Abstract
Background
Several technical devices are available to monitor and promote changes in behavior toward higher activity. In particular, smartphones are becoming the primary platform for recognizing human activity. However, the effects of behavior change techniques that promote physical, cognitive, and social activities on incident dementia in older adults remain unknown.
Objectives
This randomized controlled trial aims to examine the effects of behavior change techniques on the prevention of dementia among community-dwelling older adults using a smartphone as a behavior change tool.
Design
A randomized controlled trial.
Setting
Community in Japan.
Participants
The study cohort comprises 3,498 individuals, aged ≥60 years, randomized into two groups: the smartphone group (n = 1,749) and the control group (n = 1,749). Intervention. The smartphone group will be asked to use smartphone applications for at least 30 minutes daily to self-manage and improve their physical, cognitive, and social activities. The smartphone group will perform 60-minute group walking sessions using application-linked Nordic walking poles with cognitive stimulation twice a week during the intervention period. The walking poles are a dual-task exercise tool that works with a smartphone to perform cognitive tasks while walking, and the poles are equipped with switches to answer questions for simple calculation and memory tasks. The smartphone and control groups will receive lectures about general health that will be provided during the baseline and follow-up assessments.
Measurements
Incident dementia will be detected using cognitive tests (at baseline, after 15 months, and after 30 months) and by preparing diagnostic monthly reports based on data from the Japanese Health Insurance System. Participants without dementia at baseline who will be diagnosed with dementia over the 30-month follow-up period will be considered to have incident dementia.
Conclusions
This study has the potential to provide the first evidence of the effectiveness of information communication technology and Internet of Things in incident dementia. If our trial results show a delayed dementia onset for self-determination interventions, the study protocol will provide a cost-effective and safe method for maintaining healthy cognitive aging.
Key words: Dementia, geriatric medicine, protocols and guidelines, preventive medicine
Introduction
Dementia is a serious social problem in an aging society. The aging rate in Japan has exceeded 28%, and dementia prevention efforts have been implemented as a national strategy. Nonpharmacological interventions that address cognitive function and its effect on daily living are widely studied for preventing cognitive decline. A meta-analysis of longitudinal studies demonstrated low-to-moderate inverse associations between physical activity (PA) and cognitive decline and dementia, with overall relative risk estimates of 0.65 (95% confidence interval [CI] 0.55–0.76) and 0.86 (95% CI 0.76–0.97), respectively (1).
Despite the established PA benefits, 30% of the world’s population fail to reach the recommended PA levels (2). Adherence with PA recommendations can be affected by behavioral (motivation and personal beliefs) and environmental factors (availability of public transport and exercise venues) (3). The most commonly reported behavior change techniques to improve PA are self-monitoring, goal setting, tailoring, relapse prevention training, feedback, and strategies for increasing motivation and self-efficacy (4). Currently, several technical devices (smartphones, pedometers, heart rate monitors, and smartphone applications) are available to monitor and promote changes in behavior toward higher PA (5). They can be used separately or together with a computer, smartphone, or personal tablet computer when self-monitoring PA. Smartphones are ubiquitous and are changing the landscape of the daily lives of all generations. Many smartphones are equipped with various sensors, including GPS, accelerometers, gyroscopes, and barometers. These sensors are rich data sources for measuring the various aspects of the users’ daily lives. Due to their unobtrusiveness, low installation cost, and ease of use, smartphones are becoming the main platform for human activity recognition. Although smartphones are likely to be beneficial in improving the health of older people (6), it is unclear whether smartphone use is effective for preventing dementia in older adults.
We previously reported a significantly lower probability of dementia in individuals who engage in active lifestyle behaviors—including daily conversation, driving a car, shopping, fieldwork, and gardening using longitudinal observational data (7). Moreover, the participation in these activities was associated with reversibility of mild cognitive impairment (MCI) to normal cognition. Participants with MCI were more likely to revert if they continued driving a car; used maps to travel; read newspapers or books; and participated in cultural lessons, community meetings, sports, hobbies, gardening, and fieldwork (8). However, the effects of lifestyle activities on cognitive function improvement and the incidence of dementia in older adults remain unclear.
Several large-scale and nonpharmacological multimodal intervention studies for dementia prevention have failed to provide clear evidence of delayed onset of dementia. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), which is a two-year multicenter randomized controlled trial (RCT) conducted in Finland, investigated the effects of a multidomain intervention on cognitive impairment and disability delay in older adults who are at risk of cognitive decline (9). At the two-year interim analysis, FINGER found that the multidomain intervention improved cognitive function in older adults with cognitive impairments. The U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) by the Alzheimer’s Association sought to replicate the Finnish trial results in the United States (10). The U.S. POINTER was a 2-year clinical trial that evaluated whether lifestyle interventions that simultaneously target many risk factors protect cognitive function in older adults who are at increased risk for cognitive decline. Although these multimodal interventions may have strong effects, they are expensive and difficult to implement. Additionally, evidence from RCTs is limited, and existing evidence does not support that nonpharmacological interventions reduce the risk of developing dementia (11). There is also limited evidence on the effectiveness of nonpharmacological approaches, and the cost-effectiveness of such approaches may be negative. A major reason for not being able to articulate the effectiveness of nonpharmacological interventions in preventing dementia may be the reduction in statistical power due to the short duration of the intervention and the small sample size. Future RCTs with a prolonged intervention phase and larger sample size are required to identify effective strategies for preventing dementia in older adults.
We developed a self-monitoring smartphone application and walking pole to facilitate daily physical, cognitive, and social activities. These information and communication technology and Internet of Things tools will enable RCTs with a prolonged intervention phase and larger sample size. We designed an RCT to determine the effect of dementia prevention by behavior change techniques—including the effects of daily smartphone application use on activities of daily living, and the effects of a pole walking program on cognitive function—in community-dwelling older adults. We also need to identify older adults with limited activities to prevent the spread of coronavirus disease 2019 (COVID-19) (12) by encouraging specific activities. In this study, the intervention will be conducted using a smartphone, which allows the participants to work by themselves and promotes non-face-to-face communication. In addition, group exercise can be conducted outdoors with sufficient interpersonal distance, thus, the program can be continued while managing COVID-19 infection precautions.
Methods
The Self-Management Activity Programme for the Older (SMAFO) study is funded by the Ministry of Health, Labour and Welfare in Japan (Subsidy of Long-Term Care Insurance [large-scale demonstration project]). This proof-of-concept trial aims to determine the effects of behavior change techniques on dementia prevention via smartphone use. This study may generate high-quality, long-term population-based data to support dementia prevention strategies (e.g., cost-effective smartphone-based SMAFO). Given that major cities in Japan provide similar services, the results can be used to develop a national strategy. This is one of the largest nonpharmacological intervention studies that focus on preventing dementia in older adults. Considering that SMAFO is a low-cost intervention that is adaptable to many older people, the information that we generate may inform future strategies in countries with similar service structures.
Study design
The SMAFO study is a single-center, assessorblind RCT, parallel-group superiority trial with a 1:1 allocation based on the National Centre for Geriatrics and Gerontology-Study of Geriatric Syndromes (NCGG-SGS), which is a population-based cohort study that began in 2011. The overall goal of NCGG-SGS is to establish a screening system for geriatric syndromes and validate evidence-based interventions for preventing geriatric syndromes. Eligible NCGG-SGS participants are aged ≥60 years at recruitment and live in the survey site, which includes Obu, Nagoya, Takahama, Tokai, Toyoake, and Chita Cities. Individuals with certificated Japanese long-term care insurance (LTCI) are excluded. Japan implemented the national social LTCI system on 1 April 2000 to publicly address the issue of care. Every Japanese aged ≥65 years is eligible for the benefits strictly on the basis of physical and mental frailty or disability (13). The LTCI categorizes a person as “Support Level 1 or 2″ or “Care Levels 1, 2, 3, 4, or 5″ if he or she needs support for daily activities or continuous care, respectively. A trained local government official evaluates nursing care needs by using a questionnaire on current physical and mental status (73 items) and on the use of medical procedures (12 items). The results are used to calculate the applicant’s standardized scores for the seven dimensions of physical and mental status, estimate the time of care, and assign a care needs level based on the total estimated care minutes. The Nursing Care Needs Certification Board, which comprises physicians, nurses, and other experts in health and social services, is appointed by a mayor. The board determines whether the initial assessment is appropriate based on the applicant’s primary care physician’s statement and home visit assessor’s notes to make a final decision about LTCI certification.
This study will be performed in the three NCGG-SGS subcohorts (Chita City, Takahama City, and Midori Ward in Nagoya City). The first surveys in Chita City (2021), Takahama City (2015) and Midori Ward in Nagoya City (2013) have been completed. The NCGG-SGS participants were 6,367 adults aged ≥65 years in Chita City, 4,167 adults aged ≥60 years in Takahama City, and 5,257 older adults aged ≥70 years in Nagoya City. We will recruit candidates for intervention research for these NCGG-SGS participants. Our research group plans to conduct a survey to recruit potential participants in other areas if the required number of cases is not reached in these three cities.
Eligibility criteria
Inclusion criteria
The initial eligibility criteria for the study are as follows: (1) NCGG-SGS survey participants in Chita City, Takahama City, or Midori Ward in Nagoya City, and (2) living in any of the three cities. All potential participants meeting the eligibility criteria will be recruited via direct mail from older adults enrolled in the NCGG-SGS or non-NCGG-SGS participants aged ≥60 years as of the briefing session date living in the cohort survey site or via public relations. The participants will be asked to attend a SMAFO study briefing session and will be asked to provide a signed written consent before participation to indicate that they understand and agree with the purpose and content of SMAFO.
Exclusion criteria
Participants will be excluded if they are unwilling or cannot access trial tasks on the SMAFO, have difficulty in participating in intervention sessions or in attending sessions due to severe disability or restricted exercises by the doctor or for health problems, have dementia, are LTCI certified; have cognitive function assessment deficit, or are unable to write/converse in Japanese. Participants will undergo the Mini-Mental State Examination (MMSE) to exclude older adults with severe cognitive impairment. The MMSE scores will be set at an absolute of <23, <24, or <26 for persons with <12, 12–15, or ≥16 years of education, respectively (14).
Intervention
The SMAFO group participants are asked to use a smartphone daily and to visit one of the 9 research fields twice weekly, where the 60-minute exercise program will be performed for 30 months in a total of 200 sessions. The SMAFO participants will use the online community salon application called Online Kayoinoba App, developed to enhance physical, cognitive, and social activities by using self-monitoring techniques. This application promotes outdoor activities by automatically creating walking courses and by posting photos of favorite places. The application also has a cognitive training game to improve memory, attention, and executive function, and has functions for home exercise, health check-up, communication, information of Kayoinoba, dietary control, and self-management (Table 1). Furthermore, SMAFO participants will be encouraged to communicate by using small group chats via a simple notification service and check whether they eat a well-balanced meal every day; these functions are included in the application developed for the SMAFO study. The smartphone group will be asked to use smartphone applications for at least 30 minutes daily to self-manage and improve physical, cognitive, and social activities.
Table 1.
Functional details of the Online Kayoinoba Application
| Function | Contents |
|---|---|
| Support of outdoor activity | Neighborhood spots registered in the application will be displayed, and when users select their favorite place, the route, time required, and distance traveled to that destination will be displayed. If they reach their destination, points are earned. The system automatically creates a recommended walking course within a walkable distance from the current location, and users can choose their favorite walking course and earn points if they follow the course. There is also a function that allows users to register their favorite walking courses and share the recommended walking courses with other users. |
| Home exercise | Delivery of videos of local physical exercises in each city, town and village. Delivery of videos of dementia prevention program that combine exercise and cognitive tasks. |
| Health check-up | Home Activity Guide 2020: Browse guidelines for exercises and activities that can be practiced at home according to physical and cognitive functions. Use of the Kihon Checklist. |
| Cognitive Training | Provide cognitive training included visuospatial memory, puzzle, calculation, n-back task to improve cognitive function. |
| Communication | Chat function for communication among users and groups. Bulletin board function for event information, etc. (To be implemented by the end of 2021) |
| Information of Kayoinoba (Community place in the real world.) | Displays location information, facility information, and activities of Kayoinoba to go nationwide. |
| Dietary control | Register meals and view ratings. Allows checking the balance of users’ diet. |
| Self-management | It is equipped with functions to help users manage themselves by visualizing and ranking the usage of each application function, including the amount of activity including the number of steps taken and the frequency of home exercise. |
Two trained facilitators will check the attendance of the participants at group sessions, provide instructions on how to use the smartphone, and facilitate communication between the participants at each SMAFO site. The group session will consist of 60 minutes of walking in the park. During the first month, the intervention group will perform simple walks, and the facilitator will teach them how to use their smartphones and applications. In the second month of the intervention, the participants will walk using the Nordic walking poles. From the third month of the intervention, the participants will perform the walk under multitasking conditions that include physical and cognitive tasks; we call this the combination training “Cognicise” (15). The SMAFO participants will use an application-linked Nordic walking pole with cognitive stimulation called Cognipole. A switch is equipped to the Cognipole handgrip to answer yes/no (right-hand/left-hand button) to cognitive tasks such as simple calculation and memory presented by the smartphone while walking. The cognitive tasks have a system of gradually increasing the difficulty level according to the correct answer rate, and the results are immediately provided to participants. The Cognipole was developed for this study and is not for sale at this time, but will be available from medical device manufacturers in the future.
Adherence
The participants’ adherence to the interventions is expected to improve with free smartphone use, as well as group chatting and group sessions, during study periods. Adherence will be monitored via the application, Cognipole, and supervised walking program. If a participant in the smartphone group fails to participate, the study facilitator will contact the person via chat.
Participants in both groups will attend three 90-minute health education classes that focus on health promotion during the 30-month trial period. The instructors will provide the participants with information regarding the aging process and geriatric syndromes. The topics in this lecture are planned so that they do not promote physical, cognitive, and social activities or significantly influence the study outcomes. Additional contacts will be made with the control participants over the study period to promote participation adherence in the follow-up examinations. Participants will be permitted to continue taking their normal medications and to perform daily activities, including sports, walking, and use of smartphone.
Outcome measures
Primary outcome: incidence of dementia
In Japan, all adults aged ≥65 years have public health insurance, including health insurance for employed individuals (Employees’ Health Insurance), national health insurance for unemployed and self-employed individuals aged 65–74 years (Japanese National Health Insurance), or health care for individuals aged ≥75 years (Later-Stage Medical Care) (16). In this study, participants will be tracked monthly for new incident dementia (Alzheimer’s disease or other dementia subtypes) during the follow-up period, as recorded by the Japanese National Health Insurance and Later-Stage Medical Care systems. Dementia will be diagnosed by medical doctors according to the International Classification of Diseases, 10th Revision (17). Participants without dementia at baseline but who are later diagnosed with dementia over a 30-month follow-up period will be considered incident dementia patients.
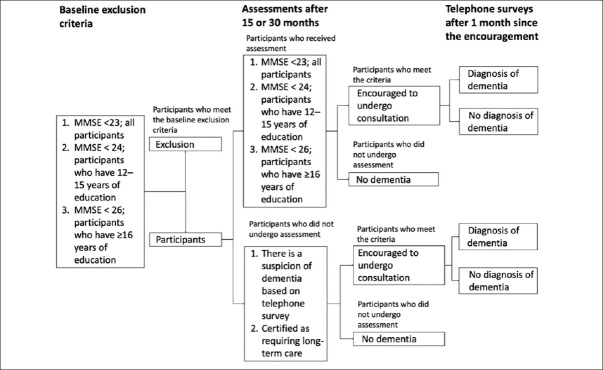
MMSE will be performed 15 months after the baseline survey and after the intervention to identify delayed dementia diagnosis for participants’ failure to visit the hospital. Participants with any less than the standard MMSE scores will be encouraged to visit a hospital: absolute scores of <23, <24, and <26 for persons with <12, 12–15, or ≥16 years of education, respectively, which are based on normative data and previous work of the New Haven Epidemiologic Catchment Area study (14). Participants who do not undergo MMSE will be confirmed by telephonic survey, and a suspicion of dementia or certification as requiring long-term care will lead to hospital consultation recommendation. Figure 1 shows the follow-up survey flow chart for the onset of dementia.
Figure 1.
Flow chart of the follow-up survey for the onset of dementia
MMSE, Mini-Mental State Examination
Secondary measurements
Before the intervention and at the 15- and 30-month intervention and control periods, secondary measurements (neuropsychological testing, completion of questionnaires, and assessments of physical function, blood test, neuroimaging assessment, incident of disability, and mortality) will be performed.
Neuropsychological testing
We will use the National Centre for Geriatrics and Gerontology-Functional Assessment Tool (NCGG-FAT), which is an iPad application, to conduct cognitive screening (18, 19). The NCGG-FAT includes the following domains: 1) memory (wordlist memory-I [immediate recognition] and II [delayed recall]), 2) attention (a tablet version of the Trail Making Test part A), 3) executive function (a tablet version of the Trail Making Test part B), 4) processing speed (a tablet version of the Digit Symbol Substitution Test), 5) working memory (digit span memory test), and 6) global cognition. The NCGG-FAT has high test-retest reliability, moderate-to-high criterion-related validity (18), and predictive validity (19) among community-dwelling older persons. Well-trained study assistants will conduct cognitive functioning assessments in community facilities, including community halls. Before the study begins, all staff will receive training from the authors regarding the protocols for administering the assessments. For all cognitive tests, we will use established standardized thresholds for each corresponding domain to define impairment in population-based cohorts comprising community-dwelling older persons (scores > 1.5 standard deviations [SDs] below age- and education-specific means). We will also assess MMSE (20) to measure the global cognitive function.
Questionnaires
Participants will be evaluated for basic activity of daily living (ADL), which includes five items, to assess basic self-care abilities, feeding, grooming, walking, bathing, and walking up stairs. The scores range from zero (complete independence; not applicable to all the items) to five (complete dependence). Participants will also be assessed for instrumental ADL by using the National Centre for Geriatrics and Gerontology-ADLs scale (21), which includes 13 items, including going out alone, taking the bus and train, handling medication, managing money, and making a meal. The scores range from 0 (low function) to 13 (high function). Self-reported PA will be assessed using the International Physical Activity Questionnaire-Short Form, which includes the frequency (days/week) and duration (minutes/day) of walking and moderate and vigorous intensity PA in the past 7 days (22, 23). Participants will be evaluated for depressive symptoms by using the 15-item Geriatric Depression Scale (GDS) (24), with scores ranging from 0 to 15; higher scores indicate a more depressed mood. The participants’ level of life satisfaction will be evaluated using the 13-item life satisfaction scale, where the scores range from 13 to 52 (higher scores indicate higher overall satisfaction) (25). Loneliness will be assessed using the 20-item version of the University of California Los Angeles Loneliness Scale version 3, with higher scores reflecting a greater degree of loneliness (26). Participants will be assessed for their dietary variety by using a dietary variety score comprising 10 food-based components; the scores range from 0 to 10, with a higher score indicating greater dietary variety (27). Participants will be assessed for social contact by using the National Centre for Geriatrics and Gerontology-Social Network Scale (NCGG-SNS), where the scores range from 0 to 64, with a higher score indicating better social network (28).
Physical function
The walking speed will be measured in seconds by using a stopwatch. The participants will walk five times on a flat and straight surface at a comfortable walking speed, and the average walking speed will be calculated. Dominant-hand grip strengths will be measured using a standard digital hand grip dynamometer (Takei Scientific Instruments Co., Ltd., Niigata City, Japan) in standing position with shoulder adducted and neutrally rotated and elbow in full extension (29). For all participants, the determination of physical frailty was based on the frailty phenotype proposed by Fried et al. (30) in the Cardiovascular Health Study. This phenotype consists of shrinking, weakness, slowness, self-reported exhaustion, and low PA.
Neuroimaging assessment
We will measure the brain structure (T1-weighted image, T2-weighted image, fluid attenuated inversion recovery image, diffusion tensor image, and phase difference enhanced image) and the resting state brain activity function (T2* BOLD-weighted image) by using 3-T magnetic resonance imaging.
Mortality and LTCI certification
We requested for new LTCI certification and mortality reports during the study periods from the administrative organization at Chita, Takahama, and Nagoya Cities.
Potential confounding factors
Numerous interrelated factors affecting the occurrence of dementia (including demographic variables, chronic medical conditions, psychosocial factors, and physical performance) are associated with dementia incidence in older persons (31, 32). We will assess outcome measurements and potential confounding factors to evaluate independent intervention effects. In this study, all multivariate models will include the following covariates unless otherwise specified: age at enrolment, sex, educational level, current smoking/drinking status, chronic medical illnesses, use of digital tools in participants’ usual life, and apolipoprotein E4 (ApoE4) carrier status. The presence of the following self-reported chronic medical illnesses will be entered into the models: history of stroke, heart disease, respiratory disease, hypertension, hyperlipidemia, and diabetes mellitus.
Procedure
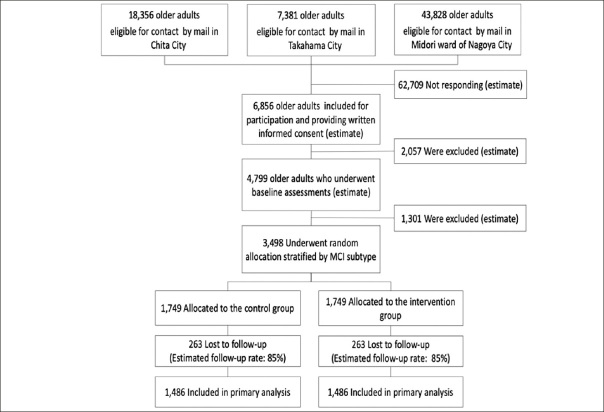
Figure 2 shows the predicted number of participants that will be included at each stage of the recruitment process. We would contact potential participants from the existing database via standard post. A two-stage screening process would be used to determine eligibility. Firstly, potential participants will be screened for major inclusion and exclusion criteria via recruitment letter. Potential participants will then be invited to attend a baseline assessment to measure secondary outcomes and confounding factors by a well-trained tester to confirm eligibility. Suitable participants will provide written informed consent prior to the collection of baseline measures.
Figure 2.
Flow chart of participants and estimated number of participants
MCI, mild cognitive impairment;
Blinding and data collection
After baseline measures have been obtained, participants will be randomized with a 1:1 allocation to one of two interventions: i) smartphone group or ii) control group. The individuals will be assigned to each study group by using a stratified randomization protocol. The participants will be stratified according to residence and cognitive status. Established standardized thresholds will be used for all cognitive tests conducted in this study to define impairment in the corresponding domain for a population-based cohort of community-dwelling older persons (scores > 1.5 SDs below age- and education-specific means). Major cognitive impairment will be characterized by deficits in more than one of the four NCGG-FAT domains. The randomization schedule will be generated by an investigator who is not involved in determining eligibility procedures (IC). Allocation concealment will be maintained by this same investigator (IC), who will only reveal group allocation to an investigator (SL) once baseline measures have been obtained.
All outcome measures will be collected by a research assistant and analyzed by a primary investigator (HS) by using the data without personally identifiable information. Due to the nature of the interventions, neither the primary investigator providing the interventions nor the participants can be blinded to treatment group allocation. In the case of an adverse event requiring the consideration of participant withdrawal, investigators who are not involved in data management and analysis will be consulted.
Study power and sample size
Power analysis has been performed and was based on the proportion of incident dementia, which was determined via meta-analysis, to estimate the incidence of dementia in community settings (33). The meta-analysis yielded a pooled incidence rate of dementia per 1,000 person-years of 17.18 (95% confidence interval [CI]: 13.90–21.23). By assuming a 40% reduction in the incidence of dementia due to this study intervention, the prevalence rates of dementia during the 30-month follow-up period will be 2.58% and 4.30% in the intervention and control groups, respectively. The 40% between-group difference is deemed realistic on the basis of previously published data (34). For instance, a meta-analysis that was conducted to assess the effects of exercise on the onset of dementia revealed that the incidence rates of dementia in exercisers and controls were 3.7% (n = 949) and 6.1% (n = 1,017), respectively (11). The meta-analysis found 39% between-group differences due to exercise interventions. When the α level is 0.05 and the power is 0.80, the required number of cases will be 2,972 (1,486 on each side). Considering a dropout rate of 15%, the required number of participants can be met by setting the number of registered cases to 3,498.
Data management and monitoring
All participant data will be coded by a research assistant, with no group identifier to maintain the blinding of the investigator responsible for data analysis (HS). All cases of nonadherence and nonretention will be electronically documented on a master spreadsheet to ensure the appropriate handling and interpretation of results. All electronic data were deidentified and stored on a password-protected computer. All hard copy data were deidentified and kept in a locked filing cabinet in a secured office. Paper-completed outcome measures will undergo double data entry by the blinded assistant into electronic spreadsheets with embedded range checkers for data value.
Statistical analyses
Student’s t-test and Pearson’s chi-squared test will be used to calculate intergroup differences in baseline characteristics. We will calculate the cumulative dementia incidence during follow-up and estimate intergroup differences by using the log-rank test. Cox proportional hazards regression models will be used to examine the intervention effects of SMAFO on dementia incidence. After adjusting for age and sex in the first model, we will use a multiple adjustment model adjusted for demographic variables, chronic medical diseases, lifestyle, psychological and physical performance, and cognitive function variables. Adjusted hazard ratios for dementia incidence and their 95% CIs will be estimated. The primary analysis will be conducted according to the intention-to-treat principle to determine whether there is a significantly reduced decline in primary and secondary outcome measures in participants in the combined program compared with those in the control group. To account for possible bias introduced by participants without 30-month data, multiple imputation analyses will be performed. The Markov chain Monte Carlo method, which is based on multivariate normality and is appropriate for measurement outcomes in this study, will be used to generate 50 imputed datasets based on age, sex, education history, ApoE4 carrier status, rating on the GDS, MCI subtype, and prior observations of outcome variables.
To evaluate differences in secondary outcome changes from the baseline and at 30-month follow-up between the SMAFO and control groups, the mixed-effects model for repeated measures with an unstructured covariance structure will be conducted. The differences between pre- and post-data for each outcome will be the dependent variables adjusted for age, sex, educational level, depressive symptoms, ApoE4 genotype carrier status, and MCI as covariates in the multivariable models. For secondary categorical variables, logistic regression analyses or chi-squared tests will be conducted as appropriate. Given that we hypothesized that the intervention effects may be affected by the baseline cognitive deficit status, we will also perform the above analyses separately for the MCI and non-MCI subgroups. All analyses will be performed using SPSS Statistics for Mac version 25.0 (IBM Corp., Armonk, NY, USA). Statistical significance threshold will be set at P <.05.
Patient and public involvement
Residents in the research field assisted in the development of this research protocol.
Study limitations
This study has several limitations. Considering that the SMAFO study participants are Japanese seniors, it is unclear if this intervention can be performed outside Japan. Despite these uncertainties, the SMAFO is relatively inexpensive to implement and is readily disseminated to other countries. Second, there is a possible selection bias for participants in this study. Participants in the study are seniors who have or are willing to use smartphones. Such seniors may have an unusually high understanding of information communication technology and high cognitive function (35). Therefore, our results may underestimate dementia onset. Third, the smartphone-based SMAFO cannot determine the effects of individual interventions. Future studies will be needed to determine the effects of individual interventions on reducing the incident dementia. Fourth, there is a risk of overestimating the onset of dementia because using MMSE results for excluding dementia may not identify very early dementia. Nevertheless, the impact on the intervention effect is expected to be negligible since participants will be randomly assigned.
Ethics and Dissemination
The Human Research Ethics Committee of the National Centre for Geriatrics and Gerontology approved this study (No. 1335). The study is registered with the University Hospital Medical Information Network Clinical Trials Registry (identification number: UMIN000041926). The annual expense for controls to obtain similar tools, including the smartphone and Cognipole, remains equal (at €900); therefore, this does not exclude them from pensioners’ privileges within the community. The study researchers will publish the articles according to the International Committee of Medical Journal Editors recommendations (36). According to the publication and data policy of the NCGG, all research information produced by this project will be made available for the scientific community and society as a whole. New results will be presented to the general public via NCGG web pages. When applicable, research results will be published in peer-reviewed, high-quality scientific journals that perform reliable scientific review processes for research papers. Anonymized data are planned to be dispensed to the scientific community in an appropriate open-access data repository.
Acknowledgments
The authors appreciate the assistance of Dr. Takehiko Doi, Dr. Kota Tsutsumimoto, Dr. Sho Nakakubo, Dr. Satoshi Kurita, and Dr. Hideaki Ishii for their professional help with the intervention protocol.
Funding: This work was supported by the Japan Agency for Medical Research and Development, grant numbers 16dk0110021h0001 and 17le0110004h0001. The funding source had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Ethics approval
The SMAFO studies have been reviewed and approved by the Ethics Committee of the National Centre for Geriatrics and Gerontology. All regulations and measures of confidentiality are handled in accordance with the Declaration of Helsinki.
Footnotes
Conflict of Interest
None.
Patient consent for publication
Not required.
Trial registration number
Clinical Trials Registry (identification number: UMIN000041926).
References
- 1.Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia?: A systematic review and metaanalysis of longitudinal studies. BMC Public Health. 2014;14:510. doi: 10.1186/1471-2458-14-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallal PC, Andersen LB, Bull FC, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–257. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 3.Franco MR, Tong A, Howard K, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. 2015;49:1268–1276. doi: 10.1136/bjsports-2014-094015. [DOI] [PubMed] [Google Scholar]
- 4.van Achterberg T, Huisman-de Waal GG, Ketelaar NA, Oostendorp RA, Jacobs JE, Wollersheim HC. How to promote healthy behaviours in patients? An overview of evidence for behaviour change techniques. Health Promot Int. 2011;26:148–162. doi: 10.1093/heapro/daq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 6.Joe J, Demiris G. Older adults and mobile phones for health: a review. J Biomed Inform. 2013;46:947–954. doi: 10.1016/j.jbi.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimada H, Makizako H, Lee S, Doi T, Lee S. Lifestyle activities and the risk of dementia in older Japanese adults. Geriatr Gerontol Int. 2018;18:1491–1496. doi: 10.1111/ggi.13504. [DOI] [PubMed] [Google Scholar]
- 8.Shimada H, Doi T, Lee S, Makizako H. Reversible predictors of reversion from mild cognitive impairment to normal cognition: a 4-year longitudinal study. Alzheimers Res Ther. 2019;11:24. doi: 10.1186/s13195-019-0480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 10.Espeland MA, Baker LD, Carrillo MC, et al. Increasing adherence in a centre-based trial of lifestyle intervention in older individuals: U.S. POINTER Trial. Innov Aging. 2018;2:809. doi: 10.1093/geroni/igy023.3011. [DOI] [Google Scholar]
- 11.de Souto Barreto P, Demougeot L, Vellas B, Rolland Y. Exercise training for preventing dementia, mild cognitive impairment, and clinically meaningful cognitive decline: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2018;73:1504–1511. doi: 10.1093/gerona/glx234. [DOI] [PubMed] [Google Scholar]
- 12.Tison GH, Avram R, Kuhar P, et al. Worldwide effect of COVID-19 on physical activity: a descriptive study. Ann Intern Med. 2020;173:767–770. doi: 10.7326/M20-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsutsui T, Muramatsu N. Care-needs certification in the long-term care insurance system of Japan. J Am Geriatr Soc. 2005;53:522–527. doi: 10.1111/j.1532-5415.2005.53175.x. [DOI] [PubMed] [Google Scholar]
- 14.Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374:523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimada H, Makizako H, Doi T, et al. Effects of combined physical and ognitive exercises on cognition and mobility in patients with mild cognitive impairment: a randomized clinical trial. J Am Med Dir Assoc. 2018;19:584–591. doi: 10.1016/j.jamda.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Ministry of Health Labour, and Welfare of Japan. Annual Health, Labour, and Welfare Report 2011–2012. 2012. http://www.mhlw.go.jp/english/wp/wp-hw6/dl/02e.pdf. Accessed 1 January 2017.
- 17.World Health Organization. International Classification of Diseases, 10th Revision (ICD-10). 1992. World Health Organization, Geneva.
- 18.Makizako H, Shimada H, Park H, et al. Evaluation of multidimensional neurocognitive function using a tablet personal computer: test-retest reliability and validity in community-dwelling older adults. Geriatr Gerontol Int. 2013;13:860–866. doi: 10.1111/ggi.12014. [DOI] [PubMed] [Google Scholar]
- 19.Shimada H, Makizako H, Park H, Doi T, Lee S. Validity of the National Center for Geriatrics and Gerontology-Functional Assessment Tool and Mini-Mental State Examination for detecting the incidence of dementia in older Japanese adults. Geriatr Gerontol Int. 2017;17:2383–2388. doi: 10.1111/ggi.13079. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Makino K, Lee S, Bae S, Shinkai Y, Chiba I, Shimada H. Predictive validity of a new Instrumental Activities of Daily Living Scale for detecting the incidence of functional disability among community-dwelling older Japanese adults: a prospective cohort study. Int J Environ Res Public Health 2020;172291. doi:10.3390/ijerph17072291 [DOI] [PMC free article] [PubMed]
- 22.Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71(Suppl2):114–120. doi: 10.1080/02701367.2000.11082794. [DOI] [PubMed] [Google Scholar]
- 23.Tomioka K, Iwamoto J, Saeki K, Okamoto N. Reliability and validity of the International Physical Activity Questionnaire (IPAQ) in elderly adults: the Fujiwara-kyo Study. J Epidemiol. 2011;21:459–465. doi: 10.2188/jea.JE20110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 25.Shimada H, Lee S, Bae S, Hotta R. A new Life Satisfaction Scale predicts depressive symptoms in a national cohort of older Japanese adults. Front Psychiatry. 2020;11:625. doi: 10.3389/fpsyt.2020.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20–40. doi: 10.1207/s15327752jpa6601_2. [DOI] [PubMed] [Google Scholar]
- 27.Kumagai S, Watanabe S, Shibata H, et al. [Effects of dietary variety on declines in high-level functional capacity in elderly people living in a community] Nihon Koshu Eisei Zasshi. 2003;50:1117–1124. [PubMed] [Google Scholar]
- 28.Bae S, Harada K, Chiba I, et al. A New Social Network Scale for Detecting Depressive Symptoms in Older Japanese Adults. Int J Environ Res Public Health. 2020;17:8874. doi: 10.3390/ijerph17238874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe T, Owashi K, Kanauchi Y, Mura N, Takahara M, Ogino T. The short-term reliability of grip strength measurement and the effects of posture and grip span. J Hand Surg Am. 2005;30:603–609. doi: 10.1016/j.jhsa.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 31.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 32.Morley JE, Morris JC, Berg-Weger M, et al. Brain health: the importance of recognizing cognitive impairment: an IAGG consensus conference. J Am Med Dir Assoc. 2015;16:731–739. doi: 10.1016/j.jamda.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiest KM, Jette N, Roberts JI, et al. The prevalence and incidence of dementia: a systematic review and meta-analysis. Can J Neurol Sci. 2016;43(Suppl1):S3–S50. doi: 10.1017/cjn.2016.18. [DOI] [PubMed] [Google Scholar]
- 34.Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/S0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 35.Gordon ML, Gatys L, Guestrin C, Bigham JP, Trister A, Patel K. App Usage Predicts Cognitive Ability in Older Adults. Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems. 2019. https://www.cs.cmu.edu/~jbigham/pubs/pdfs/2019/app-usage-older-adults.pdf
- 36.International Committee of Medical Journal Editors. Defining the role of authors and contributors 2020. http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html. Accessed 29 October 2020.