Abstract
Purpose/Objectives(s):
Early-stage endometrial cancer patients are at higher risk of noncancer mortality than of cancer mortality. Competing event models incorporating comorbidity could help identify women most likely to benefit from treatment intensification.
Methods and Materials:
67,397 women with stage I-II endometrioid adenocarcinoma after total hysterectomy diagnosed from 1988 to 2009 were identified in Surveillance, Epidemiology, and End Results (SEER) and linked SEER-Medicare databases. Using demographic and clinical information, including comorbidity, we sought to develop and validate a risk score to predict the incidence of competing mortality.
Results:
In the validation cohort, increasing competing mortality risk score was associated with increased risk of noncancer mortality (subdistribution hazard ratio [SDHR], 1.92; 95% confidence interval [CI], 1.60–2.30) and decreased risk of endometrial cancer mortality (SDHR, 0.61; 95% CI, 0.55–0.78). Controlling for other variables, Charlson Comorbidity Index (CCI) = 1 (SDHR, 1.62; 95% CI, 1.45–1.82) and CCI >1 (SDHR, 3.31; 95% CI, 2.74–4.01) were associated with increased risk of noncancer mortality. The 10-year cumulative incidences of competing mortality within low-, medium-, and high-risk strata were 27.3% (95% CI, 25.2%−29.4%), 34.6% (95% CI, 32.5%−36.7%), and 50.3% (95% CI, 48.2%−52.6%), respectively. With increasing competing mortality risk score, we observed a significant decline in omega (ω), indicating a diminishing likelihood of benefit from treatment intensification.
Conclusion:
Comorbidity and other factors influence the risk of competing mortality among patients with early-stage endometrial cancer. Competing event models could improve our ability to identify patients likely to benefit from treatment intensification.
Summary
We developed and validated a competing mortality risk score for women with stage I-II endometrial cancer that is able to discriminate effects on primary cancer-specific versus competing events. The likelihood of benefit from treatment intensification was assessed by estimating the effect of the risk score on the relative balance of cancer-specific versus all-cause mortality.
Introduction
Endometrial cancer is the most common gynecologic cancer in the United States (1). Multiple studies have found that women with early-stage endometrial cancer are at higher risk of mortality from competing noncancer causes than from their primary cancer (2–5). This is due to the favorable prognosis associated with surgical treatment (2, 4, 6) and the high prevalence of risk factors for cardiovascular disease (7, 8) and second malignancies (9). Endometrial cancer patients with comorbidities are also less likely to undergo intensive surgical treatment (10), and patients medically unfit for surgery are more likely to die of noncancer causes (11).
As the incidence of competing mortality rises, the benefit of intensifying cancer therapy diminishes. Effective methods to stratify patients according to competing mortality risk are needed to appropriately tailor the intensity of therapy for cancer patients. Traditionally, risk-stratification models have focused on the effects of treatments and risk factors on combined endpoints, such as overall survival, that pool 1 or more disease-specific events with death of any cause. This is helpful for determining the net impact of these factors on patients’ overall health, but it is problematic in early-stage endometrial cancer because the effects in question are not likely to be homogeneous with respect to the events constituting a combined endpoint.
Models of survival and event-free survival are constrained, in general, by their inability to discriminate effects on primary cancer-specific versus competing events, predisposing clinical studies to inefficiency and potentially suspect inferences regarding the effects of therapies (12, 13). By contrast, competing event models can discriminate effects of treatments and risk factors on a heterogeneous set of competing events. Such models may better aid health researchers, physicians, and patients in predicting the value of treatment intensification, and identifying cancer patients with unmet medical need, for whom interventions directed at mitigating noncancer mortality risk could be offered. Population-based competing event models have been developed in other diseases (14, 15) but are lacking in endometrial cancer. We hypothesized that comorbidity would have a strong effect on competing mortality in early-stage endometrial cancer, and we sought to validate a population-based risk score to identify patients most likely to benefit from treatment intensification.
Methods and Materials
Data source and study population
We used data from Surveillance, Epidemiology, and End Results (SEER) 17-Registries and SEER-Medicare linked databases. SEER covers approximately 28% of the cancer population in the United States (16). Medicare provides health insurance for approximately 97% of persons aged ≥65 years in the United States. SEER-Medicare links the registry data with the Medicare administrative and health care claims files for Medicare beneficiaries enrolled in fee-for-service programs (parts A and B).
We abstracted SEER data for 63,595 women with primary stage I-II endometrioid adenocarcinoma, diagnosed as the first primary malignancy from 1988 to 2006, after total hysterectomy (Fig. 1). The date of diagnosis was reported according to the date of histopathologic analysis, whether at the time of hysterectomy or endometrial biopsy. Histological classification was based on the International Classification of Diseases for Oncology, Third Edition (ICD-O-3 codes 8140, 8210, 8380, 8382, 8383, 8480, 8481, 8560, 8570) (17). Patients with type II histologies were not abstracted. A total of 4836 patients were excluded because of unknown information regarding total hysterectomy as initial treatment (n=807) or with unknown stage (n=1353), grade (n=3654), lymphadenectomy status (n=60), or a combination of these conditions. The year 2006 was selected to ensure that all women in the training cohort had adequate follow-up. Data from SEER 1988 to 2006 were extracted using SEER Stat 7.1.0.
Fig. 1.
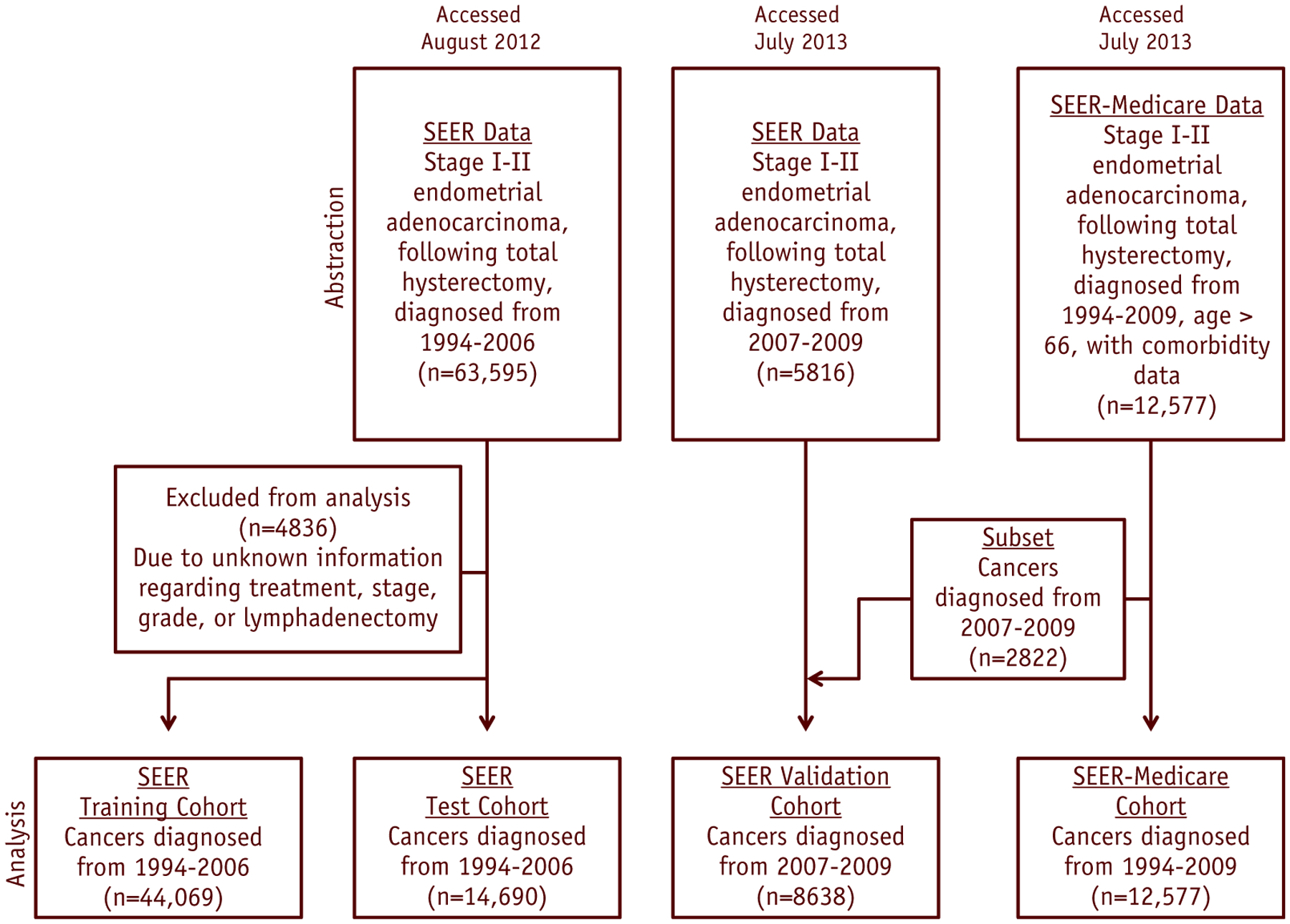
Diagram for data abstraction, exclusion, and analysis.
To ascertain comorbidity data, we abstracted records for 12,577 women from SEER-Medicare data (Fig. 1). We included patients with diagnoses made between 1994 and 2009 who met the same clinical criteria as those used earlier. We used a subset of SEER-Medicare (n=2822) and SEER (n=5816) patients with diagnoses made from 2007 to 2009 as an external validation cohort (because these patients were not in the training or test cohorts). Only women age ≥66 were included in the SEER-Medicare dataset, to ensure accurate Medicare claims for the 12-month period before diagnosis. SEER-Medicare data were extracted using SAS 9.3 software.
The following demographic and clinical variables were extracted: age at diagnosis, race, marital status, median household income, TNM stage (American Joint Committee on Cancer third edition), depth of myometrial invasion, histology, grade, and number of lymph nodes dissected. A modified Charlson Comorbidity Index (CCI) was derived with the use of Medicare claims (18). Endometrial cancer stage was recorded in SEER according to the 1988 Fédération Internationale de Gynécologie Obstétrique (FIGO) system and reclassified according to the more recent 2009 FIGO system (IA, <1/2 myometrial invasion; IB, >1/2 myometrial invasion; II, cervical stromal invasion without extrauterine or lymph node involvement). Patients with 1988 FIGO stage IIA or stage II disease not otherwise specified (NOS) that could not be recategorized as FIGO 2009 stage I or II were classified as a separate group. Grade 1 was defined as well-differentiated, grade 2 as moderately differentiated, and grade 3 as poorly differentiated or undifferentiated.
Statistical analysis
We used χ2 tests, analysis of variance, and standardized differences (19) to examine differences in categorical and continuous variables, respectively. Causes of death were classified as endometrial cancer mortality (ECM), second cancer mortality (SCM), or noncancer mortality (NCM). All-cause mortality was defined as death of any cause. Surviving patients were censored at their last date of follow-up. We calculated cumulative event probabilities using nonparametric cumulative incidence functions (20).
To develop the initial competing mortality risk score, we randomly partitioned the SEER dataset into training (75% sample) and test (25% sample) cohorts. We applied the Fine-Gray model (21) to the training cohort to estimate adjusted effects of covariates on sub-distribution hazards for each failure type. The proportional hazards assumption was assessed by the Grambsch-Therneau method (22). Goodness of fit was also assessed for proportionality of subdistributions hazards models (23).
Covariates included in each regression were age (continuous), race (black vs other), marital status (yes vs no), socioeconomic status (higher vs lower; socioeconomic status [SES] as defined by earnings above the median of median household income), stage (IA vs IB vs IIA/NOS vs II), grade (1 vs 2 vs 3), and number of lymph nodes dissected (>10 nodes vs 1–10 nodes vs 0 nodes). If a variable met the previously established significance level (P<.10) in at least 1 regression model, it was retained in the overall competing event model. We included age as a continuous variable because when we investigated varying age specifications, we did not find that it affected the ability to stratify events. Other studies have shown similar results in this population (24). A risk score for each event was computed in the training and test cohorts by taking the inner product of the coefficient vector for the given event (estimated from the training cohort) and the corresponding data vector (for general method, see Appendix eI, available at www.redjournal.org). A competing mortality risk score was obtained by subtracting the ECM risk score from the sum of the NCM and SCM risk scores.
The competing mortality risk score was partitioned into tertiles based on the distribution in the training cohort. We plotted cumulative incidences of ECM, NCM, and SCM within competing mortality risk score tertiles for women in the training cohort. We assessed the performance of the model quantitatively by using Fine-Gray regression, Gray’s test (25), and the area under the curve (AUC) (26) and visually by comparing cumulative incidences according to risk strata in the test and validation cohorts.
To test the impact of comorbidity on the competing event model, we applied both multivariable Cox proportional hazards (27) and Fine-Gray regression to the SEER-Medicare data. We used Akaike’s information criterion (AIC) to test whether CCI improves prediction beyond the SEER-trained competing mortality risk score. Then, we reestimated parameters for each of the covariates used in the initial model. We plotted cumulative incidences of ECM and NCM according to CCI and competing mortality risk strata. Gray’s test was used to test differences in cumulative incidences across strata. A final risk score, including the effect of CCI, was computed in the manner described previously.
To determine the effects of risk stratification, we calculated the ratio as follows:
| (1) |
as a function of the competing mortality risk score, where Λx represents the cumulative cause-specific hazard for event x and ΛACM represents the cumulative hazard for all-cause mortality. ω may be regarded as a measure of the potential to benefit from treatment intensification. For example, when ω is low, irrespective of one’s risk for mortality, intensifying cancer therapy would be expected to have little benefit; by contrast, at high values, the potential benefit of treatment intensification is optimized. Values of ω were estimated at 5 years. A 2-sided P value of .05 or less was considered statistically significant unless otherwise specified. Data were prepared and analyzed in R version 2.15.1 (www.R-project.org) using the “cmprsk” package.
Results
Patient characteristics
The majority of patients were white, married, and of lower socioeconomic status and had stage IA, low- to intermediate-grade disease (Table 1). According to the standardized differences and test P values, patients in the validation cohort had later-stage disease, were older, and were more likely to undergo lymphadenectomy than were patients in the training/test cohorts. The majority of patients in the SEER-Medicare dataset had a CCI of zero, were white, were unmarried, had lower socioeconomic status, and had stage IA, low- to intermediate-grade disease (Table 1). Outcomes data are provided in Appendix eII, available at www.redjournal.org.
Table 1.
Demographic and clinical characteristics
| Characteristic | SEER | SEER-medicare | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Training cohort | Test cohort | Validation cohort | All | Stand. diff.† | P value* | CCI = 0 | CCI = 1 | CCI > 1 | All | Stand. diff.† | P value* | |
| Patients, n | 44,069 | 14,690 | 8638 | 67,397 | 10,611 | 1495 | 471 | 12,577 | ||||
| Mean age at diagnosis, y (SD) | 63 (12) | 63 (12) | 69 (9) | 63 (12) | 0.33 | <.001 | 75 (6) | 75 (6) | 75 (6) | 75 (6) | 0.04 | .18 |
| Race, n (%) | 0.09 | <.001 | 0.18 | <.001 | ||||||||
| White | 38,775 (88) | 12,960 (88.2) | 7305 (84.6) | 59,040 (87.6) | 9613 (90.6) | 1284 (85.9) | 379 (80.5) | 11,276 (89.7) | ||||
| Black | 2009 (4.6) | 670 (4.6) | 651 (7.5) | 3330 (4.9) | 511 (4.8) | 117 (7.8) | 65 (13.8) | 693 (5.5) | ||||
| Other | 3285 (7.4) | 1060 (7.2) | 682 (7.9) | 5027 (7.5) | 487 (4.6) | 94 (6.3) | 27 (5.7) | 608 (4.8) | ||||
| Married, n (%) | 24,298 (55.1) | 8125 (55.3) | 4254 (49.2) | 36,677 (54.4) | 0.08 | <.001 | 4960 (46.7) | 573 (38.3) | 159 (33.8) | 5692 (45.3) | 0.14 | <.001 |
| Higher SES, n (%)‡ | 21,160 (48) | 7192 (49) | 3036 (35.1) | 31,388 (46.6) | 0.18 | <.001 | 3536 (33.3) | 382 (25.5) | 137 (29.1) | 4055 (32.2) | 0.11 | <.001 |
| Stage, n (%) | 0.24 | <.001 | 0.08 | .002 | ||||||||
| IA | 33,926 (77) | 11,211 (76.3) | 5634 (65.2) | 50,771 (75.3) | 6911 (65.1) | 901 (60.3) | 275 (58.4) | 8087 (64.3) | ||||
| IB | 6004 (13.6) | 2071 (14.1) | 1965 (22.7) | 10,040 (14.9) | 2442 (23) | 386 (25.8) | 128 (27.2) | 2956 (23.5) | ||||
| IIA/II NOS | 2963 (6.7) | 990 (6.7) | 445 (5.2) | 4398 (6.5) | 763 (7.2) | 123 (8.2) | 41 (8.7) | 927 (7.4) | ||||
| II | 1176 (2.7) | 418 (2.9) | 594 (6.9) | 2188 (3.3) | 495 (4.7) | 85 (5.7) | 27 (5.7) | 607 (4.8) | ||||
| Grade, n (%) | 0.07 | <.001 | 0.09 | <.001 | ||||||||
| 1 | 22,015 (50) | 7339 (50) | 3954 (45.8) | 33,308 (49.4) | 4729 (44.6) | 593 (39.7) | 174 (36.9) | 5496 (43.7) | ||||
| 2 | 15,719 (35.6) | 5164 (35.1) | 3173 (36.7) | 24,056 (35.7) | 3958 (37.3) | 605 (40.4) | 191 (40.6) | 4754 (37.8) | ||||
| 3 | 6335 (14.4) | 2187 (14.9) | 1511 (17.5) | 10,033 (14.9) | 1924 (18.1) | 297 (19.9) | 106 (22.5) | 2327 (18.5) | ||||
| Lymphadenectomy, n (%) | 1.61 | <.001 | 0.04 | .24 | ||||||||
| 0 nodes | 23,161 (52.6) | 7610 (51.8) | 2707 (31.3) | 33,478 (49.7) | 4882 (46) | 725 (48.5) | 222 (47.2) | 5829 (46.4) | ||||
| 1–10 nodes | 9011 (20.4) | 3110 (21.2) | 2250 (26.1) | 14,371 (21.3) | 2519 (23.7) | 356 (23.8) | 117 (24.8) | 2992 (23.8) | ||||
| >10 nodes | 11,897 (27) | 3970 (27) | 3681 (42.6) | 19,548 (29.0) | 3210 (30.3) | 414 (27.7) | 132 (28) | 3756 (29.8) | ||||
Abbreviations: CCI = Charlson Comorbidity Index; NOS = not otherwise specified; SEER = Surveillance, Epidemiology, and End Results; SES = socioeconomic status.
P values for categorical and continuous variables were generated from χ2 tests and analysis of variance, respectively.
Standardized difference compares differences in means (or proportions) in units of the pooled standard deviation; imbalances are defined as absolute values greater than 0.20 (small effect size). Standardized difference is not influenced by sample size.
Higher SES = above $47,070 annual salary.
Effects of characteristics on outcomes in the training cohort
On multivariable analysis, increasing age, black race, stage IB disease, and stage IIA/NOS disease were associated with increased risk of NCM, whereas grade 3 disease, married status, higher socioeconomic status, and lymphadenectomy were associated with decreased risk of NCM (Table 2). Increasing age, black race, more advanced stage, and increasing grade were associated with increased risk of ECM, and lymphadenectomy was the only factor associated with decreased risk of ECM (Table 2).
Table 2.
Multivariable competing risks regression analysis by cause of death
| SEER | SEER-medicare | |||
|---|---|---|---|---|
| Variable | SDHR (95% CI) | P Value | SDHR (95% CI) | P Value |
| Endometrial cancer mortality | ||||
| Age at diagnosis, per year | 1.03 (1.03–1.04) | <.001 | 1.02 (1.01–1.04) | <.001 |
| Race (referent: white/other) | ||||
| Black | 1.67 (1.41–1.97) | <.001 | 1.37 (1.05–1.80) | .02 |
| Married (referent: no) | ||||
| Yes | 0.93 (0.84–1.02) | .10 | 0.96 (0.82–1.12) | .57 |
| SES (referent: lower) | ||||
| Higher* | 0.97 (0.89–1.06) | .50 | 1.01 (0.86–1.18) | .92 |
| Stage (referent: IA) | ||||
| IB | 2.14 (1.91–2.39) | <.001 | 2.35 (1.98–2.79) | <.001 |
| IIA/II NOS | 3.17 (2.78–3.60) | <.001 | 3.48 (2.83–4.27) | <.001 |
| II | 3.46 (2.88–4.16) | <.001 | 3.78 (2.96–4.84) | <.001 |
| Grade (referent: 1) | ||||
| 2 | 2.22 (1.95–2.53) | <.001 | 2.56 (2.05–3.19) | <.001 |
| 3 | 6.10 (5.33–6.97) | <.001 | 6.40 (5.12–7.97) | <.001 |
| Lymphadenectomy (referent: 0 nodes) | ||||
| 1–10 nodes | 0.87 (0.78–0.98) | .02 | 1.13 (0.94–1.35) | .18 |
| >10 nodes | 0.81 (0.73–0.91) | .003 | 0.99 (0.83–1.18) | .93 |
| Charlson comorbidity index (referent: 0) | ||||
| 1 | NA | NA | 0.97 (0.78–1.21) | .79 |
| >1 | NA | NA | 1.09 (0.77–1.55) | .63 |
| Noncancer mortality | ||||
| Age at diagnosis, per y | 1.08 (1.08–1.09) | <.001 | 1.09 (1.08–1.10) | <.001 |
| Race (referent: white/other) | ||||
| Black | 1.28 (1.13–1.45) | <.001 | 1.13 (0.94–1.36) | .20 |
| Married (referent: no) | ||||
| Yes | 0.75 (0.71–0.79) | <.001 | 0.76 (0.69–0.83) | <.001 |
| SES (referent: lower) | ||||
| Higher* | 0.95 (0.91–1.00) | .07 | 0.94 (0.86–1.02) | .16 |
| Stage (referent: IA) | ||||
| IB | 1.12 (1.05–1.20) | <.001 | 1.05 (0.95–1.16) | .34 |
| IIA/II NOS | 1.11 (1.01–1.22) | .03 | 1.06 (0.92–1.23) | .43 |
| II | 1.04 (0.85–1.27) | .70 | 1.05 (0.84–1.31) | .67 |
| Grade (referent: 1) | ||||
| 2 | 1.02 (0.96–1.08) | .49 | 1.01 (0.92–1.10) | .92 |
| 3 | 0.93 (0.86–1.00) | .05 | 0.91 (0.81–1.03) | .14 |
| Lymphadenectomy (referent: 0 nodes) | ||||
| 1–10 nodes | 0.88 (0.82–0.94) | <.001 | 0.90 (0.81–1.00) | .06 |
| >10 nodes | 0.76 (0.71–0.81) | <.001 | 0.79 (0.71–0.88) | <.001 |
| Charlson comorbidity index (referent: 0) | ||||
| 1 | NA | NA | 1.62 (1.45–1.82) | <.001 |
| >1 | NA | NA | 3.31 (2.74–4.01) | <.001 |
| Second cancer mortality | ||||
| Age at diagnosis, per y | 1.03 (1.03–1.04) | <.001 | 1.03 (1.02–1.04) | <.001 |
| Race (referent: white/other) | ||||
| Black | 1.40 (1.17–1.66) | <.001 | 1.34 (0.97–1.83) | .07 |
| Married (referent: no) | ||||
| Yes | 0.96 (0.88–1.05) | .35 | 1.00 (0.85–1.18) | .97 |
| SES (referent: lower) | ||||
| Higher* | 1.06 (0.98–1.15) | .16 | 0.92 (0.77–1.08) | .30 |
| Stage (referent: IA) | ||||
| IB | 1.08 (0.96–1.21) | .18 | 1.17 (0.97–1.42) | .10 |
| IIA/II NOS | 1.34 (1.16–1.54) | <.001 | 1.48 (1.15–1.91) | .002 |
| II | 1.25 (0.96–1.63) | <.001 | 1.72 (1.24–2.39) | .001 |
| Grade (referent: 1) | ||||
| 2 | 1.18 (1.07–1.29) | <.001 | 1.22 (1.02–1.47) | .03 |
| 3 | 1.42 (1.26–1.60) | <.001 | 1.67 (1.35–2.07) | <.001 |
| Lymphadenectomy (referent: 0 nodes) | ||||
| 1–10 nodes | 1.02 (0.91–1.13) | .77 | 0.83 (0.68–1.01) | .06 |
| >10 nodes | 0.84 (0.76–0.94) | .002 | 0.83 (0.69–1.01) | .06 |
| Charlson Comorbidity Index (referent: 0) | ||||
| 1 | NA | NA | 1.14 (0.90–1.44) | .28 |
| >1 | NA | NA | 0.92 (0.59–1.43) | .70 |
Abbreviations: CI = confidence interval; NA = not applicable; NOS = not otherwise specified; SDHR = subdistribution hazard ratio; SEER = Surveillance, Epidemiology, and End Results; SES = socioeconomic status.
Higher SES = above $47,070 annual salary.
Training and testing of competing mortality risk score
The initial competing mortality risk score was calculated as follows:
The mean, standard deviation (SD), minimum, and maximum of R were 4.38, 1.14, −0.49, and 7.89, respectively. Patients were separated into low, medium, and high competing mortality risk strata for R<3.90, 3.91–4.88, and >4.88, respectively.
In the training cohort, the 10-year cumulative incidences of competing mortality (NCM and SCM combined) within low-, medium-, and high-risk strata were 9.7% (95% CI, 9.1%−10.3%), 16.2% (95% CI, 15.4%−17.0%), and 34.9% (95% CI, 34.0%−36.0%), respectively (P<.001). In the test cohort, the 10-year cumulative incidences of competing mortality within low-, medium-, and high-risk strata were 10.3% (95% CI, 9.2%−11.4%), 17.1% (95% CI, 15.7%−18.5%), and 35.8% (95% CI, 34.1%−37.5%), respectively (P<.001). In the test cohort, increased competing mortality risk score was associated with increased risk of NCM (SDHR, 2.04 per unit score [95% CI, 1.95–2.14], P<.001) and decreased risk of ECM (SDHR, 0.90 [95% CI, 0.84–0.96], P=.002). The risk score was also significantly associated with increased risk of SCM (SDHR, 1.22 [95% CI, 1.14–1.31], P<.001). As a categorical variable, the medium (SDHR, 1.87 [95% CI, 1.61–2.16], P<.001) and high (SDHR, 4.90 [95% CI, 4.29–5.58], P<.001) competing mortality risk strata were associated with increased risk of NCM relative to the low-risk stratum. The AUC demonstrated a higher predictive ability for noncancer mortality (0.71) than for cancer-specific mortality (0.46). Effective stratification of competing mortality events according to risk strata was observed in both the training (Fig. 2A–C) and the test (Fig. 2D–F) cohorts.
Fig. 2.
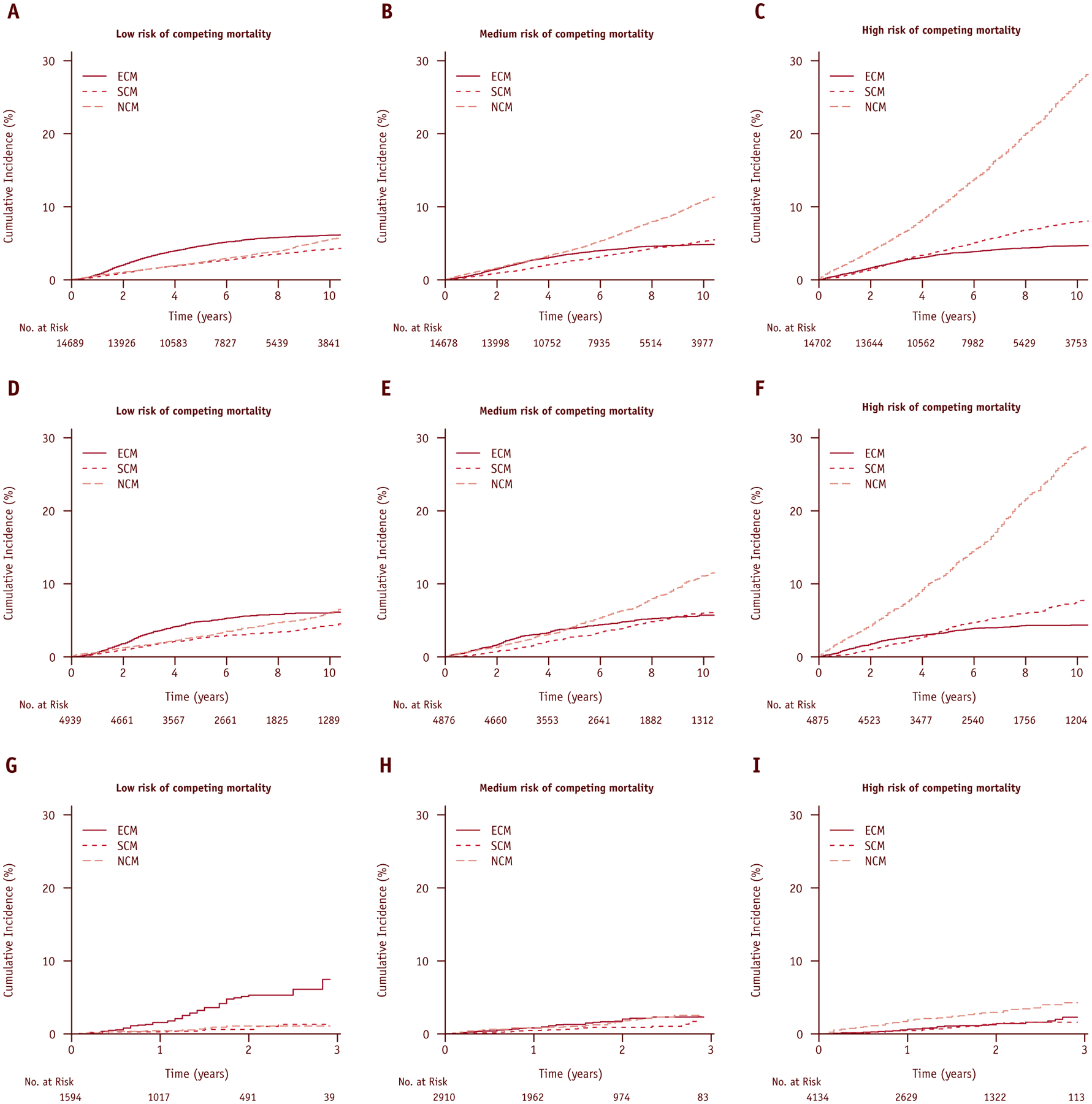
Cumulative incidence plots of cause-specific mortalities according to the SEER-trained competing mortality risk score by (A-C) training, (D-F) test, and (G-I) validation cohorts.
Validation of competing mortality risk score
In the validation cohort, the 2.5-year cumulative incidences of competing mortality within low-, medium-, and high-risk strata were 2.4% (95% CI, 1.3%−3.5%), 3.3% (95% CI, 2.4%−4.3%), and 5.6% (95% CI, 4.5%−6.7%), respectively (P<.001) (Fig. 2G–I). Increasing risk score was associated with increased risk of NCM (SDHR, 1.92 [95% CI, 1.60–2.30], P<.001) and decreased risk of ECM (SDHR, 0.61 [95% CI, 0.55–0.78], P<.001). The risk score was not significantly associated with SCM (SDHR, 1.24 [95% CI, 0.95–1.62], P=.12). As a categorical variable, medium (SDHR, 1.88 [95% CI, 0.96–3.67] P=.06) and high (SDHR, 3.40 [95% CI, 0.16–0.55], P<.001) competing mortality risk strata were associated with increased risk of NCM relative to the low-risk stratum. The AUC demonstrated a higher predictive ability for noncancer mortality (0.66) than for cancer-specific mortality (0.34).
Effects of comorbidity on competing mortality
The CCI plus the competing mortality risk score (AIC, 13,865) improved the prediction beyond the competing mortality risk score (AIC, 13,870). Increased CCI was associated with a higher incidence of NCM overall and within risk strata (Fig. 3A–C). Controlling for other variables used in the initial competing event model, CCI = 1 (SDHR, 1.62 [95% CI, 1.45–1.82]) and CCI >1 (SDHR, 3.31 [95% CI, 2.74–4.01]) were significantly associated with increased risk of NCM (Table 2). By contrast, CCI was not significantly correlated with ECM or SCM on multivariable regression (Table 2).
Fig. 3.
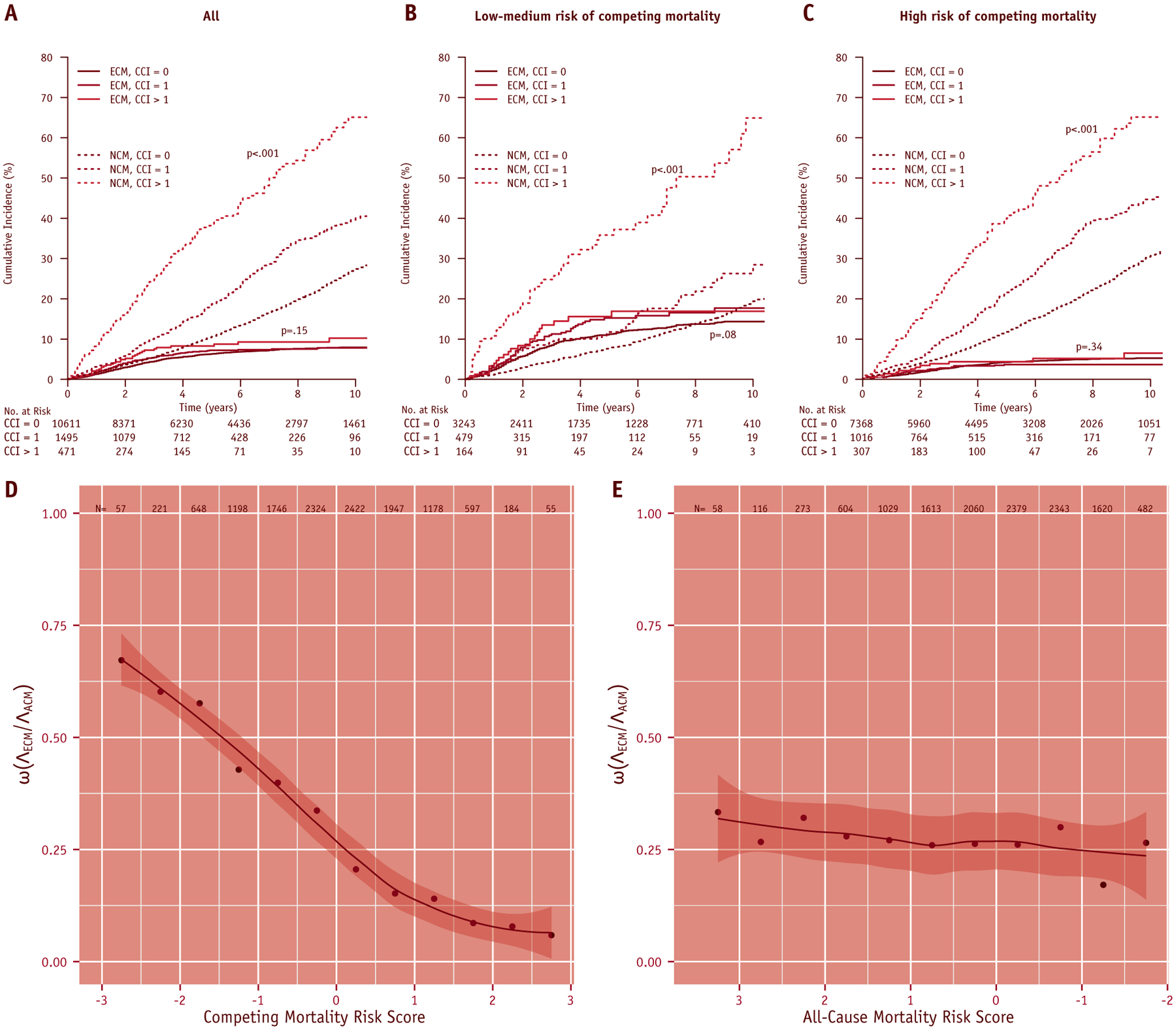
(A-C) Cumulative incidence plots with Gray’s test P values for endometrial cancer mortality (ECM) and noncancer mortality (NCM) grouped by the Charlson Comorbidity Index score according to all women in the (A) SEER-Medicare cohort and within (B) low-medium and (C) high competing mortality risk strata based on SEER-trained cutoffs. Gray’s test P values are shown. (D, E) Ratio (ω) of the cumulative hazard of endometrial cancer mortality (ΛECM) to all-cause mortality (ΛACM) at 5 years, as a (smoothed) function of (A) normalized competing event risk score or (B) normalized all-cause mortality risk score. Values of ω are calculated at intervals of one-half standard deviation of the risk score. The competing mortality risk score is better able to stratify patients based on event composition. The abscissa for all-cause mortality risk score is reversed, so that the likelihood of benefitting from treatment intensification decreases moving from left to right in both plots. CCI = Charlson Comorbidity Index; ECM = endometrial cancer mortality; NCM = noncancer morbidity; SCM = second cancer mortality.
Competing mortality risk score accounting for comorbidity
The revised competing mortality risk score, accounting for effects of comorbidity, was calculated as follows:
The mean, SD, minimum, and maximum of R′ were 5.72, 0.97, 2.91, and 9.20, respectively. The cohort was separated into low-, medium-, and high-risk strata for R′ <5.30, 5.30–6.16, and >6.16, respectively. The 10-year cumulative incidences of competing mortality within low-, medium-, and high-risk strata were 27.3% (95% CI, 25.2%−29.4%), 34.6% (95% CI, 32.5%−36.7%), and 50.3% (95% CI, 48.2%−52.6%), respectively. Increasing competing mortality risk was associated with advanced age, higher CCI, unmarried status, lower SES, early-stage low-grade disease, and a lower probability of lymphadenectomy (Table 3). Despite the fact that black women are at increased risk of competing mortality, in controlling for other factors we observed no significant racial differences across competing mortality risk strata (P=.20).
Table 3.
Demographic and clinical characteristics by competing event risk strata
| Characteristic | Low | Medium | High | P value* |
|---|---|---|---|---|
| Patients, n | 4193 | 4188 | 4196 | |
| Mean age at diagnosis, y (SD) | 72 (5) | 74 (6) | 79 (6) | <.001 |
| Race, n (%) | ||||
| White | 3724 (88.8) | 3767 (89.9) | 3785 (90.2) | .08 |
| Black | 249 (5.9) | 217 (5.2) | 227 (5.4) | .29 |
| Other | 220 (5.2) | 204 (4.9) | 184 (4.4) | .18 |
| Married, n (%) | 2461 (58.7) | 1979 (47.3) | 1252 (29.8) | <.001 |
| Higher SES, n (%)† | 1465 (34.9) | 1395 (33.3) | 1195 (28.5) | <.001 |
| Stage, n (%) | ||||
| IA | 1728 (41.2) | 2918 (69.7) | 3441 (82) | <.001 |
| IB | 1535 (36.6) | 851 (20.3) | 570 (13.6) | <.001 |
| IIA/II NOS | 561 (13.4) | 256 (6.1) | 110 (2.6) | <.001 |
| II | 369 (8.8) | 163 (3.9) | 75 (1.8) | <.001 |
| Grade, n (%) | ||||
| 1 | 429 (10.2) | 1979 (47.3) | 3088 (73.6) | <.001 |
| 2 | 2049 (48.9) | 1738 (41.5) | 967 (23.0) | <.001 |
| 3 | 1715 (40.9) | 471 (11.2) | 141 (3.4) | <.001 |
| Lymphadenectomy, n (%) | ||||
| 0 nodes | 896 (21.4) | 1970 (47.0) | 2963 (70.6) | <.001 |
| 1–10 nodes | 1380 (32.9) | 1016 (24.3) | 596 (14.2) | <.001 |
| >10 nodes | 1917 (45.7) | 1202 (28.7) | 637 (15.2) | <.001 |
| Charlson Comorbidity Index, n (%) | ||||
| 0 | 3906 (93.2) | 3649 (87.1) | 3056 (72.8) | <.001 |
| 1 | 244 (5.8) | 429 (10.3) | 822 (19.6) | <.001 |
| >1 | 43 (1.0) | 110 (2.6) | 318 (7.6) | <.001 |
Abbreviations: NOS = not otherwise specified; SD = standard deviation; SES = socioeconomic status.
P values for categorical and continuous variables were generated from χ2 tests and analysis of variance, respectively.
Higher SES = above $47,070 annual salary.
With increasing competing mortality risk score, we observed a significant decline in the proportion of the overall hazard for mortality attributable to endometrial cancer (ω). For the entire SEER-Medicare cohort, ω = 0.27. Risk stratification effectively differentiates women at increased risk of ECM relative to competing events, for any given hazard for overall mortality. By comparison, a risk score based on all-cause mortality, using the same covariates as inputs, cannot optimize the composition of events (ie, stratify according to ω) as well as the competing event model (Fig. 3D–E).
Discussion
In this study, we developed a model to stratify women with stage I-II endometrial cancer according to competing mortality risk. This model had high discriminatory ability in the test cohort and was validated in a contemporary population-based cohort, despite short follow-up times. On the basis of prior studies (28–30), we were interested in testing the hypothesis that comorbidity would be a strong predictor of competing mortality and could augment our ability to stratify patients according to risk of this event. Our observations support this hypothesis.
There are several applications of competing event models. Clinically, these can serve as tools to predict the value of treatment intensification. In particular, such models could help identify women who are more likely to benefit from interventions directed at their underlying nononcologic diseases, such as intensive primary care, or risk-adapted survivorship care plans. A recent study of overweight and obese survivors of endometrial cancer showed positive effects on weight loss and nutrient intake among women randomized to lifestyle intervention versus usual care (31). If maintained, these effects have the potential to decrease morbidity and mortality in these patients. Therefore, it is crucial to address comorbidity and other noncancer mortality risk factors, which may improve health outcomes in this population. However, prospective validation of the risk score developed in this study would be important before its widespread clinical use can be advocated.
In comparative effectiveness research, this model can be used to adjust effects of primary interest for a patient’s potential to benefit from treatment intensification. In clinical trial design, stratification by competing event risk can help ensure balance across arms of a trial (32), reducing problems with confounding that result from vagaries in random allocation. Enrichment based on competing mortality risk can also increase the power and decrease the cost of clinical trials (33), particularly when effects on competing events are not of primary interest or when a large trial is economically infeasible. Notably, we did not observe significant racial imbalances according to competing event risk strata in our study; however, our model also implies that black patients with early-stage endometrial cancer are less likely to benefit from treatment intensification, presumably as a consequence of underlying health disparities. Assuring racial and ethnic impartiality would be needed if treatment selection were to be based on this risk score.
The strengths of this study included a large population-based sample, which permitted robust training and validation measures. The SEER data contain important factors, which are essential for developing a competing event model, in addition to cause-of-death data, which are generally regarded as accurate in SEER (34). By separating the cause-specific effects of covariates before aggregating them in the prognostic model, we were able to estimate the effects of these factors on the relative balance of disease-specific versus competing events. This process is needed to determine the likely benefit of treatment intensification in competing risks settings, and it contrasts with modeling approaches that use combined endpoints, in which the effects are invariant to endpoint composition.
Several limitations of our study deserve discussion. Some important predictors are lacking in SEER (eg, body mass index, smoking history, and lymphovascular space invasion). CCI is a fairly crude instrument for measuring comorbidity, which also tends to be underreported in the Medicare data. Models incorporating more detailed metrics may perform better. Despite these limitations, we used a parsimonious model to explain a high degree of variance in competing events, and we estimate the marginal impact of this missing information to be minimal. The lack of consistency between SEER and other datasets hinders retrospective head-to-head comparisons of competing event models versus standard prognostic models. Further studies comparing this model prospectively against prevailing models in the wider population are needed. Despite a relatively homogeneous group in terms of stage, primary treatment, and histology, it is possible that variations in adjuvant treatment could affect our results, because these were not explicitly controlled for in our model.
In conclusion, we observed that multiple demographic and clinical characteristics, particularly comorbidity, influence the risk of competing mortality among patients with early-stage endometrial cancer. Competing event models could improve our ability to distinguish patients most likely to benefit from interventions directed at mitigating competing causes of mortality, as opposed to treatment intensification.
APPENDIX eI
Generalized competing event model
Let n, k, and p be the number of observations, covariates, and mutually exclusive event types, respectively. Let z be the number cause-specific events, and p-z be the number of competing events. Let d represent the k × 1 vector of covariate values, and 1m represent a m × 1 vector of 1’s. Let i be an index of natural numbers ranging from 1 to p. Let λ0i represent the cause-specific hazard for event i, and λ0 = Σ λ0i represent the hazard for any event, under a given set of experimental conditions.
We model the cause-specific hazard for event i, under an alternative set of conditions as λ1i = g(Xβi) λ0i, for an invertible function g(●), an n × k data matrix X, and a k × 1 vector of effect coefficients βi. The hazard for any event under the alternative set of conditions is λ1 = Σ λ1i = Σ g(Xβi) λ0i and the hazard ratio is expressed as:
| (2) |
in other words, the hazard ratio is a weighted average of the effects on the cause-specific hazards under the initial conditions. Here β is the k × p coefficient matrix, with each element βv,w representing the effect of covariate v on event w. Note that under the assumption of effect homogeneity with respect to the cause-specific events, βj=βk=β for all j, k ε {1,…,p}, therefore λ1 / λ0 = Σ g(Xβi) λ0i / Σ λ0i = Σ g(Xβ) λ0i / Σ λ0i = g(Xβ) Σ λ0i / Σ λ0i = g(Xβ).
Let bi be a maximum (partial) likelihood estimator for βi (e.g., using g(x) = ex (27); alternatively, we can let bi represent an analogous maximum partial likelihood estimator for sub-distribution hazards (21,35). Let B = [b1 b2 … bp] be the k × p matrix of coefficients, with each element bv,w of B representing the estimated effect of covariate v on event w. Since columns of B are interchangeable, we can order the elements of B such that the first z vectors correspond to events of interest and the remaining p-z vectors correspond to competing events, i.e. B1,z = [b1 b2 … bz] and Bz,p = [bz+1 bz+2 … bp], so B = [B1,z Bz,p]. Now using the data vector d, we construct an individual risk score as follows:
| (3) |
Note that under the assumption of effect homogeneity with respect to the cause-specific events, bj=bk=b for all j, k ε {1, …, p}, so R = cdTb for some constant c.
APPENDIX eII
Outcomes
In the SEER dataset, 44,925 women were alive at last follow-up. Median follow-up times were 81 months for surviving patients and 77 months overall (range: 0–251). The number of deaths due to endometrial cancer, non-cancer causes, and second cancers were 2639, 8137, and 3058, respectively. The median times to death from endometrial cancer, non-cancer causes, and second cancers were 31, 78, and 57 months, respectively. The 10-year cumulative incidences of all-cause mortality, ECM, SCM, and NCM were 26.3% [95% confidence interval (CI), 25.9–26.8%], 5.2% [95% CI, 5.0–5.4%], 5.9% [95% CI, 5.7–6.2%], and 15.2% [95% CI, 14.8–15.6%], respectively.
In the validation cohort, 8,290 women were alive at last follow-up. Median follow-up times were 17 months for surviving patients and 17 months overall (range: 0–35). The number of deaths due to endometrial cancer, non-cancer causes, and second cancers were 133, 147, and 68, respectively. The median times to death from endometrial cancer, non-cancer causes, and second cancers were 13, 9, and 14 months, respectively. The 2.5-year cumulative incidences of all-cause mortality, ECM, SCM, and NCM were 6.9% [95% CI, 6.1–7.7%], 2.7% [95% CI, 2.2–3.2%], 1.4% [95% CI, 1.0–1.7%], and 2.9% [95% CI, 2.3–3.4%], respectively.
In the SEER-Medicare cohort, 8,737 patients were alive at last follow-up. Median follow-up times were 60 months for surviving patients and 56 months overall (range: 0–189). The number of deaths due to endometrial cancer, non-cancer causes, and second cancers were 775, 2406, and 659, respectively. The median times to death from endometrial cancer, non-cancer causes, and second cancers were 26, 59, and 48 months, respectively. The 10-year cumulative incidences of all-cause mortality, ECM, SCM, and NCM were 55.0% [95% CI, 53.4–56.6%], 8.3% [95% CI, 7.7–8.9%], 9.2% [95% CI, 8.4–10.0%], and 37.5% [95% CI, 36.0–39.0%], respectively.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- 2.Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and post-operative radiotherapy versus surgery alone for patient with stage-1 endometrial carcinoma: Multicenter randomized trial. PORTEC study group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 2000;355:1404–1411. [DOI] [PubMed] [Google Scholar]
- 3.ASTEC study group, Kitchener H, Swart AM, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomized study. Lancet 2009;373:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nout RA, Smit VT, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet 2010;375:816–823. [DOI] [PubMed] [Google Scholar]
- 5.Ward KK, Shah NR, Saenz CC, et al. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol Oncol 2012;126:176–179. [DOI] [PubMed] [Google Scholar]
- 6.Lachance JA, Darus CJ, Rice LW. Surgical management and post-operative treatment of endometrial carcinoma. Rev Obstet Gynecol 2008;1:97–105. [PMC free article] [PubMed] [Google Scholar]
- 7.Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004;4:579–591. [DOI] [PubMed] [Google Scholar]
- 8.Arem H, Park Y, Pelser C, et al. Prediagnosis body mass index, physical activity, and mortality in endometrial cancer patients. J Natl Cancer Inst 2013;105:342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh H, Nugent Z, Demers A, et al. Risk of colorectal cancer after diagnosis of endometrial cancer: A population-based study. J Clin Oncol 2013;31:2010–2015. [DOI] [PubMed] [Google Scholar]
- 10.Coon D, Beriwal S, Heron DE, et al. High-dose-rate Rotte “Y” applicator brachytherapy for definitive treatment of medically inoperable endometrial cancer: 10-year results. Int J Radiat Oncol Biol Phys 2008;71:779–783. [DOI] [PubMed] [Google Scholar]
- 11.Podzielinski I, Randall ME, Breheny PJ, et al. Primary radiation therapy for medically inoperable patients with clinical stage I and II endometrial carcinoma. Gynecol Oncol 2012;124:36–41. [DOI] [PubMed] [Google Scholar]
- 12.Mell LK, Jeong JH. Pitfalls of using composite primary end points in the presence of competing risks. J Clin Oncol 2010;28:4297–4299. [DOI] [PubMed] [Google Scholar]
- 13.Dignam JJ, Kocherginsky MN. Choice and interpretation of statistical tests used when competing risks are present. J Clin Oncol 2008;26: 4027–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose BS, Jeong JH, Nath SK, et al. Population-based study of competing mortality in head and neck cancer. J Clin Oncol 2011;29: 3503–3509. [DOI] [PubMed] [Google Scholar]
- 15.Taghipour S, Banjevic D, Fernandes J, et al. Predictors of competing mortality to invasive breast cancer incidence in the Canadian National Breast Screening study. BMC Cancer 2012;12:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surveillance, Epidemiology, and End Results (SEER) Program Public-Use Data (1973–2008). National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2011. Available at http://www.seer.cancer.gov. Accessed August 22, 2012. [Google Scholar]
- 17.ICD-O-3-SEER site/histology validation list. Rockville, MD: SEER Program Quality Control Section; 2007. Available at http://www.seer.cancer.gov. Accessed August 22, 2012. [Google Scholar]
- 18.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000; 53:1258–1267. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korn EL, Dorey FJ. Applications of crude incidence curves. Stat Med 1992;11:813–829. [DOI] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 22.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–526. [Google Scholar]
- 23.Zhou B, Fine J, Laird G. Goodness-of-fit test for proportional subdistribution hazards model. Stat Med 2013;32:3804–3811. [DOI] [PubMed] [Google Scholar]
- 24.Mell LK, Carmona R, Gulaya S, et al. Cause-specific effects of radiotherapy and lymphadenectomy in stage I-II endometrial cancer: A population based study. J Natl Cancer Inst 2013;105:1656–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–1154. [Google Scholar]
- 26.Saha P, Heagerty PJ. Time-dependent predictive accuracy in the presence of competing risks. Biometrics 2010;66:999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox DR. Regression models and life tables. J R Stat Soc Series B Stat Methodol 1972;B34:187–220. [Google Scholar]
- 28.Cook LS, Nelson HE, Cockburn M, et al. Comorbidities and endometrial cancer survival in Hispanics and non-Hispanic whites. Cancer Causes Control 2013;24:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson SH, Atoria CL, Cote ML, et al. The impact of race and comorbidity on survival in endometrial cancer. Cancer Epidemiol Biomarkers Prev 2012;21:753–760. [DOI] [PubMed] [Google Scholar]
- 30.Truong PT, Kader HA, Lacy B, et al. The effects of age and comorbidity on treatment and outcomes in women with endometrial cancer. Am J Clin Oncol 2005;28:157–164. [DOI] [PubMed] [Google Scholar]
- 31.Kavanagh MB, Von Gruenigen VE, Courneya KS, et al. Effects of a lifestyle intervention on nutrient intake in overweight/obese endometrial cancer survivors. e-SPEN, the European e-Journal of Clinical Nutrition and Metabolism:e143–e147. Available at, www.sciencedirect.com, 2009;4. Accessed on July 27, 2013. [Google Scholar]
- 32.Zakeri K, Rose BS, Gulaya S, et al. Competing event risk stratification may improve the design and efficiency of clinical trials: Secondary analysis of SWOG 8794. Contemp Clin Trials 2013;34:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zakeri K, Rose BS, Mell LK. Improving the efficiency of clinical trials through selection models. Int J Radiat Oncol Biol Phys 2013; 84(Suppl):S45. [Google Scholar]
- 34.Lund JL, Harlan LC, Yabroff KR, et al. Should cause of death from the death certificate be used to examine cancer-specific survival? A study of patients with distant stage disease. Cancer Invest 2010;28:758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong JH, Fine JP. Parametric regression on cumulative incidence function. Biostatistics 2007;8:184–196. [DOI] [PubMed] [Google Scholar]


