Abstract
Appropriate flowering time is critical for the reproductive success of plant species. Emerging evidence indicates that calcium may play an important role in the regulation of flowering time. However, the underlying molecular mechanisms remain unclear. In this study, we demonstrate that calcium-dependent protein kinase 32 (CPK32) regulates flowering time by affecting the alternative polyadenylation of FLOWERING CONTROL LOCUS A (FCA) and altering the transcription of FLOWERING LOCUS C (FLC), a central repressor of flowering time. The knockdown of CPK32 results in an obvious late flowering phenotype and dramatically enhanced FLC transcription. CPK32 interacts with FCA, and phosphorylates the serine592 of FCA in a Ca2+-dependent manner. Moreover, the ratio of abundance of the FCA transcripts (FCA-D and FCA-P) changes significantly in the cpk32 mutant, which subsequently affects FLC expression and consequently regulates floral transition. The present evidence demonstrates that CPK32 modulates flowering time by regulating FCA alternative polyadenylation and consequent FLC expression.
Keywords: calcium signaling, CPK32, FCA, FLC, flowering time
INTRODUCTION
Flowering time is critical for flowering plant reproduction, species maintenance, adaptation and domestication, and is also a key element in crop breeding and variety selection because of its importance for crop growth vigor and yield [1,2]. Molecular analysis and genetic studies of flowering time regulation have made great strides in deciphering the regulatory mechanism of floral transition.
FLOWERING LOCUS C (FLC) is the core regulatory gene mediating flowering time in the autonomous and vernalization pathways [3,4]. Alternative polyadenylation (APA) generates mRNAs with distinct 3′ ends and has emerged as a pervasive regulatory mechanism in gene expression [5,6]. Several floral regulators modulate FLC expression through APA, such as the conserved RNA-binding proteins FLOWERING CONTROL LOCUS A (FCA) and FLOWERING LOCUS PA (FPA) [7–11]. Interaction between FCA and FLOWERING LOCUS Y (FY) is required for the efficient selection of the 3' end of FCA transcripts and FLC repression [9,12]. Integrative genome-wide analysis revealed that the heterogeneous nuclear ribonucleoprotein (hnRNP) A1-like protein 1 (HLP1) regulates flowering by modulating the APA of FCA [13]. Despite the wealth of knowledge about the FCA-FLC module in the autonomous pathway, little is known about the molecular mechanism that regulates the APA of FCA during flowering.
Plants have evolved sophisticated signaling cascades that perceive, integrate and respond to endogenous and external stimuli, thereby ensuring their survival and the proper timing of flowering for species maintenance. Calcium (Ca2+) is a ubiquitous second messenger in living organisms [14]. Previous reports have shown that the environmental factors that influence flowering time, such as light quality [15], day length, light intensity [16] and low temperatures [17,18], also affect the cytosolic Ca2+ concentration in Arabidopsis roots, leaves or seedlings.
Ca2+ signals are perceived and decoded by a series of Ca2+-binding proteins or Ca2+ transducers. Among calcium-binding proteins, Ca2+-dependent protein kinases (CPKs/CDPKs) are plant-specific protein kinases that participate in a variety of important physiological activities, including ion channel regulation [19,20], stomatal movements [21], cell expansion [22] and responses to pathogens [23]. In the present study, we present evidence for the involvement of CPK32 in flowering time regulation. The CPK32 knockdown mutant cpk32 exhibited an obvious late-flowering-time phenotype, accompanied by significant enhancement of FLC expression. It was further revealed that CPK32 interacts with FCA, and phosphorylates the serine592 of FCA in a Ca2+-dependent manner. As a result, the alternative splicing of FCA pre-mRNA is regulated, and in turn FLC transcription is regulated. The presented evidence demonstrates that CPK32 regulates FCA pre-mRNA levels to fine-tune flowering time through the autonomous pathway.
RESULTS
CPK32 is a positive regulator of plant flowering
The CPK family in the Arabidopsis genome includes 34 members, and they cluster into four subgroups based on protein sequence homology [24]. To investigate the potential function of CPKs in the floral transition in Arabidopsis, the flowering time phenotype of 32 CPK mutant lines (Supplementary Fig. S1 and Supplementary Table 1) were screened in long-day (LD) photoperiod conditions. At the time of bolting, the majority of cpk mutants showed a similar flowering time phenotype compared with the wild-type (WT) plants, except cpk23, cpk32 and cpk33 mutants, which displayed a late flowering phenotype, and the cpk28 mutant (Salk_112540, an overexpression line), which displayed an early flowering phenotype (Fig. 1A). In Arabidopsis, flowering time is closely associated with leaf number, which is widely used to quantify the time of the floral transition [12,25]. At the time of flowering, these three late flowering mutants developed more leaves than the WT plants (Fig. 1B). CPK32 belongs to subgroup III, CPK23 and CPK33 belong to subgroup II, and CPK28 belongs to subgroup IV of the CPK super-family [24]. Their divergent homology suggests that they may regulate the flowering of Arabidopsis via different mechanisms. RT-qPCR experiments revealed that transcription of FLC was dramatically enhanced in the cpk32 mutant, while FLC transcription was not changed in cpk23, cpk28 and cpk33 mutants (Fig. 1C). Considering FLC is a central repressor of flowering [3,4], further investigation was focused on characterizing CPK32 function in flowering time regulation.
Figure 1.
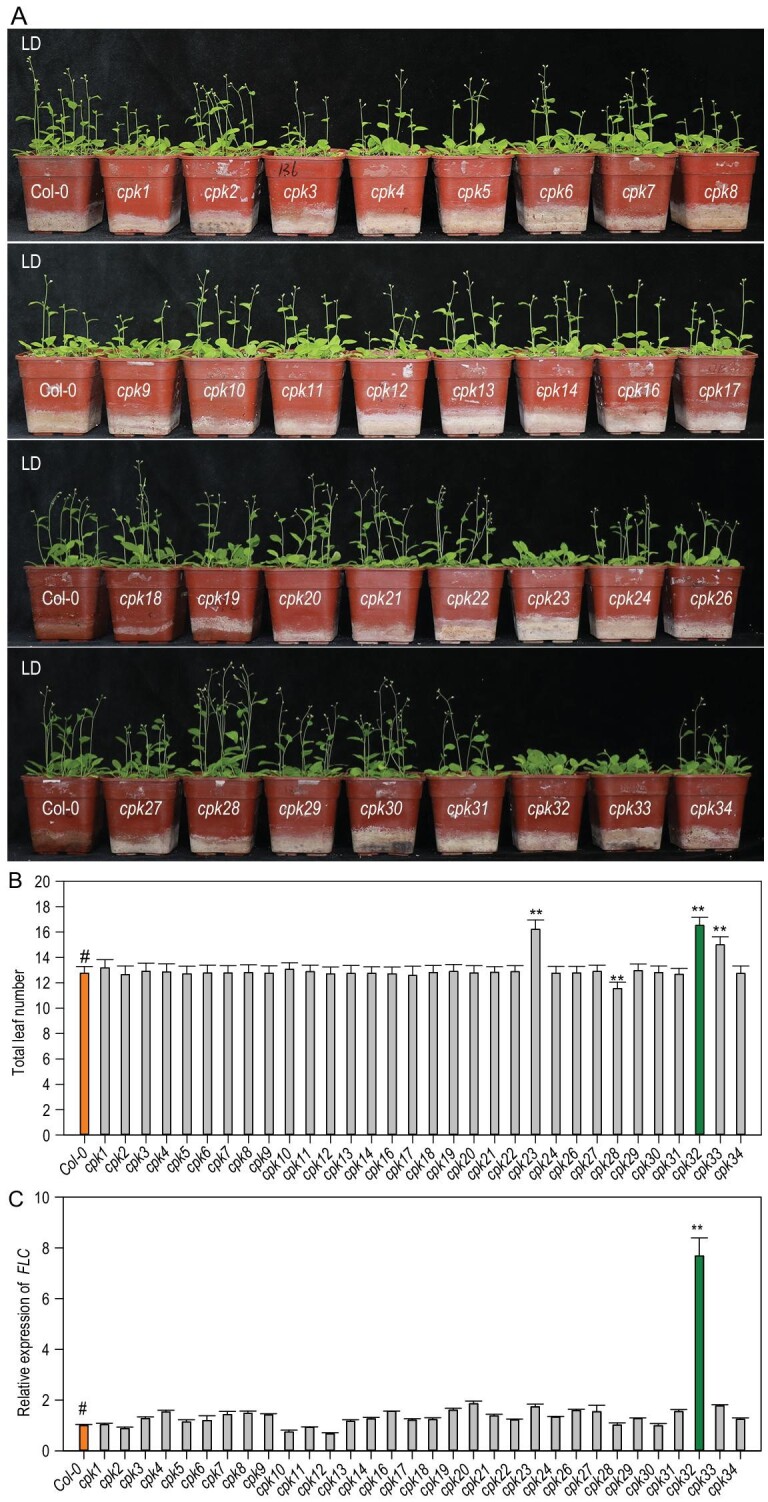
Effect of Arabidopsis CPK gene mutations on flowering time. (A) Flowering-time phenotype of Arabidopsis cpk mutants planted in LDs (16 h light/8 h dark). (B) Total leaf number per plant at flowering of cpk mutants planted shown in (A), n = 30. (C) FLC transcription in 7-day-old Arabidopsis cpk mutants, as determined by RT-qPCR assay, n = 3. (B) and (C) Experiments were repeated three times with similar results and the data are presented as the mean ± SE (Student's t-test; **P < 0.01, and ‘#’ represents control).
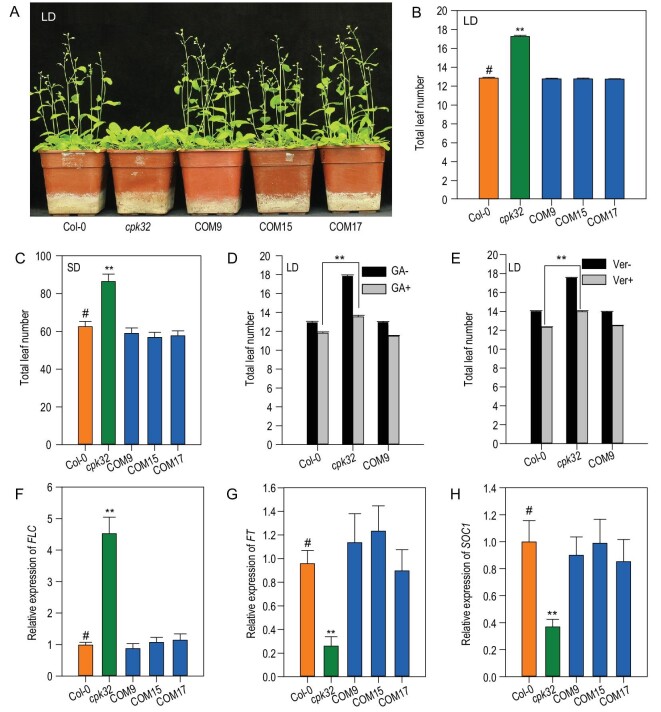
The cpk32 mutant line used in our experiments is a knock-down allele (Supplementary Fig. S2). To validate that the late-flowering-time phenotype was caused by decreased CPK32 expression, three independent complementation lines (COM9, COM15 and COM17) were generated (Supplementary Fig. S2C and D). All complementation lines displayed a flowering time similar to the WT in LD photoperiod conditions (Fig. 2A and B). These results confirmed that the decreased expression of CPK32 in the cpk32 mutant is responsible for its late-flowering-time phenotype and suggests that CPK32 may positively regulate the floral transition in Arabidopsis.
Figure 2.
CPK32 regulates flowering time through the autonomous pathway. (A) and (B) Flowering-time phenotype of Col-0, cpk32 and cpk32 complementation lines planted in soil in LDs (A) and total leaf number per plant at flowering (B). Experiments were repeated three times with similar results and the data are presented as the mean ± SE, n = 100. (C) Total leaf number for Col-0, cpk32 and the cpk32 complementation lines grown in soil in SDs. The data are presented as the mean ± SE, n = 30. (D) and (E) Total leaf number at flowering for Col-0, cpk32 and COM9 treated with (D) GA and (E) vernalization in LDs. Data are presented as the mean ± SE, n = 100. (F)–(H) RT-qPCR analysis of FLC, FT and SOC1 transcription in Col-0, cpk32 and CPK32 complementation lines of 7-day-old LD-grown seedlings. Data are presented as the mean ± SE, n = 3. Student's t-test (#, control; **, P < 0.01) was used to analyze statistical significance.
Expression profiling of CPK32
To gain insight into the function of CPK32 during floral initiation, CPK32pro : GUS transgenic lines were used and the GUS staining showed that CPK32 was expressed in embryos and emerged radicles, roots, floral organs, anthers and stigma (Supplementary Fig. S3). The results also showed that CPK32 is expressed in the shoot apex (Supplementary Fig. S3D), indicating that CPK32 may play a role in floral initiation.
CPK32 regulates flowering time through the autonomous pathway
Four major pathways regulating flowering time have been defined by classic genetics approaches in Arabidopsis [26,27]. To identify the pathway that CPK32 is involved in, the flowering-time phenotypes of the cpk32 mutant were tested under different photoperiods, gibberellic acid (GA) treatment and vernalization treatments. Compared with LD conditions, short-day (SD) conditions extended the vegetative growth period of all lines indicated with their increased leaf number, and the cpk32 mutant still flowered significantly later than the WT plants and the complementation lines (Fig. 2C). These results indicate that cpk32 mutants respond to changes in photoperiod in a similar way to WT plants, except for their delayed flowering. The experiments conducted with GA and vernalization treatments in LD photoperiod conditions showed that all plants flowered earlier than their corresponding controls, indicating that the cpk32 mutant is similar to WT in its response to GA and vernalization (Fig. 2D and E). Taken together, these data demonstrate that CPK32 does not promote floral transition through the photoperiod, GA and vernalization pathways but most likely through the autonomous pathway.
To confirm the involvement of CPK32 in the autonomous pathway, the FLC transcription in various lines was analyzed. As shown in Figs 1C and 2F, FLC transcriptional expression in the cpk32 mutants was much higher than in WT plants, while the complementation lines showed similar transcription to the WT (Fig. 2F). Consistently with this, FLOWERING LOCUS T (FT) and SUPPRESSOR OF OVEREXPRESSION OF CO 1 (SOC1), which are downstream targets of FLC in flowering-time control [28,29], were down-regulated in the cpk32 mutant compared to the WT plants and the complementation lines (Fig. 2G and H).
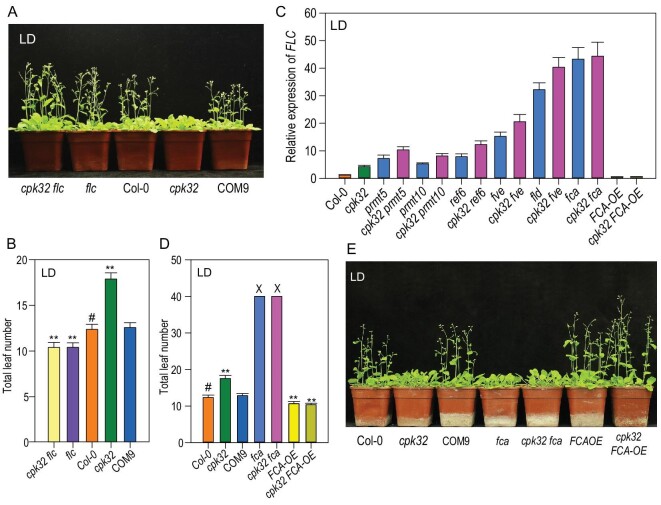
To further confirm CPK32 involvement in floral transition through its effect on FLC expression, the cpk32 flc double mutant was generated (Supplementary Fig. S2E) and it showed the similar early flowering phenotype as the flc single mutant in LD photoperiod conditions (Fig. 3A and B). These results support our hypothesis that CPK32 regulates flowering by repressing FLC expression.
Figure 3.
CPK32 regulates FLC expression through FCA. (A) Flowering-time phenotype of Col-0, cpk32, COM9, flc and cpk32 flc plants grown in soil in LDs. (B) Total leaf number for Col-0, cpk32, COM9, flc and cpk32 flc plants in LDs. The data are presented as the mean ± SE, n = 50. (C) RT-qPCR analysis of FLC transcription in the double mutants (i.e. cpk32 crossed individually with six different mutants of the autonomous pathway). Data are presented as the mean ± SE, n = 3. (D) Total leaf number of CPK32 and FCA-related materials grown in LDs. Experiments were repeated three times with similar results and the data are presented as the mean ± SE, n = 50 (‘X’ means that the counting was terminated after plants had produced over 40 total leaves before bolting). (E) Flowering-time phenotype of CPK32- and FCA-related plant materials grown in soil in LDs. Student's t-test (#, control; **, P < 0.01) was used to analyze statistical significance.
CPK32 regulates FLC expression through FCA
Previous reports have shown that a number of genes, such as FCA, FPA, FY, FLK, LUMINIDEPENDENS, FLOWERING LOCUS D (FLD), RELATIVE OF EARLY FLOWERING 6 (REF6), FVE, PROTEIN ARGININE METHYLTRANSFERASE 5 (PRMT5) and PRMT10, regulate flowering time via the autonomous pathway [27,30]. Among these genes, seven, including FLK, FLD, FVE, FPA, FY, REF6 and LUMINIDEPENDENS, showed no obvious changes in their expression in the cpk32 mutant compared to the WT (Supplementary Fig. S4A). FLC transcription in the double mutants cpk32 prmt5, cpk32 prmt10, cpk32 ref6, cpk32 fve and cpk32 fld was higher than that in corresponding single mutants, respectively, except the cpk32 fca double mutant (Fig. 3C and Supplementary Fig. S4B and C). FLC transcription in the cpk32 fca double mutant was nearly the same as that in the fca single mutant (Fig. 3C), suggesting that CPK32 may regulate FLC expression through FCA.
Previous reports have shown that FCA functions as a posttranscriptional regulator, and that the fca mutant has a late-flowering phenotype [7,25]. The cpk32 fca double mutant displayed the same phenotype as the fca single mutant in terms of flowering time, and FCA-OE and cpk32 FCA-OE plants (Supplementary Fig. S4D) showed lower FLC expression and earlier flowering-time phenotype (Fig. 3D and E). These results demonstrate that CPK32 and FCA act in the same pathway, and the upregulated expression of FLC in the cpk32 mutant is most likely mediated by FCA.
CPK32 interacts with FCA
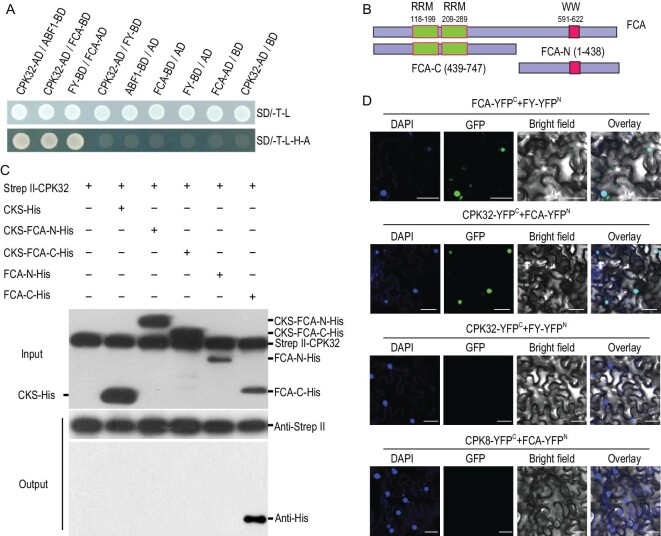
As previous reports showed that FCA interacts with FY to downregulate FLC expression [12] and CPK32 interacts with Abscisic acid responsive element-Binding Factors (ABFs) [31], FCA-FY and CPK32-ABF1 were used as the positive controls in Yeast Two-Hybrid (Y2H) experiments to test the proposed interaction between CPK32 and FCA. Indeed, CPK32 strongly interacted with FCA (Fig. 4A), while FY, FLK and FVE had no interaction with CPK32 (Supplementary Fig. S5A). All the other seven subgroup III CPK members had no interaction with FCA (Supplementary Fig. S5B).
Figure 4.
CPK32 interacts with FCA. (A) Interaction between CPK32 and FCA in yeast. CPK32-ABF1 and FCA-FY were used as positive controls and CPK32/pDEST32 as a negative control. (B) Diagram of FCA N-terminal and C-terminal truncations. (C) Pull-down assay for the interaction between CPK32 and FCA. The Strep II-CPK32 was used as a bait to pull down FCA-His fusion protein prey. Anti-Strep II and anti-His antibodies were simultaneous used to test the proteins in the input reaction. An anti-Strep II and anti-His antibody was separately used to blot the Strep II-CPK32 and the interacted truncated FCA protein. (D) BiFC assay of the CPK32 and FCA interaction in N. benthamiana leaves. The C-terminal half of YFP was fused to CPK32, and the N-terminal half of YFP was fused to FCA. FCA-YC/FY-YN was used as a positive control. DAPI was used to stain the cell nucleus. Scale bar: 50 μm.
The protein pull-down assays further confirmed the interaction between CPK32 and FCA. The N-terminal of FCA containing two RNA recognition motif (RRM) domains and the C-terminal of FCA containing the tryptophan-tryptophan (WW) domain were purified (Fig. 4B). CPK32 interacted with the C-terminal region of FCA (FCA-C-His), but not with its N-terminal region (Fig. 4C). It should be noted that CPK32 did not interact with CKS-FCA-C-His, suggesting that CKS-tag, a 29 kD fragment, may weaken the interaction between CPK32 and the FCA-C terminal. In addition, bimolecular fluorescence complementation (BiFC) assays showed specific CPK32-FCA interaction signals in the nucleus (Fig. 4D). These results demonstrate that CPK32 directly interacts with FCA both in vitro and in vivo.
CPK32 phosphorylates FCA in a Ca2+-dependent manner
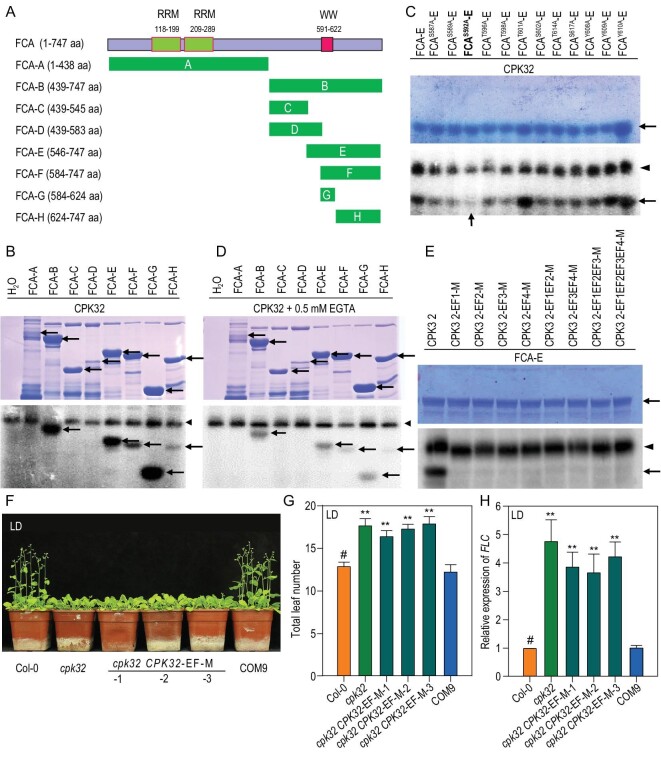
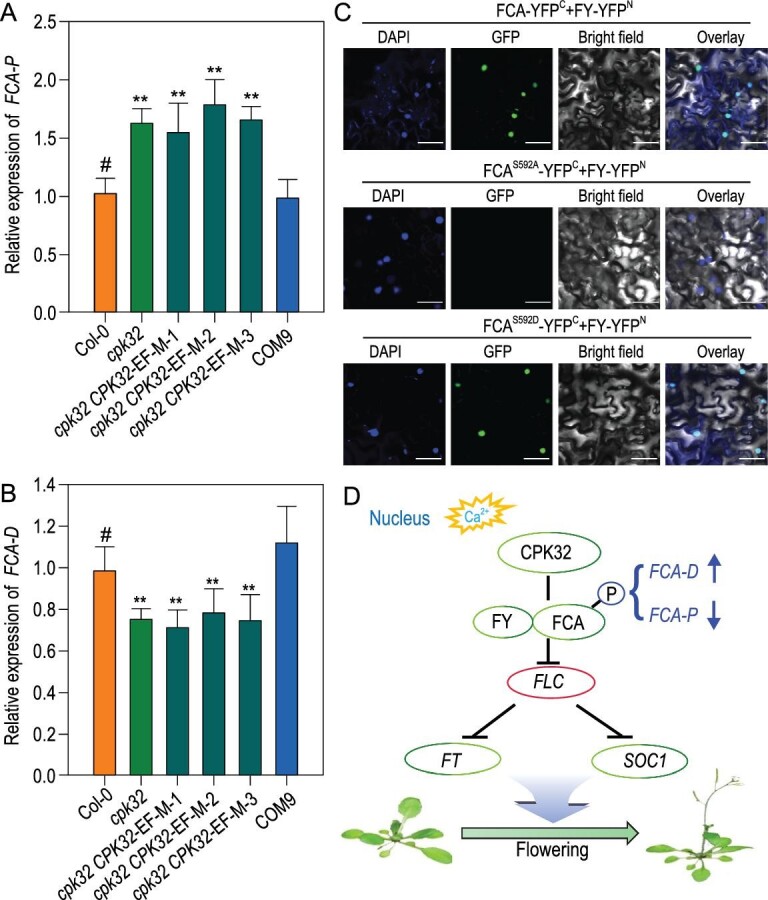
It was hypothesized that CPK32, as a protein kinase, may regulate FCA function by phosphorylation. Truncated FCA segments (Fig. 5A) were the substrates. Autoradiography showed that FCA-B, FCA-E, FCA-F, FCA-G and FCA-H fragments were phosphorylated by CPK32, and the strongest signal detected was in the FCA-G fragment (Fig. 5B), which mainly contains the WW domain required for FCA autoregulation [9]. The potential phosphorylation sites, including serine, threonine and tyrosine in the WW domain, were substituted with alanine to mimic an un-phosphorylation status for the phosphorylation assay. Autoradiography results showed that the phosphorylation signal of FCAS592A-E significantly decreased (Fig. 5C), suggesting that the serine592 is the target site of phosphorylation by CPK32.
Figure 5.
CPK32-mediated phosphorylation of FCA is dependent on Ca2+. (A) Diagram of FCA N-terminal and C-terminal truncations. The green bars represent different segments of FCA. (B) CPK32 phosphorylates FCA in vitro. Coomassie blue-stained recombinant proteins (FCA-A to FCA-H) are indicated by the arrows in the upper panel. In the lower panel, the arrowhead shows auto-phosphorylated CPK32, and the arrows show phosphorylated FCA variants. (C) Determination of phosphorylation site by CPK32 in FCA-E. As the potential phosphorylation sites, serine, threonine and tyrosine were mutated to alanine to mimic an unphosphorylated status. Coomassie blue-stained recombinant proteins of mutated FCA-E proteins are indicated by the arrow in the upper panel. In the bottom panel, the arrowhead shows auto-phosphorylated CPK32, and the arrow shows phosphorylated FCA-E. (D) EGTA weakens the phosphorylation mediated by CPK32. (E) CPK32-mediated phosphorylation of FCA is dependent on Ca2+ binding. Coomassie blue-stained recombinant proteins of FCA-E proteins are indicated by the arrow in the upper panel. In the bottom panel, the arrowhead shows auto-phosphorylated CPK32, and the arrow shows phosphorylated FCA-E. EF1, EF2, EF3 and EF4 denotes the first, second, third and fourth EF-hand respectively. (F) and (G) Flowering-time phenotype (F) and total leaf number (G) for Col-0, cpk32, COM9 and the EF-hand mutant complemented line grown in LDs. Data are presented as the mean ± SE, n = 50. (H) RT-qPCR analysis of FLC transcript levels in cpk32, the four EF-hand mutant complementation lines and COM9. Data are presented as the mean ± SE, n = 3. Student's t-test (#, control; **, P < 0.01) was used to analyze statistical significance.
Furthermore, FCA phosphorylation by CPK32 is Ca2+-dependent. Chelation of Ca2+ by addition of EGTA in the reaction medium resulted in weaker signals for all phosphorylated bands (Fig. 5D). The C terminus of CPK protein contains a Ca2+-binding domain constituted by EF-hand motifs [24]. To determine whether CPK32-mediated phosphorylation of FCA is Ca2+ binding dependent, a series of single, double and multiple EF-hand motif mutations of CPK32 were generated as described previously [32], rendering these motifs unable to bind Ca2+. Using an FCA-E fragment as the substrate, phosphorylation signals were abolished for all CPK32 proteins with mutated EF-hands (Fig. 5E). The results further demonstrated that phosphorylation of FCA by CPK32 is Ca2+ dependent.
To further confirm correlation of CPK32-mediated Ca2+ signaling and flowering-time regulation, the transgenic lines (cpk32 CPK32-EF-M) with four EF-hands mutations [32] were generated (Supplementary Fig. S6) and used to test the flowering-time phenotype. As shown in Fig. 5F and G, the cpk32 CPK32-EF-M plants displayed the same delayed flowering-time phenotype as the cpk32 mutant. Moreover, FLC expression in these EF-hand-mutated transgenic lines was similarly enhanced as in the cpk32 mutant (Fig. 5H).
CPK32 regulates the alternative polyadenylation of FCA by affecting FCA-FY interaction
Previous studies have shown that FCA regulates its own expression by promoting premature cleavage and polyadenylation of its own pre-mRNA [7]. The FCA pre-mRNA can be alternatively spliced to form four different transcripts and only the FCA-γ can complement the late-flowering phenotype of the fca mutant [8]. CPK32 phosphorylated the WW domain, which is required for FCA autoregulation in a calcium-dependent manner (Fig. 5B, D and E). To test whether CPK32 plays a role in processing FCA mRNA, the distal (FCA-D, mainly FCA-γ) and proximal (FCA-P, mainly FCA-β) polyadenylation of FCA pre-mRNA were analyzed. Compared to the WT, the cpk32 mutant had decreased levels of FCA-D transcripts and increased levels of FCA-P transcripts, while COM9 had approximately the same levels as WT (Fig. 6A and B). Obviously, disruption of CPK32 expression in the cpk32 mutant resulted in changes in the ratio of the FCA-D to FCA-P transcripts. Previous reports have shown that the functional FCA-γ (FCA-D) transcripts result in accelerated flowering [7,8], which together with the present results suggests that the late flowering-time phenotype of the cpk32 mutant may result from the decreased functional distal polyadenylation FCA transcript.
Figure 6.
CPK32-mediated floral initiation via regulation of FCA alternative polyadenylation through the autonomous pathway. (A) and (B) RT-qPCR analysis of (A) proximal (FCA-P) and (B) distal (FCA-D) polyadenylated FCA transcripts in Col-0, cpk32, EF-hand mutant complementation lines and COM9. Experiments were repeated three times with similar results. Data are presented as the mean ± SE, n = 3. The asterisks indicate a significant difference relative to Col (Student's t-test, **P < 0.01). (C) BiFC assay of the FY and mutated FCA interaction in N. benthamiana leaves. The C-terminal half of YFP was fused to FCA, and the N-terminal half of YFP was fused to FY. DAPI was used to stain the cell nucleus. Scale bar: 50 μm. (D) Working model for CPK32-mediated calcium signaling in regulation of Arabidopsis flowering time. Ca2+-activated CPK32 interacts with FCA and phosphorylates the WW domain of FCA in the nucleus. Phosphorylated FCA interacts with FY and in turn regulates the alternative splicing of FCA pre-mRNA. Changes in the ratio of functional FCA transcripts (FCA-D) to non-functional FCA transcripts (FCA-P) induces regulation of FLC expression and consequent flowering transition. The T-bar in the diagram represents negative regulation.
The transcripts of FCA-P and FCA-D in EF-hand-mutated complementation lines did not resume at WT levels (Fig. 6A and B), demonstrating that CPK32 activity plays an important role in regulating FCA pre-mRNA processing. The WW domain is required for FCA autoregulation of RNA 3′ polyadenylation and mediates the interaction with FY, and this interaction significantly affects flowering time [12]. As shown in the BiFC assays, the FCAS592D, a persistent phosphorylated FCA form, showed an interaction with FY, while the FCAS592A, an unphosphorylated form of FCA, did not show the interaction between FCA and FY (Fig. 6C). These results suggest that CPK32 may regulate flowering time by maintaining the FCA-FY interaction via phosphorylation modification.
DISCUSSION
In this study, we showed that CPK32 modulates flowering time by regulating FCA pre-mRNA processing in Arabidopsis. CPK32 interacts with FCA, phosphorylates the WW domain of FCA and regulates the APA of FCA transcripts (Figs 4–6). FCA plays an important role in alternative polyadenylation of antisense RNAs and 3′ end formation of FLC and its transcript [11,33]. As a result, FLC expression is suppressed and the inhibition on flowering transition is released (Fig. 6D).
Among CPK family members, the present data showed that CPK32 is specifically involved in flowering-time control by repressing FLC expression (Fig. 1C). We also observed a late flowering-time phenotype for the mutant cpk33 (Fig. 1A and B), which is consistent with previously reported results [34,35]. In addition to CPK32, CPK23, CPK28 and CPK33 are also involved in the regulation of flowering time (Fig. 1A and B), although these CPKs do not affect FLC expression (Fig. 1C). Further analysis of their functions would increase the understanding of the Ca2+ signaling mechanism’s role in regulation of flowering time.
CPKs have been identified as regulators involved in various physiological responses in plants [36]. Previous reports have shown that CPK32 can interact with ABFs and regulate ABA-responsive gene expression [32]. A genome-wide survey of CPK function in pollen tubes demonstrated that CPK32 overexpression caused the formation of a bulge at the tip of the pollen tube [37]. CPK32 interacted with cyclic nucleotide-gated channel 18 (CNGC18) and promoted Ca2+ entry during the elevation phase of the Ca2+ oscillations that occurred during polar growth of pollen tubes [38]. In addition, CPK32 was one of the main regulators of nitrate-CPK-NIN-LIKE PROTEIN (NLP) signaling networks [39]. CPK32 also regulated the activity of ammonium transporter 1;1 (AMT1;1) [40]. It seems that CPK32 may be a multi-functional regulator and play an important role in integrating multiple signaling processes and optimizing plant growth and development.
In conclusion, this study has revealed that the Ca2+-dependent protein kinase CPK32 functions as a positive regulator of flowering time acting through the autonomous pathway, and this finding provides new insight into the complex regulatory network of flowering time and calcium signaling in plants.
MATERIALS AND METHODS
Detailed descriptions of materials and methods are available as supporting information at NSR online.
Supplementary Material
Acknowledgements
We thank Prof. Xiao-Feng Cao (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) and Prof. Ming Yuan (China Agricultural University, China) for seeds and comments.
Contributor Information
Xidong Li, State Key Laboratory of Plant Physiology and Biochemistry, College of Biological Sciences, China Agricultural University, Beijing 100193, China.
Limei Chen, State Key Laboratory of Plant Physiology and Biochemistry, College of Biological Sciences, China Agricultural University, Beijing 100193, China; Center for Crop Functional Genomics and Molecular Breeding, China Agricultural University, Beijing 100193, China.
Li Yao, State Key Laboratory of Plant Physiology and Biochemistry, College of Biological Sciences, China Agricultural University, Beijing 100193, China; Syngenta Biotechnology China Co., Ltd., Beijing 102206, China.
Junjie Zou, State Key Laboratory of Plant Physiology and Biochemistry, College of Biological Sciences, China Agricultural University, Beijing 100193, China; Biotechnology Research Institute, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Jie Hao, State Key Laboratory of Plant Physiology and Biochemistry, College of Biological Sciences, China Agricultural University, Beijing 100193, China.
Weihua Wu, State Key Laboratory of Plant Physiology and Biochemistry, College of Biological Sciences, China Agricultural University, Beijing 100193, China; Center for Crop Functional Genomics and Molecular Breeding, China Agricultural University, Beijing 100193, China.
FUNDING
This work was supported by the National Natural Science Foundation of China (31370308 and 31921001), the China Postdoctoral Science Foundation (2018M641533) and the Chinese Universities Scientific Fund (2019TC122 and 2019TC228).
AUTHOR CONTRIBUTIONS
X.-D.L., L.-M.C. and W.-H.W. designed the experiments. X.-D.L., L.-M.C., L.Y., J.-J.Z. and J.H. performed the experiments and analyzed the data. X.-D.L. and L.-M.C. drafted the manuscript. W.-H.W. revised the manuscript.
Conflict of interest statement. None declared.
REFERENCES
- 1. Cho LH, Yoon J, An G. The control of flowering time by environmental factors. Plant J 2017; 90: 708–19. 10.1111/tpj.13461 [DOI] [PubMed] [Google Scholar]
- 2. Gaudinier A, Blackman BK.. Evolutionary processes from the perspective of flowering time diversity. New Phytol 2020; 225: 1883–98. 10.1111/nph.16205 [DOI] [PubMed] [Google Scholar]
- 3. Michaels SD, Amasino RM.. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 1999; 11: 949–56. 10.1105/tpc.11.5.949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whittaker C, Dean C.. The FLC locus: a platform for discoveries in epigenetics and adaptation. Annu Rev Cell Dev Biol 2017; 33: 555–75. 10.1146/annurev-cellbio-100616-060546 [DOI] [PubMed] [Google Scholar]
- 5. Hunt AG. The Arabidopsis polyadenylation factor subunit CPSF30 as conceptual link between mRNA polyadenylation and cellular signaling. Curr Opin Plant Biol 2014; 21: 128–32. 10.1016/j.pbi.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 6. Deng X, Cao X.. Roles of pre-mRNA splicing and polyadenylation in plant development. Curr Opin Plant Biol 2017; 35: 45–53. 10.1016/j.pbi.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 7. Macknight R, Bancroft I, Page Tet al. FCA, a gene controlling flowering time in arabidopsis, encodes a protein containing RNA-binding domains. Cell 1997; 89: 737–45. 10.1016/S0092-8674(00)80256-1 [DOI] [PubMed] [Google Scholar]
- 8. Macknight R, Duroux M, Laurie Ret al. Functional significance of the alternative transcript processing of the Arabidopsis floral promoter FCA. Plant Cell 2002; 14: 877–88. 10.1105/tpc.010456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quesada V, Macknight R, Dean Cet al. Autoregulation of FCA pre-mRNA processing controls Arabidopsis flowering time. EMBO J 2003; 22: 3142–52. 10.1093/emboj/cdg305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schomburg FM, Patton DA, Meinke DWet al. FPA, a gene involved in floral induction in Arabidopsis, encodes a protein containing RNA-Recognition motifs. Plant Cell 2007; 13:1427–36. 10.1105/tpc.13.6.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hornyik C, Terzi LC, Simpson GG.. The spen family protein FPA controls alternative cleavage and polyadenylation of RNA. Dev Cell 2010; 18: 203–13. 10.1016/j.devcel.2009.12.009 [DOI] [PubMed] [Google Scholar]
- 12. Simpson GG, Dijkwel PP, Quesada Vet al. FY is an RNA 3′ end-processing factor that interacts with FCA to control the Arabidopsis floral transition. Cell 2003; 113: 777–87. 10.1016/s0092-8674(03)00425-2 [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y, Gu L, Hou Yet al. Integrative genome-wide analysis reveals HLP1, a novel RNA-binding protein, regulates plant flowering by targeting alternative polyadenylation. Cell Res 2015; 25: 864–76. 10.1038/CR.2015.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kudla J, Becker D, Grill Eet al. Advances and current challenges in calcium signaling. New Phytol 2018; 218: 414–31. 10.1111/nph.14966 [DOI] [PubMed] [Google Scholar]
- 15. Long JC, Jenkins GI.. Involvement of plasma membrane redox activity and calcium homeostasis in the UV-B and UV-A/blue light induction of gene expression in Arabidopsis. Plant Cell 1998; 10: 2077–86. 10.1105/tpc.10.12.2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Love J, Dodd AN, Webb AAR.. Circadian and diurnal calcium oscillations encode photoperiodic information in Arabidopsis. Plant Cell 2004; 16: 956–66. 10.1105/tpc.020214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plieth C, Hansen UP, Knight Het al. Temperature sensing by plants: the primary characteristics of signal perception and calcium response. Plant J 1999; 18: 491–7. 10.1046/J.1365-313X.1999.00471.X [DOI] [PubMed] [Google Scholar]
- 18. Knight H, Knight MR.. Imaging spatial and cellular characteristics of low temperature calcium signature after cold acclimation in Arabidopsis. J Exp Bot 2000; 51: 1679–86. 10.1093/jexbot/51.351.1679 [DOI] [PubMed] [Google Scholar]
- 19. Zou JJ, Wei FJ, Wang Cet al. Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol 2010; 154: 1232–43. 10.1104/pp.110.157545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou JJ, Li XD, Ratnasekera Det al. Arabidopsis CALCIUM-DEPENDENT PROTEIN KINASE8 and CATALASE3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. Plant Cell 2015; 27: 1445–60. 10.1105/tpc.15.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geiger D, Scherzer S, Mumm Pet al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci USA 2010; 107:8023–8. 10.1073/pnas.0912030107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Myers C, Romanowsky SM, Barron YDet al. Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J 2009; 59: 528–39. 10.1111/j.1365-313X.2009.03894.x [DOI] [PubMed] [Google Scholar]
- 23. Coca M and San Segundo B. AtCPK1 calcium-dependent protein kinase mediates pathogen resistance in Arabidopsis. Plant J 2010; 63: 526–40. 10.1111/j.1365-313X.2010.04255.x [DOI] [PubMed] [Google Scholar]
- 24. Cheng SH, Willmann MR, Chen HCet al. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol 2002; 129: 469–85. 10.1104/pp.005645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koornneef M, Hanhart CJ, van der Veen JH.. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet 1991; 229: 57–66. 10.1007/BF00264213 [DOI] [PubMed] [Google Scholar]
- 26. Mouradov A, Cremer F, Coupland G.. Control of flowering time: interacting pathways as a basis for diversity. Plant Cell 2002; 14: s111–30. 10.1105/tpc.001362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Srikanth A, Schmid M.. Regulation of flowering time: all roads lead to Rome. Cell Mol Life Sci 2011; 68: 2013–37. 10.1007/s00018-011-0673-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee H, Suh SS, Park Eet al. The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev 2000; 14: 2366–76. 10.1101/gad.813600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Samach A, Onouchi H, Gold SEet al. Target reproductive development of Arabidopsis. Science 2000; 288: 1613–6. 10.1126/science.288.5471.1613 [DOI] [PubMed] [Google Scholar]
- 30. Simpson GG. The autonomous pathway: epigenetic and post-transcriptional gene regulation in the control of Arabidopsis flowering time. Curr Opin Plant Biol 2004; 7: 570–4. 10.1016/j.pbi.2004.07.002 [DOI] [PubMed] [Google Scholar]
- 31. Choi H, Park HJ, Park JHet al. Arabidopsis calcium-dependent protein kinase AtCPK32 interacts with ABF4, a transcriptional regulator of abscisic acid-responsive gene expression, and modulates its activity. Plant Physiol 2005; 139: 1750–61. 10.1104/pp.105.069757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Franz S, Ehlert B, Liese Aet al. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol Plant 2011; 4: 83–96. 10.1093/mp/ssq064 [DOI] [PubMed] [Google Scholar]
- 33. Liu F, Quesada V, Crevillén Pet al. The Arabidopsis RNA-binding protein FCA requires a lysine-specific demethylase 1 homolog to downregulate FLC. Mol Cell 2007; 28: 398–407. 10.1016/j.molcel.2007.10.018 [DOI] [PubMed] [Google Scholar]
- 34. Kawamoto N, Sasabe M, Endo Met al. Calcium-dependent protein kinases responsible for the phosphorylation of a bZIP transcription factor FD crucial for the florigen complex formation. Sci Rep 2015; 5: 8341. 10.1038/srep08341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kawamoto N, Endo M, Araki T.. Expression of a kinase-dead form of CPK33 involved in florigen complex formation causes delayed flowering. Plant Signal Behav 2015; 10: e1086856. 10.1080/15592324.2015.1086856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Atif RM, Shahid L, Waqas Met al. Insights on Calcium-Dependent Protein Kinases (CPKs) signaling for abiotic stress tolerance in plants. Int J Mol Sci 2019; 20: 5298. 10.3390/ijms20215298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou L, Fu Y, Yang Z. A genome-wide functional characterization of Arabidopsis regulatory calcium sensors in pollen tubes. J Integr Plant Biol 2009; 51: 751–61. 10.1111/j.1744-7909.2009.00847.x [DOI] [PubMed] [Google Scholar]
- 38. Zhou L, Lan W, Jiang Yet al. A calcium-dependent protein Kinase interacts with and activates a calcium channel to regulate pollen tube growth. Mol Plant 2014; 7: 369–76. 10.3389/fpls.2021.633293 [DOI] [PubMed] [Google Scholar]
- 39. Liu KH, Niu Y, Konishi Met al. Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature 2017; 545: 311–6. DOI:10.1038/nature22077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qin DB, Liu MY, Yuan Let al. Calcium-dependent protein kinase 32-mediated phosphorylation is essential for the ammonium transport activity of AMT1;1 in Arabidopsis roots. J Exp Bot 2020; 71: 5087–97. 10.1093/jxb/eraa249 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.