Abstract
Objective:
Characterize vegetable and fruit (VF) intake in a Yup’ik community using self-reported intake and skin carotenoid status (SCS) and evaluate the relationship between SCS and fish intake.
Methods:
Self-reported VF intake was measured by 24-hour recall, SCS was measured by reflection spectroscopy via the Veggie Meter, and fish intake was estimated by the nitrogen isotope ratio (NIR) for 80 participants in a remote community in southwestern Alaska. Bivariate correlations were used to assess the relationship between self-reported VF intake, SCS and the NIR.
Results:
Intake of all VF subgroups was low. The SCS was higher for males (262.7 vs. 185.3; P=0.002) and participants consuming more than one VF serving (232.5 vs. 183.0; P=0.02). It was not associated with the NIR.
Conclusions and Implications:
Increasing VF intake is one way to improve diet in Yup’ik communities and SCS is a simple and non-invasive tool to facilitate surveillance efforts.
Keywords: Biomarkers, Carotenoids, Alaska Native
INTRODUCTION
Dietary patterns high in vegetables and fruits are associated with lower rates of obesity, cardiovascular disease, some cancers, and all-cause mortality.1–4 Deep-yellow and dark-green vegetables are a particularly important part of a healthy diet because they are a source of carotenoids, pigments that play a protective role in human health as antioxidants.5 Promoting and monitoring intake levels of vegetables and fruits is critical for public health efforts. These efforts are especially important for populations that experience disproportionate levels of chronic diseases, such as Alaska Native communities.6 Studies have shown that Alaska Native People do not meet the dietary recommendations for vegetable and fruit intake set by the United Stated Department of Agriculture and consume significantly less vegetables and fruits than their counterparts in a national US sample.7
Yup’ik communities in the Yukon-Kuskokwim Delta (YKD) region of Southwest Alaska are undergoing a nutrition transition characterized by a shift from traditional to market-based food sources.8 Traditional foods include tundra plants gathered from the land and water(e.g., blueberries and salmonberries). According to one study from the 1960’s, tundra plants were consumed throughout the year and constituted 15% of the diet.9 Current estimates suggest that traditional plants constitute less than three percent of the diet of Yup’ik People.10 Fish is one traditional food group still consumed at high levels. One recent study estimated that the mean intake of omega-3 fatty acids, sourced from fish and sea mammals, is at least 20 times greater among Yup’ik People compared to the general US population.11 In comparison to traditional foods that are locally sourced, market foods are received by local bush plane service companies year round and barge services in the summer. Given the geographic and climatic challenges faced by the limited food distribution system, highly-processed shelf-stable market items are more widely available and less expensive than fresh, frozen, or canned vegetables and fruits.12
Previous nutrition studies conducted in the YKD have relied on subjective self-reported dietary assessment methods to measure vegetable and fruit intake. These methods are prone to measurement error and bias.13 The Veggie Meter is an emerging technology that objectively measures the optical density of skin carotenoids (skin carotenoid status) via reflection spectroscopy. The Veggie Meter offers advantages over other methods because it is non-invasive, rapid, and free of self-report bias. It has been validated against plasma carotenoids and a predecessor technology that uses resonance Raman spectroscopy to measure skin carotenoid status.14,15 However, the association between self-reported vegetable and fruit intake and skin carotenoid status is weak,16 and skin carotenoid status is influenced by factors such as smoking status, body mass index (BMI), and exposure to sunlight.17 In addition, skin carotenoid status only represents intake of brightly-colored vegetables and fruits so certain vegetables, like white potatoes, are not captured. In the US, carotenoids are primarily obtained from vegetables and fruit, which makes them a strong dietary biomarker of these foods,18 but they can also be obtained from other dietary sources, such as salmon and other marine foods.19 For populations with a diet rich in non-vegetable and fruit carotenoid sources (i.e., salmon), it is unclear how the relationship between vegetable and fruit intake and skin carotenoid status would be affected.
The primary goal was to compare estimates of vegetable and fruit intake based on self - report (24 hour recall) and a biomarker (skin carotenoid status) in a Yup’ik community in Southwestern Alaska. A secondary goal was to examine whether fish intake, assessed by a validated hair nitrogen isotope ratio biomarker (NIR), 20,21 was related to skin carotenoid status. This study builds on previous research in two important ways. First, it uses a biomarker of vegetable and fruit intake and examines specific sources of vegetables and fruits. Second, it measures skin carotenoid status in a population that frequently consumes carotenoids from a source other than vegetables and fruits, namely salmon and other fish.
METHODS
Study Setting
The YKD is a road-less region in southwestern Alaska that is approximately the size of Oregon. Communities are only accessible by bush plane year round, or by snow machine and boat seasonally. The YKD is the traditional homeland of Yup’ik People and 85% of residents are Yup’ik. Opportunities for wage labor are limited and more than 30% of residents fall below the national poverty level.22
Participants and Recruitment
Data were collected over two weeks in November 2018. A convenience sample was recruited by word-of mouth, posters at the grocery store, and from 3 public venues in a hub city (population ~6,500) in the YKD.22 The public venues included a regional health clinic and hospital that serve communities in the YKD and a monthly community market. Inclusion criteria were to be at least 18 years old and to reside in the YKD. There were no exclusion criteria. The majority of Yup’ik people living in the hub city are bilingual and written informed consent was obtained in English. Alaska Native race was not a requirement of enrollment in the study because the study goal was to estimate vegetable and fruit intake in the YKD food environment, where vegetable and fruit access is limited and fish intake is common. This study was approved by the University of Alaska Fairbanks Institutional Review Board and the Yukon-Kuskokwim Health Corporation Human Subjects Committee. Participants received a $25 gift card to the local grocery store as compensation for their time.
Study Procedures
Eligibility was confirmed prior to enrollment in the study and all study procedures (except the follow-up 24-hour dietary recall) were completed at enrollment. Participants completed a sixteen-item sociodemographic survey that asked about age, sex, race, current smoking status, Supplemental Nutrition Assistance Program (SNAP) participation, income, and education. While Alaska Native race was not an enrollment requirement, almost all participants identified as Alaska Native. Given the low representation (≤5% each) of other groups in the study (Asian, Hispanic, white, and other) race was reported as a dichotomous variable (Alaska Native/ not Alaska Native).
At enrollment, each participant completed the first of 2 24-hour dietary recalls face-to face. The second unannounced recall was completed over the phone within a week later. Approximately 10% of recalls were completed on the weekend. The 24-hour dietary recalls were administered by a certified interviewer using Nutrition Data System for Research (NDSR), an algorithm-driven, computer-assisted software that uses a multiple-pass approach (NDSR 2018; University of Minnesota, Minneapolis, MN).23
While 61% of study participants completed 2 24-hour recalls, 39% did not complete the second 24-hour recall because they were unable to be reached at follow-up (their phone number was no longer in service, a common occurrence in the YKD, or the participant did not return interviewers’ phone calls after they left 3 messages). There were no significant differences in self-reported total vegetable and fruit intake (P=0.93) between those who completed 1 vs. 2 24-hour dietary recalls when assessed by a t-test. Any dietary recall that was incomplete or determined to be unreliable by the interviewer was excluded from the analysis.
Total vegetable and fruit intake (servings/day) and intake of vegetable and fruit subgroup (servings/day) were calculated for each participant using the NDSR food and nutrient database (NDSR 2018). The NDSR database includes many traditional Alaska Native foods including traditional vegetables and fruits (tundra plants). Each vegetable and fruit reported in the 24-hour dietary recalls, including those in a mixed dish or juice, was assigned to a vegetable and fruit subgroup based on NDSR food group coding. White potatoes were categorized into their own subgroup. The NDSR non-citrus fruit category was split into brightly-colored fruits (e.g. blueberries, salmonberries, watermelon, and mangos) and other fruits (e.g. bananas, apples, and pears) so intake of carotenoid-rich fruits (foods represented by the skin carotenoid status) could be evaluated. The brightly-colored fruits subgroup included all tundra plants. For participants who completed 2 24-hour dietary recalls, results were averaged over the 2 recall days. All serving sizes were calculated by NDSR and are based on the recommendations made by the 2000 Dietary Guidelines for Americans when available.7 Total vegetable and fruit intake was also dichotomized as at least 1 servings of vegetables and fruits per day (yes/no).
Skin carotenoid status was measured by the Veggie Meter (Longevity Link Corp.; Salt Lake City, UT) following the protocol described by Ermakov et al.14 Participants were instructed to place their finger against the lens surface of the Veggie Meter with the help of a spring-loaded cover. Then, a white light LED source was projected onto their finger and an adjoining laptop displayed their skin carotenoid status. Three individual readings were collected for each participant and the glass lens of the Veggie Meter was cleaned with an optical cloth between each reading. Mean skin carotenoid status was calculated for each participant by averaging their 3 measurements.
The skin carotenoid status is a unit-less value that ranges from 0–800, with a higher value indicating a higher concentration of carotenoids in the skin. It is not directly related to an amount of vegetable and fruit servings, but can be used to rank intake. Skin carotenoids are a long term measure of intake and represent intake from at least the 8 past weeks.24 A validation study found that across a year of measurements, self-reported vegetable and fruit intake was correlated with skin carotenoid status measured by reflection spectroscopy (r=0.37, P<0.001).16 The same study found a high correlation between reflection spectroscopy and resonance Raman spectroscopy (r=0.86, p<0.001), and reflection spectroscopy and plasma carotenoids (r=0.70, p<0.001).16
A subset of participants provided a hair sample to measure the NIR, which has been previously validated as a biomarker of fish intake in Yup’ik communities.20,21,25 Hair was collected by cutting ~50 hairs from the back of the head as close to the scalp as possible. The 1 centimeter section of hair closest to the head was analyzed and represented the last 4 to 8 weeks of intake. Samples were cleaned and prepared for stable isotope analysis as described elsewhere.20 Analysis was completed at the Alaska Stable Isotope Facility using continuous-flow isotope ratio mass spectroscopy with a Costech ECS4010 Elemental Analyzer (Costech Scientific) interfaced to a Finnigan Delta Plus XP isotope ratio mass spectrometer via the Conflo III interface (Thermo-Finnigan). Data are presented in standard delta values as δX = (Rsample − Rstandard)/(Rstandard) · 1000‰, where R is the ratio of heavy to light isotope (15N/14N) and the standard is atmospheric nitrogen. In a validation study, the hair NIR varied from 6.9 to 15.2‰, corresponding to diets in which the % energy from fish and marine mammals ranged from 0 to 57%.25
Statistical Analysis
Descriptive statistics were calculated for all study participants. Self-reported vegetable and fruit intake was summarized as median and interquartile range and skin carotenoid status was summarized as mean and standard deviation. A kurtosis of greater than +/−3 was used as a criteria to decide which variables should be log-transformed. Total vegetable and fruit intake was transformed as log(x+1) to compensate for the high number of 0 servings and to include participants who reported 0 servings. Back-transformed data is reported in the tables for interpretation. Differences in total vegetable and fruit intake and skin carotenoid status by sociodemographic subgroups were assessed using Student’s t-test for binary variables (e.g., sex) and ANOVA for multilevel variables (e.g., age group, income range). Participants with a missing response to a single sociodemographic variable were excluded from the statistical test for that variable. Frequency of missing responses ranged from 4% (race) to 15% (income). Pearson’s correlation coefficient was used to assess the association of total vegetable and fruit intake, skin carotenoid status, and the NIR. A P-value of 0.05 was considered significant. Statistical analyses were performed using SPSS Statistics 25 (IBM Corp.; 2017 Armonk, NY).
RESULTS
Eighty-four participants enrolled in the study but 4 participants were excluded due to incomplete or unreliable 24-hour dietary recall data so the analysis of self-reported intake and skin carotenoid status was based on 80 participants. Seventy-one participants (89%) provided a hair sample, so the analysis of skin carotenoid status and NIR was based on 71 participants. The average age of participants was 47.5±15.2 years. Forty-five percent of the participants were female, 40% were current smokers, and 39% participated in SNAP (Table 1) The mean NIR was 8.8±0.8‰ (range: 7.2 to 11.2‰).
Table 1.
Comparison of Total Vegetables and Fruit Intake (servings/day) and Skin Carotenoid Status by Sociodemographic Subgroups (n=80)
| n (%) | Total Vegetable and Fruit Intakea
(servings/day) Median ± IQR |
P | Skin Carotenoid Status Mean ± SD |
P | |
|---|---|---|---|---|---|
| Age group (years) | 0.40 | 0.59 | |||
| 20–30 | 13 (16) | 2.0 ± 2.8 | 198.1 ± 136.8 | ||
| 31–50 | 29 (36) | 2.5 ± 2.9 | 214.4 ± 65.8 | ||
| 51–84 | 38 (47) | 2.6 ± 2.7 | 232.0 ± 123.3 | ||
| Sex | 0.47 | 0.002** | |||
| Male | 36 (45) | 1.9 ± 3.4 | 262.7 ± 119.6 | ||
| Female | 44 (55) | 2.9 ± 2.0 | 185.3 ± 83.8 | ||
| Race | 0.39 | 0.93 | |||
| Alaska Native | 65 (81) | 2.4 ± 2.4 | 214.6 ± 93.0 | ||
| Not Alaska Native | 12 (15) | 3.5 ± 3.8 | 211.8 ± 140.1 | ||
| No responseb | 3 (4) | 4.5 ± 5.2 | 372.1 ± 199.0 | ||
| Current Smoker | 0.21 | 0.58 | |||
| Yes | 32 (40) | 1.9 ± 2.4 | 216.4 ± 128.1 | ||
| No | 44 (55) | 2.9 ± 2.5 | 230.5 ± 77.8 | ||
| No responseb | 4 (5) | 1.5 ± 2.7 | 177.6 ± 74.7 | ||
| SNAP Participant | 0.17 | 0.86 | |||
| Yes | 31 (39) | 2.8 ± 26 | 222.5 ± 84.8 | ||
| No | 49 (61) | 1.8 ± 2.5 | 218.5 ± 121.2 | ||
| Income Range | 0.15 | 0.64 | |||
| <$10, 000 | 25 (31) | 1.9 ± 2.5 | 228.3 ± 86.9 | ||
| $10,000 – 34,999 | 11 (14) | 2.1 ± 2.9 | 196.0 ± 94.5 | ||
| $35,000–74,999 | 14 (17) | 3.2 ± 2.8 | 254.4 ± 139.3 | ||
| ≥ $75,000 | 20 (25) | 2.8 ± 2.6 | 223.4 ± 126.8 | ||
| Do not know or no responseb | 12 (15) | 2.4 ± 4.5 | 181.0 ±76.7 | ||
| Education | 0.02 * | 0.25 | |||
| <12th grade | 10 (13) | 2.9 ± 4.3 | 254.4 ± 68.7 | ||
| 12th grade/ GED | 25 (31) | 1.8 ± 2.3 | 192.0 ± 93.1 | ||
| Some college | 22 (27) | 2.5 ± 1.9 | 204.7 ± 119.7 | ||
| Graduated college | 16 (20) | 3.8 ± 1.9 | 250.5 ± 139.9 | ||
| No responseb | 7 (8) | 2.4 ± 1.7 | 250.2 ± 54.8 | ||
| At least 1 Serving of Vegetables and Fruits | 0.02* | ||||
| Yes | 60 (75) | 232.5 ± 116.1 | |||
| No | 20 (25) | 183.0 ±68.3 |
IQR indicates interquartile range; SD indicates standard deviation; SNAP indicates Supplemental Nutrition Assistance Program; GED indicates general education development.
P < .05
P < .01.
Students t-test was used for binary variables and ANOVA was used for multi-level variables.
Total vegetable and fruit intake was transformed as log(x+1) for statistical analysis;
Do not know or no response levels were not included in the statistical analysis.
Median total vegetable and fruit intake and mean skin carotenoid status is reported by sociodemographic subgroups in Table 1. Total vegetable and fruit servings per day differed significantly by education status, but did not follow a linear trend. Males had significantly higher skin carotenoid status than females (262.7 vs. 185.3; P=0.002). Skin carotenoid status was also significantly higher among participants who reported eating more than 1 serving of vegetables and fruits (232.5 vs. 183.0; P=0.02). Neither total vegetable and fruit intake nor skin carotenoid status differed by age, race, current smoking status, SNAP participation, or income.
Figure 1 shows the distribution of skin carotenoid status. The mean skin carotenoid status was 220.1±108.0 (range: 29.7 to 609.3).
Figure 1.
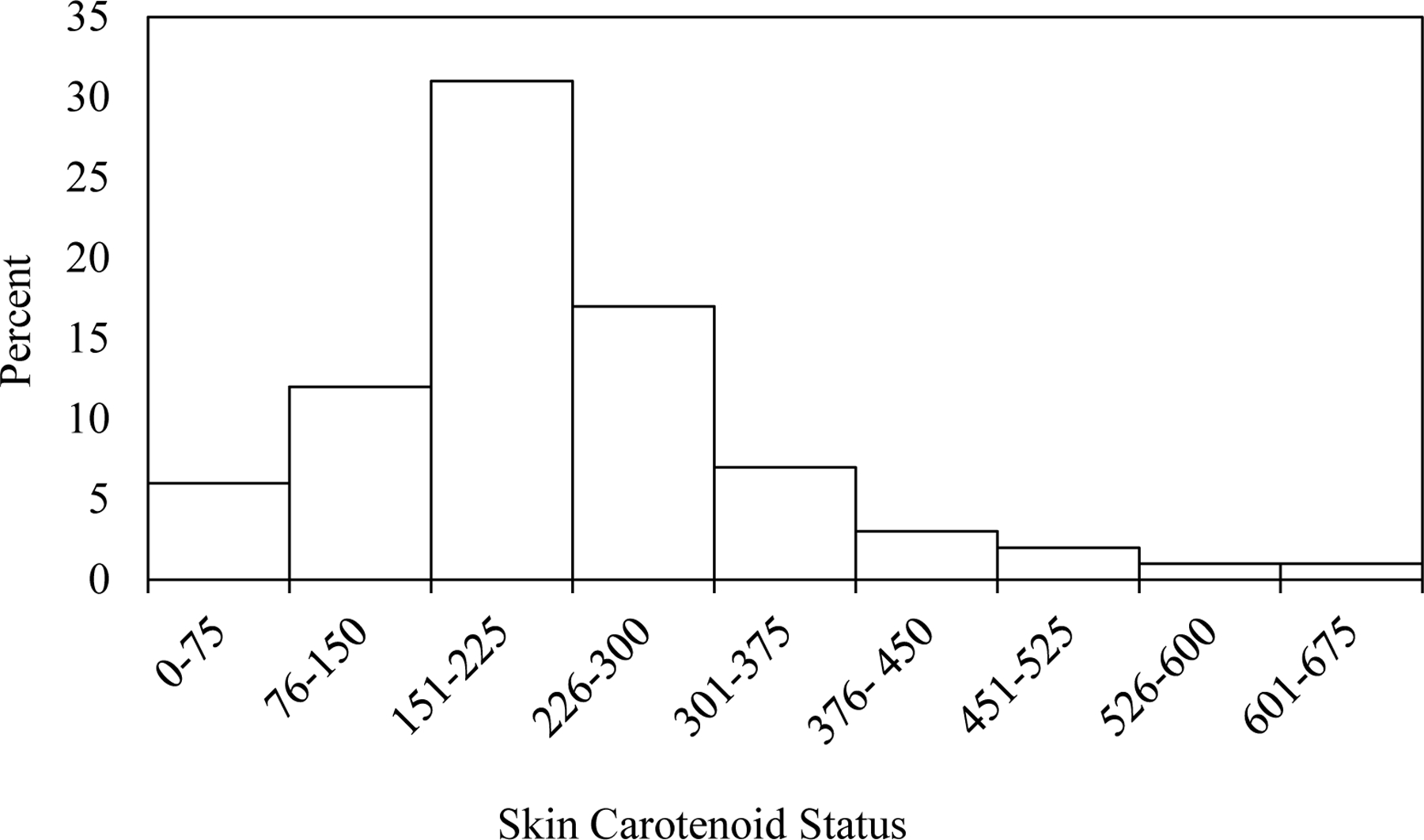
Distribution of Skin Carotenoid Status Measured by Reflection Spectroscopy (n=80)
Table 2 shows the median servings of each vegetable and fruit subgroup. The median intake of total vegetable and fruits was 2.5 ± 2.7 servings per day and 9% of participants reported no servings of any vegetables or fruits. The median intake of all vegetable and fruit groups was 0 servings per day with the exception of the subgroup “other vegetables” (which includes lettuce, onions, and peas) and the subgroup tomatoes. Only 17%, 47%, and 29% of the participants reported eating any amount of brightly-colored fruits, deep-yellow-vegetables, and dark-green vegetables, respectively.
Table 2.
Median ± IQR (Max) Servings/day of Vegetable and Fruit Subgroups and n (%) of Participants that Reported > 0 Serving of Each Vegetable and Fruit Subgroup (n=80)
| Vegetable and Fruit Subgroup | Mean reported intake (servings/day) Median ± IQR(Max) |
Participants that reported >0 serving/day n (%) |
|---|---|---|
| Total Vegetables and Fruits | 2.5 ± 2.7 (13.9) | 73 (91) |
| Other Vegetables | 0.3 ± 0.7 (4.1) | 62 (77) |
| Tomatoesa | 0.1 ± 0.5 (5.5) | 46 (57) |
| White Potatoes | 0.0 ± 0.4 (4.0) | 51 (64) |
| Other Non-Citrus Fruits | 0.0 ± 0.6 (2.3) | 28 (35) |
| Citrus Juices | 0.0 ± 0.0 (4.0) | 13 (16) |
| Brightly-Colored Fruitsa | 0.0 ± 0.0 (2.4) | 14 (17) |
| Deep-Yellow Vegetablesa | 0.0 ± 0.1 (2.7) | 38 (47) |
| Fried Potatoes and Vegetables | 0.0 ± 0.0 (1.9) | 10 (13) |
| Dark-Green Vegetablesa | 0.0 ± 0.1 (1.5) | 23 (29) |
| Legumes | 0.0 ± 0.0 (0.9) | 37 (46) |
| Other Starchy Vegetables | 0.0 ± 0.0 (0.8) | 12 (15) |
| Citrus Fruits | 0.0 ± 0.0 (1.5) | 10 (13) |
IQR indicates interquartile range; SD indicates standard deviation.
Vegetable and fruit subgroups that are carotenoid-rich.
Skin carotenoid status was not significantly correlated with either self-reported total vegetable and fruit intake (r=0.15, P=0.19) or with the NIR (r= −0.05, P=0.65).
DISCUSSION
These findings from a community-based sample of Yup’ik adults living in a remote community in Southwestern Alaska demonstrate the importance of continued monitoring of vegetable and fruit intake. Consistent with previous findings about the Yup’ik diet, vegetable and fruit intake was low among Yup’ik people in this study.26,27 Participants consumed less than half the daily recommended amounts of vegetables and fruits.7 While participants fell short of the recommended intake of all vegetable and fruit subgroups, intake of brightly-colored fruits, deep-yellow vegetables, and dark-green vegetables was particularly low. Indeed, more than half of the participants consumed 0 servings of each of these subgroups and the median intake was 0 servings for each subgroup. These carotenoid-rich vegetable and fruit subgroups are associated with a reduced risk of cardiovascular disease and some cancers,28 which underscores the importance consuming these foods frequently and at the recommended level.
The mean skin carotenoid status in the Yup’ik community was 220.1±108.0. Some studies have found higher levels of skin carotenoid status. In a multi-ethnic sample of New Zealanders, the mean skin carotenoid status was 342.0 ± 116.0,29 and among a large sample of Japanese adults it was 343.1 ± 142.1.30 In comparison, the skin carotenoid status among a sample of African Americans in North Carolina was more similar to the current study (239.0 ± 85.8).31
In this study, males had significantly higher skin carotenoid status than females (262.7 vs.185.3; P=0.002), which is consistent with one other study that used reflection spectroscopy.32 Yup’ik females tend to have greater BMI compared to Yup’ik males,33 which could be one explanation for this finding since BMI is inversely associated with skin carotenoid concentration.34 Interestingly, although males had significantly higher skin carotenoid status than females, they did not report eating more vegetables and fruits than females. It is possible that biases associated with self-reported dietary data such as differences in the under- and over-reporting habits of males and females could explain this inconsistency.35 No significant differences in skin carotenoid status were observed among other characteristics, including smoking status, which is another factor that has been associated with depleted skin carotenoids concentrations.17 One explanation could be that the study population has limited access to food sources and all participants were exposed to the same nutrition environment regardless of behavior, education, or income level.
Participants who reported consuming at least 1 serving of vegetables and fruits had significantly higher skin carotenoid status than those who reported consuming less than 1 serving (P=0.02). However, a significant relationship between skin carotenoid status and total vegetable and fruit intake was not observed when it was treated as a continuous variable. Substantial evidence exists for an association between self-reported vegetable and fruit intake and skin carotenoid status measured by optical assessment methods, such as resonance Raman spectroscopy, an older, more-widely used method.17, 36 In comparison, a recent validation study found only a weak correlation between self-reported vegetable and fruit intake measured by 24-hour dietary recall and skin carotenoid status measured by reflection spectroscopy.16 However, in the same study, skin carotenoid status measured by reflection spectroscopy was strongly correlated with plasma carotenoid concentration. One explanation for the lack of significant correlation in this study could be that measurement errors in self-reported diet could be attenuating the relationship. However, the significant difference in skin carotenoid status for participants that had at least 1 serving of vegetables and fruits compared to those who did not provides evidence that skin carotenoid status could be used to rank levels of intake.
Skin carotenoid status and level of fish intake measured by the hair NIR was not associated. This suggests that for the level of intake in this population, carotenoids from fish did not influence skin carotenoid status measured by reflection spectroscopy. The mean hair NIR in this study was lower compared to other studies in the YKD possibly indicating that fish intake was lower than expected.20,25 For Yup’ik communities that consume greater amounts of fish, either due to stronger ties to traditional food practices or due to greater access to marine foods (e.g. coastal communities), it remains unclear if carotenoids from fish would influence skin carotenoid status.
A number of limitations should be considered in the interpretation of the study findings. First, although 2 24-hour recalls were collected, this dietary assessment method is subject to error because of day-to-day variability in diet.37 In contrast, skin carotenoid status measured by optical methods is a habitual measure of intake, estimating roughly 8 weeks of intake.24 Collecting additional recalls, or a second objective measure of vegetable and fruit intake, such as plasma carotenoids, would have given a more robust estimate of habitual carotenoid-rich vegetable and fruit consumption. Second, only 61% of the study participants completed a second 24-hour dietary recall. However, there were no significant differences in self-reported total vegetable and fruit intake (P=0.93) between those who completed 1 vs. 2 24-hour dietary recalls, which reduces the likelihood of measurement bias. Third, it is possible that our study was underpowered. The logistics of flying to the remote hub community for data collection limited the number of participants that could be recruited. Future research with larger samples would help to determine whether these findings can be replicated.
IMPLICATIONS FOR RESEARCH AND PRACTICE
This study estimated vegetable and fruit intake in a sample of Yup’ik People two ways: a subjective self-reported measure and an objective biomarker. Insufficient vegetable and fruit intake remains a critical public health issue in the YKD. Nutrition education in this population could consider focusing on the sources of available nutrients, such as tundra plants and other traditional foods since Yup’ik individuals obtain key nutrients from different food sources than the general United States.10 Food assistance programs could subsidize the cost of harvesting tundra plants and berries in addition to policies that subsidize market vegetables and fruit. In addition, more research on vegetable and fruit accessibility in Yup’ik communities would shed a light on specific community need and on ways to promote access. The finding that the skin carotenoids status measured by the Veggie Meter was significantly greater for those who had at least 1 serving of vegetables and fruits compared to those who did not suggests that the non-invasive instrument could play an important role in surveillance efforts. Further research is warranted to assess the impact of factors such as BMI on skin carotenoid status and to verify the absence of a relationship between skin carotenoid status measured and fish intake across a greater variability of fish consumption.
Acknowledgements
We would like to express our thanks to Chris Desnoyers, Director of Research at the YKHC, and the YKHC Human Subjects Committee. This research would not have been possible without support from the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under three linked award numbers: RL5GM118990, TL4GM118992 and 1UL1GM118991.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rolls BJ. The relationship between dietary energy density and energy intake. Physiol Behav 2009;97:609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oude Griep LM, Verschuren WM, Kromhout D, Ocke MC, Geleijnse JM. Colours of fruit and vegetables and 10-year incidence of CHD. Br J Nutr 2011;106:1562–1569. [DOI] [PubMed] [Google Scholar]
- 3.Vainio H, Weiderpass E. Fruit and vegetables in cancer prevention. Nutr Cancer 2006;54:111–42. [DOI] [PubMed] [Google Scholar]
- 4.Bellavia A, Larsson SC, Bottai M, Wolk A, Orsini N. Fruit and vegetable consumption and all-cause mortality: a dose-response analysis. Am J Clin Nutr 2013;98:454–459. [DOI] [PubMed] [Google Scholar]
- 5.Johnson EJ. The role of carotenoids in human health. Nutr Clin Care 2002;5:56–65. [DOI] [PubMed] [Google Scholar]
- 6.Sarche M, Spicer P. Poverty and health disparities for American Indian and Alaska Native children: current knowledge and future prospects. Ann N Y Acad Sci 2008;1136:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietary Guidelines for Americans 2015–2020 Washington, DC: United States Department of Health and Human Services. 2016. [Google Scholar]
- 8.O’Brien DM, Thummel KE, Bulkow LR, et al. Declines in traditional marine food intake and vitamin D levels from the 1960s to present in young Alaska Native women. Public Health Nutr 2017;20:1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young SB, Hall ES. Contribution to the ethnobotany St. Lawrence Island Eskimo. Anthropological Papers of the University of Alaska 1969;14:43–53. [Google Scholar]
- 10.Bersamin A, Zidenberg-Cherr S, Stern JS, Luick BR. Nutrient intakes are associated with adherence to a traditional diet among Yup’ik Eskimos living in remote Alaska Native communities: the CANHR Study. Int J Circumpolar Health 2007;66:62–70 [DOI] [PubMed] [Google Scholar]
- 11.Johnson JS, Nobmann ED, Asay E, Lanier AP. Dietary intake of Alaska Native people in two regions and implications for health: the Alaska Native Dietary and Subsistence Food Assessment Project. Int J Circumpolar Health 2009;68:109–22. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg JA, Luick B, Alfred JM, et al. The Affordability of a Thrifty Food Plan-based Market Basket in the United States-affiliated Pacific Region. Hawaii J Health Soc Welf 2020;79:217–223. [PMC free article] [PubMed] [Google Scholar]
- 13.Subar AF, Freedman LS, Tooze JA, et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. J Nutr 2015;145:2639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ermakov IV, Ermakova M, Sharifzadeh M, et al. Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Arch Biochem Biophys 2018;646:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jahns L, Johnson LK, Mayne ST, et al. Skin and plasma carotenoid response to a provided intervention diet high in vegetables and fruit: uptake and depletion kinetics. Am J Clin Nutr 2014;100:930–937. [DOI] [PubMed] [Google Scholar]
- 16.Jahns L, Johnson LK, Conrad Z, et al. Concurrent validity of skin carotenoid status as a concentration biomarker of vegetable and fruit intake compared to multiple 24-h recalls and plasma carotenoid concentrations across one year: a cohort study. Nutr J 2019;18:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayne ST, Cartmel B, Scarmo S, Jahns L, Ermakov IV, Gellermann W. Resonance Raman spectroscopic evaluation of skin carotenoids as a biomarker of carotenoid status for human studies. Arch Biochem Biophys 2013;539:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids National Academy Press: Institute of Medicine, National Academy of Sciences, Food and Nutrition Board, Panel on Dietary Antioxidants and Related Compounds. 2000. [Google Scholar]
- 19.Ambati RR, Phang SM, Ravi S, Aswathanarayana RG. Astaxanthin: sources, extraction,stability, biological activities and its commercial applications--a review. Mar Drugs 2014;12:128–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nash SH, Kristal AR, Boyer BB, King IB, Metzgar JS, O’Brien DM. Relation between stableisotope ratios in human red blood cells and hair: implications for using the nitrogen isotope ratio of hair as a biomarker of eicosapentaenoic acid and docosahexaenoic acid. Am J Clin Nutr 2009;90:1642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien DM, Kristal AR, Nash SH, et al. A stable isotope biomarker of marine food intake captures associations between n-3 fatty acid intake and chronic disease risk in a Yup’ik study population, and detects new associations with blood pressure and adiponectin. J Nutr 2014;144:706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United States Census Bureau. QuickFacts Bethel city, Alaska; Bethel Census Area, Alaska. https://www.census.gov/quickfacts/fact/table/bethelcityalaska,bethelcensusareaalaska/PST045219. Accessed January 30, 2021. [Google Scholar]
- 23.Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc 1996;96:1140–1144. [DOI] [PubMed] [Google Scholar]
- 24.Scarmo S, Cartmel B, Lin H, et al. Single v. multiple measures of skin carotenoids byresonance Raman spectroscopy as a biomarker of usual carotenoid status. Br J Nutr 2013;110:911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choy K, Nash SH, Hill C, et al. The Nitrogen Isotope Ratio Is a Biomarker of Yup’ikTraditional Food Intake and Reflects Dietary Seasonality in Segmental Hair Analyses. J Nutr 2019;149:1960–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bersamin A, Luick BR, Ruppert E, Stern JS, Zidenberg-Cherr S. Diet quality among Yup’ik Eskimos living in rural communities is low: the Center for Alaska Native Health Research Pilot Study. J Am Diet Assoc 2006;106:1055–1063. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JS, Nobmann ED, Asay E. Factors related to fruit, vegetable and traditional food consumption which may affect health among Alaska Native People in Western Alaska. Int J Circumpolar Health 2012;71:17345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowles JL, Erdman JW. Carotenoids and their role in cancer prevention. Biochim Biophys Acta Mol Cell Biol Lipids 2020;1865:158613. [DOI] [PubMed] [Google Scholar]
- 29.Rush E, Amoah I, Diep T, Jalili-Moghaddam S. Determinants and Suitability of Carotenoid Reflection Score as a Measure of Carotenoid Status. Nutrients 2020;12:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obana A, Gohto Y, Gellermann W, et al. Skin Carotenoid Index in a large Japanese population sample. Scientific Rep 2019;9:9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jilcott Pitts SB, Jahns L, Wu Q, et al. A non-invasive assessment of skin carotenoid status through reflection spectroscopy is a feasible, reliable and potentially valid measure of fruit and vegetable consumption in a diverse community sample. Public Health Nutr 2018;21:1664–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGuirt JT, Jilcott Pitts SB, Gustafson A. Association between Spatial Access to Food Outlets, Frequency of Grocery Shopping, and Objectively-Assessed and Self-Reported Fruit and Vegetable Consumption. Nutrients 2018;10:1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyer BB, Mohatt GV, Plaetke R, et al. Metabolic syndrome in Yup’ik Eskimos: the Center for Alaska Native Health Research (CANHR) Study. Obesity (Silver Spring) 2007;15:2535–2540. [DOI] [PubMed] [Google Scholar]
- 34.Virtanen SM, van’t Veer P, Kok F, Kardinaal AF, Aro A. Predictors of adipose tissuecarotenoid and retinol levels in nine countries. The EURAMIC Study. Am J Epidemiol 1996;144:968–79. [DOI] [PubMed] [Google Scholar]
- 35.Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social desirability bias in dietaryself-report may compromise the validity of dietary intake measures. Int J Epidemiol 1995;24:389–398. [DOI] [PubMed] [Google Scholar]
- 36.Mayne ST, Cartmel B, Scarmo S, et al. Noninvasive assessment of dermal carotenoids as abiomarker of fruit and vegetable intake. Am J Clin Nutr 2010; 92:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson FE, Subar AF. Dietary assessment methodology. In: Coulston AM, Boushey CJ,eds. Nutrition in the Prevention and Treatment of Disease San Diego, CA: Academic Press; 2008:5–6. [Google Scholar]


