Abstract
Metastasis, accounting for ~90% of cancer-related mortality, involves the systemic spread of cancer cells from primary tumors to secondary sites such as the bone, brain, and lung. Although extensively studied, the mechanistic details of this process remain poorly understood. While common imaging modalities, including computed tomography (CT), positron emission tomography (PET), and magnetic resonance imaging (MRI), offer varying degrees of gross visualization, each lacks the temporal and spatial resolution necessary to detect the dynamics of individual tumor cells. To address this, numerous techniques have been described for intravital imaging of common metastatic sites. Of these sites, the lung has proven especially challenging to access for intravital imaging owing to its delicacy and critical role in sustaining life. Although several approaches have previously been described for single-cell intravital imaging of the intact lung, all involve highly invasive and terminal procedures, limiting the maximum possible imaging duration to 6–12 h. Described here is an improved technique for the permanent implantation of a minimally invasive thoracic optical Window for High-Resolution Imaging of the Lung (WHRIL). Combined with an adapted approach to microcartography, the innovative optical window facilitates serial intravital imaging of the intact lung at single-cell resolution across multiple imaging sessions and spanning multiple weeks. Given the unprecedented duration of time over which imaging data can be gathered, the WHRIL can facilitate the accelerated discovery of the dynamic mechanisms underlying metastatic progression and numerous additional biologic processes within the lung.
Introduction
Responsible for ~90% of deaths, metastas is isthe major cause of cancer-related mortality1. Among the major sites of clinically observed metastasis (bone, liver, lung, brain)2, the lung has proven particularly challenging for in vivo imaging via intravital microscopy. This is because the lung is a delicate organ in perpetual motion. The lungs' continuous motion, further compounded by intrathoracic cardiac motion, represents a substantial barrier to accurate imaging. Therefore, due to its relative inaccessibility to modalities for high-resolution intravital optical imaging, cancer growth within the lung has often been deemed an occult process3.
In the clinical setting, imaging technologies such as computed tomography (CT), positron emission tomography (PET), and magnetic resonance imaging (MRI) enable visualization deep within intact vital organs such as the lung4. However, while these modalities provide for excellent views of the gross organ (often even revealing pathology prior to the onset of clinical symptoms), they are of inadequate resolution to detect individual disseminated tumor cells as they advance through the early stages of metastasis. Consequently, by the time the aforementioned modalities provide any indication of metastasis to the lung, metastatic foci are already well established and proliferating. Since the tumor microenvironment plays a pivotal role in cancer progression and metastasis formation5,6, there is great interest in investigating the earliest steps of metastatic seeding in vivo. This interest is further fueled by the increased appreciation that cancer cells disseminate even before the primary tumor is detected7,8 and the increasing evidence that they survive as single cells and in a dormant state for years to decades before outgrowth into macro-metastasis9.
Previously, imaging of the lung at single-cell resolution has necessarily involved ex vivo or explant preparations10,11,12,13, limiting analyses to single time points. While these preparations do provide useful information, they do not provide any insight into the dynamics of tumor cells within the organ connected to an intact circulatory system.
Recent technological advancements in imaging have enabled intravital visualization of the intact lung at single-cell resolution over periods of up to 12 h14,15,16. This was accomplished in a murine model using a protocol that involved mechanical ventilation, resection of the ribcage, and vacuum-assisted lung immobilization. However, despite offering the first single cell-resolution images of the physiologically intact lung, the technique is highly invasive and terminal, thereby precluding further imaging sessions beyond the index procedure. This limitation, therefore, prevents its application to the study of metastatic steps that take longer than 12 h, such as dormancy and re-initiation of growth14,15,16. Further still, patterns of cellular behavior observed using this imaging approach must be cautiously interpreted, given that vacuum-induced pressure differentials are likely to cause diversions in blood flow.
To overcome these limitations, a minimally invasive Window for High-Resolution Imaging of the Lung (WHRIL) was recently developed, facilitating serial imaging over an extended period of days to weeks, without the need for mechanical ventilation17. The technique entails the creation of a 'transparent ribcage' with a sealed thoracic cavity for the preservation of normal lung function. The procedure is well-tolerated, permitting the mouse to recover without meaningful alteration to baseline activity and function. To reliably localize exactly the same lung region at each respective imaging session, a technique known as microcartography was applied to this window18. Through this window, it was possible to capture images of cells as they arrive at the vascular bed of the lung, cross the endothelium, undergo cell division, and grow into micro-metastases.
Here, the study presents a detailed description of an improved surgical protocol for implantation of the WHRIL, which simplifies the surgery while simultaneously increasing its reproducibility and quality. While this protocol was designed to enable investigation of the dynamic processes underlying metastasis, the technique may be alternatively applied to investigations of numerous processes of lung biology and pathology.
Protocol
All procedures described in this protocol have been performed in accordance with guidelines and regulations for the use of vertebrate animals, including prior approval by the Albert Einstein College of Medicine Institutional Animal Care and Use Committee.
1. Passivation of windows
Rinse the optical window frames (Supplemental Figure 2) with a 1% (w/v) solution of enzymatically-active detergent.
Inside a glass jar, submerge the optical window frames in 5% (w/v) sodium hydroxide solution for 30 min at 70 °C.
Remove and wash the window frames with deionized water.
Inside a new glass jar, submerge the optical window frames in 7% (w/v) citric acid solution for 10 min at 55 °C.
Again, remove and wash the window frames with deionized water.
Repeat step 1.2; then, remove and wash window frames with deionized water.
2. Preparation for surgery
Conduct the surgery in a hood or laminar flow cabinet. To avoid contamination of the operative field, ensure distinct, separated areas for preparation, surgery, and recovery, respectively.
In advance of the surgery, sterilize all surgical instruments in an autoclave. If subsequent procedures are planned, re-sterilize instruments using a hot bead sterilizer. For this surgical procedure, a tips-only technique is used.
Power on the heated surgical bead and bead sterilizer.
Anesthetize the mouse with 5% isoflurane in the anesthesia chamber.
To remove hair, generously apply depilatory cream to the upper-left chest incision site. After no longer than 20 s, firmly wipe away hair and depilatory cream using moistened tissue paper. Repeat as necessary to remove all hair from the surgical site.
Using 2–0 silk suture, tie a knot at the base of a 22 G catheter, leaving 2-inch long tails (see Figure 1A).
Figure 1:
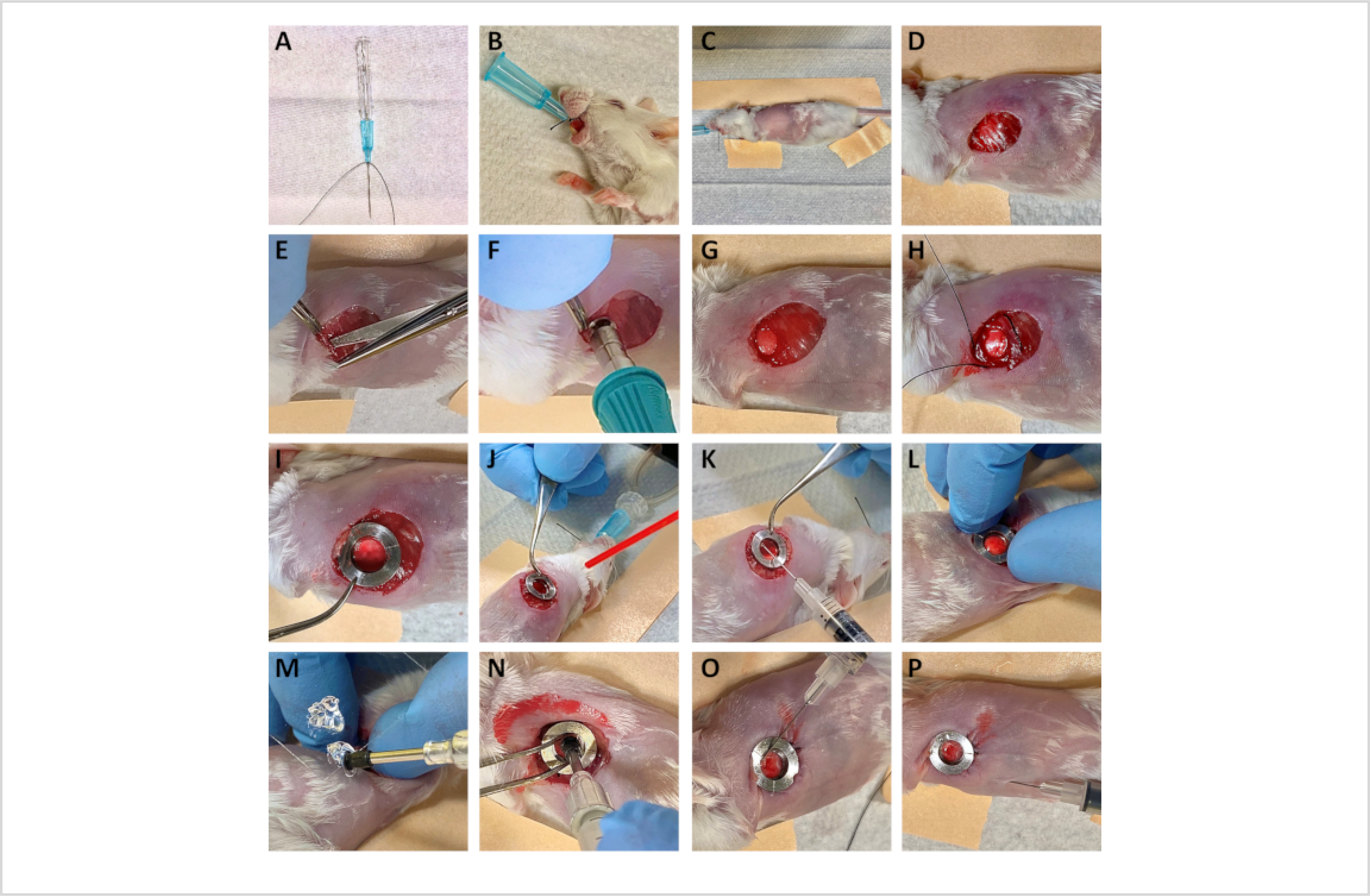
Summary of surgery for the implantation of the Window for High-Resolution Imaging of the Lung (WHRIL).
3. Lung window surgery
Wash hands using antiseptic soap.
Prior to each new surgery, don new sterile gloves.
To prevent corneal drying and damage to the mouse's eyes, apply ophthalmic ointment to both eyes.
Dilute 10 μL (0.1 mg/kg) of buprenorphine in 90 μL of sterile PBS, and then inject subcutaneously to ensure preoperative analgesia.
Intubate the mouse with the silk suture-tied 22 G catheter15. Using an inflation bulb, confirm successful intubation by noting bilateral chest rise upon bulb squeeze.
Secure the intubation catheter by tying the 2–0 silk suture around the mouse's snout (see Figure 1B).
Place the mouse onto the heated surgical stand and position it in the right lateral decubitus to expose the left thorax.
Connect ventilator to the intubation catheter.
Ensure controlled, stable ventilation on the ventilator and then lower the isofluorane to 3%. At the procedure's onset and periodically throughout the duration of the procedure, assess the adequacy of anesthesia by performing a toe pinch test.
Using paper tape, cranially and caudally secure the front and hind limbs, respectively, to the heated surgical stage. Place another piece of tape along the length of the mouse's back to maximize exposure to the surgical field (see Figure 1C).
Open all surgical instruments underneath the hood for the preservation of sterility.
Sterilize the surgical site by a generous application of antiseptic to the mouse's skin.
Using forceps, lift the skin and make an ~10 mm circular incision, ~7 mm to the left of the sternum and ~7 mm superior to the subcostal margin (Figure 1D).
Carefully identify any major vessels. If the division of vessels is necessary, cauterize at both ends with the electrocautery pen to maintain hemostasis.
Excise the soft tissue overlying the ribs.
Elevate the 6th or 7th rib using forceps. Using a single blade of the blunt micro-dissecting scissors, the rounded side towards the lung, carefully pierce the intercostal muscle between the 6th and 7th ribs to enter the intrathoracic space (Figure 1E).
Delicately discharge compressed air canister at the defect to collapse the lung and separate it from the chest wall. Fire the compressed air in short bursts to prevent iatrogenic lung injury.
Place the biopsy punch over the cutting tool (Supplementary Figure 1) and carefully maneuver the cutting tool's base through the intercostal incision (Figure 1F).
-
Orient the base of the cutting tool such that it is parallel with the chest wall. Punch a 5 mm circular hole through the rib cage (Figure 1G).
NOTE: Ensure that the exposed lung tissue is pink, without signs of damage.
Using the 5–0 silk suture, create a purse-string stitch ~1 mm from the hole, circumferentially, interlacing with the ribs (Figure 1H).
Position the window frame such that the edges of the circular defect align within the window's groove (see Figure 1I).
Securely lock the implanted window by tightly tying down the 5–0 silk suture.
Load 100 μL of cyanoacrylate gel adhesive into the 1 mL syringe.
Dry the lung by applying a steady gentle stream of compressed air for ~10–20 s (Figure 1J).
Using forceps to grip the window frame by its outside edge, gently lift to ensure separation of the lung from the undersurface of the window frame.
Dispense a thin layer of cyanoacrylate adhesive along the undersurface of the optical window frame (Figure 1K).
Increase the positive end-expiratory pressure (PEEP) on the ventilator to inflate the lung.
Holding for 10–20 s, apply gentle but firm pressure to attach the optical window frame onto the lung tissue (Figure 1L).
Dispense a 5 mm drop of the remaining cyanoacrylate gel adhesive onto a rectangular coverslip.
Pick up the 5 mm coverslip using vacuum pickups. Dip the undersurface of the coverslip into the adhesive, and then scrape off excess adhesive three times against the side of the rectangular coverslip, such that only a very thin layer remains (Figure 1M).
Carefully position the coverslip to fit inside the recess at the center of the optical window frame and is held above the lung tissue at an angle. Briefly clamp the ventilator to generate positive pressure, hyper-inflating the lung. Using a rotating motion, orient the coverslip parallel to the lung tissue to create direct apposition between the lung's surface and the undersurface of the coverslip. Maintain gentle pressure, allowing the cyanoacrylate adhesive to set (~25 s).
Use the forceps to separate the coverslip from the vacuum pickups (Figure 1N).
Using 5–0 silk suture, again create a purse-string stitch, this time <1 mm circumferentially from the cut-edge of the skin incision. Tuck any excess skin underneath the outer rim of the window frame before tying it down tightly with locking knots.
To ensure an air-tight seal between the coverslip and the window frame, dispense a small amount of liquid cyanoacrylate at the metal-glass interface (see Figure 1O).
Attach a sterile needle to a 1 mL insulin syringe. Insert the needle below the xiphoid process, advancing toward the left shoulder, entering the thoracic cavity through the diaphragm. Gently draw back on the syringe to remove any residual air from the thoracic cavity (see Figure 1P).
Remove the tape from the mouse.
Turn off isoflurane.
Continue ventilation with 100% oxygen until the mouse appears ready to awaken.
Carefully cut the 2–0 silk suture around the mouse's snout and extubate the mouse.
Transfer the mouse to a clean cage and monitor until fully recovered. Euthanize the mouse if signs of difficulty in breathing are present.
Provide postoperative analgesia by subcutaneously injecting 10 μL (0.1 mg/kg) of buprenorphine diluted in 90 μL of sterile phosphate buffered solution (PBS).
Representative Results
The steps of the surgical procedure described in this protocol are summarized and illustrated in Figure 1. Briefly, prior to surgery, mice are anesthetized and the hair over the left thorax is removed. Mice are intubated and mechanically ventilated to enable survival upon breachment of the thoracic cavity. Soft tissue overlying the ribs is excised, and a small circular defect is created, spanning the 6th and 7th ribs. The optical window frame is inserted into the defect and its bottom side (outside of the clear aperture) is adhered to the lung tissue. The window frame is then secured with a combination of sutures and adhesive, resealing the thoracic cavity and permitting the resumption of normal breathing following extubation. When successfully implanted, the lung will adhere to the optical window (which is incorporated as part of the chest wall), with intrathoracic pressure gradients preserved. This permits comfortable survival of the mouse, enabling daily imaging up to the protocol allowance (2 weeks). Intravital imaging can then be performed through the window, as previously described for other windows15,19,20.
For visualization of various cell types, biological structures, or cellular functional states, the procedure presented here can be performed on a wide range of mice that have either been genetically manipulated to express fluorescent proteins21 or injected with dyes22. The permanent nature of the window makes it compatible with techniques for relocalization of fields of view such as photoconversion23,24 or microcartography17,18. Microcartography is a triangulation technique based upon using computed transformations of coordinates of fixed fiducial marks between imaging sessions in order to predict and re-localize a region of interest. In the window created as described above, these fiducial marks are light scratches etched into the window frame (Supplemental Figure 2) that are easily identifiable under the microscope. This makes it possible to find the same field of view multiple times, even in otherwise unmarked tissue. Figure 2 demonstrates the result of these techniques in a mouse where the lung vasculature has been labeled by injection of a dye-labeled high molecular weight dextran (tetramethylrhodamine 155 kD dextran) and the same microvasculature re-localized over 3 days.
Figure 2: Microcartography enables the relocalization of fixed positions within the optical window.
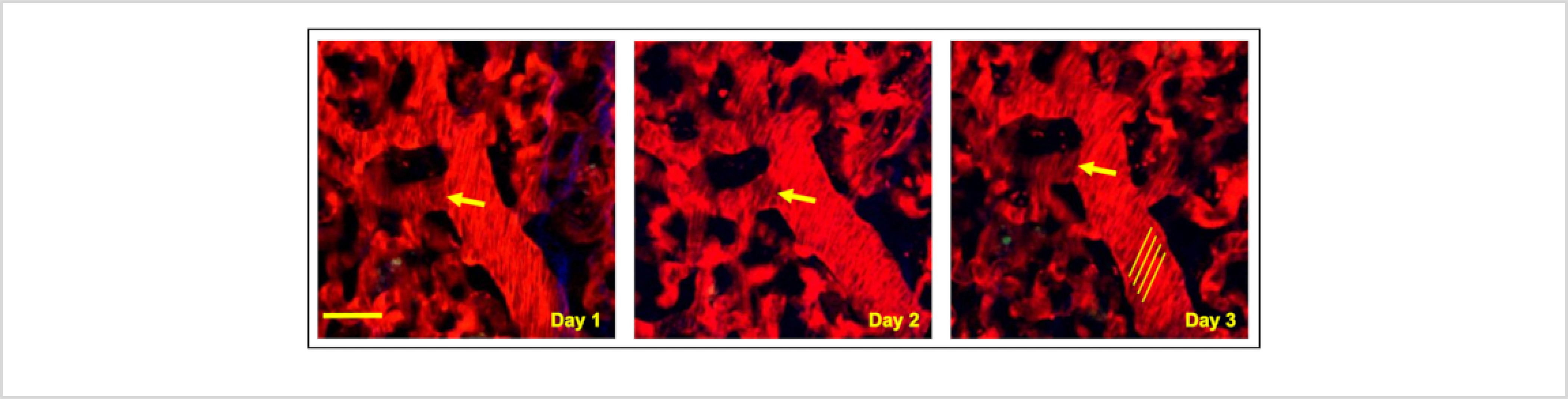
Multiphoton intravital imaging of a single region of the lung under the optically transparent coverslip shows microvasculature relocated over 3 consecutive days using microcartography. Yellow arrows indicate a clearly definable branch point from a single vessel identified each consecutive day. Yellow lines highlight shadows that unlabeled erythrocytes make when flowing in larger vessels. The angle of these lines relative to the vessel can be used to calculate erythrocyte flow rates. Red = tdTomato labeled endothelial cells and 155 kDa Tetramethylrhodamine dextran labeled blood serum, Green = GFP labeled tumor cells, Blue = second harmonic generation. Scale bar = 15 μm.
This dextran was found to be extremely useful in evaluating transient vascular openings that are induced during periods of tumor cell intravasation25,26,27. Indeed, it has been shown that, in primary breast tumors, this high molecular weight dextran is otherwise effectively sequestered to the vasculature and does not leak into the interstitium25. This is in contrast to dextrans of lower molecular weight (such as 10 kD or 70 kD), which have been shown to leak from neoangiogenic vessels passively28,29. Meanwhile, the healthy lung vasculature has been observed to be more resistant to leakage, with dextrans >10 kD only escaping to the interstitium upon insult to the organ, such as upon exposure to exosomes30 or viruses31. A variety of contrast agents also exist to measure other parameters in the lung (e.g., nuclear markers, live/dead indicators, oxidative stress reporters, blood flow velocity trackers) in addition to vascular permeability. An excellent resource cataloging them can be found in the protocol by Ueki et al.22.
The WHRIL is a technique that is very well-suited to investigating the dynamics of blood flow in the lung. This can be accomplished in several ways. First, when imaged using relatively slow frame rates (~1–10 frames per second, fps) blood flow velocities can be determined by the shadows that unlabeled erythrocytes make when flowing in larger vessels. At low fps, these shadows form lines whose angle relative to the vessel can be used to calculate erythrocyte flow rates32 (Figure 2, yellow lines). Second, shadows can also be tracked on low fps microscopes by aligning the vessels with the fast scan axis of the microscope and acquiring kymographs using rapid line scanning33,34,35. Finally, when imaging at high frame rates (>10 fps) on a microscope capable of integrating the signal over time (e.g., a spinning disk confocal equipped with a charge-coupled device (CCD) detector), individual particles can be traced directly16,17. In this situation, stationary objects appear as bright dots, and flowing objects trace out tracks through with the circulation. Cell speeds can be quantified by measuring the length of the tracks and dividing by the frame acquisition time. An example of this is given in Figure 3 and Supplemental Movie 1, where 2 μm fluorescent microspheres have been intravascularly injected into the mouse before imaging.
Figure 3: Visualization of blood flow rate.
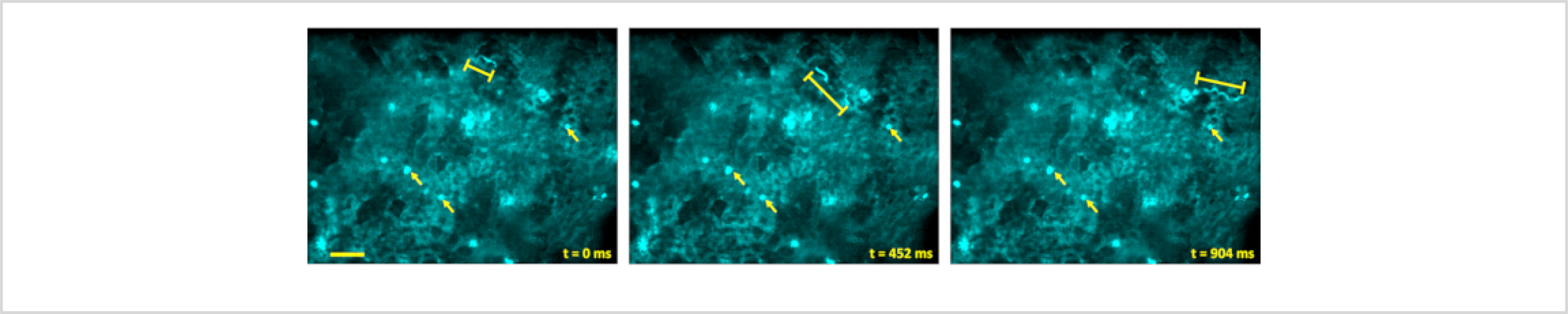
Blood flow rates can be visualized by injecting 2 μm diameter fluorescent microspheres retro-orbitally and imaging their passage through the blood vessels. When imaged on a microscope capable of integrating the signal over time (e.g., a spinning disk confocal equipped with a CCD detector), stationary microspheres appear as bright dots (arrows), and flowing spheres trace out tracks through with the circulation (bracketed lines). Scale bar = 50 μm.
With the ability to repeatedly and consistently return to the same field of view, visualization of processes that evolve over multiple days is now possible. As a demonstration of this application, the WHRIL was used to visualize the metastatic progression of breast cancer cells within the lungs17,21: that is, to track over time the fate of individual tumor cells that arrive at the lung vasculature. This concept is depicted in Figure 4A, where a single disseminated tumor cell is visualized shortly after lodging in a segment of lung micro-vasculature. Returning to that same location on subsequent days reveals the tumor cell's fate (e.g., recirculation, extravasation, etc.). Applied to the investigation of the culminating steps of metastatic progression in the lung, it was possible to visually chronicle dynamic processes, including tumor cell arrival (Figure 4B), extravasation (Figure 4C), and proliferation to form macro-metastasis (Figure 4D).
Figure 4. The WHRIL can capture each step of the metastatic cascade within the lung by directly visualizing the fate of disseminated tumor cells.
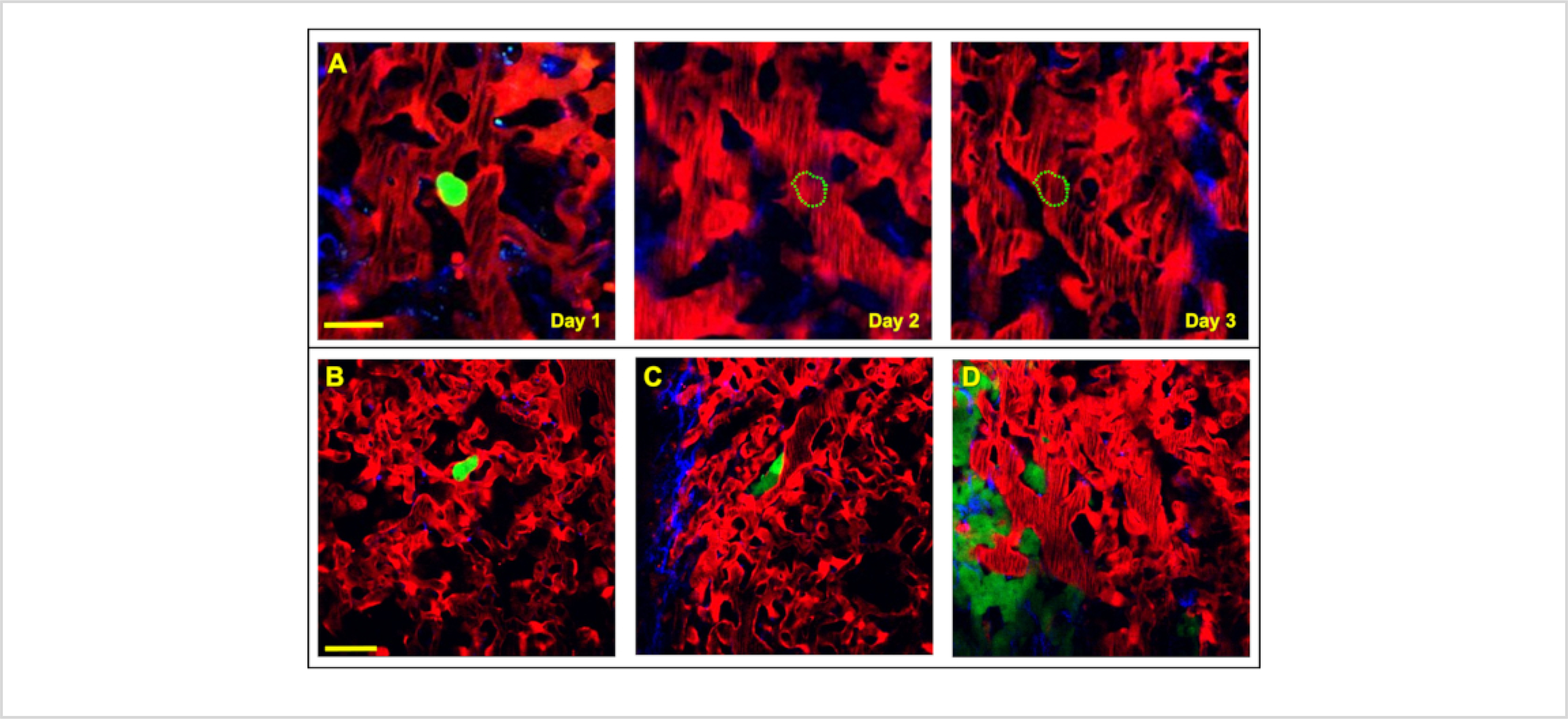
(A) Tracking the fate of disseminated tumor cells (green) is achievable with serial imaging, over several days, through the WHRIL. On Day 1, a tumor cell is observed to have arrived to and lodged in the lung vasculature. On Day 2 and Day 3 the cell is no longer present in the lung vasculature, having either recirculated or died. Scale bar = 15 μm. (B-D) Visualization of each of the stages of tumor cell metastasis in the lung. (B) An intravascular disseminated tumor cell (green) lodged in the lung vasculature after arrival. (C) Disseminated tumor cell (green) after extravasating into the lung parenchyma. (D) Tumor cells that have proliferated and grown into micro-metastases. Red = tdTomato labeled endothelial cells and 155 kDa Tetramethylrhodamine dextran labeled blood serum, Green = GFP labeled tumor cells, Blue = second harmonic generation. Scale bar = 20 μm.
Discussion
At sites of distant metastasis such as the lung, high-resolution optical imaging provides insight into the elaborate dynamics of tumor cell metastasis. By enabling in vivo visualization of single cancer cells and their interactions with the host tissue, high-resolution intravital imaging has proven instrumental to understanding the mechanisms underlying metastasis.
Described here is an improved surgical protocol for the permanent thoracic implantation of an optical window designed to enable serial imaging of the murine lung via high-resolution multiphoton microscopy. The window created using this protocol is well-tolerated and, given its ability to successfully reseal the thoracic cavity, is able to maintain the intrathoracic pressure gradients necessary for spontaneous ventilation (contrary to any other previously described window for imaging of the murine lung14,15,16,36,37). This permits the mouse to awaken from anesthesia, breathe independently, and comfortably survive with the transparent ribcage for an extended period of time spanning multiple weeks.
Using this window, it was possible to visualize, with single-cell resolution, all of the steps of metastasis, including arrival, extravasation, and growth into micrometastases.
Although the protocol requires some technical proficiency, with practice and careful attention to several key steps, the procedure can be performed with a high success rate. First, when removing hair prior to surgery, it is critical to protect the mouse's skin by removing the depilatory cream with a moistened tissue after no more than 20 s of contact. During surgery, extreme caution should be paid to avoid cutting vessels. Excessive bleeding, most commonly encountered due to the division of either the brachial or internal mammary arteries during removal of the mammary fat pad, can obscure visualization in the surgical field or lead to death through exsanguination. Newly described in this protocol is the utilization of a biopsy punch and cutting tool (Supplemental Figure 1), which considerably hasten and simplify the creation of the circular defect through the rib cage, and a window holder tool making implantation easier. The implementation of these advances significantly improves the success rate of the procedure and reduces the required level of prior surgical skill. Individual laboratories can use the drawings in the supplemental figures to manufacture these tools with either in-house or commercial machine shops. An internet search for "machine shop bidding sites" will yield several online applications that will aid in finding local commercial machine shops.
Finally, it is crucial to ensure that the lung tissue remains dry before adhesive application. The most common pitfall resulting in unsuccessful coverslip attachment is failure to ensure complete removal of moisture from the lung surface prior to apposition with the frame or cover glass. Furthermore, to ensure quality images, an extremely thin layer of glue (<10 μm) should be applied. Excess glue should be scraped off prior to placement of the cover glass.
The main limitation of IVI through the WHRIL is the relatively limited depth of penetration achievable. Therefore, pathology occurring deep within the lung is inaccessible. Despite this limitation, the technique can still yield an abundance of clinically relevant information, especially in oncologic investigations, given the described proclivity for peripherally localized lung metastases38,39,40,41. Ultimately, this imaging approach provides a considerable advantage over standard ex vivo assays and other methods for in vivo imaging, which either disconnect tissue from vital physiological processes10,11,12,13, or limit longitudinal analysis to a maximum duration of 12 h14,15,16,37,42, respectively.
For repeated imaging over this time period, several challenges must still be overcome. First, it is important to maintain the health of the skin around the implanted window, for, while the wounded tissue is not exposed, the skin around may still become inflamed or infected. Routine application of an antibiotic ointment will help prevent this. Second, with time, exudate from the cut skin may congeal under the window frame and prevent the placement of the fixturing plate used to immobilize the mouse in the microscope stage. Placing a wet tissue over the WHRIL for 10–15 min will soften this exudate and allow placement of the window frame. Third, one of the body's mechanisms for excreting excess water and maintaining homeostasis is via exhalation of vapor. Thus, too much fluid intake (mostly as a result of injection of contrast agents or tumor cell suspensions) will cause the lung surface to excrete this excess water and will result in the lung tissue detaching from the WHRIL. This can be avoided by limiting the volume of injections to a maximum of 50 μL at a time. Finally, even with the best of care, lung tissue may occasionally detach from the WHRIL due to the mouse ingesting a large volume of water or due to the mouse overexerting itself. When this occurs, detachment of the lung tissue from the WHRIL typically occurs slowly, starting at the outside edge. Thus, it may be impossible to follow some fields of view located on the first day of imaging for the full duration of the window. It was found that the best imaging results will be obtained within the first few days and that employing mosaicking techniques such as the previously published Large-Volume High-Resolution Intravital Imaging21 can minimize the impact of this limitation.
Given that the WHRIL is integrated into the chest wall of the mouse, drift during imaging is generally not a significant issue, as long as care is paid to ensure that the attachment between the window and the microscope is firm. Still, some small amount of drift may be observed during the time immediately following placement of the mouse in the microscope stage. This may come from the relaxation of the mouse's body or from the thermal expansion of the microscope components (stage plate, XY stage, objective lens) due to the environmental chamber. This drift may be avoided by allocating ~30 min for equilibration before starting the imaging procedure. This period of time allows the mouse's physiology to stabilize under the anesthesia and allows all components to come to thermal equilibrium. Any small amount of residual drift may easily be handled by computational algorithms such as StackReg43 or HyperStackReg44.
Finally, this protocol is an improvement over the prior written version for two reasons. First, the visual format allows a better conceptualization of the surgical protocol. This is particularly useful for the crucial steps where 1) the lung is dried by applying a steady gentle stream of compressed air (step 3.24, Figure 1J), 2) the coverslip is attached to the window frame's central bore in a way that prevents entrapment of bubbles (step 3.31), and 3) a small amount of liquid cyanoacrylate is added at the metal-glass interface to ensure an air-tight seal between the coverglass and the window frame (step 3.34, Figure 1O).
In conclusion, with the advent of the WHRIL, given its amenability to subcellular visualization of the same lung tissue across an extended period, investigators are newly empowered to address many unanswered questions. Specifically, the protocol outlined herein enables fundamental exploration of the dynamic processes underlying numerous pathologies, including the progression of cancer metastasis.
Supplementary Material
Supplemental Figure 1: Mechanical design drawings for the stainless-steel cutting tool used to guide the 5 mm biopsy punch.
Supplemental Figure 2: Mechanical design drawings for the stainless-steel window frame.
Supplemental Figure 3: Mechanical design drawings for the window holder tool.
Supplemental Movie 1: Video corresponding to Figure 3 showing the lung vasculature with circulating 2 μm microspheres.
Acknowledgments
This work was supported by the following grants: CA216248, CA013330, Montefiore's Ruth L. Kirschstein T32 Training Grant CA200561, METAvivor Early Career Award, the Gruss-Lipper Biophotonics Center and its Integrated Imaging Program, and Jane A. and Myles P. Dempsey. We would like to thank the Analytical Imaging Facility (AIF) at Einstein College of Medicine for imaging support.
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/62761.
Disclosures
The authors disclose no conflicts of interest.
References
- 1.Mehlen P, Puisieux A Metastasis: a question of life or death. Nature Reviews Cancer 6 (6), 449–458 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Lee YT Breast carcinoma: pattern of metastasis at autopsy. Journal of Surgical Oncology 23 (3), 175–180 (1983). [DOI] [PubMed] [Google Scholar]
- 3.Chambers AF, Groom AC, MacDonald IC Dissemination and growth of cancer cells in metastatic sites. Nature Reviews Cancer 2 (8), 563–572 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Coste A, Oktay MH, Condeelis JS, Entenberg D Intravital imaging techniques for biomedical and clinical research. Cytometry Part A 95 (5), 448–457 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeClerck YA, Pienta KJ, Woodhouse EC, Singer DS, Mohla S The tumor microenvironment at a turning point knowledge gained over the last decade, and challenges and opportunities ahead: A white paper from the NCI TME network. Cancer Research 77 (5), 1051–1059 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borriello L et al. The role of the tumor microenvironment in tumor cell intravasation and dissemination. European Journal of Cell Biology 99 (6), 151098 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosseini H et al. Early dissemination seeds metastasis in breast cancer. Nature 540 (7634), 552–558 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper KL et al. Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature 540 589–612 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Risson E, Nobre AR, Maguer-Satta V, Aguirre-Ghiso JA The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nature Cancer 1 (7), 672–680 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian B et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One 4 (8), e6562 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian BZ et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475 (7355), 222–225 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyao N et al. Various adhesion molecules impair microvascular leukocyte kinetics in ventilator-induced lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology 290 (6), L1059–1068 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Bernal PJ et al. Nitric-oxide-mediated zinc release contributes to hypoxic regulation of pulmonary vascular tone. Circulation Research 102 (12), 1575–1583 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Entenberg D et al. In vivo subcellular resolution optical imaging in the lung reveals early metastatic proliferation and motility. IntraVital 4 (3), 1–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Tirado C et al. Long-term High-Resolution Intravital Microscopy in the Lung with a Vacuum Stabilized Imaging Window. Journal of Visualized Experiments: JoVE 116, 54603 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Looney MR et al. Stabilized imaging of immune surveillance in the mouse lung. Nature Methods 8 (1), 91–96 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Entenberg D et al. A permanent window for the murine lung enables high-resolution imaging of cancer metastasis. Nature Methods 15 (1), 73–80 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunphy MP, Entenberg D, Toledo-Crow R, Larson SM In vivo microcartography and subcellular imaging of tumor angiogenesis: a novel platform for translational angiogenesis research. Microvascular Research 78 (1), 51–56 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harney AS, Wang Y, Condeelis JS, Entenberg D Extended time-lapse intravital imaging of real-time multicellular dynamics in the tumor microenvironment. Journal of Visualized Experiments: JoVE 112, e54042 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seynhaeve ALB, Ten Hagen TLM Intravital microscopy of tumor-associated vasculature using advanced dorsal skinfold window chambers on transgenic fluorescent mice. Journal of Visualized Experiments: JoVE 131, 55115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Entenberg D et al. Time-lapsed, large-volume, high-resolution intravital imaging for tissue-wide analysis of single cell dynamics. Methods 128, 65–77 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueki H, Wang IH, Zhao D, Gunzer M, Kawaoka Y Multicolor two-photon imaging of in vivo cellular pathophysiology upon influenza virus infection using the two-photon IMPRESS. Nature Protocols 15 (3), 1041–1065 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritsma L, Ponsioen B, van Rheenen J Intravital imaging of cell signaling in mice. IntraVital 1 (1), 2–10 (2012). [Google Scholar]
- 24.Kedrin D et al. Intravital imaging of metastatic behavior through a mammary imaging window. Nature Methods 5 (12), 1019–1021 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harney AS et al. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discovery 5 (9), 932–943 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karagiannis GS et al. Assessing tumor microenvironment of metastasis doorway-mediated vascular permeability associated with cancer cell dissemination using intravital imaging and fixed tissue analysis. Journal of Visualized Experiments: JoVE 148, 59633 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karagiannis GS et al. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Science Translational Medicine 9 (397) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dreher MR et al. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. Journal of the National Cancer Institute 98 (5), 335–344 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Rizzo V, Kim D, Duran WN, DeFouw DO Ontogeny of microvascular permeability to macromolecules in the chick chorioallantoic membrane during normal angiogenesis. Microvascular Research 49 (1), 49–63 (1995). [DOI] [PubMed] [Google Scholar]
- 30.Hoshino A et al. Tumour exosome integrins determine organotropic metastasis. Nature 527 (7578), 329–335 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueki H et al. In vivo imaging of the pathophysiological changes and neutrophil dynamics in influenza virus-infected mouse lungs. Proceedings of the National Academy of Sciences of the United States of America 115 (28), E6622–E6629 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornfield TE, Newman EA Measurement of retinal blood flow using fluorescently labeled red blood cells. eNeuro 2 (2) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dasari S, Weber P, Makhloufi C, Lopez E, Forestier CL Intravital microscopy imaging of the liver following leishmania infection: An assessment of hepatic hemodynamics. Journal of Visualized Experiments: JoVE 101, e52303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaigneau E, Roche M, Charpak S Unbiased analysis method for measurement of red blood cell size and velocity with laser scanning microscopy. Frontiers in Neuroscience 13, 644 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim TN et al. Line-scanning particle image velocimetry: an optical approach for quantifying a wide range of blood flow speeds in live animals. PLoS One 7 (6), e38590 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Presson RG Jr. et al. Two-photon imaging within the murine thorax without respiratory and cardiac motion artifact. American Journal of Pathology 179 (1), 75–82 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabuchi A, Mertens M, Kuppe H, Pries AR, Kuebler WM Intravital microscopy of the murine pulmonary microcirculation. Journal of Applied Physiology 104 (2), 338–346 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Travis WD Classification of lung cancer. Seminars in Roentgenology 46 (3), 178–186 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Scholten ET, Kreel L Distribution of lung metastases in the axial plane. A combined radiological-pathological study. Radiologica Clinica (Basel) 46 (4), 248–265 (1977). [PubMed] [Google Scholar]
- 40.Braman SS, Whitcomb ME Endobronchial metastasis. Archives of Internal Medicine 135 (4), 543–547 (1975). [PubMed] [Google Scholar]
- 41.Herold CJ, Bankier AA, Fleischmann D Lung metastases. European Radiology 6 (5), 596–606 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Kimura H et al. Real-time imaging of single cancer-cell dynamics of lung metastasis. Journal of Cellular Biochemistry 109 (1), 58–64 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Thevenaz P, Ruttimann UE, Unser M A pyramid approach to subpixel registration based on intensity IEEE Transactions on Image Processing: A Publication of the IEEE Signal Processing Society 7 (1), 27–41 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Sharma VP ImageJ plugin HyperStackReg V5.6 Zenodo (2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Mechanical design drawings for the stainless-steel cutting tool used to guide the 5 mm biopsy punch.
Supplemental Figure 2: Mechanical design drawings for the stainless-steel window frame.
Supplemental Figure 3: Mechanical design drawings for the window holder tool.
Supplemental Movie 1: Video corresponding to Figure 3 showing the lung vasculature with circulating 2 μm microspheres.


