Abstract
Reliable, clinic-friendly screening for Chronic postsurgical pain (CPSP) risk is unavailable. Within a prospective, observational study, we evaluated Pediatric Pain Screening Tool (PPST), a concise 9-item questionnaire, as a preoperative screening tool to identify those at higher risk for CPSP (NRS>3/10 beyond three months post-surgery) and poor function (disability/FDI/quality of life/PedsQL)) after spine fusion and Nuss procedures. Incidence of CPSP was 34.86% (38/109). We confirmed PPST scale stability, test re-test reliability (ICC=0.68;p<0.001); PPST measures were positively correlated with known CPSP risk factors (p<0.001) (preoperative pain (SCC:0.672), CASI (SCC:0.357), PROMIS pain interference (SCC:0.569), PROMIS depression (SCC:0.501), PedsQL (SCC:−0.460) and insomnia severity index (SCC0.567). Preoperative PPST and PPST physical sub-scores (median(IQR) were higher in CPSP (2(0.5,4), 1(0,2)) compared to non-CPSP ((1(0,3), 0(0,1.5)) groups (p=0.026, p=0.029) respectively. PPST scores/sub-scores positively correlated with higher FDI at 6 months but only PPST total and PPST psychosocial subscore correlated with higher FDI at 12 months. Based on ROC, optimal PPST cutoff for CPSP was 2 (63.9% sensitivity, 64.7% specificity). CPSP risk was high (48.94% risk) if PPST ≥ 2 (n=47) and medium (22.81%) if PPST<2 (n=57) after spine/pectus surgery. General and risk-strata specific, targeted psychosocial non-pharmacological interventions, need to be studied. Findings need validation in diverse, larger cohorts.
Keywords: Chronic post-surgical pain, children, adolescents, screening, questionnaire, PPST
INTRODUCTION
Chronic postsurgical pain (CPSP) is an important entity recognized as a diagnosis in International Classification of Diseases, ICD-1144; 55 and a sizable problem in children with an incidence of 14.5–38%.28 Chronic pain in children is knoen to lead to significant functional disability, poor quality of life23; 39, and increased health care costs.14; 41 Chronic pain also increases the risk of anxiety, depression and somatic complaints in children and their parents.51 Hence, ability to predict CPSP risk is critical to enable initiation of preventive strategies in high-risk patients.
The ICD-11 definition of CPSP is “pain that develops or increases in intensity after a surgical procedure and persists beyond the healing process, i.e., at least 3 months after the initiating event. The pain has to be localised to the surgical field, projected to the innervation territory of a nerve situated in this area or referred to a dermatome,… and other causes of pain have to be excluded”.44 Studies have shown that anxiety sensitivity4, parental37 and child pain catastrophizing, sleep disturbances and depression37 are important predictors of CPSP in children. We have previously reported a 37% incidence of CPSP in a cohort of spine surgical subjects recruited at a single institution.4 Risk for CPSP in this cohort was predicted by surgical duration, acute postoperative pain and childhood anxiety sensitivity index (CASI). Despite the identification of several CPSP risk factors, translation of research findings into clinical domains and preoperative interventions have been lacking. This is likely due to the burden of the administration of multiple long questionnaires to evaluating these risk factors as they are time consuming, labor intensive and hinder clinical flow. This points to an important need for a rapid and effective screening tool for CPSP risk, that is easy to administer and captures the different domains of psychosocial risk predictors of CPSP.
One such candidate is the Pediatric pain screening tool (PPST), modified from the 9-item Keele STarT Back Screening Tool which has been tested for risk stratification of musculoskeletal pain in adults 18; 19. The PPST is a brief 9-item self-report questionnaire which evaluates prognostic physical (function, pain, sleep quality) and psychosocial (anxiety, depressive symptoms, catastrophizing) constructs with a scoring format. PPST was initially developed and used for screening of children with chronic pain, to rapidly identify addressable treatment targets (e.g., sleep disruption, pain-related fear) and derive cut-off scores for grouping patients into low risk (few negative prognostic indicators, responsive to analgesia, advice, and education), medium risk (moderately unfavorable prognosis, high level of physical/functional prognostic indicators, appropriate for physiotherapy), and high risk (very unfavorable prognosis, high levels of psychosocial prognostic indicators, appropriate for physical and cognitive-behavioral therapy).48 PPST has been found useful to risk stratify children with chronic pain in outpatient pain clinics and for prediction of longitudinal outcomes in children with musculoskeletal pain.17; 46 Since even in healthy children with minimal preoperative pain undergoing surgery, similar physical (function, pain, sleep quality) and psychosocial (anxiety, depressive symptoms, catastrophizing) constructs influence acute to chronic pain transitions, we aimed to evaluate PPST as a screening tool for risk of CPSP. To our knowledge, the PPST has not been used to predict CPSP in the perioperative setting.
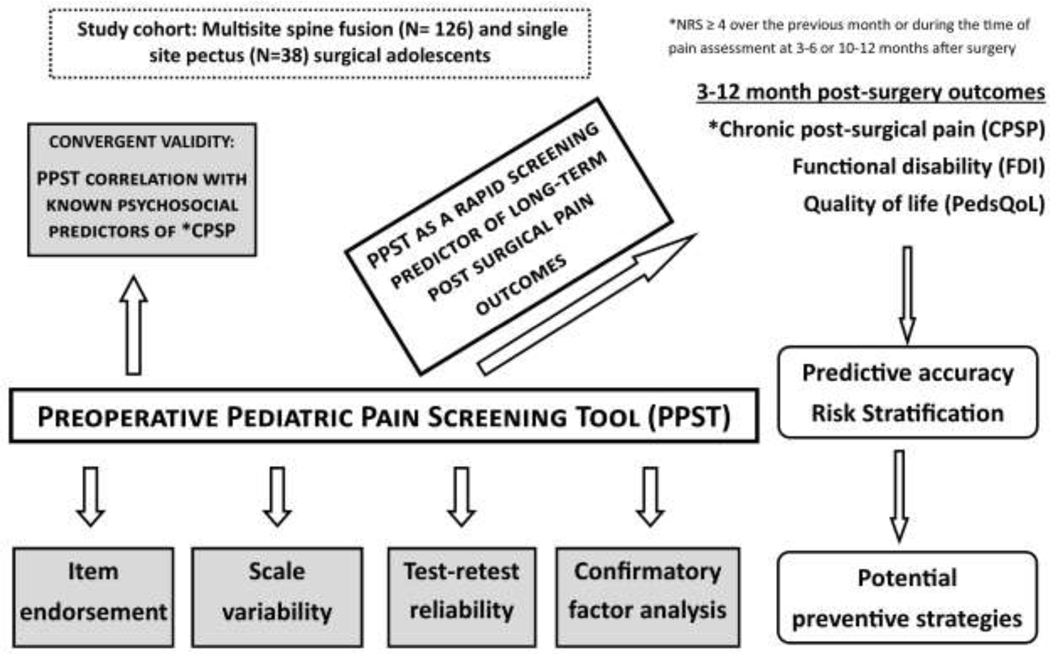
We hypothesized that PPST will independently predict CPSP and long-term functional outcomes, and PPST scores/sub-scores would demonstrate acceptable discrimination of previously identified reference standard risk factors for CPSP (Figure 1). Along with testing the above hypotheses, we aimed to derive cutoff PPST scores/sub-scores for defining physical and psychosocial risk, thereby developing a simple and easy to use decision algorithm for classifying risk for CPSP. We anticipated that the physical versus psychococial sub-scores, and possibly scoring of particular questions, will suggest a priori individualized, targeted preventive strategies. To evaluate these aims, we conducted a secondary analysis within an ongoing prospective, longitudinal genomics study in opioid naïve children undergoing major musculoskeletal surgery (spine fusion for idiopathic scoliosis and Nuss procedure for pectus excavatum) at three pediatric institutions (ClinicalTrials.gov Identifier: NCT02998138). These surgeries are associated with a high risk for CPSP, and share similar pain pathophysiology, and similar risk factors.4; 28; 38; 39; 45; 56 Since the predictive accuracy of psychosocial and perioperative predictors for CPSP was ≈ 70%, we anticipate similar accuracy for PPST as a predictor. 4
Figure 1:
Schematic diagram representing the relationships of known risk factors and the study aims to evaluate PPST as a screening tool, its convergent validity, characteristics (reliability, variability, etc.) and the final goals including risk stratification and recommendations.
MATERIALS AND METHODS
This prospective, observational, longitudinal study was conducted within a larger genomics study (ClinicalTrials.gov Identifier: NCT02998138). The multisite study was approved under a single institutional review board (IRB) at the sponsoring site. Results pertaining to psychosocial factors and epigenetic factors influencing CPSP using data from a single institution spinal fusion cohort recruited as part of the larger study have been previously published.5; 6
Participants
We prospectively recruited subjects with a diagnosis of idiopathic scoliosis undergoing posterior spine fusion in three institutions using institution specific standard anesthesia/pain protocols, and subjects with a diagnosis of pectus excavatum undergoing Nuss procedure at a single institution. Appropriate parental consent and patient approval was obtained.
Inclusion criteria:
American Society of Anesthesiologists (ASA) physical status ≤ 2 (mild systemic disease), aged 8 years and above, regardless of sex or race, with a diagnosis of idiopathic scoliosis undergoing posterior spine fusion surgery and subjects with a diagnosis of pectus excavatum undergoing Nuss procedure. These are typically adolescent conditions. Exclusion criteria: Patients with history of opioid use in the past six months, liver and renal disease, pregnant or breastfeeding women, developmental delay, cancer, and those not fluent in written and/or spoken English.
Recruitment and follow up
Eligible patients were identified from the surgical schedule and approached for potential participation in the study either in person or by telephone interview in the preoperative period. Preoperative questionnaires were completed in person or electronically via REDCap. Two sites followed the same spine fusion protocols: total intravenous anesthesia (propofol and remifentanil) followed by patient controlled analgesia (morphine/hydromorphone), muscle relaxants, acetaminophen and ketorolac. Subjects were transitioned to oral opioids by postoperative day 1 or 2. The third site used the same protocol, with the addition of preoperative gabapentin. Pectus protocol (at one site) included thoracic epidural, pregabalin, acetaminophen, ketorolac and muscle relaxants with as needed intravenous opioids followed by oral opioids. All patients were requested to complete 3–6 month and 10–12 month postoperative followup questionnaires either electronically via REDCap or by telephone interviews. Participants received incentives ($30 per participant - $10 preoperatively on recruitment and $10 on each successful patient followup) to motivate participation and completion of preoperative and followup questionnaires.
Data collection:
Perioperative data collected included demographics, surgical, anesthesia and analgesia details. Preoperatively, participants were asked to complete a set of questionnaires including PPST, PainDETECT,11 Childhood Anxiety Sensitivity Index (CASI),47 Patient Related Outcome Measures Information System - depression scale (PROMIS-DS),36 PROMIS pain interference scale (PROMIS-PIS),36 Functional Disability Inventory (FDI),52 Pediatric Quality of Life (PedsQL),49 andInsomnia Severity Index (ISI) 30and Pain Intensity Numerical Rating Scale (NRS) for average pain over last 4 weeks (0–10).50 These questionnaires are described in Table 1.
Table 1:
Questionnaires used in the study.
| Questionnaire | Description |
|---|---|
| Pediatric Pain Screening Tool (PPST)48 | Nine items in two domains- physical and psychosocial.Total score range 0 to 9. Physical subscale is focused on assessing presence of comorbid pain, functional ability and quality of life measures such as attending school, walking and sleep quality and psychosocial subscale is focused on assessing pain related fear, anxiety, catastrophizing, depression and pain inconvenience. |
| Child Anxiety Sensitivity Index (CASI)47 | 18 items with total score range 18 to 54. Refers to the degree of child’s anxiety being associated with harmful somatic, psychological and social consequences such as “feeling like throwing up, going to faint, don’t want others to know that I’m scared.” |
| Functional Disability Index (FDI)52 | 5-point Likert scale with total score range 0 to 60. 15-item scale that assesses the extent to which children experience difficulties in completing everyday specific tasks (e.g., walking to the bathroom, eating regular meals, being at school all day).52 Used in many pediatric populations, including children with chronic pain26 and post-surgical pain.25; 32 |
| Pediatric Quality of Life measure (PedsQL)49 | 23 items with total score range 0 to 92.Assess the child’s functional and mental status in the domains of health, activity, personal feelings, ability to get along with others and school problems. |
| NIH Patient-Reported Outcome Measurement Information System (PROMIS) Pediatric Short Form v2.0 Depressive Symptoms 8a36 | 8 items with total raw score range 8–40 which is converted into a T score based on a table. T score of 50 is average for US populations. Eight -item short form which assesses self-reported negative mood (sadness, guilt), views of self (self-criticism, worthlessness), social cognition (loneliness, interpersonal alienation), and decreased positive affect. Validated in 8–17 year olds, and absence of suicidal intent assessment, obviates responsibilities beyond the scope of the study.8; 24 |
| PROMIS Pediatric SF v2.0 Pain Interference 8a | Eight items uilizing a 7–day recall period. total score range 8–40. Assesses the consequences of pain in daily activities of life in social, cognitive, emotional and physical activities. Measures self-reported consequences of pain on relevant aspects of a person’s life and may include the extent to which pain hinders engagement with social, cognitive, emotional, physical, and recreational activities. Validated for ages>7 year old.1 |
| PainDETECT11 | Seven questions and visual chart to mark area of pain and radiation. Total score range 0 to 38; score > 19 indicates likely neuropathic component. Reliable screening tool for neuropathic pain, with high sensitivity, specificity and positive predictive accuracy in chronic pain conditions - |
| Insomnia Severity Index (ISI)30 | 7 questions designed to assess the nature, severity, and impact of insomnia, and monitor treatment response30; Severity of sleep onset, sleep maintenance and early morning wakening problems, sleep dissatisfaction, interference of sleep difficulties with daytime functioning, noticeability of sleep problems by others, distress caused by the sleep difficulties; cutoff score of 10 had a 86.1% sensitivity and 87.7% specificity for detecting insomnia cases; High internal consistency (Cronbach α of 0.90) |
NIH: National Institutes of Health; PROMIS: Patient-Reported Outcomes Measurement Information System All questionnaires used in this study are approved for use in children ≥ 8 years of age and have been validated in previous studies for test-retest reliability.
PPST:
The PPST is a brief, 9-item self-report questionnaire developed for rapid identification of risk for poor pain coping.48 The first four items evaluate physical (presence of widespread pain, functional ability such as walking, quality of life measures such as attending school, and, sleep quality). The next four questions evaluate psychosocial (pain associated fear, anxiety, catastrophizing, depressive symptoms and pain inconvenience) constructs. Patients were instructed to consider the previous two weeks while answering the questions. Items 1–8 require respondents to check “yes” or “no.” All “yes” responses are scored as 1. For item 9, patients check boxes with ratings from “not at all” to “a whole lot.” The ratings “a lot” and “a whole lot” are scored as 1, whereas the lower ratings of “not at all”, “a little”, and “some” are scored as 0. Summing all items, PPST total scores range from 0 to 9. Psychosocial subscale scores range from 0 to 5 and Physical subscale scores range from 0 to 4 44.
Data collected 3–6 months and 10–12 months after surgery: Patients were asked to complete the Pain Intensity (NRS), Functional Disability Index (FDI), Pediatric Quality of Life measure (PedsQL), PPST and painDETECT questionnaires at 3–6 months and 10–12 months after surgery. Electronic reminders were sent to participants every week for a maximum of three reminders followed by a telephone call if the questionnaires are not completed.
Outcomes:
Primary Outcome:
CPSP was considered to be present if participant reported a pain score of NRS ≥ 4 over the previous month or during the time of pain assessment at 6 months post-surgery or 12 months after surgery. While we recognize that the presence of any amount of pain may be significant, we used NRS ≥ 4 to identify patients with moderate to severe pain based on previous studies.4; 13
Secondary Outcomes:
Functional disability and quality of life at 3–6 months and 10–12 months as continuous variables.
Statistical analysis
Study recruitment, demographics and baseline variables:
Study recruitment and retention were determined. We compared demographics (age, sex, race), preoperative PPST, FDI and PedsQL measures between the subjects whose followup data were missing and for those we had outcomes, as these are relevant to PPST correlation with outcomes. Demographics and baseline variables were analyzed for descriptive statistics and compared between CPSP and non-CPSP subjects with outcomes using two-sample t-tests or Wilcoxon rank-sum tests, as appropriate. We compared preoperative psychosocial characteristics between the surgical groups to ensure the findings were not driven by a particular surgical group.
PPST scale variability, item endorsement and test-retest reliability:
Descriptives for PPST (total and subscales) and item endorsement were derived. PPST scores were analyzed using Wilcoxon rank-sum tests or Kruskal-Wallis Test to evaluate differences by sex, race, surgery type and surgical site. Test-retest reliability for PPST was assessed using intraclass correlation coefficient (ICC) based on a two-way mixed-effects model and preoperative and postoperative 6 month PPST within non-CPSP patients.27
Confirmatory factor analysis (CFA):
CFA was used to test whether measures of the construct were consistent with construct domain by mapping the 2 PPST sub-scores to the first 8 PPST items (PPST physical subscore: 1st 4 items and PPST psychosocial subscore to the last 4 items). Tetrachoric correlation was used for binary items and the means and variance adjusted weighted least squares (WLSMV) was used to estimate model parameters. Analysis was run in Mplus version 8.3.
Convergent validity was examined for association of PPST (total and subscales) and known risk CPSP psychosocial risk factors (preoperative CASI, PCS-C, PROMIS, FDI, pain, psychosocial (total and subscales) using Spearman or Pearson correlation coefficients.
CPSP characterization at 6 and 12 months:
Measures related to pain and function were compared between CPSP groups at 6 and 12 months using using two-sample t-tests or Wilcoxon rank-sum tests, as appropriate. In addition, nature of pain at those time points were also described as incidence % of subjects who described nature of pain by checking that character, among those who described pain.
Univariate analysis:
Pearson or Spearman correlation coefficients were used to study the relationship between PPST (total and subscales) with secondary outcomes (postoperative FDI and PedsQL total and subscales at 6 and 12 months after surgery). Associations between PPST (total, 1–4, and 5–9) and CPSP at 6 and 12 months were tested using Wilcoxon rank-sum tests.
Receiver operant characteristic (ROC) and risk stratification:
We determined risk groups based on CPSP, FDI (FDI 3–6 and 10–12 months postoperatively were combined and upper tertile was derived and used as cutoff to dichotomize postoperative FDI, where higher values indicate worse outcomes) and PedsQL scores (PedsQL 3–6 and 10–12 months postoperative scores were combined and lower tertile was derived and used as cutoff to dichotomize postoperative PedsQL, where lower values indicate worse outcomes). Logistic regression models using PPST scores to predict CPSP, PPST 1–4 physical subscores to predict FDI and PPST 5–9 psychosocial subscore to predict PedsQL psychosocial score were fitted. We generated ROC curves and calculated the area under the curve (AUC). Optimal cutoff on predicted probability and PPST total and subscores were determined by maximizing Youden’s index (Youden Index = sensitivity + specificity-1 (or weighted Youden Index considering the cost of false positive and false negative)). Finally, risk for CPSP was stratified as low (<10%), medium (10–30%) and high (>30%) based on the PPST and subscore cutoffs and distribution of CPSP within each risk stratum was calculated.
Power analysis:
Power analysis was based on accuracy in estimating AUC when predicting CPSP with PPST total.15 If we assume that, after accounting for retention, we have 100 subjects with data on CPSP, and we assume a 30–40% rate of CPSP. With a sample size of 30 (40) cases and 70 (60) controls, when the true AUC is between 0.6 and 0.7, marginal error of estimate (i.e. the difference between true AUC and its estimate) does not exceed 0.12 (0.11) with 95% confidence level. In the power calculation the variance of AUC was estimated based on binormal assumption and normal approximation was used in constructing confidence interval for AUC.
RESULTS
Subject recruitment and retention
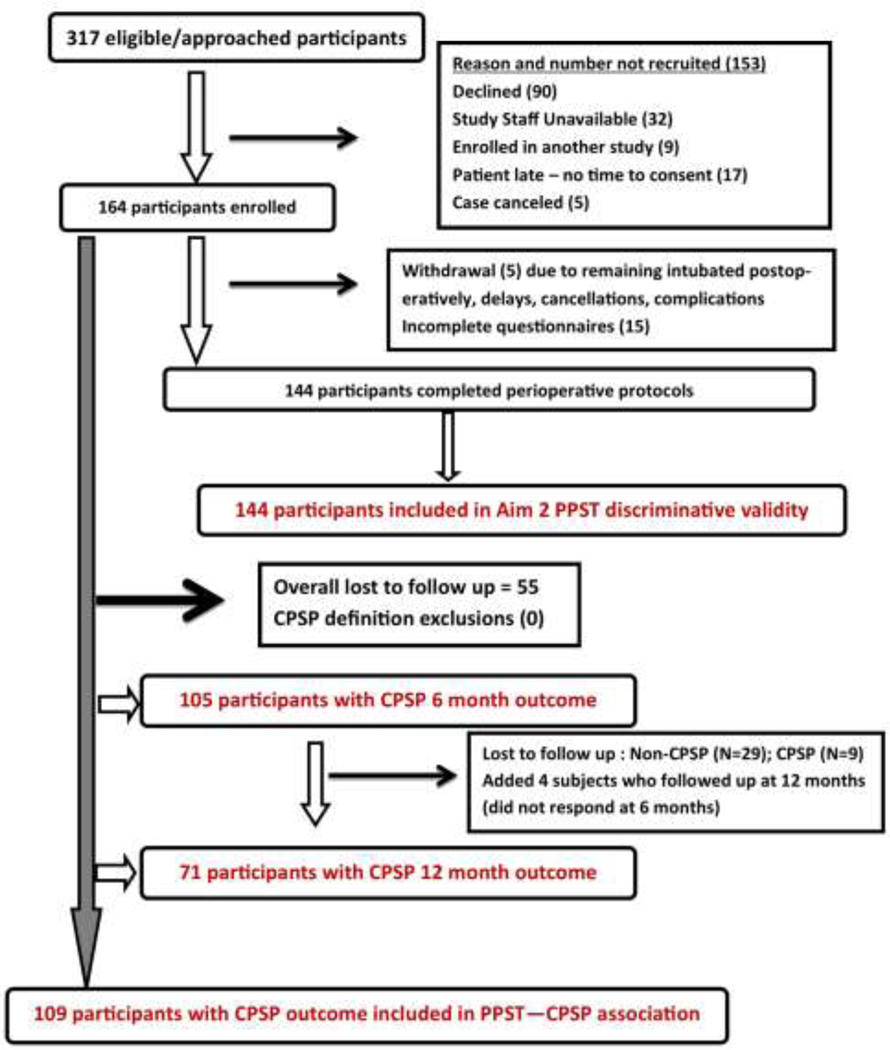
Flow diagram for recruitment is depicted in Figure 2. We approached 317 participants and consented 126 spine subjects in three sites (N=66, 48, 12 respectively at each site) and 38 pectus subjects at a single site. After accounting for withdrawals and incomplete questionnaires, 144 participants completed the study. All subjects with CPSP outcomes were included for analyses in predictive aim (N=109). All subjects who had preoperative PPST and standard reference questionnaire data were included for the convergent validity aim of the study (N=144). Majority of subjects underwent spine surgery (76.83%) while the rest underwent pectus surgery. The entire cohort had a mean age of 14.88 years (SD 3.08) (>85% subjects were between 12.06 and 17.75 years old), was 64.81% females and 80.92% White. As would be expected, the sex ratios were different for spine (81.67 % female) and pectus cohorts (78.95 % male).
Figure 2:
Recruitment workflow diagram denoting the numbers of eligicble, approached, recruited and retained subjects. Reasons for inability to recruit and withdrawal are provided. Reasons for loss to follow up are solely due to inability to reach the subject via email, phone/mail or unwillingness to complete questionnaire despite reminders. Of 109 subjects included in overall CPSP analyses, 105 were included at 6 months and 71 at 12 months.
Comparison of subjects with outcomes vs. those lost to follow-up:
Of 164 patients, 109 patients had outcomes. Hence, approximately one-third were lost followup. On comparing subjects who had outcomes to those who didn’t due to loss to follow up, the groups were comparable in all measures (age, sex, surgical type and race composition, preoperative PPST total and sub-scores and preoperative PedsQL) except FDI, with higher values (median (IQR) of 8 (3–14) in the loss to follow up group compared to 4 (0–9.5 in the group with outcomes (p=0.016). (Supplementary Table 1).
Demographics and surgical details of CPSP and non-CPSP cohorts
Baseline demographics of the CPSP cohort are described in Table 2. CPSP outcomes were determined in 109 subjects. The incidence of CPSP was 34.86% in our entire cohort. The incidence was 38.27% (31/81) for the spine cohort and 24.14% (7/28) for the pectus cohort. Female sex was associated with higher odds for CPSP (6.756 (95% CI 2.165–21.090; p<0.001) in both cohorts. Race was associated with CPSP overall but not in either surgical cohort separately. On comparing preoperative psychosocial characteristics between the surgical groups, we found following mean (standard deviation) or median (IQR) and p-values for the comparison of measures in pectus and spine groups for CASI (pectus: 30.3 (5.3); spine: 29.3 (5.7); p:0.342), PROMIS depression (pectus: 46.3 (8.7); spine: 47.8 (11.3); p=0.399), PROMIS PI (pectus: 48.2 (35.2, 54.0); spine: 40.6 (34.0, 52.7); p=0.254), and PedsQL psychosocial score (pectus: 78.3 (71.7, 90.0); 78.3 (68.3, 88.3); p=0.725) show no significant differences in preoperative psychosocial measures between the surgical groups.
Table 2.
Demographics, Preoperative Pain, Psychosocial and Functional Characteristics for CPSP and Non-CPSP Cohorts.
| Demographics | All CPSP patients (N = 109) | No CPSP (N = 71) | CPSP(N = 38) | Pvalue |
|---|---|---|---|---|
| Age (y)‡ | 14.67(13.24, 15.95) | 14.82 (13.5, 16.24) | 14.51 (13.53, 15.79) | .543 |
| Weight (Kg)‡ | 54.9 (48.3, 63.5) | 54.7 (46.2, 63.2) | 56.6 (49.95, 66.85) | .615 |
| Height (cm† | 164.96 ± 10.15 | 165.94 ± 11.09 | 162.97 ± 7.68 | .140 |
| Body Mass Index (Kg/m2)† | 20.46 ± 4.52 | 19.16 ± 3.06 | 24.37 ± 6.1 | .067 |
| Sex (Female/Male); Female %§ | 74/35; 64.81% | 39/31; 56% | 34/4; 89% | <.001 |
| Ethnicity (Non-hispanic %)§ | 96% | 97% | 94% | .599 |
| Race (Caucasian/African-American/ Other); Caucasian%§ | 88/13/8; 83.02% | 63/3/4; 90% | 25/10/1; 69% | <.001 |
| Surgery type (N, %)§ | Spine surgery (81, 74.31%) | 49, 69.02% | 32, 84.21% | .084 |
| Pectus surgery (28, 25.69%) | 22, 30.98% | 6, 15.79% | ||
| Baseline functional and pain characteristics | ||||
| PedsQL total‡ | 138.54 (94.57, 171.67) | 136.25 (109.38, 171.67) | 145.21 (89.13, 168.96) | .613 |
| PedsQL psychosocial‡ | 78.33 (70.00, 90.00) | 78.33 (68.33, 85) | 78.33 (70, 93.33) | .466 |
| PedsQL physical‡ | 84.38 (62.50, 96.88) | 71.88 (53.13, 90.63) | 84.38 (68.75, 96.88) | .155 |
| Pain scores‡ | 2.75 (1, 4) | 3.5 (1.5,5) | 1.75 (0,4) | .003* |
| Pain DETECT‡ | 2.50 (0, 7) | 3 (1,8) | 1 (0,6) | .022* |
| Medications§ | 14/28 (50%) | 5/36 (13%) | .002* | |
| Acetaminophen 11 | Acetaminophen 2 | |||
| NSAID 3 | NSAID 3 | |||
| Preoperative psychosocial characteristics | ||||
| Preop PPST total‡ | 1 (0, 3) | 2 (0.5,4) | 1 (0,3) | .026* |
| Preop PPST physical‡ | 1 (0, 2) | 1 (0,2) | 0 (0,1.5) | .029* |
| Preop PPST psychosocial‡ | 0 (0, 2) | 1 (0,2) | 0 (0,1.5) | .092 |
| CASI score‡ | 30 (26, 34) | 31 (28, 36) | 29 (24.5, 33) | .023* |
| PROMIS t-score (depression)† | 47.54 ± 9.73 | 51.9 ± 7.7 | 46.4 ± 10.0 | .022* |
| PROMIS t-score (pain interference)‡ | 47.4 (35.2, 54) | 53.1 (49.6, 57.2) | 43.7 (35.2, 53.7) | .257 |
| ISI‡ | 5 (3, 10.5) | 11.5 (6,15) | 4 (2,9) | .043* |
Abbreviation: NSAID, Non-steroidal anti-inflammatory drugs.
indicates P <.05 level of significance;
data exhibited normal distribution; shown as mean ± SD and compared using 2 sample t-tests;
data exhibited non-normal distribution; shown as median ± Interquartile range and compared using 2 sided Wilcoxon test;
shown as frequency and compared using Fisher's exact tests.
Preoperative pain, functional and psychosocial characteristics of CPSP and non-CPSP cohorts
Baseline preoperative characteristics are described in Table 2. Preoperative average pain intensity (NRS) was 2.94 (SD 2.37) for the entire cohort. Pain and PainDETECT scores and use of analgesics were significantly higher in the CPSP group before surgery, though functional measures (PedsQL, Pain interference) were not different. Nature of pain was described sharp (31%), stabbing (22%), throbbing (12.5%), crampy (28%), tightness (50%) and burning (12.5%) among those who had pain preoperatively. As expected, comparison of preoperative psychosocial characteristics showed significantly higher PPST, CASI, PROMIS depression and ISI scores in the CPSP group compared to non-CPSP group.
PPST scale variability, item endorsement and test-retest reliability
Preoperative PPST scores ranged from 0 to 7 (2.1±2.31). We did not find statistically significant PPST scale variability in the cohort. Preoperative PPST scores (median (interquartile range)) were compared between age groups (<12 years: 1 (0, 3); >12, <18 years: 1 (0,3); >18 years: 3(1,4); p=0.423), sites (site 1: 1 (0,3); Site 2: 2 (0, 4); Site 3: 3 (0, 5); p=0.739) and race (Caucasian: 1 (0,4); African-American: 1 (0,4); Other: 1(0,3.5); p-value:0.851) using Kruskall-Wallis test. Wilcoxon tests were used to compare PPST by surgery type (Pectus: 1 (0,3); Spine: 1 (0, 4); p-value:0.433) and sex (Female: 1 (0, 4); Male: 1 (0, 3); p-value:0.575).
We note that every item was endorsed to be positive (scored as 1) in a higher proportion of those who went on to develop CPSP than those who did not, except for q9 (Overall, how much has pain been a problem over the last 2 weeks?), indicating pain was not a problem in either group preoperatively (Table 3). The items with the significant difference in scoring between the groups were item 2 “I can only walk a short distance because of my pain” scored as yes in the CPSP group on the physical sub-scale, and item 6 “I worry about my pain a lot” on the psychosocial subscale. The total PPST score demonstrated acceptable test-retest reliability at 6 months [ICC = 0.68 (p<0.001)].
Table 3:
Pediatric pain screening tool items and preoperative item endorsement among those who developed and did not develop chronic post-surgical pain (CPSP)
| PPST Item | Response: Agree % | p value | |
|---|---|---|---|
| No CPSP | CPSP | ||
| 1. My pain is in more than one body part | 27.94% | 38.89% | 0.254 |
| 2. I can only walk a short distance because of my pain | 11.76% | 33.33% | 0.008 |
| 3. It is difficult for me to be at school all day | 17.65% | 22.22% | 0.573 |
| 4. It is difficult for me to fall asleep and stay asleep at night | 26.47% | 41.67% | 0.113 |
| Physical subscale score PPST 1–4 (0 vs >0) | 44.12% | 66.67% | 0.029 * |
| 5. It’s not really safe for me to be physically active | 10.29% | 13.89% | 0.585 |
| 6. I worry about my pain a lot | 23.53% | 47.22% | 0.014 * |
| 7. I feel that my pain is terrible and it’s never going to get any better | 8.82% | 13.89% | 0.424 |
| 8. In general, I don’t have as much fun as I used to | 25% | 30.56% | 0.543 |
| 9. Overall, how much has pain been a problem in the last 2 weeks? # | 16.18% | 13.89% | 0.758 |
| Psychosocial subscale score PPST5–9 (0 vs >0) | 41.18% | 58.33% | 0.095 |
responses “not at all”, “a little”, “some” were scored as disagree, “a lot”, “a whole lot” were scored as agree. PPST: Pediatric pain screening tool, CPSP: chronic postsurgical pain
P<0.05
Confirmatory factor analysis:
The standardized regression weights for item loading on PPST sub-score factors ranged from 0.610 to 0.782. Regression weights and standard errors are presented in supplementary Figure 1. Goodness of fit for CFA was confirmed using chi-square statistic (p=0.249), Root Mean Square Error of Approximation (Estimate 0.037 (95% CI 0.00–0.086)) and Comparative Fit Index (0.990). Standardized model correlation between factor 1 (ppst1–4) and factor 2 (ppst5–8) was 0.975 (two-tailed p-value <0.001 for all CFA factor loadings).
Convergent validity – PPST correlation with known standard risk factors for CPSP
We found that preoperative PPST total scores positively correlated with preoperative pain scores (SCC 0.672; p<0.001), CASI (SCC 0.357; p<0.001), preoperative PROMIS measures for depressive symptoms (SCC0.569; p<0.001), pain interference (SCC 0.501; p<0.001) and ISI (SCC 0.567; p<0.001) and negatively with PedsQL measures (SCC −0.460 to −0.614; p<0.001) (Table 4). Similarly, preoperative PPST sub-scores were also significanlty correlated with the constructs in the same direction as PPST total score. This finding supports our hypotheses that PPST score strongly correlates with known gold standards in several relevant domains.
Table 4:
Correlation of preoperative PPST score and sub-scores with known risk factors of CPSP (Convergent validity)
| Preoperative measure | PPST 1–4 physical score | PPST 5–9 psychosocial score | PPST 1–9 total score | |||
|---|---|---|---|---|---|---|
| SCC | p-value | SCC | p-value | SCC | p-value | |
| Pain score | 0.606 | <.001* | 0.628 | <.001* | 0.672 | <.001* |
| CASI | 0.389 | <.001* | 0.283 | 0.001* | 0.357 | <.001* |
| PROMIS pain interference | 0.565 | <.001* | 0.534 | <.001* | 0.569 | <.001* |
| PROMIS depression | 0.507 | <.001* | 0.490 | <.001* | 0.501 | <.001* |
| PedsQL total score | −0.439 | <.001* | −0.411 | <.001* | −0.460 | <.001* |
| PedsQL physical score | −0.581 | <.001* | −0.581 | <.001* | −0.638 | <.001* |
| PedsQL psychosocial score | −0.529 | <.001* | −0.584 | <.001* | −0.614 | <.001* |
| ISI | 0.596 | <.001* | 0.427 | 0.011* | 0.567 | <.001* |
CPSP: chronic postsurgical pain, PPST: pediatric pain screening tool, FDI: Functional disability index, PedsQL: pediatric quality of life measure, CASI: Child anxiety sensitivity index, PROMIS: Patient-Reported Outcomes Measurement Information System, SCC: Spearman correlation coefficient; ISI: Insomnia Severity Index
p value less than 0.05 was considered to be significant
Characterization of pain and functional measures at 6 and 12 months
The incidence of CPSP was 27/105 (25.7%) at 6 months and 20/71 (28.2%) at 12 months, as subjects lost to follow up between 6 and 12 months mostly were those who did not have CPSP at 6 months (N=29). Most pain and functional measures are significantly higher in CPSP groups at both time points compared to non-CPSP groups. (Table 5) Of note, median PainDETECT, FDI and PedsQL scores, as well as medication use seem to improve by 12 months compared to 6 months in both CPSP and non-CPSP groups. These findings supports our assumption that FDI and PedsQL are reflective of functional outcomes mirroring pain experiences months after surgery.
Table 5:
Comparison of pain and functional measures between patients with and without chronic postsurgical pain at 6 and 12 months
| Measures | 6 months | 12 months | ||||
|---|---|---|---|---|---|---|
| CPSP (N=27) | No CPSP (N=78) | P value | CPSP (N=20) | No CPSP (N=51) | P value | |
| b FDI | 10 (5,15) | 4 (1,7) | <0.00 1* | 4 (1,10) | 1 (0,3) | 0.00 2* |
| b PedsQL total | 125.63 (107.71, 141.46) | 156.67 (123.54,179.38) | 0.005* | 150.63 (105.42, 169.58) | 163.65 (100, 186.77) | 0.28 |
| b PedsQL psychosocial | 76.67 (70, 83.33) | 85 (76.67, 95) | <0.00 1* | 74.56 (18.98) | 86.15 (14.51) | 0.03, a |
| b PedsQL physical | 51.56 (37.5, 68.75) | 78.13 (62.5, 90.63) | <0.00 1* | 78.13 (65.63, 87.5) | 93.75 (82.81, 100) | <0.0 01* |
| b Pain scores | 5 (4,7) | 1 (1,2) | 0.035 | 4 (3,5) | 2 (0,4) | 0.00 5* |
| b Pain DETECT | 11 (9,13) | 4 (1,9) | <0.00 1* | 7 (4,11) | 3 (0,7) | 0.00 7* |
| c Medications | 3/27 | 0/78 | 0.003* | 4/20 | 2/51 | 0.83 7 |
| c Nature of pain descriptives (% within subcohort) | sharp 37.5% stabbing 12.5% tightness 37.5% burning 12.5% | sharp 13.3% stabbing 6.7% throbbing 6.7% crampy 20% tightness 33.3% burning 20% | – | sharp 19.3% stabbing 19.3% throbbing 16.1% crampy 22.6% tightness 16.1% burning 6.5% | sharp 21.1% stabbing 7.8% throbbing 15.8% crampy 23.7% tightness 21.3% burning 10.5% | – |
CPSP: chronic postsurgical pain, PPST: pediatric pain screening tool, FDI: Functional disability index, PedsQL: pediatric quality of life measure.
p value less than 0.025 was considered to be significant
data exhibited normal distribution; shown as mean ± SD and compared using two sample t-tests;
data exhibited non-normal distribution; shown as median ± Interquartile range and compared using 2 sided Wilcoxon test;
shown as frequency and compared using Fisher’s exact tests
Univariate analyses: PPST as a predictor of CPSP and functional outcomes at 6 and 12 months
PPST total and PPST physical sub-scores were only nominally significantly higher in CPSP groups at 6 and 12 months compared to non-CPSP groups at those time points. Of note, only median PPST psychosocial score (PPST 5–9) was significantly higher (p=0.02) in CPSP (2 (1,4)) vs non-CPSP (1 (0,3)) at 12 months. (Table 6). In contrast, PPST total, physical and psychosocial scores correlated with higher FDI and lower PedsQL at 6 months. Similar correlation were found for PPST total and PPST psychosocial score with functional outcomes at 12 months.
Table 6:
Correlation of PPST with outcome measures at 6 and 12 months
| Outcome | PPST 1–4 | PPST 5–9 | PPST total | PPST 1–4 | PPST 5–9 | PPST total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||||||||
| M(IQR) | p-value | M(IQR) | p-value | M(IQR) | p-value | p-value | M(IQR) | p-value | M(IQR) | p-value | ||
| CPSP (Yes) | 1(0,2) | 0.03 | 1(0,2) | 0.19 | 2(1,4) | 0.04 | 1(0,2) | 0.20 | 1(0,3) | 0.02* | 2(1,4) | 0.04 |
| CPSP (No) | 0(0,2) | 0(0,2) | 1(0,3) | 1(0,3) | 0(0,1) | 1(0,3) | ||||||
| SCC | p-value | SCC | p-value | SCC | p-value | SCC | p-value | SCC | p-value | SCC | p-value | |
| FDI | 0.350 | <0.001* | 0.266 | 0.008* | 0.351 | <0.001* | 0.247 | 0.047 | 0.328 | 0.008* | 0.347 | 0.005* |
| PedsQL total | −0.440 | <0.001* | −0.226 | 0.028 | −0.378 | <0.001* | −0.167 | 0.200 | −0.319 | 0.011* | −0.249 | 0.051 |
| PedsQL physical | −0.368 | <0.001* | −0.267 | 0.009* | −0.357 | <0.001* | −0.274 | 0.031* | −0.405 | 0.001* | −0.393 | 0.002* |
| PedsQL psychosocial | −0.495 | <0.001* | −0.380 | <0.001* | −0.481 | <0.001* | −0.434 | <0.001* | −0.407 | 0.001* | −0.466 | <0.001* |
CPSP: chronic postsurgical pain, PPST: pediatric pain screening tool, FDI: Functional disability index, PedsQL: pediatric quality of life measure. SCC: Spearman Correlation Coefficient; M(IQR): Median (Interquartile range)
p v alue less than 0.025 was considered to be significant
Receiver operating curve (ROC)
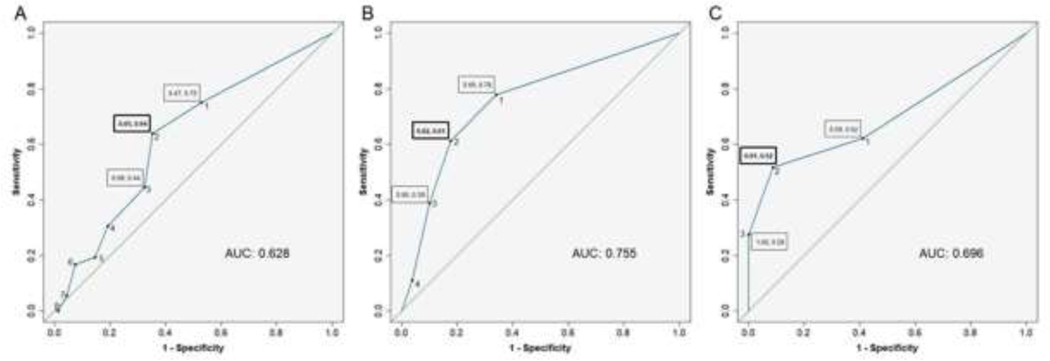
We determined ROC and Youden’s indices for prediction of CPSP by PPST total scores. Based on maximum Youden’s index, we determined that the optimal PPST cutoff for CPSP was 2 (63.9% sensitivity, 64.7% specificity). The AUC for PPST score was 0.63 reflecting fair discrimination (Figure 3A). For developing physical sub-score risk cutoff, we determined ROC and Youden’s index for PPST 1-subscore for prediction of FDI. Surgical duration was not a factor affecting FDI, hence was not included. The AUC for prediction was 0.70, and the cutoff was determined to be 2 (51.7% sensitivity, 91.2% specificity) (Figure 3B). For developing psychosocial sub-score risk cutoff, we determined ROC and Youden’s index for PPST5–9 subscore for prediction of PedsQL psychosocial score. The AUC for prediction was 0.76, and the cutoff was determined to be 2 (61.1% sensitivity, 82.3% specificity) (Figure 3C)
Figure 3.
Receiver operator characteristics (ROC) curve for CPSP prediction based on PPST total scores (Figure 3A), PPST physical subscore 1–4 risk cutoff based on functional disability index (FDI) (Figure 3B), and PPST psychosocial subscore 5–9 risk cutoff based on pediatric quality of life (PedsQL) measure (Figure 3C). Boxed numbers indicate sensitivity and specificity. Green line signifies null.
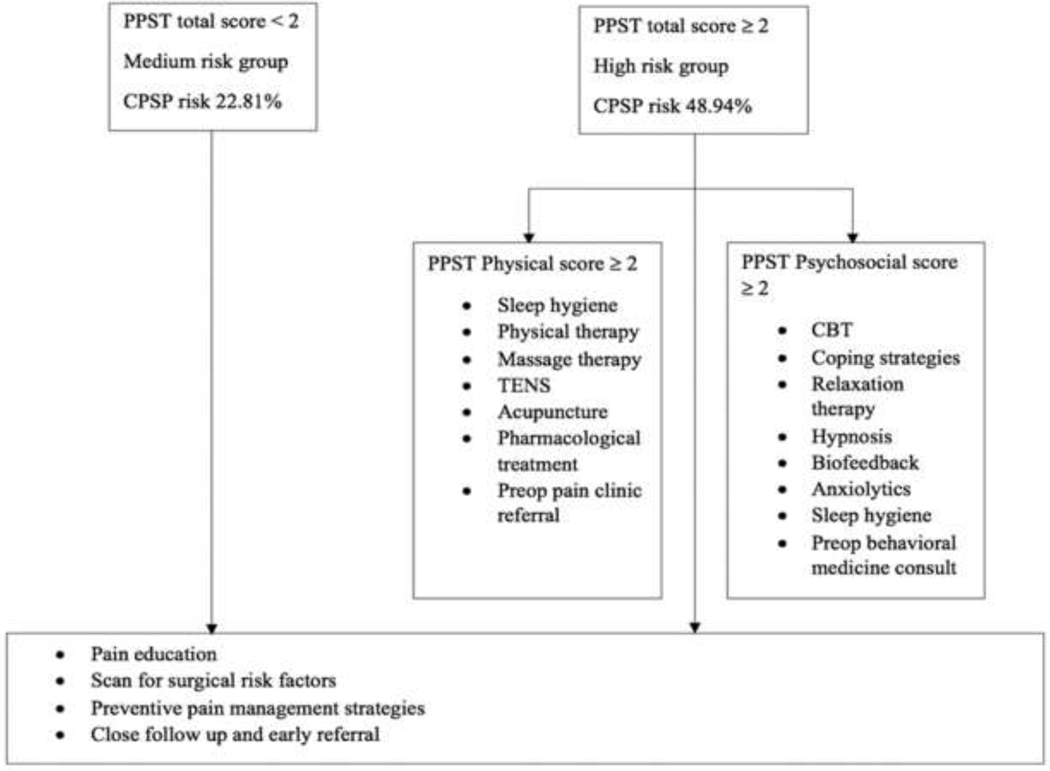
Risk stratification by PPST:
Based on cutoffs based on Youden’s index, we stratified risk for CPSP based on PPST scores < 2 and ≥2. Based on our prior definition, PPST < 2 group (N=57) still had a medium risk for CPSP (22.81%). (Figure 4) PPST ≥ 2 group (N=47) had high risk for CPSP (48.94%). The suggested interventions based on PPST scores and risk are described schematically in Figure 4.
Figure 4.
CPSP risk stratification based of PPST score and suggested interventions. CPSP- Chronic postsurgical pain; PPST- pediatric pain screening tool. It is recommended to consider increased baseline risk of CPSP for female sex (compared to male) while applying this stratification.
DISCUSSION
In this multi-institutional study, we evaluated the utility of PPST as a simple screening tool to predict risk for chronic pain and functional outcomes after posterior spinal fusion and Nuss procedures in a predominantly pediatric cohort. Importantly, preoperative PPST and PPST physical sub-scores were predictive of overall CPSP. PPST scores and subscores were all significantly correlated with functional outcomes FDI and PedsQL at 6 months; PPST total and psychosocial subscore continued to be predictive of functional outcomes at 12 months. We foresee the easy application of this tool in preoperative surgical or anesthesia consultation clinics. The PPST scoring rubric to assess risk groups could guide specific preoperative steps and form a basis for future interventional studies aimed at decreasing risk. This results of the study needs to be validated in other surgical cohorts with increased racial diversity.
The test-retest reliability of PPST as a scoring tool for chronic pain has been established by Simons et al.. Researchers asked a subset of patients to re-score the PPST at two weeks. They found intraclass coefficients = 0.75.48 We confirmed longer-term reliability of PPST in our study using preoperative PPST compared with PPST scores at 6 months in CPSP free subjects. Confirmatory factor analysis and item endorsement showed certain items (2 and 6 related to function and catastrophizing respectively) that may play a bigger role in determining the PPST sub-scores.
We reaffirmed higher preoperative pain, anxiety sensitivity, depressive symptoms and insomnia symptoms as preoperative factors for CPSP. Unlike other studies which showed preoperative functional disability in subjects who develop CPSP,40 we did not find differences in pain interference or quality of life before surgery among those who did and did not develop CPSP. We did find that FDI and quality of life measures were affected negatively at 6 months in those with CPSP, but improved from 6 to 12 months likely reflecting normalization of function and quality of life before pain resolution, which is commonly seen with chronic pain conditions.12 Importantly, psychosocial subscore continued to be predictive of functional outcomes at 12 months, while physical subscore did not. The psychosocial subscore questions of PPST evaluate depressive symptoms, pain catastrophizing, anxiety and pain unpleasantness. This might imply that psychosocial measures play a bigger role in maintenance of pain. Page et. al previously showed that anxiety sensitivity predicted maintenance of moderate/severe CPSP from 6 to 12 months after surgery.32 Thus, different factors may play a role in maintenance versus development of CPSP.
We confirmed our hypotheses that preoperative PPST and sub-scores correlated well with CASI, painDETECT, depressive symptoms, pain interference and ISI. Although PPST scores will not help quantify severity of these conditions, the findings imply that PPST as a single measure reflects increased risk associated with the constructs of preoperative pain, anxiety,32 depressive symptoms20 sleep disturbances and pain interference which are known to increase the risk of development of CPSP in adolescents.38 Although pain catastrophizing is a known risk factor for CPSP in adults, our and other studies have not found this to be a factor affecting pediatric CPSP. 4 Hence we did not include it in our evaluation although PPST item 6 conforms to this construct.
In previous studies in children with chronic musculoskeletal pain, PPST was found useful to identify patients at high risk for long-term emotional distress and disability.46 In youths with acute musculoskeletal pain, higher PPST scores at baseline predicted poor longitudinal pain outcomes such as pain persistence, pain related disability and quality of life.21 PPST also has been utilized in youths with sickle cell disease to identify those with chronic pain or at risk of poor outcomes46 and to risk stratify youths presenting with headaches.17 Similar to Simons et. al, we were able to derive a stratification rubric using PPST cutoff scores>2 to define higher risk for CPSP.48 Although this risk rubric needs further validation in larger studies involving other surgeries, we discuss below putative recommendations and suggested strategies based on risk strata.
Interestingly, patients with even low PPST scores (< 2) still had a medium risk for CPSP, as defined (10–30% risk). This can likely be explained by the inclusion of higher risk surgeries in this cohort.16 Due to a baseline medium CPSP risk for major musculoskeletal procedures, there is an imperative need to study interventions such as preoperative education about multimodal therapies and setting positive but realistic expectations of pain,2 and modulation of contributing risk factors for the prevention of CPSP. It is encouraging that Simons et. al. describe treatment responsivity of the PPST in children with chronic pain.48 Decreases in 4-month follow up PPST scores were associated with improvements in distress and functioning following multidisciplinary treatment. According to the risk constructs correlated with PPST sub-scores, we believe children with PPST total score >/= 2, physical subscore > 2 may benefit from interventions such as sleep hygiene, physical therapy and nonpharmacological therapy for preexisting pain (massage therapy, TENS, acupuncture). For those with PPST >/= 2, psychosocial subscore >2, preoperative referral to behavioral medicine clinic for cognitive behavioral therapy, coping strategies, relaxation therapy, hypnosis, biofeedback and pharmacological therapy for anxiety management, may be helpful. Although there is no current evidence for this in perioperative settings among children, a transitional pain service for identifying CPSP risk and offering coordinated multidisciplinary care in adults undergoing surgery has been described to be successful in improving outcomes.7 Adaptation of such a service to the pediatric cohort needs to be studied. In adults, we know that cognitive behavioral therapy (CBT) 35 improves sleep, chronic pain and decreases opioid consumption.3 Acceptance and commitment therapy has been shown to be helpful for CPSP prevention and management in patients with negative affective constructs, such as anxiety, depressive symptoms and pain catastrophizing.54 Perioperative cognitive behavioral therapy and relaxation therapy are effective for reducing persistent pain and physical impairment after surgery.31; 53 Sleep interventions have been shown to improve sleep in anxious youth29 and web based CBT programs have been shown to improve chronic pain outcomes. 34 The feasibility and success of technology-delivered pain self-management program for youth with chronic pain provides encouragement that this may be feasible in adolescents before and after surgery.33
The predictive accuracy of PPST for CPSP and functional outcomes ranged from AUC of 0.62 to 0.76 in our study, similar to what was described by Simons et. al for chronic pain and psychosocial outcomes. The moderate AUC for CPSP suggests involvement of other factors contributing to risk, including female sex, race, surgical duration and acute postoperative pain severity, which are not captured by PPST and may increase the predictive accuracy. 4; 10; 42 In this study, race was not consistently associated with CPSP in both surgical cohorts, but female sex significantly increased risk for CPSP in both (and combined) cohorts. This has been previously found to be an independent risk factor for CPSP9 but not in children.37 In addition, genetic factors influencing pain susceptibility may contribute further to CPSP risk.22; 43
Strengths of our study include its multisite cohort undergoing similar surgeries with similar pathophysiology of pain. This needs to be further studies in other surgeries with different pathology (for example, visceral pain) for generalizability of results. Some limitations of our study include a) missing data, although we did ensure the cohorts with and without missing data were similarly balanced for relevant factors to prevent bias; b) use of pediatric scales in a minority of subjects >18 years; this can be justified however as PPST was originally modified from a 9-item adult scale and has been used for risk stratification of musculoskeletal pain in adults,19 and c) significant loss of follow up leading to attrition of cohorts between 6 to 12 months. Incidentally, the loss to follow up group had higher preoperative FDI compared to the group that successfully were contacted for follow up, introducing potential bias in who was retained in the study and the characteristics of the outcome groups. Our findings provide a basis for future larger diverse studies to allow further stratification by race and surgical/pain characteristics, and interventions to assess efficacy. Given a median incidence 20% CPSP after major surgeries in children and AUC for prediction of risk by PPST at 0.70, PPST will enable easy delineation and cost-effective targeted interventions in a sizable proportion of high risk subjects. We believe the advantages of a concise scale which includes physical and psychosocial metrics, we believe PPST presents a practical way of preoperative risk stratification and choice of preventive strategies to minimize the risk of CPSP development.
Supplementary Material
Perspective.
The article supports Pediatric Pain Screening Tool, a simple 9-item questionnaire, as a preoperative screening tool for chronic post-surgical pain (CPSP) and function 6–12 months after spine/pectus surgeries. PPST measures correlate with known risk factors for CPSP. Risk stratification and targeted preventive interventions in high-risk subjects are proposed.
Highlights.
Chronic postsurgical pain (CPSP) is a sizable problem in pediatric patients
Lack of easy to administer screening tools makes prediction of risk less feasible
Pediatric pain screening tool (PPST) is a simple 9-item questionnaire
PPST scores are associated with CPSP and functional outcomes
PPST measures correlate with known risk factors for CPSP
PPST cut-off scores inform preoperative risk stratification and targeted interventions
Acknowledgments:
The authors thank research coordinators at all sites who helped with conducting the study including (Cincinnati Children’s: Bobbie Stubbeman, Paula Hu – CHOP; Gerri Wilson – University of Mississippi ); Susmita Kashika-Zuck PhD and Laura E. Simons PhD, faculty in behavioral medicine at Cincinnati Children’s and Stanford University respectively, for their discussions and insight. Additionally, we thank Maria Ashton MS, RPH, MBA for providing writing assistance, editing and proofreading.
Funding: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR001425 through pilot funding and R01AR075857 through NATIONAL INSTITUTE OF ARTHRITIS AND MUSCULOSKELETAL AND SKIN DISEASES. (PI:Chidambaran). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest: None of the authors have any conflicts of interest to disclose
ClinicalTrials.gov Identifier: NCT02998138
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Amtmann DA CK, Jensen MP, Chen W-H, Choi SW, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, Lai J-S. Development of a PROMIS item bank to measure pain interference. Pain;150:173–182 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bayman EO, Parekh KR, Keech J, Larson N, Vander Weg M, Brennan TJ. Preoperative Patient Expectations of Postoperative Pain Are Associated with Moderate to Severe Acute Pain After VATS. Pain Med;20:543–554 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cheatle MD, Foster S, Pinkett A, Lesneski M, Qu D, Dhingra L. Assessing and Managing Sleep Disturbance in Patients with Chronic Pain. Anesthesiology Clinics;34:379–393 2016 [DOI] [PubMed] [Google Scholar]
- [4].Chidambaran V, Ding L, Moore DL, Spruance K, Cudilo EM, Pilipenko V, Hossain M, Sturm P, Kashikar-Zuck S, Martin LJ, Sadhasivam S. Predicting the pain continuum after adolescent idiopathic scoliosis surgery: A prospective cohort study. European Journal of Pain;21:1252–1265 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chidambaran V, Zhang X, Geisler K, Stubbeman BL, Chen X, Weirauch MT, Meller J, Ji H. Enrichment of genomic pathways based on differential DNA methylation associated with chronic postsurgical pain and anxiety in children - a prospective, pilot study. J Pain; 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chidambaran V, Zhang X, Martin LJ, Ding L, Weirauch MT, Geisler K, Stubbeman BL, Sadhasivam S, Ji H. DNA methylation at the mu-1 opioid receptor gene (OPRM1) promoter predicts preoperative, acute, and chronic postsurgical pain after spine fusion. Pharmgenomics Pers Med;10:157–168 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clarke H, Katz J, Flor H, Rietschel M, Diehl SR, Seltzer Z. Genetics of chronic post-surgical pain: a crucial step toward personal pain medicine. Can J Anaesth;62:294–303 2015 [DOI] [PubMed] [Google Scholar]
- [8].Cunningham NR, Kashikar-Zuck S, Mara C, Goldschneider KR, Revicki DA, Dampier C, Sherry DD, Crosby L, Carle A, Cook KF, Morgan EM. Development and validation of the self-reported PROMIS pediatric pain behavior item bank and short form scale. Pain;158:1323–1331 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fiorelli S, Cioffi L, Menna C, Ibrahim M, De Blasi RA, Rendina EA, Rocco M, Massullo D. Chronic Pain After Lung Resection: Risk Factors, Neuropathic Pain, and Quality of Life. J Pain Symptom Manage;60:326–335 2020 [DOI] [PubMed] [Google Scholar]
- [10].Fletcher D, Stamer UM, Pogatzki-Zahn E, Zaslansky R, Tanase NV, Perruchoud C, Kranke P, Komann M, Lehman T, Meissner W, Anaesthesiology egftCTNgotESo. Chronic postsurgical pain in Europe: An observational study. Eur J Anaesthesiol;32:725–734 2015 [DOI] [PubMed] [Google Scholar]
- [11].Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin;22:1911–1920 2006 [DOI] [PubMed] [Google Scholar]
- [12].Friedrichsdorf SJ, Giordano J, Desai Dakoji K, Warmuth A, Daughtry C, Schulz CA. Chronic Pain in Children and Adolescents: Diagnosis and Treatment of Primary Pain Disorders in Head, Abdomen, Muscles and Joints. Children (Basel);3:42 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gerbershagen HJ, Rothaug J, Kalkman CJ, Meissner W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth;107:619–626 2011 [DOI] [PubMed] [Google Scholar]
- [14].Guertin JR, Pagé MG, Tarride J, Talbot D, Watt-Watson J, Choinière M. Just how much does it cost? A cost study of chronic pain following cardiac surgery. J Pain Res;11:2741–2759 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform;48:193–204 2014 [DOI] [PubMed] [Google Scholar]
- [16].Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain;154:95–102 2013 [DOI] [PubMed] [Google Scholar]
- [17].Heathcote LC, Rabner J, Lebel A, Hernandez JM, Simons LE. Rapid Screening of Risk in Pediatric Headache: Application of the Pediatric Pain Screening Tool. Journal of pediatric psychology;43:243–251 2018 [DOI] [PubMed] [Google Scholar]
- [18].Hill JC, Dunn KM, Lewis M, Mullis R, Main CJ, Foster NE, Hay EM. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum;59:632–641 2008 [DOI] [PubMed] [Google Scholar]
- [19].Hill JC, Whitehurst DG, Lewis M, Bryan S, Dunn KM, Foster NE, Konstantinou K, Main CJ, Mason E, Somerville S, Sowden G, Vohora K, Hay EM. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet;378:1560–1571 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hinrichs-Rocker A, Schulz K, Järvinen I, Lefering R, Simanski C, Neugebauer EA. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) - a systematic review. Eur J Pain;13:719–730 2009 [DOI] [PubMed] [Google Scholar]
- [21].Holley AL, Fussner L, Wilson A, Palermo T. (261) Using the pediatric pain screening tool to predictlongitudinal pain outcomes in treatment seeking youth with acute musculoskeletal pain. The Journal of Pain;18:S41 2017 [Google Scholar]
- [22].Hoofwijk DM, van Reij RR, Rutten BP, Kenis G, Buhre WF, Joosten EA. Genetic polymorphisms and their association with the prevalence and severity of chronic postsurgical pain: a systematic review. Br J Anaesth;117:708–719 2016 [DOI] [PubMed] [Google Scholar]
- [23].Hunfeld JA, Perquin CW, Duivenvoorden HJ, Hazebroek-Kampschreur AA, Passchier J, van Suijlekom-Smit LW, van der Wouden JC. Chronic pain and its impact on quality of life in adolescents and their families. J Pediatr Psychol;26:145–153 2001 [DOI] [PubMed] [Google Scholar]
- [24].Irwin DE, Stucky B, Langer MM, Thissen D, Dewitt EM, Lai JS, Varni JW, Yeatts K, DeWalt DA. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual Life Res;19:595607 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kashikar-Zuck S, Flowers SR, Claar RL, Guite JW, Logan DE, Lynch-Jordan AM, Palermo TM, Wilson AC. Clinical utility and validity of the Functional Disability Inventory among a multicenter sample of youth with chronic pain. Pain;152:1600–1607 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kashikar-Zuck S, Vaught MH, Goldschneider KR, Graham TB, Miller JC. Depression, coping, and functional disability in juvenile primary fibromyalgia syndrome. J Pain;3:412–419 2002 [DOI] [PubMed] [Google Scholar]
- [27].Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med;15:155–163 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Landman Z, Oswald T, Sanders J, Diab M, Group SDS. Prevalence and predictors of pain in surgical treatment of adolescent idiopathic scoliosis. Spine (Phila Pa 1976);36:825–829 2011 [DOI] [PubMed] [Google Scholar]
- [29].McMakin DL, Ricketts EJ, Forbes EE, Silk JS, Ladouceur CD, Siegle GJ, Milbert M, Trubnick L, Cousins JC, Ryan ND, Harvey AG, Dahl RE. Anxiety Treatment and Targeted Sleep Enhancement to Address Sleep Disturbance in Pre/Early Adolescents with Anxiety. Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53;48:S284–S297 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep;34:601–608 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nowakowski ME, McCabe RE, Busse JW. Cognitive behavioral therapy to reduce persistent postsurgical pain following internal fixation of extremity fractures (COPE): Rationale for a randomized controlled trial. Canadian Journal of Pain;3:59–68 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pagé MG, Stinson J, Campbell F, Isaac L, Katz J. Identification of pain-related psychological risk factors for the development and maintenance of pediatric chronic postsurgical pain. J Pain Res;6:167–180 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Palermo TM, de la Vega R, Dudeney J, Murray C, Law E. Mobile health intervention for self-management of adolescent chronic pain (WebMAP mobile): Protocol for a hybrid effectiveness-implementation cluster randomized controlled trial. Contemp Clin Trials;74:55–60 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Palermo TM, Murray C, Aalfs H, Abu-El-Haija M, Barth B, Bellin MD, Ellery K, Fishman DS, Gariepy CE, Giefer MJ, Goday P, Gonska T, Heyman MB, Husain SZ, Lin TK, Liu QY, Mascarenhas MR, Maqbool A, McFerron B, Morinville VD, Nathan JD, Ooi CY, Perito ER, Pohl JF, Schwarzenberg SJ, Sellers ZM, Serrano J, Shah U, Troendle D, Zheng Y, Yuan Y, Lowe M, Uc A. Web-based cognitive-behavioral intervention for pain in pediatric acute recurrent and chronic pancreatitis: Protocol of a multicenter randomized controlled trial from the study of chronic pancreatitis, diabetes and pancreatic cancer (CPDPC). Contemp Clin Trials;88:105898 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pigeon WR, Moynihan J, Matteson-Rusby S, Jungquist CR, Xia Y, Tu X, Perlis ML. Comparative effectiveness of CBT interventions for co-morbid chronic pain & insomnia: a pilot study. Behaviour research and therapy;50:685–689 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Quinn H, Thissen D, Liu Y, Magnus B, Lai JS, Amtmann D, Varni JW, Gross HE, DeWalt DA. Using item response theory to enrich and expand the PROMIS® pediatric self report banks. Health Qual Life Outcomes;12:160 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rabbitts JA, Fisher E, Rosenbloom BN, Palermo TM. Prevalence and Predictors of Chronic Postsurgical Pain in Children: A Systematic Review and Meta-Analysis. J Pain;18:605–614 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rabbitts JA, Groenewald CB, Zhou C. Subacute Pain Trajectories following major musculoskeletal surgery in adolescents: A Pilot Study. Can J Pain;4:3–12 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rabbitts JA, Zhou C, Groenewald CB, Durkin L, Palermo TM. Trajectories of postsurgical pain in children: risk factors and impact of late pain recovery on long-term health outcomes after major surgery. Pain;156:2383–2389 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rosenbloom BN, Pagé MG, Isaac L, Campbell F, Stinson JN, Wright JG, Katz J. Pediatric Chronic Postsurgical Pain And Functional Disability: A Prospective Study Of Risk Factors Up To One Year After Major Surgery. Journal of pain research;12:3079–3098 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Roth-Isigkeit A, Thyen U, Stoven H, Schwarzenberger J, Schmucker P. Pain among children and adolescents: restrictions in daily living and triggering factors. Pediatrics;115:e152–162 2005 [DOI] [PubMed] [Google Scholar]
- [42].Ruscheweyh R, Viehoff A, Tio J, Pogatzki-Zahn EM. Psychophysical and psychological predictors of acute pain after breast surgery differ in patients with and without pre-existing chronic pain. Pain;158:1030–1038 2017 [DOI] [PubMed] [Google Scholar]
- [43].Schug SA, Bruce J. Risk stratification for the development of chronic postsurgical pain. PAIN Reports;2:e627 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schug SA, Lavand’homme P, Barke A, Korwisi B, Rief W, Treede RD, Pain ITftCoC. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain;160:45–52 2019 [DOI] [PubMed] [Google Scholar]
- [45].Sieberg CB, Simons LE, Edelstein MR, DeAngelis MR, Pielech M, Sethna N, Hresko MT. Pain prevalence and trajectories following pediatric spinal fusion surgery. J Pain;14:1694–1702 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sil S, Cohen LL, Dampier C. Pediatric pain screening identifies youth at risk of chronic pain in sickle cell disease. Pediatr Blood Cancer;66:e27538 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Silverman WK, Fleisig W, Rabian B, Peterson RA. Childhood anxiety sensitivity index. J Clin Child Psychol 20:162–168 1991 [Google Scholar]
- [48].Simons LE, Smith A, Ibagon C, Coakley R, Logan DE, Schechter N, Borsook D, Hill JC. Pediatric Pain Screening Tool: rapid identification of risk in youth with pain complaints. Pain;156:1511–1518 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care;39:800–812 2001 [DOI] [PubMed] [Google Scholar]
- [50].von Baeyer CL. Numerical rating scale for self-report of pain intensity in children and adolescents: recent progress and further questions. Eur J Pain;13:1005–1007 2009 [DOI] [PubMed] [Google Scholar]
- [51].Walker LS, Greene JW. Children with recurrent abdominal pain and their parents: more somatic complaints, anxiety, and depression than other patient families? J Pediatr Psychol;14:231–243 1989 [DOI] [PubMed] [Google Scholar]
- [52].Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol;16:39–58 1991 [DOI] [PubMed] [Google Scholar]
- [53].Wang L, Chang Y, Kennedy SA, Hong PJ, Chow N, Couban RJ, McCabe RE, Bieling PJ, Busse JW. Perioperative psychotherapy for persistent post-surgical pain and physical impairment: a meta-analysis of randomised trials. Br J Anaesth;120:1304–1314 2018 [DOI] [PubMed] [Google Scholar]
- [54].Weinrib AZ, Azam MA, Birnie KA, Burns LC, Clarke H, Katz J. The psychology of chronic post-surgical pain: new frontiers in risk factor identification, prevention and management. British journal of pain;11:169–177 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Werner MU, Kongsgaard UE. I. Defining persistent post-surgical pain: is an update required? Br J Anaesth;113:1–4 2014 [DOI] [PubMed] [Google Scholar]
- [56].Wildemeersch D, D’Hondt M, Bernaerts L, Mertens P, Saldien V, Hendriks JMH, Walcarius A-S, Sterkens L, Hans GH. Implementation of an Enhanced Recovery Pathway for Minimally Invasive Pectus Surgery: A Population-Based Cohort Study Evaluating Short- and Long-Term Outcomes Using eHealth Technology. JMIR Perioper Med;1:e10996 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.