Abstract
Background
The kidney is the main site for the removal of chromogranin A (CgA). Previous studies have found that patients with renal impairment displayed elevated concentrations of CgA in plasma and that CgA concentrations reflect a deterioration of renal function. In this study, we aimed to estimate serum CgA levels and to evaluate the role of serum CgA in the early diagnosis of diabetic nephropathy (DN).
Methods
A total of 219 patients with type 2 diabetes mellitus (T2DM) were included in this cross-sectional study. These patients were classified into normoalbuminuria (n = 121), microalbuminuria (n = 73), or macroalbuminuria (n = 25) groups based on their urine albumin to creatinine ratios (UACRs). The degree of DN is reflected by UACR. A control group consisted of 45 healthy subjects. The serum CgA levels were measured by ELISA, and other key parameters were assayed.
Results
Serum CgA levels were higher in patients with T2DM than in control subjects, and a statistically significant difference among the studied subgroups regarding CgA was found (P < 0.05). The levels of serum CgA increased gradually with the degree of DN (P < 0.001). Serum CgA levels showed a moderate-intensity positive correlation with UACRs (P < 0.001). A cutoff level of 3.46 ng/ml CgA showed 69.86% sensitivity and 66.12% specificity to detect DN in the early stage.
Conclusion
The levels of serum CgA increased gradually with the degree of DN and can be used as a biomarker in the early detection of DN.
Keywords: Chromogranin A, Diabetic nephropathy, Biomarker, Microalbumin
Background
Diabetes is one of the fastest growing health challenges of the twenty-first century. According to recent estimates from the International Diabetes Federation (IDF), 463 million adults are currently living with diabetes. The IDF estimates that there will be 578 million adults with diabetes by 2030 and 700 million by 2045. Globally, 11.3% of deaths are due to diabetes [1]. Diabetic nephropathy (DN) is a common and serious complication of diabetes and has been shown to be a major cause of end-stage renal disease (ESRD), requiring costly renal replacement therapy in the form of dialysis or transplantation [2]. It is appreciated that up to 40% of patients with type 1 and type 2 diabetes mellitus (DM) present DN [3, 4]. Early detection and appropriate treatment are essential to prevent disability and death.
At present, the clinical diagnosis of DN relies mainly on the detection of urine microalbumin. Although urine microalbumin is the gold standard for the early detection of DN, its predictive power is still limited. In some cases, patients with type 2 diabetes mellitus (T2DM) have progressive loss of renal function before the onset of microalbuminuria. Thus, in patients with T2DM, microalbuminuria is not specific or sensitive enough for the early detection of DN [5, 6]. In recent years, important achievements have been made in finding associated biomarkers in various aspects of DN. Circulating TNF-α receptor (TNFR) levels, particularly TNFR1, are excellent predictors of ESRD in both Caucasians and American Pima Indians patients with T2DM with and without proteinuria. A large real-life epidemiological study confirmed serum uric acid (UA) may be a biomarker in DN patients with the non-albuminuric phenotype. In patients with T2DM and normal renal function, copeptin also predicted an early eGFR decline leading to chronic kidney disease (CKD)-3. Markers of tubular injury, such as Kidney Injury Molecule-1 (KIM-1), Neutrophil Gelatinase-Associated Lipocalin (NGAL), Liver-type Fatty Acid Binding Protein (L-FABP), Monocyte Chemoattractant Protein-1 (MCP-1), and Epidermal Growth Factor (EGF), have been extensively investigated as prognostic biomarkers in DN. Kim et al. found that serum extracellular vesicle (EV)-miRNA profile differs in T2DM patients with normoalbuminuria and micro/macroalbuminuria and miR-4449 was highly upregulated in albuminuric patients. Early studies in T2DM patients suggested that CKD-273 could predict both development and progression of albuminuria. However, they still cannot replace proteinuria [7]. The identification of new biomarkers that can be used as alternatives to or together with routine biomarkers in the early detection of DN is still urgently warranted. In addition, new DN biomarkers may also provide new insight into the pathophysiological mechanisms leading to complications, which are still not fully understood.
Chromogranin A (CgA) is the main member of the chromogranin family and is an acidic glycoprotein consisting of 439 amino acids with an approximate molecular mass of 48 kDa. CgA is used as a diagnostic marker of neuroendocrine tumors and to monitor tumor progression or regression during treatment [8]. CgA also has a role as a biomarker in neurodegenerative and neuropsychiatric diseases, hypertension, cardiovascular disease, heart failure and renal and liver failure [8, 9]. The kidney is the main site for the removal of CgA, and it is retained in serum with declining renal function [10, 11]. In patients with renal failure, serum CgA increases much more than creatinine and the other studied low-MW proteins [12]. CgA was shown to be increased in diabetic patients [13, 14], but its relationship with DN has not been clarified. In this study, we aimed to estimate serum CgA levels and to determine the sensitivity and specificity of this biomarker for the early detection of DN.
Methods
Patients and samples
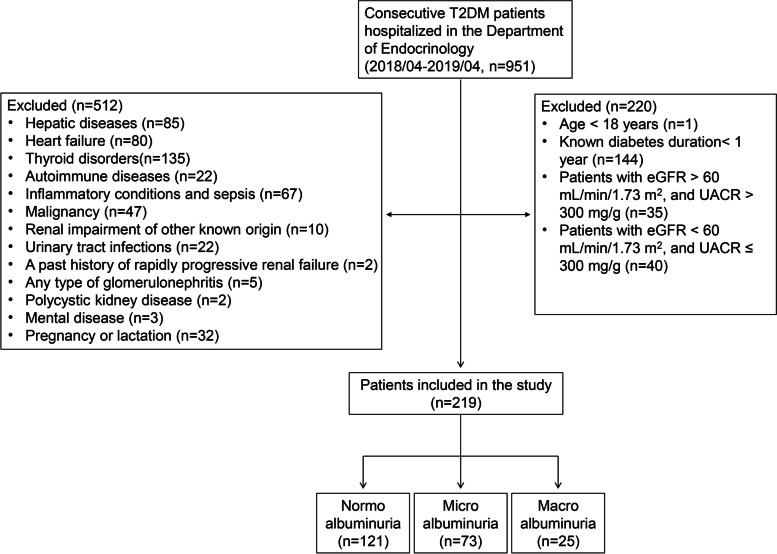
In this cross-sectional study, 219 patients with T2DM (per the 2007 American Diabetes Association diagnostic criteria) who were hospitalized in the Department of Endocrinology, Putuo Hospital, Shanghai University of Traditional Chinese Medicine, China, between April 2018 and April 2019 were selected. This study was approved by the Ethics Committee of our hospital (approval number PTEC-A-2018-11-1). All participants signed informed consent. The specific inclusion criteria were as follows: (1) age ≥ 18 years; (2) known diabetes duration ≥1 year; and (3) normoalbuminuria, microalbuminuria patients (eGFR > 60 mL/min/1.73 m2 and UACR ≤300 mg/g) and macroalbuminuria patients (eGFR < 60 mL/min/1.73 m2 and UACR > 300 mg/g). Patients with hepatic diseases, heart failure, thyroid disorders, autoimmune diseases, inflammatory conditions, sepsis, malignancy, renal impairment of other known origin than DN, urinary tract infections, a past history of rapidly progressive renal failure, any type of glomerulonephritis, polycystic kidney disease, any mental disease, pregnancy or lactation were excluded from the study (Fig. 1). In the same period, 45 healthy people who underwent physical examination in our hospital were selected as controls. All groups were matched for age and sex.
Fig. 1.
Flow chart of patient enrollment. (T2DM, type 2 diabetes mellitus; UACR, urine albumin to creatinine ratios)
All patients with T2DM were divided into three subgroups according to the urinary microalbumin to creatinine ratio (UACR): 1. patients with normoalbuminuria – UACR< 30 mg/g (n = 121), 2. patients with microalbuminuria – UACR 30–300 mg/g (n = 73), and 3. patients with macroalbuminuria – UACR> 300 mg/g (n = 25). Classification was performed according to the 2012 KDIGO (Kidney Disease: Improving Global Outcomes) guidelines [15]. Increased albuminuria (UACR ≥30 mg/g) was considered objective evidence of kidney disease. UACR 30–300 mg/g and eGFR > 60 mL/min/1.73 m2 were considered early DN; UACR> 300 mg/g and eGFR < 60 mL/min/1.73 m2 were considered advanced DN. The degree of DN is reflected by UACR.
Spot urine samples were collected at the first morning void. Blood samples were collected before eating in the morning, centrifuged (3000 rpm, 4 °C, 10 min) to acquire serum and stored at − 80 °C until use. Samples for measurements of serum creatinine (sCr), serum uric acid (sUA), blood urea nitrogen (BUN), fasting blood glucose (FBG), hemoglobin A1c (HbAlc), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and urinary microalbumin to creatinine ratio (UACR) levels were examined at the department of clinical examination using standard procedures. The estimated glomerular filtration rate (eGFR) was calculated using the abbreviated MDRD equation [16]. eGFR (mL/min per 1.73 m2) = 186 X Pcr-1.154 X age-0.203X 0.724 (if female), where Pcr is in mg/dl and age is in years. The concentration of CgA was measured using human-CgA ELISA kits (Cat# ab196271) from Abcam.
Statistical analysis
Continuous variables with a normal distribution are reported as the mean ± standard deviation (SD), whereas skewed distributed variables are expressed as the median (interquartile range). Categorical data were summarized as proportions with frequencies. Continuous variables were compared between groups using an unpaired t test, a one-way ANOVA test, the Kruskal–Wallis test or the Jonckheere-Terpstra test where appropriate, while the chi-square test or Fisher’s exact test was used to analyze the differences in categorical variables. Logistic regression analyses were used to ascertain the predictive value of the CgA level for the presence of early DN in patients with T2DM. Considering the interaction between sCr and eGFR and their similar clinical implications, only sCr was used for multivariate logistic regression. The Spearman rank correlation test was performed to analyze the relationship between 2 quantitative parameters. Statistical analyses were conducted using SPSS software (version 23.0; SPSS, Inc., Chicago, IL, United States). Statistical significance was considered 2-tailed, with a P value of < 0.05.
Results
Clinical characteristics
All enrolled T2DM patients were categorized into three subgroups according to UACR. The demographic and laboratory features of the patients as well as the healthy subjects are reported in Table 1. Our results showed that there was a significant difference among the subgroups of patients regarding SBP, known diabetes duration, UACR, sCr, BUN and eGFR. However, there was no significant difference among the subgroups of patients regarding sex, age, DBP, FBG, HbA1c, sUA, TC, TG, HDL-C and LDL-C. Comparing the clinical and laboratory data among the subgroups of patients and the healthy subjects, there was no significant difference in sex, age, TC and LDL-C levels.
Table 1.
Comparison of clinical and laboratory data among subgroups of patients with T2DM divided according to UACR and healthy subjects
| Heathy controls (n = 45) | Normo albuminuria (n = 121) | Micro albuminuria (n = 73) | Macro albuminuria (n = 25) | aP-value | bP-value | |
|---|---|---|---|---|---|---|
| Male, n (%) | 26(57.8%) | 72(59.5%) | 38(52.1%) | 15(60.0%) | 0.768 | 0.568 |
| Age (years) | 63.40 ± 10.38 | 62.51 ± 10.34 | 65.18 ± 13.52 | 64.48 ± 10.85 | 0.445 | 0.277 |
| SBP (mmHg) | 117.44 ± 7.04 | 129.50 ± 12.15&& | 132.12 ± 14.41&& | 141.60 ± 17.68&&**# | < 0.001 | < 0.001 |
| DBP (mmHg) | 74.00 ± 6.79 | 79.41 ± 7.76&& | 78.96 ± 9.47& | 79.88 ± 6.70& | 0.001 | 0.874 |
| FBG (mmol/L) | 4.81 ± 0.42 | 8.63 ± 4.14&& | 8.79 ± 3.50&& | 9.10 ± 2.76&& | < 0.001 | 0.850 |
| HbA1c (%) | 5.50 ± 0.37 | 9.41 ± 2.40&& | 9.88 ± 3.19&& | 9.45 ± 2.17&& | < 0.001 | 0.479 |
| Known diabetes duration (years) | / | 8.00(3.00, 15.00) | 10.00(6.00, 20.00)** | 18.00(13.00, 20.00)**# | / | < 0.001 |
| UACR (mg/g) | 3.59(2.04, 5.75) | 8.55(5.30, 14.07)&& | 77.69(48.49, 166.00)&&** | 834.05(614.33, 2103.99)&&**## | < 0.001 | < 0.001 |
| sCr (umol/L) | 66.73 ± 12.19 | 65.07 ± 13.82 | 72.07 ± 18.48* | 137.04 ± 49.66&&**## | < 0.001 | < 0.001 |
| BUN (mmol/L) | 5.11 ± 0.96 | 5.58 ± 1.45 | 7.08 ± 2.81&&** | 8.91 ± 4.33&&**# | < 0.001 | < 0.001 |
| sUA (ummol/L) | 308.84 ± 71.81 | 325.74 ± 94.77 | 345.77 ± 101.29& | 373.84 ± 103.01&* | 0.022 | 0.060 |
| eGFR (ml/min/1.73 m2) | 102.44 ± 21.80 | 106.44 ± 21.12 | 95.31 ± 28.75* | 47.53 ± 12.50&&**## | < 0.001 | < 0.001 |
| TC (mmol/L) | 4.67 ± 0.72 | 4.68 ± 1.59 | 4.38 ± 1.22 | 4.41 ± 1.38 | 0.421 | 0.335 |
| TG (mmol/L) | 0.94(0.75, 1.33) | 1.55(1.08, 2.43)& | 1.40(0.99, 2.12)& | 1.66(1.13, 2.63)& | < 0.001 | 0.360 |
| LDL-C (mmol/L) | 2.97 ± 0.84 | 2.93 ± 1.15 | 2.69 ± 0.84 | 2.84 ± 1.00 | 0.373 | 0.319 |
| HDL-C (mmol/L) | 1.41 ± 0.28 | 1.13 ± 0.35&& | 1.11 ± 0.31&& | 1.06 ± 0.27&& | < 0.001 | 0.660 |
| CgA (ng/mL) | 1.89(1.42, 2.47) | 2.26(1.38, 4.12)& | 4.83(2.63, 6.46)&&** | 6.35(4.91, 7.54)&&**# | < 0.001 | < 0.001 |
| ACEI/ARB, n (%) | 0(0.0%) | 34(28.1%)&& | 25(34.2%)&& | 18(72.0%)&&**# | < 0.001 | < 0.001 |
Notes
SBP systolic blood pressure, DBP diastolic blood pressure, FBG fasting blood glucose, HbA1c hemoglobin A1c, UACR urine albumin to creatinine ratio, sCr serum creatinine, BUN blood urea nitrogen, sUA serum uric acid, eGFR estimated glomerular filtration rate, TC total cholesterol, TG triglyceride, LDL-C low density lipoprotein cholesterol, HDL-C high density lipoprotein cholesterol, CgA Chromogranin A
aP-value: comparisons among the healthy controls group, the normoalbuminuria group, the microalbuminuria group and the macroalbuminuria group;
bP-value: comparisons among the normoalbuminuria group, the microalbuminuria group and the macroalbuminuria group
&P < 0.05
&&P < 0.001 vs. healthy controls
*P < 0.05
**P < 0.001 vs. normoalbuminuria group
#P < 0.05
##P < 0.001 vs. microalbuminuria group
Comparison of serum CgA levels among subgroups of patients with T2DM and healthy subjects
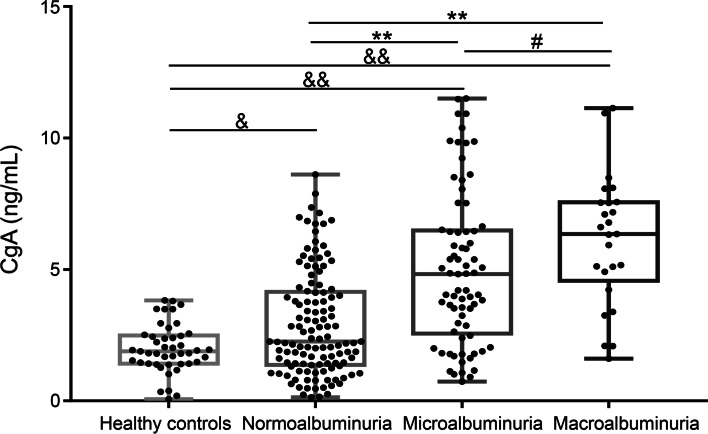
A comparison of serum CgA levels among subgroups of patients divided according to UACR and healthy subjects was performed using the Kruskal–Wallis test. The serum CgA levels were higher in patients with T2DM [3.52 (1.79, 5.67) ng/mL] than in healthy subjects [1.89 (1.42, 2.47) ng/mL] (P < 0.001), and a statistically significant difference was found among the normoalbuminuria group [2.26 (1.38, 4.12) ng/mL], the microalbuminuria group [4.83 (2.63, 6.46) ng/mL] and the macroalbuminuria group [6.35 (4.91, 7.54) ng/mL] regarding serum CgA levels (P < 0.001). A nonparametric Jonckheere-Terpstra test showed that CgA increased gradually with the degree of DN (Z = 6.567, P < 0.001). The results are shown in Fig. 2 and Table 1.
Fig. 2.
Comparison of serum CgA levels in subgroups of subjects divided according to UACR and healthy subjects. (& P < 0.05, && P < 0.001 vs. healthy controls; ** P < 0.001 vs. normoalbuminuria group; # P < 0.05 vs. microalbuminuria group)
Correlation between serum CgA levels and clinical and laboratory data
The Spearman rank correlation test demonstrated that there was a moderate-intensity positive correlation between serum CgA levels and known diabetes durations and UACRs in all subjects (P < 0.001). Weak correlations were found between serum CgA levels and FBG, HbA1c, sCr, BUN, eGFR, and HDL-C (all P < 0.05), and there was no correlation between serum CgA levels and age, SBP, DBP, sUA, TC, TG, or LDL-C in all subjects. There was a moderate-intensity positive correlation between serum CgA levels and UACRs in patients with T2DM (P < 0.001). Weak correlations were found between serum CgA levels and known diabetes duration, DBP, HbA1c, sCr, BUN, and eGFR (all P < 0.05), but there was no correlation between serum CgA levels and age, SBP, FBG, sUA, TC, TG, LDL-C, or HDL-C in patients with T2DM. The correlation coefficients between serum CgA levels and clinical and laboratory data are presented in Table 2.
Table 2.
Correlation between serum CgA and clinical and laboratory data of subjects
| Clinical and laboratory data | CgA (in all subjects) (n = 264) | CgA (in patients with T2DM) (n = 219) | ||
|---|---|---|---|---|
| r | P | r | P | |
| Known diabetes duration (years) | 0.302 | < 0.001 | 0.171 | 0.011 |
| Age (years) | 0.066 | 0.286 | 0.053 | 0.434 |
| SBP (mmHg) | 0.099 | 0.110 | −0.029 | 0.673 |
| DBP (mmHg) | −0.084 | 0.172 | −0.170 | 0.012 |
| FBG (mmol/L) | 0.240 | < 0.001 | 0.128 | 0.060 |
| HbA1c (%) | 0.285 | < 0.001 | 0.144 | 0.033 |
| UACR (mg/g) | 0.441 | < 0.001 | 0.408 | < 0.001 |
| sCr (umol/L) | 0.163 | 0.008 | 0.188 | 0.005 |
| BUN (mmol/L) | 0.229 | < 0.001 | 0.202 | 0.003 |
| sUA (ummol/L) | 0.061 | 0.322 | 0.049 | 0.470 |
| eGFR (ml/min/1.73 m2) | −0.227 | < 0.001 | −0.252 | < 0.001 |
| TC (mmol/L) | −0.118 | 0.056 | −0.109 | 0.107 |
| TG (mmol/L) | 0.035 | 0.572 | −0.049 | 0.473 |
| LDL-C (mmol/L) | −0.109 | 0.076 | −0.105 | 0.120 |
| HDL-C (mmol/L) | −0.124 | 0.044 | −0.027 | 0.689 |
SBP systolic blood pressure, DBP diastolic blood pressure, FBG fasting blood glucose, HbA1c hemoglobin A1c, UACR urine albumin to creatinine ratio, sCr serum creatinine, BUN blood urea nitrogen, sUA serum uric acid, eGFR estimated glomerular filtration rate, TC total cholesterol, TG triglyceride, LDL-C low density lipoprotein cholesterol, HDL-C high density lipoprotein cholesterol, CgA Chromogranin A
Serum CgA levels were associated with the occurrence of early DN
We performed multivariate logistic regression analyses in patients with T2DM between the normoalbuminuria group and the microalbuminuria group. As shown in Table 3, multivariable logistic regression analysis revealed that serum CgA (OR 1.403, 95% CI 1.202–1.637, P < 0.001) and BUN (OR 1.299, 95% CI 1.045–1.616, P = 0.019) were independently associated with the occurrence of early DN when adjusted for age, sex, known diabetes duration, SBP, DBP, FBG, HbAlc, TC, TG, HDL-C, LDL-C, sUA, BUN, sCr and CgA. (Enter).
Table 3.
Multivariate logistic regression analysis of factors independently associated with early DN in patients with T2DM between the normoalbuminuria group and the microalbuminuria group
| Variables | P-value | EXP(B) | 95% CI |
|---|---|---|---|
| Male | 0.252 | 0.603 | 0.254–1.434 |
| Age | 0.375 | 0.983 | 0.946–1.021 |
| Known diabetes duration | 0.164 | 1.039 | 0.985–1.096 |
| SBP | 0.468 | 1.013 | 0.978–1.049 |
| DBP | 0.538 | 1.017 | 0.963–1.074 |
| FBG | 0.564 | 0.971 | 0.877–1.074 |
| HbA1c | 0.519 | 1.050 | 0.905–1.219 |
| TC | 0.686 | 0.850 | 0.386–1.871 |
| TG | 0.637 | 1.052 | 0.851–1.302 |
| HDL-C | 0.315 | 2.176 | 0.478–9.917 |
| LDL-C | 0.927 | 0.958 | 0.385–2.384 |
| sUA | 0.816 | 0.999 | 0.994–1.004 |
| BUN | 0.019 | 1.299 | 1.045–1.616 |
| sCr | 0.097 | 1.029 | 0.995–1.064 |
| CgA | < 0.001 | 1.403 | 1.202–1.637 |
adjusting for age, gender, Known diabetes duration, SBP, DBP, FBG, HbA1c, TC, TG, HDL-C, LDL-C, sUA, BUN, sCr and CgA. (Enter)
Significance level was set at P < 0.05
CI confidence interval, SBP systolic blood pressure, DBP diastolic blood pressure, FBG fasting blood glucose, HbA1c hemoglobin A1c, sCr serum creatinine, BUN blood urea nitrogen, sUA serum uric acid, TC total cholesterol, TG triglyceride, LDL-C low density lipoprotein cholesterol, HDL-C high density lipoprotein cholesterol, CgA Chromogranin A
ROC analysis
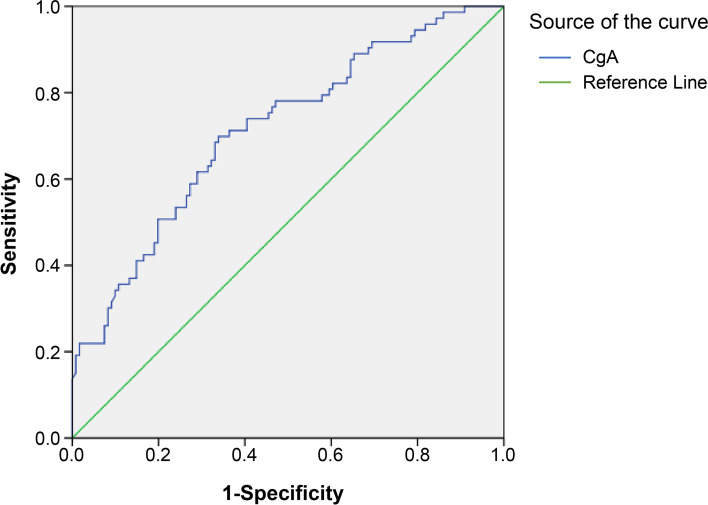
ROC analysis was performed to assess the sensitivity and specificity of serum CgA as a potential biomarker in the prediction of early DN. The ROC analysis of serum CgA yielded an AUC of 0.714 (95% CI, 0.639–0.788; P < 0.001) in the differentiation of T2DM patients with early DN (Table 4 and Fig. 3). The diagnostic sensitivity and specificity of serum CgA for early DN were 69.86 and 66.12%, respectively, when the cutoff value was 3.46 ng/mL.
Table 4.
Validity of serum CgA in prediction of early DN. (in patients with T2DM)
| Parameters | CgA | UACR |
|---|---|---|
| AUC | 0.714 | 1.000 |
| 95% CI | 0.639–0.788 | 1.000 |
| Youden index J | 0.360 | 1.000 |
| Cut-off | 3.46 | 29.67 |
| Sensitivity (%) | 69.86 | 100.00 |
| Specificity (%) | 66.12 | 100.00 |
| P-value | < 0.001 | < 0.001 |
CI confidence interval, AUC Area Under Curve, CgA Chromogranin A
Fig. 3.
Roc curve for validity of serum CgA in prediction of early DN
Discussion
DN is an important microvascular complication of diabetes mellitus. To date, there is no satisfactory method to recognize early DN. According to KDOQI definitions [17], DN diagnosis is based on increased albuminuria and decreased eGFR. There are some shortcomings in using albuminuria as a diagnostic marker for (early) DN. The change in glomerular basement membrane structure may occur earlier than the increase in albuminuria [18]. Approximately 30–45% of T2DM patients who develop kidney disease associated with a decrease in glomerular filtration do not have increased albuminuria [19, 20]. In addition, albuminuria is not specific for DN. Hypertension or obesity may also affect the filtration barrier of the glomeruli, leading to increased albuminuria in patients with T2DM [21]. However, the treatment of hypertension usually includes renin angiotensin aldosterone inhibitors, which reduce glomerular hydrostatic pressure and cause proteinuria to be in the normal range. These factors affect the accuracy of diagnosis based on current guidelines. In DN, when the kidney is damaged, the decrease in eGFR occurs quite late. Early damage is often accompanied by hyperfiltration [22]. Therefore, both routine markers of DN (albuminuria and eGFR) reflecting glomerular damage have certain limitations.
CgA is the main member of the chromogranin family, consisting of water-soluble acidic glycoproteins. CgA was first found in secretory granules from adrenal medullary chromaffin cells and is released into the circulation after splanchnic nerve stimulation together with catecholamines [23]. CgA also exists in many endocrine and neuroendocrine cells [24], nerve cells [25] and immune cells [26]. Elevated serum CgA levels have been found in many cancers [27] and neurodegenerative diseases, such as Alzheimer’s disease [28] and Parkinson’s disease [29]. A previous study showed that CgA, similar to many other low-molecular-weight (MW) proteins, is handled by the kidney [12]. The serum levels of CgA do not change significantly until GFR is reduced to 40 mL/min. Further reduction in GFR is accompanied by a progressive increase in serum values of CgA [12]. However, serum CgA increases in renal failure more than creatinine and the other studied low-MW proteins, β2-MG (β2 microglobulin) and TATI (tumor-associated trypsin inhibitor) [12]. In animal experiments, an inverse correlation suggested a decrease in GFR with increasing plasma CgA concentration [30]. The study by Chen et al. suggests CgA-stimulation of endothelial cell exocytosis of endothelin as a possible mechanism for regulation of renal function in health and disease [31].
Our study aimed to investigate the role of serum CgA in the early diagnosis of DN in patients with T2DM. Because our groups were matched regarding age and sex, the effects of these factors on the results of serum CgA in our study were excluded. In our study, we found a significant difference among the normoalbuminuria, microalbuminuria and macroalbuminuria groups regarding known diabetes duration. This finding is consistent with other previous studies [32, 33].
In our study, the level of serum CgA in patients with T2DM was much higher than that in healthy people. A previous study documented the clinical value of measurements of CgA as a potential marker for diabetes [13]. The concentrations of plasma and salivary CgA were significantly higher in the diabetic groups than in the control group [14]. Our results are consistent with their findings.
Serum concentrations of CgA correlated strongly with eGFR [34]. The relationship between CgA and GFR is very similar to that of other substances eliminated by the kidney, such as creatinine and TATI [12]. In our results, the Spearman rank correlation test demonstrated that serum CgA was negatively correlated with eGFR not only in patients with T2DM but also in all subjects. There was a moderate-intensity positive correlation between serum CgA levels and known diabetes durations and UACRs in all subjects. So the most important finding of our study is that CgA correlated not only with eGFR and the presence of diabetes, which have already been shown in previous studies, but also with UACR and this correlation appeared even stronger than others.
We found for the first time that serum CgA increased gradually with the degree of DN and that serum CgA levels were associated with the occurrence of microalbuminuria. The multivariate logistic regression analyses in patients with T2DM between the normoalbuminuria group and the microalbuminuria group revealed that CgA levels and not eGFR were independently associated with microalbuminuria, which suggests that CgA reflects progression of renal damage even at an early stage without apparent reduction of kidney function, and thus might represent a sensitive marker of DN. One study showed that serum CgA was a poor indicator of DN since patients with T2DM and a reduced glomerular filtration rate failed to show any significant increase in serum CgA [35]. In their research, patients were divided into those without DN with normoalbuminuria (n = 27), patients with DN with microalbuminuria (n = 8), and patients with macroalbuminuria (n = 42). It is noteworthy that in their study, the P value of the difference between patients with normoalbuminuria and DN was 0.07. The failure to reach a significant difference may be due to the small sample size. Moreover, other studies have found that patients with renal impairment display elevated concentrations of CgA in plasma and that CgA concentrations reflect a deterioration of renal function [12, 36]. CgA contains multiple amino acid motifs prone to endoproteolytic cleavage, resulting in multiple processing fragments. This constitutes a biochemical challenge for accurate quantification of CgA. Several assays for measurements of CgA have been developed, but since the antibodies used detect different epitopes, the results from the assays vary considerably [37]. The assay kits we used were different from those used by OA Mojiminiyi’s team [35].
ROC analysis showed the diagnostic accuracy of serum CgA in differentiating between T2DM patients with early DN and those without DN. Using a cutoff of 3.46 ng/mL for serum CgA, we found a sensitivity of 69.86%, a specificity of 66.12%, and a diagnostic accuracy of 71.4% in predicting early DN.
The in vivo isolated cleavage products of CgA in humans include vasostatin-1 and vasostatin-2, pancreastatin (PST), WE-14, cateslytin, and catestatin (CST). All of these products have significant and specific biological effects. Recent studies suggest that WE-14, CST and PST contribute to the development of different diabetes mellitus forms [38]. At the circulation level, CST is associated with chronic heart failure, myocardial infarction, malignant arrhythmia, acute coronary syndrome and unstable angina. Vasostatin-1 and vasostatin-2 exert vasodilatory effects. Vasostatin-1 is associated with multiple myeloma, carotid artery atherosclerosis, sepsis and Takayasu’s arteritis. Vasostatin-2 increased coronary pressure in Langendorff-perfused rat hearts without affecting inotropism. Vasostatin-2 is associated with ischemic chronic heart failure and coronary artery atherosclerosis [39]. Studies on the relationship between these cleavage products of CgA and DN are rare. Further investigation should be performed to determine whether these CgA cleavage products can be used for DN detection.
The findings of this study are limited by the sample size and the cross-sectional design of the study; therefore, the direction of causality cannot be determined from these results. Moreover, the selected T2DM patients did not undergo renal biopsy. Although we formulated strict standards for excluding other patients with renal dysfunction caused by nondiabetic diseases, it cannot be ruled out that there could be patients with renal diseases other than DN selected into groups. Additional prospective studies are needed to examine associations between CgA and early DN risk.
Conclusion
The results of this study show that serum CgA levels were higher in patients with T2DM than in healthy subjects and increased gradually with the degree of DN. CgA could also be used as a biomarker in the early detection of DN.
Acknowledgements
Not applicable.
Abbreviations
- DN
Diabetic nephropathy
- T2DM
Type 2 diabetes mellitus
- IDF
International Diabetes Federation
- ESRD
End-stage renal disease
- CKD
Chronic kidney disease
- TNFR
TNF-α receptor
- KIM-1
Kidney Injury Molecule-1
- NGAL
Neutrophil Gelatinase-Associated Lipocalin
- L-FABP
Liver-type Fatty Acid Binding Protein
- MCP-1
Monocyte Chemoattractant Protein-1
- EGF
Epidermal Growth Factor
- EV
Extracellular vesicle
- TGF
Transforming growth factor
- PST
Pancreastatin
- CST
Catestatin
- SD
Mean ± standard deviation
- TATI
Tumor associated trypsin inhibitor
- MW
Molecular weight
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- FBG
Fasting blood glucose
- HbA1c
Hemoglobin A1c
- UACR
Urine albumin to creatinine ratio
- sCr
Serum creatinine
- BUN
Blood urea nitrogen
- sUA
Serum uric acid
- eGFR
Estimated glomerular filtration rate
- TC
Total cholesterol
- TG
Triglyceride
- LDL-C
Low density lipoprotein cholesterol
- HDL-C
High density lipoprotein cholesterol
- CgA
Chromogranin A
- CI
Confidence interval
- AUC
Area Under Curve
Authors’ contributions
HY, HpW, JF and HW participated in the conception and design of the study, and analyzed the data of study. HY drafted the initial manuscript, JF and HW revised the manuscript. XS, AlC, XmY, YmW and BbZ participated in data collection. All authors have read and approved the final manuscript.
Funding
This work was supported in part by grants from the Key Medical Discipline Project of Shanghai Municipal Health Bureau (ZK2019A12) and the Clinical Specialty (Nephrology) of Shanghai Putuo District Health System Support (2021tszk02). The funding bodies played no role in the design of the study, collection, analysis, and interpretation of the data, or in writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study protocol was approved by the Ethics Committee of Putuo Hospital, Shanghai University of Traditional Chinese Medicine, China (approval number PTEC-A-2018-11-1). Written informed consents were obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hui Yu and Hongping Wang contributed equally to this work.
Contributor Information
Hao Wang, Email: wang402hao@163.com.
Ji Fang, Email: 13601833102@163.com.
References
- 1.IDF Diabetes Atlas (9th Edition 2019). https://www.diabetesatlas.org/ [DOI] [PubMed]
- 2.Atkins RC, Zimmet P. Diabetic kidney disease: act now or pay later. Kidney Int. 2010;77:375–377. doi: 10.1038/ki.2009.509. [DOI] [PubMed] [Google Scholar]
- 3.Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. 2014;63:S39–S62. doi: 10.1053/j.ajkd.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 4.Fiseha T, Tamir Z. Prevalence and awareness of chronic kidney disease among adult diabetic outpatients in Northeast Ethiopia. BMC Nephrol. 2020;21:129. doi: 10.1186/s12882-020-01768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uwaezuoke SN. The role of novel biomarkers in predicting diabetic nephropathy: a review. Int J Nephrol Renov Dis. 2017;10:221–231. doi: 10.2147/IJNRD.S143186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SY, Choi ME. Urinary biomarkers for early diabetic nephropathy: beyond albuminuria. Pediatr Nephrol. 2015;30:1063–1075. doi: 10.1007/s00467-014-2888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barutta F, Bellini S, Canepa S, Durazzo M, Gruden G. Novel biomarkers of diabetic kidney disease: current status and potential clinical application. Acta Diabetol. 2021;58:819–830. doi: 10.1007/s00592-020-01656-9. [DOI] [PubMed] [Google Scholar]
- 8.Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 9.D'amico MA, Ghinassi B, Izzicupo P, Manzoli L, Di Baldassarre A. Biological function and clinical relevance of chromogranin A and derived peptides. Endocr Connect. 2014;3:R45–R54. doi: 10.1530/EC-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connor DT, Pandlan MR, Carlton E, Cervenka JH, Hslao RJ. Rapid radioimmunoassay of circulating chromogranin A: in vitro stability, exploration of the neuroendocrine character of neoplasia, and assessment of the effects of organ failure. Clin Chem. 1989;35:1631–1637. doi: 10.1093/clinchem/35.8.1631. [DOI] [PubMed] [Google Scholar]
- 11.Boomsma F, Bhaggoe UM, Man in 't Veld AJ, Schalekamp MA. Sensitivity and specificity of a new ELISA method for determination of chromogranin A in the diagnosis of phaeochromocytoma and neuroblastoma. Clin Chim Acta. 1995;239:57–63. doi: 10.1016/0009-8981(95)06100-R. [DOI] [PubMed] [Google Scholar]
- 12.Tramonti G, Ferdeghini M, Annichiarico C, Norpoth M, Donadio C, Bianchi R, et al. Relationship between renal function and blood level of chromogranin A. Ren Fail. 2001;23:449–457. doi: 10.1081/JDI-100104728. [DOI] [PubMed] [Google Scholar]
- 13.Soell M, Feki A, Hannig M, Sano H, Pinget M, Selimovic D. Chromogranin A detection in saliva of type 2 diabetes patients. Bosn J Basic Med Sci. 2010;10:2–8. doi: 10.17305/bjbms.2010.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kogawa EM, Grisi DC, Falcão DP, Amorim IA, Rezende TM, da Silva IC, et al. Impact of glycemic control on oral health status in type 2 diabetes individuals and its association with salivary and plasma levels of chromogranin A. Arch Oral Biol. 2016;62:10–19. doi: 10.1016/j.archoralbio.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 15.KDIGO Chapter 1: definition and classification of CKD. Kidney Int Suppl (2011) 2013;3:19–62. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.National Kidney Foundation KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Fioretto P, Steffes MW, Mauer M. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes. 1994;43:1358–1364. doi: 10.2337/diab.43.11.1358. [DOI] [PubMed] [Google Scholar]
- 19.Macisaac RJ, Jerums G. Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens. 2011;20:246–257. doi: 10.1097/MNH.0b013e3283456546. [DOI] [PubMed] [Google Scholar]
- 20.Kim SS, Song SH, Kim IJ, Kim WJ, Jeon YK, Kim BH, et al. Nonalbuminuric proteinuria as a biomarker for tubular damage in early development of nephropathy with type 2 diabetic patients. Diabetes Metab Res Rev. 2014;30:736–741. doi: 10.1002/dmrr.2546. [DOI] [PubMed] [Google Scholar]
- 21.Duran-Perez EG, Almeda-Valdes P, Cuevas-Ramos D, Campos-Barrera E, Muñoz-Hernandez L, Gomez-Perez FJ. Treatment of metabolic syndrome slows progression of diabetic nephropathy. Metab Syndr Relat Disord. 2011;9:483–489. doi: 10.1089/met.2011.0056. [DOI] [PubMed] [Google Scholar]
- 22.Trevisan R, Dodesini AR. The Hyperfiltering kidney in diabetes. Nephron. 2017;136:277–280. doi: 10.1159/000448183. [DOI] [PubMed] [Google Scholar]
- 23.Cohn DV, Zangerle R, Fischer-Colbrie R, Chu LL, Elting JJ, Hamilton JW, et al. Similarity of secretory protein I from parathyroid gland to chromogranin A from adrenal medulla. Proc Natl Acad Sci U S A. 1982;79:6056–6059. doi: 10.1073/pnas.79.19.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaschko H, Comline RS, Schneider FH, Silver M, Smith AD. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature. 1967;215:58–59. doi: 10.1038/215058a0. [DOI] [PubMed] [Google Scholar]
- 25.Hagn C, Schmid KW, Fischer-Colbrie R, Winkler H. Chromogranin A, B and C in human adrenal medulla and endocrine tissues. Lab Investig. 1986;55:405–411. [PubMed] [Google Scholar]
- 26.Nolan JA, Trojanowski JQ, Hogue-Angeletti R. Neurons and neuroendocrine cells contain chromogranin: detection of the molecule in normal bovine tissues by immunochemical and immunohistochemical methods. J Histochem Cytochem. 1985;33:791–798. doi: 10.1177/33.8.3894497. [DOI] [PubMed] [Google Scholar]
- 27.Szarvas T, Jardin-Watelet B, Bourgoin N, Hoffmann MJ, Nyirády P, Oláh C, et al. High-soluble CGA levels are associated with poor survival in bladder cancer. Endocr Connect. 2019;8:625–633. doi: 10.1530/EC-19-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deftos JL. Chromogranin A: its role in endocrine function and as an endocrine and neuroendocrine tumor marker. Endocr Rev. 1991;12:181–187. doi: 10.1210/edrv-12-2-181. [DOI] [PubMed] [Google Scholar]
- 29.Lassmann H, Weiler R, Fischer P, Bancher C, Jellinger K, Floor E, et al. Synaptic pathology in Alzheimer’s disease: immunological data for markers of synaptic and large dense–core vesicles. Neuroscience. 1992;46:1–8. doi: 10.1016/0306-4522(92)90003-K. [DOI] [PubMed] [Google Scholar]
- 30.Mir SA, Biswas N, Cheung W, Wan J, Webster N, Macedo E, O'Connor DT, Vaingankar SM. Chromogranin A pathway: from pathogenic molecule to renal disease. J Hypertens. 2020;38:456–466. doi: 10.1097/HJH.0000000000002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Mahata M, Rao F, Khandrika S, Courel M, Fung MM, et al. Chromogranin A Regulates Renal Function by Triggering Weibel–Palade Body Exocytosis. J Am Soc Nephrol. 2009;20:1623–1632. doi: 10.1681/ASN.2008111148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sana MA, Chaudhry M, Malik A, Iqbal N, Zakiuddin A, Abdullah M. Prevalence of microalbuminuria in type 2 diabetes mellitus. Cureus. 2020;12:e12318. doi: 10.7759/cureus.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kundu D, Roy A, Mandal T, Bandyopadhyay U, Ghosh E, Ray D. Relation of microalbuminuria to glycosylated hemoglobin and duration of type 2 diabetes. Niger J Clin Pract. 2013;16:216–220. doi: 10.4103/1119-3077.110159. [DOI] [PubMed] [Google Scholar]
- 34.Mikkelsen G, Åsberg A, Hultström ME, Aasarød K, Hov GG. Reference limits for chromogranin A, CYFRA 21-1, CA 125, CA 19-9 and carcinoembryonic antigen in patients with chronic kidney disease. Int J Biol Markers. 2017;32:e461–e466. doi: 10.5301/ijbm.5000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mojiminiyi OA, Abdella N, George S. Evaluation of serum cystatin C and chromogranin A as markers of nephropathy in patients with type 2 diabetes mellitus. Scand J Clin Lab Invest. 2000;60:483–489. doi: 10.1080/003655100448464. [DOI] [PubMed] [Google Scholar]
- 36.Bech PR, Ramachandran R, Dhillo WS, Martin NM, Bloom SR. Quantifying the effects of renal impairment on plasma concentrations of the neuroendocrine neoplasia biomarkers chromogranin A, chromogranin B, and cocaine-and amphetamine-regulated transcript. Clin Chem. 2012;58:941–943. doi: 10.1373/clinchem.2011.176099. [DOI] [PubMed] [Google Scholar]
- 37.Broedbaek K, Hilsted L. Chromogranin A as biomarker in diabetes. Biomark Med. 2016;10:1181–1189. doi: 10.2217/bmm-2016-0091. [DOI] [PubMed] [Google Scholar]
- 38.Herold Z, Doleschall M, Kovesdi A, Patocs A, Somogyi A. Chromogranin-A and its role in the pathogenesis of diabetes mellitus. Endokrynol Pol. 2018;69:598–610. doi: 10.5603/EP.a2018.0052. [DOI] [PubMed] [Google Scholar]
- 39.Corti A, Marcucci F, Bachetti T. Circulating chromogranin A and its fragments as diagnostic and prognostic disease markers. Pflugers Arch. 2018;470:199–210. doi: 10.1007/s00424-017-2030-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.