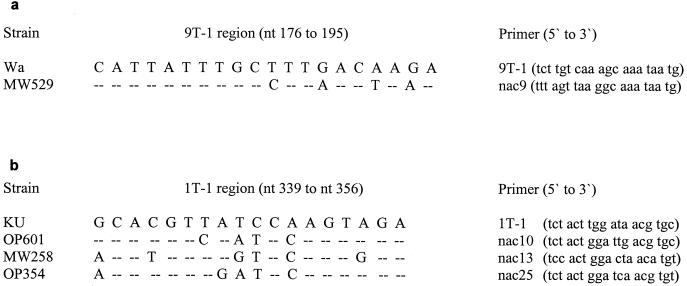Abstract
In a 2-year study of viral gastroenteritis in children in Blantyre, Malawi, the diversity of rotavirus strains was investigated by using electropherotyping, reverse transcription-PCR amplification of the VP7 and VP4 genes (G and P genotyping), and nucleotide sequencing. Of 414 rotavirus strains characterized, the following strain types were identified: P[8], G1 (n = 111; 26.8%); P[6], G8 (n = 110; 26.6%); P[8], G3 (n = 93; 22.5%); P[4], G8 (n = 31; 7.5%); P[8], G4 (n = 21; 5.1%); P[6], G3 (n = 12; 2.9%); P[6], G1 (n = 7; 1.7%); P[6], G9 (n = 3; 0.7%); P[6], G4 (n = 3; 0.7%); P[4], G3 (n = 1; 0.2%); and mixed (n = 15; 3.6%). While all strains could be assigned a G type, seven strains (1.7%) remained P nontypeable. The majority of serotype G8 strains and all serotype G9 strains had short electropherotype profiles. All remaining typeable strains had long electropherotypes. Divergent serotype G1 rotaviruses, which contained multiple base substitutions in the 9T-1 primer binding site, were commonly identified in the second year of surveillance. Serotype G2 was not identified. Overall, G8 was the most frequently identified VP7 serotype (n = 144; 34.8%) and P[8] was the most frequently detected VP4 genotype (n = 227; 54.8%). Partial sequence analysis of the VP4 gene of genotype P[8] rotaviruses identified three distinct clusters, which predominantly (but not exclusively) comprised strains belonging to a distinct VP7 serotype (G1, G3, or G4). As a result of mutations in the 1T-1 primer binding site, strains belonging to each cluster required a separate primer for efficient typing. One cluster, represented by P[8], G4 strain OP354, was highly divergent from the established Wa and F45 VP4 P[8] lineages. As is the case for some other countries, the diversity of rotaviruses in Malawi implies that rotavirus vaccines in development will need to protect against a wider panel of serotypes than originally envisioned.
In developing countries severe, dehydrating diarrhea caused by human rotavirus (HRV) results in an estimated 500,000 to 870,000 childhood deaths annually (13, 43). The routine implementation of safe and effective rotavirus vaccine programs in developing countries is expected to reduce dramatically the high level of mortality attributed to HRV, but the differing epidemiology of rotavirus in such settings may pose special challenges to successful vaccine use (6). In particular, the greater diversity of HRV strains encountered in some developing countries has implications for the formulation of rotavirus vaccines, since the original targets of these vaccines were the four most globally common HRV serotypes, G1 to G4, and some candidate vaccines were designed to provide serotype-specific (homotypic) protection (42). Thus, the first licensed vaccine, the tetravalent rhesus-human reassortant rotavirus (RRV-TV) vaccine, incorporated strains with these four most-common G-serotypes. The RRV-TV vaccine proved highly effective in preventing severe rotavirus diarrhea in infants and young children (3, 35, 49, 52, 53).
Recent evidence suggests that serotypes other than G1 to G4 are epidemiologically important in some developing countries (e.g., serotype G5 in Brazil [23, 40], serotype G9 in India [50] and Bangladesh [59], and serotype G8 in Malawi [10]). Furthermore, the recent identification of serotype G9 rotaviruses in the United States (51), the United Kingdom (8), Australia (48), and France (4) indicates that some developed countries may also harbor previously uncommon serotypes. Thus, future HRV vaccines may need to protect against additional serotypes in order to provide broadly effective protection.
Rotaviruses are nonenveloped viruses that possess a segmented double-stranded RNA (dsRNA) genome, which is enclosed in a triple-layered protein capsid. The 11 segments can be separated by using electrophoresis, and two major strain types (short and long electropherotypes) can be differentiated according to differences in the relative migration patterns of segments 10 and 11. The middle layer of the mature rotavirus particle, comprised exclusively of VP6 (encoded by RNA segment 6) specifies rotavirus group and subgroup. Two major subgroups are recognized among group A HRVs (I and II) but some HRVs possess both subgroup I and II antigens, and others possess neither antigen (31). The two outer capsid proteins, VP7 (encoded by segment 7, 8, or 9) and VP4 (encoded by segment 4), each induce neutralizing antibodies; thus, a dual typing system classifies HRVs by VP7 serotype (or G type, since VP7 is a glycoprotein) and VP4 serotype (or P type, since VP4 is cleaved by proteases). Fourteen VP7 serotypes have been identified by cross-neutralization studies, which correspond to their respective genotypes determined by molecular analyses. Eleven VP4 serotypes have similarly been identified, each of which has been assigned a “genotype” using molecular analysis (15, 45). Since at least nine further VP4 genotypes have not yet been assigned a serotype, a complete description of VP4 requires both molecular and serologic characterization. By convention, VP4 genotype is enclosed within square brackets (for a review of rotavirus structure and strain nomenclature, see reference 15).
Field studies of rotavirus strain diversity have traditionally employed VP7-specific neutralizing monoclonal antibodies in enzyme immunoassays (EIAs) to detect the common VP7 serotypes in fecal samples (56). Since a substantial number of strains remain nontypeable by EIA, and because serologic methods to detect common VP4 serotypes are not routinely available, molecular methods (reverse transcription-PCR [RT-PCR] and probe hybridization) have been developed for both VP7 and VP4 typing (17, 18, 24, 27, 39).
Although the genes encoding VP7 and VP4 segregate independently (32) and numerous combinations of G and P types are theoretically possible, early studies suggested that four strains predominate (19). Thus, HRVs with G serotypes G1, G3, and G4 are typically associated with P[8] VP4 specificity (and possess long electropherotype profiles and the subgroup II antigen), and serotype G2 HRVs normally possess the P[4] VP4 genotype (and have short electropherotype profiles and the subgroup I antigen). However, recent studies in developing countries that have extensively characterized large numbers of HRVs have demonstrated much greater strain diversity than previously appreciated (50, 58, 59). In Bangladesh, the recognition of multiple combinations of G and P types and atypical combinations of subgroup and electropherotype, especially among serotype G9 strains, indicates that genomic reassortment is a major driving force in the generation of rotavirus strain diversity (59).
As part of a recently completed 2-year study of viral gastroenteritis in Blantyre, Malawi, we sought to investigate the diversity of rotaviruses detected in children with diarrhea (10). We were especially interested in examining by nucleotide sequencing those strains that failed to be typed with established RT-PCR methods. In so doing, we gained a more complete understanding of the extent of strain diversity in this region and enhanced our ability to routinely type Malawi strains.
MATERIALS AND METHODS
Fecal specimens.
The study was conducted at the Queen Elizabeth Central Hospital, Blantyre, Malawi, from July 1997 to June 1999, as previously described (10). Fecal specimens were collected from children under 5 years old with acute, dehydrating diarrhea who either were given oral rehydration therapy at the “Under 5” clinic or were admitted to the pediatric wards where they received oral rehydration therapy and/or intravenous fluid replacement. Rotavirus testing was performed the same day as specimen collection, and fecal samples containing rotavirus were then stored at −80°C until analyzed further.
Rotavirus detection, subgrouping, and electropherotyping.
Rotavirus antigen was detected using the Rotaclone EIA kit (Meridian Diagnostics, Cincinnati, Ohio). Selected rotaviruses were subgrouped from stool specimens by EIA, using subgroup-specific monoclonal antibodies (26). Rotavirus dsRNA was extracted using a guanidine and silica method (18), modified from that of Boom et al. (5). To determine rotavirus electropherotype (short or long), the dsRNA was electrophoresed on a 10% polyacrylamide gel and then stained with silver nitrate as previously described (29). A mixed rotavirus electropherotype was defined by the presence of a typical group A rotavirus RNA profile comprising more than 11 RNA segments. Rotavirus genome profiles that could not be clearly categorized as short or long electropherotypes were labeled indeterminate.
RT-PCR genotyping.
G and P typing employed nested, multiplex RT-PCR and used consensus and type-specific primers described previously (12, 18, 24). Briefly, for G typing, consensus primers 9con1 and 9con2 were used in a first-round RT-PCR (30 cycles) to generate a 905-bp VP7 gene fragment; 9con1 was then used in a second-round PCR (20 cycles) with type-specific primers 9T-1 (G1), 9T-2 (G2), 9T-3P (G3), 9T-4 (G4), and 9T-9B (G9). Since serotype G8 strains were detected frequently in the early part of this study, the G8-specific typing primer MW8 was added to this primer cocktail (10). For P typing, consensus primers con2 and con3 were used in a first-round RT-PCR (30 cycles) to generate an 877-bp fragment of gene 4; con3 was then used in a second-round PCR (20 cycles) with type-specific primers 1T-1 (P[8]), 2T-1 (P[4]), 3T-1 (P[6]), 4T-1 (P[9]), and 5T-1 (P[10]). PCR products were resolved by electrophoresis on a 2% agarose gel, stained with ethidium bromide, and visualized under UV illumination.
Investigation of nontypeable strains.
As we began typing year 2 strains it became apparent that, using our existing approach, a large number were nontypeable with respect to their G and/or P type. In order to determine whether this reflected genetic variation in primer binding sites of common strains or uncommon G and P types, nontypeable strains were further investigated by nucleotide sequencing of first-round RT-PCR products. The RT-PCR products were purified by using spin columns (Qiagen, Chatsworth, Calif.) and sequenced by using the dideoxynucleotide chain termination method with the BigDye sequencing kit (Applied Biosystems, Inc., Foster City, Calif.) and a model 377 automated DNA sequencer (Applied Biosystems, Inc.). Sequences were aligned with DNASTAR (Madison, Wis.), and phylogenetic trees were drawn by using PHYLIP (version 3.5c; copyright, J. Felsenstein and the University of Washington) and CLUSTALX (European Molecular Biology Laboratory, Heidelberg, Germany).
For G typing, a large number of strains either gave weak G1 products or failed to give a detectable product. The full-length VP7 gene of a nontypeable strain, MW529, was amplified by using the primer pair beg9-end9 (24), and sequence analysis demonstrated 97.8% nucleotide identity to the VP7 gene of an Australian serotype G1 rotavirus, G194A (14). Significantly, four base substitutions were identified in the region of the G1 typing primer 9T-1, compared with the prototype strain Wa from which the primer was designed (Fig. 1a). Therefore, an alternative primer (nac9) was designed in the 9T-1 region, which generated consistently stronger G1-specific products when substituted for 9T-1 in the second-round PCR (data not shown). Primer nac9 did not cross-prime with Malawi serotype G3, G4, G8, or G9 strains or with standard serotype P[4], G2 strain DS-1 (data not shown).
FIG. 1.
(a) Nucleotide changes in VP7 of Malawi serotype G1 strain MW529 in the 9T-1 region compared to prototype serotype G1 strain Wa. (b) Nucleotide changes in VP4 of Malawi P[8] strains OP601, MW258, and OP354 in the IT-1 region compared to prototype genotype P[8] strain KU.
Since a large number of strains generated weak P[8] products, or failed to be P typed, con2-con3 gene 4 fragments (representing nt 11 to 887) of nine P nontypeable strains were sequenced and compared with three strains that typed with conventional P[8] typing primer 1T-1 (Table 1). Where possible, strains were selected for sequencing which were collected at different times in the study, although the choice was limited by a failure to obtain con2-con3 product for some strains. Four internal primers were used in addition to con2 and con3 to sequence each con2-con3 fragment. Sequence analysis demonstrated that the 12 strains comprised three groups of genotype P[8] viruses, each with multiple (and distinct) base changes in the region of the 1T-1 P[8] typing primer, compared with prototype strain KU (Fig. 1b). Using sequence data of representative strains OP601, MW258, and OP354, three additional P[8] typing primers were designed in the 1T-1 region (respectively, nac10, nac13, and nac25), and these were added separately to the typing primer cocktail used in the second-round PCR. Primers nac10 and nac25 did not cross-prime with Malawi genotype P[4] or P[6] strains. We did observe cross-priming of primer nac13 with Malawi genotype P[4] strains; therefore, we confirmed by nucleotide sequencing the P[8] specificity of each strain typed by this primer (data not shown).
TABLE 1.
Genotype P[8] strains sequenced in this study
| Strain designationa | G serotype | Date of collection | P[8] primerb |
|---|---|---|---|
| OP601 | G1 | 27 May 1999 | nac10 |
| MW279 | G1 | 11 February 1998 | 1T-1 |
| OP351 | G1 | 4 August 1998 | nac10 |
| MW258 | G3 | 2 February 1998 | nac13 |
| MW194 | G3 | 4 December 1997 | 1T-1 |
| MW434 | G3 | 16 June 1998 | nac13 |
| OP371 | G3 | 3 September 1998 | 1T-1 |
| OP498 | G3 | 4 January 1999 | nac10 |
| OP354 | G4 | 6 August 1998 | nac25 |
| MW670 | G4 | 17 February 1999 | nac25 |
| OP511 | G4 | 13 January 1999 | nac13 |
| OP530 | G4 | 29 January 1999 | nac25 |
Strains in boldface type represent the prototype strains from which the alternative P[8] typing primers were designed.
Primer which successfully typed the corresponding strain.
Ethical approval.
The study protocol was approved by the Malawi National Health Sciences Research Committee. The parents (or guardians) of each child gave written, informed consent prior to their child's enrolment in the study.
Nucleotide sequence accession numbers.
Nucleotide sequences representing con2-con3 fragments of the VP4 gene of the twelve genotype P[8] strains included in this report have been submitted to the EMBL Nucleotide Sequence Database. The accession numbers of the corresponding nucleotide sequences for the following strains are indicated in parentheses: MW194 (AJ302142), MW258 (AJ302143), MW279 (AJ302144), MW434 (AJ302145), MW670 (AJ302146), OP351 (AJ302147), OP354 (AJ302148), OP371 (AJ302149), OP498 (AJ302150), OP511 (AJ302151), OP530 (AJ302152), and OP601 (AJ302153).
RESULTS
G and P types.
A total of 414 rotaviruses detected in fecal specimens of children with diarrhea (221 specimens were collected in the first year of study) were characterized by RT-PCR genotyping (Table 2; Fig. 2.) A large number of strains could be typed effectively only after alternative primer design. For G typing, 97 strains (23.4%), 96 of which were collected in year 2, were typeable only with the alternative G1 primer nac9. For P typing, 82 strains (19.8%) failed to be typed with primer 1T-1 and could be clearly typed only with alternative P[8] primers nac10, nac13, and nac25: these comprised 47 serotype G1 strains (46 were collected in year 2), 22 serotype G3 strains (7 in year 2), and 13 serotype G4 strains (8 in year 2).
TABLE 2.
Electropherotypes and G and P types of Malawi rotavirus strains
| E-typea | n | No. of strains typed as:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P[6]
|
P[8]
|
P[4]
|
Mixedb | NTc | |||||||||
| G1 | G3 | G4 | G8 | G9 | G1 | G3 | G4 | G3 | G8 | ||||
| Long | 250 | 7 | 10 | 3 | 2 | 106 | 89 | 17 | 1 | 11 | 4 | ||
| Short | 133 | 98 | 3 | 0 | 29 | 2 | 1 | ||||||
| Mixed | 2 | 1 | 1 | ||||||||||
| Indeterminate | 29 | 2 | 10 | 5 | 4 | 4 | 1 | 1 | 2 | ||||
| No. (%) | 414 (100) | 7 (1.7) | 12 (2.9) | 3 (0.7) | 110 (26.6) | 3 (0.7) | 111 (26.8) | 93 (22.5) | 21 (5.1) | 1 (0.2) | 31 (7.5) | 15 (3.6) | 7 (1.7) |
E-type, electropherotype.
Mixed strains comprised (i) long e-type: P[6+8], G1 (5 strains); P[6+8], G3 (one strain); P[4+8], G3 (two strains); P[6], G3+8 (one strain); P[8], G1+3 (one strain); P[8], G1+3+8 (one strain); (ii) short e-type: P[6+8], G1 (one strain); P[4], G3+8 (one strain); (iii) mixed e-type: P[4+8], G3 (one strain); and (iv) indeterminate e-type: P[6], G3+8 (one strain).
All strains were nontypeable (NT) with respect to their P type. These comprised strains with (i) long e-type (G3, two strains; G4, one strain; G8, one strain); (ii) short e-type (G8, one strain); and (iii) indeterminate e-type (G3, one strain; G8, one strain).
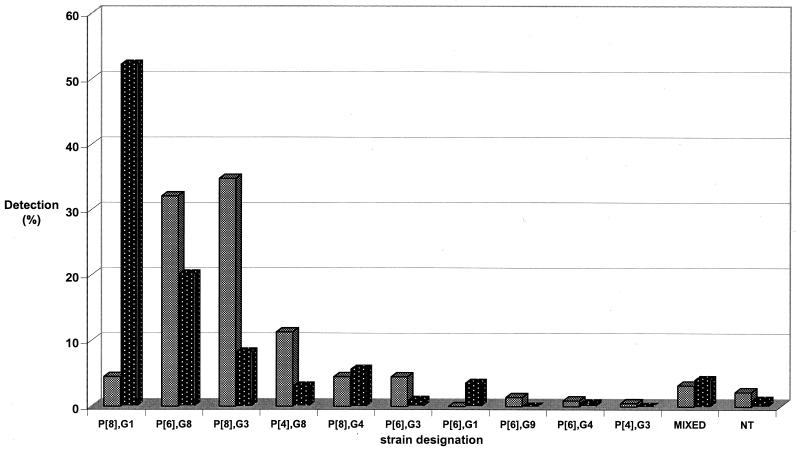
FIG. 2.
Temporal distribution of rotavirus strains in Blantyre, Malawi, from 1997 to 1999. A total of 221 strains were detected in year 1 of the study (1997 to 1998 [shaded bars]), and 193 strains were detected in year 2 (1998 to 1999 [black bars with white stippling]). NT, nontypeable.
After primer design and retesting, a total of 10 different G-P type combinations were recognized in this collection. Overall, strain P[8], G1 was the most frequently detected strain (n = 111; 26.8%), but was commonly identified only during the second year, when it represented 52.3% of strains. Two novel serotype G8 rotaviruses (strains P[6], G8 and P[4], G8) accounted for 110 (26.6%) and 31 (7.5%) of strains, respectively, and were detected frequently in both years of surveillance. Strain P[8], G3 was the third-most-common strain overall (n = 93; 22.5%). Other strains detected were P[8], G4 (n = 21; 5.1%); P[6], G3 (n = 12; 2.9%); P[6], G1 (n = 7; 1.7%); P[6], G9 (n = 3; 0.7%); P[6], G4 (n = 3; 0.7%); and P[4], G3 (n = 1; 0.2%). Fifteen strains (3.6%) comprised mixed G and/or P types. While all strains could be assigned a G type, seven strains (1.7%) remained P nontypeable.
Serotype G8 was the most commonly identified G type (n = 144; 34.8%) followed by serotypes G1 (n = 124; 30.0%), G3 (n = 113; 27.3%), G4 (n = 25; 6.0%), G9 (n = 3; 0.7%), and mixed G types (n = 5; 1.2%). Serotype G2 was not identified in any specimen.
The P[8] genotype was the most commonly identified VP4 type (n = 227; 54.8%), followed by P[6] (n = 137; 33.1%) and P[4] (n = 33; 8.0%). Ten strains (2.4%) displayed mixed P types, and seven strains (1.7%) could not be P typed.
Electropherotypes and subgroups.
An electropherotype could be assigned to 385 (93%) of the strains (Table 2). These comprised 250 (60.4%) long profiles, 133 (32.1%) short profiles, and two (0.5%) mixed profiles. No evidence of rearrangement of genome segments was identified. The short electropherotype profiles consisted of the majority of serotype G8 strains and each of three serotype G9 strains. Two P[6], G8 strains possessed long electropherotypes. All remaining typeable strains had long electropherotypes. Each strain of a representative sample of short electropherotype viruses, including serotype G8 and G9 strains, possessed subgroup I specificity (data not shown). Sufficient fecal material remained for subgrouping of one long electropherotype, P[6], G8 strain, which belonged to subgroup II. Finally, each of the three representative members of the long electropherotype, divergent P[8] strains (OP601, MW258 and OP354), belonged to subgroup II.
Phylogenetic analyses.
Phylogenetic analysis of the complete 326-amino-acid (-aa) VP7 protein of serotype G1 strain MW529 confirmed that it belonged to lineage I of serotype G1 HRVs (34) (data not shown).
The presence of three distinct clusters of P[8] strains was suggested by the typing characteristics of strains that failed to be typed with the conventional P[8] primer 1T-1. Each alternative P[8] typing primer (nac10, nac13, and nac25) effectively amplified the majority of strains with the same G-type as the strain from which the primer was designed but generally failed to give product with strains of a different G type (data not shown). However, six strains (comprising a single serotype G3 strain and five serotype G4 strains), were P typed using an alternative primer designed from a strain of a different G type. The con2-con3 RT-PCR products of two such strains, serotype G3 strain OP498 (typed by nac10) and serotype G4 strain OP511 (typed by nac13), were therefore sequenced and examined phylogenetically.
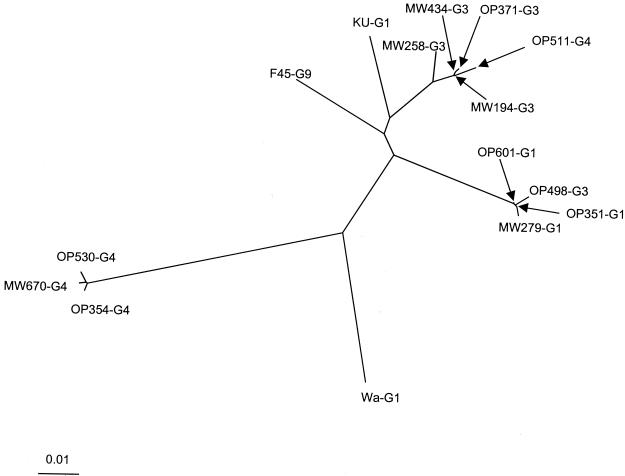
Phylogenetic analysis of partial VP4 sequences of 12 Malawi P[8] strains confirmed the presence of three clusters that each comprised strains typed by a distinct alternative primer (or by 1T-1) and predominantly (but not exclusively) comprised strains belonging to a distinct G type (G1, G3, or G4) (Fig. 3). The three groups are represented respectively by prototype strains OP601 (P[8], G1), MW258 (P[8], G3), and the highly divergent strain OP354 (P[8], G4). Malawi strains within a cluster shared greater than 98% nucleotide and amino acid identity. The VP4 sequences of serotype G3 strain OP498 and serotype G4 strain OP511 clustered with serotype G1 (OP601-like) and serotype G3 (MW258-like) rotaviruses, respectively (Fig. 3).
FIG. 3.
Neighbor-joining nucleotide distance tree for residues representing nucleotides 11 to 887 of the VP4 gene of the indicated genotype P[8] strains. The G type is indicated for each strain. Sequences were aligned by using CLUSTALX and analyzed by using the DNADIST and NEIGHBOR programs in PHYLIP. The accession numbers of the reference strains used in this analysis were L34161 (for Wa) (46), U30716 (for F45) (37), and M21014 (for KU) (57).
The VP4 gene fragments of three serotype G4 strains (represented by strain OP354), shared 99.5% nucleotide identity and 100% amino acid identity. Using >89% amino acid identity as the cutoff for strains of the same genotype (21), OP354-like strains were just classified within the P[8] genotype and displayed 89.7, 90.0, and 90.4% amino acid identities to standard genotype P[8] strains Wa, KU, and F45, respectively. They were also quite distantly related to Malawi P[8] strains that comprised the remaining two clusters (88.6 to 89.2% nucleotide identity and 90.4 to 91.4% amino acid identity to OP601-like strains and 88.3 to 89.2% nucleotide identity and 90.7 to 91.4% amino acid identity to MW258-like strains). Phylogenetic analysis (Fig. 3) confirmed that OP354-like strains form a cluster which is distinct from the two established VP4 lineages, represented by strains Wa and F45 (25). Gouvea et al. (25) described 11 aa between aa 27 and 240 which are conserved within the Wa lineage but are distinct from the F45 lineage. At these sites, OP354-like strains possess Wa-like amino acids at four positions, F45-like amino acids at five positions, and an amino acid typical of neither lineage at two positions (Fig. 4). Thus, based on partial sequence analysis of the VP4 gene, three serotype G4 Malawi strains as typified by OP354 appear to comprise a further, highly divergent P[8] lineage.
FIG. 4.
Comparison of conserved amino acids between residues 27 and 240 of VP4 of OP354-like strains compared to Wa-like and F45-like strains. (The data for Wa-like and F45-like strains are from a report by Gouvea et al. [25].)
DISCUSSION
This study has described the diversity of rotavirus strains in Blantyre from 1997 to 1999. By using nested RT-PCR amplification of VP7 and VP4 gene fragments, and by extensive investigation of rotaviruses that failed to be typed by established methods, we were able to fully type 407 of 414 (98.3%) strains. The design of additional primers enabled the effective amplification of all serotype G8 rotaviruses, all divergent serotype G1 strains, and many divergent genotype P[8] strains. Furthermore, the thorough investigation of nontypeable strains by nucleotide sequencing described the degree of divergence among these strains and their relationships to published HRV strains.
A notable finding was the marked contrast in strain distributions between the first and second years of the study, especially the detection in the second year of large numbers of divergent P[8], G1 strains. Although poorly understood, sudden changes in prevalent rotavirus serotypes are well recognized (36), and established strain typing methods may need modifying to take account not only of geographic diversity among HRVs but of temporal changes as well. In this regard, the type-specific primers currently used in RT-PCR typing methods were designed up to 10 years ago based on as few as one sequence for each G or P type, because only limited sequence data were available at that time (12, 18, 24, 27). The large amount of sequence information now available in the database, combined with the relative ease of automated compared with radioactive sequencing, should encourage the design of updated primers because of the high degree of sequence variation that has been found in this and other recent studies (33). The importance of rigorously evaluating the specificity of newly designed primers was emphasized in the present study by the finding of cross-priming between the alternative P[8] primer nac13 and local genotype P[4] strains. The close genetic relationship between P[8] and P[4] strains makes cross-priming between these genotypes more likely.
The VP7 serotypes G1 to G4 together account for approximately 80% of global strains (19, 38) but represent only 63.3% of the collection used in this study. The absence of serotype G2, combined with the high prevalence of serotype G8, largely accounts for the relative underrepresentation of the four globally common G types in Malawi. We have previously described the frequent detection of serotype G8 rotaviruses in Malawi (10), which may represent human-bovine reassortant viruses (11). Serotype G8 was detected throughout the present study and was the most commonly identified G type in this collection, representing 34.8% of all strains. Most serotype G8 strains were of the short electropherotype. We did not identify any supershort electropherotype profiles characteristic of the prototype human serotype G8 rotavirus 69M isolated in Southeast Asia (28). However, two P[6], G8 strains of long electropherotype were recognized (one strain examined belongs to subgroup II), which are more typical of serotype G8 rotaviruses detected in Europe (20). Serotype G8 may be especially common in Africa, since several recent reports from African countries other than Malawi have documented the presence of this serotype. These countries include Nigeria (1), Egypt (30), Kenya (44), South Africa (55), and Guinea-Bissau (16).
Serotype G1 is the most common global serotype and was the second most frequently detected G-type in this study, encompassing 30% of strains. The VP7 protein of a representative serotype G1 strain, MW529, was closely related to several recent Australian serotype G1 strains described by Diwakarla et al. (14) and was demonstrated by phylogenetic analysis to belong to lineage I of serotype G1 strains (34). Serotypes G3 and G4 are also globally common strains and respectively accounted for 27.3 and 6.0% of strains in our collection. Serotype G9, which appears to be an emerging global serotype (4, 8, 48, 50, 51, 59), was detected in only 0.7% of strains. A surprising finding was the total absence of serotype G2 rotaviruses, since this is the most commonly identified serotype in some studies and represents >10% of global strains (19, 38). The lack of archived stool collections from children in Malawi renders it difficult to ascertain if serotype G2 has previously circulated in the country, but this serotype has recently been described in a study of a neighboring country, Zambia (54).
The globally most prevalent P[8] genotype was the most commonly detected VP4 type in the present study (54.8% of all strains). However, sequence analysis of the VP8* subunit of VP4 identified extensive diversity among these strains, with three distinct clusters of strains that segregated largely according to G type. Because of this diversity, three P[8] typing primers were required, in addition to 1T-1, to successfully type the majority of P[8] strains. Sequence diversity within the VP8∗ subunit of P[8] strains necessitated the design of a degenerate version of the 1T-1 typing primer in a recent study from the United Kingdom (33). The observation of clusters of P[8] strains that, in general, grouped according to VP7 serotype, contradicts previous observations that found no such association (33, 41). Although VP7 and VP4 segregate independently, it is likely that the three groups of P[8] strains that possess G1, G3, or G4 VP7 specificity (represented, respectively, by strains OP601, MW258, and OP354), represent the most stable VP7-VP4 combinations, and the viruses are therefore more likely to persist in nature. Some strains were detected, however, that did not fall into one of the three clusters. For example, the VP4 sequence of a serotype G3 strain (OP498) clustered with serotype G1 (OP601-like) strains, and the VP4 sequence of a serotype G4 strain (OP511) clustered with serotype G3 (MW258-like) strains. Such strains are likely to be VP7 or VP4 reassortants between strains belonging to separate clusters.
Of particular interest is the highly divergent cluster of closely related P[8] strains represented by strain OP354. Overall sequence identity, comparison of conserved amino acid residues with Wa and F45 lineages, and phylogenetic analysis indicate that OP354-like strains form a distinct lineage. Using shorter VP8* sequences (encompassing nucleotides 250 to 624), Maunula and von Bonsdorff (41) could define three P[8] lineages, P[8]-1, P[8]-2, and P[8]-3. Furthermore, by examining amino acid residues between positions 121 and 135, 4 aa (at positions 121, 125, 131, and 135) were strictly conserved within lineages. These P[8] lineage signature motifs were identified as ISSN, VNRD, and INRN, respectively, for lineages P[8]-1, P[8]-2, and P[8]-3 (41). While OP354-like strains group phylogenetically (using short sequences) with strains representing lineage P[8]-3 described by Maunula and von Bonsdorff (data not shown), the signature motif (ISRN) differs from the lineage-defining motif for P[8]-3 (Fig. 4). Therefore, we are currently examining further the complete VP4 (including VP8* and VP5* subunits) of OP354 to more fully assess its genetic and antigenic relationship to standard serotype P1A[8] strains. Although VP7 is the surface protein represented in most current vaccines, VP4 may also be important in inducing protective immunity (60), and rotavirus vaccines containing antigen representing the most common P serotype, P1A[8], have undergone evaluation in field trials (7). Since previous studies have documented greater antigenic variability in VP4 than was indicated by genomic typing methods (47), the diversity of VP4 in Malawi may be even greater than is apparent by the genomic analysis presented here. This may be relevant to future HRV vaccine strategies in Malawi.
Because of their recognized association with asymptomatic neonatal infections, HRVs with the VP4 P[6] genotype were thought to be attenuated and unlikely to cause diarrhea (22). Our finding of large numbers of P[6] strains among children with diarrhea (this genotype was detected in 33.1% of children) confirms similar recent reports from other countries (2, 4, 8, 50, 51, 58, 59).
This study extends our understanding of the tremendous diversity of HRV strains in some countries and has direct implications for the formulation of effective rotavirus vaccines. Strain surveillance is important in countries such as Malawi to monitor the prevalent rotavirus serotypes before future rotavirus vaccine programs are instated and to enable detection of new serotypes that could emerge as a consequence of widespread vaccine use. Reduction of the huge burden of disease caused by rotavirus in Africa by the successful introduction of rotavirus vaccines would make such efforts worthwhile (9).
ACKNOWLEDGMENTS
Nigel Cunliffe is the recipient of a Wellcome Trust Research Training Fellowship in Clinical Tropical Medicine (grant 049485/Z/96).
We thank the staff of the Department of Paediatrics, College of Medicine, for their support during the course of this work; Ida Nkhonjera and Constance Magola for collecting samples; and the parents and guardians of the children involved in this study for their willing participation. We thank Jon Gentsch for providing laboratory support throughout this project and for his critical review of the manuscript. We also thank Roger Glass and Joseph Bresee for helpful comments.
REFERENCES
- 1.Adah M I, Rohwedder A, Olaleye O D, Werchau H. Nigerian rotavirus serotype G8 could not be typed by PCR due to nucleotide mutation at the 3′ end of the primer binding site. Arch Virol. 1997;142:1881–1887. doi: 10.1007/s007050050206. [DOI] [PubMed] [Google Scholar]
- 2.Adah M I, Rohwedder A, Olaleye O D, Durojaiye O A, Werchau H. Further characterization of field strains of rotavirus from Nigeria. VP4 genotype P6 most frequently identified among symptomatically infected children. J Trop Pediatr. 1997;43:267–274. doi: 10.1093/tropej/43.5.267. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein D I, Glass R I, Rodgers G, Davidson B L, Sack D A. Evaluation of rhesus rotavirus monovalent and tetravalent reassortant vaccines in US children. JAMA. 1995;273:1191–1196. [PubMed] [Google Scholar]
- 4.Bon F, Fromantin C, Aho S, Pothier P, Kohli E the AZAY group. G and P genotyping of rotavirus strains circulating in France over a three-year period: detection of G9 and P[6] strains at low frequencies. J Clin Microbiol. 2000;38:1681–1683. doi: 10.1128/jcm.38.4.1681-1683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim-Van Dillen P M E, Van Der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bresee J S, Glass R I, Ivanoff B, Gentsch J R. Current status and future priorities for rotavirus vaccine development, evaluation and implementation in developing countries. Vaccine. 1999;17:2207–2222. doi: 10.1016/s0264-410x(98)00376-4. [DOI] [PubMed] [Google Scholar]
- 7.Clark H F, Offit P A, Ellis R W, Eiden J J, Krah D, Shaw A R, Pichichero M, Treanor J J, Borian F E, Bell L M, Plotkin S A. The development of multivalent bovine rotavirus (strain WC3) reassortant vaccine for infants. J Infect Dis. 1996;174:S73–S80. doi: 10.1093/infdis/174.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- 8.Cubitt W D, Steele A D, Iturriza M. Characterisation of rotaviruses from children treated at a London hospital during 1996: emergence of strains G9P2A[6] and G3P2A[6] J Med Virol. 2000;61:150–154. doi: 10.1002/(sici)1096-9071(200005)61:1<150::aid-jmv24>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 9.Cunliffe N A, Kilgore P E, Bresee J S, Steele A D, Luo N, Hart C A, Glass R I. Epidemiology of rotavirus diarrhoea in Africa: a review to assess the need for rotavirus immunization. Bull W H O. 1998;76:525–537. [PMC free article] [PubMed] [Google Scholar]
- 10.Cunliffe N A, Gondwe J S, Broadhead R L, Molyneux M E, Woods P A, Bresee J S, Glass R I, Gentsch J R, Hart C A. Rotavirus G and P types in children with acute diarrhea in Blantyre, Malawi, from 1997 to 1998: predominance of novel P[6]G8 strains. J Med Virol. 1999;57:308–312. [PubMed] [Google Scholar]
- 11.Cunliffe N A, Gentsch J R, Kirkwood C D, Gondwe J S, Dove W, Nakagomi O, Nakagomi T, Hoshino Y, Bresee J S, Glass R I, Molyneux M E, Hart C A. Molecular and serologic characterization of novel serotype G8 human rotavirus strains detected in Blantyre, Malawi. Virology. 2000;274:309–320. doi: 10.1006/viro.2000.0456. [DOI] [PubMed] [Google Scholar]
- 12.Das B K, Gentsch J R, Cicirello H G, Woods P A, Gupta A, Ramachandran M, Kumar R, Bhan M K, Glass R I. Characterization of rotavirus strains from newborns in New Delhi, India. J Clin Microbiol. 1994;32:1820–1822. doi: 10.1128/jcm.32.7.1820-1822.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Zoysa I, Feachem R G. Interventions for the control of diarrhoeal diseases among young children: rotavirus and cholera immunization. Bull W H O. 1985;63:569–583. [PMC free article] [PubMed] [Google Scholar]
- 14.Diwakarla C S, Palombo E A. Genetic and antigenic variation of capsid protein VP7 of serotype G1 human rotavirus isolates. J Gen Virol. 1999;80:341–344. doi: 10.1099/0022-1317-80-2-341. [DOI] [PubMed] [Google Scholar]
- 15.Estes M K. Rotaviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Press; 1996. pp. 1625–1655. [Google Scholar]
- 16.Fischer T K, Steinsland H, Molbak K, Ca R, Gentsch J R, Valentiner-Branth P, Aaby P, Sommerfelt H. Genotype profiles of rotavirus strains from children in a suburban community in Guinea-Bissau, Western Africa. J Clin Microbiol. 2000;38:264–267. doi: 10.1128/jcm.38.1.264-267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flores J, Green K Y, Garcia D, Sears J, Perez-Schael I, Avendano L F, Rodriguez W B, Taniguchi K, Urasawa S, Kapikian A Z. Dot hybridization assay for distinction of rotavirus serotypes. J Clin Microbiol. 1989;27:29–34. doi: 10.1128/jcm.27.1.29-34.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentsch J R, Glass R I, Woods P, Gouvea V, Gorziglia M, Flores J, Das B K, Bhan M K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gentsch J R, Woods P A, Ramachandran M, Das B K, Leite J P, Alfieri A, Kumar R, Bhan M K, Glass R I. Review of G and P typing results from a global collection of rotavirus strains: implications for vaccine development. J Infect Dis. 1996;174:S30–S36. doi: 10.1093/infdis/174.supplement_1.s30. [DOI] [PubMed] [Google Scholar]
- 20.Gerna G, Sarasini A, Zentilin L, Di Matteo A, Miranda P, Parea M, Battaglia M, Milanesi G. Isolation in Europe of 69M-like (serotype 8) human rotavirus strains with either subgroup I or II specificity and a long RNA electropherotype. Arch Virol. 1990;112:27–40. doi: 10.1007/BF01348983. [DOI] [PubMed] [Google Scholar]
- 21.Gorziglia M, Larralde G, Kapikian A Z, Chanock R M. Antigenic relationships among human rotaviruses as determined by outer capsid protein VP4. Proc Natl Acad Sci USA. 1990;87:7155–7159. doi: 10.1073/pnas.87.18.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorziglia M, Green K, Nishikawa K, Taniguchi K, Jones R, Kapikian A Z, Chanock R M. Sequence of the fourth gene of human rotaviruses recovered from asymptomatic or symptomatic infections. J Virol. 1988;62:2978–2984. doi: 10.1128/jvi.62.8.2978-2984.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gouvea V, de Castro L, Timenetsky M do C, Greenberg H, Santos N. Rotavirus serotype G5 associated with diarrhea in Brazilian children. J Clin Microbiol. 1994;32:1408–1409. doi: 10.1128/jcm.32.5.1408-1409.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gouvea V, Glass R I, Woods P, Taniguchi K, Clark H F, Forrester B, Fang Z Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gouvea V, Lima R C C, Linhares R E, Clark H F, Nosawa C M, Santos N. Identification of two lineages (WA-like and F45-like) within the major rotavirus genotype P[8] Virus Res. 1999;59:141–147. doi: 10.1016/s0168-1702(98)00124-5. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg H, McAuliffe V, Valdesuso J, Wyatt R, Flores J, Kalica A, Hoshino Y, Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983;39:91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunasena S, Nakagomi O, Isegawa Y, Kaga E, Nakagomi T, Steele A D, Flores J, Ueda S. Relative frequency of VP4 gene alleles among human rotaviruses recovered over a 10-year period (1982–1991) from Japanese children with diarrhea. J Clin Microbiol. 1993;31:2195–2197. doi: 10.1128/jcm.31.8.2195-2197.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasegawa A, Inouye S, Matsuno S, Yamaoka K, Eko R, Suharyono W. Isolation of human rotaviruses with a distinct RNA electrophoretic pattern from Indonesia. Microbiol Immunol. 1984;28:719–722. doi: 10.1111/j.1348-0421.1984.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 29.Herring A J, Inglis N F, Ojeh C K, Snodgrass D R, Menzies J D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982;16:473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes J L, Kirkwood C D, Gerna G, Clemens J D, Rao M R, Naficy A B, Abu-Elyazeed R, Savarino S J, Glass R I, Gentsch J R. Characterization of unusual G8 rotavirus strains isolated from Egyptian children. Arch Virol. 1999;144:1381–1396. doi: 10.1007/s007050050594. [DOI] [PubMed] [Google Scholar]
- 31.Hoshino Y, Kapikian A Z. Rotavirus antigens. Curr Top Microbiol Immunol. 1994;185:179–227. doi: 10.1007/978-3-642-78256-5_7. [DOI] [PubMed] [Google Scholar]
- 32.Hoshino Y, Sereno M M, Midthun K, Flores J, Kapikian A Z, Chanock R M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci USA. 1985;82:8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iturriza-Gomara M, Green J, Brown D W G, Desselberger U, Gray J J. Diversity within the VP4 gene of rotavirus P[8] strains: implications for reverse transcription-PCR genotyping. J Clin Microbiol. 2000;38:898–901. doi: 10.1128/jcm.38.2.898-901.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin Q, Ward R L, Knowlton D R, Gabbay Y B, Linhares A C, Rappaport R, Woods P A, Glass R I, Gentsch J R. Divergence of VP7 genes of G1 rotaviruses isolated from infants vaccinated with reassortant rhesus rotaviruses. Arch Virol. 1996;141:2057–2076. doi: 10.1007/BF01718215. [DOI] [PubMed] [Google Scholar]
- 35.Joensuu J, Koskenniemi E, Pang X-L, Vesikari T. Randomised placebo-controlled trial of rhesus-human reassortant rotavirus vaccine for prevention of severe rotavirus gastroenteritis. Lancet. 1997;350:1205–1209. doi: 10.1016/S0140-6736(97)05118-0. [DOI] [PubMed] [Google Scholar]
- 36.Kapikian A Z, Chanock R M. Rotaviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Press; 1996. pp. 1657–1708. [Google Scholar]
- 37.Kirkwood C D, Bishop R F, Coulson B S. Human rotavirus VP4 contains strain-specific, serotype-specific and cross-reactive neutralization sites. Arch Virol. 1996;141:587–600. doi: 10.1007/BF01718319. [DOI] [PubMed] [Google Scholar]
- 38.Koshimura Y, Nakagomi T, Nakagomi O. The relative frequencies of G serotypes of rotaviruses recovered from hospitalized children with diarrhea: a 10-year survey (1987–1996) in Japan with a review of globally collected data. Microbiol Immunol. 2000;44:499–510. doi: 10.1111/j.1348-0421.2000.tb02525.x. [DOI] [PubMed] [Google Scholar]
- 39.Larralde G, Flores J. Identification of gene 4 alleles among human rotaviruses by polymerase chain reaction-derived probes. Virology. 1990;179:469–473. doi: 10.1016/0042-6822(90)90317-k. [DOI] [PubMed] [Google Scholar]
- 40.Leite J P G, Alfieri A A, Woods P A, Glass R I, Gentsch J R. Rotavirus G and P types circulating in Brazil: characterization by RT-PCR, probe hybridization, and sequence analysis. Arch Virol. 1996;141:2365–2374. doi: 10.1007/BF01718637. [DOI] [PubMed] [Google Scholar]
- 41.Maunula L, von Bonsdorff C-H. Short sequences define genetic lineages: phylogenetic analysis of group A rotaviruses based on partial sequences of genome segments 4 and 9. J Gen Virol. 1998;79:321–332. doi: 10.1099/0022-1317-79-2-321. [DOI] [PubMed] [Google Scholar]
- 42.Midthun K, Kapikian A Z. Rotavirus vaccines: an overview. Clin Microbiol Rev. 1996;9:423–434. doi: 10.1128/cmr.9.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller M A, McCann L. Policy analysis of the use of hepatitis B, Haemophilus influenzae type B-, Streptococcus pneumoniae-conjugate and rotavirus vaccines in national immunization schedules. Health Econ. 2000;9:19–35. doi: 10.1002/(sici)1099-1050(200001)9:1<19::aid-hec487>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 44.Nakata S, Gatheru Z, Ukae S, Adachi N, Kobayashi N, Honma S, Muli J, Ogaja P, Nyangao J, Kiplagat E, Tukei P M, Chiba S. Epidemiological study of the G serotype distribution of group A rotaviruses in Kenya from 1991 to 1994. J Med Virol. 1999;58:296–303. doi: 10.1002/(sici)1096-9071(199907)58:3<296::aid-jmv17>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 45.Okada J, Urasawa T, Kobayashi N, Taniguchi K, Hasegawa A, Mise K, Urasawa S. New P serotype of group A human rotavirus closely related to that of a porcine rotavirus. J Med Virol. 2000;60:63–69. [PubMed] [Google Scholar]
- 46.Padilla-Noriega L, Dunn S J, Lopez S, Greenberg H B, Arias C F. Identification of two independent neutralization domains on the VP4 trypsin cleavage products VP5* and VP8* of human rotavirus ST3. Virology. 1995;206:148–154. doi: 10.1016/s0042-6822(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 47.Padilla-Noriega L, Mendez-Toss M, Menchaca G, Contreras J F, Romero-Guido P, Puerto F I, Guiscafre H, Mota F, Herrera I, Cedillo R, Munoz O, Calva J, de Lourdes Guerrero M, Coulson B S, Greenberg H B, Lopez S, Arias C F. Antigenic and genomic diversity of human rotavirus VP4 in two consecutive epidemic seasons in Mexico. J Clin Microbiol. 1998;36:1688–1692. doi: 10.1128/jcm.36.6.1688-1692.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palombo E A, Masendycz P J, Bugg H C, Bogdanovic-Sakran N, Barnes G L, Bishop R F. Emergence of serotype G9 human rotaviruses in Australia. J Clin Microbiol. 2000;38:1305–1306. doi: 10.1128/jcm.38.3.1305-1306.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez-Schael I, Guntinas M J, Perez M, Pagone V, Rojas A M, Gonzalez R, Cunto W, Hoshino Y, Kapikian A Z. Efficacy of the rhesus rotavirus-based quadrivalent vaccine in infants and young children in Venezuela. N Engl J Med. 1997;337:1181–1187. doi: 10.1056/NEJM199710233371701. [DOI] [PubMed] [Google Scholar]
- 50.Ramachandran M, Das B K, Vij A, Kumar R, Bhambal S S, Kesari N, Rawat H, Bahl L, Thakur S, Woods P A, Glass R I, Bhan M K, Gentsch J R. Unusual diversity of human rotavirus G and P genotypes in India. J Clin Microbiol. 1996;34:436–439. doi: 10.1128/jcm.34.2.436-439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramachandran M, Gentsch J R, Parashar U D, Jin S, Woods P A, Holmes J L, Kirkwood C D, Bishop R F, Greenberg H B, Urasawa S, Gerna G, Coulson B S, Taniguchi K, Bresee J S, Glass R I The National Rotavirus Strain Surveillance System Collaborating Laboratories. Detection and characterization of novel rotavirus strains in the United States. J Clin Microbiol. 1998;36:3223–3229. doi: 10.1128/jcm.36.11.3223-3229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rennels M B, Glass R I, Dennehy P H, Bernstein D I, Pichichero M E, Zito E T, Mack M E, Davidson B L, Kapikian A Z. Safety and efficacy of high-dose rhesus-human reassortant rotavirus vaccines-report of the national multicenter trial. Pediatrics. 1996;97:7–13. [PubMed] [Google Scholar]
- 53.Santosham M, Moulton L H, Reid R, Croll J, Weatherholt R, Ward R, Forro J, Zito E, Mack M, Brenneman G, Davidson B L. Efficacy and safety of high dose rhesus human reassortant rotavirus vaccine in Native American populations. J Pediatr. 1997;131:632–638. doi: 10.1016/s0022-3476(97)70076-3. [DOI] [PubMed] [Google Scholar]
- 54.Steele A D, Kasolo F C, Bos P, Peenze I, Oshitani H, Mpabalwani E. Characterization of VP6 subgroup, VP7 and VP4 genotype of rotavirus strains in Lusaka, Zambia. Ann Trop Pediatr. 1998;18:111–116. doi: 10.1080/02724936.1998.11747936. [DOI] [PubMed] [Google Scholar]
- 55.Steele A D, Parker S P, Peenze I, Pager C T, Taylor M B, Cubitt W D. Comparative studies of human rotavirus serotype G8 strains recovered in South Africa and the United Kingdom. J Gen Virol. 1999;80:3029–3034. doi: 10.1099/0022-1317-80-11-3029. [DOI] [PubMed] [Google Scholar]
- 56.Taniguchi K, Urasawa T, Morita Y, Greenberg H B, Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987;155:1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- 57.Taniguchi K, Maloy W L, Nishikawa K, Green K Y, Hoshino Y, Urasawa S, Kapikian A Z, Chanock R M, Gorziglia M. Identification of cross-reactive and serotype 2-specific neutralization epitopes on VP3 of human rotavirus. J Virol. 1988;62:2421–2426. doi: 10.1128/jvi.62.7.2421-2426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Timenetsky M do C, Santos N, Gouvea V. Survey of rotavirus G and P types associated with human gastroenteritis in Sao Paulo, Brazil, from 1986 to 1992. J Clin Microbiol. 1994;32:2622–2624. doi: 10.1128/jcm.32.10.2622-2624.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unicomb L E, Podder G, Gentsch J R, Woods P A, Hasan K Z, Faruque A S G, Albert M J, Glass R I. Evidence of high-frequency genomic reassortment of group A rotavirus strains in Bangladesh: emergence of type G9 in 1995. J Clin Microbiol. 1999;37:1885–1891. doi: 10.1128/jcm.37.6.1885-1891.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ward R L, McNeal M M, Sander D S, Greenberg H B, Bernstein D I. Immunodominance of the VP4 neutralization protein of rotavirus in protective natural infections of young children. J Virol. 1993;67:464–468. doi: 10.1128/jvi.67.1.464-468.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]