Abstract
Background
Allergies have become more prevalent globally over the last 20 years. Dietary consumption of n‐3 (or omega 3) long chain polyunsaturated fatty acids (LCPUFA) has declined over the same period of time. This, together with the known role of n‐3 LCPUFA in inhibiting inflammation, has resulted in speculation that n‐3 LCPUFA may prevent allergy development. Dietary n‐3 fatty acids supplements may change the developing immune system of the newborn before allergic responses are established, particularly for those with a genetic predisposition to the production of the immunoglobulin E (IgE) antibody. Individuals with IgE‐mediated allergies have both the signs and symptoms of the allergic disease and a positive skin prick test (SPT) to the allergen.
Objectives
To assess the effect of n‐3 LCPUFA supplementation in pregnant and/or breastfeeding women on allergy outcomes (food allergy, atopic dermatitis (eczema), allergic rhinitis (hay fever) and asthma/wheeze) in their children.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (6 August 2014), PubMed (1966 to 01 August 2014), CINAHL via EBSCOhost (1984 to 01 August 2014), Scopus (1995 to 01 August 2014), Web of Knowledge (1864 to 01 August 2014) and ClinicalTrials.gov (01 August 2014) and reference lists of retrieved studies.
Selection criteria
We included randomised controlled trials (RCTs) evaluating the effect of n‐3 LCPUFA supplementation of pregnant and/or lactating women (compared with placebo or no treatment) on allergy outcomes of the infants or children. Trials using a cross‐over design and trials examining biochemical outcomes only were not eligible for inclusion.
Data collection and analysis
Two review authors independently assessed eligibility and trial quality and performed data extraction. Where the review authors were also investigators on trials selected, an independent reviewer assessed trial quality and performed data extraction.
Main results
Eight trials involving 3366 women and their 3175 children were included in the review. In these trials, women were supplemented with n‐3 LCPUFA during pregnancy (five trials), lactation (two trials) or both pregnancy and lactation (one trial). All trials randomly allocated women to either a n‐3 LCPUFA supplement or a control group. The risk of bias varied across the eight included trials in this review with only two trials with a low risk of selection, performance and attrition bias.
N‐3 LCPUFA supplementation showed a clear reduction in the primary outcome of any allergy (medically diagnosed IgE mediated) in children aged 12 to 36 months (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.44 to 0.98; two RCTs; 823 children), but not beyond 36 months (RR 0.86, 95% CI 0.61 to 1.20; one RCT, 706 children). For any allergy (medically diagnosed IgE mediated and/or parental report), no clear differences were seen in children either at 12 to 36 months (RR 0.89, 95% CI 0.71 to 1.11; two RCTs, 823 children) or beyond 36 months of age (RR 0.96, 95% CI 0.84 to 1.09; three RCTs, 1765 children).
For the secondary outcomes of specific allergies there were no clear differences for food allergies at 12 to 36 months and beyond 36 months, but a clear reduction was seen for children in their first 12 months with n‐3 LCPUFA (both for medically diagnosed IgE mediated and medically diagnosed IgE mediated and/or parental report). There was a clear reduction in medically diagnosed IgE‐mediated eczema with n‐3 LCPUFA for children 12 to 36 months of age, but not at any other time point for both medically diagnosed IgE mediated and medically diagnosed IgE mediated and/or parental report. No clear differences for allergic rhinitis or asthma/wheeze were seen at any time point for both medically diagnosed IgE mediated, and medically diagnosed IgE mediated and/or parental report.
There was a clear reduction in children's sensitisation to egg and sensitisation to any allergen between 12 to 36 months of age when mothers were supplemented with n‐3 LCPUFA.
In terms of safety for the mother and child, n‐3 LCPUFA supplementation during pregnancy did not show increased risk of postpartum haemorrhage or early childhood infections.
Authors' conclusions
Overall, there is limited evidence to support maternal n‐3 LCPUFA supplementation during pregnancy and/or lactation for reducing allergic disease in children. Few differences in childhood allergic disease were seen between women who were supplemented with n‐3 LCPUFA and those who were not.
Plain language summary
Fish oil (n‐3 or omega‐3) for pregnant mothers or breastfeeding mothers to prevent allergies in their young children
Fish and fish oil are the major sources of omega‐3 long chain fatty acids. Dietary marine omega‐3 fatty acid supplements during pregnancy may change the immune system of the newborn before allergic responses are established, particularly for those with a genetic predisposition to the production of the immunoglobulin E (IgE) antibody. Individuals with IgE‐mediated allergies have both the signs and symptoms of the allergic disease and a positive skin prick test (SPT) to the allergen.
Allergy is an important public health problem that places a burden on individuals, society and healthcare costs. Allergic diseases include food allergies, eczema (atopic dermatitis), asthma or wheeze and hay fever (allergic rhinitis). Many childhood allergies continue into adulthood.
Pregnant women, especially those from Western countries, are not eating as much fish and allergic diseases have been increasing over the time that pregnant women have been eating less fish. The unborn baby gets nutrition from his or her mother and so the mother's diet is important. Supplementing women with omega‐3 fatty acids from marine origin may be important in preventing their children from developing allergies.
In this review of randomised controlled studies, we evaluated the effects of adding marine omega‐3 fatty acids to women’s diets during pregnancy or lactation on allergic diseases in their children. We analysed eight trials that involved 3366 women and 3175 children. The women were randomly assigned to receive a marine omega‐3 supplement (as fish oil capsules, or added to foods) or no treatment during pregnancy (five trials), during breast feeding (two trials) or both pregnancy and breast feeding (one trial). Overall, the methodological quality of the trials varied, with only two trials being at low risk of bias.
Overall, the results showed little effect of maternal marine omega‐3 supplementation during pregnancy and/or breast feeding for the reduction of allergic disease in the children. However there were reductions in some outcomes such as food allergy during the baby's first year and eczema with marine omega‐3 supplementation in women with a baby at high risk of allergy. Currently, there is not enough evidence to say that omega‐3 supplements from marine origin during pregnancy and/or breast feeding for mothers will reduce allergies in their children.
In terms of safety for the mother and child, omega‐3 fatty acids supplementation from marine origin during pregnancy did not show increased risk of excessive bleeding after the baby was born (postpartum haemorrhage) or early childhood infections.
Background
Description of the condition
Over the past 20 years the prevalence of allergies in industrialised countries has increased fivefold, from approximately 4% to an estimated 20% (Asher 2006; Pawanker 2011). Allergic diseases include food allergies, atopic dermatitis (eczema), asthma and allergic rhinitis (hay fever) (Arkwright 2008). The pattern of allergy expression differs with age (Spergel 2003). Food allergies and eczema are common in children under three years of age, while asthma and allergic rhinitis are more common between the ages of three and 15 years (Saarinen 1995). Regardless of these changing patterns of allergic disease in childhood, many childhood allergies persist, with 50% of childhood asthma sufferers and 80% of allergic rhinitis sufferers continuing to experience allergic symptoms into adulthood (Asher 2006; Barbee 1998; Greisner 1998; Spergel 2010).
Atopy is defined as a genetic predisposition to the production of specific immunoglobulin E (IgE) antibodies to allergens and atopic people are more liable to have immune reactions that lead to allergies (Sears 1996). One of the principal methods of determining specific IgE sensitisation to an allergen is the skin prick test (SPT). To perform a SPT, a drop of allergen extract is placed on the skin and a small prick is made through the drop. This allows a small amount of allergen to enter the skin. If allergic to the tested allergen, a small lump (wheal) will appear at the site of testing within 15 to 20 minutes. A test is positive if there is a mean wheal diameter of 3 mm or greater (Bousquet 2012). A positive SPT to an allergen, along with clinical allergy symptoms, forms the basis for IgE‐mediated allergy diagnosis (Johansson 2001; Johansson 2004).
The risk of allergy is 30% greater if one first degree relative (parent or sibling) is atopic, but if both parents are atopic then the allergy risk increases to 70% (Sears 1996). Allergic responses are reactions to an extrinsic substance (allergen) that is mediated by an immunological response (Arkwright 2008). This immunological response may cause mild to severe reactions in different individuals and can be life threatening (Arkwright 2008). Thus allergy is an important public health problem which places a burden on individuals, society and the healthcare system (Gupta 2004).
Environmental factors seem to have had an important influence on the increasing incidence of allergies. Possible contributing factors include lack of breastfeeding, higher socio‐economic conditions with higher standards of hygiene, fewer respiratory infections, greater use of antibiotics early in life, fewer older siblings in the household, less contact with farm animals, general lack of microbial exposure and changes in dietary patterns (Gupta 2004; Strong 2005).
Among the changes in dietary patterns, it has been hypothesised that the balance of long chain polyunsaturated fatty acids (LCPUFA), specifically the n‐3 (omega 3) to n‐6 (omega 6) ratio, may be a factor in the increased incidence of childhood allergies (Calder 2000; Calder 2010b; Prescott 2004). Furthermore, maternal fish consumption during pregnancy has also reduced due to precautionary public health advice regarding the consumption of specific fish which may contain methyl mercury (Oken 2003).
Description of the intervention
Fish and fish oil are the major sources of n‐3 LCPUFA, while vegetable oils are the major source of n‐6 LCPUFA. Recent data suggest that dietary consumption of n‐3 LCPUFA has declined in Western diets to favour the intake of n‐6 fatty acids (Meyer 2003). Epidemiological data suggest that a higher fish intake during pregnancy is associated with fewer symptoms of allergic diseases in the offspring in early childhood (Calvani 2006; Romieu 2007; Sausenthaler 2007; Willers 2007). Thus, supplementing maternal diets with n‐3 LCPUFA may be an important factor in reducing the incidence of allergic diseases.
How the intervention might work
Dietary n‐3 LCPUFA supplementation during pregnancy and lactation has been suggested to modulate the immune system of the fetus, neonate or infant before allergic responses are established (Denburg 2005). The early programming of fetal immune responses to allergens possibly begins in the epithelial tissue where antigen (allergen) proteins first encounter antigen‐presenting cells (Prescott 2007). The pattern of cytokine production by antigen‐presenting cells determines the pattern of T‐helper (Th) cells differentiation (Prescott 2007). T cells producing Type 1 T cells (Th1), develop under the influence of interleukin (IL)‐12 and IL‐2, whereas T cells producing Type 2 T cells (Th2), develop in the relative absence of pro‐Th2 factors such as IL‐4 (Snijdewint 1993). The differences in T cell phenotypes also determine the pattern of B‐cell antibody production with Th2 cytokines (IL‐4, IL‐5, and IL‐13), prompting IgE production and allergic inflammation, whereas Th1 cytokines (IFN‐γ) largely inhibit this in favour of low level IgG production (Calder 2006; Calder 2010a). Th2 cytokines are also important in determining whether these immune responses result in clinically relevant diseases such as asthma, allergic rhinitis or allergic dermatitis (Calder 2003; Georas 2005; Prescott 2007). A well regulated placental balance between the Th1 and Th2 responses is important for developing a robust immune system during pregnancy (Wilczynski 2005).
Maternal n‐3 LCPUFA intervention studies support this immune programming hypothesis (Krauss‐Etschmann 2007; Lauritzen 2005; Lee 2013; Romero 2013). Two studies investigated Th1/Th2 related molecules in cord blood (Krauss‐Etschmann 2008; Romero 2013), and the other investigated cytokine production in children at two and a half years of age (Lauritzen 2005). These studies showed that allergy‐related immune parameters were lower in the offspring of women who had n‐3 LCPUFA supplementation during pregnancy or lactation (Krauss‐Etschmann 2008; Lauritzen 2005; Romero 2013). The other study also showed that maternal n‐3 LCPUFA supplementation during pregnancy was associated with balancing Th1/Th2 and modulating IFNγ and IL13 in infants (Lee 2013).
Supporting evidence from mechanistic (Denburg 2005; Prescott 2007) and small scale intervention studies show that allergy markers and allergy mechanisms are influenced by n‐3 LCPUFA (Dunstan 2003; Prescott 2007a). Also there were studies in which maternal or postnatal consumption of n‐3 LCPUFA through oily fish or fish oil had an effect on allergy outcomes in children (Hodge 1996; Romieu 2007), as well as a transient effect on allergy outcomes (Mihrshahi 2004; Oddy 2004).
Why it is important to do this review
Allergy is an important public health problem that places a burden on individuals, society and healthcare costs (Gupta 2004; Kemp 1999; Pawanker 2011). Consequently, allergy prevention is a major global challenge (Strong 2005) and the World Allergy Organization (WAO) recommends that allergy preventative strategies are urgently needed (Asher 2004). It is uncertain if n‐3 LCPUFA supplementation during pregnancy or lactation reduces allergic disease in children. For these reasons, we need clear evidence from intervention studies. Therefore, in this systematic review we aim to evaluate the effects of maternal n‐3 LCPUFA supplementation during pregnancy and/or lactation on allergy outcomes in children.The safety aspects of n‐3 LCPUFA also need to be considered; it is postulated that high doses of n‐3 LCPUFA may have antithrombotic antiplatelet properties which may lead to bleeding (Simopoulos 1991), as well as immune cell alterations which may have an effect on infections (Calder 2007).
Objectives
To assess the effect of maternal n‐3 LCPUFA supplementation during pregnancy and/or lactation on the allergy outcomes of their children.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) focusing on n‐3 LCPUFA supplementation of pregnant and/or lactating women (compared with placebo or no treatment) and assessed allergy outcomes of the infants or children. Quasi‐RCTs and RCTs using a cluster‐randomised design were eligible for inclusion but none were identified. Trials published in abstract form only were not identified for inclusion. In future updates we will only consider abstracts for inclusion if unpublished data can be obtained from the trails. Trials using a cross‐over design and trials examining biochemical outcomes only were not eligible for inclusion.
Types of participants
Women and their children, with either a normal or high risk of developing allergic disease, were included. A fetus or a child with a first degree relative with medically diagnosed allergies, or a positive a SPT, or a positive radioallergosorbent test (RAST) was defined as being at high risk of allergies. Infants were also considered at high risk of allergies if their cord blood IgE level was above 0.70 IU/mL.
Types of interventions
We considered all randomised comparisons of n‐3 LCPUFA supplementation given to pregnant or lactating women (either with or without arachidonic acid), with placebo or no supplementation as a control, regardless of dose regimens and duration of intervention. Trials in which fish was the intervention were included if appropriately controlled, for example, if the diet was appropriately adjusted to match the protein contribution of fish.
Types of outcome measures
Primary outcome measures included children with allergy, including food allergy, atopic dermatitis (eczema), asthma/wheeze, allergic rhinitis (hay fever) or any allergies (children with one or more of the allergy types). Outcomes were assessed as short term (occurring at less than 12 months of age), medium term (occurring from 12 to less than 36 months of age) and long term (36 months of age and older). Outcomes were also assessed by combining short‐term, medium‐term and long‐term results to assess the cumulative incidence.
Primary outcomes
Medically diagnosed any allergy with sensitisation, i.e. IgE‐mediated allergies where both the signs and symptoms of the allergic disease and a positive SPT and/or RAST test are present.
Medical diagnosis or parental report (using validated questionnaire) of any allergy, +/‐ IgE sensitisation.
Secondary outcomes
Secondary outcome measures included children with specific forms of allergy, including food allergy, atopic dermatitis (eczema), asthma/wheeze, allergic rhinitis (hay fever) with IgE sensitisation and +/‐ IgE sensitisation, SPT results, and parent‐reported allergies using non‐validated questionnaires. Secondary safety outcomes included infant safety (e.g. infections) and maternal safety (e.g. postpartum haemorrhage or infection) due to the theoretical risk associated with higher doses of n‐3 LCPUFA.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (6 August 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched PubMed (1966 to 01 August 2014) (Appendix 1), CINAHL via EBSCOhost (1984 to 01 August 2014) (Appendix 2), Scopus (1995 to 01 August 2014) (Appendix 3), Web of Knowledge (1864 to 01 August 2014) (Appendix 4) and ClinicalTrials.gov (01 August 2014) (Appendix 5).
Searching other resources
We searched reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Two review authors Anoja W Gunaratne (AWG) and Carmel T Collins (CTC) independently assessed the eligibility of trials identified by the search. Disagreements were resolved through discussion or, if required, by consultation with the third review author Maria Makrides (MM).
Data extraction and management
Two review authors (AWG, CTC) independently extracted the data from eligible trials using the agreed form. Discrepancies were resolved through discussion or through consultation with the third author (MM) if required. When information was unclear or incomplete, we attempted to contact authors of the original reports to provide further details. We entered data into Review Manager software 5.3 (RevMan 2014) and checked them for accuracy.
Assessment of risk of bias in included studies
Two review authors (AWG, CTC) independently assessed the risk of bias for each trial using the criteria outlined inthe Cochrane Handbook for Systematic Reviews of Interventions (Handbook) (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor (MM). MM and CTC are Investigators on two trials, Makrides 2009 and Makrides 2010 included in the review. These trials were independently assessed for risk of bias and data extracted by AWG and an independent researcher third party Karen Best (KB).
(1) Random sequence generation (checking for possible selection bias)
For each included trial, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it would produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table, computer random number generator); high risk of bias (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included trial, we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen before recruitment, during recruitment or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described the methods used, if any, to blind trial participants and personnel from knowledge of which intervention a participant received for each trial. We considered that trials were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants; low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received for each included trial. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described the completeness of data, including attrition and exclusions from the analysis, for each included trial and for each outcome or class of outcomes. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants). We also outlined the reasons for attrition or exclusion, where reported, and whether missing data were balanced across groups or related to outcomes. We included missing data in the analyses where sufficient information was reported or could be supplied by the trial authors.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups); high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation); unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described our investigation of possible selective outcome reporting bias and our results for each included trial.
We assessed the methods as having a:
low risk of bias – where it is clear that all of the trial’s pre‐specified outcomes and all expected outcomes of interest to the review were reported; high risk of bias – where not all the trial’s pre‐specified outcomes had been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; or trial failed to include results of a key outcome that would have been expected to have been reported; unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described any important concerns we had about other possible sources of bias for each included trial.
We assessed whether each trial was free of other problems that could put it at risk of bias, as low, high or unclear risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether trials were at high risk of bias according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses – seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
Results are presented as summary risk ratio with 95% confidence intervals.
Continuous data
We planned to present the results of continuous data as the mean difference, if outcomes were measured in the same way between trials. We would have used the standardised mean difference to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review. If we identify cluster‐randomised trials in future updates of this review, we will include them in the analyses along with individually‐randomised trials. We will adjust their sample size using the methods described in the Handbook (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a trial of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we will synthesis the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the trial designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not an appropriate design for this review.
Other unit of analysis issues
Trials with more than two treatment groups
Trials using one or more treatment groups (multi‐arm trials) were combined to create a single pair‐wise comparison where appropriate. We used the methods described in the Handbook (Higgins 2011) to ensure that we did not double count participants.
Dealing with missing data
We noted levels of attrition within the included trials. We used sensitivity analyses to explore the impact of including trials with high levels of missing data on the overall assessment of treatment effect. For all outcomes, we carried out analyses (as far as possible) on an intention‐to‐treat basis. This meant that we attempted to include all participants randomised to each group in the analyses with all participants analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator used for each outcome was the number randomised minus the number with missing outcomes. In studies where there were missing data, we imputed results, if the imputed results differed little from the raw data.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the T² was greater than zero or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analyses using the Review Manager software 5.2 (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that trials were estimating the same underlying treatment effect: that is, where trials were examining the same intervention and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. We did not combine trials if the average treatment effect was not clinically meaningful.
Where we used random‐effects analyses, the results are presented as the average treatment effect with 95% confidence intervals and estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
Substantial heterogeneity was investigated using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, used random‐effects analyses.
We planned to carry out the following subgroup analyses.
-
Timing of supplementation
n‐3 LCPUFA supplementation during pregnancy versus placebo or no supplementation during pregnancy
n‐3 LCPUFA supplementation during lactation versus placebo or no supplementation during lactation
n‐3 LCPUFA supplementation during pregnancy and lactation versus placebo or no supplementation during pregnancy and lactation
-
Allergy risk
Maternal n‐3 LCPUFA supplementation in women whose infants were at high risk of allergic disease versus placebo or no supplementation
Maternal n‐3 LCPUFA supplementation in women whose infants were not considered as at high risk of allergic disease versus placebo or no supplementation
-
Infant maturity
Maternal n‐3 LCPUFA supplementation in term born infants versus placebo or no supplementation
Maternal n‐3 LCPUFA supplementation in preterm born infants versus placebo or no supplementation
We restricted subgroup analyses to the primary outcomes.
Sensitivity analysis
We planned to carry out sensitivity analyses for the review's primary outcomes to investigate the effect of trial quality by removing those trials rated as ‘high' or 'unclear' risk of selection, performance or attrition bias to establish whether it was likely to impact on the findings.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Electronic searches yielded 2290 records. The Cochrane Pregnancy and Childbirth Trials Register search (06 August 2014), retrieved 144 reports. Other electronic databases, PubMed (1966 to 01 August 2014), CINAHL via EBSCOhost (1984 to 01 August 2014), Scopus (1995 to 01 August 2014), Web of Knowledge (1864 to 01 August 2014) and ClinicalTrials.gov (01 August 2014) retrieved 159, 18,1817, 140 and 12 reports respectively. Two additional reports included the doctoral thesis (submitted) of AWG and an unpublished honours dissertation supervised by MM and CTC.
Of the 2292 records, we removed duplicates and irrelevant reports and identified 141 titles and abstracts by considering the inclusion criteria. Sixteen trials (with 43 reports) were excluded, leaving eight trials (with 94 reports) for inclusion in this review. Four trials (with four reports) are ongoing (Bisgaard 2012; Duchen 2012; Laitinen 2013; Liu 2013.) See Figure 1.
1.
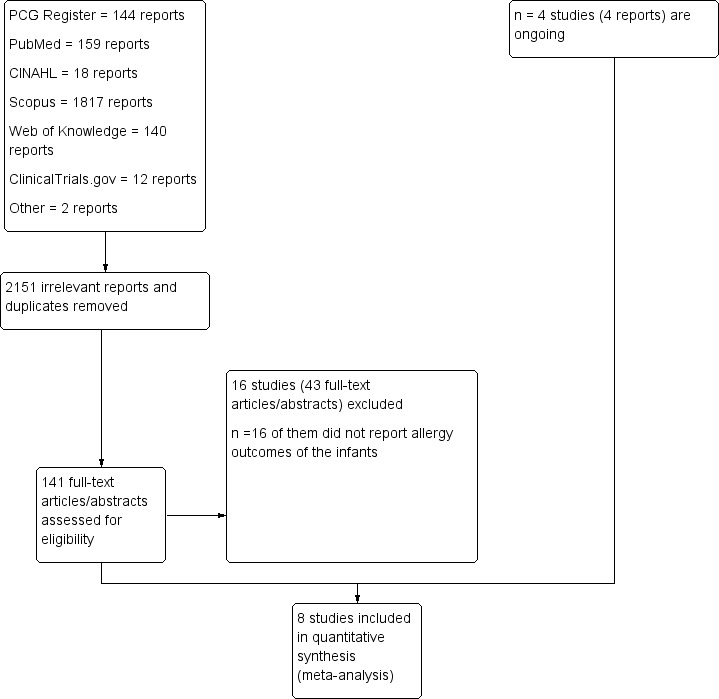
Study flow diagram.
Included studies
See: Characteristics of included studies.
We identified eight randomised controlled trials with 3366 women (3175 children) who were supplemented with oily fish, or n‐3 LCPUFA supplements during pregnancy and/or during lactation and assessed allergic outcomes in their children.
Study design
All eight trials were parallel randomised controlled trials published in English. Six trials had two parallel groups (Dunstan 2003; Furuhjelm 2009; Makrides 2009; Noakes 2012; Makrides 2010; Ramakrishnan 2010), one trial included two parallel groups and a high fish intake non‐randomised reference group (Lauritzen 2005), and one trial had three parallel groups including intervention, placebo and no oil group (Olsen 1992).
Full details of the included trials are provided in the Characteristics of included studies table.
Participants
Of the 3366 women included in the review, 667 were supplemented during the postnatal period only (Lauritzen 2005; Makrides 2009), 145 received supplementation in both the prenatal and postnatal period (Furuhjelm 2009) and the remaining 2554 women were only supplemented in the prenatal period (Dunstan 2003; Noakes 2012; Olsen 1992; Makrides 2010; Ramakrishnan 2010). Women with a fetus at high risk of allergies (n = 1072) were included in four trials (Dunstan 2003; Furuhjelm 2009; Noakes 2012; Makrides 2010) while the remainder included women with a fetus at both high and normal risk of allergies (Lauritzen 2005; Makrides 2009; Olsen 1992; Ramakrishnan 2010), see 'types of participants for definition of high risk'. In one trial, only preterm infants were included (Makrides 2009).
Sample sizes
The sample sizes of the included trials ranged from 98 (Dunstan 2003) to 1094 (Ramakrishnan 2010). Four trials had approximately 100 to 150 mothers (Dunstan 2003; Furuhjelm 2009; Lauritzen 2005; Noakes 2012) and four trials had > 500 participants (Makrides 2009; Makrides 2010; Olsen 1992; Ramakrishnan 2010).
Study location
Three trials (Dunstan 2003; Makrides 2009; Makrides 2010) were undertaken in Australia, two (Lauritzen 2005; Olsen 1992) in Denmark, one (Furuhjelm 2009) in Sweden, one (Ramakrishnan 2010) in Mexico, and one (Noakes 2012) in the UK. Most of the trials were conducted in high‐income, well‐developed industrialised countries except one trial (Ramakrishnan 2010) which was conducted in a upper‐middle income country.
Intervention
Six trials used n‐3 LCPUFA capsules (Dunstan 2003; Furuhjelm 2009; Makrides 2009; Makrides 2010; Olsen 1992; Ramakrishnan 2010), one used muesli bars containing microencapsulated fish oil as a source of n‐3 LCPUFA (Lauritzen 2005), and the remaining trial used oily fish (Noakes 2012). The daily dosage of n‐3 LCPUFA varied between 400 mg and 4500 mg; providing between 331 mg and 2070 mg of docosahexaenoic acid (DHA) and 100 mg and 1600 mg of eicosapentaenoic acid (EPA). Control groups received olive oil in three trials (Dunstan 2003; Lauritzen 2005; Olsen 1992), soy oil in two trials (Furuhjelm 2009; Makrides 2009), a blend of vegetable oils (rapeseed, sunflower, and palm in equal proportions) in one trial, (Makrides 2010), or a blend of corn and soy oil in one trial (Ramakrishnan 2010). Olsen 1992 included a third randomised group who did not receive any supplementation and the control group in Noakes 2012 also received no supplementation.
The prenatal supplementation trials commenced supplementation between 18 to 20 weeks (Ramakrishnan 2010), at 20 (Dunstan 2003; Makrides 2010; Noakes 2012) and 30 (Olsen 1992) weeks of gestation and continued until delivery. One trial supplemented in both the prenatal and postnatal period, commencing from 25 weeks' gestation and continuing until the infant reached four months of age (Furuhjelm 2009). In the two postnatal supplementation trials, supplementation commenced within one week after delivery (Lauritzen 2005; Makrides 2009). The duration of supplementation was four months in Lauritzen 2005 while in Makrides 2009 trial in preterm infants, supplementation continued until the infant reached 40 weeks postmenstrual age (a median duration of 9.4 weeks supplementation).
Outcome measures
The allergy outcomes were determined by parental reports of doctor diagnosed allergy in one trial (Lauritzen 2005), parental reports of allergy symptoms in one trial (Ramakrishnan 2010) and parental reports of allergy symptoms and parental reports of doctor diagnosed allergy in one trial (Makrides 2009). Allergy was medically diagnosed in the remaining trials (Dunstan 2003; Furuhjelm 2009; Noakes 2012; Olsen 1992; Makrides 2010). In Olsen 1992, the medical diagnosis of allergy was obtained from the Danish Medical registries. Parent reports of allergy outcome data were collected using a non‐validated questionnaire and validated International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire in Makrides 2009 trial. Parent reports of allergy outcome data were collected using a non‐validated questionnaire in Ramakrishnan 2010 trial. Additionally, Makrides 2010 trial also had parent reports of allergy outcome data using a non‐validated questionnaire. The data that were collected using non‐validated questionnaires were only included in the secondary outcomes.
The age of assessments differed in the trials. Assessments were conducted at one month and three months (Ramakrishnan 2010), six months (Furuhjelm 2009; Noakes 2012; Makrides 2010; Ramakrishnan 2010), 12 months (Dunstan 2003; Furuhjelm 2009; Makrides 2009; Makrides 2010), 18 months corrected age (Makrides 2009; Ramakrishnan 2010), 24 months (Furuhjelm 2009), 30 months (Lauritzen 2005), three to five years corrected age (Makrides 2009) (subgroup), 36 months (Makrides 2010), seven years of age (Makrides 2009), and 16 years of age (Olsen 1992). Of the five trials that used medical diagnosis of allergies (Dunstan 2003; Furuhjelm 2009; Noakes 2012; Olsen 1992; Makrides 2010), four performed skin prick tests (SPT) (Dunstan 2003; Furuhjelm 2009; Noakes 2012; Makrides 2010) and included children at high risk of allergy. Two trials (Furuhjelm 2009; Noakes 2012) reported SPT results for children under 12 months of age, three trials (Dunstan 2003; Furuhjelm 2009; Makrides 2010), reported SPT between 12 to 36 months of age, and one trial (Makrides 2010) reported SPT in children at 36 months of age.
IgE‐mediated allergies were reported in Furuhjelm 2009 and Makrides 2010. Two trials (Furuhjelm 2009; Noakes 2012) used blood samples for IgE detection in infants and one trial (Noakes 2012) reported the results at birth and six months of age while the other trial (Furuhjelm 2009) analysed the serum IgE levels at three and 12 months of age, but results were not reported.
The type of allergies reported in the trials differed. Five trials (Dunstan 2003; Furuhjelm 2009; Lauritzen 2005; Makrides 2009; Makrides 2010) reported food allergy. Six trials (Dunstan 2003; Furuhjelm 2009; Lauritzen 2005; Makrides 2009; Noakes 2012; Makrides 2010) reported eczema. All trials (Dunstan 2003; Furuhjelm 2009; Lauritzen 2005; Makrides 2009; Noakes 2012; Olsen 1992; Makrides 2010; Ramakrishnan 2010) reported asthma or wheeze. Three trials (Furuhjelm 2009; Makrides 2009; Makrides 2010) reported allergic rhinitis and four trials (Furuhjelm 2009; Makrides 2009; Olsen 1992; Makrides 2010) reported any allergy.
Two trials (Olsen 1992; Makrides 2010) reported postpartum haemorrhage. Makrides 2010 (n = 2399) was the primary trial from which the Palmer 2012 participants were recruited (see table of included studies); the incidence of postpartum haemorrhage is reported for the primary trial. Four trials (Noakes 2012; Makrides 2009; Makrides 2010; Ramakrishnan 2010) reported early childhood infections. Infection outcomes (in‐hospital proven late onset sepsis) for Makrides 2009 are reported for the whole sample (n = 657). Ramakrishnan 2010 also reported fever in infants.
Excluded studies
See: Characteristics of excluded studies.
We excluded 16 randomised controlled trials where the intervention was n‐3 LCPUFA supplementation but allergy outcomes of the infants and/or children were not reported (Bergmann 2008; Campos‐Martinez 2012; Carlson 2013 : Colombo 2004; Courville 2011; Granot 2011; Hauner 2009; Helland 2001; Innis 2007; Judge 2007; Karlsson 2010; Knudsen 2006; Krauss‐Etschmann 2007; Martin‐Alvarez 2012: Pena‐Quintana 2011; Ribeiro 2012). Many of these trials are included in the Cochrane Systematic Review currently being updated (Makrides 2006).
Characteristics of ongoing studies
See: Characteristics of ongoing studies.
There are four ongoing trials (Bisgaard 2012; Duchen 2012; Laitinen 2013; Liu 2013).
Risk of bias in included studies
Overall, the eight included studies had various levels of risk of bias for methodological quality. See Figure 2 and Figure 3 for details.
2.
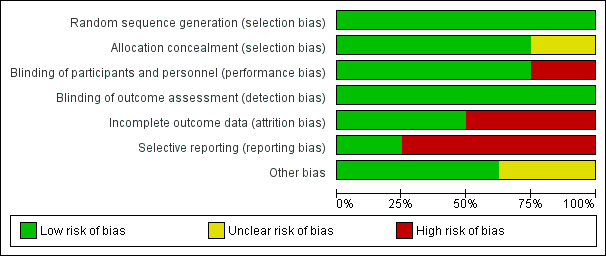
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
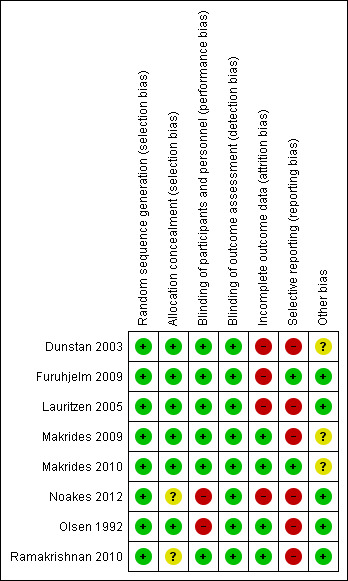
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All of the eight trials were at low risk of bias for sequence generation.
Six trials reported adequate allocation concealment methods (Dunstan 2003; Furuhjelm 2009; Lauritzen 2005; Makrides 2009; Makrides 2010: Olsen 1992) and two trials (Noakes 2012; Ramakrishnan 2010) had an unclear risk of bias as the method of allocation concealment was not described.
Blinding
Women, care providers and research personnel were blinded in six trials (Dunstan 2003; Furuhjelm 2009; Lauritzen 2005; Makrides 2009; Makrides 2010; Ramakrishnan 2010). In two trials (Noakes 2012; Olsen 1992), women who were randomised to the 'no supplement' group could not be blinded.
Outcome assessments were performed by assessors who were blinded to the trial supplementations in all eight trials (Dunstan 2003; Furuhjelm 2009; Lauritzen 2005; Makrides 2009; Makrides 2010: Noakes 2012; Olsen 1992; Ramakrishnan 2010).
Incomplete outcome data
All trials reported withdrawals and dropouts. We assessed four trials (Makrides 2009; Makrides 2010: Olsen 1992; Ramakrishnan 2010) as having a low risk of attrition bias. Makrides 2009 reported 6% of participant losses to follow‐up at 18 months, 8% at three to five years of age and 10.5% at seven years of age. Olsen 1992 reported 1% of participant losses to follow‐up at 16 years of age. Makrides 2010 reported participant follow‐up losses of 3.5% and 9.6% at one and three years of age respectively. Ramakrishnan 2010 reported 25% participants losses to follow‐up at six months, but balanced between group, and reasons for withdrawals were reported (intervention (23%) and control (24%)). Dunstan 2003, Furuhjelm 2009, Lauritzen 2005 and Noakes 2012 were assessed as having a high risk of attrition bias. Dunstan 2003 reported a loss of 15% to follow‐up and participant exclusion after randomisation, which differed between intervention (n = 12, 23%) and control (n = 3, 6.5%). More women in the intervention group discontinued treatment because of nausea (n = 7, 13.5%) than in the control (n = 1, 2%). Furuhjelm 2009 reported a 19% loss of participants to follow‐up (n = 16), with 23% in the treatment group (n = 9) and 12% in the placebo group excluded from the analysis because they did not complete the 15‐week intervention. Lauritzen 2005 had large follow‐up losses of more than 47%, with no reasons reported for the withdrawals (treatment n = 25, 40%; control n = 32, 53%). Noakes 2012 also had large follow‐up losses (30%) at six months with no reasons reported (treatment n = 16, 23%; control n = 23, 37%).
Selective reporting
All trials (Dunstan 2003; Lauritzen 2005; Makrides 2009; Noakes 2012; Olsen 1992; Ramakrishnan 2010) except two (Furuhjelm 2009; Makrides 2010) were assessed as having a high risk of reporting bias, as expected outcomes of interest to this review were not reported or were not reported completely.
Other potential sources of bias
There were no obvious other potential sources of bias identified in four trials (Furuhjelm 2009; Lauritzen 2005; Noakes 2012; Ramakrishnan 2010). There was an unclear risk of bias in the two trials that included subgroups of their original trials (Makrides 2009; Makrides 2010). In Olsen 1992 the placebo group and no oil group were combined in this review. We conducted analyses with the n‐3 LCPUFA supplementation group compared to olive oil control group separately to n‐3 LCPUFA supplementation group compared to no supplement control group, and found that although the direction of effect differed it made little difference to the meta‐analysis therefore for this review the control groups were combined. Dunstan 2003 was rated as having an unclear risk of bias because preterm infants were excluded from their analysis after randomisation.
Effects of interventions
N‐3 LCPUFA supplementation versus placebo or no supplementation
Primary outcomes ‐ any allergy
1. The effects of n‐3 LCPUFA supplementation on IgE‐mediated allergies using pooled analysis RR (M‐H, Fixed/Random, 95% CI).
| Allergy (IgE mediated) | Assessed Age | No of studies | No of participants | n‐3 LCPUFA | Placebo |
Effect Estimate RR [95% CI] |
||
| Events | Total | Events | Total | |||||
| Food allergy | < 12 months | 1 | 117 | 1 | 52 | 10 | 65 | 0.13 [0.02 to 0.95] ٭ |
| 12‐36 months | 2 | 825 | 13 | 422 | 20 | 403 | 0.58 [0.18 to 1.88] R | |
| ≥ 36 months | 1 | 706 | 14 | 368 | 9 | 338 | 1.43 [0.63 to 3.26] | |
| Eczema | < 12 months | 1 | 117 | 4 | 52 | 13 | 65 | 0.38 [0.13 to 1.11] |
| 12‐36 months | 2 | 823 | 29 | 422 | 45 | 401 | 0.61 [0.39 to 0.95] ٭ | |
| ≥ 36 months | 1 | 706 | 44 | 368 | 48 | 338 | 0.84 [0.57 to 1.23] | |
| Allergic rhinitis | < 12 months | 0 | 0 | NE | ||||
| 12‐36 months | 2 | 825 | 1 | 422 | 3 | 403 | 0.47 [0.07 to 3.06] | |
| ≥ 36 months | 1 | 706 | 18 | 368 | 20 | 338 | 0.83 [0.44 to 1.54] | |
| Asthma | < 12 months | 0 | 0 | NE | ||||
| 12‐36 months | 2 | 824 | 3 | 422 | 4 | 402 | 0.86 [0.21 to 3.49] | |
| ≥ 36 months | 1 | 706 | 6 | 368 | 5 | 338 | 1.10 [0.34 to 3.58] | |
| Any allergies | < 12 months | 0 | 0 | NE | ||||
| 12‐36 months | 2 | 823 | 36 | 422 | 52 | 401 | 0.66 [0.44 to 0.98] ٭ | |
| ≥ 36 months | 1 | 706 | 55 | 368 | 59 | 338 | 0.86 [0.61 to 1.20] | |
*significant P < 0.05, ٭٭ significant P < 0.005, NE = Not estimable , R= Random‐effects estimate
2. The effects of n‐3 LCPUFA supplementation on all allergies using pooled analysis RR (M‐H, Fixed, 95% CI).
| Allergy (IgE mediated or not) | Assessed Age | No of studies | No of participants | n‐3 LCPUFA | Placebo |
Effect Estimate RR [95% CI] |
||
| Events | Total | Events | Total | |||||
| Food allergy | < 12 months | 1 | 117 | 1 | 52 | 10 | 65 | 0.13 [0.02, 0.95] ٭ |
| 12‐36 months | 4 | 973 | 19 | 499 | 26 | 474 | 0.72 [0.40 to 1.30] | |
| ≥ 36 months | 1 | 706 | 14 | 368 | 9 | 338 | 1.43 [0.63 to 3.26] | |
| Eczema | < 12 months | 2 | 203 | 16 | 100 | 20 | 103 | 0.76 [0.22 to 2.62]R |
| 12‐36 months | 4 | 973 | 118 | 499 | 122 | 474 | 0.96 [0.69 to 1.33]R | |
| ≥ 36 months | 2 | 1237 | 122 | 628 | 131 | 609 | 0.88 [0.68 to 1.13]R | |
| Allergic rhinitis | < 12 months | 0 | 0 | NE | ||||
| 12‐36 months | 2 | 805 | 10 | 414 | 18 | 391 | 0.53 [0.25 to 1.12] | |
| ≥ 36 months | 2 | 1169 | 114 | 593 | 109 | 576 | 1.03 [0.81 to 1.30] | |
| Asthma | < 12 months | 1 | 83 | 11 | 46 | 7 | 37 | 1.26 [0.54 to 2.94] |
| 12‐36 months | 4 | 955 | 106 | 491 | 105 | 464 | 0.93 [0.73 to 1.18] | |
| ≥ 36 months | 3 | 1697 | 165 | 856 | 171 | 841 | 0.94 [0.78 to 1.13] | |
| Any allergies | < 12 months | 0 | 0 | NE | ||||
| 12‐36 months | 2 | 823 | 109 | 422 | 117 | 401 | 0.89 [0.71 to 1.11] | |
| ≥ 36 months | 3 | 1765 | 270 | 891 | 274 | 874 | 0.96 [0.84 to 1.09] | |
*significant P < 0.05, ٭٭ significant P < 0.005, NE= Not estimable , R= Random‐effects estimate
We considered any allergy that included children with one or more allergy types including food allergy, atopic dermatitis (eczema), asthma/wheeze, allergic rhinitis (hay fever) as primary outcomes of the review. Allergic disease was considered in two ways. Firstly, the effect of n‐3 LCPUFA supplementation on IgE‐mediated allergic disease, then the effect of supplementation on all allergic disease (+/‐ IgE sensitivity) was analysed, for each of the allergic diseases under study. The effect of supplementation was analysed at various points in the child's life – short term (up to 12 months of age), medium term (12 to 36 months), and long term (36 months and beyond). We were unable to report cumulative incidences due to variation in reporting between studies.
Any allergies (Analysis 1.1; Analysis 1.2)
N‐3 LCPUFA supplementation showed a clear reduction in IgE‐mediated allergies in 12 to 36 months of age children when compared with the control group (Analysis 1.1; Furuhjelm 2009; Makrides 2010, 823 children, risk ratio (RR) 0.66, 95% confidence interval (CI) 0.44 to 0.98). No clear differences were found in IgE‐mediated allergies between treatments in 36 months of age or older children (Makrides 2010, 706 children, RR 0.86, 95% CI 0.0.61 to 1.20). No included trials reported on combined IgE‐mediated allergies in infants under 12 months of age.
1.1. Analysis.
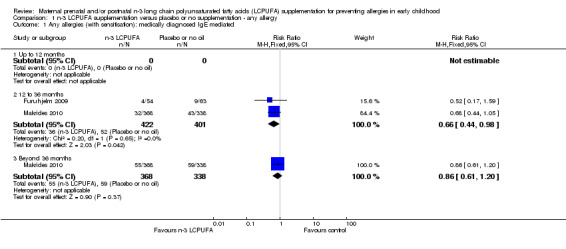
Comparison 1 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ any allergy, Outcome 1 Any allergies (with sensitisation): medically diagnosed IgE mediated.
When all allergies (+/‐ IgE sensitivity) were considered, n‐3 LCPUFA supplementation did not show clear differences in allergies in children at 12 to 36 months (Furuhjelm 2009, Makrides 2010, RR 0.89, 95% CI 0.71 to 1.11, 823 children) or 36 months and beyond (Makrides 2009, Makrides 2010, Olsen 1992, RR 0.96, 95% CI 0.84 to 1.09, 1765 children) (Analysis 1.2). No included trials reported on combined +/‐ IgE‐mediated allergies in infants under 12 months of age.
1.2. Analysis.
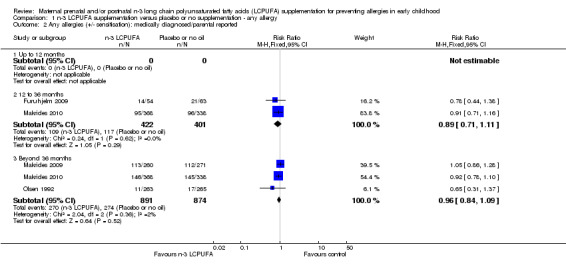
Comparison 1 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ any allergy, Outcome 2 Any allergies (+/‐ sensitisation): medically diagnosed/parental reported.
Secondary outcomes
As secondary outcomes, we considered specific forms of allergy including food allergy, atopic dermatitis (eczema), asthma/wheeze and allergic rhinitis (hay fever) (IgE‐mediated and +/‐ IgE sensitivity).
Food allergy (Analysis 2.1; Analysis 2.2)
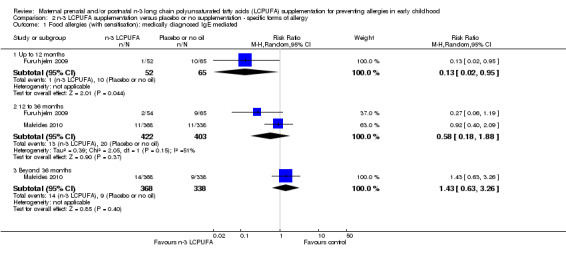
N‐3 LCPUFA supplementation reduced the incidence of IgE‐mediated food allergies in children up to 12 months of age (Furuhjelm 2009, 117 infants, RR 0.13, 95% 0.02 to 0.95; Analysis 2.1), but there were no clear differences found between the intervention and control groups at any other age (12 to 36 months Furuhjelm 2009; Makrides 2010, 825 children, average RR 0.58, 95% CI 0.18 to 1.88; > 36 months of age Makrides 2010, 706 children, RR 1.43, 95% CI 0.63 to 3.26). A random‐effects analysis was used as substantial heterogeneity was noted at the 12‐ to 36‐month time point (Tau² = 0.39; P = 0.15; I² = 51%) (Analysis 2.1). The heterogeneity may be due to the duration of the intervention, the dose used and the difference in assessment ages.
2.1. Analysis.
Comparison 2 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ specific forms of allergy, Outcome 1 Food allergies (with sensitisation): medically diagnosed IgE mediated.
When food allergies +/‐ IgE sensitivity were considered (Analysis 2.2), results showed few differences from those for IgE‐mediated allergies (Analysis 2.1) with no differences in the direction of findings from those for IgE‐mediated allergies (up to 12 months of age, Furuhjelm 2009, 117 infants, RR 0.13, 95% CI 0.02 to 0.95; between 12 to 36 months, Dunstan 2003; Furuhjelm 2009; Lauritzen 2005; Makrides 2010, 973 children, RR 0.72, 95% CI 0.40 to 1.30; > 36 months of age Makrides 2010, 706 children, RR 1.43, 95% CI 0.63 to 3.26).
2.2. Analysis.
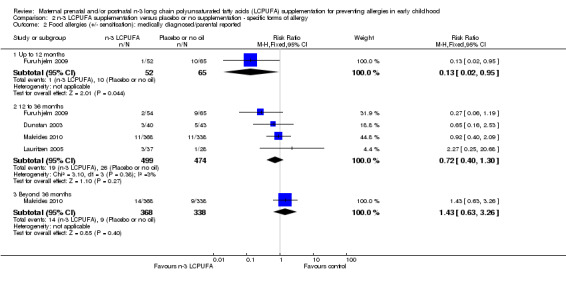
Comparison 2 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ specific forms of allergy, Outcome 2 Food allergies (+/‐ sensitisation): medically diagnosed/parental reported.
Eczema (Analysis 2.3; Analysis 2.4)
N‐3 LCPUFA supplementation reduced the incidence of IgE‐mediated eczema in children at 12 to 36 months of age (Furuhjelm 2009; Makrides 2010; 823 children, RR 0.61, 95% CI 0.39 to 0.95, Analysis 2.3). There were no clear differences between groups at the other time points (< 12 months Furuhjelm 2009; 117 children, RR 0.38, 95% CI 0.13 to 1.11 or > 36 months of age Makrides 2010, 706 children, RR 0.84, 95% CI 0.57 to 1.23)
2.3. Analysis.
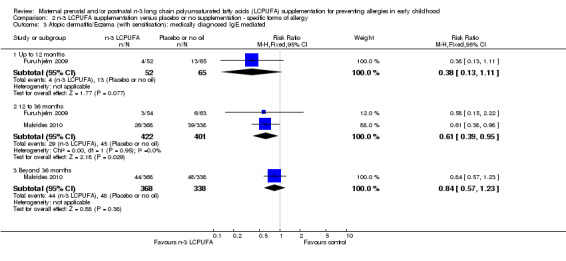
Comparison 2 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ specific forms of allergy, Outcome 3 Atopic dermatitis/Eczema (with sensitisation): medically diagnosed IgE mediated.
When eczema outcomes +/‐ IgE sensitivity were considered (Analysis 2.4), results showed few differences from those for IgE‐mediated eczema (Analysis 2.3), however the direction of effect was reversed for the 12‐ to 36‐month age group (up to 12 months of age (Furuhjelm 2009; Noakes 2012, 203 infants, average RR 0.76, 95% CI 0.22 to 2.62; between 12 to 36 months, Dunstan 2003; Furuhjelm 2009; Lauritzen 2005; Makrides 2010, 973 children, average RR 0.96, 95% CI 0.69 to 1.33; > 36 months of age, Makrides 2009; Makrides 2010, 1237 children, average RR 0.88, 95% CI 0.68 to 1.13). A random‐effects analysis was used all time points as substantial heterogeneity was noted at the 12‐month time point (Tau² = 0.57; P = 0.06; I² = 71%) (Analysis 2.4).
2.4. Analysis.
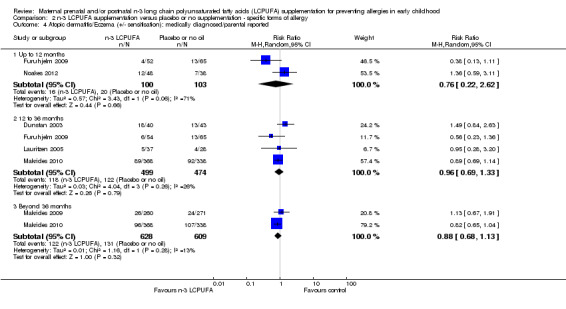
Comparison 2 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ specific forms of allergy, Outcome 4 Atopic dermatitis/Eczema (+/‐ sensitisation): medically diagnosed/parental reported.
Allergic rhinitis (Analysis 2.5; Analysis 2.6)
No clear difference was seen between n‐3 LCPUFA and control groups in either IgE‐mediated allergic rhinitis (Analysis 2.5: 12 to 36 months, Furuhjelm 2009; Makrides 2010, 825 children, RR 0.47, 95% CI 0.07 to 3.06; > 36 months of age, Makrides 2010, 706 children, RR 0.83, 95% CI 0.44 to 1.54 or allergic rhinitis +/‐ IgE sensitivity (Analysis 2.6:12 to 36 months, Furuhjelm 2009, Makrides 2010, 805 children, RR 0.53, 95% CI 0.25 to 1.12; > 36 months of age, Makrides 2009, Makrides 2010, 1169 children, RR 1.03, 95% CI 0.81 to 1.30) across any age group. No included trials reported allergic rhinitis outcomes in infants under 12 months of age.
2.5. Analysis.
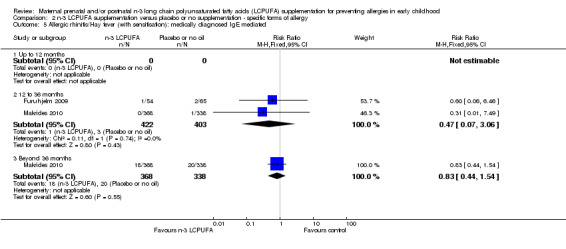
Comparison 2 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ specific forms of allergy, Outcome 5 Allergic rhinitis/Hay fever (with sensitisation): medically diagnosed IgE mediated.
2.6. Analysis.
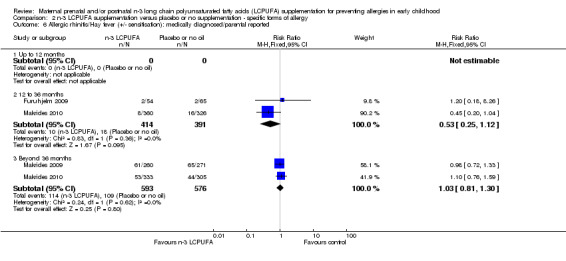
Comparison 2 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ specific forms of allergy, Outcome 6 Allergic rhinitis/Hay fever (+/‐ sensitisation): medically diagnosed/parental reported.
Asthma (Analysis 2.7; Analysis 2.8)
No clear differences were found between n‐3 LCPUFA and control groups in children with either IgE‐mediated asthma (Analysis 2.7; 12 to 36 months, Furuhjelm 2009; Makrides 2010, 824 children, RR 0.86, 95% CI 0.21 to 3.49; > 36 months of age, Makrides 2010, 706 children, RR 1.10, 95% CI 0.34 to 3.58), or asthma +/‐ IgE sensitivity (Analysis 2.8; < 12 months, Noakes 2012, 83 infants, RR 1.26, 95% CI 0.54 to 2.94; 12 to 36 months, Dunstan 2003, Furuhjelm 2009, Lauritzen 2005, Makrides 2010, 955 children, RR 0.93, 95% CI 0.73 to 1.18; > 36 months of age, Makrides 2009, Makrides 2010, Olsen 1992, 1697 children, RR 0.94, 95% CI 0.78 to 1.13) across any age group. No included trials reported IgE‐mediated asthma outcomes in infants under 12 months of age.
2.7. Analysis.
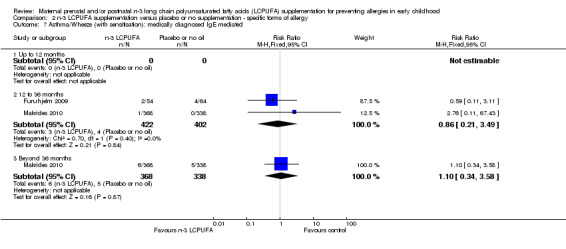
Comparison 2 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ specific forms of allergy, Outcome 7 Asthma/Wheeze (with sensitisation): medically diagnosed IgE mediated.
2.8. Analysis.
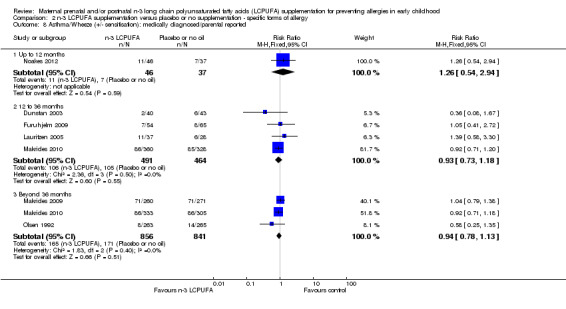
Comparison 2 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ specific forms of allergy, Outcome 8 Asthma/Wheeze (+/‐ sensitisation): medically diagnosed/parental reported.
Maternal safety (Analysis 3.1)
There was no clear difference in postpartum haemorrhage (defined as > 500 mL of blood loss post delivery) in women supplemented with n‐3 LCPUFA compared with those in the control group (Analysis 3.1; Makrides 2010; Olsen 1992, n = 2932, average RR 0.73, 95% CI 0.49 to 1.10). Given the substantial heterogeneity between trials, a random‐effects model was used (Tau² = 0.05, P = 0.11; I² = 60%; Analysis 3.1). Postpartum infection was not reported in any of the included trials.
3.1. Analysis.
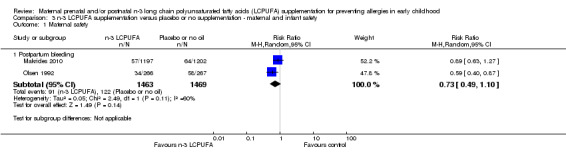
Comparison 3 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ maternal and infant safety, Outcome 1 Maternal safety.
Infant safety (Analysis 3.2)
Infant safety was assessed using early childhood infections. Four trials reported this outcome (Makrides 2009; Makrides 2010; Noakes 2012; Ramakrishnan 2010, 2280 infants) with no clear difference between the n‐3 LCPUFA and control group (RR 0.99; 95% CI 0.87 to 1.12). Ramakrishnan 2010 reported fever in 834 infants and found no differences between groups (RR 0.99; 95% CI 0.74 to 1.31; Analysis 3.2).
3.2. Analysis.
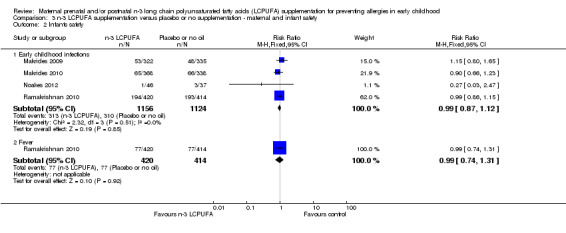
Comparison 3 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ maternal and infant safety, Outcome 2 Infants safety.
Sensitisation to allergens (Analysis 4.1 to Analysis 4.9)
See: Table 3.
3. The effects of n‐3 LCPUFA supplementation on skin prick results for allergens using pooled analysis RR (M‐H, Fixed, 95% CI).
| Skin prick results | Assessed Age | No of studies | No of participants | n‐3 LCPUFA | Placebo |
Fixed Effect estimate RR [95% CI] |
||
| Events | Total | Events | Total | |||||
| Egg | < 12 months | 2 | 203 | 7 | 100 | 17 | 103 | 0.44 [0.19 to 1.04] |
| 12‐36 months | 3 | 893 | 46 | 455 | 82 | 438 | 0.55 [0.39 to 0.77] ٭٭ | |
| ≥ 36 months | 1 | 706 | 11 | 368 | 16 | 338 | 0.63 [0.30 to 1.34] | |
| Cow’s milk | < 12 months | 2 | 205 | 5 | 102 | 7 | 103 | 0.75 [0.24 to 2.29]R |
| 12‐36 months | 3 | 897 | 9 | 457 | 13 | 440 | 0.68 [0.20 to 2.34]R | |
| ≥ 36 months | 0 | 0 | NE | |||||
| Peanut | < 12 months | 0 | 0 | NE | ||||
| 12‐36 months | 2 | 778 | 18 | 403 | 28 | 375 | 0.61 [0.34 to 1.08] | |
| ≥ 36 months | 1 | 706 | 13 | 368 | 20 | 338 | 0.60 [0.30 to 1.18] | |
| Wheat | < 12 months | 1 | 117 | 1 | 52 | 0 | 65 | 3.74 [0.16 to 89.85] |
| 12‐36 months | 2 | 783 | 3 | 401 | 2 | 382 | 1.40 [0.29 to 6.84] | |
| ≥ 36 months | 1 | 706 | 6 | 368 | 2 | 338 | 2.76 [0.56 to 13.56] | |
| Fish | < 12 months | 0 | 0 | NE | ||||
| 12‐36 months | 1 | 666 | 3 | 349 | 0 | 317 | 6.36 [0.33 to 122.65] | |
| ≥ 36 months | 1 | 706 | 2 | 368 | 1 | 338 | 1.84 [0.17 to 20.17] | |
| Inhalant allergens (pollens) | < 12 months | 0 | 0 | NE | ||||
| 12‐36 months | 2 | 779 | 2 | 400 | 5 | 379 | 0.44 [0.08 to 2.30] | |
| ≥ 36 months | 1 | 580 | 21 | 303 | 25 | 277 | 0.77 [0.44 to 1.34] | |
| House dust mite | <12 months | 0 | 0 | NE | ||||
| 12‐36 months | 2 | 738 | 3 | 384 | 4 | 354 | 0.76 [0.18 to 3.28] | |
| ≥36 months | 1 | 580 | 22 | 303 | 24 | 277 | 0.84 [0.48 to 1.46] | |
| Cat | < 12 months | 1 | 86 | 1 | 48 | 1 | 38 | 0.79 [0.05 to 12.25] |
| 12‐36 months | 2 | 738 | 8 | 384 | 7 | 354 | 1.07 [0.39 to 2.94] | |
| ≥ 36 months | 1 | 706 | 25 | 368 | 12 | 338 | 1.91 [0.98 to 3.75] | |
|
Any allergen (one or more allergen) |
< 12 months | 2 | 201 | 14 | 100 | 25 | 101 | 0.59 [0.32 to 1.09] |
| 12‐36 months | 3 | 892 | 68 | 455 | 93 | 437 | 0.70 [0.53 to 0.94] ٭ | |
| ≥ 36 months | 1 | 706 | 91 | 368 | 88 | 338 | 0.95 [0.74 to 1.22] | |
*significant P < 0.05, ٭٭ significant P < 0.005, NE = Not estimable
Sensitisation is the strongest predictor of IgE‐mediated allergy and was defined by a positive skin prick test to an allergen (de Jong 2011). Sensitisation to egg (Analysis 4.1) was reduced in the n‐3 LCPUFA group compared with the control in 12‐ to 36 month‐old children (Dunstan 2003; Furuhjelm 2009; Makrides 2010, 893 children, RR 0.55; 95% CI 0.39 to 0.77). No clear differences between groups were seen in egg sensitisation in children up to 12 months of age (Furuhjelm 2009; Noakes 2012) or in children 36 months or older (Makrides 2010).
4.1. Analysis.
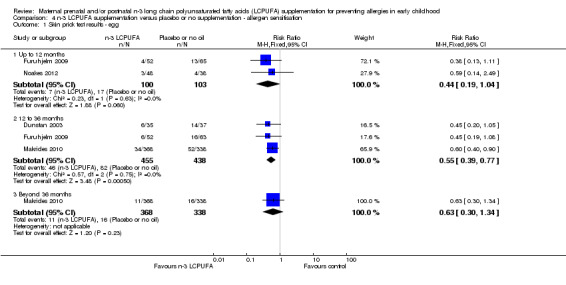
Comparison 4 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ allergen sensitisation, Outcome 1 Skin prick test results ‐ egg.
Sensitisation to cows' milk was not different between the treatment groups at any age of assessment (Analysis 4.2), although no trials contained data for children aged 36 months or older. Given the substantial heterogeneity at 12 to 36 months, a random‐effects model was used (Tau² = 0.48; P = 0.19; I² = 40%).
4.2. Analysis.
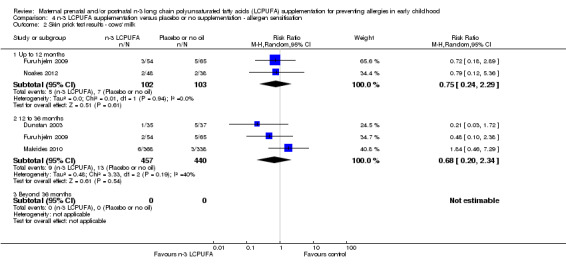
Comparison 4 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ allergen sensitisation, Outcome 2 Skin prick test results ‐ cows' milk.
There were no clear differences between groups in peanut sensitisation at any time point (Analysis 4.3; no trials in infants under 12 months of age),
4.3. Analysis.
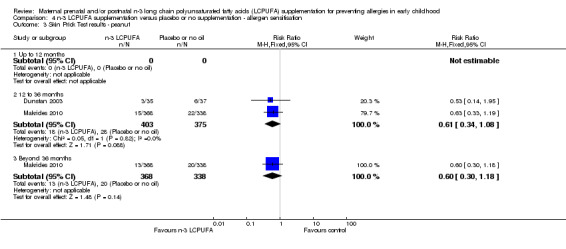
Comparison 4 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ allergen sensitisation, Outcome 3 Skin Prick Test results ‐ peanut.
The effect of n‐3 LCPUFA supplementation on wheat sensitisation was not different from the control group at any age (Analysis 4.4) and similar results were seen with sensitisation to fish (Analysis 4.5; no trials up to 12 months of age), inhalant allergens (pollens) (Analysis 4.6; no trials up to 12 months of age), dust mites (Analysis 4.7; no trials up to 12 months of age) and cats (Analysis 4.8).
4.4. Analysis.
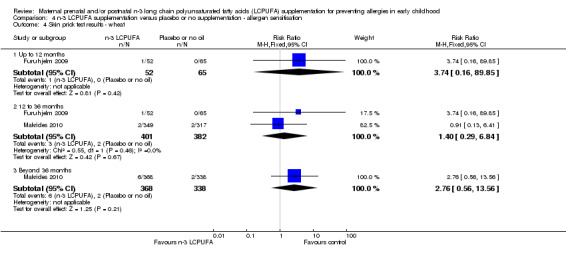
Comparison 4 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ allergen sensitisation, Outcome 4 Skin prick test results ‐ wheat.
4.5. Analysis.
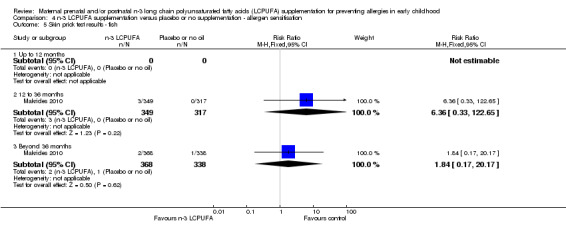
Comparison 4 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ allergen sensitisation, Outcome 5 Skin prick test results ‐ fish.
4.6. Analysis.
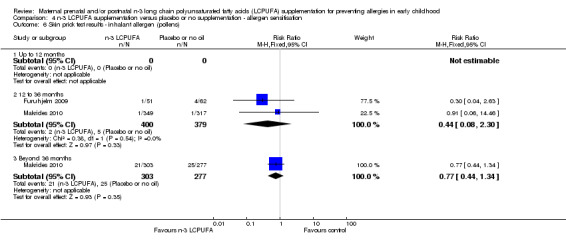
Comparison 4 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ allergen sensitisation, Outcome 6 Skin prick test results ‐ inhalant allergen (pollens).
4.7. Analysis.
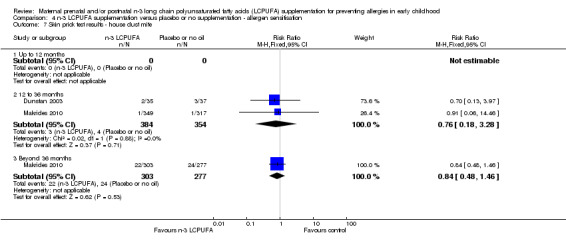
Comparison 4 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ allergen sensitisation, Outcome 7 Skin prick test results ‐ house dust mite.
4.8. Analysis.
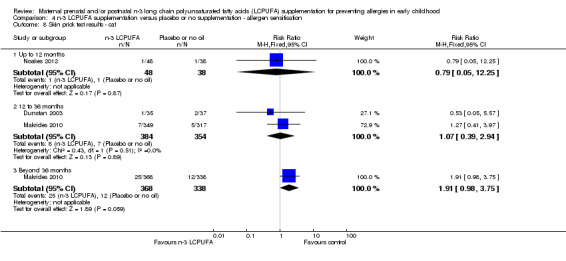
Comparison 4 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ allergen sensitisation, Outcome 8 Skin prick test results ‐ cat.
When all allergens were considered (Analysis 4.9), no clear differences were found between treatments for infants up to 12 months and beyond 36 months. However, n‐3 LCPUFA showed a clear reduction in sensitisation in 12 to 36 months of age children (Dunstan 2003; Furuhjelm 2009; Makrides 2010, 892 children, RR 0.70; 95% CI 0.53 to 0.94).
4.9. Analysis.
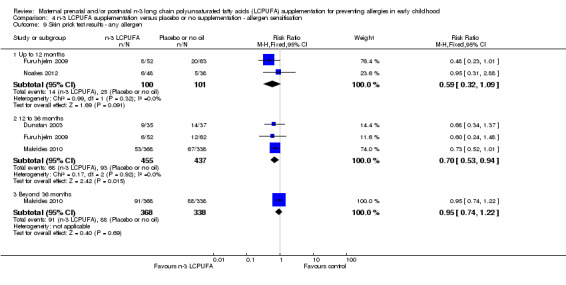
Comparison 4 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ allergen sensitisation, Outcome 9 Skin prick test results ‐ any allergen.
Parent's report of allergies from non‐validated questionnaires (Analysis 5.1 to Analysis 5.5)
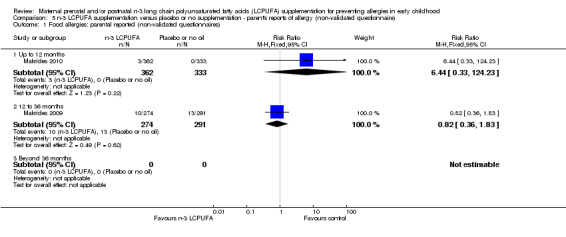
Three trials (Makrides 2009; Makrides 2010; Ramakrishnan 2010), included parents' reports of allergy collected using non‐validated questionnaires. No clear differences were found between n‐3 LCPUFA supplementation and control at any age in the incidence of parental reports of food allergies (Analysis 5.1), eczema (Analysis 5.2), allergic rhinitis (Analysis 5.3), asthma/wheeze (Analysis 5.4), or any allergies (Analysis 5.5).
5.1. Analysis.
Comparison 5 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ parent's reports of allergy (non‐validated questionnaire), Outcome 1 Food allergies: parental reported (non‐validated questionnaires).
5.2. Analysis.
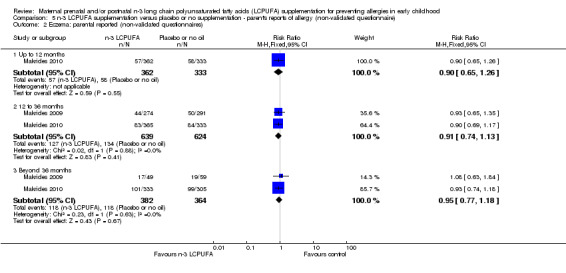
Comparison 5 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ parent's reports of allergy (non‐validated questionnaire), Outcome 2 Eczema: parental reported (non‐validated questionnaires).
5.3. Analysis.
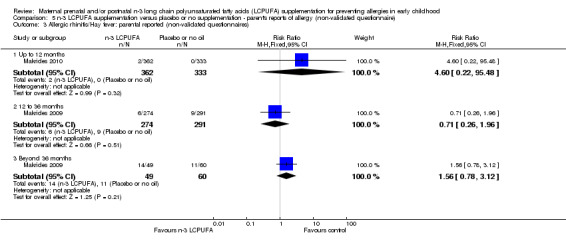
Comparison 5 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ parent's reports of allergy (non‐validated questionnaire), Outcome 3 Allergic rhinitis/Hay fever: parental reported (non‐validated questionnaires).
5.4. Analysis.
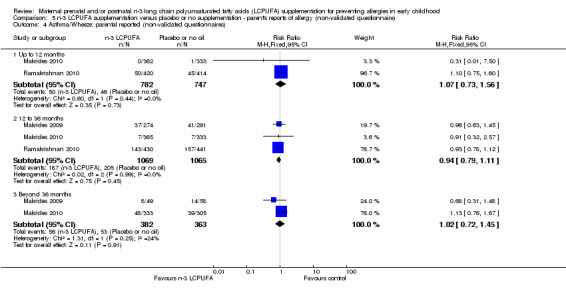
Comparison 5 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ parent's reports of allergy (non‐validated questionnaire), Outcome 4 Asthma/Wheeze: parental reported (non‐validated questionnaires).
5.5. Analysis.
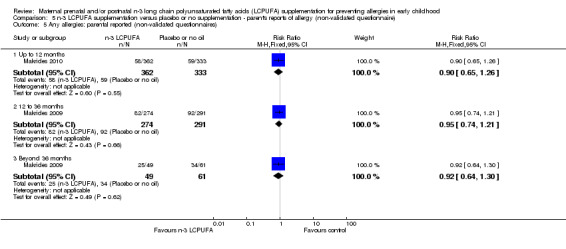
Comparison 5 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ parent's reports of allergy (non‐validated questionnaire), Outcome 5 Any allergies: parental reported (non‐validated questionnaires).
Subgroup analysis
Data were reported at last time point only in the subgroup analysis comparisons.
Timing of supplementation (Analysis 6.1 to Analysis 6.2)
Five trials included in the review confined supplementation with n‐3 LCPUFA to the prenatal period only (Dunstan 2003; Noakes 2012; Olsen 1992; Makrides 2010; Ramakrishnan 2010). Ramakrishnan 2010 collected allergy outcome data using a non‐validated questionnaire, therefore these data did not meet the inclusion criteria for the primary outcome. Two of the included trials supplemented women with n‐3 LCPUFA in the postnatal period only (Lauritzen 2005; Makrides 2009), and only one trial (Furuhjelm 2009) supplemented women with n‐3 LCPUFA through both prenatal and postnatal periods.
There were no significant subgroup differences for any of the outcomes (Analysis 6.1; Analysis 6.2).
6.1. Analysis.
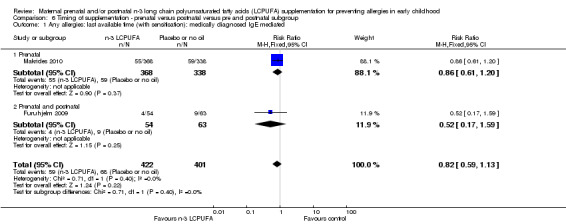
Comparison 6 Timing of supplementation ‐ prenatal versus postnatal versus pre and postnatal subgroup, Outcome 1 Any allergies: last available time (with sensitisation): medically diagnosed IgE mediated.
6.2. Analysis.
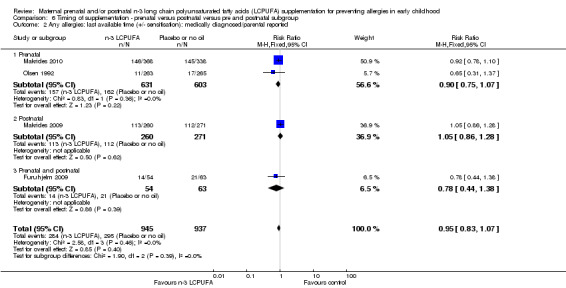
Comparison 6 Timing of supplementation ‐ prenatal versus postnatal versus pre and postnatal subgroup, Outcome 2 Any allergies: last available time (+/‐ sensitisation): medically diagnosed/parental reported.
Allergy risk of the offspring (Analysis 5.1 to Analysis 5.5)
Four trials (Dunstan 2003; Furuhjelm 2009; Noakes 2012; Makrides 2010) provided n‐3 LCPUFA supplements to women whose fetuses were at high risk of allergy development with two reporting only IgE‐mediated allergies (Furuhjelm 2009; Makrides 2010). Three trials studied the effect of n‐3 LCPUFA supplementation on allergy in women with fetuses or women with infants who were not selected on the basis of allergy risk (Lauritzen 2005; Makrides 2009; Olsen 1992). There were no significant subgroup differences for any outcome (Analysis 7.1).
7.1. Analysis.
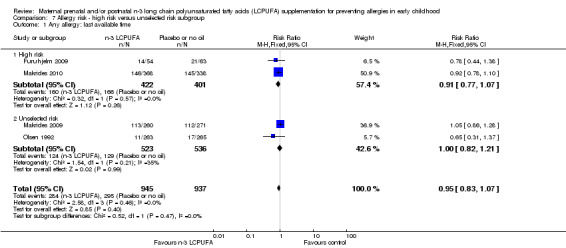
Comparison 7 Allergy risk ‐ high risk versus unselected risk subgroup, Outcome 1 Any allergy: last available time.
Infant maturity
Subgroup analyses based on infant maturity were not able to be conducted. Although data from preterm children were available for Makrides 2009, it was not possible to separate out children according to their gestational age in the remaining included trials (Furuhjelm 2009; Lauritzen 2005; Noakes 2012; Olsen 1992; Makrides 2010). Dunstan 2003 excluded preterm infants after randomisation.
Sensitivity analysis
Sensitivity analyses were conducted for the primary outcome removing trials with high or unclear risk of selection, performance or attrition bias; Makrides 2009 and Makrides 2010 were the only trials with low risk of bias across these parameters. Removing trials with high or unclear risk of bias changed the direction for IgE‐mediated any allergy at 12 to 36 months time point, but not beyond the 36 months time point, or for medically diagnosed IgE mediated and/or parental report any allergy outcome at any time points (Analysis 8.1; Analysis 8.2).
8.1. Analysis.
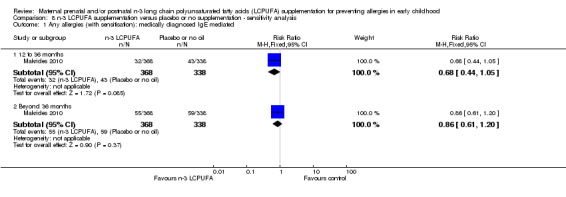
Comparison 8 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ sensitivity analysis, Outcome 1 Any allergies (with sensitisation): medically diagnosed IgE mediated.
8.2. Analysis.
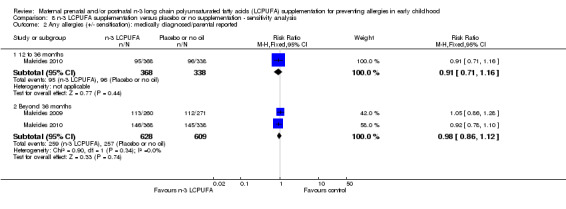
Comparison 8 n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ sensitivity analysis, Outcome 2 Any allergies (+/‐ sensitisation): medically diagnosed/parental reported.
Discussion
Summary of main results
Eight trials involving 3366 women with 3175 children were included in this review. Supplementation occurred during pregnancy, lactation or both pregnancy and lactation.
Overall, the available evidence shows that maternal n‐3 LCPUFA (long chain polyunsaturated fatty acid) supplementation showed little benefit in the reduction of childhood allergic disease. There was no clear overall effect of maternal n‐3 LCPUFA supplementation on the incidence of medically diagnosed or parental reports of allergy (+/‐ IgE sensitisation) including food allergy, eczema, allergic rhinitis, asthma/wheeze or any allergy. No reduction was observed with maternal n‐3 LCPUFA supplementation on IgE‐mediated allergic rhinitis or IgE‐mediated asthma.
However, there were reductions in some outcomes such as IgE‐mediated food allergy up to 12 months of age, IgE‐mediated eczema between one and three years of age and the risk of developing any IgE‐mediated allergic disease between one and three years of age. However, this needs to be interpreted with caution as IgE‐mediated allergies were reported in only two trials, which only focused on high‐risk populations of allergy.
There was also a reduction in the incidence of sensitisation to egg and any allergen in children in the n‐3 LCPUFA group.
Overall completeness and applicability of evidence
The majority of the evidence came from children of women supplemented with n‐3 LCPUFA during pregnancy and/or lactation and from women with a fetus at high risk of allergy (who were supplemented during pregnancy). Most of the trials included in this review were conducted in high‐income industrialised countries and the findings are therefore applicable to the most affluent societies where the burden of allergy is known to be high.
IgE‐mediated allergies, where both the signs and symptoms of the allergic disease and a positive skin prick test (SPT) and/or radioallergosorbent test (RAST) were present, were reported in two trials (Furuhjelm 2009; Makrides 2010 and all trials except three (Lauritzen 2005; Makrides 2009; Ramakrishnan 2010), used medical diagnosis of allergy for the analyses (with IgE status not tested or unknown).
Most allergic responses are mediated by IgE antibodies, specific to the trigger allergen (Johansson 2001; Sicherer 2012). However, although the presence of IgE antibodies indicates a sensitised state, the most reliable diagnosis of allergic disease should take into account clinical history as well (Sicherer 2012). Thus, diagnoses involving laboratory tests (RAST), SPT and assessment of clinical symptoms are more reliable than clinical presentation or parental reports (using validated questionnaires), or laboratory reports alone.
As asthma is difficult to diagnose in young children, authors often report 'wheeze' (Lauritzen 2005; Makrides 2010; Noakes 2012; Ramakrishnan 2010); we therefore included reports of wheeze in asthma outcomes.
The selection of women differed with respect to fish intake, with three trials targeting women with a low fish intake (Dunstan 2003; Lauritzen 2005; Noakes 2012), while fish intake was not related to inclusion criteria in other trials (Furuhjelm 2009; Makrides 2009; Makrides 2010; Olsen 1992; Ramakrishnan 2010). One of the reasons for some of the mixed results in this review may be due to the mothers baseline intake of n‐3 LCPUFA not being considered in some studies.
In the two trials where there was not an assigned control and women continued their 'usual diet' (Noakes 2012; Olsen 1992), the women in these groups may have had a high intake of dietary n‐3 LCPUFA as the benefits of dietary n‐3 LCPUFA in pregnancy was promoted in the countries in which these two trials were conducted.
In relation to diet and supplementation, some studies excluded women who were known to be allergic to fish (Dunstan 2003; Olsen 1992), as a safety precaution in case they may have reacted to fish oil capsules. Consequently, not many studies looked at fish allergy and hence data in this area may be limited (Dunstan 2003; Olsen 1992).
The evidence is incomplete for subgroup comparisons on the timing of supplementation, allergy risk of infants and maturity of infants. The trial in which n‐3 LCPUFA supplementation started during pregnancy and continued through to lactation was limited by small sample size and high risk of attrition bias (Furuhjelm 2009).
Quality of the evidence
The majority of the comparisons in this review were based on data from two trials (Furuhjelm 2009; Makrides 2010). The quality of the evidence for each of the comparisons greatly depends on the quality of these two trials. However, other primary outcome data regardless of IgE mediation were based on all included trials except Ramakrishnan 2010. The risk of bias varied across the eight included trials with only two trials (Makrides 2009; Makrides 2010) with a low risk of selection, performance and attrition bias.
Women's adherence to the supplementation may also impact on the outcomes. Blood analysis was used to check adherence to the supplementation in most trials (Dunstan 2003; Furuhjelm 2009; Lauritzen 2005; Noakes 2012; Olsen 1992; Makrides 2010), and all reported a significant n‐3 LCPUFA increase in the intervention group. The use of fish, rather than fish oil as a supplement also needs consideration as it may contribute to the energy and protein content in the maternal diet and influence outcomes in a different way. In this review we included one trial (Noakes 2012) which supplemented with fish, however this trial had been designed to overcome any additional effect of the diet by using it as a replacement for white fish, chicken and some red meat; thus minimising any additional energy and protein contributions (personal communication Noakes 2012).
Potential biases in the review process
Our search strategy was comprehensive and was not limited by language or publication status. We searched the major international and local bibliographic databases, handsearched major journals and the proceedings of major conferences in the field and set weekly current awareness alerts for a further 44 journals as well as monthly BioMed Central email alerts. We used a clear inclusion criteria and thorough quality assessment methodology to appraise each trial. Two review authors (AWG, CTC) independently screened and appraised the trials, and used pre‐designed data extraction forms to extract data. Therefore, biases in the review process are unlikely. Review authors MM and CTC were investigators on two trials (Makrides 2009; Makrides 2010) included in the review. These trials were independently assessed for inclusion, risk of bias and data extracted by AWG and an independent researcher Karen Best (KB).
Agreements and disagreements with other studies or reviews
Two systematic reviews (Klemens 2011; Kremmyda 2011), have been recently published on this topic. Kremmyda 2011 aimed to determine the effect of n‐3 LCPUFA supplementation during the perinatal period on allergies (irrespective of IgE status) in children, but did not conduct a meta‐analysis. Klemens 2011 conducted a meta‐analysis and reported that n‐3 LCPUFA supplementation during pregnancy reduced the incidence of childhood asthma but had no effect on atopic dermatitis. Their analysis did not include the most recent trials (Makrides 2009; Makrides 2010; Noakes 2012; Ramakrishnan 2010). Neither of the Klemens 2011 or Kremmyda 2011 reviews separated IgE‐mediated allergies from allergies with or without IgE mediation.
Authors' conclusions
Implications for practice.
Overall, there is limited evidence to support maternal n‐3 LCPUFA supplementation during pregnancy and/or lactation for the reduction of allergic disease in the children with few differences seen in allergic disease in children between women who were supplemented with n‐3 LCPUFA and those who were not. However, at some time points there were reductions in some outcomes such as IgE‐mediated food allergy, IgE‐mediated eczema and IgE‐mediated any allergy, with n‐3 LCPUFA supplementation in women with a fetus at high risk of allergy; therefore, further research is warranted.
Implications for research.
As the studies included in this review used differing doses, docosahexaenoic acid (DHA) to eicosapentaenoic acid (EPA) ratios and duration of n‐3 LCPUFA supplementation, and did not take into account the baseline n‐3 long chain polyunsaturated fatty acid (LCPUFA) status of the women, further research is needed to investigate the influence of these factors on childhood allergic outcomes. Trials should clearly differentiate between children at high risk of allergy and children at low risk of allergy given the suggestion of benefit to children who were at high risk of allergy. Trials should report both IgE‐mediated allergy and allergy +/‐ IgE mediation and should include follow‐up into the school years. Studies should also be conducted in other than high‐income countries to determine the generalisability of findings.
Acknowledgements
To Michael Draper, research librarian University of Adelaide, who developed extra search strategies.
To Karen Best for assessing and data extracting the Makrides 2009; and Makrides 2010 trials.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. PubMed search strategy
1. pregnancy[mh]
2. pregnan*[tiab]
3. maternal exchange*[tiab]
4. transplacental exposure*[tiab]
5. gestat*[tiab]
6. fetal Development [mesh]
7. Fetal Development [tiab]
8. Fetal Programming* [tiab]
9. fetal growth[tiab]
10. Foetal Development [tiab]
11. Foetal Programming* [tiab]
12. foetal growth[tiab]
13. Gestational Age*[tiab]
14. Fetal Age*[tiab]
15. foetal age*[tiab]
16. Breast Feeding[mh]
17. breast feeding[tiab]
18. breast fed[tiab]
19. lactating mother*[tiab]
20. breastfeeding[tiab]
21. Postpartum Period[mh]
22. Postpartum[tiab])
23. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16OR 17 OR 18 OR 19 OR 20 OR 21 OR 22
24. fish oils[mh]
25. fish oil*[tiab]
26. docosahexaenoic acid*[tiab]
27. cod liver oil[tiab]
28. omega 3 fatty acid*[tiab]
29. n 3 fatty acid*[tiab]
30. eicosapentaenoic acid[tiab]
31. n 3 pufa)
32. 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31
33. hypersensitivity[mh]
34. hypersensitivit*[tiab]
35. allerg*[tiab]
36. environmental illness*[tiab]
37. atopic dermatitis[tiab]
38. anaphyla*[tiab]
39. atopic dermatitis[tiab]
40. eczema[tiab]
41. urticaria*[tiab]
42. hives[tiab])
43. 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42
44. 23 AND 32 AND 43
45. randomized controlled trial[pt]
46. controlled clinical trial[pt]
47. randomized controlled trials[mh]
48. random allocation[mh]
49. double‐blind method[mh]
50. single‐blind method[mh]
51. clinical trial[pt]
52. clinical trials[mh]
53. clinical trial[tw]
54. ((singl*[tw] OR doubl*[tw] OR trebl*[tw] OR tripl*[tw]) AND (mask*[tw] OR blind*[tw]))
55. (placebos[mh] OR placebo*[tw] OR random*[tw] OR research design [mh:noexp] OR comparative study[pt] OR evaluation studies as topic[mh] OR follow‐up studies[mh] OR prospective studies[mh] OR control[tw] OR controlled[tw] OR prospectiv*[tw] OR volunteer*[tw])
56. 45 OR 46 OR 47 OR 48 OR 49 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55
57. NOT animals[mh] NOT (human[mh] and animals[mh])
58. 44 AND 56
59. 58 NOT 57
Appendix 2. CINAHL (via EBSCOhost) search strategy
(((singl* OR doubl* OR trebl* OR tripl*) AND (mask* OR blind*)) OR (placebo* OR random* OR "research design*" OR "comparative stud*" OR "evaluation stud*" OR "follow‐up stud*" OR control OR controlled OR prospectiv* OR volunteer*)) NOT (animals NOT human))
(pregnan* OR "maternal exchange*" OR "transplacental exposure*" OR gestat* OR "fetal development" OR "fetal programming*" OR "fetal growth" OR "foetal development" OR "foetal programming*" OR "foetal growth" OR "fetal age*" OR "foetal age*" OR "breast feeding" OR "breast fed" OR "lactating mother*" OR breastfeeding OR postpartum)
("fish oil*" OR "docosahexaenoic acid*" OR "cod liver oil" OR "omega 3 fatty acid*" OR "n 3 fatty acid*" OR "eicosapentaenoic acid" OR "n 3 pufa")
(hypersensitivit* OR allerg* OR "environmental illness*" OR "atopic dermatitis" OR anaphyla* OR eczema OR urticaria* OR hives)
1 AND 2 AND 3 AND 4
Appendix 3. Scopus search strategy
(pregnan* OR "maternal exchange" OR "transplacental exposure" OR gestat* OR "fetal development" OR "fetal programming" OR "fetal growth" OR "foetal development" OR "foetal programming" OR "foetal growth" OR "Gestational Age" OR "fetal age" OR "foetal age" OR "breast feeding" OR "breast fed" OR "lactating mother" OR breastfeeding OR postpartum)
("fish oil" OR "docosahexaenoic acid" OR "cod liver oil" OR "omega 3 fatty acid" OR "n 3 fatty acid" OR "eicosapentaenoic acid" OR "n 3 pufa")
(hypersensitivit* OR allerg* OR "environmental illness" OR "atopic dermatitis" OR anaphyla* OR "atopic dermatitis" OR eczema OR urticaria* OR hives)
("randomized controlled trial" OR "randomised controlled trial" OR "random allocation" OR "double blind" OR "single blind" OR "clinical trial*" OR ((singl* OR doubl* OR trebl* OR tripl*) AND (mask* OR blind*)) OR placebo* OR "comparative stud" OR "follow up stud" OR "prospective stud" OR rct OR "systematic review" OR "meta analys" OR metaanalys*)
1 AND 2 AND 3 AND 4
Appendix 4. Web of Knowledge search strategy
1. pregnan*
2. "maternal exchange*"
3. "transplacental exposure*"
4. gestat*
5. "Fetal Development*"
6. "Fetal Programming*"
7. "fetal growth"
8. "Foetal Development*"
9. "Foetal Programming*"
10. "foetal growth"
11. "Gestational Age*"
12. "Fetal Age*"
13. "foetal age*"
14. "breast feeding*"
15. "breast fed"
16. "lactating mother*"
17. breastfeeding
18. postpartum
19. 1 OR 2 OR 3 OR4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18
20. "fish oil*"
21. "docosahexaenoic acid*"
22. "cod liver oil*"
23. "omega 3 fatty acid*"
24. "n 3 fatty acid*"
25. "eicosapentaenoic acid*"
26. "n 3 pufa*"
27. 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26
28. hypersensitivit*
29. allerg*
30. "environmental illness*"
31. "atopic dermatitis"
32. anaphyla*
33. "atopic dermatitis"
34. eczema
35. urticaria*
36. hives
37. 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36
38. 19 AND 27 AND 372124
39. ("randomized controlled trial*" OR "controlled clinical trial*" OR "randomised controlled trial*" OR "random allocation*" OR "double blind" OR "single blind" OR "clinical trial*" OR ((singl* OR doubl* OR trebl* OR tripl*) AND (mask* OR blind*)) OR placebo* OR "comparative stud*" OR"follow up stud*" OR "prospective stud*" OR rct OR "systematic review*" OR "meta analys*" OR metaanalys*)
40. 38 AND 39
Appendix 5. ClinicalTrials.gov search strategy
http://clinicaltrials.gov
(hypersensitivity OR hypersensitive OR allergy) AND ("fish oil" OR docosahexaenoic OR "omega 3 fatty acid") AND (pregnancy OR pregnant OR postpartum OR fetal OR foetal OR "breast feeding" OR breastfeeding)
Appendix 6. Data analysis
Comparison 1: n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ any allergy
Comparison 2: Secondary Outcomes: n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ specific type of allergy
Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8
Comparison 3: Secondary Outcomes: n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ maternal and infant safety
Comparison 4: Secondary Outcomes: n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ allergen sensitisation
Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6; Analysis 4.7; Analysis 4.8; Analysis 4.9
Comparison 5: Secondary Outcomes: n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ parent's reports of allergy
Analysis 5.1; Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5
Comparison 6: Subgroup: Timing of supplementation
Comparison 7: Subgroup: Allergy risk
Comparison 8: Sensitivity analysis
Data and analyses
Comparison 1. n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ any allergy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergies (with sensitisation): medically diagnosed IgE mediated | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Up to 12 months | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 12 to 36 months | 2 | 823 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.44, 0.98] |
| 1.3 Beyond 36 months | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.20] |
| 2 Any allergies (+/‐ sensitisation): medically diagnosed/parental reported | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Up to 12 months | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 12 to 36 months | 2 | 823 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.11] |
| 2.3 Beyond 36 months | 3 | 1765 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.09] |
Comparison 2. n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ specific forms of allergy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Food allergies (with sensitisation): medically diagnosed IgE mediated | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Up to 12 months | 1 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 0.95] |
| 1.2 12 to 36 months | 2 | 825 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.18, 1.88] |
| 1.3 Beyond 36 months | 1 | 706 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.63, 3.26] |
| 2 Food allergies (+/‐ sensitisation): medically diagnosed/parental reported | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Up to 12 months | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.95] |
| 2.2 12 to 36 months | 4 | 973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.40, 1.30] |
| 2.3 Beyond 36 months | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.63, 3.26] |
| 3 Atopic dermatitis/Eczema (with sensitisation): medically diagnosed IgE mediated | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Up to 12 months | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.13, 1.11] |
| 3.2 12 to 36 months | 2 | 823 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.39, 0.95] |
| 3.3 Beyond 36 months | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.57, 1.23] |
| 4 Atopic dermatitis/Eczema (+/‐ sensitisation): medically diagnosed/parental reported | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Up to 12 months | 2 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.22, 2.62] |
| 4.2 12 to 36 months | 4 | 973 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.69, 1.33] |
| 4.3 Beyond 36 months | 2 | 1237 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.68, 1.13] |
| 5 Allergic rhinitis/Hay fever (with sensitisation): medically diagnosed IgE mediated | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Up to 12 months | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 12 to 36 months | 2 | 825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.07, 3.06] |
| 5.3 Beyond 36 months | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.44, 1.54] |
| 6 Allergic rhinitis/Hay fever (+/‐ sensitisation): medically diagnosed/parental reported | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Up to 12 months | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 12 to 36 months | 2 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.25, 1.12] |
| 6.3 Beyond 36 months | 2 | 1169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.81, 1.30] |
| 7 Asthma/Wheeze (with sensitisation): medically diagnosed IgE mediated | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Up to 12 months | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 12 to 36 months | 2 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.21, 3.49] |
| 7.3 Beyond 36 months | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.34, 3.58] |
| 8 Asthma/Wheeze (+/‐ sensitisation): medically diagnosed/parental reported | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Up to 12 months | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.54, 2.94] |
| 8.2 12 to 36 months | 4 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.73, 1.18] |
| 8.3 Beyond 36 months | 3 | 1697 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.78, 1.13] |
Comparison 3. n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ maternal and infant safety.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal safety | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Postpartum bleeding | 2 | 2932 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.49, 1.10] |
| 2 Infants safety | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Early childhood infections | 4 | 2280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.87, 1.12] |
| 2.2 Fever | 1 | 834 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.74, 1.31] |
Comparison 4. n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ allergen sensitisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Skin prick test results ‐ egg | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Up to 12 months | 2 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.19, 1.04] |
| 1.2 12 to 36 months | 3 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.39, 0.77] |
| 1.3 Beyond 36 months | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.30, 1.34] |
| 2 Skin prick test results ‐ cows' milk | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Up to 12 months | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.24, 2.29] |
| 2.2 12 to 36 months | 3 | 897 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.20, 2.34] |
| 2.3 Beyond 36 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Skin Prick Test results ‐ peanut | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Up to 12 months | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 12 to 36 months | 2 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.34, 1.08] |
| 3.3 Beyond 36 months | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.30, 1.18] |
| 4 Skin prick test results ‐ wheat | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Up to 12 months | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.74 [0.16, 89.85] |
| 4.2 12 to 36 months | 2 | 783 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.29, 6.84] |
| 4.3 Beyond 36 months | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.56, 13.56] |
| 5 Skin prick test results ‐ fish | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Up to 12 months | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 12 to 36 months | 1 | 666 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.36 [0.33, 122.65] |
| 5.3 Beyond 36 months | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.17, 20.17] |
| 6 Skin prick test results ‐ inhalant allergen (pollens) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Up to 12 months | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 12 to 36 months | 2 | 779 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.08, 2.30] |
| 6.3 Beyond 36 months | 1 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.44, 1.34] |
| 7 Skin prick test results ‐ house dust mite | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Up to 12 months | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 12 to 36 months | 2 | 738 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.18, 3.28] |
| 7.3 Beyond 36 months | 1 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.48, 1.46] |
| 8 Skin prick test results ‐ cat | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Up to 12 months | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.05, 12.25] |
| 8.2 12 to 36 months | 2 | 738 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.39, 2.94] |
| 8.3 Beyond 36 months | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.98, 3.75] |
| 9 Skin prick test results ‐ any allergen | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Up to 12 months | 2 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.32, 1.09] |
| 9.2 12 to 36 months | 3 | 892 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.53, 0.94] |
| 9.3 Beyond 36 months | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.74, 1.22] |
Comparison 5. n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ parent's reports of allergy (non‐validated questionnaire).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Food allergies: parental reported (non‐validated questionnaires) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Up to 12 months | 1 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.44 [0.33, 124.23] |
| 1.2 12 to 36 months | 1 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.36, 1.83] |
| 1.3 Beyond 36 months | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Eczema: parental reported (non‐validated questionnaires) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Up to 12 months | 1 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.26] |
| 2.2 12 to 36 months | 2 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.13] |
| 2.3 Beyond 36 months | 2 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.18] |
| 3 Allergic rhinitis/Hay fever: parental reported (non‐validated questionnaires) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Up to 12 months | 1 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.60 [0.22, 95.48] |
| 3.2 12 to 36 months | 1 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.26, 1.96] |
| 3.3 Beyond 36 months | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.78, 3.12] |
| 4 Asthma/Wheeze: parental reported (non‐validated questionnaires) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Up to 12 months | 2 | 1529 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.73, 1.56] |
| 4.2 12 to 36 months | 3 | 2134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.79, 1.11] |
| 4.3 Beyond 36 months | 2 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.72, 1.45] |
| 5 Any allergies: parental reported (non‐validated questionnaires) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Up to 12 months | 1 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.26] |
| 5.2 12 to 36 months | 1 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.74, 1.21] |
| 5.3 Beyond 36 months | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.64, 1.30] |
Comparison 6. Timing of supplementation ‐ prenatal versus postnatal versus pre and postnatal subgroup.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergies: last available time (with sensitisation): medically diagnosed IgE mediated | 2 | 823 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.59, 1.13] |
| 1.1 Prenatal | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.20] |
| 1.2 Prenatal and postnatal | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.17, 1.59] |
| 2 Any allergies: last available time (+/‐ sensitisation): medically diagnosed/parental reported | 4 | 1882 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.07] |
| 2.1 Prenatal | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.75, 1.07] |
| 2.2 Postnatal | 1 | 531 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.86, 1.28] |
| 2.3 Prenatal and postnatal | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.44, 1.38] |
Comparison 7. Allergy risk ‐ high risk versus unselected risk subgroup.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy: last available time | 4 | 1882 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.07] |
| 1.1 High risk | 2 | 823 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.07] |
| 1.2 Unselected risk | 2 | 1059 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.82, 1.21] |
Comparison 8. n‐3 LCPUFA supplementation versus placebo or no supplementation ‐ sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergies (with sensitisation): medically diagnosed IgE mediated | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 12 to 36 months | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.05] |
| 1.2 Beyond 36 months | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.20] |
| 2 Any allergies (+/‐ sensitisation): medically diagnosed/parental reported | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 12 to 36 months | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.71, 1.16] |
| 2.2 Beyond 36 months | 2 | 1237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.12] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dunstan 2003.
| Methods | Randomised controlled trial. Dunstan 2003 was the main trial with 12 publications at different times with different outcomes. All 12 publications are included in the references to included studies. |
|
| Participants | Setting: Australia. 98 pregnant atopic women whose fetus was considered to be at high risk of allergic disease. All women had a history of physician‐diagnosed allergic rhinitis and/or asthma and 1 or more positive SPT to common allergens. Exclusions: women who smoked, had other medical problems, complicated pregnancies, seafood allergy or if normal dietary intake exceeded 2 meals of fish per week. |
|
| Interventions | Intervention: 4 (1 g) n‐3 LCPUFA capsules per day comprising a total of 3.7 g of n‐3 LCPUFAs with 56.0% as DHA and 27.7% as EPA to give 2.07 g of DHA and 1.03 g of EPA (n = 52). Control: 4 (1 g) capsules of olive oil per day containing 66.6% n‐9 oleic acid and < 1% n‐3 LCPUFAs (n = 46). Duration of intervention: 20th week of gestation until delivery. |
|
| Outcomes |
Dunstan 2003 reported. Primary outcomes: allergen‐specific T‐cell responses in cord blood. Secondary outcomes: medically‐diagnosed allergies including incidence of asthma, atopic eczema, and food allergy at 1 year of age. The diagnosis of asthma was made in children with recurrent wheezing; i.e. 3 or more episodes with at least 1 episode confirmed by a paediatrician or general practitioner. Atopic eczema diagnosis was made in infants exhibiting typical skin lesions or physician‐diagnosed eczema response to topical steroids. The severity was scored according to the modified assessment clinical tool called SCORing Atopic Dermatitis (SCORAD). The SPT was performed using a standardised technique and allergen extracts (egg, milk, peanut, house dust mite, cat), the positive control was histamine and negative control glycerin, a wheel diameter of ≥ 2 mm was considered positive. |
|
| Notes | Contacted authors to determine if they diagnosed IgE‐mediated allergies ‐ no response received to date. Supported by grants from the National Health and Medical Research Council and Raine Medical Research Foundation, Australia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The groups were block‐randomised according to parity, prepregnancy BMI, age and maternal allergy". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization and allocation of capsules occurred at a different centre separate from the recruitment of participants. Capsules were administered to the participants by someone separate from those doing the allocation". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The capsules in the 2 groups were image matched, and the participants, research scientists, and paediatrician remained blinded to the groups for the duration of the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, research scientists, and paediatrician remained blinded to the groups for the duration of the trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomised n = 98 (52 intervention, 46 control). At birth 15 (15%) excluded (12 treatment, 3 control): 8 discontinued intervention due to nausea (7 treatment, 1 control), 1 cord blood not collected (control), 4 gestation < 36 weeks (3 treatment, 1 control), 2 unrelated infant disease (treatment), (85% follow‐up rate; intervention 77%, control 94%). Outcomes were reported on n = 83 (85%) at 1 year of age (fish oil group n = 40, 77% and control group n = 43, 94%). Of the 83, telephone interviewed n = 11, clinic visit and SPT n = 72. |
| Selective reporting (reporting bias) | High risk | Trial registered at anzctr.org.au Identifier: ACTRN12611000041954. Prespecified outcomes were reported in this trial according to their protocol. Unable to determine IgE‐mediated allergy from results reported although both medically diagnosed allergy and SPT results reported separately (authors contacted for information). |
| Other bias | Unclear risk | Preterm infants were excluded from their analysis after randomisation. |
Furuhjelm 2009.
| Methods | Randomised controlled trial. Furuhjelm 2009 was the main trial with 6 reports. All 6 publications are included in the references to included studies. |
|
| Participants | Setting: Sweden. 145 pregnant women who were at high risk of having a baby with atopy were recruited through antenatal care clinics during a 2‐year period in 2003–2005. Women were considered at high risk if they, or their husband or an older child had current or previous allergic symptoms, i.e. bronchial asthma diagnosed by a doctor, atopic eczema, allergic food reactions, itching and running eyes and nose on exposure to pollen, pets or other known allergens. |
|
| Interventions | Intervention: Women took 9 500 mg capsules a day containing 35% EPA, and 25% DHA, to provide 1.6 g of EPA and 1.1 g of DHA, n = 70. Control: 9 soy oil capsules a day, containing 58% LA to provide 2.5 g LA/day and 6% ALA to provide 0.28 g ALA/day, (n.=.75). Duration of intervention: 25th week of gestation to delivery, encouraged to continue during lactation (average 3 to 4 months). |
|
| Outcomes | 1. Furuhjelm 2009 reported Medically diagnosed allergy outcomes at 3, 6 and 12 months of age including: IgE antibody analysis, food allergy and eczema. Food allergy was defined as gastrointestinal symptoms, hives, aggravated eczema or wheeze following ingestion of egg or milk in the presence of detectable IgE antibodies or a positive SPT towards the particular food. Recovery from symptoms after elimination of the particular food from the diet and reoccurrence after ingestion of the food was required for the diagnosis. IgE‐associated eczema was characterised as reoccurring and itching eczematous, lichenified or nummular dermatitis according to the criteria modified by Oranje 1995 in the presence of detectable IgE antibodies or positive SPT towards egg, milk or wheat. 2. Furuhjelm 2011 reported Medically diagnosed allergy outcomes at 2 years of age including: food allergy, eczema, allergic rhinitis, asthma and any allergies with or without IgE associated. |
|
| Notes | The trial was supported financially by the Medical Research Council of Southeast Sweden (FORSS), The Östergötland County Council, The Ekhaga Foundation, Swedish Asthma and Allergy Association, The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS), The Swedish Society of Medicine and Glaxo Smith Kline, Sweden. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The mothers were randomly allocated to dietary supplementation either with Ѡ‐3 fatty acids or placebo". |
| Allocation concealment (selection bias) | Low risk | Quote: "Producer performed the block randomisation". We interpreted this as central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Active and placebo capsules could not be distinguished from each other". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The research nurses, the paediatricians and the person performing the laboratory analyses were blinded during the intervention and follow‐up". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomised n = 145 (70 intervention, 75 control). 25 did not complete the requested 15 week intervention period (16, 23% treatment and 9, 12% placebo) and were excluded from the analysis, 1 withdrew post delivery, 2 not followed as moved before 6/12 follow up (group not stated). Total 28 (19%) not included in analysis, 117 included (81%). SPT 117 (81%) at 6 months, 115 (79%) at 12 months. Medically diagnosed allergy outcomes were reported on n = 117 (81%) (fish oil group n = 52 (74%) and control group n = 65 (87%), at 6 months and at 1 year of age. Medically diagnosed allergy outcomes were reported on n = 119 (82%) (fish oil group n = 54 (77%) and control group n = 65 (87%), at 2 years of age. |
| Selective reporting (reporting bias) | Low risk | Trial registered at ClinicalTrials.gov identifier: NCT00892684. Prespecified outcomes were reported in this trial according to their protocol. Outcomes of interest to the review are reported. |
| Other bias | Low risk | No obvious risk of other bias. |
Lauritzen 2005.
| Methods | Randomised controlled trial. Lauritzen 2004 was the main trial with 9 publications at different times with different outcomes. All 9 publications are included in the references to included studies. |
|
| Participants | Setting: Denmark. Recruited from among pregnant women recruited for the Danish National Birth Cohort study based on their intake of n‐3 LCPUFA. Inclusion criteria included: an uncomplicated pregnancy, pre‐pregnancy BMI < 30 kg/m², no metabolic disorders, and the intention to breastfeed for at least 4 months. Infants had to be healthy, term and singleton with normal weight for gestation and Apgar score > 7, women with a fish intake below the population median (< 0.4 g n‐3 LCPUFA/day) were recruited for the randomised intervention trial (n = 122) and women with a fish intake in the upper quartile (> 0.8 g n‐3 LC‐PUFA/day) as a high‐fish‐intake reference group. |
|
| Interventions | Intervention: microencapsulated fish oil given in muesli bars. The fish oil supplement provided 1.5 g/day of n‐3 LCPUFA (equivalent to 4.5 g/day of fish oil) with 22.8% as DHA and 10% as EPA to provide 0.342 g per day of DHA and 0.15 g per day of EPA. As an alternative, the supplements were offered in homemade cookies or oil capsules (n = 62) Control: microencapsulated olive oil given in muesli bars . As an alternative, the olive oil supplements were offered in homemade cookies or oil capsules (n = 60). Referance group: 64 women with high fish intake (> 0.8 g n‐3 LC‐PUFA/day). Duration of intervention: 4 months postpartum. |
|
| Outcomes | 1. Lauritzen 2004 reported Primary outcomes ‐ DHA content of breast milk and infant red blood cell membranes at 2 and 4 months of age, and infant visual acuity at 2 and 4 months of age. 2. Lauritzen 2005 reported Pirmary outcome ‐ immune function assessed by interferon gamma and interleukin 10 production and plasma immunoglobulin E (IgE). Secondary outcomes ‐ parent report of doctor diagnosed allergy: food allergy, wheeze, eczema at 2.5 years of age. |
|
| Notes | The high fish intake reference group participants were not included in our meta‐analysis as this group was not randomised. The trial was funded by FOTEK‐The Danish Research and Development program for Food Technology and BASF Aktiengesellschaft. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After birth, the women with fish intakes below the 50th percentile were randomly assigned to a supplementation group by a randomisation schedule prepared by a person uninvolved in the study". |
| Allocation concealment (selection bias) | Low risk | Quote: "Owing to the non‐identical appearance of the capsules for the two groups, a person who was not otherwise involved in the project handled the capsules in order to avoid breaking the blinding of the investigators". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Investigators and families were blinded to the randomisation". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A person not otherwise involved in the study handled the capsules in order to avoid breaking the blinding of the investigators". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomised: trial entry n = 122 (intervention 62, control 60). At 2.5 years of age: n = 65 (53%) (fish oil group n = 37 (60%) and control group n = 28 (47%)). Large losses to follow‐up, all noted as ‘withdrawals’ or no reason given. |
| Selective reporting (reporting bias) | High risk | Trial registered at ClinicalTrials.gov identifier: NCT00266305. Most of the prespecified review outcomes were not reported in this trial. |
| Other bias | Low risk | No obvious risk of other bias. |
Makrides 2009.
| Methods | Randomised controlled trial ‐ the DINO trial. Unpublished data reporting outcomes for subgroup of infants whose mothers were providing breast milk at trial entry from Makrides 2009 are included in this systematic review. There are 11 publications, 1 thesis and 1 dissertation from this trial. All publications, the thesis and dissertation details are included in the references to included studies. |
|
| Participants | Setting: 5 Australian perinatal centres. Infants born before 33 weeks' gestation and within 5 days of any enteral feeds were eligible to participate (included n = 657 infants and 545 women). Excluded were infants 1) with major congenital or chromosomal abnormalities, 2) from a multiple birth in which not all live‐born infants were eligible, or 3) were in other trials of fatty acid supplementation. Lactating women in whom tuna oil was contraindicated (for example, because of bleeding disorders or therapy with anticoagulants) were also excluded. To meet the inclusion criteria for this systematic review only infants whose mothers were supplying breast milk at trial entry were included (n = 603 infants; 92% of the 657 randomised to the trial; 297/322, 92% high‐DHA and 306/335, 91%, control). A follow‐up of the first 143 infants who participated in the pilot phase was conducted at 3 to 5 years corrected age (Simmonds 2007), again only infants whose mothers were providing breast milk at trial entry are included in this systematic review (n = 125). A follow‐up of was conducted at 7 years corrected age and n = 569 children completed ISAAC questionnaires, again only infants whose mothers were providing breast milk at trial entry are included in this systematic review (n = 531). |
|
| Interventions | Intervention: lactating women whose infants were randomly assigned to the high‐DHA group consumed 6 x 500 mg DHA‐rich tuna oil capsules per day which provided 900 mg DHA and 195 mg EPA. The intent was to achieve a breast milk DHA concentration that was∼1% of total fatty acids without altering the naturally occurring concentration of AA in breast milk. If supplementary formula was required, infants were given a high‐DHA preterm formula (1% DHA and 0.6% AA). Control: lactating women with infants allocated to the standard‐DHA group consumed 6 500 mg placebo soy oil capsules with no n‐3 LCPUFA. If supplementary formula was required in this group, a standard preterm infant formula was used (0.35% DHA and 0.6% AA). Duration of the intervention: within 5 days from the infant receiving any enteral feeds until infants reached their expected date of delivery. |
|
| Outcomes | 1. Makrides 2009 reported
Primary outcome: neurodevelopment at 18 months corrected age. Secondary outcomes: infection outcomes (in‐hospital proven late onset sepsis). 2. Manley 2011 reported Secondary outcomes: parental reported food allergy, eczema, asthma and allergic rhinitis at 18 months corrected age. 3. Simmonds 2007 reported ISAAC questionnaire was used to collect parent report of allergy diagnosis and parent report of doctor diagnosis eczema, allergic rhinitis and asthma at 3 to 5 years corrected age. (The ISAAC questionnaire is not validated for this age group). 4. Gunaratne 2014 reported ISAAC questionnaire was used to collect parent report of allergy outcomes at 7 years of corrected age and parent report of allergy outcomes from birth to 7 years of corrected age for eczema, allergic rhinitis and asthma. (The ISAAC questionnaire is validated for this age group.) |
|
| Notes | This study was supported by a grant from the Australian National Health and Medical Research Council (grant 250322) and by the Channel 7 Children’s Research Foundation of South Australia Inc. Treatment and placebo capsules were donated by Clover Corp and infant formula was donated by Mead Johnson Nutritionals and Nutricia Australasia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Mother‐infant pairs were randomly assigned a unique study number through a computer‐driven telephone randomisation service according to an independently generated randomisation schedule. Stratification was by centre, birth weight (1250 grams vs 1250 grams), and infant sex. Multiple births were considered a single randomisation unit and randomisation of twins or triplets was according to the sex and birth weight of the first born infant". |
| Allocation concealment (selection bias) | Low risk | Central allocation by a computer‐driven telephone randomisation service. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "Parents, clinicians, and all research personnel were blinded to participant study group". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote "Parents, clinicians, and all research personnel were blinded to participant study group". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 603 infants whose mothers were providing breast milk at trial entry were enrolled in the trial (297 were randomised to the high DHA group and 306 to the control). 18 months corrected age: 565 (94%) completed the allergy follow up (274, 92% in the intervention and 291, 95% in the control) 3 to 5 years corrected age: 120 infants whose mothers in the pilot phase of the study were providing breast milk at trial entry (55 in intervention and 65 in control), at 3 to 5 years corrected age 110 (92%) completed the allergy follow‐up (49, 89% in the intervention and 61, 94% in the control). 7 years corrected age: 531 (88%) completed ISAAC questionnaire (260, 87.5% in the intervention and 271, 88.6% in the control) |
| Selective reporting (reporting bias) | High risk | Trial registered at anzctr.org.au Identifier: ACTRN12606000327583. Most of the prespecified review outcomes were not reported in this trial. |
| Other bias | Unclear risk | The children included in this review were a subgroup of the primary DINO trial and included children of mothers who were providing breast milk at trial entry, 92% of the infants randomised to the primary trial met this criteria. |
Makrides 2010.
| Methods | Randomised controlled trial. Makrides 2010 was the main trial with 10 publications. All 10 publications and 1 thesis are included in the references to included studies. |
|
| Participants | Setting: Australian maternity hospitals. Recruitment to primary 'DOMInO' trial (Makrides 2010): women with singleton pregnancies at less than 21 weeks’ gestation. Excluded were women already taking a prenatal supplement with DHA, with a bleeding disorder in which tuna oil was contraindicated, were taking anticoagulant therapy, had a documented history of drug or alcohol abuse, were participating in another fatty acid trial, fetus had a known major abnormality, or were unable to give written informed consent or if English was not the main language spoken at home (n = 2399). Pregnant women were approached to enter the allergy follow‐up (Palmer 2012) after randomisation into the DOMInO trial. Only Adelaide‐based women were eligible for the allergy follow‐up. Women were eligible if the unborn baby had a mother, father, or sibling with a history of any medically diagnosed allergic disease (asthma, allergic rhinitis, eczema) (n = 706). |
|
| Interventions | Intervention: 3 x 500 mg capsules daily of DHA rich fish oil concentrate, providing 800 mg of DHA and 100 mg of EPA per day. Control: 3 500 mg vegetable oil capsules daily without DHA. |
|
| Outcomes | 1. Makrides 2010 reported Primary outcome ‐ maternal postnatal depression at 6 weeks and 6 months; child neurodevelopment at 18 months of age. Secondary outcomes included a range of clinical outcomes including postpartum haemorrhage. 2. Palmer 2012 reported Primary outcome: At 1 year of age 1) IgE‐associated allergic diseases including: food allergy, eczema, asthma, allergic rhinitis and any allergy with sensitisation . 2) Medically diagnosed allergic diseases with or without IgE‐mediated allergic diseases including: food allergy, eczema, asthma/wheeze, allergic rhinitis and any allergy with or without sensitisation based on medically diagnosed allergy at the age of 1 year. Secondary outcomes included IgE sensitisation ‐ SPT at 1 year of age. Infants with respiratory tract infections between birth and 1 year. 3. Palmer 2013 reported the 3‐year follow‐up of the same children in Palmer 2012 to evaluate medically diagnosed allergy. Reported IgE‐associated allergic diseases including food allergy, eczema, asthma, allergic rhinitis and any allergy with sensitisation at 3 years of age. Obtained medically diagnosed allergic diseases with or without IgE‐mediated allergic diseases including: food allergy, eczema, asthma/wheeze, allergic rhinitis and any allergies with or without sensitisation at the age of 3 years. 4. Gunaratne 2014 reported parental reports of doctor diagnosed allergy outcomes including food allergy, eczema, asthma, allergic rhinitis and any allergy below 36 months of age. Modified ISAAC questions were used to collect parent report of doctor diagnosed eczema, allergic rhinitis and asthma from birth to 3 years of age. (The ISAAC questions are not validated for this age group.) |
|
| Notes | Supported by grants from the Australian National Health and Medical Research council and Australian Egg Corporation Limited. Treatment and placebo capsules were donated by Efamol, UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote " Women were randomly allocated to a unique number that corresponded to treatment or control through a computer driven telephone randomisation service according to an independently generated randomisation schedule, with balanced variable sized blocks. Stratification was by centre and parity". |
| Allocation concealment (selection bias) | Low risk | Computer‐driven telephone randomisation service. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To maintain the blind, both active and placebo capsules were identical in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neither the parents nor the research staff were aware of the treatment allocated. Described as "double‐blind". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 681/706 (96.5%) infants (intervention: 357/368, 97%,placebo: 324/338, 96%) attended their 1‐year medical review and 666/706 (94.3%) infants had SPT results. Missing data were imputed for all IgE‐mediated allergy outcomes and medially diagnosed food allergy, eczema and any allergy but not imputed for medically diagnosed asthma (wheeze) and allergic rhinitis. In secondary outcomes missing data were imputed for egg, cow's milk. peanut and any SPT sensitisation but not for wheat, fish, pollen, house dust mite and cat allergens. 638/706 (90.4%) infants (intervention: 333/368, 90.5%placebo: 305/338, 90.2%) attended their 3‐year medical review and 587/706 (83.1%) infants had SPT results; missing data were imputed for all IgE‐mediated allergy outcomes and medically diagnosed food allergy, eczema and any allergy but not imputed for medically diagnosed asthma (wheeze) and allergic rhinitis. In secondary outcomes missing data were imputed for egg, wheat, peanut, fish, cat and any allergens but not for pollens and house dust mite allergens. Parent reported allergy: below 12 months of age ‐ 695/706 (98.4%) (intervention: 362/368, 98.4%, placebo: 335/338, 99%), between 12‐ 36 months of age ‐ 698/706 (99%), (intervention: 365/368, 99%, placebo: 333/338, 98.5%) and at 36 months of age ‐ 638/706 (90%) (intervention: 333/368, 90.5%, placebo: 305/338, 90%) were available. Missing data were not imputed for any parent reported allergy outcomes. Results using imputed data were reported to differ little from raw data (Palmer 2012; Palmer 2013). |
| Selective reporting (reporting bias) | Low risk | Trial registered at anzctr.org.au Identifier: ACTRN12605000569606 Prespecified outcomes were reported in this trial according to their protocol. Most of the outcomes of interest to the review are reported. |
| Other bias | Unclear risk | Of the original DOMInO trial (n = 2399), a subgroup of mothers whose unborn child had a family history of allergies were included. |
Noakes 2012.
| Methods | Randomised controlled trial. Miles 2011 was the main trial with 13 reports. All 13 publications are included in the references to included studies. |
|
| Participants | Setting: United Kingdom. Women aged 18 to 40 years who were at 19 weeks' gestation with a healthy uncomplicated pregnancy who reported low habitual consumption of oily fish (2 portions/month excluding canned tuna and no use of fish oil supplements in previous 3 months) and had a family history of atopy (1 or more first‐degree relatives of the infant affected by atopy, asthma, or allergy) were recruited to the trial (n = 123). Excluded were those participating in another research study, known diabetes, autoimmune disease, learning disability, terminal illness or mental health problems. |
|
| Interventions | Intervention: women in the salmon group (n = 62) were asked to incorporate 2 portions of salmon (300 g) which contained 7.12 g total n‐3 LCPUFAs (1.14 g EPA/week and 2.32 g DHA/week) into their diet from week 20 (trial entry) until they gave birth. Women and their partners were provided with 2 portions of salmon per week to incorporate into their diet. They also received a cookbook that provided recipes for preparing and cooking salmon. Each portion of salmon was to replace a serve of protein (e.g. white fish, chicken or red meat). Control: women in the control group were asked to continue their habitual diets; these women received the information sheet that described the possible health benefits of consuming oily fish during pregnancy and the government recommendation that pregnant women consume 1 or 2 oily fish meals/week. They also received a cookbook providing recipes for healthy eating during pregnancy. |
|
| Outcomes | 1. Miles 2011 reported Primary outcome ‐ determine the effect on maternal and umbilical cord plasma n‐3 LCPUFA content. 2. Noakes 2012 reported Effect on neonatal immune responses and diagnosis, by research nurse, of eczema and wheeze at 6 months of age. Chest infections and SPT at 6 months of age. |
|
| Notes | Supported by the European Commission under Framework 6: Sustainable aqua feeds to maximize the health benefits of farmed fish for consumers (Aquamax; FOOD‐CT‐2006‐16249). 2 researches were supported by the Southampton NIHR Biomedical Research Unit in Nutrition, Diet & Lifestyle. Salmon was donated by the University of Bergen, Norway. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "The women were allocated to one of two groups according to a previously generated random number table". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers responsible for assessing outcome measures (both laboratory and clinical) remained blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Enrolled 123 (62 salmon, 61 control). At delivery: 107 (87%): 53 (85%) salmon, 54 (89%) control. Birth samples: 101 (82%): 51 (82%) salmon, 50 (82%) control. 6‐month clinic visit: 86 (70%): 48 (77%) salmon, 38 (62%) control. |
| Selective reporting (reporting bias) | High risk | Trial registered at clinicaltrials.gov as NCT00801502. Only limited data were reported on some of the prespecified review outcomes. |
| Other bias | Low risk | There is no obvious risk of other bias. |
Olsen 1992.
| Methods | Randomised controlled trial. Olsen 1992 was the main trial with 16 publications. All 16 publications are included in the references to included studies. |
|
| Participants | Setting: Denmark. Pregnant women were recruited from the midwife clinic at 30 weeks' gestation. Exluded were women with a history of placental abruption in a previous pregnancy or a serious bleeding episode in the present pregnancy, women who used prostaglandin inhibitors regularly, multiple pregnancies, allergy to fish, and regular intake of fish oil (n = 533). |
|
| Interventions | Intervention: 4 x 1 g fish oil capsules daily containing 32% EPA and 23% DHA to provide 1.28 g EPA and 0.92 g DHA. Control group 1: 4 x 1 g capsules of olive oil daily. Control group 2: no supplement. Duration of intervention: 30th week of gestation until delivery. |
|
| Outcomes | 1. Olsen 1992 reported Primary outcome ‐ pregnancy duration. Secondary outcomes included side effects and complications including postpartum bleeding. 2. Olsen 2008 reported Primary outcome ‐ asthma at 16 years of age. Secondary outcomes ‐ combined outcome of asthma, atopic dermatitis and allergic rhinitis at 16 years of age. Medically diagnosed (from a mandatory registry that recorded diagnoses from hospitals in Denmark) allergy outcomes. |
|
| Notes | For this systematic review control groups 1 and 2 were combined to compare with the intervention group (see 'Risk of bias' table). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Women were randomly assigned to the three groups in the ratio 2/1/1. Randomisation was stratified by parity and arranged in balanced blocks of between 8 and 12”. |
| Allocation concealment (selection bias) | Low risk | Quote "Sealed, opaque envelope for that study number contained a randomisation number that either identified a particular package of oil capsules or showed that the woman should receive no oil supplement". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | While group 1 and 2 were blinded, group 3 received no supplement and were therefore unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independant evaluation, registry based diagnosis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomised 533 (intervention 266; olive oil 136; and no oil 131). Assessed at 16 years 528 (99%) (intervention 263; olive oil 136; and no oil 129). |
| Selective reporting (reporting bias) | High risk | Trial registered at ClinicalTrials.gov identifier: NCT01353807. Most of the prespecified outcomes were reported in this trial according to their protocol. However, outcomes of interest to the review are not reported completely. Only limited data were reported on some of the prespecified review outcomes. |
| Other bias | Low risk | Placebo group and no oil group were combined in the review. Analyses were conducted with the n‐3 LCPUFA supplementation group compared to olive oil control group separately to n‐3 LCPUFA supplementation group compared to no supplement control group, although the direction of effect differed it made little difference to the meta‐analysis. |
Ramakrishnan 2010.
| Methods | Randomised controlled trial. Ramakrishnan 2010 was the main trial with 14 publications. All 14 publications are included in the references to included studies. |
|
| Participants | Setting: Mexico. Women were recruited at the Mexican Institute of Social Security (Instituto Mexicano del Seguro Social [IMSS]) General Hospital in Cuernavaca, Mexico, and 3 small health clinics within the IMSS system in Cuernavaca during routine prenatal care visits. Women were recruited for inclusion in the study if they were in gestation week 18 to 22, were aged 18 to 35 years, planned to deliver at the IMSS General Hospital in Cuernavaca, planned to predominantly breastfeed for at least 3 months, and planned to live in the area for 2 years after delivery. Women were excluded, if they had a (1) high‐risk pregnancy, (2) lipid metabolism/ absorption disorders, (3) regular intake of fish oil or DHA supplements, or (4) chronic use of certain medications. (n = 1094) |
|
| Interventions | Intervention: 2 200 mg capsules daily of DHA rich algal oil concentrate, providing 400 mg of DHA per day. Control: 2 200 mg corn oil and soy oil capsules daily without DHA. |
|
| Outcomes |
Ramakrishnan 2010 reported Primary outcome measures: birth size and gestational age. Imhoff‐Kunsch 2011 Outcome measures: immune function and morbidity. Parental reports of wheeze were reported at 1 month, 3 months and 6 months of age . A non‐validated questionnaire was used to collect data. Upper respiratory tract infections and fever were reported at 1 month, 3 months and 6 months of age. Escamilla‐Nunez 2014 Outcome measures: respiratory symptoms in children at 18 months of age (reported as incidence rate according to atopy). |
|
| Notes | Contacted authors to obtain incidence of 18 month allergy outcomes. The research was supported by NIH (HD‐043099) and the March of Dimes foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Ouote "All eligible women were randomly assigned to either the treatment or the control group by use of a computer generated list created by the study biostatistician at Emory University. We used block randomization to create balanced replicates of 4 treatments (2 colors for DHA and 2 for control) using a block size of 8". |
| Allocation concealment (selection bias) | Unclear risk | It is likely that participants and investigators enrolling participants could not foresee assignment because the assignment codes were placed in sealed envelopes and kept in a sealed location administered by a faculty member of the university who was not involved in the study. It is not stated if they were sequentially numbered nor if they were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and members of the study team were blinded to the treatment scheme throughout the intervention period of the study. Capsules were similar in appearance and taste. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote "Data were unblinded for the analytical study team after the last infant in the study was born and had reached the age of 6 months at which time the participants were no longer taking supplements". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1094 randomised, 834 assessed at 6 months ‐ 76% follow‐up rate, similar between intervention (77%) and control (76%) At 18 months, 869 assessed according to mother atopy and treatment group ‐ 82% follow‐up rate, similar between intervention (80%) and control (78%). 54 randomised but did not begin treatment, 67 withdrew after beginning treatment (lack of family support, moved from area, disliked flavour, heartburn, nausea). |
| Selective reporting (reporting bias) | High risk | ClinicalTrials.gov Identifier:NCT00646360. Most of the prespecified outcomes were reported in this trial according to their protocol. However, outcomes of interest to the review are reported incompletely and so cannot be used. |
| Other bias | Low risk | No obvious risk of other bias. |
AA: arachidonic acid ALA: alpha‐linolenic acid BMI: body mass index DHA: docosahexaenoic acid EPA: eicosapentaenoic acid g: gram IgE: immunoglobulin E LA: linolenic acid PUFA: polyunsaturated fatty acids SPT: skin prick test vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bergmann 2008 | Excluded because the trial did not report allergy outcome of the infants and/or children. |
| Campos‐Martinez 2012 | Excluded because this trial published as an abstract and did not report allergy outcome of the infants and/or children. |
| Carlson 2013 | Excluded because the trial did not report allergy outcome of the infants and/or children. |
| Colombo 2004 | Excluded because the trial did not report allergy outcome of the infants and/or children. |
| Courville 2011 | Excluded because this trial did not report allergy outcome of the infants and/or children. |
| Granot 2011 | Excluded because the trial did not report allergy outcome of the infants and/or children. |
| Hauner 2009 | Excluded because the trial does not report allergy outcome of the infants and/or children. |
| Helland 2001 | Excluded because the trial did not report allergy outcome of the infants and/or children. |
| Innis 2007 | Excluded because the trial did not report allergy outcome of the infants and/or children. |
| Judge 2007 | Excluded because the trial did not report allergy outcome of the infants and/or children. |
| Karlsson 2010 | Excluded because the trial did not report allergy outcome of the infants and/or children. |
| Knudsen 2006 | Excluded because the trial reported only timing of spontaneous delivery. |
| Krauss‐Etschmann 2007 | Excluded because the trial did not report allergy outcome of the infants and/or children. |
| Martin‐Alvarez 2012 | Excluded because the trial published as an abstract and did not report allergy outcome of the infants and/or children. |
| Pena‐Quintana 2011 | Excluded because this trial published as an abstract and did not report allergy outcome of the infants and/or children. |
| Ribeiro 2012 | Excluded because the trial did not report allergy outcome of the infants and/or children. |
Characteristics of ongoing studies [ordered by study ID]
Bisgaard 2012.
| Trial name or title | Fish oil supplementation during pregnancy for prevention of asthma, eczema and allergies in childhood: interventional trial in the COPSAC2010 |
| Methods | Randomised controlled trial. |
| Participants | Pregnant women from week 24. |
| Interventions | n‐3 fatty acid start from week 24 gestation to 1 week after delivery. Olive oil start from week 26 gestation to 1 week after delivery. |
| Outcomes | Primary outcome measures: development af wheezy disorder from 0 to 3 years of age [time frame: 3 years]. Development of eczema from 0 to 3 years of age [time frame: 3 years]. Sensitisation at 18 months of age [time frame: 18 months] Secondary outcome measures: development of asthma exacerbations from 0 to 3 years of age [time frame: 3 years]. Infections from 0 to 3 years of age [time frame: 3 years]. Growth [time frame: 0 to 3 years of age]. Cognitive, language and motor development [time frame: 2½ years]. |
| Starting date | November 2008. |
| Contact information | Hans Bisgaard, MD, DMSc, Copenhagen University Hospital of Copenhagen, Gentofte, Denmark, 2820. |
| Notes | ClinicalTrials.gov identifier: NCT00798226. |
Duchen 2012.
| Trial name or title | Combined dietary supplementation with Lactobacillus reuteri and omega‐3 PUFA during pregnancy and postnatally in relation to development of IgE‐associated disease during infancy |
| Methods | Randomised controlled trial. |
| Participants | Pregnant women. |
| Interventions | Dietary supplement: placebo. Dietary supplement: omega‐3 fatty acids. Dietary supplement: refined coconut and peanut oil without L. reuteri. Dietary supplement: L. reuteri. |
| Outcomes | Primary outcome measures: IgE‐associated disease [time frame: 2 years of age]. A food reaction is defined as gastrointestinal symptoms, hives, aggravated eczema or wheezing following ingestion of a certain food with recovery after food elimination from the diet and reoccurrence of symptoms after ingestion of the particular food. Eczema is characterised as reoccurring, itching eczematous and lichenified or nummular dermatitis. Doctor diagnosed wheezing at least 3 times during the first 2 years is required for the diagnosis of asthma. If specific positive SPT or serum IgE antibodies is present, the food reaction, eczema in defined as IgE associated. Secondary outcome measures: maternal gastrointestinal function [time frame: 20th gestational week to 6 months postpartum]. Maternal gastrointestinal function will be addressed by validated diary cards. The mothers will record every single stool, stool consistency, and corresponding defecatory symptoms (urgency, straining, and feeling of incomplete evacuation) for 7 days at gestational week 25 and 35. Stool consistency will be defined by the Bristol Stool Form Scale. The mothers will also record every meal, and episodes (start and ending time) of abdominal pain and bloating. |
| Starting date | March 2012. |
| Contact information | Karel M Duchén, MD, PhD, Allergicentrum, Universitetssjukhuset, Linköping, Sweden, 58185. Tel +46‐10‐103 1355. |
| Notes | ClinicalTrials.gov Identifier: NCT01542970. |
Laitinen 2013.
| Trial name or title | Nutrition and pregnancy intervention study |
| Methods | Randomised controlled trial. |
| Participants | Pregnant obese women. |
| Interventions | Dietary supplement: comparison of probiotics, fish oil and their combination to placebo. |
| Outcomes | Primary outcome measures: prevalence of GDM [time frame: assessed at gestational weeks 24‐28].
Fasting glucose levels [time frame: assessed at the third trimester of pregnancy].
Prevalence of allergy in child [time frame: assessed at 12 and 24 months of age].
Secondary outcome measures: need for medication for management of gestational diabetes mellitus GDM (insulin or metformin) [time frame: during pregnancy]. Body composition of mother [time frame: during and after pregnancy]. Immunologic and metabolic markers [time frame: during and after pregnancy]. Fecal microbiota [time frame: before, during and after intervention]. Other outcome measures: body composition, growth, development and metabolic markers of the child [time frame: 0 to 24 months of age]. |
| Starting date | September 2013. |
| Contact information | Kirsi Laitinen, Adjunct professor, Turku University Hospital, Turku, Finland, 20521, + 358 02 333 6063, kirsi.laitinen@utu.fi |
| Notes | ClinicalTrials.gov identifier: NCT01922791. |
Liu 2013.
| Trial name or title | The effects of polyunsaturated fatty acids (PUFA) on allergic/atopic dermatitis |
| Methods | November 2013. |
| Participants | Pregnant women between 16 and 20 weeks. |
| Interventions | Dietary supplement: DHA + EPA. Dietary supplement: high olive oil. Dietary supplement: DHA. |
| Outcomes | Primary outcome measures: lipid analysis [time frame: baseline, delivery, within 1 week after delivery, 6 weeks postpartum, 4 months postpartum]. Metabolomics study of PUFA [time frame: baseline, delivery, within 1 week after delivery, 6 weeks postpartum, 4 months postpartum, 12 months postpartum]. Skin prick test to common allergens [time frame: 4 months postpartum, 12 months postpartum]. Clinical assessment of IgE‐mediated allergic eczema [time frame: 4 months postpartum, 12 months postpartum]. Secondary outcome measures: fatty acid desaturase (FADS) phenotypes [time frame: baseline]. Immunoglobulin E (IgE) antibodies [time frame: baseline]. Immunological biomarkers [time frame: 4 months postpartum, 12 months postpartum]. Medically‐confirmed adverse events collected throughout the study period [time frame: 12 months postpartum]. |
| Starting date | November 2013. |
| Contact information | Huimin Liu, International Peace Maternity and Child Health Hospital of China welfare Institute, Shanghai, Shanghai, China, 200030, 86‐20‐82156129, huimin.liu@mjn.com |
| Notes | ClinicalTrials.gov Identifier:NCT01936194. |
GDM: gestational diabetes mellitus DHA: docosahexaenoic acid EPA: eicosapentaenoic acid PUFA: polyunsaturated fatty acids SPT: skin prick test
Differences between protocol and review
Due to different methods of reporting across trials, we deemed it inappropriate to conduct analyses of cumulative allergies across time points.
In the protocol we specified the primary outcome only as allergy. We have refined this for the review, with any allergy being our primary outcome (medically diagnosed IgE mediated; and medically diagnosed IgE mediated and/or parental report). Specific forms of allergy (food allergy, eczema, allergic rhinitis and asthma/wheeze) are now secondary outcomes.
Trials that reported 'wheeze' were included in the allergy outcomes for asthma, with the outcome being changed to asthma/wheeze.
The data were reported at last time point in the subgroup analysis comparisons.
Maternal safety was added as a secondary safety outcome (e.g. postpartum haemorrhage or infection) due to the theoretical risk of harm associated with higher doses of n‐3 LCPUFA.
In the Objectives, we made it clearer that the aim of this review was to assess the effect of maternal n‐3 LCPUFA supplementation in mothers during pregnancy and/or lactation on the allergy outcomes of in their children.
Contributions of authors
AWG and MM were responsible for conceiving the review. AWG, MM and CTC designed, developed and wrote the review. MM and CTC provided a methodological perspective and a clinical perspective and provided general advice on the review. AWG and CTC were responsible for co‐ordinating the review.
Sources of support
Internal sources
Women’s & Children’s Health Research Institute, The University of Adelaide, Adelaide, Australia.
External sources
NHMRC Center for Research Excellence in Food for Future Austalians (ID App1035530), Australia.
Maria Makrides was supported by a Fellowship from National Health Medical Research Council (NHMRC) (ID App1061704), Australia.
Carmel T Collins was supported by a Postdoctoral Research Fellowship from the MS McLeod Research Fund of the Women's and Children's Hospital Foundation, Australia.
Declarations of interest
Maria Makrides (MM) serves on scientific advisory boards for Nestle, Fonterra and Nutricia. Associated honoraria are paid to the Women's and Children's Health Research Institute to support conference travel and continuing education for post graduate students and early career researchers. MM is an investigator on two trials included in this review (Makrides 2009; Makrides 2010).
Carmel T Collins is an investigator on two trials included in this review (Makrides 2009; Makrides 2010).
Anoja W Gunaratne (AWG) was involved in the follow‐up at 7 years corrected age of Makrides 2009 and at 3 years of age of Makrides 2010.
The Makrides 2009 and Makrides 2010 trials were independently assessed for inclusion, risk of bias and data extracted by AWG and a third party (Karen Best).
New
References
References to studies included in this review
Dunstan 2003 {published data only}
- Barden AE, Dunstan JA, Beilin LJ, Prescott SL, Mori TA. n ‐ 3 fatty acid supplementation during pregnancy in women with allergic disease: effects on blood pressure, and maternal and fetal lipids. Clinical Science 2006;111(4):289‐94. [DOI] [PubMed] [Google Scholar]
- Barden AE, Mori TA, Dunstan JA, Taylor AL, Thornton CA, Croft KD, et al. Fish oil supplementation in pregnancy lowers f2‐isoprostanes in neonates at high risk of atopy. Free Radical Research 2004;38(3):233‐9. [DOI] [PubMed] [Google Scholar]
- Denburg JA, Hatfield HM, Cyr MM, Hayes L, Holt PG, Sehmi R, et al. Fish oil supplementation in pregnancy modifies neonatal progenitors at birth in infants at risk of atopy. Pediatric Research 2005;57(2):276‐81. [DOI] [PubMed] [Google Scholar]
- Dunstan JA, Mitoulas LR, Dixon G, Doherty DA, Hartmann PE, Simmer K, et al. The effects of fish oil supplementation in pregnancy on breast milk fatty acid composition over the course of lactation: a randomized controlled trial. Pediatric Research 2007;62(6):689‐94. [DOI] [PubMed] [Google Scholar]
- Dunstan JA, Mori TA, Barden A, Beilin LJ, Holt PG, Calder PC, et al. Effects of n‐3 polyunsaturated fatty acid supplementation in pregnancy on maternal and fetal erythrocyte fatty acid composition. European Journal of Clinical Nutrition 2004;58:429‐37. [DOI] [PubMed] [Google Scholar]
- Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, et al. Fish oil supplementation in pregnancy modifies neonatal allergen‐specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. Journal of Allergy and Clinical Allergy Immunology 2003;112(6):1178‐84. [DOI] [PubMed] [Google Scholar]
- Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, et al. Maternal fish oil supplementation in pregnancy reduces interleukin‐13 levels in cord blood of infants at high risk of atopy. Clinical and Experimental Allergy 2003;33(4):442‐8. [DOI] [PubMed] [Google Scholar]
- Dunstan JA, Roper J, Mitoulas L, Hartmann PE, Simmer K, Prescott SL. The effect of supplementation with fish oil during pregnancy on breast milk immunoglobulin A, soluble CD14, cytokine levels and fatty acid composition. Clinical and Experimental Allergy 2004;34(8):1237‐42. [DOI] [PubMed] [Google Scholar]
- Dunstan JA, Simmer K, Dixon G, Prescott SL. Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: a randomised controlled trial. Archives of Disease in Childhood Fetal and Neonatal Edition 2008;93(1):F45‐F50. [DOI] [PubMed] [Google Scholar]
- Hatfield HM, Dunstan JA, Hayes L, Sehmi R, Holt PM, Denberg JA, et al. Dietary N‐3 polyunsaturated fatty acid (PUFA) supplementation during pregnancy is associated with changes in cord blood (CB) progenitor numbers and responsiveness to IL‐5 in infants at risk of atopy [abstract]. Journal of Allergy and Clinical Immunology 2003;111(2 Suppl).):S320. [Google Scholar]
- Mattes E, McCarthy S, Gong G, Eekelen JA, Dunstan J, Foster J, et al. Maternal mood scores in mid‐pregnancy are related to aspects of neonatal immune function. Brain, Behavior, and Immunity 2009;23(3):380‐8. [DOI] [PubMed] [Google Scholar]
- Prescott SL, Barden AE, Mori TA, Dunstan JA. Maternal fish oil supplementation in pregnancy modifies neonatal leukotriene production by cord‐blood‐derived neutrophils. Clinical Science 2007;113(10):409‐16. [DOI] [PubMed] [Google Scholar]
Furuhjelm 2009 {published data only}
- Furuhjelm C, Jenmalm MC, Falth‐Magnusson K, DuchIn K. Th1 and Th2 chemokines, vaccine‐induced immunity, and allergic disease in infants after maternal ω‐3 fatty acid supplementation during pregnancy and lactation. Pediatric Research 2011;69(3):259‐64. [DOI] [PubMed] [Google Scholar]
- Furuhjelm C, Warstedt K, Fageras M, Falth‐Magnusson K, Larsson J, Fredriksson M, et al. A randomised placebo controlled study of maternal n‐3 fatty acid supplementation during pregnancy and lactation. Allergy 2008;63(Suppl 88):53. [Google Scholar]
- Furuhjelm C, Warstedt K, Fageras M, Falth‐Magnusson K, Larsson J, Fredriksson M, et al. A randomised placebo controlled study of omega‐3‐fatty acid supplementation during pregnancy and lactation. Skin sensitisation in the children. Allergy 2007;62(Suppl 83):46. [Google Scholar]
- Furuhjelm C, Warstedt K, Fageras M, Falth‐Magnusson K, Larsson J, Fredriksson M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega‐3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatric Allergy and Immunology 2011;22(5):505‐14. [DOI] [PubMed] [Google Scholar]
- Furuhjelm C, Warstedt K, Larsson J, Fredriksson M, Bottcher MF, Falth‐Magnusson K, et al. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatrica 2009;98(9):1461‐7. [DOI] [PubMed] [Google Scholar]
- Warstedt K, Furuhjelm C, Duchen K, Falth‐Magnusson K, Fageras M. The effects of omega‐3 fatty acid supplementation in pregnancy on maternal eicosanoid, cytokine, and chemokine secretion. Pediatric Research 2009;66(2):212‐7. [DOI] [PubMed] [Google Scholar]
Lauritzen 2005 {published data only}
- Asserhoj M, Nehammer S, Matthiessen J, Michaelsen KF, Lauritzen L. Maternal fish oil supplementation during lactation may adversely affect long‐term blood pressure, energy intake, and physical activity of 7‐year‐old boys. Journal of Nutrition 2009;139(2):298‐304. [DOI] [PubMed] [Google Scholar]
- Cheatham CL, Nerhammer AS, Asserhoj M, Michaelsen KF, Lauritzen L. Fish oil supplementation during lactation: effects on cognition and behavior at 7 years of age. Lipids 2011;46(7):637‐45. [DOI] [PubMed] [Google Scholar]
- Jorgensen MH, Michaelsen KF, Lauritzen L. Long chain polyunsaturated fatty acids and liver biochemistry in breast‐fed infants. Journal of Pediatric Gastroenterology and Nutrition 2005;40(5):631‐2. [Google Scholar]
- Larnkjaer A, Christensen JH, Michaelsen KF, Lauritzen L. Maternal fish oil supplementation during lactation does not affect blood pressure, pulse wave velocity, or heart rate variability in 2.5‐y‐old children. Journal of Nutrition 2006;136(6):1539‐44. [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Hoppe C, Straarup EM, Michaelsen KF. Maternal fish oil supplementation in lactation and growth during the first 2.5 years of life. Pediatric Research 2005;58(2):235‐42. [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Jorgensen MH, Mikkelsen TB, Skovgaard M, Straarup EM, Olsen SF, et al. Maternal fish oil supplementation in lactation: effect on visual acuity and n‐3 fatty acid content of infant erythrocytes. Lipids 2004;34(3):195‐206. [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Jorgensen MH, Olsen SF, Straarup EM, Michaelsen KF. Maternal fish oil supplementation in lactation: effect on developmental outcome in breast‐fed infants. Reproduction, Nutrition, Development 2005;45(5):535‐47. [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Kjaer TM, Fruekilde MB, Michaelsen KF, Frokiaer H. Fish oil supplementation of lactating mothers affects cytokine production in 2 1/2‐year‐old children. Lipids 2005;40(7):669‐76. [DOI] [PubMed] [Google Scholar]
- Ulbak J, Lauritzen L, Hansen HS, Michaelsen KF. Diet and blood pressure in 2.5‐y‐old Danish children. American Journal of Clinical Nutrition 2004;79(6):1095‐102. [DOI] [PubMed] [Google Scholar]
Makrides 2009 {unpublished data only}
- Atwell K, Collins CT, Sullivan TR, Ryan P, Gibson RA, Makrides M, et al. Respiratory hospitalisation of infants supplemented with docosahexaenoic acid as preterm neonates. Journal of Paediatric Child Health 2013;49(1):E17‐E22. [DOI] [PubMed] [Google Scholar]
- Collins CT, Gibson RA, Miller J, McPhee AJ, Willson K, Smithers LG, et al. Carbohydrate intake is the main determinant of growth in infants born <33 weeks' gestation when protein intake is adequate. Nutrition 2008;24:451‐7. [DOI] [PubMed] [Google Scholar]
- Collins CT, Makrides M, Gibson RA, McPhee AJ, Davis PG, Doyle LW, et al. Pre and post‐term growth in pre‐term infants supplemented with higher‐dose DHA: a randomised controlled trial. British Journal of Nutrition 2011;105(11):1635‐43. [DOI] [PubMed] [Google Scholar]
- Gunaratne AW. Effects of n‐3 LCPUFA Supplementation for Pregnant and Lactating Women in Preventing Allergic Disease in Early Childhood [PhD thesis‐submitted]. University of Adelaide, 2014 July. [Google Scholar]
- Hawkes JS, Bryan DL, Makrides M, Neumann MA, Gibson RA. A randomized trial of supplementation with docosahexaenoic acid‐rich tuna oil and its effects on the human milk cytokines interleukin 1beta, interleukin 6, and tumor necrosis factor a. American Journal of Clinical Nutrition 2002;75(4):754‐60. [DOI] [PubMed] [Google Scholar]
- Makrides M, Gibson RA, McPhee AJ, Collins CT, Davis PG, Doyle LW, et al. Neurodevelopmental outcomes of preterm infants fed high‐dose docosahexaenoic acid: a randomised controlled trial. JAMA 2009;301(2):175‐82. [DOI] [PubMed] [Google Scholar]
- Manley BJ, Makrides M, Collins CT, McPhee AJ, Gibson RA, Ryan P, et al. High‐dose docosahexaenoic acid supplementation of preterm infants: respiratory and allergy outcomes. Pediatrics 2011;128(1):e71‐e77. [DOI] [PubMed] [Google Scholar]
- Simmonds L. Behavioural and Allergic Disease Assessment at Pre‐School Age: A Follow‐up Study of Preterm Infants Fed Docosahexaenoic Acid Supplementation in the Neonatal Period [dissertation]. University of Adelaide, 2007 Nov. [Google Scholar]
- Smithers LG, Collins CT, Simmonds LA, Gibson RA, McPhee A, Makrides M. Feeding preterm infants milk with a higher dose of docosahexaenoic acid than that used in current practice does not influence language or behavior in early childhood: a follow‐up study of a randomised controlled trial. American Journal of Clinical Nutrition 2010;91(3):628‐34. [DOI] [PubMed] [Google Scholar]
- Smithers LG, Gibson RA, Makrides M. Long‐chain polyunsaturated fatty acid supplementation for infants born preterm. NeoReviews 2007;8:143‐51. [Google Scholar]
- Smithers LG, Gibson RA, McPhee A, Makrides M. Effect of two doses of docosahexaenoic acid (DHA) in the diet of preterm infants on infant fatty acid status: results from the DINO trial. Prostaglandins Leukotrins and Essential Fatty Acids 2008;79(3‐5):141‐6. [DOI] [PubMed] [Google Scholar]
- Smithers LG, Gibson RA, McPhee A, Makrides M. Higher dose of docosahexaenoic acid in the neonatal period improved visual acuity of preterm infants: results of a randomised controlled trial. American Journal of Clinical Nutrition 2008;88(4):1049‐56. [DOI] [PubMed] [Google Scholar]
- Smithers LG, Makrides M, Gibson RA. Human milk fatty acids from lactating mothers of preterm infants: A study revealing wide intra‐ and inter‐individual variation. Prostaglandins, Leukotrienes and Essential Fatty Acids 2010;83(1):9‐13. [DOI] [PubMed] [Google Scholar]
Makrides 2010 {published and unpublished data}
- Gunaratne AW. Effects of n‐3 LCPUFA Supplementation for Pregnant and Lactating Women in Preventing Allergic Disease in Early Childhood [PhD thesis‐submitted]. University of Adelaide, 2014 July. [Google Scholar]
- Makrides M. A randomised trial of DHA in pregnancy to prevent postnatal depression symptoms and enhance neurodevelopment in children: the DOMINO trial. Australian Clinical Trials Register (http://www.actr.org/actr) [accessed 6 December 2005] 2005.
- Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J. Effect of docosahexaenoic acid (DHA) supplementation during pregnancy on neurodevelopment of young children: The domino trial. Journal of Paediatrics and Child Health 2011;47(Suppl 1):89. [Google Scholar]
- Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P, et al. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA 2010;304(15):1675‐83. [DOI] [PubMed] [Google Scholar]
- Makrides M, Gould JF, Gawlik NR, Yelland LN, Smithers LG, Anderson PJ, et al. Four‐year follow‐up of children born to women in a randomized trial of prenatal DHA supplementation. JAMA 2014;311(17):1802‐4. [DOI] [PubMed] [Google Scholar]
- Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, et al. Effect of n‐3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants' allergies in first year of life: randomised controlled trial. BMJ 2012;344(e184):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, et al. Effect of n‐3 polyunsaturated fatty acid supplementation in pregnancy on early childhood allergic disease: Randomized controlled trial. Journal of Paediatrics and Child Health 2013;49 Suppl(2):56. [Google Scholar]
- Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy 2013;68(11):1370‐6. [DOI] [PubMed] [Google Scholar]
- Ryan P, Griffith E, McDermott B, Makrides M, Gibson R, for the DOMINO Steering Group. Data management tools in the DOMINO trial: DHA in pregnancy to prevent postnatal depressive symptoms and enhance neurodevelopment in children. Clinical Trials 2007;4(4):426. [Google Scholar]
- Smithers LG, Gibson RA, Makrides M. Maternal supplementation with docosahexaenoic acid during pregnancy does not affect early visual development in the infant: a randomized controlled trial. American Journal of Clinical Nutrition 2011;93(6):1293‐9. [DOI] [PubMed] [Google Scholar]
- Zhou SJ, Yelland L, McPhee AJ, Quinlivan J, Gibson RA, Makrides M. Fish‐oil supplementation in pregnancy does not reduce the risk of gestational diabetes or preeclampsia. American Journal of Clinical Nutrition 2012;95(6):1378‐84. [DOI] [PubMed] [Google Scholar]
Noakes 2012 {published data only}
- Garcia‐Rodriguez C, Helmersson‐Karlqvis J, Mesa M, Miles E, Noakes P, Vlachava M, et al. Increased intake of salmon decreases F2‐isoprostanes in pregnant women. Annals of Nutrition and Metabolism 2011;58(Suppl 3):122‐3. [Google Scholar]
- Garcia‐Rodriguez C, Mesa M, Olza J, Vlachava M, Kremmyda L, Diaper N, et al. Farmed salmon supplementation enhances the enzymatic defence system. Annals of Nutrition and Metabolism 2011;58(Suppl 3):89‐90. [Google Scholar]
- Garcia‐Rodriguez CE, Helmersson‐Karlqvist J, Mesa MD, Miles EA, Noakes PS, Vlachava M, et al. Does increased intake of salmon increase markers of oxidative stress in pregnant women? The salmon in pregnancy study. Antioxidants and Redox Signaling 2011;15(11):2819‐23. [DOI] [PubMed] [Google Scholar]
- Garcia‐Rodriguez CE, Mesa MD, Olza J, Vlachava M, Kremmyda LS, Diaper ND, et al. Does consumption of two portions of salmon per week enhance the antioxidant defence system in pregnant women?. Antioxidants and Redox Signaling 2012;16(12):1401‐6. [DOI] [PubMed] [Google Scholar]
- Garcia‐Rodriguez CE, Olza J, Aguilera CM, Mesa MD, Miles EA, Noakes PS, et al. Plasma inflammatory and vascular homeostasis bio markers increase during human pregnancy but are not affected by oily fish intake. Journal of Nutrition 2012;142(7):1191‐6. [DOI] [PubMed] [Google Scholar]
- Helmersson‐Karlqvist J, Miles EA, Vlachava M, Kremmyda LS, Noakes PS, Diaper ND, et al. Enhanced prostaglandin F2alpha formation in human pregnancy and the effect of increased oily fish intake: results from the Salmon in Pregnancy Study. Prostaglandins Leukotrienes and Essential Fatty Acids 2012;86(1‐2):35‐8. [DOI] [PubMed] [Google Scholar]
- Miles EA, Noakes PS, Kremmyda LS, Vlachava M, Diaper ND, Rosenlund G, et al. The Salmon in Pregnancy Study: study design, subject characteristics, maternal fish and marine n‐3 fatty acid intake, and marine n‐3 fatty acid status in maternal and umbilical cord blood. American Journal of Clinical Nutrition 2011;94(6 Suppl):1986S‐92S. [DOI] [PubMed] [Google Scholar]
- Noakes PS, Vlachava M, Kremmyda LS, Diaper ND, Miles EA, Erlewyn‐Lajeunesse M, et al. Increased intake of oily fish in pregnancy: effects on neonatal immune responses and on clinical outcomes in infants at 6 mo. American Journal of Clinical Nutrition 2012;95(2):395‐404. [DOI] [PubMed] [Google Scholar]
- Rossary A, Farges M, Lamas B, Miles EA, Noakes PS, Kremmyda L, et al. Increased consumption of salmon during pregnancy partly prevents the decline of some plasma essential amino acid concentrations in pregnant women. Clinical Nutrition 2014;33(2):267‐73. [DOI] [PubMed] [Google Scholar]
- Rossary A, Farges MC, Vlachava M, Kremmyda LS, Diaper ND, Noakes PS, et al. Does salmon consumption by pregnant women change amino acids availability?. Clinical Nutrition Supplement 2011;6(1):109. [Google Scholar]
- Urwin HJ, Miles EA, Noakes PS, Kremmyda L, Vlachava M, Diaper ND, et al. Effect of salmon consumption during pregnancy on maternal and infant faecal microbiota, secretory IgA and calprotectin. British Journal of Nutrition 2014;111(5):733‐84. [DOI] [PubMed] [Google Scholar]
- Urwin HJ, Miles EA, Noakes PS, Kremmyda LS, Vlachava M, Diaper ND, et al. Salmon consumption during pregnancy alters fatty acid composition and secretory IgA concentration in human breast milk. Journal of Nutrition 2012;142(8):1603‐10. [DOI] [PubMed] [Google Scholar]
- Elsen LW, Noakes PS, Maarel MA, Kremmyda LS, Vlachava M, Diaper ND, et al. Salmon consumption by pregnant women reduces ex vivo umbilical cord endothelial cell activation. American Journal of Clinical Nutrition 2011;94(6):1418‐25. [DOI] [PubMed] [Google Scholar]
Olsen 1992 {published data only}
- Boris J, Jensen B, Salvig JD, Secher NJ, Olsen SF. A randomized controlled trial of the effect of fish oil supplementation in late pregnancy and early lactation on the n‐3 fatty acid content in human breast milk. Lipids 2004;39(12):1191‐6. [DOI] [PubMed] [Google Scholar]
- Olsen SF, Osterdal ML, Salvig JD, Mortensen LM, Rytter D, Secher NJ, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry‐based follow‐up from a randomized controlled trial. American Journal of Clinical Nutrition 2008;88(1):167‐75. [DOI] [PubMed] [Google Scholar]
- Olsen SF, Osterdal ML, Salvig JD, Weber T, Tabor A, Secher NJ. Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: a randomised clinical trial with fish oil. European Journal of Clinical Nutrition 2007;61(8):976‐85. [DOI] [PubMed] [Google Scholar]
- Olsen SF, Secher NJ. Fish oil and preeclampsia. 8th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1992 November 8‐12; Buenos Aires, Argentina. 1992:94.
- Olsen SF, Secher NJ, Sorensen JD, Grant A. Gestational age and fish oil supplementation in third trimester: a population‐based randomized controlled trial. International Journal of Gynecology and Obstetrics 1991;36 Suppl:31. [Google Scholar]
- Olsen SF, Sorensen JD, Secher NJ, Hedegaard M, Henriksen TB, Hansen HS, et al. Randomised controlled trial of effect of fish‐oil supplementation on pregnancy duration. Lancet 1992;339:1003‐7. [DOI] [PubMed] [Google Scholar]
- Olsen SF, Søorensen JD, Secher NJ, Hedegaard M, Henriksen TB, Hansen HS, et al. Fish oil supplementation and duration of pregnancy. A randomized controlled trial [Fiskeolietilskud og graviditetsvarighed. En randomiseret kontrolleret undersogelse.]. Ugeskrift for Laeger 1994;156(9):1302‐7. [PubMed] [Google Scholar]
- Rytter D, Bech BH, Christensen JH, Schmidt EB, Henriksen TB, Olsen SF. Intake of fish oil during pregnancy and adiposity in 19‐y‐old offspring: Follow‐up on a randomized controlled trial. American Journal of Clinical Nutrition 2011;94(3):701‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytter D, Christensen JH, Bech BH, Schmidt EB, Henriksen TB, Olsen SF. The effect of maternal fish oil supplementation during the last trimester of pregnancy on blood pressure, heart rate and heart rate variability in the 19‐year‐old offspring. British Journal of Nutrition 2012;108(8):1475‐83. [DOI] [PubMed] [Google Scholar]
- Rytter D, Schmidt EB, Bech BH, Christensen JH, Henriksen TB, Olsen SF. Fish oil supplementation during late pregnancy does not influence plasma lipids or lipoprotein levels in young adult offspring. Lipids 2011;46(12):1091‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvig JD, Olsen SF, Secher NJ. Effects of fish oil supplementation in late pregnancy on blood pressure: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 1996;103:529‐33. [DOI] [PubMed] [Google Scholar]
- Sorensen JD, Olsen SF, Pedersen AK, Boris J, Secher NJ, FitzGerald GA. Effects of fish oil supplementation in the third trimester of pregnancy on prostacyclin and thromboxane production. American Journal of Obstetrics and Gynecology 1993;168:915‐22. [DOI] [PubMed] [Google Scholar]
- Sorensen JD, Olsen SF, Pedersen AK, Boris J, Secher NJ, FitzGerald GA. Effects of fish oil supplementation in the third trimester of pregnancy on prostacyclin and thromboxane production. International Journal of Gynecology and Obstetrics 1993;43:229. [DOI] [PubMed] [Google Scholar]
- Sorensen JD, Olsen SF, Secher NJ. Effects of fish oil supplementation in late pregnancy on blood pressure: a randomised controlled study. Acta Obstetricia et Gynecologica Scandinavica Supplement 1995;73:110. [Google Scholar]
- Sorensen JD, Olsen SF, Secher NJ, Jespersen J. Effects of fish oil supplementation in late pregnancy on blood lipids, serum urate, coagulation and fibrinolysis A randomised controlled study. Fibrinolysis 1994;8:54‐60. [Google Scholar]
- Houwelingen AC, Sorensen JD, Hornstra G, Simonis MMG, Boris J, Olsen SF, et al. Essential fatty acid status in neonates after fish‐oil supplementation during late pregnancy. British Journal of Nutrition 1995;74:723‐31. [DOI] [PubMed] [Google Scholar]
Ramakrishnan 2010 {published data only}
- Escamilla‐Nunez MC, Barraza‐Villarreal A, Hernandez‐Cadena L, Navarro‐Olivos E, Sly PD, Romieu I. Omega‐3 fatty acid supplementation during pregnancy and respiratory symptoms in children. Chest 2014; Vol. 146, issue 2:373‐82. [DOI] [PMC free article] [PubMed]
- Hernandez E, Barraza‐Villarreal A, Escamilla‐Nunez MC, Hernandez‐Cadena L, Sly PD, Neufeld LM, et al. Prenatal determinants of cord blood total immunoglobulin E levels in Mexican newborns. Allergy & Asthma Proceedings 2013;34(5):e27‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoff‐Kunsch B, Stein AD, Martorell R, Parra‐Cabrera S, Romieu I, Ramakrishnan U. Prenatal docosahexaenoic acid supplementation and infant morbidity: Randomized controlled trial. Pediatrics 2011;128(3):e505‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoff‐Kunsch B, Stein AD, Villalpando S, Martorell R, Ramakrishnan U. Docosahexaenoic acid supplementation from mid‐pregnancy to parturition influenced breast milk fatty acid concentrations at 1 month postpartum in Mexican women. Journal of Nutrition 2011;141(2):321‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Barraza‐Villarreal A, Hernandez‐Vargas H, Sly PD, Biessy C, Ramakrishnan U, et al. Modulation of DNA methylation states and infant immune system by dietary supplementation with omega‐3 PUFA during pregnancy in an intervention study. American Journal of Clinical Nutrition 2013;98(2):480‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan U, Di A, Schnaas L, Stein AD, Wang M, Martorell R, et al. Effect of prenatal supplementation with docosahexanoic acid on infant development: A randomized placebo‐controlled trial in Mexico. FASEB Journal 2010;24(Suppl):Abstract no 227.6. [Google Scholar]
- Ramakrishnan U, Stein AD, Parra‐Cabrera S, Wang M, Imhoff‐Kunsch B, Juirez‐Mirquez S, et al. Effects of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: randomized, double‐blind, placebo‐controlled trial in Mexico. Food and Nutrition Bulletin 2010;31(2 Suppl):S108‐16. [DOI] [PubMed] [Google Scholar]
- Romieu I, Barraza‐Villarreal A, Hernandez‐Cadena L, Escamilla‐Nunez C, Sly P, Neulfeld L, et al. Supplementation with omega‐3 fatty acids and atopy symptoms in infants: A randomized controlled trial. American Journal of Epidemiology 2008;167(11):S23. [Google Scholar]
- Romieu I, Barraza‐Villarreal A, Hernandez‐Cadena L, Escamilla‐Nunez C, Sly P, Neulfeld L, et al. Supplementation with omega‐3 fatty acids and atopy symptoms in infants: a randomized controlled trial. European Respiratory Society Annual Congress; 2008 October 4‐8; Berlin, Germany. 2008:346s.
- Romieu I, Hernandez E, Barraza‐Villarreal A, Escamilla‐Nunez C, Sly P, Neufeld L, et al. Predictors of cord blood ige levels in child: preliminary results of a randomized controlled trial of Omega‐3 PUFA supplementation during pregnancy [Abstract]. Journal of Allergy and Clinical Immunology 2009;123(2 Suppl 1):S193. [Google Scholar]
- Romieu I, Lee H, Barraza A, Biessy C, Duarte‐Salles T, Sly P, et al. Dietary supplementation with polyunsaturated fatty acid during pregnancy modulates DNA methylation at IGF2/H19 imprinted genes and growth of infants. FASEB Journal 2014;28(1 Suppl 1):1120.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AD, Wang M, Martorell R, Neufeld L, Flores‐Ayala R, Rivera J, et al. Postnatal growth following maternal gestational supplementation with docosahexanoic acid (DHA): Randomized placebo‐controlled trial in Mexico. FASEB Journal 2010;24(Suppl):Abstract no 277.5. [Google Scholar]
- Stein AD, Wang M, Martorell R, Neufeld LM, Flores‐Ayala R, Rivera JA, et al. Growth to age 18 months following prenatal supplementation with docosahexaenoic acid differs by maternal gravidity in Mexico. Journal of Nutrition 2011;141(2):316‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AD, Wang M, Rivera JA, Martorell R, Ramakrishnan U. Auditory‐and visual‐evoked potentials in Mexican infants are not affected by maternal supplementation with 400 mg/d docosahexaenoic acid in the second half of pregnancy. Journal of Nutrition 2012;142(8):1577‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bergmann 2008 {published data only}
- Bergmann RL, Bergmann KE, Richter R, Haschke‐Becher E, Henrich W, Dudenhausen JW. Does docosahexaenoic acid (DHA) status in pregnancy have any impact on postnatal growth? Six‐year follow‐up of a prospective randomized double‐blind monocenter study on low‐dose DHA supplements. Journal of Perinatal Medicine 2012;40:677‐84. [DOI] [PubMed] [Google Scholar]
- Bergmann RL, Haschke‐Becher E, Klassen‐Wigger P, Bergmann KE, Richter R, Dudenhausen JW, et al. Supplementation with 200 mg/day docosahexaenoic acid from mid‐pregnancy through lactation improves the docosahexaenoic acid status of mothers with a habitually low fish intake and of their infants. Annals of Nutrition and Metabolism 2008;52(2):157‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann RL, Haschke‐Becher, Bergmann KE, Dudenhausen JW, Haschke F. Low dose docosahexaenoic acid supplementation improves the DHA status of pregnant women [abstract]. Pediatric Academic Societies Annual Meeting; 2006 April 29‐May 2; San Francisco, CA, USA. 2006.
- Bergmann RL, Richter R, Bergmann KE, Dudenhausen JW, Haschke F. 21 months old infants are leaner if their mothers received low dose DHA supplements during pregnancy and lactation. European Journal of Pediatrics 2006;165 Suppl 1:114. [Google Scholar]
Campos‐Martinez 2012 {published data only}
- Campos‐Martinez A, Serrano‐ L, Medina‐ M, Ochoa‐ J, Pena‐Caballero M. Levels of docosahexaenoic acid in pregnant women and their children after taking a fish oil enriched diet. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(S2):92. [Google Scholar]
Carlson 2013 {published data only}
- Carlson SE. DHA supplementation and pregnancy outcome. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 21 March 2006] 2006.
- Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, et al. DHA supplementation and pregnancy outcomes. American Journal of Clinical Nutrition 2013;97(4):808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colombo 2004 {published data only}
- Colombo J, Kannass KN, Shaddy DJ, Kundurthi S, Maikranz JM, Anderson CJ, et al. Maternal DHA and the development of attention in infancy and toddlerhood. Child Development 2004;75(4):1254‐67. [DOI] [PubMed] [Google Scholar]
Courville 2011 {published data only}
- Courville AB, Harel O, Lammi‐Keefe CJ. Consumption of a DHA‐containing functional food during pregnancy is associated with lower infant ponderal index and cord plasma insulin concentration. British Journal of Nutrition 2011;106(2):208‐12. [DOI] [PubMed] [Google Scholar]
Granot 2011 {published data only}
- Granot E, Jakobovich E, Rabinowitz R, Levy P, Schlesinger M. DHA supplementation during pregnancy and lactation affects infants' cellular but not humoral immune response. Mediators of Inflammation 2011;2011:493925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauner 2009 {published data only}
- Brunner S, Schmid D, Huttinger K, Much D, Bruderl M, Sedlmeier EM, et al. Effect of a dietary intervention to reduce the n‐6/n‐3 fatty acid ratio on the maternal and cord blood leptin axis and relation of leptin to body composition in the offspring ‐ Results of the infat‐study (pepo‐consortium of the competence network obesity). Obesity Facts 2012;5(Suppl 2):10. [Google Scholar]
- Hauner H, Much D, Vollhardt C, Brunner S, Schmid D, Sedlmeier EM, et al. Effect of reducing the n‐6:n‐3 long‐chain PUFA ratio during pregnancy and lactation on infant adipose tissue growth within the first year of life: An open‐label randomized controlled trial. American Journal of Clinical Nutrition 2012;95(2):383‐94. [DOI] [PubMed] [Google Scholar]
- Hauner H, Vollhardt C, Schneider KT, Zimmermann A, Schuster T, Amann‐Gassner U. The impact of nutritional fatty acids during pregnancy and lactation on early human adipose tissue development. Rationale and design of the INFAT study. Annals of Nutrition and Metabolism 2009;54(2):97‐103. [DOI] [PubMed] [Google Scholar]
- Much D, Brunner S, Vollhardt C, Schmid D, Sedlmeier EM, Bruderl M, et al. Effect of dietary intervention to reduce the n‐6/n‐3 fatty acid ratio on maternal and fetal fatty acid profile and its relation to offspring growth and body composition at 1 year of age. European Journal of Clinical Nutrition 2013;67(3):282‐8. [DOI] [PubMed] [Google Scholar]
Helland 2001 {published data only}
- Helland IB, Saarem K, Saugstad OD, Drevon CA. Fatty acid composition in maternal milk and plasma during supplementation with cod liver oil. European Journal of Clinical Nutrition 1998;52(11):839‐45. [DOI] [PubMed] [Google Scholar]
- Helland IB, Saugstad OD, Saarem K, Houwelingen AC, Nylander G, Drevon CA. Supplementation of n‐3 fatty acids during pregnancy and lactation reduces maternal plasma lipid levels and provides DHA to the infants. Journal of Maternal‐Fetal and Neonatal Medicine 2006;19(7):397‐406. [DOI] [PubMed] [Google Scholar]
- Helland IB, Saugstad OD, Smith L, Saarem K, Solvoll K, Ganes T, et al. Similar effects on infants of n‐3 and n‐6 fatty acids supplementation to pregnant and lactating women. Pediatrics 2001;108(5):E82. [DOI] [PubMed] [Google Scholar]
- Helland IB, Smith L, Blomen B, Saarem K, Saugstad OD, Drevon CA. Effect of supplementing pregnant and lactating mothers with n‐3 very‐long‐chain fatty acids on children's IQ and body mass index at 7 years of age. Pediatrics 2008;122(2):e472‐9. [DOI] [PubMed] [Google Scholar]
- Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very‐long‐chain n‐3 fatty acids during pregnancy and lactation augments children's iq at 4 years of age. Pediatrics 2003;111(1):e39‐44. [DOI] [PubMed] [Google Scholar]
Innis 2007 {published data only}
- Innis SM. N‐3 fatty acid requirements for human development. http://clinicaltrials.gov/ct2/show/NCT00620672 (accessed 9 April 2008).
- Innis SM, Friesen RW. Essential N‐3 fatty acids in pregnant women and early visual acuity maturation in term infants. American Journal of Clinical Nutrition 2008;87(3):548‐57. [DOI] [PubMed] [Google Scholar]
- Innis SM, Friesen RW. Maternal DHA supplementation in pregnancy: a double blind randomized prospective trial of maternal N‐3 fatty acid status, human milk fatty acids and infant development. Pediatric Academic Societies Annual Meeting; 2007 May 5‐8; Toronto, Canada. 2007.
- Mulder KA, Innis SM, Richardson KJ. Cognitive performance of children 5‐6 years of age is associated with children's docosahexaenoic acid (DHA), but not dietary DHA or maternal DHA in pregnancy. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2014 May 3‐6; Vancouver, Canada. 2014:Abstract no: 3680.6.
- Mulder KA, King DJ, Innis SM. Omega‐3 fatty acid deficiency in infants before birth identified using a randomized trial of maternal DHA supplementation in pregnancy. Plos One 2014;9(1):e83764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Judge 2007 {published data only}
- Judge MP, Harel O, Lammi‐Keefe CJ. A docosahexaenoic acid‐functional food during pregnancy benefits infant visual acuity at four but not six months of age. Lipids 2007;42(2):117‐22. [DOI] [PubMed] [Google Scholar]
Karlsson 2010 {published data only}
- Karlsson T, Birberg‐Thornberg U, Duchen K, Gustafsson PA. LC‐PUFA supplemented to mothers during pregnancy and breast‐feeding improves cognitive performance in their children four years later ‐ an rct study. 9th Congress of the ISSFAL; 29 May‐2 June 2010; Maastricht, The Netherlands 2010:113.
Knudsen 2006 {published data only}
- Knudsen VK, Hansen HS, Osterdal ML, Mikkelsen TB, Mu H, Olsen SF. Fish oil in various doses or flax oil in pregnancy and timing of spontaneous delivery: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2006;113(5):536‐43. [DOI] [PubMed] [Google Scholar]
Krauss‐Etschmann 2007 {published data only}
- Broekaert I, Campoy C, Iznaola C, Hoffman B, Mueller‐Felber W, Koletzko BV. Visual evoked potentials in infants after dietary supply of docosahexaenoic acid and 5‐methyl‐tetrahydrofolate during pregnancy. Journal of Pediatric Gastroenterology and Nutrition 2004;39(Suppl 1):S33. [Google Scholar]
- Campoy C, Escolano‐Margarit MV, Ramos R, Parrilla‐Roure M, Csabi G, Beyer J, et al. Effects of prenatal fish‐oil and 5‐methyltetrahydrofolate supplementation on cognitive development of children at 6.5 y of age. American Journal of Clinical Nutrition 2011;94(6 Suppl):1880S‐8S. [DOI] [PubMed] [Google Scholar]
- Campoy C, Marchal G, Decsi T, Cruz M, Szabo E, Demmelmair H, et al. Spanish pregnant women's plasma phospholipids LC‐PUFAs concentrations and its influence on their newborns. Journal of Pediatric Gastroenterology and Nutrition 2004;39(Suppl 1):S11. [Google Scholar]
- Decsi T, Campoy C, Koletzko B. Effect of n‐3 polyunsaturated fatty acid supplementation in pregnancy: the nuheal trial. Advances in Experimental Medicine and Biology 2005;569:109‐13. [DOI] [PubMed] [Google Scholar]
- Demmelmair H, Klingler M, Campoy C, Decsi T, Koletzko B. Low eicosapentaenoic acid concentrations in fish oil supplements do not influence the arachidonic acid contents in placental lipids. Journal of Pediatric Gastroenterology and Nutrition 2004;39(Suppl 1):S11. [Google Scholar]
- Demmelmair H, Klingler M, Campoy C, Diaz J, Decsi T, Veszpremi B, et al. The influence of habitual diet and increased docosahexaenoic acid intake during pregnancy on the fatty acid composition of individual placental lipids [abstract]. Journal of Pediatric Gastroenterology and Nutrition 2005;40(5):622‐3. [Google Scholar]
- Dolz V, Campoy C, Molloy A, Scott J, Marchal G, Decsi T, et al. Homocysteine, folate & methylenetetrahydrofolate reductase (MTHFR) 677 ‐ T poly‐morphism in Spanish pregnant woman and in their offspring [abstract]. Journal of Pediatric Gastroenterology and Nutrition 2005;40(5):623‐4. [Google Scholar]
- Escolano‐Margarit MV, Campoy C, Ramirez‐Tortosa MC, Demmelmair H, Miranda MT, Gil A, et al. Effects of fish oil supplementation on the fatty acid profile in erythrocyte membrane and plasma phospholipids of pregnant women and their offspring: a randomised controlled trial. British Journal of Nutrition 2013;109(9):1614‐56. [DOI] [PubMed] [Google Scholar]
- Escolano‐Margarit MV, Ramos R, Beyer J, Csabi G, Parrilla‐Roure M, Cruz F, et al. Prenatal DHA status and neurological outcome in children at age 5.5 years are positively associated. Journal of Nutrition 2011;141(6):1216‐23. [DOI] [PubMed] [Google Scholar]
- Klingler M, Blaschitz A, Campoy C, Cano A, Molloy AM, Scott JM, et al. The effect of docosahexaenoic acid and folic acid supplementation on placental apoptosis and proliferation. British Journal of Nutrition 2006;96(1):182‐90. [DOI] [PubMed] [Google Scholar]
- Krauss‐Etschmann S, Hartl D, Heinrich J, Thaqi A, Prell C, Campoy C, et al. Association between levels of toll‐like receptors 2 and 4 and CD14 mRNA and allergy in pregnant women and their offspring. Clinical Immunology 2006;118(2‐3):292‐9. [DOI] [PubMed] [Google Scholar]
- Krauss‐Etschmann S, Hartl D, Rzehak P, Heinrich J, Shadid R, Carmen Ramirez‐Tortosa M, et al. Decreased cord blood IL‐4, IL‐13, and CCR4 and increased TGF‐beta levels after fish oil supplementation of pregnant women. Journal of Allergy and Clinical Immunology 2008;121(2):464‐70. [DOI] [PubMed] [Google Scholar]
- Krauss‐Etschmann S, Shadid R, Campoy C, Hoster E, Demmelmair H, Jimenez M, et al. Effects of fish‐oil and folate supplementation of pregnant women on maternal and fetal plasma concentrations of docosahexaenoic acid and eicosapentaenoic acid: a European randomized multicenter trial. American Journal of Clinical Nutrition 2007;85(5):1392‐400. [DOI] [PubMed] [Google Scholar]
Martin‐Alvarez 2012 {published data only}
- Martin‐Alvarez E, Guerrero‐Montenegro B, Romero‐Paniagua MT, Lara‐Villoslada F, Hurtado‐Suazo JA. Maternal docosahexaenoic acid supplementation during pregnancy and lactation can modulate oxidative stress in the term newborn. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(S2):40. [Google Scholar]
Pena‐Quintana 2011 {published data only}
- Pena‐Quintana L, Pena M, Rodriguez‐Santana Y, Hurtado JA, Ochoa J, Sanjurjo P, et al. Consumption of a dairy product enriched with fish oil during pregnancy maintains the dha status of the mother and increase dha concentration in cord blood. Journal of Pediatric Gastroenterology and Nutrition 2011;52(Suppl 1):E84. [Google Scholar]
Ribeiro 2012 {published data only}
- Ribeiro P, Carvalho FD, Abreu Ade A, Sant'anna Mde T, Lima RJ, Carvalho Pde O. Effect of fish oil supplementation in pregnancy on the fatty acid composition of erythrocyte phospholipids and breast milk lipids. International Journal of Food Sciences and Nutrition 2012;63(1):36‐40. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Bisgaard 2012 {published data only}
- Bisgaard H. Fish oil supplementation during pregnancy for prevention of asthma, eczema and allergies in childhood: interventional trial in the cCOPSAC2010 (Copenhagen studies on asthma in childhood) birth cohort. http://clinicaltrials.gov/ct2/show/NCT00798226 (accessed 15 September 2012).
Duchen 2012 {published data only}
- Duchen K. Combined dietary supplementation with lactobacillus reuteri and omega‐3 PUFA during pregnancy and postnatally in relation to development of IgE‐associated disease during infancy. http://clinicaltrials.gov/ct2/show/NCT01542970 (accessed 15 September 2012).
Laitinen 2013 {published data only}
- Laitinen K. Nutrition and pregnancy intervention study. ClinicalTrials.gov (http://clinicaltrials.gov/ct2/show/NCT01922791) (accessed 5 February 2014). NCT01922791 2013.
Liu 2013 {published data only}
- Liu Z, Yin H. The effect of polyunsaturated fatty acids (PUFA) on allergic/atopic dermatitis. http://clinicaltrials.gov/ct2/show/NCT01936194 (verified September 2013) 2014.
Additional references
Arkwright 2008
- Arkwright PD, David TJ. Allergic disorders. In: McIntosh N, Helms PJ, Smyth RL, Logan S editor(s). Forfar and Arneil's Test Book of Paediatrics. 7th Edition. Churchill Livingstone, 2008:1519‐39. [Google Scholar]
Asher 2004
- Asher I, Baena‐Cagnani C, Boner A, Canonica GW, Chuchalin A, Custovic A, et al. World Allergy Organization guidelines for prevention of allergy and allergic asthma. International Archives of Allergy and Immunology 2004;135(1):83‐92. [DOI] [PubMed] [Google Scholar]
Asher 2006
- Asher MI, Montefort S, Björkstén B, Lai CKW, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhino‐conjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multi country cross‐sectional surveys. Lancet 2006;368(9537):733‐43. [DOI] [PubMed] [Google Scholar]
Barbee 1998
- Barbee RA, Murphy S. The natural history of asthma. Journal of Allergy and Clinical Immunology 1998;102(4 Pt 2):S65‐S72. [DOI] [PubMed] [Google Scholar]
Bousquet 2012
- Bousquet, J. Heinzerling, L. Bachert, C. Papadopoulos, N. G.Bousquet, P. J.Burney, P. G, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy 2012;67(1):18‐24. [DOI] [PubMed] [Google Scholar]
Calder 2000
- Calder PC, Miles EA. Fatty acids and atopic disease. Pediatrics Allergy and Immunology 2000;13:29‐36. [DOI] [PubMed] [Google Scholar]
Calder 2003
Calder 2006
- Calder PC. n‐3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. American Journal of Clinical Nutrition 2006;83(6):1505s‐19s. [DOI] [PubMed] [Google Scholar]
Calder 2007
- Calder PC. Immunomodulation by omega‐3 fatty acids. Prostaglandins Leukotrine and Essential Fatty Acids 2007;77(5–6):327–35. [DOI] [PubMed] [Google Scholar]
Calder 2010a
- Calder PC. Does early exposure to long chain polyunsaturated fatty acids provide immune benefits?. Journal of Pediatrics 2010;156(6):869‐71. [DOI] [PubMed] [Google Scholar]
Calder 2010b
- Calder PC, Kremmyda LS, Vlachava M, Noakes PS, Miles EA. Is there a role for fatty acids in early life programming of the immune system?. Proceedings of the Nutrition Society 2010;69(3):373‐80. [PUBMED: 20462467] [DOI] [PubMed] [Google Scholar]
Calvani 2006
- Calvani M, Alessandri C, Sopo SM, Panetta V, Pingitore G, Tripodi S, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatric Allergy and Immunology 2006;17(2):94‐102. [DOI] [PubMed] [Google Scholar]
de Jong 2011
- Jong AB, Dikkeschei LD, Brand PL. Sensitization patterns to food and inhalant allergens in childhood: a comparison of non‐sensitized, monosensitized, and polysensitized children. Pediatric Allergy Immunology 2011;22(2):166‐71. [DOI] [PubMed] [Google Scholar]
Denburg 2005
- Denburg JA, Hatfield HM, Cyr MM, Hayes L, Holt PG, Sehmi R, et al. Fish oil supplementation in pregnancy modifies neonatal progenitors at birth in infants at risk of atopy. Pediatric Research 2005;57(2):276‐81. [DOI] [PubMed] [Google Scholar]
Escamilla‐Nunez 2014
- Escamilla‐Nunez MC, Barraza‐Villarreal A, Hernandez‐Cadena L, Navarro‐Olivos E, Sly PD, Romieu I. Omega‐3 fatty acid supplementation during pregnancy and respiratory symptoms in children. Chest 2014;146(2):373‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furuhjelm 2011
- Furuhjelm C, Warstedt K, Fageras M, Falth‐Magnusson K, Larsson J, Fredriksson M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega‐3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatric Allergy and Immunology 2011;22(5):505‐14. [DOI] [PubMed] [Google Scholar]
Georas 2005
- Georas SN, Guo J, Fanis U, Casolaro V. T‐helper cell type‐2 regulation in allergic disease. European Respiratory Journal 2005;26(6):1119‐37. [DOI] [PubMed] [Google Scholar]
Greisner 1998
- Greisner WA, Settipane RJ, Settipane GA. Natural history of hay fever: a 23‐year follow‐up of college students. Allergy and Asthma Proceedings 1998;19(5):271‐5. [DOI] [PubMed] [Google Scholar]
Gunaratne 2014
- Gunaratne AW. Effects of n‐3 LCPUFA Supplementation for Pregnant and Lactating Women in Preventing Allergic Disease in Early Childhood [PhD thesis (submitted)]. University of Adelaide, 2014 July. [Google Scholar]
Gupta 2004
- Gupta R, Sheikh A, Strachan DP, Anderson HR. Burden of allergic disease in the UK: secondary analyses of national databases. Clinical and Experimental Allergy 2004;34(4):520‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodge 1996
- Hodge L, Salome CM, Peat JK, Haby MM, Xuan W, Woolcock AJ. Consumption of oily fish and childhood asthma risk. Medical Journal of Australia 1996;164(3):137‐40. [DOI] [PubMed] [Google Scholar]
Imhoff‐Kunsch 2011
- Imhoff‐Kunsch B, Stein AD, Martorell R, Parra‐Cabrera S, Romieu I, Ramakrishnan U. Prenatal docosahexaenoic acid supplementation and infant morbidity: randomized controlled trial. Pediatrics 2011;128(3):e505‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johansson 2001
- Johansson SGO, O’B Hourihane J, Bousquet J, Bruijnzeel‐Koomen C, Dreborg S, Haahtela T, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy 2001;56:813‐24. [DOI] [PubMed] [Google Scholar]
Johansson 2004
- Johansson SGO, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Journal of Allergy and Clinical Immunology 2004;113(5):832‐36. [DOI] [PubMed] [Google Scholar]
Kemp 1999
- Kemp AS. Atopic eczema: its social and financial costs. Journal of Paediatrics and Child Health 1999;35(3):229‐31. [DOI] [PubMed] [Google Scholar]
Klemens 2011
- Klemens CM, Berman DR, Mozurkewich EL. The effect of perinatal omega‐3 fatty acid supplementation on inflammatory markers and allergic diseases: a systematic review. BJOG: an international journal of obstetrics and gynaecology 2011;118(8):916‐25. [DOI] [PubMed] [Google Scholar]
Krauss‐Etschmann 2008
- Krauss‐Etschmann S, Hartl D, Rzehak P, Heinrich J, Shadid R, Ramírez‐Tortosa MDC, et al. Decreased cord blood IL‐4, IL‐13, and CCR4 and increased TGF‐beta levels after fish oil supplementation of pregnant women. Journal of Allergy and Clinical Immunology 2008;121(2):464‐70. [DOI] [PubMed] [Google Scholar]
Kremmyda 2011
Lauritzen 2004
- Lauritzen L, Jorgensen MH, Mikkelsen TB, Skovgaard M, Straarup EM, Olsen SF, et al. Maternal fish oil supplementation in lactation: effect on visual acuity and n‐3 fatty acid content of infant erythrocytes. Lipids 2004;34(3):195‐206. [DOI] [PubMed] [Google Scholar]
Lee 2013
- Lee HS, Barraza‐Villarreal A, Hernandez‐Vargas H, Sly PD, Biessy C, Ramakrishnan U, et al. Modulation of DNA methylation states and infant immune system by dietary supplementation with ω‐3 PUFA during pregnancy in an intervention study. American Journal of Clinical Nutrition 2013;98:480‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Makrides 2006
- Makrides M, Duley L, Olsen SF. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre‐eclampsia or intrauterine growth restriction. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD003402.pub2] [DOI] [PubMed] [Google Scholar]
Manley 2011
- Manley BJ, Makrides M, Collins CT, McPhee AJ, Gibson RA, Ryan P, et al. DINO Steering Committee. High‐dose docosahexaenoic acid supplementation of preterm infants: respiratory and allergy outcomes. Pediatrics 2011;128(1):e71‐7. [DOI] [PubMed] [Google Scholar]
Meyer 2003
- Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, Howe PR. Dietary intakes and food sources of omega‐6 and omega‐3 polyunsaturated fatty acids. Lipids 2003;38(4):391‐8. [DOI] [PubMed] [Google Scholar]
Mihrshahi 2004
- Mihrshahi S, Peat JK, Webb K, Oddy W, Marks GB, Mellis CM. Effect of omega‐3 fatty acid concentrations in plasma on symptoms of asthma at 18 months of age. Pediatric Allergy and Immunology 2004;15(6):517‐22. [DOI] [PubMed] [Google Scholar]
Miles 2011
- Miles EA, Noakes PS, Kremmyda LS, Vlachava M, Diaper ND, Rosenlund G, et al. The Salmon in Pregnancy Study: study design, subject characteristics, maternal fish and marine n‐3 fatty acid intake, and marine n‐3 fatty acid status in maternal and umbilical cord blood. American Journal of Clinical Nutrition 2011;94(6 Suppl):1986S‐92S. [DOI] [PubMed] [Google Scholar]
Oddy 2004
- Oddy WH, Klerk NH, Kendall GE, Mihrshahi S, Peat JK. Ratio of omega‐6 to omega‐3 fatty acids and childhood asthma. Journal of Asthma 2004;41(3):319‐26. [DOI] [PubMed] [Google Scholar]
Oken 2003
- Oken E, Kleinman KP, Berland WE, Simon SR, Rich‐Edwards JW, Gillman MW, et al. Decline in fish consumption among pregnant women after a national mercury advisory. Obstetrics and Gynecology 2003;102(2):346‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olsen 2008
- Olsen SF, Osterdal ML, Salvig JD, Mortensen LM, Rytter D, Secher NJ, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry‐based follow‐up from a randomized controlled trial. American Journal of Clinical Nutrition 2008;88(1):167‐75. [DOI] [PubMed] [Google Scholar]
Oranje 1995
- Oranje AP. Development of childhood eczema and its classification. Pediatric Allergy and Immunology 1995;6 Suppl 7:31‐5. [DOI] [PubMed] [Google Scholar]
Palmer 2012
- Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, et al. Effect of n‐3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants' allergies in first year of life: randomised controlled trial. BMJ 2012;344(e184):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 2013
- Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy 2013;68(11):1370‐6. [DOI] [PubMed] [Google Scholar]
Pawanker 2011
- Pawanker RCG, Holgate ST, Lockey RF, editors. WAO White Book On Allergy. World Allergy Organization (WAO), 2011. [Google Scholar]
Prescott 2004
- Prescott SL, Calder PC. N‐3 polyunsaturated fatty acids and allergic disease. Current Opinion in Clinical Nutrition and Metabolic Care 2004;7:123‐9. [DOI] [PubMed] [Google Scholar]
Prescott 2007
- Prescott SL, Dunstan JA. Prenatal fatty acid status and immune development: the pathways and the evidence. Lipids 2007;42(9):801‐10. [DOI] [PubMed] [Google Scholar]
Prescott 2007a
- Prescott SL, Barden AE, Mori TA, Dunstan JA. Maternal fish oil supplementation in pregnancy modifies neonatal leukotriene production by cord‐blood‐derived neutrophils. Clinical Science (Lond) 2007;113(10):409‐16. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romero 2013
- Romero VC, Somers EC, Stolberg V, Clinton C, Chensue S, Djuric Z, et al. Developmental programming for allergy: a secondary analysis of the Mothers, Omega‐3, and Mental Health Study. American Journal of Obstetrics and Gynecology 2013;208(4):316.e.1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romieu 2007
- Romieu I, Torrent M, Garcia‐Esteban R, Ferrer C, Ribas‐Fitó N, Antó JM, et al. Maternal fish intake during pregnancy and atopy and asthma in infancy. Clinical and Experimental Allergy 2007;37(4):518‐25. [DOI] [PubMed] [Google Scholar]
Saarinen 1995
- Saarinen UM, Kajosaari M. Breastfeeding as prophylaxis against atopic disease: prospective follow‐up study until 17 years old. Lancet 1995;346(8982):1065‐9. [DOI] [PubMed] [Google Scholar]
Sausenthaler 2007
- Sausenthaler S, Koletzko S, Schaaf B, Lehmann I, Borte M, Herbarth O, et al. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. American Journal of Clinical Nutrition 2007;85(2):530‐7. [DOI] [PubMed] [Google Scholar]
Sears 1996
- Sears MR, Holdaway MD, Flannery EM, Herbison GP, Silva PA. Parental and neonatal risk factors for atopy, airway hyper‐responsiveness, and asthma. Archives of Disease in Childhood 1996;75(5):392‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sicherer 2012
- Sicherer SH, Wood RA. Allergy testing in childhood: using allergen‐specific IgE tests. Pediatrics 2012;129(1):193‐7. [DOI] [PubMed] [Google Scholar]
Simmonds 2007
- Simmonds L. Behavioural and Allergic Disease Assessment at Pre‐School Age: A Follow‐up Study of Preterm Infants Fed Docosahexaenoic Acid Supplementation in the Neonatal Period [dissertation]. University of Adelaide, 2007 Nov. [Google Scholar]
Simopoulos 1991
- Simopoulos AP. Omega‐3 fatty acids in health and disease and in growth and development. American Journal of Clinical Nutrition 1991;54:438‐63. [DOI] [PubMed] [Google Scholar]
Snijdewint 1993
- Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. Journal of Immunology 1993;150(12):5321‐9. [PubMed] [Google Scholar]
Spergel 2003
- Spergel JM, Paller AS. Atopic dermatitis and the atopic march. Journal of Allergy and Clinical Immunology 2003;112(6 Suppl):S118‐27. [DOI] [PubMed] [Google Scholar]
Spergel 2010
- Spergel JM. From atopic dermatitis to asthma: the atopic march. Annals of Allergy Asthma and Immunology 2010;105(2):99‐106. [DOI] [PubMed] [Google Scholar]
Strong 2005
- Strong K, Mathers C, Leeder S, Beaglehole R. Preventing chronic diseases: how many lives can we save?. Lancet 2005;366(9496):1578‐82. [DOI] [PubMed] [Google Scholar]
Wilczynski 2005
- Wilczynski JR. Th1/Th2 cytokines balance‐yin and yang of reproductive immunology. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;122(2):136‐43. [DOI] [PubMed] [Google Scholar]
Willers 2007
- Willers SM, Devereux G, Craig LC, McNeill G, Wijga AH, Abou El‐Magd W, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5‐year‐old children. Thorax 2007;62(9):773‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Gunaratne 2012
- Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n‐3 fish oil supplementation for preventing allergies in early childhood. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010085] [DOI] [PMC free article] [PubMed] [Google Scholar]


