Abstract
Significant changes have occurred in the policy landscape surrounding cannabis legalization, production, and use around the globe and across the United States. With widespread availability of novel cannabis and cannabis-based products, there is an urgent need to understand their safety and effectiveness for medical indications. Three primary barriers contribute to the difficulty in initiating research geared toward answering the most pressing public health questions: the US regulatory status of cannabis and cannabinoids, sources for cannabis and cannabinoid study medications, and limited funding and resources to support studies. Despite these hurdles, research is rapidly increasing, and recent changes in the United States have paved the way for exciting new work. Here, challenges and barriers to cannabis and cannabinoid research are described from the perspectives of the National Institute on Drug Abuse, National Institutes of Health; the US Food and Drug Administration; and 2 clinical researchers. Barriers specifically to studying cannabis, cannabinoids, and cancer are emphasized.
Significant changes have taken place in the policy landscape surrounding cannabis legalization, production, and use around the globe and across the United States. Over the last couple of decades, 35 states and the District of Columbia have legalized cannabis for medical conditions; of these, 15 states and the District of Columbia (1) have also legalized adult use of cannabis. These landmark changes in policy have impacted cannabis use patterns and the perceived levels of risk.
However, despite this changing landscape, evidence regarding the short- and long-term health effects of cannabis use remains inconclusive. Several research studies have examined cannabis use in many forms, however, often these research conclusions are not appropriately translated and/or communicated to policy makers, health-care providers, state health officials, and other stakeholders who have been charged with influencing and enacting policies, procedures, and laws related to cannabis use (2,3). Other relevant challenges include the availability of cannabinoid-based study medications, federal regulations, and other constraints associated with clinical trials.
Oncologists frequently discuss the clinical use of cannabis with their patients although most feel they lack an adequate knowledgebase to advise effectively (4). The National Academies of Sciences, Engineering and Medicine’s report on the Health Effects of Cannabis and Cannabinoids found strong evidence in support of the use of cannabinoids for chemotherapy-induced nausea and vomiting as well as pain (5). Despite those findings, many oncologists prefer to recommend approved pharmaceuticals with larger bodies of supporting evidence. Increasingly patients are hearing of people healing their malignancies with highly concentrated cannabis oils (6). Although there is a significant body of preclinical evidence suggesting anticancer effects of cannabinoids, translation to clinical benefit has not yet occurred. Hence, oncologists and cancer researchers are likely to be particularly interested in seeing cannabis research advance.
This review highlights challenges and barriers to cannabis and cannabinoid research from the perspectives of administrators from the National Institute on Drug Abuse, National Institutes of Health (NIDA/NIH); the US Food and Drug Administration (FDA); and clinical researchers. Barriers specifically to studying cannabis, cannabinoids, and cancer are emphasized.
Current Regulatory Status of Cannabis and Cannabinoids: An Overview
Federal restrictions on clinical cannabis research result from its legal status as defined by the Controlled Substances Act [CSA (7)] and international treaties. However, federal and state laws conflict, with diverse state regulations allowing personal possession and recreational and medical use. Laws and regulations on federal cannabinoid research have been changing recently, and more changes are expected (for up-to-date information from the US Drug Enforcement Administration [DEA], refer to deadiversion.usdoj.gov and the NIDA Drug Supply program at https://www.drugabuse.gov/research/research-data-measures-resources/nida-drug-supply-program).
Cannabis remains a federal schedule I controlled substance. Schedule I substances include those determined to have high potential for abuse, no currently accepted medical use, and a lack of accepted safety for use under medical supervision. Additionally, the 1961 Single Convention on Narcotic Drugs makes it illegal to grow, possess, or distribute cannabis except under strict conditions. One of those restrictions is that nations may designate a single source of research marijuana. NIDA has served as the single source in the United States since 1968. However, because of a recent re-interpretation of the Single Convention requirements, the DEA recently published a new rule that will potentially allow the approval of additional growers and producers of cannabis for research (8). Currently, an estimated 41 applications are pending. It is anticipated that NIDA’s Drug Supply Program will remain one of the licensed producers.
Conflicting federal and state cannabis regulations hinder research in several ways, including the inability of researchers to access products that are legal in their state, a lack of standardization and quality control of cannabis and cannabis-derived products within and across states, and no national oversight of this standardization and quality control or the industry.
Recent and Pending Legislative Action
The Agriculture Improvement Act of 2018 [also known as the Farm Bill (9)] removed hemp (cannabis containing no more than 0.3% on a dry-weight basis) from the CSA schedule and re-affirmed the FDA’s regulatory authority for hemp-derived medications, dietary supplements, and food additives (10).
The US House of Representatives–passed Marijuana Opportunity Reinvestment and Expungement Act of 2020 (11) would have removed cannabis from the CSA schedule; eliminated criminal penalties for its manufacture, distribution, or possession; established a 5% cannabis tax to support War on Drugs compensation; protected the federal benefits and immigration status of people convicted of cannabis-related offenses; and reviewed sentences and expunged federal cannabis convictions. The US Senate–passed Cannabidiol and Marihuana Research Expansion Act (12) would have removed plant-derived and synthetic cannabidiol (CBD) from the CSA schedule, streamlined the process of obtaining DEA registration to conduct research with marijuana, and required the DEA to act on pending applications. As of July 2021, no recent federal legislation on cannabis has passed both houses of Congress and been signed into law by the president, although many bills have been introduced, and some have passed either the House or the Senate (for updates, see https://www.govtrack.us/congress/bills/subjects/drug_abuse/1759#sort=-introduced_date&text=cannabis&congress=__ALL__&terms=__ALL__&terms2=__ALL__).
Research Procedures and Barriers: A Single Source for Cannabis
The administrative challenges for cannabinoid research include the single domestic source requirement for cannabis, complex and lengthy registration processes, and schedule I classification of nonintoxicating cannabis components such as CBD. Scientific challenges include the complexity of cannabis plants (containing >100 cannabinoids and other components); difficulty in designing blinded, controlled studies (particularly for driving after drug exposure); and the inability to study products available from dispensaries in states where they exist.
Researchers who order cannabis from NIDA for human research in the United States must obtain FDA Investigational New Drug authorization, DEA schedule I registration, and institutional review board (IRB) approval. Despite misconceptions, NIDA has no role in determining qualifications. If researchers receive FDA, DEA, and IRB approval, NIDA fulfills orders for cigarettes and bulk cannabis in various THC and CBD concentrations, plus placebos. NIDA’s research cannabis is consistent, reproducible, pesticide free, and herbicide free. Although the cannabis provided by NIDA tracks the average THC potency of the cannabis generally available, NIDA does recognize the need for greater varieties of products, including improved placebos and more formulations (eg, extracts), a larger range of potencies, and variable terpene content (13).
Role of the FDA in the Regulation of Cannabis Products
There is broad public interest in expanding the availability of cannabis-based products for both medical and nonmedical use. In responding to this demand, the mission of the FDA is focused on advancing public health by overseeing the investigation, approval, and production of safe, effective, and high-quality medical products, including those that are synthesized chemically or derived from the cannabis plant.
As described above, the Agricultural Improvement Act of 2018 (9) had an important impact on the FDA’s actions in this area. Importantly, the Farm Bill stipulates that the FDA’s authorities under the Federal Food, Drug, and Cosmetic Act are unchanged, so that hemp-based drug products will be subject to the same authorities and requirements as any other drug product. To date, the consequences of FDA regulation of cannabis and cannabinoids, including hemp, include the approval of 4 drug products. Three of these products are synthetic THC or similar to THC and are approved to treat nausea from cancer chemotherapy. The fourth product, Epidiolex, is made from highly purified CBD from cannabis. It is approved for certain rare seizure disorders and, more recently, for tuberous sclerosis complex. The marketing approval of Epidiolex, particularly in light of the DEA’s placement of FDA-approved CBD-containing products in schedule V according to the CSA (14), shows that the clinical development of cannabis-derived medicines derives from the concerted evolution of biomedical knowledge and regulatory flexibility.
Beyond the 4 FDA approvals mentioned above, the agency has more generally performed a scientific assessment of cannabis-derived CBD and concluded that, although CBD is psychoactive, it does not have the same abuse potential as THC (which remains on schedule I of the CSA) (15). In addition to reviewing marketing applications for drugs and their indications, the FDA also regulates clinical studies with cannabis and cannabinoids. For these avenues of research, the investigator submits an Investigational New Drug application to the FDA for review. The application includes a detailed description of the study protocol and information about the investigational drug, including a summary of previous human experience with the investigational drug; animal pharmacology and toxicology; chemistry, manufacturing, and controls information on the investigational drug; and evidence that it was manufactured according to current good manufacturing practices (16). Given that state legalization may have facilitated the municipal sale and use of many new cannabis-derived products around the United States, it is important for investigators to be aware of FDA regulations before engaging in clinical studies with cannabis and cannabinoids.
As the FDA works to regulate cannabis-derived products appropriately, there is a great deal that is unknown about CBD, and even less is known about the dozens of cannabinoids and other compounds present in cannabis extracts. For example, little is known about the effects of long-term human use of CBD and the impact of CBD in susceptible populations: children, pregnant women, and the elderly. Based on data from the drug development program for Epidiolex and from the published literature, there are known toxicities of concern related to CBD use (15). For example, there are signals of potential liver injury, potential male reproductive toxicity, and clinically important drug-drug interactions. We do not know exactly how serious these signals are, and it is important that we continue efforts to assess them. Going forward, we also need to identify ways of answering the many remaining questions about the safety of CBD, as well as the many other compounds found in cannabis.
With so much yet to be learned, the FDA is committed to supporting scientific cannabinoid research and development. To support human drug development of cannabis and cannabis-derived compounds, the FDA has created several resources to aide investigators as they develop their clinical studies and use real-world data to fill the scientific gaps of knowledge. Some examples of these resources include information about the conduct of clinical studies and how to request formal meetings (16-19), considerations for using botanicals (20), a frequently asked questions website (21), recently released draft guidance about manufacturing cannabis-derived drugs (22), and the newly published FDA Voices Blog (23). The FDA’s research agenda is aimed at supporting studies to develop the data that is needed to understand how cannabinoids can be used safely in drug products and other consumer goods, such as dietary supplements, cosmetics, and pet foods. The FDA also is exploring policy options to enable broader availability of safe, effective, and high-quality cannabinoid products. There is substantial interest in US Congress legislation, and the FDA is actively offering assistance to state and nongovernmental partners in understanding the evolving cannabinoid landscape. The FDA also continues to take enforcement actions whenever violative marketing of cannabinoid products is identified. For example, during the COVID-19 pandemic, the FDA had to take action in multiple instances where makers of CBD products made antiviral, curative claims despite the lack of any supporting evidence on their safety or efficacy.
In summary, the FDA has a well-defined and multifaceted role in the cannabinoid space. Its role has been strengthened and clarified in some respects through recent activities on the legislative level. Most importantly, the FDA continues to support the scientific assessment of cannabis-derived compounds. Because of broad interest in expanding the availability of these products, the FDA is considering many different regulatory options for responding to this interest, always informed by our commitment to protect patients and advance our national public health interests.
General Challenges for the Clinical Researcher
The clinical researcher striving to respond to public health priorities related to the surge in cannabis and cannabinoid use is met with a number of regulatory hurdles. With rapid expansion of new products, novel methods of use, and growing populations using these products for medical indications or for nonmedical use, these restrictions are a major contributing factor to the limited data published addressing the most urgent issues. Apart from questions on the potential effectiveness of products on the market for certain indications, a more immediate concern relates to the safety of these products. There are increasingly popular product categories and modes of delivery that are available for purchase in state-regulated dispensaries that have yet to be tested under controlled conditions. Some of these products are hypothesized to have potentially significant negative effects, such as high-potency extracts geared toward delivering efficient intoxicating effects, as well as products such as specific minor cannabinoids and terpenes that, based on preclinical literature, may be safe but have yet to be assessed in humans. In an effort to elucidate both the safety and the potential therapeutic uses of these products for a range of indications for which they are already approved in an overwhelming majority of the United States, researchers must work tirelessly through institutional, regulatory, funding, and drug supply hurdles, all of which significantly influence the scientific impact, public health relevancy, and efficiency of investigations.
Given the diverse nature of cannabis and cannabinoid research, differences in state laws, and varied institutional regulations, every scientist will likely have a unique experience when initiating cannabis and cannabinoid work. In fact, with rapid changes in oversight and regulations, one cannot necessarily predict how to navigate regulatory hurdles for future studies based on one’s own previous experiences. The following account is from personal experience working with cannabis and cannabinoids in 2 states and 2 institutions. This account highlights the arduous path of starting a research program focusing on controlled administration of cannabis and cannabinoids in humans and demonstrates that the pathway to embarking on this research is demanding both of the researcher’s time and resources. This experience is important to stress as more researchers become interested in delving into this field but are required to start from scratch to get projects off the ground. Because of the time, expenses, and regulatory knowledge required to get a single study started in this field, researchers will frequently opt not to pursue work in this area. Consequently, although more issues need to be addressed by diverse experts, the field will likely continue to be limited to those institutions and researchers who have historically pursued this work.
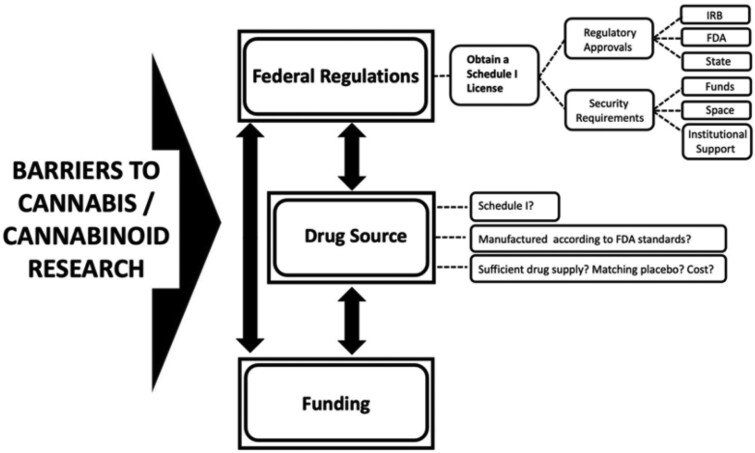
Below is a description of the 3 primary hurdles to conducting cannabis and cannabinoid research in the United States. Although the US regulatory status of cannabis and cannabinoids is a US-specific barrier, the other barriers—the paucity of funding available for investigations and the availability of test medications that can be studied—are global and limit the expansion of work in the field. Similarly, although some barriers are unique to this field, it should be noted that clinical research in general is difficult and burdensome, regardless of the study medication under investigation. However, many hurdles detailed below are unique to studying cannabis and cannabinoids in the United States. These challenges are intertwined with one another, creating a situation where overcoming one obstacle requires success in surmounting the others, as depicted in Figure 1.
Figure 1.
Primary obstacles in pursuing cannabis and cannabinoid research. Federal regulations, drug source, and funding are significant barriers to conducting cannabis and cannabinoid research, which can lead to significant delays in study onset. As depicted by the double-headed arrows, these challenges are intertwined with one another, creating a situation where overcoming one obstacle requires success in surmounting the others. IRB = institutional review board; FDA = US Food and Drug Administration.
US Regulatory Status of Cannabis and Cannabinoids
As mentioned earlier, the most obvious regulatory hurdle in conducting cannabis and cannabinoid research is the schedule I status of cannabis (with more than 0.3% THC) and specific cannabinoids. Although there has been movement in the field to relax regulations, including the landmark change decontrolling CBD derived from hemp, many changes have, in fact, made the regulatory landscape more confusing and difficult to navigate for the researcher. For example, in the case of CBD, according to the DEA interim final rule that outlines DEA’s amendments to the CSA made by the Agriculture Improvement Act of 2018 (24), products with CBD specifically derived from the plant that contain less than 0.3% delta-9-THC are now decontrolled, yet synthetic CBD remains on schedule I according to the CSA. Despite the fact that the molecule is the same, its origins define its regulatory standing. Another example of these confusing laws relates to the classification of delta-9-THC, which has 3 distinct schedules based on the source of production and the formulation. For example, dronabinol, synthetic delta-9-THC, is categorized on 3 different schedules: oral capsules of the synthetic delta-9-THC dronabinol (ie, Marinol) is classified as schedule III, yet the FDA-approved liquid dronabinol (Syndros) is schedule II, and dronabinol not packaged according to FDA formulations (ie, active pharmaceutical ingredient) is schedule I (25). Currently, all forms of delta-9-THC derived from the cannabis plant (ie, not synthetic) are schedule I. These are a few examples of the confusing nature of cannabinoid scheduling that require near mastery to successfully identify whether a proposed trial with a potential study medication requires a schedule I DEA license. Without proper guidance and support from people in the field, a researcher is likely to get lost in the regulatory quagmire that is rapidly evolving.
Once it has been determined that the test material is indeed schedule I, the investigator is required to apply and be approved for schedule I registration. Prior to applying for this license, 2 significant milestones must be met: approval of a research protocol that employs the schedule I substance for which the license is being sought and identification of a storage facility that will meet DEA requirements (26).
Regulatory Approvals. A study assessing the effects of cannabis and/or cannabinoid administration is required to be submitted to and approved by the IRB and the FDA. Protocols submitted to the IRB must be justified scientifically, meet the ethical principles of the Belmont Report (27), and include steps to minimize risk. This protocol is also submitted to the FDA as an Investigational New Drug application alongside detailed information regarding the chemistry, manufacturing, and control data of the agent to be studied as described in Section titled Role of the FDA in the Regulation of Cannabis Products. Finally, state regulatory approvals must also be obtained. Some states have separate controlled substance licensing requirements. For example, in New York State, the investigator must apply for and receive a license from the Bureau of Narcotic Enforcement. Other states may require that protocols be approved by the state board of medical examiners. For example, in the state of California, the Research Advisory Panel of California, under the state attorney general’s office, reviews and authorizes studies involving schedules I and II substances to ensure the safety and protection of participating human research subjects, in addition to the security provisions in place for the controlled substances used in the study. This panel also evaluates the scientific validity of the studies. Therefore, studies can be rejected if deemed to “produce conclusions of little scientific value, or would not justify the exposure of California subjects to the risk of research” (28). If any of these bodies request modifications to the protocol, amendments must be submitted to and reviewed by the other agencies. This approval process can take several months even if no modifications are required.
Application to the DEA for a schedule I license includes the above-mentioned approved regulatory documents; the investigator’s qualifications, including a curriculum vitae; a description of the research project, including its statement of purpose; the name and amount of substances to be used; a description of and the number of research subjects; drug doses to be administered; mode of administration; and the duration of the study. Details regarding where the study will be conducted and security provisions for storing the drug must also be included. Finally, an institutional letter of support must be provided, as well as an indication of approved funding provided, if applicable (26). Once the application is received, the DEA communicates with the FDA to determine the merits of the study and the qualifications and competency of the submitting investigator, which is a process that takes at least 30 days. The local DEA inspector will then make an appointment to inspect the facility. Security issues noted during the inspection need to be resolved before the license is granted.
Security Requirements for Schedule I Material. Requirements for schedule I drug storage are complicated, and the specifics outlined in Title 21 of the Code of Federal Regulations may not realistically correspond to an investigator’s study needs. For example, drugs may be required to be frozen to maintain stability (26). The Code of Federal Regulations does not provide an adequate solution for how this drug should be stored. As such, researchers develop ways to maintain the integrity of their drug product while still adhering to the DEA code. In general, small amounts of the drug must be stored in a safe or steel cabinet that weighs at least 750 pounds and complies with the following specifications: protected for “30 man-minutes against surreptitious entry, 10 man-minutes against forced entry, 20 man-hours against lock manipulation, and 20 man-hours against radiological techniques.” If the safe or cabinet is not 750 pounds, it needs to be bolted or cemented to the floor or wall. The safe or steel cabinet should be equipped with an alarm that signals a central protection agency, a police agency, or a 24-hour control station operated by the registrant in the event of an unauthorized entry. Guidance for practitioners (clinical researchers) is modified to state that “Controlled substances listed in Schedule I shall be stored in a securely locked, substantially constructed cabinet” (26), however, there is not a detailed account of what type of safe meets this definition and may be left up to the local DEA jurisdiction and agent. The storage facility is required to be in a space that is only accessible to a minimum number of specifically authorized employees. This stipulation requires that the institution secure space for the researcher that cannot be accessible to anyone other than the investigator and employees authorized to have access to the drug.
These requirements place a significant burden on the investigator embarking on a path to researching cannabis and cannabinoids. The security provisions can only be met with institutional support and commitment and with sufficient funding to establish a secure drug storage area that adheres to the DEA’s standards.
Source of the Study Drug
Whereas the schedule I status of cannabis and many cannabinoids is a significant barrier to research, identifying a drug for clinical studies continues to be a principal obstacle, regardless of the drug’s scheduling status. The challenges related to the single source of cannabis are outlined earlier in this paper (Section Research Procedures and Barriers: A Single Source for Cannabis). Although the limitations related to a single source of cannabis is clearly related to its schedule I status, a challenge that is frequently overlooked is identifying sources of any cannabinoid study drug independent of the drug’s scheduling. These challenges lie in the issues raised in Section 4.0, addressing the need for a study drug to meet the FDA’s standards for human study. Therefore, even if cannabis and schedule I cannabinoids shed their classification and are removed from the CSA, researchers would still bear the burden of identifying a product that adheres to the FDA’s Good Manufacturing Practice requirements. For example, hemp-derived CBD was recently removed from the CSA. This evolution should have improved research in this area substantially, yet there are few manufacturers that make plant-derived CBD according to the FDA’s standards. When a manufacturer of clinical-grade, hemp-derived CBD is identified, the product can only be considered for use if the manufacturer agrees to provide the materials needed for the researcher’s FDA Investigational New Drug submission and provides enough study drug to cover the needs of the investigation at a cost that is not prohibitively expensive for a typical research budget. In addition, for a placebo-controlled study, a matched placebo is also required, ideally manufactured by the company providing the study drug. As such, providing a study drug for clinical trials is time intensive and resource heavy for the manufacturer, and few manufacturers are creating these materials for direct sale to customers and researchers. This presents a conundrum where the types of cannabis products available to the public continue to increase, yet research is limited to only a handful of cannabinoids, modes of delivery, and doses. Another issue with respect to feasibility of clinical trials with schedule I material is the lack of clear federal guidance on the limitations or restrictions for taking such study medication across state lines. Researchers would be advised to check with individual state governments if such scenarios are planned.
Because of the various scheduling and availability issues of cannabis and cannabinoids, identifying the source of a drug for a particular study is absolutely integral when developing the protocol and applying for funding (Figure 1). In fact, given the limitations of study drug availability, a study’s premise, objective, and design are usually crafted based on what is available.
Funding
The ultimate limitation to research in this field is the availability of funding. As noted before, funding is difficult to obtain for nearly all areas of research, but this is especially true for cannabis and cannabinoid research. Until recently, there were very few opportunities to fund research dedicated to the therapeutic effects of cannabis and cannabinoids. The NIH Research, Condition, and Disease Categorization (29) system tracks expenditures by the NIH institutes. In 2019, the NIH budget for research projects was approximately $9.6 billion, with an overall funding rate of 19% (30). Despite laws restricting research, the NIH cannabis and cannabinoid research portfolio is significant and growing and includes the provision of cannabis materials for research. The cannabinoid research category includes the endocannabinoid system, CBD, and therapeutic cannabinoids. NIH cannabinoid research support increased from $111.3 million for 285 projects in 2015 to $189 million for 408 projects in 2019, with more than a doubling of funds dedicated toward cannabis and cannabinoid therapeutics from 2015 to 2019, from $21 million to $46.5 million (Table 1), about 0.5% of the overall NIH research budget. Of the 27 NIH components, 20 supported some cannabinoid research in 2019. NIDA was the primary source of support, with $118.7 million for 258 projects. Noteworthy changes include the National Center for Complementary and Integrative Health research on the potential therapeutic benefits of minor cannabinoids and terpenes and the National Cancer Institute workshop and research funds dedicated to cannabinoids and cancer.
Table 1.
NIH cannabinoid research investment by Research, Condition, and Disease Categorization (RCDC), fiscal years 2015-2019
| RCDC categorya | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|
| Cannabinoid | $111 275 219 | $115 167 703 | $139 903 453 | $146 551 293 | $188 912 542 |
| Cannabidiol | $9 035 446 | $11 667 081 | $15 059 130 | $19 397 279 | $30 661 833 |
| Endocannabinoid | n/a | $51 217 092 | $62 870 455 | $62 628 836 | $73 139 271 |
| Therapeutic cannabinoids | $21 214 163 | $28 174 758 | $36 290 698 | $37 322 692 | $46 461 827 |
The Research, Condition, and Disease Categorization system tracks National Institutes of Health (NIH) research expenditures year by year in standardized groups defined by NIH experts. The cannabinoid research RCDC category is the “master” category for all NIH-supported cannabis research. It includes 3 subsets: cannabidiol, endocannabinoid, and therapeutic cannabinoid research. Individual projects may be included in multiple research categories. For example, a study investigating the potential therapeutic benefits of cannabidiol would be categorized under the cannabinoid, cannabidiol, and therapeutic cannabinoids RCDC categories. Studies investigating endogenous cannabinoids would be included in the cannabinoid and endocannabinoid systems RCDC studies.
In addition to NIH, additional sources for funding have become available for cannabis and cannabinoid research. For example, in 2000, $3 million per year for 3 years was appropriated to the California state-funded Center for Medicinal Cannabis Research (CMCR) based at the University of California, San Diego, through legislation calling for a research program to oversee medical research of cannabis and cannabinoids. This center, now funded by revenue from taxes on adult-use cannabis sales, was initially created to conduct and support clinical trials on the efficacy of cannabis. The research agenda expanded to include supporting clinical trials on the efficacy of cannabis and cannabinoids to determine optimal dosing, timing, and modes of administration; comparing the efficacy and safety of various delivery methods; assessing the safety and toxicity of cannabis in the medically ill; and conducting limited preclinical studies. Although funding is available only to investigators at institutions based in California, submissions are high, with 55 applications received in the past 2 years. Yet, similar to NIH funding rates, the CMCR awards are very competitive, with a 12% funding rate (personal communication with Thomas Marcotte, co-director of the CMCR). The volume of grants submitted demonstrates the eagerness of researchers to do work in the field, and the limited success rate exemplifies the difficulty in obtaining funds. In addition to state-funded research, private philanthropy and foundation support are other sources for supporting cannabis and cannabinoid research for specific conditions.
Without funding, it is impossible to cover the expenses associated with the study, among which are personnel, participant expenses, study medication, and the costs to maintain regulatory approvals and drug storage security (Figure 1). With limited funding opportunities and the highly competitive nature of those that exist, a proposal’s impact and novelty are weighed alongside the study’s feasibility and potential for success in trial initiation and completion. A key component of study feasibility for cannabis and cannabinoid studies is the existing infrastructure needed for this type of research, including institutional support for this research, investigator expertise, and a schedule I license, if required for the study medication proposed in the grant application. As such, to obtain funding, it is optimal for the researcher to demonstrate experience in the field and have the support necessary to have successfully applied for and obtained a schedule I license. This is nearly impossible for most new investigators given that obtaining a schedule I license requires funding to support 1) the secure drug storage space and 2) a study that is submitted for IRB, FDA, and state regulatory approvals. These mutually dependent conditions create a situation that shuts out new investigators, especially those based at institutions that do not have infrastructure in place to support clinical studies with schedule I substances.
Experience of a Clinician-Researcher Studying the Therapeutic Effects of Cannabis in Cancer
Embarking on Clinical Cannabis Research: Cannabis in HIV and AIDS
Until recently, NIH did not have pathways specifically dedicated to provide funds to study the therapeutic effects of cannabis; however, funds were set aside to investigate the potential adverse effects of the plant. Hence, 25 years ago, to assess whether cannabis could be useful in patients with AIDS wasting, Donald I. Abrams and colleagues in the Department of Medicine at San Francisco General Hospital, California, proposed a clinical trial that was funded to primarily determine the safety of adding cannabis to HIV protease inhibitors, which also allowed for the potential study of the therapeutic effects of cannabis in this population (31-33).
A second study funded by the CMCR 20 years ago sought to determine the effects of inhaled cannabis on neuropathic pain in patients with HIV-related peripheral neuropathy. This trial was designed to enroll 16 participants in a pilot phase to assess the activity of inhaled cannabis and calculate the sample size needed for a follow-up randomized controlled trial if the initial results were encouraging. The study involved 9-day inpatient stays in the San Francisco General Hospital Clinical Research Center. Inpatient studies were favored for research involving this schedule I substance to ensure that the participants were using cannabis as described in the study protocol and not diverting it to family or friends. Participants were not allowed to have visitors or leave the Clinical Research Center ward. To standardize the inhaled dosing, the Foltin uniform puff procedure was employed (34). To anchor the participants’ subjective description of their pain, the heat and capsaicin experimental pain model was performed to provide a more objective measurement. This method involved heating an area of the forearm to 40°C and then applying capsaicin cream, creating an area of allodynia and hypesthesia that was mapped with a brush and a piece of foam while the subject looked off in another direction. These areas were measured before and after exposure to the study drug. The trial was successfully completed with 50 participants enrolled in the randomized trial (35).
Cannabis and Cancer Research
Simultaneous with funding awarded to assess the effects of cannabis on HIV neuropathy, the Abrams team was awarded a CMCR grant to study cannabis in combination with opioids in patients with breast and prostate cancer with painful bone metastases. This study also involved 9-day inpatient stays in the San Francisco General Hospital Clinical Research Center, and most of the study procedures were identical to those used in the HIV neuropathy study. However, in the time that it took to complete the neuropathy study, only 3 participants enrolled in the cancer pain study. In an effort to increase accrual, eligibility was expanded to include any cancer patient with any pain. Ultimately, the funding for the cancer pain study was withdrawn. Barriers to enrollment of cancer patients in this trial were considered. It was suggested that cancer patients may not be interested in spending unnecessary inpatient time (eg, in the Clinical Research Center to participate in a trial). The IRB expressed concern about inflicting experimental pain models on cancer patients. In addition, patients in San Francisco have long had access to cannabis without having to consent to a trial and risk getting randomly assigned to receive a placebo.
In an effort to bypass the need for inpatient Clinical Research Center admission, an outpatient study to examine the effects of cannabinoids on delayed chemotherapy-induced nausea and/or vomiting was designed and favorably reviewed for funding by the CMCR nearly 2 decades ago. Patients who had experienced delayed nausea after the first cycle of chemotherapy were then randomly assigned to receive true cannabis cigarettes and placebo dronabinol, placebo cigarettes and active dronabinol, or placebo cigarettes and placebo dronabinol. The target sample size was 81. After enrolling the first 8 patients in this study, aprepitant was licensed and improved for this precise indication. Local oncologists lost interest in referring patients to a trial where a placebo was possible in view of the new available effective treatment option. Having only enrolled 10% of the accrual target, trial funding was withdrawn.
The question of possible synergy between cannabinoids and opioids still loomed as a compelling area of investigation despite the failure of the initial attempt to study it. In an effort to be sensitive to the potential concerns of cancer patients regarding the smoked method of cannabis administration, use of the Volcano vaporizer as a smokeless delivery system for cannabis was explored. In healthy volunteers, the dose-dependent subjective effects and pharmacokinetics of smoked and vaporized cannabis were compared. Findings demonstrated that vaporization was a safe and effective delivery system and likely had reduced respiratory risk compared with smoked cannabis (36). The Abrams team then submitted a proposal to NIDA to do a pharmacokinetic interaction study in patients with cancer on sustained-release morphine or sustained-released oxycodone to determine whether it was safe to add vaporized cannabis to the regimen. The study focused on the safety of the drug combination and was quickly funded. After screening 218 cancer patients who expressed interest, only 1 had met the eligibility criteria and enrolled in the trial. The most frequent reasons that potential participants were deemed ineligible were because they were not taking the correct opioid analgesic, or more commonly, they were taking the sustained-release morphine or oxycodone preparations 3 or 4 times a day, which would not allow for the 12-hour opioid kinetics curve desired. Rather than forfeit funding because of lack of accrual, the protocol was modified after several months to eliminate cancer-related pain as an entry criterion and included any participants with any pain as long as they took the sustained-release opioid twice a day. With the expansion of the eligibility criteria beyond cancer patients, the study was successfully completed (37).
Cannabis and Sickle Cell Disease
More recently, a colleague of Dr Abrams, Kalpna Gupta, PhD, works with transgenic mice with the human sickle hemoglobin gene that experience pain. In her laboratory, she found that cannabinoids ameliorate the chronic hypoxia-reoxygenation -evoked acute pain in the mice. Approximately 8 years ago (approximately 2013), she was seeking a collaborator interested in doing a human proof of principle study to accompany a grant that she was submitting to the National Heart, Lung, and Blood Institute. Having completed the opioid-cannabinoid pharmacokinetic interaction study, the Abrams team felt that a trial in sickle cell pain would be easily designed using a similar protocol as most of the participants would be on opioid analgesics. By this time, CBD had come bursting onto the scene as the most favored cannabinoid. A 4-arm trial was envisioned comparing THC-dominant cannabis, CBD-dominant cannabis, a balanced blend, and a placebo. However, funding was only available to support 2 arms, and 1 had to be a placebo. Eager to evaluate a CBD-containing product, the team requested that NIDA provide a balanced strain, and they received a 4.4%THC to 4.9% CBD chemovar.
The goal of this inpatient randomized, double-blind, placebo-controlled crossover trial was to determine the analgesic and subjective effects of cannabis in sickle cell patients maintained on opioid analgesics. This study required approvals from multiple regulatory bodies as described in Section General Challenges for the Clinical Researcher, and more than 1 year elapsed from the time the protocol was submitted to the IRB for approval when enrollment began. Nearly 3 years later, only 23 of the target 35 patients had completed both arms of the crossover trial; similar to cancer patients, patients with sickle cell disease also found the inpatient component difficult (38).
With so many products available to patients currently, a common questions is, “What is the right ratio of THC: CBD to study?” Or, should it be cannabigerol or perhaps cannabinol? How can an investigator decide what chemovar or mode of delivery should be studied in a prospective clinical trial? It would almost seem that by the time the decision is made, a newer option may have become most favored in the free market of medicinal cannabis products. In summary, cannabis and cannabinoid research requires navigating many hurdles. The schedule I status of cannabis creates excessive regulatory hurdles, and potential participants have increased opportunities to access medicinal cannabis and cannabis-derived products without participating in a clinical trial. These factors contribute to the difficulty in initiating a study and enrolling enough participants to successfully complete a trial.
Conclusion
With widespread availability of novel cannabis and cannabis-based products, there is an urgent need to understand their safety and potential effectiveness for medical indications. Three primary barriers contribute to the difficulty in initiating research geared toward answering the most pressing public health questions: the US regulatory status of cannabis and cannabinoids, sources for cannabis and cannabinoid study medications, and funding to support studies. These barriers are especially difficult to navigate for researchers new to studying cannabinoids and therefore prevent the multidisciplinary work that the field needs. In addition to these barriers, research related to cannabis and cannabinoids in cancer patients exemplifies difficulties in successfully completing these trials even once these primary hurdles are overcome. Although these barriers are daunting, some recent changes in the United States have paved the way for exciting new work in the field; the removal of hemp-derived CBD from the CSA and the growing availability of funding for the study of cannabis and cannabinoids as therapeutics are 2 such developments. A primary way to navigate these barriers is through collaborations with researchers who are experienced in the clinical cannabis and cannabinoid field, whether they are at the investigator’s home institution or elsewhere. Additionally, many universities have resources for clinical researchers to guide them through regulatory hurdles, and agencies like the FDA can also provide guidance and support. Although clinical research with cannabis and cannabinoids is difficult, strong national and international collaborations and communities of researchers have made significant strides in this field, pushing science forward in innovative and impactful ways. In particular, international partnerships prove to have significant potential to enhance research collaborations by leveraging opportunities in other countries and potentially avoiding difficult regulations that hinder development.
Funding
Part of this work was supported by the Semel Charitable Foundation. No novel data are presented in this manuscript, therefore no data are available in association with this manuscript.
Notes
Role of the funder: Part of this work was supported by the Semel Charitable Foundation, which had no role in conceiving or drafting this manuscript.
Disclosures: The authors have no competing interests in relation to the work described. Over the past 3 years, ZDC has served as a consultant to GB Sciences and Beckley Canopy Therapeutics and has served on the scientific advisory board of FSD Pharma. DIA is a scientific advisor to Cannformatics, Lumen, and Maui Grown Therapies. The other authors have no conflicts of interest to disclose.
Author contributions: All authors drafted sections of the text and reviewed the final draft. ZDC edited and compiled the complete and final draft.
Disclaimers: The views and opinions expressed in this manuscript are those of the authors only and do not necessarily represent the views, official policy, or position of the US Department of Health and Human Services or any of its affiliated institutions or agencies.
Prior presentations: Ideas discussed in this manuscript were presented at the 2020 NCI Cannabis, Cannabinoids, and Cancer Research Symposium.
Contributor Information
Ziva D Cooper, UCLA Cannabis Research Initiative, Jane and Terry Semel Institute for Neuroscience and Human Behavior, Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California, Los Angeles, CA, USA; Department of Anesthesiology and Perioperative Medicine, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Donald I Abrams, Department of Oncology, Zuckerberg San Francisco General Hospital and Trauma Center, University of California, San Francisco, USA.
Steven Gust, Office of the Director, National Institute on Drug Abuse, National Institutes of Health, Rockville, MD, USA.
Alejandro Salicrup, Research and Training Branch, Center for Global Health and Office of Cancer Complementary and Alternative Medicine, Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health, Rockville, MD, USA.
Douglas C Throckmorton, Center for Drug Evaluation and Research, U.S. Food and Drug Administration, Rockville, MD, USA.
References
- 1.Britannica ProCon. Legal Medical Marijuana States and DC. https://medicalmarijuana.procon.org/legal-medical-marijuana-states-and-dc/. Updated June 22, 2021. Accessed December 27, 2021.
- 2.The Lancet Oncology. Cannabis: high time for evidence-based policies. Lancet Oncol. 2017;18(1):1. [DOI] [PubMed] [Google Scholar]
- 3. Pacula RL, Smart R. Medical marijuana and marijuana legalization. Annu Rev Clin Psychol. 2017;13(1):397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braun IM, Wright A, Peteet J, et al. Medical oncologists’ beliefs, practices, and knowledge regarding marijuana used therapeutically: a nationally representative survey study. J Clin Oncol. 2018;36(19):1957–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: National Academies Press (US; ); 2017. http://www.ncbi.nlm.nih.gov/books/NBK423845/. Accessed September 14, 2017. [Google Scholar]
- 6. Shi S, Brant AR, Sabolch A, Pollom E. False news of a cannabis cancer cure. Cureus. 2019;11(1):e3918. doi:10.7759/cureus.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unites States Drug Enforcement Administration. The Controlled Substances Act. https://www.dea.gov/controlled-substances-act. Accessed February 22, 2021.
- 8.Unites States Drug Enforcement Administration. Controls to Enhance the Cultivation of Marihuana for Research in the United States, 85 FR 82333. https://www.federalregister.gov/documents/2020/12/18/2020-27999/controls-to-enhance-the-cultivation-of-marihuana-for-research-in-the-united-states. Published December 18, 2020. Accessed February 22, 2021.
- 9.United States Congress. H.R.2 - Agriculture Improvement Act of 2018. https://www.congress.gov/bill/115th-congress/house-bill/2. Published December 20, 2018. Accessed February 22, 2021.
- 10.United States Food and Drug Administration. Scientific Data and Information about Products Containing Cannabis or Cannabis-Derived Compounds; Public Hearing. https://www.fda.gov/news-events/fda-meetings-conferences-and-workshops/scientific-data-and-information-about-products-containing-cannabis-or-cannabis-derived-compounds. Published May 21, 2019. Accessed February 22, 2021.
- 11.United States Congress. Marijuana Opportunity Reinvestment and Expungement Act of 2020. https://www.congress.gov/bill/116th-congress/house-bill/3884. Published November 27, 2020. Accessed February 22, 2021.
- 12.United States Congress. Cannabidiol and Marijuana Research Expansion Act. https://www.congress.gov/bill/116th-congress/senate-bill/2032/text?format=txt. Published December 15, 2020. Accessed February 22, 2021.
- 13. Volkow ND. Hearing on Cannabis Policies for the New Decade. https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2020/hearing-on-cannabis-policies-for-the-new-decade. Published January 15, 2020. Accessed April 14, 2021.
- 14.United States Drug Enforcement Administration. Schedules of Controlled Substances: Placement in Schedule V of Certain FDA-Approved Drugs Containing Cannabidiol; Corresponding Change to Permit Requirements. 83 Federal Register 48950. https://www.federalregister.gov/documents/2018/09/28/2018-21121/schedules-of-controlled-substances-placement-in-schedule-v-of-certain-fda-approved-drugs-containing. Published September 28, 2018. Accessed July 9, 2021. [PubMed]
- 15.United States Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy; 2018. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms. Accessed April 14, 2021.
- 16.United States Food and Drug Administration. Investigational new drug application (IND). https://www.fda.gov/drugs/types-applications/investigational-new-drug-ind-application. Accessed February 23, 2021.
- 17.United States Food and Drug Administration. Investigational New Drug Applications Prepared and Submitted by Sponsor-Investigators. https://www.fda.gov/media/92604/download. Accessed February 23, 2021.
- 18.United States Food and Drug Administration. Formal meetings between FDA and sponsors or applicants of PDUFA products guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/formal-meetings-between-fda-and-sponsors-or-applicants-pdufa-products-guidance-industry. Accessed February 23, 2021.
- 19.United States Food and Drug Administration. Pre-IND consultation meeting. https://www.fda.gov/drugs/investigational-new-drug-ind-application/pre-ind-consultation-program. Accessed February 23, 2021.
- 20.United States Food and Drug Administration. Botanical Drug Development Guidance for Industry. https://www.fda.gov/media/93113/download. Accessed February 23, 2021.
- 21.United States Food and Drug Administration. FDA regulation of cannabis and cannabis-derived products, including cannabidiol (CBD). https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd. Accessed February 23, 2021.
- 22.United States Food and Drug Administration. Cannabis and Cannabis-Derived Compounds: Quality Considerations for Clinical Research. https://www.fda.gov/media/140319/download. Accessed February 23, 2021.
- 23.United States Food and Drug Administration. Better Data for a Better Understanding of the Use and Safety Profile of Cannabidiol (CBD) Products. https://www.fda.gov/news-events/fda-voices/better-data-better-understanding-use-and-safety-profile-cannabidiol-cbd-products. Accessed February 23, 2021.
- 24.Drug Enforcement Administration. Implementation of the Agriculture Improvement Act of 2018. . https://www.deadiversion.usdoj.gov/fed_regs/rules/2020/fr0821.htm. Published August 21, 2020. Accessed February 20, 2021.
- 25.Drug Enforcement Administration. Schedules of controlled substances: placement of FDA-approved products of oral solutions containing dronabinol [(-)-delta-9-trans-tetrahydrocannabinol (delta-9-thc)] in schedule II. https://www.federalregister.gov/documents/2017/03/23/2017-05809/schedules-of-controlled-substances-placement-of-fda-approved-products-of-oral-solutions-containing. Published March 23, 2017. Accessed February 22, 2020. [PubMed]
- 26.Drug Enforcement Administration. Title 21 Code of Federal Regulations Part 1301 — Registration of Manufacturers, Distributors, and Dispensers of Controlled Substances. https://www.deadiversion.usdoj.gov/21cfr/cfr/1301/1301_71.htm. Accessed February 22, 2020.
- 27.National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report. 1979.
- 28.State of California Department of Justice. Research Advisory Panel. https://oag.ca.gov/research. Accessed February 22, 2021.
- 29.National Institutes of Health Research Portfolio Online Reporting Tools (RePORT. Estimates of funding for various research, condition, and disease categories (RCDC). https://report.nih.gov/funding/categorical-spending#/. Published February 24, 2020. Accessed February 22, 2021.
- 30.NIH RePORT. Funding success rates. https://report.nih.gov/funding/nih-budget-and-spending-data-past-fiscal-years/success-rates. Accessed February 22, 2021.
- 31. Kosel BW, Aweeka FT, Benowitz NL, et al. The effects of cannabinoids on the pharmacokinetics of indinavir and nelfinavir. AIDS. 2002;16(4):543–550. [DOI] [PubMed] [Google Scholar]
- 32. Bredt BM, Higuera-Alhino D, Shade SB, Hebert SJ, McCune JM, Abrams DI. Short-term effects of cannabinoids on immune phenotype and function in HIV-1-infected patients. J Clin Pharmacol. 2002;42(S1):82S–89S. [DOI] [PubMed] [Google Scholar]
- 33. Abrams DI, Hilton JF, Leiser RJ, et al. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann Intern Med. 2003;139(4):258–266. [DOI] [PubMed] [Google Scholar]
- 34. Foltin RW, Brady JV, Fischman MW. Behavioral analysis of marijuana effects on food intake in humans. Pharmacol Biochem Behav. 1986;25(3):577–582. [DOI] [PubMed] [Google Scholar]
- 35. Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68(7):515–521. [DOI] [PubMed] [Google Scholar]
- 36. Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharmacol Ther. 2007;82(5):572–578. [DOI] [PubMed] [Google Scholar]
- 37. Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90(6):844–851. [DOI] [PubMed] [Google Scholar]
- 38. Abrams DI, Couey P, Dixit N, et al. Effect of inhaled cannabis for pain in adults with sickle cell disease: a randomized clinical trial. JAMA Netw Open. 2020;3(7):e2010874. [DOI] [PMC free article] [PubMed] [Google Scholar]