Abstract
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive and often fatal interstitial lung disease (ILD); other ILDs have a progressive, fibrotic phenotype (PF-ILD). Antifibrotic agents can slow but not stop disease progression in patients with IPF or PF-ILD. c-Jun N-terminal kinases (JNKs) are stress-activated protein kinases implicated in the underlying mechanisms of fibrosis, including epithelial cell death, inflammation and polarisation of profibrotic macrophages, fibroblast activation and collagen production. CC-90001, an orally administered (PO), one time per day, JNK inhibitor, is being evaluated in IPF and PF-ILD.
Methods and analysis
This is a phase 2, randomised, double-blind, placebo-controlled study evaluating efficacy and safety of CC-90001 in patients with IPF (main study) and patients with PF-ILD (substudy). Both include an 8-week screening period, a 24-week treatment period, up to an 80-week active-treatment extension and a 4-week post-treatment follow-up. Patients with IPF (n=165) will be randomised 1:1:1 to receive 200 mg or 400 mg CC-90001 or placebo administered PO one time per day; up to 25 patients/arm will be permitted concomitant pirfenidone use. Forty-five patients in the PF-ILD substudy will be randomised 2:1 to receive 400 mg CC-90001 or placebo. The primary endpoint is change in per cent predicted forced vital capacity from baseline to Week 24 in patients with IPF.
Ethics and dissemination
This study will be conducted in accordance with Good Clinical Practice guidelines, Declaration of Helsinki principles and local ethical and legal requirements. Results will be reported in a peer-reviewed publication.
Trial registration number
Keywords: interstitial fibrosis
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive and often fatal fibrosing interstitial lung disease (ILD).1 Median survival for patients with IPF is 3–5 years.2 3 IPF is diagnosed in the clinic by the pathological and radiographic pattern known as usual interstitial pneumonia (UIP) without any identifiable cause.4 Pirfenidone and nintedanib are antifibrotic therapies used to slow the progression of IPF, but they do not halt lung function decline.5–7 In addition, patients may discontinue treatment because of gastrointestinal (eg, nausea, diarrhoea, vomiting and dyspepsia) or skin-related (eg, photosensitivity reactions) adverse events (AEs) with pirfenidone5 8 9 or gastrointestinal AEs (eg, diarrhoea, nausea and vomiting) with nintedanib.7 10 11 Due to these limitations, treatment with pirfenidone or nintedanib may be outweighed by safety and tolerability risks for some patients,12 emphasising the need for more effective and well-tolerated treatment options in IPF.
Non-IPF ILDs can also manifest a progressive fibrosing phenotype and are also similarly characterised by worsening respiratory symptoms, declining lung function, progression on imaging, as well as early mortality.13 14 A proportion of the patients with a progressive fibrotic ILD (PF-ILD) may have a high-resolution CT (HRCT) pattern that is indeterminate for UIP or not consistent with UIP.13 15 Pirfenidone and nintedanib have been studied in patients with a progressive fibrosing phenotype. Patients with unclassifiable progressive ILD treated with pirfenidone experienced a lower mean change in forced vital capacity (FVC) compared with those who received placebo over 24 weeks.16 In the INBUILD study, patients with non-IPF PF-ILD treated with nintedanib had a lower annual rate of decline in FVC over the 52-week study period than those who received placebo.17 These findings, along with the recent approval of nintedanib in the USA and European Union for patients with chronic fibrosing ILD with a progressive phenotype,10 11 suggest a benefit of antifibrotic treatment in this broader patient population.
c-Jun N-terminal kinase (JNK) is a stress-activated protein kinase that has been implicated in the pathogenesis of lung fibrosis. JNK is induced by cellular stresses, resulting in epithelial cell injury or death, inflammation and fibrosis.18–21 Once activated, JNK isoforms (JNK1, JNK2 and JNK3) phosphorylate substrates, including transcription factors, to regulate the expression of genes involved in inflammatory and fibrotic processes.21 22 In the lungs of patients with IPF, multiple cell types, including vascular endothelial cells, smooth muscle cells, alveolar epithelial cells, alveolar macrophages and lymphocytes, exhibit increased levels of activated JNK, with levels corresponding to the degree of fibrosis.20 Data from preclinical models of fibrosis show that the inhibition or deletion of JNK, in particular the JNK1 isoform, has been shown to have antifibrotic effects,18 21 23–25 suggesting that inhibition of JNK1 may represent an important therapeutic target in various forms of pulmonary fibrosis.
CC-930 was a first-generation JNK inhibitor with a JNK2 bias.26 In preclinical models, CC-930 reduced the levels of biomarkers considered to be associated with IPF, including collagen 1A1 gene expression, airway mucin 5B expression and matrix metalloproteinase 7 (MMP-7) expression.27 In a phase 2 study in patients with IPF, CC-930 orally administered (PO) at three dosages (50 mg or 100 mg one time per day or 100 mg two times per day) showed preliminary evidence of FVC stabilisation and dose-dependent modulation of biomarkers MMP-7 and surfactant protein D (SP-D); however, chronic dosing of CC-930 at 100 mg two times per day led to elevations in liver enzymes,27 and development was discontinued.
CC-90001 is a second-generation, oral, one time per day JNK inhibitor with a strong bias for JNK1 that has demonstrated anti-inflammatory effects and antifibrotic activity in clinical and/or non-clinical studies.28 29 Phase 1 studies in healthy participants and patients with pulmonary fibrosis showed that CC-90001 was safe and well tolerated with no clinically meaningful elevations in liver enzymes. In a phase 1b open-label study, 10 of 12 patients with pulmonary fibrosis who received 200 mg or 400 mg CC-90001 PO one time per day and completed 12 weeks of treatment experienced a mean FVC change from baseline of +168 mL (95% CI: +71 to +276).30 Although the study was small and an open-label design, it provided an impetus to further explore CC-90001 in patients with pulmonary fibrosis. This phase 2 study will evaluate the clinical efficacy, tolerability and safety of the JNK inhibitor CC-90001 in patients with IPF and PF-ILD. The study objectives and design are presented below.
Methods
IPF study design and interventions
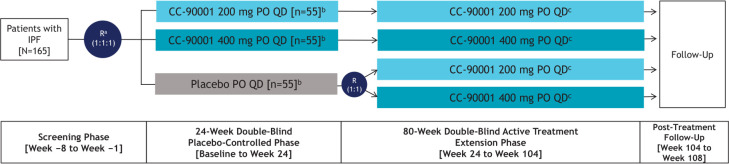
This is a phase 2, randomised, double-blind, placebo-controlled, multicentre, multinational clinical study investigating the efficacy, safety and tolerability of CC-90001 in patients with IPF. The trial will consist of an 8-week screening phase; a 24-week double-blind, placebo-controlled treatment phase; up to an 80-week double-blind, active-treatment extension phase; and a 4-week post-treatment follow-up (figure 1). Approximately 165 patients with IPF will be randomised 1:1:1 to receive 200 mg or 400 mg CC-90001 or placebo administered PO one time per day in the 24-week, double-blind, placebo-controlled treatment phase; up to 25 patients per treatment arm will be permitted concomitant pirfenidone use. Randomisation will be stratified by previous history of pirfenidone or nintedanib use and by concomitant pirfenidone use. In the double-blind, active-treatment extension phase, patients will continue to receive their assigned CC-90001 dosage, and patients from the placebo arm will be re-randomised 1:1 to receive 200 mg or 400 mg CC-90001 PO one time per day. Patients not receiving concomitant pirfenidone in the initial 24-week, double-blind, placebo-controlled phase will be permitted to receive concomitant pirfenidone in the extension phase if deemed appropriate by the investigator.
Figure 1.
IPF study design. a Randomisation will be stratified by previous history of pirfenidone or nintedanib use and by concomitant pirfenidone use status. b Up to 25 patients per treatment arm receiving pirfenidone will be enrolled and randomised. c Patients not receiving concomitant pirfenidone in the initial 24-week, double-blind, placebo-controlled phase will have the option to receive concomitant pirfenidone in the up to 80- week, double-blind, active treatment extension phase. IPF, idiopathic pulmonary fibrosis; PO, oral; QD, one time per day; R, randomisation.
PF-ILD exploratory substudy design and interventions
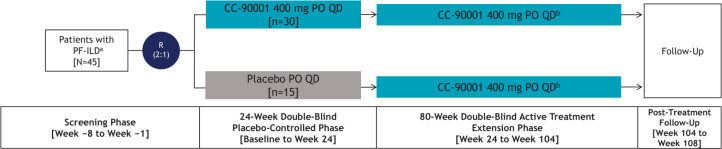
An exploratory randomised, double-blind, placebo-controlled, multicentre, multinational clinical substudy investigating the efficacy, safety and tolerability of CC-90001 in patients with PF-ILD will also be initiated. Patients not treated with pirfenidone who were screened and did not receive a confirmed IPF diagnosis following central reading of HRCT and lung biopsy, if obtained, will be considered for the substudy. Similar to the IPF study, the substudy will consist of an 8-week screening phase; a 24-week double-blind, placebo-controlled treatment phase; an up to 80-week double-blind, active-treatment extension phase; and a 4-week post-treatment follow-up (figure 2). Approximately 45 patients with PF-ILD will be randomised 2:1 to receive the intended dose of 400 mg CC-90001 or placebo administered PO one time per day in the 24-week, double-blind, placebo-controlled phase. All patients will receive 400 mg CC-90001 PO one time per day in the active-treatment extension phase.
Figure 2.
PF-ILD exploratory substudy design. a Eligible patients include those not receiving pirfenidone who were screened and did not receive a confirmed IPF diagnosis following central reading of HRCT and lung biopsy (if obtained). b The decision to receive additional treatments for pulmonary fibrosis (eg, pirfenidone) during the up to 80-week, active treatment extension phase is at the discretion of the investigator. HRCT, high-resolution CT; IPF, idiopathic pulmonary fibrosis; PF-ILD, progressive fibrotic interstitial lung disease; PO, oral; QD, one time per day; R, randomisation.
Patient population
Patient eligibility criteria for the IPF study and the PF-ILD substudy are shown in box 1. In summary, eligible patients for the IPF study will include adults age ≥40 years with a diagnosis of IPF supported by HRCT as summarised in table 1. Patients must also have an FVC (% predicted) ≥45% and ≤95% at screening, haemoglobin-corrected diffusing capacity of carbon monoxide (Dlco; % predicted) ≥25% and ≤90%, a 6-minute walk test (6-MWT) result of ≥150 m, and no features supporting an alternative diagnosis on transbronchial biopsy, bronchoalveolar lavage or surgical lung biopsy (if performed prior to screening). Patients who meet the IPF criteria above, excluding the UIP criteria summarised in table 1, and who have features of diffuse fibrosing lung disease >10% on centrally read HRCT as well as investigator-documented ≥5% annualised relative decline in FVC in the past 24 months from the first screening visit, will be eligible to participate in the PF-ILD substudy.
Box 1. Patient eligibility criteria.
Inclusion criteria
All patients
Age ≥40 years.
FVC (% predicted) ≥45% and ≤95%.
Haemoglobin-corrected Dlco (% predicted) ≥25% and ≤90%.
6-MWT result of ≥150 m.
No features supporting an alternative diagnosis on transbronchial biopsy, bronchoalveolar lavage or SLB, if performed prior to screening.
IPF study
IPF diagnosis supported by HRCT and lung biopsy (if available; see table 1).
Patients receiving concomitant pirfenidone therapy must receive and agree to maintain the same dose of pirfenidone therapy for at least 8 weeks prior to screening and through Week 24.*
PF-ILD substudy
Non-IPF diagnosis supported by HRCT and lung biopsy (if available; see table 1).
Features of diffuse fibrosing lung disease >10% on centrally read HRCT.
Investigator-documented ≥5% annualised relative decline in FVC in past 24 months from first screening visit.
Exclusion criteria
All patients
Medical conditions
Extent of emphysema on centrally read chest HRCT greater than the extent of fibrotic changes (eg, honeycombing and reticular changes).
Change in FVC (mL) between screening and Day 1 of >10% (relative difference).
Any significant medical condition, laboratory abnormality or psychiatric illness that would prevent study participation.
Any condition, including the presence of laboratory abnormalities, that places the patient at an unacceptable risk with study participation.
Significant clinical worsening of pulmonary fibrosis between screening and baseline (visit 2).
History of the following: deep vein thrombosis or pulmonary embolism within 6 months of screening visit; cardiac valve replacement requiring chronic anticoagulation therapy; clinical diagnosis of any connective tissue disease, including, but not limited to, scleroderma, polymyositis/dermatomyositis, systemic lupus erythematosus and rheumatoid arthritis; end-stage renal disease requiring dialysis; severe hepatic impairment or end-stage liver disease; Gilbert’s syndrome; malignancy (exceptions: excised and cured basal/squamous cell skin carcinomas or cervical carcinoma in situ with no recurrence in 5 years).
Alcohol or drug abuse within 6 months prior to screening.
Use of tobacco products (cigarettes, pipes and cigars), vapes, e-cigarettes or marijuana within 3 months of screening and/or unwillingness to avoid the use of these products throughout the study.
Pregnancy or lactation.
Laboratory tests
White blood cell count <3.5×109/L or >14×109/L.
Platelet count <120×109/L.
Serum creatinine >1.5 mg/dL (>132.6 µmol/L).
AST/SGOT >1.5×ULN; ALT/SGPT >1.5×ULN.
Total bilirubin >2 mg/dL (>34.2 µmol/L).
Haemoglobin <100 g/L.
QTcF >450 msec.
Lung function
Any condition other than pulmonary fibrosis that the investigator deems likely to result in the death of the patient within the next year.
Patients likely to have lung transplantation during the first 24 weeks of the study (being on transplantation list is acceptable for participation).
Impairment (other than dyspnoea) limiting the ability to comply with study requirements (eg, pulmonary function tests, 6-MWT).
Evidence of clinically relevant airway obstruction (ie, FEV1/FVC <0.7) at screening and/or significant respiratory disorder/pathology (eg, pulmonary arterial hypertension requiring treatment, asthma, tuberculosis, sarcoidosis, hypersensitivity pneumonitis, aspergillosis, asbestosis, neoplastic disease, cystic fibrosis or other ILD) other than IPF.†
Prior therapy
Use of any medications that are substrates of one or more of the transporters P-gp, BCRP, OAT3, OATP1B1, OATP1B3 and OCT2 and have a narrow therapeutic index (eg, digoxin, mycophenolate mofetil).
Within 12 weeks of randomisation: use of any cytokine modulator/biological, such as etanercept, adalimumab, efalizumab, infliximab or rituximab.
Within 4 weeks prior to screening visit: use of nintedanib, endothelium receptor antagonists (eg, bosentan, ambrisentan), interferon γ-1b, imatinib mesylate, N-acetylcysteine, azathioprine, cyclophosphamide, methotrexate, mycophenolate mofetil, cyclosporine, oral steroids (eg, prednisone >12.5 mg/day or equivalent) and/or pirfenidone (in patients in the IPF study who were not assigned to receive concomitant treatment or patients in the PF-ILD substudy).
Within 2 weeks of first dose of the study drug or during the study: use of drugs that are known to cause hepatotoxicity, such as, but not limited to, acetaminophen (paracetamol) at dosages of >3 g/day and niacin at dosages of >2 g/day.
Within 24 hours of the screening visit: use of an inhaled long-acting bronchodilator.
Within 8 hours of screening visit: use of short-acting bronchodilator.
Use of any investigational product within 1 month of screening or 5 PD/PK half-lives (whichever is longer).
Infections
History of congenital and/or acquired immunodeficiencies.
History of hepatitis B and/or hepatitis C, including patients considered successfully treated/cured.
Active or history of recurrent bacterial, viral, fungal, mycobacterial or other infections (including, but not limited to, atypical mycobacterial disease and herpes zoster), or any major episode of infection requiring hospitalisation or treatment with intravenous or oral antibiotics within 4 weeks of the screening visit and at any time during the screening phase, up through the first dose of the study drug.
History of active or latent TB infection, unless there is medical record documentation of successful completion of a standard course of treatment considered appropriate, based on local prevalence of multidrug resistant TB and consistent with WHO guidelines.
Patients who have had household contact with a person with active TB and did not receive appropriate and documented prophylaxis for TB.
Table 1.
IPF diagnostic criteria based on HRCT and lung biopsy
| UIP pattern on HRCT | Histopathological criteria for UIP in IPF* | ||||
| Lung biopsy not available or non-diagnostic | Definite UIP-IPF |
Probable UIP-IPF |
Indeterminate UIP-IPF |
Features most consistent with an alternative diagnosis | |
| Typical | Eligible | Eligible | Eligible | Eligible | Ineligible |
| Probable, age >60 years | Eligible | Eligible | Eligible | Eligible | Ineligible |
| Probable, age ≤60 years | Ineligible | Eligible | Eligible | Ineligible | Ineligible |
| Indeterminate | Ineligible | Eligible | Eligible | Ineligible | Ineligible |
| Most consistent with non-IPF diagnosis | Ineligible | Ineligible | Ineligible | Ineligible | Ineligible |
Endpoints
The primary endpoint is change in percentage point difference in FVC (% predicted) from baseline to Week 24 in the IPF study. Key secondary and exploratory endpoints for the IPF study and PF-ILD substudy include safety and tolerability, disease progression and changes in the following: Dlco, 6-MWT, quantitative lung fibrosis (QLF; that is, data-driven textural analysis) score based on HRCT in a subset of subjects, patient-reported outcomes, acute exacerbations and biomarkers. Pharmacokinetic and pharmacodynamic analyses in relation to treatment response will also be conducted. All primary and secondary endpoints and key exploratory endpoints for the IPF study and PF-ILD substudy and their definitions are shown in box 2.
Box 2. IPF study and PF-ILD substudy key endpoints.
Primary endpoint
Change in percentage point difference in FVC (% predicted) from baseline to Week 24 (IPF cohort).
Secondary endpoints
Efficacy (IPF cohort)
Absolute change and rate of decline in FVC (mL) from baseline through Week 24.
Change in 6-MWT results and dyspnoea rating on Borg scale from baseline through Weeks 24, 52, 76, 104; and from Week 24 through Weeks 52 and 104.
Disease progressiona from baseline through Week 24.
Change in HRQoL, as measured by the SGRQ and UCSD-SOBQ, from baseline through Week 24.
Safety and tolerability (IPF and PF-ILD cohorts)
AEs.
SAEs.
Clinical laboratory findings.
Key exploratory endpoints
Efficacy
-
Change in percentage point difference in FVC (% predicted), absolute change in FVC (mL) and annualised rate of decline in FVC (mL) from:
Baseline through Week 104 and from Week 24 through Week 104 (IPF cohort).
Baseline through Weeks 24 and 104, and from Week 24 through Week 104 (PF-ILD cohort).
Change in 6-MWT results and dyspnoea rating on Borg scale from baseline through Weeks 24, 52, 76, 104; and from Week 24 through Weeks 52 and 104 (PF-ILD cohort).
Disease progression* from baseline through Weeks 24 and 104, and from Week 24 through Week 104 (PF-ILD cohort).
Change in lung volume (TLC and FRC) from baseline through Week 24 (IPF and PF-ILD cohorts).
Change in haemoglobin-corrected Dlco (% predicted) and absolute haemoglobin-corrected Dlco from baseline through Weeks 24 and 104, and Week 24 through Week 104 (IPF and PF-ILD cohorts).
-
Change in QLF score based on HRCT from:
Baseline through Weeks 24 and 104, and Week 24 through Week 104 (IPF cohort and PF-ILD cohorts).
Change in HRQoL as measured by the SF-36v2 questionnaire from baseline through Week 24 (IPF and PF-ILD cohorts).
-
Change in HRQoL as measured by SGRQ and UCSD-SOBQ from:
Baseline through Week 104 and Week 24 through Week 104 (IPF cohort).
Baseline through Weeks 24 and 104, and Week 24 through Week 104 (PF-ILD cohort).
-
Time to the following events:
Disease progression* from baseline through Week 104, and Week 24 through Week 104 (IPF cohort); from baseline through Weeks 24 and 104, and Week 24 through Week 104 (PF-ILD cohort).
First acute exacerbation† from baseline through Weeks 24 and 104, and Week 24 through Week 104 (IPF and PF-ILD cohorts).
All-cause mortality (IPF and PF-ILD cohorts).
PK/PD (IPF and PF-ILD cohorts)
Change in predose CC-90001 concentrations and the relationship between exposure and efficacy (FVC and Dlco) from Week 4 through Week 24.
Biomarkers (IPF and PF-ILD cohorts)
-
Change in the following parameters from multiple time points:
Plasma and serum biomarkers (through Week 104).
Whole blood RNA gene expression (through Week 104).
PG markers (through Week 24).
Statistical analyses
The full analysis set (FAS) will include all randomised patients who receive at least one dose of the study drug. Data from the IPF study and PF-ILD substudy will be analysed separately using the FAS population. The primary endpoint will be analysed by a mixed-effect model for repeated measures (MMRM). The MMRM model will contain change from baseline FVC (% predicted) value as the dependent variable and treatment group, time (Weeks 1, 4, 8, 12, 16, 20 and 24), interactions (treatment-by-time, pirfenidone-by-time, treatment-by-pirfenidone-by-time) and stratification factor (previous exposure to either pirfenidone or nintedanib (yes/no)) as fixed effects and baseline FVC value as a covariate, and time will be treated as a repeated effect. For secondary endpoints, descriptive statistics for change from baseline, frequencies or median time to event in efficacy measures will be summarised by visit for each treatment group. Planned subgroup analyses will assess whether the treatment effect is consistent across patients with or without concurrent pirfenidone therapy. A Cochran-Mantel-Haenszel test will be used for the pairwise comparisons of the proportions of patients who experience disease progression at Week 24 between the two active treatment groups±pirfenidone and the placebo group±pirfenidone. A Kaplan-Meier estimate for the survival function will be provided for all time-to-event endpoints by active-treatment groups±pirfenidone and the placebo group ±pirfenidone. Similar analyses will be performed for the PF-ILD substudy, and the results will be compared descriptively.
Analyses of safety data will be based on the safety set, defined as all randomised patients who take at least one dose of study treatment. Treatment-emergent AEs, study drug-related AEs, serious AEs, physical examination findings, vital signs, clinical laboratory test results and ECG results will be summarised using descriptive statistics for continuous variables and frequency distributions for categorical variables.
Sample size assumptions and rationale
The primary objective of the study is to evaluate the effect of CC-90001 in patients with IPF who are or are not receiving pirfenidone. Approximately 165 patients (with roughly 45% receiving concurrent pirfenidone) randomised evenly between the two active and one placebo arms provide 75% power for testing an overall difference of 2.2% points or more in the Week 24 mean change from baseline of FVC (% predicted) value between either active-treatment group and the placebo group (two-sided α=0.1; SD=5%).
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Ethics and dissemination
This study will be conducted in accordance with the principles derived from international guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences, Good Clinical Practice, International Council for Harmonisation, ethical principles underlying European Union Directive 2001/20/EC, USA CFR, Title 21, Part 50 (21CFR50) and applicable local requirements. All patients will provide written informed consent. Study results will be monitored by an independent Data Monitoring Committee (DMC). The DMC will review data and make recommendations to the study sponsor on whether the study should be allowed to continue, be modified or terminated. Final efficacy and safety results will be reported in a peer-reviewed publication.
Discussion
JNKs are stress-activated protein kinases that play an important role in the pathological mechanisms underlying the fibrotic process, including epithelial cell death, inflammation and polarisation of profibrotic macrophages, and fibroblast activation and collagen production.18–21 In preclinical models of lung fibrosis, deletion or inhibition of the JNK1 isoform resulted in an attenuated fibrotic response.18 21 23–25 Furthermore, during an open-label phase 1b study, patients with pulmonary fibrosis treated with CC-90001 had an overall lack of FVC decline and reductions in tenascin-C, a matrix protein associated with fibrosis, over 12 weeks.30 CC-90001 was generally well tolerated, with all treatment-emergent AEs being mild or moderate in intensity, and no serious AEs were reported.30 Based on these findings, the phase 2 study described in this article was designed to further investigate the efficacy and safety of CC-90001 in patients with IPF or PF-ILD in a controlled setting.
Several aspects of this study were designed to reflect how patients with pulmonary fibrosis are being diagnosed and treated within real-world settings. Similar to other clinical trials in IPF, the inclusion criteria in the main study require the presence of probable or typical UIP based on a central reading; however, owing to the clinical significance of other progressive forms of pulmonary fibrosis, patients with PF-ILD who do not have these pathological features are eligible to be enrolled into an exploratory substudy. As antifibrotics have shown the ability to slow FVC decline in PF-ILD,16 17 CC-90001 will be evaluated within this patient population to further address their unmet needs. Additionally, as IPF is often characterised as a relentless and irreversible disease, the combination of CC-90001 and pirfenidone will be permitted to allow for the investigation of potential additive improvements and provide insight into the utility of this combination regimen.
The IPF study and PF-ILD substudy are similarly designed. Both have two blinded phases: a 24-week, double-blind, placebo-controlled phase, followed by an up to 80-week, double-blind, active-treatment extension phase. The 24-week duration in the double-blind phase is based on the apparent benefits in FVC observed as early as 24 weeks in clinical trials of pirfenidone and nintedanib in patients with IPF.5 7 8 The extension phase can provide additional long-term safety and efficacy information up to 104 weeks for CC-90001 and for the combination of CC-90001 and pirfenidone. Furthermore, in the extension phase, patients randomised to receive placebo will have an opportunity to receive CC-90001 and initiate pirfenidone if deemed appropriate by the investigator, in all participants not receiving concurrent pirfenidone. Given that the intended patient population for this trial exhibits a progressive disease phenotype and the currently approved treatments only slow disease progression, the active treatment extension phase will provide long-term data on the durability of response with CC-90001 (alone or with pirfenidone).
In addition to standard clinical measures of efficacy, such as FVC, Dlco and 6-MWT, the IPF study and PF-ILD substudy will assess a range of other secondary and exploratory endpoints that will contribute to the body of knowledge about pulmonary fibrosis and aid in guiding optimal patient selection for future studies. One such exploratory endpoint is measurement of the extent of pulmonary fibrosis assessed by QLF score on HRCT.31 32 Because structural progression may be evident on HRCT scans before reaching the threshold for spirometric progression, this quantitative analysis may serve as a supplementary tool to measure clinical outcomes in patients with pulmonary fibrosis. Additional endpoints of interest relate to the assessment of patients’ quality of life. The St. George’s Respiratory Questionnaire,33 University of California, San Diego Shortness of Breath Questionnaire34 and SF-36v233 instruments that will be used during the study rely on patient-reported metrics to directly measure treatment benefits that are important to patients such as the impact of their disease on daily living activities. There is also an unmet need to identify optimal IPF therapies for patients based on genetic polymorphisms, as well as to identify biomarkers that can be used to monitor disease progression and predict therapeutic response. Thus, these studies will assess genetic polymorphisms and blood biomarkers. Specifically, the pharmacogenetic data will investigate genes associated with progressive pulmonary fibrosis and those involved in the JNK pathway. The pharmacodynamic effects of CC-90001 on relevant biomarkers, such as MMP-7,35 plasminogen activator inhibitor-1,36 SP-D37 and tenascin C,38 will be analysed for correlations with disease activity and clinical outcomes.
Conclusions
CC-90001 is an oral, one time per day, JNK inhibitor with a strong bias for JNK1; CC-90001 treatment has been associated with a lack of FVC decline in patients with pulmonary fibrosis in a phase 1b study. This phase 2 study will investigate the efficacy, safety and tolerability of CC-90001 (alone and in combination with pirfenidone) in patients with IPF, as well as in an exploratory substudy in patients with PF-ILD, reflecting a real-world patient population. This study will assess the clinical utility of JNK inhibition as a treatment option in pulmonary fibrosis.
Footnotes
Contributors: Medical writing support was provided by Apurva Davé, PhD, and Kendall Foote, PhD, of Medical Expressions (Chicago, IL) and funded by Bristol Myers Squibb. All authors were involved in the study design, reviewed and revised drafts of the manuscript, and approved the final draft.
Funding: This work was supported by Bristol Myers Squibb.
Competing interests: ZP, DJS, GSH, AG, TFR and SG were all employees of Celgene when the study was designed and conducted prior to acquisition by Bristol Myers Squibb. TFR is a current employee of Repertoire Immune Medicines. DAL has received grants from NHLBI and personal fees from Boehringer Ingelheim, Parexel and Veracyte. PWN has served as a consultant for Boehringer Ingelheim, Bristol Myers Squibb, Celgene (prior to acquisition by Bristol Myers Squibb), InterMune, Moerae Matrix, Roche and Takeda. LR has received research grants from InterMune and personal fees from Boehringer Ingelheim, Bristol Myers Squibb, Celgene (prior to acquisition by Bristol Myers Squibb), DynaMed, Fibrogen, ImmuneWorks, InterMune, Nitto, Promedior (acquired by Roche), Roche, Sanofi and Shionogi.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med 2018;378:1811–23. 10.1056/NEJMra1705751 [DOI] [PubMed] [Google Scholar]
- 2.Tran T, Šterclová M, Mogulkoc N, et al. The European MultiPartner IPF registry (EMPIRE): validating long-term prognostic factors in idiopathic pulmonary fibrosis. Respir Res 2020;21:11. 10.1186/s12931-019-1271-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan SD, Shlobin OA, Weir N, et al. Long-Term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest 2011;140:221–9. 10.1378/chest.10-2572 [DOI] [PubMed] [Google Scholar]
- 4.Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society white paper. Lancet Respir Med 2018;6:138–53. 10.1016/S2213-2600(17)30433-2 [DOI] [PubMed] [Google Scholar]
- 5.King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083–92. 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 6.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J 2016;47:243–53. 10.1183/13993003.00026-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071–82. 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 8.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (capacity): two randomised trials. Lancet 2011;377:1760–9. 10.1016/S0140-6736(11)60405-4 [DOI] [PubMed] [Google Scholar]
- 9.ESBRIET (pirfenidone) [package insert]. South San Francisco, CA: Genentech, Inc; 2019. [Google Scholar]
- 10.OFEV (nintedanib) [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2020. [Google Scholar]
- 11.OFEV (nintedanib) [summary of product characteristics]. Ingelheim am Rhein, Germany: Boehringer Ingelheim International GmbH; 2019. [Google Scholar]
- 12.Galli JA, Pandya A, Vega-Olivo M, et al. Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice: tolerability and adverse drug reactions. Respirology 2017;22:1171–8. 10.1111/resp.13024 [DOI] [PubMed] [Google Scholar]
- 13.Cottin V, Hirani NA, Hotchkin DL, et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018;27. 10.1183/16000617.0076-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wijsenbeek M, Kreuter M, Olson A, et al. Progressive fibrosing interstitial lung diseases: current practice in diagnosis and management. Curr Med Res Opin 2019;35:2015–24. 10.1080/03007995.2019.1647040 [DOI] [PubMed] [Google Scholar]
- 15.Flaherty KR, Brown KK, Wells AU, et al. Design of the PF-ILD trial: a double-blind, randomised, placebo-controlled phase III trial of nintedanib in patients with progressive fibrosing interstitial lung disease. BMJ Open Respir Res 2017;4:e000212. 10.1136/bmjresp-2017-000212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2020;8:147–57. 10.1016/S2213-2600(19)30341-8 [DOI] [PubMed] [Google Scholar]
- 17.Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 2019;381:1718–27. 10.1056/NEJMoa1908681 [DOI] [PubMed] [Google Scholar]
- 18.Alcorn JF, van der Velden J, Brown AL, et al. C-Jun N-terminal kinase 1 is required for the development of pulmonary fibrosis. Am J Respir Cell Mol Biol 2009;40:422–32. 10.1165/rcmb.2008-0174OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett BL. C-Jun N-terminal kinase-dependent mechanisms in respiratory disease. Eur Respir J 2006;28:651–61. 10.1183/09031936.06.00012106 [DOI] [PubMed] [Google Scholar]
- 20.Yoshida K, Kuwano K, Hagimoto N, et al. MAP kinase activation and apoptosis in lung tissues from patients with idiopathic pulmonary fibrosis. J Pathol 2002;198:388–96. 10.1002/path.1208 [DOI] [PubMed] [Google Scholar]
- 21.van der Velden JL, Alcorn JF, Chapman DG, et al. Airway epithelial specific deletion of Jun-N-terminal kinase 1 attenuates pulmonary fibrosis in two independent mouse models. PLoS One 2020;15:e0226904. 10.1371/journal.pone.0226904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeke A, Misheva M, Reményi A, et al. JNK signaling: regulation and functions based on complex protein-protein partnerships. Microbiol Mol Biol Rev 2016;80:793–835. 10.1128/MMBR.00043-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee VY, Schroedl C, Brunelle JK, et al. Bleomycin induces alveolar epithelial cell death through JNK-dependent activation of the mitochondrial death pathway. Am J Physiol Lung Cell Mol Physiol 2005;289:L521–8. 10.1152/ajplung.00340.2004 [DOI] [PubMed] [Google Scholar]
- 24.Lin C-H, Yu M-C, Tung W-H, et al. Connective tissue growth factor induces collagen I expression in human lung fibroblasts through the Rac1/MLK3/JNK/AP-1 pathway. Biochim Biophys Acta 2013;1833:2823–33. 10.1016/j.bbamcr.2013.07.016 [DOI] [PubMed] [Google Scholar]
- 25.Kluwe J, Pradere J-P, Gwak G-Y, et al. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology 2010;138:347–59. 10.1053/j.gastro.2009.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plantevin Krenitsky V, Nadolny L, Delgado M, et al. Discovery of CC-930, an orally active anti-fibrotic JNK inhibitor. Bioorg Med Chem Lett 2012;22:1433–8. 10.1016/j.bmcl.2011.12.027 [DOI] [PubMed] [Google Scholar]
- 27.van der Velden JLJ, Ye Y, Nolin JD, et al. JNK inhibition reduces lung remodeling and pulmonary fibrotic systemic markers. Clin Transl Med 2016;5:36. 10.1186/s40169-016-0117-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett B, Blease K, Ye Y. CC-90001, a second generation Jun N-terminal kinase (JNK) inhibitor for the treatment of idiopathic pulmonary fibrosis. C38 understanding therapeutics in IPF, 2017: A5409. [Google Scholar]
- 29.Nagy MA, Hilgraf R, Mortensen DS, et al. Discovery of the c-Jun N-Terminal Kinase Inhibitor CC-90001. J Med Chem 2021;64:18193–208. 10.1021/acs.jmedchem.1c01716 [DOI] [PubMed] [Google Scholar]
- 30.Greenberg S, Horan G, Bennett B. Evaluation of the JNK inhibitor, CC-90001, in a phase 1B pulmonary fibrosis trial. Eur Respir J 2017;50:OA474. 10.1183/1393003.congress-2017.OA474 [DOI] [Google Scholar]
- 31.Humphries SM, Yagihashi K, Huckleberry J, et al. Idiopathic pulmonary fibrosis: data-driven textural analysis of extent of fibrosis at baseline and 15-month follow-up. Radiology 2017;285:270–8. 10.1148/radiol.2017161177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humphries SM, Swigris JJ, Brown KK, et al. Quantitative high-resolution computed tomography fibrosis score: performance characteristics in idiopathic pulmonary fibrosis. Eur Respir J 2018;52:1801384.. 10.1183/13993003.01384-2018 [DOI] [PubMed] [Google Scholar]
- 33.Swigris JJ, Brown KK, Behr J, et al. The SF-36 and SGRQ: validity and first look at minimum important differences in IPF. Respir Med 2010;104:296–304. 10.1016/j.rmed.2009.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gries KS, Esser D, Wiklund I. Content validity of CASA-Q cough domains and UCSD-SOBQ for use in patients with idiopathic pulmonary fibrosis. Glob J Health Sci 2013;5:131–41. 10.5539/gjhs.v5n6p131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosas IO, Richards TJ, Konishi K, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med 2008;5:e93. 10.1371/journal.pmed.0050093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senoo T, Hattori N, Tanimoto T, et al. Suppression of plasminogen activator inhibitor-1 by RNA interference attenuates pulmonary fibrosis. Thorax 2010;65:334–40. 10.1136/thx.2009.119974 [DOI] [PubMed] [Google Scholar]
- 37.Greene KE, King TE, Kuroki Y, et al. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J 2002;19:439–46. 10.1183/09031936.02.00081102 [DOI] [PubMed] [Google Scholar]
- 38.Estany S, Vicens-Zygmunt V, Llatjós R, et al. Lung fibrotic tenascin-C upregulation is associated with other extracellular matrix proteins and induced by TGFβ1. BMC Pulm Med 2014;14:120. 10.1186/1471-2466-14-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung JH, Oldham JM, Montner SM, et al. CT-Pathologic correlation of major types of pulmonary fibrosis: insights for revisions to current guidelines. AJR Am J Roentgenol 2018;210:1034–41. 10.2214/AJR.17.18947 [DOI] [PubMed] [Google Scholar]