Abstract
Since the initial outbreak of coronavirus disease 2019 (COVID-19), extensive research has emerged from across the globe to understand the pathophysiology of this novel coronavirus. Transmission of this virus is a subject of particular interest as researchers work to understand which protective and preventative measures are most effective. Despite the well understood model of aerosol-respiratory mediated transmission, the exact mechanism underlying the inoculation, infection and spread of COVID-19 is currently unknown. Given anatomical positioning and near constant exposure to aerosolized pathogens, the eye may be a possible gateway for COVID-19 infection. This critical review explores the possibility of an ocular-systemic or ocular–nasal–pulmonic pathway of COVID-19 infection and includes novel insights into the possible immunological mechanisms leading to cytokine surge.
Keywords: ACE2, COVID-19, Eye, Nasolacrimal, Ocular infection
Introduction
During late 2019, a novel coronavirus was identified as the cause of an outbreak of pneumonia cases in Wuhan, China. The virus rapidly spread throughout China and ultimately the entire world in early 2020. In February 2020, the World Health Organization (WHO) designated the disease coronavirus disease 19 or COVID-19. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the name for the actual virus responsible for the COVID-19 disease. Previously, it was referred to as 2019-nCoV. On January 30th, 2020, the WHO declared COVID-19 a public health emergency of international concern (PHEIC) [1]. As of November 2021, the Center for Disease Control and Prevention (CDC) has reported 48,160,971 total cases and 776,703 total deaths in the United States due to COVID-19. This recent outbreak and the ensuing political, economic, and biopsychosocial implications emphasize the need to understand transmissibility so that appropriate protective and preventive measures may be implemented in communities and healthcare settings worldwide. Research regarding COVID-19 is rapidly evolving and several hypotheses regarding various mechanisms of transmissibility have been put forward. While most of the current literature focuses on respiratory transmission of aerosolized virus, this article focuses specifically on existing research that pertains to the transmissibility of COVID-19 through the human eye, especially through the conjunctiva, cornea, sclera, and nasolacrimal ducts.
Coronaviruses were first identified in the late 1960’s and have been traditionally associated with the common cold and other respiratory tract infections. Coronaviruses are single-stranded, positive-sense, enveloped and the largest known RNA viruses with a genome of approximately 30 kilobases [2]. They belong to the subfamily of Coronavirinae in the family Coronaviridae of the order Nidovirales with four known genera namely α-, β-, γ- and δ-coronavirus [3–5]. Coronaviruses from all four genera exhibit zoonotic origin and transmit freely between human and animal hosts. The origin of α and β genera have been traced to bat species, while the γ and δ genera are mainly derived from avian and swine species [6]. SARS‐CoV‐2, like severe acute respiratory syndrome (SARS-CoV) and Middle East Respiratory Syndrome (MERS-CoV), is predominantly spread via the respiratory tract through droplet transmission [7]. Droplet transmission refers to the direct projection of large droplets onto mucous membranes of a susceptible host when an infected patient releases droplets as they sneeze, talk, or cough [8]. SARS-CoV-2 and SARS-CoV-1 belong to the β-coronavirus genus. Coronaviruses are so named due to the characteristic crown of electron density that is evident on transmission electron micrographs. This crown appearance is due to densely packed proteins on the viral envelope, called spike proteins (SP), which are responsible for priming angiotensin-converting enzyme 2 (ACE2) receptors on host cell membranes [9].
Potential modes of transmission
As the cases of COVID-19 increase worldwide, it is becoming evident that presentation and severity of disease varies widely among patients. On average, an infected person remains asymptomatic for a period of 5 days which is referred to as a latent phase. Usually, manifestation of symptoms begins within 11.5 days. Up to 18–33% of viral carriers remain asymptomatic throughout the course of the disease; however, these patients are most likely to contribute to rampant silent disease transmission [10]. Asymptomatic carriers are most likely to be “super-spreaders”, which is a term used by a recent study from India [11]. A seminal study, conducted by Princeton, Johns Hopkins University, and the University of California, Berkeley found that 71% of COVID-19 positive individuals failed to transmit disease to any direct contacts, while 8% of infected individuals accounted for 60% of new infections.
COVID-19 infection classically presents as symptoms of fever, cough, dyspnea, shortness of breath, myalgia, and fatigue [12]. Rhinorrhea, gastrointestinal symptoms, anosmia, and ageusia are other symptoms that have been reported with varying frequency. While most COVID-19 patients develop mild-to-moderate symptoms, 10–20% of patients require hospitalization of which 10–40% require intensive care unit (ICU) support [13]. Ultimately, up to 0.3–8% of infected individuals succumb to the disease, usually secondary to acute respiratory distress.
Respiratory droplets carry live viruses encapsulated in packets of mucus, saliva, and water. The size of the packets greatly dictates the fate of the virus in the environment. Larger globules tend to travel shorter distances and quickly settle [14, 15]. On the other hand, smaller globules evaporate faster in the form of aerosols and linger in the air. This allows these particles to drift larger distances from the initial site of release. Variation in temperature, humidity and airflow greatly impact the distance travelled by aerosolized SARS-CoV-2; however, the three-hour viability of SARS-CoV-2 in the air poses the greatest threat to any close contact with infected individuals [8, 16, 17]. Additional studies indicate that human coronaviruses survive and maintain their infectious properties for extended periods of time on inanimate surfaces or fomites. Kampf et al. revealed that coronaviruses persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days; however, can be inactivated by surface disinfection procedures within 1 min [18].
Researchers at two independent laboratories have isolated live SARS‐CoV‐2 from the stool specimens of COVID-19 patients [19]. This phenomenon is possibly explained by the high expression of ACE2 receptors in the epithelia of the small intestine [20, 21]. Therefore, the method of fecal‐oral or fecal‐droplet transmission cannot be ignored and warrants further research. Regarding vertical transmission, retrospective findings suggest that there is currently no evidence for intrauterine infection in women who develop COVID-19 in third trimester pregnancy. In addition, breast milk from six patients of the same study tested negative for SARS-CoV-2 indicating minimal possibilities of transmission through breast feeding [22]. However, in a separate study, from Wuhan, China, two newborns were confirmed to have COVID-19 shortly after delivery suggesting that transmission is possible from mothers to infants. In these cases, whether transmission occurred during pregnancy, or immediately after delivery, remains unknown [7]. COVID-19 offers a greater risk to patients with preexisting chronic clinical conditions that cause immunosuppression or inflammatory response. Pathological events that render patients more likely to suffer complications from SARS-CoV-2 include obesity, atherosclerosis, diabetes, asthma, and hypertension. These complications are susceptible to all organ systems, including the eye. However, no information is available whether these comorbidities increase the ocular transmissibility of SARS-CoV-2 [23].
A seminal study evaluated the impact of age, sex, and ethnicity on immune responses to various pathogens. Generally, immune responses very between genders, change throughout life and are influenced by age and reproductive status. These differences render men and women susceptible to different autoimmune diseases, malignancies, and infectious diseases such as COVID-19. Overall, females seem to have heightened immunity to pathogens, contributing to lower intensity and prevalence of infections; however, with increase in the symptomatology and severity among females compared to males [24].
The current literature suggests that males have a higher risk of complications and mortality related to COVID-19. It is postulated that elevated estrogen levels in females reduce the severity and mortality of COVID-19 through an increased innate and humoral response. From an ethnic standpoint, African American and Hispanic populations have shown increased rates of infection and hospitalization compared to other ethnic groups. These differences are likely attributed to a higher prevalence of comorbidities and decreased access to care [25]. Overall, the vast and various modes of possible COVID-19 transmission remain unknown.
Primary target organs and major symptoms
While COVID-19 is a systemic disease, the most obvious and perhaps the most severe effects of SARS-CoV-2 occur in the respiratory system. Due to variability of presentations and severity of disease, lung complications from SARS-CoV-2 range from mild pneumonia (81%) to severe hypoxia (14%), shock and multi-organ failure (5%) and death (2.3%) [26]. The ACE2 receptor is found throughout the body which allows the virus to affect a multitude of organs and systems (Table 1). The ACE2 receptor is predominantly expressed on the apical side of type II pneumocytes in the alveoli. This provides an abundance of binding sites for SARS-CoV-2 and suggests why the lungs are particularly vulnerable to infection [27, 28]. Research on SARS-CoV-2 revealed that the virus promotes endothelial dysfunction, vascular leak, and pulmonary microthrombi through mechanisms such as inflammation, hypoxia, oxidative stress, mitochondrial dysfunction, and DNA damage [29–32].
Table 1.
Effect of COVID-19 on primary target organs
| System | Symptoms/Presentation | Sequelae |
|---|---|---|
| Pulmonary | Cough, apnea, hypoxia, pneumonia | Alveolar damage, fibrosis, edema, hemorrhage, pneumocyte hyperplasia and hyaline scar tissue formation |
| Cardiovascular | Palpitations, chest tightness | Myocarditis, elevated blood pressure |
| Gastrointestinal/Hepatobiliary | Abdominal pain, loss of appetite, diarrhea, vomiting | Transaminitis, bowel ischemia |
| Vascular and Hematologic | Muscle/body aches, DVT, headache | Thrombotic events secondary to hypercoagulable state |
| Neurologic | Dizziness, impaired consciousness, ageusia, anosmia, neuropathy, seizure | Stroke |
| Renal | Proteinuria | Sepsis related kidney injury |
| Ocular | Conjunctivitis, dry eye, blurred vision, foreign body sensation | None |
Unfortunately, findings on the long-term implications of COVID-19 on the lungs is scarce. Given the inflammatory effects of the disease, the most likely long-term complications include alveolar damage, fibrosis, edema, hemorrhage, pneumocyte hyperplasia and hyaline scar tissue formation. Another organ system with an abundance of ACE2 is the heart and vascular system. In acute infection, the virus causes direct damage to cardiac tissue as evidenced by isolated cases of COVID-19-induced myocarditis [33–36]. In addition, SARS-CoV-2 interferes with the normal physiology of the ACE2 receptor, which normally functions to catalyze the conversion of angiotensin II to angiotensin 1–7 and to protect the cardiovascular system with antioxidant and anti-inflammatory properties [37–39]. In addition, COVID-19 patients commonly present with gastrointestinal complaints such as diarrhea, cramping abdominal pain, loss of appetite, vomiting and nausea. Regarding hepatic complications, while direct cases of acute liver failure secondary to SARS-CoV-2 have not been reported, it is difficult to differentiate the independent hepatic effects of the virus from complications of antiviral drug therapies and disease sequelae such as sepsis and hypoxia [40]. As previously stated, the presence of SARS-CoV-2 in stool of affected patients suggests the possibility of fecal–oral transmission.
Recent data suggests that SARS-CoV-2 affects the vascular and hematologic systems. Infected individuals are believed to be susceptible to hypercoagulability and have an increased risk for thrombotic events. One study showed approximately 33% of COVID-19 patients later developed pulmonary embolisms (PE) as evidenced on CT scans [41]. Another similar study by Klok et al. showed that nearly 30% of COVID-19 patients admitted to the ICU suffered from PE, deep vein thrombosis (DVT) and strokes despite aggressive anticoagulative therapy [42]. It has been hypothesized that COVID-19 predisposes patients to both venous and arterial thromboembolic disease due to systemic inflammation, hypoxia, immobilization, and diffuse intravascular coagulation (DIC). For this reason, it has become standard of care to begin prompt prophylactic anticoagulation with low molecular weight heparin in these patients [43]. Some studies have suggested using D-dimer levels as a metric for COVID-19 disease progression and mortality [12].
Anosmia and ageusia are two symptoms that have recently been associated with COVID-19 infection. Although the mechanism linking COVID-19 infection and these loss of sensations remains to be illuminated, the American Academy of Otolaryngology—Head and Neck Surgery and the British Association of Otorhinolaryngology have suggested to consider these as significant symptoms for screening in otherwise asymptomatic carriers of disease. In particular, the sudden onset of olfactory dysfunction could represent an early indicator of COVID-19 infection [44]. Other neurological complications of SARS-CoV-2 coincide with the hypercoagulable state of the disease. Even in younger patients with COVID-19 who were treated with prophylactic anticoagulants, there have been cases of strokes leading to profound neurological injury [45]. Based on data from a retrospective study, some of the neurological symptoms associated with COVID-19 include dizziness (16.8%), headache (13.1%), impaired consciousness (7.5%), ageusia (5.6%) anosmia (5.1%), acute cerebrovascular disease (2.8%), neuropathy (2.7%), vision impairment (1.4%), ataxia (0.5%), and seizure (0.5%) [46].
The most frequent renal abnormality in patients with COVID-19 is mild-to-moderate proteinuria, which is explained by the cytokine storm phase of the disease that results in kidney injury comparable in presentation to sepsis related acute kidney injuries (AKI) [47]. Further research is warranted to delineate the precise impact of COVID-19 on kidney function. However, based on preliminary data in hospitalized patients with COVID-19, it is possible to conclude that kidney disease on admission and kidney injury during hospitalization is associated with increased mortality and progression of disease. Also, the early detection and management of renal sequelae could reduce the chance of death in COVID-19 patients [47].
Possible transmission through the eye
The eyes could be directly impacted by the SARS-CoV-2 virus and serve as a gateway to the rest of the body. A recent study examined 216 children with confirmed COVID-19 status and concluded that 49 (22.7%) had ocular manifestations. The most common of these symptoms include conjunctival discharge, itchiness, and conjunctival congestion. Ultimately, these ocular symptoms were not severe, and all children eventually recovered with no lasting visual or ocular complications [48]. Another cross-sectional study considered the ocular and visual sequelae of 534 adult COVID-19 cases revealing that 25 patients (4.68%) presented with conjunctival congestion with an average duration of 4.9 ± 2.6 days. Other ocular symptoms related to COVID-19 status include dry eyes (112, 20.97%), blurred vision (68, 12.73%), and foreign body sensation (63, 11.80%). Importantly, these ocular symptoms proved to be self-limiting and short-lived [49]. Another study revealed that around one-third of patients with COVID-19 had ocular abnormalities and tended to have more susceptibility to COVID-19. Of note, it was found that only 1 of the 38 patients in this retrospective study presented with conjunctivitis as the first symptom suggesting limited diagnostic or screening value in this finding [50].
A case report by Cheema et al. details the case of a healthy 29-year-old woman who presented with right eye conjunctivitis, photophobia, and clear watery discharge. Though her presenting symptom was keratoconjunctivitis and mild respiratory symptoms without fever, she was, however, tested positive for COVID-19. Considering such cases, eye care professionals recommend a high index of suspicion of SARS-CoV-2 when evaluating patients with viral conjunctivitis. Also, other reports suggest conjunctivitis as an early presentation of COVID-19 [51]. Taken together, it is reasonable to conclude that SARS-CoV-2 does indeed affect the eye. Most often, these infections result in conjunctivitis, some blurred vision, dry eyes, or foreign body sensation. Ultimately, most ocular manifestations seem relatively short lived and have not proven to cause long term effects and have minimal diagnostic value. Nevertheless, eye care professionals need to remain vigilant for COVID-19 when evaluating patients with viral conjunctivitis, especially in high-risk patients with prior exposure.
On average, the total ocular surface area of an adult human ranges between 1600 and 1869 mm2 per eye [52]. This estimate, which includes the cornea, conjunctiva and fornices, represents a maximal absorptive area of 3738 mm2 when accounting for both eyes. After incorporating the surface area of the eyelids and brows, roughly 4000 mm2 and 3000 mm2 respectively, the total ocular surface area surpasses 10,000 mm2. This is two orders of magnitude greater than the nares and mouth without considering the surface area attributed to the hair of the eyebrows or eyelashes, for which estimates have not been made [53]. As this large area frequently contacts respiratory droplets, contaminated fingers and fomites, it is important to consider possible transmission of SARS-CoV-2 through the eye.
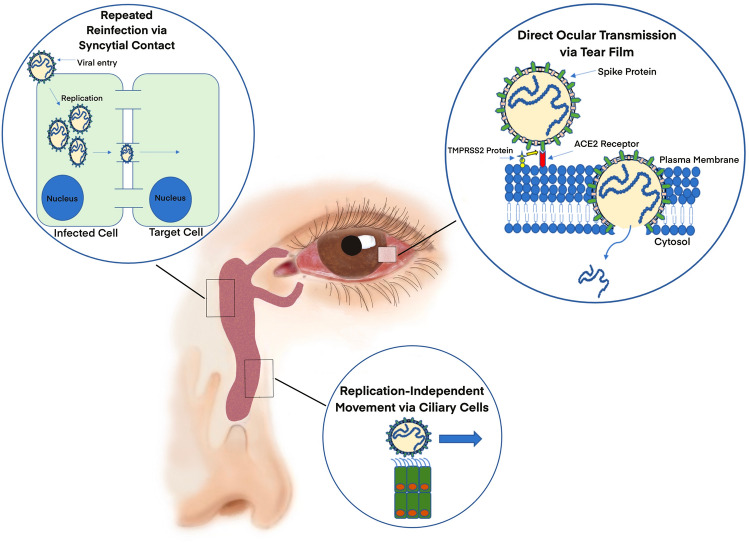
There are two existing hypotheses for COVID-19 transmission through the eye (Fig. 1). The first considers the conjunctiva as a direct inoculation site of infected droplets. The second centers around the nasolacrimal system as a conduit for viral migration to the gastrointestinal and respiratory tracts [1]. The conjunctiva regularly encounters pathogens and, following the contact, the eye serves as both a nidus of replication and a site for systemic uptake. Before draining into the nasolacrimal system, a small volume of tears is absorbed by the conjunctiva, cornea, or sclera which would allow viral particles to enter the globe or circulatory system. However, most tears are drained into the inferior meatus of the nose to be eventually swallowed into the gastrointestinal tract or drained into the respiratory tract [54].
Fig. 1.
Proposed mechanisms of ocular COVID-19 transmission. The conjunctiva may serve as a direct inoculation site of infected droplets. Given a sufficiently high viral load, some virus may be able to evade the antimicrobial agents of the tear film and directly gain access to conjunctival and corneal cells before eventually entering general circulation. TMPRSS2 proteins found on the surface of ocular cells activate the virus spike protein through proteolytic cleavage. Once activated, the spike protein can bind to ACE2 receptor and enter a host cell. Other hypotheses focus on nasolacrimal transmission of SARS-CoV-2 from the eye to the lungs in humans. Virus may do this by spanning the length of the nasolacrimal duct through repeated reinfection and replication of neighboring cells. Alternatively, the virus may travel in a replication-independent manner via ciliary movement by the cells which line the nasolacrimal duct
Interestingly, ACE2 receptors and transmembrane protease serine 2 (TMPRSS2) proteins have been found on stem cells of the corneal limbus [55]. The presence of these proteins suggests that SARS-CoV-2 can enter the cells of the ocular surface [56]. While this mechanism has not been observed in humans, it has been shown in some animal models that beta-coronaviruses caused supraocular and intraocular infections such as conjunctivitis, uveitis, retinitis, and optic neuritis, which suggests possible penetration of the ocular globe [1, 57]. Other viruses, such as cytomegalovirus, have been shown to cause full-blown retinopathy, however, no cases of retinopathy to-date have been specifically linked to SARS-CoV-2 infection. While the evidence for ACE2 receptors and TMPRSS2 proteins on the ocular surface seems conclusive, there remains some debate on whether the tear film of infected COVID-19 patients contains viral particles. One cross-sectional study showed that SARS-CoV-2 RNA was detected in tears of 24% of patients with laboratory-proven moderate to severe COVID-19. The method used to evaluate the tears was conjunctival swab and reverse transcription polymerase chain reaction (RT-PCR) [58]. In contrast, another study reported that, of the 29 COVID-19 patients studied, ocular symptoms were common in patients with COVID-19. However, tear analysis revealed the absence of SARS-CoV-2 [59]. Interestingly, both studies used similar methods of tear analysis, namely Schirmer paper strips to obtain tear samples and RT-PCR to assess for viral RNA.
A prospective study followed the disease course of seventeen COVID-19 patients. Of the 17 patients recruited, all tested positive for COVID-19 and none demonstrated ocular symptoms except for one patient who developed conjunctival injection and chemosis during hospitalization. A total of 64 samples were obtained using the traditional Schirmer method over the study period, with 12, 28, and 24 samples obtained from the first, second, and third week of initial symptoms, respectively. All samples showed negative results for SARS-CoV-2 on RT-PCR. Furthermore, this study found no evidence of SARS-CoV-2 shedding in tears during the disease and the patients from this study had no active ocular symptoms.
Overall, further research is warranted to confirm whether SARS-CoV-2 RNA or the virus itself can be confidently isolated and cultured from ocular secretions. Preliminary data suggest that it is unusual to find SARS-CoV-2 in the tear film of most COVID-19 patients warranting specific and sensitive instrumentation for designing appropriate diagnostic tools. However, exceptions may include patients with active conjunctivitis or a particularly high viral load [57]. Additionally, the presence of antimicrobial peptides such as immunoglobulins, lysozyme, and lactoferrin, as well as normal components of bacterial flora that neutralize viral particles decrease the viral load. Moreover, the mucin layer in normal tears prevents binding of the virus to epithelial receptors which promotes swift clearance through nasolacrimal drainage [54].
The nasolacrimal system is designed to drain excess ocular fluid from the eye surface, providing an anatomical bridge from the eye to the nasopharynx. The epithelial cells, which line the nasolacrimal duct, possess microvilli that permit both secretion and reabsorption of tear fluid [60]. To date, no study has directly linked a COVID-19 case specifically to nasolacrimal transmission of SARS-CoV-2 from the eye to the lungs in humans. However, research has shown that other viruses, such as respiratory syncytial virus (RSV), are able to infect the eye and subsequently the lungs through nasolacrimal transmission. There are two proposed mechanisms. In the first, the virus travels the span of the nasolacrimal duct through repeated reinfection and replication of neighboring cells by diffusion or syncytial contact. This is the slower of the two proposed methods as infection is dependent on viral replication time. In the second proposed mechanism, the virus travels in a replication-independent manner, perhaps transported by the ciliary cells which line the nasolacrimal duct [61]. The second mechanism is the most plausible since most respiratory viruses, after infecting the eyes, travel rapidly and normally impact the lungs within 1 day. Given the 7–8-h replication cycle of SARS-CoV-2, it is unlikely to infect all the mucosal cells en route to the lungs. However, viral replication likely occurs to some extent to amplify the viral load in the eyes and respiratory tract [61]. Further confirmatory studies are required to validate these mechanisms regarding COVID-19 infection.
ACE2 receptors in the eye
Currently it is established that the ACE2 receptor and TMPRSS2 protease are two important factors for the viral entry of SARS-CoV-2 in humans. SARS-CoV-2 initially uses heparan sulfate proteoglycans as anchors before binding to ACE2 and TMPRSS2. TMPRSS2 primes the spike protein on SARS-CoV-2 for interaction with ACE2, which facilitates infection of cells [62]. ACE2 receptors were previously studied and found in human retina and choroid tissues [63]. While current research regarding the expression of these two receptors have been studied extensively in the respiratory tract and other organs, research is lacking pertaining to the ocular surfaces as potential infection sites. Since the eye is a mucosal surface that constantly interacts with the external environment, it is an important site for possible infection and transmission of SARS-CoV-2. It is critical to study where, and to what extent, ACE2 and TMPRSS2 is found in the eye to properly determine the likelihood of ocular transmissibility of SARS-CoV-2 infection.
Earlier reports evaluating the presence of ACE2 and TMPRSS2 in ocular structures resulted in contradictory findings. Sungnak et al. used single-cell RNA sequencing (scRNA-seq) datasets from healthy donors to assess the abundance of ACE2 and TMPRSS2 in different tissues. Their results suggested low expression of both ACE2 and TMPRSS2 in ocular structures including the conjunctiva and cornea [55]. Similarly, microarray and RNA-sequence expressions revealed that ACE2 was downregulated in conjunctival tissue and corneal tissue [64]. The expression of TMPRSS2 was expressed only in conjunctiva with heterogeneous expressions throughout the tissue.
Lange et al. studied 38 conjunctival samples that included both healthy and diseased tissues with abnormalities such as melanoma, squamous cell carcinoma and papilloma [65]. Through high-throughput RNA sequencing, they found an insignificant level of ACE2 and TMPRSS2 expression in the conjunctiva. Subsequent immunohistochemical staining of healthy conjunctival samples confirmed there was negligible ACE2 and TMPRSS2 expression. Zhang et al. stated that there was significant upregulation of both ACE2 and TMPRSS2 in the conjunctiva epithelial with some, albeit less, expression on corneal epithelial cells [65]. Lange et al. attributed this difference to the types of polyclonal antibodies used which allegedly had much less specific binding. In response to Lange et al., Grajewski et al. used the same anti-ACE2 antibody, under slightly modified conditions, (i.e., used basic antigen retrieval buffer, different dilutions, and different incubation periods of primary antibodies) to show that ACE2 expression in healthy donor conjunctiva was obtainable by immunohistochemical staining [66]. Similar to the findings by Zhang et al., Grajewski et al. found the expression of ACE2 proteins to be significant in the conjunctival epithelial cells and minimal expression of ACE2 in conjunctival stroma; however, there was immunopositivity in epithelium.
A study by Li et al. used RT-qPCR, and immune assays to assess the expression of ACE2 in surgical waste conjunctival tissue biopsies [67]. The study revealed significant differences in levels of ACE2 in normal conjunctival compared to diseased tissue. In general, there was overexpression of ACE2 in conjunctival tissue with nevi, cysts, papillomas, polyps, and conjunctivitis. ACE2 expression among the diseased conjunctival tissues were not significantly different. Contrastingly, Ma et al. reported consistent expression of ACE2 and TMPRSS2 in the conjunctival and pterygium cells [68]. Due to significantly higher corneal expression of TMPRSS2 and ACE2, it is reasonable to conclude that cornea, rather than conjunctiva, is the most likely infection site for SARS-CoV-2. However, in conditions of disease or inflammation, the conjunctiva may also be a potential route for SARS-CoV-2 entry.
Collins et al. reported scRNA-seq data of human adult cornea and conjunctiva tissues revealing that ACE2 was expressed mainly in the limbal and conjunctival superficial epithelium [69]. Specifically, ACE2 was highly expressed in limbal, peripheral corneal epithelium, superficial conjunctival epithelium, and superficial central corneal epithelium. Also, TMPRSS2 was expressed in fewer cells, predominantly in basal and superficial conjunctival epithelium. These findings were confirmed by the highest co-expression of ACE2 and TMPRSS2 found in basal conjunctival epithelial cells, with lower levels at limbal and peripheral corneal epithelium, superficial conjunctiva, and superficial corneal epithelium. Collins et al. studied the expression of ACE2 and TMPRSS2 in fetal and embryonic tissue, regarding the role of inflammatory signaling pathway with infection of SARS-CoV-2. Through analysis of overlapping genes associated with ACE2 and TMPRSS2, the possible transcriptional pathway is activated by pro-inflammatory signals and inhibited in a feedback loop regulating the immune response on the ocular surface have been elucidated. This suggests that SARS-CoV-2 exploits inflammatory drive for the upregulation of ACE2 and TMPRSS2, promoting infection through the ocular surfaces. scRNA-sequence and bulk RNA-sequence analysis on conjunctival tissue revealed that developing human fetuses do not express SARS-CoV-2 entry genes. However, it is yet unclear if the two genes are expressed in conjunctival or corneal epithelium in children.
Zhou et al. examined corneal epithelial samples from healthy myopic patients who underwent photorefractive keratectomy, post-mortem human globes from non-diabetic patients without ocular disease, and from diabetic individuals with diabetic retinopathy [70]. Overall, ACE2 and TMPRSS2 expressions has been shown to be distributed throughout the ocular structures suggesting the possible route of entry and infection by COVID-19.
Interplay of cytokines in eye inflammation
Due to its similarity to SARS-CoV and MERS-CoV, researchers investigated previously implicated cytokines as a starting point for SARS-CoV-2 related cytokine interactions (Table 2). In SARS-CoV and MERS-CoV infected patients with extensive pulmonary involvement and inflammation, increased levels of IL-1β, IL-6, IL-12, and CXCL10 and CCL2 were reported [71–74]. Similarly, early reports regarding COVID-19 infected patients, who required intensive care or intubation, displayed higher levels of these cytokines than cases with less severe clinical course or non-ICU patients [12, 75–77]. These increased levels of cytokines and chemokines has been related to Th1 activation and subsequent cytokine storm. However, in comparison with previous SAR-CoV infections, SARS-CoV-2 showed high levels of IL-4 and IL-10, suggesting Th2 activation related to attempts to suppress the hyperactive inflammatory responses in cytokine storm [75, 77]. Ultimately in both cases, there are overwhelming levels of proinflammatory cytokines and chemokines that cause a sustained inflammatory response resulting in the most severe sequelae including ARDS, multi-organ failure, and death [75, 77].
Table 2.
The role of major cytokines in inflammation
| Cytokine | Source of secretion | Type | Function |
|---|---|---|---|
| IL-1 |
• Macrophages • Monocytes • Epithelial cells • Keratocytes |
Proinflammatory |
• Activates myeloid response through macrophages and Th17 • Activation of IL-1β to further increase proinflammatory cytokines |
| IL-2 | • T-cells and subtypes | Proinflammatory | • Effector T-cell and Treg proliferation and differentiation |
| IL-4 | • T-helper (Th) cells | Proinflammatory and anti-inflammatory |
• B-lymphocyte activation and differentiation • Production of IgE isotype • Decreases CD8 + Memory T-cells |
| IL-6 |
• Macrophages • T-cells • Endothelial cells • APC • Neutrophils |
Proinflammatory and anti-inflammatory |
• Induces acute-phase protein expression and release • Increases antibody production • Regenerative and anti-inflammatory via conventional signaling • Proinflammatory response via trans-signaling |
| IL-7 |
• Thymic stromal cells • Bone marrow stromal cells • Dendritic cells |
Proinflammatory |
• T-cell development with all CD4 + T-cell subgroups requiring this cytokine for peripheral homeostasis • Activates T-cell and increases proinflammatory cytokines • Decreases Transforming growth factor Beta (TGF-B) |
| IL-10 |
• Regulatory T-cells (Treg) • Th9 cells |
Anti-inflammatory |
• Inhibits Th1 cells • Inhibits proinflammatory cytokine release • Blocks dendritic cell maturation • Decreases MHC complex expression |
| IL-12 |
• Dendritic cells • Macrophages • Monocytes • B-cells |
Proinflammatory |
• Activates Th1 and Th17 cells • Induces IFN-γ production in T-cells and NK cells • NK cell chemotaxis |
| IL-17 |
• Th17 cells • NK cells • ILC3 |
Proinflammatory |
• Works with IL-22 and TNF-α to induce antimicrobial peptide production • Neutrophilic chemotaxis and activation |
| IL-18 |
• Monocytes • Macrophages • Dendritic cells |
Proinflammatory |
• Activates Th1 pathway • Enhanced CD8 + T-cell and NK cell cytotoxicity through FasL upregulation • Induces IFN-γ production • Synergistically works with IL-12 • Proinflammatory alarmin cytokine |
| IFN-γ |
• Th1 cells • ILC1 • NK cells • Cytotoxic T-cell |
Proinflammatory |
• Activates macrophages and upregulates antigen presentation • Proinflammatory cytokine that induces anti-viral peptide production and expression |
| TNF-α |
• Macrophages • T-cells • NK cells • Mast cells |
Proinflammatory |
• Increases vascular permeability for immune cell chemotaxis • Works synergistically with IL-1β and IL-6 for proinflammatory responses • NK cell differentiation |
| M-CSF |
• Monocytes • Fibroblasts • Endothelial cells |
Proinflammatory |
• Proliferation of hematopoietic cells into myeloid cells • Synergistically works with IL-1 and IL-3 for myeloid differentiation |
| G-CSF |
• Macrophages • Endothelial cells |
Proinflammatory |
• Proliferation and activation of polymorphonuclear granulocyte cells (PMNS) • PMN chemotaxis and phagocytosis upregulation |
| GM-CSF |
• Th17 cells • Fibroblasts • Hematopoietic cells • Endothelial cells • Epithelial cells |
Proinflammatory |
• Proliferation and activation of macrophages, eosinophils, neutrophils, monocytes, and dendritic cells • Increases production of proinflammatory cytokine • Upregulates antigen presentation and phagocytosis • Promotes chemotaxis and leukocyte adhesion for increased immune response |
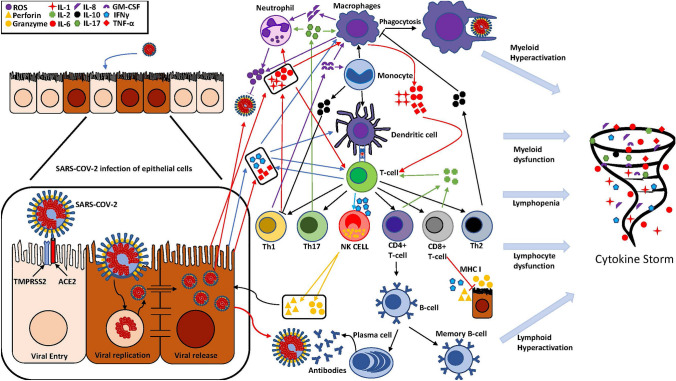
In SARS-CoV-2 related infections, it is believed that dysfunctional immune effector cells, excessive immune infiltration, excessive immune effector cell activation, excessive cytokine release, and activation of IL-6 signaling pathway are the main cause of tissue damage, especially those related to ARDS [71, 72, 76, 78, 79]. Some of the implicated cytokines include interferons (IFN-γ), tumor necrosing factor (TNF-α), granulocyte colony stimulating factors (G-CSF), and interleukins (IL-1β, IL-2, IL-4, IL-6, IL-7, IL-10, IL-12, IL-13, IL-17) [75, 77]. Increased proinflammatory cytokines and chemokines subsequently attract more neutrophils, monocytes, macrophages, and T-cells that further amplify the cytokine release causing a storm of proinflammatory cytokines [75, 79]. While the exact cells and mechanisms that initiates the cytokine storm in COVID-19 are currently unclear, it is reasonable that cells that cause cytokine release such as dendritic cells, macrophages, and endothelial cells are the primary suspects [77]. In SARS-CoV-2 associated infection of the eye, the exact underlying mechanism of cytokine storm is currently unknown; however, similar mechanism of cytokine related damage in the lungs from ARDS could be possible in the eye, which warrants further investigation [78].
Cytokine storm (Fig. 2) largely contributes to SARS-CoV-2 mortality and the neutralization of certain cytokines in the cytokine storm may ultimately improve patient outcomes [75]. For instance, IL-1α and IL-1β are elevated in severe COVID-19 infections [75, 77]. It is believed that IL-1 levels are related to virulence and significantly elevated serum levels are observed in severe SARS-CoV-2 infection, similar to previously studied SARS-CoV and MERS-CoV [75]. Since keratocytes and corneal epithelial cells express IL-1α, the cornea could potentially be an important site for complications and contribution to cytokine storm in severe COVID-19 patients [80].
Fig. 2.
SARS-CoV-2 infection of epithelial cells with cytokine pathways in dysfunctional myeloid and lymphoid immune responses leading to cytokine storm. ROS Reactive Oxygen Species, IL Interleukin, GM-CSF Granulocyte Macrophage Colony Stimulating Factor, IFN Interferon, TNF Tumor Necrosis Factor, NK Natural Killer, MHC Major Histocompatibility complex, Th T helper
Elevated IL-6 has been reported extensively in early cases of SARS-CoV and SARS-CoV-2 and is associated with poor patient prognosis [75]. Since IL-6 is induced by IL-1β, higher serum levels of these two cytokines are directly correlated with SARS-CoV-2 morbidity and mortality [75]. Recent reports suggest that elevated IL-6 levels accelerate the inflammatory process and in turn contributes to the cytokine storm, which worsen the prognosis of COVID-19 infections. Hence, IL-6 has been suggested to be an important target cytokine for cytokine storm. However, the results regarding the inhibition of IL-6 and improvement from COVID19 infections are yet to be unveiled [71, 75, 77].
Several other interleukins including IL-2, IL-4, IL-7, IL-12, IL13, and IL-17 are involved in inflammatory reactions through proliferation and differentiation of effector immune cells (Table 2) [71, 75–77]. IL-2 is intimately involved in the proliferation and differentiation of T-cells into effector and memory T-cells and the activation of B-cells and NK cells. IL-2 is involved in prevention of autoimmune diseases while maintaining a sufficient immune response to virally infected cells [75]. IL-7 is involved in differentiation of CD4 + T-cell sub-population increasing the inflammatory cytokine production [75]. IL-4 is secreted by activated T-helper cells and involves B-cell activation and differentiation, and the production of IgE. Also, IL-4 is necessary for Th2 differentiation and is heavily involved in anti-parasitic and allergic reactions [75]. Similarly, IL-13 is important regulator to the Th1 immune response that is heavily involved in allergic and anti-parasitic reactions. IL-13, functions to activate mast cells and is involved in mobilization of eosinophils [75].
IL-12 is key for differentiation of Th1 and Th17 cells and chemotaxis of NK cells. In addition, IL-12 induces IFN-γ production through a positive feedback mechanism, which is important for inhibition of viral replication [75]. IL-17 produced by Th17 cells are similarly involved in inflammatory and autoimmune processes. In synergy with IL-22 and TNF-α, IL-17 accelerates the induction of antimicrobial peptides [75]. In SARS-CoV-2 infection, it has been reported that there are significantly elevated levels of proinflammatory cytokines, which has been correlated with significantly worse prognosis [71, 75–77]. These interleukins play a major role in the proinflammatory cascade as they involve proliferation and differentiation of many effector immune cells leading to further release and exacerbation of cytokine release syndrome (CRS) [75, 77].
CSFs participate in the inflammatory response and is responsible for proliferation and differentiation of hematopoietic stem cells into the myeloid response during viral infections. Early reports in SARS-CoV-2 infection have reported elevated levels of CSFs in acute phases of infection; however, in more severe clinical cases, it has been suggested that elevated CSF levels causes a hyperactive myeloid response contributing to cytokine storm [71, 75, 77]. Elevated CSF levels are also indicative of viral load and is associated with significant tissue damage in the lungs. As such, CSF could be a target of inhibition to prevent cytokine storm and hyperactivation of myeloid cells during COVID-19 infection.
While antigen presenting cells (APCs) and immune cells are directly involved in increased cytokines, there are various processes and conditions such as meibomian gland dysfunction and dry eyes that alter the protective structures of the eye leading to damage on the ocular surface epithelium [81, 82]. This damage leads to cytokine surge driving secondary inflammation and exacerbation of proinflammatory responses. Previous studies show that APC density in the center of the cornea is increased in inflammatory conditions such as viral infection, graft rejections, bacterial infections, and after procedures such as photorefractive keratectomy [81, 82]. Inflammatory conditions such as Sjogren’s displayed tenfold increase in inflammatory cytokines such as IL-1, IL-6, and IL-8 with a concomitant decrease in epidermal growth factor (EGF) level and hyperosmolar tears leading to the upregulation of tear cytokines [78, 81]. In mice models, CD4 + T-cells promoted severe inflammation in the lacrimal glands, cornea, and conjunctiva leading to decreased tear production and goblet cell loss, which further amplified the inflammatory response [82, 83]. Also, tear deficient mice upregulated IFN-γ, which further lead to apoptosis of goblet cells inhibiting the protection of tear film. Moreover, Th17 cells that are resistant to Tregs trigger ocular surface autoimmunity and IL-17 produced by Th17 cells facilitate the inflammatory cycle through recruiting of neutrophils and hyperactive secretion of cytokines that leads to dry eye [81].
Immunology of the eye against infection
The ocular immune system defends the eye against infection and damage from the external environment [82]. An important innate defense mechanism by the eyes is the tear film which prevents against desiccation of the ocular surface, washes away foreign particles, and provides antimicrobial defense (Fig. 3) [80, 83]. The top portion of the tear-film includes the lipid secretions from the meibomian glands that prevents evaporation of the tears. The middle aqueous layer originates from the lacrimal glands to lubricate the ocular surface and contains many antimicrobial peptides and proteins such as immunoglobulins (predominantly IgA), lactoferrin, lysozymes, lipocalin, complement, secretory phospholipase A2 (sPLA2) and beta-lysin [80]. Goblet cells produce the bottom mucosal layer, which functions to keep the epithelial cells of the eye moist and contains mucin, immunoglobulins, and leukocytes as a last line defense [82, 84, 85]. Mucin located in the mucosal layer, acts as an adhesive to many pathogenic agents. Ultimately these tear-film layers work together to provide important lubricant and microbial peptides, which defends the eye against microbial agents. Dysfunctional and blocked tear ducts result in inflammatory responses in the meibomian glands that lead to meibomian gland dysfunction, which increases risks for chronic dry eyes or infections [83, 86].
Fig. 3.
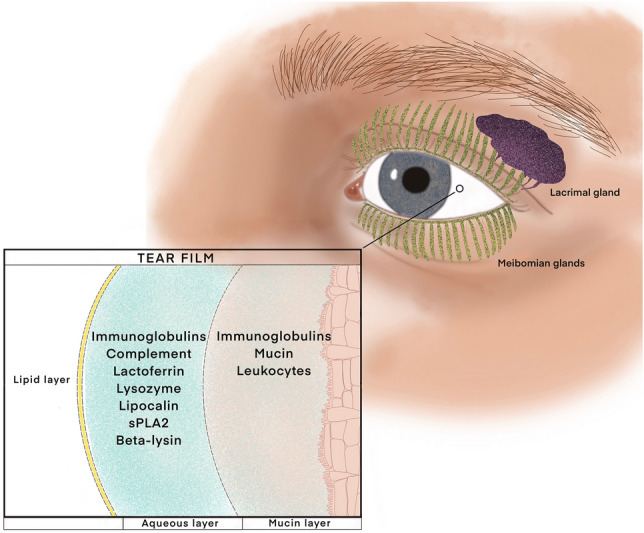
The tear film protects the ocular surface from desiccation as well as infection from microbes. Meibomian glands secrete the top, lipid portion of the tear film which mostly prevents against evaporation. The lacrimal glands secrete the middle aqueous layer which also lubricates the ocular surface and contains many antimicrobial factors such as immunoglobulins, lactoferrin, lysozymes, lipocalin, complement, secretory phospholipase A2 and beta-lysin. Goblet cells produce the bottom mucosal layer which contains mucin, immunoglobulins, and leukocytes as a last line defense against pathogens
Underneath the initial defense of the tear film, corneal epithelial cells and keratocytes produce cytokines that serve to activate the immune system to protect the eye against infections. IL-1α is present in both corneal epithelial cells and keratocytes, which get released when these cells rupture due to damage to the cell membranes [80]. IL-1α release leads to enhanced immune trafficking and proinflammatory immune responses. Production of IL-1α and TNF-α induces the secretion of IL-6 from corneal epithelial cells and keratocytes with subsequently production of macrophage inflammatory protein (MIP), which is a potent chemoattractant for neutrophils and T-cells [80]. Keratocytes produce defensins, which provides broad spectrum antimicrobial defenses; however, possess additional anti-inflammatory benefits such as increased epithelial healing [80]. Other antimicrobial proteins located in the cornea and conjunctiva include the complement system, which helps with opsonization of microbial agents and chemotaxis for recruitment of immune cells [80].
Outside of the passive defense elements in the cornea and conjunctiva, early responding non-specific immune cells are some of the most important defenses our eye uses to control and fight infections [76]. Neutrophils reside in corneal endothelium after migrating through the limbal vasculature by diapedesis [80, 84, 86]. The neutrophil-derived interferon-α (IFN-α) plays an important role in viral immunity as mice models without interferon exhibited elevated viral titer levels [80]. Similarly, macrophages play an important role in phagocytosis and antigen presenting to T-cells for activation of the adaptive inflammatory response [87, 88]. Macrophages are thought to be located in both conjunctiva and corneal stroma [80]. Macrophages play an important role in initial defense connecting the innate and adaptive immune systems. Corneal and conjunctival Langerhans cells provide a similar and important role in antigen recognition, processing, and presentation to T-cells [80]. Viral detection and activation of Langerhans cells, ultimately leads to production of cytokines, chemokines, antimicrobial peptides, and a pro-inflammatory response [82, 84, 89]. Also, Langerhans cells exhibit the initial anti-viral defense in release of IFN-γ and cytokine release [88].
Generally, myeloid response located in peripheral tissues recognize a wide range of pathogens through pattern-recognition receptors (PRR) and toll-like receptors (TLRs) leading to direct or indirect elimination of the pathogens [90]. During the infection of RNA viruses including SARs-CoV-2, TLR and PRR are activated through interactions with viral single stranded RNA (ssRNA) or double stranded (dsRNA). PRR and TLR activation causes downstream signaling cascades that lead to expression of anti-viral proteins and cytokines such as TNF-α, IL1, IL6, IL18, and IFN I/III [76]. The most important of these are the interferons, which have been shown to play important roles in anti-viral defenses through enhancing production of anti-viral proteins, activation of natural killer (NK) cells, and presentation of viral proteins to T-cells through upregulation of major histocompatibility complex class I (MHC I) [76, 80]. Similarly, SARS-CoV-2 has been shown to be sensitive to IFN I and IFN III pre-treatments; however, the specific mechanisms and interactions are still unknown [76]. As IFN I and IFN III play an important role in viral defenses, studying previous coronaviruses, such as SARS-CoV and MERS-CoV, have shown that most coronaviruses have developed mechanisms to inhibit IFN I signaling [76]. Similarly, an impaired and reduced IFN I signaling has been seen in COVID-19 patients with severe SARS-CoV-2 infections compared to patients with mild or moderate cases [76]. While failure of an early IFN I response leads to more severe disease correlation, it has also been observed that incubation timing is also important as IFN induces ACE2 upregulation causing more pathological effects and cytokine release in COVID-19 infection [76].
Therefore, myeloid responses from macrophages and APCs such as Langerhans and dendritic cells play an important role in the initial response to viral infections. It is reasonable to believe that the reduced IFN I signaling cascades seen in severe cases of coronavirus, especially those in SARS-CoV-2, is due to mechanisms developed by coronavirus to evade or disrupt activation of anti-viral defenses in myeloid cells. One mechanism suggested that coronavirus escapes the immune system through protection of their RNA by gaining membrane-bound shields against interactions with host PRRs or TLRs [76]. Another mechanism involves disguising viral RNA with guanosine caps and methylated 5′ ends to resemble our own host mRNA to avoid detection through PRRs and TLRs [76]. Other mechanisms developed could be the direct interference with downstream signaling from PRR and TLR activation, which ultimately causes increased viral replication through reduced IFN I and IFN III transcription and release [76]. While initially protective in the early stages of infection, it seems that unregulated activation of myeloid cells such as neutrophils and macrophages cause increased inflammatory cascades, cytokine release, and tissue damage. In previous studies involving murine SARS-CoV-1, impaired IFN-I signaling leads to increased myeloid response by monocyte and macrophages that promoted increased cytokine release and recruitment of pro-inflammatory immune cells [76]. When the myeloid response systems become dysfunctional or hyperactivated, severity of SARS-CoV-2 infection occurs through increased viral loads and CRS [76]. While the current research in COVID-19 focuses mainly on lung involvement and severity of lung pathology, further investigations are warranted to confidently demonstrate myeloid responses and their effect on the severity of SARS-CoV-2 infections in the eye.
Importantly, viral mediated activation of inflammasomes, such as NLRP3, play a key response in initial responders such as macrophages and dendritic cells [76, 91]. There are several molecules that lead to the activation of NLRP3 where different viral structures are predominant resulting in a proinflammatory response. Activation of NLRP3 leads to activation of procaspase 1, which in turn leads to the proteolytic activation of IL-1β and IL-18 to initiate pyroptosis [76, 91]. NLRP3 activation has been suggested as a mechanism for other viral infections such as influenza, adenovirus, HSV, and SARS-CoV-1 [76, 91]. Previously it was shown that defective NLRP3 led to defective inflammatory responses, increased CD4 + infiltrates, and higher levels of neutrophils with concomitantly increased levels of IL-1β, IL-18, proinflammatory cytokines, and chemokines. Therefore, NLRP3 plays an aggravative effect on inflammatory responses as defective NLRP3 lead to increased susceptibility of viral infections and increased viral loads [91]. As inflammasome contributes to proinflammatory responses, it is reasonable that inflammasome activation likely is contributory to cytokine storm and CRS noticed in COVID-19 patients. More studies are warranted to unveil the influence of inflammasome activations on SARS-CoV-2 infection of the eye.
Along with the myeloid response, innate lymphoid cells (ILCs) play an important part in viral defense. ILC is divided into cytotoxic NK cells and non-cytotoxic helper ILCs. NK cells are lymphocytes that are devoid of T-cell receptors (TCR) or B-cell receptors (BCR) that function in clearing abnormal cells such as tumors, virally infected cells, antibody-mediated cell destruction, and undifferentiated cells [76, 80]. Previous studies have shown that NK cells are recruited to lung tissue in influenza and conjunctival stroma in dry eye disease [76, 92]. NK cells secrete TNF-α and IFN-α for inflammatory response through activation of phagocytosis [80, 88]. NK cell are first responders to viral eye infections as they are destined to signal through IFN-γ and cytotoxic molecules such as perforin and granzymes, which allow for rapid targeting of pathologic antigens. The cytotoxicity is achieved through activation of Fc receptor recognition of antibodies bound to viral antigens and/or ligand recognition on NK cell activating receptors [76]. Like the myeloid response, NK cells are important for early containment and elimination of viral threats in the eye. However, early reports regarding NK mediated ADCC and NK cell dysfunction in COVID-19 patients has suggested its contributory role to cytokine storm. Multiple early reports of COVID-19 revealed reduced NK cells in the peripheral blood, which has been associated with increased COVID-19 severity and morbidity [76]. In addition, the NK cells harvested ex vivo from the peripheral blood of COVID-19 patients have been shown to have reduced cytotoxicity, chemokine production, and reduced granzyme B, granulysin, IFN-γ, and TNF-α production [76]. Similarly, COVID-19 patients with higher plasma concentrations of IL-6 and TNF-α displayed reduced NK cell numbers and impaired cytolytic functions, suggesting that proinflammatory states with large myeloid responses contribute to impaired NK cell function and reduced elimination of SARS-CoV-2 infected cells [76].
Helper ILCs such as tissue resident type 1 innate lymphoid cells (ILC1) play an early role in host defense against viral infections. Shown in previous mouse cytomegalovirus (MCMV) models, the ILCs are the primary producers of IFN-γ prior to initiation of immune response [90]. Tissue resident XCR1 dendritic cells produce early IL-12 to increase ILC1 production of IFN-γ via STAT-4 dependent manner [90]. ILC1 production of IFN-γ is critical to limit viral replication and infection as evident from increased viral load and decreased IFN-γ production in the absence of ILC1 [90]. Previous studies found that ILC2 were involved in restoration of damaged lung tissues following influenza infection [93]. Similarly, in ILC2 depleted mice models that suffered corneal abrasions, it was revealed that improvement in corneal epithelial wound healing occurred upon reintroduction of ILC2 [93]. ILCs play an important role in host defense against viral infections and tissue healing, the reports on their role in SARS-CoV-2 infections are unavailable. Since the different subsets of ILCs are involved in both proinflammatory and anti-inflammatory processes during viral infections in the lungs and eye, it is important to study their interactions in SARS-CoV-2 infections. Further research is warranted to examine their roles in SARS-CoV-2 eye infections and clinical course or increased inflammatory response with CRS [76, 90].
Initial viral infection and activation of TCRs lead to virus-specific effector T cells differentiation, which help to establish an effective immune response against the acute viral infections and facilitate memory response for an efficient secondary response. Langerhans cells in corneal tissues present viral antigens through MHC I or MHC class II receptors to activate CD8 + cytotoxic or CD4 + T-helper cells, respectively. Once activated, CD8 + T-cells act to eliminate infected cells and reduce viral load, while CD4 + T-cells secretes cytokines to activate other effectors cells, such as aiding B-cells in antibody production or recruitment of macrophages to prevent viral threats [80]. CD8 + T cells play a critical role in defending intracellular pathogens, especially viral infected cells, during the later phase of the acute viral infections [94]. During acute phases, the CD8 + T-cells proliferation and upregulation of granzymes and perforins bolster their cytolytic function. In addition, CD8 + secretes IFN-γ to enhance the clearance and processing of viral peptides by APCs, which serves to increase overall viral peptides loading onto MHC I to further facilitate viral elimination [88]. When MHC I is downregulated or virus-MHC I interaction is disrupted, viral clearance suffers resulting in increased viral load [94]. In contrast, CD4 + cells play a regulatory role through secretions of cytokines that recruit other effector cells such as macrophages for inflammatory responses or Tregs in anti-inflammatory response. CD4 + cells differentiate into Th1, Th2, Th17, and Tregs to modulate the immune response depending on the needs and the cytokines in the environment. As T-cells play an important role in bridging the innate and adaptive immune system, it is important to understand its role in COVID19 infection. Many studies regarding COVID-19 as well as SARS-CoV-1 have reported lymphopenia and severely reduced amounts of CD4 + and CD8 + T-cells in more severe cases of COVID-19 compared to mild and moderate counterparts [76]. There is an association between decreased CD8 + T-cell levels and severity and mortality of COVID-19 patients in ICU. Also, lymphopenia is associated with higher IL-6, IL-10, and TNF-α levels. However, unlike viral infection of T-cells in MERS-CoV, the mechanism for lymphopenia in SARS-CoV-2 remains unknown. Cytokines such as IFN-I and TNF-α result in the retention of T-cells into lymphoid organs and attachment onto endothelium, thus preventing their circulation in the blood [76]. However, recent studies have suggested that IL-6 and Fas-FasL interactions trigger lymphocyte death causing low levels of lymphocytes in peripheral blood obtained from COVID-19 patients [76].
Furthermore, dysfunctional, or hyperactive T-cell response has been implicated in the CRS as the CD4 + T-cells resulted in IL-6 and IFN-γ production in patients with severe SARS-CoV-2 infections. Also, previous reports have suggested that CD8 + T-cells are less cytotoxic, with dysfunctional production of Granzyme B and degranulation in severe COVID19 [76]. Contrastingly, other reports have suggested increased Granzyme B and perforin levels in severely sick cases of COVID-19. In addition, Treg dysfunction and reduced Treg numbers were observed in severe COVID-19 patients [76]. Moreover, the loss of Treg anti-inflammatory response coupled with increased myeloid, lymphoid, and T-cell hyperactivity is a recipe for CRS and inflammatory overdrive leading to increased severity and mortality in SARS-CoV-2 infections.
Conclusions
Based on the existing literature, we assert that the eyes act as an additional entry point for SARS-CoV-2. While current data is controversial on the feasibility of viral RNA isolation from the tear films of COVID-19 patients, SARS-CoV-2 is likely to be found in the ocular secretions of high-viral load patients with active conjunctivitis. The infected ocular fluid serves as a nidus of replication and vector for transmission to conjunctival, corneal, scleral, and nasolacrimal tissue. Like other respiratory diseases, SARS-CoV-2 can persist in the tear film despite antimicrobial peptides and progresses to the respiratory or gastrointestinal tracts through the nasolacrimal duct. Further investigations are recommended to unveil the ocular transmission of SARS-CoV-2 and to identify novel targets for intervention. Nonetheless, the possibilities of SARS-CoV-2 transmission cannot be neglected and is necessary to develop strategies to prevent virus spread via ocular route during this pandemic era.
Author contributions
Conception and design: FGT, DKA; Literature search, collection of the scientific information, analysis and interpretation of the data: GD, KL, FGT, and DKA; Drafting of the article: GD, KL, FGT, and DKA; Critical revision and editing of the article for important intellectual content: FGT, DRW, DKA; Final approval of the submitted article: GD, KL, FGT, DRW, and DKA.
Funding
This research work was supported by NIH-NHLBI Grants R01HL147662 and R01HL144125 of DK Agrawal. The content of this original review article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Data availability
Not applicable; all information is gathered from published articles.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? a review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28:391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madhugiri R, Fricke M, Marz M, Ziebuhr J. Coronavirus cis-acting RNA elements. Adv Virus Res. 2016;96:127–163. doi: 10.1016/bs.aivir.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snijder EJ, van der Meer Y, Zevenhoven-Dobbe J, Onderwater JJ, van der Meulen J, Koerten HK, Mommaas AM. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J Virol. 2006;80:5927–5940. doi: 10.1128/jvi.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woo PC, Lau SK, Lam CS, Lau CC, Tsang AK, Lau JH, Bai R, Teng JL, Tsang CC, Wang M, Zheng BJ, Chan KH, Yuen KY. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol. 2012;86:3995–4008. doi: 10.1128/jvi.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han Q, Lin Q, Ni Z, You L. Uncertainties about the transmission routes of 2019 novel coronavirus. Influenza Other Respir Viruses. 2020;14:470–471. doi: 10.1111/irv.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayaweera M, Perera H, Gunawardana B, Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res. 2020;188:109819. doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atri D, Siddiqi HK, Lang JP, Nauffal V, Morrow DA, Bohula EA. COVID-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020 doi: 10.2807/1560-7917.Es.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laxminarayan R, Wahl B, Dudala SR, Gopal K, Mohan C, Neelima S, Jawahar Reddy KS, Radhakrishnan J, Lewnard JA. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science. 2020 doi: 10.1126/science.abd7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grayson SA, Griffiths PS, Perez MK, Piedimonte G. Detection of airborne respiratory syncytial virus in a pediatric acute care clinic. Pediatr Pulmonol. 2017;52:684–688. doi: 10.1002/ppul.23630. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Wei J, Li Y, Ooi A. Evaporation and dispersion of respiratory droplets from coughing. Indoor Air. 2017;27:179–190. doi: 10.1111/ina.12297. [DOI] [PubMed] [Google Scholar]
- 16.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323:1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 18.Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan Q, Pan Y, Wu Q, Liu S, Song X, Xie Z, Liu Y, Zhao L, Wang Z, Zhang Y, Wu Z, Guan L, Lv X. Anal swab findings in an infant with COVID-19. Pediatr Investig. 2020;4:48–50. doi: 10.1002/ped4.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christian MD, Poutanen SM, Loutfy MR, Muller MP, Low DE. Severe acute respiratory syndrome. Clin Infect Dis. 2004;38:1420–1427. doi: 10.1086/420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/s0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiappetta S, Sharma AM, Bottino V, Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. Int J Obes. 2020;44:1790–1792. doi: 10.1038/s41366-020-0597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 25.Kopel J, Perisetti A, Roghani A, Aziz M, Gajendran M, Goyal H. Racial and Gender-Based Differences in COVID-19. Front Public Health. 2020 doi: 10.3389/fpubh.2020.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 27.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020 doi: 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guzzi PH, Mercatelli D, Ceraolo C, Giorgi FM. Master regulator analysis of the SARS-CoV-2/human interactome. J Clin Med. 2020 doi: 10.3390/jcm9040982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luftig MA. Viruses and the DNA damage response: activation and antagonism. Annu Rev Virol. 2014;1:605–625. doi: 10.1146/annurev-virology-031413-085548. [DOI] [PubMed] [Google Scholar]
- 33.Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, Wang LF, Gao H, Wang Y, Dong CF, Li YJ, Xie XJ, Feng C, Liu L. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48:773–777. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doyen D, Moceri P, Ducreux D, Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395:1516. doi: 10.1016/s0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, Liu FF, Zhang K, Zhang C. Angiotensin-converting enzyme 2 and angiotensin 1–7: novel therapeutic targets. Nat Rev Cardiol. 2014;11:413–426. doi: 10.1038/nrcardio.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1–7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol. 2008;28:25–33. doi: 10.1159/000108758. [DOI] [PubMed] [Google Scholar]
- 39.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/circresaha.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gavriatopoulou M, Korompoki E, Fotiou D, Ntanasis-Stathopoulos I, Psaltopoulou T, Kastritis E, Terpos E, Dimopoulos MA. Organ-specific manifestations of COVID-19 infection. Clin Exp Med. 2020 doi: 10.1007/s10238-020-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Léonard-Lorant I, Delabranche X, Séverac F, Helms J, Pauzet C, Collange O, Schneider F, Labani A, Bilbault P, Molière S, Leyendecker P, Roy C, Ohana M. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-Dimer levels. Radiology. 2020;296:E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agbuduwe C, Basu S. Haematological manifestations of COVID-19: from cytopenia to coagulopathy. Eur J Haematol. 2020;105:540–546. doi: 10.1111/ejh.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lao WP, Imam SA, Nguyen SA. Anosmia, hyposmia, and dysgeusia as indicators for positive SARS-CoV-2 infection. World J Otorhinolaryngol Head Neck Surg. 2020 doi: 10.1016/j.wjorl.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yaghi S, Ishida K, Torres J, Grory BM, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, Sanger M, Kim S, Scher E, Dehkharghani S, Wachs M, Tanweer O, Volpicelli F, Bosworth B, Lord A, Frontera J. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, Anheim M, Meziani F. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma N, Li P, Wang X, Yu Y, Tan X, Chen P, Li S, Jiang F. Ocular manifestations and clinical characteristics of children with laboratory-confirmed COVID-19 in Wuhan, China. JAMA Ophthalmology. 2020;138:1079–1086. doi: 10.1001/jamaophthalmol.2020.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen L, Deng C, Chen X, Zhang X, Chen B, Yu H, Qin Y, Xiao K, Zhang H, Sun X. Ocular manifestations and clinical characteristics of 534 cases of COVID-19 in China: a cross-sectional study. medRxiv. 2020 doi: 10.1101/2020.03.12.20034678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, Wu K. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheema M, Aghazadeh H, Nazarali S, Ting A, Hodges J, McFarlane A, Kanji JN, Zelyas N, Damji KF, Solarte C. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19) Can J Ophthalmol. 2020;55:e125–e129. doi: 10.1016/j.jcjo.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watsky MA, Jablonski MM, Edelhauser HF. Comparison of conjunctival and corneal surface areas in rabbit and human. Curr Eye Res. 1988;7:483–486. doi: 10.3109/02713688809031801. [DOI] [PubMed] [Google Scholar]
- 53.Coroneo MT. The eye as the discrete but defensible portal of coronavirus infection. Ocul Surf. 2020 doi: 10.1016/j.jtos.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belser JA, Rota PA, Tumpey TM. Ocular tropism of respiratory viruses. Microbiol Mol Biol Rev. 2013;77:144–156. doi: 10.1128/mmbr.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Napoli PE, Nioi M, d’Aloja E, Fossarello M. The ocular surface and the coronavirus disease 2019: does a dual ‘ocular route’ exist? J Clin Med. 2020 doi: 10.3390/jcm9051269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arora R, Goel R, Kumar S, Chhabra M, Saxena S, Manchanda V, Pumma P. Evaluation of SARS-CoV-2 in tears of patients with moderate to severe COVID-19. Ophthalmology. 2020 doi: 10.1016/j.ophtha.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92:589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paulsen F. The human nasolacrimal ducts. Adv Anat Embryol Cell Biol. 2003;170:1–106. doi: 10.1007/978-3-642-55643-2_1. [DOI] [PubMed] [Google Scholar]
- 61.Bitko V, Musiyenko A, Barik S. Viral infection of the lungs through the eye. J Virol. 2007;81:783–790. doi: 10.1128/jvi.01437-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willcox MD, Walsh K, Nichols JJ, Morgan PB, Jones LW. The ocular surface, coronaviruses and COVID-19. Clin Exp Optom. 2020;103:418–424. doi: 10.1111/cxo.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nuzzi R, Carucci LL, Tripoli F. COVID-19 and ocular implications: an update. J Ophthalmic Inflamm Infect. 2020;10:20. doi: 10.1186/s12348-020-00212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leonardi A, Rosani U, Brun P. Ocular surface expression of SARS-CoV-2 receptors. Ocul Immunol Inflamm. 2020;28:735–738. doi: 10.1080/09273948.2020.1772314. [DOI] [PubMed] [Google Scholar]
- 65.Lange C, Wolf J, Auw-Haedrich C, Schlecht A, Boneva S, Lapp T, Horres R, Agostini H, Martin G, Reinhard T, Schlunck G. Expression of the COVID-19 receptor ACE2 in the human conjunctiva. J Med Virol. 2020 doi: 10.1002/jmv.25981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grajewski RS, Rokohl AC, Becker M, Dewald F, Lehmann C, Fätkenheuer G, Cursiefen C, Klein F, Heindl LM. A missing link between SARS-CoV-2 and the eye?: ACE2 expression on the ocular surface. J Med Virol. 2020 doi: 10.1002/jmv.26136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li S, Li D, Fang J, Liu Q, Sun X, Xu G, Cao W. COVID-19 receptor ACE2 is expressed in human conjunctival tissue, expecially in diseased conjunctival tissue. medRxiv. 2020 doi: 10.1101/2020.05.21.20109652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma D, Chen CB, Jhanji V, Xu C, Yuan XL, Liang JJ, Huang Y, Cen LP, Ng TK. Expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in human primary conjunctival and pterygium cell lines and in mouse cornea. Eye (Lond) 2020;34:1212–1219. doi: 10.1038/s41433-020-0939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collin J, Queen R, Zerti D, Dorgau B, Georgiou M, Djidrovski I, Hussain R, Coxhead JM, Joseph A, Rooney P, Lisgo S, Figueiredo F, Armstrong L, Lako M. Co-expression of SARS-CoV-2 entry genes in the superficial adult human conjunctival, limbal and corneal epithelium suggests an additional route of entry via the ocular surface. Ocul Surf. 2020 doi: 10.1016/j.jtos.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surf. 2020;18:537–544. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keddie S, Ziff O, Chou MKL, Taylor RL, Heslegrave A, Garr E, Lakdawala N, Church A, Ludwig D, Manson J, Scully M, Nastouli E, Chapman MD, Hart M, Lunn MP. Laboratory biomarkers associated with COVID-19 severity and management. Clin Immunol. 2020;221:108614. doi: 10.1016/j.clim.2020.108614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hodges G, Pallisgaard J, Schjerning Olsen AM, McGettigan P, Andersen M, Krogager M, Kragholm K, Køber L, Gislason GH, Torp-Pedersen C, Bang CN. Association between biomarkers and COVID-19 severity and mortality: a nationwide Danish cohort study. BMJ Open. 2020;10:e041295. doi: 10.1136/bmjopen-2020-041295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, Levantovsky R, Malle L, Moreira A, Park MD, Pia L, Risson E, Saffern M, Salomé B, Esai Selvan M, Spindler MP, Tan J, van der Heide V, Gregory JK, Alexandropoulos K, Bhardwaj N, Brown BD, Greenbaum B, Gümüş ZH, Homann D, Horowitz A, Kamphorst AO, Curotto de Lafaille MA, Mehandru S, Merad M, Samstein RM. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsai A, Diawara O, Nahass RG, Brunetti L. Impact of tocilizumab administration on mortality in severe COVID-19. Sci Rep. 2020;10:19131. doi: 10.1038/s41598-020-76187-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ciabattini A, Garagnani P, Santoro F, Rappuoli R, Franceschi C, Medaglini D. Shelter from the cytokine storm: pitfalls and prospects in the development of SARS-CoV-2 vaccines for an elderly population. Semin Immunopathol. 2020 doi: 10.1007/s00281-020-00821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Akpek EK, Gottsch JD. Immune defense at the ocular surface. Eye. 2003;17:949–956. doi: 10.1038/sj.eye.6700617. [DOI] [PubMed] [Google Scholar]
- 81.Yamaguchi T. Inflammatory response in dry eye. Investig Ophthalmol Visual Sci. 2018;59:DES192–DES199. doi: 10.1167/iovs.17-23651. [DOI] [PubMed] [Google Scholar]
- 82.Na KS, Hwang KY, Lee HS, Chung SH, Mok JW, Joo CK. Wakayama symposium: interface between innate and adaptive immunity in dry eye disease. BMC Ophthalmol. 2015;15(Suppl 1):159. doi: 10.1186/s12886-015-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mizoguchi S, Iwanishi H, Arita R, Shirai K, Sumioka T, Kokado M, Jester JV, Saika S. Ocular surface inflammation impairs structure and function of meibomian gland. Exp Eye Res. 2017;163:78–84. doi: 10.1016/j.exer.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mohammed I, Said DG, Dua HS. Human antimicrobial peptides in ocular surface defense. Prog Retin Eye Res. 2017;61:1–22. doi: 10.1016/j.preteyeres.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 85.Fini ME, Jeong S, Gong H, Martinez-Carrasco R, Laver NMV, Hijikata M, Keicho N, Argüeso P. Membrane-associated mucins of the ocular surface: new genes, new protein functions and new biological roles in human and mouse. Prog Retin Eye Res. 2020;75:100777. doi: 10.1016/j.preteyeres.2019.100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Callaghan RJ. The pathogenesis of Staphylococcus aureus eye infections. Pathogens. 2018 doi: 10.3390/pathogens7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Santeford A, Wiley LA, Park S, Bamba S, Nakamura R, Gdoura A, Ferguson TA, Rao PK, Guan JL, Saitoh T, Akira S, Xavier R, Virgin HWT, Apte RS. Impaired autophagy in macrophages promotes inflammatory eye disease. Autophagy. 2016;12:1876–1885. doi: 10.1080/15548627.2016.1207857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang J, Liu H, Wei B. Immune response of T cells during herpes simplex virus type 1 (HSV-1) infection. J Zhejiang Univ Sci B. 2017;18:277–288. doi: 10.1631/jzus.B1600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller D, Iovieno A. The role of microbial flora on the ocular surface. Curr Opin Allergy Clin Immunol. 2009;9:466–470. doi: 10.1097/ACI.0b013e3283303e1b. [DOI] [PubMed] [Google Scholar]
- 90.Weizman OE, Adams NM, Schuster IS, Krishna C, Pritykin Y, Lau C, Degli-Esposti MA, Leslie CS, Sun JC, O’Sullivan TE. ILC1 confer early host protection at initial sites of viral infection. Cell. 2017;171:795–808.e12. doi: 10.1016/j.cell.2017.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gimenez F, Bhela S, Dogra P, Harvey L, Varanasi SK, Jaggi U, Rouse BT. The inflammasome NLRP3 plays a protective role against a viral immunopathological lesion. J Leukoc Biol. 2016;99:647–657. doi: 10.1189/jlb.3HI0715-321R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Y, Chauhan SK, Saban DR, Sadrai Z, Okanobo A, Dana R. Interferon-γ-secreting NK cells promote induction of dry eye disease. J Leukoc Biol. 2011;89:965–972. doi: 10.1189/jlb.1110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu J, Xiao C, Wang H, Xue Y, Dong D, Lin C, Song F, Fu T, Wang Z, Chen J, Pan H, Li Y, Cai D, Li Z. Local group 2 innate lymphoid cells promote corneal regeneration after epithelial abrasion. Am J Pathol. 2017;187:1313–1326. doi: 10.1016/j.ajpath.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 94.Berry R, Watson GM, Jonjic S, Degli-Esposti MA, Rossjohn J. Modulation of innate and adaptive immunity by cytomegaloviruses. Nat Rev Immunol. 2020;20:113–127. doi: 10.1038/s41577-019-0225-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable; all information is gathered from published articles.
Not applicable.