Abstract
Hypothesis
Broad-spectrum antimicrobials are needed to mitigate the issues of antibiotic-resistant infections. It is highly important to formulate new antimicrobials by combining agents with different mechanistic and broader microbial targets. A combined antimicrobial solution could be a highly critical step towards developing the strategy to prevent polymicrobial infections. Herein, we have investigated the interaction and antimicrobial potential of a solution that contains cerium oxide nanoparticles (CNP) and a nitric oxide (NO) donor, S-nitroso-N-acetylpenicillamine (SNAP). It is hypothesized that these two agents induce synergistic effects and would provide broad antimicrobial effects since CNP is known to be an effective antifungal agent while NO released by SNAP is known to be a potent bactericidal agent.
Experiments
Different concentrations of SNAP and CNP were combined in a solution and tested for colloidal stability, NO release, mammalian cell cytotoxicity, and antimicrobial efficacy against Staphylococcus aureus, Escherichia coli, and Candida albicans, accounting for Gram-positive bacteria, Gram-negative bacteria, and fungi, respectively.
Findings
SNAP and CNP combined in equimolar solution of 3 mM were found to be highly effective for all microbes tested compared to higher amounts of the treatments required individually. These results hold a promising outlook toward the development of broad-spectrum antimicrobial coatings and films with the potential to prevent polymicrobial infections and further enhance biomedical device usage and applications.
Keywords: nitric oxide, antimicrobial, cerium oxide nanoparticle, nanoceria, antifungal, bactericidal, infection
Graphical Abstract
Introduction
In a world where biomedical innovation is constantly on the rise, it is indeed surprising that hospital-acquired infections (HAIs) are the fourth leading cause of death in the U.S. [1]. Several microbes implicated as the main culprits of HAI are Staphylococcus aureus (S. aureus), Escherichia coli (E. coli) and Candida albicans (C. albicans). These bacteria and fungi, among others, infect patients in various ways but most commonly in the form of a bloodstream infection, ventilator-associated pneumonia, catheter-associated urinary tract infection, and/or surgical site infection [2]. To make these matters worse, what once were easily treatable infections are currently much more challenging to eradicate due to the emergence of antibiotic-resistant organisms [3, 4]. These newly adapted microbes are incredibly difficult to treat and often cause extended hospital stays and increased healthcare costs due to more complex and unique treatment options [3]. Patients infected with drug-resistant bacteria are also more prone to developing severe complications brought about by the infection, including death [4]. Antimicrobial resistance is a naturally occurring phenomenon that usually progresses gradually through minor genetic changes in the microorganism making it more resilient. However, the overuse and misuse of antibiotics has sped up the process quite rapidly by killing off good bacteria that fight infections in our bodies and allowing drug-resistant microbes to multiply and spread [5]. In fact, more than 2.8 million people are infected with antibiotic-resistant bacteria every year in the U.S., leading to at least 35,000 deaths annually [3].
Antibiotics are divided into several classes depending on their mode of action for antimicrobial activity. Mechanisms include inhibition of cell wall synthesis, protein synthesis, DNA replication, and folic acid metabolism [6]. These single mechanism drugs, though highly potent when used correctly, can easily lead to development of antibiotic resistant strains of microbes when patients do not complete their antibiotic regimen or antibiotics are mis-prescribed. In those cases, the microbes that survive the initial attack of the antibiotic reproduce, pass on their resilient genes, and create a new strain that is more difficult to treat. To combat these emerging superbugs, researchers have been exploring multi-mechanistic strategies that target microorganisms in multiple ways, decreasing the chance of microbial adaptation and reproduction before they are killed. Many of these strategies employ antimicrobial nanoparticles to conquer invading pathogens.
Nanoparticles have caught the attention of many researchers looking to combat antimicrobial resistant microorganisms as they permit targeted delivery, controlled release, more effective administration, relatively easy modification of drug release characteristics, blood circulation half-life, solubility, and diffusivity [7]. Recent developments include nanomaterials highly specific for gastrointestinal tract visualization as contrast agents and nanofiber based nanomaterials against multiple drug resistant bacterial infections [8, 9]. Nanoparticles are also preferred over small antimicrobials because they have prolonged effectivity and induce minimal toxicity into the surrounding environment [10]. In recent years, Cerium oxide nanoparticles (CNP) have received much attention for their therapeutic potential due to the high antioxidative behavior in biological systems. CNP application as nanomedicine for neuroprotection [11–14] and in cancer biology [15–18] is very well explored. The antioxidative nature of CNP is due to the presence of mixed valence states of Ce+3 and Ce+4 ions [19], which help in scavenging reactive oxygen species (ROS) and reactive nitrogen species (RNS) and is highly effective in ameliorating oxidative stress and reducing chronic inflammation [20, 21]. These antioxidative CNP behave as a multi-enzyme complex unit and mimic the activity of superoxide dismutase (SOD) scavenging superoxide anions and catalase scavenging hydrogen peroxide in the surrounding environment. The redox active nature and antioxidative scavenging properties of CNP have promoted them as a fascinating and highly lucrative material for therapeutics and biomedical applications [22–24]. In fact, nanotechnology has been used to develop ultrastructural nanoscale materials with potential antimicrobial properties [10, 25]. Nanoceria have been found to be highly effective antimicrobials against both Gram-positive [26, 27] and Gram-negative bacteria [28, 29], and have even demonstrated an effectiveness against C. albicans [30]. The antimicrobial effects of CNP are mainly due to the oxidoreductive properties generated based on the route of synthesis and thus, the oxidative ratio can be modified based on synthesis process. However, other CNP based antimicrobial mechanisms such as increased surface adsorption leading to toxicity might also play an important role in the overall antimicrobial action.
To increase antimicrobial potential of infection treatments outside the realm of traditional antibiotics, combination therapy research has grown exponentially. Combination approaches involving multiple antibiotics has been discussed [31], but there are often drug-drug interactions, and this treatment may only expedite the process of developing multi-drug-resistant organisms that are even more difficult to eradicate. There are also cases of antibiotics combined with other classes of antimicrobials such as phages [32, 33], but some combinations actually hampered the antibiotic’s effectiveness, and the diversity of phages requires extensive research on their success against different pathogens. Interestingly, combination therapies with CNP have also brought about challenges. When combined with iron oxide nanoparticles (Fe2O3), CNP were unable to inhibit bacterial and biofilm growth. Upon further combination of the solution with the antibiotic ciprofloxacin, the antibacterial activity was all but diminished entirely [29]. Thus, it is necessary to find a combination treatment that does not involve single-mechanism antibiotics nor decrease antimicrobial efficacy of the treatments individually or in tandem.
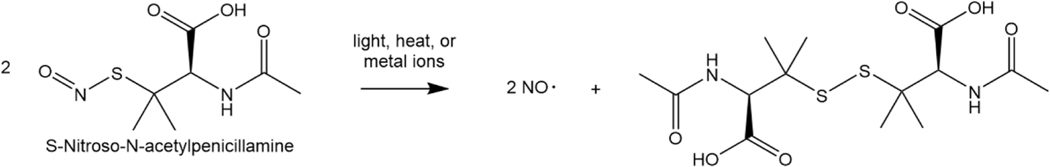
One antimicrobial strategy that has previously been combined with other antimicrobials such as copper nanoparticles [34, 35], zinc oxide nanoparticles [36, 37], antimicrobial peptides, and antibiotics [38] all without hampering and even providing synergistic antimicrobial effects is the molecule nitric oxide (NO). Nitric oxide is a small molecule produced endogenously for various biological roles ranging from neurotransmission [39, 40] to vasodilation [41, 42] to anti-inflammation [43]. Nitric oxide is also produced by macrophages to kill invading pathogens [44], a property rendering it advantageous for use in the infection sector of the biomedical industry. As a gaseous molecule, NO is able to permeate bacterial biofilms and penetrate bacterial membranes directly [45]. The mechanism of killing lies in NO as well as the many reactive nitrogen species (RNS) and reactive oxygen species (ROS) formed in side reactions with NO that have the ability to modify lipids, proteins, and DNA, equipping the molecule with multiple strategies of attack [46]. To harness and employ the potency of NO, NO donor molecules such as diazeniumdiolates or S-nitrosothiols (RSNOs) have been created that can be dissolved in solution or swollen into polymers and release local doses of NO [40]. S-Nitroso-N-acetylpenicillamine (SNAP) is a RSNO NO donor that releases NO through a reaction catalyzed by light, heat, or metal ions and produces two molecules of NO for every two molecules of SNAP, followed by a disulfide bond formation between the precursor SNAP molecules (Figure 1). The NO releasing molecule has been utilized in many biomedical applications such as urinary catheters [47, 48], vascular catheters [49–51], insulin cannulas [52], endotracheal tubes [53], orthopedic implants [54], and numerous biomedical device coatings, which has consistently shown its antimicrobial ability against pathogens including Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Staphylococcus epidermidis, Proteus mirabilis, as well as multidrug-resistant strains of microbes.
Figure 1. Nitric oxide release mechanism from SNAP molecule.
The nitric oxide release from SNAP is catalyzed by light, heat, or metal ions and results in two NO molecules for every two SNAP molecules. A disulfide bridge formed between the two precursor SNAP molecules prevents further nitrosation.
Herein, this project was aimed at investigating the interaction of SNAP and CNP and evaluating the combined broad-spectrum antimicrobial effects by testing them individually and in combination against S. aureus, E. coli, and C. albicans. Both NO donor (SNAP) and cerium oxide nanoparticles (CNP) were synthesized in the labs and tested for their physicochemical properties before being evaluated for in vitro antimicrobial effects. This proof-of-concept study demonstrates the possibility of having broad-spectrum activity at a lower concentration of antimicrobial agents when two different antimicrobials are combined, which will help to evaluate other combinations that can potentially have better results without having side-effects caused by most high dose requiring antimicrobials. This type of combination antimicrobial solution can also be studied and developed further into medical device coatings to ensure efficacy against polymicrobial infections, a major threat to target-specific antimicrobials.
Materials and Methods
Materials
Cerium nitrate hexahydrate (99.999%), hydrogen peroxide (3% solution), N-acetyl-D-penicillamine (NAP, > 99.0%), sodium nitrite (> 99.0%), concentrated sulfuric acid (conc. H2SO4, 18 M), and phosphate buffered saline (PBS) were bought from Sigma Aldrich (St Louis, MO). Concentrated hydrochloric acid (conc. HCl, 12.1 M), and methanol (> 99.8%) were purchased from Fisher-Scientific (Hampton, NH). Milli-Q filter was used to obtain de-ionized (DI) water for the aqueous solution preparations. Luria Agar (LA) and Miller and Luria broth (LB) were obtained from Fischer BioReagents (Fair Lawn, NJ). Staphylococcus aureus (ATCC 6538, S. aureus), Escherichia coli (ATCC 11303, E. coli), and Candida albicans (ATCC MYA 4441, C. albicans) were used for all microbial experiments. 3T3 mouse fibroblast cells (ATCC® CRL-1658TM) were cultured from stocks initially acquired from American Type Culture Collection (ATCC Manassas, VA, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), trypsin-EDTA, and PenStrep solution were purchased from Fisher Scientific (Waltham, MA, USA). FDA-approved fetal bovine serum, Cell Counting Kit-8 (CCK-8) solution, and all other cell culture supplies were obtained from VWR (Atlanta, GA, USA).
Synthesis of S-nitroso-N-acetyl-D-penicillamine (SNAP, nitric oxide donor)
The nitric oxide donor, S-nitroso-N-acetyl-penicillamine, was synthesized using a modified protocol from a previously published report [55]. In short, sodium nitrite was dissolved in DI water to make a 1 M solution. In a separate beaker, NAP was dissolved in methanol and an equimolar concentration of DI water. Conc. H2SO4 and conc. HCl were added to the NAP solution. This provides the acidic conditions for sodium nitrite to react with NAP. The sodium nitrite solution was then added to the NAP solution and was left to react in an ice bath for 8 h. At the end of the reaction, SNAP crystals were collected by vacuum filtration. The wet SNAP crystals were dried in a vacuum desiccator and stored in the freezer for later use.
Synthesis of cerium oxide nanoparticles (CNP)
Cerium oxide nanoparticles (CNP) were synthesized using the wet chemistry approach as described in our previous publication [56]. Briefly, a quantified amount of cerium nitrate hexahydrate was dissolved in DI water and stoichiometric amount of hydrogen peroxide (H2O2) was added to induce the oxidation of cerium ions, leading to formation of ultra-small CeO2 nanoparticles. To avoid the precipitation of the solution, the pH was strictly maintained below 4 during the reaction.
Physicochemical and biological activity characterization of CNP
The synthesized CeO2 nanoparticles were characterized for their physicochemical properties using several techniques. High-resolution transmission electron microscopy (HRTEM, Philips Tecnai operating at 300 kV) was employed to characterize the precise size of synthesized CNP. The optical and electronic properties of these samples were characterized using a UV-visible spectrophotometer (PerkinElmer Lambda 7505). The hydrodynamic size and the surface charge (zeta potential) were estimated in DI water (pH = 7.4) using a zeta sizer (Nano-ZS from Malvern Instruments). Surface area of the particles was determined by vacuum degassing of CNP at 80 °C for 6 h followed by measurement using a Quantachrome Nova-e surface area analyzer. X-ray photoelectron spectroscopy (XPS) was carried out using Thermo fisher ESCALAB 250xi spectrometer with a monochromatic Al Kα x-ray source. The deconvolution of Ce3d envelope was performed to further verify the concentration of Ce3+/Ce4+ oxidation states. The biocatalytic activity was analyzed using a commercially available superoxide dismutase (SOD) assay kit (Sigma Aldrich, Kit #19160–1KTF).
Incorporation of NO donor and cerium oxide into a nutrient media
The CNP suspension and NO donor were added to LB broth to make the broad-spectrum antimicrobial mixture. Different concentrations of the compounds were made for analyses. Cerium oxide nanoparticle molar mass (172 g/mol) and SNAP molar mass (220.25 g/mol) were used to calculate the suspensions (Table 1). The molar mass of CNP was used for calculations of the colloidal concentration due to the assumption that all initial reagents were converted into nanoparticle form as there were not washing steps during synthesis and therefore no loss of cerium molecule from the initial concentration.
Table 1.
Concentrations of SNAP and CNP used in NO release tests and microbial studies
| Solution | SNAP conc. (mM) | CNP conc. (mM) | Microbes Tested |
|---|---|---|---|
| SNAP only | 3 | --- | S. aureus, E. coli, C. albicans |
| 3.5 | --- | ||
| 4 | --- | ||
| 4.5 | --- | ||
| 5 | --- | ||
| CNPonly | --- | 3 | S. aureus, E. coli, C. albicans |
| --- | 3.5 | ||
| --- | 4 | ||
| --- | 4.5 | ||
| --- | 5 | ||
| Combination Treatments | 3 | 3 | S. aureus, E. coli, C. albicans |
| 3 | 3.5 | C. albicans | |
| 3 | 4 | ||
| 3 | 4.5 | ||
| 3 | 5 | ||
| 3.5 | 3 | C. albicans | |
| 3.5 | 3.5 | S. aureus, E. coli, C. albicans | |
| 3.5 | 4 | C. albicans | |
| 3.5 | 4.5 | ||
| 3.5 | 5 |
CNP colloidal stability in LB media
The colloidal stability of CNP and SNAP-CNP in LB media was studied to observe the effect of LB media components on the aggregation properties and zeta surface charge of the particles. DLS and zeta potential measurements were taken with increasing CNP concentrations (1 mM, 3 mM, and 5 mM) and with the addition of SNAP in DI water to first determine if there is a correlation between concentration and stability. Colloidal suspensions of increasing CNP concentration as well as a SNAP-CNP suspension were then incubated in LB media for time points of 0 min, 10 min, 1 h, and 30 h and hydrodynamic size and zeta potential were measured at each time point. All measurements were obtained within a few minutes ( < 5 min) after mixing the nanoparticles with SNAP molecules as the biomolecules begin interacting with the nanoparticle surface from microseconds onward [57]. Three readings were taken for each sample with n = 3 sample size.
Nitric oxide analysis
SNAP in the suspension releases NO under physiological conditions, and this release was measured and recorded using Sievers chemiluminescence NO analyzers® (NOA 280i, GE Analytical, Boulder, CO, USA). The sample holder maintained a dark environment for the samples to prevent catalysis of the NO production by any light source. Each of the samples containing only SNAP was prepared by dissolving different concentrations of SNAP in 300 μL of ethanol –to ensure a steady and accurate reading of NO release rather than rapid bursts due to the breakup of undissolved SNAP crystals –followed by the addition of 2.7 mL of LB media. The final 10% concentration of ethanol in the SNAP samples is not expected to affect NO release as it has been shown that SNAP has a similar half-life in ethanol and PBS [58], ethanol is not a catalyst, and is only used to ensure total dissolution of SNAP crystals in the LB media. The dissolution of SNAP crystals in ethanol was only used for NOA testing and not for bacterial and fungal studies due to the interference of the antimicrobial activity of ethanol. LB broth and the suspension of cerium oxide nanoparticles were then added. Once a baseline of NO flux without the sample was established in the NO analyzer, the sample was then placed in the sample holder which was maintained at 37 °C by a temperature-regulated water jacket. Nitric oxide released by the sample in the sample holder was swept and purged by a continuous supply of high purity nitrogen maintained at a constant flow rate of 200 mL min−1 towards the chemiluminescence detection chamber. The voltage signal produced was converted to a ppb NO concentration and displayed on the analyzer’s screen. Using the raw data in ppb form and NOA constant (mol ppb−1 s−1), the data in ppb are normalized for the volume of the sample and converted to NO flux units (×10−10 mol mL−1 min−1). Data was collected in the time intervals mentioned and samples were stored in glass vials at 37 °C in dark conditions between measurements. The instrument operating parameters were a cell pressure of 7.4 Torr, a supply pressure of 6.1 psig. and a temperature of −12 °C.
Microbial growth kinetics studies
To determine the antimicrobial potential of SNAP, CNP, and SNAP-CNP, bacterial and fungal suspensions were incubated with varying concentrations of the treatments for 30 h under constant agitation and their optical densities (ODs) recorded in 30-minute intervals. A custom curve-smoothing program was utilized for CNP growth curves to minimize wavering absorbance values attributed by the aggregation of CNP in media. The program takes in the bacterial and fungal growth data and passes it through one iteration of a moving average filter to normalize jagged curves.
Bacterial suspensions were created after LB broth was inoculated with the bacteria (S. aureus or E. coli) and grown to mid-log phase (14 h, 37 °C). Fungal solutions were inoculated with C. albicans and incubated in yeast media in the same conditions. The starting absorbance for each microbe was then adjusted to 0.1 (λ = 600 nm) using a UV-vis spectrophotometer (Thermo-Scientific Genesys 10S UV-Vis) before beginning the study. For each concentration of antimicrobial being tested, 1 mL of bacterial or fungal suspension was combined with 1 mL of the antimicrobial solution and pipetted into a 96-well plate for reading. A blank was prepared for each sample with the antimicrobial compound and the media (without microbes) to calculate final absorbances. CNP and SNAP concentrations of 3, 3.5, 4, 4.5, and 5 mM were used to investigate antimicrobial potential. These specific concentrations were chosen based on the results of preliminary antimicrobial studies revealing the microbial range of sensitivity to both compounds between 3 mM and 5 mM. To scope out the optimal combination of antimicrobial strategies that would inhibit microbial growth, concentrations of 3 mM SNAP + 3 mM CNP and 3.5 mM SNAP + 3.5 mM CNP were tested against bacterial growth, while a wider array of combination concentrations was tested against fungal growth.
Mammalian cell cytotoxicity
3T3 mouse fibroblast cells were revived from cryopreservation and maintained in DMEM supplemented with 10% FBS and 1% PenStrep in 75 cm2 tissue culture treated flasks. Cells were incubated in a humified incubator with 5% CO2 at 37 ºC. Culture medium was changed every two days. Once 70% culture confluency was reached, cells were prepped for cytotoxicity experiments. 3T3 cells were washed, detached with 0.25% (w/v) Trypsin 0.53 mM EDTA solution, and resuspended in DMEM medium. Cells were then seeded in 96-well tissue culture treated polystyrene plates with 10,000 cells per well with 100 μL of medium. After incubation for 24 h and ensuring 70% plate confluency, cells were resuspended in two parts consisting of 500 μL of medium and 500 μL of nanoparticles and/or SNAP dissolved in medium. All treatment solutions were sonicated for ten minutes prior to exposure and filtered using a 0.22 μm filter. After treatment, cells were incubated for varied lengths of time: 1, 2, and 24 h. Afterwards, cells were washed with PBS, resuspended in media, and treated with 10 μL of CCK-8 solution per well. Cells were incubated for an additional 1 h then read for absorbance at 450 nm with background reading at 650 nm. Cells were treated at n = 5 per treatment type.
Statistical Analysis
All data is represented as mean ± standard deviation. Statistical comparison between suspensions was analyzed using an unpaired t test. Values of p < 0.05 were deemed significant.
Results
Physiochemical and biocatalytic characterization of CNP
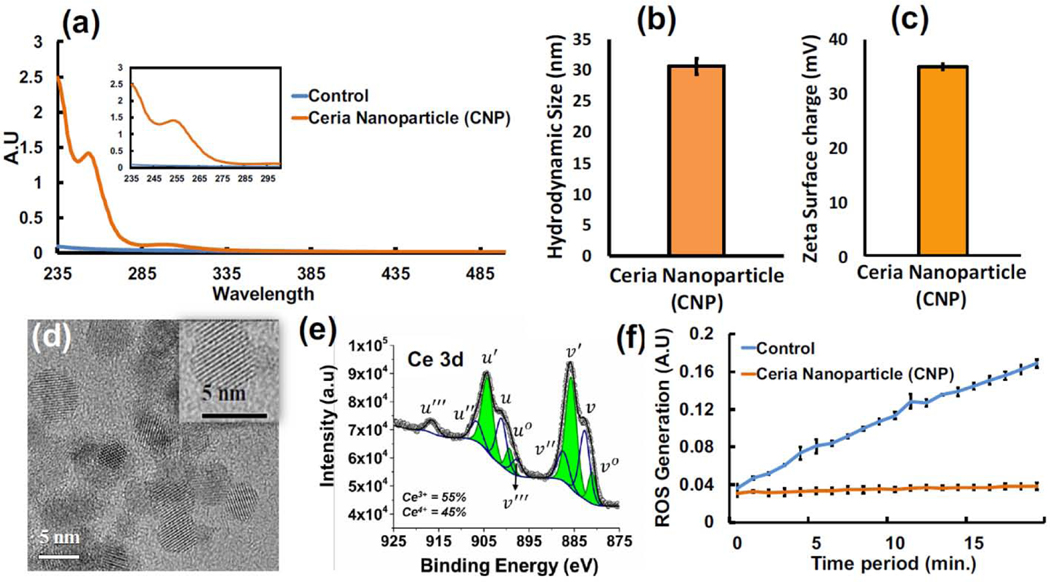
Uniform sized CNP were synthesized using the wet chemistry approach, confirmed by UV-Visible spectroscopy where the peak at 253 nm indicates a higher ratio of Ce3+/Ce4+ oxidative state (Figure 2a). HRTEM images revealed CNP to have a precise size of 5 nm (Figure 2d), indicating the ultra-small size of the synthesized nanoparticle. The mass of an individual CNP was calculated using the density of CNP (7.22 g/cm3) and the volume of a sphere () calculated using the diameter acquired from TEM. The assumption of perfectly spherical particles and the knowledge that mass = density × volume lead to the calculation that one CNP has an approximate mass of 4.725 × 10−19 g. To determine the approximate number of CNP in a 3 mM solution, the molarity (3 mM), molar mass of CNP (172.1 g/mol), and mass of a single CNP (4.725 × 10−19 g) were multiplied to acquire a value of 1.0927 × 1018 CNP per liter of DI water. The hydrodynamic size of CNP in DI water was measured with DLS, and the diameter was found to be 30.6 ±1.3 nm (Figure 2b). Surface area measurements of CNP were calculated using a BET surface area analyzer and the value obtained was 178 m2/g. The zeta potential of 35 ± 0.6 mV (Figure 2c) indicates a highly stable suspension due to the degree of electrostatic repulsion between adjacent particles and resistivity to aggregation.
Figure 2. Ceria Nanoparticle Characterization.
(a) UV-Visible spectroscopy of CNP prepared using wet chemistry, where signature peak at 253nm indicates the surface chemistry dictating more Ce3+ oxidative state. (b) The hydrodynamic size of the synthesized CNPs measured with DLS. (c) The surface zeta charge measurement indicates the CNP suspension to be highly stable. (d) HRTEM image indicates the presence of homogenous suspension of CNP in the size range of 5 nm. (e) Deconvoluted and peak fitted Ce3d spectral envelop of CNP sample shows the variations in spin-orbital component of Ce 3d5/2 and Ce 3d3/2. In Ce3d envelop, v0, v’, u0 and u’ peaks are the characteristic peak of Ce3+ and v, v”, v’’’, u, u”, and u’’’ are belongs to Ce4+ (f) Superoxide dismutase (SOD) mimetic assay used to determine the reactive oxygen species (ROS) scavenging potential. Here the CNP produce high SOD behavior indicating a high antioxidative nature in scavenging ROS molecules. DLS, Zeta analysis, and SOD assay were performed in triplicate (n = 3) and are presented as the mean value ± standard deviation (SD).
Many of the chemical and biological properties of CNP are determined by the Ce3+/4+ ratio. For instance, a higher Ce3+/4+ ratio allows for superoxide scavenging potential of CNP, whereas a lower ratio is associated with scavenging of NO radicals by CNP, attributed to a reduced number of oxygen vacancies on the surface of the particles. Deconvoluted XPS Ce 3d spectra of CNP are shown in Figure 2e where peaks in the spectra can be distinguished and ascribed to unique oxidation states. Peaks at ~880, 885, 898, and 903 eV belong to Ce3+ oxidation states and peaks at ~882, 888, 899, 901, 908, and 916 eV belong to Ce4+ [59]. XPS results confirm that the present material contains both Ce3+ and Ce4+ valence states. The relative concentration of Ce3+ (or Ce4+) oxidation states on the surface of CNPs are calculated using the ratio of the total integral area under the fitted peak of Ce3+ (or Ce4+) to the total area of all peaks, and the concentration of Ce3+ was found to be higher (55%) than the Ce4+ (45%) oxidation state. Biocatalytic activity of synthesized CNP was analyzed using the superoxide dismutase mimetic assay and found to be highly active in dismutating the generated superoxide anions and scavenging the oxidative radicals compared to a control solution without CNP (Figure 2f).
CNP colloidal stability in LB media
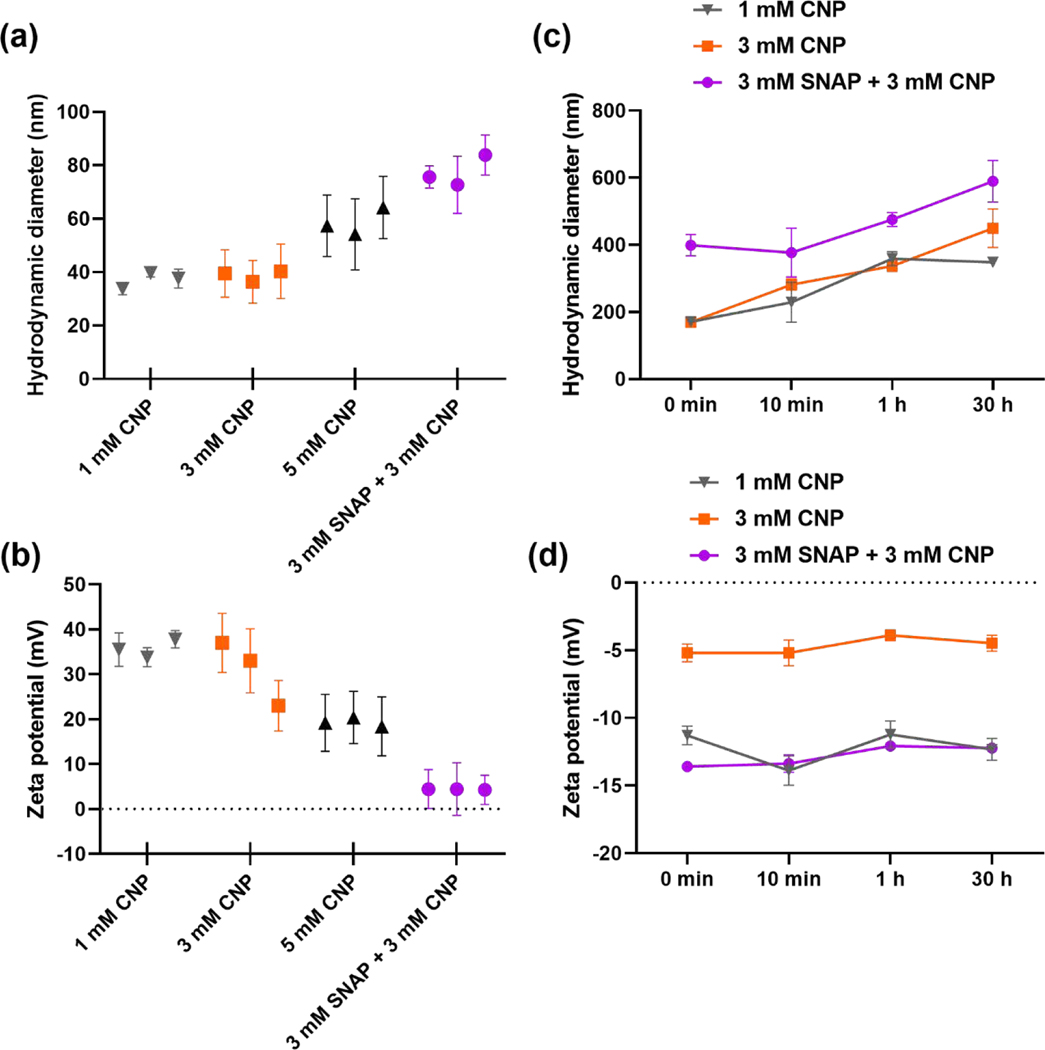
The colloidal stability of CNP as well as SNAP-CNP solutions is an important factor and can largely interfere with their antimicrobial potential. To investigate the impact of CNP concentration on colloidal stability, the hydrodynamic size and zeta potential at 1 mM, 3 mM, and 5 mM CNP concentrations were investigated in DI water. With increasing CNP concentration there is an increase in hydrodynamic diameter from 37.03 ± 2.99 nm for 1 mM CNP to 38.73 ± 2.06 nm for 3 mM CNP followed by 58.58 ± 5.12 for 5 mM CNP (Figure 3a). The addition of 3 mM SNAP to the 3 mM CNP solution further increased the hydrodynamic size to 77.39 ± 5.792, demonstrating that interaction of SNAP with CNP surface increased the hydrodynamic size of the particles. This trend was also observed in the zeta potential measurements of the suspensions of particles as the increase in CNP concentration decreased the zeta charge of the particles (Figure 3b). The 1 mM CNP solution measured 35.7 ± 2.01 mV, 3 mM was 31 ± 7.21 mV, and 5 mM measured 19.33 ± 1.01 mV. The SNAP-CNP solution demonstrated the lowest zeta potential of 4.38 ± 0.11 mV, indicating the combination produces a relatively unstable colloidal suspension.
Figure 3. CNP colloidal stability in LB media.
(a) Hydrodynamic size measurements of CNP and SNAP-CNP in DI water show an increase in diameter with increasing concentration. (b) Zeta potential measurements in DI water reveal decreasing colloidal stability with the increasing CNP content and the addition of SNAP. (c) CNP and SNAP-CNP incubated in LB media for different time points displays an increase in hydrodynamic size with higher concentrations and incubation times. (d) Zeta potential measurements of CNP and SNAP-CNP incubated in LB media reveal no time dependency on colloidal stability, but a stabilizing effect on CNP with the addition of SNAP. Three readings were taken for each sample with n = 3.
After the investigation of CNP concentration and the addition of SNAP on colloidal stability, the same concentrations were immersed in LB media and hydrodynamic size and zeta potential were measured to determine the impact of LB media components on aggregation of CNP. At 0 min of immersion the hydrodynamic size of 1 mM CNP and 3 mM CNP were similar at 171.25 ± 10.54 nm and 169.75 ± 5.87 nm, respectively (Figure 3c). The addition of SNAP to CNP further increased the diameter to 399.1 ± 31.96 nm. From 10 min to 1 h incubation in LB media the diameter increased for all three tested solutions: 1 mM CNP increased from 229.20 ± 59.68 nm to 359.25 ± 21.14 nm, 3 mM rose from 281.4 ± 16.83 nm to 337.0 ± 15.84 nm, and 3 mM SNAP-CNP jumped from 377.3 ± 72.83 nm to 475.35 ± 21.14 nm (Figure 3c). The 30 h readings continued to rise for 3 mM CNP (449.4 ± 57.7 nm) and 3 mM SNAP-CNP (589.35 ± 62.3 nm), but 1 mM CNP decreased slightly to 348.25 ± 15.34 nm.
Zeta potential measurements for CNP and SNAP-CNP incubated in LB media displayed interesting results, particularly with the addition of SNAP to the solution. Upon initial immersion in LB media, 1 mM CNP had a surface charge of –11.3 ± 0.7 mV, 3 mM CNP measured –5.2 ± 0.66 mV, and the addition of 3mM SNAP decreased the charge to −13.6 ± 0.17 mV (Figure 3d). Unlike the hydrodynamic size, the zeta potential did not change significantly over the 30 h period, and the 3 mM SNAP-CNP solution retained the lowest potential, indicating it as the most stable of the 3 solutions tested and least likely to aggregate.
Nitric oxide analysis
Nitric oxide release analysis using the chemiluminescence nitric oxide analyzer (NOA) is the gold standard for measuring NO release and has high reliability and reproducibility. It does not have the shortcomings of including nitrates and nitrites instead of only measuring NO, unlike other detection methods like the Griess assay. The detection method utilizes the following reaction between NO and ozone (O3):
The electronically excited nitrogen dioxide emits a photon in the red and near-infrared region of the electromagnetic spectrum that is detected by the red-sensitive photomultiplier tube. Once the signal is multiplied, it is converted to a ppb or ppm reading. Chemiluminescent NOAs have a detection limit of ~ 1 picomole measurement of NO from liquid samples, allowing for even minute amounts of NO release to be measured. Any simultaneous chemiluminescent reactions taking place because of the incorporation of CNP will not be measured or influence the NO reading due to the light filter on the reaction chamber designed to block all photons not contributed by the NO and O3 reaction.
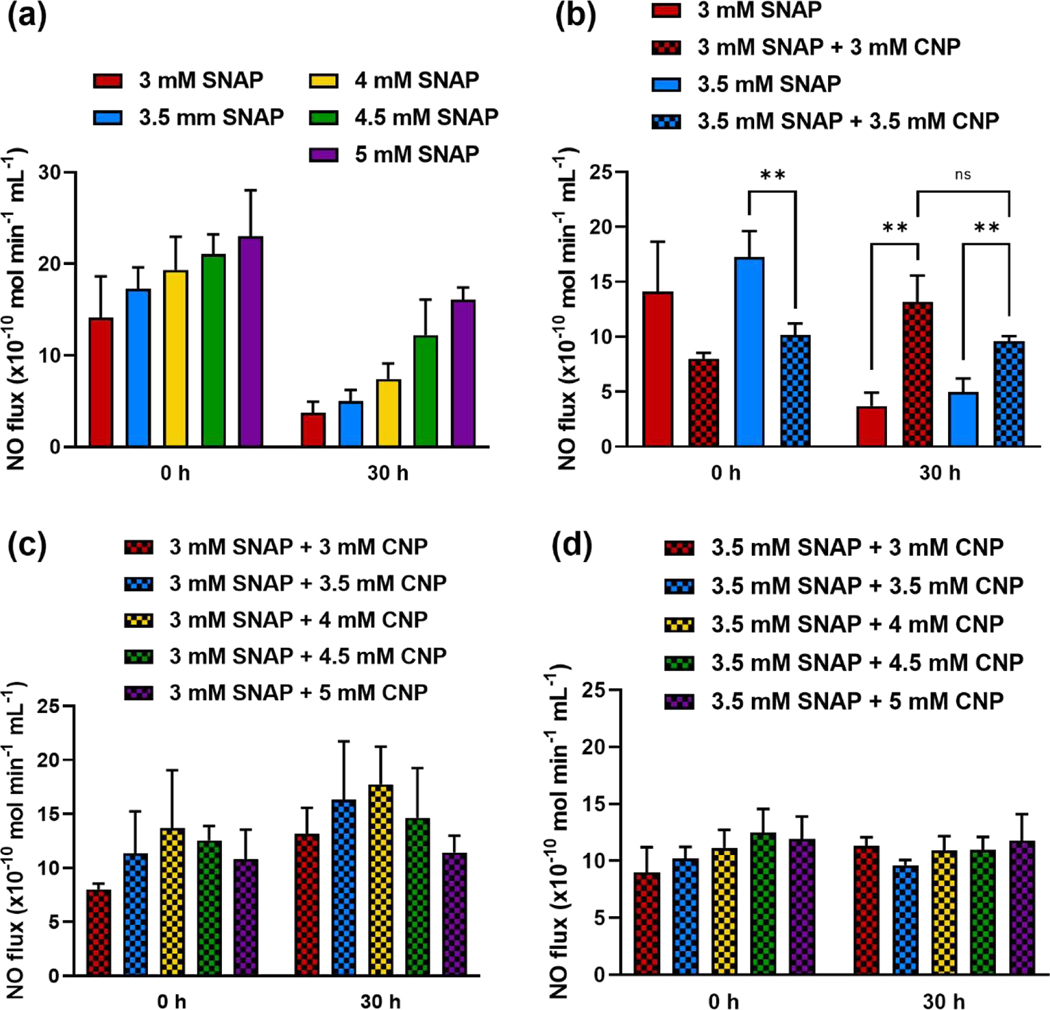
To first determine the NO flux associated with the SNAP concentrations used, solutions of SNAP in LB media were tested in the NOAs at hour 0 and hour 30, corresponding with the time limits of the bacteria and fungus studies. At hour 0, samples reached a stable NO flux after about 30 min ranging from 14.11 to 23.04 (× 10−10 mol min−1 mL−1) (Figure 4a). After being stored at 37 °C in the dark for 30 h, the same samples released a NO flux within the range of 3.70 to 16.06 (× 10−10 mol min−1 mL−1) (Figure 4a), depending on the concentration of SNAP in the sample. There was a direct positive trend of SNAP concentration versus NO flux at both time points and among all SNAP samples. The decrease in NO release at hour 30 is expected and is due to the diminishing of the NO reservoir present in the SNAP molecules after the elapsed time.
Figure 4. Nitric oxide release characteristics of NO donor alone and in combination with CNP.
(a) NO release of samples at 3, 3.5, 4, 4.5, and 5 mM of SNAP in LB media shows a proportional increase in NO release as SNAP content is increased, as well as the reduction in NO release after 30 h for all concentrations. (b) Comparing SNAP to the combination of SNAP-CNP reveals the minimized burst release at 0 h and prolonged NO activity of the suspension at 30 h. (c) NOA measurements of 3 mM SNAP-CNP combinations and (d) 3.5 mM SNAP-CNP combinations reveal a stabilized NO release over the 30-h period. Data represent mean ± SD (n = 3). ** indicates a statistical significance (p < 0.01).
The combination of SNAP and CNP in LB media was then assessed to determine the effect of CNP on NO release from the donor molecule. Five concentrations of CNP were added to solutions of 3 mM and 3.5 mM SNAP in media. Direct comparison of 3 mM SNAP with 3 mM SNAP-CNP and 3.5 mM SNAP with 3.5 mM SNAP-CNP demonstrates a decrease in NO flux at hour 0, but an increase of NO flux at hour 30 when CNP is added (Figure 4b). In fact, 3 mM SNAP-CNP released 76.29% less NO than 3 mM SNAP at hour 0 but released 71.86% more NO than 3 mM SNAP at hour 30. Similarly, 3.5 mM SNAP-CNP released 69.66% less than 3.5 mM SNAP alone at hour 0 but 48.09% more at hour 30. It is hypothesized that the decreased burst release and increased NO release lifespan is due to the biocatalytic superoxide scavenging potential of CNP. When SNAP produces NO in solution, the NO radicals react with other molecules, forming alternate reactive oxygen species (ROS) and reactive nitrogen species (RNS). These reactive species can attach to thiol groups present in the solution from the NO donor decomposition, react with oxygen, and act catalytically to break down the NO donor at a faster rate [60, 61]. When CNP scavenge free ROS in the solution, they increase the lifespan of the SNAP molecule by decreasing the rate of decomposition leading to NO release, allowing for substantial NO release from the solution even at 30 h (Figure 4).
This trend also follows when assessing NO release from 3 mM and 3 mM SNAP mixed with all 5 doses of CNP. The lowest SNAP concentrations were chosen to demonstrate the influence of even the smallest amount of NO donor with the addition of the nanoparticles. Figure 4c–d displays the NO release of the 3 mM SNAP-CNP combination suspensions where at hour 0 the NO fluxes from 3 mM SNAP combinations range from 8.01 to 13.71 (× 10−10 mol min−1 mL−1) with 3 mM SNAP + 4 mM CNP attributing the greatest NO release. Unlike SNAP alone which has a slight burst release and then a decreasing NO flux as time progresses, the addition of CNP leads to a slightly increased flux at hour 30 with a range of 11.42 to 17.74 (× 10−10 mol min−1 mL−1). The 3.5 mM SNAP-CNP combinations also display the consistent NO release levels with a flux of 9.00 to 12.47 (× 10−10 mol min−1 mL−1) at hour 0 and a flux of 9.62 to 11.76 (× 10−10 mol min−1 mL−1) at hour 30.
Bacterial growth kinetics studies
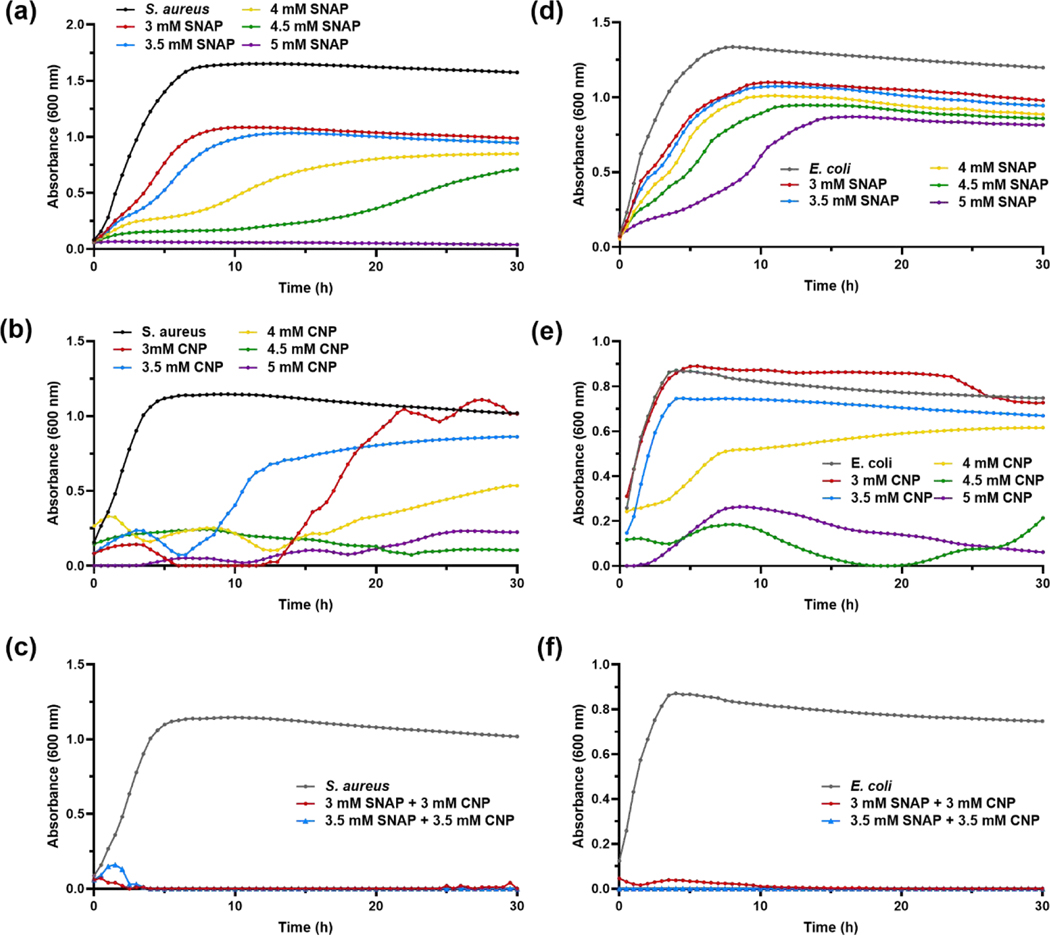
Both NO and CNP have been previously studied for their antibacterial properties, but the combination has never been investigated for antimicrobial activity. S. aureus and E. coli growth kinetics were measured and recorded for 30 h while treated with SNAP alone, CNP alone, and the combination strategy of SNAP and CNP to determine antibacterial efficacy. Figure 5a–c displays results for the Gram-positive S. aureus growth in different conditions. As shown in Figure 5a, SNAP was able to decrease overall bacterial growth of S. aureus and stunt the progression of growth with concentrations as low as 3 mM, with increasing concentrations attributing to higher antibacterial activity. The 5 mM treatment was the only concentration able to completely eradicate S. aureus propagation, but Figure 5a demonstrates that increased SNAP concentration leads to an increased inhibition time span. Figure 5b shows that CNP treatment appeared to inhibit S. aureus growth for the first 10–15 h, but bacteria treated with 3 mM and 3.5 mM CNP quickly multiplied to nearly reach the untreated bacterial growth by hour 20. Only the two highest concentrations of 4.5 mM and 5 mM had a substantial impact on the Gram-positive bacteria. From Figure 5c we see that a combination treatment proved to be the most successful at preventing S. aureus generation. Both 3 mM SNAP-CNP and 3.5 mM SNAP-CNP killed bacteria in all wells and did not allow for a secondary burst in bacterial growth, while the control wells grew exponentially and quickly reached the stationary phase within 6 h.
Figure 5. Bacterial growth studies.
(a-c) S. aureus and (d-f) E. coli treatment with (a, d) SNAP, (b, e) CNP, and (c, f) SNAP-CNP display the synergistic effect of the SNAP-CNP treatment compared to SNAP and CNP alone at the same concentrations. Data represent the mean for each time point (n = 3).
E. coli growth curves showed similar trends. Figure 5d demonstrates the slight decrease in absorbance, corresponding to microbial growth, as SNAP concentrations (therefore NO release) are increased. Although it is unable to completely inhibit proliferation, 5 mM SNAP slows E. coli growth up to 5 h. CNP was slightly more effective at killing E. coli, but only at 4.5 mM CNP concentration or higher (Figure 5e). Nevertheless, both SNAP-CNP concentrations were bactericidal against Gram-negative E. coli, as shown in Figure 5f. Unlike even the highest treatment of CNP, the combination strategy killed the bacteria immediately, rather than allowing low levels of E. coli to multiply.
Fungal growth kinetics studies
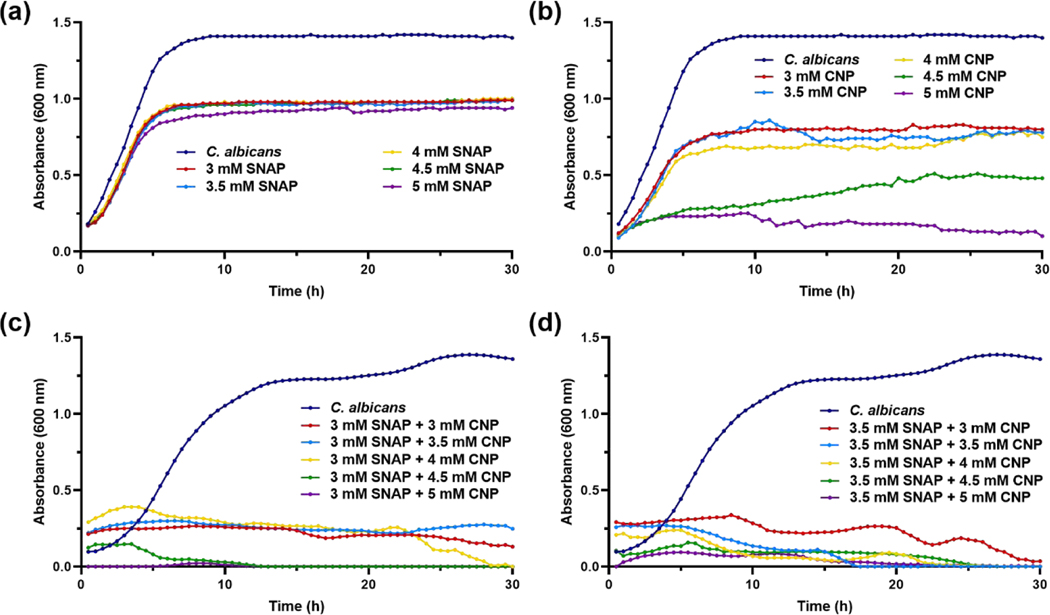
Nitric oxide alone is known to have potent antibacterial effects against both Gram-negative and Gram-positive bacteria, but as C. albicans and other fungi have an inducible NO defense mechanism [62], it may not be enough to eradicate the infectious microbe. To increase the broad-spectrum activity of NO released from SNAP, the donor molecule was mixed into a suspension with varying concentrations of CNP in LB media. However, each treatment, SNAP and CNP, was tested independently against C. albicans before the combination treatment to determine the presence and extent of a synergistic effect. As shown in Figure 6a, SNAP did not have any significant effect on the growth of the fungus even at the highest dose. CNP, known to be an effective antifungal treatment, are successful in suppressing C. albicans growth at concentrations of 4 mM –5 mM, but are ineffective at the lower doses (Figure 6b). The combination strategy of SNAP-CNP versus C. albicans proves to be much more potent, as 3 mM and 3.5 mM SNAP-CNP treatments are the most competent at all doses (Figure 6c–d).
Figure 6. Fungal growth studies.
C. albicans treated with (a) SNAP, (b) CNP, and (c-d) SNAPCNP display the ineffectiveness of SNAP, range of efficacy with CNP, and complete eradication with the combination treatment. Data represent the mean for each time point (n = 3).
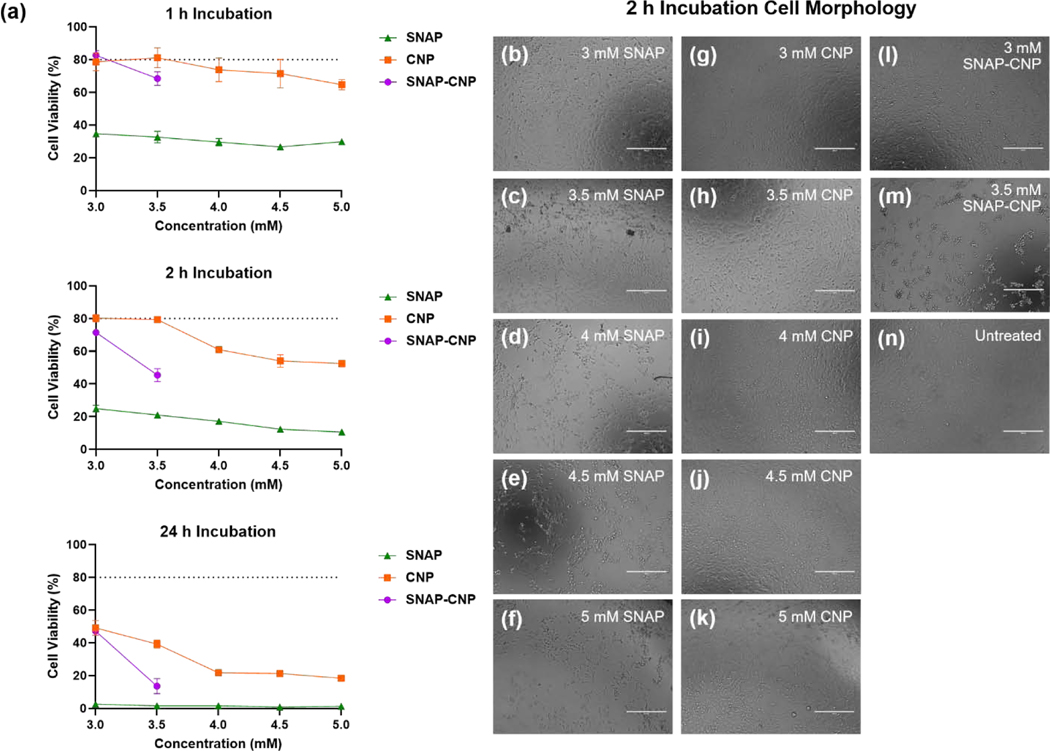
Mammalian cell cytotoxicity
Since the combination of SNAP and CNP was successful in decreasing microbial viability, it is important to verify that the treatment is biocompatible with mammalian cells, indicating a potential solution in effectively treating infections in vivo. Different concentrations of SNAP, CNP, and SNAP-CNP were incubated with 3T3 mouse fibroblast cells for 1 h, 2 h, and 24 h to determine the time and concentration dependent toxicity of the solutions (Figure 7a). At all time points, SNAP alone is toxic at concentrations of 3 mM to 5 mM and it is determined that toxicity is more dependent on time of exposure rather than concentration. Nanoceria treatment of fibroblasts is the most cytocompatible at all time points and concentrations, though there is no significant difference between 3 mM CNP and 3 mM SNAP-CNP after 24 h of incubation. There is a decrease in cell viability with increasing CNP concentrations, but after 4 mM CNP there is little change in toxicity. The combination of SNAP and CNP appears to stabilize the toxicity of SNAP alone, likely through the ROS scavenging activity of CNP decreasing the oxidative and nitrosative stress on the cells caused by SNAP. Fibroblast morphology after 2 h of incubation reveals the toxic nature of the SNAP solutions and CNP concentrations greater than 3.5 mM (Figure 7b–n). Morphological changes in the cells such as loss of striation are seen in most treatments but are more pronounced in the 3.5 SNAP-CNP treatment when compared to the 3 mM SNAP-CNP treated and untreated cells. The 3mM SNAP-CNP is the most promising treatment as it is composed of both antimicrobial treatments and does not confer greater toxicity than CNP alone. However, the cytotoxicity even after 2 h is of concern as a patient treatment and alternative methods of delivering the combination therapy is necessary.
Figure 7. Mammalian cell cytotoxicity of SNAP, CNP, and SNAP-CNP.
(a) Fibroblast cell viability decreases with increasing incubation times, revealing the highly toxic nature of SNAP and the ROS scavenging capabilities of CNP alone and in combination with SNAP. Data represent the mean for each concentration at each time point (n = 5). (b) Cell morphology after 2 h of incubation with CNP, SNAP, or SNAP-CNP treatments reveals the toxicity of higher concentrations. Scale bar = 400 μm
Discussion
Numerous biomedical applications have been reported with the incorporation of CNP that are synthesized using different routes, as the physiochemical and biocatalytic properties entirely depend on the route of synthesis. These different methods include the wet chemical process [63], hydrolysis process [64], sol-gel synthesis [65], microemulsion process, sonochemical synthesis [66], spray pyrolysis [67], and gas condensation methods [68]. Size, surface zeta potential, dispersion stability, and surface chemistry are the different attributes associated with nanoparticle synthesis and can influence the CNP functional interaction, reaction chemistry, and antimicrobial potency. In CNP, irrespective of the dynamic physical properties, it is the Ce+3/Ce+4 ratio which regulates the surface chemistry and influences the catalytic functional activity. Here, manipulating the surface charge ratio of CNP can alter the functional catalytic activity of synthesized CNP. In this study, we synthesized ultra-small cerium oxide nanoparticles (CNP) and characterized them for their different physiochemical properties. These CNP were prepared by wet chemical methodology, which leads to uniformly distributed 5 nm CNP as visualized using the high-resolution electron microscope (Figure 2d). The hydrodynamic diameter (30.6 ± 1.3 nm, Figure 2b) and zeta potential measurements (35 ± 0.6 mV, Figure 2c) confirm the highly dispersed and stable nature of the colloidal suspension in DI water. As per a published report, the specific surface area of CNP is 166 m2/g when calculated using the Sauter formula [69]. Measurements using a BET surface area analyzer found synthesized CNP to have a surface area of 178 m2/g, which is quite close to the theoretical value. The size of the nanoparticle is important as it plays a critical role in antimicrobial activity. Smaller nanoparticles have a larger surface area associated with it which has higher interaction opportunities with its surrounding environment. Smaller sized CNP are more efficient in inducing antimicrobial activity by the surface-based redox chemistry. It is also important to note that smaller nanoparticles utilize simpler diffusion, endocytosis, and exocytosis routes through the cell membranes of microbial systems, inducing more potent potential effects [70–72]. On the other hand, the larger or more aggregated nanoparticle might interact with negatively charged bacterial cell membranes, interfering with inherent functional activity of the membrane and leading to potential antimicrobial effects of the nanoparticle [73].
The biocatalytic activity of CNP was also investigated, as we have previously shown that CNP can scavenge oxidative radicals in biological systems [74, 75]. Superoxide dismutase mimetic activity analyzed for this CNP was found to be high due to the high Ce+3/Ce+4 ratio (Figure 2e–f) and is in accordance with our previously published results, where we have dissected that higher Ce+3/Ce+4 ratio of surface chemistry on CNP leads to high scavenging of superoxide radicals and high catalytic activity.
Before investigating the antimicrobial efficacy of the combination of SNAP and CNP, the colloidal stability of the particles must be understood based on concentration, molecule-particle interaction, and LB media components adsorption. As shown by the hydrodynamic size measurements, 1 mM, 3 mM, and 5 mM CNP in DI water revealed an increase in diameter with an increase in concentration (Figure 3a). This is expected, as an increase in concentration leads to greater particle-particle interaction within the solution and further aggregation as well. The introduction of SNAP to the CNP solution also increased the hydrodynamic diameter, which could be due to the charge neutralization that decreases the electrostatic repulsion between nanoparticles leading to aggregation of CNP. It was shown previously that the interaction of nanoparticles with biological molecules passivates the surface and alters the stability [76]. Similar observations were made in the zeta potential measurements of CNP and SNAP-CNP in DI water. As CNP concentration was increased and as SNAP was added, the zeta potential decreased closer to zero (Figure 3b). It is important to note that a steady suspension of CNP in DI water followed by agglomeration after the addition of SNAP is going to be profoundly identified with the zeta potential change. However, agglomeration is certainly not a basic result of a zeta potential shift to zero. Stabilization or agglomeration of the CNP suspension could be incited by changes in surface chemistry, including surface associations among biomolecules and CNP. SNAP molecules with marginally certain charge centers cannot be effectively adsorbed onto the surfaces of positively charged CNP. Rather, they can be lightly bound to the NP surface by means of association between Ce3+/Ce4+ and oxygen functional groups. Due to the noteworthy surface charge decrease of CNP when combined with SNAP, it was considered that there was conceivable interplay between CNP and SNAP which made SNAP-CNP assemblies, prompting unstable colloidal suspensions and leading to a lower zeta potential. There are other important biological factors influencing colloidal stability which need to be considered such as exposure time, temperature, pH, physiochemical properties of nanoparticles (size, surface charge, surface functional groups, hydrophobic/hydrophilic behavior), relative ratio of NP to physiological media, etc.. However, in this study the antimicrobial efficacy was not affected by the colloidal stability. In fact, further stabilization of the suspension may lead to increased antimicrobial potential, though ideal stability was not achieved in these studies.
After gaining an insight into the effect of increasing CNP concentration and adding SNAP to the aggregation tendencies of the solutions, two concentrations of CNP and one concentration of SNAP-CNP were added to LB media to investigate the interaction of media components with the ultra-small CNP. After immediate dispersion in LB media, the hydrodynamic size of 1 mM CNP, 3 mM CNP, and 3 mM SNAP-CNP rose sharply compared to solutions of DI water, with 3 mM SNAP-CNP measuring about double the diameter of CNP alone in solution (Figure 3c). This effect is likely due to the media components adsorbing onto the surface of CNP and SNAP molecules, forming a corona and greatly increasing the measured hydrodynamic size. This has been shown to occur when NP were exposed to fetal bovine serum and PBS in a previous study [77]. The diameter continued to increase after LB exposure times of 10 min, 1 h, and 30 h for 3 mM CNP and 3 mM SNAP-CNP whereas 1 mM CNP stabilized in size after 1 h, which indicates that the nanoparticle concentration as well as incubation time plays a vital role in stability. Interestingly, the zeta potential measurements revealed that this larger 3 mM SNAP-CNP particle had the most negative zeta potential after immediate dispersion and after 30 h of incubation in LB media (Figure 3d). The increase from 1 mM CNP to 3 mM CNP revealed a more destabilized colloidal suspension as the zeta potential rose from –11.3 mV to –5.2 mV, respectively. However, upon the addition of SNAP, the zeta potential decreased to –13.6 mV, indicating increased colloidal stability when SNAP was added. Taking these results into account, it is hypothesized that a more stable suspension could be developed by combining SNAP with 1 mM, or even lower CNP concentrations in LB media, leading to a stable zeta potential that is made increasingly stable with SNAP inclusion, though further studies are needed to confirm this. In any case, similar observations of charge inversion have been recorded with hydroxide core formation around gold nanoparticles adsorbed with bovine serum albumin [78]. The changes in aggregation and colloidal stability raise interesting concerns about the antimicrobial efficacy of the combination, but microbial studies demonstrate the potency of the treatments despite aggregation and protein corona formation when CNP are exposed to LB media. Moreover, reports have been presented where high colloidal stability have been obtained at high particle concentration in cell culture medium owing to controlled and robust protein corona formation around the nanoparticle surface [79]. Similarly, here LB media components form a protein corona around the CNP and SNAP-CNP particles, though since it is uncontrolled and seemingly random, ideal stability is not achieved. Although addition of SNAP interferes with the zeta surface charge in the presence of LB media components, the most stable charge measured was –13.6 mV, well higher than the recognized value of ~ ± 30 mV required for complete stability. In future studies, higher stability can be achieved by adding biocompatible stabilizing reagents (e.g., cetyltrimethylammonium bromide (CTAB), citrate, polyacrylic acid, etc.) to the CNP. However, the concentration of stabilizing agents on the NP surface need to be optimized for the ligand exchange process with SNAP molecules. Also, CTAB has associated cytotoxicity risks and should be avoided if possible. Other possible stabilizing methods that have been shown effective are the precipitation and redispersion of CNP with poly(acrylic acid) [80], and dextran-coating of CNP [81]. Each of these techniques should be explored and optimized for antibacterial effect and SNAP interaction before utilized further. In any case, creating a more stable SNAP-CNP solution will in theory provide an even greater antimicrobial capacity due to the greater surface area/volume ratio and thus increased particle-microbe interaction, providing antimicrobial effects beyond the substantial results found with the current unstable combination treatment. It is also important to note that no flocculation was observed in any of the concentrations and at all time points.
The antimicrobial activity of CNP has revealed a wide range of success depending on the synthesis method, Ce3+/Ce4+ ratio, and nanoparticle size, but has been shown to be effective against bacteria and fungi under the right synthesis conditions. Additionally, SNAP, the chosen NO donor, has a history of high bactericidal action against both Gram-negative and Gram-positive bacteria due to its multi-mechanistic approach. The unique antimicrobial talents of both components have never been harnessed in one application, creating a broad-spectrum treatment requiring lower doses of each therapy. Moreover, the addition of CNP to the SNAP solution subdues the burst release of NO at hour 0, leading to a higher NO release at hour 30 (Figure 4b). We hypothesize that this protective effect on the SNAP molecule is due to the ROS scavenging capabilities of CNP which are due to the high Ce3+/Ce4+ ratio [21]. Nitric oxide released from the SNAP molecule interacts with oxygen and other molecules in the solution to form ROS and RNS that can react with free thiol groups. These thiol radicals can react with the SNAP molecule to increase the decomposition rate and NO release from the donor [60, 61]. When CNP is added to the solution is it able to scavenge free ROS so that the SNAP molecule decomposition is catalyzed by heat alone rather than radical attack.
Figure 5a–c shows the capability of the combination against a Gram-positive bacterium, S. aureus where although both SNAP and CNP can stall the growth of the microbe at the highest concentration tested, 5 mM, the combination treatment stops proliferation entirely at the very lowest dose, 3 mM. In fact, the growth curve reveals that the microbe is killed within the first 3 h of exposure to the mixed suspension, whereas the untreated bacteria propagate rapidly, reaching the stationary phase in about 5 h. The E. coli growth studies display similar results (Figure 5d–f). E. coli treated with SNAP alone showed almost no inhibition at the chosen doses. This is not a surprising find, as NO is generally not as potent against Gram-negative bacteria due to the outer membrane resisting penetration by the molecule. Similar to the outcome of CNP treated S. aureus, E. coli growth was inhibited by concentrations above 4.5 mM, though not killed entirely. However, upon exposure to 3 mM SNAP-CNP and 3.5 mM SNAP-CNP bacterial growth was thwarted completely within the first hour, demonstrating the potency of the combination dose when the individual treatments at that dose had almost no effect on the microbe. Further proof of this phenomenon is shown when the suspensions are tested against C. albicans, a common infection fungus. In Figure 6, SNAP once again has a negligible effect against proliferation of the microbe, while CNP is bactericidal at concentrations of 4 mM and above. However, when SNAP and CNP are combined, the suspension halts growth of the fungus entirely.
The secret of the effectiveness of the combined treatments lies in their individual multi-mechanistic antimicrobial approaches. CNP induces the antimicrobial effects through different mechanisms involving but not limited to the adsorption to outer cellular surfaces, oxidoreduction mechanism based on its surface valance state, and by inducing toxicity through its intracellular interaction. Electrostatic interaction induces the adsorption phenomenon which might lead CNP to interfere with the bacterial cellular surface transport or modify the numerous ion transport pumps necessary for bacterial growth and function. Further, the oxidation reduction of CNP might take place at the outer surface of the microbes, interfering the Ce+3/Ce+4 ratio which may meddle with the cellular surface proteins and lipids, inhibiting the functionality of the membrane. This oxido-reduction process also interferes with and hampers the electron gradient within the antimicrobial system, leading to electron transport inhibition. It is important to note that no reduction of Ce4+ ions of CNP takes place within the in vitro cellular medium [82, 83]. Reports also indicate that CNP interfere with the cellular gene expression leading to the impairment of the bacterial respiration. This has been observed in E.coli where low levels of essential enzymes like succinate dehydrogenase and cytochrome b terminal oxidase have exhibited low levels of expression after treatment with CNP [84]. Similarly, CNP interaction with C. albicans interferes with the cell wall enzymatic activity, inhibiting its growth and acting as a potent antifungal agent [30].
Nitric oxide’s repertoire of antimicrobial mechanisms begins with NO and its byproducts including peroxynitrites, nitrogen dioxides, hydroxides, and dinitrogen trioxides, among others [46]. These reactive nitrogen and oxygen intermediates inflict devastating nitrosative and oxidative stress to various parts of microbes. DNA deamination can occur, as well as damage to DNA repair systems, obliterating the organism’s ability to correct alterations. NO can also oxidize proteins and lipids, inhibit metabolic enzymes, and impede signal transduction, preventing further microbial growth, and most likely killing the organism altogether [46]. Membrane transporters can also be obstructed or destroyed through thiol nitrosation and nitrosation of amines. With the wide-spread attack of NO and its intermediates, no known microbes have yet developed resistance to NO, unlike most widely used antibiotics.
The combined antimicrobial efficacy of SNAP and CNP is due to multiple facets. Undoubtedly, there are two antimicrobial mechanisms at work rather than one treatment with a single mechanism. Nitric oxide itself has multiple instruments of antimicrobial attack and CNP’s role in antimicrobial activity helps to strengthen the overall attack. However, there is another facet at work in the combination system that is attributed to its success. As mentioned above, the high Ce3+/Ce4+ ratio characteristic of the synthesized CNP grant ROS scavenging capabilities, whereas a low Ce3+/Ce4+ ratio allows for RNS (including NO) scavenging abilities that have been demonstrated in previous studies [21]. Nonetheless, rather than scavenging all of the NO produced from the SNAP molecules, CNP scavenging ROS prevents those oxygen radicals from reacting to form thiol radicals that break down the SNAP molecule and increase the rate of SNAP decomposition. Therefore, the ROS-protected SNAP molecule has a more prolonged NO release compared to SNAP without CNP at the same concentration. Thus, some of the synergistic antimicrobial effects of the combination therapy may be due to the increased NO release from the preserved SNAP molecules.
Not only did the antioxidative nature of CNP increase NO release from the SNAP molecules, but it also decreased the mammalian cell cytotoxicity compared to SNAP alone (Figure 7a). After incubation times ranging from 1 h to 24 h, 3 mM –5mM SNAP solutions were highly toxic to mouse fibroblast cells. Ceria nanoparticles in that same range were significantly less toxic, but still revealed relatively low cell viability even after only 2 h, which may partially be due to aggregation and settling of CNP on top of the cells in the well plate. However, this result is not surprising due to the Ce3+/Ce4+ ratio of the synthesized particles. Previous studies have shown that the main indicator of toxicity is the percentage of surface Ce3+ sites on the surface of CNP, revealing that a surface content of 58% Ce3+ is highly toxic [85]. Since the CNP synthesized for this study have a Ce3+ surface content of 55% (Figure 2e), it is not surprising that cell viability was so low after treatment. The fascinating result of the study was that adding CNP to SNAP greatly increased cell viability compared to just SNAP. In fact, at 3mM, there is no significant difference between cell viability at 1 h and 24 h of treatment between CNP alone and the SNAP-CNP combination (Figure 7a). This stability of cell viability is likely the cause of CNP scavenging excess ROS and RNS created by NO radicals reacting with oxygen and other molecular species.
Although it is a compelling finding, the cell viability is too low to utilize the current solution as an antimicrobial treatment. Fortunately, CNP have been successfully stabilized by coatings [81] and dispersion in materials such as bioglass [86] and polymer scaffolds [87–90] in previous studies, allowing for antioxidative effects without cytotoxicity observed by direct contact with mammalian cells. Many medical-grade polymers have also been successfully impregnated with SNAP and other NO donors which demonstrate a prolonged release of NO anywhere from a few hours to several months [36, 47, 50, 51, 53, 91–97]. Quite recently, our lab has investigated the combination of SNAP and nanoparticles in polymer composites for antimicrobial purposes with promising results [34–36, 98].
To our knowledge, this is the very first study investigating the interaction of a NO donor with CNP and exploring the antimicrobial potential of this combination. Future studies are aimed at incorporating SNAP and CNP into a polymer scaffold and investigating the antioxidant and antimicrobial potential of the composite. Ideally, the combination therapy will allow for lower doses of SNAP and CNP impregnated in the polymer for treating infections and can be utilized in urinary and vascular catheters, insulin cannulas, and for other biomedical devices or device coatings. With the dual-action antimicrobial SNAP-CNP treatment, medical devices could be transformed into broad-spectrum antimicrobial biomaterials, fighting infectious bacteria and fungi.
Conclusion
Overall, we have shown that combining the nitric oxide donor, S-Nitroso-N-acetyl-penicillamine (SNAP), with highly antioxidative cerium oxide nanoparticles (CNP) induces a synergistic effect as highly antimicrobial solutions. Despite the decreased colloidal stability of the combination suspension, the treatments were efficacious in inhibiting the growth kinetics of S. aureus, E. coli and C. albicans, microbes responsible for devastating hospital-acquired infections in many patients. Although there have been mixed results regarding the antimicrobial efficacy of CNP in the past due to the many synthesis parameters that can alter their biological activity, the ultrasmall CNP synthesized in this study provide effective antimicrobial action. Furthermore, the combination of SNAP and CNP lead to greater microbial killing, unlike previous studies showing CNP use in tandem with the antibiotic ciprofloxacin [29] as well as magnesium and potassium salts [81] resulting in lower and even abolished antimicrobial activity. The success of the combination therapy is due to the protective effect on the SNAP molecules owing to the ROS scavenging potential of CNP, allowing for prolonged NO release and more potent antimicrobial effects at lower doses. Metal nanoparticles such as copper [34, 35] and zinc oxide [36, 37] have been shown in the past to boost NO release from biomedical polymers, but through the catalytic decomposition of the donor molecules. This is the first study to display an increased and extended NO release from SNAP through the preservation of the NO donor by metal nanoparticles and opens new doors to investigating NO donors in combination with antioxidative therapies for enhanced NO release. The results obtained suggest the potential application of NO combined with CNP for further broad-spectrum antimicrobial testing and biomedical application of antimicrobial coatings on medical devices for numerous therapeutic applications. Just as SNAP has been combined with nanoparticles in polymers films in previous studies, our future studies hope to determine the efficacy of the SNAP-CNP treatment embedded in a medical-grade polymer. This will lead to a flurry of research that focuses on combining such small molecules and particles that synergistically produce antimicrobial efficacy at lower treatment doses.
Acknowledgement
The authors gratefully acknowledge the support of Material Characterization Facility (MCF) provided by the Advanced Materials Processing and Analysis Center and Material Science and Engineering Department for the characterization of different material employed in this manuscript.
Funding
This work was supported by National Institutes of Health (NIH) grant R01HL134899 and National Science Foundation (NSF) MRI XPS: ECCS:1726636 for XPS measurements.
Footnotes
Authorship Contribution Statement
Lori M. Estes: Writing –original draft, Investigation, Methodology, Validation, Visualization. Priyadarshini Singha: Investigation, Methodology, Writing –review and editing. Sushant Singh: Investigation, Visualization, Writing –review and editing. Tamil S. Sakthivel: Investigation, Validation, Writing –review and editing. Mark Garren: Investigation, Visualization, Writing –review and editing. Ryan Devine: Investigation, Writing –review and editing. Elizabeth Brisbois: Conceptualization, Supervision, Writing –review and editing. Sudipta Seal: Conceptualization, Funding acquisition, Supervision, Resources. Hitesh Handa: Conceptualization, Funding acquisition, Supervision, Resources, Writing –review and editing.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].October News, Clinical Infectious Diseases 53(7) (2011) i–ii. [Google Scholar]
- [2].Health Care-Associated Infections, 2020. https://health.gov/hcq/prevent-hai.asp. 2020).
- [3].About Antibiotic Resistance, 2019. https://www.cdc.gov/drugresistance/about.html. 2020).
- [4].Antimicrobial resistance. https://www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resistance. 2020).
- [5].C.f.D.C.a. Prevention, How Antibiotic Resistance Happens, 2020. https://www.cdc.gov/drugresistance/about/how-resistance-happens.html.
- [6].Kapoor G, Saigal S, Elongavan A, Action and resistance mechanisms of antibiotics: A guide for clinicians, Journal of anaesthesiology, clinical pharmacology 33(3) (2017) 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang L, Gu F, Chan J, Wang A, Langer R, Farokhzad O, Nanoparticles in medicine: therapeutic applications and developments, Clinical pharmacology & therapeutics 83(5) (2008) 761–769. [DOI] [PubMed] [Google Scholar]
- [8].Liu Z, Liu J, Wang R, Du Y, Ren J, Qu X, An efficient nano-based theranostic system for multi-modal imaging-guided photothermal sterilization in gastrointestinal tract, Biomaterials 56 (2015) 206–218. [DOI] [PubMed] [Google Scholar]
- [9].Yang X, Yang J, Wang L, Ran B, Jia Y, Zhang L, Yang G, Shao H, Jiang X, Pharmaceutical Intermediate-Modified Gold Nanoparticles: Against Multidrug-Resistant Bacteria and Wound-Healing Application via an Electrospun Scaffold, ACS Nano 11(6) (2017) 5737–5745. [DOI] [PubMed] [Google Scholar]
- [10].Sanvicens N, Marco MP, Multifunctional nanoparticles –properties and prospects for their use in human medicine, Trends in Biotechnology 26(8) (2008) 425–433. [DOI] [PubMed] [Google Scholar]
- [11].Cimini A, D’Angelo B, Das S, Gentile R, Benedetti E, Singh V, Monaco AM, Santucci S, Seal S, Antibody-conjugated PEGylated cerium oxide nanoparticles for specific targeting of Abeta aggregates modulate neuronal survival pathways, Acta Biomater 8(6) (2012) 2056–67. [DOI] [PubMed] [Google Scholar]
- [12].Eitan E, Hutchison ER, Greig NH, Tweedie D, Celik H, Ghosh S, Fishbein KW, Spencer RG, Sasaki CY, Ghosh P, Das S, Chigurapati S, Raymick J, Sarkar S, Chigurupati S, Seal S, Mattson MP, Combination therapy with lenalidomide and nanoceria ameliorates CNS autoimmunity, Exp Neurol 273 (2015) 151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dowding JM, Song W, Bossy K, Karakoti A, Kumar A, Kim A, Bossy B, Seal S, Ellisman MH, Perkins G, Self WT, Bossy-Wetzel E, Cerium oxide nanoparticles protect against Abeta-induced mitochondrial fragmentation and neuronal cell death, Cell Death Differ 21(10) (2014) 1622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen J, Patil S, Seal S, McGinnis JF, Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides, Nat Nanotechnol 1(2) (2006) 142–50. [DOI] [PubMed] [Google Scholar]
- [15].Das S, Chigurupati S, Dowding J, Munusamy P, Baer DR, McGinnis JF, Mattson MP, Self W, Seal S, Therapeutic potential of nanoceria in regenerative medicine, MRS Bulletin 39(11) (2014) 976–983. [Google Scholar]
- [16].Sack M, Alili L, Karaman E, Das S, Gupta A, Seal S, Brenneisen P, Combination of conventional chemotherapeutics with redox-active cerium oxide nanoparticles--a novel aspect in cancer therapy, Mol Cancer Ther 13(7) (2014) 1740–9. [DOI] [PubMed] [Google Scholar]
- [17].Alili L, Sack M, von Montfort C, Giri S, Das S, Carroll KS, Zanger K, Seal S, Brenneisen P, Downregulation of tumor growth and invasion by redox-active nanoparticles, Antioxid Redox Signal 19(8) (2013) 765–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sack-Zschauer M, Karaman-Aplak E, Wyrich C, Das S, Schubert T, Meyer H, Janiak C, Seal S, Stahl W, Brenneisen P, Efficacy of Different Compositions of Cerium Oxide Nanoparticles in Tumor-Stroma Interaction, J Biomed Nanotechnol 13(12) (2017) 1735–1746. [DOI] [PubMed] [Google Scholar]
- [19].Xu C, Qu X, Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications, Npg Asia Materials 6 (2014) e90. [Google Scholar]
- [20].Korsvik C, Patil S, Seal S, Self WT, Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles, Chemical Communications (10) (2007) 1056–1058. [DOI] [PubMed] [Google Scholar]
- [21].Dowding JM, Dosani T, Kumar A, Seal S, Self WT, Cerium oxide nanoparticles scavenge nitric oxide radical ( NO), Chem Commun (Camb) 48(40) (2012) 4896–8. [DOI] [PubMed] [Google Scholar]
- [22].Celardo I, Pedersen JZ, Traversa E, Ghibelli L, Pharmacological potential of cerium oxide nanoparticles, Nanoscale 3(4) (2011) 1411–20. [DOI] [PubMed] [Google Scholar]
- [23].Li M, Shi P, Xu C, Ren J, Qu X, Cerium oxide caged metal chelator: anti-aggregation and anti-oxidation integrated H2O2-responsive controlled drug release for potential Alzheimer’s disease treatment, Chemical Science 4(6) (2013) 2536–2542. [Google Scholar]
- [24].Xu C, Lin Y, Wang J, Wu L, Wei W, Ren J, Qu X, Nanoceria-triggered synergetic drug release based on CeO(2) -capped mesoporous silica host-guest interactions and switchable enzymatic activity and cellular effects of CeO(2), Adv Healthc Mater 2(12) (2013) 1591–9. [DOI] [PubMed] [Google Scholar]
- [25].Ramasamy M, Lee J, Recent Nanotechnology Approaches for Prevention and Treatment of Biofilm-Associated Infections on Medical Devices, BioMed Research International 2016 (2016) 1851242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gopinath K, Karthika V, Sundaravadivelan C, Gowri S, Arumugam A, Mycogenesis of cerium oxide nanoparticles using Aspergillus niger culture filtrate and their applications for antibacterial and larvicidal activities, Journal of Nanostructure in Chemistry 5(3) (2015) 295–303. [Google Scholar]
- [27].Patil SN, Paradeshi JS, Chaudhari PB, Mishra SJ, Chaudhari BL, Bio-therapeutic Potential and Cytotoxicity Assessment of Pectin-Mediated Synthesized Nanostructured Cerium Oxide, Applied Biochemistry and Biotechnology 180(4) (2016) 638–654. [DOI] [PubMed] [Google Scholar]
- [28].Arumugam A, Karthikeyan C, Haja Hameed AS, Gopinath K, Gowri S, Karthika V, Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties, Materials Science and Engineering: C 49 (2015) 408–415. [DOI] [PubMed] [Google Scholar]
- [29].Masadeh MM, Karasneh GA, Al-Akhras MA, Albiss BA, Aljarah KM, Al-Azzam SI, Alzoubi KH, Cerium oxide and iron oxide nanoparticles abolish the antibacterial activity of ciprofloxacin against gram positive and gram negative biofilm bacteria, Cytotechnology 67(3) (2015) 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Babenko L, Zholobak N, Shcherbakov A, Voychuk S, Lazarenko L, Spivak MY, Antibacterial activity of cerium colloids against opportunistic microorganisms in vitro, Мікробіологічний журнал (74,№ 3) (2012) 54–62. [PubMed] [Google Scholar]
- [31].Worthington RJ, Melander C, Combination approaches to combat multidrug-resistant bacteria, Trends in biotechnology 31(3) (2013) 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tagliaferri TL, Jansen M, Horz HP, Fighting Pathogenic Bacteria on Two Fronts: Phages and Antibiotics as Combined Strategy, Frontiers in cellular and infection microbiology 9 (2019) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Torres-Barceló C, Hochberg ME, Evolutionary Rationale for Phages as Complements of Antibiotics, Trends in microbiology 24(4) (2016) 249–256. [DOI] [PubMed] [Google Scholar]
- [34].Pant J, Goudie MJ, Hopkins SP, Brisbois EJ, Handa H, Tunable Nitric Oxide Release from S-Nitroso-N-acetylpenicillamine via Catalytic Copper Nanoparticles for Biomedical Applications, ACS Appl Mater Interfaces 9(18) (2017) 15254–15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Douglass ME, Goudie MJ, Pant J, Singha P, Hopkins S, Devine R, Schmiedt CW, Handa H, Catalyzed Nitric Oxide Release via Cu Nanoparticles Leads to an Increase in Antimicrobial Effects and Hemocompatibility for Short-Term Extracorporeal Circulation, ACS Applied Bio Materials (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Singha P, Workman CD, Pant J, Hopkins SP, Handa H, Zinc-oxide nanoparticles act catalytically and synergistically with nitric oxide donors to enhance antimicrobial efficacy, Journal of Biomedical Materials Research Part A (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Doverspike JC, Zhou Y, Wu J, Tan X, Xi C, Meyerhoff ME, Nitric oxide releasing two-part creams containing S-nitrosoglutathione and zinc oxide for potential topical antimicrobial applications, Nitric oxide : biology and chemistry 90 (2019) 1–9. [DOI] [PubMed] [Google Scholar]
- [38].Ren H, Wu J, Colletta A, Meyerhoff ME, Xi C, Efficient eradication of mature Pseudomonas aeruginosa biofilm via controlled delivery of nitric oxide combined with antimicrobial peptide and antibiotics, Frontiers in microbiology 7 (2016) 1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Knowles RG, Moncada S, Nitric oxide synthases in mammals, Biochem J 298 ( Pt 2) (1994) 249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang T, Zelikin AN, Chandrawati R, Progress and Promise of Nitric Oxide-Releasing Platforms, Adv Sci (Weinh) 5(6) (2018) 1701043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Marsh N, Marsh A, A short history of nitroglycerine and nitric oxide in pharmacology and physiology, Clinical and Experimental Pharmacology and Physiology 27(4) (2000) 313–319. [DOI] [PubMed] [Google Scholar]
- [42].Moncada S, Higgs E, The discovery of nitric oxide and its role in vascular biology, British journal of pharmacology 147(S1) (2006) S193–S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bogdan C, Nitric oxide and the immune response, Nature immunology 2(10) (2001) 907. [DOI] [PubMed] [Google Scholar]
- [44].Nathan CF, Hibbs JB Jr, Role of nitric oxide synthesis in macrophage antimicrobial activity, Current opinion in immunology 3(1) (1991) 65–70. [DOI] [PubMed] [Google Scholar]
- [45].Anggard E, Nitric oxide: mediator, murderer, and medicine, The Lancet 343(8907) (1994) 1199–1206. [DOI] [PubMed] [Google Scholar]
- [46].Fang FC, Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity, The Journal of clinical investigation 99(12) (1997) 2818–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Colletta A, Wu J, Wo Y, Kappler M, Chen H, Xi C, Meyerhoff ME, S-Nitroso-N-acetylpenicillamine (SNAP) impregnated silicone foley catheters: a potential biomaterial/device to prevent catheter-associated urinary tract infections, ACS biomaterials science & engineering 1(6) (2015) 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Homeyer K, Goudie M, Singha P, Handa H, Liquid-infused nitric oxide-releasing silicone Foley urinary catheters for prevention of catheter-associated urinary tract infections, ACS Biomaterials Science & Engineering (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Brisbois EJ, Kim M, Wang X, Mohammed A, Major TC, Wu J, Brownstein J, Xi C, Handa H, Bartlett RH, Meyerhoff ME, Improved Hemocompatibility of Multilumen Catheters via Nitric Oxide (NO) Release from S-Nitroso-N-acetylpenicillamine (SNAP) Composite Filled Lumen, ACS Appl Mater Interfaces 8(43) (2016) 29270–29279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pant J, Goudie MJ, Chaji SM, Johnson BW, Handa H, Nitric oxide releasing vascular catheters for eradicating bacterial infection, Journal of biomedical materials research. Part B, Applied biomaterials (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Brisbois EJ, Davis RP, Jones AM, Major TC, Bartlett RH, Meyerhoff ME, Handa H, Reduction in thrombosis and bacterial adhesion with 7 day implantation of S-nitroso-N-acetylpenicillamine (SNAP)-doped Elast-eon E2As catheters in sheep, Journal of Materials Chemistry B 3(8) (2015) 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wang X, Jolliffe A, Carr B, Zhang Q, Bilger M, Cui Y, Wu J, Wang X, Mahoney M, Rojas-Pena A, Nitric oxide-releasing semi-crystalline thermoplastic polymers: preparation, characterization and application to devise anti-inflammatory and bactericidal implants, Biomaterials science 6(12) (2018) 3189–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Homeyer KH, Singha P, Goudie MJ, Handa H, S-nitroso-N-acetylpenicillamine (SNAP) Impregnated Endotracheal Tubes for Prevention of Ventilator-Associated Pneumonia, Biotechnology and Bioengineering (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Reger NA, Meng WS, Gawalt ES, Antimicrobial Activity of Nitric Oxide-Releasing Ti-6Al-4V Metal Oxide, Journal of functional biomaterials 8(2) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Liu Q, Singha P, Handa H, Locklin J, Covalent Grafting of Antifouling Phosphorylcholine-Based Copolymers with Antimicrobial Nitric Oxide Releasing Polymers to Enhance Infection-Resistant Properties of Medical Device Coatings, Langmuir (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Das S, Singh S, Dowding JM, Oommen S, Kumar A, Sayle TX, Saraf S, Patra CR, Vlahakis NE, Sayle DC, Self WT, Seal S, The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments, Biomaterials 33(31) (2012) 7746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Docter D, Westmeier D, Markiewicz M, Stolte S, Knauer S, Stauber R, The nanoparticle biomolecule corona: lessons learned–challenge accepted?, Chemical Society Reviews 44(17) (2015) 6094–6121. [DOI] [PubMed] [Google Scholar]
- [58].Wo Y, Li Z, Colletta A, Wu J, Xi C, Matzger AJ, Brisbois EJ, Bartlett RH, Meyerhoff ME, Study of crystal formation and nitric oxide (NO) release mechanism from S-nitroso-N-acetylpenicillamine (SNAP)-doped CarboSil polymer composites for potential antimicrobial applications, Composites Part B: Engineering 121 (2017) 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Seal S, Jeyaranjan A, Neal CJ, Kumar U, Sakthivel TS, Sayle DC, Engineered defects in cerium oxides: tuning chemical reactivity for biomedical, environmental, & energy applications, Nanoscale 12(13) (2020) 6879–6899. [DOI] [PubMed] [Google Scholar]
- [60].Williams DLH, The chemistry of S-nitrosothiols, Accounts of chemical research 32(10) (1999) 869–876. [Google Scholar]
- [61].Hu T-M, Chou T-C, The kinetics of thiol-mediated decomposition of S-nitrosothiols, The AAPS journal 8(3) (2006) E485–E492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ullmann BD, Myers H, Chiranand W, Lazzell AL, Zhao Q, Vega LA, Lopez-Ribot JL, Gardner PR, Gustin MC, Inducible defense mechanism against nitric oxide in Candida albicans, Eukaryotic cell 3(3) (2004) 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chen H-I, Chang H-Y, Synthesis of nanocrystalline cerium oxide particles by the precipitation method, Ceramics International 31(6) (2005) 795–802. [Google Scholar]
- [64].Hirano M, Fukuda Y, Iwata H, Hotta Y, Inagaki M, Preparation and Spherical Agglomeration of Crystalline Cerium(IV) Oxide Nanoparticles by Thermal Hydrolysis, Journal of the American Ceramic Society 83(5) (2000) 1287–1289. [Google Scholar]
- [65].He H-W, Wu X-Q, Ren W, Shi P, Yao X, Song Z-T, Synthesis of crystalline cerium dioxide hydrosol by a sol–gel method, Ceramics International 38(Supplement 1) (2012) S501–S504. [Google Scholar]
- [66].Yu JC, Zhang L, Lin J, Direct sonochemical preparation of high-surface-area nanoporous ceria and ceria–zirconia solid solutions, Journal of Colloid and Interface Science 260(1) (2003) 240–243. [DOI] [PubMed] [Google Scholar]
- [67].Feng X, Sayle DC, Wang ZL, Paras MS, Santora B, Sutorik AC, Sayle TXT, Yang Y, Ding Y, Wang X, Her Y-S, Converting Ceria Polyhedral Nanoparticles into Single-Crystal Nanospheres, Science 312(5779) (2006) 1504. [DOI] [PubMed] [Google Scholar]
- [68].Sun C, Li H, Chen L, Nanostructured ceria-based materials: synthesis, properties, and applications, Energy & Environmental Science 5(9) (2012) 8475–8505. [Google Scholar]
- [69].Nath B, Barbhuiya T, Studies on the density and surface area of nanoparticles from Camellia sinensis, A natural source, J. Chem. Pharm. Res 6(11) (2014) 608–610. [Google Scholar]
- [70].Mukha IP, Eremenko A, Smirnova N, Mikhienkova A, Korchak G, Gorchev V, Chunikhin AY, Antimicrobial activity of stable silver nanoparticles of a certain size, Applied biochemistry and microbiology 49(2) (2013) 199–206. [DOI] [PubMed] [Google Scholar]
- [71].Varner K, Sanford J, El-Badawy A, Feldhake D, Venkatapathy R, State of the science literature review: everything nanosilver and more, US Environmental Protection Agency, Washington DC 363 (2010). [Google Scholar]
- [72].Sotiriou GA, Pratsinis SE, Antibacterial activity of nanosilver ions and particles, Environmental science & technology 44(14) (2010) 5649–5654. [DOI] [PubMed] [Google Scholar]
- [73].Padmavathy N, Vijayaraghavan R, Interaction of ZnO nanoparticles with microbes—a physio and biochemical assay, Journal of biomedical nanotechnology 7(6) (2011) 813–822. [DOI] [PubMed] [Google Scholar]
- [74].Pirmohamed T, Dowding JM, Singh S, Wasserman B, Heckert E, Karakoti AS, King JES, Seal S, Self WT, Nanoceria exhibit redox state-dependent catalase mimetic activity, Chemical Communications 46(16) (2010) 2736–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Heckert EG, Karakoti AS, Seal S, Self WT, The role of cerium redox state in the SOD mimetic activity of nanoceria, Biomaterials 29(18) (2008) 2705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Singh S, Dosani T, Karakoti AS, Kumar A, Seal S, Self WT, A phosphate-dependent shift in redox state of cerium oxide nanoparticles and its effects on catalytic properties, Biomaterials 32(28) (2011) 6745–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sharma G, Kodali V, Gaffrey M, Wang W, Minard KR, Karin NJ, Teeguarden JG, Thrall BD, Iron oxide nanoparticle agglomeration influences dose rates and modulates oxidative stress-mediated dose–response profiles in vitro, Nanotoxicology 8(6) (2014) 663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Schubert J, Radeke C, Fery A, Chanana M, The role of pH, metal ions and their hydroxides in charge reversal of protein-coated nanoparticles, Physical Chemistry Chemical Physics 21(21) (2019) 11011–11018. [DOI] [PubMed] [Google Scholar]
- [79].Tebbe M, Kuttner C, Männel M, Fery A, Chanana M, Colloidally stable and surfactant-free protein-coated gold nanorods in biological media, ACS applied materials & interfaces 7(10) (2015) 5984–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sehgal A, Lalatonne Y, Berret J-F, Morvan M, Precipitation− Redispersion of Cerium Oxide Nanoparticles with Poly (acrylic acid): Toward Stable Dispersions, Langmuir 21(20) (2005) 9359–9364. [DOI] [PubMed] [Google Scholar]
- [81].Shah V, Shah S, Shah H, Rispoli FJ, McDonnell KT, Workeneh S, Karakoti A, Kumar A, Seal S, Antibacterial activity of polymer coated cerium oxide nanoparticles, PLoS One 7(10) (2012) e47827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Thill A, Zeyons O, Spalla O, Chauvat F, Rose J, Auffan M, Flank AM, Cytotoxicity of CeO2 Nanoparticles for Escherichia coli. Physico-Chemical Insight of the Cytotoxicity Mechanism, Environmental Science & Technology 40(19) (2006) 6151–6156. [DOI] [PubMed] [Google Scholar]
- [83].Zeyons O, Thill A, Chauvat F, Menguy N, Cassier-Chauvat C, Oréar C, Daraspe J, Auffan M, Rose J, Spalla O, Direct and indirect CeO2 nanoparticles toxicity for Escherichia coli and Synechocystis, Nanotoxicology 3(4) (2009) 284–295. [Google Scholar]
- [84].Pelletier DA, Suresh AK, Holton GA, McKeown CK, Wang W, Gu B, Mortensen NP, Allison DP, Joy DC, Allison MR, Brown SD, Phelps TJ, Doktycz MJ, Effects of Engineered Cerium Oxide Nanoparticles on Bacterial Growth and Viability, Applied and Environmental Microbiology 76(24) (2010) 7981–7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pulido-Reyes G, Rodea-Palomares I, Das S, Sakthivel TS, Leganes F, Rosal R, Seal S, Fernández-Piñas F, Untangling the biological effects of cerium oxide nanoparticles: the role of surface valence states, Scientific reports 5(1) (2015) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Karakoti AS, Tsigkou O, Yue S, Lee PD, Stevens MM, Jones JR, Seal S, Rare earth oxides as nanoadditives in 3-D nanocomposite scaffolds for bone regeneration, Journal of Materials Chemistry 20(40) (2010) 8912–8919. [Google Scholar]
- [87].Mandoli C, Pagliari F, Pagliari S, Forte G, Di Nardo P, Licoccia S, Traversa E, Stem cell aligned growth induced by CeO2 nanoparticles in PLGA scaffolds with improved bioactivity for regenerative medicine, Advanced Functional Materials 20(10) (2010) 1617–1624. [Google Scholar]
- [88].Jain A, Behera M, Mahapatra C, Sundaresan NR, Chatterjee K, Nanostructured Polymer Scaffold Decorated with Cerium Oxide Nanoparticles Toward Engineering an Antioxidant and Anti-hypertrophic Cardiac Patch, Materials Science and Engineering: C (2020) 111416. [DOI] [PubMed] [Google Scholar]
- [89].Xu Z, Xu Y, Basuthakur P, Patra C, Ramakrishna S, Liu Y, Thomas V, Nanda HS, Fibro-porous PLLA/Gelatin Composite Membrane Doped with Cerium Oxide Nanoparticles as Bioactive Scaffolds for future Angiogenesis, Journal of Materials Chemistry B (2020). [DOI] [PubMed] [Google Scholar]
- [90].Saha P, Maharajan A, Dikshit PK, Kim BS, Rapid and reusable detection of hydrogen peroxide using polyurethane scaffold incorporated with cerium oxide nanoparticles, Korean Journal of Chemical Engineering 36(12) (2019) 2143–2152. [Google Scholar]
- [91].Hopkins SP, Pant J, Goudie MJ, Schmiedt C, Handa H, Achieving long-term biocompatible silicone via covalently immobilized S-nitroso-N-acetylpenicillamine (SNAP) that exhibits 4 months of sustained nitric oxide release, ACS applied materials & interfaces 10(32) (2018) 27316–27325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Brisbois EJ, Major TC, Goudie MJ, Meyerhoff ME, Bartlett RH, Handa H, Attenuation of thrombosis and bacterial infection using dual function nitric oxide releasing central venous catheters in a 9 day rabbit model, Acta biomaterialia 44 (2016) 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Cai W, Wu J, Xi C, Meyerhoff ME, Diazeniumdiolate-doped poly (lactic-co-glycolic acid)-based nitric oxide releasing films as antibiofilm coatings, Biomaterials 33(32) (2012) 7933–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Devine R, Singha P, Handa H, Versatile biomimetic medical device surface: hydrophobin coated, nitric oxide-releasing polymer for antimicrobial and hemocompatible applications, Biomaterials science 7(8) (2019) 3438–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Gierke GE, Nielsen M, Frost MC, S-Nitroso-N-acetyl-D-penicillamine covalently linked to polydimethylsiloxane (SNAP–PDMS) for use as a controlled photoinitiated nitric oxide release polymer, Science and technology of advanced materials 12(5) (2011) 055007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Goudie MJ, Pant J, Handa H, Liquid-infused nitric oxide-releasing (LINORel) silicone for decreased fouling, thrombosis, and infection of medical devices, Sci Rep 7(1) (2017) 13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Singha P, Pant J, Goudie MJ, Workman CD, Handa H, Enhanced antibacterial efficacy of nitric oxide releasing thermoplastic polyurethanes with antifouling hydrophilic topcoats, Biomaterials science 5(7) (2017) 1246–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mondal A, Douglass M, Hopkins SP, Singha P, Tran M, Handa H, Brisbois EJ, Multifunctional S-Nitroso-N-acetylpenicillamine-Incorporated Medical-Grade Polymer with Selenium Interface for Biomedical Applications, ACS applied materials & interfaces 11(38) (2019) 34652–34662. [DOI] [PMC free article] [PubMed] [Google Scholar]