Abstract
Purpose
To identify the defining lung ultrasound (LUS) findings of COVID-19, and establish its association to the initial severity of the disease and prognostic outcomes.
Method
Systematic review was conducted according to the PRISMA guidelines. We queried PubMed, Embase, Web of Science, Cochrane Database and Scopus using the terms ((coronavirus) OR (covid-19) OR (sars AND cov AND 2) OR (2019-nCoV)) AND ((“lung ultrasound”) OR (LUS)), from 31st of December 2019 to 31st of January 2021. PCR-confirmed cases of SARS-CoV-2 infection, obtained from original studies with at least 10 participants 18 years old or older, were included. Risk of bias and applicability was evaluated with QUADAS-2.
Results
We found 1333 articles, from which 66 articles were included, with a pooled population of 4687 patients. The most examined findings were at least 3 B-lines, confluent B-lines, subpleural consolidation, pleural effusion and bilateral or unilateral distribution. B-lines, its confluent presentation and pleural abnormalities are the most frequent findings. LUS score was higher in intensive care unit (ICU) patients and emergency department (ED), and it was associated with a higher risk of developing unfavorable outcomes (death, ICU admission or need for mechanical ventilation). LUS findings and/or the LUS score had a good negative predictive value in the diagnosis of COVID-19 compared to RT-PCR.
Conclusions
The most frequent ultrasound findings of COVID-19 are B-lines and pleural abnormalities. High LUS score is associated with developing unfavorable outcomes. The inclusion of pleural effusion in the LUS score and the standardisation of the imaging protocol in COVID-19 LUS remains to be defined.
Keywords: SARS-CoV-2, COVID-19, Lung, Ultrasound, Systematic review
1. Introduction
A new variant of coronavirus, SARS-CoV-2, has become responsible for the worst global pandemic since the influenza A H1N1 pandemic in 1918 [1]. The first case of human infection was described on December 2019 in the Chinese city of Wuhan [2]. On account of that, SARS-CoV-2 has caused >100 million new cases and 2 million deaths globally from coronavirus 19 disease (COVID-19) until January 31st [3]. Its effect on society as well as the measures taken by governments to try to contain its spread, have pushed the economy into its worst recession since World War II, with an estimated contraction of 5.2% of the World Gross Domestic Product by 2020 [4].
The impact of these statistics has forced researchers around the world into an accelerated search for the natural history of the disease to develop possible tools for the management of the disease. The diagnosis of COVID-19 and the establishment of prognostic markers have become to major focus, in order to determine which patients require admission and to adapt therapeutic measures accordingly. Real-time reverse transcriptase polymerase chain reaction (RT-PCR) and antibody serology have become the reference methods in the diagnosis of infection, but do not provide information on disease severity and prognosis [5].
In this context, thoracic imaging has proven to be a useful diagnostic tool. Although thoracic computed tomography (CT) has been the most studied technique, there is evidence that lung ultrasound (LUS) may be an effective alternative for diagnosing the disease [6]. This imaging modality is quick, cost-effective, and does not require ionising radiation. In addition, it can be repeated as many times as necessary to monitor disease progression, and be performed at the patient's bedside (point-of-care ultrasound, POCUS) [7]. However, this evidence remains weak, with no up-to-date systematic review to support its use.
At present, there is an increasing literature on the LUS use in COVID, both in the diagnosis and prognosis of the disease. However, the available information is dispersed, without a detailed compilation of COVID-associated findings and their association with initial disease severity and prognostic outcomes. The purpose of this article is to identify the defining LUS findings of COVID-19, and to establish its association to the initial severity of the disease and prognostic outcomes.
2. Material and methods
2.1. Search strategy
This study was designed according to the 2015 PRISMA (Preferred Reporting Items for Systematic review and Meta-Analysis) guidelines [8] and has been registered in the PROSPERO database under the number CRD42021237210.
The information used in this article was extracted from the electronic databases of PubMed, Embase, Web of Science, Cochrane Database of Systematic Reviews and Scopus. A combination of MeSH and associated terms (COVID-19, ultrasonography) with other methodological terms (lung, LUS) were used in the search, resulting in the terms ((coronavirus) OR (covid-19) OR (sars AND cov AND 2) OR (2019-nCoV)) AND ((“lung ultrasound”) OR (LUS)). This has been enriched by the possibility to analyse the bibliography of relevant studies to add additional publications.
2.2. Articles selection
The studies included span the time frame from 31st of December 2019 to 31st of January 2021. We established as inclusion criteria confirmed cases of SARS-CoV-2 infection in humans by SARS-CoV-2 RT-PCR and/or serology or antigen test. Participants were adults aged 18 years or older, with no other limitations on the included population. As exclusion criteria, we established the following: only original studies conducted in a significant patient sample of at least 10 participants with the characteristics described above were included; studies without a significant sample (e.g. case report), non-original studies (e.g. reviews) and secondary research (narrative review, systematic review and meta-analysis) were excluded. No other limitations by article type, language or publication status (preprint, peer-reviewed or already published) was settled.
2.3. Extraction of data
For data processing, the reference and document management tool Mendeley® and the calculation spreadsheet programme Microsoft Excel® were used. Two independent operators (J.G. and E.G.), were involved in the search, selection and inclusion, with no communication of results between them during the process. In a first search, we screened by title and abstract of the article, and in a second phase according to the full text. Discrepancies between the two researchers were resolved by a third researcher (A.B.).
Data were collected in a data template common to both investigators. The variables collected from the selected studies were title, authors, date, type of study, total number of participants and number of PCR-confirmed COVID-19 cases, characteristics of the included patients (age, sex, BMI, clinical severity, associated comorbidity or any other selection criteria), setting (hospital, primary care, emergency department), time of LUS acquisition, presence or absence of blinding evaluation of LUS images, transducer used, number of fields scanned, ultrasound findings (pulmonary B lines, pleural thickening, pleural irregularity, subpleural consolidation, pulmonary consolidation, pleural effusion, lung ultrasound score (LUS score)) and LUS performance in diagnosing COVID-19 cases and predicting clinical outcomes.
The LUS score is a severity score used in COVID-19 pneumonia, assigning a range of 0–3 to each of the lung fields analysed. Soldati et al. [9] made a standardisation proposal establishing the findings associated with each point, being 0 normal, 1 presence of 3 or more B-lines or pleural irregularity, 2 confluence of B-lines or subpleural consolidation and 3 pulmonary consolidation. Only articles that met this definition were pooled in the study, in order to make their results comparable. For the purpose of comparison, in the cases where studies did not scan the 12 lung fields (dividing each hemithorax into 3 by the midclavicular anterior and posterior line and subdividing each of these fields into upper and lower fields) as proposed by Soldati et al. [9], but instead chose to analyse a smaller or greater number of lung fields, an adapted LUS score was calculated. Specifically, the LUS score of each individual study was multiplied by 12/number of lung areas scanned, so as to obtain the equivalent LUSS if 12 anatomical zones had been scanned.
Clinical outcomes were often varied among different studies, so we collected the following: in-hospital death (or death after a certain follow-up time), need for mechanical intubation, intensive care unit (ICU) admission, development of acute respiratory distress syndrome (ARDS). During the selection phase, in case of overlapping population samples, the most recent study was chosen.
We assessed the quality of the studies included with the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool [10]. Definitions and judgment criteria for each domain were established according the guidelines of the Cochrane Collaboration's “Cochrane Handbook of Systematic Reviews of Interventions” [11]. Two independent reviewers (P.L. and J.P.) evaluated the risk of bias in the included studies by means of a tailored QUADAS-2 tool. Disagreements were discussed and resolved with a senior reviewer (E.G.). Specifically, high risk in each of the 4 domains assessed in QUADAS-2 was defined as follows. In domain 1 (patient selection), high risk of bias was assigned if study design was case–control or if enrolment was non-consecutive; and high risk regarding applicability if the setting of the study or the severity of COVID-19 disease was not clear in all patients of the study. Domain 2 (index test) was established as high risk of bias if LUS images were interpreted without blinding to SARS-CoV-2 PCR, other imaging techniques or clinical data, depending on the study approach, or if a threshold was used that was not pre-specified; and high risk regarding applicability if the LUS acquisition and analysis method was not clear or was not the same for all patients. Domain 3 (reference standard) high risk of bias was determinated if the reference standard was unlikely to correctly classify COVID-19 patients; and high risk regarding applicability if no alterations were made to the definition of a positive SARS-CoV-2 PCR (in terms of cycle threshold or otherwise). Lastly, domain 4 (flow and timing) was settled as high risk of bias if LUS acquisition was not performed within 5 days of the reference standard.
3. Results
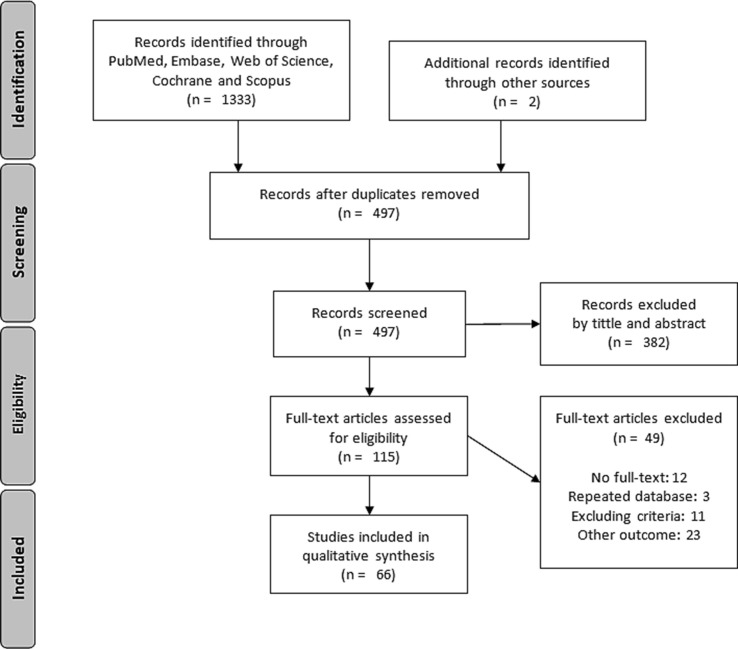
According to the established search criteria, we found 1333 articles in the 6 databases described above, to which 2 more articles were added from the bibliography of these studies. 835 duplicate articles were removed. Of the remaining 497 articles, 381 were excluded based on title and abstract information. Finally, of the 115 full-text articles assessed for eligibility, 66 articles were included in our qualitative synthesis and 49 were excluded (Fig. 1 ).
Fig. 1.
Article selection flow diagram according to PRISMA guidelines [8].
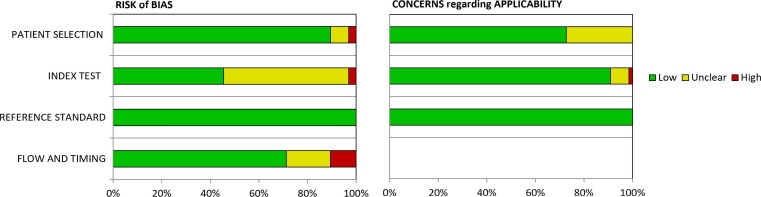
Quality of included studies, as evaluated by QUADAS-2, is shown in Fig. 2 and Appendix 1. Risk of bias was high in 2 articles ([12], [13]) for patient selection, in 2 articles for index test ([14], [15]) and in 7 articles for flow and timing ([16], [17], [18], [19], [20], [21], [22]). There were high applicability concerns only in one study, specifically in the index test domain ([23]). All studies were considered as low-risk in the reference standard domain, since all of them used SARS-CoV-2 RT-PCR as gold standard. None of the articles included were classified as high risk in two or more categories of the 4 domains.
Fig. 2.
Quality of included studies as established in the QUADAS-2 tool [10].
These 66 studies are distributed as follows: 41 were conducted in Europe, 18 in Asia, 5 in North America and 2 in South America. Among them, 25 (37.9%) were prospective cohorts, 24 (36.4%) retrospective cohorts, 2 (3.0%) retrospective case-control studies and 2 (3.0%) cross-sectional studies; while 13 (19.7%) of them did not make it explicit (although it seems clear that these were also observational studies). According to the area of acquisition, 22 (33.3%) were conducted in the emergency department (ED), 13 (19.7%) in hospitalisation (ICU and ward), 12 (18.2%) in hospitalisation ward, 11 (16.7%) in ICU, 3 (4.5%) in pregnancy hospitalisation ward, 2 (3.0%) in nursing homes, 1 (1.5%) in rehabilitation unit, 1 in ED and ICU and 1 in screening tents. Most images were taken within 24 h of admission (40 articles, 60.6%). The most commonly used protocol consisted in scanning 12 lung areas (35, 53.0%), scanning <12 areas in 16 studies (24.2%) and >12 in 8 studies (12.1%), unspecified in the remaining 7 studies (10.6%). The convex probe was the main probe most commonly used (41 articles, 62.1%), followed by the linear probe (7, 10.6%) and the phased array probe (4, 6.1%); in 14 articles it was not detailed (21.2%). Only 34 (51.5%) articles established a protocol for blinded ultrasound evaluators. The data of the included studies are detailed in Table 1 and Appendix 2.
Table 1.
Characteristics of included articles.
| Author and publication date | Country and study design | COVID-19 cases and clinical setting | Time of LUS acquisition | Blinding |
Age (mean or median, years) |
Male cases (%) |
BMI (mean or median, kg/m2) |
Scanned regions, main probe and frequency (Hz) |
|---|---|---|---|---|---|---|---|---|
|
Lu[24], 15/04/2020 |
China, Retrospective cohort |
30, Wards |
Within 24 h | Yes, NA | 52 | 53 | 22.5 | 12, Convex, 5–2 |
|
Yasukawa[25], 24/04/2020 |
United States, Retrospective cohort |
10, Wards |
Within 24 h | No | 53 | 70 | NA | NA, Phased array |
|
Xing[16], 28/04/2020 |
China, NA |
20, Hospital |
After a median time of >5 days | No | NA | 60 | NA | 10, NA |
|
Tan[12], 05/06/2020 |
China, Retrospective case–control |
12, ED |
Within 24 h | No | 61 | 33 | NA | 10, Convex, 3.5–5 |
|
Bar[13], 10/06/2020 |
France, Retrospective case–control |
31, ED |
Within 24 h | Yes, PCR | 67 | 35 | 30.0 | 6, Convex |
|
Pare[26], 19/06/2020 |
United States, Retrospective cohort |
27, ED |
Within 24 h | Yes, PCR | 53 | 59 | 31.7 | NA, Convex |
|
Nouvenne[27], 22/06/2020 |
Italy, NA |
26, Wards |
Within 24 h | Yes, CT | 64 | 54 | NA | 8, Convex, 3.5–5 |
|
Yassa[28], 30/06/2020 |
Turkey, Prospective cohort |
43, Pregnancy ward |
Following a routine fetal assessment | Yes, PCR and clinical data | NA | 0 | NA | 14, Convex, 1–8 |
|
Møller-Sørensen[29], 02/07/2020 |
Denmark, NA |
10, ICU |
Within 24 h | Yes, NA | 53 | 60 | NA | 6, Linear, 12 |
|
Ye[17], 09/07/2020 |
China, Retrospective cohort |
23, Wards |
NA | Yes, Clinical data | 60 | 52 | NA | 12, Convex, 1–5.5 |
|
Deng[30], 14/07/2020 |
China, Retrospective cohort |
128, Hospital |
Within 24 h | Yes, Clinical data | 65 | 59 | NA | 8, Convex, 3.5–5 |
|
Bonadia[31], 15/07/2020 |
Italy, Prospective cohort |
41, ED |
Within 24 h | No | 60 | 78 | NA | 14, Convex, 6 |
|
Dargent[32], 21/07/2020 |
France, NA |
10, ICU |
Within 24 h | No | 56 | 80 | 32.0 | 12, NA |
|
Zhang[18], 22/07/2020 |
China, Retrospective cohort |
28, Hospital |
After a median time of >5 days | No | 60 | 50 | NA | NA, Convex, 1–5 |
|
Yassa[33], 28/07/2020 |
Turkey, Prospective cohort |
23, Pregnancy ward |
Within 24 h | No | NA | 0 | NA | 14, Convex, 1–8 |
|
Veronese[34], 29/07/2020 |
Italy, NA |
48, Nursing home |
Within 5 days | No | 84 | 19 | NA | 12, NA |
|
Zieleskiewicz[35], 29/07/2020 |
France, Retrospective cohort |
100, ED and ICU |
Within 24 h | No | 61 | 65 | NA | 12, NA |
|
Favot[36], 31/07/2020 |
United States, Retrospective cohort |
40, ED |
Within 24 h | Yes, NA | 69 | 60 | 31.0 | 8, Convex |
|
Gaspardone[19], 08/08/2020 |
Italy, Prospective cohort |
70, Rehabilitation unit |
At discharge | No | 68 | 69 | 25.6 | 12, Convex |
|
Ottaviani[37], 12/08/2020 |
France, Prospective cohort |
21, Wards |
NA | Yes, CT and clinical data | 65 | 76 | NA | 12, Convex, 5–18 |
|
Alharthy[38], 14/08/2020 |
Saudi Arabia, Prospective cohort |
89, ICU |
Within 24 h | No | 43 | 84 | 26.5 | 12, Phased array, 2–4 |
|
Thomaz[39], 21/08/2020 |
Brazil, Cross-sectional |
409, Screening tents |
NA | No | 41 | 33 | NA | 12, Linear, 7.5–10 |
|
Lichter[40], 28/08/2020 |
Israel, Retrospective cohort |
120, Hospital |
Within 24 h | Yes, NA | 65 | 62 | NA | 12, Phased array |
|
Iodice[41], 01/09/2020 |
Italy, Prospective cohort |
29, Hospital |
Within 24 h | No. Yes, CT | 60 | 90 | NA | NA, Convex, 3.5–5 |
|
Dini[42], 02/09/2020 |
Italy, NA |
94, Nursing home |
NA | No | NA | NA | NA | 12, Convex, 3.5 |
|
Gil[43], 04/09/2020 |
Spain, Cross-sectional |
27, ED |
Within 24 h | No | 48 | 33 | NA | 13, Convex, 2–5 |
|
Battista[44], 07/09/2020 |
Italy, Prospective cohort |
44, ED |
Within 24 h | Yes, PCR | 66 | 66 | NA | 12, Convex, 2.5–5 |
|
Narinx[45], 10/09/2020 |
Belgium, NA |
15, ED |
Within 24 h | Yes, PCR | 50 | 60 | NA | 6, Convex, 5 |
|
Kalafat[46], 11/09/2020 |
Turkey, Retrospective cohort |
82, Pregnancy ward |
NA | Yes, PCR | 28 | 0 | 27.6 | 6, NA |
|
Li[47], 15/09/2020 |
China, Retrospective cohort |
91, Hospital |
NA | No | 59 | 68 | NA | 12, Linear, 4–12 |
|
Castelao[48], 16/09/2020 |
Spain, Prospective cohort |
63, Wards |
Within 5 days | Yes, Chest radiography and clinical data, but not PCR status | 61 | 68 | NA | 12, Convex, 2–5 |
|
Cocconcelli[20], 16/09/2020 |
Italy, Retrospective cohort |
102, Hospital |
After a median time of >5 days | No | 68 | 74 | 25.0 | 12, Convex, 1–8 |
|
Brahier[49], 17/09/2020 |
Switzerland, Prospective cohort |
80, ED |
Within 24 h | Yes, Clinical data | 62 | 58 | NA | 10, NA |
|
Ramos-Hernández[50], 21/09/2020 |
Spain, Prospective cohort |
44, Wards |
Within 5 days | No | 69 | 68 | NA | 8, Convex |
|
Zhu[51], 21/09/2020 |
China, Prospective cohort |
27, Hospital |
Within 5 days | No | 63 | 59 | NA | 10, Convex, 1.5–5 |
|
Rojatti[52], 25/09/2020 |
Italy, Retrospective cohort |
41, ICU |
Within 24 h | No | 62 | 78 | 26.7 | 8, Convex |
|
Marggrander[23], 01/10/2020 |
Germany, Retrospective cohort |
17, Hospital |
NA | No | 51 | 65 | NA | NA, Convex, 3–5 |
|
Bosso[14], 03/10/2020 |
Italy, NA |
26, ED |
NA | Yes, PCR | 66 | 69 | NA | 12, Linear |
|
Colombi[53], 08/10/2020 |
Italy, Retrospective cohort |
341, ED |
Within 24 h | No | NA | NA | NA | 12, Convex, 1–8 |
|
Haaksma[54], 08/10/2020 |
Netherlands, NA |
61, ICU |
NA | Yes, Clinical data | 66 | 90 | 28.5 | 12, Linear, 5–10 |
|
Pivetta[55], 12/10/2020 |
Italy, Prospective cohort |
107, ED |
Within 24 h | Yes, PCR | 63 | 55 | NA | 12, Convex, 3–5 |
|
Lieveld[56], 15/10/2020 |
Netherlands, Prospective cohort |
86, ED |
Within 24 h | Yes, PCR and CT | 63 | 58 | NA | 12, NA |
|
Zhu[21], 16/10/2020 |
China, Retrospective cohort |
48, Hospital |
After a median time of >5 days | Yes, CT and clinical data | 63 | 54 | NA | 12, Convex, 3.5–5 |
|
Bosevski[57], 22/10/2020 |
Macedonia, NA |
17, ICU |
NA | No | 57 | NA | NA | NA |
|
Sorlini[58], 22/10/2020 |
Italy, Retrospective cohort |
287, ED |
Within 24 h | No | NA | NA | NA | 12, NA |
|
Jalil[59], 26/10/2020 |
United States, Retrospective cohort | 36, Hospital |
Within 24 h | Yes, PCR, clinical data and chest radiography | 63 | 53 | 30.5 | 8, NA |
| Gibbons[60], 29/10/2020 | United States, Prospective cohort |
83, ED |
Within 24 h | No | 56 | 43 | NA | 8, NA |
|
Li[61], 03/11/2020 |
China, NA |
42, ICU |
Within 24 h | No | 68 | 43 | NA | NA, Convex, 5 |
|
Zotzmann[62], 03/11/2020 |
Germany, Retrospective cohort |
20, ICU |
NA | Yes, CT | 62 | 70 | 28.3 | 8, Convex, 5–1 |
|
Perrone[63], 06/11/2020 |
Italy, NA |
52, Wards |
Within 24 h | Yes, Clinical data | 64 | 54 | 25.3 | 14, Convex, 3.5–5 |
|
Alharthy[64], 13/11/2020 |
Saudi Arabia, Prospective cohort |
171, ICU |
Within 24 h | No | 47 | 79 | 26.4 | 12, Phased array, 2–4 |
|
Haak[65], 18/11/2020 |
Netherlands, Prospective cohort |
24, ED |
Within 24 h | Yes, PCR and clinical data | NA | NA | NA | 12, Convex, 2–6 |
|
Allegorico[66], 01/12/2020 |
Italy, Retrospective cohort |
42, ED |
Within 24 h | No | 69 | 69 | NA | 12, NA |
|
Recinella[67], 03/12/2020 |
Italy, NA |
37, Wards |
Within 24 h | No | 82 | 51 | 23.7 | 12, Linear, 5–8 |
|
Schmid[68], 07/12/2020 |
Germany, Retrospective cohort |
39, ED |
Within 24 h | Yes, PCR, clinical data and CT | 61 | 56 | 26.3 | 12, NA |
|
Zanforlin[69], 07/12/2020 |
Italy, Retrospective cohort |
46, ED |
Within 24 h | No | 60 | 57 | NA | 20, Convex, 5–2 |
|
Wangüemert Pérez[70], 17/12/2020 |
Spain, Retrospective cohort |
45, Wards |
Within 24 h | No | 82 | 44 | NA | 12, Convex, 3–5 |
|
Ji[22], 22/12/2020 |
China, Prospective cohort |
280, Hospital |
After a median time of >5 days | Yes, Clinical data | 55 | 50 | 23.1 | 12, Convex, 1–6 |
|
Speidel[15], 25/12/2020 |
Switzerland, Prospective cohort |
11, Wards |
Within 24 h | Yes, PCR and clinical data | 76 | 73 | NA | 12 Convex, 4 |
|
Ibrahim[71], 04/01/2021 |
Kuwait, Prospective cohort |
77, ICU |
Within 24 h | Yes, PCR | 53 | 83 | NA | 12, Convex, 3.5 |
|
Bock[72], 07/01/2021 |
Denmark, Prospective cohort |
12, ED |
Within 24 h | Yes, PCR and chest radiography but not to clinical data | 68 | 58 | NA | 14, Convex, 1–5 |
|
Garcia de Alencar[73], 11/01/2021 |
Brazil, Prospective cohort |
180, ED |
Within 24 h | Yes, CT and clinical data | 60 | 58 | NA | 12, Convex, 2–5 |
|
Boero[74], 24/01/2021 |
Italy, Retrospective cohort |
274, ED |
Within 5 days | No | NA | NA | NA | 12, Convex, 2–9 |
|
Avdeev[75], 25/01/2021 |
Russia, Prospective cohort |
22, Wards |
NA | Yes, Clinical data | 49 | 73 | 28.7 | 14, NA |
|
Heldeweg[76], 25/01/2021 |
Netherlands, Prospective cohort |
34, ICU |
NA | Yes, CT | 63 | 88 | 28.2 | 12, Linear, 10–5 |
|
Seiler[77], 26/01/2021 |
Sweden, Prospective cohort |
72, Hospital |
Within 5 days | No | 65 | 60 | 28.0 | 12, Convex, 2–6 |
*BMI: Body Mass Index; ED: Emergency Department; ICU: Intensive Care Unit; NA: Not Available.
The pooled population studied included a total of 4687 patients with confirmed COVID-19. Among them, 43.7% were male (data not available in 22.1%), the mean age was 58 years and the mean body mass index (BMI) was 26.2 kg/m2. 64.6% were hospitalised (30.4% in ward or intermediate care unit, 17.4% in ICU and 3.2% in pregnancy ward) and 15.5% were followed up outside the hospital (11.0% at home, 3.0% in nursing home and 1.5% in rehabilitation units). In 19.9% of patients it was not specified (ED patients that were either admitted to hospital or followed at home) (Appendix 3).
The ultrasound findings in the included articles are summarised in Table 2 and detailed in Appendix 4. The findings examined in the largest number of patients were at least 3 B-lines (1828, 39.27%) and confluent B-lines (1753, 37.40%), subpleural consolidation (1400, 29.87%), pleural effusion (2055, 43.84%) and bilateral or unilateral distribution (1615, 34.46%); while the least investigated were separated B-lines (386, 8.24%), white lung (118, 2.52%), fragmented pleural line (341, 7.28%), air bronchogram (228, 4.86%), and pneumothorax (492, 10.50%). According to these studies, among the findings studied in more patients, the most frequent findings in patients with COVID-19 were the presence of B lines (91%), in particular in ICU patients (99%) as well as their confluent presentation (80%), whereas at least 3 B-lines was more common in the ED (83%); followed by pleural abnormalities (84%). However, pleural abnormalities were even more common in ward patients (100%), but with a significant presence in ICU particularly irregular pleural lines (88% and 81% respectively). Consolidations, indicative of more severe disease, were less frequent in all studies (43%) and in ED (38%), and more common in ward and ICU (77%); as were subpleural (30% and 42% vs. 60% and 43% respectively) but not pulmonary consolidations (33% and 0% vs. 36% and 0%). Pleural effusion was an infrequent finding (14%), although slightly more frequent in ward (20%) and ICU (26%) patients, and the distribution of abnormal LUS findings was eminently bilateral (59% overall) in ED, ward and ICU (83%, 92% and 79% respectively).
Table 2.
Lung ultrasound findings in COVID-19.
|
All studied patients* N/n (%) |
Emergency department N/n (%) |
Wards N/n (%) |
Intensive care unit N/n (%) |
|
|---|---|---|---|---|
| B-lines | ||||
| Any | 867/952 (91) | 172/178 (97) | 29/31 (94) | 118/119 (99) |
| At least 3 | 1158/1828 (63) | 349/421 (83) | 104/137 (76) | 58/79 (73) |
| Separated | 236/386 (61) | 22/40 (55) | 5/30 (17) | 174/260 (67) |
| Confluent | 713/1753 (41) | 88/132 (67) | 32/56 (57) | 223/280 (80) |
| White lung | 35/118 (30) | 10/12 (83) | 20/77 (26) | NA |
| Pleural abnormalities | ||||
| Any | 394/468 (84) | 21/27 (78) | 37/37 (100) | 184/208 (88) |
| Pleural thickening | 327/574 (57) | 136/175 (78) | 39/121 (32) | 29/39 (74) |
| Irregular pleural lines | 293/623 (47) | 36/95 (38) | 10/10 (100) | 83/102 (81) |
| Fragmented pleural line | 79/341 (23) | 15/24 (63) | 33/37 (89) | NA |
| Consolidations | ||||
| Any | 304/707 (43) | 161/423 (38) | 65/84 (77) | 40/52 (77) |
| Subpleural | 424/1400 (30) | 33/79 (42) | 70/117 (60) | 42/109 (39) |
| Pulmonary | 221/669 (33) | 0/12 (0) | 37/103 (36) | 0/17 (0) |
| Other | ||||
| Pleural effusion | 288/2055 (14) | 56/481 (12) | 57/285 (20) | 92/349 (26) |
| Air bronchogram | 27/228 (12) | 17/44 (39) | 4/56 (7) | NA |
| Pneumothorax | 30/492 (6) | 0/12 (0) | 1/30 (3) | 7/131 (5) |
| Distribution | ||||
| Bilateral | 956/1615 (59) | 376/451 (83) | 143/156 (92) | 70/89 (79) |
| Unilateral | 291/1615 (18) | 37/451 (8) | 10/156 (6) | 19/89 (21) |
Including pregnancy wards, nursing homes, rehabilitation units and patients followed at home.
The adapted LUS score (assuming 12 anatomical zones were scanned, as described above) was higher in ICU patients (22.52) and in the ED (15.10) than in the ward (13.98). Even so, these three hospital services had higher scores than the total number of patients including non-hospital patients (11.27). The included studies also describe the utility of the LUS findings and/or the LUS score in the diagnosis of COVID-19, using RT-PCR as the gold standard. This indicator has a remarkable sensitivity (from 89.48% in all patients to 97.4% in ICU patients) with a moderate specificity (from 62.55% in the ED to 76.32% in the ward, except in ICU with 90.63%). The resulting positive predictive value is also moderate (from 52.63% in ward to 75.66% in overall patients, excluding ICU with 96.15%), while the negative predictive value is considerable (from 84.9% in ED to 93.55% in ICU) (Table 3 and Appendix 5).
Table 3.
LUS findings and diagnostic performance.
|
All studied patients* (n = 2543) |
Emergency department (n = 580) |
Wards (n = 342) |
Intensive care unit (n = 179) |
|
|---|---|---|---|---|
| LUS score (mean) | 11.27 | 15.10 | 13.98 | 22.52 |
|
All studied patients* (n = 2894) |
Emergency Department (n = 2169) |
Wards (n = 49) |
Intensive care unit (n = 109) |
|
|
Sensitivity % (95% CI) |
89.48 (87.80–91.00) |
90.83 (89.05–92.41) |
90.91 (58.72–99.77) |
97.40 (90.93–99.68) |
|
Specificity % (95% CI) |
70.16 (67.71–72.53) |
62.55 (59.44–65.59) |
76.32 (59.76–88.56) |
90.62 (74.98–98.02) |
| Positive Predictive Value % (95% CI) | 75.66 (74.13–77.13) |
74.64 (73.04–76.17) |
52.63 (37.87–66.95) |
96.15 (89.48–98.66) |
| Negative Predictive Value % (95% CI) | 86.55 (84.67–88.23) |
84.90 (82.37–87.13) |
96.67 (81.61–99.48) |
93.55 (78.61–98.28) |
Including pregnancy wards, nursing homes, rehabilitation units and patients followed at home.
A total 16 of the 66 included articles studied the role of LUS score in the prediction of relevant clinical outcomes. Recinella et al. [67] found a hazard ratio (HR) of death for total LUSS12 (LUS score for 12 quadrants) in univariate analysis of 1.168 (95% CI 1.049–1.301). Lichter et al. [40] also calculated the unadjusted HR of death for total LUSS12, which was equal to 1.08 (95% CI 1.02–1.16). Moreover, Garcia de Alencar et al. [74] found an odds ratio (OR) of death for total LUSS12 of 1.13 (95% CI 1.07–1.21), and adjustment by age did not change results and Wangüemert Pérez et al. [71] found an OR of death for total LUSS12 of 1.57 (95% CI 1.10 –2.23) adjusted by sex and age-adjusted Charlson index. All four of these results are similar and statistically significant. Another way of presenting these results is by showing the mean LUS score in patients who died and those who survived. Rojatti et al. [52] shows that LUSS8 was 13.9 ± 2.8 in non-survivors and 10.5 ± 3.6 in survivors (p-value = 0.029), Bosso et al. [14] shows that LUSS12 was 20.9 ± 6.5 in non-survivors and 15.6 ± 4.5 in survivors (p-value < 0.01), and finally Garcia de Alencar et al. [74] shows that LUSS12 was 21.6 ± 4.9 in non-survivors and 16.7 ± 4.9 in survivors (p-value < 0.001). Rojatti et al. results are surprisingly similar to those of Bosso, if we multiply the LUSS8 presented in Rojatti et al. by 12/8 to obtain what the equivalent LUSS12 might have been, we obtain a mean LUSS12 for non-survivors of 20.85 and 15.75 for survivors. Likewise, Bonadia et al. [31] a nLUSS (normalized LUSS) median of 1.43 (IQR: 1.31–1.69) in patients who died and of 1 (IQR: 0.27–1.4) in patients who were discharged. If we multiply this result by 12, to obtain its equivalent LUSS12, we obtain 17.16 in dead patients and 12.00 in patients who were discharged.
Another common reported clinical outcome was the need for invasive mechanical ventilation (IMV) or need for non-invasive respiratory support (NIRS). Lichter et al. [40] reported a HR of mechanical intubation for total LUSS12 in univariate analysis of 1.2 (95% CI 1.1–1.3), and Garcia de Alencar et al. [74] found an OR of 1.17 (95% CI 1.09–1.26). Ji et al. [22] found a HR of ARDS development for total LUSS12 of 1.049 (95% CI 1.023–1.076); adjusted for age, lymphocytes count and comorbidity. Meanwhile, Castelao et al. [48] found the mean LUSS12 in patients with NIRS 23.5 ± 5.3 and of 13.0 ± 7.2 in patients without NIRS (p-value < 0.001). García de Alencar et al. [74] found the mean LUSS12 in IMV patients to be 21.3 ± 4.9 and in not-IMV patients to be 15.2 ± 7.1 (p-value < 0.001) and in Seiler et al. [78], the LUSS12 was equal to 20.0 in IMV patients and 12.0 in non-IMV patients (p-value < 0.0001). Seiler et al. also found that a LUSS cut-off point of 19.5 had an area under the curve (AUC) of 0.80 (95% CI 0.70–0.90), sensitivity 57% (95% CI 34–77), specificity 82% (95% CI 68–91) for prediction of need of IMV.
Finally, there are some articles, like Perrone et al. [63], which group unfavourable outcomes into the same category, in this case: high-flow oxygen support, ICU admission, death. For these 3 grouped events, they find an adjusted HR of 1.17 (95% CI 1.05–1.29) for total LUSS14; adjusted for comorbidities (>2), age (>65 years), sex (male), and body mass index (≥25 kg/m2). Boero et al. [75] considers need for IMV, ICU admission, and death as unfavourable outcomes and finds a relative risk (RR) of unfavourable outcome for LUSS12 > 15 of 2.05 (95% CI 1.52–2.77).
According to index test risk of bias, among the studies assessing the diagnostic accuracy of LUS (21 in total), 13 had “low” risk of bias and 8 had “unclear” or “high” risk in one of the QUADAS-2 domains. To assess whether the risk of bias could alter the results of this review, for each domain of QUADAS-2 we compared the diagnostic accuracy of LUS in studies that had “low” risk of bias and studies that had “high” or “unclear” risk of bias. As can be seen in Table 4 , sensitivity and specificity results were similar (p value < 0.05 only found in one comparaison, where studies with “low” risk of bias in time and flow domain showed higher sensitivity for COVID-19 diagnosis).
Table 4.
LUS diagnostic performance according to the QUADAS-2 risk of bias.
|
Low risk n (%) |
High or unclear risk n (%) |
p value | ||
|---|---|---|---|---|
| Patient selection | Sensitivity | 20 (89.3) | 1 (96.8) | 0.180 |
| Specificity | 20 (70.1) | 1 (62.3) | 0.144 | |
| Index test | Sensitivity | 13 (87.3) | 8 (90.9) | 0.064 |
| Specificity | 13 (71.0) | 8 (69.3) | 0.051 | |
| Time and Flow | Sensitivity | 19 (90.5) | 2 (77.5) | 0.001* |
| Specificity | 19 (70.2) | 2 (69.0) | 0.803 | |
p < 0.05.
With regard to the comparison between CT scores and LUSS, 5 of the included articles describe the Pearson's r correlation between them. These articles were Nouvenne [27] (r = 0.650), Deng [30] (0.891), Ottaviani [37] (0.935), Zhu [21] (0.820) and Heldeweg [77] (0.795). All these values correspond to moderate to high correlations and they also proved to be statistically significant, with all p values < 0.01. As for studies that simultaneously evaluate LUS and CT diagnostic accuracy, in most of them LUS has higher sensitivity but lower specificity. Sensitivity and specificity between both of them were described in Battista [44] (CT 93.0% and 90.0% vs. LUS 68.0% and 79.0%), Narinx [45] (CT 80.0% and 86.7% vs. LUS 93.3% and 21.3%) and Lieveld [56] (CT 90.0–95.0% and 43.0–69.0% vs. LUS 93.0–94.0% and 7.0–31.0%) respectively.
4. Discussion
Lung ultrasound is a developing technique, not yet as widely implemented as other thoracic imaging modalities (computed tomography, X-ray or even echocardiography [78]) but increasingly used. In particular, its use to study pulmonary involvement in COVID-19 has increasingly risen. Recently, other systematic reviews have been published in an attempt to synthesise the available information and establish stronger evidence in this field [79], [80], [81], [82]. However, these studies have only partially described lung ultrasound findings, focusing on the most common findings without exploring the frequency of occurrence of other phenomena. The association of each of these findings with the clinical patient profile and the area of acquisition is yet to be described.
The data collected shows a predominance of B-lines, pleural alterations and bilateral distribution in COVID-19, consistent with previous studies that describe SARS-CoV-2 pneumonia as bilateral, peripheral and patchy lung involvement with posterior and inferior predominance [83], [84]. Findings of confluent B-lines, irregularity and pleural thickening are more frequent in ICU patients, which corresponds to higher LUS scores. However, subpleural and pleural consolidations are more frequent on ward. Although subpleural consolidation may reasonably be more represented on the ward, as it is indicative of moderate-severe infection, the higher proportion of pulmonary consolidation relative to ICU is likely to be a bias in the selected articles, due to a variable description of this finding. White lung is possibly over-represented in the ED given the low sample of patients in which it was sought. Pleural effusion is an infrequent finding but is more common in ward and ICU patients, suggesting that its presence may be associated with more severe disease (as the most severe patients are admitted to these two departments and as described in other studies [85]) or with complications of the disease and its management (such as superinfection, prolonged ICU stay or need for mechanical ventilation). However, this finding is not included in the LUS score.
LUS score has classically been the prognostic index used during the COVID-19 pandemic, developed by Soldati [9] and in line with previous work by Rouby [86] and Soummer [87]. However, the included studies have also employed other protocols such as the original Bedside Lung Ultrasound in Emergency (BLUE) protocol [88], the modified lung ultrasound (MLUS) scoring system [12] or adapted LUS scores at the criteria of each author [29], [33], [63]. It is clear that the LUS score is higher in the ICU, although the fact that it is higher in the ED than in the ward may be due to the possibility that ED patients may be referred to one or the other location, or be discharged. In all of the 16 articles that study the role of LUS score in the prediction of relevant clinical outcomes, the data shows that a higher value of baseline LUS scores is associated with a higher risk of developing unfavorable outcomes, such as death, ICU admission or need for mechanical ventilation. A good correlation between CT results and LUSS is also observed, as well as a higher sensitivity but lower specificity.
It seems that LUS is most reliable to rule out severe lung involvement, given its high sensitivity (89.5%) and negative predictive value (86.6%). It must be considered that this negative predictive value was calculated for an average prevalence of 50.9% of COVID-19 confirmed cases in the pooled population of studies that assessed the validity of LUS as a diagnostic tool in suspected cases, using PCR tests for SARS-CoV-2 as the gold standard. This should not suppose a problem, however, given that the lower expected prevalence of COVID-19 cases in the future will only increase the negative predictive value of LUS. As LUS can reasonably rule out COVID-19 in hospitalised patients, it could enable early detection of non-infected patients after an outbreak on the ward, for instance. Nonetheless, it is also true that LUS specificity (70.2%) and positive predictive value (75.7%) might be too low for standardized clinical practice. Furthermore, if COVID-19 prevalence falls below 25% in suspected cases, the positive predictive value will be <50%. Therefore, LUS could allow early management of a patient with indeterminate radiographic findings or a high clinical suspicion of a false negative RT-PCR, but this statement might only hold true in the pandemic context, when the prevalence of COVID-19 was high.
It is worth mentioning the great variability in the way these data are acquired and presented. Unfortunately, the lack of a standard in the type of probe used, the number of lung fields to be analysed and the reference severity index, have not made possible the quantitative synthesis of these studies in a meta-analysis of prognostic variables as intended, making it necessary to use narrative synthesis. Given the heterogeneity in measurement of the LUS score, the selection of the prognostic factors studied and the statistical method applied to calculate the final results, we found no way to even group the prognostic results in a table.
Although it is important to note that, the 7 studies that assessed the role of LUS score in predicting mortality all found a positive association, which was significant when statistical inference tests where applied. Similarly, the 4 studies that investigated the role of LUS score in predicting need for invasive ventilation or need for non-invasive respiratory support, all found a significant association. These results support the utility of LUS not only in early detection of pulmonary involvement in the suspicion of COVID-19, but also in assessing the risk of complications. Therefore, it could potentially be a useful tool in bedside monitoring of disease severity in hospitalised patients.
Some of the potential limitations of this work are those inherent to review studies, with the quality of a review being equal to the studies it includes. In this case, only 25 of the included studies (37.9%) were prospective, and the rest may incur biases such as selection bias. Also the low sample of patients in which some of the findings are described (e.g. separated B-lines, white lung, fragmented pleural line, air bronchogram or pneumothorax) and the absence of an acquisition standard may lead to information bias.
Among the strengths of the study, the fact that the results collected include a wide variety of care areas and up to 4687 patients, showing a more reliable picture of the different manifestations of COVID-19 (a disease characterised by its high clinical variability) may be highlighted [89]. These different areas have been analysed separately, allowing to identify the ultrasound findings reported in each of them (not only the most frequent ones) and the performance of the LUS score in these care environments. The time of acquisition and the existence or not of blinding in each of these studies has also been made explicit.
This study also raises some questions for future work and proposes some recommendations based on the results obtained. Other studies should homogenise the LUS study protocol in COVID: define the type of probe to be used, establish the 12 lung fields as the study standard as suggested [90], contrast the LUS score proposed by Soldati et al. with other scoring systems and confirm its advantage over them. Also, it should come towards an agreement on further studies about the best cut-off points for LUS to categorise the severity of patients according to the severity of initial lung involvement of the disease and expected prognosis; and define the prognostic value of pleural effusion in COVID. Furthermore, the utility of performing a radiological test after lung ultrasound in those patients in which lung ultrasound is less effective (particularly to rule out false positives in phases of the pandemic with low prevalence of COVID-19 infection), and other diagnosis are suspected that require a more detailed anatomical studied should also be studied. We propose the use of the variable death at 30 days and the combined variable poor prognosis (non-invasive mechanical ventilation, invasive mechanical ventilation, ICU admission and death at 30 days) as main prognostic variables.
5. Conclusions
The most frequent ultrasound findings of COVID-19 are the presence of B-lines and pleural abnormalities. LUS score is associated with ICU admission, need for mechanical ventilation and death. The inclusion of pleural effusion in the LUS score and the standardisation of the imaging protocol in COVID-19 LUS remains to be defined.
CRediT authorship contribution statement
Jaime Gil-Rodríguez: Conceptualization, Methodology, Investigation, Writing – original draft, Supervision, Project administration. Javier Pérez de Rojas: Conceptualization, Methodology, Formal analysis, Investigation, Writing – review & editing. Pablo Aranda-Laserna: Investigation, Formal analysis, Writing – review & editing, Visualization. Alberto Benavente-Fernández: Conceptualization, Validation, Writing – review & editing. Michel Martos-Ruiz: Writing – review & editing, Visualization. José-Antonio Peregrina-Rivas: . Emilio Guirao-Arrabal: Conceptualization, Methodology, Investigation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
To Alejandra Ciria García, for her assistance editing the text.
Appendix 1. Quality of included studies as established in the four domains described in the QUADAS-2 tool
| Author and publication date |
Risk of bias |
Concerns regarding applicability |
|||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
|
Lu, 15/04/2020 |
Low | Low | Low | Low | Low | Low | Low |
|
Yasukawa, 24/04/2020 |
Low | Unclear | Low | Low | Low | Low | Low |
|
Xing, 28/04/2020 |
Unclear | Unclear | Low | High | Low | Low | Low |
|
Tan, 05/06/2020 |
High | Unclear | Low | Low | Unclear | Low | Low |
|
Bar, 10/06/2020 |
High | Low | Low | Low | Unclear | Low | Low |
|
Pare, 19/06/2020 |
Low | Low | Low | Low | Low | Unclear | Low |
|
Nouvenne, 22/06/2020 |
Low | Low | Low | Low | Low | Low | Low |
|
Yassa, 30/06/2020 |
Low | Low | Low | Low | Low | Low | Low |
|
Møller-Sørensen, 02/07/2020 |
Unclear | Low | Low | Low | Low | Low | Low |
|
Ye, 09/07/2020 |
Low | Low | Low | High | Low | Low | Low |
|
Deng, 14/07/2020 |
Low | Low | Low | Low | Low | Low | Low |
|
Bonadia, 15/07/2020 |
Low | Unclear | Low | Low | Low | Low | Low |
|
Dargent, 21/07/2020 |
Unclear | Unclear | Low | Low | Low | Low | Low |
|
Zhang, 22/07/2020 |
Low | Unclear | Low | High | Unclear | Unclear | Low |
|
Yassa, 28/07/2020 |
Low | Unclear | Low | Low | Low | Low | Low |
|
Veronese, 29/07/2020 |
Low | Unclear | Low | Low | Low | Low | Low |
|
Zieleskiewicz, 29/07/2020 |
Low | Unclear | Low | Low | Unclear | Low | Low |
|
Favot, 31/07/2020 |
Low | Low | Low | Low | Low | Low | Low |
|
Gaspardone, 08/08/2020 |
Low | Unclear | Low | High | Low | Low | Low |
|
Ottaviani, 12/08/2020 |
Low | Low | Low | Unclear | Low | Low | Low |
|
Alharthy, 14/08/2020 |
Low | Unclear | Low | Low | Low | Low | Low |
|
Thomaz, 21/08/2020 |
Low | Unclear | Low | Unclear | Low | Low | Low |
|
Lichter, 28/08/2020 |
Low | Low | Low | Low | Unclear | Low | Low |
|
Iodice, 01/09/2020 |
Low | Low | Low | Low | Unclear | Unclear | Low |
|
Dini, 02/09/2020 |
Low | Unclear | Low | Unclear | Low | Low | Low |
|
Gil, 04/09/2020 |
Low | Unclear | Low | Low | Unclear | Low | Low |
|
Battista, 07/09/2020 |
Low | Low | Low | Low | Low | Low | Low |
|
Narinx, 10/09/2020 |
Low | Low | Low | Low | Unclear | Low | Low |
|
Kalafat, 11/09/2020 |
Low | Low | Low | Unclear | Low | Low | Low |
|
Li, 15/09/2020 |
Low | Unclear | Low | Unclear | Unclear | Low | Low |
|
Castelao, 16/09/2020 |
Low | Low | Low | Low | Low | Low | Low |
|
Cocconcelli, 16/09/2020 |
Low | Unclear | Low | High | Low | Low | Low |
|
Brahier, 17/09/2020 |
Low | Low | Low | Low | Low | Low | Low |
|
Ramos-Hernández, 21/09/2020 |
Unclear | Unclear | Low | Low | Low | Low | Low |
|
Zhu, 21/09/2020 |
Low | Unclear | Low | Low | Unclear | Low | Low |
|
Rojatti, 25/09/2020 |
Low | Unclear | Low | Low | Low | Low | Low |
|
Marggrander, 01/10/2020 |
Low | Unclear | Low | Unclear | Unclear | High | Low |
|
Bosso, 03/10/2020 |
Low | High | Low | Unclear | Low | Low | Low |
|
Colombi, 08/10/2020 |
Low | Unclear | Low | Low | Unclear | Low | Low |
|
Haaksma, 08/10/2020 |
Low | Low | Low | Unclear | Low | Low | Low |
|
Pivetta, 12/10/2020 |
Low | Low | Low | Low | Low | Low | Low |
|
Lieveld, 15/10/2020 |
Low | Low | Low | Low | Low | Low | Low |
|
Zhu, 16/10/2020 |
Low | Low | Low | High | Low | Low | Low |
|
Bosevski, 22/10/2020 |
Unclear | Unclear | Low | Unclear | Low | Unclear | Low |
|
Sorlini, 22/10/2020 |
Low | Unclear | Low | Low | Unclear | Low | Low |
|
Jalil, 26/10/2020 |
Low | Low | Low | Low | Low | Low | Low |
| Gibbons, 29/10/2020 | Low | Unclear | Low | Low | Unclear | Low | Low |
|
Li, 03/11/2020 |
Low | Unclear | Low | Low | Low | Unclear | Low |
|
Zotzmann, 03/11/2020 |
Low | Low | Low | Unclear | Low | Low | Low |
|
Perrone, 06/11/2020 |
Low | Unclear | Low | Low | Low | Low | Low |
|
Alharthy, 13/11/2020 |
Low | Unclear | Low | Low | Low | Low | Low |
|
Haak, 18/11/2020 |
Low | Low | Low | Low | Low | Low | Low |
|
Allegorico, 01/12/2020 |
Low | Unclear | Low | Low | Unclear | Low | Low |
|
Recinella, 03/12/2020 |
Low | Unclear | Low | Low | Low | Low | Low |
|
Schmid, 07/12/2020 |
Low | Low | Low | Low | Low | Low | Low |
|
Zanforlin, 07/12/2020 |
Low | Unclear | Low | Low | Unclear | Low | Low |
|
Wangüemert Pérez, 17/12/2020 |
Low | Unclear | Low | Low | Low | Low | Low |
|
Ji, 22/12/2020 |
Low | Low | Low | High | Low | Low | Low |
|
Speidel, 25/12/2020 |
Low | High | Low | Low | Low | Low | Low |
|
Ibrahim, 04/01/2021 |
Low | Low | Low | Low | Low | Low | Low |
|
Bock, 07/01/2021 |
Low | Low | Low | Low | Low | Low | Low |
|
Garcia de Alencar, 11/01/2021 |
Low | Low | Low | Low | Unclear | Low | Low |
|
Boero, 24/01/2021 |
Low | Unclear | Low | Low | Low | Low | Low |
|
Avdeev, 25/01/2021 |
Low | Unclear | Low | Unclear | Low | Low | Low |
|
Heldeweg, 25/01/2021 |
Low | Low | Low | Unclear | Low | Low | Low |
|
Seiler, 26/01/2021 |
Low | Unclear | Low | Low | Unclear | Low | Low |
Appendix 2. Characteristics of included articles
| Characteristics | Number of studies (%) |
|---|---|
| Continent | |
| Europe | 41 (62.1) |
| Asia | 18 (27.3) |
| North America | 5 (7.6) |
| South America | 2 (3.0) |
| Study design | |
| Prospective cohort | 25 (37.9) |
| Retrospective cohort | 24 (36.4) |
| Retrospective case–control | 2 (3.0) |
| Cross-sectional | 2 (3.0) |
| Not specified | 13 (19.7) |
| Clinical setting where LUS was performed | |
| ED | 22 (33.3) |
| Hospital (wards and ICU) | 13 (19.7) |
| Wards | 12 (18.2) |
| ICU | 11 (16.7) |
| ED and ICU | 1 (1.5) |
| Pregnancy wards | 3 (4.5) |
| Home | 1 (1.5) |
| Nursing home | 2 (3.0) |
| Rehabilitation unit | 1 (1.5) |
| Number of COVID-19 patients included | |
| 10–29 | 23 (34.8) |
| 30–99 | 31 (47.0) |
| 100–500 | 12 (18.2) |
| Time of LUS acquisition | |
| Withing 24 h of admission | 40 (60.6) |
| Withing 5 days of admission | 6 (9.1) |
| After a median time of >5 days | 5 (7.6) |
| At discharge | 1 (1.5) |
| Following fetal assessment | 1 (1.5) |
| Not specified | 13 (19.7) |
| Blinded operators | |
| Yes | 34 (51.5) |
| No | 32 (48.5) |
| Type of blinding | |
| To clinical data | 16 (47.1) |
| To PCR status | 15 (44.1) |
| To CT scan or chest radiography | 12 (35.3) |
| Not specified | 4 (11.8) |
| Chest anatomical areas scanned | |
| <12 | 16 (24.2) |
| 12 | 35 (53.0) |
| >12 | 8 (12.1) |
| Not specified | 7 (10.6) |
| Main type of ultrasound probe used | |
| Convex | 41 (62.1) |
| Linear | 7 (10.6) |
| Phased array | 4 (6.1) |
| Not specified | 14 (21.2) |
*ED: Emergency Department, ICU: Intensive Care Unit specified
Appendix 3. Pooled characteristics of COVID-19 confirmed patients
| Characteristics | Results |
|---|---|
| COVID-19 confirmed cases (n) | 4687 |
| Age (mean years; n) | 58 (3581) |
| Number of studies according to mean/median age (N, %) | |
| ≥25 and <50 years | 7 (10.4) |
| ≥50 and <70 years | 47 (70.1) |
| ≥70 and <90 years | 4 (6.0) |
| NA | 8 (11.9) |
| Sex (N, %) | |
| Male | 2048 (43.7) |
| Female | 1602 (34.2) |
| NA | 1037 (22.1) |
| BMI (mean kg/m2; n) | 26.2 (1346) |
| Hospitalised (N, %) | 3028 (64.6) |
| In wards or intermediate care unit | 1423 (30.4) |
| In ICU | 816 (17.4) |
| Not specified (in wards or ICU) | 641 (13.7) |
| In pregnancy wards | 148 (3.2) |
| Not hospitalised (N, %) | 727 (15.5) |
| Followed at home | 515 (11.0) |
| In nursing home | 142 (3.0) |
| In rehabilitation unit | 70 (1.5) |
| Not specified (ED patients that were either admitted to hospital or followed at home) (N, %) | 932 (19.9) |
*N: Number of articles, n: Number of patients, NA: Not available.
Appendix 4. Detailed data of lung ultrasound findings from the included studies.
| Study | COVID-19 cases (N) | B-lines (%) | At least 3 B-lines (%) | Separated B-lines (%) | Confluent B-lines (%) | White lung (%) | Pleural abnormalities (%) | Pleural thickening (%) | Irregular pleural lines (%) | Fragmented pleural line (%) | Consolidation (%) | Subpleural consolidation (%) | Pulmonary consolidation (%) | Pleural effusion (%) | Air bronchogram (%) | Pneumothorax (%) | Bilateral (%) | Unilateral (%) | LUS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lu | 30 | 90 | 17 | 50 | 10 | 10 | 20 | 3 | 7 | 3 | 73 | 17 | 10.6 | ||||||
| Yasukawa | 10 | 100 | 50 | 100 | 100 | 50 | 10 | 0 | |||||||||||
| 20 | 100 | 55 | 90 | 100 | 50 | 10 | 100 | 0 | |||||||||||
| Tan | 12 | 100 | 33 | 83 | 100 | 75 | 50 | 42 | 0 | 8 | 0 | ||||||||
| Pare | 27 | 89 | 85 | 78 | 78 | 37 | |||||||||||||
| Nouvenne | 26 | 27 | 65 | 65 | 50 | 4 | 8 | 100 | 0 | 15.0 | |||||||||
| Møller-Sørensen | 10 | 100 | 100 | 100 | 50 | ||||||||||||||
| Ye | 23 | 17 | 6.8 | ||||||||||||||||
| Deng | 128 | 100 | 66 | 95 | 50 | 9 | 5 | 2 | 12.6 | ||||||||||
| Bonadia | 41 | 13.4 | |||||||||||||||||
| Dargent | 10 | 22.0 | |||||||||||||||||
| Zhang | 28 | 100 | 61 | 68 | 4 | ||||||||||||||
| Veronese | 48 | 3.0 | |||||||||||||||||
| Zieleskiewicz | 100 | 96 | 32 | 32 | 6 | 0 | 85 | 11 | 15.3 | ||||||||||
| Favot | 40 | 55 | 90 | 45 | 85 | 5 | |||||||||||||
| Gasparedone | 70 | 7.5 | |||||||||||||||||
| Ottaviani | 21 | 90 | 62 | ||||||||||||||||
| Alharthy | 89 | 100 | 7 | 8 | 79 | 27 | 22 | 3 | 79 | 21 | |||||||||
| Thomaz | 722 | 41 | 20 | 5 | 28 | 28 | 1.7 | ||||||||||||
| Lichter | 120 | 83 | 78 | 8 | 15.0 | ||||||||||||||
| Iodice | 29 | 100 | 17 | 97 | 100 | 0 | |||||||||||||
| Battista | 44 | 100 | 86 | 45 | 18 | 39 | 75 | 25 | |||||||||||
| Narinx | 15 | 93 | |||||||||||||||||
| Kalafat | 82 | 9.2 | |||||||||||||||||
| Li | 91 | 65 | 62 | 7 | 53 | 43 | 22 | ||||||||||||
| Castelao | 63 | 83 | 5 | 94 | 6 | 15.3 | |||||||||||||
| Cocconcelli | 25 | 7.0 | |||||||||||||||||
| Brahier | 80 | 50 | 60 | 70 | 25 | 25 | 79 | 6 | 10.0 | ||||||||||
| Ramos-Hernández | 44 | 75 | 25 | 59 | 30 | 7.0 | |||||||||||||
| Zhu | 27 | 11.8 | |||||||||||||||||
| Rojatti | 34 | 11.2 | |||||||||||||||||
| Marggrander | 17 | 53 | 29 | 18 | 18 | ||||||||||||||
| Bosso | 26 | 18.1 | |||||||||||||||||
| Haaksma | 24 | 19.0 | |||||||||||||||||
| Zhu | 48 | 71 | 21 | 13 | 4 | 58 | 21 | 5.3 | |||||||||||
| Bosevski | 17 | 47 | 53 | 0 | 6 | ||||||||||||||
| Sorlini | 287 | 92 | 39 | 8 | 86 | 7 | |||||||||||||
| Jalil | 36 | 67 | 92 | 92 | 58 | 0 | |||||||||||||
| Gibbons | 83 | 98 | 33 | ||||||||||||||||
| Li | 42 | 86 | 76 | 71 | 24 | 10 | |||||||||||||
| Zotzmann | 20 | 95 | 70 | 55 | 95 | 90 | 50 | ||||||||||||
| Perrone | 52 | 48 | 23.8 | ||||||||||||||||
| Alharthy | 171 | 67 | 83 | 91 | 27 | ||||||||||||||
| Allegorico | 42 | 14.0 | |||||||||||||||||
| Recinella | 37 | 100 | 32 | 100 | 41 | 89 | 59 | 46 | 27 | 97 | 3 | 12.0 | |||||||
| Zanforlin | 46 | 2 | |||||||||||||||||
| Wangüemert Pérez | 45 | 9.7 | |||||||||||||||||
| Ji | 280 | 89 | 63 | 12 | 35 | 11 | 16 | 2 | |||||||||||
| Ibrahim | 77 | 27.0 | |||||||||||||||||
| Bock | 12 | 92 | 58 | 75 | 75 | 67 | 17 | ||||||||||||
| Garcia de Alencar | 180 | 18.7 | |||||||||||||||||
| Boero | 211 | 13.4 | |||||||||||||||||
| Avdeev | 22 | 17.8 | |||||||||||||||||
| Seiler | 72 | 96 | 88 | 42 | 6 | 15.7 |
Appendix 5. Sensitivity and specificity of LUS in the diagnosis of COVID-19 (using RT-RCP as the gold standard)
| Study | LUS sensitivity (%) | LUS specificity (%) | Criteria |
Population characteristics (mean n, age, BMI; % male) |
|
|---|---|---|---|---|---|
| COVID + | COVID− | ||||
| Bar | 96.8% | 62.3% | ≥3 B-lines at the upper site; consolidation and thickened pleura at the lower site; and thickened pleura. | 31, 66.8 yr, 30.0 kg/m2; 35% | 69, 68.7 yr, 26.4 kg/m2; 44% |
| Pare | 88.9% | 56.3% | Any B-lines were detected. | 27, 53.0 yr, 31.7 kg/m2; 59.3% | 16, 50.0 yr, 31.3 kg/m2; 31.3% |
| Yassa | 72.1% | 77.8% | LUS score ≥ 1. | 43 pregnant women | 9 pregnant women |
| Yassa | 73.9% | 94.1% | LUS score ≥ 1. | 23 pregnant women | 273 pregnant women |
| Favot | 70.0% | 75.0% | Nondependent bilateral pulmonary edema (bilateral B-lines with superior count ≥ inferior count and no pleural effusions). | 40, 69 yr, 31 kg/m2; 60% | 16, 63 yr, 29 kg/m2; 63% |
| Dini | 78.7% | 57.1% | LUS score ≥ 1. | 94 | 56 |
| Gil | 100.0% | 80.6% | Any pattern not compatible with A-lines in all intercostal areas. | 27, 48 yr; 33.3% | 31, 45 yr; 16.1% |
| Battista | 68.2% | 78.9% | Not specified. | 44, 66 yr; 65.9% | 19, 61 yr; 63.2% |
| Narinx | 93.3% | 21.3% | ≥ 3 B-lines. | 15, 49.9 yr; 60.0% | 75, 50.5 yr; 42.7% |
| Bosso | 73.1% | 90.3% | LUS score > 12.5. | 26, 66 yr; 69.2% | 27, 65 yr; 70.4% |
| Colombi | 93.5% | 28.3% | Not specified. | 341 | 145 |
| Pivetta | 94.4% | 95.0% | Focal or diffuse interstitial syndrome plus with spared areas, subpleural consolidations, and irregular or thickened pleural line. | 107, 62.8 yr; 54.1% | 121, 50.3 yr; 43.7% |
| Lieveld | 91.9% | 71.0% | ≥3 or more B-lines and/or consolidation in two or more zones unilaterally or in one or more zones bilaterally. | 86, 63.4 yr; 58.1% | 100, 64.1 yr; 58.0% |
| Sorlini | 92.0% | 64.9% | (A) Interstitial lung syndrome1 (B) Interstitial lung pattern2 (C) White lung (coalescent B lines) in two or more zones. (D) Subpleural consolidations. | 287 | 97 |
| Jalil | 86.1% | 90.9% | Multifocal confluent B-lines, irregular pleura, and the absence of a moderate or large pleural effusion. | 36, 62.5 yr, 30.5 kg/m2; 52% | 33, 65 yr, 29 kg/m2; 42% |
| Gibbons | 97.6% | 33.3% | ≥3 B-lines was considered positive. Additionally, the presence of a single confluent B-line encompassing a third or more of the visualized distal intercostal space was considered positive3. | 83, 56 yr; 43.4% | 27, 64 yr; 55.6% |
| Haak | 95.8% | 59.4% | Irregular pleural line, multiple or confluent B-lines, subpleural consolidations and small pleural effusions. | 24 | 69 |
| Schmid | 76.9% | 77.1% | Bilateral patchy distribution of one of the following: pleural line irregularity OR ≥ 3 B-Lines per intercostal space OR small subpleural consolidation (<1.5 cm) OR no or small pleural effusion unilateral appearance of two or more of the criteria above. | 39, 61 yr, 26.3 kg/m2; 56.4% | 96, 60 yr, 26.3 kg/m2; 53.1% |
| Speidel | 90.9% | 76.3% | LUS score ≥ 8. | 11, 76.0 yr; 73% | 38, 69.5 yr; 47% |
| Ibrahim | 97.4% | 90.6% | (A) Patchy distribution of multiple coalesced and separated B-lines with the light beam sign, with bilateral and well-demarcated separation from large “spared” areas. (B) The pleural is sliding and might appear irregular and fragmented. (C) Multiple small subpleural consolidations are limited to the periphery of the lungs. | 77, 53 yr; 83% | 32, 68 yr; 50% |
| Bock | 91.7% | 64.8% | Lung sliding, lung pulse, lung point, multiple B-lines (≥3 per intercostal space), or thickened or fragmented visceral pleura were present. | 12, 68 yr; 58% | 71, 63 yr; 46% |
References
- 1.Centers for Disease Control and Prevention, History of 1918 Flu Pandemic | Pandemic Influenza (Flu) | CDC, (n.d.).
- 2.Enaa J., Wenzel R.P. A Novel Coronavirus Emerges. Rev. Clínica Española. 2020;220:115–116. doi: 10.1016/j.rce.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johns Hopkins Coronavirus Resource Center, COVID-19 Map, (n.d.). https://coronavirus.jhu.edu/map.html (accessed 10 June 2021).
- 4.M.A. Kose, F. Ohnsorge, C. Pazarbasioglu, C. Arteta, J. Baffes, A. Dieppe, J.-D. Guénette, A. Kabundi, S. Kasyanenko, S.K. Celik, G. Kindberg-Hanlon, P. Kirby, M. Maliszewska, H. Matsuoka, P. Nagle, Y. Okawa, C. Okou, F.U. Ruch, R. Steinbach, N. Sugawara, E. Vashakmadze, D. Vorisek, C.M. Wheeler, L.S. Ye, S. Yu, Y. Chen, Z. Chen, H. Doytchinova, F. Jiang, Y. L, M.H. Macadangdang, J.R.R. Norfleet, I.C. Oymak, V. Papagianni, M.F.S.E. Pereira, S. Shi, K. Temaj, X. Wang, J. Wu, H. Zhao, J. Zhou, R.K. Danda, J.R.R. Norfleet, S. Shi, G. Littler, I. Chand, M. Felsenthal, A. Viveros, G. Littler, A. Maximiliano, Global Economic Propects, 2020.
- 5.Jarrom D., Elston L., Washington J., Prettyjohns M., Cann K., Myles S., Groves P. Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: a rapid systematic review. BMJ Evidence-Based Med. 2020:1–13. doi: 10.1136/bmjebm-2020-111511. [DOI] [PubMed] [Google Scholar]
- 6.Islam N., Ebrahimzadeh S., Salameh J.-P., Kazi S., Fabiano N., Treanor L., Absi M., Hallgrimson Z., Leeflang M.MG., Hooft L., van der Pol C.B., Prager R., Hare S.S., Dennie C., Spijker R., Deeks J.J., Dinnes J., Jenniskens K., Korevaar D.A., Cohen J.F., Van den Bruel A., Takwoingi Y., van de Wijgert J., Damen J.A., Wang J., McInnes M.DF. Thoracic imaging tests for the diagnosis of COVID-19. Cochrane Database Syst. Rev. 2021;2021(3) doi: 10.1002/14651858.CD013639.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smallwood N., Dachsel M. Point-of-care ultrasound (POCUS): unnecessary gadgetry or evidence-based medicine? Clin. Med. J. R. Coll. Phys. Lond. 2018;18(3):219–224. doi: 10.7861/clinmedicine.18-3-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349 doi: 10.1136/bmj.g7647. g7647–g7647. [DOI] [PubMed] [Google Scholar]
- 9.Soldati G., Smargiassi A., Inchingolo R., Buonsenso D., Perrone T., Briganti D.F., Perlini S., Torri E., Mariani A., Mossolani E.E., Tursi F., Mento F., Demi L. Proposal for International Standardization of the Use of Lung Ultrasound for Patients With COVID-19. J. Ultrasound Med. 2020;39:1413–1419. doi: 10.1002/jum.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whiting P.F. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011;155(8):529. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 11.J. Higgins, S. Green, Manual Cochrane de revisiones sistemáticas de intervenciones. Version 5.1.0, 2011.
- 12.Tan G., Lian X., Zhu Z., Wang Z., Huang F., Zhang Y., Zhao Y., He S., Wang X., Shen H., Lyu G. Use of Lung Ultrasound to Differentiate Coronavirus Disease 2019 (COVID-19) Pneumonia From Community-Acquired Pneumonia. Ultrasound Med. Biol. 2020;46:2651–2658. doi: 10.1016/j.ultrasmedbio.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bar S., Lecourtois A., Diouf M., Goldberg E., Bourbon C., Arnaud E., Domisse L., Dupont H., Gosset P. The association of lung ultrasound images with COVID-19 infection in an emergency room cohort. Anaesthesia. 2020;75(12):1620–1625. doi: 10.1111/anae.15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosso G., Allegorico E., Pagano A., Porta G., Serra C., Minerva V., Mercurio V., Russo T., Altruda C., Arbo P., De Sio C., Dello Vicario F., Numis F.G. Lung ultrasound as diagnostic tool for SARS-CoV-2 infection, Intern. Emerg. Med. 2021;16(2):471–476. doi: 10.1007/s11739-020-02512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speidel V., Conen A., Gisler V., Fux C.A., Haubitz S. Lung Assessment with Point-of-Care Ultrasound in Respiratory Coronavirus Disease (COVID-19): A Prospective Cohort Study. Ultrasound Med. Biol. 2021;47(4):896–901. doi: 10.1016/j.ultrasmedbio.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing C., Li Q., Du H., Kang W., Lian J., Yuan L. Lung ultrasound findings in patients with COVID-19 pneumonia. Crit. Care. 2020;24:174. doi: 10.1186/s13054-020-02876-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye R., Zhou X., Shao F., Xiong L., Hong J., Huang H., Tong W., Wang J., Chen S., Cui A., Peng C., Zhao Y., Chen L. Feasibility of a 5G-Based Robot-Assisted Remote Ultrasound System for Cardiopulmonary Assessment of Patients With Coronavirus Disease 2019. Chest. 2021;159(1):270–281. doi: 10.1016/j.chest.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y., Xue H., Wang M., He N., Lv Z., Cui L. Lung Ultrasound Findings in Patients With Coronavirus Disease (COVID-19) Am. J. Roentgenol. 2021;216(1):80–84. doi: 10.2214/AJR.20.23513. [DOI] [PubMed] [Google Scholar]
- 19.Gaspardone C., Meloni C., Preda A., Romagnolo D., Brugliera L., Castellazzi P., Tettamanti A., Conte C., Secchi A., Maranta F., Iannaccone S., Cianflone D. Lung Ultrasound in <scp>COVID</scp> -19 A Role Beyond the Acute Phase? J. Ultrasound Med. 2021;40:503–511. doi: 10.1002/jum.15425. [DOI] [PubMed] [Google Scholar]
- 20.Cocconcelli E., Biondini D., Giraudo C., Lococo S., Bernardinello N., Fichera G., Barbiero G., Castelli G., Cavinato S., Ferrari A., Saetta M., Cattelan A., Spagnolo P., Balestro E. Clinical Features and Chest Imaging as Predictors of Intensity of Care in Patients with COVID-19. J. Clin. Med. 2020;9:2990. doi: 10.3390/jcm9092990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu F., Zhao X., Wang T., Wang Z., Guo F., Xue H., Chang P., Liang H., Ni W., Wang Y., Chen L., Jiang B. Ultrasonic Characteristics and Severity Assessment of Lung Ultrasound in COVID-19 Pneumonia in Wuhan, China: A Retrospective, Observational Study. Engineering. 2021;7(3):367–375. doi: 10.1016/j.eng.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji L., Cao C., Gao Y., Zhang W., Xie Y., Duan Y., Kong S., You M., Ma R., Jiang L., Liu J., Sun Z., Zhang Z., Wang J., Yang Y., Lv Q., Zhang L., Li Y., Zhang J., Xie M. Prognostic value of bedside lung ultrasound score in patients with COVID-19. Crit. Care. 2020;24:700. doi: 10.1186/s13054-020-03416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marggrander D.T., Borgans F., Jacobi V., Neb H., Wolf T. Lung Ultrasound Findings in Patients with COVID-19. SN Compr. Clin. Med. 2020;2(11):2151–2157. doi: 10.1007/s42399-020-00553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu W., Zhang S., Chen B., Chen J., Xian J., Lin Y., Shan H., Shan H., Su Z.Z., Su Z.Z., Su Z.Z. A Clinical Study of Noninvasive Assessment of Lung Lesions in Patients with Coronavirus Disease-19 (COVID-19) by Bedside Ultrasound. Ultraschall Der Medizin. 2020;41:300–307. doi: 10.1055/a-1154-8795. [DOI] [PubMed] [Google Scholar]
- 25.Yasukawa K., Minami T. Point-of-Care Lung Ultrasound Findings in Patients with COVID-19 Pneumonia. Am. J. Trop. Med. Hyg. 2020;102:1198–1202. doi: 10.4269/ajtmh.20-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pare J., Camelo I., Mayo K., Leo M., Dugas J., Nelson K., Baker W., Shareef F., Mitchell P., Schechter-Perkins E. Point-of-care Lung Ultrasound Is More Sensitive than Chest Radiograph for Evaluation of COVID-19. West. J. Emerg. Med. 2020;21:771–778. doi: 10.5811/westjem.2020.5.47743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nouvenne A., Zani M., Milanese G., Parise A., Baciarello M., Bignami E., Odone A., Sverzellati N., Meschi T., Ticinesi A. Lung Ultrasound in COVID-19 Pneumonia: Correlations with Chest CT on Hospital admission. Respiration. 2020;99(7):617–624. doi: 10.1159/000509223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yassa M., Mutlu M.A., Birol P., Kuzan T.Y., Kalafat E., Usta C., Yavuz E., Keskin I., Tug N. Lung ultrasonography in pregnant women during the COVID-19 pandemic: an interobserver agreement study among obstetricians. Ultrasonography. 2020;39:340–349. doi: 10.14366/usg.20084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Møller-Sørensen H., Gjedsted J., Lind Jørgensen V., Lindskov Hansen K. COVID-19 Assessment with Bedside Lung Ultrasound in a Population of Intensive Care Patients Treated with Mechanical Ventilation and ECMO. Diagnostics. 2020;10:447. doi: 10.3390/diagnostics10070447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng Q., Zhang Y., Wang H., Chen L., Yang Z., Peng Z., Liu Y.a., Feng C., Huang X., Jiang N., Wang Y., Guo J., Sun B., Zhou Q. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single-center study. Acad. Radiol. 2020;27(10):1363–1372. doi: 10.1016/j.acra.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonadia N., Carnicelli A., Piano A., Buonsenso D., Gilardi E., Kadhim C., Torelli E., Petrucci M., Di Maurizio L., Biasucci D.G., Fuorlo M., Forte E., Zaccaria R., Franceschi F. Lung Ultrasound Findings Are Associated with Mortality and Need for Intensive Care Admission in COVID-19 Patients Evaluated in the Emergency Department. Ultrasound Med. Biol. 2020;46(11):2927–2937. doi: 10.1016/j.ultrasmedbio.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dargent A., Chatelain E., Kreitmann L., Quenot J.-P., Cour M., Argaud L., Adrish M. Lung ultrasound score to monitor COVID-19 pneumonia progression in patients with ARDS. PLoS One. 2020;15(7):e0236312. doi: 10.1371/journal.pone.0236312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yassa M., Yirmibes C., Cavusoglu G., Eksi H., Dogu C., Usta C., Mutlu M., Birol P., Gulumser C., Tug N. Outcomes of universal SARS-CoV-2 testing program in pregnant women admitted to hospital and the adjuvant role of lung ultrasound in screening: a prospective cohort study. J. Matern. Neonatal Med. 2020;33(22):3820–3826. doi: 10.1080/14767058.2020.1798398. [DOI] [PubMed] [Google Scholar]
- 34.Veronese N., Sbrogiò L.G., Valle R., Marin L., Boscolo Fiore E., Tiozzo A. Prognostic Value of Lung Ultrasonography in Older Nursing Home Residents Affected by COVID-19. J. Am. Med. Dir. Assoc. 2020;21(10):1384–1386. doi: 10.1016/j.jamda.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zieleskiewicz L., Markarian T., Lopez A., Taguet C., Mohammedi N., Boucekine M., Baumstarck K., Besch G., Mathon G., Duclos G., Bouvet L., Michelet P., Allaouchiche B., Chaumoître K., Di Bisceglie M., Leone M. Comparative study of lung ultrasound and chest computed tomography scan in the assessment of severity of confirmed COVID-19 pneumonia. Intensive Care Med. 2020;46(9):1707–1713. doi: 10.1007/s00134-020-06186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Favot M., Malik A., Rowland J., Haber B., Ehrman R., Harrison N. Point-of-Care Lung Ultrasound for Detecting Severe Presentations of Coronavirus Disease 2019 in the Emergency Department: A Retrospective Analysis. Crit. Care Explor. 2020;2:1–7. doi: 10.1097/CCE.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ottaviani S., Franc M., Ebstein E., Demaria L., Lheure C., Debray M.P., Khalil A., Crestani B., Borie R., Dieudé P. Lung ultrasonography in patients with COVID-19: comparison with CT. Clin. Radiol. 2020;75(877):e1–877.e6. doi: 10.1016/j.crad.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alharthy A., Faqihi F., Abuhamdah M., Noor A., Naseem N., Balhamar A., Al Saud A.A.A.S.B.A., Brindley P.G., Memish Z.A., Karakitsos D., Blaivas M. Prospective Longitudinal Evaluation of Point-of-Care Lung Ultrasound in Critically Ill Patients With Severe COVID-19 Pneumonia. J. Ultrasound Med. 2021;40:443–456. doi: 10.1002/jum.15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mafort T.T., Lopes A.J., Costa C.H., Cal M.S., Lopes M.C., Silva B.R.A., Faria L.F., Faria A.C., Costa W., Salles R.E.B., Castro M.C.S., Rufino R. Changes in lung ultrasound of symptomatic healthcare professionals with COVID-19 pneumonia and their association with clinical findings. J. Clin. Ultrasound. 2020;48:515–521. doi: 10.1002/jcu.22905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lichter Y., Topilsky Y., Taieb P., Banai A., Hochstadt A., Merdler I., Gal Oz A., Vine J., Goren O.r., Cohen B., Sapir O., Granot Y., Mann T., Friedman S., Angel Y., Adi N., Laufer-Perl M., Ingbir M., Arbel Y., Matot I., Szekely Y. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020;46(10):1873–1883. doi: 10.1007/s00134-020-06212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iodice V., Pisaturo M., Fusco F.M., Tambaro O., Parrella G., Flumeri G.D., Viglietti R., Pisapia R., Palmiero G., Bignardi E., Coppola M., Rescigno C., Sangiovanni V. Use of lung ultrasound in COVID-19: comparison with ultra-high-resolution computed tomography among 29 patients at “D. Cotugno” hospital, Naples, Italy. Infez. Med. 2020;28:346–350. [PubMed] [Google Scholar]
- 42.Dini F.L., Bergamini C., Allegrini A., Scopelliti M., Secco G., Miccoli M., Boni S., Brigada R., Perlini S. Bedside wireless lung ultrasound for the evaluation of COVID-19 lung injury in senior nursing home residents. Monaldi Arch. Chest Dis. 2020;90:523–527. doi: 10.4081/monaldi.2020.1446. [DOI] [PubMed] [Google Scholar]
- 43.Gil-Rodrigo A., Llorens P., Martínez-Buendía C., Luque-Hernández M.-J., Espinosa B., Ramos-Rincón J.M. Diagnostic yield of point-of-care ultrasound imaging of the lung in patients with COVID-19. Emergencias Rev. La Soc. Esp. Med. Emergencias. 2020;32:340–344. [PubMed] [Google Scholar]
- 44.Fonsi G.B., Sapienza P., Brachini G., Andreoli C., De Cicco M.L., Cirillo B., Meneghini S., Pugliese F., Crocetti D., Fiori E., Mingoli A. Is Lung Ultrasound Imaging a Worthwhile Procedure for Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia Detection? J. Ultrasound Med. 2021;40(6):1113–1123. doi: 10.1002/jum.15487. [DOI] [PubMed] [Google Scholar]
- 45.Narinx N., Smismans A., Symons R., Frans J., Demeyere A., Gillis M. Feasibility of using point-of-care lung ultrasound for early triage of COVID-19 patients in the emergency room. Emerg. Radiol. 2020;27(6):663–670. doi: 10.1007/s10140-020-01849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalafat E., Yassa M., Koc A., Tug N., Baydemir K., Benlioglu C., Oz O.F., Aslan B., Oruc B.B., Ozkavukcu E., Birol P., Budak D., Yavuz E., Cavusoglu G., Mutlu M.A., Yirmibes C., Kuzan T. Utility of lung ultrasound assessment for probable SARS-CoV-2 infection during pregnancy and universal screening of asymptomatic individuals. Ultrasound Obstet. Gynecol. 2020;56:624–626. doi: 10.1002/uog.23099. [DOI] [PubMed] [Google Scholar]
- 47.Li S., Qu Y.-L., Tu M.-Q., Guo L.-Y., Zhang Q.-L., Lv C.-Y., Guo R.-J. Application of lung ultrasonography in critically ill patients with COVID-19. Echocardiography. 2020;37(11):1838–1843. doi: 10.1111/echo.14849. [DOI] [PubMed] [Google Scholar]
- 48.Castelao J., Graziani D., Soriano J.B., Izquierdo J.L. Findings and Prognostic Value of Lung Ultrasound in COVID-19 Pneumonia. J. Ultrasound Med. 2021;40:1315–1324. doi: 10.1002/jum.15508. [DOI] [PubMed] [Google Scholar]
- 49.Brahier T., Meuwly J.-Y., Pantet O., Brochu Vez M.-J., Gerhard Donnet H., Hartley M.-A., Hugli O., Boillat-Blanco N. Lung Ultrasonography for Risk Stratification in Patients with Coronavirus Disease 2019 (COVID-19): A Prospective Observational Cohort Study. Clin. Infect. Dis. 2021;73(11):e4189–e4196. doi: 10.1093/cid/ciaa1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramos Hernández C., Botana Rial M., Pazos Area L.A., Núñez Fernández M., Pérez Fernández S., Rubianes González M., Crespo Casal M., Fernández Villar A. Predicción de evolución desfavorable en pacientes hospitalizados por COVID-19 mediante ecografía pulmonar. Arch. Bronconeumol. 2021;57:47–54. doi: 10.1016/j.arbres.2020.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu S.-T., Tao F.-Y., Xu J.-H., Liao S.-S., Shen C.-L., Liang Z.-H., Shi B.-B., Li Q. Utility of Point-of-Care Lung Ultrasound for Clinical Classification of COVID-19. Ultrasound Med. Biol. 2021;47(2):214–221. doi: 10.1016/j.ultrasmedbio.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rojatti M., Regli I.B., Zanforlin A., Ferretti E., Falk M., Strapazzon G., Gamper M., Zanon P., Bock M., Rauch S. Lung Ultrasound and Respiratory Pathophysiology in Mechanically Ventilated COVID-19 Patients—an Observational Trial. SN Compr. Clin. Med. 2020;2(11):1970–1977. doi: 10.1007/s42399-020-00536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colombi D., Petrini M., Maffi G., Villani G.D., Bodini F.C., Morelli N., Milanese G., Silva M., Sverzellati N., Michieletti E. Comparison of admission chest computed tomography and lung ultrasound performance for diagnosis of COVID-19 pneumonia in populations with different disease prevalence. Eur. J. Radiol. 2020;133:109344. doi: 10.1016/j.ejrad.2020.109344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haaksma M.E., Heldeweg M.L.A., Lopez Matta J.E., Smit J.M., van Trigt J.D., Nooitgedacht J.S., Elzo Kraemer C.V., van de Wiel M., Girbes A.R.J., Heunks L., van Westerloo D.J., Tuinman P.R. Lung ultrasound findings in patients with novel SARS-CoV-2. ERJ Open Res. 2020;6:00238–02020. doi: 10.1183/23120541.00238-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pivetta E., Goffi A., Tizzani M., Locatelli S.M., Porrino G., Losano I., Leone D., Calzolari G., Vesan M., Steri F., Ardito A., Capuano M., Gelardi M., Silvestri G., Dutto S., Avolio M., Cavallo R., Bartalucci A., Paglieri C., Morello F., Richiardi L., Maule M.M., Lupia E., Baldassa F., Baron P., Bianchi G., V B., Conterno A., Del Rizzo P., Fascio Pecetto P., Giachino F., Iannaccone A., Ferrera P., Riccardini F., Sacchi C., Sozzi M., Totaro S., Visconti P., Risi F., Basile F., Baricocchi D., Beux A., Beux V., Bima P., Cara I., Chichizola L., Dellavalle F., Grosso F., Labarile G., Oddi M., Ottino M., Pia I., Scategni V., Surra A. Lung Ultrasonography for the Diagnosis of SARS-CoV-2 Pneumonia in the Emergency Department. Ann. Emerg. Med. 2021;77(4):385–394. doi: 10.1016/j.annemergmed.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lieveld A.W.E., Kok B., Schuit F.H., Azijli K., Heijmans J., van Laarhoven A., Assman N.L., Kootte R.S., Olgers T.J., Nanayakkara P.W.B., Bosch F.H. Diagnosing COVID-19 pneumonia in a pandemic setting: Lung Ultrasound versus CT (LUVCT) – a multicentre, prospective, observational study. ERJ Open Res. 2020;6(4):00539-2020. doi: 10.1183/23120541.00539-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bosevski M., Janusevski F., Kapsarov K. Utility of Combined Echocardiography and Lung Ultrasound for Coronavirus Disease-19 Intensive Care Unit Patients: Case Series, Open Access Maced. J. Med. Sci. 2020;8(T1):408–410. doi: 10.3889/oamjms.2020.5382. [DOI] [Google Scholar]
- 58.Sorlini C., Femia M., Nattino G., Bellone P., Gesu E., Francione P., Paternò M., Grillo P., Ruffino A., Bertolini G., Cariati M., Cortellaro F. The role of lung ultrasound as a frontline diagnostic tool in the era of COVID-19 outbreak. Intern. Emerg. Med. 2021;16(3):749–756. doi: 10.1007/s11739-020-02524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jalil B.A., Khan A., Kugasia I.R., Ijaz M. Lung ultrasound in early SARS-CoV-2 pneumonia and the LUS-CoV criteria. Baylor Univ. Med. Cent. Proc. 2021;34(1):1–4. doi: 10.1080/08998280.2020.1834658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gibbons R.C., Magee M., Goett H., Murrett J., Genninger J., Mendez K., Tripod M., Tyner N., Costantino T.G. Lung Ultrasound vs. Chest X-Ray Study for the Radiographic Diagnosis of COVID-19 Pneumonia in a High-Prevalence Population. J. Emerg. Med. 2021;60(5):615–625. doi: 10.1016/j.jemermed.2021.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li R., Liu H., Qi H., Yuan Y., Zou X., Huang H., Wan J., Lv Z., Ouyang Y., Pan S., Zhao X., Shu H., Shang Y. Lung ultrasound assessment of acute respiratory distress syndrome caused by coronavirus disease 2019: An observational study, Hong Kong. J. Emerg. Med. 2021;28:8–14. doi: 10.1177/1024907920969326. [DOI] [Google Scholar]
- 62.Zotzmann V., Lang C.N., Wengenmayer T., Bemtgen X., Schmid B., Mueller-Peltzer K., Supady A., Bode C., Duerschmied D., Staudacher D.L. Combining lung ultrasound and Wells score for diagnosing pulmonary embolism in critically ill COVID-19 patients. J. Thromb. Thrombolysis. 2021;52(1):76–84. doi: 10.1007/s11239-020-02323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perrone T., Soldati G., Padovini L., Fiengo A., Lettieri G., Sabatini U., Gori G., Lepore F., Garolfi M., Palumbo I., Inchingolo R., Smargiassi A., Demi L., Mossolani E.E., Tursi F., Klersy C., Di Sabatino A. A New Lung Ultrasound Protocol Able to Predict Worsening in Patients Affected by Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. J. Ultrasound Med. 2021;40(8):1627–1635. doi: 10.1002/jum.15548. [DOI] [PubMed] [Google Scholar]
- 64.Alharthy A., Abuhamdah M., Balhamar A., Faqihi F., Nasim N., Ahmad S., Noor A., Tamim H., Alqahtani S.A., Bin Abdulaziz Al Saud A.A.A.S., Kutsogiannis D.J., Brindley P.G., Memish Z.A., Karakitsos D., Blaivas M. Residual Lung Injury in Patients Recovering From COVID-19 Critical Illness. J. Ultrasound Med. 2021;40:1823–1838. doi: 10.1002/jum.15563. [DOI] [PubMed] [Google Scholar]
- 65.Haak S.L., Renken I.J., Jager L.C., Lameijer H., van der Kolk B. (Britt) Y. Diagnostic accuracy of point-of-care lung ultrasound in COVID-19. Emerg. Med. J. 2021;38:94–99. doi: 10.1136/emermed-2020-210125. [DOI] [PubMed] [Google Scholar]
- 66.Allegorico E., Buonerba C., Bosso G., Pagano A., Porta G., Serra C., Dolce P., Minerva V., Dello Vicario F., Altruda C., Arbo P., Russo T., De Sio C., Franco N., Ruffa G., Mormile C., Cannavacciuolo F., Mercurio V., Gervasio G., Di Costanzo G., Ragozzino A., Scafuri L., Facchini G., Numis F. The use of chest ultrasonography in suspected cases of COVID-19 in the emergency department. Futur. Sci. OA. 2021;7:FSO635. doi: 10.2144/fsoa-2020-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Recinella G., Marasco G., Tufoni M., Brizi M., Evangelisti E., Maestri L., Fusconi M., Calogero P., Magalotti D., Zoli M. Clinical Role of Lung Ultrasound for the Diagnosis and Prognosis of Coronavirus Disease Pneumonia in Elderly Patients: A Pivotal Study. Gerontology. 2021;67(1):78–86. doi: 10.1159/000512209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmid B., Feuerstein D., Lang C.N., Fink K., Steger R., Rieder M., Duerschmied D., Busch H.-J., Damjanovic D. Lung ultrasound in the emergency department – a valuable tool in the management of patients presenting with respiratory symptoms during the SARS-CoV-2 pandemic. BMC Emerg. Med. 2020;20:96. doi: 10.1186/s12873-020-00389-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zanforlin A., Strapazzon G., Falk M., Gallina V., Viteritti A., Valzolgher L., La Guardia M., Ferro F., Pagani L., Vezzali N. Lung Ultrasound in the Emergency Department for Early Identification of COVID-19 Pneumonia. Respiration. 2020;100:1–9. doi: 10.1159/000512782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wangüemert Pérez A.L., Figueira Gonçalves J.M., Hernández Pérez J.M., Ramallo Fariña Y., Del Castillo Rodriguez J.C. Prognostic value of lung ultrasound and its link with inflammatory biomarkers in patients with SARS-CoV-2 infection. Respir. Med. Res. 2021;79:100809. doi: 10.1016/j.resmer.2020.100809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bitar Z.I., Shamsah M., Maadarani O., Bamasood O.M., Bitar A.Z., Alfoudri H., Tran Q.K. Lung Ultrasound and Sonographic Subpleural Consolidation in COVID-19 Pneumonia Correlate with Disease Severity. Crit. Care Res. Pract. 2021;2021:1–6. doi: 10.1155/2021/6695033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ask B., Annmarie T.L., Christian B.L., Stefan P. Lung ultrasound as a prognostic tool in emergency patients clinically suspected of COVID-19. Dan. Med. J. 2021;68:A07200551. [PubMed] [Google Scholar]
- 73.de Alencar J.C.G., Marchini J.F.M., Marino L.O., da Costa Ribeiro S.C., Bueno C.G., da Cunha V.P., Lazar Neto F., Brandão Neto R.A., Souza H.P. Lung ultrasound score predicts outcomes in COVID-19 patients admitted to the emergency department. Ann. Intensive Care. 2021;11:6. doi: 10.1186/s13613-020-00799-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boero E., Rovida S., Schreiber A., Berchialla P., Charrier L., Cravino M.M., Converso M., Gollini P., Puppo M., Gravina A., Fornelli G., Labarile G., Sciacca S., Bove T., Karakitsos D., Aprà F., Blaivas M., Vetrugno L. The COVID-19 Worsening Score (COWS)—a predictive bedside tool for critical illness. Echocardiography. 2021;38(2):207–216. doi: 10.1111/echo.14962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Avdeev S.N., Nekludova G.V., Trushenko N.V., Tsareva N.A., Yaroshetskiy A.I., Kosanovic D. Lung ultrasound can predict response to the prone position in awake non-intubated patients with COVID-19 associated acute respiratory distress syndrome. Crit. Care. 2021;25:35. doi: 10.1186/s13054-021-03472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heldeweg M.L.A., Lopez Matta J.E., Haaksma M.E., Smit J.M., Elzo Kraemer C.V., de Grooth H.-J., de Jonge E., Meijboom L.J., Heunks L.M.A., van Westerloo D.J., Tuinman P.R. Lung ultrasound and computed tomography to monitor COVID-19 pneumonia in critically ill patients: a two-center prospective cohort study. Intensive Care Med. Exp. 2021;9:1. doi: 10.1186/s40635-020-00367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seiler C., Klingberg C., Hårdstedt M. Lung Ultrasound for Identification of Patients Requiring Invasive Mechanical Ventilation in COVID-19. J Ultrasound Med. 2021;9999:1–13. doi: 10.1002/jum.15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mongodi S., De Luca D., Colombo A., Stella A., Santangelo E., Corradi F., Gargani L., Rovida S., Volpicelli G., Bouhemad B., Mojoli F. Quantitative Lung Ultrasound: Technical Aspects and Clinical Applications. Anesthesiology. 2021;134:949–965. doi: 10.1097/ALN.0000000000003757. [DOI] [PubMed] [Google Scholar]
- 79.Pecho-Silva S., Navarro-Solsol A.C., Taype-Rondan A., Torres-Valencia J., Arteaga-Livias K., Herriman D.A., Acosta-Pinzas K., Valenzuela-Rodriguez G., Barboza J.J., Panduro-Correa V. Pulmonary Ultrasound in the Diagnosis and Monitoring of Coronavirus Disease (COVID-19): A Systematic Review. Ultrasound Med. Biol. 2021;47(8):1997–2005. doi: 10.1016/j.ultrasmedbio.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barssoum K., Victor V., Salem A., Kumar A., Mubasher M., Hassib M., Magdi M., Renjithlal S., Abdelazeem M., Shariff M., Idemudia O., Ibrahim M., Mohamed A., Thakkar S., Patel H., Diab M., Szeles A., Ibrahim F., Jha R., Chowdhury M., Akula N., Kalra A., Nanda N.C. Echocardiography, lung ultrasound, and cardiac magnetic resonance findings in COVID-19: A systematic review. Echocardiography. 2021:1–40. doi: 10.1111/echo.15152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peixoto A.O., Costa R.M., Uzun R., Fraga A.M.A., Ribeiro J.D., Marson F.A.L. Applicability of lung ultrasound in COVID-19 diagnosis and evaluation of the disease progression: A systematic review. Pulmonology. 2021;27(6):529–562. doi: 10.1016/j.pulmoe.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pillai K., Hewage S., Harky A. The Role of the Lung Ultrasound in Coronavirus Disease 2019: A Systematic Review. J. Med. Ultrasound. 2020;28:207. doi: 10.4103/JMU.JMU_87_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Volpicelli G., Gargani L. Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J. 2020;12:1–3. doi: 10.1186/S13089-020-00171-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smargiassi A., Soldati G., Torri E., Mento F., Milardi D., Del Giacomo P., De Matteis G., Burzo M.L., Larici A.R., Pompili M., Demi L., Inchingolo R. Lung Ultrasound for COVID-19 Patchy Pneumonia: Extended or Limited Evaluations? J. Ultrasound Med. 2021;40:521–528. doi: 10.1002/jum.15428. [DOI] [PubMed] [Google Scholar]
- 85.Izcovich A., Ragusa M.A., Tortosa F., Lavena Marzio M.A., Agnoletti C., Bengolea A., Ceirano A., Espinosa F., Saavedra E., Sanguine V., Tassara A., Cid C., Catalano H.N., Agarwal A., Foroutan F., Rada G., Lazzeri C. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS One. 2020;15(11):e0241955. doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rouby J.-J., Arbelot C., Gao Y., Zhang M., Lv J., An Y., Chunyao W., Bin D., Valente Barbas C.S., Dexheimer Neto F.L., Prior Caltabeloti F., Lima E., Cebey A., Perbet S., Constantin J.-M., Arbelot C., Rouby J.-J., Brisson H., Deransy R., Vezinet C., Garçon P., El Hadj Kacem N., Lemesle D., Monsel A., Lu Q., Langeron O., Gay F., Lucena B., Malbouisson L., Carvalho Carmona M.J., Neves J., de Tarso Roth P., de Dalcin G., Schettino P.P., Biestro A., Cristovao D., Salluh J. Training for Lung Ultrasound Score Measurement in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2018;198:398–401. doi: 10.1164/rccm.201802-0227LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soummer A., Perbet S., Brisson H., Arbelot C., Constantin J.-M., Lu Q., Rouby J.-J. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit. Care Med. 2012;40(7):2064–2072. doi: 10.1097/CCM.0b013e31824e68ae. [DOI] [PubMed] [Google Scholar]
- 88.Lichtenstein D.A., Mezière G.A. Relevance of Lung Ultrasound in the Diagnosis of Acute Respiratory Failure*: The BLUE Protocol. Chest. 2008;134(1):117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mehta O.P., Bhandari P., Raut A., Kacimi S.E.O., Huy N.T. Coronavirus Disease (COVID-19): Comprehensive Review of Clinical Presentation. Front. Public Heal. 2021:1034. doi: 10.3389/FPUBH.2020.582932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mento F., Perrone T., Macioce V.N., Tursi F., Buonsenso D., Torri E., Smargiassi A., Inchingolo R., Soldati G., Demi L. On the Impact of Different Lung Ultrasound Imaging Protocols in the Evaluation of Patients Affected by Coronavirus Disease 2019. J. Ultrasound Med. 2021;40(10):2235–2238. doi: 10.1002/jum.15580. [DOI] [PMC free article] [PubMed] [Google Scholar]