully covered self-expanding metal stent; ERCP, endoscopic retrograde cholangiopancreatography; OTW, over the guidewire; NOTES, natural orifice transluminal endoscopic surgery; TTS, through the scope.
Other than the EDGE-specific adverse events, there were three cases of post-ERCP pancreatitis, with one identified as a major adverse event, one as a minor adverse event, and one in which severity was not reported [12, 17]. There were also three cases of sphincterotomy-associated bleeding that were defined as minor adverse events. An additional adverse event reported in a case report was LAMS migration while waiting for fistula maturation [23].
Weight change following EDGE was reported in four of the included studies. Of these, three studies found weight loss (−1.1 kg, −2.9 kg, and −1.4 kg), while one found weight gain (+ 1.7 kg) when assessed at various time points after EDGE [9, 11, 12].
Discussion
This systematic review preliminarily demonstrates that in expert hands EDGE is a technically feasible method for accessing the descending duodenum to perform ERCP across a host of indications in patients with RYGB anatomy, with a rate of major adverse events similar to other methods for accessing the descending duodenum in patients with RYGB anatomy. However, many deficiencies remain in our understanding of the optimal technical approach and clinical outcomes for this procedure.
The main advantage of EDGE compared with other modalities for ERCP in patients with RYGB anatomy is that EDGE can be completely performed with endoscopy, and it allows ERCP to be completed using a standard duodenoscope. Based on this review, EDGE is being performed successfully across a spectrum of indications including choledocholithiasis and other more challenging pancreaticobiliary diseases, such as acute and chronic pancreatitis. The critical component of EDGE, namely creation of the gastrogastrostomy or jejunogastrostomy, has also been used for histological sampling of gastroduodenal lesions and to evaluate and treat extraluminal pathology with EUS [26].
Based on the published observational data, EDGE has a high (99%) technical success rate for both EDGE and the subsequent ERCP. Such a high technical success rate should be scrutinized, as it is higher than the technical success rate of ERCP in patients with standard foregut anatomy [27]. Nevertheless, it is reasonable to assume that any differences between the technical success of ERCP as part of EDGE and ERCP for patients with standard foregut anatomy are largely attributable to the technical limitations of EDGE. As a completely endoscopic method, the technical success of EDGE is far superior to that reported for enteroscopy-assisted ERCP and is comparable to that of the hybrid endoscopic and laparoscopic procedure [28–30]. It is critical to consider, however, that observational studies of novel procedures tend to overestimate success rates and underestimate risk because (i) these procedures are performed by experts with particular interest and expertise in complex endoscopy, (ii) adverse events are likely under-reported, and (iii) sample sizes are very small. An important next step in the evidence-based evolution of this procedure is rigorous investigation aiming to more precisely estimate the technical outcomes and risks, especially as EDGE becomes more widely applied in clinical practice. Such estimates are critical toward accurately informing patients of the risk-benefit ratio of the procedure and for designing comparative studies.
Given the high technical success rates for both EDGE and laparoscopy-assisted ERCP, two issues must be reconciled prior to deciding which technique is to be preferred. First, in patients who require a cholecystectomy, laparoscopy-assisted ERCP allows combined cholecystectomy to be performed in a single session [2] and whether the advantages of EDGE justify two additional procedures in this context must be explored. Second, EDGE obviates the administrative obstacles of scheduling a two-physician procedure, when the ERCP provider is not a general surgeon.
Certain technical aspects of EDGE, mainly pertaining to fistula creation, are still being refined to mitigate complications and increase success rates. One area of interest is the optimal size of the LAMS for fistula creation. The majority of patients in this study had a 15-mm LAMS during EDGE. In 2018, a 20-mm LAMS became commercially available. It is unclear what effect the 20-mm LAMS will have on issues such as stent migration and the endoscopists comfort level to perform same-session ERCP. It has been theorized that this new stent will reduce stent migration and may lead to higher rates of same-session ERCP, which has only occurred in 38% of EDGE procedures.
Whether same-session ERCP is advantageous in non-urgent cases, as a repeat procedure is necessary to remove the LAMS once the fistula has matured, remains to be defined. Presently, removing the LAMS during the index EDGE procedure poses significant risk owing to the defect created in the excluded stomach, akin to a free perforation. A single-session procedure, whereby an EDGE is performed, the biliary intervention is completed, and the defect is closed in the same session, would offer many patient-centered and economic benefits. In the absence of a robust method to close the perforation in the excluded stomach during the index EDGE procedure, removing the LAMS at the index procedure should currently be avoided.
Early studies of the EDGE procedure reported placement of the LAMS over a guidewire, after access to the excluded stomach had been achieved with a needle puncture, followed by guidewire placement and dilation of the fistula tract [4, 5, 20]. Cautery-assisted LAMSs do not require placement over a guidewire and can be placed by a “freehand” puncture technique. This technique has the advantage of not committing the endoscopist to a specific access point, potentially optimizing the site of fistula creation. Based on the limited available data, there does not appear to be any advantage of the guidewire approach as long as the stomach is fully distended before puncture. The choice to perform EDGE over a guidewire should be based on the endoscopist’s preference; a guidewire potentially adds a level of security during fistula tract creation, is inexpensive, and adds little in terms of procedure time.
Securing the proximal portion of the LAMS to the luminal wall to reduce the risk of stent migration has been reported [1, 31]. This has the potential to reduce the risk of stent migration, improving the safety profile of EDGE for same-session ERCP. No data exist to support or detract from this practice and further studies are needed to determine the benefit of this practice.
There is a paucity of data pertaining to the location of the fistula (gastrogastric or jejunogastric) and its association with clinical outcomes, with none of the studies included in this systematic review specifically addressing this issue. Although this has not been specifically reported, it is possible that acid exposure from the excluded stomach to the jejunum vs. gastric pouch could have varying clinical implications. Additionally, the complexity and success of endoscopic or surgical repair for persistent fistulas likely vary according to location.
Despite the high technical success rate reported in the literature, EDGE is a challenging procedure with the potential for major adverse events such as bleeding and perforation. Although the technique is similar to that of transmural drainage of a pancreatic fluid or necrotic collection, the excluded stomach is not confined to the retroperitoneum and is rarely engorged with fluid to facilitate endosonographic localization.
An important issue with EDGE is stent migration, occurring in 11% of cases in this study. The majority of stent migrations may be salvaged during the initial procedure; however, when multiple devices are required to preserve the fistula tract, such as a covered esophageal stent or double-pigtail stent with its delivery system, the facility costs for EDGE increase. Because of the lack of reporting in published studies on any additional management decisions, such as admission to hospital or antibiotic therapy, it is difficult to gauge if this truly represents a clinically significant adverse event. Only one patient in this systematic review required surgery for a misdeployed stent that resulted in a perforation, clearly representing a severe adverse event. In addition, a case report has described the migration of a LAMS into the excluded stomach that happened weeks after the initial EDGE, which required an additional procedure to access the excluded stomach and remove the stent, qualifying as a moderate adverse event. Given its frequency, future studies in this field should aim to understand the frequency of significant stent migration – probably best described as a migration that requires the use of additional devices or procedures to address it – and how best to mitigate it.
When including the patient with an abdominal perforation related to stent maldeployment, 5.9% of patients (10/169) suffered moderate or severe adverse events, as described by the ASGE lexicon. Two of these events were recognized intraprocedurally: one perforation and one episode of bleeding. The remaining complications were delayed, with in one case a persistent fistula only being discovered 7 months later.
The clinical significance of a persistent fistula is unclear, although weight regain represents the principal concern. Multiple approaches have been described to close these fistulas, including closure by primary intent. It is not known if these fistulas need to be actively closed and, if so, what is the best method. Weight loss plateaus after RYGB after 1–2 years and reporting on changes in weight post-EDGE has been inconsistent with three studies showing weight loss and one study showing weight gain. Until the clinical significance of a persistent fistula can be determined, prospective follow-up on patients undergoing EDGE to ensure fistula closure and assess changes in weight are essential to determine the relevance of these two factors.
The main strength of this study is the comprehensive and detailed examination and synthesis of the existing studies on EDGE that has allowed a preliminary understanding of the outcomes and the identification of important knowledge gaps. An additional strength is the method used to select studies for inclusion, which ensured that patients included in multiple studies were not analyzed more than once. The main limitation of this study is the small number of cases that were included and the likely selection bias of these cases. As mentioned above, observational studies of this nature tend to overestimate success rates and underestimate risk because the procedures are performed by experts and the sample sizes are usually small. As such, the results of this study should be interpreted with this risk for bias in mind. However, this early systematic review has allowed the identification of knowledge gaps to help frame reporting strategies in future studies (Table 4).
Table 4.
Gaps in our knowledge of the outcomes of endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography (EDGE).
| 1 | Additional risk of same-session ERCP vs. delayed ERCP |
| 2 | Clinical significance of stent migration outside of stent mal-deployment requiring separate intervention such as surge |
| 3 | Clinical significance of a persistent gastrogastric or jejunogastric fistula in terms of weight gain and additional complications |
| 4 | Method required, if any, to close gastrogastric or jejunogastric fistulas and best time to assess for fistula closure on follow-up |
| 5 | Clinical significance of the location of fistula (gastrogastrostomy vs. jejunogastrostomy) |
| 6 | ERCP-specific complication rate of EDGE and how it compares to standard ERCP complication rates in normal anatomy and in laparoscopy assisted-ERCP |
ERCP, endoscopic retrograde cholangiopancreatography.
In conclusion, EDGE is a promising alternative approach to facilitate the performance of ERCP for patients with prior RYGB. This nascent procedure requires more rigorous study, with a particular emphasis on the systematic documentation of technical success and the management of minor and major adverse events, along with comparative effectiveness studies with alternate approaches. Akin to laparoscopic surgery, where all ports are closed at the completion of surgery, EDGE should use novel devices that facilitate single-session EDGE and tract closure. In the interim, the unbiased publication of “real-world” experience with EDGE will help providers decide when this approach should be offered in clinical practice.
Supplementary Material
Competing interests
B.J. Elmunzer is a consultant for Takeda Pharmaceuticals. E.M. Forster receives research support from BMS, Janssen, and Takeda, and is a consultant for AbbVie. G.A. Cote receives research support from Kangen Pharmaceuticals and Boston Scientific Corporation; he is a consultant for Boston Scientific Corporation, Olympus Corporation of the Americas, and Abbvie. The remaining authors declare that they have no conflict of interest.
Footnotes
Appendix 1s
Supplementary material is available under https://doi.org/10.1055/a-1376-2394
References
- [1].Moran RA, Ngamruengphong S, Sanaei O et al. EUS-directed transgastric access to the excluded stomach to facilitate pancreaticobiliary interventions in patients with Roux-en-Y gastric bypass anatomy. Endosc Ultrasound 2019; 8: 139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Forster E, Elmunzer BJ. Endoscopic retrograde cholangiopancreatography in patients with Roux-en-Y gastric bypass. Am J Gastroenterol 2020; 115: 155–157 [DOI] [PubMed] [Google Scholar]
- [3].Wang TJ, Ryou M. Evolving techniques for endoscopic retrograde cholangiopancreatography in gastric bypass patients. Curr Opin Gastroenterol 2018; 34: 444–450 [DOI] [PubMed] [Google Scholar]
- [4].Ngamruengphong S, Nieto J, Kunda R et al. Endoscopic ultrasound-guided creation of a transgastric fistula for the management of hepatobiliary disease in patients with Roux-en-Y gastric bypass. Endoscopy 2017; 49: 549–552 [DOI] [PubMed] [Google Scholar]
- [5].Tyberg A, Nieto J, Salgado S et al. Endoscopic ultrasound (EUS)-directed transgastric endoscopic retrograde cholangiopancreatography or EUS: mid-term analysis of an emerging procedure. Clin Endosc 2017; 50: 185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269 [DOI] [PubMed] [Google Scholar]
- [7].Binmoeller KF, Shah J. A novel lumen-apposing stent for transluminal drainage of nonadherent extraintestinal fluid collections. Endoscopy 2011; 43: 337–342 [DOI] [PubMed] [Google Scholar]
- [8].Cotton PB, Eisen GM, Aabakken L et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010; 71: 446–454 [DOI] [PubMed] [Google Scholar]
- [9].Amateau SK, Lim CH, McDonald NM et al. EUS-guided endoscopic gastrointestinal anastomosis with lumen-apposing metal stent: feasibility, safety, and efficacy. Obes Surg 2018; 28: 1445–1451 [DOI] [PubMed] [Google Scholar]
- [10].Bukhari M, Kowalski T, Nieto J et al. An international, multicenter, comparative trial of EUS-guided gastrogastrostomy-assisted ERCP versus enteroscopy-assisted ERCP in patients with Roux-en-Y gastric bypass anatomy. Gastrointest Endosc 2018; 88: 486–494 [DOI] [PubMed] [Google Scholar]
- [11].James TW, Baron TH. Endoscopic ultrasound-directed transgastric ERCP (EDGE): a single-center US experience with follow-up data on fistula closure. Obes Surg 2019; 29: 451–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kedia P, Tarnasky PR, Nieto J et al. EUS-directed transgastric ERCP (EDGE) versus laparoscopy-assisted ERCP (LA-ERCP) for Roux-en-Y gastric bypass (RYGB) anatomy: a multicenter early comparative experience of clinical outcomes. J Clin Gastroenterol 2019; 53: 304–308 [DOI] [PubMed] [Google Scholar]
- [13].Wang TJ, Thompson CC, Ryou M. Gastric access temporary for endoscopy (GATE): a proposed algorithm for EUS-directed transgastric ERCP in gastric bypass patients. Surg Endosc 2019; 33: 2024–2033 [DOI] [PubMed] [Google Scholar]
- [14].de Benito SM, Carbajo AY, Sanchez-Ocana HR et al. Endoscopic ultrasound-directed transgastric ERCP in patients with Roux-en-Y gastric bypass using lumen-apposing metal stents or duodenal self-expandable metal stents. A European single-center experience. . Rev Esp Enferm Dig 2020; 112: 211–215 [DOI] [PubMed] [Google Scholar]
- [15].Kochhar GS, Mohy-Ud-Din N, Grover A et al. EUS-directed transgastric endoscopic retrograde cholangiopancreatography versus laparoscopic-assisted ERCP versus deep enteroscopy-assisted ERCP for patients with RYGB. Endosc Int Open 2020; 8: E877–E882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Krafft MR, Fang W, Nasr JY. Shortened-interval dual-session EDGE reduces the risk of LAMS dislodgement while facilitating timely ERCP. Dig Dis Sci doi: 10.1007/s10620-020-06551-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tyberg A, Kedia P, Tawadros A et al. EUS-directed transgastric endoscopic retrograde cholangiopancreatography (EDGE): the first learning curve. J Clin Gastroenterol 2020; 54: 569–572 [DOI] [PubMed] [Google Scholar]
- [18].Crismale JF, Riff BP, Schwartz M et al. Closure of an iatrogenic gastrogastric fistula created during EUS-directed transgastric ERCP. VideoGIE 2016; 1:61–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Duloy A, Hammad H, Shah RJ. An adverse event of EUS-directed transgastric ERCP: stent-in-stent technique to bridge the peritoneal gap. VideoGIE 2019; 4: 508–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kedia P, Sharaiha RZ, Kumta NA et al. Internal EUS-directed transgastric ERCP (EDGE): game over. Gastroenterology 2014; 147: 566–568 [DOI] [PubMed] [Google Scholar]
- [21].Ligresti D, Amata M, Granata A et al. Single session EUS-guided temporary gastro-gastrostomy and ERCP following gastric bypass. Obes Surg 2018; 28: 886–888 [DOI] [PubMed] [Google Scholar]
- [22].Mendoza LA. EUS-directed transgastric ERCP. VideoGIE 2018; 3: 175–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Simons-Linares CR, Chahal P. ERCP through gastrogastric fistula in a patient with Roux-en-Y gastric bypass anatomy. Obes Surg 2019; 29: 1370–1371 [DOI] [PubMed] [Google Scholar]
- [24].Vallabh H, Poushanchi B, Hsueh W et al. EUS-directed transgastric ERCP (EDGE) with use of a 20-mm × 10-mm lumen-apposing metal stent in a patient with Roux-en-Y gastric bypass. VideoGIE 2018; 3: 262–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xu MM, Carames C, Novikov A et al. One-step endoscopic ultrasound-directed gastro-gastrostomy ERCP for treatment of bile leak. Endoscopy 2017; 49: 715–716 [DOI] [PubMed] [Google Scholar]
- [26].Krafft MR, Hsueh W, James TW et al. The EDGI new take on EDGE: EUS-directed transgastric intervention (EDGI), other than ERCP, for Roux-en-Y gastric bypass anatomy: a multicenter study. Endosc Int Open 2019; 7: E1231–E1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Colton JB, Curran CC. Quality indicators, including complications, of ERCP in a community setting: a prospective study. Gastrointest Endosc 2009; 70: 457–467 [DOI] [PubMed] [Google Scholar]
- [28].Abbas AM, Strong AT, Diehl DL et al. Multicenter evaluation of the clinical utility of laparoscopy-assisted ERCP in patients with Roux-en-Y gastric bypass. Gastrointest Endosc 2018; 87: 1031–1039 [DOI] [PubMed] [Google Scholar]
- [29].Banerjee N, Parepally M, Byrne TK et al. Systematic review of transgastric ERCP in Roux-en-Y gastric bypass patients. Surg Obes Relat Dis 2017; 13: 1236–1242 [DOI] [PubMed] [Google Scholar]
- [30].Dhindsa BS, Dhaliwal A, Mohan BP et al. EDGE in Roux-en-Y gastric bypass: How does it compare to laparoscopy-assisted and balloon enteroscopy ERCP: a systematic review and meta-analysis. Endosc Int Open 2020; 8: E163–E171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Irani S, Yang J, Khashab MA. Mitigating lumen-apposing metal stent dislodgment and allowing safe, single-stage EUS-directed transgastric ERCP. VideoGIE 2018; 3: 322–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


