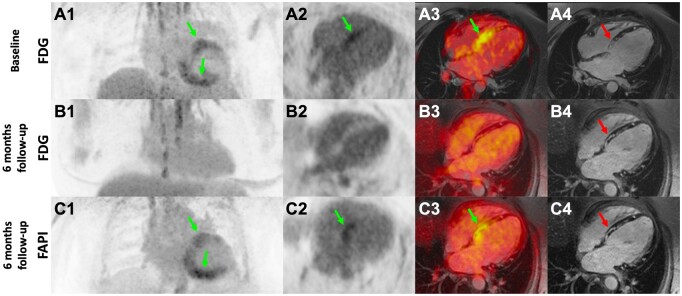
One year after being diagnosed with Stage I pulmonary sarcoidosis by lymph node extraction, a 58-years old female patient without a history of heart disease presented with shortness of breath, fatigue, N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels of 11 865 pg/mL (<125 pg/mL), and a markedly reduced left ventricular ejection fraction (LVEF) of 29%. A hybrid magnetic resonance imaging (MRI) technique with 2-[18F]fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET) showed intense FDG uptake (Panels A1–3, light green arrows) and late gadolinium enhancement (LGE; Panel A4, red arrow) in the septal and posterior wall of the left ventricle, confirming the diagnosis of cardiac sarcoidosis (CS). Heart failure medication with spironolactone, sacubitril/valsartan, bisoprolol, and high doses of corticosteroids (methylprednisolone 80 mg/day) was initiated. In addition, the patient was provided with an external defibrillation vest for markedly reduced systolic function and active inflammation in cardiac sarcoidosis.
The patient’s condition deteriorated and PET/MRI 3 months later remained unchanged; therefore, medical therapy was escalated with azathioprine according to the clinical routine with an initial starting dose of 50 mg/day. This dosage was gradually increased to 150 mg/day while methylprednisolone was reduced to a maintenance dose of 7.5 mg/day.
Six months after diagnosis, the patient’s symptoms improved significantly, accompanied by a drop of NT-proBNP to 570 pg/mL and recovery of LVEF (43%). Follow-up FDG–PET revealed no tracer uptake (Panels B1–B3), suggesting complete remission of CS. Magnetic resonance imaging findings were stable showing persistent LGE (Panel B4). An additional PET-MRI was performed using 68Gallium-FAPI-46 (FAPI), an inhibitor of fibroblast protein-alpha, expressed by activated fibroblasts in fibrotic remodelling processes. FAPI–PET (Panels C1–C3, light green arrows) demonstrated pronounced tracer uptake indicating intense fibroblast activation in the basal septum/posterior wall. These areas matched the regions of LGE/FDG uptake from 6 months before (Panel C4, red arrow), suggesting ongoing cardiac remodelling hence questioning the discontinuation of the immunomodulatory therapy. This hypothesis of ongoing remodelling was underlined by the need for an implantable cardioverter/defibrillator implantation for several episodes of slow ventricular tachycardias despite improved LVEF about 9 months after the initial diagnosis. As the clinical condition improved under azathioprine and imaging suspected ongoing remodelling, we decided to continue the combined immunosuppressive therapy for a minimum duration of 2 years. Further, routine in-office visits including assessment of clinical condition, echocardiography, electrocardiogram, and laboratory panels including NT-proBNP were scheduled every 3 months. In order to reduce azathioprine toxicity, immunosuppression was reduced to alternating doses of 100 mg/150 mg. At the end of the 2-year period, the next FDG–PET scan (in combination with MRI) is scheduled before the decision on (dis)continuation of the dual immunosuppression as serial FDG-PET scans are suggested to be useful to monitor the clinical course of the disease. Now, about 1 year after initial sarcoidosis diagnosis, the patient is free of ventricular tachycardia recurrence under moderate beta-blocker dosage (bisoprolol 5 mg/day) and a combined immunosuppression with azathioprine in alternating doses of 100 mg/150 mg and methylprednisolone 7.5 mg/day.
Our findings suggest a potential role of FAPI-PET in the treatment guidance of immunomodulatory therapy in CS. Future studies will have to evaluate whether FAPI-PET has prognostic implications.
Figure 1.
Hybrid 2-FDG PET/MRI showed intense FDG uptake (Panels A1–A3, light green arrows) and patchy LGE (Panel A4, red arrow) in the septal and posterior wall of the left ventricle in the first scan at our centre, confirming the diagnosis of CS. Follow-up FDG-PET 6 months after immunosuppressive escalation revealed no increased tracer uptake in the initially involved areas, suggesting complete remission of the inflammation in the context of CS (Panels B1–B3, light green arrows). Late gadolinium enhancement MRI, however, demonstrated persistent LGE (Panel B4, red arrow). The PET scan using FAPI, an inhibitor of fibroblast protein-alpha, demonstrated pronounced tracer uptake as a surrogate for intense fibroblast activation in the initially inflamed basal septum/posterior wall (Panels C1–C3). These areas matched the regions of LGE/FDG uptake from 6 months before (Panels A4, B4, C4).
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Contributor Information
Johannes Siebermair, Department of Cardiology and Vascular Medicine, West German Heart and Vascular Center Essen, University of Duisburg-Essen, Hufelandstrasse 55, 45147, Essen, Germany; DZHK (German Centre for Cardiovascular Research), Partner Site Munich Heart Alliance, Munich, Germany.
Lukas Kessler, Department of Nuclear Medicine, University Hospital Essen, University of Duisburg-Essen, Hufelandstrasse 55, 45147, Essen, Germany.
Jana Kupusovic, Department of Cardiology and Vascular Medicine, West German Heart and Vascular Center Essen, University of Duisburg-Essen, Hufelandstrasse 55, 45147, Essen, Germany.
Tienush Rassaf, Department of Cardiology and Vascular Medicine, West German Heart and Vascular Center Essen, University of Duisburg-Essen, Hufelandstrasse 55, 45147, Essen, Germany.
Christoph Rischpler, DZHK (German Centre for Cardiovascular Research), Partner Site Munich Heart Alliance, Munich, Germany; Department of Nuclear Medicine, University Hospital Essen, University of Duisburg-Essen, Hufelandstrasse 55, 45147, Essen, Germany.