Abstract
Background
It has been reported that neural tube defects (NTD) can be prevented with periconceptional folic acid supplementation. The effects of different doses, forms and schemes of folate supplementation for the prevention of other birth defects and maternal and infant outcomes are unclear.
Objectives
This review aims to examine whether periconceptional folate supplementation reduces the risk of neural tube and other congenital anomalies (including cleft palate) without causing adverse outcomes in mothers or babies. This is an update of a previously published Cochrane review on this topic.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 August 2015). Additionally, we searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (31 August 2015) and contacted relevant organisations to identify ongoing and unpublished studies.
Selection criteria
We included all randomised or quasi‐randomised trials evaluating the effect of periconceptional folate supplementation alone, or in combination with other vitamins and minerals, in women independent of age and parity.
Data collection and analysis
Two review authors independently assessed the eligibility of studies against the inclusion criteria, extracted data from included studies, checked data entry for accuracy and assessed the risk of bias of the included studies. We assessed the quality of the body of evidence using the GRADE approach.
Main results
Five trials involving 7391 women (2033 with a history of a pregnancy affected by a NTD and 5358 with no history of NTDs) were included. Four comparisons were made: 1) supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials); 2) supplementation with folic acid alone versus no treatment or placebo (one trial); 3) supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials); and 4) supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials). The risk of bias of the trials was variable. Only one trial was considered to be at low risk of bias. The remaining studies lacked clarity regarding the randomisation method or whether the allocation to the intervention was concealed. All the participants were blinded to the intervention, though blinding was unclear for outcome assessors in the five trials.
The results of the first comparison involving 6708 births with information on NTDs and other infant outcomes, show a protective effect of daily folic acid supplementation (alone or in combination with other vitamins and minerals) in preventing NTDs compared with no interventions/placebo or vitamins and minerals without folic acid (risk ratio (RR) 0.31, 95% confidence interval (CI) 0.17 to 0.58); five studies; 6708 births; high quality evidence). Only one study assessed the incidence of NTDs and showed no evidence of an effect (RR 0.07, 95% CI 0.00 to 1.32; 4862 births) although no events were found in the group that received folic acid. Folic acid had a significant protective effect for reoccurrence (RR 0.34, 95% CI 0.18 to 0.64); four studies; 1846 births). Subgroup analyses suggest that the positive effect of folic acid on NTD incidence and recurrence is not affected by the explored daily folic acid dosage (400 µg (0.4 mg) or higher) or whether folic acid is given alone or with other vitamins and minerals. These results are consistent across all four review comparisons.
There is no evidence of any preventive or negative effects on cleft palate (RR 0.73, 95% CI 0.05 to 10.89; three studies; 5612 births; low quality evidence), cleft lip ((RR 0.79, 95% CI 0.14 to 4.36; three studies; 5612 births; low quality evidence), congenital cardiovascular defects (RR 0.57, 95% CI 0.24 to 1.33; three studies; 5612 births; low quality evidence), miscarriages (RR 1.10, 95% CI 0.94 to 1.28; five studies; 7391 pregnancies; moderate quality evidence) or any other birth defects (RR 0.94, 95% CI 0.53 to 1.66; three studies; 5612 births; low quality evidence). There were no included trials assessing the effects of this intervention on neonatal death, maternal blood folate or anaemia at term.
Authors' conclusions
Folic acid, alone or in combination with vitamins and minerals, prevents NTDs, but does not have a clear effect on other birth defects.
Keywords: Female, Humans, Infant, Pregnancy, Dietary Supplements, Folic Acid, Folic Acid/administration & dosage, Neural Tube Defects, Neural Tube Defects/prevention & control, Preconception Care, Randomized Controlled Trials as Topic, Vitamin B Complex, Vitamin B Complex/administration & dosage
Plain language summary
Folic acid supplements before conception and in early pregnancy (up to 12 weeks) for the prevention of birth defects
Folic acid is a synthetic form of folate used in supplements and fortified staple foods (like wheat and maize flour) to reduce the occurrence of neural tube defects (NTDs). These include spina bifida (or cleft spine), where there is an opening in one or more of the bones (vertebrae) of the spinal column, and anencephaly where the head (cephalic) end of the neural tube fails to close. Supplementation with folic acid is internationally recommended to women from the moment they are trying to conceive until 12 weeks of pregnancy. Another option recommended by the World Health Organization (WHO) is that women of reproductive age take intermittent (weekly) iron and folic acid supplements, especially in populations where the prevalence of anaemia is above 20%. Supplementation may also reduce other birth defects such as cleft lip, with or without cleft palate, and congenital cardiovascular defects. Recently, 5‐methyl‐tetrahydrofolate (5‐MTHF) has been proposed as an alternative to folic acid supplementation. This is because most dietary folate and folic acid are metabolised to 5‐MTHF. Some women have gene characteristics which reduce folate concentration in blood.
This review confirms that folic acid supplementation prevents the first and second time occurrence of NTDs and shows there is not enough evidence to determine if folic acid prevents other birth defects. Information about the safety of other current and alternative supplementation schemes and any possible effects on other outcomes for mothers and babies is also lacking. This review of five trials, involving 7391 pregnancies (2033 with a history of a pregnancy affected by a NTD and 5358 with no history of NTDs), shows the protective effect of daily folic acid supplementation in doses ranging from 0.36 mg (360 µg) to 4 mg (4000 µg) a day, with and without other vitamins and minerals, before conception and up to 12 weeks of pregnancy, for preventing the recurrence of these defects. There were insufficient data to evaluate the effects on other outcomes such as cleft lip and palate, miscarriages or any other birth defects. More research is needed on different types of supplementation programmes and the use of different types of supplements (such as 5‐methyl‐tetrahydrofolate ‐5‐MTHF), particularly in countries where folic acid fortification of staple foods like wheat or maize flour is not mandatory and where the prevalence of NTDs is still high. The overall quality of the evidence for neonatal outcomes was high for NTDs, whereas, it was of low quality for other neonatal outcomes. The overall quality of the evidence for maternal outcomes was rated as moderate.
Summary of findings
Summary of findings for the main comparison. Supplementation with folate versus no intervention, placebo or other micronutrients without folate (neonatal outcomes).
| Population: all women who become pregnant or were 12 or less weeks' pregnant at the time of the intervention Setting: the studies took place in nine high‐income countries and one low‐middle‐income country Intervention: supplementation with folate Comparison: no intervention, placebo or other micronutrients without folate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no intervention, placebo or other micronutrients without folate | Risk with supplementation with folate | |||||
| Neural tube defects (All) | Study population | RR 0.31 (0.17 to 0.58) | 6708 (5 RCTs) | ⊕⊕⊕⊝ high1,2 | ||
| 13 per 1000 | 4 per 1000 (2 to 7) | |||||
| Moderate | ||||||
| 35 per 1000 | 11 per 1000 (6 to 20) | |||||
| Cleft lip (All) | Study population | RR 0.79 (0.14 to 4.36) | 5612 (3 RCTs) | ⊕⊕⊝⊝ low1,3 | ||
| 1 per 1000 | 1 per 1000 (0 to 6) | |||||
| Moderate | ||||||
| 2 per 1000 | 2 per 1000 (0 to 9) | |||||
| Cleft palate (All) | Study population | RR 0.73 (0.05 to 10.89) | 5612 (3 RCTs) | ⊕⊕⊝⊝ low1,3 | ||
| 1 per 1000 | 1 per 1000 (0 to 8) | |||||
| 0 per 1000 | 0 per 1000 (0 to 0) |
|||||
| Congenital cardiovascular anomalies (All) | Study population | RR 0.57 (0.24 to 1.33) | 5612 (3 RCTs) | ⊕⊕⊝⊝ low1,3 | ||
| 5 per 1000 | 3 per 1000 (1 to 7) | |||||
| Moderate | ||||||
| 5 per 1000 | 3 per 1000 (2 to 10) | |||||
| Other congenital anomalies (All) | Study population | RR 0.94 (0.53 to 1.66) | 5612 (3 RCTs) | ⊕⊕⊝⊝ low4,5 | ||
| 16 per 1000 | 15 per 1000 (8 to 26) | |||||
| Moderate | ||||||
| 17 per 1000 | 16 per 1000 (8 to 26) | |||||
| Neonatal death | not estimable | not estimable | not pooled | 0 (0 study) | No trial assessed this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Most studies contributing data had design limitations (selection bias was unclear).
2 Authors refrained from downgrading the quality of the evidence due to the large magnitude of effect.
2 Wide confidence interval crossing the line of no effect & few events.
3 Wide confidence interval crossing the line of no effect.
4 Statistical Heterogeneity (I² > 60%). Variation in direction.
Summary of findings 2. Supplementation with folate versus no intervention, placebo or other micronutrients without folate (maternal outcomes).
| Population: all women who become pregnant or were 12 or less weeks' pregnant at the time of the intervention Setting: the studies took place in nine high‐income countries and one low‐middle‐income country Intervention: supplementation with folate Comparison: no intervention, placebo or other micronutrients without folate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no intervention, placebo or other micronutrients without folate | Risk with supplementation with folate | |||||
| Maternal anaemia at or near term (All) | Study population | not pooled | 0 (0 study) | No trial assessed this outcome. | ||
| not pooled | not pooled | |||||
| Maternal red blood cell folate at or near term (All) | not estimable | The mean maternal red blood cell folate at or near term (All) in the intervention group was 0 undefined more (0 more to 0 more) | not pooled | 0 (0 study) | No trial assessed this outcome. | |
| Serum folate at or near term | not estimable | not estimable | not pooled | 0 (0 study) | No trial assessed this outcome. | |
| Miscarriage (All) | Study population | RR 1.10 (0.94 to 1.28) | 7391 (5 RCTs) | ⊕⊕⊕⊝ moderate1 | ||
| 99 per 1000 | 109 per 1000 (93 to 127) | |||||
| Moderate | ||||||
| 96 per 1000 | 106 per 1000 (90 to 123) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Most studies contributing data had design limitations (selection bias was unclear).
Background
The main focus of this review is on the provision of oral supplements of folate (with or without other vitamins and minerals) before pregnancy and in early pregnancy (before 12 weeks’ gestation) to reduce the occurrence and recurrence of congenital anomalies, particularly neural tube defects (NTDs): spina bifida, anencephaly, encephalocoele, hydranencephaly, iniencephaly, schizencephaly, and other birth defects. We limited the review to the use of supplements in the form of capsules (hard and soft), modified release capsules, tablets (coated and uncoated) and suspensions, therefore excluding other ways of provision of folate. Other Cochrane reviews and protocols focus on related topics such as oral iron with or without folic acid during pregnancy (Peña‐Rosas 2015a); intermittent iron and folic acid during pregnancy (Peña‐Rosas 2015b); treatment for iron deficiency and anaemia (Reveiz 2011); the use of various vitamin and multivitamin/micronutrient supplements for women during pregnancy (Haider 2015), the effectiveness of oral folate supplementation alone during pregnancy on haematological and biochemical parameters during pregnancy and on pregnancy outcomes (Lassi 2013), the effects of intermittent iron and folic acid supplementation in menstruating women (Fernandez‐Gaxiola 2011), or the effects or harms of folate supplementation on folate status and health outcomes (physical, psychological, and neurocognitive) among women of reproductive age outside the periconceptional period (Tsang 2015). This is an update of a previously published systematic review on this topic (De‐Regil 2010).
Introduction
Folate is a water‐soluble B vitamin present in legumes, leafy green vegetables (such as spinach and turnip greens) and some fruits (such as citrus fruits and juices). Folic acid is the synthetic and most stable form of folate and the form often used in supplements and in fortified foods (Bailey 2015). The bioavailability of folic acid is approximately 70% higher than that of folate naturally contained in foods, although there are wide variations depending on the methodology used in the measurement (McNulty 2004). Serum and red blood cell folate can increase in response to folic acid supplements in a dose‐response manner up to an intake of 400 μg (0.4 mg) daily (Duffy 2014). It is estimated that non pregnant non lactating women would require an intake of natural food folate intake of at least 450 μg dietary folate equivalents daily to prevent NTDs (Marchetta 2015).
Folate status in populations is generally assessed using static biochemical tests that directly measure folate in serum or in red blood cells (Pfeiffer 2013). The cut‐off currently suggested to define deficiency is < 6.8 nmol/L for serum folate, an indicator sensitive to recent usual intake; and < 100 nmol/L for red blood cell folate, an indicator of folate storage (WHO 2015a). There are no universally accepted cut‐off points to define deficiency during pregnancy, as concentrations decline over gestation and recover at delivery (WHO 2015a), probably due to a physiologic haemodilution. However, the optimal folate level associated with lowest risk of NTDs is 906 nmol/L or above (WHO 2014), a concentration that can be reached within four weeks at supplemental daily doses (Brämswig 2009). Thus, women with red blood cell concentrations below this threshold would be considered folate insufficient or to have sub‐optimal folate status. Folate concentrations measurements differ depending on the method used for assessment including radioimmunoassay and microbiological assays, particularly at the lower range of concentrations (CDC 2008a; Life Sciences Research Office 1994). The microbiological method remains as the recommended method to quantify folate, although there are many newly developed radioimmunoassays (WHO 2015a).
Description of the condition
An estimated 276,000 babies die within four weeks of birth every year worldwide from congenital anomalies (about 7% of all neonatal deaths), and congenital anomalies can also be important causes of chronic illness and disability in many countries (WHO 2015b). Congenital anomalies are structural or functional anomalies (e.g. metabolic disorders) that occur during intrauterine life and can be identified prenatally, at birth or later in life (WHO 2015b). Major anomalies involve structural changes that have significant medical, social or cosmetic consequences for the affected individual, and commonly require medical intervention (WHO 2014). NTDs are major congenital anomalies that arise during the structural development of the neural tube, a process that is completed within 28 days after conception. NTDs are among the most common of the congenital anomalies (WHO/CDC/ICBDSR 2014).
In 1991, one randomised controlled trial demonstrated that periconceptional folic acid supplementation prevented the recurrence of NTDs (MRC 1991) and in 1992, another showed that a multiple micronutrient supplement containing folic acid prevented the occurrence of NTDs (Czeizel 1992). The latter results were confirmed in a public health campaign among women preparing for marriage conducted between 1993 and 1995 in China, after which the risk of NTDs among the fetuses or infants of the women who took a folic acid supplement more than 80% of the time decreased by between 40% and 85% (Berry 1999). It is estimated that in low‐ and middle‐income countries 2.55/1000 live births, still births and terminations are affected by a NTD, with 1.04 affected by spina bifida, 1.03 by anencephaly and 0.21 by encephalocoele (Lo 2014).
Insufficient periconceptional folate and folic acid intake is associated with a number of congenital anomalies that may also relate to genetic and environmental factors (IOM 2003) (i.e. gene defects, chromosomal disorders, multifactorial inheritance, environmental teratogens, maternal infectious diseases or illnesses, exposure to medicines/chemicals and recreational drugs, high dose of radiation) operating before conception or during pregnancy (WHO 2015b). Congenital anomalies can cause lifelong problems affecting health, growth, and learning and may be immediately apparent after birth, or manifest later in life (Murray 1997; WHO 1999; WHO 2000). Environmental factors, including folate status, are thought to contribute to about 5% to 10% of total congenital anomalies (IOM 2003). in Europe, the regulatory agencies have assessed the evidence and approved claims indicating that Increasing maternal folate status by supplemental folate intake is a beneficial physiological effect in the context of reducing the risk of NTDs (European Food Safety Authority NDA Panel 2013).
While maternal intake of folate and folic acid is specifically associated with a decreased risk for NTDs, they may also provide protection for other selected congenital anomalies. There is suggestive evidence of protection from cardiovascular defects, Down syndrome, limb defects, cleft lip with or without cleft palate, urinary tract anomalies and congenital hydrocephalus (Coppede 2009; Eskes 2006; Goh 2006; Wilcox 2007). In the case of orofacial clefts, there are several similarities with NTDs: their occurrence at a similar time during embryogenesis, their involvement with the midline of the embryo, their near identical population characteristics and similar gene contributions. There is also some evidence of a suggested protective effect of folic acid use, especially for cleft lip with or without cleft palate (Wehby 2010), although this remains controversial, possibly because of the differences in dosage and type of supplementation (e.g. folic acid alone or with other micronutrients) used among studies (Botto 2004; Botto 2006). No effects have been shown in preventing pyloric stenosis, undescended testis or hypospadias. Approximately half of the congenital anomalies are limited to a single organ, and the other half frequently present additional congenital anomalies, such as heart malformations (Shibuya 1998).
Suspicion of a NTD may be raised by a maternal serum screening test during the second trimester of pregnancy which detects an elevated concentration of alpha fetoprotein. The diagnosis is confirmed by ultrasound examination during the second trimester of pregnancy. Cleft lip and palate can be identified on detailed ultrasound examination, but if there is only involvement of the palate, diagnosis by ultrasound can be difficult, and often not established until after birth. Unfortunately, these tests are not yet routinely done in most developing countries. Affected infants have difficulty with feeding and later with speech development, hearing and tooth formation. Stigmatisation and discrimination may pose lifelong problems. Malnutrition and infection resulting from cleft lip or cleft palate, or both, can lead to severe illness and, in some cases, death (Shibuya 1998). Recent evidence also shows that low maternal folate status during early pregnancy is associated with a higher risk of emotional and behavioural problems (Roza 2010; Steenweg‐de Graaff 2012), and possibly hyperactivity/inattention problems during childhood (Schlotz 2010).
The impact of folate insufficiency on congenital anomalies in different populations varies with each healthcare system. It partly relates to the use and coverage of preventive strategies including education and awareness of the importance of folic acid intake among women of reproductive age, access to and/or distribution of pre‐pregnancy folic acid supplements, and/or fortification of staple foods with folic acid, in some cases with mandatory regulations for fortification of staple foods such as wheat (CDC 2008b) and maize flours (Peña‐Rosas 2014). Recent evidence demonstrates that public health policies which include folic acid fortification of staple foods are likely to result in a large‐scale prevention of NTDs (Botto 2005; Berry 2010; De Wals 2007). It would seem reasonable to implement both interventions fully, especially in countries with a high prevalence of birth defects.
Several gene polymorphisms affect folate metabolism and are associated with reduced folate absorption and therefore increased folate needs. Some of the most studied mutations are the methylene‐tetrahydrofolate reductase (MTHFR) gene and the reduced folate carrier (RFC1) gene (Chango 2000). The former affects 8% to 35% of the population, depending on ethnicity (Botto 2000; Guéant‐Rodriguez 2006). In the absence of a folate‐sufficient diet, these mutations are associated with increased risk of NTDs and conotruncal defects in the offspring (Van Beynum 2006; Van der Put 1998).
Folic acid intake may also affect fetal and child growth. Observational and controlled trials have showed a positive effect of periconceptional folic acid supplementation on fetal growth (Iyengar 1975; Relton 2005; Rolschau 1999), although the evidence for this association remains controversial.
Description of the intervention
After the first consistent evidence about the protective effect of folic acid supplementation against NTDs emerged, the United States Public Health Service recommended daily supplementation with 400 μg (0.4 mg) of folic acid for all women who could get pregnant (Berry 1999). Supplementation with 400 μg (0.4 mg) of folic acid is internationally recommended to women from the moment they are trying to conceive until 12 weeks of pregnancy (WHO 2006). Despite policies in several countries on the periconceptional folic acid supplementation (Gomes 2015), many women still do not follow the recommendations, particularly women from low socioeconomic status (Branum 2013; Friberg 2015).
For women with a previous history of delivery of a baby with a NTD, have diabetes, or are receiving an anticonvulsant treatment, the recommended daily dose is 5000 μg (5 mg) of folic acid in addition to dietary advice to increase food folate intake (IOM 2003; WHO 2006). Daily supplementation with 400 μg (0.4 mg) of folic acid in addition to iron is routinely recommended for all pregnant women in areas of high prevalence of malnutrition to prevent anaemia (WHO 2006). The World Health Organization (WHO) also recommends intermittent iron and folic acid supplementation as a public health intervention in menstruating women living in settings where anaemia is highly prevalent, to improve their haemoglobin (Hb) concentrations and iron status and reduce the risk of anaemia (WHO 2011) although it is recognised that there was limited evidence for the effective dose of folic acid in intermittent supplementation, thus basing the recommendation for the folic acid dosage in the rationale of providing seven times the recommended supplemental dose to prevent NTDs (400 µg or 0.4 mg daily). Further limited experimental evidence suggests this dose can improve red cell folate concentrations to levels associated with a reduced risk of NTDs.
Recently, the use of 5‐methyl‐tetrahydrofolate (5‐MTHF) has been proposed as an alternative to folic acid supplementation. The rationale for this is that most dietary folate and folic acid are metabolised to 5‐MTHF during its passage across the intestinal mucosa. The 5‐MTHF may be an adequate alternative for supplementation in the presence of MTHFR gene mutation. Four controlled trials using different doses have shown that supplementation with 5‐MTHF is at least as effective as folic acid in improving folate status in women of childbearing age (Houghton 2006; Lamers 2004; Venn 2002; Venn 2003). This form of folate may also be less likely to mask haematological symptoms of severe vitamin B12 deficiency and it exhibits a lower interaction potential with antifolate antimalarial drugs (Nduati 2008; Pietrzik 2010), specifically sulphadoxine‐pyrimethamine (SP), which might be affected by folic acid supplementation (Carter 2005; English 2006; Van Eijk 2008).
How the intervention might work
The main function of folate is as coenzyme in one‐carbon transfer during the methylation cycle, a process essential for the syntheses of nucleic acids, which form part of DNA and the neurotransmitters. From these reactions it is immediately apparent why folate is so important to gene expression. Folate also plays an important role in protein synthesis and metabolism and other processes related to cell multiplication and tissue growth (WHO 2015a). The main consequence of folate deficiency in adults is megaloblastic anaemia, characterised by abnormally large red‐cell precursors in the bone marrow and larger than normal red cells in the peripheral blood.
The methylation of homocysteine to produce methionine (both essential amino acids) uses 5‐MTHF as the methyl donor in the reaction. In folate deficiency, homocysteine accumulates in the serum resulting in negative effects for health. Elevated circulating homocysteine concentrations have been associated with an increased risk in cardiovascular disease (Refsum 2008) and late pregnancy complications such as pre‐eclampsia (Makedos 2007; Patrick 2004; Tamura 2006), and possibly NTDs. Therefore, elevated plasma homocysteine may be a risk factor or, alternatively, merely a marker of risk that needs to be determined (WHO 2015a).
Additional potentially undesired effects of pre‐pregnancy folic acid supplementation come from the possible association of the use of multivitamins containing folic acid and an increase in twin pregnancies found in the Swedish study (Ericson 2001), although these findings were confounded by in vitro fertilisation (Berry 2005; Vollset 2005). Further studies have confirmed that there is no impact of supplementation with 400 µg (0.4 mg) of folic acid and fortification of staple foods with folic acid (Kucik 2004; Shaw 2003; Signore 2005), on twinning.
From the safety perspective, an observational study on the fetal origins of disease proposed that normal to high maternal folate status coupled with low vitamin B12 status was associated with higher adiposity and insulin resistance in Indian babies (Yajnik 2008), which could probably have a long‐term effect in the fetus later in life. Additional potentially undesired effects of folic acid supplementation come from the ambiguous findings on the effects of folic acid supplementation on colonic lesions (Fife 2009; Jaszewski 2008; Wu 2009). However, a recent meta‐analysis with individual‐participant data from 50,000 participants receiving folic acid supplementation for 5.2 years on average concluded that folic acid supplementation does not substantially increase or decrease the incidence of site‐specific cancer during the first five years of treatment.
Why it is important to do this review
There seems to be sufficient evidence of known benefits of folic acid supplementation on NTDs but not on other congenital anomalies or on benefits to the mother. Furthermore, the best schemes (intermittent or daily), dose and/or form (5‐MTHF, folic acid or other) for providing oral folate supplements to women during the periconceptional period are not yet established. Additionally, concerns raised by some authors about the potential harm associated with high levels of folic acid, merit exploration (Cole 2007; Mason 2007).
In countries where the prevalence of NTDs is high, the cost‐effectiveness of an integrated approach with supplementation and fortification may be more favourable. However, it is important to consider that countries with mandatory wheat flour fortification have achieved a significant increase in folate intake and a significant decline in the prevalence of NTDs, but the relative decline depends on the initial NTD rate: countries with lower NTD prevalence may have much smaller reductions in NTD rates with folic acid fortification (Heseker 2009).
Objectives
This review aims to examine whether periconceptional folate supplementation reduces the risk of neural tube and other congenital anomalies (including cleft palate) without causing adverse outcomes in mothers or babies.
Methods
Criteria for considering studies for this review
Types of studies
We have included randomised trials only. We had planned to include quasi‐randomised and cluster‐randomised trials if they were otherwise eligible but none were identified. Other levels of evidence (e.g. cohort or case‐control studies) have not been included in meta‐analyses nor have they contributed to the results or conclusions, but we have considered such evidence in the discussion where relevant.
Types of participants
All women who become pregnant or were 12 or less weeks' pregnant at the time of the intervention, independent of their age and parity or history of neural tube defect‐affected pregnancy. We have not included trials in which supplementation was continued beyond the first 12 weeks of gestation (even when supplementation was initiated during the periconceptional period or in the first 12 weeks of pregnancy). We did not include studies targeting women during their second or third semesters of pregnancy.
Types of interventions
Oral supplements of folate alone (FA) and with other vitamins and minerals given on a daily or intermittent (one, two or three times a week on non‐consecutive days) basis and compared with receiving a placebo, no supplementation or other vitamins and minerals but no folate.
For the purpose of this review, oral folate supplementation refers to the delivery of folate compounds (e.g. folic acid, 5MTHF or folinic acid) directly to the oral cavity, either as a tablet, capsule, dispersible tablet or liquid.
Where data were available we planned to compare:
supplementation with any folate versus no treatment/placebo/other micronutrients without folate;
supplementation with folate alone versus no intervention/placebo;
supplementation with folate plus other micronutrients versus other micronutrients (no folate).
We excluded multiple micronutrient powders for point‐of‐use fortification of foods, fortification of staple foods, water, condiments or seasonings with folic acid and other micronutrients or the provision of oral contraceptives that contain folic acid in this review.
Types of outcome measures
Primary outcomes
Infant
Neural tube defects (any)
Cleft lip
Cleft palate
Congenital cardiovascular anomalies
Other congenital anomalies (excluding neural tube defects, cleft lip, cleft palate and cardiovascular defects)
Neonatal deaths (death occurring within the first 28 days after birth)
Maternal
Anaemia at or near term (defined as haemoglobin (Hb) less than 110 g/L at 34 weeks of gestation or more)
Red blood cell folate at or near term (nmol/L at 34 weeks of gestation or more)
Serum folate at or near term (nmol/L at 34 weeks of gestation or more))
Miscarriage (as defined by trial authors)
Secondary outcomes
Infant
Spina bifida
Anencephaly
Encephaloceles
Hydranencephaly
Iniencephaly
Schizencephaly
Stillbirths (as defined by trial authors)
Low birthweight (< 2500 g)
Very low birthweight (< 1500 g)
Macrosomia (birthweight greater than 4000 g)
Preterm birth (less than 37 weeks' gestation
Infant optimal health status at birth (as defined by trial authors)
Admission to special care for any cause (as defined by trial authors)
Infant insulin resistance (as defined by trial authors)
Apgar at one minute after birth (> eight)
Apgar score at five minutes after birth (> eight)
Maternal
Multiple pregnancy (defined as a pregnancy with two or more fetuses)
Homocysteine at or near term (µmol/L at 34 weeks' gestation or more)
Serum vitamin B6 concentration at or near term (nmol/L at 34 weeks' gestation or more)
Serum vitamin B12 concentration at or near term (pmol/L at 34 weeks' gestation or more)
Pre‐eclampsia (defined as gestational hypertension ‐ blood pressure higher than 140/90 mmHg ‐ and proteinuria ‐ more than 300 mg of protein in a 24‐hour urine sample)
Pregnancy termination for fetal anomaly (as defined by trial authors)
Any side effects (as defined by trial authors)
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 August 2015).
For full search methods used to populate the PCG Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in The Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Trials Search Co‐ordinator searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
In addition, we searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (31 August 2015).
Searching other resources
We contacted the WHO headquarters and regional offices, the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR) March of Dimes, the nutrition section of the United Nations Children's Fund (UNICEF), the World Food Programme (WFP), the US Centers for Disease Control and Prevention (CDC)'s National Center on Birth Defects and Developmental Dissabilities, US Agency for International Development (USAID) micronutrient programme, Micronutrient Initiative (MI), the Global Alliance for Improved Nutrition (GAIN), Hellen Keller International (HKI), Sight and Life Foundation to identify ongoing trials and unpublished reports (correspondence is available upon request).
We did not apply any language or date restrictions. For those articles written in other language than English and Spanish, we commissioned their translation into English to assess them for eligibility according to the prespecified selection criteria. We did not handsearch printed journals but screened previously published reviews to identify other possible studies.
We did not apply any language or date restrictions
Data collection and analysis
For methods used in the previous version of this review, see 'De‐Regil 2010'.
For this update, the following methods were used for assessing the new reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors (JPPR and LMD) independently assessed the titles and abstracts of all the studies we identified as a result of the search strategy against the inclusion criteria and obtained full‐text reports of all relevant or potentially relevant studies. We resolved any disagreement through discussion or, if required, we consulted a third author (AFG).
In this version of the review all studies were reported in journal articles. Had we identified trials published only as abstracts, or study reports containing insufficient information on methods, we would have attempted to contact the trial authors to obtain further details of study design and results; if there was insufficient information for us to be able to assess risk of bias, studies would await assessment until further information was published, or made available to us.
Data extraction and management
We designed an electronic form to extract data and record information on the study setting and participants (inclusion and exclusion criteria), details of study methods and risk of bias. The review authors (JPPR, LMD, TD, AFG and PRS) independently extracted the data from eligible studies using the agreed form. We entered data into Review Manager software (RevMan 2014) and carried out checks for accuracy. We resolved discrepancies through discussion.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (JPPR and LMD) independently assessed risk of bias for each included study using the domain‐based evaluation criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor (PRS).
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. For this type of intervention, where different regimens were compared, it would be theoretically possible to blind study participants and staff by providing both active and placebo tablets to women allocated to intermittent regimens and placebo tablets to women in no supplementation arms of trials.
Blinding was assessed separately for different outcomes or classes of outcomes and we have noted where there was partial blinding. We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We assessed losses to follow‐up and post‐randomisation exclusions systematically for each trial. We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis.
We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes.
We assessed the methods as:
low risk of bias;
high risk of bias;
unclear.
We considered follow‐up to be adequate if more than 80% of participants initially randomised in a trial were included in the analysis and any loss was balanced across groups, unclear if the percentage of initially randomised participants included in the analysis was unclear, and inadequate if less than 80% of those initially randomised were included in the analysis, or if loss was imbalanced in different treatment groups.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We assessed whether each study was free of other problems that could put it at risk of bias. We have noted for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of further bias;
high risk of further bias;
unclear whether there is a risk of further bias.
(7) Overall risk of bias
We assessed the overall risk of bias at two levels: within studies (within domains) and across studies. We have made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011) and for primary outcomes, we explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of evidence using GRADE
For the assessment across studies, we employed the GRADE approach as outlined in the GRADE Handbook and used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) to create 'Summary of findings' (SoF) tables (set out in Table 1; Table 2). The primary neonatal and maternal outcomes for the main comparison have been listed with estimates of relative effects along with the number of participants and studies contributing data for those outcomes. These tables provide outcome‐specific information concerning the overall quality of evidence from studies included in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes we considered. We only included primary outcomes in the 'Summary of findings' tables. For each individual outcome, two review authors independently assessed the quality of the evidence using the GRADE approach (Balshem 2010).
For assessments of the overall quality of evidence for each outcome that included pooled data from included trials, we downgraded the evidence from 'high quality' by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias. This assessment was limited only to the trials included in this review and, as we did not consider there was a serious risk of indirectness or publication bias, we did not downgrade in these domains.
Measures of treatment effect
Dichotomous data
We presented results as average risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
No continuous data were analysed in this update. In future updates, if appropriate, we will use the mean difference (MD) with 95% CIs if outcomes are measured in the same way between trials. We will use the standardised mean difference (SMD) to combine trials that measure the same outcome (e.g. serum folate concentration) but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials. If in future updates of this review we identify any such trials, we will adjust the standard error of the effect estimate from cluster trials using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Meta‐analyses will be carried out using the generic inverse‐variance method available in RevMan (RevMan 2014). We will use an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs, and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a separate meta‐analysis.
Cross‐over trials
Cross‐over trials are not an appropriate study design for the interventions considered in this review and have been excluded.
Studies with more than two treatment groups
For studies with more than two intervention groups (multi‐arm studies), we included the directly relevant arm only. When we identified studies with various relevant arms, we combined groups to create a single pair‐wise comparison (Higgins 2011) and included the disaggregated data in the corresponding subgroup category.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
We planned to carry out analyses, as far as possible, on an intention‐to‐treat (ITT) basis, i.e. by attempting to include all participants randomised to each group in the analyses, so the denominator for each outcome in each trial would be the number randomised minus any participants whose outcomes are known to be missing. In those studies where women were recruited before conception for outcomes relating to pregnancy, we have taken a pragmatic approach and included in the denominators only those women known to become pregnant (for maternal outcomes) and the number of informative pregnancies (for neonatal outcomes).
Assessment of heterogeneity
We examined the forest plots from meta‐analysis and the risk of bias to look for methodological heterogeneity among studies, and used the I² and Tau² statistics to quantify the level of heterogeneity among the trials in each analysis. Where we identified substantial heterogeneity (an I² greater than approximately 50%), we noted this in the text and explored it by pre‐specified subgroup analysis. We would advise caution in the interpretation of those results where there are high levels of unexplained heterogeneity.
Assessment of reporting biases
Where we suspected reporting bias (seeSelective reporting (reporting bias) above), we attempted to contact study authors, to ask them to provide missing outcome data. We have not explored possible publication bias by producing funnel plots as too few studies contributed data to the review. In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014).
Because of our experience in conducting other reviews in this area, we anticipated high heterogeneity amongst trials and we pooled trial results using a random‐effects model and were cautious in our interpretation of the pooled results. We have indicated in the text that the random‐effects model gives the average treatment effect. For statistically significant results where there are high levels of heterogeneity ((an I² greater than 50%), we have given the values of I², Tau² and the P value of the Chi² test for heterogeneity and have provided an estimate of the 95% range of underlying intervention effects (prediction interval (PI)).
Subgroup analysis and investigation of heterogeneity
Where data were available we had planned to carry out subgroup analysis for primary outcomes:
by scheme: daily supplementation; or intermittent supplementation;
by daily dose: 400 µg/d (0.4 mg/d) or less of folic acid; more than 400 µg (0.4 mg) folic acid per day;
by timing of supplementation: before pregnancy; during first trimester only; periconceptional period;
by history of a pregnancy affected by a neural tube defect (recurrence): yes; no; or unknown/unreported/mixed;
by folate compound: folic acid; 5MTHF; other compound; or unknown/unreported/mixed;
by micronutrient composition: folate alone; folate + iron; folate + other micronutrient (except iron); or folate + multiple micronutrients;
by malaria setting at the time of the study: yes; no; or unknown/unreported/mixed.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2014) and to report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We considered a study to be of high quality if it was assessed as low risk of bias in both the randomisation and allocation concealment and in either blinding or loss to follow‐up. We planned to conduct a sensitivity analysis by quality of the study but it was not possible as only one study was considered to be of high quality (Kirke 1992).
If, in future updates of the review, if cluster‐randomised trials are included, we will carry out sensitivity analysis using a range of intra cluster correlation (ICC) values.
Results
Description of studies
Results of the search
See: Figure 1
1.
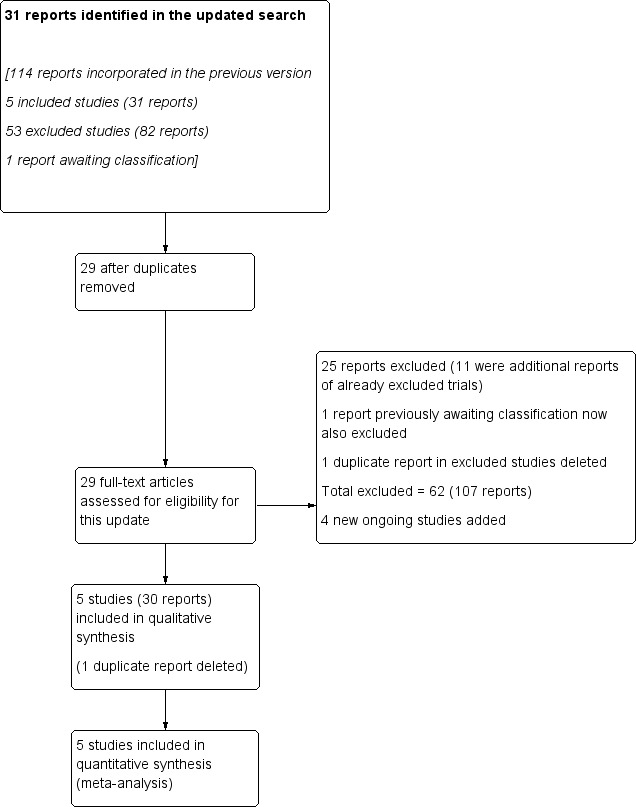
Study flow diagram.
The updated search retrieved 31 new reports to assess. Two were duplicates. Twenty‐five of these reports were excluded, 11 of which were additional reports of already excluded trials. We also identified four Ongoing studies. Additionally, we deleted two exact duplicate references: one from an included study (Czeizel 1994), and one from an excluded study (Christian 2003).
Five trials (30 references) were included and four trials are still ongoing and results are expected in 2015 (see Figure 1 and Characteristics of ongoing studies for details).
Included studies
We included five trials involving 7391 women in the review, all of which met the pre‐stated inclusion criteria. Most of the studies focused on infant outcomes and few of the results reported related to the maternal outcomes we had pre‐specified in the protocol. All of the studies that we included were published before 2001. Sample sizes varied among trials.
Settings
Four studies had study participants from high‐income countries: Wales (Laurence 1981), Australia, Canada, France, Hungary, Israel, Russia and the UK (MRC 1991), Ireland (Kirke 1992). Hungary (Czeizel 1994). One study had study participants from a lower middle‐income country: India (ICMR 2000).
None of the studies reported on malaria incidence or testing at any point during the study, though the locations of the studies make it very unlikely that malaria was a significant issue.
Participants
History of pregnancy with neural tube defects
Four trials evaluated neural tube defects (NTD) recurrence (n = 2033) (ICMR 2000; Kirke 1992; Laurence 1981; MRC 1991), and only one evaluated first‐time NTD occurrence (n = 5358) (Czeizel 1994).
Interventions
Micronutrient composition
One study used folate alone (Laurence 1981); three studies used folate + multiple micronutrients (Czeizel 1994; ICMR 2000; MRC 1991); and one study used folate alone in one group and folate + multiple micronutrients in another group (Kirke 1992).
Dose of folic acid
In one trial, women received less than 400 µg (0.4 mg) of folic acid per day (Kirke 1992), while in the remaining studies women consumed 800 µg (0.8 mg) (Czeizel 1994); 2000 µg (2.0 mg) per day (Laurence 1981) and 4000 µg (4.0 mg) per day (ICMR 2000; MRC 1991).
Folate compound
All studies used folic acid. We did not identify any randomised controlled trials which examined the use of 5‐MTHF.
Regimen
In all studies, participants were supplemented daily. In one trial, supplements were divided into two equal doses (ICMR 2000) and in another, the supplements were divided into three equal doses (Kirke 1992). We did not identify any randomised controlled trials which examined intermittent supplementation.
Timing of supplementation
All trials gave the supplements in the periconceptional period. In all trials women started supplementation before pregnancy and discontinued them after 12 weeks of pregnancy.
Comparison group
There were three studies with two arms. One trial compared folic acid supplementation with a placebo group (Laurence 1981). Two trials compared folic acid plus multiple micronutrients versus multiple micronutrients (Czeizel 1994; ICMR 2000), although the control groups received different formulations. Czeizel 1994 provided in comparison group 1 mg copper; 1 mg manganese; 7.5 mg zinc; 7.5 mg (calcium ascorbate) vitamin C and lactose 736.27 mg. ICMR 2000 provided iron (120 mg ferrous sulphate and 240 mg calcium phosphate).
One trial had three arms (Kirke 1992). One arm provided folic acid only while women in the second group received Pregnavite Forte® containing 4000 international units (IU) vitamin A; 400 IU vitamin D (calciferol); 1.5 mg vitamin B1 (thiamine hydrochloride); 1.5 mg vitamin B2 (riboflavin); 1 mg vitamin B6 (pyridoxine hydrochloride); 15 mg niacin (nicotinamide); 40 mg vitamin C; 480 mg calcium phosphate, and iron (252 mg ferrous sulphate) daily. A third group received Pregnavite Forte F® containing 360 µg (0.36 mg) folic acid in addition to 4000 IU vitamin A; 400 IU vitamin D (as calciferol); 1.5 mg vitamin B1 (thiamine hydrochloride); 1.5 mg vitamin B2 (riboflavin); 1 mg vitamin B6 (pyridoxine hydrochloride); 15 mg niacin (nicotinamide); 40 mg vitamin C; 480 mg calcium phosphate, and iron (252 mg ferrous sulphate) daily.
One trial included four comparison groups: one with folic acid with iron and calcium, one with folic acid plus iron, calcium and multiple micronutrients, another with iron, calcium and multiple micronutrients without folic acid, and a control group that only received iron and calcium but did not receive multiple micronutrients nor folic acid (MRC 1991). In this study the iron and calcium in the capsules was provided as ferrous sulphate and dicalcium phosphate. In the another included study (Kirke 1992), there were three comparison groups: one with folic acid; another with multiple micronutrients and another with multiple micronutrients and folic acid.
See Characteristics of included studies tables for a detailed description of the studies and their interventions.
Supervision and co‐interventions
In only one study were participants delineated by geography. Residents of Glamorgan and Gwent in south Wales who were qualified to join the study were visited by medically qualified field workers in their homes for the enrolment and an initial assessment of their diet (Laurence 1981). The participants were then instructed to report to the authors within six weeks of a missed period. They were revisited at six months and at the end of pregnancy.
One study was set in 33 centres across seven countries: 17 in the UK and 16 in Australia, Canada, France, Hungary, Israel and Russia (MRC 1991). All centres had capability for antenatal diagnosis of NTDs. The potency of the capsules was checked every three months.
One study was conducted in 12 Irish hospitals and co‐ordinated with the Health Research Board (Kirke 1992). Eligible participants (women who had a previous pregnancy with a child with NTD, not pregnant at the time of enrolment and were planning a further pregnancy) were block randomised (12 women per block) and stratified by hospital. Otherwise eligible women who were pregnant when first contacted constituted the non‐randomised control group. The stability of the supplements was assessed regularly during the study period.
One study was part of the Hungarian Optimal Family Planning Programme (HOFPP) where a qualified nurse performed or supervised the reproductive health check‐up, the preparation for conception, the initiation of the intervention and the follow‐up visits up to the 12th week of pregnancy (Czeizel 1994).
One study was carried out in five human genetics or paediatrics centres in Bangalore, Lucknow, Mumbai, New Delhi and Pune, India (ICMR 2000). The reproductive history, history of addictions, intake of medicines, consanguinity and a three‐generation pedigree chart were ascertained during enrolment and a physical examination was performed.
Intervention settings and health worker cadre
Compliance to folate intake was assessed using blood tests in two studies (Kirke 1992; Laurence 1981), and with both blood and urine radioimmunoassay tests in one study (MRC 1991). In the two remaining studies, compliance to folate intake was assessed using diary or tick cards and the number of capsules returned (Czeizel 1994; ICMR 2000).
Pregnancy was ascertained by stopping of menstrual period and follow‐up in three studies (Kirke 1992; Laurence 1981; MRC 1991); by a urinary human chorionic gonadotrophin (HCG) test after the first missed period in one study (ICMR 2000); and, by maternal serum test after the first missed period and an ultrasound at two weeks of gestation in one study (Czeizel 1994).
NTDs were detected using maternal serum alpha fetoprotein levels and ultrasound at the 16th week of gestation and further confirmed by amniotic fluid AFP and acetyl cholinesterase by amniocentesis and repeat ultrasound in one study (ICMR 2000). In one study, antenatal diagnosis of NTDs was available in all study centres, outcomes of all completed pregnancies were recorded and reports of NTDs were corroborated by independent reports such as a necropsy report of description of lesion at the trial centre in London (MRC 1991). In three studies, the NTDs were confirmed by examination by a medical personnel such as an obstetrician (Czeizel 1994), a pathologist (Kirke 1992), or the study's medically qualified fieldworker (Laurence 1981).
Excluded studies
We excluded 62 studies: 34 studies because their study design or scope did not match the objectives of this review (cohort or case‐control studies, or reviews; five studies were performed in non‐pregnant women; 11 studies gave folic acid to all participants; and, eight studies had interventions that were out of scope of this review (two studies were on fortification, two were on dietary advise, and four were on folic acid supplementation that started in the first trimester of gestation but that continued throughout pregnancy).
SeeCharacteristics of excluded studies tables for a detailed description of the studies and the reasons for exclusion.
Risk of bias in included studies
Allocation
Sequence generation: three trials adequately randomised the participants to the treatment groups (Czeizel 1994; Kirke 1992; Laurence 1981); one of them used block randomisation (Kirke 1992). Two multi‐centre trials did not report, or did not state clearly the method used to generate the randomisation sequence (ICMR 2000; MRC 1991).
Allocation concealment: two trials reported using sealed envelopes, opaque bottles or similar pills when allocating the women to treatment groups (ICMR 2000; Kirke 1992). In this last study pills were changed after one year resulting in a partial loss of blinding. The three remaining studies were reported as blinded, but were unclear in their method of concealment of treatment allocation.
Blinding
All of these trials were described as "double‐blind" although it was not clear whether outcome assessors were blind to group allocation. Women in all treatment groups received capsules or tablets containing active treatment ingredients or placebo although it was not always clear whether the preparations were of identical appearance.
Incomplete outcome data
For those women becoming pregnant, loss to follow‐up ranged from 1% (Czeizel 1994; MRC 1991) to 20% (Kirke 1992). The remaining two trials lost less than 10% of the randomised participants.
We noted in the methods section above, that for pregnancy outcomes we would include in the denominators only those women with confirmed pregnancies. In all studies women were randomised before conception and not all women became pregnant. For example, in the study by Czeizel 1994 of 7905 randomised, 5502 had confirmed pregnancy (69.6%); so 30% of those randomised did not become eligible to experience pregnancy and birth outcomes. Of these confirmed pregnancies, 49 were lost of follow‐up and a further 95 were later found to be not pregnant ("chemical pregnancies"). Overall, we included 5358 evaluated pregnancies from this study. We are aware that this makes results more difficult to interpret. Where data were available, we have provided information on the number of women randomised and the numbers with confirmed pregnancies in the Characteristics of included studies tables.
In some cases, we did not find it simple to determine the denominators as detailed information on attrition at different stages was not reported. For some competing/overlapping outcomes (e.g. perinatal deaths, miscarriages and pregnancy termination for fetal anomaly), we have reported figures provided in the trial reports but we advise caution in interpreting such data.
Selective reporting
Assessing selective reporting bias was difficult as we did not have access to study protocols. We were not able to explore possible reporting bias as too few studies have been included to allow us to carry out meaningful analyses.
Other potential sources of bias
In one study (Czeizel 1994), the randomisation code was broken twice: once to analyse the teratogenic effect of vitamin A and at the end of the trial to analyse the effect of the supplementation. One study (ICMR 2000) was terminated before expected as a consequence of the publication of the results from another trial (MRC 1991); however the calculated sample size was almost achieved.
SeeCharacteristics of included studies tables for assessments of the methodological quality of each included trial and Figure 2 and Figure 3 for summaries of the quality of included studies.
2.
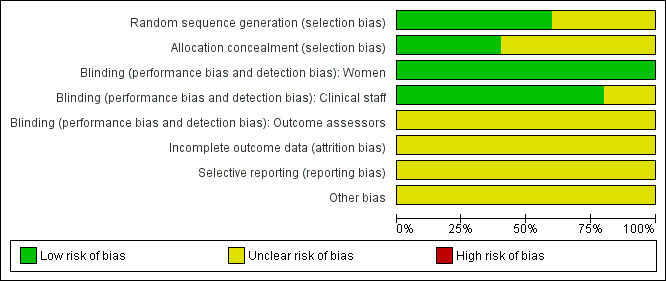
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
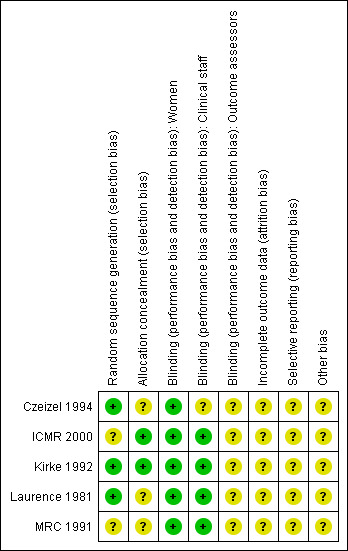
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
The summary of results is organised by comparisons and by infant and maternal outcomes. See the Data and analyses section for detailed results on primary and secondary outcomes.
Four of the five trials included in the review recruited women with a history of NTDs and subgroup analyses suggested no clear differences in trials examining first occurrence and recurrence of NTDs. However, to aid interpretation of results, along with reporting overall findings from trials, we have described the results from the trials examining first occurrence and recurrence of NTDs separately.
Only one out of the five studies (Kirke 1992) was considered to be of high quality as per our criteria.
(1) Supplementation with any folate versus no treatment, placebo or other micronutrients without folate (five trials)
Primary outcomes
Infant outcomes
Neural tube defects (NTDs) (all)
Five trials (Czeizel 1994; ICMR 2000; Kirke 1992; Laurence 1981; MRC 1991) with 6708 births examined the prevalence of NTDs in women receiving folic acid supplementation (alone or in combination with other vitamins and minerals) compared with women receiving placebo or other vitamins and minerals (not including folic acid). In all of these trials, supplementation started before pregnancy, continued throughout the first trimester, and involved daily supplementation. Periconceptional folate supplementation (alone, or in combination with other vitamins and minerals) reduces the prevalence of NTDs in comparison with no intervention, placebo or multiple micronutrients without folic acid (risk ratio (RR) 0.31, 95% confidence interval (CI) 0.17 to 0.58) (Analysis 1.1).
1.1. Analysis.
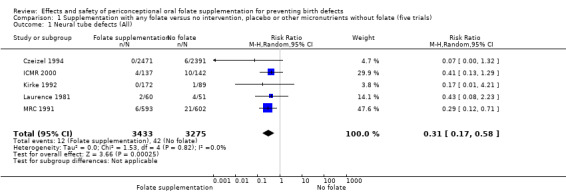
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 1 Neural tube defects (All).
Four trials recruited women with a history of NTDs (ICMR 2000; Kirke 1992; Laurence 1981; MRC 1991) and supplementation was associated with a reduction in the recurrence of a pregnancy affected by a NTD (RR 0.34, 95% CI 0.18 to 0.64; n = 1846) (Analysis 1.5). In the single trial (4862 births) (Czeizel 1994) recruiting women with no history of NTDs, and examining the first occurrence of NTDs, results favoured the group receiving supplementation, (RR 0.07, 95% CI 0.00 to 1.32) (Analysis 1.5); however, no cases of NTDs were observed in the supplemented group.
1.5. Analysis.
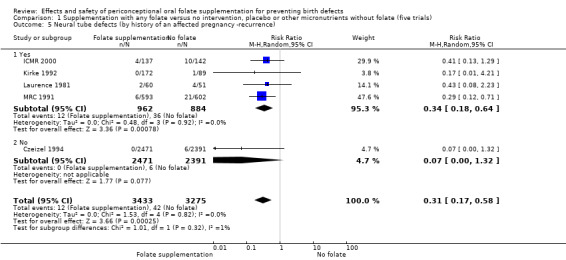
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 5 Neural tube defects (by history of an affected pregnancy ‐recurrence).
In one of the trials involving 261 births (Kirke 1992) the daily supplementation dose was 360 µg (0.36 mg); the results from this study favoured the intervention group (RR 0.17, 95% CI 0.01 to 4.21) (Analysis 1.3). In the remaining four trials (Czeizel 1994; ICMR 2000; Laurence 1981; MRC 1991), the daily dose of folic acid was more than 400 µg (0.4 mg) and NTDs were reduced in the intervention group (RR 0.32, 95% CI 0.17 to 0.60; n = 6447 births) (Analysis 1.3). There was no evidence of a difference between subgroups, although only one study was included in one of the subgroups (Test for subgroup differences: Chi² = 0.14, df = 1 (P = 0.71), I² = 0%).
1.3. Analysis.
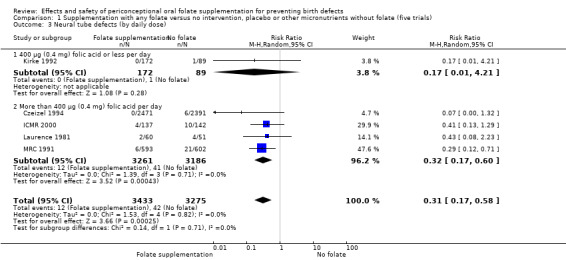
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 3 Neural tube defects (by daily dose).
Cleft lip
In the three trials (Czeizel 1994; Kirke 1992; MRC 1991) reporting the number of babies affected by cleft lip, there was no evidence of a difference between groups; overall eight babies were affected by cleft lip, four in each treatment group (Analysis 1.9).
1.9. Analysis.
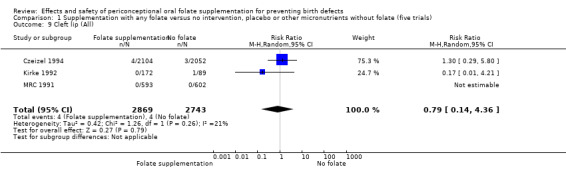
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 9 Cleft lip (All).
Cleft palate
Overall, only three babies were affected with cleft palate in the studies examining this outcome (Czeizel 1994; Kirke 1992; MRC 1991); there was no significant difference between the group receiving folic acid supplementation and comparison groups (RR 0.73, 95% CI 0.05 to 10.89; n = 5612 births) (Analysis 1.17).
1.17. Analysis.
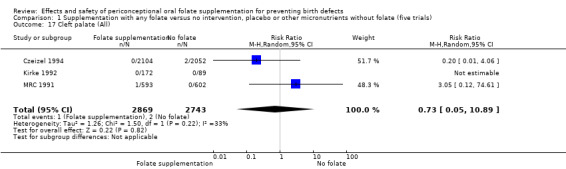
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 17 Cleft palate (All).
Congenital cardiovascular anomalies
This outcome was reported in three trials (Czeizel 1994; Kirke 1992; MRC 1991). There was no significant evidence of a difference between experimental and control groups in the number of babies with congenital cardiovascular defects irrespective of dose of drug, or history of NTDs (Analysis 1.25).
1.25. Analysis.
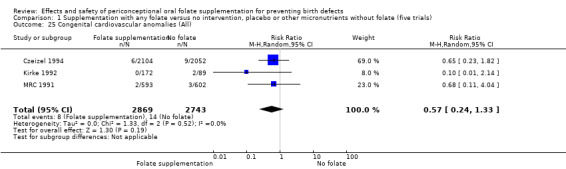
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 25 Congenital cardiovascular anomalies (All).
Other birth defects (excluding NTDs, cleft lip, cleft palate and cardiovascular defects)
Three trials reported the number of babies with other birth defects (Czeizel 1994; Kirke 1992; MRC 1991). Overall, when results from these three trials were pooled, there was no significant evidence of a difference between groups (RR 0.94, 95% CI 0.53 to 1.66; n = 5612 births) (Analysis 1.33).
1.33. Analysis.
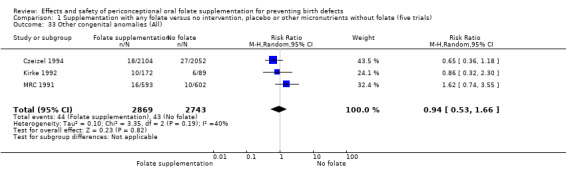
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 33 Other congenital anomalies (All).
Maternal outcomes
Maternal anaemia at term (defined as haemoglobin (Hb) less than 110 g/L); red blood cell folate at term; serum folate at term
No trials reported on these outcomes.
Miscarriage
All five trials examined the rate of miscarriage in women with confirmed pregnancy receiving daily folic acid supplements compared with the comparison groups (Czeizel 1994; ICMR 2000; Kirke 1992; Laurence 1981; MRC 1991); the number of miscarriages was less in the group receiving supplements containing folic acid, (RR 1.10, 95% CI 0.94 to 1.28; n = 7391 pregnancies). There were no significant differences between experimental and comparison groups for any of the subgroups we examined (Analysis 1.41).
1.41. Analysis.
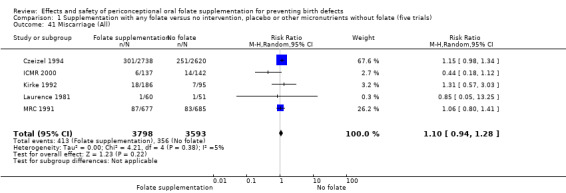
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 41 Miscarriage (All).
Secondary outcomes
Infant outcomes
Spina bifida
Three studies (Czeizel 1994; ICMR 2000; Laurence 1981) reported six spina bifida cases from 4546 births. Although the numbers of spina bifida cases were less in the folate supplemented groups, there was no significant difference between treatment groups (RR 0.32, 95% CI 0.06 to 1.62) (Analysis 1.49).
1.49. Analysis.
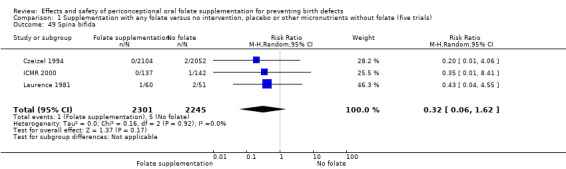
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 49 Spina bifida.
Anencephaly
Four studies reported 17 cases of anencephaly (Czeizel 1994; ICMR 2000; Kirke 1992; MRC 1991), with no significant difference between the two treatment groups (RR 0.36, 95% CI 0.13 to 1.03; n = 4807 births) (Analysis 1.50).
1.50. Analysis.
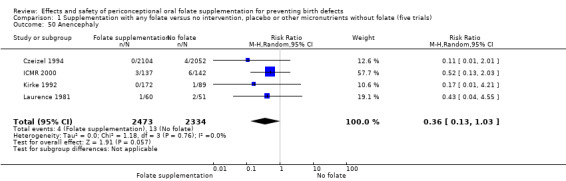
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 50 Anencephaly.
Stillbirths
Stillbirths were reported in four trials (Czeizel 1994; ICMR 2000; Kirke 1992; MRC 1991). There was no significant evidence of any difference between treatment groups in the pooled analysis (RR 1.05, 95% CI 0.54 to 2.05), or in any subgroup analyses (Analysis 1.51).
1.51. Analysis.
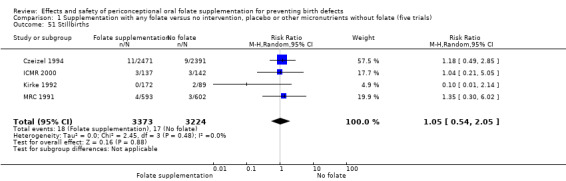
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 51 Stillbirths.
Pregnancy termination for fetal anomaly
Overall, in the four studies examining this outcome (Czeizel 1994; ICMR 2000; Laurence 1981; MRC 1991) there were 50 pregnancies terminated for fetal anomaly; 11/3612 in the experimental group and 39/3498 in the control group. The difference between groups was significant in the pooled analysis (RR 0.29, 95% CI 0.15 to 0.56; n = 7110). In all of these studies daily supplementation was greater than 400 µg (0.4 mg) and women started supplements before pregnancy (Analysis 1.52).
1.52. Analysis.
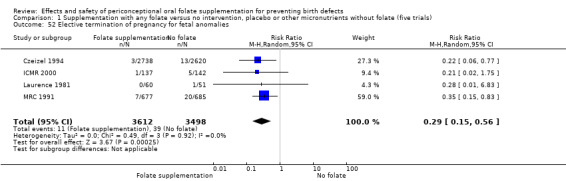
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 52 Elective termination of pregnancy for fetal anomalies.
Low birthweight (less than 2500 g)
The number of babies with low birthweight was examined in two trials (Czeizel 1994; ICMR 2000). There was no significant evidence of a difference between treatment groups (RR 1.13, 95% CI 0.84 to 1.52; n = 5048 births), (Analysis 1.53).
1.53. Analysis.
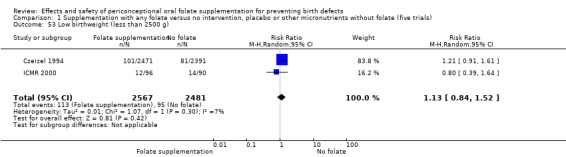
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 53 Low birthweight (less than 2500 g).
Macrosomia (birthweight greater than 4000 g)
Macrosomia was reported by one study (Czeizel 1994) and did not show significant difference between treatment groups (RR 0.98, 95% CI 0.79 to 1.22; n = 4862 births) (Analysis 1.54).
1.54. Analysis.
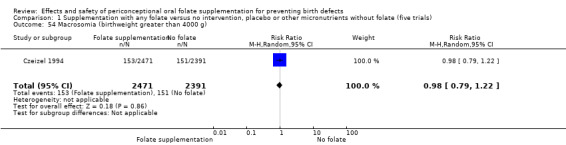
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 54 Macrosomia (birthweight greater than 4000 g).
Preterm births (less than 37 weeks' gestation)
One study reported on preterm births (Czeizel 1994) and showed no significant difference between treatment groups (RR 1.14, 95% CI 0.93 to 1.41; n = 4862 births) (Analysis 1.54).
Other infant outcomes
No trials reported results for our other pre‐specified secondary outcomes.
Very low birthweight (less than 1500 g)
Infant optimal health status at birth (as defined by trial authors)
Neonatal deaths
Admission to special care for any cause (as defined by trial authors)
Infant insulin resistance (as defined by trial authors)
Apgar at one minute after birth (eight or greater)
Apgar score at five minutes after birth (eight or greater)
Long‐term outcomes (as defined by trial authors)
Maternal
Multiple pregnancy
The number of women affected by multiple pregnancy was examined in four trials (Czeizel 1994; ICMR 2000; Kirke 1992; MRC 1991). Overall, there was no evidence of any significant difference between women receiving supplements with folic acid and those in the controls (RR 1.38, 95% CI 0.89 to 2.14; n = 7280 pregnancies) (Analysis 1.56).
1.56. Analysis.
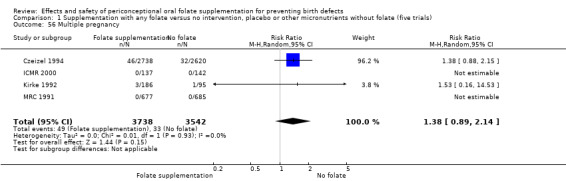
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 56 Multiple pregnancy.
Side effects
Only one study reported findings on side effects like nausea, vomiting, constipation or diarrhoea (Czeizel 1994), and the number of reported cases was very low in both the group of women receiving folic acid with multiple micronutrients and the control group during the pre‐pregnancy and pregnancy period.
Other maternal outcomes
No trials reported on our other pre‐specified secondary outcomes.
Homocysteine at term (µmol/L)
Serum vitamin B6 concentration at term (nmol/L)
Plasma or serum vitamin B12 concentration at term (pmol/L)
Pre‐eclampsia (defined as gestational hypertension (blood pressure higher than 140/90 mmHg) and proteinuria (more than 300 mg of protein in a 24‐hour urine sample)
(2) Supplementation with folic acid alone versus no treatment or placebo (one trial)
Primary outcomes
Infant outcomes
Neural tube defects (NTDs) (all)
One trial (Laurence 1981) involving 111 births was included in this comparison. There was no significant difference in the risk of NTDs between those receiving folic acid alone and those receiving placebo (RR 0.43, 95% CI 0.08 to 2.23). (Analysis 2.1).
2.1. Analysis.
Comparison 2 Supplementation with folic acid alone versus no treatment or placebo (one trial), Outcome 1 Neural tube defects (All).
Maternal outcomes
Miscarriage
One trial (111 pregnancies) examined the rate of miscarriage in women with confirmed pregnancies receiving folic acid supplements compared with controls (Laurence 1981); there was no significant evidence of a difference between treatment groups in the number of women having miscarriage (RR 0.85, 95% CI 0.05 to 13.25) (Analysis 2.2).
2.2. Analysis.
Comparison 2 Supplementation with folic acid alone versus no treatment or placebo (one trial), Outcome 2 Miscarriage (All).
Secondary outcomes
Infant outcomes
Spina bifida
One trial reported on the rate of spina bifida among women receiving folic acid daily during the periconceptional period compared with those receiving placebo (Laurence 1981) and showed no evidence of a significant difference between treatment groups (RR 0.43, 95% CI 0.04 to 4.55) (Analysis 2.3).
2.3. Analysis.
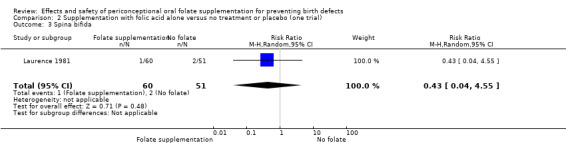
Comparison 2 Supplementation with folic acid alone versus no treatment or placebo (one trial), Outcome 3 Spina bifida.
Anencephaly
One trial reported on the rate of anencephaly among women receiving daily doses of 4.0 mg folic acid during the periconceptional period compared with those receiving placebo (Laurence 1981) and showed no evidence of a significant difference between treatment groups (RR 0.43, 95% CI 0.04 to 4.55) (Analysis 2.4).
2.4. Analysis.
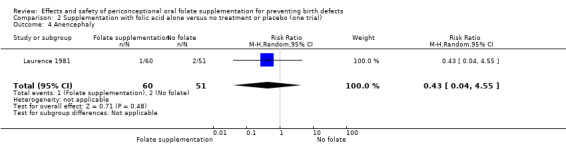
Comparison 2 Supplementation with folic acid alone versus no treatment or placebo (one trial), Outcome 4 Anencephaly.
Other secondary outcomes
No trials reported on our other pre‐specified secondary outcomes.
Maternal
Pregnancy termination for fetal anomaly
Laurence 1981 reported one pregnancy termination due to NTD in the group that did not receive folate while in the experimental group there were zero cases (RR 0.28, 95% CI 0.01 to 6.83) (Analysis 2.5)
2.5. Analysis.
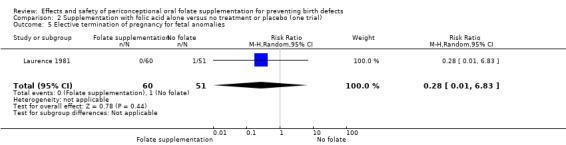
Comparison 2 Supplementation with folic acid alone versus no treatment or placebo (one trial), Outcome 5 Elective termination of pregnancy for fetal anomalies.
No trials reported on other pre‐specified secondary outcomes.
(3) Supplementation with folic acid plus other micronutrients versus other micronutrients without folic acid (four trials)
Primary outcomes
Infant outcomes
Neural tube defects (NTDs) (all)
Four of the trials (Czeizel 1994; ICMR 2000; Kirke 1992; MRC 1991) (6512 births) included comparisons of women receiving folic acid with other micronutrients compared with women receiving other micronutrients without folic acid. There was a significant difference between groups favouring those who received the folic acid supplementation (RR 0.31, 95% CI 0.16 to 0.60). In all of these trials supplementation started before pregnancy, continued throughout the first trimester and involved daily supplementation (Analysis 3.1).
3.1. Analysis.
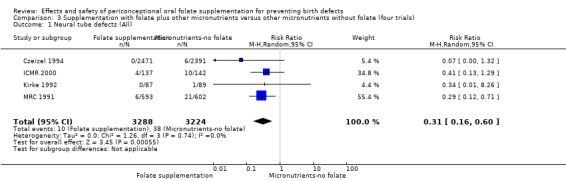
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 1 Neural tube defects (All).
Three of these trials involved women with a previous history of NTD‐affected pregnancy (ICMR 2000; Kirke 1992; MRC 1991). There were fewer recurrences of NTDs among those who received folate compared to those who did not (RR 0.33, 95% CI 0.17 to 0.66); n = 1650 births). In one trial with no history of NTDs (Czeizel 1994), there was no significant difference in risk of incidence of NTDs (RR 0.07, 95% CI 0.00 to 1.32; n = 4862 births) though there were no cases of NTDs in the group receiving folate (Analysis 3.5).
3.5. Analysis.
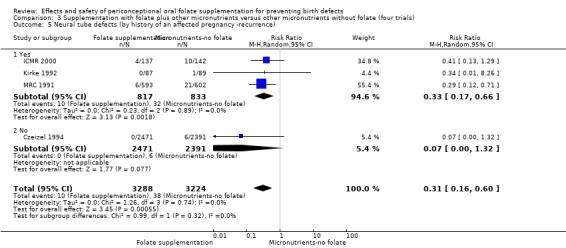
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 5 Neural tube defects (by history of an affected pregnancy ‐recurrence).
One trial (Kirke 1992) supplemented folic acid at a daily dose of 360 µg (0.36 mg) and did not show a significant difference between treatment groups (RR 0.34, 95% CI 0.01 to 8.26; n = 176 births). Three trials gave doses of 4.0 mg daily folic acid and found a difference in the risk of NTDs among treatment groups (RR 0.31, 95% CI 0.15 to 0.61; n = 6336 births) (Analysis 3.3). There was no evidence of difference between subgroups (Test for subgroup differences: Chi² = 0.00, df = 1 (P = 0.95), I² = 0%).
3.3. Analysis.
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 3 Neural tube defects (by daily dose).
Cleft lip
In the two trials (Czeizel 1994; Kirke 1992) reporting the number of babies affected by cleft lip, there was no evidence of a difference between groups; overall eight babies were affected by cleft lip, four in each treatment group (Analysis 3.9).
3.9. Analysis.
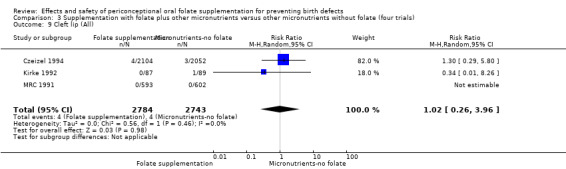
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 9 Cleft lip (All).
Cleft palate
Three babies (one in the intervention group and one in the control group) were affected with cleft palate in the three studies examining this outcome (Czeizel 1994; Kirke 1992; MRC 1991); there was no evidence of a difference between groups (RR 0.73, 95% CI 0.05 to 10.89; n = 5527 births) (Analysis 3.17).
3.17. Analysis.
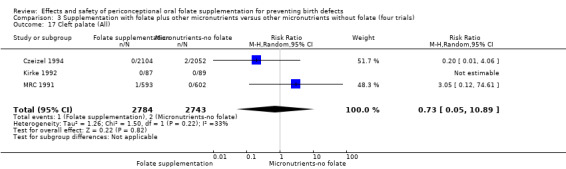
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 17 Cleft palate (All).
Congenital cardiovascular anomalies
This outcome was reported in three trials (Czeizel 1994; Kirke 1992; MRC 1991). There was no evidence of a difference between experimental and control groups in the number of babies with congenital cardiovascular defects irrespective of dose of drug, or history of NTDs (Analysis 3.25).
3.25. Analysis.
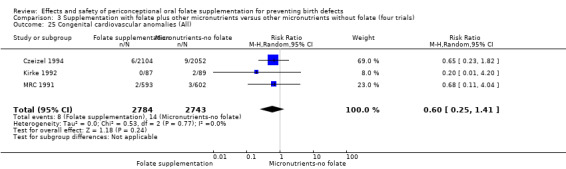
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 25 Congenital cardiovascular anomalies (All).
Other birth defects (excluding neural tube defects, cleft lip, cleft palate and cardiovascular defects)
Three trials reported the number of babies with other birth defects (Czeizel 1994; Kirke 1992; MRC 1991). There was no significant evidence of a difference between groups (RR 0.98, 95% CI 0.54 to 1.77; n = 5527 births) (Analysis 3.36). In the Czeizel 1994 trial which recruited women with no history of NTDs, the difference between groups was not significant (RR 0.65, 95% CI 0.36 to 1.18; n = 4156 births) as were the pooled results from the two studies (Kirke 1992; MRC 1991), which recruited women with previous history of NTDs (RR 1.39, 95% CI 0.74 to 2.62; n = 1371 births) (Analysis 3.37).
3.36. Analysis.
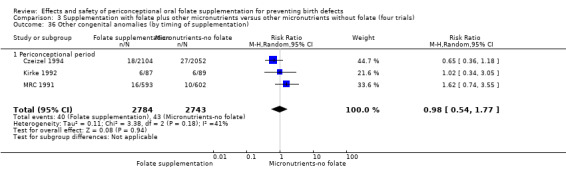
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 36 Other congenital anomalies (by timing of supplementation).
3.37. Analysis.
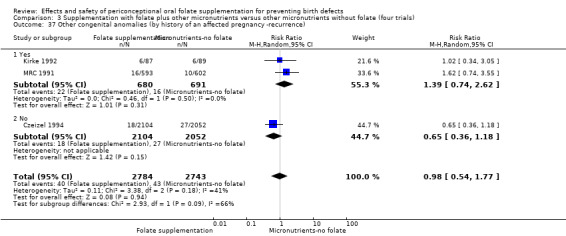
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 37 Other congenital anomalies (by history of an affected pregnancy ‐recurrence).
Maternal outcomes
Maternal anaemia at term (defined as Hb less than 110 g/L); red blood cell folate at term (nmol/L); serum folate at term (nmol/L)
No trials reported on these outcomes.
Miscarriage
Four trials reported the number of women with confirmed pregnancy suffering miscarriage (Czeizel 1994; ICMR 2000; Kirke 1992; MRC 1991); there were no differences between groups (RR 1.08, 95% CI 0.87 to 1.32; n = 7187 pregnancies) (Analysis 3.41). There were no significant differences between groups for any of the subgroups we examined (Analysis 3.42 to Analysis 3.48).
3.41. Analysis.
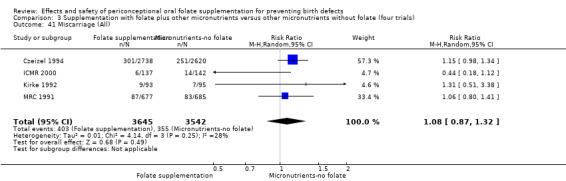
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 41 Miscarriage (All).
3.42. Analysis.
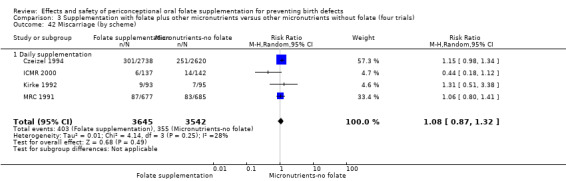
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 42 Miscarriage (by scheme).
3.48. Analysis.
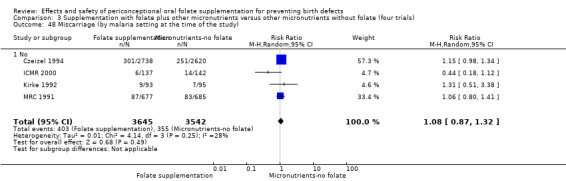
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 48 Miscarriage (by malaria setting at the time of the study).
Secondary outcomes
Infant outcomes
Spina bifida
Two trials (Czeizel 1994; ICMR 2000) reported three cases of spina bifida, all in the control group, though no significant differences between groups were seen (RR 0.26, 95% CI 0.03 to 2.31; n = 4435 births) (Analysis 3.49).
3.49. Analysis.
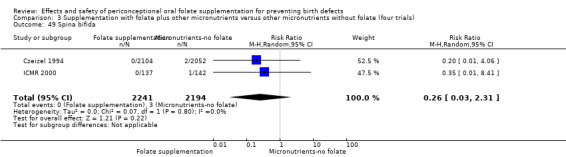
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 49 Spina bifida.
Anencephaly
Three studies (Czeizel 1994; ICMR 2000; Kirke 1992) reported 10 cases of anencephaly (three in the treatment group with folate and seven in the control group though the pooled results showed no evidence of a difference (RR 0.38, 95% CI 0.12 to 1.22; n = 4611 births) (Analysis 3.50).
3.50. Analysis.
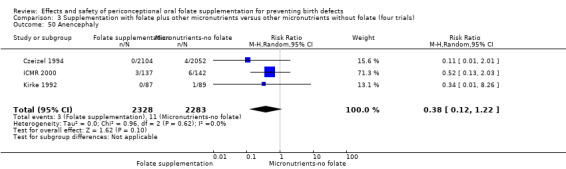
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 50 Anencephaly.
Stillbirths
Stillbirths were reported in four trials included in this comparison (Czeizel 1994; ICMR 2000; Kirke 1992; MRC 1991). There was no significant evidence of any difference between treatment groups in the pooled analysis (RR 1.09, 95% CI 0.56 to 2.12; n = 6512 births) (Analysis 3.51).
3.51. Analysis.
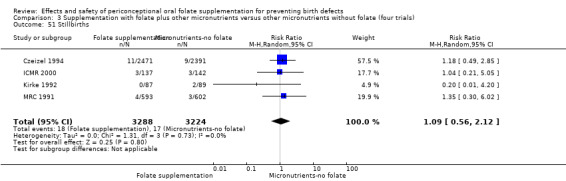
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 51 Stillbirths.
Pregnancy termination for fetal anomaly
Overall, in the three studies examining this outcome (Czeizel 1994; ICMR 2000; MRC 1991) there were 49 pregnancies terminated for fetal anomaly; 11/3552 in the women who received folic acid with other micronutrients and 38/3447 in the control group who received other micronutrients supplements without folic acid. The difference between groups was significant in the pooled analysis (RR 0.29, 95% CI 0.15 to 0.57; n = 6999) (Analysis 3.52).
3.52. Analysis.
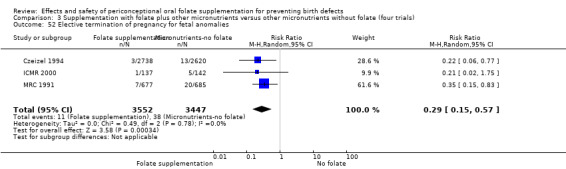
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 52 Elective termination of pregnancy for fetal anomalies.
Low birthweight (less than 2500 g)
The number of babies with low birthweight was examined in two trials (5048 births) (Czeizel 1994; ICMR 2000). There was no significant evidence of a difference between treatment groups (Analysis 3.53).
3.53. Analysis.
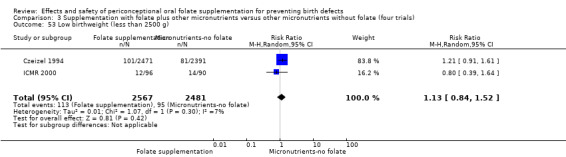
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 53 Low birthweight (less than 2500 g).
Macrosomia (birthweight greater than 4000 g)
One study (Czeizel 1994) reported 304 macrosomic births out of 4862 with no significant difference between treatment groups (RR 0.98, 95% CI 0.79 to 1.22) (Analysis 3.54).
3.54. Analysis.
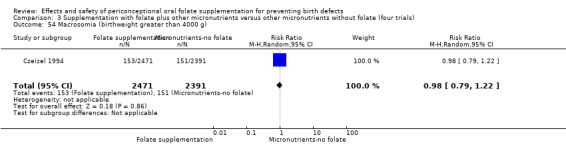
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 54 Macrosomia (birthweight greater than 4000 g).
Preterm birth (less than 37 weeks' gestation)
In one study (Czeizel 1994), there was no significant difference in risk of preterm births between treatment groups (RR 1.14, 95% CI 0.93 to 1.41; n = 4862 births) (Analysis 3.55).
3.55. Analysis.
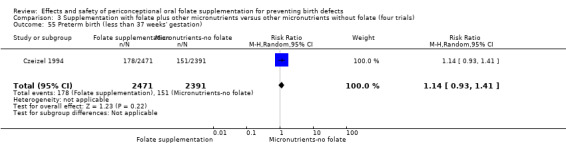
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 55 Preterm birth (less than 37 weeks' gestation).
Other infant outcomes
No trials reported results for our other pre‐specified secondary outcomes.
Maternal
Multiple pregnancy
The number of women affected by multiple pregnancy was examined in three trials (Czeizel 1994; Kirke 1992; MRC 1991). There was no significant evidence of a difference between women in the two treatment groups (RR 1.39, 95% CI 0.90 to 2.17; n = 7187 pregnancies) (Analysis 3.56).
3.56. Analysis.
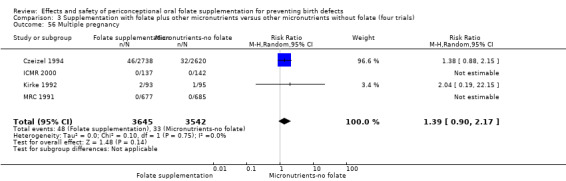
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 56 Multiple pregnancy.
Other maternal outcomes
No trials reported on our other pre‐specified secondary outcomes.
(4) Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials)
Primary outcomes
Infant outcomes
Neural tube defects (NTDs) (all)
Two of the trials (Kirke 1992; MRC 1991) (1371 births) included comparisons of women receiving folic acid with micronutrients compared with women receiving the same micronutrients but without folic acid. There was a difference between groups favouring those who received the folic acid supplementation (RR 0.29, 95% CI 0.12 to 0.70) (Analysis 4.1). In all of these trials supplementation started before pregnancy, continued throughout the first trimester, involved daily supplementation and selected women with a history of a previous pregnancy affected by NTD.
4.1. Analysis.
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 1 Neural tube defects (All).
Subgroup analysis according to dose of the folic acid did not show significant differences between subgroups (Analysis 4.2 to Analysis 4.8).
4.2. Analysis.
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 2 Neural tube defects (by scheme).
4.8. Analysis.
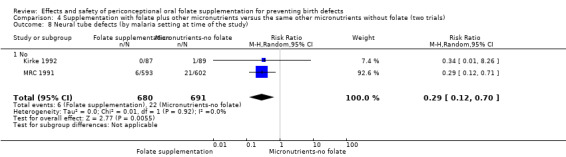
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 8 Neural tube defects (by malaria setting at time of the study).
Cleft lip
In the two trials (Kirke 1992; MRC 1991) reporting the number of babies affected by cleft lip, there was only one case noted in the control group in one study (Kirke 1992). There was no evidence of a difference between groups (RR 0.34, 95% CI 0.01 to 8.26; n = 1371 births) (Analysis 4.9).
4.9. Analysis.
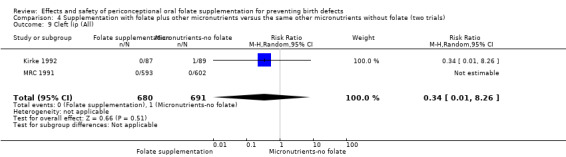
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 9 Cleft lip (All).
Cleft palate
One case of cleft palate in the folate supplementation group was found in one study (MRC 1991), although two studies reported this outcome (Kirke 1992; MRC 1991); there was no significant difference between groups (RR 3.05, 95% CI 0.12 to 74.61; n = 1371 births) (Analysis 4.17).
4.17. Analysis.
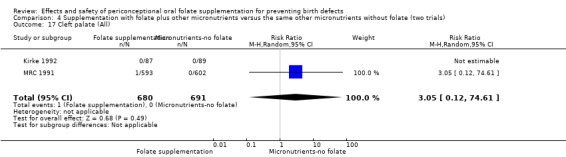
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 17 Cleft palate (All).
Congenital cardiovascular anomalies
This outcome was reported in two trials (Kirke 1992; MRC 1991) and found two affected births in the folate supplementation group out of 680 births and five affected in the control group out of 691 births. There was no evidence of a difference between experimental and control groups in the number of babies with congenital cardiovascular defects irrespective of dose of drug, or history of NTDs (Analysis 4.25 to Analysis 4.32).
4.25. Analysis.
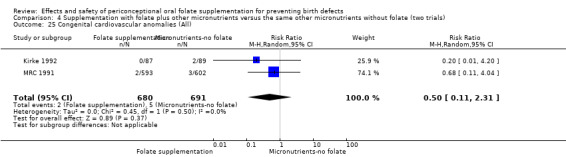
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 25 Congenital cardiovascular anomalies (All).
4.32. Analysis.
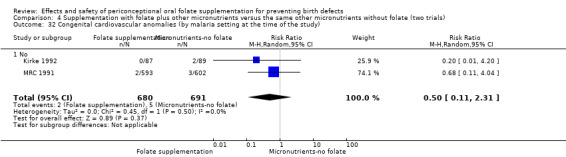
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 32 Congenital cardiovascular anomalies (by malaria setting at the time of the study).
Other congenital anomalies (excluding neural tube defects, cleft lip, cleft palate and cardiovascular anomalies)
Two trials reported the number of babies with other birth defects (Kirke 1992; MRC 1991). There was no significant evidence of a difference between groups (RR 1.39, 95% CI 0.74 to 2.62; n = 1371 births) (Analysis 4.33).
4.33. Analysis.
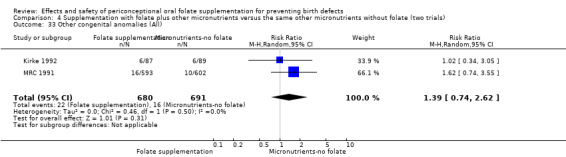
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 33 Other congenital anomalies (All).
Maternal outcomes
Maternal anaemia at term (defined as Hb less than 110 g/L); red blood cell folate at term (nmol/L); serum folate at term (nmol/L)
No trials reported on these outcomes.
Miscarriage
Two trials reported the number of women with confirmed pregnancy suffering miscarriage (Kirke 1992; MRC 1991); while the number of miscarriages was higher in the group receiving folic acid, the difference between groups was not significant (RR 1.08, 95% CI 0.82 to 1.41; n = 1550 pregnancies)). There were no significant differences between groups for any of the subgroups we examined (Analysis 4.42 to Analysis 4.48).
4.42. Analysis.
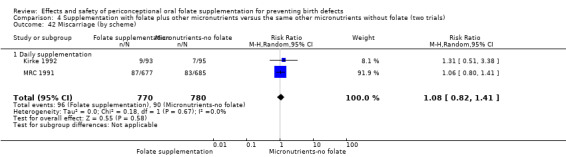
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 42 Miscarriage (by scheme).
4.48. Analysis.
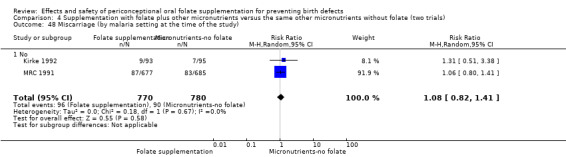
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 48 Miscarriage (by malaria setting at the time of the study).
Secondary outcomes
Infant outcomes
Anencephaly
One case of anencephaly was reported in the control group in one study (Kirke 1992). There was no significant difference in risk of anencephaly between the treatment groups (RR 0.34, 95% CI 0.01 to 8.26; n = 176 births) (Analysis 4.49).
4.49. Analysis.
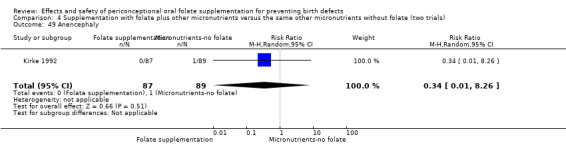
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 49 Anencephaly.
Stillbirths
Stillbirths were reported in two trials included in this comparison (Kirke 1992; MRC 1991). There was no significant evidence of any difference between treatment groups in the pooled analysis (RR 0.84, 95% CI 0.16 to 4.28; n = 1371 births) (Analysis 4.50).
4.50. Analysis.
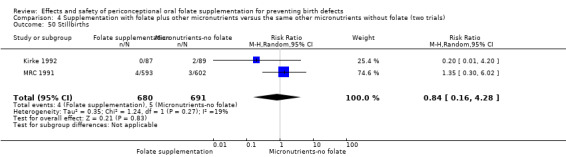
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 50 Stillbirths.
Pregnancy termination for fetal anomalies
Overall, in the one study examining this outcome (MRC 1991), there were 27 pregnancies terminated for fetal anomaly; 7/677 in the women who received folic acid with other micronutrients and 20/685 in the control group who received other micronutrients supplements without folic acid. The was a difference between groups in the pooled analysis (RR 0.35, 95% CI 0.15 to 0.83; n = 1362) (Analysis 4.51).
4.51. Analysis.
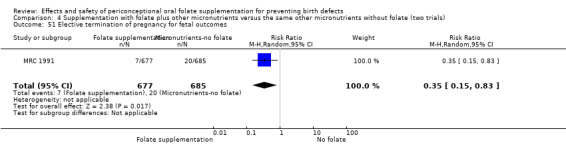
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 51 Elective termination of pregnancy for fetal outcomes.
Other infant outcomes
No trials reported results for our other pre‐specified secondary outcomes.
Maternal
Multiple pregnancy
The number of women affected by multiple pregnancy was reported in two trials (Kirke 1992; MRC 1991). There was no significant evidence of a difference between women in the two treatment groups (RR 2.04, 95% CI 0.19 to 22.15; n = 1550 pregnancies) (Analysis 4.52).
4.52. Analysis.
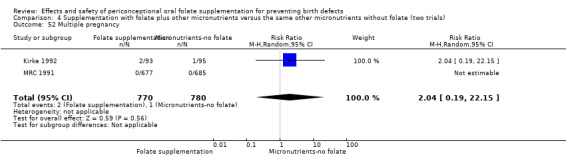
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 52 Multiple pregnancy.
Other maternal outcomes
No trials reported on our other pre‐specified secondary outcomes.
Discussion
Summary of main results
Folic acid alone or plus other vitamins and minerals prevents the incidence and recurrence of neural tube defects (NTDs). Overall, this positive response does not differ by folic acid dose (above 360 µg/day or 0.360 mg/day), or by whether folic acid was given alone or accompanied by other micronutrients.
The evidence does not support any protective or negative effect of folic acid supplementation on other congenital anomalies or maternal outcomes, including miscarriage.
No included trials assessed biochemical outcomes including maternal blood folate or anaemia at term.
Overall completeness and applicability of evidence
This review addresses the effects and safety of folic acid supplementation or folic acid with other micronutrients versus no treatment/placebo or other micronutrients (without folic acid) during the periconceptional period and the first trimester of pregnancy. This review updates an earlier Cochrane Review (De‐Regil 2010).
To reduce the risk of NTDs for women capable of becoming pregnant, the recommendation is to take 400 μg (0.4 mg) of folic acid daily from fortified foods, supplements, or both, in addition to consuming food folate from a varied diet beginning at least one month before conception (IOM 1998; WHO/UNICEF/UNU 2001). Expert panels suggest that supplemental intake in this population should range between 400 μg (0.4 mg) and 800 μg (0.8 mg) (US Preventive Services Task Force 2009). The first recommendations were based on the amount needed to maintain an adequate haematological status, and the protective role of folic acid against NTDs was later confirmed with a public health supplementation trial carried out in China from 1993 to 1995 which showed a reduction in the occurrence of NTDs of 40% to 85% (from the south and north of the country, respectively) (Berry 1999). The results of this review clearly show that periconceptional folic acid supplementation reduces the recurrence of NTDs and although there is only one trial assessing first time occurrence of NTDs (with wide confidence intervals that cross the null value) no events were found among women supplemented with folic acid (Czeizel 1994). The evidence from early clinical trials designed to study the effect of folic acid supplementation around the time of conception on NTDs is so strong that this probably accounts for why we only found one additional randomised controlled trial looking at this matter (ICMR 2000) and few observational studies published from 1995 to date that reinforce these findings (US Preventive Services Task Force 2009). There are no trials exploring the effect of 5MTHF during the periconceptional period, but it is very likely that the benefits of this intervention observed during the reproductive age and lactation (Houghton 2006; Lamers 2004) might extend to pregnancy.
Questions remain about the best dose and periodicity of folic supplementation as there is still a high prevalence of birth defects worldwide. Doses ranging from 360 μg (0.36 mg) (Kirke 1992) to 6000 μg (6 mg) (Chen 2008) a day have proven to be effective in preventing both occurrence and recurrence of NTDs; this wide response to supplementation may be determined by the baseline blood folate concentrations in each population. Improving nutrition surveillance in order to find the appropriate dose and supplementation scheme is crucial to promote a cost‐effective public health policy that can reach a larger number of people.
There are no clinical trials that review the effect of weekly periconceptional folate supplementation for the prevention of birth defects. The weekly iron and folic acid supplementation programme (WIFS) studies (Fernandez‐Gaxiola 2011; WHO 2011) have proven that weekly supplementation is an effective preventive intervention to control iron deficiency among childbearing age women, with good compliance. There is no information on the impact of such schemes on birth defects. Two published studies have evaluated weekly folic acid supplementation before pregnancy (not randomised controlled trials). In the first study, carried out in Mexico, women received 5000 µg (5 mg) folic acid for three months, and their red blood cell and plasma folate concentrations significantly increased (Martinez‐de Villareal 2001). This strategy was also associated with a 50% decrease in the incidence of anencephaly and spina bifida cases, and a significant reduction in infant mortality and disability after two years (Martinez‐de Villareal 2002). In the second study, conducted in New Zealand, a weekly single supplement of 2800 µg (2.8 mg) of folic acid taken for 12 weeks increased women’s red blood cell folate to concentrations associated with a reduced risk of bearing a child with a NTD (Norsworthy 2004).
New studies have documented that MTHFR polymorphism may be considered a susceptible gene for cardiovascular birth defects (Brandalize 2009; Marinho 2009). If MTHFR is associated with congenital heart defects (i.e. Tetralogy of Fallot), this may in part explain the lack of effect of periconceptional folic acid supplementation on cardiovascular malformations, and may warrant further study for opportunities for preventing other birth defects.
This review found a protective effect against birth defects with the use of folic acid combined with other micronutrients. From a metabolic perspective, it is clear that niacin, thiamin, vitamin B6, and vitamin B12 influence reactions that include folate. The possibility that other micronutrients apart from folic acid may have an additional protective effect has been a matter considered in several more recent studies (Czeizel 2004; Goh 2006; Thompson 2009).
All the trials included in this review were performed before many countries introduced mandatory flour fortification with folic acid. In the US, for example, the flour fortification programme was designed so that typical folic acid intake would be increased by approximately 100 µg/day and that the risk of intakes greater than 1000 µg/day (the FDA's tolerable upper level of daily intake) would be minimal. Results showed that the typical intake of folic acid from fortified foods is more than twice as high as originally predicted (Quinlivan 2003). Even though the effectiveness of mandatory folic acid fortification programmes has been documented by a decline in the prevalence of NTDs in the United States, Canada, Costa Rica, Chile, and South Africa (Berry 2010), fortified foods (mandatory and not) might be providing relatively large amounts of folic acid to the entire population and some authors have suggested that the recommendation of supplementing 400 µg/day of folic acid in addition to dietary folate to women planning to become pregnant deserves to be reviewed. However, they acknowledge the lack of certainty on the minimum dose of supplemental folic acid that is effective for reducing NTDs occurrence (Dary 2009).
The force of the results makes this an intervention that can be applied in most settings for reducing NTDs rates. However, the women in the trials included in this review had Caucasian, Anglo‐Saxon, Anglo‐American and Indian backgrounds and the evidence suggests that the presence of gene polymorphisms (i.e. MTHFR gene mutation) is more prevalent among people of Latin origins (Guéant‐Rodriguez 2006), thus the findings may slightly differ across all population groups. Also, there is still a need to understand the applicability of these results in endemic malaria regions where antifolate antimalarial drugs are being used, and in women living with HIV/AIDS, receiving or not receiving antiretroviral therapy.
Women in the trials included in this review were actively planning pregnancy and attending for preconception care. It is estimated that 80 million women each year have unwanted or unintended pregnancies (Glasier 2006), and women seeking preconception care may be more affluent and more educated, or be seeking care as they are at high risk after previous pregnancy complications (Czeizel 1999b; Elsinga 2006). These women may be more likely to comply with prescribed supplementation schemes, and the same level of compliance and effect may not be achieved by other groups.
Quality of the evidence
Quality of the individual trials
The included trials were rated as unclear or low risk of bias considering allocation concealment and blinding, and pooling results in meta‐analysis resulted in consistent protective results. Having said this, in trials where only a subset of the original randomised sample became eligible to experience many of the outcomes measured, results may be at risk of bias and not simple to interpret. In all the trials included in this review, studies recruited non‐pregnant women (albeit women who may have been planning pregnancy) and inevitably only a proportion of those randomised had confirmed pregnancy during the study periods. As the focus of the review was on pregnancy outcomes, we decided that denominators would include only those women who became pregnant.
Restricting the analyses to pregnant women may have led to the size of the treatment effect for some outcomes being either over or under‐estimated. It is possible that women who became pregnant may have been different in a number of respects from those that did not, and that the intervention may have had a different effect on those women that did or did not become pregnant. It is possible that the intervention may have an impact on the ability to become pregnant or to sustain a pregnancy during the very early stages (before pregnancy confirmation) although there is no strong evidence from the studies included in this review or in the literature to suggest that this was likely to have been the case. One study (Czeizel 1994) suggested small but statistically significant differences in confirmed pregnancy rates in women in the intervention and control groups (71.3% versus 67.9% respectively); however, this finding may have occurred by chance or been confounded by the use of in vitro fertilisation by some women (Berry 2004). The possible difference between groups in one trial (Czeizel 1994) was not borne out in other studies examining conception rates in the two treatment groups as the numbers of women with confirmed pregnancy were very similar in intervention and control groups (ICMR 2000; Kirke 1992; MRC 1991).
Interpreting the results from the review for potentially overlapping outcomes may not be straightforward and there was insufficient information in trial reports for us to be clear about whether outcomes were mutually exclusive. It was likely, but not certain, that the same fetus may have been counted in more than one outcome. For example, where NTDs were detected, these same pregnancies may have been included in the figures for pregnancy termination, but we cannot assume that all detected anomalies led to pregnancy termination. Results for outcomes such as pregnancy termination for fetal anomaly therefore need to be interpreted with caution.
Overall quality of the evidence for primary outcomes
For the main comparison supplementation with folate versus no intervention, placebo or other micronutrients without folate, the overall quality of the evidence for neonatal outcomes was high for NTDs, whereas, it was of low quality for cleft lip, cleft palate, congenital cardiovascular anomalies, and other congenital anomalies (seeTable 1). In spite of some concerns about an unclear risk of selection bias for trials reporting on NTDs, we refrained from downgrading the quality of the evidence due to the large magnitude of the effect. In addition to the methodological concerns, the quality of the body of evidence for cleft lip, cleft palate, congenital cardiovascular and other congenital anomalies were further downgraded due to very wide confidence intervals, encompassing both large benefit and harm. The overall quality of the evidence for maternal outcomes was rated as moderate for miscarriage (seeTable 2).
Potential biases in the review process
There were a number of potential biases in the review process. We attempted to minimise bias in several ways: two review authors independently assessed eligibility for inclusion, and data extraction, assessments of risk of bias and data entry were checked by two authors. However, carrying out reviews is not an exact science and may require a number of subjective judgements; it is possible that a different review team may have reached different decisions regarding assessments of eligibility and risk of bias. We would encourage readers to examine the Characteristics of included studies tables to assist in the interpretation of results.
Agreements and disagreements with other studies or reviews
The conclusions of this update do not change in comparison with the previous Cochrane Review (De‐Regil 2010). Our results are consistent with Blencowe 2010 and Ramakrishnan 2012 and in showing a protective effect of daily folic acid supplementation (alone or in combination with other micronutrients) in preventing NTDs, with a similar effect for recurrence. However, Blencowe 2010 reported a different (and significant) effect on first occurrence of NTDs due to the inclusion of observational studies.
Authors' conclusions
Implications for practice.
The beneficial effects of folic acid supplementation for preventing birth defects depend on the timing of the intervention (before pregnancy and up to 12 weeks of pregnancy). Adequate targeting is needed to ensure the effectiveness of periconceptional folate supplementation for the prevention on birth defects.
Implications for research.
The included trials were published before international recommendations for periconceptional folic acid supplementation emerged. There is a need for clinical trials using the current internationally recommended doses to determine the effect of folic acid supplementation on birth defects. Considering the high compliance of the weekly iron supplementation regimen, it is worth assessing the feasibility and impact of this scheme on birth defects. Recent studies suggest periconceptional micronutrient supplementation may have a potential protective effect against paediatric cancers and folic acid may be conferring the protective effect (Goh 2007); these areas also merit further research attention.
What's new
| Date | Event | Description |
|---|---|---|
| 7 September 2015 | New search has been performed | Search updated. A new author joined the review team and another author stepped down. Methods were updated and random‐effects models were used for all meta‐analyses. GRADE 'Summary of findings' tables were also added for primary outcomes. |
| 7 September 2015 | New citation required but conclusions have not changed | No new trials met the inclusion criteria and thus the conclusions did not change. |
Acknowledgements
We would like to thank Dr Therese Dowswell for her contribution to co‐writing and for providing technical support and guidance on the previous version of this review. We are also grateful to Dr Erika Ota for helping us produce the 'Summary of findings' tables.
The World Health Organization (WHO) and Luz Maria De‐Regil and Ana Cecilia Fernandez‐Gaxiola retain copyright and all other rights in their respective contributions to the manuscript of this updated review as submitted for publication.
Erika Ota helped with the preparation of the 'Summary of findings' tables. Erika Ota's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), WHO. The named authors alone are responsible for the views expressed in this publication.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Additional search terms for the International Clinical Trials Registry Platform
folic acid AND women; folate AND women; folate AND periconception; folic acid supplementation AND women
Data and analyses
Comparison 1. Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neural tube defects (All) | 5 | 6708 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.58] |
| 2 Neural tube defects (by scheme) | 5 | 6708 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.58] |
| 2.1 Daily supplementation | 5 | 6708 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.58] |
| 3 Neural tube defects (by daily dose) | 5 | 6708 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.58] |
| 3.1 400 µg (0.4 mg) folic acid or less per day | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 4.21] |
| 3.2 More than 400 µg (0.4 mg) folic acid per day | 4 | 6447 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.17, 0.60] |
| 4 Neural tube defects (by timing of supplementation) | 5 | 6708 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.58] |
| 4.1 Periconceptional period | 5 | 6708 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.58] |
| 5 Neural tube defects (by history of an affected pregnancy ‐recurrence) | 5 | 6708 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.58] |
| 5.1 Yes | 4 | 1846 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.18, 0.64] |
| 5.2 No | 1 | 4862 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.32] |
| 6 Neural tube defects (by folate compound) | 5 | 6708 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.58] |
| 6.1 Folic acid | 5 | 6708 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.58] |
| 7 Neural tube defects (by micronutrient composition) | 5 | 6623 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.18, 0.63] |
| 7.1 Folate alone | 2 | 709 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.08, 0.73] |
| 7.2 Folate + multiple micronutrients | 4 | 5914 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.18, 0.85] |
| 8 Neural tube defects (by malaria setting at time of the study) | 5 | 6708 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.58] |
| 8.1 No | 5 | 6708 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.58] |
| 9 Cleft lip (All) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.14, 4.36] |
| 10 Cleft lip (by scheme) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.14, 4.36] |
| 10.1 Daily supplementation | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.14, 4.36] |
| 11 Cleft lip (by daily dose) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.14, 4.36] |
| 11.1 400 µg (0.4 mg) folic acid or less per day | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 4.21] |
| 11.2 More than 400 µg (0.4 mg) folic acid per day | 2 | 5351 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.29, 5.80] |
| 12 Cleft lip (by timing of supplementation) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.14, 4.36] |
| 12.1 Periconceptional period | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.14, 4.36] |
| 13 Cleft lip (by history of an affected pregnancy ‐recurrence) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.14, 4.36] |
| 13.1 Yes | 2 | 1456 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 4.21] |
| 13.2 No | 1 | 4156 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.29, 5.80] |
| 14 Cleft lip (by folate compound) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.14, 4.36] |
| 14.1 Folic acid | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.14, 4.36] |
| 15 Cleft lip (by micronutrient composition) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 15.1 Folate alone | 1 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Folate + multiple micronutrients | 3 | 4929 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 16 Cleft lip (by malaria setting at the time of the study) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.14, 4.36] |
| 16.1 No | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.14, 4.36] |
| 17 Cleft palate (All) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 18 Cleft palate (by scheme) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 18.1 Daily supplementation | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 19 Cleft palate (by daily dose) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 19.1 More than 400 µg (0.4 mg) folic acid per day | 2 | 5351 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 19.2 400 µg (0.4 mg) folic acid or less per day | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Cleft palate (by timing of supplementation) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 20.1 Periconceptional period | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 21 Cleft palate (by history of an affected pregnancy ‐recurrence) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 21.1 Yes | 2 | 1456 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 21.2 No | 1 | 4156 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.06] |
| 22 Cleft palate (by folate compound) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 22.1 Folic acid | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 23 Cleft palate (by micronutrient composition) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.76] |
| 23.1 Folate alone | 1 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 3.02 [0.12, 73.84] |
| 23.2 Folate + multiple micronutrients | 3 | 5014 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.06] |
| 24 Cleft palate (by malaria setting at the time of the study) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 24.1 No | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 25 Congenital cardiovascular anomalies (All) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.33] |
| 26 Congenital cardiovascular anomalies (by scheme) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.33] |
| 26.1 Daily supplementation | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.33] |
| 27 Congenital cardiovascular anomalies (by daily dose) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.33] |
| 27.1 400 µg (0.4 mg) folic acid or less per day | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 2.14] |
| 27.2 More than 400 µg (0.4 mg) folic acid per day | 2 | 5351 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.27, 1.60] |
| 28 Congenital cardiovascular anomalies (by timing of supplementation) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.33] |
| 28.1 Periconceptional period | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.33] |
| 29 Congenital cardiovascular anomalies (by history of an affected pregnancy ‐recurrence)) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.33] |
| 29.1 Yes | 2 | 1456 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.08, 2.11] |
| 29.2 No | 1 | 4156 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.23, 1.82] |
| 30 Congenital cardiovascular anomalies (by folate compound) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.33] |
| 30.1 Folic acid | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.33] |
| 31 Congenital cardiovascular anomalies (by micronutrient composition) | 2 | 5351 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.27, 1.60] |
| 31.1 Folate alone | 1 | 1195 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 4.04] |
| 31.2 Folate + multiple micronutrients | 1 | 4156 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.23, 1.82] |
| 32 Congenital cardiovascular anomalies (by malaria setting at the time of the study) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.33] |
| 32.1 No | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.33] |
| 33 Other congenital anomalies (All) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.53, 1.66] |
| 34 Other congenital anomalies (by scheme) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.53, 1.66] |
| 34.1 Daily supplementation | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.53, 1.66] |
| 35 Other congenital anomalies (by daily dose) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.53, 1.66] |
| 35.1 400 µg (0.4 mg) folic acid or less per day | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.32, 2.30] |
| 35.2 More than 400 µg (0.4 mg) folic acid per day | 2 | 5351 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.40, 2.42] |
| 36 Other congenital anomalies (by timing of supplementation) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.53, 1.66] |
| 36.1 Periconceptional period | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.53, 1.66] |
| 37 Other congenital anomalies (by history of an affected pregnancy ‐recurrence) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.53, 1.66] |
| 37.1 Yes | 2 | 1456 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.69, 2.34] |
| 37.2 No | 1 | 4156 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.36, 1.18] |
| 38 Other congenital anomalies (by folate compound) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.53, 1.66] |
| 38.1 Folic acid | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.53, 1.66] |
| 39 Other congenital anomalies (by micronutrient composition) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.58, 1.51] |
| 39.1 Folate alone | 1 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.34, 4.64] |
| 39.2 Folate + other micronutrients | 3 | 5014 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.50, 1.71] |
| 40 Other congenital anomalies (by malaria setting at the time of the study) | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.53, 1.66] |
| 40.1 No | 3 | 5612 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.53, 1.66] |
| 41 Miscarriage (All) | 5 | 7391 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.94, 1.28] |
| 42 Miscarriage (by scheme) | 5 | 7391 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.94, 1.28] |
| 42.1 Daily supplementation | 5 | 7391 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.94, 1.28] |
| 43 Miscarriage (by daily dose) | 5 | 7391 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.94, 1.28] |
| 43.1 400 µg (0.4 mg) folic acid or less per day | 1 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.57, 3.03] |
| 43.2 More than 400 µg (0.4 mg) folic acid per day | 4 | 7110 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.86, 1.31] |
| 44 Miscarriage (by timing of supplementation) | 5 | 7391 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.94, 1.28] |
| 44.1 Periconceptional period | 5 | 7391 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.94, 1.28] |
| 45 Miscarriage (by history of an affected pregnancy ‐recurrence) | 5 | 7391 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.94, 1.28] |
| 45.1 Yes | 4 | 2033 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.68, 1.40] |
| 45.2 No | 1 | 5358 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.98, 1.34] |
| 46 Miscarriage (by folate compound) | 5 | 7391 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.94, 1.28] |
| 46.1 Folic acid | 5 | 7391 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.94, 1.28] |
| 47 Miscarriage (by micronutrient compound) | 5 | 7283 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.90, 1.28] |
| 47.1 Folate alone | 2 | 795 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.66, 1.44] |
| 47.2 Folate + multiple micronutrients | 4 | 6488 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.74, 1.42] |
| 48 Miscarriage (by malaria setting at the time of the study) | 5 | 7370 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.77, 1.33] |
| 48.1 No | 5 | 7370 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.77, 1.33] |
| 49 Spina bifida | 3 | 4546 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.06, 1.62] |
| 50 Anencephaly | 4 | 4807 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.13, 1.03] |
| 51 Stillbirths | 4 | 6597 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.54, 2.05] |
| 52 Elective termination of pregnancy for fetal anomalies | 4 | 7110 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.15, 0.56] |
| 53 Low birthweight (less than 2500 g) | 2 | 5048 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.84, 1.52] |
| 54 Macrosomia (birthweight greater than 4000 g) | 1 | 4862 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 55 Preterm birth (less than 37 weeks' gestation) | 1 | 4862 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.93, 1.41] |
| 56 Multiple pregnancy | 4 | 7280 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.89, 2.14] |
1.2. Analysis.
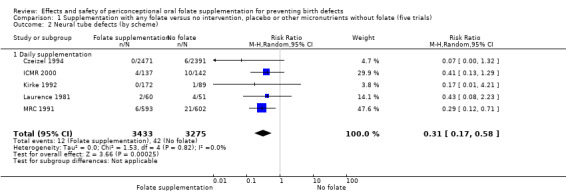
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 2 Neural tube defects (by scheme).
1.4. Analysis.
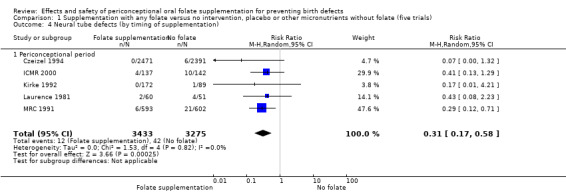
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 4 Neural tube defects (by timing of supplementation).
1.6. Analysis.
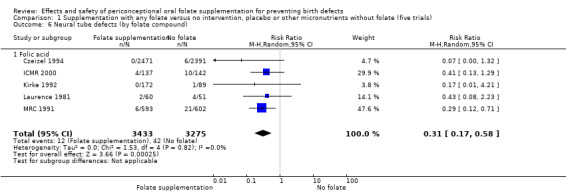
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 6 Neural tube defects (by folate compound).
1.7. Analysis.
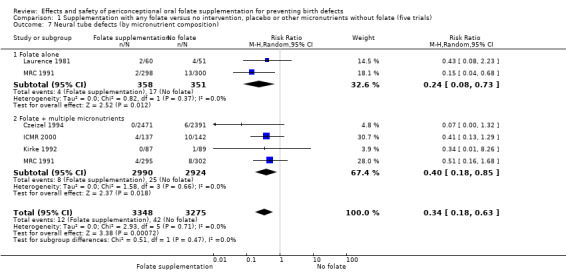
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 7 Neural tube defects (by micronutrient composition).
1.8. Analysis.
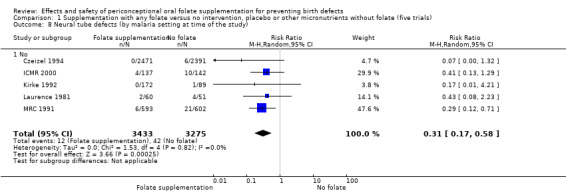
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 8 Neural tube defects (by malaria setting at time of the study).
1.10. Analysis.
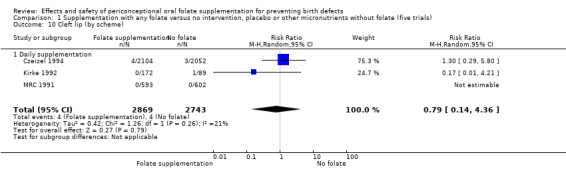
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 10 Cleft lip (by scheme).
1.11. Analysis.
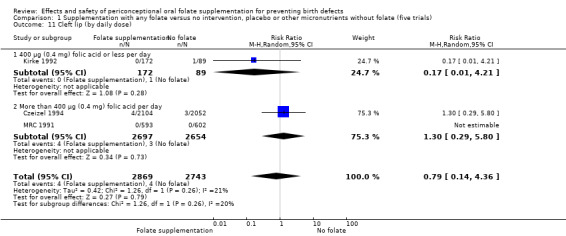
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 11 Cleft lip (by daily dose).
1.12. Analysis.
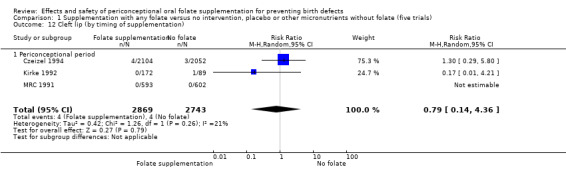
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 12 Cleft lip (by timing of supplementation).
1.13. Analysis.
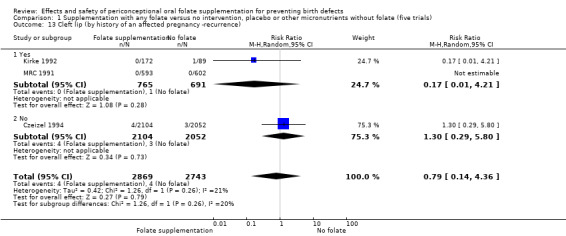
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 13 Cleft lip (by history of an affected pregnancy ‐recurrence).
1.14. Analysis.
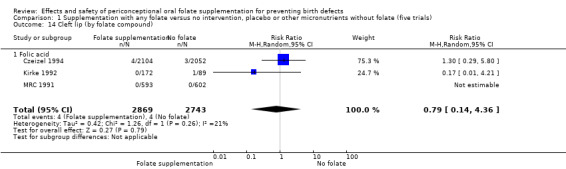
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 14 Cleft lip (by folate compound).
1.15. Analysis.
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 15 Cleft lip (by micronutrient composition).
1.16. Analysis.
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 16 Cleft lip (by malaria setting at the time of the study).
1.18. Analysis.
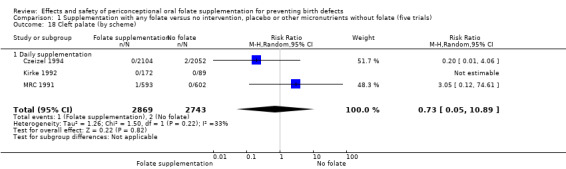
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 18 Cleft palate (by scheme).
1.19. Analysis.
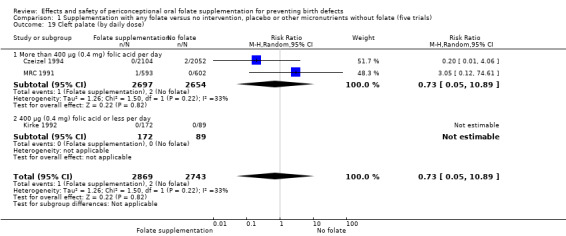
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 19 Cleft palate (by daily dose).
1.20. Analysis.
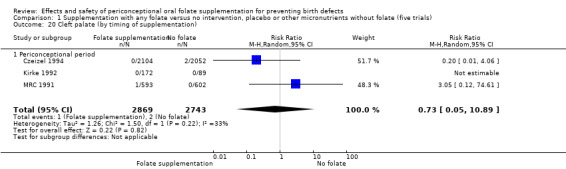
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 20 Cleft palate (by timing of supplementation).
1.21. Analysis.
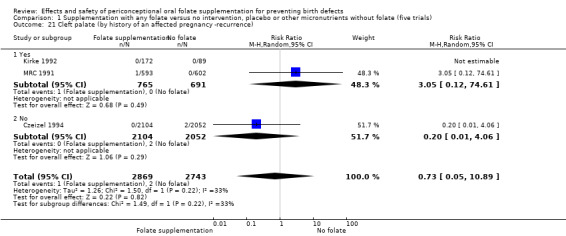
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 21 Cleft palate (by history of an affected pregnancy ‐recurrence).
1.22. Analysis.
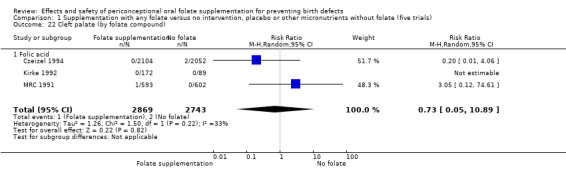
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 22 Cleft palate (by folate compound).
1.23. Analysis.
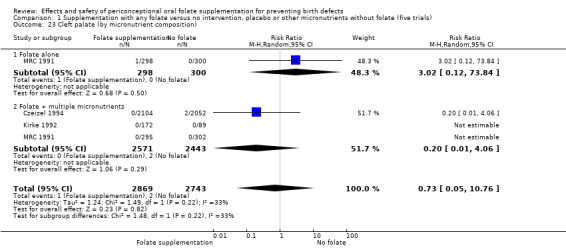
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 23 Cleft palate (by micronutrient composition).
1.24. Analysis.
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 24 Cleft palate (by malaria setting at the time of the study).
1.26. Analysis.
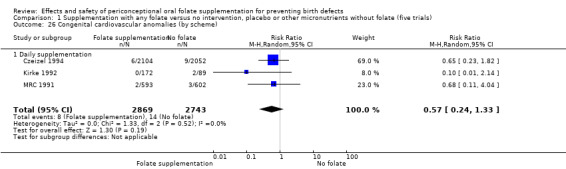
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 26 Congenital cardiovascular anomalies (by scheme).
1.27. Analysis.
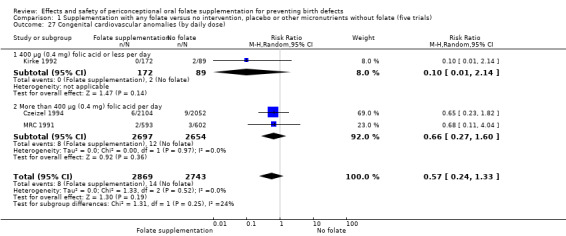
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 27 Congenital cardiovascular anomalies (by daily dose).
1.28. Analysis.
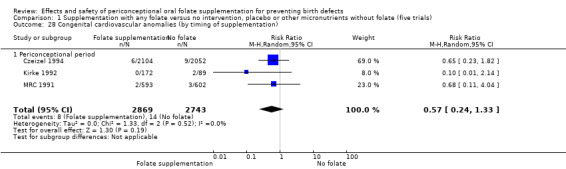
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 28 Congenital cardiovascular anomalies (by timing of supplementation).
1.29. Analysis.
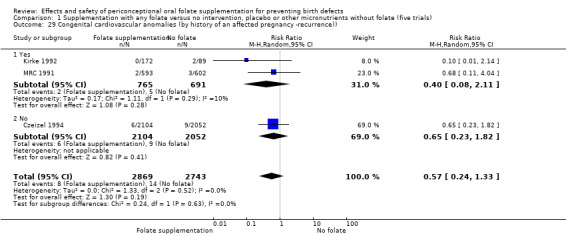
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 29 Congenital cardiovascular anomalies (by history of an affected pregnancy ‐recurrence)).
1.30. Analysis.
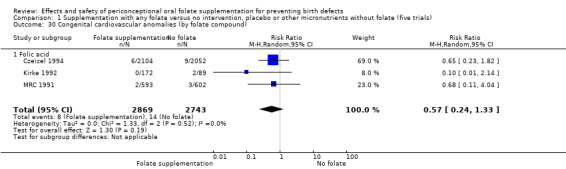
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 30 Congenital cardiovascular anomalies (by folate compound).
1.31. Analysis.
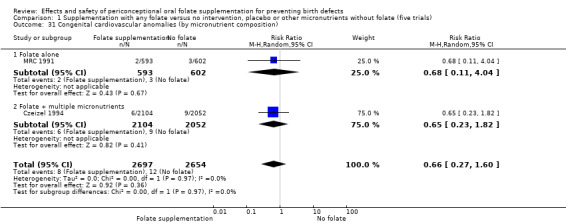
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 31 Congenital cardiovascular anomalies (by micronutrient composition).
1.32. Analysis.
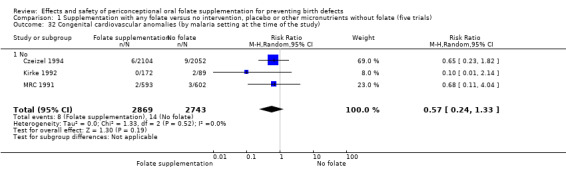
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 32 Congenital cardiovascular anomalies (by malaria setting at the time of the study).
1.34. Analysis.
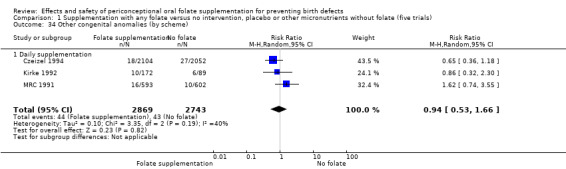
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 34 Other congenital anomalies (by scheme).
1.35. Analysis.
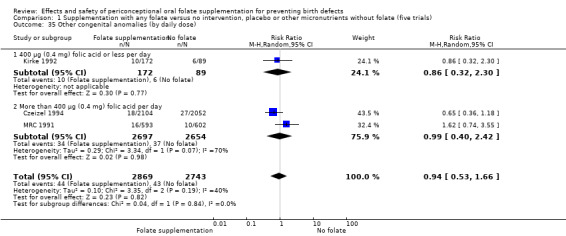
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 35 Other congenital anomalies (by daily dose).
1.36. Analysis.
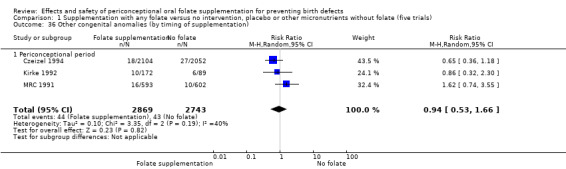
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 36 Other congenital anomalies (by timing of supplementation).
1.37. Analysis.
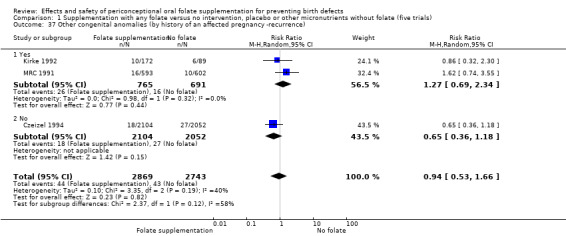
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 37 Other congenital anomalies (by history of an affected pregnancy ‐recurrence).
1.38. Analysis.
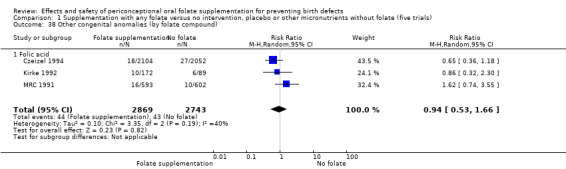
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 38 Other congenital anomalies (by folate compound).
1.39. Analysis.
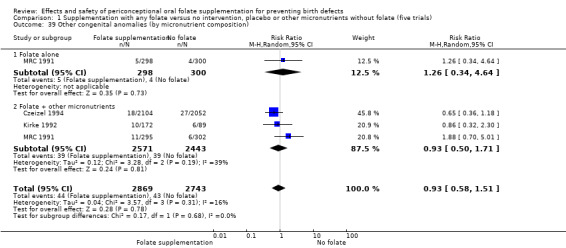
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 39 Other congenital anomalies (by micronutrient composition).
1.40. Analysis.
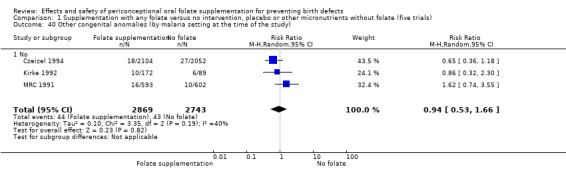
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 40 Other congenital anomalies (by malaria setting at the time of the study).
1.42. Analysis.
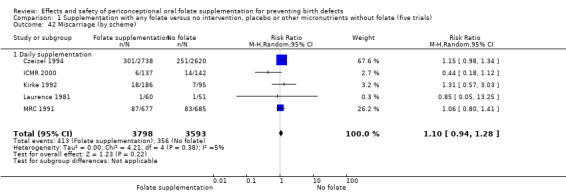
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 42 Miscarriage (by scheme).
1.43. Analysis.
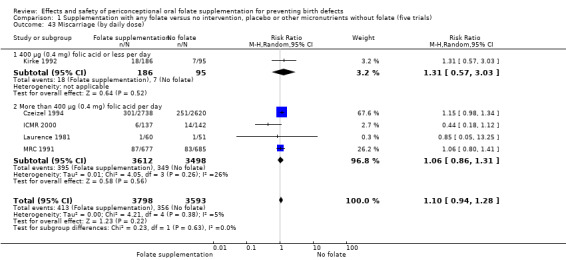
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 43 Miscarriage (by daily dose).
1.44. Analysis.
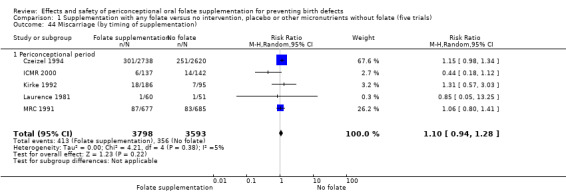
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 44 Miscarriage (by timing of supplementation).
1.45. Analysis.
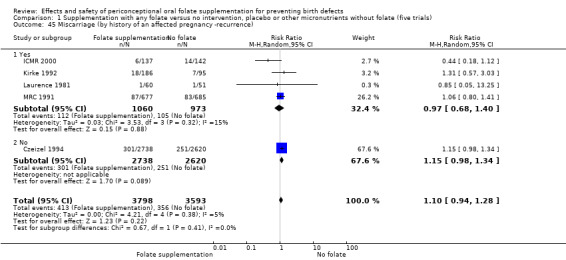
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 45 Miscarriage (by history of an affected pregnancy ‐recurrence).
1.46. Analysis.
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 46 Miscarriage (by folate compound).
1.47. Analysis.
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 47 Miscarriage (by micronutrient compound).
1.48. Analysis.
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 48 Miscarriage (by malaria setting at the time of the study).
1.55. Analysis.
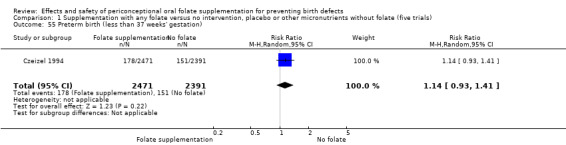
Comparison 1 Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (five trials), Outcome 55 Preterm birth (less than 37 weeks' gestation).
Comparison 2. Supplementation with folic acid alone versus no treatment or placebo (one trial).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neural tube defects (All) | 1 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.08, 2.23] |
| 2 Miscarriage (All) | 1 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.05, 13.25] |
| 3 Spina bifida | 1 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.04, 4.55] |
| 4 Anencephaly | 1 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.04, 4.55] |
| 5 Elective termination of pregnancy for fetal anomalies | 1 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 6.83] |
Comparison 3. Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neural tube defects (All) | 4 | 6512 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.60] |
| 2 Neural tube defects (by scheme) | 4 | 6512 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.60] |
| 2.1 Daily supplementation | 4 | 6512 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.60] |
| 3 Neural tube defects (by daily dose) | 4 | 6512 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.60] |
| 3.1 400 µg (0.4 mg) folic acid or less per day | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 3.2 More than 400 µg (0.4 mg) folic acid per day | 3 | 6336 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.15, 0.61] |
| 4 Neural tube defects (by timing of supplementation) | 4 | 6512 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.60] |
| 4.1 Periconceptional period | 4 | 6512 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.60] |
| 5 Neural tube defects (by history of an affected pregnancy ‐recurrence) | 4 | 6512 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.60] |
| 5.1 Yes | 3 | 1650 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.17, 0.66] |
| 5.2 No | 1 | 4862 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.32] |
| 6 Neural tube defects (by folate compound) | 4 | 6513 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.60] |
| 6.1 Folic acid | 4 | 6513 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.60] |
| 7 Neural tube defects (by micronutrient composition) | 4 | 6512 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.16, 0.64] |
| 7.1 Folate alone | 1 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.04, 0.68] |
| 7.2 Folate + multiple micronutrients | 4 | 5914 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.18, 0.85] |
| 8 Neural tube defects (by malaria setting at time of the study) | 4 | 6512 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.60] |
| 8.1 No | 4 | 6512 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.60] |
| 9 Cleft lip (All) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 10 Cleft lip (by scheme) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 10.1 Daily supplementation | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 11 Cleft lip (by daily dose) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 11.1 400 µg (0.4 mg) folic acid or less per day | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 11.2 More than 400 µg (0.4 mg) folic acid per day | 2 | 5351 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.29, 5.80] |
| 12 Cleft lip (by timing of supplementation) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 12.1 Periconceptional period | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 13 Cleft lip (by history of an affected pregnancy ‐recurrence) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 13.1 Yes | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 13.2 No | 1 | 4156 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.29, 5.80] |
| 14 Cleft lip (by folate compound) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 14.1 Folic acid | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 15 Cleft lip (by micronutrient composition) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 15.1 Folate alone | 1 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Folate + multiple micronutrients | 3 | 4929 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 16 Cleft lip (by malaria setting at the time of the study) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 16.1 No | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.96] |
| 17 Cleft palate (All) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 18 Cleft palate (by scheme) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 18.1 Daily supplementation | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 19 Cleft palate (by daily dose) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 19.1 400 µg (0.4 mg) folic acid or less per day | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 More than 400 µg (0.4 mg) folic acid per day | 2 | 5351 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 20 Cleft palate (by timing of supplementation) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 20.1 Periconceptional period | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 21 Cleft palate (by history of an affected pregnancy ‐recurrence) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 21.1 Yes | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 21.2 No | 1 | 4156 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.06] |
| 22 Cleft palate (by folate compound) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 22.1 Folic acid | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 23 Cleft palate (by micronutrient composition) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.76] |
| 23.1 Folate alone | 1 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 3.02 [0.12, 73.84] |
| 23.2 Folate + multiple micronutrients | 3 | 4929 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.06] |
| 24 Cleft palate (by malaria setting at the time of the study) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 24.1 No | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.89] |
| 25 Congenital cardiovascular anomalies (All) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.41] |
| 26 Congenital cardiovascular anomalies (by scheme) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.41] |
| 26.1 Daily supplementation | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.41] |
| 27 Congenital cardiovascular anomalies (by daily dose) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.41] |
| 27.1 400 µg (0.4 mg) folic acid or less per day | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.20] |
| 27.2 More than 400 µg (0.4 mg) folic acid per day | 2 | 5351 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.27, 1.60] |
| 28 Congenital cardiovascular anomalies (by timing of supplementation) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.41] |
| 28.1 Periconceptional period | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.41] |
| 29 Congenital cardiovascular anomalies (by history of an affected pregnancy ‐recurrence)) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.41] |
| 29.1 Yes | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.31] |
| 29.2 No | 1 | 4156 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.23, 1.82] |
| 30 Congenital cardiovascular anomalies (by folate compound) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.41] |
| 30.1 Folic acid | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.41] |
| 31 Congenital cardiovascular anomalies (by micronutrient composition) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.41] |
| 31.1 Folate alone | 1 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.06, 16.02] |
| 31.2 Folate + multiple micronutrients | 3 | 4929 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.23, 1.40] |
| 32 Congenital cardiovascular anomalies (by malaria setting at the time of the study) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.41] |
| 32.1 No | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.41] |
| 33 Other congenital anomalies (All) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.54, 1.77] |
| 34 Other congenital anomalies (by scheme) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.54, 1.77] |
| 34.1 Daily supplementation | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.54, 1.77] |
| 35 Other congenital anomalies (by daily dose) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.54, 1.77] |
| 35.1 400 µg (0.4 mg) folic acid or less per day | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.34, 3.05] |
| 35.2 More than 400 µg (0.4 mg) folic acid per day | 2 | 5351 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.40, 2.42] |
| 36 Other congenital anomalies (by timing of supplementation) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.54, 1.77] |
| 36.1 Periconceptional period | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.54, 1.77] |
| 37 Other congenital anomalies (by history of an affected pregnancy ‐recurrence) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.54, 1.77] |
| 37.1 Yes | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.74, 2.62] |
| 37.2 No | 1 | 4156 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.36, 1.18] |
| 38 Other congenital anomalies (by folate compound) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.54, 1.77] |
| 38.1 Folic acid | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.54, 1.77] |
| 39 Other congenital anomalies (by micronutrient composition) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.59, 1.60] |
| 39.1 Folate alone | 1 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.34, 4.64] |
| 39.2 Folate + other micronutrients | 3 | 4929 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.51, 1.86] |
| 40 Other congenital anomalies (by malaria setting at the time of the study) | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.54, 1.77] |
| 40.1 No | 3 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.54, 1.77] |
| 41 Miscarriage (All) | 4 | 7187 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.87, 1.32] |
| 42 Miscarriage (by scheme) | 4 | 7187 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.87, 1.32] |
| 42.1 Daily supplementation | 4 | 7187 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.87, 1.32] |
| 43 Miscarriage (by daily dose) | 4 | 7187 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.87, 1.32] |
| 43.1 400 µg (0.4 mg) folic acid or less per day | 1 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.51, 3.38] |
| 43.2 More than 400 µg (0.4 mg) folic acid per day | 3 | 6999 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.81, 1.35] |
| 44 Miscarriage (by timing of supplementation) | 4 | 7187 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.87, 1.32] |
| 44.1 Periconceptional period | 4 | 7187 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.87, 1.32] |
| 45 Miscarriage (by history of an affected pregnancy ‐recurrence) | 4 | 7187 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.87, 1.32] |
| 45.1 Yes | 3 | 1829 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.56, 1.53] |
| 45.2 No | 1 | 5358 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.98, 1.34] |
| 46 Miscarriage (by folate compound) | 4 | 7187 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.87, 1.32] |
| 46.1 Folic acid | 4 | 7187 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.87, 1.32] |
| 47 Miscarriage (by micronutrient compound) | 4 | 7187 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.92, 1.29] |
| 47.1 Folate alone | 1 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.66, 1.45] |
| 47.2 Folate + multiple micronutrients | 4 | 6503 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.85, 1.39] |
| 48 Miscarriage (by malaria setting at the time of the study) | 4 | 7187 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.87, 1.32] |
| 48.1 No | 4 | 7187 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.87, 1.32] |
| 49 Spina bifida | 2 | 4435 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.31] |
| 50 Anencephaly | 3 | 4611 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.12, 1.22] |
| 51 Stillbirths | 4 | 6512 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.56, 2.12] |
| 52 Elective termination of pregnancy for fetal anomalies | 3 | 6999 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.15, 0.57] |
| 53 Low birthweight (less than 2500 g) | 2 | 5048 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.84, 1.52] |
| 54 Macrosomia (birthweight greater than 4000 g) | 1 | 4862 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 55 Preterm birth (less than 37 weeks' gestation) | 1 | 4862 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.93, 1.41] |
| 56 Multiple pregnancy | 4 | 7187 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.90, 2.17] |
3.2. Analysis.
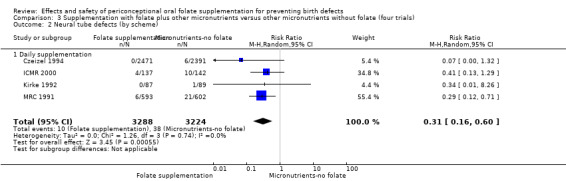
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 2 Neural tube defects (by scheme).
3.4. Analysis.
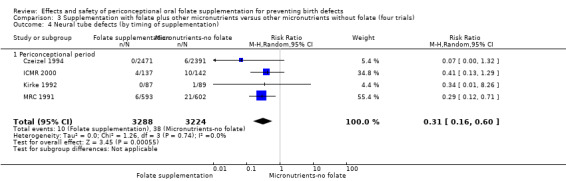
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 4 Neural tube defects (by timing of supplementation).
3.6. Analysis.
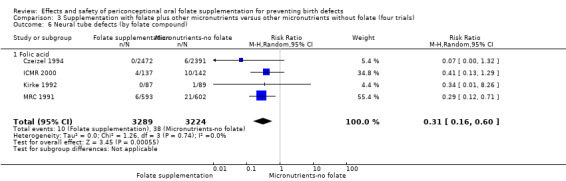
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 6 Neural tube defects (by folate compound).
3.7. Analysis.
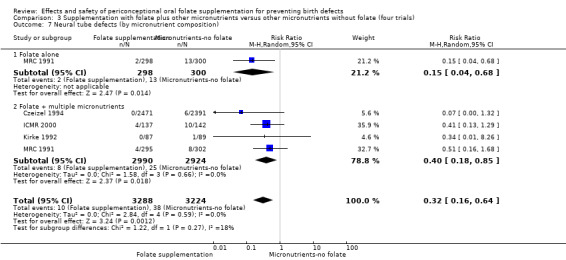
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 7 Neural tube defects (by micronutrient composition).
3.8. Analysis.
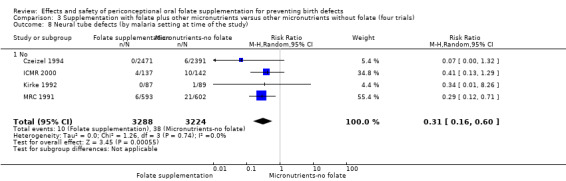
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 8 Neural tube defects (by malaria setting at time of the study).
3.10. Analysis.
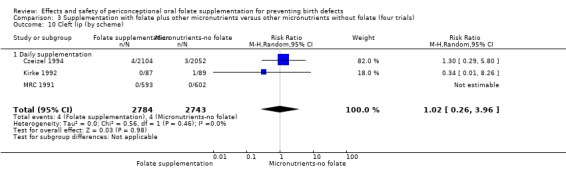
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 10 Cleft lip (by scheme).
3.11. Analysis.
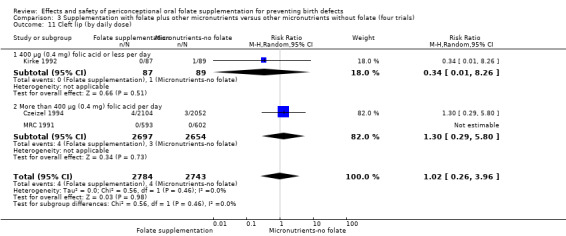
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 11 Cleft lip (by daily dose).
3.12. Analysis.
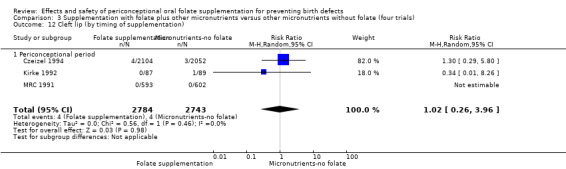
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 12 Cleft lip (by timing of supplementation).
3.13. Analysis.
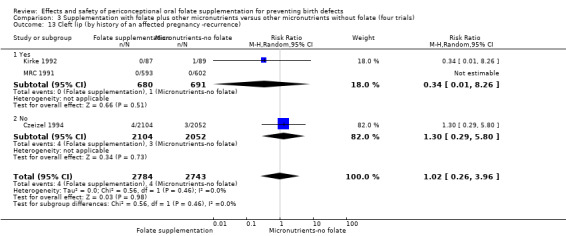
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 13 Cleft lip (by history of an affected pregnancy ‐recurrence).
3.14. Analysis.
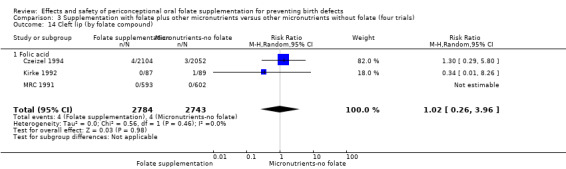
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 14 Cleft lip (by folate compound).
3.15. Analysis.
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 15 Cleft lip (by micronutrient composition).
3.16. Analysis.
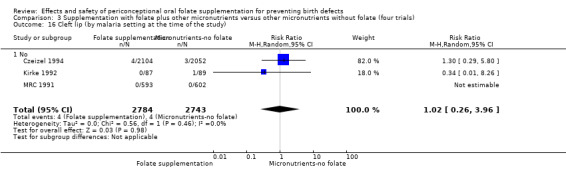
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 16 Cleft lip (by malaria setting at the time of the study).
3.18. Analysis.
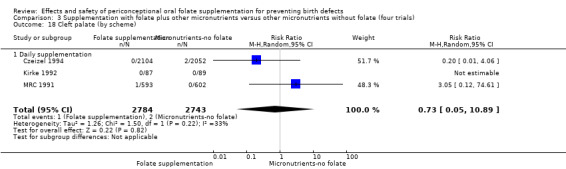
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 18 Cleft palate (by scheme).
3.19. Analysis.
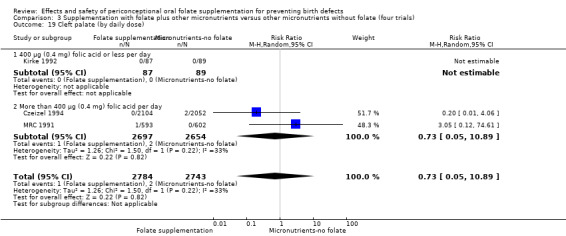
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 19 Cleft palate (by daily dose).
3.20. Analysis.
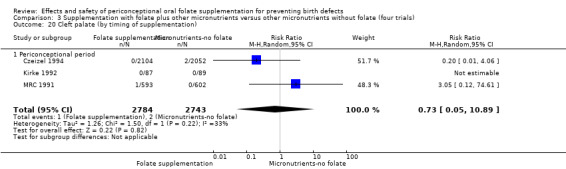
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 20 Cleft palate (by timing of supplementation).
3.21. Analysis.
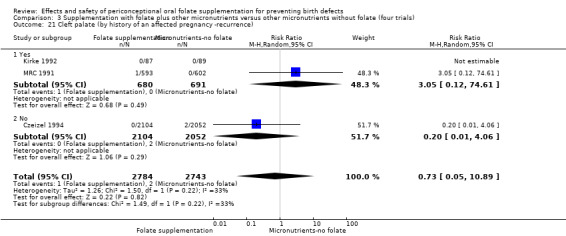
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 21 Cleft palate (by history of an affected pregnancy ‐recurrence).
3.22. Analysis.
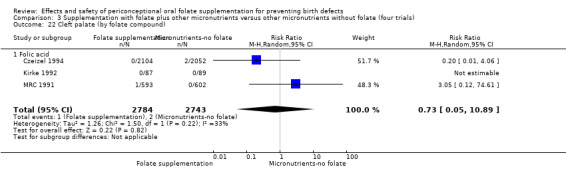
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 22 Cleft palate (by folate compound).
3.23. Analysis.
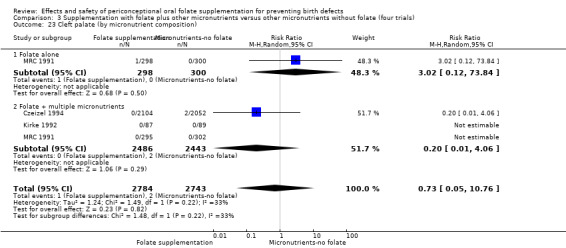
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 23 Cleft palate (by micronutrient composition).
3.24. Analysis.
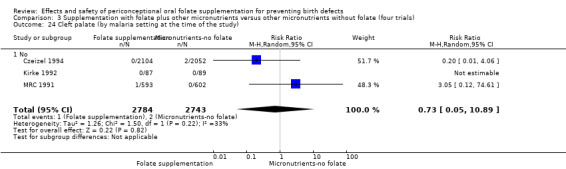
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 24 Cleft palate (by malaria setting at the time of the study).
3.26. Analysis.
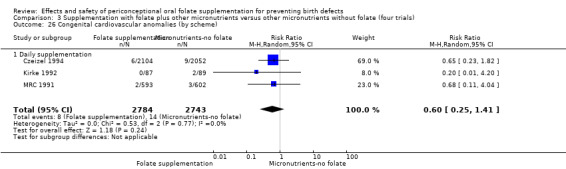
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 26 Congenital cardiovascular anomalies (by scheme).
3.27. Analysis.
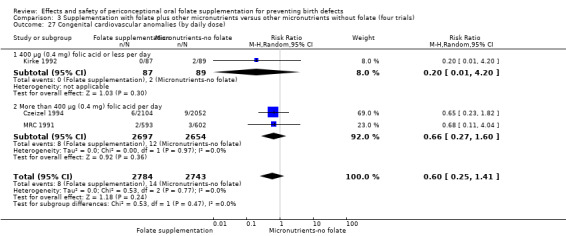
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 27 Congenital cardiovascular anomalies (by daily dose).
3.28. Analysis.
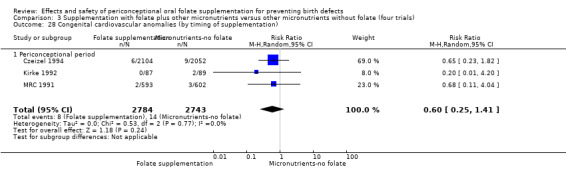
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 28 Congenital cardiovascular anomalies (by timing of supplementation).
3.29. Analysis.
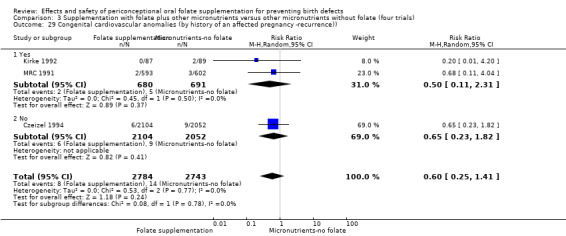
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 29 Congenital cardiovascular anomalies (by history of an affected pregnancy ‐recurrence)).
3.30. Analysis.
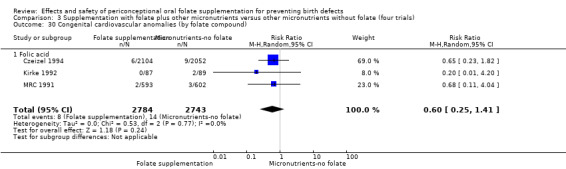
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 30 Congenital cardiovascular anomalies (by folate compound).
3.31. Analysis.
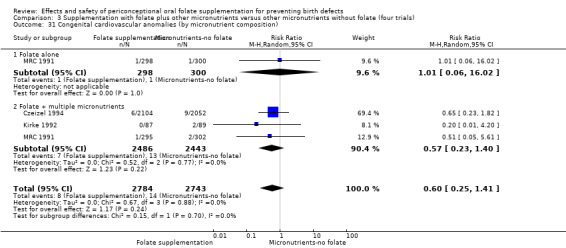
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 31 Congenital cardiovascular anomalies (by micronutrient composition).
3.32. Analysis.
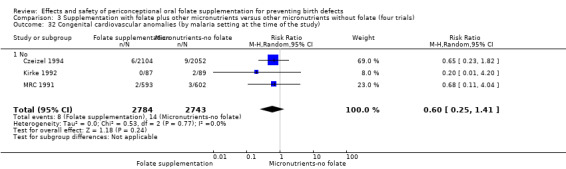
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 32 Congenital cardiovascular anomalies (by malaria setting at the time of the study).
3.33. Analysis.
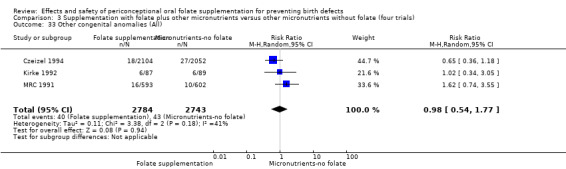
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 33 Other congenital anomalies (All).
3.34. Analysis.
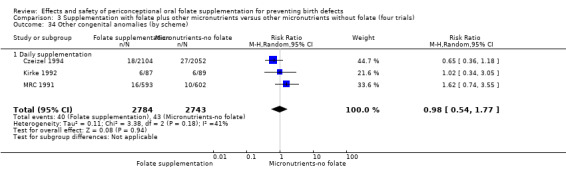
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 34 Other congenital anomalies (by scheme).
3.35. Analysis.
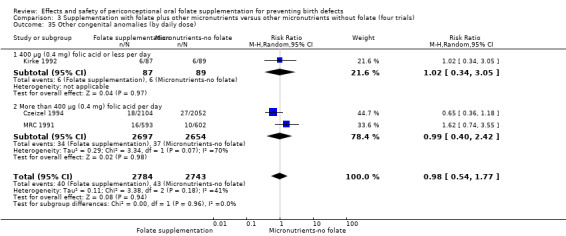
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 35 Other congenital anomalies (by daily dose).
3.38. Analysis.
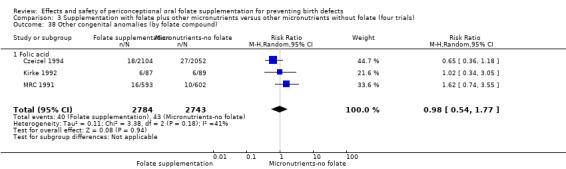
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 38 Other congenital anomalies (by folate compound).
3.39. Analysis.
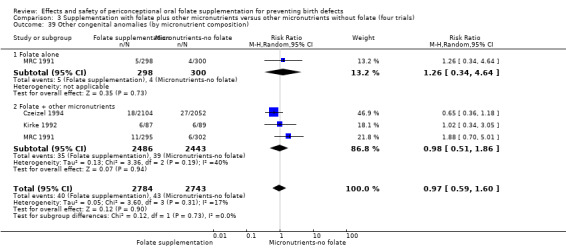
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 39 Other congenital anomalies (by micronutrient composition).
3.40. Analysis.
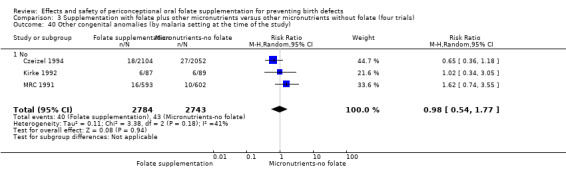
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 40 Other congenital anomalies (by malaria setting at the time of the study).
3.43. Analysis.
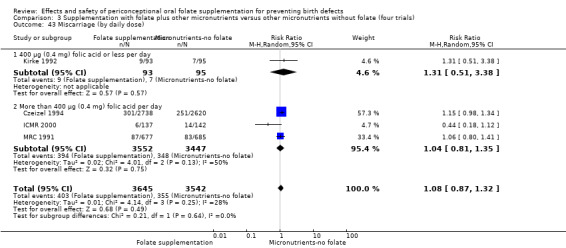
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 43 Miscarriage (by daily dose).
3.44. Analysis.
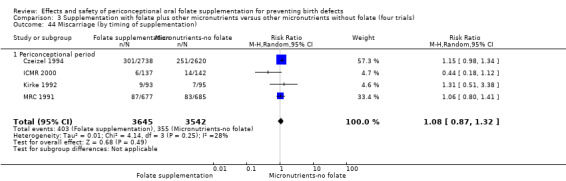
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 44 Miscarriage (by timing of supplementation).
3.45. Analysis.
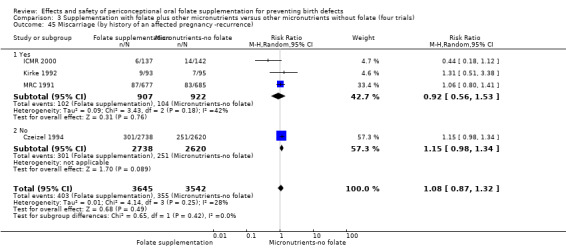
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 45 Miscarriage (by history of an affected pregnancy ‐recurrence).
3.46. Analysis.
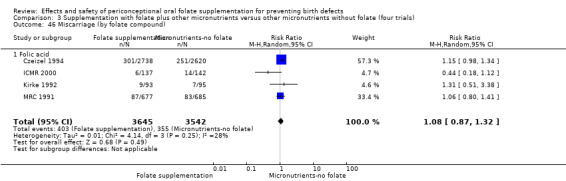
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 46 Miscarriage (by folate compound).
3.47. Analysis.
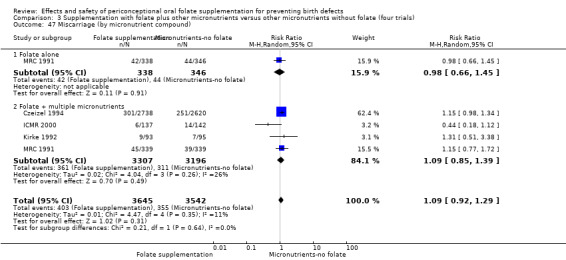
Comparison 3 Supplementation with folate plus other micronutrients versus other micronutrients without folate (four trials), Outcome 47 Miscarriage (by micronutrient compound).
Comparison 4. Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neural tube defects (All) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 2 Neural tube defects (by scheme) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 2.1 Daily supplementation | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 3 Neural tube defects (by daily dose) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 3.1 400 µg (0.4 mg) folic acid or less per day | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 3.2 More than 400 µg (0.4 mg) folic acid per day | 1 | 1195 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.71] |
| 4 Neural tube defects (by timing of supplementation) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 4.1 Periconceptional period | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 5 Neural tube defects (by history of an affected pregnancy ‐recurrence) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 5.1 Yes | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 6 Neural tube defects (by folate compound) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 6.1 Folic acid | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 7 Neural tube defects (by micronutrient composition) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.13, 0.78] |
| 7.1 Folate alone | 1 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.04, 0.68] |
| 7.2 Folate + multiple micronutrients | 2 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.16, 1.48] |
| 8 Neural tube defects (by malaria setting at time of the study) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 8.1 No | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 9 Cleft lip (All) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 10 Cleft lip (by scheme) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 10.1 Daily supplementation | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 11 Cleft lip (by daily dose) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 11.1 400 µg (0.4 mg) folic acid or less per day | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 11.2 More than 400 µg (0.4 mg) folic acid per day | 1 | 1195 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Cleft lip (by timing of supplementation) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 12.1 Periconceptional period | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 13 Cleft lip (by history of an affected pregnancy ‐recurrence) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 13.1 Yes | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 14 Cleft lip (by folate compound) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 14.1 Folic acid | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 15 Cleft lip (by micronutrient composition) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 15.1 Folate alone | 1 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Folate + multiple micronutrients | 2 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 16 Cleft lip (by malaria setting at the time of the study) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 16.1 No | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 17 Cleft palate (All) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 18 Cleft palate (by scheme) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 18.1 Daily supplementation | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 19 Cleft palate (by daily dose) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 19.1 400 µg (0.4 mg) folic acid or less per day | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 More than 400 µg (0.4 mg) folic acid per day | 1 | 1195 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 20 Cleft palate (by timing of supplementation) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 20.1 Periconceptional period | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 21 Cleft palate (by history of an affected pregnancy ‐recurrence) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 21.1 Yes | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 22 Cleft palate (by folate compound) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 22.1 Folic acid | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 23 Cleft palate (by micronutrient composition) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 3.02 [0.12, 73.84] |
| 23.1 Folate alone | 1 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 3.02 [0.12, 73.84] |
| 23.2 Folate + multiple micronutrients | 2 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Cleft palate (by malaria setting at the time of the study) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 24.1 No | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 74.61] |
| 25 Congenital cardiovascular anomalies (All) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.31] |
| 26 Congenital cardiovascular anomalies (by scheme) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.31] |
| 26.1 Daily supplementation | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.31] |
| 27 Congenital cardiovascular anomalies (by daily dose) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.31] |
| 27.1 400 µg (0.4 mg) folic acid or less per day | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.20] |
| 27.2 More than 400 µg (0.4 mg) folic acid per day | 1 | 1195 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 4.04] |
| 28 Congenital cardiovascular anomalies (by timing of supplementation) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.31] |
| 28.1 Periconceptional period | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.31] |
| 29 Congenital cardiovascular anomalies (by history of an affected pregnancy ‐recurrence)) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.31] |
| 29.1 Yes | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.31] |
| 30 Congenital cardiovascular anomalies (by folate compound) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.31] |
| 30.1 Folic acid | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.31] |
| 31 Congenital cardiovascular anomalies (by micronutrient composition) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.35] |
| 31.1 Folate alone | 1 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.06, 16.02] |
| 31.2 Folate + multiple micronutrients | 2 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.05, 2.35] |
| 32 Congenital cardiovascular anomalies (by malaria setting at the time of the study) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.31] |
| 32.1 No | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.31] |
| 33 Other congenital anomalies (All) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.74, 2.62] |
| 34 Other congenital anomalies (by scheme) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.74, 2.62] |
| 34.1 Daily supplementation | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.74, 2.62] |
| 35 Other congenital anomalies (by daily dose) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.74, 2.62] |
| 35.1 400 µg (0.4 mg) folic acid or less per day | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.34, 3.05] |
| 35.2 More than 400 µg (0.4 mg) folic acid per day | 1 | 1195 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.74, 3.55] |
| 36 Other congenital anomalies (by timing of supplementation) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.74, 2.62] |
| 36.1 Periconceptional period | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.74, 2.62] |
| 37 Other congenital anomalies (by history of an affected pregnancy ‐recurrence) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.74, 2.62] |
| 37.1 Yes | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.74, 2.62] |
| 38 Other congenital anomalies (by folate compound) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.74, 2.62] |
| 38.1 Folic acid | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.74, 2.62] |
| 39 Other congenital anomalies (by micronutrient composition) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.73, 2.62] |
| 39.1 Folate alone | 1 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.34, 4.64] |
| 39.2 Folate + other micronutrients | 2 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.69, 2.97] |
| 40 Other congenital anomalies (by malaria setting at the time of the study) | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.74, 2.62] |
| 40.1 No | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.74, 2.62] |
| 41 Miscarriage (All) | 2 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.41] |
| 42 Miscarriage (by scheme) | 2 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.41] |
| 42.1 Daily supplementation | 2 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.41] |
| 43 Miscarriage (by daily dose) | 2 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.41] |
| 43.1 400 µg (0.4 mg) folic acid or less per day | 1 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.51, 3.38] |
| 43.2 More than 400 µg (0.4 mg) folic acid per day | 1 | 1362 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.80, 1.41] |
| 44 Miscarriage (by timing of supplementation) | 2 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.41] |
| 44.1 Periconceptional period | 2 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.41] |
| 45 Miscarriage (by history of an affected pregnancy ‐recurrence) | 2 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.41] |
| 45.1 Yes | 2 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.41] |
| 46 Miscarriage (by folate compound) | 2 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.41] |
| 46.1 Folic acid | 2 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.41] |
| 47 Miscarriage (by micronutrient compound) | 2 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.41] |
| 47.1 Folate alone | 1 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.66, 1.45] |
| 47.2 Folate + multiple micronutrients | 2 | 866 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.81, 1.70] |
| 48 Miscarriage (by malaria setting at the time of the study) | 2 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.41] |
| 48.1 No | 2 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.41] |
| 49 Anencephaly | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 50 Stillbirths | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.16, 4.28] |
| 51 Elective termination of pregnancy for fetal outcomes | 1 | 1362 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.15, 0.83] |
| 52 Multiple pregnancy | 2 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.19, 22.15] |
4.3. Analysis.
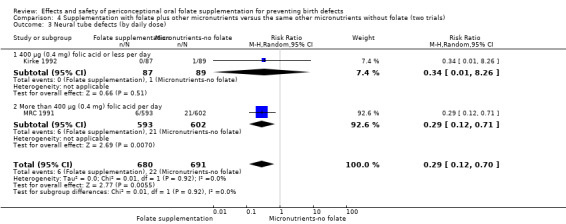
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 3 Neural tube defects (by daily dose).
4.4. Analysis.
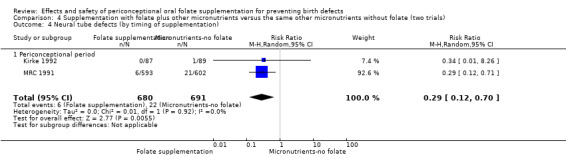
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 4 Neural tube defects (by timing of supplementation).
4.5. Analysis.
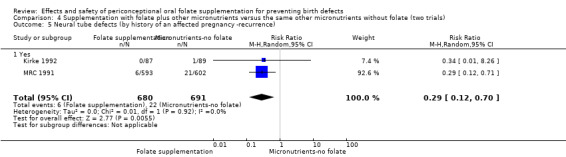
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 5 Neural tube defects (by history of an affected pregnancy ‐recurrence).
4.6. Analysis.
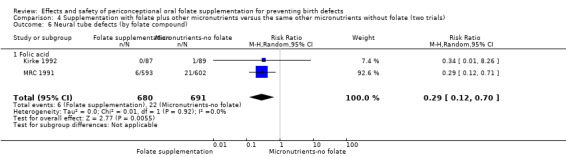
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 6 Neural tube defects (by folate compound).
4.7. Analysis.
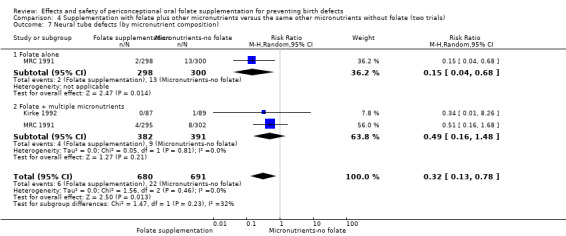
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 7 Neural tube defects (by micronutrient composition).
4.10. Analysis.
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 10 Cleft lip (by scheme).
4.11. Analysis.
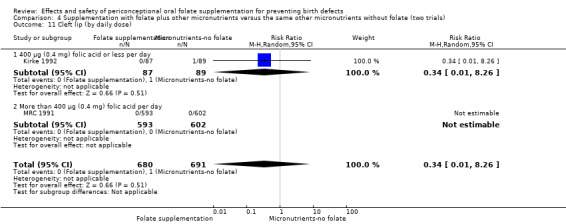
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 11 Cleft lip (by daily dose).
4.12. Analysis.
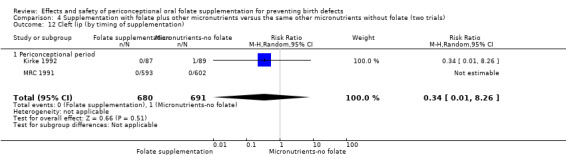
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 12 Cleft lip (by timing of supplementation).
4.13. Analysis.
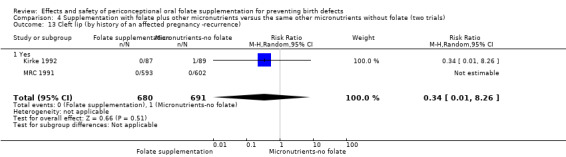
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 13 Cleft lip (by history of an affected pregnancy ‐recurrence).
4.14. Analysis.
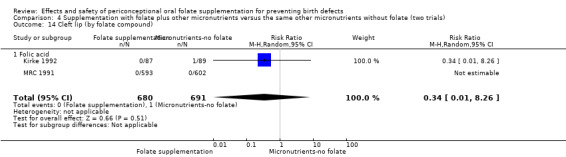
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 14 Cleft lip (by folate compound).
4.15. Analysis.
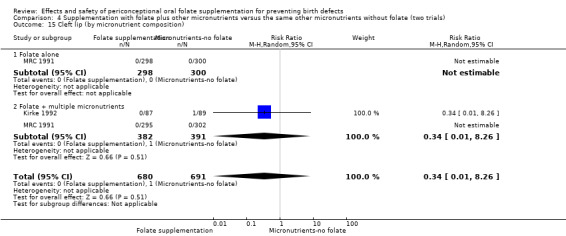
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 15 Cleft lip (by micronutrient composition).
4.16. Analysis.
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 16 Cleft lip (by malaria setting at the time of the study).
4.18. Analysis.
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 18 Cleft palate (by scheme).
4.19. Analysis.
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 19 Cleft palate (by daily dose).
4.20. Analysis.
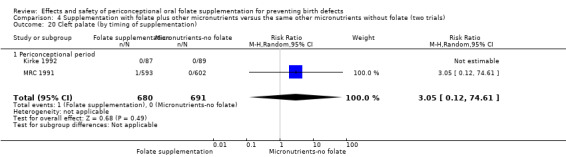
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 20 Cleft palate (by timing of supplementation).
4.21. Analysis.
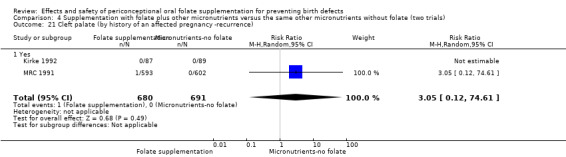
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 21 Cleft palate (by history of an affected pregnancy ‐recurrence).
4.22. Analysis.
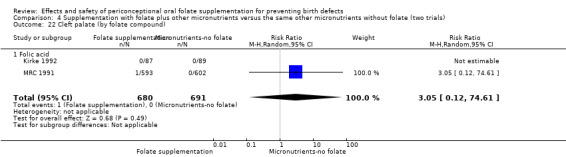
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 22 Cleft palate (by folate compound).
4.23. Analysis.
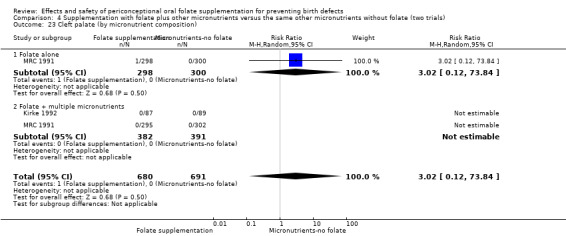
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 23 Cleft palate (by micronutrient composition).
4.24. Analysis.
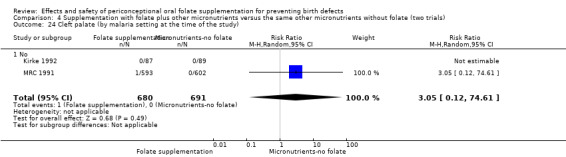
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 24 Cleft palate (by malaria setting at the time of the study).
4.26. Analysis.
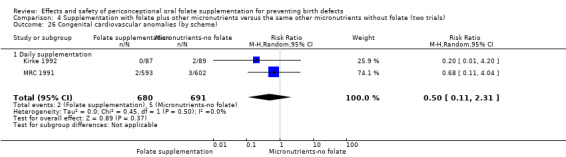
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 26 Congenital cardiovascular anomalies (by scheme).
4.27. Analysis.
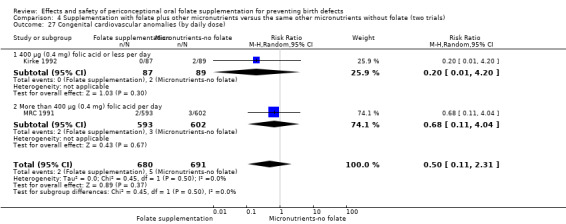
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 27 Congenital cardiovascular anomalies (by daily dose).
4.28. Analysis.
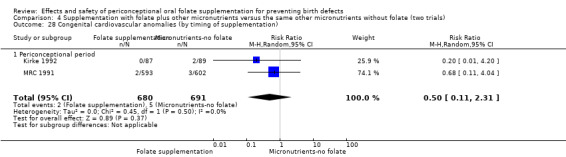
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 28 Congenital cardiovascular anomalies (by timing of supplementation).
4.29. Analysis.
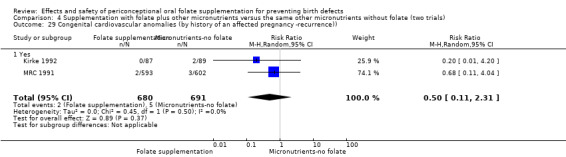
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 29 Congenital cardiovascular anomalies (by history of an affected pregnancy ‐recurrence)).
4.30. Analysis.
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 30 Congenital cardiovascular anomalies (by folate compound).
4.31. Analysis.
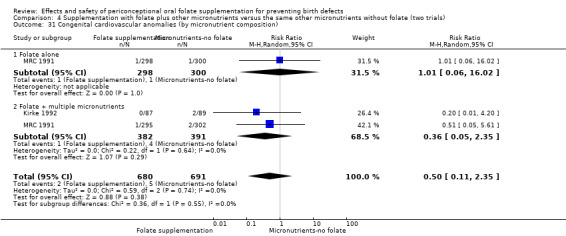
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 31 Congenital cardiovascular anomalies (by micronutrient composition).
4.34. Analysis.
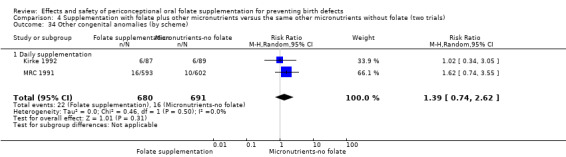
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 34 Other congenital anomalies (by scheme).
4.35. Analysis.
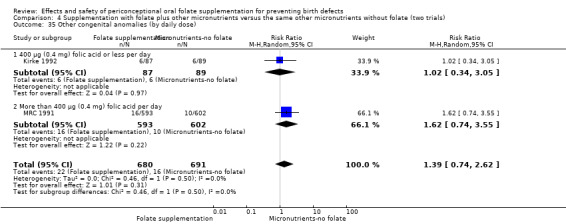
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 35 Other congenital anomalies (by daily dose).
4.36. Analysis.
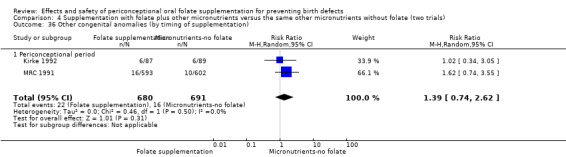
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 36 Other congenital anomalies (by timing of supplementation).
4.37. Analysis.
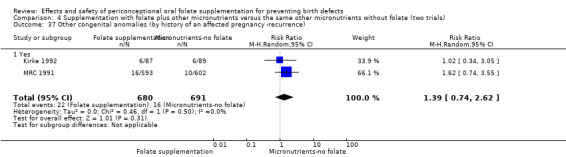
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 37 Other congenital anomalies (by history of an affected pregnancy ‐recurrence).
4.38. Analysis.
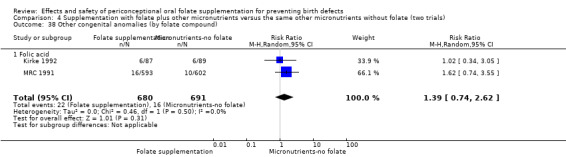
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 38 Other congenital anomalies (by folate compound).
4.39. Analysis.
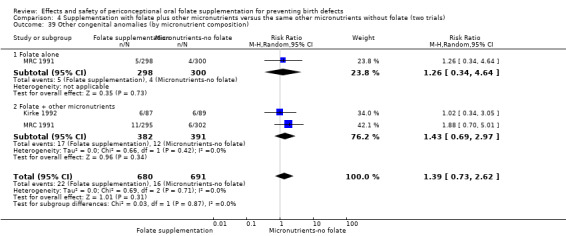
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 39 Other congenital anomalies (by micronutrient composition).
4.40. Analysis.
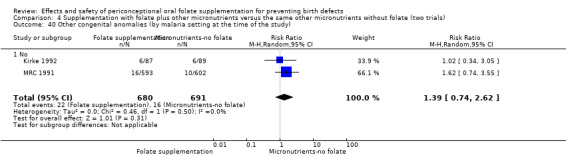
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 40 Other congenital anomalies (by malaria setting at the time of the study).
4.41. Analysis.
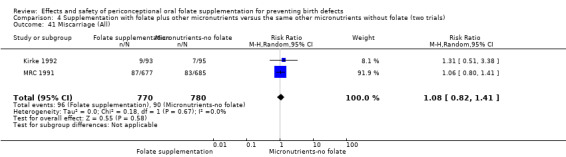
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 41 Miscarriage (All).
4.43. Analysis.
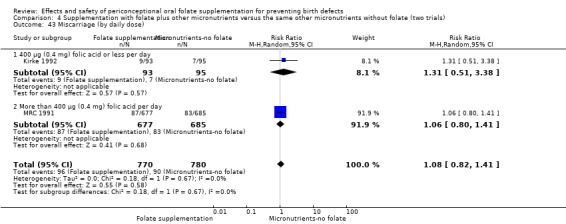
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 43 Miscarriage (by daily dose).
4.44. Analysis.
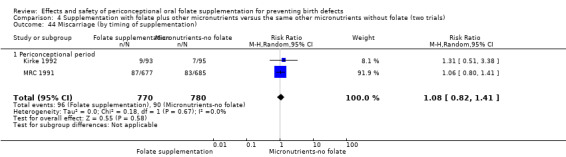
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 44 Miscarriage (by timing of supplementation).
4.45. Analysis.
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 45 Miscarriage (by history of an affected pregnancy ‐recurrence).
4.46. Analysis.
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 46 Miscarriage (by folate compound).
4.47. Analysis.
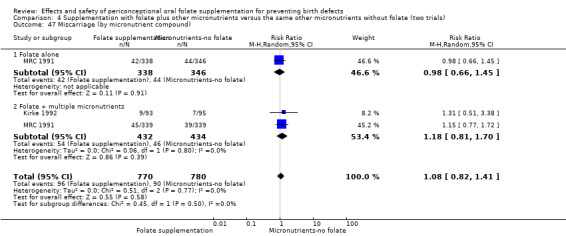
Comparison 4 Supplementation with folate plus other micronutrients versus the same other micronutrients without folate (two trials), Outcome 47 Miscarriage (by micronutrient compound).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Czeizel 1994.
| Methods | RCT 2‐arm parallel‐group design. | |
| Participants | 5502 pregnant women (out of the 7905 participants) attending prenatal care at the Hungarian Family Planning Program (HFPP) in Hungary. Inclusion criteria were: (I) no delayed conception or infertility (i.e. no conception after more than 12 months of sexual activity without contraception), (II) not currently pregnant, (III) voluntary participation and a promise of compliance. In the first 4 years of the HFPP, there were 2 other criteria: age under 35 (women over 35 were referred to a genetic counselling clinic) and no previous wanted pregnancy. Of the 5502 women, 49 were lost to follow‐up and 95 had false‐positive pregnancy results ("chemical pregnancies"). Thus, 5358 women were included in the meta‐analysis. |
|
| Interventions | Women were randomly assigned to 1 of 2 groups: Group 1 (n = 2738 women): women received a supplement (Elevit Pronatal®) containing 800 µg (0.8 mg) folic acid, in addition to 6000 IU (until the end of 1989) and 4000 IU (in 1990 to 1991) vitamin A; 1.6 mg vitamin B1; 1.8 mg vitamin B2; 19 mg nicotinamide; 2.6 mg vitamin B6; 10 mg pantothenic acid (as calcium pantothenate); 0.2 mg biotin; 4.0 pg vitamin B12; 100 mg vitamin C; 500 IU vitamin D; 15 mg vitamin E (as alpha‐tocopherol‐tocopherol acetate); 125 mg calcium; 125 mg phosphorus; 100 mg magnesium; 60 mg elemental iron; 1 mg copper; 1 mg manganese; and 7.5 mg zinc. Group 2 (control; n = 2620 women): women received a supplement containing 1 mg copper; 1 mg manganese; 7.5 mg zinc; 7.5 mg (calcium ascorbate) vitamin C and lactose 736.27 mg. A single tablet in either group was to be taken daily for 1 month before planned conception until 12 weeks of pregnancy (confirmed by sensitive pregnancy test after the first missed menstrual period and by ultrasonography within 2 weeks). Women were asked to ingest the tablet each day before recording the basal body temperature and to leave unused tablets in the box. |
|
| Outcomes | Maternal: weight gain, increased appetite, lack of appetite, nausea and vomiting, vertigo, heartburn, constipation, diarrhoea, ectopic pregnancy, miscarriage, spontaneous abortion, multiple birth, twin birth. Infant: NTDs and other congenital anomalies (congenital limb deficiency, cardiovascular congenital abnormalities, congenital pyloric stenosis, cleft lip, cleft palate) stillbirths, infant mortality, prenatally terminated fetuses, birthweight, low birthweight, gestational age, weight, length, and head circumference at 8 to 16 months of age, functional development tests at 8 to 16 months. |
|
| Notes | Exclusion criteria not clear. The final database included 5358 women with evaluated pregnancy. Summary characteristics of the study:
Source of funding: Hoffmann‐La Roche supplied the supplements. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were asked about whether they agreed to their allocation on the basis of a randomisation table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. It was not clear whether staff carrying out recruitment were aware of randomisation group. |
| Blinding (performance bias and detection bias) Women | Low risk | Women were informed about the "blind' use of 1 of 2 kinds of tablets. |
| Blinding (performance bias and detection bias) Clinical staff | Unclear risk | Not clear if staff were blinded. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | Certificates were filled in by mothers and signed by physicians. If certificates were not sent back, one of the co‐workers visited the participants at home. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of 7905 women randomised 69.6% had confirmed pregnancies. Loss to follow‐up for women with confirmed pregnancies 1%. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent. |
| Other bias | Unclear risk | In a comment in Czeizel 1992 it is stated that randomisation was broken twice; first to evaluate teratogenic effect of vitamin A and the second at the end of the trial in 1991. |
ICMR 2000.
| Methods | RCT 2‐arm parallel‐group design. | |
| Participants | 466 women with a history of giving birth to a child with open NTD and planning to have another child from 5 centres: Bangalore, Mumbai, Lucknow, New Delhi and Pune, in India. Previous NTDs included anencephaly, encephalocoele, meningocoele/meningomyelocoele, cranio‐rachischisis, and their combinations, including complicating hydrocephalus; but isolated cases of hydrocephalus were not included in the trial. Women with a history of giving birth to a child with closed spina bifida, women with a history of diabetes mellitus or abnormal fasting and post‐prandial blood sugar, epilepsy, congenital anomalies indicative of a genetic syndrome in previous NTD, or vitamin intake during 3 months prior to the enrolment and pregnancy were excluded. Of the 466 women registered in the study, 90 were immediately lost to follow‐up, 71 did not become pregnant, and 26 were lost to follow‐up after pregnancy. Thus, 279 women were included in the meta‐analysis. |
|
| Interventions | Women were randomly assigned to 1 of 2 groups. Group 1 (n = 137 women): received a supplement containing 4000 µg (4 mg) folic acid, iron (120 mg ferrous sulphate), 240 mg calcium phosphate; 10 mg zinc; 4000 IU vitamin A; 2.5 mg vitamin B1; 2.5 mg vitamin B2; 2 mg vitamin B6; 40 mg vitamin C; 400 IU vitamin D and 15 mg niacin (nicotinamide). Group 2 (control; n = 142 women): received a supplement containing iron (120 mg ferrous sulphate and 240 mg calcium phosphate). Both tablets were to be taken by mouth daily. Supplementation started at least 1 month before conception up to 12 weeks of pregnancy. Compliance with supplement intake was checked with the help of a dietary card maintained by the women and number of capsules returned. |
|
| Outcomes | Maternal: parity, spontaneous abortion, induced abortion. Infant: livebirth, stillbirth, NTD (anencephaly, complicated spina bifida, other combination). |
|
| Notes | Summary characteristics of the study:
Source of funding: M/s Unichem Limited, Mumbai provided the supplements; funding from the Indian Council of Medical Research, New Delhi. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised. |
| Allocation concealment (selection bias) | Low risk | Containers were given a random number and sent to each centre. |
| Blinding (performance bias and detection bias) Women | Low risk | Both capsules were identical and packed in similar containers. |
| Blinding (performance bias and detection bias) Clinical staff | Low risk | Participants and care providers reported as blinded. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | All women were instructed to inform the outcome of pregnancy personally or by post. In absence of the report a social worker contacted the participant to record the outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 466 women were randomised and 305 had a confirmed pregnancy during the study period (65.5%). Loss to follow‐up after pregnancy confirmation less than 10%. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent. |
| Other bias | Unclear risk | After publication of MRC (1991) trial the study was stopped. Comment: calculated sample size (250 per arm, including 20% of losses to follow‐up) was almost completed. |
Kirke 1992.
| Methods | RCT 3‐arm parallel‐group design plus a second non‐randomised control group. | |
| Participants | 354 women with a previously affected pregnancy, who were not pregnant but planning to have another pregnancy attending 12 hospitals in Ireland. Women with conditions likely to result in impaired absorption from the gastrointestinal tract were excluded. Of the 354 women randomised to 1 of the 3 arms, 281 became pregnant, 70 did not become pregnant and 3 had unknown pregnancy status. The meta‐analysis thus includes 281 women, of whom, there were 261 known infant outcomes. |
|
| Interventions | Women were randomly assigned to 1 of 3 groups. Group 1 (n = 84 women): received supplement of 360 µg (0.36 mg) folic acid only (0.12 mg/tablet) daily. Group 2 (n = 88 women): received supplements (Pregnavite Forte®) containing 4000 IU vitamin A; 400 IU vitamin D (calciferol); 1.5 mg vitamin B1 (thiamine hydrochloride); 1.5 mg vitamin B2 (riboflavin); 1 mg vitamin B6 (pyridoxine hydrochloride); 15 mg niacin (nicotinamide); 40 mg vitamin C; 480 mg calcium phosphate, and iron (252 mg ferrous sulphate) daily. Group 3 (n = 85 women): received supplements (Pregnavite Forte F®) containing 360 µg (0.36 mg) folic acid in addition to 4000 IU vitamin A; 400 IU vitamin D (as calciferol); 1.5 mg vitamin B1 (thiamine hydrochloride); 1.5 mg vitamin B2 (riboflavin); 1 mg vitamin B6 (pyridoxine hydrochloride); 15 mg niacin (nicotinamide); 40 mg vitamin C; 480 mg calcium phosphate, and iron (252 mg ferrous sulphate) daily. There was a fourth non‐randomised group that served as negative control. It was excluded from the analysis. Participants were instructed to take 1 tablet 3 times daily for at least 2 months before conception and until the date of the third missed period. Compliance was based on tablet counts and blood tests. |
|
| Outcomes | Maternal: spontaneous abortion, ectopic pregnancy. Fetal/infant: livebirth, stillbirth, occurrence and recurrence of NTD (major malformations: anencephalus, cleft lip, bilateral corneal ectasia with agenesis of the corpus callosum, polycystic kidneys with cleft lip, congenital mitral insufficiency, polydactyly, pyloric stenosis, urethral obstruction, cystic fibrosis, hydrocephalus without spina bifida, oesophageal atresia, and transposition of the great vessels; minor malformations: congenital dislocation of the hip, talipes, and scaphocephaly). |
|
| Notes | Summary characteristics of the study:
Source of funding: Antigen Pharmaceuticals and Glaxo (Ireland) Ltd provided study tablets; Beecham Pharmaceuticals (UK) gave initial support; Hoffman La Roche (Switzerland) and Glaxo (Ireland) Ltd carried out regular assays of stability of study tablets; financial support from Health Research Board, Children's Research Centre of Our Lady's Hospital for Sick Children, the Coombe, National Maternity and Rotunda Hospitals, and the Irish Association for Spina Bifida and Hydrocephalus. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was employed, 12 participants per block, stratified by hospital. |
| Allocation concealment (selection bias) | Low risk | Randomisation: achieved by using consecutively numbered, opaque, sealed envelopes. |
| Blinding (performance bias and detection bias) Women | Low risk | Women initially received similarly presented white pills. After 1 year, supplements with multiple micronutrients with folic acid were changed to commercially available tablet (colour purple) and the tablets containing multiple micronutrients without folic acid, produced by a different supplier were white. Authors stated that "It is felt that this partial loss of blinding did not materially affect the study outcome". |
| Blinding (performance bias and detection bias) Clinical staff | Low risk | Study reported as double‐blind. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | Not clear if outcome assessors were aware of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the 354 women randomised 281 had confirmed pregnancies (79.3%). |
| Selective reporting (reporting bias) | Unclear risk | Not apparent. |
| Other bias | Unclear risk | Not apparent. |
Laurence 1981.
| Methods | RCT 2‐arm parallel‐group design. | |
| Participants | 905 women resident in Glamorgan and Gwent, Wales who had a pregnancy complicated by a fetal NTD (anencephaly, encephalocoele, and spina bifida cystica) between 1954 and 1969 were traced through malformation registers, maternal and paediatric records, local authority records, and other sources. Those under 35 years of age at the time of the study were visited in their homes by medically qualified field workers and invited to participate. Of the 905 women, 111 women agreed to participate in the intervention trial and who subsequently became pregnant. |
|
| Interventions | Women were randomly assigned to 1 of 2 groups. Group 1 (n = 60 women): received supplements containing 4000 µg (4.0 mg) folic acid daily (each tablet contained 2000 µg or 2.0 mg folic acid). Group 2 (n = 51 women): received placebo. Women were asked to take a tablet twice a day starting from the time contraceptive precautions were stopped. Compliance among women in group 1 was monitored at the sixth to ninth week of estimated gestation; if the serum folate concentration at this stage was higher than 10 µg/L the woman's account of taking the tablets during the earlier part of the pregnancy could be accepted as valid. If the serum folate concentration was below 10 µg/L the woman was classified as non‐compliant. Compliance was not tested among women in group 2. |
|
| Outcomes | Maternal: diet, serum and red blood cell folate concentrations at 6 to 9 weeks of pregnancy, miscarriage, termination. Infant: livebirth, NTD (anencephaly, spina bifida cystica). |
|
| Notes | Exclusion criteria not clear. Summary characteristics of the study:
Source of funding: Action for the Crippled Child, the Manpower Services Commission, and Tenovus; the supply of folic acid and placebo from Glaxo Laboratories Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Women were allocated to receive treatment or placebo by random numbers. |
| Blinding (performance bias and detection bias) Women | Low risk | Women did not know the content of the tablets. |
| Blinding (performance bias and detection bias) Clinical staff | Low risk | Study reported as double‐blind. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | It was not clear whether outcome assessors were blind to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | (Not clear, some discrepancies in figures in different papers) 218 women were randomised and there were 123 pregnancies reported (56.4%). The main study report describes outcomes for 111 women who had confirmed pregnancies. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent. |
| Other bias | Unclear risk | Not apparent. |
MRC 1991.
| Methods | 2 by 2 factorial RCT. | |
| Participants | 1817 participating women from 7 countries (UK, Hungary, Israel, Australia, Canada, Russia and France) at high risk of having a pregnancy with a NTD, because of a previous affected pregnancy (not associated with the autosomal recessive disorder Meckel’s syndrome), who were planning another pregnancy and were not already taking micronutrient supplements. Women were excluded if they had epilepsy in case the folic acid supplementation adversely affected their treatment. Of the 1817, 1362 became pregnant and were included in the meta‐analysis. Of these, 1195 had pregnancies in which the fetus could be classified as having a NTD or not ("informative pregnancies"). |
|
| Interventions | Women were randomly assigned to 1 of 4 groups Group 1 (n = 298 women): received 4000 µg (4 mg) folic acid; iron (120 mg ferrous sulphate) and 240 dicalcium phosphate daily. Group 2 (n = 295 women): received 4000 µg (4 mg) folic acid; iron (120 mg ferrous sulphate); 240 di‐calcium phosphate; 4000 IU vitamin A; 400 IU vitamin D; 1.5 mg vitamin B1;1.5 mg vitamin B2; 10 mg vitamin B6; 40 mg vitamin C and 15 mg niacin (nicotinamide). Group 3 (n = 302 women): received iron (120 mg ferrous sulphate); 240 di‐calcium phosphate; 4000 IU vitamin A; 400 IU vitamin D; 1.5 mg vitamin B1;1.5 mg vitamin B2; 10 mg vitamin B6; 40 mg vitamin C and 15 mg niacin (nicotinamide) daily with no folic acid. Group 4 (control; n = 300 women): received iron (120 mg ferrous sulphate) and 240 di‐calcium phosphate daily. Women were asked to take a single capsule each day from the date of randomisation until 12 weeks of pregnancy (estimated from the first day of the last menstrual period). The capsules used in the study were packaged in 2‐week calendar "blister" packs. |
|
| Outcomes | Maternal: serum folic acid at last visit before becoming pregnant, miscarriage, ectopic pregnancy, termination of pregnancy. Infant: any fetal malformation (i.e. anencephaly, spina bifida cystica, or encephalocoele), sex, birthweight, head circumference. |
|
| Notes | Summary characteristics of the study:
Source of funding: Medical Research Council. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation: method not clear. |
| Allocation concealment (selection bias) | Unclear risk | Women were allocated at random to each of the 4 groups. Separate set of random allocations were used for each centre. |
| Blinding (performance bias and detection bias) Women | Low risk | Neither the doctor nor the patient knew which regimen had been allocated. |
| Blinding (performance bias and detection bias) Clinical staff | Low risk | Double‐blind trial. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of 1817 women randomised 1195 had confirmed pregnancies (65.7%). Subsequent loss to follow‐up 7%. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent. |
| Other bias | Unclear risk | Not apparent. |
IU: international unit NTD: neural tube defect RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Angeles‐Agdeppa 2005 | 744 pregnant and non‐pregnant women of reproductive age from the Philippines participated in this community‐based longitudinal study. The non‐pregnant participants were encouraged to buy and take weekly iron‐folic acid supplements with 60 mg elemental iron and 3500 µg (3.5 mg) (though a social marketing campaign); those who were pregnant in the first 20 weeks of gestation were given free weekly iron‐folic acid supplements until 3 months postpartum; those who were pregnant with at least 20 weeks of gestation were given free daily iron‐folic acid tablets with 60 mg iron and 0.4 mg folic acid. All study participants were given folic acid. The types of interventions and participants are out of scope for this review. |
| Atukorala 1994 | 95 pregnant women aged 17 to 45 years at 14 to 24 weeks' gestation from tea plantations in 5 regions of Sri Lanka attending antenatal clinics participated in this cohort and received iron‐fortified food (thriposha) and were advised to consume 50 g/day or supplements containing 60 mg elemental iron (as ferrous sulphate) + 250 µg (0.25 mg) folic acid. The participants were at least at the 14th week of gestation (none of the participants were recruited in the first trimester) and the interventions were given throughout the rest of pregnancy (in the second and third trimesters). The types of participants, types of interventions and type of study are out of the scope of this review. |
| Bailey 2005 | This is a review paper and not an intervention trial. This meta‐analysis included studies from 1995 to 2000. The type of study is out of scope of this review. |
| Berger 2005 | 864 pregnant women in Vietnam were given iron‐folic acid supplements at either daily (60 mg elemental iron and 250 µg (0.25 mg) folic acid or weekly (120 mg iron and 3500 µg (3.5 mg) folic acid frequency. The interventions were started pre‐pregnancy and continued until 3 months postpartum. All study participants were given folic acid. The types of interventions and participants are out of scope for this review. |
| Binns 2006 | 587 women who delivered their infants in 1 of 2 maternity hospitals in Australia and who completed a self‐administered questionnaire while in hospital or within 3 days of discharge participated in the study. This is not a randomised trial. This is a cross‐sectional study that documented the prevalence of mothers taking folic acid as supplements or added in fortified foods, and explored determinants of folic acid intake. The study design, types of participants and types of interventions are out of the scope of this review. |
| Bortolus 2014 | This is a study among women of reproductive age in Italy and The Netherlands who intend to become pregnant in the next 12 months. Participants are randomised to receive either 4000 µg (4 mg) or 400 µg (0.4 mg) of folic acid until the 12th week of gestation (from pre‐pregnancy until the end of the first trimester). The participants from Italy stop taking folic acid after the first trimester. Those from The Netherlands are assigned to take either 200 µg (0.2 mg) or 800 µg (0.8 mg) of folic acid until the end of the pregnancy (during the second and third trimesters). All the participants take folic acid. The types of interventions are out of scope of this review. |
| Botto 2006 | This is a retrospective cohort study of births monitored through 13 birth defects registries monitoring rates of NTDs from 1988 to 1998 in Norway, Finland, Northern Netherlands, England and Wales, Ireland, France (Paris, Strasbourg, and Central East), Hungary, Italy (Emilia Romagna and Campania), Portugal, and Israel. The aims were to evaluate the effectiveness of policies and recommendations on folic acid aimed at reducing the occurrence of NTDs by comparing the incidence of NTDs before and after the year of the recommendation in each country. The study design is out of the scope of this review. |
| Canfield 2005 | This is a secondary data‐analysis using data reported from states to the National Birth Defects Prevention Network and examined the effect of enriched cereal‐grains products fortification with folic acid on birth defects in the United States. Periods were 1995 to 1996 (pre‐fortification) and 1999 to 2000 (post‐fortification). The results suggest some modest benefit from the folic acid fortification on the prevalence of a number of non‐NTD birth defects. The study design is out of the scope of this review. |
| Cavalli‐Sforza 2005 | This is a review of studies on compliance and purchase behaviour of women of reproductive age after a social mobilisation and communication campaign on weekly iron‐folic acid supplementation. This is not an intervention trial. The type of study is out of scope for this review. |
| Chen 2008 | 52,043 women residing in these counties who were planning a pregnancy were included in the trial. A population‐based community intervention study was carried out in Henan, Guizhou, Hunan, and Jilin provinces of China. Ten intervention counties and 8 control counties were selected from these provinces. Women from intervention counties received a supplement (Forceval®) containing 400 µg (0.4 mg) folic acid; 563 IU vitamin A; 200 IU vitamin D2; 1.4 mg vitamin B1; 1.4 mg vitamin B2; 3 µg vitamin B12; 60 mg vitamin C; 8 mg vitamin E, 100 µg biotin (bioepiderm); 14 mg niacin (niacinamide); 4 mg pantothenic acid, 100 mg calcium; 10 mg iron; 2 mg copper (cuprum); 10 mg zinc, 77 mg phosphorus, 30 mg magnesium, 3 mg manganese, 30 µg selenium, 100 µg molybdenum, and 4 mg potassium. Women in the control counties did not receive supplementation. Participants were followed up for 2 years. NTDs were recorded for all births from 28 weeks of gestation to 7 days after birth. 9 NTDs were recorded from 25,444 pregnancies (NTD birth prevalence of 0.35/1000 pregnancies) in the intervention group and 48 NTDs among 26,599 pregnancies (NTD birth prevalence of 1.80/1000 pregnancies) in the control group. The protective rate was 80.4%. This is not a randomised trial. The type of study is out of the scope of this review. |
| Christian 2003 | 4926 pregnant women in rural Nepal participated in a cluster‐randomised, double‐masked, controlled trial with 5 arms. The following groups were evaluated: group 1 received 400 µg (0.4 mg) folic acid and 1000 µg retinol equivalents (RE) vitamin A; group 2 received 400 µg (0.4 mg) folic acid; 60 mg elemental iron and 1000 µg retinol equivalents (RE) vitamin A; group 3 received 400 µg (0.4 mg) folic acid; 60 mg elemental iron; 30 mg zinc and 1000 µg retinol equivalents (RE) vitamin A; group 4 received 400 µg (0.4 mg) folic acid; 60 mg elemental iron; 30 mg zinc; 10 mg vitamin D; 10 mg vitamin E; 1.6 mg vitamin B1; 1.8 mg vitamin B2; 20 mg niacin; 2.2 mg vitamin B6; 2.6 mg vitamin B12; 100 mg vitamin C; 65 mg vitamin K; 2 mg copper; 100 mg magnesium and 1000 µg retinol equivalents (RE) vitamin A; and group 5, 1000 µg retinol equivalents (RE) vitamin A alone as the control. All participating women were offered deworming treatment (albendazole 400 mg single dose) in the second and third trimester. Short‐ and long‐term effects of antenatal supplementation were evaluated, showing a protective effect of iron‐folic acid supplementation on infant mortality at age 7 years. Supplementation started at recruitment and continued until 3 months postpartum in the case of live births of 5 weeks or more after a miscarriage or stillbirth. In each of the intervention groups, 46% to 50% of the participants started supplements at less than 9 weeks of gestation, 41% to 46% at 9 to 16 weeks of gestation, and 8% to 10% on at least 17 weeks of gestation. Supplements were continued through to 3 months postpartum. The types of participants are out of the scope of this review. |
| Daly 1995 | 80 non‐pregnant women attending Coombe Women's Hospital, Ireland were randomised to 1 of 4 groups: control, dietary advice, fortified milk (76 µg [0.076 mg] folic acid/100 mL), and fortified milk plus dietary advice. Milk fortification was effective to raise serum and red cell folate. None of the participants became pregnant. The types of interventions and types of participants are out of the scope of this review. |
| Daly 1997 | 121 women of childbearing age employees in the Coombe Women's Hospital, Ireland were randomly allocated to 0, 100 µg (0.1 mg)/day, 200 µg (0.2 mg)/day, or 400 µg (0.4 mg)/day of folic acid. Red cell folate and plasma homocysteine were measured at baseline and after 10 weeks supplementation. Compliance was monitored by having the women sign a dated sheet when taking the tablet. 95 women completed the 6‐month study. Double‐blind randomised controlled trial to find the lowest folic acid dose that effectively reduces plasma homocysteine levels in premenopausal women. None of the participants became pregnant. The types of participants are out of the scope of this review. |
| Doyle 2001 | 55 women who had given birth to a low birthweight baby (less than 2500 g), and who planned to have a further pregnancy, were recruited to a prospective study in East London, UK. The participants were assigned into 3 groups: group 1 were assessed to have adequate diets and were given dietary advice; group 2 were assessed to have inadequate diets and were given a vitamin‐mineral supplement with 400 µg (0.4 mg) folic acid and dietary advice; and group 3 were assessed to have inadequate diet and were given dietary advice. MM supplementation started at 3 months postpartum and follow‐up lasted 6 months. None of the participants became pregnant during the duration of the study. The type of participants are out of the scope of this review. |
| Drazkowski 2002 | Case reports from 4 women with epilepsy identified with low B12 levels using data from electronic medical records of the Barrow Neurologic Institute, Epilepsy Specialty Clinic, United States. They received supplements of either 4 or 5 mg synthetic folic acid and parenteral B12. 2 were supplemented up for more than 18 months; the other 2 were supplemented for an unknown length of time. All cases resolved their B12 deficiency symptoms after parenteral B12 administration. The type of study, participants and interventions are out of the scope of the review. |
| Eichholzer 2006 | This is a review that addresses supplementation and fortification as public health policies. This is not an intervention trial. The type of study is out of scope of this review. |
| Ejidokun 2000 | Qualitative study using focus group discussions, observational data and in‐depth interview to identify community perspectives and attitudes to pregnancy, anaemia, iron and folate supplements during pregnancy amongst women (n = 23), and 2 healthcare providers in Lagos, Nigeria. Maternal anaemia was not perceived as a priority health problem by pregnant women. This is not an intervention trial. The type of study, participants and interventions are out of the scope of this review. |
| Elbourne 2002 | This is not an intervention trial. This paper addresses methodological issues relating to the meta‐analysis of trials with cross‐over designs. The type of study is out of scope of this review. |
| Ellison 2004 | 31 women with singleton pregnancy were randomised to receive 400 µg (0.4 mg) folic acid once daily until 16 weeks' gestation or until the end of pregnancy. Authors found that folic acid supplementation throughout pregnancy maintains plasma homocysteine concentration. All participants received folic acid. The type of participants and type of interventions are out of the scope of this review. |
| Eskes 2000 | This paper reviews the relationship between low vitamin status (folic acid, vitamin B6 and B12), hyperhomocysteinaemia, the MTHFR gene mutation C677T, and thrombotic factors like Protein C, Protein S, antithrombin III, factor V Leiden and Activated Protein C, either alone or in combination as high‐risk factors for obstetrical vascular disease. This is not an intervention trial. The type of study is out of scope of this review. |
| Field 1991 | This paper reviews the effect of folic acid in NTD in humans and animals. This is not an intervention trial. The type of study is out of scope of this review. |
| Geisel 2003 | This paper reviews the effect of folic acid in NTD in humans, and ways in which knowledge of folic acid can be increased. This is not an intervention trial. The type of study is out of scope of this review. |
| Gunaratna 2015 | 802 non‐pregnant women in Tanzania were randomly assigned to receive either 1) folic acid; 2) folic acid and iron; or 3) folic acid, iron and multivitamins. All treatment groups received folic acid. New pregnancies were withdrawn from the study. The type of participants and interventions are out of scope of this review. |
| Hague 2003 | This paper discusses the effects of changing levels of homocysteine in pregnancy. This is not an intervention trial. The type of study is out of scope of this review. |
| Hayes 1996 | This is a case‐control study examining factors associated with orofacial clefts. Participants were mothers with babies with cleft lip with or without CLP, controls were mothers of babies with other congenital anomalies (except NTDs). The type of study is out of the scope of this review. |
| Itikala 2001 | Case‐control study to evaluate the relation between regular multivitamin use and the birth prevalence of orofacial clefts. There was a 48% risk reduction for cleft lip with or without CLP among mothers who used multivitamins during the periconceptional period or who started multivitamin use during the first postconceptional month. No reductions for cleft lip with or without CLP or CLP alone were found for women who began multivitamin use in the second or third month after conception. The type of study is out of the scope of the review. |
| Johnston 2008 | This paper reviews the effectiveness of cereal grain fortification with folic acid. This is not an intervention trial. The type of study is out of scope of this review. |
| Khambalia 2009 | 88 women in rural Bangladesh, women were randomised before pregnancy to receive daily 60 mg elemental iron (as ferrous fumarate) and 400 µg (0.4 mg) folic acid or folic acid only. Both treatment groups received folic acid. Once pregnancy was confirmed women were withdrawn from the study and received routine care which included folic acid supplements. The types of interventions and types of participants are out of scope of this review. |
| Lee 2005 | 131 apparently healthy pregnant women were assigned to 1 of 5 groups: group 1 (control) received no supplement; group 2 received 30 mg elemental iron (as ferrous sulphate) plus 175 µg (0.17 mg) folic acid daily from the first trimester until delivery; group 3 received 60 mg elemental iron (as ferrous sulphate) plus 350 µg (0.35 mg) folic acid from the first trimester until delivery; group 4 received 30 mg elemental iron (as ferrous sulphate) plus 175 µg (0.17 mg) folic acid daily from the 20th week gestation until delivery and group 5 received 60 mg elemental iron (as ferrous sulphate) plus 350 µg (0.35 mg) folic acid from the 20th week gestation until delivery. The participants in the groups 2 and 3 received their supplements starting in the first trimester (mean ± SD = 9.1 ± 2.3 weeks) and continued until delivery. The type of study and type of participants are out of the scope of this review. |
| Li 2014 | 4052 women in China undergoing prenatal consultations were divided into 2 ethnic groups, Han or Mongolian, and were further assigned into 1 of 4 groups: group 1 received folic acid tablets before pregnancy and drank liquid milk (containing folate which ranged in concentration from 1.68 to 5.69 µg/100ml); group 2 did not take folic acid tablets before pregnancy but drank milk daily once pregnancy was confirmed; group 3 received folic acid but did not drink milk throughout the trial; and, group 4 (control) did not take folic acid tablets and did not drink milk throughout the trial. Women who received folic acid tablets (groups 1 and 3) and/or milk (groups 1 and 2) continued their interventions throughout pregnancy. The type of intervention is out of the scope of the review. |
| Mandishona 1999 | 112 women aged between 12 and 50 years from a population of 425 rural people participating in ongoing family genetic studies in the Murehwa and Zaka districts of Zimbabwe and in Mpumalanga Province, South Africa to assess the effect of consumption of a traditional beer, rich in iron, in the regular diet for preventing iron deficiency. The type of study, participants and interventions are out of the scope of this review. |
| Manizheh 2009 | 246 nulliparous women attending outpatient antenatal clinic in Alzahra Hospital in Iran were randomised to receive daily folic acid from early pregnancy; either 500 µg (0.5 mg) per day or 5000 µg (5 mg) per day. Folic acid was started in the first trimester and supplements continued throughout pregnancy. All participants received folic acid. The types of interventions and participants are out of scope of this review. |
| Mathews 1999 | The authors merged 2 studies in order to investigate the association between folic acid supplementation and twinning: the MRC Vitamin study, a randomised controlled trial of folic acid supplementation (4 mg/day) among women in whom a previous pregnancy had been affected by NTD; and the Prospective Study of Nutrition, Smoking and Pregnancy Outcome, an observational study that gathered detailed information on periconceptional nutrition from nulliparous women in the UK (women who had undergone infertility treatment were excluded). This is an analysis of secondary data. None of the outcomes of interest for this review were included in this study. The type of study is out of the scope of this review. |
| McNulty 2013 | 119 women attending antenatal clinic in Northern Ireland and who reported taking folic acid supplements during the first trimester were assigned in the second trimester to receive either 400 µg (0.4 mg) of folic acid or a placebo everyday until the end of pregnancy. The types of intervention and participants are out of the scope of the review. |
| Melli 2008 | 203 nulliparous pregnant women with a singleton pregnancy in their first trimester, attending the antenatal outpatient clinic in Tabriz, Iran with no history of hypertension and folic acid supplementation. Women were divided into 2 groups: group 1 was given 5000 µg (5 mg) per day and group 2 received 500 µg (0.5 mg/day) folic acid. In addition to the plasmatic homocysteine concentrations during the first trimesters and at delivery, the incidence of pregnancy‐induced hypertension, pre‐eclampsia and eclampsia and were compared. The incidence of any type of hypertension was 2% with regimen 1 compared to 11% with routine regimen. All the study participants received folic acid. The type of intervention is out of the scope of this review. |
| Molster 2007 | The paper reported the results of a survey of knowledge, attitudes and behaviour with regard to food fortification with folic acid amongst a randomly selected sample aged 18 years or older in Australia. This is not an intervention trial. The type of study is out of scope of this review. |
| Nelen 2000 | This is a case‐control study where homocysteine (fasting and afterload), folate (serum and red cells), pyridoxal 5*‐phosphate, and cobalamin concentrations were measured in 123 white women who had at least 2 consecutive spontaneous early pregnancy losses each and compared with 104 healthy controls from the University Hospital Nijmegen St. Radboud, The Netherlands. Elevated homocysteine and reduced serum folate concentrations were risk factors for recurrent spontaneous early pregnancy losses. The type of study is out of the scope of this review. |
| Nguyen 2009 | 40 non‐pregnant women aged between 18 and 45 years, who had not taken folic acid supplements, from the Motherisk Program, The Hospital for Sick Children, Canada were randomly assigned to 1 of 2 groups: group 1 received a daily supplement (PregVit®) containing 1100 µg (1.1 mg) folic acid; 2700 IU b‐carotene; 30 IU vitamin E; 12 µg vitamin B12; 120 mg vitamin C; 250 IU vitamin D; 3 mg thiamine; 300 mg calcium; 3.4 mg riboflavin; 20 mg niacinamide; 10 mg vitamin B‐6; 5 mg pantothenic acid; 50 mg magnesium; 0.15 mg iodine; 35 mg iron (as ferrous fumarate); 2 mg copper and 15 mg zinc; or group 2 received 5000 µg (5 mg) folic acid 5 mg (PregVit‐folic 5®); 2700 IU b‐carotene; 30 IU vitamin E; 12 µg vitamin B12; 120 mg vitamin C; 250 IU vitamin D; 3 mg thiamine; 300 mg calcium; 3.4 mg riboflavin; 20 mg niacinamide; 10 mg vitamin B6; 5 mg pantothenic acid; 50 mg magnesium; 0.15 mg iodine; 35 mg iron (as ferrous fumarate); 2 mg copper and 15 mg zinc. The women were instructed to take the supplement for 30 weeks. Plasma and red blood cell folate concentrations were measured at baseline and at weeks 2, 4, 6, 12, and 30. The use of 5 mg folic acid among women of childbearing age produced higher blood folate concentrations, with a faster rate of folate accumulation, compared with 1.1 mg folic acid. None of the participants became pregnant. All participants were given folic acid. The types of participants and interventions are out of the scope of this review. |
| Pitkin 2007 | This is not an intervention trial. This review includes prospective and retrospective studies. In the clinical trials section the conclusion is that folic acid supplementation was useful to prevent occurrence (only 1 trial) and recurrence of NTD. The type of study is out of scope of this review. |
| Pritchard 1991 | This paper describes the causes of abruptio placentae. It was excluded because the type of study is out of the scope of this review. |
| Ramakrishnan 2003 | A randomised, double‐blind clinical trial in semi‐rural Mexico to compare the effects of MMs supplements with those of iron supplements during pregnancy on birth size. 873 pregnant women were recruited before 13 weeks of gestation and randomly assigned to 1 of 2 groups: group 1 received supplements containing 2150 IU vitamin A; 309 IU vitamin D3; 5.73 IU vitamin E; 0.93 mg thiamine; 1.87 mg riboflavin; 15.5 mg niacin; 215 µg (0.21 mg) folic acid; 1.94 mg vitamin B6; 2.04 µg vitamin B12; 66.5 mg vitamin C; 12.9 mg zinc; 62.4 mg elemental iron (as ferrous sulphate) and 252 mg magnesium; group 2 received 60 mg elemental iron (as ferrous sulphate). The supplements were provided 6 days a week at home. Routine antenatal care was provided to both groups until delivery. These findings suggest that MM supplementation during pregnancy does not lead to greater infant birth size than iron‐only supplementation. The supplementation was started at a mean ± SD gestational age of 9.24 ± 2.51 weeks in the first group (MMs) and 9.31 ± 3.00 weeks in the second group (iron‐only supplements). Supplements were continued until delivery. The study was excluded because supplementation time surpassed 12 weeks of pregnancy. The type of intervention is out of scope of this review. |
| Ray 2007 | A population‐based case‐control study in Ontario, Canada (89 NTD cases and 422 controls). The outcome was serum holotranscobalamin (holoTC) at 15 to 20 weeks' gestation. There was a trend of increasing risk with lower levels of holoTC. The type of study and intervention are out of the scope of this review. |
| Ray 2008 | A cross‐sectional study among 10,622 women in Ontario, Canada. Authors determined the prevalence of biochemical B12 deficiency and found that 1 in 20 women may be deficient in B12 in early pregnancy. The type of study is out of the scope of this review. |
| Robbins 2005 | 232 non‐pregnant women from 2 clinics in Arkansas, United States were assigned randomly to receive brief folic acid counselling, a reminder phone call, and 30 folic acid tablets containing 400 µg (0.4 mg) of folic acid (n = 162 women; intervention group) or to receive counselling about other preventive health behaviours and a folic acid informational pamphlet (n = 160 women; control group). Self‐reported folic acid use was compared at baseline and at 2 months. Weekly folic acid intake increased in the intervention group by 68%, compared with 20% in the control group. No significant differences were found in daily intake. None of the participants became pregnant during the duration of this study. The type of participants and interventions are out of the scope of this review. |
| Rolschau 1999 | 8184 Danish female citizens resident in the county of Funen, Denmark planning a pregnancy or already pregnant were offered a free supplement of folic acid of either 1000 µg (1 mg) folic acid or 2500 µg (2.5 mg) folic acid in a double‐blind randomised study to determine whether a supplement of folic acid given preconceptionally or early in pregnancy had any influence on birthweight, incidence of preterm labour, low birthweight and small‐for‐gestational age. Folic acid given preconceptionally or in the first half of pregnancy slightly increased birthweight and a decreased the incidence of preterm labour, infants with low birthweight and small‐for‐gestational age. All the study participants received folic acid. The types of interventions are out of the scope of this review. |
| Rosenthal 2008 | 140 female factory workers aged 18 to 49 years in Choloma, Honduras were randomly assigned to 1 of 2 groups: group 1 received a daily dosage of 1000 μg (1 mg) folic acid; group 2 received a once weekly dosage of 5000 μg (5 mg). Serum folate and red blood cell folate levels were determined by radioimmunoassay at baseline, 6 weeks and 12 weeks. Although both folate supplementation regimens increased serum and red blood cell folate levels significantly among the women studied, blood folate levels that are considered to be protective of NTD were reached faster with the daily dosage of 1000 μg (1 mg) folic acid. Both groups received folic acid at different doses and regimens. The types of interventions are out of the scope of this review. |
| Sayers 1997 | A cross‐sectional community‐based survey was conducted in Dublin, Ireland to document the knowledge and behaviour of 335 women of childbearing age to periconceptional folic acid. Approximately two‐thirds (213/ 335, 63.6%) had heard of folic acid. Knowledge was significantly associated with higher social class and higher education; few were advised to take folic acid before pregnancy. The type of study, participants and interventions are out of the scope of this review. |
| Schorah 1993 | The authors studied the impact of folate fortification of food on folate intake in women of childbearing age. Folic acid intake was measured by a 7‐day weighed procedure from 1986 to 1988. The results show that the consumption of fortified cereals considerably increased the intake of folic acid in the 10 women consuming fortified cereals compared to the intake of the 14 women not consuming fortified cereals. The type of study, participants and interventions are out of the scope of this review. |
| Schwarz 2008 | 446 English‐speaking women, aged 18 to 45 years from 2 urgent clinics in San Francisco, United States were randomly assigned to receive computerised counselling about either periconceptional folate supplements (intervention group) or emergency contraception (control group). After 6 months the women in the intervention group were more likely to know that folate prevents birth defects, that folate is most important in early pregnancy, and to report the recent use of a folate supplement. The types of participants and interventions are out of the scope of this review. |
| Shaw 1995 | This is a case‐control study to investigate if periconceptional use of multivitamins containing folic acid was associated with a reduced risk of orofacial clefts (n = 734 per group). Women who used multivitamins containing periconceptional folic acid had a 25% to 50% reduction in risk for offspring with orofacial clefts compared to women who did not use such vitamins. Maternal daily consumption of cereal containing folic acid produced similar results. The type of study is out of the scope of this review. |
| Shaw 2006 | Population‐based case‐control study investigating whether periconceptional intakes of supplemental folic acid, dietary folate, and several other nutrients were associated with orofacial clefts. There was no association detected. The type of study is out of the scope of this review. |
| Shrimpton 2002 | Commentary about the advantages of supplementation in different age groups. This is not an intervention trial. The type of study is out of scope of this review. |
| van der Put 1998a | Case‐control study which suggests that the combined heterozygosity for the 2 methylenetetrahydrofolate reductase (MTHFR) gene common mutations accounts for a proportion of folate‐related NTDs. The type of study, participants, and interventions are out of the scope of this review. |
| van Rooij 2004 | Case‐control study (n = 174) to investigate the association between maternal folate intake by supplement and food and the risk of cleft lip with or without CLP. Dietary folate intake reduced CLP risk independently in a dose‐response manner. The largest risk reductions were found in those mothers who had a diet of more than 200 µg folate per day in combination with a folic acid supplement. The type of study is out of the scope of this review. |
| Wald 2004 | A letter to the editor addressing the expediency of folic acid fortification to prevent NTDs versus masking vitamin B12 status. The study was excluded because the letter does not contain results from clinical trials. The type of study is out of scope of this review. |
| Walsh 2007 | Cross‐sectional population study looking at blood folate status of over 400 sequential primigravid Caucasian women with a singleton pregnancy, at less than or equal to 20 weeks' gestation. The type of study is out of the scope of the review. |
| Watson 1999 | This study explores different methods of communicating information to increase folate awareness in women of childbearing age, who participated in a community randomised trial. The type of interventions is out of the scope of this review. |
| Wehby 2012 | 2508 non‐pregnant women of reproductive age from craniofacial clinics in Brazil who had nonsyndromic or isolated oral clefts or had at least 1 natural child with nonsyndromic or isolated oral clefts were assigned to receive either a single pill of 4000 µg (4 mg) folic acid or 400 µg (0.4 mg) of folic acid daily to be continued until the end of the first trimester. From these, there were 234 live births with recorded infant outcomes. All study participants took folic acid. The type of intervention is out of the scope of the review. |
| Wen 2005 | Review of 65 studies to examine the biological basis of why folic acid may have health effects beyond its proven effect of reducing NTDs; and to explore controversial policies of folic acid supplementation and food fortification. This is not an intervention trial. The type of study is out of scope of this review. |
| Westphal 2004 | This is a placebo‐controlled study among 30 women to determine the effects of taking for 3 months a commercial supplement that includes folate (amount unspecified) on progesterone level, basal body temperature, menstrual cycle and pregnancy rate. None of the participants became pregnant during the duration of the supplementation. None of the outcomes of interest for this review were reported in the study. The type of interventions is out of the scope of this review. |
| Wilcox 2007 | National population based case‐control study to measure the possible association of facial clefts with maternal intake of folic acid supplements, multivitamins, and folates in diet. Results show that 400 µg (0.4 mg) folic acid supplementation daily during early pregnancy was associated with a reduced risk of isolated cleft lip with or without CLP, especially among women with folate rich diets who also took folic acid supplements and multivitamins. Folic acid provided no protection against CLP. The type of study is out of the scope of the review. |
| Zeng 2008 | 5828 pregnant women in 2 rural counties in Shaanxi Province, in north west China participated in this study. Villages were randomly assigned to 1 of 3 groups: group 1 received supplements containing 400 µg (0.4 mg) folic acid; group 2 received 30 mg elemental iron, 400 µg (0.4 mg) folic acid; and group 3 received 30 mg elemental iron, 400 µg (0.4 mg) folic acid; 15 mg zinc; 2 mg copper; 65 µg selenium; 150 µg iodine; 800 µg vitamin A; 1.4 mg vitamin B1 (thiamine); 1.4 mg vitamin B2 (riboflavin); 1.9 mg vitamin B6; 2.6 µg vitamin B12; 5 µg vitamin D; 70 mg vitamin C; 10 mg vitamin E; and 18 mg niacin. Authors found that antenatal supplementation with iron‐folic acid was associated with longer gestation and a reduction in early neonatal mortality compared with folic acid. MMs supplements were associated with modest increase in birthweight compared with folic acid. All women received folic acid. The type of interventions is out of the scope of this review. |
CLP: cleft palate MM: multiple micronutrient MTHFR: methylenetetrahydrofolate reductase NTD: neural tube defect SD: standard deviation
Characteristics of ongoing studies [ordered by study ID]
Bailey 2014.
| Trial name or title | Folic acid supplementation in pregnant women: dose response. |
| Methods | Randomised, double‐blind intervention trial. |
| Participants | Healthy women with singleton pregnancy at < 10 weeks' gestation at enrolment. |
| Interventions | Daily supplement of 800 µg (0.8 mg) or 400 µg (0.4 mg) of folic acid daily. |
| Outcomes | Gene‐specific DNA methylation, RBC folate, serum folate, serum folic acid, Apgar score, cell‐type specific DNA methylation, infant birthweight, infant head circumference, infant length. |
| Starting date | May 2014. |
| Contact information | Dorothy B Hausman Tel: +706 542 4871 Email: dhausman@uga.edu |
| Notes | Sponsor: Cornell University, Emory University, University of Florida. |
Hekmatdoost 2011.
| Trial name or title | Comparison of the effect of folic acid and 5‐methyltetrahydrofolate (5MTHF) on serum folate and homocysteine levels, and abortion rates in women suffering from recurrent abortion. |
| Methods | Randomised, double‐blind. placebo‐controlled trial. |
| Participants | Women aged 18‐50 willing to participate in the study and filling out the informed consent form, history of 3 or more idiopathic abortions, the last one should be more than 6 months ago, no history of in taking folate supplements or high folate containing foods during previous 6 months, All previous abortions should be idiopathic without any anatomic, cytologic, hormonal, septic pathologic finding, no special diets. Exclusion criteria: elective or induced abortion, molar or ectopic pregnancies, presence of any malignancy, chromosomal abnormality, uterine abnormality, thyroid, kidney, or liver dysfunction, glucose intolerance, seizure, alcohol or drug abuse, taking any drug affecting homocysteine metabolism, not following the study protocol. |
| Interventions | Intervention 1: 5 methyltetrahydrofolate (Intervention, 5 mg, 7 months). Intervention 2: folic acid (control, 5 mg, 7 months). |
| Outcomes | Primary: changes in recurrent abortion successful treatment. Secondary: serum folate level, serum homocysteine levels at 8 weeks. Method of measurement: ELISA |
| Starting date | 22 June 2010, Recruiting. |
| Contact information | Azita Hekmatdoost Address: West Arghavan, Shahrak Gharb Tehran, Islamic Republic of Iran. Phone: +98 2122930824 Email: a_hekmat2000@yahoo.com Affiliation: Dep Human Nutrition, Shahid Beheshti University of Medical Sciences Tehran, Islamic Republic of Iran |
| Notes | Sponsor: Avicenna Research Institute and National Institute of Nutrition Research. |
Koren 2013.
| Trial name or title | Optimizing periconceptional and prenatal folic acid supplementation. |
| Methods | Randomised controlled intervention trial, open‐label masking. |
| Participants | Women 18‐45 years of age, not taking multivitamins or folic acid in the previous 6 months and planning a pregnancy. |
| Interventions | Twice daily supplement of PregVit® containing 1.1 mg of folic acid (group 1) or PregVit‐folic 5® containing 5 mg of folic acid (group 2). |
| Outcomes | RBC and serum folate levels. |
| Starting date | December 2006. |
| Contact information | Gideon Koren Director of the Mother Risk Program, Clinical Pharmacology and Toxicology The Hospital for Sick Childen |
| Notes | Sponsor: The Hospital for Sick Children and Duchesnay Inc. |
Linet 2011.
| Trial name or title | Follow‐up study of late effects of periconceptional folic acid in mothers and offspring in the community intervention program population: The Chinese children and families study. |
| Methods | Observational study design: time perspective: retrospective. |
| Participants | 500 Chinese mothers, fathers and their child aged 14‐16 years who participated in the community intervention programme from 1993 to 1996 living in Laoting and Tai ang counties, China and who provide consent. Excluison criteria: not having been a singleton birth, missing data on pill usage, or missing data on gender. |
| Interventions | The women and children who participated in the joint China‐U.S. Community Intervention Program (CIP) trial (N = 243,779 women treated or not treated with folic acid in the periconceptional period and their offspring) represent unique cohorts whose periconceptional exposure to folic acid is well documented. This study will follow a sample of 22,000 CIP mothers and their offspring (currently 14 to 17 years of age), associated with periconceptional folic acid supplements. |
| Outcomes | Vital status, medical history, and lifestyle habits, growth, physical development during the puberty period, selected serious morbidity and mortality in offspring and risks of serious health outcomes and mortality in mothers. |
| Starting date | May 2011. |
| Contact information | Dr. Martha Linet +1 (301) 496‐6600 Email: linetm@mail.nih.gov |
| Notes | Sponsor: National Cancer Institute (NCI). |
ELISA: enzyme‐linked immunosorbent assay RBC: red blood cell
Differences between protocol and review
In comparison to the previous version of this review the following changes have been made.
We reduced the number of comparisons, from eight to four and included the form of folate (folic acid or 5MTHF as subgroup analysis). We removed the following subgroup analyses.
By assisted reproduction: assisted versus non‐assisted reproduction.
By mandatory folic acid fortification: places with mandatory flour fortification versus non‐flour fortification or not mandatory.
We updated the methods section as per the current Pregnancy Childbirth standards. These include updating the methods on subgroup analysis. We have changed the analytical methods from fixed‐effect to random‐effects, independently of the magnitude of the heterogeneity.
We have included 'Summary of findings' tables and a PRISMA flowchart.
Contributions of authors
Luz Maria De‐Regil, Ana Cecilia Fernandez‐Gaxiola and Juan Pablo Peña‐Rosas participated in the previous version of the review. For this update Juan Pablo Pena‐Rosas and Luz Maria De‐Regil updated the methods and screened new eligible studies for inclusion. Pura Rayco‐Solon reviewed the results section, updated the PRISMA flow chart and described the excluded studies. Luz Maria De‐Regil helped in the production of the GRADE evidence profiles for the critical outcomes. All authors contributed to the final preparation of this version.
Disclaimer: Juan Pablo Peña‐Rosas and Pura Rayco‐Solon are currently staff members of the WHO. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the WHO.
Sources of support
Internal sources
The University of Liverpool, UK.
Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
Micronutrient Initiative, Canada.
External sources
-
National Institute for Health Research (NIHR), UK.
TD was supported in a previous update by a grant from the National Institute for Health Research (NIHR). UK NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS prioritised, centrally managed, pregnancy and childbirth systematic reviews: CPGS02
-
The Bill & Melinda Gates Foundation, USA.
WHO acknowledges the financial support from The Bill & Melinda Gates Foundation for the development of systematic reviews of the evidence on the effects of nutrition interventions.
-
Centers for Disease Control and Prevention (CDC), USA.
The World Health Organization gratefully acknowledges the financial contribution of the National Center on Birth Defects and Developmental Disabilities (NCBDDD) towards the preparation of this updated version of the review.
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
Luz Maria De‐Regil: I am a full time staff of the Micronutrient Initiative, an International NGO that supports research projects and large scale programmes to improve vitamin and mineral consumption among the most vulnerable populations.
Juan Pablo Peña‐Rosas and Pura Rayco‐Solon: The World Health Organization gratefully acknowledges the financial contribution of the Bill & Melinda Gates Foundation, Micronutrient Initiative, the Centers for Disease Control and Prevention (CDC), the US Agency for International development (USAID), the Global Alliance for Improved Nutrition (GAIN) and Harvest Plus towards the work in the area of nutrition. Donors do not fund specific guidelines and do not participate in any decision related to the guideline development process, including the composition of research questions, membership of the guideline groups, conduct and interpretation of systematic reviews, or formulation of recommendations.
Ana C Fernández‐Gaxiola: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Czeizel 1994 {published data only}
- Czeizel A, Fritz G. Randomized trial of periconceptional vitamins. JAMA 1989;262:1634. [Google Scholar]
- Czeizel A, Rode K. Trial to prevent first occurrence of neural tube defects by periconceptional multivitamin supplementation. Lancet 1984;2(8393):40. [DOI] [PubMed] [Google Scholar]
- Czeizel AE. Controlled studies of multivitamin supplementation on pregnancy outcomes. Annals of the New York Academy of Sciences 1993;678:266‐75. [DOI] [PubMed] [Google Scholar]
- Czeizel AE. Limb‐reduction defects and folic acid supplementation. Lancet 1995;345(8954):932. [DOI] [PubMed] [Google Scholar]
- Czeizel AE. Nutritional supplementation and prevention of congenital abnormalities. Current Opinion in Obstetrics & Gynecology 1995;7(2):88‐94. [PubMed] [Google Scholar]
- Czeizel AE. Prevention of congenital abnormalities by periconceptional multivitamin supplementation. BMJ 1993;306:1645‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE. Reduction of urinary tract and cardiovascular defects by periconceptional multivitamin supplementation. American Journal of Medical Genetics 1996;62(2):179‐83. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dobo M. Postnatal somatic and mental development after periconceptional multivitamin supplementation. Archives of Disease in Childhood 1994;70(3):229‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural‐tube defects by periconceptional vitamin supplementation. New England Journal of Medicine 1992;327:1832‐5. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dudas I, Fritz G, Tecsoi A, Hanck A, Kunovits G. The effect of periconceptional multivitamin‐mineral supplementation on vertigo, nausea and vomiting in the first trimester of pregnancy. Archives of Gynecology and Obstetrics 1992;251:181‐5. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dudas I, Metneki J. Pregnancy outcomes in a randomised controlled trial of periconceptional multivitamin supplementation. Archives of Gynecology and Obstetrics 1994;255:131‐9. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Medveczky E. Periconceptional multivitamin supplementation and multimalformed offspring. Obstetrics & Gynecology 2003;102:1255‐61. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Metneki J, Dudas I. Higher rate of multiple births after periconceptional vitamin supplementation. New England Journal of Medicine 1994;330(23):1687‐8. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Metneki J, Dudas I. The effect of preconceptional multivitamin supplementation on fertility. International Journal for Vitamin and Nutrition Research 1996;66:55‐8. [PubMed] [Google Scholar]
- Czeizel AE, Metneki J, Dudas I. The higher rate of multiple births after periconceptional multivitamin supplementation: an analysis of causes. Acta Geneticae Medicae et Gemellologiae 1994;43:175‐84. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Rockenbauer M, Susansky E. No change in sexual activity during preconceptional multivitamin supplementation. British Journal of Obstetrics and Gynaecology 1996;103:569‐73. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Vargha P. Periconceptional folic acid/multivitamin supplementation and twin pregnancy. American Journal of Obstetrics and Gynecology 2004;191(3):790‐4. [DOI] [PubMed] [Google Scholar]
- Czeizel E, Dudas I. Prevention of the first occurrence of anencephaly and spina bifida with periconceptional multivitamin supplementation (conclusion). Orvosi Hetilap 1994;135(42):2313‐7. [PubMed] [Google Scholar]
- Dobo M, Czeizel AE. Long‐term somatic and mental development of children after periconceptional multivitamin supplementation. European Journal of Pediatrics 1998;157:719‐23. [DOI] [PubMed] [Google Scholar]
- Dudas I, Rockenbauer M, Czeizel AE. The effect of preconceptional multivitamin supplementation on the menstrual cycle. Archives of Gynecology and Obstetrics 1995;256(3):115‐23. [DOI] [PubMed] [Google Scholar]
- Metneki J, Dudas I, Czeizel AE. Higher rate of multiple births after periconceptual multivitamin supplementation. Orvosi Hetilap 1996;137(43):2401‐5. [PubMed] [Google Scholar]
ICMR 2000 {published data only}
- Indian Council of Medical Research (ICMR) Collaborating Centres, Central Technical Co‐ordinating Unit. Multicentric study of efficacy of periconceptional folic acid containing vitamin supplementation in prevention of open neural tube defects from India. Indian Journal of Medical Research 2000;112:206‐11. [PubMed] [Google Scholar]
Kirke 1992 {published data only}
- Kirke PN, Daly LE, Elwood JH. A randomized trial of low dose folic acid to prevent neural tube defects. Archives of Disease in Childhood 1992;67:1442‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laurence 1981 {published data only}
- Laurence KM. Prevention of neural tube defects by improvement in maternal diet and preconceptional folic acid supplementation. Progress in Clinical and Biological Research 1985;163:383‐8. [PubMed] [Google Scholar]
- Laurence KM. The role of maternal nutrition and folic acid therapy before conception to prevent neural tube defects [Die Rolle der mütterlichen Ernährung und Folinsaure‐Therapie vor der Empfangnis zur Verhutung von Nervenkanal‐Defekten]. Monatsschrift fur Kinderheilkunde 1982;130:646. [Google Scholar]
- Laurence KM, Campbell H, James NE. The role of improvement in the maternal diet and preconceptional folic acid supplementation in the prevention of neural tube defects. Prevention of spina bifida and other neural tube defects. Academic Press, 1983:85‐125. [Google Scholar]
- Laurence KM, James N, Miller M, Campbell H, Tennant GB. A double randomised controlled trial for preconceptional folic acid supplementation for the prevention of recurrence of neural tube defects in high risk pregnancies. Journal of Medical Genetics 1982;19(1):66. [Google Scholar]
- Laurence KM, James N, Miller MH, Tennant GB, Campbell H. Double blind randomised controlled trial of folate treatment before conception to prevent recurrence of neural tube defects. BMJ (Clinical Research Edition) 1981;282:1509‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
MRC 1991 {published data only}
- Anon. Folate supplements prevent recurrence of neural tube defects. Nutrition Review 1992;50(Suppl 1):22‐4. [DOI] [PubMed] [Google Scholar]
- Anon. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet 1991;338(8760):131‐7. [PubMed] [Google Scholar]
References to studies excluded from this review
Angeles‐Agdeppa 2005 {published data only}
- Angeles‐Adgeppa I, Paulino LS, Ramos AC, Etorma UM, Cavalli‐Sforza T, Milani S. Government‐industry partnership in weekly iron‐folic acid supplementation for women of reproductive age in the Philippines: impact on iron status. Nutrition Reviews 2005;63(12):S116‐S125. [DOI] [PubMed] [Google Scholar]
Atukorala 1994 {published data only}
- Atukorala TM, Silva LD, Dechering WH, Dassenaeike TS, Perera RS. Evaluation of effectiveness of iron‐folate supplementation and anthelminthic therapy against anemia in pregnancy ‐ a study in the plantation sector of Sri Lanka. American Journal of Clinical Nutrition 1994;60(2):286‐92. [DOI] [PubMed] [Google Scholar]
Bailey 2005 {published data only}
- Bailey LB, Berry RJ. Folic acid supplementation and the occurrence of congenital heart defects, orofacial clefts, multiple births, and miscarriage. American Journal of Clinical Nutrition 2005;81(5):1213S‐17S. [DOI] [PubMed] [Google Scholar]
Berger 2005 {published data only}
- Berger J, Thanh HTK, Cavalli‐Sforza T, Smitasiri S, Khan NC, Milani S, et al. Community mobilization and social marketing to promote weekly iron‐folic acid supplementation in women of reproductive age in Vietnam: impact on anemia and iron status. Nutrition Reviews 2005;63(12):S95‐S108. [DOI] [PubMed] [Google Scholar]
Binns 2006 {published data only}
- Binns C, Scott J, Nwafor N, Graham K, Oddy W, Lee A, et al. Which mothers take folic acid and folate containing foods?. Asia Pacific Journal of Clinical Nutrition 2006;15(3):335‐40. [PubMed] [Google Scholar]
- Oddy WH, Miller M, Payne JM, Serna P, Bower CI. Awareness and consumption of folate‐fortified foods by women of childbearing age in Western Australia. Public Health Nutrition 2007;10(10):989‐95. [DOI] [PubMed] [Google Scholar]
Bortolus 2014 {published data only}
- Blom F. A randomized controlled trial on the effects of periconceptional and prenatal folic acid supplementation on congenital anomalies and preterm birth. Netherlands Trial Register (http://www.trialsregister.nl) [accessed 30 July 2015] 2011.
- Bortolus R. Randomized clinical trial to evaluate the efficacy of high dose of folic acid to prevent the occurrence of congenital malformations. International Clinical Trials Registry Platform (accessed 2 Aug 2011) http://clinicaltrials.gov/show/NCT01244347 2009.
- Bortolus R, Blom F, Filippini F, Poppel MN, Leoncini E, Smit DJ, et al. Prevention of congenital malformations and other adverse pregnancy outcomes with 4.0 mg of folic acid: community‐based randomized clinical trial in Italy and the Netherlands. BMC Pregnancy and Childbirth 2014;14(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italian Medicines Agency. Efficacy of folic acid at high doses in preventing congenital anomalies. Randomized clinical trial in fertile women who plan a pregnancy: folic acid 4 mg vs 0.4 mg. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2008‐004334‐25/IT#C (accessed 12 August 2015). EUCTR2008‐004334‐25‐IT.
Botto 2006 {published data only}
- Botto LD, Lisi A, Bower C, Canfield MA, Dattani N, Vigan C, et al. Trends of selected malformations in relation to folic acid recommendations and fortification: an international assessment. Birth Defects Research 2006;76(10):693‐705. [DOI] [PubMed] [Google Scholar]
Canfield 2005 {published data only}
- Canfield MA, Collins JS, Botto LD, Williams LJ, Mai CT, Kirby RS, et al. Changes in the birth prevalence of selected birth defects after grain fortification with folic acid in the United States: findings from a multi‐state population‐based study. Birth Defects Research 2005;73(10):679‐89. [DOI] [PubMed] [Google Scholar]
Cavalli‐Sforza 2005 {published data only}
- Cavalli‐Sforza T, Berger J, Smitasiri S, Viteri F. Weekly iron‐folic acid supplementation of women of reproductive age: impact overview, lessons learned, expansion plans, and contributions toward achievement of the Millennium Development Goals. Nutrition Reviews 2005;63(12):S152‐S158. [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen G, Song X, Ji Y, Zhang L, Pei L, Chen J, et al. Prevention of NTDs with periconceptional multivitamin supplementation containing folic acid in China. Birth Defects Research 2008;82(8):592‐6. [DOI] [PubMed] [Google Scholar]
Christian 2003 {published data only}
- Christian P. Personal communication 28 September 2007.
- Christian P, Darmstadt GL, Wu L, Khatry SK, LeClerq SC, Katz J, et al. The effect of maternal micronutrient supplementation on early neonatal morbidity in rural Nepal: a randomised, controlled, community trial. Archives of Disease in Childhood 2008;93(8):660‐4. [DOI] [PubMed] [Google Scholar]
- Christian P, Jiang T, Khatry SK, LeClerq SC, Shrestha SR, West KP Jr. Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. American Journal of Clinical Nutrition 2006;83(4):788‐94. [DOI] [PubMed] [Google Scholar]
- Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ 2003;326(7389):571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P, Khatry SK, LeClerq SC, Dali SM. Effects of prenatal micronutrient supplementation on complications of labor and delivery and puerperal morbidity in rural Nepal. International Journal of Gynecology & Obstetrics 2009;106(1):3‐7. [DOI] [PubMed] [Google Scholar]
- Christian P, Murray‐Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, et al. Prenatal micronutrient supplementation and intellectual and motor function in early school‐aged children in Nepal. JAMA 2010;304(24):2716‐23. [DOI] [PubMed] [Google Scholar]
- Christian P, Shrestha J, LeClerq SC, Khatry SK, Jiang T, Wagner T, et al. Supplementation with micronutrients in addition to iron and folic acid does not further improve the hematologic status of pregnant women in rural Nepal. Journal of Nutrition 2003;133(11):3492‐8. [DOI] [PubMed] [Google Scholar]
- Christian P, Stewart CP, LeClerq SC, Wu L, Katz J, West KP Jr, et al. Antenatal and postnatal iron supplementation and childhood mortality in rural Nepal: a prospective follow‐up in a randomized, controlled community trial. American Journal of Epidemiology 2009;170(9):1127‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P, West KP, Khatry SK, Leclerq SC, Pradhan EK, Katz J, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster‐randomized trial in Nepal. American Journal of Clinical Nutrition 2003;78(6):1194‐202. [DOI] [PubMed] [Google Scholar]
- Christian PS, Darmstadt GL, Wu L, Khatry SK, Leclerq SC, Katz J, et al. The impact of maternal micronutrient supplementation on early neonatal morbidity in rural Nepal: a randomized, controlled, community trial. Archives of Disease in Childhood. Fetal and Neonatal Edition (http://fn.bmj.com/content/early/2007/08/03/adc.2006.114009.full.pdf+html) (accessed August 2007). [DOI: 10.1136/adc.2006.114009] [DOI] [PubMed]
- Katz J, Christian P, Dominici F, Zeger SL. Treatment effects of maternal micronutrient supplementation vary by percentiles of the birth weight distribution in rural Nepal. Journal of Nutrition 2006;136(5):1389‐94. [DOI] [PubMed] [Google Scholar]
- Kulkarni B, Christian P, LeClerq SC, Khatry SK. Determinants of compliance to antenatal micronutrient supplementation and women's perceptions of supplement use in rural Nepal. Public Health Nutrition 2010;13(1):82‐90. [DOI] [PubMed] [Google Scholar]
- Stewart CP, Christian P, LeClerq SC, West KP Jr, Khatry SK. Antenatal supplementation with folic acid + iron + zinc improves linear growth and reduces peripheral adiposity in school‐age children in rural Nepal. American Journal of Clinical Nutrition 2009;90(1):132‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CP, Christian P, Schulze KJ, Arguello M, Leclerq SC, Khatry SK, et al. Low maternal vitamin B‐12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. Journal of Nutrition 2011;141(10):1912‐7. [DOI] [PubMed] [Google Scholar]
- Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KP Jr, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6‐ to 8‐year‐old children in rural Nepal. Journal of Nutrition 2009;139(8):1575‐81. [DOI] [PubMed] [Google Scholar]
- Stewart CP, Katz J, Khatry SK, LeClerq SC, Shrestha SR, West KP Jr, et al. Preterm delivery but not intrauterine growth retardation is associated with young maternal age among primiparae in rural Nepal. Maternal & Child Nutrition 2007;3(3):174‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daly 1995 {published data only}
- Daly SF, Cahill E, Turner MJ. Can fortified milk raise folic acid levels in non pregnant women?. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7, Dublin, Ireland. 1995:Abstract no: 456.
Daly 1997 {published data only}
- Daly S, Mills JL, Molloy AM, Conley M, Lee YJ, Kirke PN, et al. Minimum effective dose of folic acid for food fortification to prevent neural tube defects. Lancet 1997;350:1666‐9. [DOI] [PubMed] [Google Scholar]
- Daly S, Mills JL, Molloy AM, Conley M, McPartlin J, Lee YJ, Young PB, Kirke PN, Weir DG, Scott JM. Low‐dose folic acid lowers plasma homocysteine levels in women of child‐bearing age. QJM 2002;95(11):733‐40. [DOI] [PubMed] [Google Scholar]
Doyle 2001 {published data only}
- Doyle W, Srivastava A, Crawford MA, Bhatti R, Brooke Z, Costeloe KL. Inter‐pregnancy folate and iron status of women in an inner‐city population. British Journal of Nutrition 2001;86(1):81‐7. [DOI] [PubMed] [Google Scholar]
Drazkowski 2002 {published data only}
- Drazkowski J, Sirven J, Blum D. Symptoms of B12 deficiency can occur in women of child bearing age supplemented with folate. Neurology 2002;58(10):1572‐3. [DOI] [PubMed] [Google Scholar]
Eichholzer 2006 {published data only}
- Eichholzer M, Tonz O, Zimmermann R. Folic acid: a public‐health challenge. Lancet 2006;367(9519):1352‐61. [DOI] [PubMed] [Google Scholar]
Ejidokun 2000 {published data only}
- Ejidokun OO. Community attitudes to pregnancy, anaemia, iron and folate supplementation in urban and rural Lagos, south‐western Nigeria. Midwifery 2000;16(2):89‐95. [DOI] [PubMed] [Google Scholar]
Elbourne 2002 {published data only}
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Vaillancourt JM. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
Ellison 2004 {published data only}
- Ellison J, Clark P, Walker ID, Greer IA. Effect of supplementation with folic acid throughout pregnancy on plasma homocysteine concentration. Thrombosis Research 2004;114:25‐7. [DOI] [PubMed] [Google Scholar]
Eskes 2000 {published data only}
- Eskes TK. Homocysteine and human reproduction. Clinical and Experimental Obstetrics and Gynecology 2000;27(3‐4):157‐67. [PubMed] [Google Scholar]
Field 1991 {published data only}
- Field B. Neural tube defects. The role of vitamin supplementation in their prevention. Current Therapeutics 1991;32(3):11‐3. [Google Scholar]
Geisel 2003 {published data only}
- Geisel J. Folic acid and neural tube defects in pregnancy: a review. Journal of Perinatal and Neonatal Nursing 2003;17(4):268‐79. [DOI] [PubMed] [Google Scholar]
Gunaratna 2015 {published data only}
- Gunaratna NS, Masanja H, Mrema S, Levira F, Spiegelman D, Hertzmark E, et al. Multivitamin and iron supplementation to prevent periconceptional anemia in rural Tanzanian women: A randomized, controlled trial. PLoS ONE 2015; Vol. 10, issue 4:e0121552. [DOI] [PMC free article] [PubMed]
Hague 2003 {published data only}
- Hague WM. Homocysteine and pregnancy. Best Practice & Research in Clinical Obstetrics & Gynaecology 2003;17(3):459‐69. [DOI] [PubMed] [Google Scholar]
Hayes 1996 {published data only}
- Hayes C, Werler MM, Willett WC, Mitchell AA. Case‐control study of periconceptional folic acid supplementation and oral clefts. American Journal of Epidemiology 1996;143(12):1229‐34. [DOI] [PubMed] [Google Scholar]
Itikala 2001 {published data only}
- Itikala PR, Watkins ML, Mulinare J, Moore CA, Liu Y. Maternal multivitamin use and orofacial clefts in offspring. Teratology 2001;63(2):79‐86. [DOI] [PubMed] [Google Scholar]
Johnston 2008 {published data only}
- Johnston RB Jr. Will increasing folic acid in fortified grain products further reduce neural tube defects without causing harm? Consideration of the evidence. Pediatric Research 2008;63(1):2‐8. [DOI] [PubMed] [Google Scholar]
Khambalia 2009 {published data only}
- Khambalia A. Periconceptional Iron Supplementation and Iron and Folate Status Among Pregnant and Non‐Pregnant Women in Rural Bangladesh [thesis]. Toronto: University of Toronto, 2009. [Google Scholar]
- Khambalia A, O'Connor D, Zlotkin S. Reduced anemia and improved iron and folate status before and after pregnancy among females in rural Bangladesh is related to length of adherence to periconceptional iron and folic acid supplementation. Faseb Journal 2014;23(Suppl):Abstract no: 917.6. [Google Scholar]
- Khambalia A, O'Connor DL, Zlotkin S. Periconceptional iron and folate status is inadequate among married, nulliparous women in rural Bangladesh. Journal of Nutrition 2009;139(6):1179‐84. [DOI] [PubMed] [Google Scholar]
- Khambalia AZ, O'Connor DL, Macarthur C, Dupuis A, Zlotkin SH. Periconceptional iron supplementation does not reduce anemia or improve iron status among pregnant women in rural Bangladesh. American Journal of Clinical Nutrition 2009;90(5):1295‐302. [DOI] [PubMed] [Google Scholar]
Lee 2005 {published data only}
- Lee JI, Lee JA, Lim HS. Effect of time of initiation and dose of prenatal iron and folic acid supplementation on iron and folate nutriture of Korean women during pregnancy. American Journal of Clinical Nutrition 2005;82(4):843‐9. [DOI] [PubMed] [Google Scholar]
Li 2014 {published data only}
- Li YF, Hu NS, Tian XB, Li L, Wang SM, Xu XB, et al. Effect of daily milk supplementation on serum and umbilical cord blood folic acid concentrations in pregnant Han and Mongolian women and birth characteristics in China. Asia Pacific Journal of Clinical Nutrition 2014;23(4):567‐74. [DOI] [PubMed] [Google Scholar]
Mandishona 1999 {published data only}
- Mandishona EM, Moyo VM, Gordeuk VR, Khumalo H, Saungweme T, Gangaidzo IT, et al. A traditional beverage prevents iron deficiency in African women of child bearing age. European Journal of Clinical Nutrition 1999;53(9):722‐5. [DOI] [PubMed] [Google Scholar]
Manizheh 2009 {published data only}
- Manizheh SM, Mandana S, Hassan A, Amir GH, Mahlisha KS, Morteza G. Comparison study on the effect of prenatal administration of high dose and low dose folic acid. Saudi Medical Journal 2009;30(1):88‐97. [PubMed] [Google Scholar]
Mathews 1999 {published data only}
- Mathews F, Murphy M, Wald NJ, Hackshaw A. Twinning and folic acid use. Lancet 1999;353:291‐2. [DOI] [PubMed] [Google Scholar]
McNulty 2013 {published data only}
- McNulty B, McNulty H, Marshall B, Ward M, Molloy AM, Scott JM, et al. Impact of continuing folic acid after the first trimester of pregnancy: findings of a randomized trial of folic acid supplementation in the second and third trimesters. American Journal of Clinical Nutrition 2013;98(1):92‐8. [DOI] [PubMed] [Google Scholar]
Melli 2008 {published data only}
- Melli MS, Shojaiee M, Sheshvan MK. Prenatal administration of high dose and low dose folic acid on maternal plasma homocysteine concentration and its relationship to the development of preeclampsia. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):82. [Google Scholar]
Molster 2007 {published data only}
- Molster C, Bower C, O'Leary P. Australian survey on community knowledge and attitudes regarding the fortification of food with folic acid. Birth Defects Research 2007;79(9):664‐70. [DOI] [PubMed] [Google Scholar]
Nelen 2000 {published data only}
- Nelen WL, Blom HJ, Steegers EA, Heijer M, Thomas CM, Eskes TK. Homocysteine and folate levels as risk factors for recurrent early pregnancy loss. Obstetrics & Gynecology 2000;95(4):519‐24. [DOI] [PubMed] [Google Scholar]
Nguyen 2009 {published data only}
- Gonzalez‐Casanova I, Nguyen P, Harding K, Reinhart G, Nguyen H, Truong T, et al. Predictors of adherence to preconception and prenatal micronutrient supplementation in Vietnam. FASEB Journal 2015; Vol. 29, issue 1 Suppl:[abstract no: 729.12].
- Nguyen P, Tam C, O'Connor DL, Kapur B, Koren G. Steady state folate concentrations achieved with 5 compared with 1.1 mg folic acid supplementation among women of childbearing age. American Journal of Clinical Nutrition 2009;89:844‐52. [DOI] [PubMed] [Google Scholar]
- Nguyen PH, Lowe AE, Martorell R, Nguyen H, Pham H, Nguyen S, et al. Rationale, design, methodology and sample characteristics for the Vietnam pre‐conceptual micronutrient supplementation trial (PRECONCEPT): a randomized controlled study. BMC Public Health 2012; Vol. 12:898. [DOI] [PMC free article] [PubMed]
- Ramakrishnan U. Impact of pre‐pregnancy micronutrient supplementation on maternal and child outcomes. http://clinicaltrials.gov (accessed 20 September 2012) 2012.
- Shere M, Nguyen P, Tam C, Stern S, Kapur B, O'Connor D, et al. Optimizing periconceptional folic acid supplementation: steady‐state folate pharmacokinetics in pregnancy. FASEB Journal 2014;28(1 Suppl):LB416. [Google Scholar]
- Shere M, Nguyen P, Tam C, Stern S, Kapur B, O'Connor DL, et al. Pregnancy‐induced changes in the long‐term pharmacokinetics of 1.1mg vs. 5mg folic acid: a randomized clinical trial. Journal of Clinical Pharmacology 2015;55(2):159‐67. [DOI] [PubMed] [Google Scholar]
- Shere M, Nguyen P, Tam C, Stern S, Kapur B, O'Connor DL, et al. Steady‐state folate pharmacokinetics in pregnancy: A randomized clinical trial in pregnant women supplementing with 1.1mg vs. 5mg folic acid. Journal of Population Therapeutics and Clinical Pharmacology 2014;21(2):e280. [Google Scholar]
Pitkin 2007 {published data only}
- Pitkin RM. Folate and neural tube defects. American Journal of Clinical Nutrition 2007;85(1):285S‐8S. [DOI] [PubMed] [Google Scholar]
Pritchard 1991 {published data only}
- Pritchard JA, Cunningham FG, Pritchard SA, Mason RA. On reducing the frequency of severe abruptio placentae. American Journal of Obstetrics & Gynecology 1991;165(5 Pt 1):1345‐51. [DOI] [PubMed] [Google Scholar]
Ramakrishnan 2003 {published data only}
- Garcia‐Guerra A, Neufeld LM, Hernandez‐Cordero S, Rivera J, Martorell R, Ramakrishnan U. Prenatal multiple micronutrient supplementation impact on biochemical indicators during pregnancy and postpartum. Salud Publica de Mexico 2009;51(4):327‐35. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U, Gonzalez‐Cossio T, Neufeld L M, Rivera J, Martorell R. Effect of prenatal multiple micronutrient supplements on maternal weight and skinfold changes: a randomized double‐blind clinical trial in Mexico. Food & Nutrition Bulletin 2005;26(3):273‐80. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U, Gonzalez‐Cossio T, Neufeld LM, Rivera J, Martorell R. Multiple micronutrient supplementation during pregnancy does not lead to greater infant birth size than does iron‐only supplementation: a randomised controlled trial in a semirural community in Mexico. American Journal of Clinical Nutrition 2003;77(3):720‐5. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U, Neufeld LM, Gonzalez‐Cossio T, Villalpando S, Garcia‐Guerra A, Rivera J, et al. Multiple micronutrient supplements during pregnancy do not reduce anemia or improve iron status compared to iron‐only supplements in semirural Mexico. Journal of Nutrition 2004;134(4):898‐903. [DOI] [PubMed] [Google Scholar]
Ray 2007 {published data only}
- Ray JG, Wyatt PR, Thompson MD, Vermeulen MJ, Meier C, Wong PY, et al. Vitamin B12 and the risk of neural tube defects in a folic‐acid‐fortified population. Epidemiology 2007;18(3):362‐6. [DOI] [PubMed] [Google Scholar]
Ray 2008 {published data only}
- Ray JG, Goodman J, O'Mahoney PR, Mamdani MM, Jiang D, O'Mahoney PRA. High rate of maternal vitamin B12 deficiency nearly a decade after Canadian folic acid flour fortification. Qjm 2008;101(6):475‐7. [DOI] [PubMed] [Google Scholar]
Robbins 2005 {published data only}
- Robbins J M, Tilford JM, Bird TM, Cleves MA, Reading JA, Hobbs CA, et al. Hospitalizations of newborns with folate‐sensitive birth defects before and after fortification of foods with folic acid [erratum appears in Pediatrics. 2006 Dec;118(6):2608]. Pediatrics 2006;118(3):906‐15. [DOI] [PubMed] [Google Scholar]
- Robbins JM, Cleves MA, Collins HB, Andrews N, Smith LN, Hobbs CA, et al. Randomized trial of a physician‐based intervention to increase the use of folic acid supplements among women. American Journal of Obstetrics & Gynecology 2005;192(4):1126‐32. [DOI] [PubMed] [Google Scholar]
- Robbins JM, Hopkins SE, Mosley BS, Casey PH, Cleves MA, Hobbs CA, et al. Awareness and use of folic acid among women in the lower Mississippi Delta. Journal of Rural Health 2006;22(3):196‐203. [DOI] [PubMed] [Google Scholar]
Rolschau 1999 {published data only}
- Rolschau J, Kristoffersen K, Ulrich M, Grinsted P, Schaumburg E, Foged N. The influence of folic acid supplement on the outcome of pregnancies in the county of Funen in Denmark. Part I. European Journal of Obstetric & Gynecology and Reproductive Biology 1999;87(2):105‐10; discussion 103‐4. [PubMed] [Google Scholar]
- Ulrich M, Kristoffersen K, Rolschau J, Grinstead P, Schaumburg E, Foged N. The influence of folic acid supplement on the outcome of pregnancies in the county of Funen in Denmark. Part II. Congenital anomalies A randomised study. European Journal of Obstetrics & Gynecology and Reproductive Biology 1999;87:111‐3. [PubMed] [Google Scholar]
- Ulrich M, Kristoffersen K, Rolschau J, Grinsted P, Schaumburg E, Foged N. The influence of folic acid supplement on the outcome of pregnancies in the county of Funen in Denmark. Part III. Congenital anomalies. An observational study. European Journal of Obstetrics & Gynecology and Reproductive Biology 1999;87(2):115‐8; discussion 103‐4. [PubMed] [Google Scholar]
Rosenthal 2008 {published data only}
- Rosenthal J, Milla G, Flores A, Yon M, Pfeiffer C, Umaña E, et al. Effect of different dosage and administration schedules of folic acid on blood folate levels in a population of Honduran women of reproductive age. Public Health Nutrition 2008;11(8):822‐30. [DOI] [PubMed] [Google Scholar]
Sayers 1997 {published data only}
- Sayers GM, Hughes N, Scallan E, Johnson Z. A survey of knowledge and use of folic acid among women of child‐bearing age in Dublin. Journal of Public Health Medicine 1997;19(3):328‐32. [DOI] [PubMed] [Google Scholar]
Schorah 1993 {published data only}
- Schorah CJ, Wild J. Fortified foods and folate intake in women of child‐bearing age. Lancet 1993;341(8857):1417. [DOI] [PubMed] [Google Scholar]
Schwarz 2008 {published data only}
- Schwarz EB, Sobota M, Gonzales R, Gerbert B. Computerized counseling for folate knowledge and use: a randomized controlled trial. American Journal of Preventive Medicine 2008;35(6):568‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaw 1995 {published data only}
- Shaw GM, Lammer EJ, Wasserman CR, O'Malley CD, Tolarova MM. Risks of orofacial clefts in children born to women using multivitamins containing folic acid periconceptionally. Lancet 1995;346(8972):393‐6. [DOI] [PubMed] [Google Scholar]
Shaw 2006 {published data only}
- Shaw GM, Carmichael SL, Laurent C, Rasmussen SA. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology 2006;17(3):285‐91. [DOI] [PubMed] [Google Scholar]
Shrimpton 2002 {published data only}
- Shrimpton R, Schultink W. Can supplements help meet the micronutrient needs of the developing world?. Proceedings of the Nutrition Society 2002;61(2):223‐9. [DOI] [PubMed] [Google Scholar]
van der Put 1998a {published data only}
- Put NM, Gabreels F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural‐tube defects?. American Journal of Human Genetics 1998;62(5):1044‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Rooij 2004 {published data only}
- Rooij IA, Ocke MC, Straatman H, Zielhuis GA, Merkus HM, Steegers‐Theunissen RP, et al. Periconceptional folate intake by supplement and food reduces the risk of nonsyndromic cleft lip with or without cleft palate. Preventive Medicine 2004;39(4):689‐94. [DOI] [PubMed] [Google Scholar]
Wald 2004 {published data only}
- Wald NJ. Folic acid and the prevention of neural‐tube defects. New England Journal of Medicine 2004;350(2):101‐3. [DOI] [PubMed] [Google Scholar]
Walsh 2007 {published data only}
- Walsh T, O'Broin S, Cooley S, Donnelly J, Collins C, McMillan H, et al. Maternal folate status and neural tube defects in Ireland: the need for a national food fortification program. Irish Medical Journal 2007;100(5):469‐72. [PubMed] [Google Scholar]
Watson 1999 {published data only}
- Watson LF, Watson MJ, Halliday JL, Bell RJ. Consequences of surveying folate awareness. Health Expectations 2002;5(1):38‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson LW. The epidemiology of folate and neural tube defects: a community intervention and ongoing issues. Australasian Epidemiologist 2003;10:6‐8. [Google Scholar]
- Watson M, Watson L, Bell R, Halliday J. The increasing knowledge of the role of periconceptional folate in Victorian women of child‐bearing age: follow‐up of a randomised community intervention trial. Australian & New Zealand Journal of Public Health 2001;25(5):389‐95. [PubMed] [Google Scholar]
- Watson M, Watson L, Bell R, Halliday J, Burford N, Brennecke S. Increasing knowledge of the role of folate in women of child‐bearing age in Victoria ‐ a randomised trial. 2nd Annual Congress of the Perinatal Society of Australia & New Zealand; 1998 March 30‐April 4; Alice Springs, Australia. 1998:114.
- Watson MJ, Watson LF, Bell RJ, Halliday JL, Burford N, Brennecke SP. A randomized community intervention trial to increase awareness and knowledge of the role of periconceptional folate in women of child‐bearing age. Health Expectations 1999;2:255‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wehby 2012 {published data only}
- Javois L. Birth defects treatment and prevention program: oral cleft prevention program (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006).
- Vila‐Nova C, Wehby GL, Queiros FC, Chakraborty H, Felix TM, Goco N, et al. Periconceptional use of folic acid and risk of miscarriage ‐ findings of the Oral Cleft Prevention Program in Brazil. Journal of Perinatal Medicine 2013;41(4):461‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehby GL, Felix TM, Goco N, Richieri‐Costa A, Chakraborty H, Souza J, et al. High dosage folic acid supplementation, oral cleft recurrence and fetal growth. International Journal of Environmental Research and Public Health 2013;10(2):590‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehby GL, Goco N, Moretti‐Ferreira D, Felix T, Richieri‐Costa A, Padovani C, et al. Oral cleft prevention program (OCPP). BMC Pediatrics 2012;12:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wen 2005 {published data only}
- Wen SW, Walker M. An exploration of health effects of folic acid in pregnancy beyond reducing neural tube defects. Journal of Obstetrics & Gynaecology Canada: JOGC 2005;27(1):13‐9. [DOI] [PubMed] [Google Scholar]
Westphal 2004 {published data only}
- Westphal LM, Polan ML, Trant AS. Double‐blind, placebo‐controlled study of FertilityBlend: a nutritional supplement for improving fertility in women. Clinical and Experimental Obstetrics and Gynecology 2006;33(4):205‐8. [PubMed] [Google Scholar]
- Westphal LM, Polan ML, Trant AS, Mooney SB. A nutritional supplement for improving fertility in women: a pilot study. Journal of Reproductive Medicine 2004;49(4):289‐93. [PubMed] [Google Scholar]
Wilcox 2007 {published data only}
- Wilcox AJ, Lie RT, Solvoll K, Taylor J, McConnaughey DR, Abyholm F, et al. Folic acid supplements and risk of facial clefts: national population based case‐control study. BMJ 2007;334(7591):464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2008 {published data only}
- Li Q, Yan H, Zeng L, Cheng Y, Liang W, Dang S, et al. Effects of maternal multimicronutrient supplementation on the mental development of infants in rural western China: follow‐up evaluation of a double‐blind, randomized, controlled trial. Pediatrics 2009;123(4):e685‐92. [DOI] [PubMed] [Google Scholar]
- Zeng L, Dibley MJ, Cheng Y, Dang S, Chang S, Kong L, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ 2008;337:a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Bailey 2014 {published data only}
- Bailey LB. Folic acid supplementation in pregnant women: dose response (FAPREG). ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 27 September 2014] 2014.
Hekmatdoost 2011 {published data only}
- Hekmatdoost A. Comparison of the effect of folic acid and 5‐methyltetrahydrofolate (5MTHF) on serum folate and homocysteine levels, and abortion rates in women suffering from recurrent abortion. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 8 July 2011).
Koren 2013 {published data only}
- Koren G. Optimizing periconceptional and prenatal folic acid supplementation. ISRCTN Registry (http://www.isrctn.com/) (accessed 9 September 2015).
Linet 2011 {unpublished data only}
- Linet M. Follow‐up study of late effects of periconceptional folic acid in mothers and offspring in the community intervention program population: the Chinese children and families study. http://clinicaltrials.gov/show/NCT01365975 (accessed 5 December 2013).
Additional references
Bailey 2015
- Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF, Mills JL, et al. Biomarkers of nutrition for development‐folate review. Journal of Nutrition 2015;145(7):1636S‐1680S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2010
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Berry 1999
- Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, et al. Prevention of neural‐tube defects with folic acid in China. China‐U.S. collaborative project for neural tube defect prevention. New England Journal of Medicine 1999;341(20):1485‐90. [DOI] [PubMed] [Google Scholar]
Berry 2004
- Berry RJ, Kihlberg R, Devine D. Impact of misclassification of in vitro fertilisation in studies of folic acid and twinning: modelling using population based Swedish vital records. BMJ 2004;330:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berry 2005
- Berry RJ, Kihlberg R. Folic acid supplementation is not associated with an increase in dizygotic twinning. Early Human Development 2005;81(5):465‐7. [DOI] [PubMed] [Google Scholar]
Berry 2010
- Berry RJ, Bailey L, Mulinare J, Bower C. Folic Acid Working Group. Fortification of flour with folic acid. Food Nutrition Bulletin 2010;31(Suppl 1):S22‐35. [DOI] [PubMed] [Google Scholar]
Blencowe 2010
- Blencowe H1, Cousens S, Modell B, Lawn J. Folic acid to reduce neonatal mortality from neural tube disorders. International Journal of Epidemiology 2010;Apr;39(Suppl 1):10‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Botto 2000
- Botto LD, Yang Q. 5,10‐Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. American Journal of Epidemiology 2000;151(9):862‐77. [DOI] [PubMed] [Google Scholar]
Botto 2004
- Botto LD, Olney RS, Erickson JD. Vitamin supplements and the risk for congenital anomalies other than neural tube defects. American Journal of Medical Genetics Part C. Seminars in Medical Genetics 2004;125C(1):12‐21. [DOI] [PubMed] [Google Scholar]
Botto 2005
- Botto LD, Lisi A, Robert‐Gnansia E, Erickson JD, Vollset SE, Mastroiacovo P, et al. International retrospective cohort study of neural tube defects in relation to folic acid recommendations: are the recommendations working?. BMJ 2005;330:571‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandalize 2009
- Brandalize AP, Bandinelli E, dos Santos PA, Roisenberg I, Schüler‐Faccini L. Evaluation of C677T and A1298C polymorphisms of the MTHFR gene as maternal risk factors for Down syndrome and congenital heart defects. American Journal of Medical Genetics. Part A 2009;149A(10):2080‐7. [DOI] [PubMed] [Google Scholar]
Branum 2013
- Branum AM, Bailey R, Singer BJ. Dietary supplement use and folate status during pregnancy in the United States. Journal of Nutrition 2013;143(4):486‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brämswig 2009
- Brämswig S, Prinz‐Langenohl R, Lamers Y, Tobolski O, Wintergerst E, Berthold HK, et al. Supplementation with a multivitamin containing 800 microg of folic acid shortens the time to reach the preventive red blood cell folate concentration in healthy women. International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift fur Vitamin‐ und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition 2009;79(2):61‐70. [DOI] [PubMed] [Google Scholar]
Carter 2005
- Carter JY, Loolpapit MP, Lema OE, Tome JL, Nagelkerke NJ, Watkins WM. Reduction of the efficacy of antifolate antimalarial therapy by folic acid supplementation. American Journal of Tropical Medicine and Hygiene 2005;73(1):166‐70. [PubMed] [Google Scholar]
CDC 2008a
- Centers for Disease Control and Prevention. National Report on Biochemical Indicators of Diet and Nutrition in the US Population 1999‐2002. Water soluble vitamins and related biochemical compounds. Atlanta, GA: Department of Health and Human Services, 2008. [Google Scholar]
CDC 2008b
- Centers for Disease Control and Prevention (CDC). Trends in wheat‐flour fortification with folic acid and iron ‐ worldwide, 2004 and 2007. MMWR. Morbidity and Mortality Weekly Report 2008;57(1):8‐10. [PubMed] [Google Scholar]
Chango 2000
- Chango A, Emery‐Fillon N, Courcy GP, Lambert D, Pfister M, Rosenblatt DS, et al. A polymorphism (80G‐>A) in the reduced folate carrier gene and its associations with folate status and homocysteinemia. Molecular Genetics and Metabolism 2000;70(4):310‐5. [DOI] [PubMed] [Google Scholar]
Cole 2007
- Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 2007;297(21):2351‐9. [DOI] [PubMed] [Google Scholar]
Coppede 2009
- Coppede F. The complex relationship between folate/homocysteine metabolism and risk of Down syndrome. Mutation Research 2009;682(1):54‐70. [DOI] [PubMed] [Google Scholar]
Czeizel 1992
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural‐tube defects by periconceptional vitamin supplementation. New England Journal of Medicine 1992;327(26):1832‐5. [DOI] [PubMed] [Google Scholar]
Czeizel 1999b
- Czeizel AE. Ten years of experience in periconceptional care. European Journal of Obstetrics & Gynecology and Reproductive Biology 1999;84:43‐9. [DOI] [PubMed] [Google Scholar]
Czeizel 2004
- Czeizel AE. The primary prevention of birth defects: multivitamins or folic acid?. International Journal of Medical Sciences 2004;1(1):50‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dary 2009
- Dary O. Nutritional interpretation of folic acid interventions. Nutrition Reviews 2009;67(4):235‐44. [DOI] [PubMed] [Google Scholar]
De Wals 2007
- Wals P, Tairou F, Allen MI, Uh SH, Lowry RB, Dibbald B, et al. Reduction in neural‐tube defects after folic acid fortification in Canada. New England Journal of Medicine 2007;357(2):135‐42. [DOI] [PubMed] [Google Scholar]
Duffy 2014
- Duffy ME, Hoey L, Hughes CF, Strain JJ, Rankin A, Souverein OW, et al. Biomarker responses to folic acid intervention in healthy adults: A meta‐analysis of randomized controlled trials. American Journal of Clinical Nutrition 2014;99(1):96–106. [DOI] [PubMed] [Google Scholar]
Elsinga 2006
- Elsinga J, Pal‐de Bruin K, Cessie S, Jong‐Potjer L, Verloove‐Vanhorick S, Assendelft W. Preconception counselling initiated by general practitioners in the Netherlands: reaching couples contemplating pregnancy [ISRCTN53942912]. BMC Family Practice 2006;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
English 2006
- English M, Snow RW. Iron and folic acid supplementation and malaria risk. Lancet 2006;367(9505):90‐1. [DOI] [PubMed] [Google Scholar]
Ericson 2001
- Ericson A, Källén B, Aberg A. Use of multivitamins and folic acid in early pregnancy and multiple births in Sweden. Twin Research : the official journal of the International Society for Twin Studies 2001;4(2):63‐6. [DOI] [PubMed] [Google Scholar]
Eskes 2006
- Eskes TK. Abnormal folate metabolism in mothers with Down syndrome offspring: review of the literature. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2006;124(2):130‐3. [DOI] [PubMed] [Google Scholar]
European Food Safety Authority NDA Panel 2013
- European Food Safety Authority NDA Panel (EFSA Panel on Dietetic Products, Nutrition, Allergies). Scientific Opinionon the substantiation of a health claim related to increasing maternal folate status by supplemental folate intake and reduced risk of neural tube defects pursuant to article 14 of regulation (EC) No 1924/2006. European Food Safety Authority Journal 2013;11(7):3328. [Google Scholar]
Fernandez‐Gaxiola 2011
- Fernández‐Gaxiola AC, De‐Regil LM. Intermittent iron supplementation for reducing anaemia and its associated impairments in menstruating women. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009218.pub2] [DOI] [PubMed] [Google Scholar]
Fife 2009
- Fife J, Raniga S, Hider PN, Frizelle FA. Folic acid supplementation and colorectal cancer risk; a meta‐analysis. Colorectal Disease 2009;Oct 27:Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Friberg 2015
- Friberg AK, Jørgensen FS. Few Danish pregnant women follow guidelines on periconceptional use of folic acid. Danish Medical Journal 2015;61(3):A5019. [PubMed] [Google Scholar]
Glasier 2006
- Glasier A, Gülmezoglu AM, Schmid GP, Moreno CG, Look PF. Sexual and reproductive health: a matter of life and death. Lancet 2006;368(9547):1595‐607. [DOI] [PubMed] [Google Scholar]
Goh 2006
- Goh YI, Bollano E, Einarson TR, Koren G. Prenatal multivitamin supplementation and rates of congenital anomalies: a meta‐analysis. Journal of Obstetrics and Gynaecology Canada: JOGC 2006;28:680‐9. [DOI] [PubMed] [Google Scholar]
Goh 2007
- Goh YI, Bollano E, Einarson TR, Koren G. Prenatal multivitamin supplementation and rates of pediatric cancer: a meta‐analysis. Clinical Pharmacology and Therapeutics 2007;81(5):685‐91. [DOI] [PubMed] [Google Scholar]
Gomes 2015
- Gomes S, Lopes C, Pinto E. Folate and folic acid in the periconceptional period: recommendations from official health organizations in thirty‐six countries worldwide and WHO. Public Health Nutrition 2015 Apr 16;[Epub ahead of print]:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guéant‐Rodriguez 2006
- Guéant‐Rodriguez RM, Guéant JL, Debard R, Thirion S, Hong LX, Bronowicki JP, et al. Prevalence of methylenetetrahydrofolate reductase 677T and 1298C alleles and folate status: a comparative study in Mexican, West African, and European populations. American Journal of Clinical Nutrition 2006;83(3):701‐7. [DOI] [PubMed] [Google Scholar]
Haider 2015
- Haider BA, Bhutta ZA. Multiple‐micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD004905.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heseker 2009
- Heseker HB, Mason JB, Selhub J, Rosenberg IH, Jacques PF. Not all cases of neural‐tube defect can be prevented by increasing the intake of folic acid. British Journal of Nutrition 2009;102(2):173‐80. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Review Manager (RevMan). Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org: The Cochrane Collaboration, 2011. [Google Scholar]
Houghton 2006
- Houghton LA, Sherwood KL, Pawlosky R, Ito S, O'Connor DL. [6S]‐5‐Methyltetrahydrofolate is at least as effective as folic acid in preventing a decline in blood folate concentrations during lactation. American Journal of Clinical Nutrition 2006;83(4):842‐50. [DOI] [PubMed] [Google Scholar]
IOM 1998
- Institute of Medicine. Folate. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academy Press, 1998:196‐303. [PubMed] [Google Scholar]
IOM 2003
- Bale JR, Stoll BJ, Lucas AO, editors. Reducing birth defects: meeting the challenge in the developing world. Washington: National Academies Press, 2003. [PubMed] [Google Scholar]
Iyengar 1975
- Iyengar L, Rajalakshmi K. Effect of folic acid supplement on birth weights of infants. American Journal of Obstetrics and Gynecology 1975;122(3):332‐6. [DOI] [PubMed] [Google Scholar]
Jaszewski 2008
- Jaszewski R, Misra S, Tobi M, Ullah N, Naumoff JA, Kucuk O, et al. Folic acid supplementation inhibits recurrence of colorectal adenomas: a randomized chemoprevention trial. World Journal Gastroenterology 2008;28(14):4492‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kucik 2004
- Kucik J, Correa A. Trends in twinning rates in metropolitan Atlanta before and after folic acid fortification. Journal of Reproductive Medicine 2004;49(9):707‐12. [PubMed] [Google Scholar]
Lamers 2004
- Lamers Y, Prinz‐Langenohl R, Moser R, Pietrzik K. Supplementation with [6S]‐5‐methyltetrahydrofolate or folic acid equally reduces plasma total homocysteine concentrations in healthy women. American Journal of Clinical Nutrition 2004;79(3):473‐8. [DOI] [PubMed] [Google Scholar]
Lassi 2013
- Lassi ZS, Salam RA, Haider BA, Bhutta ZA. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD006896.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Life Sciences Research Office 1994
- Life Sciences Research Office, Center for Food Safety and Applied Nutrition. Assessment of folate methodology used in the Third National Health and Nutrition Survey (NHANES 1988‐1994). Washington DC: U.S. Food and Drug Administration, Department of Health and Human Services, 1994. [Google Scholar]
Lo 2014
- Lo A, Polšek D, Sidhu S. Estimating the burden of neural tube defects in low‐ and middle‐income countries. Journal of Global Health 2014;4(1):010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Makedos 2007
- Makedos G, Papanicolaou A, Hitoglou A, Kalogiannidis I, Makedos A, Vrazioti V, et al. Homocysteine, folic acid and B12 serum levels in pregnancy complicated with preeclampsia. Archives of Gynecology & Obstetrics 2007;275(2):121‐4. [DOI] [PubMed] [Google Scholar]
Marchetta 2015
- Marchetta CM, Devine OJ, Crider KS, Tsang BL, Cordero AM, Qi YP, et al. Assessing the association between natural food folate intake and blood folate concentrations: a systematic review and Bayesian meta‐analysis of trials and observational studies. Nutrients 2015;7(4):2663‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2009
- Marinho C, Alho I, Guerra A, Rego C, Areias J, Bicho M. The methylenetetrahydrofolate reductase gene variant (C677T) as a susceptibility gene for tetralogy of Fallot. Revista Portuguesa de Cardiologia 2009;28(7‐8):809‐12. [PubMed] [Google Scholar]
Martinez‐de Villareal 2001
- Martinez‐de Villareal L, Limón‐Benavides C, Valdez‐Leal R, Sánchez‐Peña MA, Villareal‐Pérez JZ. Impact of weekly administration of folic acid on folic acid blood levels. Salud Publica de Mexico 2001;43(2):103‐7. [PubMed] [Google Scholar]
Martinez‐de Villareal 2002
- Martinez‐de Villareal L, Pérez JZ, Vázquez PA, Herrera RH, Campos M del R, López RA, et al. Decline of neural tube defects cases after a folic acid campaign in Nuevo León, México. Teratology 2002;66(5):249‐56. [DOI] [PubMed] [Google Scholar]
Mason 2007
- Mason JB, Dickstein A, Jacques PF, Haggarty P, Selhub J, Dallal G, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiology, Biomarkers & Prevention 2007;16(7):1325‐9. [DOI] [PubMed] [Google Scholar]
McNulty 2004
- McNulty H, Pentieva K. Folate bioavailability. Proceedings of the Nutrition Society 2004;63(4):529‐36. [DOI] [PubMed] [Google Scholar]
Murray 1997
- Murray CJ, Lopez AD. Regional patterns of disability‐free life expectancy and disability‐adjusted life expectancy: Global Burden of Disease Study. Lancet 1997;349(9062):1347‐52. [DOI] [PubMed] [Google Scholar]
Nduati 2008
- Nduati E, Diriye A, Ommeh S, Mwai L, Kiara S, Masseno V, et al. Effect of folate derivatives on the activity of antifolate drugs used against malaria and cancer. Parasitology Research 2008;102(6):1227‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norsworthy 2004
- Norsworthy B, Skeaff CM, Adank C, Green TJ. Effects of once‐a‐week or daily folic acid supplementation on red blood cell folate concentrations in women. European Journal of Clinical Nutrition 2004;58(3):548‐54. [DOI] [PubMed] [Google Scholar]
Patrick 2004
- Patrick TE, Powers RW, Daftary AR, Ness RB, Roberts JM. Homocysteine and folic acid are inversely related in black women with preeclampsia. Hypertension 2004;43(6):1279‐82. [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2014
- Peña‐Rosas JP, Garcia‐Casal MN, Pachón H, Mclean MS, Arabi M. Technical considerations for maize flour and corn meal fortification in public health: consultation rationale and summary. Annals of the New York Academy of Sciences 2014;1312:1‐7. [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2015a
- Peña‐Rosas JP, De‐Regil LM, Dowswell T, Viteri FE. Daily oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD004736.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peña‐Rosas 2015b
- Peña‐Rosas JP, De‐Regil LM, Gomez Malave H, Flores‐Urrutia MC, Dowswell T. Intermittent oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD009997.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfeiffer 2013
- Pfeiffer C, Schleicher R, Caldwell K. Biochemical indices. In: Caballero OB editor(s). Enclyclopedia of Human Nutrition. 3rd Edition. Waltham, MA: Academic Press, 2013:156–74. [Google Scholar]
Pietrzik 2010
- Pietrzik K, Bailey L, Shane B. Folic acid and L‐5‐methyltetrahydrofolate: comparison of clinical pharmacokinetics and pharmacodynamics. Clinical Pharmacokinetics 2010;49(8):535‐48. [DOI] [PubMed] [Google Scholar]
Quinlivan 2003
- Quinlivan EP, Gregory JF 3rd. The impact of food fortification on folic acid intake in Canada. Canadian Journal of Public Health 2003;94(2):154. [PubMed] [Google Scholar]
Ramakrishnan 2012
- Ramakrishnan U, Grant F, Goldenberg T, Zongrone, A, Martorell R. Effect of women's nutrition before and during early pregnancy on maternal and infant outcomes: a systematic review [Effect]. Paediatric and Perinatal Epidemiology 2012;26(Suppl 1):285–301. [DOI] [PubMed] [Google Scholar]
Refsum 2008
- Refsum H, Smith AD. Are we ready for mandatory fortification with vitamin B‐12?. American Journal of Clinical Nutrition 2008;88(2):253‐4. [DOI] [PubMed] [Google Scholar]
Relton 2005
- Relton CL, Pearce MS, Parker L. The influence of erythrocyte folate and serum vitamin B12 status on birth weight. British Journal of Nutrition 2005;93(5):593‐9. [DOI] [PubMed] [Google Scholar]
Reveiz 2011
- Reveiz L, Gyte GML, Cuervo LG, Casasbuenas A. Treatments for iron‐deficiency anaemia in pregnancy. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD003094.pub3] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan). 5.3. Copenhagen: The Cochrane Collaboration, 2014.
Roza 2010
- Roza SJ, Batenburg‐Eddes T, Steegers EA, Jaddoe VW, Mackenbach JP, Hofman A, et al. Maternal folic acid supplement use in early pregnancy and child behavioural problems: The Generation R Study. British Journal of Nutrition 2010;103(3):445‐52. [DOI] [PubMed] [Google Scholar]
Schlotz 2010
- Schlotz W, Jones A, Phillips DI, Gale CR, Robinson SM, Godfrey KM. Lower maternal folate status in early pregnancy is associated with childhood hyperactivity and peer problems in offspring. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2010;51(5):594‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaw 2003
- Shaw GM, Carmichael SL, Nelson V, Selvin S, Schaffer DM. Food fortification with folic acid and twinning among California infants. American Journal of Medical Genetics. Part A 2003;119A(2):137‐40. [DOI] [PubMed] [Google Scholar]
Shibuya 1998
- Shibuya K, Murray C. Congenital anomalies. In: Murray C, Lopez A editor(s). Health Dimensions of Sex and Reproduction. Boston: Harvard School of Public Health, 1998:463‐73. [Google Scholar]
Signore 2005
- Signore C, Mills JL, Cox C, Trumble AC. Effects of folic acid fortification on twin gestation rates. Obstetrics and Gynecology 2005;105(4):757‐62. [DOI] [PubMed] [Google Scholar]
Steenweg‐de Graaff 2012
- Steenweg‐de Graaff J, Roza SJ, Steegers EA, Hofman A, Verhulst FC, Jaddoe VW, Tiemeier H. Maternal folate status in early pregnancy and child emotional and behavioral problems: the Generation R Study. American Journal of Clinical Nutrition 2012;95(6):1413‐21. [DOI] [PubMed] [Google Scholar]
Tamura 2006
- Tamura T, Picciano MF. Folate and human reproduction. American Journal of Clinical Nutrition 2006;83(5):993‐1016. [DOI] [PubMed] [Google Scholar]
Thompson 2009
- Thompson MD, Cole DE, Ray JG. Vitamin B12 and neural tube defects: the Canadian experience. American Journal of Clinical Nutrition 2009;89(2):697S‐701S. [DOI] [PubMed] [Google Scholar]
Tsang 2015
- Tsang B, Sandalinas F, De‐Regil LM. Folate supplementation in women of reproductive age. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011766] [DOI] [Google Scholar]
US Preventive Services Task Force 2009
- US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Annals of Internal Medicine 2009;150(9):626‐31. [DOI] [PubMed] [Google Scholar]
Van Beynum 2006
- Beynum IM, Kapusta L, Heijer M, Vermeulen SH, Kouwenberg M, Daniëls O, Blom HJ. Maternal MTHFR 677C>T is a risk factor for congenital heart defects: effect modification by periconceptional folate supplementation. European Heart Journal 2006;27(8):981‐7. [DOI] [PubMed] [Google Scholar]
Van der Put 1998
- Put NM, Gabreels F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural‐tube defects?. American Journal of Human Genetics 1998;62(5):1044‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Eijk 2008
- Eijk AM, Ouma PO, Williamson J, Ter Kuile FO, Parise M, Otieno K, et al. Plasma folate level and high‐dose folate supplementation predict sulfadoxine‐pyrimethamine treatment failure in pregnant women in Western Kenya who have uncomplicated malaria. Journal of Infectious Diseases 2008;198(10):1550‐3. [DOI] [PubMed] [Google Scholar]
Venn 2002
- Venn BJ, Green TJ, Moser R, McKenzie JE, Skeaff CM, Mann J. Increases in blood folate indices are similar in women of childbearing age supplemented with [6S]‐5‐methyltetrahydrofolate and folic acid. Journal of Nutrition 2002;132(11):3353‐5. [DOI] [PubMed] [Google Scholar]
Venn 2003
- Venn BJ, Green TJ, Moser R, Mann JI. Comparison of the effect of low‐dose supplementation with L‐5‐methyltetrahydrofolate or folic acid on plasma homocysteine: a randomized placebo‐controlled study. American Journal of Clinical Nutrition 2003;77(3):658‐62. [DOI] [PubMed] [Google Scholar]
Vollset 2005
- Vollset SE, Gjessing HK, Tandberg A, Rønning T, Irgens LM, Baste V, et al. Folate supplementation and twin pregnancies. Epidemiology 2005;16(2):201‐5. [DOI] [PubMed] [Google Scholar]
Wehby 2010
- Wehby GL, Murray JC. Folic acid and orofacial clefts: a review of the evidence. Oral Diseases 2010;16(1):11‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization. Human genetics: services for the prevention and management of genetic disorders and birth defects in developing countries. Report of a joint WHO/WOAPBD Meeting. Geneva: WHO, 1999. [DOI] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. Primary health care approaches for the prevention and control of congenital genetic disorders. Report of a WHO meeting. Geneva: WHO, 2000. [Google Scholar]
WHO 2006
- World Health Organization. Prevention of neural tube defects. Standards for maternal and neonatal care. www.who.int/reproductive‐health/publications/standards/neural_tube_defects.pdf (accessed 22 January 2009).
WHO 2011
- World Health Organization. Guideline Intermittent iron supplementation for menstruating women. Geneva: World Health Organization, 2011 (accessed 26 December 2013). [Google Scholar]
WHO 2014
- World Health Organization. Guideline: optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects. Geneva: World Health Organization, 2015. [PubMed] [Google Scholar]
WHO 2015a
- World Health Organization. Serum and red blood cell folate concentrations for assessing folate status in populations. Vol. WHO/NMH/NHD/EPG/15.01, Geneva: World Health Organization, 2015. [Google Scholar]
WHO 2015b
- World Health Organization. Congenital anomalies. Congenital anomalies. Fact sheet N°370. Geneva: World Health Organization, 2015. [Google Scholar]
WHO/CDC/ICBDSR 2014
- World Health Organization, Centers for Disease Control and Prevention and International Clearinghouse for Birth Defects Surveillance and Research. Birth defects surveillance: a manual for programme managers. Geneva: World Health Organization, 2014. [Google Scholar]
WHO/UNICEF/UNU 2001
- WHO, UNICEF, UNU. Iron deficiency anaemia: assessment, prevention and control. A guide for programme managers. Report from a the WHO/UNICEF/UNU consultation, 6‐10 December 1993. Geneva: World Health Organization, 2001.
Wu 2009
- Wu K, Platz EA, Willett WC, Fuchs CS, Selhub J, Rosner BA, et al. A randomized trial on folic acid supplementation and risk of recurrent colorectaladenoma. American Journal of Clinical Nutrition. 2009 90;90(6):1623‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yajnik 2008
- Yajnik CS, Deshmukh US. Maternal nutrition, intrauterine programming and consequential risks in the offspring. Reviews in Endocrine & Metabolic Disorders 2008;9(3):203‐11. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
De‐Regil 2009
- Regil LM, Fernández‐Gaxiola AC, Dowswell T, Peña‐Rosas JP. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007950] [DOI] [PMC free article] [PubMed] [Google Scholar]
De‐Regil 2010
- De‐Regil LM, Fernández‐Gaxiola AC, Dowswell T, Peña‐Rosas JP. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD007950.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lumley 2001
- Lumley J, Watson L, Watson M, Bower C. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001056] [DOI] [PubMed] [Google Scholar]


