Practitioners have long recognized the involvement of inflammation in certain acute cardiovascular diseases such as endocarditis, myocarditis, and pericarditis. Attention to the participation of immune and inflammatory mechanisms in chronic cardiovascular diseases has generally lagged. Yet, these pathways contribute to a broad swath of clinically important cardiovascular conditions, both acute and chronic (Figure 1). Understanding of these complex mechanisms can aid specialists in cardiovascular research and practice immeasurably by providing new concepts and illuminating novel diagnostic and therapeutic strategies. The collection of essays presented in this focused issue of Cardiovascular Research aims to promote this goal.
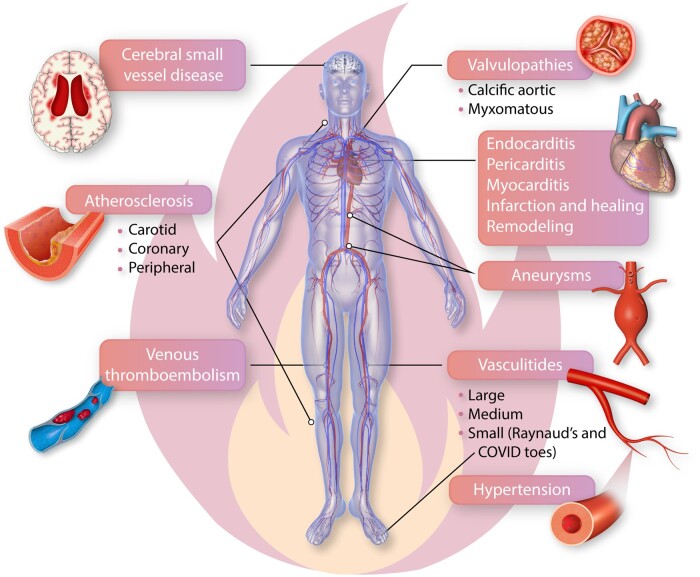
Figure 1.
Immune and inflammatory mechanisms mediate cardiovascular diseases from head to toe. Inflammation and immune mechanisms participate in many diseases that affect all aspects of the cardiovascular system from the heart to the vascular tree, including veins and arteries of all calibres. Disease of the small vessels in the intracerebral circulation can contribute to microinfarcts, cognitive decline and represent a major contributor to dementing illnesses. We have long recognized the participation of inflammation in acute cardiac diseases such as endocarditis, pericarditis, and acute myocarditis but information has recently massed regarding the role of immune and inflammatory pathways in myocardial infarction in various phases of healing, and as modifiers of the remodelling process after myocardial injury of ischaemic and non-ischaemic origin. Innate and adaptive immunity both participate in all phases of the pathogenesis of atherosclerosis, a major cause of myocardial disease, of peripheral arterial disease, and of ischaemic strokes. We now recognize that valve abnormalities such as calcific aortic stenosis and myxomatous disease of the mitral and tricuspid valves traditionally ascribed to ‘senile’ or degenerative changes increasingly involve fundamental contributions of immune and inflammatory mechanisms. Aneurysmal disease also involves inflammatory and immune mechanisms in many cases. Hypertension, depicted here by an arteriole with an expanded media-intima, involves T cells and innate immune mediators as well. Autoimmune disease of arteries, the vasculitides, target specific vascular territories, often related to the size and wall structure of affected vessels. Large vessel vasculitides manifest as wall-destructive disease of the aorta and the major aortic branches. Small vessel vasculitides lead to capillary obstruction, haemorrhage, and tissue ischaemia and can involve autoantibody-mediated pathology. Microvascular abnormalities contribute to Raynaud’s phenomenon common in the hands, and the recently reported syndrome of ‘COVID toes’. Immune and inflammatory mechanisms affect not only arteries in the microcirculation but also in the venous system. Venous thromboembolism involves dysregulation of inflammation, as recognized early on by Virchow. Pulmonary embolism is an all-too-common sequela of venous thrombosis. Thus, from head to toe, inflammation and immunity contribute to cardiovascular diseases encompassing the gamut from common to rarer condition. Understanding of immune and inflammatory mechanisms will stimulate new research and should enable the development of novel therapeutics.
Myocardium
The participation of immune and inflammatory mechanisms in acute myocarditis, infectious or otherwise, is well established. We have gained considerable understanding of the pathogenesis of acute viral myocarditis.1 Originally ascribed mainly to enteroviruses such as coxsackie B, parvovirus, and herpesviridae have emerged as prominent etiologic agents in viral myocardial diseases. More recently, human immune deficiency virus and SARS-Co-V2 have entered the lexicon of viral causes of myocarditis. Whether the cardiac injury commonly seen in COVID-19 will cause long-term abnormalities of cardiac contraction, rhythm, or autonomic control remains an area of concern that requires further observation and investigation. As an emerging and unifying concept, chronic cardiac remodelling and fibrosis, common to many myocardial diseases, involve inflammatory mechanisms. For example, these processes commonly involve transforming growth factor beta and proteinases under the control of inflammatory mediators.
Following ischaemic injury, both innate and adaptive immunity critically regulate the clearance of injured cells and repair responses. The recent burst in biologic understanding of myocardial healing may lead to novel therapies to modulate adverse remodelling and overzealous fibrosis during the healing of infarcted tissue.2 Such interventions might forestall some of the ravages of ischaemic cardiomyopathy, a major challenge to function and quality of life following myocardial infarction.
More chronic infectious diseases of the myocardium such as Chagasic cardiomyopathy and rheumatic involvement no doubt involve inflammation and malfunctioning immune responses. Sarcoidosis, characterized by the formation of tissue-destructive non-caseating granulomata, clearly depends on inflammatory pathways, but the drivers and specific therapies remain elusive.
Pericardium
Elevation of biomarkers of inflammation commonly characterize bouts of acute pericarditis. Physicians have used non-steroidal anti-inflammatory drugs to treat acute pericarditis for generations. Glucocorticoid treatment, although often effective in quelling the inflammatory aspects of acute pericarditis, has often proven difficult to taper, highlighting the need for more acceptable therapies to avoid recrudescent disease. More recently, the non-specific anti-inflammatory agent colchicine has shown promise for managing pericarditis, both acute and recurrent. The success of anti-cytokine therapy has underscored the involvement of innate immunity in pericarditis, particularly in refractory recurrent episodes.
Cardiac valves
Valvular heart disease also critically involves innate and adaptive immunity.3 Inflammatory mechanisms clearly wreak valvular damage in acute infective endocarditis. The involvement of inflammation and immunity in rheumatic heart disease has long been recognized. Valvular and cardiac damage in rheumatic fever may result from antigenic mimicry between Streptococcal and cardiac antigens. Recognition is now increasing regarding the participation of inflammation in sterile valvulopathies as well. Certain other valvular diseases, classically ascribed to ‘degeneration’, have traditionally ignored the operation of immunity and inflammation in their pathogenesis. Yet, we now recognize that disorders of valves ranging from myxomatous degeneration to calcific disease of the aortic valve (and other cardiac structures) likely depend on immune and inflammatory mechanisms.
Atherosclerosis
For over a century, most regarded atherosclerosis as a bland lipid storage disease. Initial cell biologic views of the pathogenesis of atherosclerosis focused on smooth muscle proliferation and platelet products, without invoking the involvement of inflammatory processes. The concepts of atherogenesis and the complications that lead to clinical catastrophes such as the acute coronary syndromes and stroke have broadened remarkably in recent decades to encompass the fundamental contributions of the immune system and of maladaptive inflammatory pathways.4–6 Indeed, inflammation participates in all phases of the life cycle of the atherosclerotic plaque from inception up to the acute thrombotic complications, which have become a leading cause of morbidity and mortality worldwide. Both innate immune pathways and adaptive immune effectors including multiple subtypes of T and B lymphocytes, and a growing array of mononuclear phagocyte subtypes participate in atherosclerosis and its complications.
The specialized case of allograft vasculopathy, a common contributor to the loss of transplanted organs, can occur in the absence of traditional risk factors, illustrating a predominantly immunologically driven form of arteriosclerosis.7 We stand at the threshold of an era in which we can deploy increasingly selective anti-inflammatory interventions to forestall some of the complications of atherosclerotic disease. Indeed, the statin class of drugs, whose use has revolutionized preventive cardiology, relies in part on anti-inflammatory actions independent of lipid lowering. The construct of viewing atherosclerosis as a disease of impaired resolution of inflammation has heightened our appreciation of the specific pro-resolving mediators that may mitigate inflammation with less impairment of host defences than direct anti-inflammatory treatments.8
Arteries of all sizes—from head to toe
Inflammatory processes also contribute to diseases of arteries of all sizes. For example, small vessel disease in the cerebral circulation may contribute to dementing illnesses.9 Arterioles regulate systemic hypertension, a condition increasingly linked to inflammation and immunity.10 Large vessel atherosclerotic disease of the extracranial arteries that perfuse the brain contributes importantly to ischaemic strokes, a major challenge to quality of life and a strain on the health care system. Aneurysmal disease affecting all levels of the arterial tree involves inflammatory pathways as well. The vasculitides, quintessential autoimmune diseases, can affect large vessels, medium size arteries, smaller arterioles, or microvessels. Disease mechanisms in large vessel vasculitides, e.g. giant cell arteritis and Takayasu arteritis, differ fundamentally from those affecting small vessels, e.g. vasculitic glomerulonephritis. Large vessel vasculitis links tightly to granulomatous inflammation, predominantly involving T cells and macrophages. Autoantibodies and immune complex pathology contribute to small vessel vasculitides. The mechanisms of loss of self-tolerance and of autoimmunity directed against blood vessels remain incompletely defined.
Conclusion
In sum, our appreciation of and knowledge regarding immune and inflammatory participation in cardiovascular diseases has burgeoned. Indeed, most cardiovascular conditions involve inflammatory responses dependent on cells, mediators, and pathways of the immune system, turning protective mechanisms against the host. Emerging concepts are linking the age dependency of cardiovascular disease to altered immune responses, captured in the term ‘inflammaging’. This increasing recognition has opened new vistas in the fundamental mechanisms of disease, which hold great interest for the research community. Moreover, the application of our expanded understanding of immunity and inflammation in the cardiovascular system promises to yield novel therapies of ever-increasing precision. The collection of contributions in this focused issue of cardiovascular research should thus aid both the research and clinical communities reap the fruits of these advances.
Conflict of interest: P.L. is unpaid as a consultant to, or a participant for clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Carista, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Norvo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron. P.L. is a member of the scientific advisory board for Amgen, Caristo, Cartesian, CSL Behring, DalCor Pharmaceuticals, Dewpoint, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, PlaqueTec, and XBiotech, Inc. P.L.’s laboratory has received research funding in the last 2 years from Novartis. P.L. is on the Board of Directors of XBiotech, Inc. P.L. has a financial interest in XBiotech, a company developing therapeutic human antibodies. P.L’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. All other authors declared no conflict of interest.
Funding
P.L. receives funding support from the National Heart, Lung, and Blood Institute (1R01HL134892), the American Heart Association (18CSA34080399), the RRM Charitable Fund, and the Simard Fund. Z.M. receives funding from the British Heart Foundation (CH/10/001/27642, RG/15/11/31593).
Data availability
No new data were generated or analysed in support of this research.
Contributor Information
Peter Libby, Department of Medicine, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, 77 Avenue Louis Pasteur, Boston, MA 02115, USA.
Ziad Mallat, Division of Cardiovascular Medicine, Department of Medicine, University of Cambridge, Box 157 Hills Rd Cambridge CB2 0QQ, UK.
Cornelia Weyand, Department of Medicine, Stanford University School of Medicine, 300 Pasteur Dr, Palo Alto, CA 94304, USA; Department of Medicine, Mayo Clinic College of Medicine and Science, 200 1st Street SW, Rochester, MN 55905, USA.
The opinions expressed in this article are not necessarily those of the Editors of Cardiovascular Research or of the European Society of Cardiology.
References
- 1. Schultheiss HP, Baumeier C, Pietsch H, Bock T, Poller W, Escher F. Cardiovascular consequences of viral infections: from COVID to other viral diseases. Cardiovasc Res 2021;117:2610–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Porsch F, Mallat Z, Binder CJ. Humoral immunity in atherosclerosis and myocardial infarction: from B cells to antibodies. Cardiovasc Res 2021;117:2544–2562. [DOI] [PubMed] [Google Scholar]
- 3. Bartoli-Leonard F, Zimmer J, Aikawa E. Innate and adaptive immunity: the understudied driving force of heart valve disease. Cardiovasc Res 2021;117:2506–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vallejo J, Cochain C, Zernecke A, Ley K. Heterogeneity of immune cells in human atherosclerosis revealed by scRNA-Seq. Cardiovasc Res 2021;117:2537–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Libby P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res 2021;117:2525–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hansson GK. The heart of immunology—immune mechanisms in cardiovascular medicine. Cardiovasc Res 2021;117:e166–e168. [DOI] [PubMed] [Google Scholar]
- 7. Pober JS, Chih S, Kobashigawa J, Madsen JC, Tellides G. Cardiac allograft vasculopathy: current review and future research directions. Cardiovasc Res 2021;117:2624–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fredman G. Atherosclerosis is a major human killer and non-resolving inflammation is a prime suspect. Cardiovasc Res 2021;117:2563–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans LE, Taylor JL, Smith CJ, Pritchard HAT, Greenstein AS, Allan SM. Cardiovascular comorbidities, inflammation, and cerebral small vessel disease. Cardiovasc Res 2021;117:2575–2588. [DOI] [PubMed] [Google Scholar]
- 10.Murray EC, Nosalski R, MacRitchie N, Tomaszewski M, Maffia P, Harrison DG, Guzik TJ. Therapeutic targeting of inflammation in hypertension: from novel mechanisms to translational perspective. Cardiovasc Res 2021;117:2589–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.