Abstract
Background:
For the 720,000 Americans expected to experience an new acute cardiac event this year, cardiac rehabilitation is an important part of recovery. Symptoms of depression and anxiety undermine recovery efforts leaving recovering patients at risk for diminished functional capacity and heightened risk of mortality. Poor emotion regulation can worsen symptoms of depression and anxiety and hinder recovery efforts.
Objective:
The purpose of this randomized control trial was to evaluate the early efficacy testing of a theoretically based emotion regulation treatment (RENEwS) designed to assist acute cardiac event survivors in cardiac rehabilitation to optimize recovery.
Methods:
Acute cardiac event survivors in cardiac rehabilitation (n=30, 83% men) were randomized to 5 one-hour, in-person group sessions of RENEwS or a phone-based attention-control group. Participants completed measures of depression and anxiety symptoms at three timepoints. Moderate to vigorous physical activity (MVPA) was objectively measured for 7 days at each time point using waist-worn actigraphy monitors. Between-group differences were calculated using ANOVA with Cohen’s f effect sizes calculated to evaluate initial efficacy.
Results:
There was no statistically significant difference in depression, anxiety or MVPA over time based on group assignment (all p > .05). Compared to attention-control participants, RENEwS participants, preliminary effects showed greater reductions in depression (Cohen’s f = .34) and anxiety symptoms (Cohen’s f = .40) but only modest improvements in MVPA from baseline to 5 months (Cohen’s f = .08).
Conclusions:
Findings show that RENEwS is a promising emotion regulation intervention to enhance cardiac rehabilitation and potentially decrease symptoms of depression and anxiety.
Keywords: Emotional Regulation, Depression, Anxiety, Cardiac Rehabilitation, Exercise
It is estimated that every 40 seconds someone in the US experiences a myocardial infarction (MI), an estimated 805,000 people this year.1 Depression and anxiety are risk factors for MI, are prevalent in people with cardiovascular disease, and greatly complicate the recovery of patients following acute cardiac events (e.g., myocardial infarction [MI] or revascularization).2–5 In addition to improving physical health, cardiac rehabilitation (CR) programs seek to improve the quality of life and psychological well-being of people with heart disease through physical activity training and feedback,6,7 education, and psychological support.8–10 CR programs effectively improve survival in patients following acute cardiac events.8,11,12 As an integral part of recovery, national practice guidelines recommend comprehensive CR programs that incorporate components of psychosocial education.13–15 Unfortunately, inconsistent delivery and content in CR programs complicate understanding the benefits of and the effectiveness of CR interventions on cardiac or psychological morbidity.16–18 Further, the gains in self-management activities following CR, particularly physical activity,5,14 are often not sustained,19 which can further negatively impact quality of life2–4 and survival.20,21
Psychological distress is a complicated response characterized by internal suffering that can range from self-focused processing of negative emotions and stressors, to highly intensely aversive and prolonged emotional states such as depression and anxiety.22,23 Psychological distress increases the likelihood of unhealthy habits,24 interferes with life-style and treatment engagement.25–27 worsens cardiac morbidity,18 and reduces overall mortality.28 With respect to recovery from an acute cardiac event, people with co-occurring depressive symptoms are less likely to engage in self-management behaviors29,30 such as exercise, given that psychological distress profoundly undermines physical activity.14,31 Consequently, the mortality risk after MI32 increases among people with co-occurring psychological distress.
Theoretical Framework
Contemporary theories of emotion regulation33 provide a framework to understand normative and disordered emotion functioning, and point to potential novel targets of treatment. In his emotion regulation process model, Gross adopts a functional perspective that views optimal regulation as the coordination of attentional, cognitive, and behavioral responses to emotionally evocative situations.33 Individuals are constantly adjusting and/or course correcting their appraisals of a situation and corresponding behavioral responses to fit their personal goals.33 Emotion regulation reflects a dynamic fine tuning of our attentional and cognitive capacities to help us determine the most appropriate behavioral response given the external and internal stimuli present in a given moment.33,34 Optimal emotion regulation results in an economic use of resources, matching the amount of effort exerted (e.g., attentional, cognitive, behavioral) to the situation, and emitting the least taxing response necessary to achieve the desired goal.35 Engagement in this type of self-management of emotions is a normal daily response to adjust emotional states in a contextually appropriate way.36 Maladaptive emotion regulation, however, can worsen psychological symptoms of depression and anxiety.23
Maladaptive emotion regulation is associated with a greater risk for the development of cardiovascular diseases.23,37,38 This link between emotion regulation and cardiovascular disease is an indication of why a greater proportion of individuals recovering from acute cardiac events may be at particular risk for developing symptoms of depression and anxiety. The inability to effectively regulate emotions triggered by an acute cardiac event may be one important mechanism that prevents a full recovery. Individuals select effective emotion regulation strategies based on past experiences when coping with stressful and life-threatening events.39 Unfortunately, when situations are new, such as a first acute cardiac event, familiar regulation strategies are likely to be ineffective. When under heightened distress, perhaps brought on by the burden of a cardiac event, individuals increasingly prioritize escaping or avoiding negative psychological symptoms,36 which may in turn, undermine effective self-management necessary to attend to other aspects of life such as moderate-to-vigorous physical activity (MVPA) recommended by CR programs.17
Emotion regulation training is one possible intervention to reduce the burden of distress and the accompanying depression and anxiety symptoms and develop new or improve upon adaptive emotion regulation strategies. Interventions that target reduced emotion regulation capacity have shown efficacy in a variety of chronic medical and psychological conditions including distress disorders,34,40 HIV,41 and caregivers of individuals with cancer.42 Theoretically, by employing effective emotion regulation strategies, resources can be preserved23 and redistributed to meet current goals.
A wide variety of emotion regulation strategies exist targeting specific components of the emotion generative or regulating processes.34,43,44 Individual strategies target a different points along the temporal emotional cascade that occurs during a distress episode. One especially exemplary emotion regulation intervention is Emotion Regulation Therapy (ERT), a reformulated cognitive behavior therapy that systematically addresses the multiple components of the distress context.34,45–47 In particular, ERT integrates principles from traditional (e.g., self-monitoring, exposure, imaginal rehearsal)48,49 and contemporary cognitive behavioral therapies (e.g., acceptance & mindfulness meditation practices)50–52 to help patients resolve threat and reward deficits while overcoming the challenges posed by worry, rumination, self-criticism, and other forms of negative self-referential cognitions.23,53
Although emotion regulation interventions have proven efficacy in individuals with generalized anxiety disorder with or without co-occuring symptoms of depression,34 limited evidence is available to support its efficacy in improving depression or anxiety among adults after an acute cardiac event.44,54 Compared with healthy people, people with cardiovascular disease are more likely to use fewer, less diverse, and maladaptive emotion regulation strategies.43 Emotion regulation treatments addressing multiple strategies targeted toward individuals with depression and anxiety demonstrated lasting symptom improvements and increased quality of life among adults with generalized anxiety disorder with or without co-occuring symptoms of depression.34,40 Treatments that target emotion regulation deficits may enhance CR outcomes (self-management of MVPA) by decreasing negative psychological symptoms but this premise needs validation through testing.36,55
The RENEwS (Regulating Emotions to improve self-management of Nutrition, Exercise, and Stress) intervention was specifically designed to improve emotion regulation in individuals recovering from a first acute cardiac event (MI or revascularization) using attention and metacognitive regulation skills training. This new intervention uses emotion regulation as the mechanism to decrease symptoms of depression and anxiety44 in order to improve self-management of MVPA.
The aims of this pilot study were to test the preliminary efficacy of an emotion regulation intervention in CR patients recovering from a first acute cardiac event. Specifically, we sought to determine the preliminary efficacy of the RENEwS program to improve depression and anxiety symptoms and MVPA as compared to an attention control group for people recovering from an acute cardiovascular event (Figure 1).
Figure 1. RENEwS Conceptual Framework.
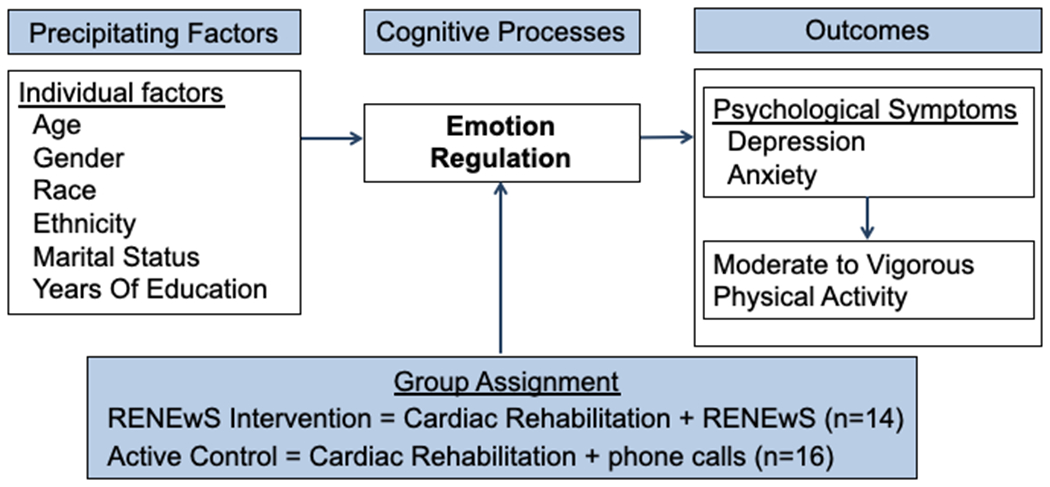
Methods
Research Design and Sample
A two-arm randomized controlled trial design was used in this pilot study. Institutional review board approval was obtained before the start of study procedures. The sample consisted of 30 participants (meeting the 12 per group rule of thumb 56 as an adequate sample size for a pilot study with an anticipated attrition of 20%). As this pilot was part of a parent study (P30NR015326), participants were excluded if they were not able to complete fMRI scans, such as implanted cardiac defibrillators. Participants were recruited from a single phase II CR program in a large Midwestern city. Inclusion criteria were: 1) first cardiac revascularization or MI (verified bymedical records); 2) initiated phase II CR within the past 2 months; 3) planned to complete 12 weeks of phase II CR; 4) lived independently; and 5) were at least 40 years of age (RENEwS intervention components are tailored to middle age and older adults). Exclusion criteria were: 1) did not speak English; 2) had an implanted cardiac defibrillator; and 3) had experienced a cardiac arrest.
Procedures
A convenience sample of participants was recruited by posting flyers in the CR clinics and providing flyers to patients in their first month of CR. Research staff screened medical records for eligible patients and discussed the study with interested potential participants. An appointment was scheduled for consent and baseline measurements in a private area of the CR clinic. Participants were randomized into groups of 14 intervention and 16 attention control participants. Block randomization in three groups of 10 occurred, concealing the randomization sequence in opaque sealed envelopes. Envelopes were prepared using a random number generator by a research assistant. Once a participant completed baseline data collection, randomization was completed by opening envelopes sequentially. Data were collected at baseline, at completion of the intervention (which corresponded to completion of both CR and the intervention), and at 5-months after baseline. To maximize retention during the study, parking passes were provided for study-related sessions, and study personnel sent email, text, or phone reminders to participants about intervention and data-collection sessions. Participants received a monetary gift at each data collection session.
Intervention and Control Protocols
The RENEwS intervention was designed to improve the use of emotion regulation strategies and is described more fully elsewhere.57 Briefly, the content of the intervention included training on emotional awareness, selecting and attending to stressors, and modulating emotional reactions. It consisted of five sessions delivered weekly, 60-minute group sessions using didactic presentation of material, active technique practicing, group discussions, and home practice worksheets. All RENEwS intervention participants received a binder with printed materials. Of the intervention group, 57% completed the in person sessions, and 43% did not. Reasons for not completing the in-person sessions included participant death, study withdrawl, and scheduling conflicts that prohibited attendance.57 To monitor intervention fidelity, a research assistant attended the RENEwS sessions and documented the implementation of key elements based on an investigator-developed fidelity checklist.58 The control group received their usual CR and five weekly phone calls from a trained nurse research assistant for attention control. Control calls lasted approximately 5 to 10 minutes with the nurse discussing healthy living exclusive of mental health and physical activity.
Measures
Demographic Characteristics
Demographic information (i.e., age, gender, race, ethnicity, marital status, and years of education) was obtained through self-report.
Symptoms of Depression and Anxiety
Symptoms of depression and anxiety were measured with a self-reported questionnaire. Participants completed the 7 depression and 7 anxiety items from the short-form of the Depression Anxiety Stress Scale (DASS-21; α= 0.82).59,60 Possible scores range from 0 to 42 for each subscale, with higher scores indicating more depression and anxiety symptoms. DASS scores can be interpreted by severity, with scores below 9 and 7 as the reference for normal levels of depressive and anxious symptoms, respectively. Mild symptoms for each depression and anxiety are between 10 to 13 and 8 to 9, moderate from 14-20 and 10-14, severe from 21-27 and 15-19, and extremely severe over 28 and 20.60 The Cronbach’s α was 0.82 for the depression and 0.62 for anxiety subscales in this sample. The DASS is responsive to change, including minimal clinically important differences in patients with sub-clinical diagnoses of depression or anxiety, while not confounded by disease severity improvements.61 This scale has demonstrated validity in samples of people recovering from cardiovascular hospital admissions and similarly structured pulmonary rehabilitation.61,62
Moderate to Vigorous Physical Activity (MVPA)
Moderate to vigorous physical activity, an intensity similar or greater than a brisk walk, was measured in minutes using actigraphy. Participants were instructed to wear an ActiGraph wGT3X-BT monitor for seven days at each measurement point on their non-dominant waist (hip). This device has high sensitivity (70%) and specificity (93-100%) for measuring MVPA and is validated in individuals participating in CR.63 Wear time was validated following the Troiano algorithm with a minimum wear time of 480 minutes a day, on at least one weekday and one weekend day.64 Energy expenditure (kilocalories) and metabolic equivalents (METs) were determined based on combination adult algorithms and a minimum bout length epoch of ten minutes, drop time of two minutes, and a minimum of 1952 counts per minute.65 Unit calibration was conducted to reduce inter-instrument variability.66
Data Collection
Participants completed all survey instruments in REDCap on a tablet computer at baseline, post-intervention (2 months), and follow-up (5 months). Research assistants helped participants place the waist-worn activity monitors, explained how to use the monitors, and answered any questions about their wear. Participants were given a reminder sheet about wear instructions and the date to return the monitors and a self-addressed, stamped envelope to return the monitor.
Statistical Analysis
Descriptive statistics were computed for sociodemographic and clinical variables to describe the sample and examine the baseline equivalencies of these variables between groups (Table 1). Descriptive statistics were computed for dependent variables of depression, anxiety, and MVPA at the three timepoints. Graphical analyses were completed to evaluate dependent variables for linearity and Mauchly’s test of sphericity was conducted. To test the study aim and evaluate differences in depression and anxiety between the RENEwS and attention control groups, repeated measures ANOVA with group (RENEwS vs. attention control) as a between subject factor and time (baseline and post-intervention) with the dependent measures of depression, anxiety, and MVPA were computed. Significance level was set at p < .05.
Table 1.
Descriptive Statistics for Sociodemographic and Clinical Variables
| Control | Intervention | p-value | |
|---|---|---|---|
|
| |||
| Mean ± SD/Freq (%) | Mean ± SD/Freq (%) | ||
| Number of participants (N) | 16 | 14 | |
| Age (yrs) | 66.8 ± 8.4 | 61.4 ± 6.5 | 0.06 |
| Male (%) | 15 (93.8) | 10 (71.4) | 0.25 |
| Race (%) | 0.44 | ||
| African American/Black | 2 (12.5) | 4 (28.6) | |
| Asian/Pacific Islander | 1 (6.2) | 0 (0.0) | |
| Native American Indian | 1 (6.2) | 0 (0.0) | |
| White (non-Hispanic) | 12 (75.0) | 10 (71.4) | |
| Education (%) | 0.40 | ||
| Did not finish high school | 1 (6.2) | 0 (0.0) | |
| HS Diploma/GED | 2 (12.5) | 0 (0.0) | |
| Some college | 5 (31.2) | 6 (42.9) | |
| 4-year degree or higher | 8 (50.0) | 8 (57.1) |
|
| Cardiac event type | 0.17 | ||
| Myocardial Infarction | 12 (75) | 7 (50) | |
| Revascularization | 4 (25) | 7 (50) | |
Note. Data are represented as Mean ± Standard Deviation and Frequency (%).
We did not seek to identify efficacy between groups with statistical significance as this study was not powered for efficacy testing but rather a pilot study.67 As our sample size was small, we utilized Cohen’s f effect size68 to characterize findings along with more traditional assessment of probability values. Total depression and anxiety scores from the DASS and mean daily minutes of MVPA were used in the between- and within-group analyses. Between-group differences were calculated using Cohen’s f statistics, providing a more conservative estimate69 of the effect size of RENEwS relative to the attention control on improving symptoms of depression, anxiety, and MVPA. We assumed missing outcome values in the models were missing at random. Exploratory within-group differences were calculated by identifying participants above and below mean depression at baseline to describe the effect on depression scores. Similarly, we identified participants above and below mean anxiety at baseline to describe the effect on anxiety scores. Mean group scores for both depression and anxiety are compared to standardized cut-points.59 High and low baseline groupings (by mean symptoms for depression and anxiety) were then used to describe the relative impact on MVPA. All statistical analyses for this study were conducted using statistical software SPSS 26.
Results
Table 1 shows the descriptive statistics for the participants, including baseline equivalencies between the groups. Participants were representative of the patients in this CR center, being mostly male, white, and having higher education. No significant differences were found in demographic or clinical variables between the RENEwS and attention control groups at baseline.
Significant differences were noted in baseline anxiety and depression between the RENEwS and attention control groups at baseline (see Table 2), with RENEwS intervention participants reporting greater symptoms than those in the attention control group. Examining the data descriptively, depressive symptoms decreased from baseline to intervention completion for the RENEwS group, but not in the attention control group. The initial decrease in depressive symptoms in the RENEwS group was not sustained, but stayed below baseline. Anxiety symptoms decreased for both groups from baseline to intervention completion. At 5 months, symptoms of anxiety returned to near baseline levels for the attention control group while the intervention group anxiety symptoms remained below what was reported at baseline. Participants in both groups increased in mean daily MVPA between baseline and intervention completion, with additional MVPA gains experienced in the RENEwS group and a drop in minutes of MVPA in the attention control group at the 5-month follow-up.
Table 2.
Descriptive Statistics for Dependent Variables
| n | Mean ± SD |
Baseline Differences p-value | Cronbach’s α | |||
|---|---|---|---|---|---|---|
| Baseline | RENEwS Completion | 5 Months | ||||
| Depressive Symptoms | ||||||
| Control | 16 | 2.62 ± 2.99 | 4.00 ± 3.19 | 4.00 ± 3.07 | 0.04 | 0.82 |
| Intervention | 14 | 6.57 ± 6.63 | 4.22 ± 4.18 | 5.33 ± 5.39 | ||
| Anxiety Symptoms | ||||||
| Control | 16 | 4.12 ± 3.54 | 3.50 ± 3.83 | 4.33 ± 4.08 | 0.01 | 0.62 |
| Intervention | 14 | 8.43 ± 5.03 | 4.00 ± 3.16 | 4.89 ± 2.26 | ||
| Moderate to Vigorous Physical Activity (Minutes) | ||||||
| Control | 11 | 21.60 ± 16.01 | 24.21 ±17.15 | 23.21 ± 20.37 | 0.92 | |
| Intervention | 7 | 22.30 ± 6.76 | 23.91 ± 11.71 | 25.52 ± 14.88 | ||
Note. Data are represented as Mean ± Standard Deviation. Bolded p-values are significant at the 0.05 level.
The aim of this study was to determine the preliminary efficacy of the RENEwS program to improve depression and anxiety symptoms and MVPA as compared to an attention control group. Mauchly’s Test of Sphericity indicated that the assumption of sphericity had not been violated for any of the outcome variables (Depression, χ2(2, N = 21) = 4.98, p = .08; Anxiety, χ2(2, N = 21) = 2.27, p = .32; MVPA, χ2(2, N = 18) = 3.65, p = .16.). None of the group by time interactions reached statistical significance: Depression: F (2,18) = 1.50, p = .25; Wilk’s Λ = 0.86, partial η2 = .14; Anxiety: F (2,18) = 2.14, p = .15; Wilk’s Λ = 0.81, partial η2 = .19; and MVPA: F (2,15) = 1.30, p = .88; Wilk’s Λ = 0.98, partial η2 = .02. However, in this admittedly underpowered study, the group by time interactions associated with Depression (f = .40) and Anxiety (f = .48) correspond to large effects, whereas the group by time interaction for MVPA (Cohen’s f = .14) corresponds to a small effect.
Between group differences at baseline and inclusion of individuals with low symptoms of depression and anxiety complicate interpreting the data. To help describe the effect of RENEwS in the group by time interactions post-hoc analysis was completed for those with low versus high symptoms of depression and anxiety, among the RENEwS group only. Exploratory within-group analyses for individuals in the RENEwS intervention indicated encouraging clinical improvement, particularly for participants with elevated symptoms of depression and anxiety at the outset of the study. Participants with elevated depression symptoms at the outset of the study evidenced reductions in symptom severity into the normative range at post-intervention (See Figure 2). Depression levels were maintained in the mild range of impairment at follow-up. For participants with low baseline levels of depression severity, depression levels remained low throughout the study. The trajectory for anxiety symptoms in participants with high baseline symptoms of anxiety decreased from moderate symptoms to normal symptoms by post-intervention and were maintained at a normal level at follow-up (See Figure 3). For participants with comparatively low baseline levels of anxiety symptoms, symptom levels remained in the normative range throughout the study. Finally, exploratory analyses reveal some potentially meaningful RENEwS-linked effects with respect to MVPA. Among participants with elevated depression symptoms, mean minutes of MVPA increased at each time point, whereas among participants with low levels of depression at baseline, MVPA evidenced a slight decrease over time (See Figure 4). Similarly, among participants with baseline elevations of anxiety, mean minutes of MVPA started very low and decreased slightly at post-intervention, followed by a sharp increase at follow-up (See Figure 5). Conversely, among individuals with low levels of baseline anxiety, mean minutes of MVPA increased at post-intervention that returned to near baseline at follow-up.
Figure 2. Intervention Group Mean Depression Change Over Time.
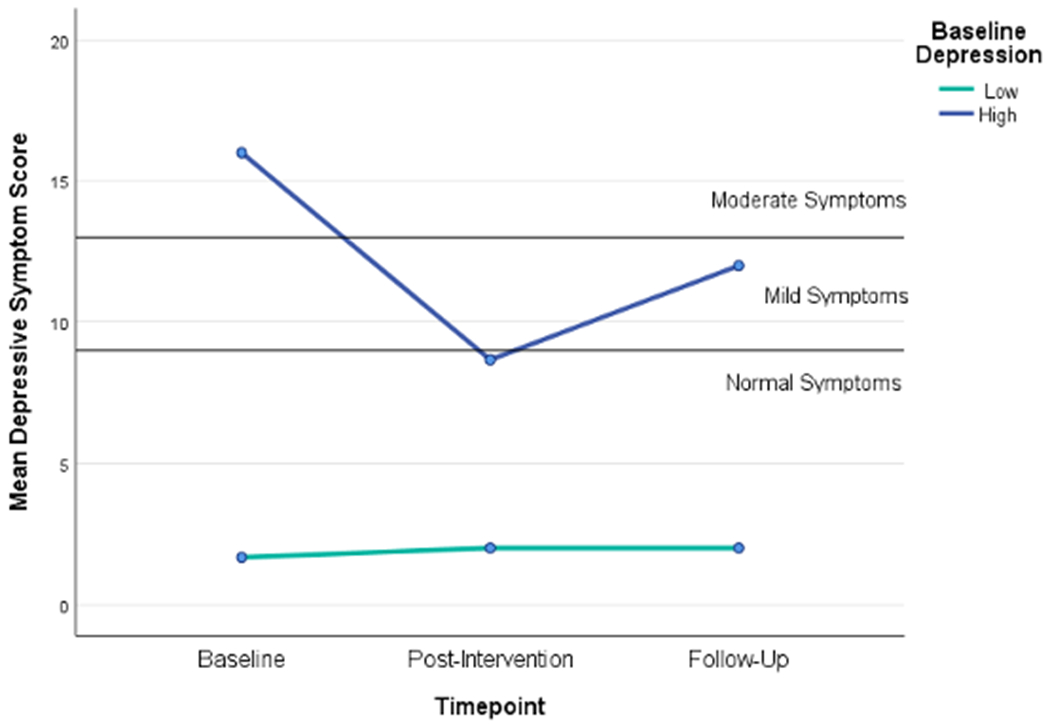
Note. Data are represented as mean changes in depressive symptoms for individuals grouped as those above (high) and below (low) mean baseline symptoms of depression in the RENEwS intervention group (n=14); Depression was assessed using the short-form of the Depression Anxiety Stress Scale (DASS-21).59,60
Figure 3. Intervention Group Mean Anxiety Change Over Time.

Note. Data are represented as mean changes in anxiety symptoms for individuals grouped as those above (high) and below (low) mean symptoms of anxiety in the RENEwS intervention group (n=14); Anxiety was assessed using the short-form of the Depression Anxiety Stress Scale (DASS-21).59,60
Figure 4. Intervention Group Daily MVPA Change Over Time by Depressive Symptom Level.

Note. Data are represented as mean minutes of daily MVPA for individuals grouped as those above (high) and below (low) mean symptoms of depression in the RENEwS intervention group (n=14); MVPA was assessed with actigraphy; MVPA = moderate-to-vigorous physical activity; Depression was assessed using the short-form of the Depression Anxiety Stress Scale (DASS-21).59,60
Figure 5. Intervention Group Daily MVPA Change Over Time by Anxiety Symptom Level.

Note. Data are represented as mean minutes of daily MVPA for individuals grouped as those above (high) and below (low) mean symptoms of anxiety in the RENEwS intervention group (n=14); MVPA was assessed with actigraphy; MVPA = moderate-to-vigorous physical activity; Anxiety was assessed using the short-form of the Depression Anxiety Stress Scale (DASS-21).59,60
Discussion
Previous literature has documented the importance of including psychological support during recovery from a cardiac event.70–72 Supportive treatments are a key component of recovery following a cardiac event. This pilot study focused on evaluating the initial efficacy of a new emotion regulation intervention to address depression and anxiety symptoms that often impede self-management behaviors such as physical activity. The RENEwS intervention described here is an example of one support program for CR patients, with promising preliminary efficacy findings.
Exploration of potential efficacy using both between-group and within-group analyses indicates that the RENEwS intervention does address and sustain decreases in symptoms of depression and anxiety over participating in a cardiac rehabilitation programs that does not include an emotion regulation component. However, levels of depression and anxiety were not equivalent at baseline, which complicates interpretation. Although between group differences did not reach statistical significance, it is important to note the large effect sizes for between group change over time in symptoms of depression and anxiety prompted further inquiry. What we did identify is that for individuals in the RENEwS group with elevated symptoms of depression and anxiety, an evidenced reduction in symptoms severity occurred. Further, among intervention group participants with elevated depression and anxiety symptoms, a positive impact on MVPA is evident. As both minimizing symptoms of depression and anxiety and improving minutes in MVPA are associated with improvements in morbidity and mortality for people with CVD,73–75 these are important preliminary findings and suggest proceeding with further refinement and testing of the RENEwS intervention. Of note, the participants in this study were not Larger, additional studies are needed to determine if depression and anxiety mediate the effects of RENEwS on MVPA in CR patients.
CR is an important contributor to improved outcomes for people recovering from a cardiac event,11,12 with adherence to completing CR negatively impacted by symptoms of depression and anxiety.17 Lifesaving CR support focuses on improving physical activity to meet the American Heart Association recommendations, with supplementary education directed at stress management, improving medication adherence, improving dietary habits, and smoking cessation.76 Although comprehensive phase II CR programs provide some supportive information for reducing depression and anxiety, the need exists to address these issues more directly in individuals with heightened symptoms. Although there is heterogeneity in supplementary programs targeted to improve psychological distress, generally, these programs reduce symptoms of depression, but not anxiety, and reduce recurrent cardiac events.18 Preliminary effect size results indicate that the RENEwS program may be a beneficial supplement to CR in the reduction of both symptoms of anxiety and depression, particularly for individuals with heightened symptoms when entering CR programs. This theoretically based program uniquely offers a variety of emotion regulation strategies, allowing participants to select and use strategies they find most useful in the reduction of feelings of distress.
Limitations
This analysis provides support and guidance for further development of emotion regulation interventions for participants in CR. As this was a pilot study with a small sample size, the determination of confounding variables, such as the number of completed CR sessions, participant engagement or social support, or the proposed mediation of emotion regulation was not possible. There were noteworthy group differences in baseline symptoms of depression and anxiety that cannot be explained. Additionally, due to this small sample at a single CR center, it is unknown if the results are generalizable to the larger population of those recovering from acute cardiac events.
Conclusions
In summary, to prepare for testing this new intervention (RENEwS) in a full trial of its effects, this report describes a carefully constructed assessment study of potential efficacy on important outcomes in the CR population. The results of the study aims as well as the post-hoc analysis demonstrate a need for refinements in future study to direct RENEwS toward participants of CR with symptoms of depression and anxiety. These preliminary data are a first step toward developing and testing an emotion regulation intervention that can be an easily accessed and scalable component of CR programs to improve important outcomes of engagement in MVPA and reduced depression and anxiety symptoms.
Funding Acknowledgement:
Kelly L. Wierenga was supported in this work as The Nurses Charitable Trust of Greater Miami Scholar of the American Nurses Foundation and by NINR grants T32NR015433 and P30NR015326. David M. Fresco was supported by NHLBI Grant R01HL119977, NINR Grant P30NR015326, NCCIH Grant R61AT009867, NIMH Grant R01MH118218, NICHD Grant R21HD095099, and NCI Grant R01CA244185. Megan Alder was supported by NINR grant P30NR015326 and T32NR015433. This secondary analysis was from a P30 pilot study in the SMART Center: Brain-Behavior Connections in Self-Management Science directed by Dr. Shirley Moore at CWRU (P30NR015326). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. There are no conflicts of interest to disclose.
Contributor Information
Kelly L. Wierenga, Assistant Professor, Indiana University School of Nursing.
David M. Fresco, Professor of Psychiatry, Research Professor, Institute for Social Research, The University of Michigan.
Megan Alder, Predoctoral Trainee, Frances Payne Bolton School of Nursing, Case Western Reserve University.
Abdus Sattar, Associate Professor, Department of Population and Quantitative Health Sciences, Case Western Reserve University.
Shirley M. Moore, Edward J. and Louise Mellen Professor of Nursing Emerita, Distinguished University Professor, Frances Payne Bolton School of Nursing, Case Western Reserve University.
Literature Cited
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics - 2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Newman S Engaging patients in managing their cardiovascular health. Heart. 2004;90 (Suppl 4):iv9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luyster FS, Hughes JW, Gunstad J. Depression and anxiety symptoms are associated with reduced dietary adherence in heart failure patients treated with an implantable cardioverter defibrillator. J Cardiovasc Nurs. 2009;24(1):10–17. [DOI] [PubMed] [Google Scholar]
- 4.Olafiranye O, Jean-Louis G, Zizi F, Nunes J, Vincent MT. Anxiety and cardiovascular risk: Review of epidemiological and clinical evidence. Mind Brain J Psychiatry. 2011;2(1):32–37. [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen BE, Edmondson D, Kronish IM. State of the art review: Depression, stress, anxiety, and cardiovascular disease. Am J Hypertens. 2015;28(11):1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozemek C, Strath SJ, Riggin K, Harber MP, Imboden MT, Kaminsky LA. Pedometer Feedback Interventions Increase Daily Physical Activity in Phase III Cardiac Rehabilitation Participants. J Cardiopulm Rehabil Prev. 2020;40(3):183–188. [DOI] [PubMed] [Google Scholar]
- 7.Vogel J, Auinger A, Riedl R, Kindermann H, Helfert M, Ocenasek H. Digitally enhanced recovery: Investigating the use of digital self-tracking for monitoring leisure time physical activity of cardiovascular disease (CVD) patients undergoing cardiac rehabilitation. PLoS One. 2017;12(10):e0186261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther. 2012;2(1):38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalal HM, Doherty P, Taylor RS. Cardiac rehabilitation. BMJ. 2015;351:h5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beniamini Y, Rubenstein JJ, Zaichkowsky LD, Crim MC. Effects of high-intensity strength training on quality-of-life parameters in cardiac rehabilitation patients. Am J Cardiol. 1997;80(7):841–846. [DOI] [PubMed] [Google Scholar]
- 11.Anderson L, Oldridge N, Thompson DR, et al. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2016;67(1):1–12. [DOI] [PubMed] [Google Scholar]
- 12.Reich B, Benzer W, Harpf H, et al. Efficacy of extended, comprehensive outpatient cardiac rehabilitation on cardiovascular risk factors: A nationwide registry. Eur J Prev Cardiol. 2020:2047487319898958. [DOI] [PubMed] [Google Scholar]
- 13.Balady GJ, Ades PA, Bittner VA, et al. Referral, Enrollment, and Delivery of Cardiac Rehabilitation/Secondary Prevention Programs at Clinical Centers and Beyond: A Presidential Advisory From the American Heart Association. Circulation. 2011;124(25):2951–2960. [DOI] [PubMed] [Google Scholar]
- 14.Wurst R, Kinkel S, Lin J, Goehner W, Fuchs R. Promoting physical activity through a psychological group intervention in cardiac rehabilitation: a randomized controlled trial. J Behav Med. 2019;42(6):1104–1116. [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal JA, Wang JT, Babyak M, et al. Enhancing standard cardiac rehabilitation with stress management training: background, methods, and design for the enhanced study. J Cardiopulm Rehabil Prev. 2010;30(2):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West RR, Jones DA, Henderson AH. Rehabilitation after myocardial infarction trial (RAMIT): multi-centre randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart. 2012;98(8):637–644. [DOI] [PubMed] [Google Scholar]
- 17.Rao A, Zecchin R, Newton PJ, et al. The prevalence and impact of depression and anxiety in cardiac rehabilitation: A longitudinal cohort study. Eur J Prev Cardiol. 2020;27(5):478–489. [DOI] [PubMed] [Google Scholar]
- 18.Albus C, Herrmann-Lingen C, Jensen K, et al. Additional effects of psychological interventions on subjective and objective outcomes compared with exercise-based cardiac rehabilitation alone in patients with cardiovascular disease: A systematic review and meta-analysis. Eur J Prev Cardiol. 2019;26(10):1035–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dibben GO, Dalal HM, Taylor RS, Doherty P, Tang LH, Hillsdon M. Cardiac rehabilitation and physical activity: systematic review and meta-analysis. Heart. 2018;104(17):1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alves AJ, Viana JL, Cavalcante SL, et al. Physical activity in primary and secondary prevention of cardiovascular disease: Overview updated. World J Cardiol. 2016;8(10):575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahtinen M, Toukola T, Junttila MJ, et al. Effect of Changes in Physical Activity on Risk for Cardiac Death in Patients With Coronary Artery Disease. Am J Cardiol. 2018;121(2):143–148. [DOI] [PubMed] [Google Scholar]
- 22.Brosschot JF, Verkuil B, Thayer JF. Generalized Unsafety Theory of Stress: Unsafe Environments and Conditions, and the Default Stress Response. Int J Environ Res Public Health. 2018;15(3):464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mennin DS, Fresco DM. Advancing Emotion Regulation Perspectives on Psychopathology: The Challenge of Distress Disorders. Psychol Inq. 2015;26(1):80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clancy F, Prestwich A, Caperon L, O’Connor DB. Perseverative Cognition and Health Behaviors: A Systematic Review and Meta-Analysis. Front Hum Neurosci. 2016;10:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sims M, Diez-Roux AV, Gebreab SY, et al. Perceived discrimination is associated with health behaviours among African-Americans in the Jackson Heart Study. J Epidemiol Community Health. 2016;70(2):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health. 2010;100(5):933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright KD, Jack AI, Friedman JP, et al. Neural processing and perceived discrimination stress in African Americans. Nurs Res. 2020;69(5):331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May HT, Horne BD, Knight S, et al. The association of depression at any time to the risk of death following coronary artery disease diagnosis. Eur Heart J - Qual Care Clin Outcomes. 2017;3(4):296–302. [DOI] [PubMed] [Google Scholar]
- 29.Ziegelstein RC. Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med. 2000;160(12):1818–1823. [DOI] [PubMed] [Google Scholar]
- 30.Gostoli S, Roncuzzi R, Urbinati S, Morisky DE, Rafanelli C. Unhealthy behaviour modification, psychological distress, and 1-year survival in cardiac rehabilitation. Br J Health Psycho. 2016;21(4):894–916. [DOI] [PubMed] [Google Scholar]
- 31.Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry. 2009;31(4):306–315. [DOI] [PubMed] [Google Scholar]
- 32.Bush DE, Ziegelstein RC, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88(4):337–341. [DOI] [PubMed] [Google Scholar]
- 33.Gross JJ, ed Handbook of Emotion Regulation. Second ed. New York: The Guilford Press; 2015. [Google Scholar]
- 34.Mennin DS, Fresco DM, O’Toole MS, Heimberg RG. A randomized controlled trial of emotion regulation therapy for generalized anxiety disorder with and without co-occurring depression. J Consult Clin Psychol. 2018;86(3):268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gross JJ. The extended process model of emotion regulation: Elaborations, applications, and future directions. Psychol Inq. 2015;26(1):130–137. [Google Scholar]
- 36.Sheppes G, Suri G, Gross JJ. Emotion regulation and psychopathology. Annu Rev Clin Psychol. 2015;11(1-440):379–405. [DOI] [PubMed] [Google Scholar]
- 37.Renna ME, Fresco DM, Mennin DS. Emotion Regulation Therapy and Its Potential Role in the Treatment of Chronic Stress-Related Pathology Across Disorders. Chronic Stress. 2020;4:2470547020905787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johansson P, Andersson G, Jaarsma T, Lundgren J, Westas M, Mourad G. Psychological distress in patients with cardiovascular disease: time to do something about it? Eur J Cardiovasc Nurs. 2021. [DOI] [PubMed] [Google Scholar]
- 39.Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16(11):693–700. [DOI] [PubMed] [Google Scholar]
- 40.Renna M, Quintero J, Fresco D, Mennin D. Emotion Regulation Therapy: A mechanism-targeted treatment for disorders of distress. Front Psychol. 2017;8(98). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parsons JT, Rendina HJ, Moody RL, Gurung S, Starks TJ, Pachankis JE. Feasibility of an emotion regulation intervention to improve mental health and reduce HIV transmission risk behaviors for HIV-positive gay and bisexual men with sexual compulsivity. AIDS Behav. 2017;21(6):1540–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Toole MS, Mennin DS, Applebaum A, et al. A randomized controlled trial of emotion regulation therapy for psychologically distressed caregivers of cancer patients. JNCI Cancer Spectr. 2019;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bahremand M, Alikhani M, Zakiei A, Janjani P, Aghei A. Emotion risk-factor in patients with cardiac diseases: The role of cognitive emotion regulation strategies, positive affect and negative affect (a case-control study). Glob J Health Sci. 2015;8(1):173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loucks EB, Schuman-Olivier Z, Britton WB, et al. Mindfulness and cardiovascular disease risk: State of the evidence, plausible mechanisms, and theoretical framework. Curr Cardiol Rep. 2015;17(12):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Applebaum AJ, Panjwani AA, Buda K, et al. Emotion regulation therapy for cancer caregivers-an open trial of a mechanism-targeted approach to addressing caregiver distress. Transl Behav Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renna ME, Quintero JM, Soffer A, et al. A Pilot Study of Emotion Regulation Therapy for Generalized Anxiety and Depression: Findings From a Diverse Sample of Young Adults. Behav Ther. 2018;49(3):403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fresco DM. An Open Trial of Emotion Regulation Therapy via Telehealth for Distressed Adults During the COVID-19 Pandemic. Presented remotely at the annual meeting of the Integrative Medicine and Health Symposium April 11, 2021. [Google Scholar]
- 48.Beck AT, Rush AJ, Shaw B, Emery G. Cognitive Therapy of Depression. New York: Guilford; 1979. [Google Scholar]
- 49.Borkovec TD, Alcaine O, Behar E. Avoidance theory of worry and generalized anxiety disorder. Generalized anxiety disorder: Advances in research and practice. 2004;2004. [Google Scholar]
- 50.Hayes SC, Pistorello J, Levin ME. Acceptance and commitment therapy as a unified model of behavior change. Couns Psychol. 2012;40(7):976–1002. [Google Scholar]
- 51.Linehan M DBT Skills Training Manual. New York, NY: Guilford Press; 2014. [Google Scholar]
- 52.Segal ZV, Teasdale J. Mindfulness-Based Cognitive Therapy for Depression. 2nd ed. New York, NY: Guilford Publications; 2018. [Google Scholar]
- 53.Mennin DS, Fresco DM. Emotion regulation therapy. In: Gross JJ. Handbook of Emotion Regulation. 2nd ed. New York, NY: The Guilford Press; 2014:469–490. [Google Scholar]
- 54.Appleton AA, Buka SL, Loucks EB, Gilman SE, Kubzansky LD. Divergent associations of adaptive and maladaptive emotion regulation strategies with inflammation. Health Psychol. 2013;32(7):748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984. [Google Scholar]
- 56.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4(4):287–291. [Google Scholar]
- 57.Wierenga KL, Fresco DM, Alder ML, Moore SM. Feasibility of an Emotion Regulation Intervention for Patients in Cardiac Rehabilitation. West J Nurs Res. 2021;43(4):338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Resnick B, Inguito P, Orwig D, et al. Treatment fidelity in behavior change research: A case example. Nurs Res. 2005;54(2):139–143. [DOI] [PubMed] [Google Scholar]
- 59.Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44(2):227–239. [DOI] [PubMed] [Google Scholar]
- 60.Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2nd ed. Sydney, N.S.W.: Psychology Foundation of Australia; 1995. [Google Scholar]
- 61.Yohannes AM, Dryden S, Hanania NA. Validity and Responsiveness of the Depression Anxiety Stress Scales-21 (DASS-21) in COPD. Chest. 2019;155(6):1166–1177. [DOI] [PubMed] [Google Scholar]
- 62.Allabadi H, Alkaiyat A, Alkhayyat A, et al. Depression and anxiety symptoms in cardiac patients: a cross-sectional hospital-based study in a Palestinian population. BMC Public Health. 2019;19(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johansson E, Larisch LM, Marcus C, Hagstromer M. Calibration and Validation of a Wrist- and Hip-Worn Actigraph Accelerometer in 4-Year-Old Children. PLoS One. 2016;11(9):e0162436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Troiano RP. Large-scale applications of accelerometers: new frontiers and new questions. Med Sci Sports Exerc. 2007;39(9):1501. [DOI] [PubMed] [Google Scholar]
- 65.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. [DOI] [PubMed] [Google Scholar]
- 66.Boerema ST, van Velsen L, Schaake L, Tonis TM, Hermens HJ. Optimal sensor placement for measuring physical activity with a 3D accelerometer. Sensors. 2014;14(2):3188–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45(5):626–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohen J A Power Primer. Psychological Bulletin. 1992;112(1):155–159. [DOI] [PubMed] [Google Scholar]
- 69.Lakens D Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4(863). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pogosova N, Saner H, Pedersen SS, et al. Psychosocial aspects in cardiac rehabilitation: From theory to practice. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation of the European Society of Cardiology. Eur J Prev Cardiol. 2014;22(10):1290–1306. [DOI] [PubMed] [Google Scholar]
- 71.Chauvet-Gelinier JC, Bonin B. Stress, anxiety and depression in heart disease patients: A major challenge for cardiac rehabilitation. Ann Phys RehabilMed. 2017;60(1):6–12. [DOI] [PubMed] [Google Scholar]
- 72.Braun LT, Wenger NK, Rosenson RS. Cardiac Rehabilitation Programs. UpToDate Web site. https://www.uptodate.com/contents/cardiac-rehabilitation-programs. Published 2017. Updated September 24, 2020. Accessed February 7, 2019.
- 73.Wierenga KL, Lehto RH, Given B. Emotion regulation in chronic disease populations: An integrative review. Res Theory Nurs Pract. 2017;31(3):247–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giles GE, Eddy MD, Brunyé TT, et al. Endurance exercise enhances emotional valence and emotion regulation. Front Hum Neurosci. 2018;12:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ter Hoeve N, Sunamura M, Stam HJ, et al. Effects of two behavioral cardiac rehabilitation interventions on physical activity: A randomized controlled trial. Int J Cardiol. 2018;255:221–228. [DOI] [PubMed] [Google Scholar]
- 76.Thomas RJ, Beatty AL, Beckie TM, et al. Home-Based Cardiac Rehabilitation: A Scientific Statement From the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140(1):e69–e89. [DOI] [PubMed] [Google Scholar]


