Abstract
Background:
No risk-stratification strategies exist for patients with recurrent oropharyngeal cancer (OPC).
Methods:
Retrospective analysis using data from prospective NRG Oncology clinical trials RTOG 0129 and 0522. Eligibility criteria included known p16 status and smoking history, and locoregional/distant recurrence. Overall survival (OS) was measured from date of recurrence. Recursive partitioning analysis was performed to produce mutually exclusive risk groups.
Results:
Hundred and fifty-four patients were included with median follow-up after recurrence of 3.9 years (range 0.04–9.0). The most important factors influencing survival were p16 status and type of recurrence, followed by surgical salvage and smoking history (≤20 vs. >20 pack-years). Three significantly different risk groups were identified. Patients in the low-, intermediate-, and high-risk groups had 2-year OS after recurrence of 81.1% (95%CI 68.5–93.7), 50.2% (95%CI 36.0–64.5), and 20.8% (95%CI 10.5–31.1), respectively.
Conclusion:
Patient and tumor characteristics may be used to stratify patients into risk groups at the time of OPC recurrence.
Keywords: human papillomavirus, Radiation Therapy Oncology Group, recurrent oropharyngeal cancer, recursive partitioning analysis, survival
1 |. INTRODUCTION
The incidence of oropharyngeal cancer (OPC) is rising in the United States, which is driven by human papillomavirus (HPV)-mediated cancers.1–5 Despite the improved prognosis associated with positive tumor HPV status,6,7 disease recurrence affects up to a quarter of patients with HPV-mediated OPC within 3 years of primary treatment completion.7,8 Existing data demonstrate that HPV-positivity and surgical salvage are each independently associated with improved survival for recurrent OPC6,9; however, this survival benefit may not be shared among all patients with recurrent HPV-positive disease or patients submitted to salvage surgery. Other data show that patients with distant recurrence have worse survival than those with locoregional recurrence, though there is also variability within this subset.9,10
There are no formal risk stratification strategies that may be employed at the time of recurrence beyond clinical assessment of resectability. Therefore, we defined risk groups for survival for patients with recurrent OPC who were previously treated with primary radiation with concurrent chemotherapy, using the patient populations from NRG Oncology RTOG 0129 and 0522.
2 |. METHODS
2.1 |. Protocol and treatment
This was a retrospective study combining data from prospective NRG Oncology clinical trials RTOG 0129 and 0522.7,8 RTOG 0129 was a phase III trial comparing standard fractionation radiotherapy to accelerated fractionation radiotherapy, each with concurrent cisplatin, among patients with advanced head and neck cancer. Standard fractionation radiotherapy (SFX) consisted of 70 Gy in 35 fractions (2 Gy per fraction) over 7 weeks and accelerated fractionation by concomitant boost radiotherapy (AFX-C) consisted of 72 Gy delivered in 42 fractions over 6 weeks, which included twice-a-day irradiation for 12 treatment days; cisplatin was given at a dose of 100 mg/m2 of body-surface area on Days 1 and 22 in the AFX-C group and on Days 1, 22, and 43 in SFX group.7 RTOG 0522 was a phase III trial assessing the addition of cetuximab to radiation therapy with concurrent cisplatin for patients with advanced head and neck cancer.8 Radiotherapy consisted of intensity-modulated radiotherapy of 70 Gy in 35 fractions (2 Gy per fraction) over 6 weeks with 6 fractions/week; chemotherapy consisted of intravenous cisplatin (100 mg/m2 of body surface area on Days 1 and 22) with or without cetuximab (intravenous 400 mg/m2 the week before radiotherapy, then 250 mg/m2 weekly during radiotherapy).8 Eligibility criteria for both RTOG 0129 and 0522 included: stage III-IV disease (except patients with T1N1 disease; AJCC 5th edition staging was used for RTOG 0129 and AJCC 6th edition for RTOG 0522),11,12 Zubrod performance status 0–1, age ≥18 years, and adequate hematopoietic, hepatic, and renal function.
The patient’s history of cigarette smoking in pack-years was taken at study enrollment by standardized questionnaire. Patients were followed by clinical exams with or without imaging studies quarterly for 2 years, biannually through year five, and then annually.
Patients eligible for this analysis had OPC with evaluable p16 expression status (a surrogate marker for HPV tumor status), available cigarette smoking history in pack-years, and documented local, regional, or distant disease recurrence.
2.2 |. Laboratory analysis
HPV status was inferred using tumor p16 expression. Immunohistochemistry was performed using a mouse monoclonal antibody (MTM Laboratories, Heidelberg, Germany) and the Ventana XT autostainer using the 1-view secondary detection kit (Ventana).16 Tumor p16-status was categorized positive if strong and diffuse nuclear and cytoplasmic staining was present in ≥70% of tumor cells.16
2.3 |. Statistical analysis
Survival after recurrence was defined as death due to any cause and measured from date of first recurrence. Recursive partitioning analysis (RPA) was performed with STREE software13 including age, sex, race, Zubrod score, anemia, pack-years, T category, N category, p16, cisplatin cycles, salvage surgery, and type of recurrence; patients with simultaneous locoregional and distant recurrence were considered to have had distant recurrence. The RPA produces mutually exclusive combinations of patient and tumor characteristics called terminal nodes. Terminal nodes with similar 2-year post-recurrence survival rates were combined into groups of statistically significantly different risk. The investigators’ clinical judgment was used to modify the RPA-derived cutoffs for the continuous variable pack-years. Survival rates were estimated by the Kaplan–Meier method and risk groups were pairwise compared (low- vs. intermediate-risk and intermediate- vs. high-risk) by two-sided log-rank test.14 Hazard ratios (HR) comparing intermediate- and high-risk groups to low-risk group were estimated using Cox model. Time to recurrence for the final risk groups was measured from randomization. In the low- and intermediate-risk groups, patient characteristics were compared by Kruskal–Wallis test or chi-square test between groups receiving and not receiving salvage surgery and the probability of salvage surgery was estimated by logistic regression.
3 |. RESULTS
Out of 547 patients with OPC enrolled in RTOG 0129 and 0522 with evaluable p16 tumor status and known tobacco smoking pack-years, there were 154 patients who had documented local-regional or distant recurrence and were included in this analysis (n = 77 from each trial). Characteristics of patients at the time of trial enrollment are summarized in Table 1. Patients were predominantly male (89.6%) and white (87.7%) with median age of 55 years (interquartile range [IQR] 51–61) and median tobacco smoking history of 23.8 pack years (IQR 4.5–45).
TABLE 1.
Patient and tumor characteristics for the study population at the time of enrollment on clinical trial
| RTOG 0129/0522 (n = 154) | |
|---|---|
| Trial | |
| RTOG 0129 | 77 (50.0%) |
| RTOG 0522 | 77 (50.0%) |
| Age at randomization (years) | |
| Median (IQR) | 55 (51–61) |
| Sex | |
| Male | 138 (89.6%) |
| Female | 16 (10.4%) |
| Race | |
| Asian | 1 (0.6%) |
| Black or African American | 17 (11.0%) |
| White | 135 (87.7%) |
| Unknown | 1 (0.6%) |
| Zubrod PS at randomization | |
| 0 | 91 (59.1%) |
| 1 | 63 (40.9%) |
| Hemoglobin at randomization (g/dl) | |
| Median (IQR) | 14.2 (13.1–15.1) |
| Anemic at randomization | |
| No | 105 (68.2%) |
| Yes | 49 (31.8%) |
| Smoking history: pack-years | |
| Median (IQR) | 23.75 (4.5–45) |
| Primary site at randomization | |
| Tonsillar fossa, tonsil | 53 (34.4%) |
| Base of tongue | 76 (49.4%) |
| Other oropharynx | 25 (16.2%) |
| p16 status | |
| p16-negative | 58 (37.7%) |
| p16-positive | 96 (62.3%) |
| T classification at randomization | |
| T2 | 59 (38.3%) |
| T3 | 41 (26.6%) |
| T4 | 54 (35.1%) |
| N classification at randomization | |
| N0 | 6 (3.9%) |
| N1 | 15 (9.7%) |
| N2a | 13 (8.4%) |
| N2b | 53 (34.4%) |
| N2c | 46 (29.9%) |
| N3 | 21 (13.6%) |
| AJCC stage at randomizationa | |
| III | 9 (5.8%) |
| IV | 145 (94.2%) |
| On protocol cisplatin cycles | |
| 0 | 3 (1.9%) |
| 1 | 8 (5.2%) |
| 2 | 117 (76.0%) |
| 3 | 26 (16.9%) |
| Salvage surgery | |
| No | 115 (74.7%) |
| Yes | 39 (25.3%) |
| First recurrence type | |
| Local | 33 (21.4%) |
| Regional | 44 (28.6%) |
| Local and regional | 7 (4.5%) |
| Local and distant | 6 (3.9%) |
| Regional and distant | 2 (1.3%) |
| Distant | 62 (40.3%) |
Abbreviations: AJCC, American Joint Committee on Cancer; IQR, interquartile range; PS, performance status.
5th edition for RTOG 0129 and 6th edition for RTOG 0522.
The median follow-up after randomization for surviving patients was 7.9 years (range 2.6–9.2) in RTOG 0129, 4.5 years (range 0.6–7.0) in RTOG 0522, and 4.8 years (range 0.6–9.2) overall. The median follow-up for surviving patients after recurrence was 6.8 years (range 0.7–9.0) in RTOG 0129, 3.2 years (range 0.04–5.7) in RTOG 0522, and 3.9 years (range 0.04–9.0) overall. Estimated 2-year overall survival after recurrence was 45.8% (95%CI 37.7–53.9); see Figure 1.
FIGURE 1.

Kaplan–Meier estimates of overall survival after recurrence for the entire cohort
We undertook a RPA which identified three groups with significantly different risk for death (Figure 2(A)). p16 status was the factor with greatest influence upon overall survival after recurrence; p16-positive tumors were associated with a 58% reduction in risk of death (HR 0.42, 95%CI 0.28–0.63). Type of recurrence had a similar effect on survival to p16 tumor status, with a 56% reduction in risk of death (HR for locoregional vs. distant recurrence 0.44 [95%CI 0.30–0.66]).
FIGURE 2.
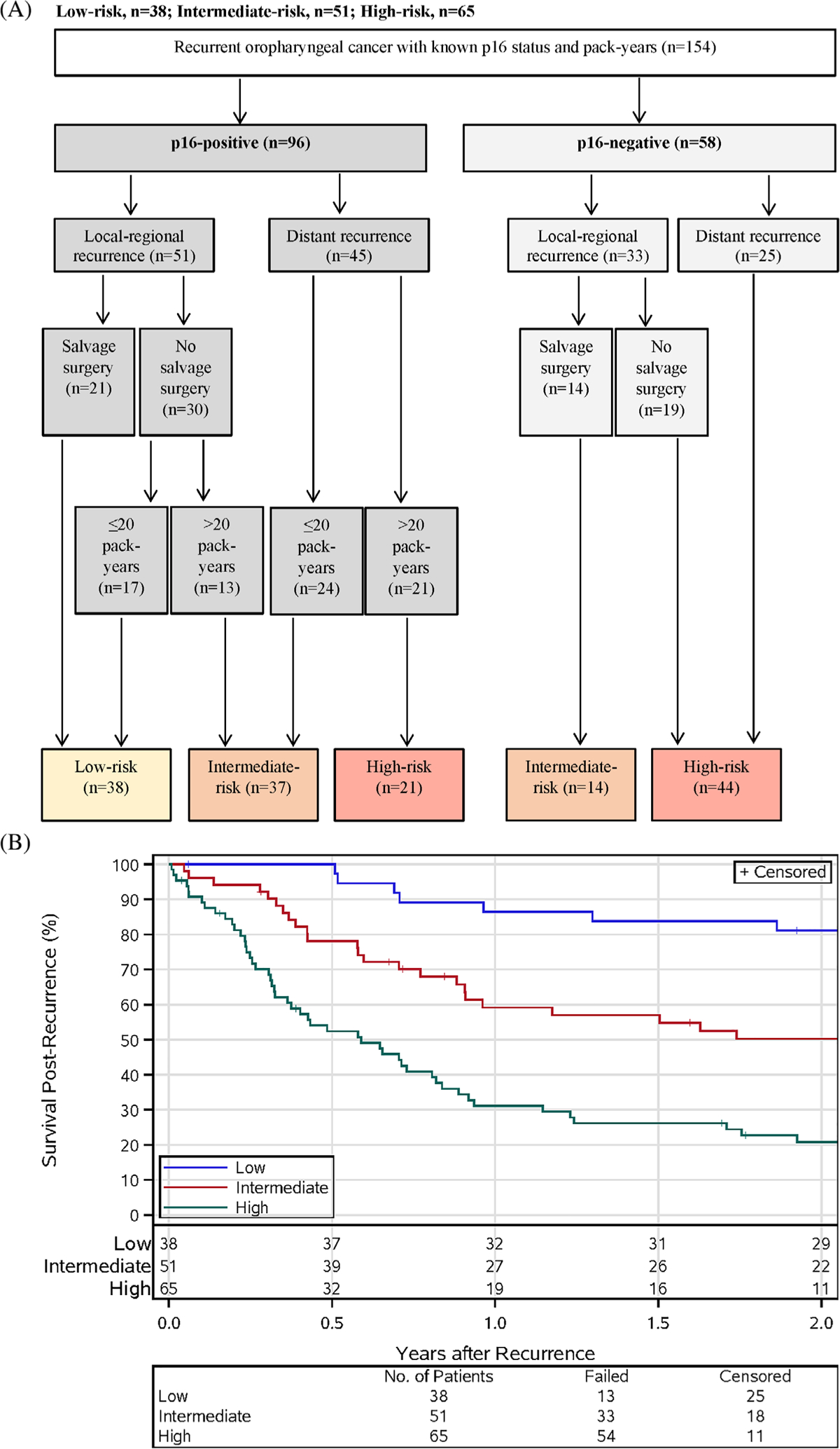
Survival after recurrence by risk group. (A) The risk groups based on post-recurrence survival. Variables included in the final risk groups were p16 tumor status, type of disease recurrence (local-regional vs. distant), receipt of salvage surgery, and smoking history. (B) Kaplan–Meier estimates of survival post-recurrence by risk group
In the RPA (Figure 2(A)), patients with p16-positive disease could be separated into those with low-, intermediate-, or high-risk of death. Patients with p16-positive cancers and local-regional recurrence who underwent salvage surgery were in the low-risk group; those with p16-positive cancers with local-regional recurrence who did not receive salvage surgery could be stratified into either the low- or intermediate-risk groups based upon tobacco smoking pack-year history (≤20 vs. >20 pack-years). Patients with p16-positive cancers with distant recurrence were assigned to either the intermediate- or high-risk groups based on tobacco smoking pack-year history.
Patients with p16-negative cancers who experienced recurrence were categorized by either intermediate- or high-risk of death. Patients with p16-negative cancers with local-regional recurrence were considered intermediate-risk if they underwent salvage surgery but high-risk if they did not. Patients with p16-negative cancers with distant recurrence comprised the high-risk group.
Patients in the low-, intermediate-, and high-risk groups had 2-year survival after recurrence of 81.1% (95% CI 68.5–93.7), 50.2% (95%CI 36.0–64.5), and 20.8% (95% CI 10.5–31.1), respectively (Figure 2(B)). Survival after recurrence for the low- and intermediate-risk groups (p = 0.0009) and the intermediate- and high-risk groups differed significantly (p = 0.0001). The hazard ratios comparing the intermediate- and high-risk groups to the low-risk group are 2.80 (95%CI 1.47–5.33) and 6.90 (95%CI 3.68–12.94). In the low-, intermediate-, and high-risk groups, the median times to recurrence were 6.6 months (IQR 4.5–11.1), 7.2 months (IQR 5.3–18.6), and 9.4 months (IQR 6.6–21.3). All patients in the low-risk group had local-regional recurrence, which typically occurs earlier than distant recurrence.
Patient and tumor characteristics of patients at the time of enrollment on their clinical trial are compared by risk group in Table 2. Consistent with the RPA, the proportion of p16-positive patients was lower (100% vs. 72.5% vs. 32.3%) in the high- and intermediate-risk groups and the proportion of patients with distant recurrence was greater in the higher risk groups (0% vs. 47.1% vs. 70.8%). The percentage who underwent salvage surgery was lower in the higher-risk groups (55.3% vs. 33.3% vs. 1.5%) while median pack-years was higher (5.1 vs. 16 vs. 40). Among characteristics that were not part of the final risk groups, distributions of age, hemoglobin/anemia, and T category differed significantly by risk group. Median age was higher in the intermediate- and high-risk groups relative to the low-risk group (52.5 vs. 56 vs. 58). Median hemoglobin was lower, and percentage with anemia higher, with assignment to higher risk group (14.6/15.8% vs. 14.2/35.3% vs. 14/38.5%). Distributions of T category differed by risk group but not in a consistent pattern.
TABLE 2.
Patient and tumor characteristics at the time of enrollment on clinical trial by risk group
| Low-risk (n = 38) | Intermediate-risk (n = 51) | High-risk (n = 65) | p-value | |
|---|---|---|---|---|
| Trial | 0.7486 | |||
| RTOG 0129 | 17 (44.7%) | 26 (51.0%) | 34 (52.3%) | |
| RTOG 0522 | 21 (55.3%) | 25 (49.0%) | 31 (47.7%) | |
| Age at randomization (years) | 0.0007 | |||
| Median (IQR) | 52.5 (49–55) | 56 (50–63) | 58 (54–63) | |
| Sex | 0.4291 | |||
| Male | 36 (94.7%) | 44 (86.3%) | 58 (89.2%) | |
| Female | 2 (5.3%) | 7 (13.7%) | 7 (10.8%) | |
| Race | 0.2976 | |||
| Asian | 0 (0.0%) | 1 (2.0%) | 0 (0.0%) | |
| Black or African American | 1 (2.6%) | 7 (13.7%) | 9 (13.8%) | |
| White | 36 (94.7%) | 43 (84.3%) | 56 (86.2%) | |
| Unknown | 1 (2.6%) | 0 (0.0%) | 0 (0.0%) | |
| Zubrod PS at randomization | 0.2046 | |||
| 0 | 27 (71.1%) | 27 (52.9%) | 37 (56.9%) | |
| 1 | 11 (28.9%) | 24 (47.1%) | 28 (43.1%) | |
| Hemoglobin at randomization (g/dl) | 0.0459 | |||
| Median (IQR) | 14.6 (14–15.3) | 14.2 (12.9–15) | 14.0 (12.7–14.9) | |
| Anemic at randomization | 0.0472 | |||
| No | 32 (84.2%) | 33 (64.7%) | 40 (61.5%) | |
| Yes | 6 (15.8%) | 18 (35.3%) | 25 (38.5%) | |
| Smoking history: pack-years | <0.0001 | |||
| Median (IQR) | 5.1 (0–18) | 16 (2.6–33) | 40 (27–54.1) | |
| Primary site at randomization | 0.3517 | |||
| Tonsillar fossa, tonsil | 15 (39.5%) | 18 (35.3%) | 20 (30.8%) | |
| Base of tongue | 18 (47.4%) | 28 (54.9%) | 30 (46.2%) | |
| Other oropharynx | 5 (13.2%) | 5 (9.8%) | 15 (23.1) | |
| p16 status | <0.0001 | |||
| p16-negative | 0 (0.0%) | 14 (27.5%) | 44 (67.7%) | |
| p16-positive | 38 (100.0%) | 37 (72.5%) | 21 (32.3%) | |
| T classification at randomization | 0.0363 | |||
| T2 | 18 (47.4%) | 21 (41.2%) | 20 (30.8%) | |
| T3 | 7 (18.4%) | 19 (37.3%) | 15 (23.1%) | |
| T4 | 13 (34.2%) | 11 (21.6%) | 30 (46.2%) | |
| N classification at randomization | 0.6935 | |||
| N0 | 2 (5.3%) | 2 (3.9%) | 2 (3.1%) | |
| N1 | 3 (7.9%) | 5 (9.8%) | 7 (10.8%) | |
| N2a | 2 (5.3%) | 5 (9.8%) | 6 (9.2%) | |
| N2b | 18 (47.4%) | 16 (31.4%) | 19 (29.2%) | |
| N2c | 9 (23.7%) | 16 (31.4%) | 21 (32.3%) | |
| N3 | 4 (10.5%) | 7 (13.7%) | 10 (15.4%) | |
| AJCC stage at randomizationa | 0.5786 | |||
| III | 1 (2.6%) | 4 (7.8%) | 4 (6.2%) | |
| IV | 37 (97.4%) | 47 (92.2%) | 61 (93.8%) | |
| On protocol cisplatin cycles | 0.0677 | |||
| 0 | 1 (2.6%) | 0 (0.0%) | 2 (3.1%) | |
| 1 | 1 (2.6%) | 1 (2.0%) | 6 (9.2%) | |
| 2 | 33 (86.8%) | 41 (80.4%) | 43 (66.2%) | |
| 3 | 3 (7.9%) | 9 (17.6%) | 14 (21.5%) | |
| Salvage surgery | <0.0001 | |||
| No | 17 (44.7%) | 34 (66.7%) | 64 (98.5%) | |
| Yes | 21 (55.3%) | 17 (33.3%) | 1 (1.5%) | |
| First recurrence type | <0.0001 | |||
| Local | 14 (36.8%) | 8 (15.7%) | 11 (16.9%) | |
| Regional | 21 (55.3%) | 18 (35.3%) | 5 (7.7%) | |
| Local and regional | 3 (7.9%) | 1 (2.0%) | 3 (4.6%) | |
| Local and distant | 0 (0.0%) | 2 (3.9%) | 4 (6.2%) | |
| Regional and distant | 0 (0.0%) | 1 (2.0%) | 1 (1.5%) | |
| Distant | 0 (0.0%) | 21 (41.2%) | 41 (63.1%) |
Note: Distributions of age, hemoglobin, and pack-years were compared by risk group by Kruskal–Wallis test; all others were tested by chi-square test; for race, all non-white categories were combined; for N stage, N0–2a were combined; for cisplatin cycles, 0–1 were combined; for first recurrence type, local, regional, and local-regional were combined as were local-distant, regional-distant, and distant.
Abbreviations: AJCC, American Joint Committee on Cancer; IQR, interquartile range; PS, performance status.
5th edition for RTOG 0129 and 6th edition for RTOG 0522.
We performed additional analyses to compare characteristics of patients who did and did not receive salvage surgery in the low- and intermediate-risk groups (Table S1, Supporting Information). The only differences between patients who did and did not receive salvage surgery were p16 status, type of the first recurrence, and time to recurrence. We evaluated factors associated with the likelihood of salvage surgery (Table S2), which indicated that p16-negative status, locoregional recurrence (compared to distant recurrence; adjusted odds ratio [aOR] 5.54, 95%CI 1.37–22.44), and shorter time to recurrence (probability of salvage surgery goes down as time to recurrence goes up in months; aOR 0.88, 95%CI 0.80–0.97) were each associated with a higher probability of salvage surgery. An odds ratio for p16 status was unable to be calculated, since all patients with p16-negative local-regional recurrence in the intermediate-risk group received salvage surgery.
4 |. DISCUSSION
For the first time, we have characterized prognostic risk groups for North American patients with recurrent OPC. Approximately one-quarter of patients with HPV-mediated OPC experience recurrence of disease.7,8 Present treatment strategies for recurrent and metastatic OPC, including surgical salvage, (re-)irradiation, and systemic therapies, each have associated risks. Risk stratification may help clinicians and patients compare and contrast the risks and benefits of treatment of recurrence in an effort to facilitate patient counseling.
Historically, survival after recurrent OPC was poor (estimated 5-year survival after recurrence of 26%),15 with marked improvement observed in contemporary studies (51%),16 likely reflecting the rise in incidence of HPV-mediated tumors. Indeed, HPV-positive tumor status and surgical salvage have each emerged as independent predictors of improved survival after recurrence.9,17–19
These previous findings are clarified by this analysis. While p16-positivity and receipt of surgical salvage remain independent predictors of overall survival at the time of recurrence, it is not true that all patients with p16-positive disease or who undergo resection of their recurrence share the same prognosis. Indeed, heterogeneity in survival is found among patients with p16-positive cancers with recurrent disease and among OPC patients who receive surgical salvage. The elaboration of risk-stratification herein helps to explain variation in survival for patients with recurrent OPC and may provide a framework for their clinical decision making when counseling patients at the time of recurrence.
Low-risk patients are p16-positive with either low cumulative tobacco exposure or who can be salvaged surgically. However, this analysis highlights that patients in intermediate- or high-risk groups, even those with p16-positive disease, have limited 2-year overall survival (20%–50%). Therefore, for these intermediate- or high-risk group patients, including patients with p16-positive disease and distant metastases, the median survival time should be discussed when exploring therapeutic options.
Salvage surgery can have significant functional consequences, and newer treatments such as immunotherapy may pose attractive alternatives with less potential morbidity.20 However, despite the possible consequences and complications inherent to salvage surgery, this RPA emphasizes surgical salvage as an important factor associated with improved survival for both p16-positive and p16-negative patient populations who have local-regional recurrence.
An important consideration in interpreting this RPA regards the limited ability to comment on specific patient resectability. For patients with local-regional recurrence, the extent and location of recurrent disease dictate the ability to offer salvage surgery. In the current analysis, this raises the question of whether resectability alone drove the choice to pursue surgical salvage and, therefore, the differences in survival, or whether there were additional patient- or tumor-specific factors that differed between groups. We are unable to elaborate on specific resectability considerations because our study population comprises patients from a multi-cooperative group, and treatment after recurrence was determined by individual institutions. However, we did seek to compare patient- and tumor-specific variables between groups who did and did not undergo surgical salvage, and analyzed factors associated with the probability of salvage (Tables S1 and S2), similar to previous investigators.21 We found that the overall demographic characteristics were similar between groups, with the exception of p16 status, the pattern of recurrence, and time to recurrence. Salvage surgery was more likely for patients with p16-negative status, those with local-regional recurrence, and a shorter time to recurrence. These findings are consistent with the fact that salvage surgery is generally offered only to patients with local-regional relapse. The remainder of the comparisons between the patients who did and did not receive surgical salvage were similar, suggesting that determination of resectability is likely a driver in the decision to offer salvage surgery.
There are additional limitations to this study. First, the population analyzed was limited to those with documented p16 status and smoking history, which excluded some enrolled patients and may introduce bias. However, survival after recurrence for patients included in analysis did not significantly differ from those excluded (log-rank p = 0.92; see Figure S1). Second, the analytic population was limited to patients enrolled in prospective clinical trials with advanced AJCC 5th or 6th stage III or IV disease treated with primary radiotherapy with concurrent chemotherapy, so may not apply to all patients diagnosed with OPC, such as patients with lower overall disease stage or who received primary surgical treatment. Another notable limitation is that several potentially important factors were not available for analysis, which might have allowed for additional nuances in risk stratification. For example, greater detail about recurrence patterns (such as the presence of oligometastatic disease or details about site of recurrence in relation to previously irradiated tissue volumes) might have provided additional insights. Similarly, more information about salvage treatments beyond the use of surgical salvage—for example, re-irradiation or the use of stereotactic body radiation therapy or surgical resection for oligometastatic disease—may have allowed for additional risk stratification. Also, because clinical characteristics were measured at the time of trial enrollment and not at the time of recurrence, some clinically relevant changes might not have been detected (such as changes in smoking status or Zubrod performance score). Finally, other retrospective studies have identified candidate factors that may negatively affect survival after recurrence, including gastrostomy-tube dependence,22 increasing T or N category at the time of recurrence,22,23 positive surgical margins and alcohol abuse,24 and a disease-free interval of <12 months.25 These factors were not explored in this RPA, but may indeed influence survival.
Nevertheless, this analysis is the first to our knowledge to define risk stratification groups at the time of OPC local-regional or distant metastatic recurrence, using data from two large prospective randomized clinical trials. The risk models proposed here introduce a stratification framework that shows that p16 status and type of disease recurrence are the most important factors affecting prognosis after recurrence, followed by receipt of salvage surgery and tobacco smoking history. This RPA suggests that, in contrast to the historically poor survival portended by recurrent OPC, there is a low-risk group that has 2-year post-recurrence survival of higher than 80%. Future directions should include external validation of the risk models described here in an external cohort, as well as following each risk group forward to assess whether the differences in survival between groups remain durable beyond 2 years.
Supplementary Material
Funding information
National Cancer Institute, Grant/Award Numbers: U10CA180822, U10CA180868, UG1CA189867
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
Data will be made available per the NCTN Data Archiving Rules (https://nctn-data-archive.nci.nih.gov/).
REFERENCES
- 1.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3235–3242. doi: 10.1200/JCO.2015.61.6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. World-wide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550–4559. doi: 10.1200/JCO.2013.50.3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Windon MJ, D’Souza G, Rettig EM, et al. Increasing prevalence of human papillomavirus-positive oropharyngeal cancers among older adults. Cancer. 2018;124(14):2993–2999. doi: 10.1002/cncr.31385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35(5):747–755. doi: 10.1002/hed.22015 [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32(30):3365–3373. doi: 10.1200/JCO.2014.55.1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32(27):2940–2950. doi: 10.1200/JCO.2013.53.5633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo T, Qualliotine JR, Ha PK, et al. Surgical salvage improves overall survival for patients with HPV-positive and HPV-negative recurrent locoregional and distant metastatic oropharyngeal cancer. Cancer. 2015;121(12):1977–1984. doi: 10.1002/cncr.29323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang SH, Perez-Ordonez B, Weinreb I, et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol. 2013;49(1):79–85. doi: 10.1016/j.oraloncology.2012.07.015 [DOI] [PubMed] [Google Scholar]
- 11.Fleming ID. AJCC Cancer Staging Manual. 5th ed. Lippincott-Raven; 1997. [Google Scholar]
- 12.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. Vol 1. 6th ed. American Joint Committee on Cancer; 2002. [Google Scholar]
- 13.STREE. Survival analysis trees. http://c2s2.yale.edu/software/stree
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomeplete observations. J Am Stat Assoc. 1958;53(282):457–481. doi: 10.2307/2281868 [DOI] [Google Scholar]
- 15.Goodwin WJ. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper Aerodigestive tract: When do the ends justify the means? Laryngoscope. 2000;110(S93):1–18. doi: 10.1097/00005537-200003001-00001 [DOI] [PubMed] [Google Scholar]
- 16.Jayaram SC, Muzaffar SJ, Ahmed I, Dhanda J, Paleri V, Mehanna H. Efficacy, outcomes, and complication rates of different surgical and nonsurgical treatment modalities for recurrent/residual oropharyngeal carcinoma: a systematic review and meta-analysis: oropharyngeal cancer treatment survival outcomes. Head Neck. 2016;38(12):1855–1861. doi: 10.1002/hed.24531 [DOI] [PubMed] [Google Scholar]
- 17.Fakhry C, Zhang Q, Nguyen-Tân PF, et al. Development and validation of nomograms predictive of overall and progression-free survival in patients with oropharyngeal cancer. J Clin Oncol. 2017;35(36):4057–4065. doi: 10.1200/JCO.2016.72.0748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermorken JB, Psyrri A, Mesía R, et al. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: retrospective analysis of the phase III EXTREME trial. Ann Oncol. 2014;25(4):801–807. doi: 10.1093/annonc/mdt574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Argiris A, Li S, Ghebremichael M, et al. Prognostic significance of human papillomavirus in recurrent or metastatic head and neck cancer: an analysis of Eastern Cooperative Oncology Group trials. Ann Oncol. 2014;25(7):1410–1416. doi: 10.1093/annonc/mdu167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. doi: 10.1016/j.oraloncology.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gañán L, López M, García J, Esteller E, Quer M, León X. Management of recurrent head and neck cancer: variables related to salvage surgery. Eur Arch Otorhinolaryngol. 2016;273(12): 4417–4424. doi: 10.1007/s00405-016-4093-3 [DOI] [PubMed] [Google Scholar]
- 22.Sweeny L, Rosenthal EL, Clemons L, Stevens TM, Cook McIntosh ER, Carroll WR. Outcomes after surgical salvage for recurrent oropharyngeal squamous cell carcinoma. Oral Oncol. 2016;60:118–124. doi: 10.1016/j.oraloncology.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 23.Bachar GY, Goh C, Goldstein DP, O’Sullivan B, Irish JC. Long-term outcome analysis after surgical salvage for recurrent tonsil carcinoma following radical radiotherapy. Eur Arch Otorhinolaryngol. 2009;267(2):295. doi: 10.1007/s00405-009-1070-0 [DOI] [PubMed] [Google Scholar]
- 24.Nichols AC, Kneuertz PJ, Deschler DG, et al. Surgical salvage of the oropharynx after failure of organ-sparing therapy. Head Neck. 2011;33(4):516–524. doi: 10.1002/hed.21480 [DOI] [PubMed] [Google Scholar]
- 25.Zafereo ME, Hanasono MM, Rosenthal DI, et al. The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer. 2009;115(24):5723–5733. doi: 10.1002/cncr.24595 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available per the NCTN Data Archiving Rules (https://nctn-data-archive.nci.nih.gov/).


