Introduction
The number of deaths owing to coronavirus disease 2019 (COVID-19) is escalating. Apart from the known clinical presentation of respiratory failure precipitated by COVID-19, the cardiovascular complications induced by this viral infection have raised essential concerns. A retrospective study reported that the cardiovascular system was the most sensitive tissue after the respiratory tract in COVID-19 (Sandoval et al., 2020). As in other viral infections, it is well-known that cardiovascular complications can increase the severity of COVID-19, leading to the aggravation of clinical conditions (Clerkin et al., 2020). The National Health Commission of China informed that almost 12% of patients had a myocardial injury in hospitalization (Zheng et al., 2020). A retrospective study of 138 hospitalized COVID-19 patients reported 10% acute cardiac injury and 16% arrhythmia (Wang et al., 2020). In addition, coagulopathy is another life-threatening problem causing arterial and venous thromboembolism in COVID-19 patients (Bikdeli et al., 2020).
It is well established that aged patients with COVID-19 are more likely to progress to severe disease (Liu et al., 2020, Li et al., 2020, Sun, 2020). A large study conducted with 4021 COVID-19 participants showed that patients over 60 years old had a significantly higher mortality rate than patients under 60 years (Yang et al., 2020). Another study stated robust relationships between older age and acute respiratory distress syndrome and death (Wu et al., 2020). The studies mentioned above have been performed during hospitalization, and data on post-discharge results are limited. Moreover, there is no data about long-term cardiovascular outcomes in very elderly (≥80 years) COVID-19 patients. Therefore, the primary goal of this research was to describe the long-term (>6 months) cardiovascular outcomes in very elderly COVID-19.
Materials and Methods
Study design and participants
The researchers screened a total of 565 hospitalized patients aged ≥80 years with confirmed COVID-19 between January 1, 2020, and February 1, 2021, from the hospital medical record. COVID-19 confirmation was assessed by the reverse transcription-polymerase chain reaction of nasopharyngeal swab samples, thoracic computed tomography, antibody testing, and symptoms according to the WHO recommendations (Organization, 2020). The exclusion criteria were: immobility, the existence of renal failure (glomerular filtration rate <60 mL/min), history of pulmonary thromboembolism (PTE) or deep vein thrombosis (DVT), and cognitive impairment, <6 months since discharge, and <2 days hospital length of stay. After the exclusion, 212 patients were found eligible for the study. Then, we called the subjects for evaluation in a multidisciplinary outpatient clinic. Consequently, 124 participants were included in the study (Fig. 1 ). According to the hospitalization status, the patients were divided into two groups: intensive care unit (ICU) (n = 48, 39%) and ward (n = 76, 61%). The local ethics committee approved this study, and all participants or their health care proxies provided written informed consent.
Fig. 1.
Study sample. One hundred twenty-four very elderly (≥ 80 years) COVID-19 patients were invited to the outpatient clinic six months after discharge. It was classified into two as those hospitalized in ICU and those in the ward. COVID-19, coronavirus disease 2019; GFR, glomerular filtration rate; DVT, deep vein thrombosis; PE, pulmonary embolism; CUS, compression ultrasonography; CTA, computed tomography pulmonary angiogram; ICU, intensive care unit.
Data collection
Data during hospitalization and until arrival at the outpatient clinic were obtained from the hospital's electronic medical record. Patient data collected included demographics, comorbidities, complications, length of hospitalization, medications, laboratory findings, and completion of hospitalization (death or discharge). The first value was used for laboratory data related to inflammation and thrombosis. Patients were invited to the outpatient clinic for the sixth month of data. The researchers questioned symptoms (dyspnea, chest pain, palpitations, dizziness), medications, and newly occurring disease after discharge. Physical examination, including blood pressure, oxygen saturation, heart rate, were performed. Coagulation and inflammation-based laboratory parameters (D-dimer, fibrinogen, international normalized ratio [INR], platelet count, C-reactive protein [CRP], erythrocyte sedimentation rate, ferritin), and high sensitive troponin I were evaluated. Electrocardiography (ECG), transthoracic echocardiography, lower extremity venous compression ultrasonography (CUS), and computed tomography pulmonary angiogram (CTA) were performed on all participants.
Outcomes
The primary outcome was the incidence of cardiovascular complications (arrhythmias, ischemic heart disease, myocarditis, PTE, and DVT) upon systematic screening six months after discharge. The secondary outcome comprised the association of coagulation and inflammation-based laboratory parameters during the patients' hospital stay with the cardiovascular complications. According to European Society of Cardiology guidelines, myocardial infarction was diagnosed utilizing clinical criteria, troponin elevation, electrocardiographic changes, or angiographic images (Thygesen et al., 2019). Myocarditis was defined as compatible clinic and symptoms, and at least one of the following: (I) elevated cardiac biomarkers; (II) ECG alterations suggestive of cardiac damage; or (III) cardiac dysfunction on echocardiogram or cardiac magnetic resonance. PTE and DVT were validated radiographically. The researchers defined arrhythmias as atrial fibrillation (AF), supraventricular or ventricular tachyarrhythmias, and conduction disorders.
Statistical Analysis
Statistical analyses were accomplished using the SPSS version 22.0 software package (IBM SPSS, USA), and figures were created employing MedCalc version 15.8 statistical software (Ostend Belgium). Kolmogorov-Smirnov test assessed the distribution of variables. Continuous variables were given as mean±standard deviation or median (interquartile range, IQR), and categorical variables were expressed as percentages (%). Comparisons of the variables across the groups were performed using a Student t-test or Mann-Whitney U test and Chi-square (χ2) test for categorical variables. Also, we did the Cox proportional hazard regression model to determine independent risk factors for 6-month cardiovascular outcomes. The Spearman correlation analysis was utilized to determine the correlations of relevant biomarkers. The statistical significance threshold was adjusted as p <0.05.
Results
Of the 565 screened very elderly COVID-19 patients, 124 subjects were eligible for analysis, and this group constituted the study population. Table 1 depicts the patients' characteristics. The entire group's mean age was 84.2±3.4, and the majority were female (n = 65, 52.4%). The median hospitalization length of all patients was 12 (IQR, 5-22) days. ICU patients stayed longer than ward patients in the hospital (16 days [IQR, 7-22] vs. 7 days [IQR, 5-13], P = 0.005, respectively). The median time after discharge was 189 days (IQR, 178-201). There was no significant difference between the treatments applied to the two groups during their hospitalization (Table 1). ICU patients' left ventricular ejection fraction was significantly lower than ward patients (54.0±8.9 vs. 57.0±6.1, P = 0.03, respectively). The mean pulmonary artery pressure was significantly higher in the ICU group than the ward group (35.0±12.8 vs. 27.7±14.0, P = 0.005, respectively) (Table 1).
Table 1.
Characteristic of the participants.
| Total n = 124 | ICU n(%)=48 (39) | Ward n(%)=76 (61) | P-value* | |
| Age, year | 84.2 ± 3.4 | 84.4 ± 3.5 | 84.2 ± 3.8 | 0.85 |
| Female, n (%) | 65 (52.4) | 25 (52) | 40 (53) | 0.95 |
| BMI, kg/m² | 25.3 ± 2.6 | 25.7 ± 2.9 | 25.3 ± 2.6 | 0.54 |
| History, n (%) | ||||
| CAD | 29 (23) | 14 (29) | 15 (20) | 0.22 |
| Hypertension | 83 (67) | 34 (71) | 49 (65) | 0.46 |
| Diabetes Mellitus | 18 (14) | 11 (23) | 7 (9) | 0.03 |
| LVEF,% | 55.9 ± 7.1 | 54.0 ± 8.9 | 57.0 ± 6.1 | 0.03 |
| PAP, mmHg | 30.7 ± 14.2 | 35.0 ± 12.8 | 27.7 ± 14.0 | 0.005 |
| Treatments, n (%) | ||||
| HQ | 96 (77) | 38 (79) | 58 (76) | 0.67 |
| Oseltamivir | 111 (89) | 42 (88) | 69 (90) | 0.49 |
| Azithromycin | 44 (35) | 18 (37) | 26 (34) | 0.18 |
| Clarithromycin | 31 (25) | 11 (23) | 20 (26) | 0.35 |
| Moxifloxacin | 53 (42) | 23 (47) | 30 (40) | 0.06 |
| Ceftriaxone | 78 (63) | 32 (66) | 46 (60) | 0.09 |
| Laboratory data | ||||
| WBC-1, 10^3/µL | 7.5 ± 3.9 | 8.7 ± 4.8 | 6.7 ± 2.8 | 0.002 |
| WBC-2, 10^3/µL | 6.2 ± 1.8 | 6.5 ± 2.1 | 6.1 ± 1.5 | 0.24 |
| Ferritin-1, µg/L | 214 (121–484) | 379 (147–998) | 184 (113–328) | 0.003 |
| Ferritin-2, µg/L | 72 (38–118) | 76 (43–174) | 68 (38–108) | 0.13 |
| D-dimer-1, ng/mL | 384 (165–849) | 521 (253–1843) | 267 (165–628) | 0.009 |
| D-dimer-2, ng/mL | 224 (99–340) | 298 (211–513) | 160 (79–290) | <0.001 |
| Troponin-1, pg/mL | 9 (4–17) | 15 (6–34) | 6 (4–13) | <0.001 |
| Troponin-2, pg/mL | 4 (2–7) | 5 (2–8) | 3 (2–6) | 0.01 |
| CRP-1, mg/dL | 46 (8–79) | 61 (30–146) | 34 (7–69) | 0.001 |
| CRP-2, mg/dL | 3 (2–6) | 5 (2–7) | 3 (2–4) | 0.01 |
| ESR-1, mm/h | 13 (8–22) | 15 (8–28) | 10 (6–15) | 0.008 |
| ESR-2, mm/h | 5 (3–8) | 7 (4–9) | 5 (3–7) | 0.004 |
| Fibrinogen-1, mg/dL | 305 (253–360) | 301 (245–365) | 317 (258–357) | 0.87 |
| Fibrinogen-2, mg/dL | 265 (193–310) | 264 (194–312) | 267 (192–310) | 0.91 |
ICU, intensive care unit; BMI, body mass index: CAD, coronary artery disease; LVEF, left ventricle ejection fraction; PAP, pulmonary artery pressure; WBC, wight blood cell; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HQ, hydroxychloroquine. The first values in the laboratory data show the results during hospitalization and the second values at the sixth month. * The P-value compares the ICU and ward patients. Values are presented as the mean ± SD, median (IQR), or n (%).
Evaluation of inflammation and thrombosis markers
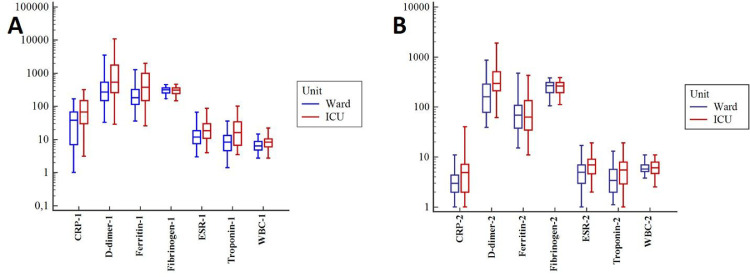
Inflammatory markers during hospitalization, including wight blood cells (WBC), ferritin, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), were significantly higher in ICU patients than the ward patients; however, only CRP and ESR were significantly higher in the ICU group at the sixth-month measurement (Fig. 2 ). The ICU group had significantly higher D-dimer levels during the hospital stay and at the sixth month than the ward group. (P = 0.009 and P < 0.001, respectively) (Table 1). Likewise, troponin levels were significantly higher in the ICU patients than the ward patients at the hospitalization (P < 0.001) (Fig. 2A) and the sixth-month assessment (P = 0.01) (Fig. 2B). However, there was no difference in fibrinogen concentrations between the two groups at hospitalization or the 6th-month follow-up.
Fig. 2.
Comparison of parameters related to inflammation and coagulation measured during hospitalization of the groups (Panel A) and the identical parameters checked in the outpatient clinic six months after discharge (Panel B). CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, wight blood cell.
Outcomes
Among 124 very elderly COVID-19 patients, 32 had cardiovascular complications (26%) (Table 2 ). New-onset AF was the most common cardiovascular abnormality in the entire group (n = 11, 9%). The clinically and radiographically confirmed PTE and DVT rates were 9 (7%) and 5 (5%). Also, ten patients (8%) had myocardial ischemia, and three (2%) had myocarditis. The majority of these patients were followed up in the intensive care unit (ICU) (n = 24, P < 0.001) (Table 2).
Table 2.
Distribution of cardiovascular outcomes according to subjects' hospital stay unit.
| Total n = 124 | ICU n(%)=48(39) | Ward n(%)=76 (61) | |
| Outcomes, n (%) | 32 (26) | 24 (50) | 8 (10) |
| Myocardial ischemia | 10 (8) | 7 (15) | 3 (4) |
| Myocarditis | 3 (2) | 3 (6) | 0 (0) |
| DVT | 5 (5) | 4 (8) | 1 (1) |
| PTE | 9 (7) | 7 (15) | 2 (3) |
| AF | 11 (9) | 9 (19) | 2 (2) |
ICU, intensive care unit; DVT, Deep vein thrombosis; PTE, Pulmonary thromboembolism; AF, Atrial fibrillation.
Atrial Fibrillation
We identified eight of the eleven AF patients at the outpatient clinic control. AF in the other three patients had been noticed earlier due to the development of cerebrovascular events. Two of these occurred in the first month of discharge, and one occurred in the third month. Cerebrovascular events were seen in one patient receiving antiplatelet (transient ischemic attack) and two patients (transient ischemic attack:1 and stroke:1) not receiving blood thinners. At the time of outpatient clinic assessment, of the 11 persistent AF patients, two were using anticoagulants (warfarin:1 and rivaroxaban:1), five were using antiplatelets (acetylsalicylic acid), and four were not on blood thinners. Nine of eleven AF cases were ICU patients. Non-vitamin K antagonist oral anticoagulants (NOAC) were given to all AF patients.
Pulmonary Thromboembolism
We detected six of the nine PTE patients by CTA at the outpatient clinic. Segmental PTE had already developed in one of these six patients during hospitalization, and he was using an anticoagulant (warfarin) at admission to the outpatient clinic. The other five patients had no previous PTE and were not using antiplatelet or anticoagulants. PTE transpired in two of the remaining three patients one month after discharge and one patient four months after discharge. All but 1 of the patients had subsegmental PTE. Seven of the nine PTE patients were followed up in the ICU.
Deep Vein Thrombosis
Events documented by CUS at the outpatient clinic included two patients with proximal lower extremity DVT and three with distal lower extremity DVT. Four of the five DVT cases were ICU patients. Two of the patients were receiving acetylsalicylic acid; however, the other three were not using blood thinners. Three patients with DVT also had PTE. NOACs were prescribed to all PTE and DVT patients.
Myocardial Ischemia
Clinically, cardiac biomarkers, electrocardiographically and angiographically confirmed events included four patients with non-ST elevation myocardial infarction (MI), four patients with unstable angina, and two with ST-elevation MI. Six of the ten patients were not on blood thinners during the events. Seven of ten patients were hospitalized at the ICU. One unstable angina and one non-ST elevation MI patient' was using acetylsalicylic acid, one ST-elevation MI patient' was using clopidogrel, and one unstable angina patient' was using edoxaban at the time of events. Coronary stenting was performed on seven patients, while medical follow-up was decided on three patients. Two myocardial ischemia patients' also had AF.
Myocarditis
All myocarditis cases were observed in ICU patients within one month after discharge. Pericarditis and pericardial effusion were also present in two cases, and one resulted in atrial fibrillation. The other two were recovered without complications.
Predictors of cardiovascular outcomes
At univariable Cox analysis, WBC, D-dimer, troponin, CRP, and ICU hospitalization were associated with long-term cardiovascular complications (Table 3 ). After adjusting significant variables in the univariable analysis and relevant clinical variables, elevated D-dimer levels (hazard ratio [HR] = 1.13, 95% confidence interval [CI] 1.10-1.22; P = 0.003), and ICU follow-up (HR = 4.70, 95% CI 1.59-13.86; P = 0.001) were independently associated with cardiovascular outcomes (Table 3).
Table 3.
Cox regression analyses for cardiovascular outcomes.
| Univariable (unadjusted) HR (95% CI) P-value | Multivariable (adjusted) HR (95% CI) P-value | |
| Age | 1.05 (0.96–1.14) 0.26 | 1.08 (0.97–1.19) 0.13 |
| Gender | 1.21 (0.60–2.43) 0.59 | 0.86 (0.40–1.83) 0.69 |
| BMI | 1.01 (0.89–1.14) 0.85 | |
| Hypertension | 0.84 (0.38–1.82) 0.66 | |
| Diabetes Mellitus | 0.46 (0.19–1.08) 0.07 | |
| Ferritin | 1.00 (0.99–1.00) 0.09 | |
| WBC | 1.07 (1.02–1.14) 0.03 | 1.01 (0.94–1.09) 0.64 |
| Fibrinogen | 1.00 (0.99–1.00) 0.23 | |
| D-dimer | 1.20 (1.16–1.28) <0.001 | 1.13 (1.10–1.22) 0.003 |
| Troponin | 1.08 (1.04–1.11) <0.001 | 1.01 (0.99–1.02) 0.18 |
| CRP | 1.01 (1.00–1.02) 0.001 | 1.01 (0.99–1.04) 0.22 |
| ESR | 1.01 (0.99–1.03) 0.34 | |
| ICU | 1.74 (1.33–2.89) <0.001 | 4.70 (1.59–13.86) 0.001 |
| HQ | 0.98 (0.96–1.02) 0.61 | |
| Oseltamivir | 1.24 (0.35–2.78) 0.54 | |
| Azithromycin | 1.00 (0.99–1.01) 0.22 | |
| Clarithromycin | 0.89 (0.58–1.34) 0.40 | |
| Moxifloxacin | 0.33 (0.10–1.19) 0.15 | |
| Ceftriaxone | 0.55 (0.26–1.49) 0.38 | |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; WBC, wight blood cell; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ICU, intensive care unit; HQ, hydroxychloroquine. Biomarkers refer to values during hospitalization.
Correlations of coagulation and inflammatory parameters
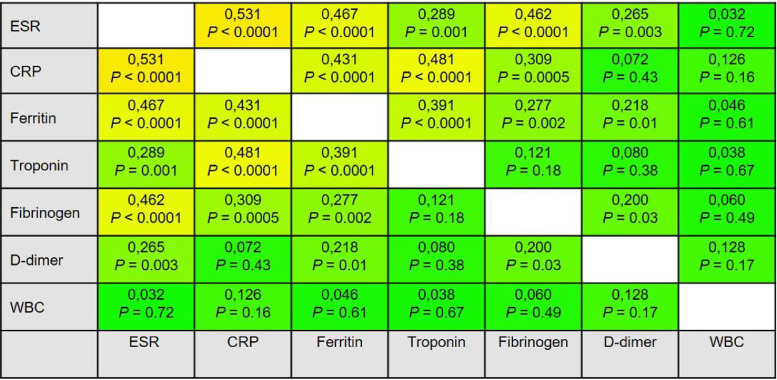
Fig. 3 illustrates the correlation matrix for the coagulation and inflammatory parameters. Troponin had a mild to moderate significant correlation between ESR (r = +0.289, P = 0.001), CRP (r = +0.481, P < 0.0001), and Ferritin (r = +0.391, P < 0.0001). D-dimer was weakly correlated with ESR (r = +0.265, P = 0.003), Ferritin (r = +0.218, P = 0.01), and fibrinogen (r = +0.200, P = 0.03). There was no significant correlation between troponin an D-dimer (r = +0.080, P = 0.38). While there was a significant moderately correlation between CRP and troponin (r = +0.481, P < 0.0001), no significant correlation was found between CRP and D-dimer (r = +0.072, P = 0.43). Another notable finding was that WBC had no significant correlation with any of the relevant parameters.
Fig. 3.
The correlation matrix displays the power of correlation between the coagulation and inflammatory parameters. The correlation coefficients and P-values are given in the cells. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, wight blood cell.
Discussion
In the present study, to our knowledge, we report the first study in the literature describing the long-term (>6 months) cardiovascular complications of very elderly (≥80 years) COVID-19 patients. The researchers observed cardiovascular outcomes in 26% (n = 32) of the participants, and the majority were ICU patients. Also, we found that ICU follow-up and elevated D-dimer levels during hospitalization were significantly predictive for long-term cardiovascular outcomes.
Although numerous studies have reported in-hospital cardiovascular complications of elderly COVID-19 patients, there is no adequate data regarding post-discharge abnormalities (Kunutsor and Laukkanen, 2020, Wang et al., 2020, Chen et al., 2020). Wang et al. published that in-hospital cardiovascular complications were acute cardiac injury (21%), arrhythmia (10.4%), and heart failure (17.4%) (Wang et al., 2020). A previous review reported that the most frequent cardiovascular complications of acute COVID-19 infection were heart failure, myocardial injury, and cardiac arrhythmias (Kunutsor and Laukkanen, 2020). Moreover, Chen and colleagues analyzed 274 elderly COVID-19 patients retrospectively and informed that acute cardiac injury and heart failure were the most common cardiac complications during aggravation of COVID-19 (Chen et al., 2020). In this study, the most prevalent cardiovascular complications were AF, myocardial ischemia, and PTE, patients recovered from COVID-19. It is, of course, these complications were expected at these ages. However, this rate was notably higher in the group that survived COVID-19 more severely. Another point to be noted was that a significant portion of these complications occurred in the first month after discharge.
Most of the patients who detected pulmonary embolisms were subclinical in the present study. Some of these patients had dyspnea and pleuritic chest pain, but their hemodynamics were stable. Within this framework, in addition to high-resolution tomography, contrast-enhanced tomography may also be helpful in the evaluation of these patients. Pertinently, the British Thoracic Society guidelines advise imaging with high-resolution CT and CTA at 12 weeks for patients diagnosed with COVID-19 pneumonia (Overview 2021). Likewise, DVT was detected incidentally in each patient. However, it is unclear whether the patients had had thrombosis before, as there were no apparent symptoms. Nevertheless, it is noteworthy that DVT was significantly higher in those hospitalized in the ICU. Therefore, routine CUS may be considered in those who severely survive the disease.
There have been several reports regarding cardiac arrhythmias, including conduction disturbances, atrial, and malignant ventricular arrhythmias during the acute inflammatory phase of COVID-19 (Wu et al., 2020, Guo et al., 2020, Capecchi et al., 2019). Many causes, such as myocardial inflammation, fever, severe hypoxemia, electrolyte disturbances, drugs may be responsible for the variety of arrhythmias. We did not notice any notable rhythm disorder other than AF. The authors considered that other types of arrhythmias were resulted in death or occurred intermittently. Also, we, interestingly, did not observe echocardiographic findings precipitating AF such as rheumatic valve disease and left atrial enlargement except for two of these patients. Additionally, since 8 of 11 AF patients were found incidentally in the outpatient clinic evaluation, it reveals the necessity of close cardiac monitoring after discharge.
Modin and colleagues informed that the incidence of myocardial infarction was five times higher during the 14 days after COVID-19 diagnosis than the control group (Modin et al., 2020). Additionally, a retrospective study documented that the rate of in-hospital myocardial ischemia was 9% among 3334 elderly COVID-19 patients. Similarly, the incidence of myocardial ischemia in this study was 8%, and the majority were patients followed up in the ICU. Moreover, Myhre et al. reported that they found cardiac pathology in 21% of the patients with COVID-19 in the cardiac magnetic resonance in the sixth month. However, they did not find a significant relationship between biochemical parameters or illness severity with cardiac pathology in MR (Myhre et al., 2021). The low number of intensive care patients, the low average age of the participants, and the less comorbid conditions might have caused the differences in the results.
In the present study, another clinical form of myocardial damage was myocarditis. Huang and colleagues stated that 5 of 41 (12%) COVID-19 patients had a myocardial injury during hospitalization, and most of them were critically ill (Huang et al., 2020). Additionally, Hua et al. published a myocarditis case complicated with pericarditis, pericardial effusion, and consequent cardiac tamponade during hospitalization in a COVID-19 patient (Hua et al., 2020). We detected pericarditis and pericardial effusion in two of the three cases of myocarditis occurring in the first month of discharge, and one of them progressed to atrial fibrillation. We observed a lower rate and less complicated myocarditis cases than the acute period studies. However, the evolution of pericarditis and pericardial effusion in two of three myocarditis cases and the progression of one of them to atrial fibrillation reveals the need for long-term follow-up.
Recent studies have reported the association between troponin levels and ICU admission, in-hospital mortality, and severity of COVID-19 inflammation (Guo et al., 2020, Huang et al., 2020, Shi et al., 2020). However, Trecarichi et al. did not detect a significant relationship between troponin levels and in-hospital mortality in very elderly COVID-19 patients (Trecarichi et al., 2020). Likewise, Ucciferri and colleagues did not find a significant difference in troponin levels between COVID-19 and Non-COVID-19 patients at admission to the emergency department (Ucciferri et al., 2021). We observed a significant relation between troponin and long-term cardiovascular outcomes; however, we did not find an independent association in the multivariable analysis. Conversely, elevated D-dimer levels supplied the most evident link to long-term cardiovascular outcomes in this study. Furthermore, D-dimer was the only biomarker independently associated with long-term cardiovascular complications in our study. In alignment with those above, a previous study reported that D-dimer could predict the clinical progression in COVID-19 patients (Biamonte et al., 2021). Also, numerous studies have demonstrated the association between raised D-dimer levels and in-hospital morbidity and mortality (Wang et al., 2020, Guo et al., 2020, Zhou et al., 2020). Therefore, patients with high D-dimer levels should be paid attention both during hospitalization and for long-term complications. In this context, closer follow-up and prescription of anticoagulants or antiplatelets may be considered for these patients.
Another independent predictor of cardiovascular outcomes in our study was ICU hospitalization. It was not surprising that long-term cardiovascular complications were more frequent in patients with severe COVID-19. However, CRP, WBC, ESR, ferritin, and troponin levels, which were significantly higher in ICU patients, were not detected as independent predictors, such as D-dimer, suggested to the authors that coagulative changes might play a role in their long-term complications. Importantly, troponin during hospitalization was moderately and significantly correlated with CRP, ESR, and ferritin; however, it was not correlated with D-dimer and fibrinogen. These findings made us think that troponin increased due to inflammatory alterations rather than coagulative. Pertinently, Ucciferri et al. revealed that drugs playing a role in the inflammatory process were increasingly prominent in treating COVID-19 (Ucciferri et al., 2020).
This study has several limitations: (i) Since the design of our study was to evaluate the alive patients, data on those who died in this process were not presented. Hence, cardiovascular complications were likely to be higher than detected. (ii) Absence of a control group (non-COVID-19 very elderly hospitalized patients) due to pandemic. Therefore, we classified according to the severity of the disease. (iii) We did not evaluate heart failure due to the patients' lack of baseline echocardiographic assessments. (iv) Also, the small number of patients might have limited the results.
Taken together, this is the first prospective study presenting long-term cardiovascular outcomes in very elderly COVID-19 patients whose follow-up, management, and clinical evaluation are challenging. We demonstrate long-term cardiovascular complications in 26% of the patients and, high D-dimer levels and being followed in the ICU were independently predictive of these complications. These findings designate the need for ongoing research of the long-term cardiovascular outcomes of COVID-19.
Declarations of interest
none
Declaration of Competing Interest
All authors state that; This paper is not under consideration elsewhere. Any part of this paper has not been previously published. All authors have read and approved the manuscript. There is no potential for conflict of interest.
References
- Biamonte F., Botta C., Mazzitelli M., Rotundo S., Trecarichi E.M., Foti D., et al. Combined lymphocyte/monocyte count, D-dimer and iron status predict COVID-19 course and outcome in a long-term care facility. Journal of Translational Medicine. 2021;19(1):79. doi: 10.1186/s12967-021-02744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardio. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi P.L., Laghi-Pasini F., El-Sherif N., Qu Y., Boutjdir M., Lazzerini P.E. Autoimmune and inflammatory K(+) channelopathies in cardiac arrhythmias: Clinical evidence and molecular mechanisms. Hear Rhyth. 2019;16(8):1273–1280. doi: 10.1016/j.hrthm.2019.02.017. [DOI] [PubMed] [Google Scholar]
- Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BM. 2020;368:M1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., et al. COVID-19 and Cardiovascular Disease. Circulation. 2020;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardio. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua A., O'Gallagher K., Sado D., Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart. 2020;41(22):2130. doi: 10.1093/eurheartj/ehaa253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet [Internet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. Available from: Https://www.sciencedirect.com/science/article/pii/S0140673620301835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunutsor S.K., Laukkanen J.A. Cardiovascular complications in COVID-19: A systematic review and meta-analysis. J. Infect. 2020;81(2):E139–E141. doi: 10.1016/j.jinf.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Liu Y., Jing X., Wang Y., Miao M., Tao L., et al. Mortality risk of COVID-19 in elderly males with comorbidities: A multi-country study. Aging (Albany NY. 2020;13(1):27–60. doi: 10.18632/aging.202456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infec. 2020;80(6):E14–E18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modin D., Claggett B., Sindet-Pedersen C., Lassen M.C.H., Skaarup K.G., Jensen J.U.S., et al. Acute COVID-19 and the Incidence of Ischemic Stroke and Acute Myocardial Infarction. Circulation. 2020;142(21):2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre P.L., Heck S.L., Skranes J.B., Prebensen C., Jonassen C.M., Berge T., et al. Cardiac pathology 6 months after hospitalization for COVID-19 and association with the acute disease severity. Am Heart. 2021;242:61–70. doi: 10.1016/j.ahj.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W.H. World Health Organization; 2020. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: Interim guidance, 2 March 2020. [Google Scholar]
- Overview, COVID-19 rapid guideline: Managing COVID-19 | Guidance | NICE [Internet]. [cited (2021). Sep 16];Available from: Https://www.nice.org.uk/guidance/ng191.
- Sandoval Y., Januzzi J.L.J., Jaffe A.S. Cardiac Troponin for Assessment of Myocardial Injury in COVID-19: JACC Review Topic of the Week. Journal of the American College of Cardiology. 2020;76(10):1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z.-.H. Clinical outcomes of COVID-19 in elderly male patients. J. Geriatr. Cardiol. 2020;17(5):243–245. doi: 10.11909/j.issn.1671-5411.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A. Fourth universal definition of myocardial infarction (2018) Eur Heart J [Internet. 2019;40(3):237–269. doi: 10.1093/eurheartj/ehy462. Available from: Https://doi.org/10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- Trecarichi E.M., Mazzitelli M., Serapide F., Pelle M.C., Tassone B., Arrighi E., et al. Clinical characteristics and predictors of mortality associated with COVID-19 in elderly patients from a long-term care facility. Scientific reports. 2020;10(1):20834. doi: 10.1038/s41598-020-77641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucciferri C., Caiazzo L., Di Nicola M., Borrelli P., Pontolillo M., Auricchio A., et al. Parameters associated with diagnosis of COVID-19 in emergency department. Immunity, Inflamm Di. 2021;9(3):851–861. doi: 10.1002/iid3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucciferri C., Vecchiet J., Falasca K. Role of monoclonal antibody drugs in the treatment of COVID-19. World J Clin case. 2020;8(19):4280–4285. doi: 10.12998/wjcc.v8.i19.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAM. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., He W., Yu X., Hu D., Bao M., Liu H., et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J Infec. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Me. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.-.I., Postema P.G., Arbelo E., Behr E.R., Bezzina C.R., Napolitano C., et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Hear Rhyth. 2020;17(9):1456–1462. doi: 10.1016/j.hrthm.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Lu, Q.-B., Liu, M.-J., Wang, Y.-X., Zhang, A.-R., & Jalali, N. et al. (2020).Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv [Internet;2020.02.10.20021675. Available from: Http://medrxiv.org/content/early/2020/02/21/2020.02.10.20021675.abstract.
- Zheng Y.-.Y., Ma Y.-.T., Zhang J.-.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol [Internet. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. Available from: Https://doi.org/10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet (London, England. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]