Abstract
Background
Immune checkpoint inhibitors (ICIs) have improved outcomes for many types of cancer. However, ICI therapies are associated with the development of myocarditis, an immune-mediated adverse event associated with a high mortality rate. Therefore, prompt diagnosis and early intervention are of outmost importance. There is limited data on the application of cardiovascular magnetic resonance (CMR)-based modified Lake Louise Criteria (mLLC) with the use of relaxometry techniques for the diagnosis of ICI myocarditis.
Case summary
Four cancer patients undergoing ICI treatment presented with various clinical symptoms and troponin elevation to emergency/ambulatory clinics within 10–21 days after ICI initiation. On the suspicion of possible ICI-related myocarditis all patients underwent CMR within a few days after admission. Applying mLLC including relaxometry techniques, all patients met ‘non-ischaemic injury criteria’, while 3/4 patients met ‘oedema criteria’. In most patients, quantitative mapping revealed substantially increased T1 values, while T2 values were only mildly increased or normal. In two patients with follow-up, CMR demonstrated improvement in findings after immunosuppressive treatment. However, there was only limited agreement between the degree of high-sensitive troponin levels and T1/T2 levels.
Discussion
The application of mLLC with T1/T2 mapping appears useful in the CMR diagnosis of acute ICI myocarditis with non-ischaemic myocardial injury criteria being the most common finding. The sensitivity of native T1 appears higher than T2 mapping in the acute diagnosis as well as in the assessment of treatment response. As troponin elevations may persist for some time with ICI myocarditis, CMR may represent an alternate strategy to monitor treatment response.
Keywords: Magnetic resonance imaging, Myocarditis, Immune checkpoint inhibitors, Left ventricular function, Case series
Learning points.
Application of modified Lake Louise Criteria appears useful in the cardiovascular magnetic resonance diagnosis of immune checkpoint inhibitor (ICI) myocarditis across a wide variety of clinical presentations/lab results.
Non-ischaemic myocardial injury may be the most common finding in patients with acute ICI myocarditis.
Introduction
Immune checkpoint inhibitor (ICI)-related myocarditis is a rare (incidence ∼1%) immune-related adverse event (irAE).1 While uncommon, it is associated with major adverse cardiac events in 20–40% of patients.1–3 Cardiovascular magnetic resonance (CMR) is the reference imaging test for the diagnosis of myocarditis.4 The CMR approach has evolved since publication of the initial Lake Louise Criteria (LLC) with added quantitative approaches (e.g. T1/T2 mapping) resulting in improved diagnostic accuracy.5–9 Recent work has demonstrated that in patients with ICI myocarditis, abnormalities in T2-weighted imaging and late gadolinium enhancement (LGE) were absent in >50% of the patients.3 However, the diagnostic value of modified LLC (mLLC) for ICI myocarditis is unknown. With the rapidly increasing use of ICI cancer therapy, there is a need for improved diagnosis of ICI myocarditis. Given the non-invasive nature of CMR, its application is attractive.
In this case series, we illustrate four representative patients who underwent CMR with suspected ICI-related myocarditis; Research ethics board (REB) approval and written informed consent was obtained (all patients deceased). We focus on CMR based mLLC for the diagnosis9 through illustrating: (i) the value of multi-parametric and quantitative CMR, (ii) the regionality of the myocardial T1/T2 changes, (iii) the relationship between troponin levels and CMR changes, and (iv) the potential role of CMR in assessing recurrence/treatment responsiveness.
Timeline
| Age/sex | Cancer diagnosis | Time post-immune checkpoint inhibitor initiation | Symptoms | hsTnI [high-sensitivity troponin I (local upper limit of normal, ULN: 26 ng/L)] | BNP [brain natrium peptide (local ULN: <99.9 pg/mL)] | Treatment | Remarks | |
|---|---|---|---|---|---|---|---|---|
| Case 1 | 52/male | Melanoma (metastatic) | Day 21 (Presentation) | Fatigue, shortness of breath on exertion | 19 ng/L | 108 pg/mL | endomyocardial biopsy (EMB) confirmation | |
| Day 23 (cardiovascular magnetic resonance, CMR) | 5 ng/L | 110 pg/mL |
|
|||||
| Day 37 | 99 ng/L | |||||||
|
|
|
Improved EF, reduced effusions | |||||
| Case 2 | 60/female | Gynecological Cancer (metastatic) | Day 13 (presentation) | Generalized weakness, muscle pain, fatigue | 168 ng/L | Not available | 1 mg/kg IV mPRED |
|
| Day 15 (CMR) | 12 122 ng/L | Not available |
|
|||||
| Day 26 (discharge) | 26 ng/L | |||||||
| Case 3 | 49/female | Breast cancer (metastatic) | Day 13 (presentation) | Fever, cough | 1829 ng/L | 405 pg/mL | EMB declined | |
| Day 23 (CMR) | 855 ng/L | Not available |
|
|||||
| Day 25 (discharge) | ||||||||
| Case 4 | 74/female | Gynecological cancer (metastatic) | Day 17 (presentation) | General pain, progressive muscle weakness, diplopia | 3744 ng/L | 26 pg/mL |
|
|
| Day 20 (CMR) | 4717 ng/L | Not available |
|
|||||
| 750 mg MMF 2× day | ||||||||
| Day 30 (discharge) |
Case presentation
Case 1
A 52-year-old male with metastatic melanoma undergoing therapy with an anti-cell death ligand 1 (anti-PD-L1) antibody in combination with an investigational ICI presented with fatigue and marked shortness of breath on exertion 21 days from the first treatment. Oxygen saturation (room air) was 96% and his electrocardiogram (ECG) was normal. Chest computed tomography (CT) identified mild bilateral pleural effusions, compression atelectasis, new moderate pericardial effusion, and minor pulmonary oedema.
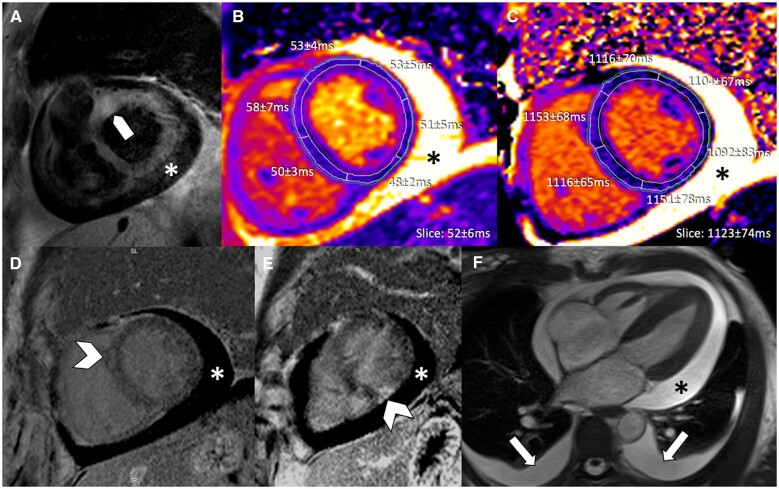
The high-sensitivity troponin I (hsTnI) was 19 ng/L [upper limit of normal (ULN) 26 ng/L, pre-treatment <2 ng/L], creatine Kinase (CK) was 14 (ULN ≤240 U/L), and brain natrium peptide (BNP) was 108 pg/mL (ULN <99.9 pg/mL). Echocardiography showed preserved left ventricular ejection fraction (LVEF) without signs of tamponade. CMR (1.5 T) was performed 2 days after symptom onset (hsTnI at time of CMR: 5 ng/L, CK <10 U/L, and BNP 110 pg/mL). The exam demonstrated focal signal abnormalities on T2-weighted imaging (Figure 1A), and regional variability in T2 values on T2 mapping [T2-prep steady state free-precession (SSFP)] slightly exceeding ULN (up to 58 ms; local normal: 45–57 ms) (Figure 1B). Native T1-mapping [modified Look-Locker inversion recovery (MOLLI); 5(3)3] demonstrated significantly elevated T1 (segmental: up to 1153 ms; local normal: 953–1053 ms) (Figure 1C). Post-contrast (0.15 mmol/kg) imaging, identified minor areas of LGE (Figure 1D and E). Cine SSFP demonstrated a moderate pericardial and pleural effusion (Figure 1F) with normal LVEF (56%). Due to discrepancy between troponin levels and CMR findings, the patient underwent endomyocardial biopsy (EMB), which demonstrated interstitial infiltration by macrophages (CD68) and lymphocytes (CD3/CD8) consistent with myocarditis. The patient was treated with corticosteroids (3 days of 2 mg/kg IV methylprednisolone, followed by a taper of oral prednisone staring at 150 mg/day). Despite treatment, hsTnI levels rose peaking at 99 ng/L on Day 16 after symptoms onset. Repeat CMR confirmed initial findings with stable LGE, improvement in LVEF (59%), and reduction in pericardial and pleural effusions. However, given the rising troponin, patient received 5 days of 1 g of methylprednisolone followed by oral prednisone (2 mg/kg) and was discharged with declining hsTnI levels (36 ng/L).
Figure 1.
Initial multi-contrast cardiovascular magnetic resonance image panel in patient with immune checkpoint inhibitor myocarditis (high-sensitivity troponin I at time of cardiovascular magnetic resonance: 5 ng/L). (A) T2-weighted SPAIR, (B) T2 mapping, (C) native T1 mapping, (D, E) phase sensitive inversion recovery late gadolinium enhancement imaging, and (F) cine steady state free precession. Focal basal myocardial oedema (block arrow), focal late gadolinium enhancement (arrowheads), moderate pericardial effusion (*), and mild bilateral pleural effusion (arrows) are demonstrated.
On tapering oral steroids, he was readmitted 79 days after initial presentation (hsTnI 84–109 ng/L); repeat CMR confirmed stable LGE/focal elevated signal on T2-weigthed imaging, normal LVEF (67%), resolution of pericardial effusion, and no elevation in myocardial T1/T2 values. Patient received 350 mg infliximab IV and mofetil mycophenolate (MMF) (1000 mg twice a day), while oral prednisone was continued; ICI therapy was never re-started.
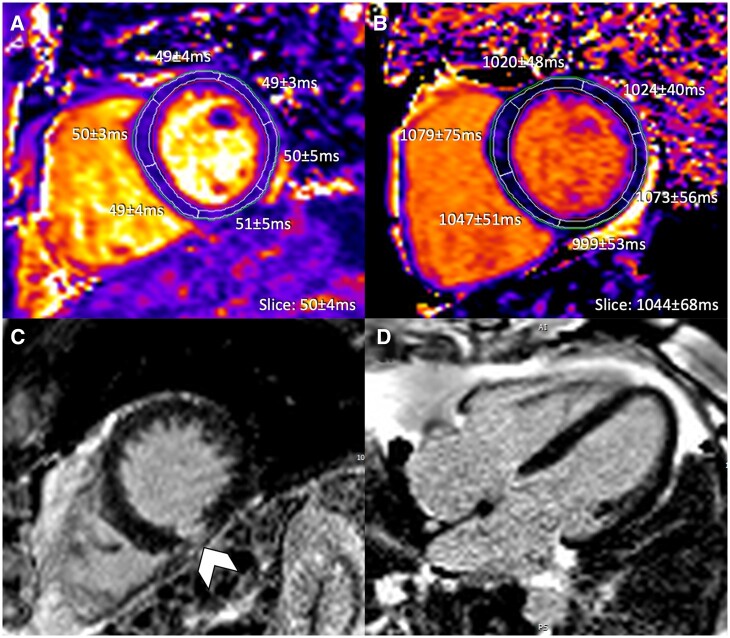
Patient had routine follow-up CMR 6 months after the initial diagnosis demonstrating normalization of T2 values (slice 50 ± 4 ms, max: 51 ms) and a prominent interval reduction in T1 (slice: 1044 ± 68 ms) (Figure 2A and B). Focal LGE changes were stable (Figure 2C and D); ECG and hsTnI values (9 ng/L) were normal.
Figure 2.
Repeat cardiovascular magnetic resonance of same patient as displayed in Figure 1 after 6 months of steroid treatment demonstrating (A) T2 mapping, (B) native T1 mapping, and (C, D) phase sensitive inversion recovery late gadolinium enhancement imaging. Pericardial effusion has resolved, residual focal late gadolinium enhancement demonstrated (arrowhead).
Despite initial discordant hsTnI values and CMR findings, T1 mapping demonstrated significant abnormalities that resolved with aggressive immunosuppression. The main T1/T2 criteria of mLLC for diagnosis of myocarditis were met (Table 1).9 Nevertheless, there was a larger increase in myocardial T1 vs. T2 values compared to normal ranges.
Table 1.
Summary of presence/absence of main and supportive criteria according to modified Lake Louise Criteria in presented cases.
| Main criteria |
Supportive criteria |
|||||||
|---|---|---|---|---|---|---|---|---|
| T2-based imaging (oedema)a | T1-based imaging (non-ischaemic injury) | Pericarditis | Systolic dysfunction | |||||
| Case 1 | Regional high T2 signal intensity | + | Increase in native T1 relaxation timeb | + | Pericardial effusion (cine images) | + | Regional wall motion abnormalities | − |
| Increase in T2 relaxation time | + | LGE with non-ischaemic distribution | + | Abnormal LGE, T2w or T1w imaging | − | Global hypokinesia | − | |
| Case 2 | Regional high T2 signal intensity | + | Increase in native T1 relaxation timeb | + | Pericardial effusion (cine images) | + | Regional wall motion abnormalities | − |
| Increase in T2 relaxation time | + | LGE with non-ischaemic distribution | + | Abnormal LGE, T2w or T1w imaging | + | Global hypokinesia | − | |
| Case 3 | Regional high T2 signal intensity | − | Increase in native T1 relaxation time and ECV | + | Pericardial effusion (cine images) | + | Regional wall motion abnormalities | − |
| Increase in T2 relaxation time | − | LGE with non-ischaemic distribution | + | Abnormal LGE, T2w or T1w imaging | + | Global hypokinesia | − | |
| Case 4 | Regional high T2 signal intensity | + | Increase in native T1 relaxation timeb | − | Pericardial effusion (cine images) | − | Regional wall motion abnormalities | − |
| Increase in T2 relaxation time | − | LGE with non-ischaemic distribution | + | Abnormal LGE, T2w or T1w imaging | − | Global hypokinesia | − | |
| Total | Cases with↑T2 SI | 3/4 | Cases with non-ischaemic LGE | 3/4 | Cases with pericardial effusion | 3/4 | Cases with abnormal regional wall motion | 0/4 |
| Cases with↑T2 time | 2/4 | Cases with↑native T1 | 4/4 | Abnormal LGE, T2w or T1w imaging | 2/4 | Cases with global hypokinesia | 0/4 | |
Performed imaging protocols did not include assessment of T2 SI ratios.
No post-contrast T1 mapping performed for ECV calculation.
Case 2
A 60-year-old female undergoing combination treatment including anti-PD-L1 antibody in the setting of metastatic gynaecological cancer presented 13 days after first administration complaining of generalized weakness, muscle pain, and fatigue. She had a fever, elevated creatinine kinase (CK) (2260 U/L), and hsTnI (168 ng/L). Due to progressive hsTnI elevation (peak: 15 463 ng/L), despite normal ECG, a coronary angiogram was performed ruling out obstructive coronary artery disease (CAD).
Cardiovascular magnetic resonance (1.5 T) was performed 2 days after initial presentation and post coronary angiogram. At the time of CMR, hsTnI was 12 122 ng/L and the first methylprednisolone dose (1 mg/kg) was administered 48 h prior. Cardiovascular magnetic resonance identified focal signal elevation on T2-weighted imaging (Figure 3A) and T2 values were increased (up to 58 ms) (Figure 3B). Corresponding T1 was increased (up to 1115 ms) with regional variation (Figure 3C). LGE imaging demonstrated multiple high intensity subepicardial to mid-myocardial areas of enhancement (Figure 3D and E). The LVEF was 56% and there was a trivial pericardial effusion (Figure 3F). Endomyocardial biopsy demonstrated interstitial infiltrates of CD3 positive T-lymphocytes. After biopsy confirmation, she received 2 days of 1 g IV methylprednisolone followed by oral prednisone (2 mg/kg daily). She was discharged 13 days after initial presentation with an hsTnI of 26 ng/L. Immune checkpoint inhibitor therapy was never re-started. Follow-up CMR (3 T) performed 8.5 months after initial presentation revealed improvement in LGE and signal elevation on T2-weighted imaging (Figure 4A and B); respective T1/T2 values were normal (Figure 4C and D).
Figure 3.
Myocarditis from anti-PD-L1 treatment with a very high-sensitivity troponin I level (12 122 ng/L at time of cardiovascular magnetic resonance). (A) T2-weighted SPAIR, (B) T2 mapping, (C) native T1 mapping, (D, E) phase sensitive inversion recovery late gadolinium enhancement imaging, and (F) cine steady state free precession. Focal mid-ventricular inferior/inferoseptal myocardial oedema (block arrows), high signal intensity focal subepicardial late gadolinium enhancement (arrowheads), and mild pericardial effusion (*) are demonstrated.
Figure 4.
Follow-up cardiovascular magnetic resonance of same patient as displayed in Figure 3, 8.5 months after initial presentation and steroid treatment demonstrating (A, B) phase sensitive inversion recovery late gadolinium enhancement, (C) native T1 mapping, and (D) T2 mapping. Small pericardial effusion has resolved, and focal late gadolinium enhancement has improved (arrowheads). Segmental and global T1 and T2 values are within local normal ranges (Note: Follow-up performed at 3 T).
In this patient, CMR demonstrated a clear increase in T1 albeit a smaller increase in T2 in the context of high hsTnI and acute ICI myocarditis.
Case 3
A 49-year-old female with metastatic triple-negative breast cancer presented 13 days after initiation treatment with anti-PD-L1 antibody with fever and cough. A non-contrast-enhanced chest CT excluded underlying pneumonia/-onitis. After fluctuating symptoms and initiation of antibiotics and anti-inflammatory therapy, she was admitted to intensive care unit 6 days later with a clinical picture suspicious of haemophagocytic lymphohistiocytosis/cytokine release syndrome. Her ECG showed small QRS complexes and non-specific ST-T wave abnormalities; hsTnI was 1829 ng/L; BNP was 405 pg/mL. Repeat CT excluded pulmonary embolism but demonstrated bilateral pleural effusions and diffuse pulmonary oedema. Due to the suspicion of myocarditis, MMF was added and titrated to 1000 mg twice daily.
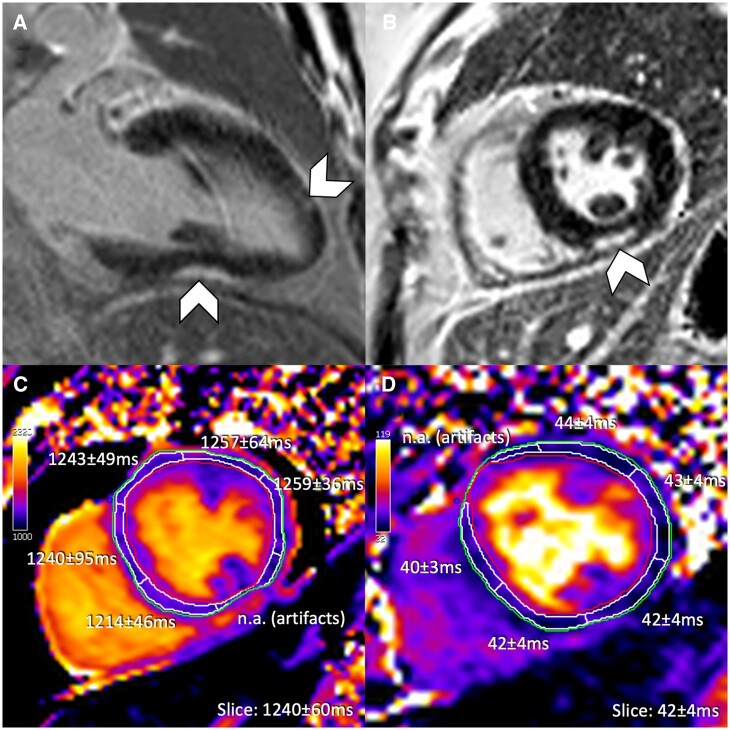
After clinical stabilization (Day 10 post-admission), the patient underwent CMR (1.5 T) for further evaluation (hsTnI at CMR: 855 ng/L). While pre-contrast T2-weighted imaging showed no abnormal signal intensity (Figure 5A) and global and regional T2 times (max. 56 ms) were upper normal (Figure 5B), native T1 values reached as high as 1192 ms (Figure 5C). In post-contrast imaging, subtle sub-epicardial basal inferior-lateral wall LGE with adjacent pericardial enhancement (Figure 5D) was identified. The extracellular volume fraction ranged from 33.2% to 40.2% (local volunteer cohort: 19.2–29.2%). Cine SSFP confirmed a small pericardial effusion on two-chamber view (Figure 5E) and small bilateral pleural effusions (Figure 5F); with LVEF of 56%.
Figure 5.
Anti-PD-L1 antibody-induced myocarditis and pericarditis with moderate to high elevations in high-sensitivity troponin I. (855 ng/L at time of cardiovascular magnetic resonance). (A) T2-weighted SPAIR, (B) T2 mapping, (C) native T1 mapping, (D) phase sensitive inversion recovery late gadolinium enhancement imaging, and (E, F) cine steady state free precession. Very subtle subepicardial late gadolinium enhancement with additional pericardial enhancement (arrowheads), mild pericardial effusion (*), and mild pleural effusions are demonstrated.
Despite lack of EMB confirmation (patient declined), ICI myocarditis was diagnosed based on European Society of Cardiology (ESC) criteria.10 Patient was discharged on oral prednisone (50 mg/day) and MMF 1000 mg twice per day. Immune checkpoint inhibitor therapy was never re-started. Again, in this patient, we note significantly elevated T1 values with upper normal T2 values in the setting of pre-treatment with steroids 10 days before CMR. In fact, in this case, T2-based criteria for diagnosis of myocarditis according to mLLC were not met (Table 1).
Case 4
A 74-year-old female with metastatic gynaecological cancer presented 17 days after initiation of anti-PD-L1 antibody therapy complaining of general pain, progressive muscle weakness and diplopia. Her hsTnI was 3744 ng/L, BNP 26 pg/mL, and the ECG demonstrated new ST-segment depression in inferolateral leads thus meeting ESC clinical criteria for myocarditis10; coronary angiogram excluded obstructive CAD.
For confirmation of possible myocarditis, CMR (1.5 T) was performed 3 days after initial presentation (hsTnI at time of CMR: 4717 ng/L) with patient having been on 1 g methylprednisolone for 3 days prior.
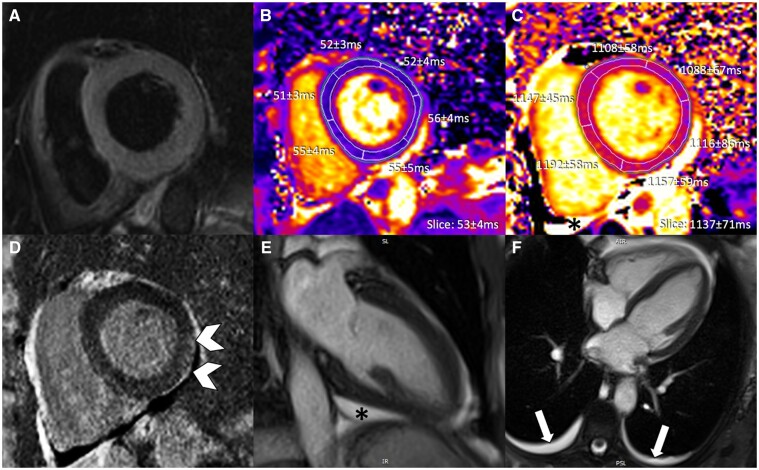
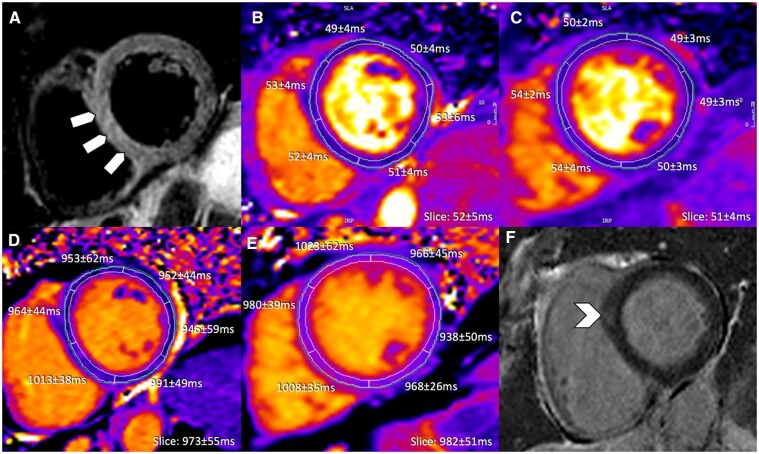
T2-weighted imaging demonstrated subtle high signal intensity within the interventricular septum (Figure 6A) suggesting myocardial oedema. Despite significant regional heterogeneity of T1/T2 values (global and segmental) at various slice positions, overall values were within normal ranges (Figure 6B and C). The highest/lowest segmental T2 and T1 times were 54/49 ms and 1023/938 ms, respectively. There was mild mid-wall LGE at the basal anterior septum (Figure 6F) while LVEF was normal (58%).
Figure 6.
Anti-PD-L1 therapy-mediated myocarditis with moderate increase in high-sensitivity troponin I (4717 ng/L at time of cardiovascular magnetic resonance). (A) T2-weighted SPAIR, (B, C) T2 mapping, (D, E) native T1 mapping, and (F) phase sensitive inversion recovery late gadolinium enhancement imaging. Diffuse septal oedema (block arrows) and only subtle focal septal late gadolinium enhancement (arrowhead) visualized.
After 5 g of methylprednisolone, the patient was switched to oral prednisone (50 mg/day). Due to persistent hsTnI elevation (607 ng/L), she was also started on MMF 750 mg twice per day with further increase of oral prednisone to 70 mg/day. She was discharged 13 days after initial presentation and ICI therapy was never re-started.
While conventional qualitative CMR techniques delineated changes that fulfilled mLLC, both quantitative approaches (T1/T2 mapping) failed to identify regional elevations despite high hsTnI elevation. This case illustrates the fact that using site-based normal ranges, some patients with clinical diagnosis of myocarditis10 may not show abnormal quantitative T1/T2 on a single applied slice.
Discussion
This case series illustrates the important CMR contribution in the assessment of ICI myocarditis. Using the mLLC, all patients met the ‘non-ischaemic myocardial injury criteria’ and 75% met the ‘oedema criteria’ (Table 1). Although LGE was present in all patients with elevated T1 values, the degree of elevation was higher than expected given the subtle LGE in some of the cases (e.g. Patient 3). While T2 criteria were met in three cases based on elevated signal intensities on T2-weighted techniques, T2 values were only mildly elevated in two cases (Table 1). Our findings are consistent with a recent meta-analysis in patients with non-ICI myocarditis demonstrating the potential advantage of T1 over T2 mapping, especially with respect to sensitivity and negative predictive value.11 While the increase in T1 may illustrate a combined effect of progressive myocardial fibrosis in addition to acute injury, the lack of significant T2 elevations may reflect the fact that imaging was performed after initiation of steroid therapy and a potential greater impact of steroid therapy on T2 changes. Interestingly, there was significant heterogeneity in segmental T1/T2 values in our patients possibly reflecting heterogeneous myocardial injury pattern in ICI myocarditis that warrants further exploration. It must be noted that T1/T2 mapping coverage is commonly limited (single slice in 3/4 of our cases) and does not match left ventricular coverage of LGE/T2-weighted imaging thus possibly missing regional abnormalities.
Pericardial abnormalities supportive mLLC were identified in most of our patients; however, none demonstrated LVEF/regional wall motion abnormality. Therefore, this case series, along with published studies,1,3 suggests that an optimal CMR protocol should include functional imaging, T2-weighted and LGE imaging (ventricular coverage) enhanced by T1/T2 mapping (ideally with ventricular coverage).
Interestingly, troponin levels at the time of CMR varied widely amongst our patients; significantly elevated T1 values were identified at normal/moderately elevated hsTn levels (5 ng/L, 855 ng/L; ULN <26 ng/L) with normal T1 in a patient with significantly elevated hsTnI (4717 ng/L; Patient 4). This suggests that markedly elevated hsTnI levels alone may not be a useful gatekeeper to CMR in patients with ICI myocarditis, but decision should be driven by clinical suspicion.
Two cases also demonstrate the responsiveness of CMR tissue characteristics to immunosuppression. While native T1 was still elevated despite CMR being performed within 3–10 days after initiation of corticosteroid therapy, prolonged treatment resulted in reduced T1/T2 values. As troponin elevations can persist in some patients with ICI myocarditis,12 CMR may also be an alternate strategy to monitor treatment response and to decide timing of stopping immunosuppression.
In conclusion, mLLC appear useful in the diagnosis of ICI myocarditis across a wide variety of clinical and laboratory findings. Our case series suggests that non-ischaemic myocardial injury may be the most common sign in patients with acute ICI myocarditis with greater sensitivity of native T1 compared to T2 mapping; T1 mapping may also have a role in identifying treatment response.
Notice: Only ICI drug classes are provided without specific drug names as per clinical study institutional policy.
Lead author biography

Dr Bernd J. Wintersperger is Professor of Radiology at the Department of Medical Imaging, University of Toronto and the Director of Magnetic Resonance Imaging and Director of Cardiac Imaging Operations of the Department of Medical Imaging (JDMI) at the Peter Munk Cardiac Centre, University Health Network, Toronto. Dr Bernd J. Wintersperger’s research interest mainly focuses on CMR with special emphasis on quantitative methods, quantitative tissue characterization, and innovative accelerated imaging methods. Other aspects involve cardiovascular MRI at high-field strength (3 T) and clinical applications of novel contrast agents. Dr Bernd J. Wintersperger has >180 PubMed listed publications.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case series including images and associated text has been obtained from the patients’ next-of-kin in line with COPE guidance.
Conflict of interest: B.J.W. has received research support and speaker’s honorarium from Siemens Healthineers and is consulting for Bayer AG. B.J.W. is an inventor of the IG fitting method owned by UHN (US10314548B2). T.G.N. has received grant support from Astra Zeneca. P.T. has received consultancy fees from Amgen, Takeda, and BI. The University Health Network (UHN) has a master research agreement (MRA) with Siemens Healthineers.
Funding: O.C.-A. was supported in part by Hold’em for Life Oncology Clinician Scientist Award. C.P.H. was funded by a grant from the German Research Foundation (419344766). P.T. was supported by a Canada Research Chair in Cardio-oncology.
Supplementary Material
Contributor Information
Bernd J Wintersperger, Department of Medical Imaging, Peter Munk Cardiac Center, University Health Network, 585 University Avenue, Toronto, Ontario M5G 2N2, Canada; Department of Medical Imaging, University of Toronto, 263 McCaul St, Toronto, Ontario M5T 1W7, Canada.
Oscar Calvillo-Argüelles, Division of Cardiology, Ted Rogers Program in Cardiotoxicity Prevention, Peter Munk Cardiac Center, University Health Network, 585 University Avenue, Toronto, Ontario M5G 2N2, Canada.
Stephanie Lheureux, Division of Medical Oncology and Hematology, Princess Margaret Cancer Center, University of Toronto, 610 University Ave, Toronto, Ontario M5G 2C1, Canada.
Christian P Houbois, Department of Medical Imaging, Peter Munk Cardiac Center, University Health Network, 585 University Avenue, Toronto, Ontario M5G 2N2, Canada; Department of Medical Imaging, University of Toronto, 263 McCaul St, Toronto, Ontario M5T 1W7, Canada.
Anna Spreafico, Division of Medical Oncology and Hematology, Princess Margaret Cancer Center, University of Toronto, 610 University Ave, Toronto, Ontario M5G 2C1, Canada.
Philippe L Bedard, Division of Medical Oncology and Hematology, Princess Margaret Cancer Center, University of Toronto, 610 University Ave, Toronto, Ontario M5G 2C1, Canada.
Tomas G Neilan, Cardio-Oncology Program and Cardiovascular Imaging Research Center (CIRC), Division of Cardiology, Department of Medicine, 55 Fruit Street Boston, Massachusetts 02114, USA.
Paaladinesh Thavendiranathan, Department of Medical Imaging, Peter Munk Cardiac Center, University Health Network, 585 University Avenue, Toronto, Ontario M5G 2N2, Canada; Division of Cardiology, Ted Rogers Program in Cardiotoxicity Prevention, Peter Munk Cardiac Center, University Health Network, 585 University Avenue, Toronto, Ontario M5G 2N2, Canada.
References
- 1. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang L, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J 2020;41:1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT et al. ; International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 2009;53:1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thavendiranathan P, Walls M, Giri S, Verhaert D, Rajagopalan S, Moore S et al. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ Cardiovasc Imaging 2012;5:102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferreira VM, Piechnik SK, Armellina ED, Karamitsos TD, Francis JM, Choudhury RP et al. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14:42–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Ntusi N et al. T(1) mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging 2013;6:1048–1058. [DOI] [PubMed] [Google Scholar]
- 8. Dabir D, Vollbrecht TM, Luetkens JA, Kuetting DLR, Isaak A, Feisst A et al. Multiparametric cardiovascular magnetic resonance imaging in acute myocarditis: a comparison of different measurement approaches. J Cardiovasc Magn Reson 2019;21:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158–3176. [DOI] [PubMed] [Google Scholar]
- 10. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 11. Pan JA, Lee YJ, Salerno M. Diagnostic performance of extracellular volume, native T1, and T2 mapping versus Lake Louise Criteria by cardiac magnetic resonance for detection of acute myocarditis: a meta-analysis. Circ Cardiovasc Imaging 2018;11:e007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu S, Chan J, Brinc D, Gandhi S, Izenberg A, Delgado D et al. Immune checkpoint inhibitor-associated myocarditis with persistent troponin elevation despite abatacept and prolonged immunosuppression. JACC Cardiooncol 2020;2:800–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.