Abstract
Among patients with methicillin-resistant Staphylococcus aureus bacteremia, vancomycin-associated acute kidney injury increased as a function of the day 2 area under the curve (AUC), even for daily AUCs within the recommended therapeutic range (400–600). Further data are needed to determine if daily AUCs <400 can be maintained without compromising efficacy.
Keywords: MRSA, outcomes, pharmacokinetics, vancomycin
Vancomycin remains the standard of care for serious methicillin-resistant Staphylococcus aureus (MRSA) infections, but optimal dosing is constrained by a tradeoff between clinical effectiveness and vancomycin-associated acute kidney injury (VA-AKI) at higher vancomycin exposures [1]. Consensus guidelines on vancomycin dosing recommend targeting a daily area under the curve (AUC) of 400–600 mg × hour/L to minimize VA-AKI while preserving effectiveness. However, patients with vancomycin exposures within this range may still be at risk of VA-AKI [2–6]. To quantify this risk, we evaluated the associations between the day 2 vancomycin AUC and VA-AKI among patients who were enrolled in Prospective Observational Evaluation of the Association between Initial Vancomycin Exposure and Failure Rates among Adult Hospitalized Patients with MRSA Bloodstream Infections (PROVIDE) [4].
METHODS
Patients were included in PROVIDE if they were aged ≥18 years, hospitalized, received vancomycin within 48 hours of index MRSA blood culture, continued vancomycin for at least 72 hours, had at least 2 vancomycin blood concentrations during the first 5 days of vancomycin, and had available 30-day outcome data. Patients from PROVIDE were included in the primary of this study if they had a (1) serum creatinine (SCR) on or before the day of the first vancomycin dose, (2) a baseline creatinine clearance (CLCR) ≥30 mL/minute, and (3) subsequent SCR value(s) from initiation of vancomycin to 48 hours postcompletion. Patients with severe renal impairment (CLCR <30 mL/minute) at start of vancomycin were excluded to focus on patients without significant baseline renal dysfunction [7, 8].
All baseline data elements collected in PROVIDE were included (Table 1) [4]. Vancomycin day 2 AUC values were estimated post hoc using the maximal a posteriori probability procedure in ADAPT 5 [9, 10]. We selected day 2 AUC as the primary exposure variable since it is indicative of near-steady-state conditions. Since most VA-AKI events occurred after >5 days of vancomycin therapy in PROVIDE [4], day 2 AUC avoids the confounding of AKI causing increased vancomycin AUCs. Day 2 vancomycin AUC values were consolidated into 3 categories: <400, 400–600, and >600 to reflect AUCs below, within, and above the recommended therapeutic range.
Table 1.
Comparison of Baseline Characteristics Between Patients by Day 2 Area Under the Curve Categories and Presence and Absence of Vancomycin-Associated Acute Kidney Injury Outcomes
| Characteristic | AUC <400 (n = 48) |
AUC 400–600 (n = 81) |
AUC >600 (n = 101) |
KDIGO (n = 73) |
No KDIGO (n = 157) | RIFLE (n = 55) |
No RIFLE (n = 175) |
KDIGO Stage II/RIFLE Injury (n = 30) | No KDIGO Stage II/RIFLE Injury (n = 200) | KDIGO Stage III/RIFLE Failure (n = 14) | No KDIGO Stage III/RIFLE Failure (n = 216) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male sex | 29 (60.4) | 48 (59.3) | 68 (67.3) | 47 (64.4) | 98 (62.4) | 34 (61.8) | 111 (63.4) | 14 (46.7)a | 131 (65.5) | 9 (64.3) | 136 (63.0) |
| White race | 30 (62.5) | 56 (69.1) | 67 (66.3) | 45 (61.6) | 108 (68.8) | 32 (58.2)b | 121 (69.1) | 17 (56.7) | 136 (68.0) | 10 (71.4) | 143 (66.2) |
| Weight, kg, mean (SD) | 76.5 (21.4)a | 78.3 (22.4) | 89.8 (27.0) | 82.7 (23.2) | 83.1 (25.9) | 81.8 (22.9) | 83.4 (25.6) | 75.9 (21.2)b | 84.0 (25.4) | 78.9 (21.7) | 83.3 (25.2) |
| Body mass index, mean (SD) | 26.8 (6.3)a | 26.2 (6.3) | 30.1 (9.1) | 28.0 (7.4) | 28.0 (8.0) | 28.0 (7.7) | 28.0 (7.9) | 26.8 (7.6) | 28.2 (7.9) | 27.2 (6.2) | 28.1 (7.9) |
| Age, years, mean (SD) | 57.0 (18.6)b | 62.2 (16.0) | 57.2 (15.9) | 63.0 (14.3) | 57.0 (17.3) | 62.0 (14.0)b | 58.0 (17.3) | 60.9 (14.8) | 58.7 (16.9) | 56.9 (13.4)b | 59.1 (16.8) |
| Residence in ICU at time of index blood culture collection | 7 (14.6) | 17 (21.0) | 24 (23.8) | 17 (23.3) | 31 (19.7) | 13 (23.6) | 35 (20.0) | 6 (20.0) | 42 (21.0) | 3 (21.4) | 45 (20.8) |
| Type of MRSA: hospital/healthcare acquired | 30 (62.5)b | 55 (67.9) | 79 (78.2) | 55 (75.3) | 109 (69.4) | 43 (78.2)b | 121 (69.1) | 24 (80.0) | 140 (70.0) | 11 (78.6) | 153 (70.8) |
| Residence in healthcare institution for >72 hours in past 180 days | 22 (45.8)a | 39 (48.1) | 66 (65.4) | 39 (53.4) | 88 (56.1) | 28 (50.9) | 99 (56.6) | 15 (50.0) | 112 (56.0) | 7 (50.0) | 120 (55.6) |
| Length of hospital stay, days, prior to index culture, median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| APACHE II score, mean (SD) | 9.8 (5.0)a | 11.9 (5.0) | 11.9 (5.4) | 11.8 (5.6) | 11.3 (5.1) | 11.4 (5.7) | 11.5 (5.1) | 11.4 (5.8) | 11.5 (5.2) | 12.9 (7.5) | 11.4 (5.1) |
| Estimated creatinine clearance at baseline, mL/min, mean (SD) | 94.5 (46.1) | 88.0 (49.0) | 99.4 (62.2) | 94.1 (61.3) | 94.5 (51.5) | 103.6 (65.6) | 91.4 (50.0) | 110.3 (77.9)b | 92.0 (50.1) | 129.8 (108.1)b | 92.1 (48.9) |
| Diabetes mellitus | 12 (25.0)b | 24 (29.6) | 41 (40.6) | 25 (34.2) | 52 (33.1) | 18 (32.7) | 59 (33.7) | 10 (33.3) | 67 (33.5) | 5 (35.7) | 72 (33.3) |
| Heart failure (class II–IV) | 3 (6.3) | 10 (12.3) | 10 (9.9) | 5 (6.8) | 18 (11.5) | 1 (1.8)a | 22 (12.6) | 0 (0.0)b | 23 (11.5) | 0 (0)b | 23 (10.6) |
| COPD | 5 (10.4)b | 17 (21.0) | 16 (15.8) | 12 (16.4) | 26 (16.6) | 5 (9.1)b | 33 (18.9) | 0 (0)a | 38 (19.0) | 0 (0)b | 38 (17.6) |
| Transplanted organ | 3 (6.3) | 5 (6.2) | 4 (4.0) | 3 (4.1) | 9 (5.7) | 2 (3.6) | 10 (5.7) | 2 (6.7) | 10 (5.0) | 0 (0) | 12 (5.6) |
| Active malignancy | 9 (18.8) | 14 (17.3) | 15 (14.9) | 13 (17.8) | 25 (15.9) | 9 (16.4) | 29 (16.6) | 3 (10.0) | 35 (17.5) | 0 (0)b | 38 (17.6) |
| Receipt of immunosuppressive drugs in last 30 days | 11 (22.9) | 12 (14.9) | 15 (14.9) | 8 (11.0)b | 30 (19.1) | 7 (12.7) | 31 (17.7) | 2 (6.7)b | 36 (18.0) | 0 (0)b | 38 (17.6) |
| Decubitus ulcers (stage II–IV) | 5 (10.4) | 6 (7.4) | 15 (14.9) | 4 (5.5)b | 22 (14.0) | 4 (7.7) | 22 (12.6) | 0 (0)a | 26 (13.0) | 0 (0)b | 26 (12.0) |
| Cerebrovascular accident | 7 (14.6) | 6 (7.4) | 12 (11.9) | 6 (8.2) | 19 (12.1) | 3 (5.5)b | 22 (12.6) | 0 (0)a | 25 (12.5) | 0 (0)b | 25 (11.6) |
| Surgery requiring >48 hours hospitalization in 30 days prior to date of index culture | 5 (10.4) | 8 (9.9) | 16 (15.8) | 12 (16.4) | 17 (10.8) | 10 (18.2)b | 19 (10.9) | 7 (23.3)b | 22 (11.0) | 3 (21.4) | 26 (12.0) |
| Presence of infective endocarditis | 12 (25.0) | 21 (25.9) | 32 (31.7) | 24 (32.9) | 41 (26.1) | 16 (29.1) | 49 (28.0) | 7 (23.3) | 58 (29.0) | 4 (28.6) | 61 (28.2) |
| Preexisting valvular heart disease | 5 (10.4) | 11 (13.6) | 9 (8.9) | 4 (5.5)b | 21 (13.4) | 3 (5.5)b | 22 (12.6) | 2 (6.7) | 23 (11.5) | 1 (7.1) | 24 (11.1) |
| Previous infective endocarditis | 1 (2.1) | 1 (1.2) | 4 (4.0) | 1 (1.4) | 5 (3.2) | 0 (0)b | 6 (3.4) | 0 (0) | 6 (3.0) | 0 (0) | 6 (2.8) |
| Cardiac prosthetic device (eg, pacemaker, cardioverter-defibrillator, prosthetic valve) | 3 (6.3) | 5 (6.2) | 10 (9.9) | 4 (5.5) | 14 (8.9) | 2 (3.6)b | 16 (9.1) | 0 (0)b | 18 (9.0) | 0 (0) | 18 (8.3) |
| Prosthetic joints | 5 (10.4) | 5 (6.2) | 6 (5.9) | 5 (6.8) | 11 (7.0) | 2 (3.6) | 14 (8.0) | 0 (0)b | 16 (8.0) | 0 (0) | 16 (7.4) |
| Intravascular prosthetic material (eg, grafts, stents) | 6 (12.5) | 9 (11.1) | 12 (11.9) | 11 (15.1) | 16 (10.2) | 7 (12.7) | 20 (11.4) | 3 (10.0) | 24 (12.0) | 0 (0)b | 27 (12.5) |
| Receipt of antibiotic for at least 48 hours in the 30 days prior to index culture | 13 (27.1) | 31 (38.3) | 40 (39.6) | 22 (30.1)b | 62 (39.5) | 16 (29.1)b | 68 (38.9) | 8 (26.7) | 76 (38.0) | 3 (21.4) | 81 (37.5) |
| Receipt of vancomycin ≥48 hours in 30 days prior to index culture | 2 (4.2) | 9 (11.1) | 13 (12.9) | 11 (15.1)b | 13 (8.1) | 8 (14.5) | 16 (9.1) | 4 (13.3) | 20 (10.0) | 2 (14.3) | 22 (10.2) |
| Polymicrobial BSI | 2 (4.2) | 5 (6.2) | 11 (10.9) | 7 (9.6) | 11 (7.0) | 4 (7.3) | 14 (8.0) | 3 (10.0) | 15 (7.5) | 0 (0) | 18 (8.3) |
| Source of bacteremia infection–possibly to definitely | |||||||||||
| Intravenous catheter | 12 (25.0) | 20 (24.7) | 34 (33.7) | 24 (32.9) | 42 (26.8) | 20 (36.4)b | 46 (26.3) | 12 (40.0)b | 54 (27.0) | 4 (28.6) | 62 (28.7) |
| Urinary tract | 6 (12.5) | 9 (11.1) | 16 (15.8) | 6 (8.2)b | 25 (15.9) | 5 (9.1) | 26 (14.9) | 3 (10.0) | 28 (14.0) | 2 (14.3) | 29 (13.4) |
| Osteoarticular (bone and joint) | 8 (16.7) | 16 (19.8) | 16 (15.8) | 12 (16.4) | 28 (17.8) | 6 (10.9)b | 34 (19.4) | 2 (6.7)b | 38 (19.0) | 2 (14.3) | 38 (17.6) |
| Skin and soft tissue | 26 (54.2) | 39 (48.1) | 42 (41.6) | 34 (46.6) | 73 (46.5) | 29 (52.7) | 78 (44.6) | 18 (60.0)b | 89 (44.5) | 6 (42.9) | 101 (46.8) |
| Abdominal source | 5 (10.4) | 5 (6.2) | 8 (7.9) | 7 (9.6) | 11 (7.0) | 7 (12.7)b | 11 (6.3) | 4 (13.3) | 14 (7.0) | 2 (14.3) | 16 (7.4) |
| Central nervous system | 1 (2.1) | 4 (4.9) | 2 (2.0) | 2 (2.7) | 5 (3.2) | 1 (1.8) | 6 (3.4) | 1 (3.3) | 6 (3.0) | 0 (0) | 7 (3.2) |
| Respiratory tract | 4 (8.3)b | 15 (18.5) | 22 (21.8) | 14 (19.2) | 27 (17.2) | 11 (20.0) | 30 (17.1) | 9 (30.0)b | 32 (16.0) | 5 (25.7)b | 36 (16.7) |
| Other | 7 (14.6) | 12 (14.8) | 19 (18.8) | 11 (15.1) | 27 (17.2) | 6 (10.9)b | 32 (18.3) | 4 (13.3) | 34 (17.0) | 3 (21.4) | 35 (16.2) |
| Receipt of β-lactam during first 7 days of vancomycin >24 hours | 25 (52.1) | 41 (50.6) | 62 (61.4) | 44 (60.3) | 84 (53.5) | 32 (58.2) | 96 (54.9) | 14 (46.7) | 114 (57.0) | 6 (42.9) | 122 (56.5) |
| Receipt of aminoglycoside during first 7 days of vancomycin >24 hours | 1 (2.1) | 3 (3.7) | 4 (4.0) | 4 (5.5) | 4 (2.5) | 4 (7.3)b | 4 (2.3) | 4 (13.3)a | 4 (2.0) | 3 (21.4)a | 5 (2.3) |
| Receipt of clindamycin during first 7 days of vancomycin >24 hours | 1 (2.1) | 3 (3.7) | 4 (4.0) | 3 (4.1) | 5 (3.2) | 3 (5.5) | 5 (2.9) | 2 (6.7) | 6 (3.0) | 2 (14.3)a | 6 (2.8) |
| Receipt of fluoroquinolone during first 7 days of vancomycin >24 hours | 2 (4.2) | 5 (6.2) | 7 (6.9) | 8 (11.0)a | 6 (3.8) | 6 (10.9)b | 8 (4.6) | 4 (13.3)b | 10 (5.0) | 4 (28.6)a | 10 (4.6) |
| Receipt of rifampin during first 7 days >24 hours | 0 (0) | 4 (4.9) | 5 (5.0) | 5 (6.8)b | 4 (2.5) | 3 (5.5) | 6 (3.4) | 1 (3.3) | 8 (4.0) | 1 (7.1) | 8 (3.7) |
| Duration of vancomycin, median (IQR) | 11.0 (7.25–22.25) | 15.0 (8.5–24.0) | 14.0 (0.0–29.5) | 15.0 (9.0–28.0)b | 13.0 (7.0–24.5) | 15.0 (9.0–20.0) | 14.0 (7.0–28.0) | 14.5 (9.0–25.0) | 14.0 (7.0–25.75) | 12.5 (7.0–24.75) | 14.5 (8.0–25.75) |
Data are presented as No. (%), unless otherwise indicated.
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; AUC, area under the curve; BSI, bloodstream infection; COPD, chronic obstructive pulmonary disease; KDIGO, Kidney Disease: Improving Global Outcomes; ICU, intensive care unit; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; RIFLE, modified Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; SD, standard deviation.
P < .05.
P < .2.
Occurrences of VA-AKI were based on the KDIGO (Kidney Disease: Improving Global Outcomes) [11] and RIFLE (modified Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) classifications [12, 13] and were assessed from day 2 of vancomycin to 48 hours post–vancomycin completion. The following KDIGO/RIFLE classifications were evaluated: (1) KDIGO stage 1 (increase in baseline SCR by ≥0.3 mg/dL within 48 hours or increase in baseline SCR by ≥1.5 mg/dL within 7 days); (2) RIFLE Risk (increase in baseline SCR by ≥1.5 mg/dL); (3) KDIGO stage 2/RIFLE Injury (increase in baseline SCR by ≥2 mg/dL); and (4) KDIGO stage 3/RIFLE Failure (increase in baseline SCR by ≥3 mg/dL or SCR ≥4.0 mg/dL). The SCR observed on vancomycin day 1 was used as the baseline SCR for classifying the occurrence of VA-AKI. When SCR was not collected on a given day, the SCR measurement from the day prior was carried forward for determining the occurrence of KDIGO stage 1. These AKI classifications were examined as occurrence of these AKI endpoints has been significantly associated with mortality [11].
Bivariate associations between baseline covariates and day 2 vancomycin AUC categories (ie, <400, 400–600, and >600) and baseline covariates and VA-AKI outcomes were assessed using parametric (ie, Student t test or 1-way analysis of variance) or nonparametric test (ie, Pearson χ2 or independent samples median test), depending on nature of comparison. Tests for trends were used to determine association between the day 2 AUC categories and VA-AKI. A sensitivity test for trends analysis was performed that excluded patients with RIFLE Risk on day 2 of vancomycin to avoid those that were potentially already in the causal pathway to AKI. Two sets of logistic regressions were performed to quantify the association between day 2 vancomycin AUC and VA-AKI outcomes after adjusting for potential confounding variables. In the first, day 2 vancomycin AUC was included as a continuous variable. In the second, day 2 AUC was included as a 3-level rank order categorical variable. For both sets, the day 2 AUC variable was forced into the model first. All potential confounding variables (baseline covariates presence in >5% of study population and associated with VA-AKI outcomes or vancomycin day 2 AUC at P < .2) were then included at model entry and a backward stepping approach was used to remove variables with P >.1. All calculations were computed using IBM SPSS Statistics 27 software (IBM Corporation, Armonk, New York).
RESULTS
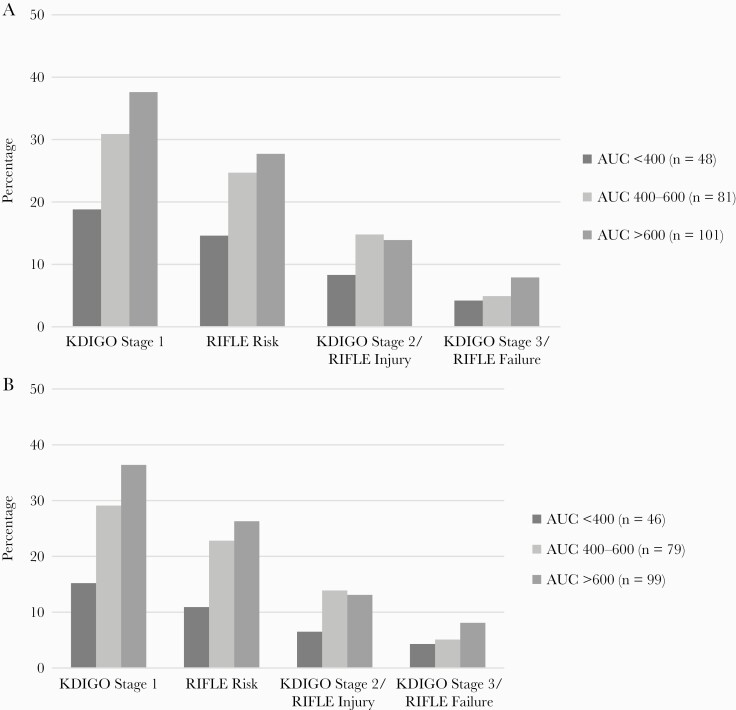
Of the 265 evaluable patients in PROVIDE, 230 had a baseline CLCR ≥30 mL/minute and a subsequent SCR value. The incidences of KDIGO stage 1, RIFLE Risk, KDIGO stage 2/RIFLE Injury, and KDIGO stage 3/RIFLE Failure were 31.7%, 23.9%, 13.0%, and 6.1%, respectively. Comparison of baseline characteristics between day 2 AUC categories and presence or absence of VA-AKI outcomes are shown in Table 1. Baseline weight, body mass index, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and prior residence in a healthcare facility were the only variables that significantly differed between day 2 AUC categories. In the test for trends analysis (Figure 1A), a significant association was observed between the day 2 AUC categories and KDIGO stage 1 (P = .02) but not RIFLE (P = .1), KDIGO stage 2/RIFLE Injury (P = .43), or KDIGO stage 3/RIFLE Failure (P = .32). Similar test for trends associations between day 2 AUC and VA-AKI outcomes were observed in the sensitivity analysis that excluded patients who experienced RIFLE risk on day 2 (Figure 1B). In the multivariate logistic regression analyses (Table 2), significant associations were observed between day 2 vancomycin AUC, coded as a continuous variable, and all VA-AKI outcomes. Day 2 AUC, coded a 3-level rank order categorical variable, was significantly associated with KDIGO stage 1 and RIFLE Risk in the second set of multivariate logistic regression analyses.
Figure 1.
Association between occurrence of KDIGO (Kidney Disease: Improving Global Outcomes) and RIFLE (modified Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) classifications and day 2 vancomycin area under the curve (AUC) with rank-ordered categories of <400, 400–600, and >600 in the primary analysis (n = 230; A) and sensitivity analysis that excluded patients who experienced RIFLE Risk on day 2 of vancomycin (n = 223; B). A, Test for trends: KDIGO stage 1: P = .02; RIFLE Risk: P = .10; KDIGO stage 2/RIFLE Injury: P = .43; KDIGO stage 3/RIFLE Injury: P = .32. B, Test for trends: KDIGO stage 1: P = .01; RIFLE Risk: P = .05; KDIGO stage 2/RIFLE Injury: P = .34; KDIGO stage 3/RIFLE Injury: P = .34.
Table 2.
Association Between Day 2 Area Under the Curve, Coded as Either a Continuous or Rank Order Ordinal Variable, and Vancomycin-Associated Acute Kidney Injury in Multivariate Regression Analyses
| Classification and Variable | Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | ||
| KDIGO stage 1 | |||||||
| AUCa | Continuous | 1.001 | 1.000–1.002 | .05 | 1.001 | 1.000–1.003 | .04 |
| AUC categoryb | <400 | Reference | Reference | ||||
| 400–600 | 1.59 | 1.09–2.33 | .02 | 1.81 | 1.18–2.78 | .007 | |
| >600 | 2.44 | 1.18–5.40 | .02 | 3.20 | 1.38–7.69 | .007 | |
| RIFLE Risk | |||||||
| AUCc | Continuous | 1.001 | 1.000–1.003 | .05 | 1.002 | 1.000–1.003 | .02 |
| AUC categoryd | <400 | Reference | Reference | ||||
| 400–600 | 1.42 | .94–2.12 | .10 | 1.70 | 1.07–2.71 | .03 | |
| >600 | 2.00 | .88–4.57 | .10 | 2.87 | 1.13–7.29 | .03 | |
| KDIGO stage 2/RIFLE Injury | |||||||
| AUCe | Continuous | 1.002 | 1.000–1.003 | .03 | 1.002 | 1.000–1.003 | .02 |
| AUC categoryf | <400 | Reference | Reference | ||||
| 400–600 | 1.23 | .73–2.05 | .43 | 1.47 | .79–2.72 | .22 | |
| >600 | 1.51 | .54–4.21 | .43 | 2.16 | .63–7.40 | .22 | |
| KDIGO stage 3/RIFLE Failure | |||||||
| AUCg | Continuous | 1.003 | 1.001–1.005 | .006 | 1.002 | 1.000–1.005 | .03 |
| AUC categoryh | <400 | Reference | Reference | ||||
| 400–600 | 1.46 | .69–3.13 | .34 | 1.42 | .65–3.09 | .38 | |
| >600 | 2.14 | .47–9.79 | .34 | 1.91 | .42–9.18 | .38 | |
Abbreviations: AUC, area under the curve; CI, confidence interval; KDIGO, Kidney Disease: Improving Global Outcomes; OR, odds ratio; RIFLE, modified Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease.
Adjusted for age, quinolone use during the first 7 days of vancomycin therapy, prior receipt of antibiotics, prior receipt of vancomycin, preexisting valvular heart disease, presence of decubitus ulcers, and urinary tract source.
Adjusted for age, quinolone use during the first 7 days of vancomycin therapy, prior receipt of antibiotics, prior receipt of vancomycin, preexisting valular heart disease, presence of decubitus ulcers, and urinary tract source.
Adjusted for age, recent residence in healthcare facility, presence of heart failure, quinolone use during the first 7 days of vancomycin therapy, recent cerebral vascular accident, intravenous catheter source, race (White vs non-White), and presence of chronic obstructive pulmonary disease.
Adjusted for age, recent residence in healthcare facility, presence of heart failure, quinolone use during the first 7 days of vancomycin therapy, abdominal source, intravenous catheter source, race (White vs non-White), recent cerebral vascular accident, and presence of chronic obstructive pulmonary disease.
Adjusted for age, weight, sex, recent residence in healthcare facility, skin and soft tissue source, intravenous catheter source, receipt of immunosuppressive drugs, creatinine clearance at baseline, and quinolone use during the first 7 days of vancomycin therapy.
Adjusted for age, weight, sex, skin and soft tissue source, intravenous catheter source, receipt of immunosuppressive drugs, creatinine clearance at baseline, and quinolone use during the first 7 days of vancomycin therapy.
Adjusted for quinolone use during the first 7 days of vancomycin therapy.
Adjusted for quinolone use during the first 7 days of vancomycin therapy.
DISCUSSION
Among patients with MRSA bacteremia, we found that the odds of VA-AKI outcomes increased as a function of the daily day 2 AUC and several VA-AKI outcomes were significantly more pronounced in patients with daily day 2 AUC values within the recommended therapeutic range of 400–600. Our findings provide some nuance to prior analyses that identified a single nephrotoxicity threshold at daily AUC in excess of 600–800 [2, 6, 14–18]. Few studies have examined the VA-AKI risk across the AUC continuum as performed in this study. Our findings align with those of Combination Antibiotics for Methicillin Resistant Staphylococcus aureus (CAMERA-2) randomized clinical trial which examined the risk of VA-AKI during the first 7 days of vancomycin therapy among adult patients with MRSA bacteremia who received vancomycin alone or in combination with a β-lactam [3]. In CAMERA-2, the risk of VA-AKI during the first 7 days of therapy was higher among those with daily AUC >400.
Importantly, the lowest effective daily vancomycin AUC is unknown. The recommendation for the lower bound of the therapeutic range of 400 in the revised consensus guidelines is based, in part, on the “Desirability of Outcome Ranking” analysis from PROVIDE [4], which found that patients in the lowest 2 AUC quantiles (AUCs of 93–515) had the best global outcomes. However, it is unclear whether efficacy is maintained at day 2 AUC values <400 as few patients had AUCs below this threshold. Confirmation of the minimum AUC values that still cure the infection would be beneficial from a global outcomes perspective (ie VA-AKI risk reduction within the range that confers maximal efficacy). Until further studies are conducted, this can most reliably be achieved within the current guideline recommendations by targeting AUCs at the lower end of the range (eg, 400–500) for patients with serious MRSA infections as the totality of data indicate that VA-AKI increases along the vancomycin AUC continuum [2–6]. Because of intrapatient variability, targeting the lower range may functionally avoid overshooting and protect against supratherapeutic concentrations (eg, AUC >800).
This study is subject to limitations inherent to the observational study design. Few baseline covariates differed between day 2 AUC groups and few variables were associated with the VA-AKI outcomes, but unmeasured cofounders may have potentially contributed to the observed findings. For example, data on other known nephrotoxins were not collected as part of this study. However, it is likely these variables would have been evenly distributed across AUC groups. Duration of vancomycin is a known predictor of VA-AKI [2, 4, 19, 20], but time-to-event analyses were not performed since the focus was to evaluate the association between day 2 AUC values and VA-AKI. Of note, therapy duration was similar between AUC groups and was not found to confound the observed day 2 AUC VA-AKI findings. Similarly, loading and daily doses were not considered (although they would be reflected in the AUC values) as the focus was to evaluate the association between day 2 AUC values and VA-AKI. This was a study of adult, nonneutropenic, nondialysis patients with a baseline CLCR ≥30 mL/minute and may not be applicable to other populations. We restricted the population to those with a CLCR ≥30 mL/minute to ensure a more homogenous population of patients without severe renal impairment at baseline [7, 8]. Last, there is imprecision in the observed study findings given the limited size of some AUC categories.
In conclusion, this analysis of adult hospitalized patients with MRSA bloodstream infections showed that VA-AKI increased as a function of the day 2 AUC, even within the recommended AUC therapeutic range of 400–600. Although caution should be exercised when applying the findings given the observational nature of the study, clinicians should consider the benefits vs risks of maintaining daily AUC at the higher end of the newly recommended AUC range. There is also a critical need to determine if daily AUC <400 can be maintained in practice without compromising clinical outcomes.
Notes
Acknowledgments. The authors acknowledge the members of the Prospective Observational Evaluation of the Association between Initial Vancomycin Exposure and Failure Rates among Adult Hospitalized Patients with MRSA Bloodstream Infections (PROVIDE) study team for their participation in the study.
Patient consent. The PROVIDE study was conducted with a waiver of informed consent, consistent with the US Code of Federal Regulations Title 45 Part 46d; the institutional review board at each site approved the study.
Data sharing. Researchers interested in accessing the clinical trial data presented herewith are encouraged to submit a research proposal and publication plan. The proposal and plan will be reviewed by the Antibacterial Resistance Leadership Group publications committee and/or appropriate study team members. If approved and upon receipt and approval of a signed data access/use agreement, individual participant data necessary to complete the proposed analysis will be made available. Related documents, including the study protocol, statistical analysis plan, and data dictionary, may also be shared. Access to data will only be granted to researchers who provide a methodologically and scientifically sound proposal. Proposed analyses that are duplicative of ongoing or proposed analyses may not be supported. To submit a proposal, please complete a proposal at https://arlg.org/how-to-apply/protocol-concept. Alternatively, visit dcri.org/data-sharing. There may be costs associated with data sharing that researchers would be expected to cover.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. Research reported herein was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH (award number UM1AI104681). V. F. was supported by a midcareer mentoring award (number K24-AI093969) from the NIH. M. S. was supported in part by the NIAID (award number R21AI149026).
Potential conflicts of interest. T. P. L. has served as a consultant for Allergan and DoseMe; consultant, scientific advisor, and speaker’s bureau for Melinta; consultant and scientific advisor for Motif and Paratek; speaker for Sunovion; and consultant and grant recipient for Merck & Co. M. S. has received personal fees from Achaogen, Paratek, Merck and Co, Nevakar, SuperTransMedical, AbbVie, Bayer, and Takeda; has received research grants from Allecra, Nevakar, SuperTrans Medical, and Merck and Co; has provided legal consulting for Chambless, Higdon, Richardson, Katz & Griggs, LLP and Taylor English Duma, LLP; and holds a patent (US10688195B2). J. J. C. has served as consultant and scientific advisor for Accelerate. H. C. has received stock from Merck and Moderna and served as a Data and Safety Monitoring Committee (DSMC) member for Merck. V. F. has received personal fees from Novartis, Debiopharm, Genentech, Achaogen, Affinium, The Medicines Co, MedImmune, Bayer, Basilea, Affinergy, Janssen, Contrafect, Regeneron, Destiny, Amphliphi Biosciences, Integrated Biotherapeutics, C3J, Armata, Valanbio, Akagera, Aridis, and Roche; has received grants from NIH, MedImmune, Allergan, Pfizer, Advanced Liquid Logics, Theravance, Novartis, Merck, Medical Biosurfaces, Locus, Affinergy, Contrafect, Karius, Genentech, Regeneron, Basilea, and Janssen; and other financial interest: from UpToDate, Valanbio, the Infectious Diseases Society of America, and ArcBio, outside the submitted work. In addition, V. F. has a patent for sepsis diagnostics pending. T. L. H. has been a consultant for Basilea Pharmaceutica (ceftobiprole), Genentech (immunotherapeutic), and Lysovant (lysin), and has received royalties from UpToDate.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2020; 77:835–64. [DOI] [PubMed] [Google Scholar]
- 2. Lodise TP, Patel N, Lomaestro BM, et al. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 2009; 49:507–14. [DOI] [PubMed] [Google Scholar]
- 3. Liu J, Tong SYC, Davis JS, et al. CAMERA2 Study Group. Vancomycin exposure and acute kidney injury outcome: a snapshot from the CAMERA2 study. Open Forum Infect Dis 2020; 7:ofaa538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycin exposure and failure rates among adult hospitalized patients with methicillin-resistant Staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis 2020; 70:1536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Avedissian SN, Pais GM, O’Donnell JN, et al. Twenty-four hour pharmacokinetic relationships for intravenous vancomycin and novel urinary biomarkers of acute kidney injury in a rat model. J Antimicrob Chemother 2019; 74:2326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chavada R, Ghosh N, Sandaradura I, Maley M, Van Hal SJ.. Establishment of an AUC0-24 threshold for nephrotoxicity is a step towards individualized vancomycin dosing for methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2017; 61:e02535-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gounden V, Bhatt H, Jialal I.. Renal function tests. Treasure Island, FL: StatPearls; 2021. [Google Scholar]
- 8. US Food and Drug Administration. Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. 1998.https://www.fda.gov/media/71334/download. Accessed 1 December 2020. [Google Scholar]
- 9. Neely MN, Youn G, Jones B, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother 2014; 58:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D\'Argenio DZ, Schumitzky A, Wang X.. ADAPT 5 user\'s guide: pharmacokinetic/pharmacodynamic systems analysis software. Los Angeles, CA: Biomedical Simulations Resource; 2009. [Google Scholar]
- 11.[No authors]. Section 2: AKI definition. Kidney Int Suppl (2011) 2012; 2:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bellomo R, Ronco C, Kellum JA, et al. Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zasowski EJ, Murray KP, Trinh TD, et al. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother 2018; 62:e01684-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suzuki Y, Kawasaki K, Sato Y, et al. Is peak concentration needed in therapeutic drug monitoring of vancomycin? A pharmacokinetic-pharmacodynamic analysis in patients with methicillin-resistant Staphylococcus aureus pneumonia. Chemotherapy 2012; 58:308–12. [DOI] [PubMed] [Google Scholar]
- 16. Aljefri DM, Avedissian SN, Rhodes NJ, et al. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis 2019; 69:1881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jumah MTB, Vasoo S, Menon SR, De PP, Neely M, Teng CB.. Pharmacokinetic/pharmacodynamic determinants of vancomycin efficacy in enterococcal bacteremia. Antimicrob Agents Chemother 2018; 62:e01602-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsutsuura M, Moriyama H, Kojima N, et al. The monitoring of vancomycin: a systematic review and meta-analyses of area under the concentration-time curve-guided dosing and trough-guided dosing. BMC Infect Dis 2021; 21:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lodise TP, Drusano G.. Vancomycin area under the curve-guided dosing and monitoring for adult and pediatric patients with suspected or documented serious methicillin-resistant Staphylococcus aureus infections: putting the safety of our patients first. Clin Infect Dis 2021; 72:1497–501. [DOI] [PubMed] [Google Scholar]
- 20. van Hal SJ, Paterson DL, Lodise TP.. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 2013; 57:734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]