Abstract
Dolutegravir (DTG) is an antiretroviral drug of the integrase strand transfer inhibitor (INSTI) class used to treat human immunodeficiency virus infection. It is the recommended first-line regimen for most people, including women of childbearing age. However, some human and animal studies have suggested that DTG causes birth defects, although its developmental toxicity remains controversial. Here, we investigated the adverse effects of DTG using pluripotent stem cell-based in vitro morphogenesis models that have previously been validated as effective tools to assess the developmental toxicity of various chemicals. DTG diminished the growth and axial elongation of the morphogenesis model of mouse pluripotent stem cells at exposures of 2 μM and above in a concentration-dependent manner. Concomitantly, DTG altered the expression profiles of developmental regulator genes involved in embryonic patterning. The adverse effects were observed when the morphogenesis model was exposed to DTG at early stages of development, but not at later stages. The potency and molecular impact of DTG on the morphogenesis model were distinct from other INSTIs. Last, DTG altered the growth and gene expression profiles of the morphogenesis model of human embryonic stem cells at 1 μM and above. These studies demonstrate that DTG impairs morphological and molecular aspects of the in vitro morphogenesis models in a manner dependent on dose and timing of exposure through mechanisms that are unrelated to its action as an INSTI. This finding will be useful for interpreting the conflicting outcomes regarding the developmental toxicity of DTG in human and animal studies.
Keywords: dolutegravir, developmental toxicity, in vitro, pluripotent stem cell, morphogenesis
Deciding which medications to take during pregnancy is difficult, because one needs to consider not only their therapeutic efficacy but also their potential toxicity on developing embryos. For many medications, however, sufficient information on developmental toxicity is often unavailable. For example, in the case of dolutegravir (DTG), an antiretroviral medication used to treat human immunodeficiency virus (HIV) infection, there is inconsistent information regarding its developmental toxicity. In 2018, the Food and Drug Administration (FDA) warned of a risk of birth defects from DTG (FDA, 2018). The warning was based on an observational study in Botswana, which found a significantly higher incidence of severe neural tube defects (NTDs) in babies born to women who received DTG before pregnancy or early in the first trimester (Zash et al., 2018). The warning was troubling to the HIV community, because DTG, which is an integrase strand transfer inhibitor (INSTI), was a preferred first-line antiretroviral agent. However, contrary to the original Botswana study, subsequent investigations at different geographical locations reported either no increase or only a slight increase in the NTD incidence associated with DTG (Money et al., 2019; Pereira et al., 2021; Vannappagari et al., 2019; Zash et al., 2019). Currently, the World Health Organization (WHO) recommends DTG as a preferred HIV treatment for all populations, including women of childbearing age, because the overall reproductive risk of the medication appears small enough to be outweighed by its benefit (WHO, 2019). Regardless, it has been suggested that DTG interferes with folate receptors, which may contribute to the pathogenesis of NTD and other birth defects (Cabrera et al., 2019; Mohan et al., 2021). Furthermore, it remains unknown whether other INSTIs, many of which are also used as anti-HIV medications, may pose reproductive risks similar to DTG (Chouchana et al., 2019; Gantner et al., 2019; Rasi et al., 2019; Sibiude et al., 2021). Thus, it is necessary to further investigate how DTG may potentially impact embryonic development in relation to its dose and timing of exposure, and also what molecular pathways DTG may affect. Such information could provide mechanistic insights into the potential developmental toxicity of DTG and other anti-HIV medications.
In vitro models of embryogenesis, particularly those constructed from pluripotent stem cells, have been explored to study the developmental toxicity of various chemicals (Seiler et al., 2006; Theunissen and Piersma, 2012; Worley et al., 2018). Pluripotent stem cells, which are originally derived from either undifferentiated embryonic cells or reprogrammed adult somatic cells, can be propagated indefinitely, and induced to differentiate into a wide range of cell types in vitro. For developmental toxicity assessment, disturbances in stem cell differentiation by chemical exposures are interpreted as potential adverse effects on embryogenesis. Even though stem cell-based in vitro models represent only limited aspects of embryogenesis, they can offer valuable opportunities for developmental toxicity research. In comparison to human- or animal-based studies, in vitro assays are relatively fast, economical, and expandable to high-throughput screening, not to mention devoid of ethical concerns for animal welfare. Furthermore, the in vitro nature of stem cell-based models is more amenable to various manipulations to analyze the molecular pathways of the adverse effects. Because of these benefits, in vitro assays may be used to gain mechanistic insights that help interpret or design more complex in vivo studies.
One of the in vitro systems used for developmental toxicity research is a 3D morphogenesis model of mouse P19C5 pluripotent stem cells (Lau and Marikawa, 2014; Li and Marikawa, 2015). When P19C5 cells are aggregated by hanging drop culture, they differentiate and transform from a spherical mass to an elongated shape in 4 days. This morphological transformation recapitulates key aspects of gastrulation, such as germ layer formation and axial elongation, as demonstrated by the distinct spatial and temporal gene expression patterns that are comparable to those in gastrulating embryos. The shape changes and gene expression profiles in P19C5 cell aggregates are dependent on the core signaling pathways controlling embryonic patterning and elongation, namely Wnt, Nodal, Fgf, Bmp, Notch, and retinoic acid (RA; Li and Marikawa, 2015), further corroborating that the morphogenesis of P19C5 cell aggregates represents gastrulation. The P19C5 morphogenesis model has been validated as an effective tool for developmental toxicity assessment in reference to the Daston list of chemical exposures. The Daston list is comprised of 39 chemical exposures, for which in vivo concentrations have been determined that adversely affect embryonic development in rats (Daston et al., 2014). Growth and axial elongation of P19C5 cell aggregates are significantly altered by the adverse exposures in the Daston list, but not by the nonadverse exposures, with an overall concordance of up to 82.9% (Warkus and Marikawa, 2017). Moreover, analyses of how chemical exposures alter the gene expression profiles in the morphogenesis model have yielded mechanistic clues for the actions of developmentally toxic chemicals, such as valproic acid (Li and Marikawa, 2016), fluoxetine (Warkus and Marikawa, 2018), and methoxyacetic acid (Li and Marikawa, 2020). Thus, the P19C5 morphogenesis model is a useful in vitro platform to study the developmental toxicity of chemical exposures.
In this study, we used the P19C5 morphogenesis model to investigate the potential developmental toxicity of DTG. Many studies in vertebrate models have suggested that defects in axial elongation contribute to the pathogenesis of NTDs (Butler and Wallingford, 2017; Ueno and Greene, 2003). Because DTG is suspected to cause NTDs, application of the morphogenesis model that displays axial elongation would be particularly relevant to assess the developmental toxicity of DTG. Here, we examined how the morphogenesis of P19C5 cell aggregates was affected by DTG in relation to its concentration and timing of exposure. The impact of DTG on gene expression profiles was also investigated, and compared with that of other INSTIs that are indicated for HIV treatment. Last, we explored another in vitro morphogenesis model, which is made from human embryonic stem cells, to examine the dose-dependent impact of DTG.
MATERIALS AND METHODS
Chemicals
All chemicals used in this study were commercially obtained. DTG (No. 22191), bictegravir (BIC; No. 26532), elvitegravir (EVG; No. 17798), and raltegravir (RAL; No. 16071) are from Cayman Chemical (San Diego, California). Folic acid (No. F7876), folinic acid (leucovorin; No. PHR1541), and dimethyl sulfoxide (DMSO; No. D2650) are from Sigma-Aldrich (St Louis, Missouri). DTG, BIC, EVG, RAL, and folic acid were dissolved in DMSO at 10 mM as stocks, and leucovorin was dissolved in water at 2 mM. All stocks were stored at −20°C.
Cell culture and 3D cell aggregates
P19C5 mouse embryonal carcinoma cells (Lau and Marikawa, 2014) were maintained in culture medium that consists of 90% Minimum Essential Medium Alpha with nucleosides and GlutaMAX Supplement (LifeTechnologies, Carlsbad, California), 2.5% fetal bovine serum, 7.5% newborn calf serum, 50 U/ml penicillin, and 50 μg/ml streptomycin. Three-dimensional cell aggregates were generated by hanging drop culture, according to the method described previously (Lau and Marikawa, 2014). Briefly, cells were dissociated with TrypLE Express (LifeTechnologies) and suspended at a density of 10 cells/μl in the culture medium containing a final concentration of 1% DMSO, with or without a test chemical. Drops (20 μl each) of cell suspension were deposited on the inner surface of Petri dish lids (16 drops per dish), which were then inverted to make hanging drops.
H9 line (WA09, National Institutes of Health registration number 0062) of human embryonic stem cells was obtained commercially (WiCell Research Institute, Madison, Wisconsin). Cells were maintained in the feeder-free culture medium mTeSR1 (Stemcell Technologies, Vancouver, British Columbia) with 40 U/ml penicillin and 40 μg/ml streptomycin in flasks that had been precoated with iMatrix-511 (Takara Bio, Mountain View, California). Three-dimensional cell aggregates were generated in round-bottom wells, according to the method described previously (Marikawa et al., 2020). Briefly, cells were dissociated with TrypLE Express and suspended at a density of 20 cells/μl in culture medium consisting of 80% Minimum Essential Medium Alpha with nucleosides and GlutaMAX, 20% 5× Supplement of mTeSR1, 10 μM CHIR99021 (Calbiochem, La Jolla, California), 2 μM SB431542 (Stemcell Technologies), and 2 nM RA (Sigma-Aldrich), with or without a test chemical. Fifty microliter of cell suspension was placed in each well of 96-well round-bottom plates (ultra-low attachment; Corning, Corning, New York) to generate human embryonic stem cell aggregates with CHIR99021, SB431542, and RA, which we refer to hereafter as HESCA-CSR (Marikawa et al., 2020). All cells and aggregates were cultured at 37°C in 4.5% CO2 in humidified air.
Morphometric analyses
Three-dimensional cell aggregates were removed from hanging drops at day 4 of culture and placed together in a dish filled with phosphate-buffered saline for photography. Image files in JPEG format were opened in the ImageJ program (https://imagej.nih.gov/ij/index.html; last accessed September 20, 2021). The circumference of individual day 4 aggregates was traced using the polygon selection tool, and the area, elongation distortion index (EDI), and aspect ratio (AR) were measured as the morphometric parameters of aggregates. Area was used as a proxy for the overall size of aggregates, whereas EDI and AR were used to gauge the extent of axial elongation. EDI is calculated as 1/circularity − 1, which becomes larger when the shape of an aggregate is more elongated or distorted (Marikawa et al., 2009). AR is the ratio of the major-to-minor axis of an ellipse that most tightly fits the circumference of an aggregate, and it becomes larger for aggregates that are more elongated along a single axis. For each set of experiments, in which 16 aggregates were generated per experimental treatment in parallel with 16 control aggregates (ie, 1% DMSO only), the morphometric parameters were normalized relative to the average values of control aggregates to calculate relative area, relative EDI, and relative AR, which are expressed as percentages (ie, control = 100%). These relative values were compiled from 3 sets of independent replicates, and their averages are presented in graphs with error bars of 95% CI.
Cell proliferation assay
The impact of DTG on cell proliferation was evaluated using the CellTiter-Glo Luminescent Cell Viability Assay system, which determines the number of live cells in culture by measuring the amount of ATP as a quantitative proxy for the number of metabolically active cells (Promega, Madison, Wisconsin). P19C5 cells were seeded in 96-well plates at density of 100 cells/well, and were cultured in maintenance medium (100 μl/well) containing a serial dilution of DTG (0.5, 1, 2, 4, and 8 μM) or vehicle only (0.08% DMSO) as a control. After 3 days of culture, cells were treated with CellTiter-Glo Reagent for measurement of luminescence, as readout of ATP amount, according to the manufacturer’s instruction, using Gene Light 55 Luminometer (Microtech, Chiba, Japan). Cell seeding density was optimized through a series of pilot experiments to confirm that cell numbers at the end of 3 days of culture were proportionate to intensities of luminescence. Relative cell number was calculated based on ratio of the luminescence intensity in each set of experiment, and the averages of 3 independent sets are presented in a graph with error bars of SD.
Quantitative reverse transcription-polymerase chain reaction
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed to determine the relative expression levels of developmental regulator genes, which are summarized in Supplementary Table. For the mouse morphogenesis model, cell suspension before aggregation (day 0) and cell aggregates at days 1–4 of hanging drop culture were harvested. For the human morphogenesis model, cell aggregates at days 3 and 5 of culture were harvested. Total RNA was extracted from these samples using TRI reagent (LifeTechnologies) and Direct-zol RNA MicroPrep Kit (Zymo Research, Irvine, California), and processed for complementary DNA synthesis using M-MLV Reverse Transcriptase (Promega) and oligo-dT (18) primer. qPCR was performed using the CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, California) with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) as follows: initial denaturation at 94°C for 5 min, followed by up to 45 cycles of 94°C for 15 s, 60°C for 20 s, and 72°C for 40 s. Data files were opened in CFX Manager software (Bio-Rad) and Ct values were transferred to the Excel program. Actb/ACTB, which encodes β-Actin, was used as a housekeeping gene to normalize the expression levels of other genes. Actb/ACTB has been effectively used as a housekeeping gene in previous studies, and the transcript level of Actb is mostly stable from days 0 to 4 of aggregation culture in a manner comparable or superior to other commonly used housekeeping genes, such as Gapdh, Hmbs, Hprt, Tbp, and Ywhaz, based on the previous microarray and RNA-seq analyses data (Kim and Marikawa, 2018; Li and Marikawa, 2020; Marikawa et al., 2020). Sequences of the primers used are shown in Supplementary Table. Relative expression levels were calculated for each set of experiments, as previously described (Warkus and Marikawa, 2018), and the averages of 3 or 4 replicates are presented with error bars of SDs.
To assess the impact of DTG on gene expressions in HESCA-CSR, ALERT (Altered Level of Embryogenesis Regulator Transcript) score was employed, which was formulated previously (Marikawa et al., 2020). Briefly, ALERT score represents the number of genes (out of 15 specific developmental regulator genes), whose transcript levels were altered by ≥2-fold (either increase or decrease) with statistical significance (p < .05) in response to a given chemical exposure. In the previous validation study examining 20 chemicals, the presence of positive ALERT scores correlated with in vivo developmental toxicity of chemical exposures with concordance of 92–94% (Marikawa et al., 2020).
Statistical analyses
Average values of relative area, relative EDI, and relative AR, which were derived from the morphometric measurement of a total of 46–48 aggregates for each group, were analyzed by 2-sample t test to compare between 2 specified groups. p-values of < .01 were deemed statistically significant. Average values of gene expression levels, which were calculated based on 3–4 sets of replicates, were analyzed by 2-sample t test to compare between 2 specified groups. For most gene expression analyses, p-values of < .02 and .05 were deemed statistically significant for the mouse and human morphogenesis models, respectively. A less stringent criterion was chosen for the human morphogenesis model, because its development appears to be more heterogeneous than the mouse morphogenesis model, as reported in the previous study (Marikawa et al., 2020). For the expression analyses of the mouse morphogenesis model during the course of 4 days of culture, in which a total of 32 genes at 4 different stages were examined, p-values were adjusted for the false discovery rate to calculate q-values (Benjamini and Hochberg, 1995; Storey, 2003; Yekutieli and Benjamini, 1999). q-values of < 0.05 were deemed statistically significant.
RESULTS
DTG Impairs Development of the Mouse In Vitro Morphogenesis Model
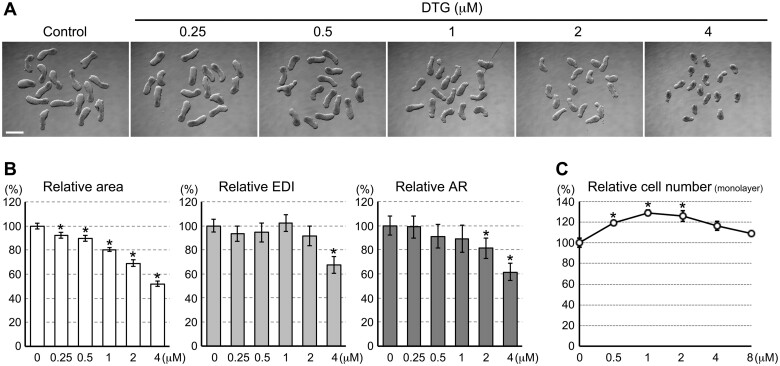
Three-dimensional aggregates of mouse P19C5 pluripotent stem cells were cultured for 4 days in the presence of varying concentrations of DTG, and the morphology of the resulting aggregates was examined based on the 3 morphometric parameters (see Materials and Methods section): area, EDI, and AR. Area is to measure the overall size of aggregates, whereas EDI and AR are to gauge the extent of elongation. Overall, DTG treatments resulted in smaller and less elongated aggregates in a concentration-dependent manner (Figure 1A). Specifically, over the range of 0.25–4 μM, relative area decreased progressively when aggregates were exposed to higher concentrations of DTG (Figure 1B). Relative EDI was significantly lower than control when aggregates were treated with 4 μM of DTG, whereas relative AR was significantly reduced with 2 and 4 μM (Figure 1B). Thus, both the size and elongation were diminished by DTG at 2 μM and above.
Figure 1.
Dolutegravir (DTG) impairs the P19C5 mouse morphogenesis model in a dose-dependent manner. A, Representative images of cell aggregates at day 4 that have been treated with DTG at the indicated concentrations. Scale bar = 500 μm. B, Morphometric parameters of day 4 cell aggregates treated with DTG. Graphs show averages of relative area, relative elongation distortion index (EDI), and relative aspect ratio (AR) with error bars of 95% CI, based on measurement of 46–48 aggregates for each condition. Asterisks indicate significant reduction (p < .01; 2-sample t test) compared with the corresponding control (0 μM). C, Impact of DTG on the number of metabolically active cells in monolayer culture after 3 days of treatment. Graph shows averages of relative cell number with error bars of SD. Asterisks indicate significant increase (p < .01; 2-sample t test) compared with the control (0 μM).
A reduction in aggregate size implicates that DTG may possess a cytotoxic property to stop cell division or to cause cell death. To test this possibility, we examined the impact of DTG on the proliferation of P19C5 cells in monolayer culture, which is the condition that maintains the pluripotent state. However, none of the concentrations tested (0.5–8 μM) decreased relative cell numbers after 3 days of monolayer culture (Figure 1C), indicating that DTG did not diminish cell division or induce cell death under the undifferentiating condition. This suggests that DTG is not generally cytotoxic up to 8 μM. Note that relative cell numbers were slightly (by 20–30%) but significantly (p < .01) increased by DTG at 0.5, 1, and 2 μM. This implies that DTG may promote proliferation of undifferentiated P19C5 cells at these concentrations.
The Morphogenetic Impact of DTG Is Not Due to Folic Acid Deficiency
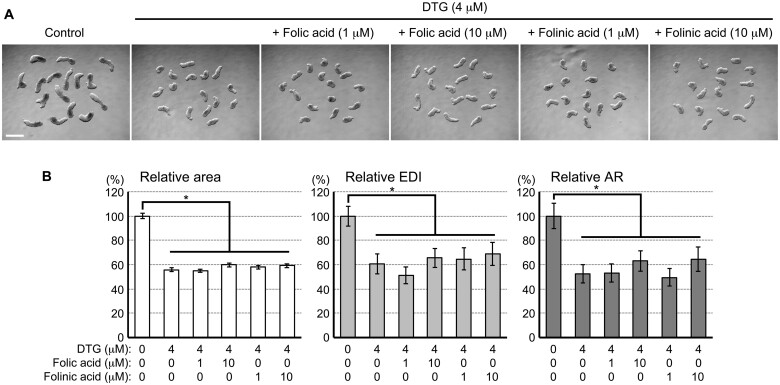
Previously, a study using zebrafish embryos showed that the deleterious impact of DTG (100 μM) is completely negated by the supplementation of folic acid (60 ng/ml or 0.136 μM), suggesting that folic acid deficiency underlies the developmental toxicity of DTG (Cabrera et al. 2019). To test whether the morphological effects of DTG on the P19C5 morphogenesis model are also due to folic acid deficiency, cell aggregates were cultured with DTG (4 μM) and folic acid (1 or 10 μM), and examined for morphology at day 4. Aggregates treated with DTG plus folic acid were smaller and less elongated than the control in a manner comparable with those treated with DTG alone (Figure 2A). None of the morphometric parameters (area, EDI, and AR) was significantly restored by the supplementation of folic acid at either concentration (Figure 2B). Similarly, supplementation with folinic acid, which is a reduced form of folic acid that does not require activation by dihydrofolate reductase, did not significantly restore the size or elongation of DTG-treated aggregates (Figs. 2A and 2B). These results suggest that the morphological impairment of the P19C5 morphogenesis model by DTG is not due to folic acid deficiency.
Figure 2.
The morphogenetic impact of dolutegravir (DTG) is not due to folic acid deficiency. A, Representative images of cell aggregates at day 4 that have been treated with DTG with or without folic acid or folinic acid at the indicated concentrations. Scale bar = 500 μm. B, Morphometric parameters of day 4 cell aggregates. Graphs show averages of relative area, relative elongation distortion index (EDI), and relative aspect ratio (AR) with error bars of 95% CI, based on measurement of 46–48 aggregates for each condition. All 3 parameters are significantly reduced in DTG-treated groups compared with the corresponding control group, as indicated with asterisks (p < .01; 2-sample t test). However, no significant difference from the DTG-treated groups was observed due to supplementation of folic acid or folinic acid.
DTG Alters the Expression Profiles of Developmental Regulator Genes
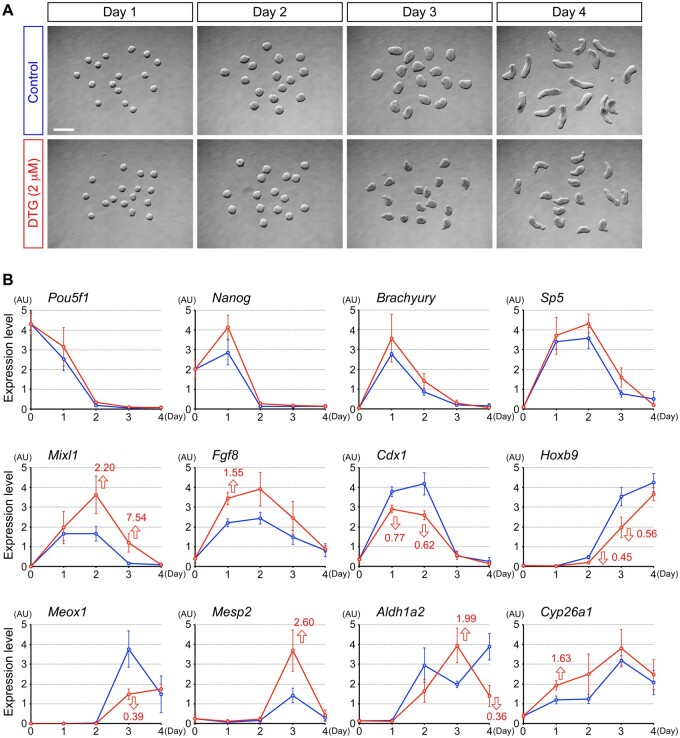
To gain mechanistic insights into the molecular pathways that are affected by DTG, temporal expression profiles of genes encoding regulators of cell differentiation and axial patterning were compared between control and DTG-treated P19C5 cell aggregates. DTG at 2 μM was selected for this analysis, as it was the lowest concentration that significantly affected both the size (area) and elongation (AR) of day 4 aggregates (Figure 1B). Regulator genes examined were those involved in the pluripotency maintenance (Pou5f1 and Nanog), mesendoderm differentiation (Brachyury, Sp5, Mixl1, and Fgf8), axial patterning (Cdx1 and Hoxb9), somitogenesis (Meox1 and Mesp2), and RA metabolism (Aldh1a2 and Cyp26a1). A previous study showed that all of these genes exhibit distinct temporal expression profiles in P19C5 cell aggregates in a manner dependent on the core signaling pathways, such as Wnt, Nodal, Fgf, Notch, and RA (Li and Marikawa, 2015).
Expression profiles of many of these regulator genes were significantly altered by DTG, although the timing and manner of alterations were different depending on the genes (Figure 3). For example, at day 1, the Fgf8 level was higher in DTG-treated aggregates than control, whereas the Cdx1 level was lower. At day 2, Mixl1 was elevated by DTG, while Hoxb9 was reduced. At day 3, the Meox1 level was decreased by DTG, while the Mesp2 level was increased. Regulators of RA metabolism, Aldh1a2 and Cyp26a1, were also distinctly altered by DTG treatment (Figure 3). Thus, DTG at 2 μM dysregulated the expressions of critical regulators of cell differentiation and axial patterning.
Figure 3.
Dolutegravir (DTG) alters temporal gene expression profiles of developmental regulators. A, Representative images of control and DTG-treated cell aggregates at different stages. Scale bar = 500 μm. B, Quantitative reverse transcription-polymerase chain reaction analysis of temporal gene expression patterns over the course of 4 days of culture. Graphs show averages of relative expression levels with error bars of SD, presented in arbitrary unit (AU). Blue and red lines correspond to control and DTG-treated aggregates, respectively. Red numbers with open arrows indicate fold differences with statistical significance (q < 0.05; 2-sample t test with the false discovery rate adjustment) between control and DTG-treated group at a given day.
Note that the expression profiles of additional genes were also examined, including those involved in the initiation of the primitive streak formation (Wnt3), the differentiation of neuromesodermal progenitors (Wnt3a and Tbx6), segmentation clock (Notch1, Dll1, Lfng, and Hes7), somitogenesis (Foxc2), axial patterning (Hoxa1 and Hoxc6), and the etiology of NTD in the mouse embryo (Pax3, Celsr1, Vangl2, Atp2c1, Casp3, Hectd1, Luzp1, Nup50, Palld, and Sec24b). However, their expressions were not significantly altered by DTG (Supplementary Figure).
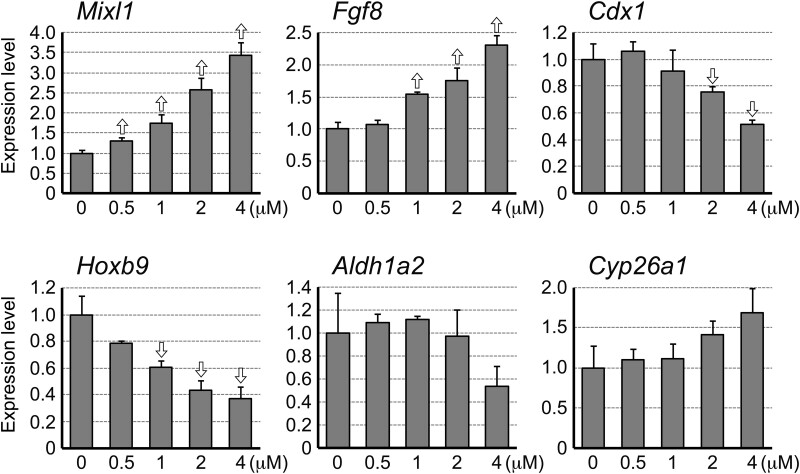
To determine the dose-dependent impact of DTG on gene expression profiles, aggregates treated with varying concentrations (0.5, 1, 2, and 4 μM) were examined for the levels of selected genes (Mixl1, Fgf8, Cdx1, Hoxb9, Aldh1a2, and Cyp26a1) at day 2 (Figure 4). The Mixl1 expression was significantly elevated even by the lowest concentration of DTG tested, while the extent of elevation was larger by higher concentrations. DTG at 1 μM and above significantly altered the expressions of Fgf8 (elevated) and Hoxb9 (reduced), whereas the Cdx1 level was significantly reduced at 2 μM and above. In contrast, the expression levels of Aldh1a2 and Cyp26a1 were not significantly altered by any concentrations tested. Thus, the impact of DTG on the expression levels of the critical regulator genes was detectable at as low as 0.5 μM (Mixl1) and 1 μM (Mixl1, Fgf8, and Hoxb9), even though the impact was more pronounced at higher concentrations.
Figure 4.
Dolutegravir (DTG) alters gene expression levels in a dose-dependent manner. Quantitative reverse transcription-polymerase chain reaction analysis of day 2 aggregates. Graphs show averages of relative expression levels with error bars of SD. Open arrows indicate statistically significant differences (p < .02; 2-sample t test) compared with the corresponding control (0 μM).
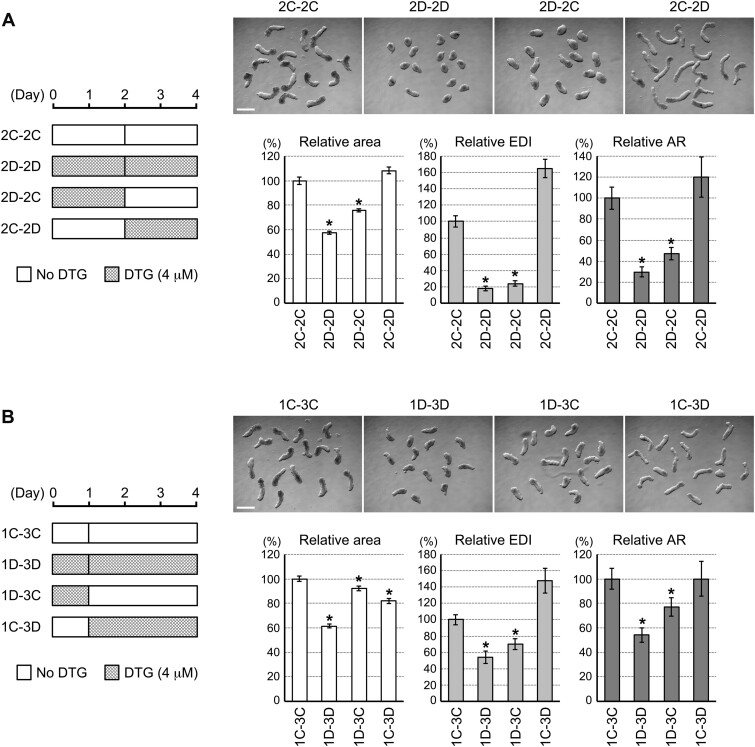
Morphological Effects of DTG Depend on Timing of Exposure
The impact of DTG on the gene expressions was already evident at days 1 and 2 of aggregation culture, as described earlier. Such early disturbance in the gene expressions may be responsible for the morphological defects (ie, reduced size and elongation) that would become apparent later at day 4 (Figure 2A). To test this possibility, we examined the morphology of aggregates that were first exposed to DTG (4 μM) for 2 days, and then moved to control medium (vehicle only) for 2 more days of culture (denoted as the 2D-2C culture regimen in Figure 5A). For a comparison, aggregates were cultured in reversed order, ie, they were first in control medium for 2 days and then moved to the DTG-containing medium (denoted as 2C-2D). Additional aggregates were also prepared that were moved from control medium to control medium (2C-2C) or moved from DTG-containing medium to DTG-containing medium (2D-2D). When their morphologies were compared at day 4, all the morphometric parameters (area, EDI, and AR) of 2D-2C aggregates were significantly lower than 2C-2C aggregates in a manner comparable to 2D-2D aggregates (Figure 5A). In contrast, none of the morphometric parameters of 2C-2D aggregates was lower than 2C-2C aggregates. Thus, exposure to DTG during the first 2 days appeared to be responsible for the morphogenetic impairment.
Figure 5.
Morphological impact of dolutegravir (DTG) depends on timing of exposure. A, The 2–2 day treatment regimen. (Left) The schematic diagrams depicting how the first and last 2 days are exposed to DTG in each group. (Top) Representative images of day 4 aggregates that correspond to the left diagram. Scale bar = 500 μm. (Bottom) Graphs showing averages of relative area, relative elongation distortion index (EDI), and relative aspect ratio (AR) with error bars of 95% CI. Asterisks indicate significant reduction (p < .01; 2-sample t test) compared with the corresponding control (2C-2C). B, The 1–3 day treatment regimen. The same descriptions as in (A).
To further narrow down the period that is most sensitive to DTG exposure, we examined aggregates that were exposed to DTG (4 μM) only during the first day of the 4-day culture (1D-3C) and those that were treated in reversed order (1C-3D; Figure 5B). All 3 morphometric parameters of 1D-3C aggregates were significantly lower than control (1C-3C) aggregates. For 1C-3D aggregates, only area was lower than the control, whereas EDI and AR were not reduced (Figure 5B).
These results suggest that the morphogenetic potential of P19C5 aggregates is most sensitively impaired by exposure to DTG during the first day of development.
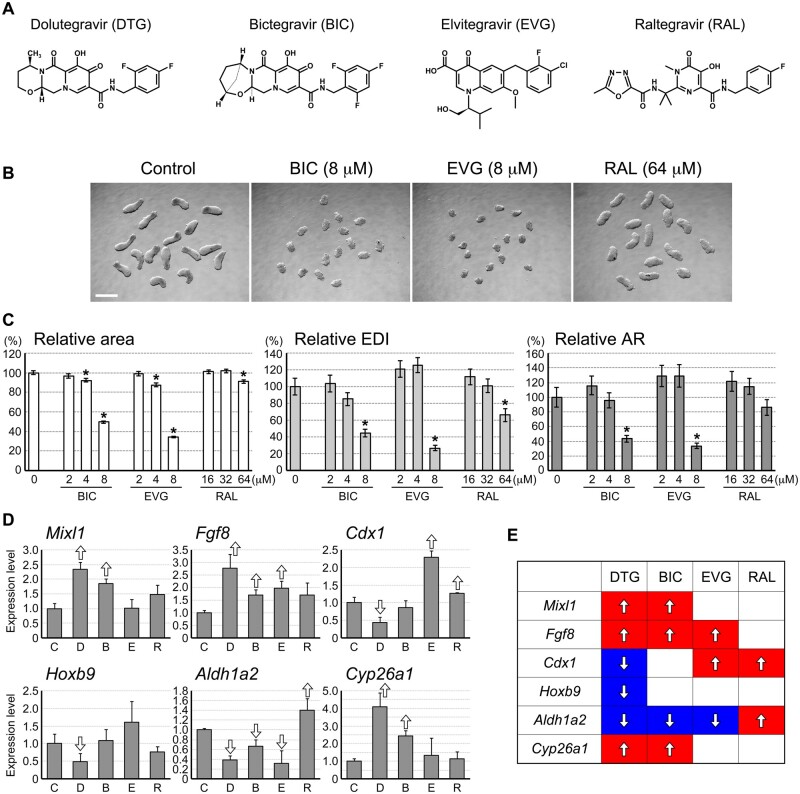
Morphogenesis Model Is Differentially Affected by INSTIs
The P19C5 morphogenesis model was impaired by DTG, which is an anti-HIV drug of the INSTI class. To assess whether the adverse effects of DTG are linked to its property as an INSTI, we examined the effects of other anti-HIV drugs of the same class, namely BIC, EVG, and RAL on the P19C5 morphogenesis model. With regards to the chemical structure, BIC is the most similar to DTG, whereas RAL is the least similar (Figure 6A). The ranges of concentrations used for BIC, EVG, and RAL were selected based on a series of pilot studies to include low levels that caused no morphological impact and high levels that elicited significant morphological impact. Treatment with these INSTIs diminished the size and elongation of aggregates at the concentrations that were higher than the adverse effect levels of DTG (Figure 6B). For BIC and EVG, the lowest concentration that significantly reduced all 3 morphometric parameters was 8 μM (Figure 6C), whereas it was 4 μM for DTG (Figure 1B). In contrast, a much higher concentration (64 μM) was needed for RAL to significantly reduce area and EDI (Figure 6C).
Figure 6.
Morphogenesis model is differentially affected by integrase strand transfer inhibitors (INSTIs). A, Chemical structures of INSTIs. B, Representative images of day 4 aggregates treated with bictegravir (BIC), elvitegravir (EVG), and raltegravir (RAL). C, Morphometric parameters of day 4 cell aggregates. Graphs show averages of relative area, relative elongation distortion index (EDI), and relative aspect ratio (AR) with error bars of 95% CI. Asterisks indicate significant reduction (p < .01; 2-sample t test) compared with the corresponding control (0 μM). D, Quantitative reverse transcription-polymerase chain reaction analysis of day 2 aggregates that have been treated with vehicle only control (C), DTG (D; 4 μM), BIC (B; 8 μM), EVG (E; 8 μM), and RAL (R; 64 μM). Graphs show averages of relative expression levels with error bars of SD. Open arrows indicate statistically significant differences (p < .02; 2-sample t test) compared with the corresponding control (0 μM). E, Comparisons of gene expression change in response to exposure to INSTIs, based on the data in (D). Red and blue indicate up- and down-regulation compared with the control level, respectively.
We then compared the impact of these INSTIs on gene expressions at the concentrations that impaired morphogenesis, namely DTG at 4 μM, BIC at 8 μM, EVG at 8 μM, and RAL at 64 μM. INSTI-treated aggregates at day 2 were specifically examined, because multiple genes were altered by DTG in a dose-dependent manner at this stage (Figure 4). Each INSTI exhibited distinct impact on the expression levels of Mixl1, Fgf8, Cdx1, Hoxb9, Aldh1a2, and Cyp26a1 (Figure 6D). The impact of BIC was the most similar to DTG, such that both INSTIs altered Mixl1, Fgf8, Aldh1a2, and Cyp26a1 in the same direction (Figs. 6D and 6E). EVG affected Fgf8 and Aldh1a2 similarly to DTG, while it altered the Cdx1 level in the opposite direction. No similarity was observed between RAL and DTG (Figs. 6D and 6E). Thus, different INSTIs differentially affected the P19C5 morphogenesis model, although similarities in the chemical structures correlated with the pattern of gene expression impact.
DTG Dysregulates Gene Expressions in the Morphogenesis Model of Human Embryonic Stem Cells
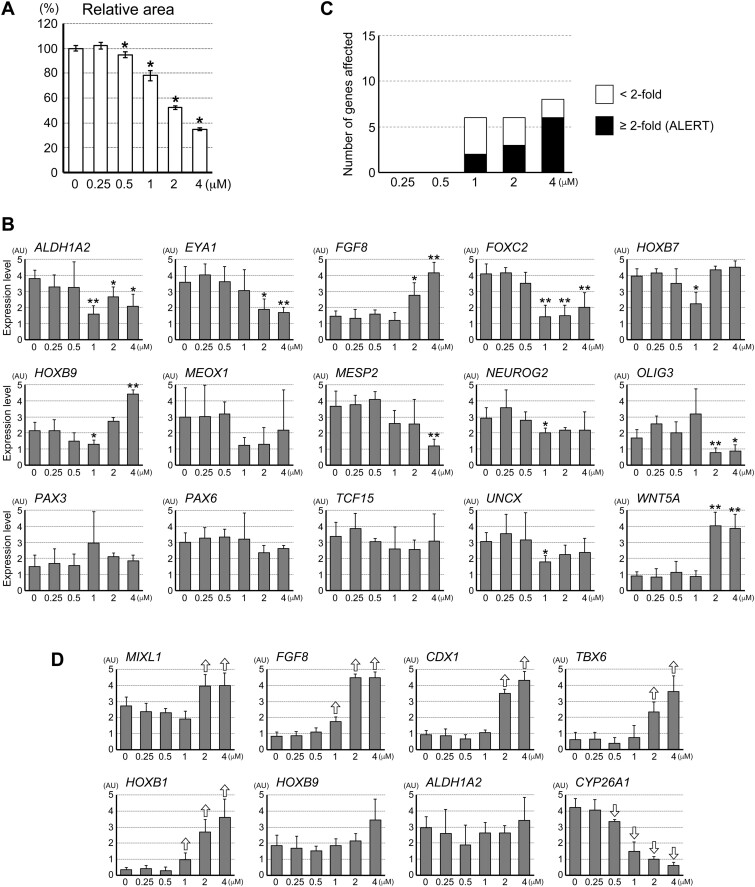
Recently, an in vitro morphogenesis model of human embryonic stem cells has been reported for the purpose of assessing developmental toxicity of chemical exposures (Marikawa et al., 2020). This model, termed HESCA-CSR, is made of aggregates of H9 human embryonic stem cells that are cultured with a unique set of signaling modulators (see Materials and Methods section), and exhibits size and shape changes over 5 days of culture. The morphogenesis of HESCA-CSR is accompanied by dynamic changes in molecular profiles, including the up-regulation of genes associated with paraxial mesoderm and neuroectoderm differentiation. The gene expression profiles in HESCA-CSR are significantly altered by exposures to various developmental toxicants at in vivo relevant concentrations (Marikawa et al., 2020). Here, we explored HESCA-CSR as a platform to evaluate the potential impact of DTG on human embryonic cells, with a particular focus on its dose-response relationship.
After 5 days of culture with DTG, the overall size of HESCA-CSR was smaller than the control in a concentration-dependent manner (Figure 7A). A reduction in the relative area was significant even at 0.5 μM, although it was more pronounced at higher concentrations. This suggests that DTG impairs the growth of human H9 cell aggregates, which was also the case for the mouse P19C5 cell aggregates (Figure 1B). Note that the morphometric parameters for elongation, namely EDI and AR, were not evaluated for HESCA-CSR due to inter- and intraexperimental variability, as reported previously (Marikawa et al., 2020).
Figure 7.
Dolutegravir (DTG) dysregulates gene expressions in the morphogenesis model of human embryonic stem cells. A, The overall size of human embryonic stem cell aggregates after 5 days of culture with varying concentrations of DTG. Graph showing averages of relative area with error bars of 95% CI, based on the measurement of 30 aggregates for each condition. Asterisks indicate significant reduction (p < .01; 2-sample t test) compared with the control (0 μM). B, Quantitative reverse transcription-polymerase chain reaction analyses of the transcript levels of the 15 embryogenesis regulator genes in day 5 human cell aggregates that have been treated with DTG. Graphs show averages of relative expression levels with error bars of SD, presented in arbitrary unit (AU). Asterisks denote transcript levels that are altered (either up- or down-regulated) compared with the control (0 μM) with statistical significance (p < .05; 2-sample t test). Note that all of the gene expression reductions with p < .05 have q-values smaller than 0.1 with the false discovery rate adjustment. One asterisk corresponds to an alteration by <2-fold, whereas 2 asterisks correspond to an alteration by ≥2-fold. C, Graph showing the numbers of genes that are affected by DTG. Open bars correspond to genes that are altered by < 2-fold, whereas closed bars correspond to those altered by ≥2-fold. The latter is referred to as the Altered Level of Embryogenesis Regulator Transcript score, according to the previous study (Marikawa et al., 2020). D, Quantitative reverse transcription-polymerase chain reaction analyses of the transcript levels of the 8 embryogenesis regulator genes in day 3 human cell aggregates that have been treated with DTG. Open arrows indicate statistically significant differences (p < .05; 2-sample t test) compared with the corresponding control (0 μM).
We then assessed the effects of DTG on the gene expression profiles in HESCA-CSR. In accordance with the previous validation study (Marikawa et al., 2020), a selected set of 15 genes (ALDH1A2, EYA1, FGF8, FOXC2, HOXB7, HOXB9, MEOX1, MESP2, NEUROG2, OLIG3, PAX3, PAX6, TCF15, UNCX, and WNT5A) were examined after 5 days of culture with varying concentrations of DTG. All of these 15 genes play essential roles in embryo patterning. Out of the 15 genes, the transcript levels of 6, 6, and 8 genes were significantly altered by DTG at 1, 2, and 4 μM, respectively (Figs. 7B and 7C). ALERT scores, which count only those genes with ≥2-fold changes (Materials and Methods), were 2 (1 μM), 3 (2 μM), and 6 (4 μM; Figure 7C).
We also examined an earlier stage, day 3, for the transcript levels of selected genes, namely MIXL1, FGF8, CDX1, TBX6, HOXB1, HOXB9, ALDH1A2, and CYP26A1, as some of the homologous genes were sensitively affected by DTG at early stages in the mouse morphogenesis model (Figure 4). None of these genes were affected by DTG at 0.25 μM. The CYP26A1 expression was reduced by 0.5 μM of DTG, while the extent of reduction was more pronounced at higher concentrations. 1 μM of DTG altered the expressions of 3 genes (FGF8, HOXB1, and CYP26A1), while 2 and 4 μM affected 6 genes (MIXL1, FGF8, CDX1, TBX6, HOXB1, and CYP26A1). Thus, the impact of DTG on the gene expressions of the developmental regulators was detectable at as low as 0.5 μM with more pronounced alterations at higher concentrations. Thus, the human morphogenesis model exhibited dose-response to DTG with the sensitivity similar to the mouse morphogenesis model. Note, however, that there were similarities and differences between the human and mouse models in terms of the type of genes and the direction of alterations. For example, Mixl1/MIXL1 and Fgf8/FGF8 were up-regulated by DTG in both the models, whereas Cdx1/CDX1 was differently affected, ie, down-regulated in the mouse model and up-regulated in the human model.
DISCUSSION
DTG is an anti-HIV medication that is part of the preferred first-line treatment option worldwide. However, the potential for DTG to cause birth defects is still unclear and controversial among studies in human (Money et al., 2019; Pereira et al., 2021; Vannappagari et al., 2019; Zash et al., 2018, 2019) and in model animals (Mohan et al., 2021; Stanislaus et al., 2020). In this study, we investigated the effects of DTG on the in vitro morphogenesis models of mouse and human pluripotent stem cells, which have been validated previously as effective assay tools to detect developmental toxicity (Marikawa et al., 2020; Warkus and Marikawa, 2017).
The morphogenesis models were adversely impacted by DTG at concentrations as low as 1 μM, and the extent of impact increased with higher concentrations. The key question is how clinically relevant are these concentrations. Human pharmacokinetic studies of healthy volunteers or HIV patients, some of which are pregnant women, have shown that the peak plasma concentration (Cmax) of DTG ranges from 4.5 to 10.9 μM (Cottrell et al., 2013; Elliot et al., 2016; Min et al., 2010; Mulligan et al., 2018; Nguyen et al., 2019; Song et al., 2013). Although these concentrations may appear comparable to those used in this study, it is crucial to take into account the role of plasma protein binding. In the plasma, DTG displays extensive protein binding with >99% bound to serum proteins, such as albumin (Cottrell et al., 2013; Gelé et al., 2020; Song et al., 2013). Therefore, <1% of the total plasma concentration is free (unbound) to contribute to its pharmacological actions. The serum albumin concentrations in the culture media for the morphogenesis models are about 4- to 10-fold lower than the plasma level, as the mouse cell culture medium contained 10% serum and the human cell culture medium contained 195 μM of serum albumin (the albumin concentrations in the human plasma are 500–700 μM; Tayyab and Feroz, 2021). Thus, due to the extremely high affinity between DTG and the serum albumin, about 4- to 10-fold higher fractions of DTG are estimated to be unbound in the culture media than in the plasma. Therefore, 1 μM in vitro may be pharmacologically equivalent to that of 4–10 μM in vivo. Because DTG’s Cmax ranges from 4.5 to 10.9 μM, the concentrations that affected the morphogenesis models in this study are clinically relevant. Nonetheless, there may be additional factors that affect the protein binding or other properties of DTG in vitro, which necessitates further investigations, including measurement of unbound DTG concentrations in the culture media.
Susceptibility to developmental toxicants varies with the developmental stage at the time of exposure (Friedman, 2010; Ujházy et al., 2012; Wilson, 1973). This study showed that the mouse morphogenesis model was most susceptible to DTG exposure during the first day of culture. According to the gene expression profiles, the first day of P19C5 cell aggregate development corresponds to the pre- and early gastrulation stages, during which the pluripotent epiblast is about to form the primitive streak (approximately the fifth to sixth day of mouse embryonic development; Li and Marikawa, 2015). In contrast, exposure to DTG at later stages, particularly between days 2 and 4, did not diminish the growth or elongation of the mouse morphogenesis model. This raises the possibility that embryonic development is impaired by DTG only when exposure occurs before or at the onset of gastrulation. Such stage-dependent nature of DTG’s developmental toxicity may provide an insight into the apparent contradiction between the 2 animal studies: one showing the lack of developmental toxicity from DTG exposure (Stanislaus et al., 2020), whereas the other showing increased rates of birth defects, including NTD (Mohan et al., 2021). There are various differences in the experimental settings between the 2 studies (eg, the former used the rat and rabbit, while the latter used the mouse), so that direct comparisons of their outcomes are difficult. However, one of the differences is related to the timing of exposure. In the rat and rabbit study (Stanislaus et al., 2020), DTG is administered daily to pregnant animals, starting from the gestation day 6, which roughly corresponds to the time of implantation in rats (Schneider and Norton, 1979) and the initiation of gastrulation in rabbits (Fischer et al., 2012). In contrast, in the mouse study (Mohan et al., 2021), DTG administration is initiated from the first day of gestation. It is possible that developmental toxicity is absent in the rat and rabbit study because DTG may not have accumulated sufficiently to affect the most susceptible early developmental stage owing to the later drug administration compared with the mouse study. A recent study reported no developmental toxicity in rat whole embryos that were cultured with DTG from gestation day 9 at 12.6 to 22.2 μM (Posobiec et al., 2021). The lack of developmental toxicity in this whole embryo culture study may also be due to the absence of DTG exposure to the most susceptible developmental stage, which is already passed by gestation day 9.
The antagonism of folate receptor has been hypothesized as a mechanism by which DTG exerts developmental toxicity (Cabrera et al., 2019). This hypothesis is supported by the experiment using zebrafish embryos, in which the toxic effects of DTG are completely negated by supplementation of folate (Cabrera et al., 2019). Folate, or vitamin B9, serves as an essential cofactor in various biochemical reactions, including de novo synthesis of nucleotides. The link between low folate status and the occurrence of birth defects, particularly NTDs, has been well established in both human and animal studies (Berry et al., 1999; Burren et al., 2008; van Gool et al., 2018; Wlodarczyk et al., 2006). However, whether DTG blocks cellular uptake of folate and causes its deficiency in developing embryos is still unclear, according to other in vitro and human studies (Chandiwana et al., 2021; Zamek-Gliszczynski et al., 2019). In this study, the deleterious impact of DTG on the mouse morphogenesis model was not suppressed by folate supplementation. Note that the in vitro morphogenesis models may not be suitable to investigate the effects of folate deficiency, because the culture media contain a substantial amount of nucleosides (Materials and Methods section ), which may conceal the inhibitory impact of folate deficiency on nucleotide biosynthesis. Regardless, our study implicates the presence of an additional mechanism, by which DTG interferes with morphogenesis in a folate-independent manner.
Transcript levels of various genes were altered by DTG in the mouse and human morphogenesis models. Many of these genes are essential regulators of body patterning and morphogenesis, and their dysregulations may contribute to embryonic malformations. Genes that were affected by DTG in both the mouse and human models include Mixl1/MIXL1, Fgf8/FGF8, Cdx1/CDX1, Aldh1a2/ALDH1A2, and Cyp26a1/CYP26A1. Notably, transcriptional regulations of these genes are dependent on RA signaling (Li and Marikawa, 2015). RA signaling plays instructive roles in axial patterning and morphogenesis, and its deficiency as well as excessive activation can result in severe malformations, including NTD (Piersma et al., 2017). Whether DTG affects RA signaling directly or indirectly is not clear based on transcriptional analysis alone, warranting further investigations. Nonetheless, various malformations in addition to NTD are found in mouse fetuses that have been exposed to DTG, such as facial anomaly, microphthalmia, and vascular defects (Mohan et al., 2021). This implicates that the impact of DTG is pleiotropic and is not restricted to the machineries controlling the neural tube closure. Because RA signaling is involved in facial, ocular, and vascular development (Pawlikowski et al., 2019; Thompson et al., 2019; Williams and Bohnsack, 2019), it is possible that altered RA signaling may be involved in the pathogenesis of various malformations found in the DTG-exposed embryos.
The INSTIs are the newest class of antiretroviral drugs approved for the treatment of HIV infection (Scarsi et al., 2020). Among the 4 INSTIs tested in this study (DTG, BIC, EVG, and RAL), DTG was the most potent and RAL was the least potent in impairing the mouse morphogenesis model. Specifically, the lowest concentrations that diminished the growth and elongation were 2 and 64 μM for DTG and RAL, respectively (Figs. 1 and 6). In addition, as the protein binding of RAL (73.3–83.9%; Barau et al., 2013) is less than that of DTG (>99%), the concentration of unbound RAL is expected to be even higher than DTG in the culture medium. As the half maximal inhibitory concentrations, or IC50, that reduce the viral integrase activity are comparable between these drugs based on the in vitro strand transfer assay (2.7 and 3.3 nM for DTG and RAL, respectively; Kobayashi et al., 2011), it is likely that the potency as an INSTI (ie, the therapeutic action) is unrelated to the adverse morphogenetic impact. Note that Cmax of BIC, EVG, and RAL in common therapeutic regimens are 7.7–13.5 μM (Gallant et al., 2017), 5.3–6.2 μM (Ramanathan et al., 2013), and 1.9–5.1 μM (Martínez-Rebollar et al., 2013), respectively. Thus, the adverse effect level of RAL on the P19C5 morphogenesis model (ie, 64 μM) is much higher than those found in the plasma, and is unlikely to emerge in clinical settings. However, the apparent lack of developmental toxicity in the morphogenesis model does not indicate the absence of pregnancy risks by any means. Human epidemiologic studies have shown that use of RAL during pregnancy does not significantly increase the occurrence of birth defects (Gantner et al., 2019; Rasi et al., 2019), although 1 study has reported a nonsignificant trend for an association between exposure to RAL at conception and birth defects (Sibiude et al., 2021). Clearly, more studies, including those in human and model animals, are needed to evaluate the developmental toxicity of RAL and other INSTIs. Nonetheless, in vitro studies can offer opportunities to elucidate the relationship between chemical structures and developmental toxicity, as this study showed similar effects on the gene expression profiles between DTG and BIC, which are structurally the most similar to each other. Such information may help identify structural determinants that are responsible for eliciting the toxicity, and may contribute to engineering safer drugs.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful to Dr Vernadeth B. Alarcon for reading the article and providing valuable comments.
FUNDING
The National Institute of Child Health and Human Development at the National Institutes of Health (NIH) to Y.M. (R03 HD101735 and R03 HD102502) and the NIH Centers of Biomedical Research Excellence Phase 3 to the Institute for Biogenesis Research (P30 GM131944).
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Lauren Kirkwood-Johnson, Developmental and Reproductive Biology Graduate Program, Institute for Biogenesis Research, University of Hawaii John A. Burns School of Medicine, Honolulu, Hawaii 96813, USA.
Nana Katayama, Developmental and Reproductive Biology Graduate Program, Institute for Biogenesis Research, University of Hawaii John A. Burns School of Medicine, Honolulu, Hawaii 96813, USA.
Yusuke Marikawa, Developmental and Reproductive Biology Graduate Program, Institute for Biogenesis Research, University of Hawaii John A. Burns School of Medicine, Honolulu, Hawaii 96813, USA.
REFERENCES
- Barau C., Furlan V., Yazdanpanah Y., Fagard C., Molina J. M., Taburet A. M., Barrail-Tran A. (2013). Characterization of binding of raltegravir to plasma proteins. Antimicrob. Agents Chemother. 57, 5147–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300. [Google Scholar]
- Berry R. J., Li Z., Erickson J. D., Li S., Moore C. A., Wang H., Mulinare J., Zhao P., Wong L. Y., Gindler J., et al. (1999). Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl. J. Med. 341, 1485–1490. [DOI] [PubMed] [Google Scholar]
- Burren K. A., Savery D., Massa V., Kok R. M., Scott J. M., Blom H. J., Copp A. J., Greene N. D. (2008). Gene-environment interactions in the causation of neural tube defects: Folate deficiency increases susceptibility conferred by loss of Pax3 function. Hum. Mol. Genet. 17, 3675–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. T., Wallingford J. B. (2017). Planar cell polarity in development and disease. Nat. Rev. Mol. Cell Biol. 18, 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera R. M., Souder J. P., Steele J. W., Yeo L., Tukeman G., Gorelick D. A., Finnell R. H. (2019). The antagonism of folate receptor by dolutegravir: Developmental toxicity reduction by supplemental folic acid. AIDS 33, 1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandiwana N. C., Chersich M., Venter W. D. F., Akpomiemie G., Hill A., Simmons B., Lockman S., Serenata C. M., Fairlie L., Moorhouse M. A. (2021). Unexpected interactions between dolutegravir and folate: Randomized trial evidence from South Africa. AIDS 35, 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchana L., Beeker N., Treluyer J. M. (2019). Is there a safety signal for dolutegravir and integrase inhibitors during pregnancy? J. Acquir. Immune Defic. Syndr. 81, 481–486. [DOI] [PubMed] [Google Scholar]
- Cottrell M. L., Hadzic T., Kashuba A. D. (2013). Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin. Pharmacokinet. 52, 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daston G. P., Beyer B. K., Carney E. W., Chapin R. E., Friedman J. M., Piersma A. H., Rogers J. M., Scialli A. R. (2014). Exposure-based validation list for developmental toxicity screening assays. Birth Defects Res. B Dev. Reprod. Toxicol. 101, 423–428. [DOI] [PubMed] [Google Scholar]
- Elliot E., Amara A., Jackson A., Moyle G., Else L., Khoo S., Back D., Owen A., Boffito M. (2016). Dolutegravir and elvitegravir plasma concentrations following cessation of drug intake. J. Antimicrob. Chemother. 71, 1031–1036. [DOI] [PubMed] [Google Scholar]
- FDA. (2018). FDA Drug Safety Communication: FDA to evaluate potential risk of neural tube birth defects with HIV medicine dolutegravir. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-evaluate-potential-risk-neural-tube-birth-defects-hiv-medicine. Accessed September 3, 2021.
- Fischer B., Chavatte-Palmer P., Viebahn C., Navarrete Santos A., Duranthon V. (2012). Rabbit as a reproductive model for human health. Reproduction 144, 1–10. [DOI] [PubMed] [Google Scholar]
- Friedman J. M. (2010). The principles of teratology: Are they still true? Birth Defects Res. A Clin. Mol. Teratol. 88, 766–768. [DOI] [PubMed] [Google Scholar]
- Gallant J. E., Thompson M., DeJesus E., Voskuhl G. W., Wei X., Zhang H., White K., Cheng A., Quirk E., Martin H. (2017). Antiviral activity, safety, and pharmacokinetics of bictegravir as 10-day monotherapy in HIV-1-infected adults. J. Acquir. Immune Defic. Syndr. 75, 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner P., Sylla B., Morand-Joubert L., Frange P., Lacombe K., Khuong M. A., Duvivier C., Launay O., Karmochkine M., Arvieux C., Coferal-IMEA048 Study Group., et al. (2019). “Real life” use of raltegravir during pregnancy in France: The Coferal-IMEA048 cohort study. PLoS One 14, e0216010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelé T., Gouget H., Furlan V., Becker P. H., Taburet A. M., Lambotte O., Barrail-Tran A. (2020). Characteristics of dolutegravir and bictegravir plasma protein binding: A first approach for the study of pharmacologic sanctuaries. Antimicrob. Agents Chemother. 64, e00895-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I. Q., Marikawa Y. (2018). Embryoid body test with morphological and molecular endpoints implicates potential developmental toxicity of trans-resveratrol. Toxicol. Appl. Pharmacol. 355, 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Yoshinaga T., Seki T., Wakasa-Morimoto C., Brown K. W., Ferris R., Foster S. A., Hazen R. J., Miki S., Suyama-Kagitani A., et al. (2011). In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob. Agents Chemother. 55, 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C. G., Marikawa Y. (2014). Morphology-based mammalian stem cell tests reveal potential developmental toxicity of donepezil. Mol. Reprod. Dev. 81, 994–1008. [DOI] [PubMed] [Google Scholar]
- Li A. S., Marikawa Y. (2015). An in vitro gastrulation model recapitulates the morphogenetic impact of pharmacological inhibitors of developmental signaling pathways. Mol. Reprod. Dev. 82, 1015–1036. [DOI] [PubMed] [Google Scholar]
- Li A. S., Marikawa Y. (2016). Adverse effect of valproic acid on an in vitro gastrulation model entails activation of retinoic acid signaling. Reprod. Toxicol. 66, 68–83. [DOI] [PubMed] [Google Scholar]
- Li A. S. W., Marikawa Y. (2020). Methoxyacetic acid inhibits histone deacetylase and impairs axial elongation morphogenesis of mouse gastruloids in a retinoic acid signaling-dependent manner. Birth Defects Res. 112, 1043–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikawa Y., Chen H. R., Menor M., Deng Y., Alarcon V. B. (2020). Exposure-based assessment of chemical teratogenicity using morphogenetic aggregates of human embryonic stem cells. Reprod. Toxicol. 91, 74–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikawa Y., Tamashiro D. A., Fujita T. C., Alarcón V. B. (2009). Aggregated P19 mouse embryonal carcinoma cells as a simple in vitro model to study the molecular regulations of mesoderm formation and axial elongation morphogenesis. Genesis 47, 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Rebollar M., Muñoz A., Pérez I., Hidalgo S., Brunet M., Laguno M., González A., Calvo M., Loncà M., Blanco J. L., et al. (2013). Pharmacokinetic study of dual therapy with raltegravir 400 mgice daily and Darunavir/Ritonavir 800/100 mg once daily in HIV-1-infected patients. Ther. Drug Monit. 35, 552–556. [DOI] [PubMed] [Google Scholar]
- Min S., Song I., Borland J., Chen S., Lou Y., Fujiwara T., Piscitelli S. C. (2010). Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. Antimicrob. Agents Chemother. 54, 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan H., Lenis M. G., Laurette E. Y., Tejada O., Sanghvi T., Leung K. Y., Cahill L. S., Sled J. G., Delgado-Olguín P., Greene N. D. E., et al. (2021). Dolutegravir in pregnant mice is associated with increased rates of fetal defects at therapeutic but not at supratherapeutic levels. EBioMedicine 63, 103167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money D., Lee T., O'Brien C., Brophy J., Bitnun A., Kakkar F., Boucoiran I., Alimenti A., Vaudry W., Singer J., et al. ; Canadian Perinatal HIV Surveillance Program. (2019). Congenital anomalies following antenatal exposure to dolutegravir: A Canadian surveillance study. BJOG 126, 1338–1345. [DOI] [PubMed] [Google Scholar]
- Mulligan N., Best B. M., Wang J., Capparelli E. V., Stek A., Barr E., Buschur S. L., Acosta E. P., Smith E., Chakhtoura N., et al. ; IMPAACT P1026s Protocol Team. (2018). Dolutegravir pharmacokinetics in pregnant and postpartum women living with HIV. AIDS 32, 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen B., Foisy M. M., Hughes C. A. (2019). Pharmacokinetics and safety of the integrase inhibitors elvitegravir and dolutegravir in pregnant women with HIV. Ann. Pharmacother. 53, 833–844. [DOI] [PubMed] [Google Scholar]
- Pawlikowski B., Wragge J., Siegenthaler J. A. (2019). Retinoic acid signaling in vascular development. Genesis 57, e23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G. F. M., Kim A., Jalil E. M., Fernandes Fonseca F., Shepherd B. E., Veloso V. G., Rick F., Ribeiro R., Pimenta M. C., Beber A., et al. ; National Cohort Study of Dolutegravir and Pregnancy Outcomes in Brazil. (2021). Dolutegravir and pregnancy outcomes in women on antiretroviral therapy in Brazil: A retrospective national cohort study. Lancet HIV 8, e33–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersma A. H., Hessel E. V., Staal Y. C. (2017). Retinoic acid in developmental toxicology: Teratogen, morphogen and biomarker. Reprod. Toxicol. 72, 53–61. [DOI] [PubMed] [Google Scholar]
- Posobiec L. M., Chapman S. P., Murzyn S. F., Rendemonti J. E., Stanislaus D. J., Romach E. H. (2021). No developmental toxicity observed with dolutegravir in rat whole embryo culture. Birth Defects Res. August 28. doi:10.1002/bdr2.1949 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Ramanathan S., Mathias A., Wei X., Shen G., Koziara J., Cheng A., Kearney B. P. (2013). Pharmacokinetics of once-daily boosted elvitegravir when administered in combination with acid-reducing agents. J. Acquir. Immune Defic. Syndr. 64, 45–50. [DOI] [PubMed] [Google Scholar]
- Rasi V., Cortina-Borja M., Peters H., Sconza R., Thorne C. (2019). Brief report: Surveillance of congenital anomalies after exposure to raltegravir or elvitegravir during pregnancy in the United Kingdom and Ireland, 2008-2018. J. Acquir. Immune Defic. Syndr. 80, 264–268. [DOI] [PubMed] [Google Scholar]
- Scarsi K. K., Havens J. P., Podany A. T., Avedissian S. N., Fletcher C. V. (2020). HIV-1 integrase inhibitors: A comparative review of efficacy and safety. Drugs 80, 1649–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider B. F., Norton S. (1979). Equivalent ages in rat, mouse and chick embryos. Teratology 19, 273–278. [DOI] [PubMed] [Google Scholar]
- Seiler A. E., Buesen R., Visan A., Spielmann H. (2006). Use of murine embryonic stem cells in embryotoxicity assays: The embryonic stem cell test. Methods Mol. Biol. 329, 371–395. [DOI] [PubMed] [Google Scholar]
- Sibiude J., Le Chenadec J., Mandelbrot L., Dollfus C., Matheron S., Lelong N., Avettand-Fenoel V., Brossard M., Frange P., Reliquet V., et al. ; EPF study group. (2021). Risk of birth defects and perinatal outcomes in HIV-infected women exposed to integrase strand inhibitors during pregnancy. AIDS 35, 219–226. [DOI] [PubMed] [Google Scholar]
- Song I. H., Borland J., Savina P. M., Chen S., Patel P., Wajima T., Peppercorn A. F., Piscitelli S. C. (2013). Pharmacokinetics of single-dose dolutegravir in HIV-seronegative subjects with moderate hepatic impairment compared to healthy matched controls. Clin. Pharmacol. Drug Dev. 2, 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaus D. J., Posobiec L. M., Laffan S. B., Solomon H. M., Ziejewski M. K., Romach E. H. (2020). Absence of developmental and reproductive toxicity in animals exposed to dolutegravir. Birth Defects Res. 112, 245–261. [DOI] [PubMed] [Google Scholar]
- Storey J. D. (2003). The positive false discovery rate: A Bayesian interpretation and the q-value. Ann. Stat. 31, 2013–2035. [Google Scholar]
- Tayyab S., Feroz S. R. (2021). Serum albumin: Clinical significance of drug binding and development as drug delivery vehicle. Adv. Protein Chem. Struct. Biol. 123, 193–218. [DOI] [PubMed] [Google Scholar]
- Theunissen P. T., Piersma A. H. (2012). Innovative approaches in the embryonic stem cell test (EST). Front. Biosci. (Landmark Ed ). 17, 1965–1975. [DOI] [PubMed] [Google Scholar]
- Thompson B., Katsanis N., Apostolopoulos N., Thompson D. C., Nebert D. W., Vasiliou V. (2019). Genetics and functions of the retinoic acid pathway, with special emphasis on the eye. Hum. Genomics 13, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno N., Greene N. D. (2003). Planar cell polarity genes and neural tube closure. Birth Defects Res. C Embryo Today 69, 318–324. [DOI] [PubMed] [Google Scholar]
- Ujházy E., Mach M., Navarová J., Brucknerová I., Dubovický M. (2012). Teratology - Past, present and future. Interdiscip. Toxicol. 5, 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool J. D., Hirche H., Lax H., De Schaepdrijver L. (2018). Folic acid and primary prevention of neural tube defects: A review. Reprod. Toxicol. 80, 73–84. [DOI] [PubMed] [Google Scholar]
- Vannappagari V., Thorne C, for APR and EPPICC. (2019). Pregnancy and neonatal outcomes following prenatal exposure to dolutegravir. J. Acquir. Immune Defic. Syndr. 81, 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warkus E. L. L., Marikawa Y. (2017). Exposure-based validation of an in vitro gastrulation model for developmental toxicity assays. Toxicol. Sci. 157, 235–245. [DOI] [PubMed] [Google Scholar]
- Warkus E. L. L., Marikawa Y. (2018). Fluoxetine inhibits canonical Wnt signaling to impair embryoid body morphogenesis: Potential teratogenic mechanisms of a commonly used antidepressant. Toxicol. Sci. 165, 372–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2019). WHO recommends dolutegravir as preferred HIV treatment option in all populations. Availablet at: https://www.who.int/news/item/22-07-2019-who-recommends-dolutegravir-as-preferred-hiv-treatment-option-in-all-populations. Accessed September 3, 2021.
- Williams A. L., Bohnsack B. L. (2019). What's retinoic acid got to do with it? Retinoic acid regulation of the neural crest in craniofacial and ocular development. Genesis 57, e23308. [DOI] [PubMed] [Google Scholar]
- Wilson J. G. (1973). Environment and Birth Defects. Academic Press, New York and London. [Google Scholar]
- Wlodarczyk B. J., Tang L. S., Triplett A., Aleman F., Finnell R. H. (2006). Spontaneous neural tube defects in splotch mice supplemented with selected micronutrients. Toxicol. Appl. Pharmacol. 213, 55–63. [DOI] [PubMed] [Google Scholar]
- Worley K. E., Rico-Varela J., Ho D., Wan L. Q. (2018). Teratogen screening with human pluripotent stem cells. Integr. Biol. (Camb.) 10, 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekutieli D., Benjamini Y. (1999). Resampling-based false discovery rate controlling multiple test procedures for correlated test statistics. J. Stat. Planning Inference 82, 171–196. [Google Scholar]
- Zamek-Gliszczynski M. J., Zhang X., Mudunuru J., Du Y., Chen J. L., Taskar K. S., Huang J., Huang Y., Romach E. H. (2019). Clinical extrapolation of the effects of dolutegravir and other HIV integrase inhibitors on folate transport pathways. Drug Metab. Dispos. 47, 890–898. [DOI] [PubMed] [Google Scholar]
- Zash R., Makhema J., Shapiro R. L. (2018). Neural-tube defects with dolutegravir treatment from the time of conception. N. Engl. J. Med. 379, 979–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zash R., Holmes L., Diseko M., Jacobson D. L., Brummel S., Mayondi G., Isaacson A., Davey S., Mabuta J., Mmalane M., et al. (2019). Neural-tube defects and antiretroviral treatment regimens in Botswana. N. Engl. J. Med. 381, 827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.