Abstract
Glucocorticoids display remarkable anti-inflammatory activity, but their use is limited by on-target adverse effects including insulin resistance and skeletal muscle atrophy. We used a chemical systems biology approach, Ligand Class Analysis (LCA), to examine ligands designed to modulate glucocorticoid receptor activity through distinct structural mechanisms. These ligands displayed diverse activity profiles, providing the variance required to identify target genes and coregulator interactions that were highly predictive of their effects on myocyte glucose disposal and protein balance. Their anti-inflammatory effects were linked to glucose disposal but not muscle atrophy. This approach also predicted selective modulation in vivo, identifying compounds that were muscle sparing or anabolic for protein balance and mitochondrial potential. LCA defined the mechanistic links between the ligand-receptor interface and ligand-driven physiological outcomes, a general approach that can be applied to any ligand-regulated allosteric signaling system.
Introduction
Glucocorticoids (GCs) are among the most prescribed medicines due to their remarkable anti-inflammatory effects. Efforts to develop improved GCs have been hampered by poor understanding of the structural and molecular mechanisms through which glucocorticoid receptor (GR) ligands drive diverse phenotypic outcomes such as effects on inflammation, glucose disposal, or skeletomuscular atrophy1,2. Compounds that selectively target specific tissues are called selective modulators, and is a common feature of allosteric signaling of nuclear receptors3, G protein-coupled receptors, and other small molecule drug targets.
The adrenal gland secretes GCs in a circadian fashion and in an acute response to nutrient deprivation or other stressors. In skeletal muscle, GCs stimulate catabolic signaling that opposes the insulin/PI3K/AKT signaling pathway, inhibits glucose uptake, and releases amino acids by inhibiting protein synthesis and stimulating protein degradation2. Upon GC binding, GR dissociates from inhibitory heat shock protein complexes, translocates to the nucleus, oligomerizes upon binding to DNA, and recruits an ensemble of coregulators, including chromatin-remodeling and histone-modifying enzymes that regulate gene expression. Several GR coregulators and target genes have been identified in skeletal muscle4–7, but factors that drive selective modulation are unclear.
We developed a chemical systems biology approach called Ligand Class Analysis (LCA) to identify molecular mechanisms that drive selective modulation8–10. With LCA, we characterize a series of related compounds that display a full range of variance in phenotypes such as insulin-mediated glucose uptake or protein synthesis and use that variance to define molecular predictors of the phenotype. This enabled us to identify ligand-dependent gene expression and protein-interaction profiles that predict the molecular phenotypes of ligand classes that perturb receptor structure differently8–10. We then overexpressed or knocked down predictive genes or interacting proteins to identify factors required for ligand-dependent phenotypes.
In this work, we evaluated the physiological effects of GCs during overnight nutrient deprivation when they coordinate acute metabolic adaptations, including glucose disposal and protein balance. We tested a GC series with different modifications on the steroidal scaffold11, providing distinct strategies to perturb GR structure and dynamics. Using a machine learning approach, we found that GCs selectively modulate protein balance in skeletal muscle versus glucose disposal and the inflammatory response. This identified coregulators and target genes that drive these activities, and enabled discovery of orally available GCs with muscle-sparing activity profiles in vivo.
Results
Structure-based design of GCs
We designed a series of GCs to test three different structural effects of PF-0251802 (PF802) (1), a selective modulator, the pro-drug of which reached clinical trials (Extended Data Fig. 1a)12,13. PF802 (1) drives antagonism by disrupting the dexamethasone (Dex) (2)-bound GR conformation (Extended Data Fig. 1b), using its benzyl group which is similar to the carbon-11 (C11) substitution in the antagonist, RU-486 (3) (Extended Data Fig. 1c). To increase affinity, PF802 (1) has a trifluoromethyl group attached at the steroid C17-equivalent position, while RU-486 (3) and momethasone furoate (4) have other substitutions at this site (Extended Data Fig. 1c-d). PF802 (1) also contains a methylpyridinyl acetamide group attached at the steroid C3-equivalent position. This group presumably enters the solvent channel underneath the coregulator binding site, as first seen with deacylcortivazol (5) (Extended Data Fig. 1e), to alter the shape of the surface and the ensemble of interacting coregulators, thus driving selective modulation as we previously showed for estrogen receptor ligands9,14.
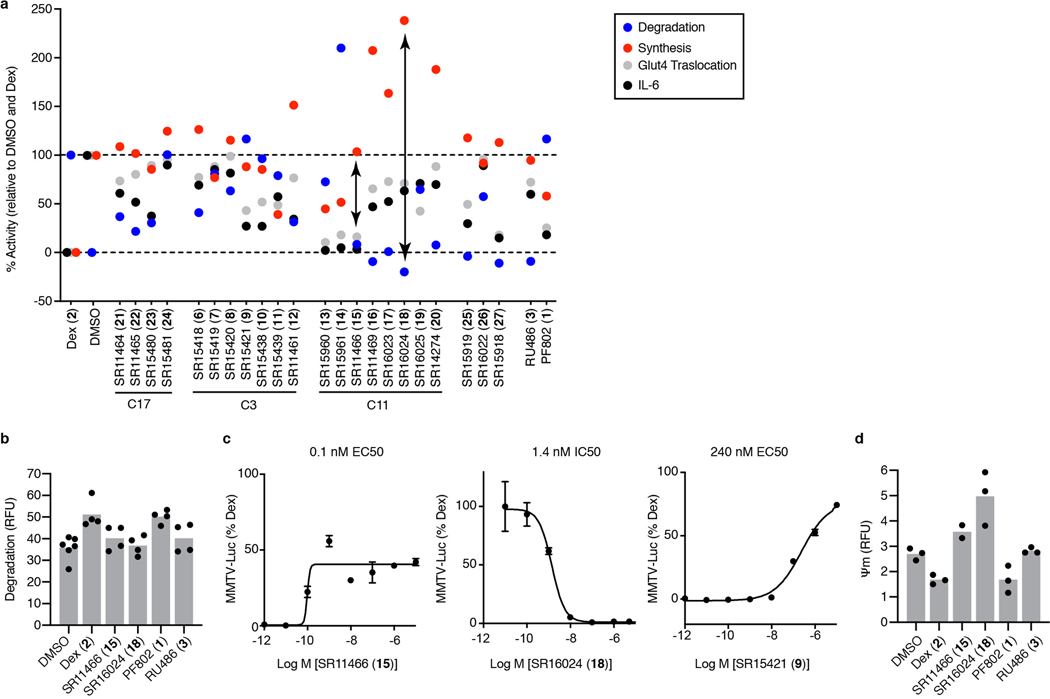
To understand how different substitutions control GR activity, we generated 22 compounds based on a steroidal core, which enabled us to direct substitutions within the ligand-binding pocket (Extended Data Fig. 1f). These included substitutions of the methylpyridinyl acetamide at C3 to perturb the AF2 surface (6–12)(Fig. 1a); substitutions at C11 to drive agonism/antagonism (13–20) (Fig. 1b); substitutions at C17 to optimize affinity (21–24) (Fig. 1c); and other modifications (25–27) (Fig. 1d). We published the synthesis and preliminary structure-activity relationships for these compounds11.
Figure 1. Structure-based design approach and glucocorticoid profiling platform.
a-d) Glucocorticoids used in this study.
e) Compound profiling and computational strategy. Effects on the independent variables were used in a machine learning approach, Random Forest, to identify predictors of skeletal muscle phenotypes, the dependent variables, described in Figure 2, Supplementary Table 2, and below.
f) Relationships among GR-mediated phenotypes. Lines indicate significant Pearson correlation between variables using Bonferroni pAdj < 0.0071.
Development of GC profiling platform
We developed a compound profiling platform to examine ligand regulation of: 1) GR nuclear localization; 2) structure; 3) target gene expression in skeletal muscle; 4) association with coregulators; 5) control glucose disposal; 6) protein balance; 7) anti-inflammatory effects such as the suppression of IL-6 secretion, and whether ligands can selectively modulate these activities (Fig. 1e). We assayed ligand-induced nuclear translocation of a GFP-GR fusion protein. To probe for ligand-selective changes in surface structure, we assayed the effects of the compounds on the interaction of full length GR with an array of 154 peptides derived from nuclear receptor coregulators using the MARCoNI FRET assay15. We completed nascent RNA expression profiling of C2C12 myotubes, one hour after vehicle or Dex (2) treatment and selected 28 differentially expressed genes as potential regulators of glucose disposal and protein balance (see Supplementary Information, Supplementary Table 1 for selection rationale). We compared ligand-dependent regulation of these genes in myotubes using the nanoString nCounter for direct multiplexed transcript counting without PCR amplification, enabling highly quantitative and reproducible assays16. These datasets comprise the independent variables used in a machine learning approach to identify ligand-selective regulators of glucose disposal and protein balance in skeletal muscle, the dependent variables.
To assay physiologically relevant effects of GCs on skeletal muscle, myotubes were treated in the context of nutrient deprivation and a brief insulin challenge. One of the barriers to understanding GC action in skeletal muscle is that effects on protein balance required 50–100 μM Dex (2) in vitro 17, despite a ~5 nM Kd for GR. Effects on protein balance and Glut4 translocation also vary in vivo, depending upon the stressor. For example, GCs induce skeletal muscle atrophy and insulin resistance during fasting, while exercise has opposite effects18,19. We found that overnight serum-deprivation enabled robust GC-induced signal to noise after a brief insulin challenge with maximal effects of Dex (2) in the 1–10 nM range (Methods, Extended Data Fig. 2a–g). This suggests that GR acts by inhibiting the insulin/PI3K/AKT signaling pathway20.
We developed an assay for effects of the compounds on phosphorylation of AKT at Thr308 (pAKT), due to its joint role in coordinating protein synthesis and glucose disposal (Extended Data Fig. 2a–b). To measure effects of the compounds on glucose disposal, we measured the rate limiting step of insulin-mediated translocation of the glucose transporter, Glut4, to the cell surface. (Extended Data Fig. 2c). For protein balance, we developed two assays: 1) Degradation: GC-induced protein degradation assayed by the release of tritiated phenylalanine (Extended Data Fig. 2d); and 2) Synthesis: effects of the ligands on insulin-stimulated protein synthesis assayed using the SUnSET assay, which measures the incorporation of puromycin into nascent polypeptide chains (Extended Data Fig. 2e). We assessed the relationship between protein balance and mitochondrial potential (Ψm) measured by high content imaging after staining with a MitoTracker dye, where accumulation of the dye is dependent on membrane potential and modulated by the GR ligands. (Extended Data Fig. 2f–h). We also compared ligand-dependent effects on IL-1β-induced secretion of IL-6 by A549 lung cells, a standard assay for the inflammatory response. Ligand activity in these assays were used as dependent variables to identify predictive coregulator interactions and target genes, and selective modulators.
Correlation analyses of GC effects
We used the Pearson correlation, r, to understand the relationships between effects of the ligands on the GC profiling assays (Fig. 1f). We identified two clusters of variables, connected by the effects of the ligands on pAKT. In the first cluster, effects of the ligands were significantly intercorrelated between effects on Synthesis, Degradation, pAKT, and ψm, suggesting transcriptional control via a common set of coregulators and target genes (Fig. 1f, Extended Data Fig. 2i, Supplementary_Data shows the Pearson correlation matrix and P values). In the second cluster, GC effects on IL-6 and Glut4 were correlated to pAKT and GR nuclear translocation (Fig. 1f). However, GC effects on pAKT and GR nuclear translocation were not correlated (Extended Data Fig. 2j), indicating that some ligand-dependent outcomes of GR signaling (e.g. Glut4, IL-6) involve a graded response to the amount of nuclear GR, while others require a threshold amount of nuclear GR. These results suggest that GCs modulate different transcriptional signaling pathways—coregulators and target genes—that coordinate some processes but allow others to be dissociated.
Machine learning defines top predictors of GC action
We performed predictive modeling of each GR-mediated phenotype (dependent variable) using Random Forest, a classification and regression tree algorithm21 (Fig. 1e). To identify a statistically significant set of gene expression and peptide interaction predictors for each phenotype, we used Boruta, a Random Forest-based wrapper algorithm22. We iteratively compared the importance of each feature (gene or peptide) in the dataset to a “shadow” dataset obtained by shuffling the dependent and independent variables. In each of 500 iterative decision rounds, a feature was selected if it was more significant than all the shadow features. We then identified a minimal set of predictors by applying a forward selection strategy. The set of significant features were tested one-by-one, starting with the most significant, for their ability to improve the model. Features that improved the model were retained, while those that worsened the model were removed. With this approach, we were able to predict between 47% (Degradation) and 84% (Glut4) of the variance in the dependent variables (Fig. 2a, Supplementary Table 2, Supplementary_Data).
Figure 2. Machine learning reveals top predictors of selective modulation and common signaling.
a) Composition of minimal predictive models defined by machine learning. The predictive capacity of the model (y-axis) for a GR-mediated phenotype i.e. dependent variable (x-axis), is also indicated. See Supplementary Table 2 for the full list of predictors.
b) Linear regression (scatter plots) demonstrates their predictive power (r2), and its associated p-value. Each point represents the effects of a distinct ligand.
c) Linear regression comparing the predictive power of the indicated peptide interactions for the indicated phenotypes and target genes. See Supplementary Table 3 for r2 values.
d) The Glut4-predictive power, r2, of target genes and peptide interactions observed with all compounds (1–3 and 6–27) were rank ordered, and then compared to the r2 observed within the C11-substituted (13–20) or C3-substituted (6–12) compound series.
e) Linear regression demonstrating the Glut4-predictive power of Bcl2l1 within the C11-substituted compounds series (13–20). Each datapoint represents the effects of a distinct C11-substituted compound on Glut4 translocation and Bcl2l1 expression.
See also Methods, Supplementary Dataset, and Extended Data Fig. 3.
This analysis revealed the degree to which target genes and interacting peptides functioned as selective predictors or coordinated effects of GCs across the different biological processes (Fig. 2a–b, Supplementary Table 2, Extended Data Fig. 3a–b). We used linear regression to define the statistical power of individual predictors, where the r2 statistic (mathematically equivalent to the square of the Pearson correlation) defines the percent of variance in the dependent variable that is predicted by the independent variable. Ligand interaction patterns with GR and NCOR1 or NCOR2 were selective for predicting Synthesis, while NCOA1 and NCOA2 interaction profiles and expression of the Bcl2l2 selectively predicted Glut4. In contrast, expression of Sgk1 and Socs2 were common regulators of both processes (Fig. 2b, Extended Data Fig. 3b). Bcl2l1 encodes Bcl-xL, a mitochondrial, anti-apoptotic BCL-2 family protein that inhibits glucose metabolism and mitochondrial-mediated secretion of insulin by pancreatic β-cells 23, suggesting that it also contributes to glucose disposal in skeletal muscle.
Ligand-mediated NCOR2 peptide interaction with GR significantly predicted Synthesis, but not Glut4 or the top Glut4-predictive target genes (Fig. 2c, Supplementary Table 3). There was a robust connection between the coregulator peptides that predicted Glut4, showing very low r2 with the protein balance assays, but very high predictive power for the specific genes that best predicted Glut4 activity (Fig. 2c), supporting a coordinating network of genes and coregulators. While the MARCoNI assay is best viewed as a probe surface structure, all of the peptides in Figure 2c represent bona fide nuclear receptor interaction motifs. The variance in ligand activity profiles across assays allowed LCA to identify some common and some selective transcriptional networks underlying GR-mediated phenotypes in skeletal muscle.
The variance in ligand activity profiles also allowed LCA to identify highly significant predictors from very small changes in gene expression, such as the Bcl2l1 and Socs2 genes where most of the compounds had very little activity individually, but collectively proved highly predictive (Fig. 2b, Extended Data Fig. 3ab). Much of the gene expression data displayed an inflection point (Extended Data Fig. 3c). When we removed the data below the inflection point, Fkbp5, Blc2l1, Tsc22d3 still significantly predicted Glut4 translocation (Extended Data Fig. 3d), suggesting that insulin-mediated glucose disposal can be fine-tuned by very small changes in target gene expression. This demonstrates that subtle changes in gene expression are sufficient to identify transcriptional networks underlying GR-mediated phenotypes using this approach.
We also found that substitutions on the steroidal scaffold that were designed to differentially perturb GR structure modulated distinct transcriptional networks. A comparison of ligand-dependent effects on Glut4 revealed a dramatic increase in predictive power by the C11-substituted compounds compared to the whole compound set, while the C3-substituted compounds exhibited a widespread reduction in r2 (Fig. 2d–e, Extended Data Fig. 3e, compare to Fig. 2b). This was evident despite their similar variances in the skeletal muscle profiling assays (Extended Data Fig. 3f), supporting the idea that C3- and C11-substituted compounds modulate GR-mediated phenotypes through different transcriptional networks and structural mechanisms, which we explore below with molecular dynamics simulations.
Validation of predictors with gene perturbation studies
FKPB5 is a good candidate to coordinate metabolic effects of GCs at an organismal level, as it facilitates pAKT dephosphorylation by the phosphatase, PHLPP124. In addition, Fkbp5-knockout mice show greater skeletal muscle insulin sensitivity and reduced adiposity on a high fat diet25. We electroporated GFP and Fkbp5 or an atrophy-inducing control (Foxo1) expression plasmid into contralateral tibialis anterior (TA) muscles. The Fkbp5- or Foxo1-transduced muscles were approximately 10% smaller than control (Fig. 3a). Electroporation with Foxo1 or Fkbp5 inhibited insulin-induced de novo protein synthesis and pAKT in vivo (Fig. 3b-c, Supplementary Fig. 1a), demonstrating that Fkbp5 regulates protein balance in skeletal muscle.
Figure 3. Functional validation of the predictive target gene, Fkbp5, and GR coregulators.
a) Weight of Tibias Anterior (TA) muscles transduced with GFP (control), Fkbp5, or Foxo1 genes in 2 independent experiments. Left panel, bars represent the mean, and n = 3 except for GFP where n = 2 TA muscles per condition. Right panel, each pair of datapoints represent TA muscles from the same mouse, n = 4 mice. Also see Methods.
b–c) Whole lysates of transduced TA muscles were analyzed by Western blot and subsequent quantitation. b) In vivo SUnSET assay for Synthesis. Bars represent the mean; n = 2, except for GFP where n = 4 biologically independent samples. c) Insulin-induced pAKT levels. Bars represent the mean; for GFP, n = 2 and for Fkbp5, n = 3 biologically independent samples. Also see Supplementary Fig. 1a.
d) SUnSET assay or e) in-cell Western for pAKT in C2C12 myotubes expressing the indicated shRNAs. Boxes represent the range; n = 6, except for Dex/shCtrl where n = 4 biologically independent samples. 1-way ANOVA, Sidak’s multiple comparisons test, adjusted p-values, *padj = 0.0235, **padj = 0.0018, ***padj = 0.0007, ****padj = 0.0004, ‡padj < 0.0001. e) Insulin-induced pAKT levels in C2C12 myotubes expressing the indicated shRNAs. Bars represent the range; n = 6, except for Dex/shCtrl where n = 4 biologically independent samples. 1-way ANOVA, Sidak’s multiple comparisons test, adjusted p-values, *padj = 0.0385, **padj = 0.0045, ***padj = 0.0001, ‡padj < 0.0001. Also see Supplementary Fig. 1b.
Knockdown of Ncor1 or Ncor2 enhanced Dex-dependent inhibition of Synthesis (Fig. 3d, Supplementary Fig. 1b), consistent with the peptide binding data showing that GR interaction with NCOR1 and NCOR2 peptides were stimulated by anabolic compounds (Fig. 2b). In contrast, knockdown of Ncoa6 and Pelp1 fully reversed the inhibitory effect of Dex (2), while knockdown of Ncoa22, but not Ncoa1, partially reversed this effect (Fig. 3d, Supplementary Fig. 1b-c). In contrast, pAKT was regulated by Ncoa1, as well as Pelp1 (Fig. 3e). These data support a model where GR recruits specific coregulators to distinct target gene sets that can have overlapping or different effects on skeletal muscle metabolism and protein balance.
Selective modulators with improved activity profiles
We identified two GCs that selectively modulate inflammation and protein balance (Fig. 4a–d). Compounds 13–15 in the C11 series inhibited IL-6 with efficacies comparable to Dex (2) (Extended Data Fig. 4a). SR11466 (15) is a partial GR agonist (EC50 = 0.1 nM) that showed no effects on protein balance, and a slight improvement (i.e. less inhibition) compared to Dex (2) in the Glut4 assay (Fig. 4a–c, Extended Data Fig. 4b-c). We also identified SR16024 (18) as a full GR antagonist (IC50 = 1.4 nM) that stimulated Synthesis but did not suppress Degradation or increase Glut4 translocation (Fig. 4b-c, Extended Data Fig. 4b-c). 15 and 18 increased ψm, with the latter doubling ψm (Fig. 4d, Extended Data Fig. 4d).
Figure 4. In vitro characterization of selective GR modulators with muscle-sparing activities.
a) IL-1β-induced secretion of IL-6 by A549 cells treated with the indicated compounds were compared by AlphaLISA. Left panel bars represent the mean; right panel datapoints represent mean ± SEM; n = 3, except for the vehicle (DMSO) where n = 6 biologically independent samples.
b) Effects of the indicated compounds on Synthesis in C2C12 myotubes were compared by SUnSET assay. Bars represent the mean; n = 3 biologically independent samples.
c) SR16024 (18) does not inhibit myotube surface expression of Glut4. Effects of the indicated compounds on Glut4 in L6 myotubes. Bars represent the mean; n = 3 biologically independent samples.
d) C2C12 myoblasts treated with the indicated compounds were stained with MitoTracker dye. Images are representative of 18 images per condition i.e. 2 independent experiments with similar results x 3 fields per well x 3 biologically independent wells per condition.
e) The effects of all tested compounds on IL-1β-induced secretion of IL-6 by A549 cells (y-axis) and primary human osteoblast mineralization (x-axis). Datapoints represent the mean effects of a distinct compound, For IL-6, n = 3 and for mineralization, n = 4 biologically independent samples.
f) Dose curves of indicated compounds in the mineralization assay. Data are mean ± SEM, n = 4 biologically independent samples. Also see Extended Data Fig. 2, Extended Data Fig. 4, and Methods.
As a further test for selective modulation, the compounds were profiled in primary human osteoblasts for effects on mineralization during 4 weeks of differentiation from mesenchymal stem cells. 15 showed a slight improvement in the inhibition of mineralization compared to Dex (2) and PF802 (1), while 18 showed a better profile (Fig. 4e). We also noticed SR15421 (9), a lower affinity compound with EC50 of 240 nM (Extended Data Fig. 4c), that was anti-inflammatory with less impact on mineralization (Fig. 4e, Supplementary Fig. 2). 9 is part of the C3-substituted series, where modifying the position of the methyl and the nitrogen on the methylpyridine had profound effects on inhibition of IL-6 secretion (Fig. 4e, Supplementary Fig. 2). Dose response curves reveal a moderately, but significantly improved effect of 15 on mineralization compared to Dex (2) (2-way ANOVA, drug p = 0.0018, drug x time p = 1.4 × 10−4), while 9 and 18 inhibited mineralization only at the 10 μM dose (Fig. 4f). The mineralization data showed very low correlations with the other assays, except for Synthesis (Pearson r = 0.54, p = 4 × 10−3), supporting the joint regulation of bone and muscle mass by GCs. These data demonstrate that it is possible to find GCs with improved bone- and muscle-sparing properties compared to Dex (2) using our in vitro profiling platform.
We opted to test the two high affinity ligands further in animal studies based on their favorable pharmacokinetics and on-target mechanism of action (Fig. 5a–b, Extended Data Fig. 5a–f, Extended Data Fig. 6a, Supplementary Fig. 3). In vivo, 15 strongly suppressed LPS-induced TNFα levels in the blood, while 18 was not inhibitory (Fig. 5c). We also assessed loss of lean mass following a larger dose of LPS, which was significantly worsened by Dex (2). Both 15 and 18 were significantly better than Dex (2), while 18 was protective against loss of lean mass compared to vehicle (Fig. 5d). The differential in vivo effects of 15 on inflammation and proteostasis demonstrate that it is a bona fide selective modulator, while 18 displayed stronger muscle sparing activities.
Figure 5. In vivo compound profiling and validation of on-target mechanism of action.
a) Effect of the indicated compounds on Synthesis were compared by SUnSET assay in C2C12 myotubes. Datapoints are mean ± SEM, n = 4 biologically independent samples.
b) IL-1β-induced IL-6 production by A549 cells treated with the indicated compounds was compared by AlphaLISA. Datapoints are mean ± SEM, n = 3 biologically independent samples.
c) SR11466 (15) blocks the LPS-induced inflammatory response in mice. Plasma TNFα levels of mice treated with the indicated GR ligands (10 mg/kg Dex or SR16024, or 50 mg/kg SR1166) overnight before a 1-hr LPS challenge of 1.5 mg per mouse. Bars represent the mean, n = 5 mice per group.
d) Changes in lean mass of mice treated as described in panel c were determined by whole-body NMR after an additional 18-hr LPS treatment of 30 mg per mouse. Bars represent the mean, n = 10 mice per group (5 × 2 experiments). 1-way ANOVA, Sidak’s multiple comparisons test, adjusted p-values, *padj = 4.5 ×10−5, **padj = 3.8 ×10−6, ***padj = 1.6 ×10-9.
e) Glucose production was compared by lactate-tolerance test (LTT) after overnight fast. Data are mean ± SEM, n = 5 mice per group. See also Extended Data Fig. 5 and Methods.
A lactate tolerance test was then administered to probe for differences in liver gluconeogenesis following an overnight fast. Here again, Dex (2) caused significantly greater loss of lean mass after the fast; both 15 and 18 were significantly better than Dex (2) (Extended Data Fig. 6b); and 18 attenuated the loss of overall body weight from the fast (Extended Data Fig. 6c). The lactate tolerance test showed that Dex (2) and 15 increased the glucose production rate, while 18 did not (Fig. 5e), suggesting that selective GC effects on glucose uptake in skeletal muscle and gluconeogenesis in the liver may utilize common signaling mechanisms, such as ligand-selective coregulator recruitment, to integrate organismal stress responses.
Correlated changes in GR structure drive suppression of IL-6
C3 isomers with differences in the location of the methyl and nitrogen in the pyridine ring showed large differences in anti-inflammatory effects, demonstrating that the methylpyridinyl acetate binding under the AF-2 surface is an important allosteric regulator of GR. To understand the mechanism, we performed all atom molecular dynamics (MD) simulations comparing the isomers 7 and 9, which differ only in the placement of the methyl on the pyridine (Fig. 1a) but showed very different effects on suppression of IL-6 (Supplementary Fig. 2), where 9 suppresses inflammation but 7 does not. Molecular docking demonstrated that the compounds bound similarly to Dex (2), with the pyridine extending off the A-ring into a solvent channel, in between helix 3 (h3) and h5 (Methods, Extended Data Fig. 7a). In three independent 1 μs simulations of ligand-bound and ligand-free (apo) GR LBD, we observed no significant structural changes in the conformation of helix 12 (h12). However, ligand anti-inflammatory activity (Dex (2) > 9 >7 > apo) was associated with a multi-Å decrease in the distance between the C-terminus of h11 and the N-terminus of h3, and a decrease in the heterogeneity of this h3/h11 interface (Fig. 6a–c). We also assessed patterns of correlated motion and found that the 7 showed less correlation between residues h3 and h11/h12 residues compared to Dex (2), with 9 again showing intermediate effects (Extended Data Fig. 7b).
Figure 6. C3 substitutions in the steroid scaffold alter allosteric communication between ligand and activity.
a) Ribbon diagram of the GR-LBD illustrates the C-terminus of helix 11 (h11) and N-terminus of h3 where we observed ligand-induced conformational effects in three 1 μs molecular dynamics simulations.
b) Distance distribution plots of the Cα distance between Met560 (h3) and Thr739 (h11).
c) Backbone Cα, C’, N, and O RMSD distribution plots of the h3 and h11 regions colored magenta in (a).
d) Dynamical network analysis of suboptimal pathways for correlated motion between E755 and R614. Red arrows indicate pathways found with Dex-bound GR.
e) Histogram showing the suboptimal pathlengths with the indicated ligands.
f) Distance distribution plots of Q570 (Cδ) and R611 (Cζ) side chain atom distances as a proxy to determine the relative populations inward R611 conformations that can interact with ligand. Inserts show R611 inward (left) and outward (right) side chain conformations extracted from the Dex-bound simulations.
We used dynamical network analysis26 to further probe for effects of the C3 substitutions on correlated motion between h12 and the solvent channel, by connecting pairs of nodes with their edges if they have satisfied a distance requirement (<4.5 Å) for ≥75% of the simulation time. Edge distance is inversely proportional to the pairwise correlations between two nodes; thus, a short path length indicates a strong correlation/communication. A pair of distal nodes are connected by the optimal (shortest) path and the suboptimal (longer) paths with the length defined as the sum of edge distances along the path. We analyzed the top 1,000 suboptimal paths between residue Glu755 and Arg614 as two nodes to study the allosteric communication between h12 and the C-terminus of h5, where the modifications on the steroidal A ring extend to the solvent channel. With apo GR, this communication was through h11 and along h5, while with Dex (2) it wrapped around the ligand-binding pocket from h12 to h3 and then through the β-sheet (Fig. 6d), enabled by the Dex-induced closing of the distance between h3 and h11 (Fig. 6b). With Dex (2), this was associated with much longer path lengths (Fig. 6e), representing overall weaker allosteric communication. With 9, there were less of these extended path lengths, while 7 more closely resembled the apo GR (Fig. 6d-e). An analysis of residues contacting the C3-substituted pyridine showed that differential positioning of the Arg611 side chain on h5, which can form H-bonds with the pyridine or A-ring ketone of Dex (2), can drive the orientation of the steroid core to control the dynamics and surface structure and associated receptor activity (Fig. 6f, Extended Data Fig. 7c–e).
Discussion
We used structure-based design and LCA to reveal how GCs modulate different transcriptional networks (coregulators and target genes) to physiologically control glucose disposal and protein balance during acute nutrient deprivation, and how different classes of these ligands with substitutions at C3 or C11 utilize these signaling pathways. By examining closely related compounds, we determined that protein balance and Ψm were intercorrelated but not correlated with effects on Glut4 translocation and suppression of IL-6. Using a physiologically relevant skeletal muscle profiling platform optimized to study insulin signaling during nutrient deprivation, we identified SR11466 (15) as a partial agonist that was not catabolic, demonstrating in vivo selective modulation of anti-inflammatory activity. 15 was slightly anabolic for protein balance, while maintaining strong anti-inflammatory activity in vivo. Such compounds may be useful treatments for cachexia, muscular dystrophies, back pain or osteoarthritis. We also identified SR16024 (18) as a highly anabolic GC that inhibits fasting- and LPS-induced weight loss, while stimulating Ψm and protein synthesis in response to insulin. Further studies are needed to evaluate the long-term effects of 18, but GR antagonists have been tested clinically for depression27, and may have efficacy in muscular dystrophies and enzalutamide-resistant prostate cancers that have switched from androgen to glucocorticoid dependency28. While preliminary, the lack of SR15421 (9) effects on mineralization suggests that it may also be possible reduce the osteoporotic effects of agonist GCs using physiologically relevant profiling assays.
The unbiased nature of LCA enabled the identification of a number of unexpected signaling and biophysical properties of GR. Insulin receptor signaling pathways control anabolic effects and glucose disposal, which can be disconnected by transcriptional control of pathway-selective regulators, often with subtle changes in gene expression. Covariance among assays reflects common underlying signaling mechanisms driven by common receptor conformations, coregulators, and target genes. The correlation between effects on bone and skeletal muscle was not surprising, but the connection between anti-inflammatory and metabolic effects of GCs in different cell types was unexpected and may point to an organismal level of evolutionary connection between the underlying signaling pathways. GC coordination of the inflammatory response often coincides with immune differentiation29,30, which requires metabolic adaptations 31,32. This suggests that GCs may use a common set of coregulators to coordinate anti-inflammatory effects with modulation of metabolism. LCA provides a mechanism to identify common signaling mechanisms among different cell types and processes and how they are utilized by structurally distinct classes of ligands.
GC-regulated transcription is controlled via underlying biophysical properties of GR, such as oligomerization, nuclear translocation, and on/off rates of interactions with an ensemble of response elements, coregulators, and collaborating transcription factors. We have shown here that LCA predicts that ligand-selective surface conformers—identified from a structural peptide interaction assay—can predict differential effects of the ligands, which we validated with knockdown of selected coregulators. We and others have observed a similar dichotomy where NCOA2 is required for NF-κB-induced cytokine production, but then switches to a repressor upon recruitment of GR to the promoter33,34. This is representative of a more general phenomena of receptors using many coregulators to regulate a single gene, but also some shared and some differential coregulator usage across genes14,35,36. The selective association of GR nuclear translocation with glucose disposal and not protein balance was also surprising. This suggests that GCs modulate insulin dependent Glut4 translocation via a graded transcriptional program that is highly sensitive to the amount of nuclear GR, and control protein balance through transcriptional responses that require a threshold amount of nuclear GR. It is remarkable that changing the position of a single methyl in the solvent channel drove such divergent effects of the C3 compounds on inflammation, while the molecular dynamics simulation revealed this region to be an important allosteric regulator of GR structure and function. Collectively our finding show that complex biology can be simulated by a set of simple biophysical models9,37 and their importance identified with LCA.
Online Methods
Quantification and statistical analyses
All statistics were done using GraphPad Prism unless otherwise stated. Random Forest/Boruta were done with R. For Pearson correlation, the p-value (two-tail) tested the null hypothesis that the true population correlation coefficient for that pair of variables is zero. Two-tailed unpaired Student’s t-test was done to compare only two groups. One-way or two-way ANOVA was used for comparisons of more than two groups with correction for multiple hypothesis testing. All values were expressed as the mean ± S.E.M. The compound injections were done by one person and the determination of weight/body composition done by another person blinded to the treatment groups. For the high content imaging assay of myotube diameter and the in cell western blots, images were visually inspected in a blinded fashion for the presence of intense foci or speckles and those images were not included in the analyses. For the IL-6 dose curves, data were identified through the linear regression function in GraphPad Prism.
Machine learning:
Each Boruta (version 6.0)22 and randomForest package (version 4.6)21 prediction was performed with three different random seeds on Z score transformed data. Variables that were confirmed in 2 or more runs were selected as predictors. Sample R scripts are available at: https://github.com/jnwachuk/ML_in_GR_signaling_networks.
Nascent RNA-seq
2×106 C2C12 myoblasts were grown in 15 cm dishes to 90% confluency and differentiated in media with 2% steroid-free FBS. After 5 days, myotubes are treated with 1 uM Dex or vehicle, in duplicate plates, for 2 hr. Nuclei were isolated, washed, and lysed with a 10% NP40 buffer and centrifuged. The pellet was washed 2x with TRIzol™ reagent (Invitrogen) to remove unbound RNA. The chromatin pellet was then dissolved in Trizol at 65°C and RNA was isolated using standard protocols (e.g. Qiashredder and RNAEasy column purification). Library preparation was performed using TruSeq stranded total RNA kit (Illumina) and sequenced on NextSeq500 (Illumina). A total of 30–40 million 2×75bp paired-end mapped reads were obtained per sample. The sequencing reads (fastq files) were mapped to the mm9 genome using Tophat 38. The number of reads falling into each gene defined in the RefSeq gene annotations was quantified using HTSeq-count 39. The DESeq software 39 was used to detect DEGs.
Nanostring nCounter gene expression analyses
Myoblasts were differentiated for 5 days and then switched to charcoal stripped serum for 24 hr, and then treated with 1 μM GCs for 6 hrs. RNA was isolated with RNeasy (Qiagen). Gene expression analyses were performed using the manufacturer’s instructions.
Animal studies
All animal procedures were approved by the Scripps Research Institute IACUC. Male C57BL/6J mice, 8–10 wk old, were acquired from the Jackson Laboratories (Bar Harbor, ME) and used for all conditions. Mice were housed under a 12-h light/dark cycle with ad libitum access to food and water unless otherwise stated. Before all surgical procedures, mice were anesthetized with gaseous isoflurane (Baxter, USP, USA). Before tissue extraction, the mice were euthanized with CO2 and cervical dislocation.
LPS-induced muscle atrophy:
Mice were treated with 1.5 μg LPS (Sigma L2880) for 1 hr and then blood drawn for determination of TNFα (ELISA, R&D Systems, MTA00B). Mice were injected with 30 μg LPS. Body composition was determined after 23 hrs using time domain nuclear magnetic resonance (TD-NMR) in a Bruker minispec.
Lactate tolerance test:
Mice were administered GCs at 10 mg/kg IP and fasted overnight. The next morning, lactate (1.5 g/kg) was injected intraperitoneally and blood glucose assayed with Accu-check glucose monitor.
Electric-pulse-mediated gene transfer and in vivo SUnSET assay:
Plasmid DNA (50 μg) was transfected into mouse tibialis anterior (TA) muscles by electroporation as previously described42. 1 hr before electroporation mice were anesthetized and a small incision was made through the skin and then injected with 30 ul of 0.5 U/Al hyaluronidase and plasmid DNA. Plasmids (Expression Ready MGC cDNA Library) were purified with an EndoFree plasmid kit (Qiagen, Valencia, CA, USA), and resuspended in 71 mM sterile PBS. 7 days later, mice were anesthetized and injected i.p. with 0.040 mol/g puromycin dissolved in 100 μl of PBS. 30 min after injection, muscles were extracted and frozen in liquid N2.
Western blot analysis
Whole cell extracts were prepared in 20 mM HEPES, pH 7.4, 1% TX-100,1x phosphoSTOP complete inhibition cocktail tablets [Roche], and 1X Complete EDTA-Free protease inhibitor cocktail [Roche]). Frozen muscle tissue was homogenized in lysis buffer T-PER® Tissue Protein Extraction reagent (Thermo Scientific) by a polytron homogenizer and sonication. Protein concentration was determined using the protein assay reagent (Bio-Rad). Sixty to eighty μg of protein was separated by SDS-PAGE and transferred to nitrocellulose membranes for immunoblotting.
Cell-based assays
C2C12 myoblasts, L6 myoblasts, and A549 cells (ATCC) were cultured in high glucose DMEM supplemented with glutamine and 10% FBS. For steroid-free culture conditions, cells were grown in charcoal:dextran-stripped FBS (Gemini Bio-products, cat no. 100–119). All assays were performed in C2C12 cells (ATCC) except for the Glut4 translocation assay, which was performed in rat L6 myocytes.
pAKT:
C2C12 myoblasts were seeded at 70% confluency and 48 hrs latter washed with PBS and switch to differentiation media consisting of phenol-free DMEM supplemented with glutamine and charcoal stripped serum (Sigma 18l289). Partial media changes were performed every 48 hrs to prevent lifting of the tubes from the wells. After six days in differentiation media, the cells were serum starved, treated with ligands. The next day, cells were stimulated with 100 nM insulin for 30 min., fixed in 3.7% formaldehyde for 20 min, permeabilized with TBS + 0.1% Triton X-100, washed in TBS and incubated for 2 h in blocking solution (Rockland MB-070), then overnight in primary antibodies at 4°C. Then the plates were washed 5 times for 5 minutes with TBS-T and then incubated with secondary antibodies for 1 hr wash and analyzed using a LI-COR Odyssey imaging system.
Glut4 translocation:
Low passage, L6 rat myoblast (ATCC), were cultured in high glucose DMEM supplemented with glutamine and 10% serum (proliferation media). On day 1 of differentiation, 2,500 cells were seeded on 384-well plates in 50 μl of proliferation media that was replaced every other day. On day 5 cells were washed and replaced with differentiation media, which was then replaced daily. After 3 days of differentiation the cells were ligand treated and serum starved overnight. Next day we replace the medium with 100 μl DMEM ± 1 μM insulin (Sigma I0516) without serum or glucose and incubate for 1 hour and then fixed with 3.7% formaldehyde for 20 minutes. Non-permeabilized cell were then washed in TBS and blocked for 2 hours (Rockland MB-070) then incubated in primary anti-Glut-4 antibody in blocking buffer for 48 hours at 4 °C. Then the plates were washed 5 times for 5 minutes with TBS-T and then incubated with secondary antibody for 1 hr wash and analyzed using a LI-COR Odyssey imaging system.
Protein synthesis (in vitro SUnSET assay):
C2C12 myoblasts were cultured and differentiated in 384-well plates as described above. After differentiation, the different ligands were added with a Biomek pin tool dispenser in serum-free DMEM overnight, next treated with 100 nM insulin. After 60 min, 1 μM puromycin was added to all wells, and the cells were incubated for an additional 30 min. The in cell western (LI-COR) was used to quantitate uptake of puromycin. Cells were fixed with 3.7% formaldehyde for 20 minutes. Non-permeabilized cell were then washed in TBS and blocked for 2 hours (Rockland MB-070) then incubated in primary anti-puromycin antibody in blocking buffer for 48 hours at 4 °C. Then the plates were washed 5 times for 5 minutes with TBS-T and then incubated with secondary antibody for 1 hr wash and analyzed using a LI-COR Odyssey imaging system.
Protein degradation:
Rates of protein degradation were determined by measuring the release of TCA‐soluble radioactivity from proteins prelabeled with [3H]‐phenylalanine. In brief C2C12 cells were seed in 24 well plates and after completing differentiation, myotubes were labeled with 1.0 μCi/ml of L‐[3,5‐3H]‐phenylalanine for 48 h dedifferentiation media. Cells were then treated for 24 h with ligands in DMEM containing 2 mM unlabeled phenylalanine. After treatment, the culture medium was transferred into a microcentrifuge tube containing 100 μl of bovine serum albumin (10 mg/ml) and TCA was added to a final concentration of 10% (w/v). Samples were incubated at 4° C for 1 hr followed by centrifugation for 5 min. The supernatant was used for determination of TCA‐soluble radioactivity. The protein precipitates were dissolved with a tissue solubilizer (Solvable™). Cell monolayers were washed with ice‐cold PBS and solubilized with 0.5 M NaOH containing 0.1% Triton X‐100. Radioactivity in the cell monolayer and TCA soluble and insoluble fractions were measured using a Packard TRI‐CARB 1600 TR liquid scintillation analyzer. Protein degradation was expressed as the percentage protein degraded over the 24 hr period and was calculated as 100 times the TCA‐soluble radioactivity in the medium divided by the TCA‐soluble plus the TCA‐insoluble radioactivity in the medium plus the cell layer (i.e., myotube) radioactivity43.
Mitochondrial potential (Ψm):
Primary or C2C12 myotubes or mycocytes in black 96- or 384-well tissue culture plates with clear bases (Greiner Bio-One, North America, Inc.) were stained for 15 min with 200 nM MitoTracker® Orange CM-H2TMRos dye (Invitrogen™ by ThermoFisher Scientific) for measuring Ψm. The cells were rinsed with steroid-free DMEM to remove un-incorporated dye, and then incubated in the differentiation media for 45 min to reach peak fluorescence. Cells were fixed in 4% formaldehyde for 20 min, stained with 300 nM DAPI for 5 min, permeabilized in PBS containing 0.1% Triton X-100 for 20 min, blocked for 1 h with 1x TBS containing 0.1% Tween-20 (TBS-T) and 2.5% normal goat serum, and incubated at 4 °C overnight with an AlexaFluor® 488-conjugated anti-skeletal muscle myosin (F59) antibody (Santa Cruz Biotechnology, Inc. cat no. sc-32732 AF488). The next day, the myotubes were washed 4 times with TBS-T to remove unbound antibodies and rinsed twice with PBS. The stained myotubes were imaged at 10–20X magnification on the IN Cell Analyzer 6000 platform (GE Healthcare). For each treatment condition, a stack of 24–27 images containing an average of 50–100 myotubes per image were analyzed using the IN Cell Developer Toolbox image analysis software, with a customized segmentation protocol for myotubes. The average (mean) diameter and mitochondrial potential (i.e. MitoTracker staining density x area) of myotubes in these images were then calculated.
GR nuclear translocation:
The cell line used for these experiments (3617) expresses green fluorescent protein (GFP)-tagged GR (GFP-GR) from a chromosomal locus under control of the tetracycline-repressible promoter 44. Cytoplasm-to-nuclear translocation of the GFP-GR in response to different compounds was determined as previously described 45. Cells were plated on a 384-well plate in DMEM medium containing 10% charcoal stripped serum (Hyclone, Logan, UT) without tetracycline (to allow the expression of the GFP-GR) at density of 2,500 cells per well. Cells were treated with compounds or a diluent as control for 30 min, fixed with paraformaldehyde, the nuclei stained with 4ʹ,6-diamindino-2-phenylindole (DAPI) and Perkin Elmer Opera Image Screening System was used for fully automated collection of images. An image analysis pipeline was customized using the Columbus software (PerkinElmer) to automatically segment the nucleus using the DAPI channel and then construct a ring region (cytoplasm) around the nucleus mask for each cell in the digital micrographs. Translocation was calculated as a ratio of the mean GFP-GR intensity in nucleus and cytoplasm, and each value was further normalized to the value for the control (i.e., DMSO) sample on the same plate.
Osteoblast mineralization:
Human mesenchymal stem cells (MSCs) at passage 2 were maintained in growth media consisted of alpha-MEM (Life Technologies, 32561−−037) supplemented with 17% FBS (Sigma-Aldrich, 12303C), 2mM L-glutamine (Life Technologies, 25030–081), and 100 units/mL penicillin/streptomycin (Life Technologies, 15140–122). Effect of glucocorticoid receptor modulators on osteogenic differentiation of MSCs was quantified using Alizarin Red S (Sigma-Aldrich, A5533) staining. Briefly, human MSCs were seeded in the middle 8 wells of 48-well plates at plating density of 10,000 cells per well. PBS (Life Technologies, 10010–031) was added to surrounding wells to reduce evaporation of media in the middle wells during prolonged incubation. 24 h later, growth media was replaced by osteogenic induction media (OIM) consisted of low-glucose DMEM (Life Technologies, 10567–014) supplemented with 10% FBS (Sigma-Aldrich, 12303C), 50 μg/ml L-ascorbic-2-phosphate (Sigma-Aldrich, A8960), 10mM β-glycerolphosphate (Sigma-Aldrich, G9891), and glucocorticoid modulators. OIM containing test compounds was replaced every week. At the end of 2 weeks, monolayers were washed with PBS (Life Technologies, 10010–031), incubated in Richard-Allan Scientific™ Neutral Buffered Formalin (10%) (Thermo Scientific, 5725) for 2 h at room temperature, washed with deionized water and stained with Alizarin Red S for 30 min at room temperature. Monolayers were then rinsed 3 × with deionized water until clear, stain was then extracted with 10% (w/v) cetylpyridinium chloride (Sigma-Aldrich, C0732) in 10 mM sodium phosphate (Sigma, S5011 and S5136), pH 7.0 for 15 min at room temperature and the amount of extracted dye quantified spectroscopically at 562 nm. Spectroscopic analysis performed using a synergy™ NEO HTS Multi-Mode Microplate Reader (BioTek Instruments, Inc.).
IL-6 secretion:
IL-1β (R&D systems) was dissolved in phosphate-buffered saline plus 0.1% bovine serum albumin (Sigma). A549 cells were plated in DMEM containing charcoal stripped serum in 384 well plates. The next day, cells were treated for 24 with GCs at 1 μM and then treated with 1 ng/ml IL-1β for 24 hrs. supernatants were collected and IL-6 assayed with AlphaLISA (PerkinElmer) using the manufacturer’s instructions.
Micro array Assay for Real-time Coregulator-Nuclear receptor Interaction (MARCoNI)
This method has been previously described 15. Assay mixes with 1 nM GST-tagged GR (aa 521–777, Invitrogen #PV4689), 25 nM Alexa-488 conjugated GST antibody (Fisher Scientific, #10368552), 5 mM DTT, in Coregulator buffer F (Invitrogen, #PV4547) in the presence of solvent only (2% DMSO) or 1 μM compound as indicated, were prepared on ice. Experiments were conducted on a PamStation96 (PamGene) using 2 cycles per minute at room temperature. In short, PamChip arrays (PamGene #88101) were blocked for 20 cycles with 1% Bovine serum albumin (Calbiochem #126609) in Tris-buffered saline (TBS), incubated for 40 minutes with 25 μl assay mix using 3 arrays per mix and finally rinsed twice with TBS. Binding on array (tif) images were quantified with BioNavigator software (PamGene). A modulation index, i.e. the log-transformed ratio of binding in presence over absence (solvent) of compound, was calculated for each interaction. Significance of the modulation was assessed using Student’s t-Test and FDR post hoc correction.
Antibodies
Anti-puromycin antibody: 3RH11 (Kerafast, Inc. cat no. EQ0001), or clone 4G11 (MilliporeSigma, cat no. MABE342). Anti-AMPKα Antibody (Cell Signaling Technology, Inc. #2532). Phospho-AMPKα (Thr172) (40H9) (Cell Signaling Technology, Inc. #2535), Anti-AKT1 (2H10) antibody (Cell Signaling Technology, Inc. #2967), Anti-pAKT-T308 (C31E5E) antibody (Cell Signaling Technology, Inc. #2965). Glut4 (3G10A3) antibody (Thermo Fisher cat no. MA5–17176). Secondary antibodies. Anti-mouse IgG goat Antibody, DyLight™ 800 (Cell Signaling Technology, cat. no. 5257). anti-Rabbit IgG (H+L) goat Antibody, DyLight 800 (Thermo Fisher Scientific cat no. SA5–10036)
Lentiviral vectors and transduction
Lentiviruses were produced from sets of four mouse piLenti-siRNA-GFP lentiviral vectors (Applied Biological Materials, Inc. Richmond, BC, Canada) targeting Pelp1 (cat no. i029598), Ncoa2 (cat no. i033105), Ncoa1 (cat no. i038883), Ncoa6 (cat no. i042804), Ncor1 (cat no. i044954), or Ncor2 (cat no. i042611), or the Control piLenti-siRNA-GFP lentiviral vector (cat no. LV015-G). HEK-293T cells in 3× 10 cm dishes per construct, were co-transfected with 10 μg lentiviral vector, 7.5 μg psPAX2, and 2.5 μg pVSV-G per dish using ProFection® kit (Promega) according to manufacturer’s protocol. At ~15 h post-transfection, the media was replaced with 2.5 ml fresh media. Conditioned media containing lentiviral particles (LVM) were collected every 12 h for another 48 h, pooled, and stored at 4 °C. LVM were passed through a 0.45-micron filter to remove cell debris, and then centrifuged at 4,000 RPM overnight to pellet the viruses. The pellets were resuspended in 50 μl sterile PBS and added with 8 μg/ml polybrene to day-1 C2C12 myoblasts.
Luciferase co-transfection reporter assays
HEK-293T cells were seeded in a 10 cm dish containing 10 ml phenol red-free DMEM (Thermo Fisher Scientific; cat no. 31053028) containing 10% charcoal-stripped FBS (Thermo Fisher Scientific, cat. no. A3382101). The next day, the cells were co-transfected with 10 μg of ARR3-tk-Luciferase or MMTV-Luciferase reporter plasmid and 5 μg of AR or GR expression plasmid, respectively, using 45 μl of TransIT®-LT1 reagent (Mirus Bio LLC., cat. no. MIR 2300). After 24 h, the cells were transferred to a 384-well plate (Greiner Bio-One, cat. no. 781080). The next day, the test compounds were added using a Biomek NXP 100-nl pintool (Beckman Coulter, Inc.). Luciferase activity was measured 24 h later, using the britelite plus reporter gene assay system (PerkinElmer, cat. no. 6066761).
Molecular dynamics simulations
Initial structures used in the simulations were built from two GR LBD crystal structures, PDB 4DUC.pdb (dexamethasone-bound) and 3K23.pdb (all other states). The Modeller46 extension within UCSF Chimera47 was used to fill in missing parts of GR LBD in the PDB files. For apo-GR LBD, the ligand in 3K23.pdb was stripped, which was then used to dock 7 and 9 using AutoDock Vina48. The protonation states of residues at pH 7.4 were determined using the H++ server49. Ligands were parameterized using ANTECHAMBER to generate force modification files using PARMCHK2 for use in TLEAP to generate topology and coordinate files using the AmberTools18 package (http://ambermd.org/tutorials/basic/tutorial4b). The ff14SB force field was used to describe the GR LBD. The structures were solvated in a truncated octahedral box of TIP3P water molecules with the 10 Å spacing between the protein, and neutralized with K+ and Cl− ions added to 50 mM. Minimization, equilibration, and execution of the production simulations were performed as previously described (PMID 30409975) in triplicate for 1 μs each. Analysis of trajectories was performed on the last 0.75 μs of the trajectories using CPPTRAJ and PYTRAJ51 including the h3-h11 distance distribution (PYTRAJ dist command of M560 and T739 Cα atoms); h3 + h11 heterogeneity (PYTRAJ rmsd command of backbone Cα, C’, N, and O atoms) of N-terminus of helix 3 (residues 556–565) and N-terminus of helix 11 (residues 736–743); and differential correlation analysis (PYTRAJ atomiccorr command) to generate correlation plots from the simulations of dexamethasone, 7, and 9, then the correlation matrices from the latter two were subtracted from the dexamethasone correlation matrix.
Dynamic network analyses were carried out by selecting Cα and C2 atoms in the protein and ligand, respectively, as nodes for networks construction. Node pairs are connected by edges if they satisfied a distance requirement (<4.5 Å) for at least 75% of the simulation time. Carma and NetworkView plugin in VMD produced the dynamic network by calculating the cartesian covariance and correlation between two nodes52,53. The edge distances were derived from pairwise correlations as a measure of communication within the network. Suboptimal paths between the C-terminus of h5 and h12 were identified using the Floyd-Warshall algorithm and analyzed by subopt program in the VMD NetworkView plugin.
Data Availability Statement
The raw and processed nascent RNA-seq dataset generated in this study has been deposited in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO), with the accession code GSE149453. Other mRNAseq data cited in this work was downloaded from the GEO with accession code GSE12463654. Sample R scripts used are available at https://github.com/jnwachuk/ML_in_GR_signaling_networks. Compound profiling assay data, results of correlation and machine learning analyses are in Supplemental_Data1.xls
Extended Data
Extended Data Fig. 1. Structure-based design of GR ligands.
a) Chemical structure of the selective GR modulator, PF802.
b) Crystal structure of the dexamethasone (Dex) bound GR ligand-binding domain (LBD). Helix 12 is colored red and the NCOA2 coregulator peptide binding in the AF2 binding surface is colored coral. Carbons-3, −11, and −17 are indicated in the chemical structure (pdb entry 1M2Z).
c) The bulky dimethylanaline group attached at C11 in RU-486 displaces h12 from the agonist position to disrupt the AF2 surface and generate antagonism (pdb entry 1NHZ).
d) Substitutions at C3, as seen with the furoate group in momethasone furoate or the propyne in RU-486 target a small internal pocket to increase affinity (pdb entry 4P6W).
e) Substitutions at C3 of the steroidal A-ring enter the solvent channel underneath the AF2 surface, potentially changing the shape of the surface and the ensemble of interacting coregulators (pdb entry 3BQD).
f) Model of SR11466 (15) bound to the GR LBD
Extended Data Fig. 2. Quantitative phenotyping assays for GC action in skeletal muscle.
a-e) Myotubes were nutrient-deprived, pre-treated with DMSO, RU-486, or Dex, and treated with insulin as outlined in Methods.
a) Effect of insulin on pAKT levels in C2C12 myotubes were compared by In-Cell Western assay (ICW) 48 h after treatment with RU-486 or Dex.
b) Quantitation of pAKT in C2C12 myotubes compared by ICW. Bars represent the mean; n = 3, except for DMSO where n = 36 biologically independent samples.
c) ICW for surface expression of Glut4 on L6 myotubes. Bars represent the mean; n = 3 biologically independent samples.
d) C2C12 myotubes were assayed for protein degradation by release of tritiated phenylalanine. Bars represent the mean; for DMSO, n = 6, and for Dex, n = 4 biologically independent samples.
e) ICW for protein synthesis by insulin-induced incorporation of puromycin into C2C12 myotube surface proteins. Bars represent the mean; n = 3 biologically independent samples.
f-g) High-content imaging and analysis of C2C12 myoblasts stained with MitoTracker™ dye. Images are representative of 18 images per condition; i.e. 3 fields x 3 biologically independent samples per condition in each of 2 independent experiments.
h) Assay reproducibility from screening 22 compounds on two separate occasions. The Pearson correlation coefficient, r, and its associated p-value is indicated. Each datapoint represents the mean effect of a distinct compound. Also see Methods.
i–j) Linear regression demonstrates the predictive power (r2), and associated p-value for the indicated variables. i) Ψm and pAKT predict Synthesis (p = 6 ×10−5 and 8 ×10−5, respectively).
j) GR nuclear translocation selectively predicts Glut4 (p = 2 ×10−5) but not pAKT. Each datapoint represents the effects of a distinct ligand.
Extended Data Fig. 3. Relationships among specific genes, peptide interaction assays, and GR-mediated phenotypes.
a-e) Linear regression was performed for the indicated assay pairs, where each point represents a different compound.
a) Effects of the ligands on Socs2 expression predicts Glut4 translocation, Ψm, and insulin-stimulated protein synthesis.
b) Ligand-dependent expression of Bcl2l1, which encodes the mitochondrial anti-apoptotic protein, Bcl-xL does not predict effects of on Ψm.
c) Fkbp5 expression as a predictor of Glut4 translocation shows an inflection point (arrow).
d) The Glut4 data was truncated below the inflection point shown in c).
e) GR interaction with an NCOR2 peptide predicts protein synthesis in the C11 subset of ligands.
f) The C11- and C3-substituted compounds showed similar variance in the skeletal muscle profiling assays. Blue dashed line, vehicle; red dashed line, Dex. Each datapoint represents the mean effect of a distinct compound, n=3 biologically independent samples. The error bars represent the mean ± SD of each compound series. For C11, n=8 distinct compounds; for C3, n=7 distinct compounds. See also Fig. 2a-e
Extended Data Fig. 4. Compound structure- activity relationships.
a) Individual compound data for protein degradation, insulin- stimulated protein synthesis, and Glut4 translocation in myotubes, as well as effects on IL-1β-stimulated secretion of IL-6 by A549 cells. Lead compounds are indicated with arrows. Among the 3 compounds with full suppression of IL-6 (13,14,15), only 15 did not inhibit protein synthesis or stimulate protein degradation. 18 showed the greatest anabolic effects, with stimulation of protein synthesis and inhibition of protein degradation.
b) Protein degradation in myotubes assayed as described in Extended Data Fig. 2 and Methods. Bars represent the mean, n = 4, except for DMSO where n = 6 biologically independent samples.
c) 293T cells were co-transfected with a GR expression plasmid and MMTV-luciferase reporter. The next day cells were treated with the indicated compounds for 24 h and probed for luciferase activity. For SR16024, was the cells were cotreated with 1 nM Dex. Data are mean ± SEM, n = 3 biologically independent samples.
d) Mitochondrial potential of myotubes assayed as described in Extended Data Fig. 2 and Methods. Bars represent the mean; n = 3, except for 15 where n = 2 biologically independent samples.
Extended Data Fig. 5. On-target mechanism of action studies.
a) Reporter activity in steroid-deprived 293T cells co- transfected with an androgen-responsive ARR3-tk-luc reporter and an androgen receptor (AR) expression plasmid or empty vector control, and then treated with the indicated compounds for 24 h. Dose curves for compounds that stimulated AR activity (left) and the indicated compounds (right) are shown. None of the compounds showed activity with the empty vector control. 18 and 19 are isomers differing only in the position of the chlorine on the benzyl substitution. Datapoints are mean ± SEM; n = 3 biologically independent samples.
b) Linear regression demonstrating that ligand-specific AR activity profiles do not correlate with protein synthesis.
c) Expression of steroid receptor mRNAs in A549 cells. Only Ar which encodes AR, and Nr3c1 which encodes GR were detected by qPCR. Bars represent the mean; n = 3 independent samples. Also see Supplementary Fig. 3.
d) Representative qPCR amplification plots for Pgr, Ar, and Nr3c1 in A549 versus MCF7 cells. Pgr, which encodes the progesterone receptor, is not expressed in A549 cells.
e) AR antagonists do not reverse the effects of Dex on IL-6 secretion. IL-6 levels in A549 cell media were measured by AlphaLISA after overnight exposure to the indicated conditions. DHT, 5α-dihydrotestosterone; BIC, bicalutamide; ENZ, enzalutamide. For the controls (left), bars represent the mean; n = 6 biologically independent samples. For dose curves, datapoints are mean ± SEM; n = 3 biologically independent samples.
f) Luciferase assay showing the effects of 1 nM DHT, 1 μM BIC and 1 μM ENZ on AR activity, demonstrating that the antagonists have cellular activity. Bars represent the mean; n =3 biologically independent samples.
Extended Data Fig. 6. In vivo compound profiling.
a) Mouse pharmacokinetics studies of the indicated compounds. Data are mean ± SEM; n = 3 biologically independent samples.
b-c) Changes in the lean mass and body weights of male C57BL/6 mice treated with (10 mg/kg Dex or SR16024, or 50 mg/kg SR11466) and fasted overnight. Bars represent the mean; n = 5 mice per group (in each of 2 independent experiments). 1-way ANOVA, Sidak’s multiple comparisons test, adjusted p-values are indicated.
Extended Data Fig. 7. Docking and molecular dynamics simulations.
a) Ribbon diagram of GR LBD bound to the indicated ligands. 7 and 9 were docked with Autodock Vina.
b) Differential analysis of correlated motion between Cα atoms from the simulations with the indicated ligands subtracted from Dex.
c) Formation of a hydrogen bond with R611 differentially shifts the position of the ligands.
d) Formation of the hydrogen bond R611-induced changes in surface structure (red arrows). With Dex, there was a shift in h12 and the C-terminus of h3. With 7, the C-terminus of h11 and N-terminus of h3 were shifted further apart, and away from h12. This destabilization of the h12 interface with h3 and h11 explains why this compound is an antagonist, a mechanism we have called “indirect antagonism.” With 9, there was a rotation of both ends of h3.
e) Usage of amino acid residue and edge in the suboptimal pathways between h12 E755 and h5 R614, demonstrating that Dex preferentially utilized R611 instead of W610 as a pathway for correlated motion. Also see Online Methods.
Supplementary Material
Acknowledgements.
N.E.B. was supported by the BallenIsles Men’s Golf Association. K.W.N. was supported by G.S. Humane Corp. W.M. Keck Foundation Grant to EAO. D.J.K. was supported by NIH DK124870. T. I. is supported by grants from the National Institute of Health, the Department of Defense, The National Science, and by start-up funds provided to The Scripps Research Institute from the State of Florida.
Footnotes
Competing Financial Interest Statement. We declare no competing financial interests in this work.
References
- 1.Watson ML et al. A cell-autonomous role for the glucocorticoid receptor in skeletal muscle atrophy induced by systemic glucocorticoid exposure. Am J Physiol Endocrinol Metab 302, E1210–20 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodine SC & Furlow JD Glucocorticoids and Skeletal Muscle. Adv Exp Med Biol 872, 145–76 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Nettles KW & Greene GL Ligand control of coregulator recruitment to nuclear receptors. Annu Rev Physiol 67, 309–33 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Kuo T et al. Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc Natl Acad Sci U S A 109, 11160–5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen SL, Dowhan DH, Hosking BM & Muscat GE The steroid receptor coactivator, GRIP-1, is necessary for MEF-2C-dependent gene expression and skeletal muscle differentiation. Genes Dev 14, 1209–28 (2000). [PMC free article] [PubMed] [Google Scholar]
- 6.Tobimatsu K et al. Overexpression of the transcriptional coregulator Cited2 protects against glucocorticoid-induced atrophy of C2C12 myotubes. Biochem Biophys Res Commun 378, 399–403 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Amat R, Solanes G, Giralt M & Villarroya F SIRT1 is involved in glucocorticoid-mediated control of uncoupling protein-3 gene transcription. J Biol Chem 282, 34066–76 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Nwachukwu JC et al. Systems Structural Biology Analysis of Ligand Effects on ERalpha Predicts Cellular Response to Environmental Estrogens and Anti-hormone Therapies. Cell Chem Biol 24, 35–45 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Nwachukwu JC et al. Predictive features of ligand-specific signaling through the estrogen receptor. Mol Syst Biol 12, 864 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasan S et al. Ligand-binding dynamics rewire cellular signaling via estrogen receptor-alpha. Nat Chem Biol 9, 326–32 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin Z et al. Synthesis of novel steroidal agonists, partial agonists, and antagonists for the glucocorticoid receptor. Bioorg Med Chem Lett 27, 347–353 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Hu X et al. The antagonists but not partial agonists of glucocorticoid receptor ligands show substantial side effect dissociation. Endocrinology 152, 3123–34 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Stock T, Fleishaker D, Wang X, Mukherjee A & Mebus C Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study. Int J Rheum Dis 20, 960–970 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nwachukwu JC et al. Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. Elife 3, e02057 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aarts JM et al. Robust array-based coregulator binding assay predicting ERalpha-agonist potency and generating binding profiles reflecting ligand structure. Chem Res Toxicol 26, 336–46 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Geiss GK et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26, 317–25 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Stitt TN et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14, 395–403 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Goodyear LJ & Kahn BB Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 49, 235–61 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Magomedova L & Cummins CL Glucocorticoids and Metabolic Control. Handb Exp Pharmacol 233, 73–93 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Kuo T, Harris CA & Wang JC Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol Cell Endocrinol 380, 79–88 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breiman L Random Forests. Machine Learning 45, 5–32 (2001). [Google Scholar]
- 22.Kursa MB Robustness of Random Forest-based gene selection methods. BMC Bioinformatics 15, 8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luciani DS et al. Bcl-2 and Bcl-xL suppress glucose signaling in pancreatic beta-cells. Diabetes 62, 170–82 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pei H et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell 16, 259–66 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balsevich G et al. Stress-responsive FKBP51 regulates AKT2-AS160 signaling and metabolic function. Nat Commun 8, 1725 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowerman S & Wereszczynski J Detecting Allosteric Networks Using Molecular Dynamics Simulation. Methods Enzymol 578, 429–47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Block T et al. Mifepristone Plasma Level and Glucocorticoid Receptor Antagonism Associated With Response in Patients With Psychotic Depression. J Clin Psychopharmacol 37, 505–511 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arora VK et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 155, 1309–22 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu B et al. Epigenetic landscapes reveal transcription factors that regulate CD8(+) T cell differentiation. Nat Immunol 18, 573–582 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coppo M, Chinenov Y, Sacta MA & Rogatsky I The transcriptional coregulator GRIP1 controls macrophage polarization and metabolic homeostasis. Nat Commun 7, 12254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geltink RIK, Kyle RL & Pearce EL Unraveling the Complex Interplay Between T Cell Metabolism and Function. Annu Rev Immunol 36, 461–488 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van den Bossche J, O’Neill LA & Menon D Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol 38, 395–406 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Hudson WH et al. Cryptic glucocorticoid receptor-binding sites pervade genomic NF-kappaB response elements. Nat Commun 9, 1337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chinenov Y et al. Role of transcriptional coregulator GRIP1 in the anti-inflammatory actions of glucocorticoids. Proc Natl Acad Sci U S A 109, 11776–81 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stallcup MR & Poulard C Gene-Specific Actions of Transcriptional Coregulators Facilitate Physiological Plasticity: Evidence for a Physiological Coregulator Code. Trends Biochem Sci 45, 497–510 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stashi E, York B & O’Malley BW Steroid receptor coactivators: servants and masters for control of systems metabolism. Trends Endocrinol Metab 25, 337–47 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voss TC et al. Dynamic exchange at regulatory elements during chromatin remodeling underlies assisted loading mechanism. Cell 146, 544–54 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods-only References
- 38.Trapnell C, Pachter L & Salzberg SL TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–11 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anders S & Huber W Differential expression analysis for sequence count data. Genome Biol 11, R106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mei S et al. Cistrome Data Browser: a data portal for ChIP-Seq and chromatin accessibility data in human and mouse. Nucleic Acids Res 45, D658–D662 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heikkinen S, Argmann CA, Champy MF & Auwerx J Evaluation of glucose homeostasis. Curr Protoc Mol Biol Chapter 29, Unit 29B 3 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Bruno NE et al. Creb coactivators direct anabolic responses and enhance performance of skeletal muscle. EMBO J 33, 1027–43 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong DH & Forsberg NE Effects of dexamethasone on protein degradation and protease gene expression in rat L8 myotube cultures. Mol Cell Endocrinol 108, 199–209 (1995). [DOI] [PubMed] [Google Scholar]
- 44.Walker D, Htun H & Hager GL Using inducible vectors to study intracellular trafficking of GFP-tagged steroid/nuclear receptors in living cells. Methods 19, 386–93 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Stavreva DA et al. Prevalent glucocorticoid and androgen activity in US water sources. Sci Rep 2, 937 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eswar N et al. Comparative protein structure modeling using Modeller. Curr Protoc Bioinformatics Chapter 5, Unit-5 6 (2006). [DOI] [PMC free article] [PubMed]
- 47.Pettersen EF et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–12 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Trott O & Olson AJ AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31, 455–61 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anandakrishnan R, Aguilar B & Onufriev AV H++ 3.0: automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucleic Acids Res 40, W537–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hopkins CW, Le Grand S, Walker RC & Roitberg AE Long-Time-Step Molecular Dynamics through Hydrogen Mass Repartitioning. J Chem Theory Comput 11, 1864–74 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Roe DR & Cheatham TE 3rd. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J Chem Theory Comput 9, 3084–95 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Humphrey W, Dalke A & Schulten K VMD: visual molecular dynamics. J Mol Graph 14, 33–8, 27–8 (1996). [DOI] [PubMed] [Google Scholar]
- 53.Glykos NM Software news and updates. Carma: a molecular dynamics analysis program. J Comput Chem 27, 1765–8 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Manjur A, Lempiainen JK, Malinen M, Palvimo JJ & Niskanen EA IRF2BP2 modulates the crosstalk between glucocorticoid and TNF signaling. J Steroid Biochem Mol Biol 192, 105382 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw and processed nascent RNA-seq dataset generated in this study has been deposited in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO), with the accession code GSE149453. Other mRNAseq data cited in this work was downloaded from the GEO with accession code GSE12463654. Sample R scripts used are available at https://github.com/jnwachuk/ML_in_GR_signaling_networks. Compound profiling assay data, results of correlation and machine learning analyses are in Supplemental_Data1.xls