Abstract
Objective: This study used connected pen to determine missed bolus dose (MBD) frequency during masked and unmasked continuous glucose monitoring (CGM) periods and examined its link with time-in-range (TIR), time-above-range (TAR), time-below-range (TBR), and key participant characteristics in people with diabetes.
Methods: This was a 12-week, single-arm, exploratory, two-period study for people with type 1 diabetes (T1D) or type 2 diabetes (T2D). The primary objective was to estimate the average number of MBD during masked and real-time CGM use. The secondary objective was to estimate the average percent TIR and its relationship to MBD. An exploratory objective was to investigate the participant characteristics that were associated with MBD. Data were analyzed for differences in MBD by diabetes type and other participant characteristics, by CGM period, and by hypoglycemic fear scores.
Results: Participants (n = 64; T1D, n = 38; T2D, n = 26) were 48 ± 11.9 years old and 44% were female. From the masked to the unmasked period, MBD, %TAR, %TBR, and glycated hemoglobin decreased significantly (0.74 MBD/day to 0.62 MBD/day, P = 0.008; 53.6%–48.1%, P = 0.004; 4.49%–2.93%, P < 0.001; mean 8.8%–8.4%, P < 0.001, respectively), while %TIR increased significantly (41.9%–49.0%, P < 0.001). MBD/day was negatively associated with TIR (P = 0.016) and positively associated with TAR (P = 0.015) for T1D and positively associated with TBR (P = 0.024) for T2D in the masked period only. MBD was significantly associated with fear of hypoglycemia for T2D, but not T1D.
Conclusions: MBD is associated with reduced TIR when CGM is masked and tailored therapeutic approaches are needed for T1D and T2D populations.
Keywords: Connected pen, Missed bolus dose, Continuous glucose monitoring
Introduction
Adherence to a basal-bolus insulin injection regimen is recognized as a challenging part of life for people with diabetes. Peyrot et al. found that one-third of people with type 1 diabetes (T1D) or type 2 diabetes (T2D) reported insulin injection omission or problematic adherence at least 1 day, with an average of 3.3 days, in the past month,1 and these problems have been associated with poor glycemic control.2,3 Furthermore, significant associations have been found with frequency of insulin boluses and glycated hemoglobin (HbA1c),4 and with number of weekly missed mealtime doses and HbA1c.5
A global survey of people with T1D (n = 110) and T2D (n = 1420) found that 35% reported a mean of 3.41 days of insulin omission/nonadherence per month. Associated factors were lifestyle burden, difficulty with injection or the insulin regimen, and psychosocial factors, such as dissatisfaction with the flexibility of injection timing.6 Moreover, a survey of 502 insulin-taking adults found that >50% reported intentional insulin omission and 20% noted omitting regularly. Behavioral and social factors such as interference with activities and embarrassment were significant risk factors.7
Before the introduction of connected or “smart” insulin pens, adherence data could only be obtained by self-reports or for people using insulin pumps. However, broad, systematic reviews of insulin practices, for example, claims and questionnaires, can be unreliable.8 Connected insulin pens, which collect both the dose amount and timing, allow for a more accurate assessment of insulin dosing behavior. A recent study using NovoPen® 6 demonstrated an increase in time-in-range (TIR) with use of the connected pen, as well as a decrease in missed bolus doses (MBDs) from 25% to 14%.9 Another study compared a connected insulin pen device (Insulclock) with a standard pen for basal doses and found that the connected pen group had an improvement in glycemic outcomes, but not in adherence.10 Neither these nor other reports using connected pens11,12 found behavioral factors related to dose adherence.
To improve insulin dosing and overall health outcomes for people with diabetes, it is critical to understand underlying behavioral factors and to use objective measures of glycemia and dosing behavior rather than self-report or to limit the population to those on insulin pumps. We conducted an exploratory study at community-based research sites to objectively estimate MBD frequency in people with T1D or T2D using continuous glucose monitoring (CGM) and an investigational connected pen device. We analyzed the data for differences in MBDs by real-time CGM (rtCGM) use, diabetes type, and by other participant characteristics. To our knowledge, this is the first multicenter study to objectively evaluate frequency of MBD and associated behavioral factors in a community-based population on multiple daily injection therapy.
Materials and Methods
Participants and study design
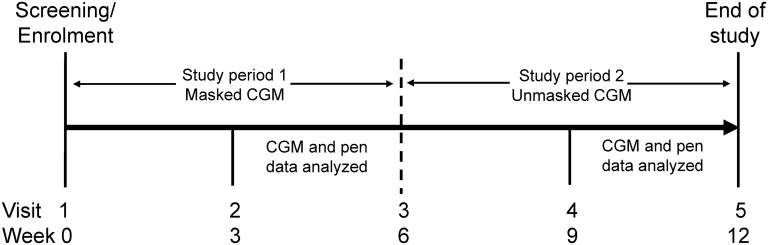
This was a 12-week, multicenter, single-arm, outpatient, exploratory study with two study periods (Fig. 1). Participants included were ≥21 to ≤65 years of age (T1D) or ≥35 to ≤65 years of age (T2D) at screening; using ≥3 doses of mealtime bolus insulin/day (e.g., insulin lispro [U-100]/[U-200], insulin aspart, or insulin glulisine), where each bolus dose was <40 U; taking a stable insulin dose regimen for the 3 months before study entry; had an HbA1c ≥8% in the last 6 months; had not used CGM or flash glucose monitoring during the last 3 months; and were able to stay on or switch to insulin lispro U-100 for the duration of the trial per the investigator's judgement. The study was conducted at six research sites in the United States. All participants were required to give informed consent for participation in the study and before any study-specific procedures. The protocol was approved by local Ethics Review Boards and was conducted according to International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki. The study is registered at clinicaltrials.gov as NCT03368807.
FIG. 1.
Study design. CGM, continuous glucose monitoring.
Outcomes
The study objectives were to determine MBD frequency during masked and unmasked CGM periods and to examine its link with TIR, time-above-range (TAR), time-below-range (TBR), and key participant characteristics. The primary objective was to estimate the average number of MBD during masked and rtCGM use. The secondary objective was to estimate the average percent TIR and its relationship to MBD. An exploratory objective was to investigate the participant characteristics that were associated with MBD. We defined a MBD as no insulin dose from 2 h before through 4 h after the start of a glucose excursion, where a glucose excursion was defined as a >70 mg/dL (>3.9 mmol/L) rise within 2 h, not preceded by a value <70 mg/dL (<3.9 mmol/L). We excluded overnight (12am–6am) glucose data in this analysis.
Procedures
Participants who entered the study taking a rapid-acting insulin analog other than insulin lispro U-100 were switched to insulin lispro U-100. Participants were trained in the use of the investigational, reusable connected pen, which captured bolus insulin dose data for download; a glucose meter; and a commercially available CGM device (Dexcom G5® system). The connected pen uses standard Lilly 3-mL Humalog U-100 glass cartridge. The pen has a display and memory function, allowing for insufficient insulin detection in the cartridge, last dose display (time since last dose and units delivered), a full dose history in memory, and “Injection Complete” prompt. At visit 1 (week 0) after training, participants began to use the pen to inject insulin lispro U-100 and continued their prestudy basal insulin and concomitant antihyperglycemic therapy. HbA1c was measured during the time of enrollment, at week 6, and at week 12. During the study, participants had their glucose levels monitored via CGM, which was masked during study period 1 (weeks 1–6) and unmasked during study period 2 (weeks 7–12).
At visit 2 (week 3) and visit 3 (week 6), study site personnel reviewed the masked CGM and pen data and, if necessary, made dose and alarm adjustments based on their medical judgement. Following the completion of visit 3 (week 6) assessments, participants began study period 2 and were unmasked to their CGM data. At visit 4 (week 9), participants had their unmasked CGM data and pen data reviewed by the study site personnel and any required adjustments to their CGM device alarms or insulin regimens were performed by study site personnel. At visit 5 (week 12), participation in the study was completed, and all pen and CGM data were collected.
At baseline, key participant information, including age, diabetes type, gender, and ethnicity, was collected. Furthermore, to examine participants' potential psychosocial concerns about hypoglycemia, we administered the Hypoglycemia Fear Survey (HFS) II,13 an evaluation of the fear of hypoglycemia using a Likert scale of 0 (“never”) to 4 (“almost always”) to rate 11 items (with a worry subscale and 2 aspects of behavior subscale: “avoidance” and “maintaining high glucose”).
Statistical analyses
Eighty participants were screened to ensure a final sample size of 50 (∼25 participants each with T1D or T2D), which was considered sufficient for this exploratory study. The statistical analysis was performed in two parts. The first part was primarily descriptive (mean, standard deviation, minimum, median, and maximum for continuous variables; frequency and percentage for categorical variables). The second part involved modeling of the relationship between factors and MBD. MBD and %TIR, %TAR, and %TBR were calculated for weeks 3–6 for the masked period and weeks 9–12 for the umasked period. We performed a pairwise comparison at the participant level on the missed doses and glycemic outcomes over study periods. In addition to the overall comparison, we also did the comparison among participants with T1D or T2D, respectively. Spearman's method was applied to examine the association between MBD and glycemic outcomes.
We used a negative binomial regression to examine the association of MBD with baseline characteristics and baseline hypoglycemia fear scores in the masked period, where the logarithm of number of days with at least one identified excursion was used as an offset term. Then, we applied a generalized linear mixed model (GLMM) to examine the association of MBD with baseline characteristics and scores in both study periods. More specifically, a GLMM of the negative binomial family was used for those participants who had evaluable CGM data and identified excursions in both periods. Participant-level random intercept terms were introduced for modeling the latent correlation between the paired MBD in both periods. Similarly, the logarithm of number of days with at least one excursion was considered as the offset term. The incidence rate ratios were estimated by the exponentialized covariate coefficient estimates. The tuning parameters were selected based on Bayesian information criteria. The statistical significance level was set to be 5%. We used the R stats14 package for the descriptive analyses and the MASS15 and lme416 package for the regression analyses.
Results
Participants
Seventy-nine participants were enrolled. One participant showed neither pen nor CGM use and was excluded from the safety population (n = 78). Ten participants had fewer than 2 weeks of unmasked CGM use and/or had a major protocol violation and were excluded from the evaluable population (n = 64) (Supplementary Fig. S1). Demographic and clinical characteristics for the evaluable population are given in Table 1. Concomitant medications for the T2D population were sodium-glucose transporter 2 inhibitors (n = 6; 7.7%), including canagliflozin (n = 2; 2.6%) dapagliflozin (n = 2; 2.6%), empagliflozin (n = 3; 3.8%), and glucagon-like peptide-1 receptor agonists (n = 10; 12.8%), including albiglutide (n = 1, 1.3%), dulaglutide (n = 3; 3.8%), exenatide (n = 2; 2.6%), and liraglutide (n = 4; 5.1%). Four participants had insufficient data for the CGM metrics and were excluded from the CGM population (n = 64).
Table 1.
Demographics and Baseline Characteristics of the Evaluable Population
| T1D (n = 38) | T2D (n = 26) | P | All, N = 64 | |
|---|---|---|---|---|
| Age, years | 42.3 ± 11.6 | 55.7 ± 6.8 | <0.001 | 47.7 ± 11.9 |
| Men | 25 (62) | 13 (52) | 0.18 | 38 (56) |
| Hispanic or Latino | 5 (13.2) | 6 (23.1) | 0.49 | 11 (17.2) |
| Race | 0.046 | |||
| White | 38 (100) | 20 (76.9) | 62 (90.6) | |
| Black or African American | 0 (0.0) | 3 (11.5) | 3 (4.7) | |
| American Indian or Alaska Native | 0 (0.0) | 1 (3.8) | 1 (1.6) | |
| Asian | 0 (0.0) | 1 (3.8) | 1 (1.6) | |
| Multiple | 0 (0.0) | 1 (3.8) | 1 (1.6) | |
| BMI, kg/m2 | 27.6 ± 5.5 | 34.4 ± 4.8 | <0.001 | 30.4 ± 6.2 |
| HbA1c, % | 9.4 ± 2.0 | 9.7 ± 1.4 | 0.52 | 9.6 ± 1.8 |
| Diabetes duration, years | 22.1 ± 11.6 | 16.5 ± 7.3 | 0.009 | 19.8 (10.4) |
| Preenrollment bolus insulin | ||||
| Lispro U-100 | 22 (57.9) | 13 (50.0) | 35 (54.7) | |
| Aspart | 15 (39.5) | 13 (50.0) | 28 (43.8) | |
| Glulisine | 1 (2.6) | 0 (0.0) | 1 (1.6) | |
Evaluable population, N = 64. Data are mean ± SD or n (%).
BMI, body mass index; HbA1c, glycated hemoglobin; SD, standard deviation; T1D, type 1 diabetes; T2D, type 2 diabetes.
MBDs and glycemic outcomes
From the masked to the unmasked period, MBD frequency (Supplementary Figure S2), %TAR, %TBR, and HbA1c decreased significantly (0.74 MBD/day to 0.62 MBD/day, P = 0.008; 53.6%–48.1%, P = 0.004; 4.49%–2.93%, P < 0.001; mean 8.8%–8.4%, P < 0.001, respectively), while %TIR increased significantly (41.9%–49.0%, P < 0.001). In the masked period, MBD/day was negatively associated with TIR (P = 0.016) and positively associated with TAR (P = 0.015) for T1D and positively associated with TBR (P = 0.024) for T2D; for the combined group only, MBD/day was positively associated with HbA1c (P = 0.017). During the unmasked period, there were no significant associations between MBD and any glycemic outcome for either T1D or T2D (Table 2).
Table 2.
Missed Doses, Glycemic Measures, and Correlations in Overall, Type 1 Diabetes, and Type 2 Diabetes Evaluable Patient Population
| Parameter | Overall (n = 64) |
T1D (n = 38) |
T2D (n = 26) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Masked CGM | Unmasked CGM | P | Masked CGM | Unmasked CGM | P | Masked CGM | Unmasked CGM | P | |
| MBD per day | 0.74 ± 0.4 | 0.62 ± 0.3 | 0.008 | 0.70 ± 0.4 | 0.64 ± 0.3 | 0.237 | 0.80 ± 0.4 | 0.60 ± 0.3 | 0.010 |
| MBD per month | 22.1 ± 11.3 | 18.6 ± 9.3 | 0.008 | 20.9 ± 11.0 | 19.11 ± 9.3 | 0.237 | 23.9 ± 11.8 | 17.9 ± 9.5 | 0.010 |
| %TIR 70–180 mg/dL | 41.9 ± 18.7 | 49.0 ± 19.5 | <0.001 | 38.31 ± 18.5 | 44.46 ± 20.4 | 0.014 | 47.07 ± 18.2 | 55.63 ± 16.4 | 0.008 |
| %TAR >180 mg/dL | 53.6 ± 21.2 | 48.1 ± 20.8 | 0.004 | 55.80 ± 22.23 | 51.95 ± 22.5 | 0.161 | 50.49 ± 19.7 | 42.40 ± 16.9 | 0.010 |
| %TBR <70 mg/dL | 4.49 ± 5.0 | 2.93 ± 3.6 | <0.001 | 5.90 ± 5.70 | 3.59 ± 3.84 | <0.001 | 2.44 ± 2.9 | 1.97 ± 3.0 | 0.590 |
| HbA1c, % | 8.77 ± 1.4 | 8.37 ± 1.4 | <0.001 | 8.76 ± 1.64 | 8.38 ± 1.52 | 0.002 | 8.79 ± 1.1 | 8.36 ± 1.1 | 0.003 |
| Correlations | — | — | — | ||||||
| MBD/day with TIR | −0.241 (0.055) | −0.188 (0.138) | — | −0.387 (0.016) | −0.144 (0.389) | — | −0.044 (0.833) | −0.138 (0.502) | — |
| MBD/day with TAR | 0.229 (0.069) | 0.150 (0.238) | — | 0.392 (0.015) | 0.103 (0.539) | — | −0.068 (0.741) | 0.073 (0.723) | — |
| MBD/day with TBR | −0.030 (0.811) | 0.127 (0.317) | — | −0.188 (0.259) | 0.070 (0.674) | — | 0.442 (0.024) | 0.289 (0.152) | — |
| MBD/day with HbA1c | 0.297 (0.017) | 0.175 (0.166) | 0.317 (0.053) | 0.122 (0.467) | 0.214 (0.294) | 0.156 (0.446) | |||
Values in boldface indicate statistical significance.
CGM population, n = 64; Data are mean ± SD or r (P).
CGM, continuous glucose monitoring; MBD, missed bolus dose; r, correlation coefficient; TIR, time-in-range; TAR, time-above-range; TBR, time-below-range.
Correlates to MBD
During the masked period, MBD frequency for T2D was positively associated with the two HFS behavior subscales, “avoidance” and “maintaining high glucose,” but was negatively associated with the worry subscale. During the unmasked period, only the association with the worry subscale persisted. There were no associations between MBD and the HFS subscales for T1D participants during the masked or unmasked periods (Tables 3 and 4).
Table 3.
Effect of Measured Variables on Missed Bolus Dose in Masked Continuous Glucose Monitoring (Period 1)
| Predictor variables | T1D (n = 38) |
T2D (n = 26) |
||
|---|---|---|---|---|
| IRR | P | IRR | P | |
| Age | 1.001 | 0.881 | 0.978 | 0.081 |
| Gender: female to male | 0.916 | 0.535 | 0.705 | 0.017 |
| BMI | 0.965 | 0.025 | 0.994 | 0.667 |
| HbA1c | 1.122 | 0.006 | 1.071 | 0.162 |
| Duration of diabetes | 1.013 | 0.072 | 0.996 | 0.792 |
| HFS | ||||
| Worry | 0.982 | 0.352 | 0.940 | 0.004 |
| Avoidance | 0.962 | 0.269 | 1.140 | 0.005 |
| Maintain high | 0.949 | 0.165 | 1.075 | 0.040 |
Values in boldface indicate statistical significance.
HFS, hypoglycemic fear survey; IRR, incidence rate ratio.
Table 4.
Effect of Measured Variables on Missed Bolus Dose in Unmasked Continuous Glucose Monitoring (Period 2)
| Predictor variables | T1D (n = 38) |
T2D (n = 26) |
||
|---|---|---|---|---|
| IRR | P | IRR | P | |
| Age | 0.995 | 0.529 | 0.960 | 0.018 |
| Gender: female to male | 1.147 | 0.365 | 1.501 | 0.160 |
| BMI | 0.977 | 0.162 | 0.968 | 0.110 |
| HbA1c | 1.000 | 0.997 | 0.913 | 0.209 |
| Duration of diabetes | 1.001 | 0.861 | 0.992 | 0.723 |
| HFS | ||||
| Worry | 0.996 | 0.859 | 0.945 | 0.032 |
| Avoidance | 0.965 | 0.348 | 1.040 | 0.521 |
| Maintain high | 0.968 | 0.442 | 1.107 | 0.078 |
Values in boldface indicate statistical significance.
Discussion
In this community-based study, participants missed a mean of 22.2 bolus doses per month during the masked period and this decreased significantly once rtCGM was introduced in the unmasked period. In parallel, we observed that more MBD was associated with decreased TIR, but only during the masked period. Thus there was no longer an association between MBD and TIR once rtCGM was introduced. This may suggest that rtCGM contributes to an attenuation of this relationship between MBD and key glycemic outcomes, perhaps due to other self-management changes that participants undertook when rtCGM became available. This finding would benefit from further investigation in future studies.
Previous studies have also shown improved glycemic outcomes with the use of rtCGM with17 or without18 MDI therapy. Our study included the use of connected pen, which allowed us to determine if improvements in glycemic control with CGM were associated with changes in insulin dosing, in addition to improvements in self-management behavior. Of note, although we observed a marked improvement in TIR, it was still far from optimal (49% with rtCGM vs. 42% without), therefore, rtCGM may be only the first step toward optimization of insulin therapy.
The underlying factors associated with MBD were different between the T1D and T2D cohorts. In the T2D cohort, fear of hypoglycemia was a significant predictor of MBD. Higher scores on the behavior subscales were positively associated with MBD, as was, interestingly, scoring lower on the worry subscale, suggesting that engaging in behaviors that consistently prevent hypoglycemia reduces the associated worry. Hypoglycemia-related anxiety has been shown to be associated with more hypoglycemia in insulin-requiring T2D, as well as with greater emotional distress. Hypoglycemia confidence has been positively associated with glycemic control.19 Until now it has been unclear what hypoglycemic fear-related mechanism could lead to poorer glycemic outcomes. Our study demonstrates that MBD is related to decreased TIR and that more-frequent MBD may be associated with fear of hypoglycemia for people with T2D.
Surprisingly, there was no association of MBD with hypoglycemic fear for the T1D cohort, indicating that other behavioral factors are at play.
The study has several limitations. It had a small sample size as it was an exploratory study. The results may not be representative of all people with diabetes as enrollment was limited to people with a baseline HbA1c >8.0% to enrich the cohort with individuals likely having problematic dosing adherence. In addition, it is difficult to interpret the change in HbA1c values; the HbA1c reduction is unlikely to be attributable solely to use of the connected pen. Other contributors to the observed HbA1c reduction may include unblinded CGM use in the second phase of the study and the impact of participation in a clinical trial on diabetes self-management (study effect). Finally, we cannot assess dosing practices specific to meals or compare actual dose versus recommended dose because we did not require participants to perform any manual logging. This was by design so that the logging did not influence adherence behavior.
Conclusions
These data highlight the heterogeneity of the behavioral factors underlying MBD and point to the potential utility of connected insulin pens as a diagnostic tool to evaluate insulin dosing practices. Fear of hypoglycemia in the T1D population did not appear to be a significant contributor to missing doses, which was somewhat surprising based on our prior clinical experience. Perhaps there are other behavioral influences that supersede even fear in this population. Our findings support the need for tailored therapeutic approaches to reduce MBD and increase TIR in both T1D and T2D populations. Rather than only measuring dosing adherence, connected pen data could be utilized as the starting point to uncover the factors that affect MBD frequency and to inform patient-clinician discussions around individual barriers to optimal dosing.
Supplementary Material
Acknowledgments
The authors thank Nany Gulati of Eli Lilly Services India Private Limited for writing and editorial contributions in preparation of this article.
Author Disclosure Statement
S.E., W.W., J.-L.P., J.J. are employees and stockholders of Eli Lilly and Company; X.H., E.M., H.W. were employed at Eli Lilly and Company at the time when this study was conducted. WP has served as consultant to Eli Lilly and Company and Dexcom.
Funding Information
This work was funded by Lilly Innovation.
Supplementary Material
References
- 1. Peyrot M, Barnett AH, Meneghini LF, et al. : Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med 2012;29:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Currie CJ, Peyrot M, Morgan CL, et al. : The impact of treatment noncompliance on mortality in people with type 2 diabetes. Diabetes Care 2012;35:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Currie CJ, Peyrot M, Morgan CL, et al. : The impact of treatment non-compliance on mortality in people with type 1 diabetes. J Diabetes Complications 2013;27:219–223. [DOI] [PubMed] [Google Scholar]
- 4. Patton SR, Clements MA, Fridlington A, et al. : Frequency of mealtime insulin bolus as a proxy measure of adherence for children and youths with type 1 diabetes mellitus. Diabetes Technol Ther 2013;15:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burdick J, Chase HP, Slover RH, et al. : Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics 2004;113e221–e224. [DOI] [PubMed] [Google Scholar]
- 6. Peyrot M, Barnett AH, Meneghini LF, et al. : Factors associated with injection omission/non-adherence in the Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabetes Obes Metab 2012;14:1081–1087. [DOI] [PubMed] [Google Scholar]
- 7. Peyrot M, Rubin RR, Kruger DF, et al. : Correlates of insulin injection omission. Diabetes Care 2010;33:240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stolpe S, Kroes MA, Webb N, et al. : A systematic review of insulin adherence measures in patients with diabetes. J Manag Care Spec Pharm 2016;22:1224–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adolfsson P, Hartvig NV, Kaas A, et al. : Increased time in range and fewer missed bolus injections after introduction of a smart connected insulin pen. Diabetes Technol Ther 2020;22:709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramos C, Galindo RJ, Alam MM, et al. : 996-P: A randomized study to evaluate the efficacy of insulclock pen device in insulin-treated patients with uncontrolled type 2 diabetes. Diabetes 2020;69(Suppl 1)996-P. [Google Scholar]
- 11. Toschi E, Slyne C, Greenberg JM, et al. : Examining the relationship between pre- and postprandial glucose levels and insulin bolus timing using bluetooth-enabled insulin pen cap technology and continuous glucose monitoring. Diabetes Technol Ther 2020;22:19–24. [DOI] [PubMed] [Google Scholar]
- 12. Munshi MN, Slyne C, Greenberg JM, et al. : Nonadherence to insulin therapy detected by bluetooth-enabled pen cap is associated with poor glycemic control. Diabetes Care 2019;42:1129–1131. [DOI] [PubMed] [Google Scholar]
- 13. Grabman J, Bailey KV, Schmidt K, et al. : An empirically derived short form of the Hypoglycaemia Fear Survey II. Diabet Med 2017;34:500–504. [DOI] [PubMed] [Google Scholar]
- 14. R Core Team: R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. [Google Scholar]
- 15. Venables WN, Ripley BD: Modern Applied Statistics with S, 4th ed. New York: Springer, 2002. [Google Scholar]
- 16. Bates D, Maechler M, Bolker B, et al. : Fitting linear mixed-effects models using lme4. J Stat Softw 2015;67:1–48. [Google Scholar]
- 17. Beck RW, Riddlesworth TD, Ruedy KJ, et al. : Effect of initiating use of an insulin pump in adults with type 1 diabetes using multiple daily insulin injections and continuous glucose monitoring (DIAMOND): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:700–708. [DOI] [PubMed] [Google Scholar]
- 18. Vigersky RA, Fonda SJ, Chellappa M, et al. : Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 2012;35:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Polonsky WH, Fisher L, Hessler D, et al. : Identifying the worries and concerns about hypoglycemia in adults with type 2 diabetes. J Diabetes Complications 2015;29:1171–1176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.