Abstract
Background
Coffee consumption has been associated with a reduced risk of some cancers, but the evidence for renal cell carcinoma (RCC) is inconclusive. We investigated the relationship between coffee and RCC within a large cohort.
Methods
Coffee intake was assessed at baseline in the National Institutes of Health–American Association of Retired Persons Diet and Health Study. Among 420 118 participants eligible for analysis, 2674 incident cases were identified. We fitted Cox-regression models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for coffee consumption vs non-drinkers.
Results
We observed HRs of 0.94 (95% CI 0.81, 1.09), 0.94 (0.81, 1.09), 0.80 (0.70, 0.92) and 0.77 (0.66, 0.90) for usual coffee intake of <1, 1, 2–3 and ≥4 cups/day, respectively (Ptrend = 0.00003). This relationship was observed among never-smokers (≥4 cups/day: HR 0.62, 95% CI 0.46, 0.83; Ptrend = 0.000003) but not ever-smokers (HR 0.85, 95% CI 0.70, 1.05; Ptrend = 0.35; Pinteraction = 0.0009) and remained in analyses restricted to cases diagnosed >10 years after baseline (HR 0.65, 95% CI 0.51, 0.82; Ptrend = 0.0005). Associations were similar between subgroups who drank predominately caffeinated or decaffeinated coffee (Pinteraction = 0.74).
Conclusion
In this investigation of coffee and RCC, to our knowledge the largest to date, we observed a 20% reduced risk for intake of ≥2 cups/day vs not drinking. Our findings add RCC to the growing list of cancers for which coffee consumption may be protective.
Keywords: Coffee, caffeinated coffee, decaffeinated coffee, renal cell carcinoma, cancer, clear cell tumour, smoking, cohort
Key Messages
The epidemiologic evidence to date relating coffee consumption to risk of renal cell carcinoma (RCC) has been inconclusive.
In this prospective analysis of coffee intake and RCC, the largest of its kind to date, we observed a 20% reduced risk for intake of ≥2 cups/day vs not drinking. The relationship was observed among never-smokers only and did not differ between caffeinated- and decaffeinated-coffee drinkers.
Our findings add RCC to the growing list of cancers for which coffee consumption may be protective.
Introduction
The health effects of coffee, one of the most popular beverages in the world, has been the subject of great debate. Although earlier studies led to concern over cardiovascular-disease risks,1,2 recent findings suggest that coffee consumption may be associated with reductions in cardiovascular disease, type 2 diabetes and overall mortality.3–6 In the case of cancer, the evidence has been similarly controversial. In 1991, the International Agency for Research on Cancer (IARC) classified coffee as a possible human carcinogen (Group 2B).7 However, following a 2016 re-evaluation of the scientific literature, IARC downgraded its classification of coffee, concluding that there is inadequate evidence of carcinogenicity (Group 3).8 The IARC panel also noted evidence of inverse associations with risk of cancers of the endometrium and liver.8 Since that time, additional findings suggesting reduced risks of these and possibly other cancers have been reported.9–13
In the case of renal cell carcinoma (RCC), the epidemiologic evidence to date relating coffee consumption to risk has been inconclusive. A recent meta-analysis14 was null, although, in an analysis restricted to cohort studies, a weak inverse relationship was observed. To clarify this relationship, we investigated the association between coffee consumption and RCC risk in the National Institutes of Health–American Association of Retired Persons (NIH-AARP) Diet and Health Study—a large prospective cohort.
Methods
Study population
The NIH-AARP Diet and Health Study has been described.15 Between 1995 and 1996, a comprehensive questionnaire assessing diet and lifestyle was mailed to 3.5 million AARP members who were aged 50–71 years and resided in one of six US states (California, Florida, Louisiana, New Jersey, North Carolina and Pennsylvania) or two US metropolitan areas (Atlanta, Georgia and Detroit, Michigan). Of the 566 398 participants who completed the questionnaire and provided informed consent, we excluded individuals with questionnaire data that originated from a spouse or other surrogate correspondent (n = 15 760), with any self-reported cancer or diagnosed with cancer (except non-melanoma skin cancer) prior to baseline (n = 51 346), with only a death record for cancer (n = 4268), with no follow-up time (n = 21), with self-reported end-stage renal disease (n = 970), with missing information on coffee intake (n = 2247) and who resided outside the eligible states and metropolitan areas (n = 9). We also excluded persons with self-reported history of heart disease (n = 67 901) and poor health at baseline (n = 3758) to avoid potential bias from changes in coffee-drinking habits arising due to illness.3 The resulting analytic cohort included 420 118 participants (240 095 men and 180 023 women). The NIH-AARP Diet and Health Study was approved by the Special Studies Institutional Review Board of the National Cancer Institute.
Cohort follow-up and case ascertainment
Incident cases of RCC were identified through probabilistic record linkage to the eight original state cancer registries and those of three additional states (Arizona, Texas and Nevada) subsequently added to capture participants who moved to those states during follow-up. Participants were followed from baseline (1995–1996) until the date of first RCC, the date of death, the end of study follow-up (31 December 2011) or the date the participant moved out of the registry area (7.6% of subjects moved out), whichever came first. We defined RCC as a first primary malignancy with the International Classification of Disease for Oncology (ICD-O, 3rd edition) topography code C649 and histology code 8140–8575.
Assessment of coffee intake and other covariates
Participants completed a self-administered 124-item food-frequency questionnaire (FFQ), as previously described.15 In an earlier validation of the FFQ, coffee consumption was highly correlated with intake assessed using two non-consecutive 24-hour dietary-recall questionnaires (Spearman coefficient = 0.80).16 Usual coffee intake over the last 12 months was assessed using 10 frequency categories, ranging from none to ≥6 cups/day. Those who reported consuming coffee also provided information on whether they drank caffeinated or decaffeinated coffee more than half the time. We used this information to divide participants into coffee-drinking categories ranging from none to ≥4 cups/day and further categorized coffee drinkers as predominately caffeinated- or decaffeinated-coffee drinkers. Information on the use of sweetener or milk/creamer when drinking coffee or tea was also obtained. We also extracted information on demographic, health and lifestyle characteristics from the baseline questionnaire such as age, sex, body mass index (BMI), marriage status, education, smoking, self-reported history of diabetes, total energy intake, alcohol consumed in the past 12 months, self-reported health, healthy eating index (HEI; a measure of diet quality)17 and state of residence. Self-reported history of hypertension was extracted from a subsequent questionnaire mailed in 1996–1997 and completed by a subset of subjects in our analytic cohort (n = 259 847).
Statistical analysis
We summarized demographic and lifestyle characteristics associated with RCC by coffee intake. We fit Cox proportional-hazards models stratifying by age category at baseline (<55, 55–59, 60–64, 65–69 and ≥70 years) to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for coffee intake with RCC using attained age as the underlying time metric.
To account for missing data among covariates (0.5% for alcohol consumption, 0.8% for marital status, 1.3% for race/ethnicity, 1.5% for self-reported health, 2.4% for BMI, 2.8% for education, 3.8% for smoking), we conducted multiple imputation using fully conditional specification implemented by the multivariate imputation by chained equations algorithm using R package ‘mice’.18 We obtained 10 imputations from the models including coffee consumption, kidney cancer and aforementioned covariates. HRs were obtained for each of the 10 imputed data sets [function ‘with()’] and were averaged over the 10 data sets with the overall variance estimated using the function ‘pool()’.
For our analysis, we fit models adjusting only for sex as a covariate as well as full multivariable models additionally adjusting for race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic and Asian, Pacific Islander or American Indian/Alaskan Native), BMI (<25, ≥25 and <30, and ≥30 kg/m2), marriage status (married or living as married, widowed/divorced/separated, never married), education (less or completed high school, post-high school or some college, college and postgraduate), smoking (never smoked, quit ≤20 cigarettes/day, quit >20 cigarettes/day, currently smoking ≤20 cigarettes/day, currently smoking >20 cigarettes/day), self-reported history of diabetes (yes/no), total energy intake (calories), alcohol consumption during the prior 12 months (yes/no) and state of residence. We also ran multivariable models additionally adjusting for self-reported history of hypertension within the subset of participants who completed the follow-up questionnaire capturing this condition. We estimated HRs for RCC with categories of coffee intake (<1, 1, 2–3, ≥4 cups/day) using no coffee intake as the reference group. We also modelled coffee intake as a continuous variable based on the midpoint of coffee-intake categories (including values of 0.21 and 5 for participants in the categories of <1 and ≥4 cups/day, respectively) and calculated a Wald statistic as a test for trend. We tested the proportional-hazards assumption using statistical tests based on the scaled Schoenfeld residuals and observed no indication of a violation after stratifying the baseline hazard by age category.
We conducted stratified analyses and tests of interaction to explore possible modification of the association between coffee intake and RCC by sex and race/ethnicity (e.g. from possible differences in pro- or anti-neoplastic hormonal and biologic pathways affected by coffee, or in behaviours correlated with coffee intake) as well as other risk factors (e.g. by affecting pro- or anti-neoplastic biologic pathways that are also affected by coffee). We tested for interaction by inserting model parameters specifying interaction between coffee intake (modelled as for the trend test) and other variables, including sex, race/ethnicity (non-Hispanic White, other), smoking status (never- or ever-smokers), BMI (<25 and ≥25 kg/m2), self-reported history of diabetes, self-reported history of hypertension and alcohol consumption in the multivariable-adjusted model. We further conducted stratified analyses across levels of the aforementioned factors. In addition, we performed subgroup analyses by consumption of predominately caffeinated vs decaffeinated coffee to assess the impact of caffeine on our findings and by RCC subtype (clear cell vs non-clear cell histology) to explore possible effects specific to pathways dysregulated in clear cell RCC. To test for relative-risk heterogeneity across tumour subtypes, we performed a case-only analysis (clear cell vs non-clear cell) using logistic regression.19 Lastly, we conducted sensitivity analyses by repeating analyses for different follow-up periods (≤5, >5 and ≤10, and >10 years after baseline), restricting to coffee drinkers (i.e. using <1 cup/day as the referent category), with additional adjustment for HEI and self-reported health, and stratifying on whether coffee drinkers reported adding any sweetener or milk/creamer. We used RStudio Version 1.3.1093 (http://www.rstudio.com/) for all statistical analyses. All statistical tests were two-sided.
Results
Among 420 118 participants who were cancer-free at baseline, 2674 incident cases of RCC (1981 men and 693 women) were identified. The median age at baseline was 62 years and the majority were male (57%), non-Hispanic White (91%), married (68%) and had attained more than post-high school or some college education (72%) (Table 1). Nearly 90% reported drinking coffee and, of those, 57% reported drinking ≥2 cups/day. At baseline, persons with higher coffee intake were more likely to be male, non-Hispanic White, current or former smokers and alcohol drinkers, and they were less likely to have a college education.
Table 1.
Summary of selected characteristics of study participants by coffee consumption (cups/day) in the NIH-AARP Diet and Health Study, 1995–2011 (n = 420 118)
| Coffee intake (cups/day) |
P for difference | |||||
|---|---|---|---|---|---|---|
| Characteristic | None (n = 43 652) |
<1 (n = 67 810) |
1 (n = 68 220) |
2–3 (n = 172 520) |
≥4 (n = 67 916) |
|
| Age at baseline, mean (SD) | 61.0 (5.5) | 61.6 (5.4) | 62.5 (5.3) | 61.9 (5.3) | 61.0 (5.4) | <0.0001 |
| Total energy intake (calories), mean (SD) | 1825.4 (968.0) | 1774.0 (951.2) | 1795.3 (956.8) | 1859.6 (894.0) | 2093.6 (1226.1) | <0.0001 |
| Sex, n (%) | <0.0001 | |||||
| Male | 21 966 (50.3%) | 36 415 (53.7%) | 35 571 (52.1%) | 101 696 (58.9%) | 44 447 (65.4%) | |
| Female | 21 686 (49.7%) | 31 395 (46.3%) | 32 649 (49.7%) | 70 824 (41.1%) | 23 469 (34.6%) | |
| Race, n (%) | <0.0001 | |||||
| Non-Hispanic White | 38 736 (88.7%) | 57 679 (85.1%) | 59 754 (87.6%) | 161 138 (93.4%) | 64 964 (95.7%) | |
| Non-Hispanic Black | 2942 (6.7%) | 5664 (8.4%) | 3802 (5.6%) | 3922 (2.3%) | 692 (1.0%) | |
| Hispanic | 662 (1.5%) | 1548 (2.3%) | 2067 (3.0%) | 3232 (1.9%) | 830 (1.2%) | |
| Asian, Pacific Islander or American Indian/Alaskan Native | 704 (1.6%) | 1826 (2.7%) | 1608 (2.4%) | 2269 (1.3%) | 646 (1.0%) | |
| Missing | 608 (1.4%) | 1093 (1.6%) | 989 (1.4%) | 1959 (1.1%) | 784 (1.2%) | |
| BMI at current age (kg/m2), n (%) | <0.0001 | |||||
| <25 | 16 118 (36.9%) | 24 464 (36.1%) | 24 885 (36.5%) | 60 447 (35.0%) | 23 718 (34.9%) | |
| ≥25 and <30 | 16 478 (37.7%) | 26 444 (39.0%) | 27 514 (40.3%) | 73 924 (42.8%) | 29 015 (42.7%) | |
| ≥30 | 9918 (22.7%) | 15 015 (22.1%) | 14 021 (20.6%) | 34 469 (20.0%) | 13 722 (20.2%) | |
| Missing | 1138 (2.6%) | 1887 (2.8%) | 1800 (2.6%) | 3680 (2.1%) | 1461 (2.2%) | |
| Marital status, n (%) | <0.0001 | |||||
| Married or living as married | 27 961 (64.1%) | 43 097 (63.6%) | 45 491 (66.7%) | 120 424 (69.8%) | 47 482 (69.9%) | |
| Widowed, divorced or separated | 12 589 (28.8%) | 20 189 (29.8%) | 18 637 (27.3%) | 43 237 (25.1%) | 17 217 (25.3%) | |
| Never married | 2782 (6.4%) | 3917 (5.8%) | 3501 (5.1%) | 7645 (4.4%) | 2754 (4.1%) | |
| Missing | 323 (0.7%) | 610 (0.9%) | 593 (0.9%) | 1222 (0.7%) | 468 (0.7%) | |
| Education, n (%) | <0.0001 | |||||
| Less or completed high school | 10 360 (23.7%) | 16 135 (23.8%) | 18 320 (26.9%) | 42 973 (24.9%) | 17 804 (26.2%) | |
| Post-high school or some college | 13 716 (31.4%) | 21 418 (31.6%) | 21 949 (32.2%) | 57 693 (33.4%) | 23 489 (34.6%) | |
| College and postgraduate | 18 407 (42.2%) | 28 247 (41.7%) | 25 896 (38.0%) | 66 998 (38.8%) | 24 764 (36.5%) | |
| Missing | 1169 (2.7%) | 2010 (3.0%) | 2055 (3.0%) | 4856 (2.8%) | 1859 (2.7%) | |
| Current (last 12 months) use of alcohol on baseline (not including food sources), n (%) | <0.0001 | |||||
| Yes | 24 869 (57.0%) | 50 202 (74.0%) | 52 544 (77.0%) | 141 146 (81.8%) | 52 656 (77.5%) | |
| Missing | 170 (0.4%) | 297 (0.4%) | 299 (0.4%) | 816 (0.5%) | 323 (0.5%) | |
| Smoking, n (%) | <0.0001 | |||||
| Never smoked | 25 394 (58.2%) | 31 160 (46.0%) | 28 895 (42.4%) | 54 820 (31.8%) | 13 872 (20.4%) | |
| Quit, ≤ 20 cigarettes/day | 8844 (20.3%) | 19 283 (28.4%) | 20 733 (30.4%) | 51 942 (30.1%) | 15 288 (22.5%) | |
| Quit, >20 cigarettes/day | 5261 (12.1%) | 10 132 (14.9%) | 10 928 (16.0%) | 37 712 (21.9%) | 18 162 (26.7%) | |
| Currently smoking, ≤ 20 cigarettes/day | 1788 (4.1%) | 3270 (4.8%) | 3810 (5.6%) | 14 756 (8.6%) | 9897 (14.6%) | |
| Currently smoking, >20 cigarettes/day | 914 (2.1%) | 1146 (1.7%) | 1166 (1.7%) | 6727 (3.9%) | 8090 (11.9%) | |
| Missing | 1451 (3.3%) | 2819 (4.2%) | 2688 (3.9%) | 6563 (3.8%) | 2607 (3.8%) | |
| Self-reported history of diabetes, n (%) | 3422 (7.8%) | 5361 (7.9%) | 5296 (7.8%) | 11 747 (6.8%) | 4585 (6.8%) | <0.0001 |
| Self-reported history of hypertensiona, n (%) | 9486 (34.2%) | 15 874 (38.0%) | 16 516 (39.4%) | 38 078 (35.6%) | 12 607 (30.3%) | <0.0001 |
| Self-reported health, n (%) | <0.0001 | |||||
| Excellent | 8810 (20.2%) | 12 415 (18.3%) | 12 436 (18.2%) | 34 657 (20.1%) | 13 858 (20.4%) | |
| Very good | 15 965 (36.6%) | 24 982 (36.8%) | 25 732 (37.7%) | 67 844 (39.3%) | 25 828 (38.0%) | |
| Good | 14 161 (32.4%) | 22 903 (33.8%) | 23 344 (34.2%) | 55 863 (32.4%) | 22 215 (32.7%) | |
| Fair | 3985 (9.1%) | 6414 (9.5%) | 5585 (8.2%) | 11 795 (6.8%) | 5068 (7.5%) | |
| Missing | 731 (1.7%) | 1096 (1.6%) | 1123 (1.6%) | 2361 (1.4%) | 947 (1.4%) | |
NIH-AARP, National Institutes of Health–American Association of Retired Persons; BMI, body mass index; SD, standard deviation.
Information on self-reported history of hypertension was extracted from a subsequent questionnaire mailed in 1996–1997 (N = 259 847; non-drinkers: 27 764, <1 cup/day: 41 803, 1 cup/day: 41 871, 2–3 cups/day: 106 868, 4+ cups/day: 41 541).
We conducted analysis of variance tests for continuous variables and chi-squared tests for categorical variables to generate P for difference between groups.
We summarized in Table 2 our findings relating coffee intake to RCC risk, both overall and by usual caffeine content. In Cox-regression models, stratifying the baseline hazard by age category and adjusting for sex only, we observed a weak inverse relationship with increasing coffee consumption (HR 0.85, 95% CI 0.73, 0.99 for ≥4 cups/day of intake vs non-drinkers; Ptrend = 0.002). When we fit the multivariable model, the inverse relationship became stronger (HR 0.77, 95% CI 0.66, 0.90; Ptrend = 0.00003), mainly due to adjustment for smoking (9% change in HR). Additional adjustment for history of hypertension in the subset with information on this condition led to virtually identical findings (HR 0.75, 95% CI 0.61, 0.91 for ≥4 cups/day of intake vs non-drinkers; Ptrend = 0.0002). We observed a slightly stronger association with intake for people reporting consumption of caffeinated coffee more than half the time (HR 0.73, 95% CI 0.62, 0.86; Ptrend = 0.00003) compared with usual consumption of decaffeinated coffee (HR 0.85, 95% CI 0.66, 1.09; Ptrend = 0.21), although a test of interaction did not support effect modification by caffeine content (Pinteraction = 0.74).
Table 2.
HRs and 95% CIs of renal cell carcinoma for daily coffee consumption in the NIH-AARP Diet and Health Study, overall and stratified by usual coffee content
| Coffee intake (cups/day) |
|||||||
|---|---|---|---|---|---|---|---|
| Coffee content | None | <1 | 1 | 2–3 | ≥4 | P trend | P interaction |
| All coffee (n = 420 118) | |||||||
| No. of cases | 289 | 453 | 467 | 1050 | 415 | ||
| Age and sex-adjusted HR (95% CI) | 1 | 0.96 (0.83, 1.12) | 0.97 (0.84, 1.13) | 0.84 (0.74, 0.96) | 0.85 (0.73, 0.99) | 0.002 | |
| Multivariable-adjusted HR (95% CI)a | 1 | 0.94 (0.81, 1.09) | 0.94 (0.81, 1.09) | 0.80 (0.70, 0.92) | 0.77 (0.66, 0.90) | 0.00003 | |
| Caffeinated coffee (n = 287 735) | |||||||
| No. of cases | 289 | 199 | 273 | 707 | 315 | ||
| Age and sex-adjusted HR (95% CI) | 1 | 0.97 (0.81, 1.16) | 0.94 (0.80, 1.11) | 0.80 (0.70, 0.92) | 0.84 (0.71, 0.98) | 0.005 | |
| Multivariable-adjusted HR (95% CI)a | 1 | 0.93 (0.78, 1.12) | 0.91 (0.76, 1.07) | 0.75 (0.65, 0.87) | 0.73 (0.62, 0.86) | 0.00003 | |
| Decaffeinated coffee (n = 160 615) | |||||||
| No. of cases | 289 | 216 | 180 | 313 | 84 | ||
| Age and sex-adjusted HR (95% CI) | 1 | 0.95 (0.80, 1.13) | 1.04 (0.86, 1.25) | 0.95 (0.81, 1.11) | 0.86 (0.67, 1.10) | 0.23 | |
| Multivariable-adjusted HR (95% CI)a | 1 | 0.96 (0.80, 1.15) | 1.03 (0.85, 1.24) | 0.95 (0.80, 1.12) | 0.85 (0.66, 1.09) | 0.21 | 0.74 |
HR, hazard ratio; CI, confidence interval; NIH-AARP, National Institutes of Health–American Association of Retired Persons; HR, hazard ratio; CI, confidence interval.
Adjusted for sex, race (non-Hispanic White, non-Hispanic Black, Hispanic and Asian, Pacific Islander or American Indian/Alaskan Native), BMI (<25, ≥25 and <30, and ≥30 kg/m2), marriage status (married or living as married, widowed/divorced/separated, never married), education (less or completed high school, post-high school or some college, college and postgraduate), smoking (never smoked, quit ≤20 cigarettes/day, quit >20 cigarettes/day, currently smoking ≤20 cigarettes/day, currently smoking >20 cigarettes/day), history of diabetes (yes/no), total energy intake (calories, continuous), alcohol consumption in the past 12 months (yes/no), state of residence (CA, FL, GA, LA, MI, NC, NJ, PA).
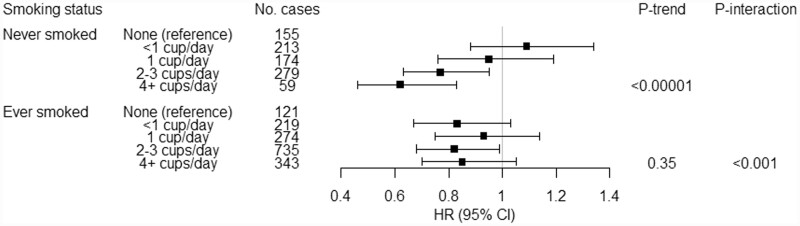
We also investigated associations across strata of study characteristics. When we stratified analysis by smoking status, we observed an inverse association among never-smokers but not ever-smokers (Figure 1; Pinteraction = 0.0009). The association with coffee was apparent among men but not among women, although a test of interaction did not support effect modification by sex (Supplementary Table 1, available as Supplementary data at IJE online). The association with coffee also did not differ across categories of race/ethnicity, BMI, histories of self-reported diabetes or hypertension, or alcohol consumption (Supplementary Table 1, available as Supplementary data at IJE online). When we repeated our analysis using <1 cup/day of intake as the reference category, our findings did not materially change (Supplementary Table 2, available as Supplementary data at IJE online). The inverse association was present across different periods of follow-up and was particularly strong for cases diagnosed >10 years after baseline (HR 0.65, 95% CI 0.51, 0.82 for ≥4 cups/day of intake vs non-drinkers; Ptrend = 0.0005; Supplementary Table 3, available as Supplementary data at IJE online). When we conducted separate analyses by tumour subtype, we observed a stronger inverse association for clear cell RCC (n = 926 cases; HR 0.64, 95% CI 0.49, 0.82 for ≥4 cups/day; Ptrend = 0.0002) compared with non-clear cell RCC (n = 1748 cases; HR 0.87, 95% CI 0.71, 1.06; Ptrend = 0.002), although, in a test of heterogeneity across subtypes, the P-value was 0.13 (Supplementary Table 4, available as Supplementary data at IJE online). Additional adjustment for HEI or self-reported health did not change our findings (Supplementary Table 5, available as Supplementary data at IJE online). When we conducted a sensitivity analysis by stratifying on use of sweetener or milk/creamer, we observed the same results (Supplementary Table 6, available as Supplementary data at IJE online).
Figure 1.
Association of daily coffee consumption with renal cell carcinoma stratified by smoking status in the NIH-AARP Diet and Health Study. NIH-AARP, National Institutes of Health–American Association of Retired Persons; HR, hazard ratio; CI, confidence interval. Adjusted for sex, race (non-Hispanic White, non-Hispanic Black, Hispanic and Asian, Pacific Islander or American Indian/Alaskan Native), BMI (<25, ≥25 and <30, ≥30 kg/m2), marriage status (married or living as married, widowed/divorced/separated, never married), education (less or completed high school, post-high school or some college, college and postgraduate), self-reported history of diabetes (yes/no), total energy intake (calories, continuous), alcohol consumption in the past 12 months (yes/no), state of residence (CA, FL, GA, LA, MI, NC, NJ, PA).
Discussion
In this large prospective US cohort, we observed an inverse relationship between coffee intake and RCC after adjustment for important risk factors, with consumption of ≥2 cups/day associated with an ∼20% lower risk over 16 years of follow-up. This association was observed among non-smokers but not ever-smokers and remained apparent in sensitivity analyses excluding the first 10 years of follow-up and assessing high vs low intake among coffee drinkers. The association did not materially differ between subgroups who reported drinking caffeinated coffee more than half of the time and those who reported predominantly drinking decaffeinated coffee.
Our findings provide the strongest epidemiologic evidence to date supporting an inverse relationship between coffee consumption and RCC. Past studies of this relationship have been inconsistent; a meta-analysis including 16 case–control and 6 cohort studies did not observe a relationship between coffee consumption and RCC risk.14 Case–control studies of dietary factors such as coffee are subject to recall bias, since exposures are assessed retrospectively, and to selection bias, if controls are unrepresentative of the source population for cases, the effects of which may substantially and unpredictably bias risk estimates. When the meta-analysis was restricted to cohort studies, a 12% lower relative risk per cup of coffee was observed, which is similar in magnitude to the effects that we observed herein. Since the publication of that meta-analysis,14 three recent cohort investigations have been reported.20–22 Weak inverse associations between coffee intake and renal cancer were observed within the Cancer Prevention Study-II (1922 renal-cancer deaths; ≥6 cups/day: HR 0.93, 95% CI 0.77, 1.11) and the Multiethnic Cohort Study (838 incident cases; ≥4 cups/day: HR 0.89, 95% CI 0.64, 1.23).20,21 An analysis of coffee intake and cancer incidence in the UK Biobank did not observe an association between reported coffee intake and 337 incident renal cancers; however, in a Mendelian-randomization analysis of a genetic instrument for coffee consumption and 1012 renal cancers, a 10% lower risk per predicted cup of coffee was observed (odds ratio 0.90, 95% CI 0.67, 1.20).22 In summary, the totality of the published cohort evidence is suggestive of an inverse association between coffee intake and RCC, similar to that observed in our study.
In analyses stratified on smoking status, we found an inverse association with coffee intake among never-smokers only. The null effect among smokers may reflect the presence of residual confounding from smoking, which is positively associated with both coffee intake and RCC risk. Past studies of coffee and renal cancer did not observe evidence of an interaction with smoking status,20,23,24 although most were limited by case numbers in stratified analyses. We also observed stronger evidence of an inverse association with coffee intake vs non-drinkers among men than among women, although a test of interaction did not support effect modification. Given the absence of supporting evidence at this time, our findings from these subgroup analyses should be interpreted with caution.
There are several potential biologic mechanisms through which coffee intake might lower the risk of RCC. First, coffee contains many antioxidants and anticarcinogenic compounds.25,26 In particular, coffee-derived diterpenes, cafestol and kahweol have been shown to induce phase-II enzymes involved in carcinogen detoxification, reduce the expression of phase-I enzymes responsible for carcinogen activation and stimulate intracellular antioxidant defence mechanisms.26 In addition, polyphenolic compounds in coffee have been found experimentally to prevent DNA damage caused by free radicals or carcinogenic agents.27 Second, coffee intake is associated with reduced risk of chronic kidney disease (CKD),28–30 a risk factor for RCC, and higher estimated glomerular filtration rate (eGFR).31 Similarly, a large Mendelian-randomization analysis found a genetic instrument for coffee intake to be associated with reduced risks of CKD categories 3 to 5 (G3-G5) and albuminuria, and a positive association with eGFR.32 Third, coffee intake has been associated with a lower risk of developing type 2 diabetes,33 a suspected RCC risk factor, by improving insulin sensitivity.34 Fourth, the diluting effect due to coffee intake may reduce the risk of RCC by increasing urine volume and thereby diluting the concentration of carcinogens in contact with renal epithelial cells.23 Our finding of a similar inverse association between subgroups of primarily caffeinated and decaffeinated coffee suggests that any protective benefits from coffee are not driven by biologic effects of caffeine itself.
The prospective design is a major strength of our study, which minimized the potential for bias from reverse causation from cancer-induced changes in coffee-drinking habits. The consistency of the observed association across different periods of follow-up and with additional adjustment for self-reported health status at baseline also argue against reverse causation as an explanation for our findings. Also important is its large size; with >2600 incident cases, it is the largest investigation of coffee intake and RCC conducted to date, nearly equalling the total number of cases among cohorts included in the meta-analysis of coffee and renal cancer (n = 2736).14 Importantly, this large size yielded robust statistical power to detect an inverse association of the magnitude observed in our analysis, unlike nearly all previous studies of coffee and RCC. Additional strengths include the availability of data on several risk factors for RCC, most notably BMI, smoking and hypertension, which provided an opportunity to control for potential confounding in the analysis, as well as data on the typical consumption of caffeinated vs decaffeinated coffee, which was not assessed in many prior cohort investigations.
Our study also had limitations. Coffee consumption was self-reported, with participants asked their typical coffee consumption over the last 12 months. Although we were unable to estimate lifetime cumulative coffee consumption, coffee consumption has been shown to be relatively stable over time.35 The limited available information on consumption of caffeinated vs decaffeinated coffee weakened our investigations restricted to these beverage types. In addition, we lacked data on the type of coffee preparation (espresso, boiled or filtered), which is important because constituents of coffee may differ, depending on the method.36 Given that filtered coffee is predominantly consumed in the USA,37 our findings are most likely reflective of the association between filtered coffee and RCC risk. Lastly, our study population was almost entirely non-Hispanic White (91%), precluding analyses of other individual racial/ethnic groups.
Conclusion
In our investigation, to our knowledge the largest of its kind to date, we found an ∼20% reduced risk of RCC for coffee intake of ≥2 cups/day, with evidence for a graded association with lower intake. Our findings add RCC to the growing list of cancers for which coffee consumption may be protective. Additional experimental and epidemiologic research investigating the potential benefits of coffee on risk of RCC and other cancers in diverse populations is warranted.
Supplementary data
Supplementary data are available at IJE online.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology & Genetics.
Supplementary Material
Acknowledgements
Cancer-incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia. Cancer-incidence data from California were collected by the California Cancer Registry, California Department of Public Health’s Cancer Surveillance and Research Branch, Sacramento, California. Cancer-incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, Lansing, Michigan. The Florida cancer-incidence data used in this report were collected by the Florida Cancer Data System (Miami, Florida) under contract with the Florida Department of Health, Tallahassee, Florida. The views expressed herein are solely those of the authors and do not necessarily reflect those of the FCDC or FDOH. Cancer-incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Health Sciences Center School of Public Health, New Orleans, Louisiana. Cancer-incidence data from New Jersey were collected by the New Jersey State Cancer Registry, The Rutgers Cancer Institute of New Jersey, New Brunswick, New Jersey. Cancer-incidence data from North Carolina were collected by the North Carolina Central Cancer Registry, Raleigh, North Carolina. Cancer-incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Cancer-incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services, Phoenix, Arizona. Cancer-incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, Austin, Texas. Cancer-incidence data from Nevada were collected by the Nevada Central Cancer Registry, Division of Public and Behavioral Health, State of Nevada Department of Health and Human Services, Carson City, Nevada. We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. We also thank Dr Ruth Pfeiffer from the Biostatistics Branch, Division of Cancer Epidemiology & Genetics, National Cancer Institute for her assistance in data imputation, Sigurd Hermansen and Kerry Grace Morrissey from Westat for study-outcomes ascertainment and management, and Leslie Carroll at Information Management Services for data support and analysis. The NIH-AARP Diet and Health Study was approved by the Special Studies Institutional Review Board of the National Cancer Institute (NIH-AARP: OH95-C-N025).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Contributor Information
Jongeun Rhee, Division of Cancer Epidemiology and Genetics, Occupational and Environmental Epidemiology Branch, National Cancer Institute, Rockville, MD, USA and .
Erikka Loftfield, Division of Cancer Epidemiology and Genetics, Metabolic Epidemiology Branch, National Cancer Institute, Rockville, MD, USA.
Neal D Freedman, Division of Cancer Epidemiology and Genetics, Metabolic Epidemiology Branch, National Cancer Institute, Rockville, MD, USA.
Linda M Liao, Division of Cancer Epidemiology and Genetics, Metabolic Epidemiology Branch, National Cancer Institute, Rockville, MD, USA.
Rashmi Sinha, Division of Cancer Epidemiology and Genetics, Metabolic Epidemiology Branch, National Cancer Institute, Rockville, MD, USA.
Mark P Purdue, Division of Cancer Epidemiology and Genetics, Occupational and Environmental Epidemiology Branch, National Cancer Institute, Rockville, MD, USA and .
Conflict of interest
None declared.
References
- 1. Jick H, Miettinen OS, Neff RK, the Boston Collaborative Drug Surveillance Program, Boston University Medical Center et al. Coffee and myocardial infarction. N Engl J Med 1973;289:63–7. [DOI] [PubMed] [Google Scholar]
- 2. Nichols AB. Coffee drinking and acute myocardial infarction. Lancet 1973;301:480–81. [DOI] [PubMed] [Google Scholar]
- 3. Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. N Engl J Med 2012;366:1891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grosso G, Micek A, Godos J et al. Coffee consumption and risk of all-cause, cardiovascular, and cancer mortality in smokers and non-smokers: a dose-response meta-analysis. Eur J Epidemiol 2016;31:1191–205. [DOI] [PubMed] [Google Scholar]
- 5. Carlström M, Larsson SC. Coffee consumption and reduced risk of developing type 2 diabetes: a systematic review with meta-analysis. Nutr Rev 2018;76:395–417. [DOI] [PubMed] [Google Scholar]
- 6. Larsson SC, Orsini N. Coffee consumption and risk of stroke: a dose-response meta-analysis of prospective studies. Am J Epidemiol 2011;174:993–1001. [DOI] [PubMed] [Google Scholar]
- 7. International Agency for Research on Cancer. Coffee, tea, mate, methylxanthines and methylglyoxal. IARC Monogr Eval Carcinogenic Risks Hum 1991;51:217–33. [PMC free article] [PubMed] [Google Scholar]
- 8. Loomis D, Guyton KZ, Grosse Y et al. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol 2016;17:877–78. [DOI] [PubMed] [Google Scholar]
- 9. Tamura T, Wada K, Konishi K et al. Coffee, green tea, and caffeine intake and liver cancer risk: a prospective cohort study. Nutr Cancer 2018;70:1210–16. [DOI] [PubMed] [Google Scholar]
- 10. Gan Y, Wu J, Zhang S et al. Association of coffee consumption with risk of colorectal cancer: a meta-analysis of prospective cohort studies. Oncotarget 2017;8:18699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larsson SC, Giovannucci EL, Wolk A. Coffee consumption and risk of gallbladder cancer in a prospective study. J Natl Cancer Inst 2017;109:1–3. [DOI] [PubMed] [Google Scholar]
- 12. Caini S, Masala G, Saieva C et al. Coffee, tea and melanoma risk: findings from the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2017;140:2246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sánchez-Quesada C, Romanos-Nanclares A, Navarro AM et al. Coffee consumption and breast cancer risk in the SUN project. Eur J Nutr 2020;59:3461–71. [DOI] [PubMed] [Google Scholar]
- 14. Wijarnpreecha K, Thongprayoon C, Thamcharoen N, Panjawatanan P, Cheungpasitporn W. Association between coffee consumption and risk of renal cell carcinoma: a meta-analysis. Intern Med J 2017;47:1422. [DOI] [PubMed] [Google Scholar]
- 15. Schatzkin A, Subar AF, Thompson FE et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health–American Association of Retired Persons Diet and Health Study. Am J Epidemiol 2001;154:1119–25. [DOI] [PubMed] [Google Scholar]
- 16. Thompson FE, Kipnis V, Midthune D et al. Performance of a food-frequency questionnaire in the us NIH–AARP (National Institutes of Health–American Association of Retired Persons) Diet and Health Study. Public Health Nutr 2008;11:183–95. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Agriculture Food and Nutrition Service. Healthy Eating Index (HEI). https://www.fns.usda.gov/resource/healthy-eating-index-hei (25 January 2020, date last accessed).
- 18. van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 19. Begg CB, Zhang Z. Statistical analysis of molecular epidemiology studies employing case-series. Cancer Epidemiol Biomarkers Prev 1994;3:173–75. [PubMed] [Google Scholar]
- 20. Gapstur SM, Anderson RL, Campbell PT et al. Associations of coffee drinking and cancer mortality in the cancer prevention study-II. Cancer Epidemiol Biomarkers Prev 2017;26:1477–86. [DOI] [PubMed] [Google Scholar]
- 21. Park S-Y, Freedman ND, Haiman CA, Le Marchand L, Wilkens LR, Setiawan VW. Prospective study of coffee consumption and cancer incidence in non-white populations. Cancer Epidemiol Biomarkers Prev 2018;27:928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ong J-S, Law MH, An J et al. Association between coffee consumption and overall risk of being diagnosed with or dying from cancer among> 300 000 UK Biobank participants in a large-scale Mendelian randomization study. Int J Epidemiol 2019;48:1447–56. [DOI] [PubMed] [Google Scholar]
- 23. Lee JE, Hunter DJ, Spiegelman D et al. Intakes of coffee, tea, milk, soda and juice and renal cell cancer in a pooled analysis of 13 prospective studies. Int J Cancer 2007;121:2246–53. [DOI] [PubMed] [Google Scholar]
- 24. Allen N, Balkwill A, Beral V, Green J, Reeves G, for the Million Women Study Collaborators. Fluid intake and incidence of renal cell carcinoma in UK women. Br J Cancer 2011;104:1487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gómez-Ruiz JÁ, Leake DS, Ames JM. In vitro antioxidant activity of coffee compounds and their metabolites. J Agric Food Chem 2007;55:6962–69. [DOI] [PubMed] [Google Scholar]
- 26. Cavin C, Holzhaeuser D, Scharf G, Constable A, Huber W, Schilter B. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem Toxicol 2002;40:1155–63. [DOI] [PubMed] [Google Scholar]
- 27. Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res 2008;52:507–26. [DOI] [PubMed] [Google Scholar]
- 28. Jhee JH, Nam KH, An SY et al. Effects of coffee intake on incident chronic kidney disease: a community-based prospective cohort study. Am J Med 2018;131:1482–90. [DOI] [PubMed] [Google Scholar]
- 29. Hu EA, Selvin E, Grams ME, Steffen LM, Coresh J, Rebholz CM. Coffee consumption and incident kidney disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 2018;72:214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bigotte Vieira M, Magriço R, Viegas Dias C, Leitão L, Neves JS. Caffeine consumption and mortality in chronic kidney disease: a nationally representative analysis. Nephrol Dial Transplant 2019;34:974–80. [DOI] [PubMed] [Google Scholar]
- 31. Herber-Gast G-CM, Essen H, Verschuren WM et al. Coffee and tea consumption in relation to estimated glomerular filtration rate: results from the population-based longitudinal Doetinchem Cohort Study. Am J Clin Nutr 2016;103:1370–77. [DOI] [PubMed] [Google Scholar]
- 32. Kennedy OJ, Pirastu N, Poole R, et al. Coffee consumption and kidney function: a mendelian randomization study. Am J Kidney Dis. 2019. https://www.sciencedirect.com/science/article/pii/S0272638619310339 (1 April 2020, date last accessed). [DOI] [PubMed]
- 33. Van Dam RM, Feskens EJ. Coffee consumption and risk of type 2 diabetes mellitus. Lancet 2002;360:1477–78. [DOI] [PubMed] [Google Scholar]
- 34. Ärnlöv J, Vessby B, Risérus U. Coffee consumption and insulin sensitivity. JAMA 2004;291:1199–201. [DOI] [PubMed] [Google Scholar]
- 35. Nilsson LM, Wennberg M, Lindahl B, Eliasson M, Jansson J-H, Van Guelpen B. Consumption of filtered and boiled coffee and the risk of first acute myocardial infarction; a nested case/referent study. Nutr Metab Cardiovasc Dis 2010;20:527–35. [DOI] [PubMed] [Google Scholar]
- 36. Nilsson LM, Johansson I, Lenner P, Lindahl B, Van Guelpen B. Consumption of filtered and boiled coffee and the risk of incident cancer: a prospective cohort study. Cancer Causes Control 2010;21:1533–44. [DOI] [PubMed] [Google Scholar]
- 37. Jee SH, He J, Appel LJ, Whelton PK, Suh I, Klag MJ. Coffee consumption and serum lipids: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol 2001;153:353–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.