Abstract
Background & Purpose:
African Americans (AAs) are twice as likely to develop dementia than Whites, which may be driven by poorer dementia knowledge and lifestyle factors. This article provides the rationale and protocol for a pilot clinical trial examining a tailored multi-domain lifestyle modification intervention in middle-aged and older AAs. This study will explore the feasibility and efficacy of individualized Cognitive Prescriptions (CogRx) which target five domains: physical activity, cognitive activity, diet, sleep, and social activity. Theoretical underpinnings include Social Cognitive Theory and the Health Belief Model, which suggest that tailored risk factor information, goal-setting, and outcome expectations along with addressing self-efficacy and barriers will promote behavior change.
Study Design:
This study plans to enroll 150 community-dwelling AA participants aged 45–65 without significant cognitive impairment. After baseline assessment including data-driven assessment of deficiencies in each of the five CogRx domains, participants are randomized with equal allocation to either: psychoeducation + CogRx, psychoeducation only, or no-contact control. The psychoeducation and CogRx groups receive general psychoeducation on dementia prevalence, prognosis, and risk factors, while the CogRx group also receives information on their risk factor profile and develops a tailored 3-month intervention plan, consisting of simple evidence-based strategies to implement. The CogRx condition receives text-messaging reminders and adherence queries and provides feedback on this program.
Conclusion:
This study tests a novel multi-domain dementia prevention intervention and has several strengths, including enrolling middle-aged AAs with a focus on prevention, assessing adherence and self-efficacy, tailoring the intervention, and examining dementia knowledge. The goal is to yield new perspectives on person-centered dementia prevention approaches in diverse populations, and ultimately impact clinical and public health recommendations for maintaining cognitive health, thereby reducing disparities in dementia. Modifications to study design due to COVID-19 and future directions are discussed.
Keywords: lifestyle, health behaviors, health literacy, minorities
Introduction
As of 2021, an estimated 6.2 million Americans age 65 and older are living with Alzheimer’s disease (AD) which is expected to double by 2060.1, 2 African Americans (AAs) are twice as likely to have AD than Whites.3, 4 Identifying and implementing lifespan preventative approaches for dementia in this at-risk population is crucial, as AD pathology may begin over a decade prior to symptoms.5–8 Addressing modifiable risk factors (eg, diabetes, midlife hypertension, midlife obesity, smoking, depression, cognitive inactivity, and physical inactivity) may prevent or delay up to 40% of AD and all-cause dementia cases.2, 9 A large systematic review from the Agency for Healthcare Research and Quality and the National Institute on Aging led to a consensus report stating areas with the most evidence for therapeutic benefit were cognitive training, physical activity, and hypertension management, and also identified priorities for future intervention work on dietary, social activity, and sleep interventions.10 Importantly, racial disparity in AD risk among AAs largely stems from socioeconomic risk factors, health behaviors, and medical conditions.2, 11, 12 Specifically, macro-level social mediators (eg, low socioeconomic status, discrimination) early in life cascade to health behaviors and health outcomes that may ultimately affect cognitive outcomes.13, 14 While these factors likely operate similarly in AAs and Whites, many of these risk factors are more common in AAs, particularly physical inactivity and presence of vascular comorbidities.15, 16 Work is needed to determine optimal interventions targeting lifestyle behaviors among middle-aged AAs that may reduce dementia risk.
While studies support lifestyle factor interventions delivered singly (e.g., cognitive training, physical activity), a comprehensive, tailored approach may be the most ecologically-valid and therapeutic.17 For example one of the first studies of its kind, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) randomised controlled trial examined a two year multidomain intervention (diet, exercise, cognitive training, vascular risk monitoring) in 591 participants compared to 599 controls and found promising effects on cognitive function18. Yet overall, existing multi-domain behavioral modification cognitive interventions19–23 have several disadvantages, including: 1) only enrolling older, clinical samples, which may not generalize to diverse populations and focus on improvement of existing dysfunction rather than prevention; 2) failure to target social activity and sleep; 3) overreliance on supplements and medications; 4) no assessment of adherence; 5) not tailoring intervention to deficient areas; 6) not examining mental and psychological outcomes; 7) employing highly structured lab-based protocols; 8) not examining dementia knowledge; and 9) lack of underlying theoretical models. Furthermore, very few multi-domain approaches have specifically targeted AAs, despite their increased vulnerability to dementia and evidence that AAs may have poorer knowledge and unique concerns and beliefs about dementia and AD, including not being aware of the higher risk in AAs.24–27 Rovner and colleagues recently conducted a randomized controlled trial among AAs aged 65+ with mild cognitive impairment and found a significant reduction in risk of memory decline in n=77 participants receiving a behavioral activation intervention (designed to increase cognitive, physical, and/or social activity) as compared to n=87 receiving supportive therapy (an attention control treatment)28. While groundbreaking, further work is needed among middle-aged AAs who have not yet developed cognitive impairment, and for whom interventions may be most beneficial.
The purpose of this article is to provide the rationale and describe the protocol for a clinical trial of an intervention approach designed to address weaknesses in prior work. Study progress, pitfalls, and modifications to the study due to the COVID-19 pandemic are also discussed. The current study is the among the first to explore the feasibility and efficacy of an individualized psychoeducation dementia risk reduction approach, dubbed Cognitive Prescriptions (CogRx), that aims to overcome prior studies’ limitations and synergize their strengths in a population vulnerable to dementia. This approach is grounded in Social Cognitive Theory29 and the Health Belief Model30, in that it focuses on the notion that cognitive factors (eg, knowledge, beliefs, self-efficacy) are key determinants of behavior change, and that in order to change behavior, perceived susceptibility, severity, benefits, and barriers must be addressed. These models are consistently used in behavior change studies among AAs31, including the prior study by Rovner and colleages.28 Informing participants of their deficient areas, educating them on outcome expectations, and facilitating them with developing short-term, realistic goals may enhance motivation and self-efficacy by framing how these changes are in their overall self-interest for successful aging. Figure 1 contains detail on the conceptual framework for this study.
FIGURE 1.
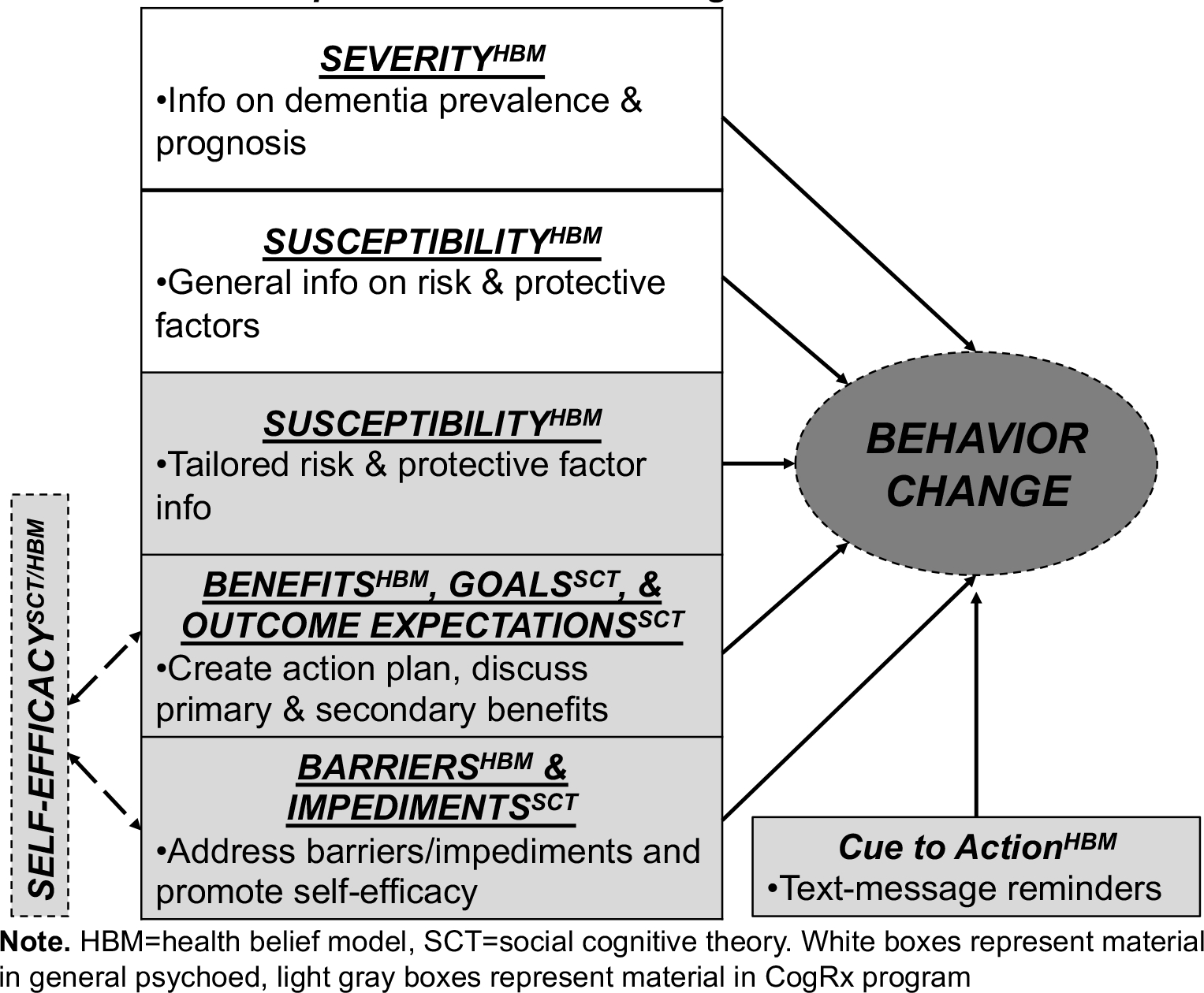
Conceptual Framework for CogRx
Material and methods
Overview of Study Design
The CogRx study employs a pre-post experimental design (Figure 2) which plans to enroll 150 community-dwelling AA participants aged 45–65 (see Study Progress and Modifications below for current numbers) from the greater Birmingham, AL area. Participants complete a baseline assessment including cognitive testing and data-driven assessment of deficiencies in each of the five CogRx domains (physical activity, cognitive activity, diet, sleep, social activity). Participants are randomized to either: psychoeducation + CogRx, psychoeducation only, or no-contact control. The psychoeducation and CogRx groups return to receive general psychoeducation on dementia prevalence, prognosis, and risk factors, while the CogRx group also receives personalized information on risk factor profile and develops a tailored 3-month intervention plan, consisting of simple evidence-based strategies to implement at home. Motivational reminders as well as adherence and self-efficacy questions are administered via text-messaging over the 3-month period. Participants return for 3-month and 6-month follow-ups.
FIGURE 2.
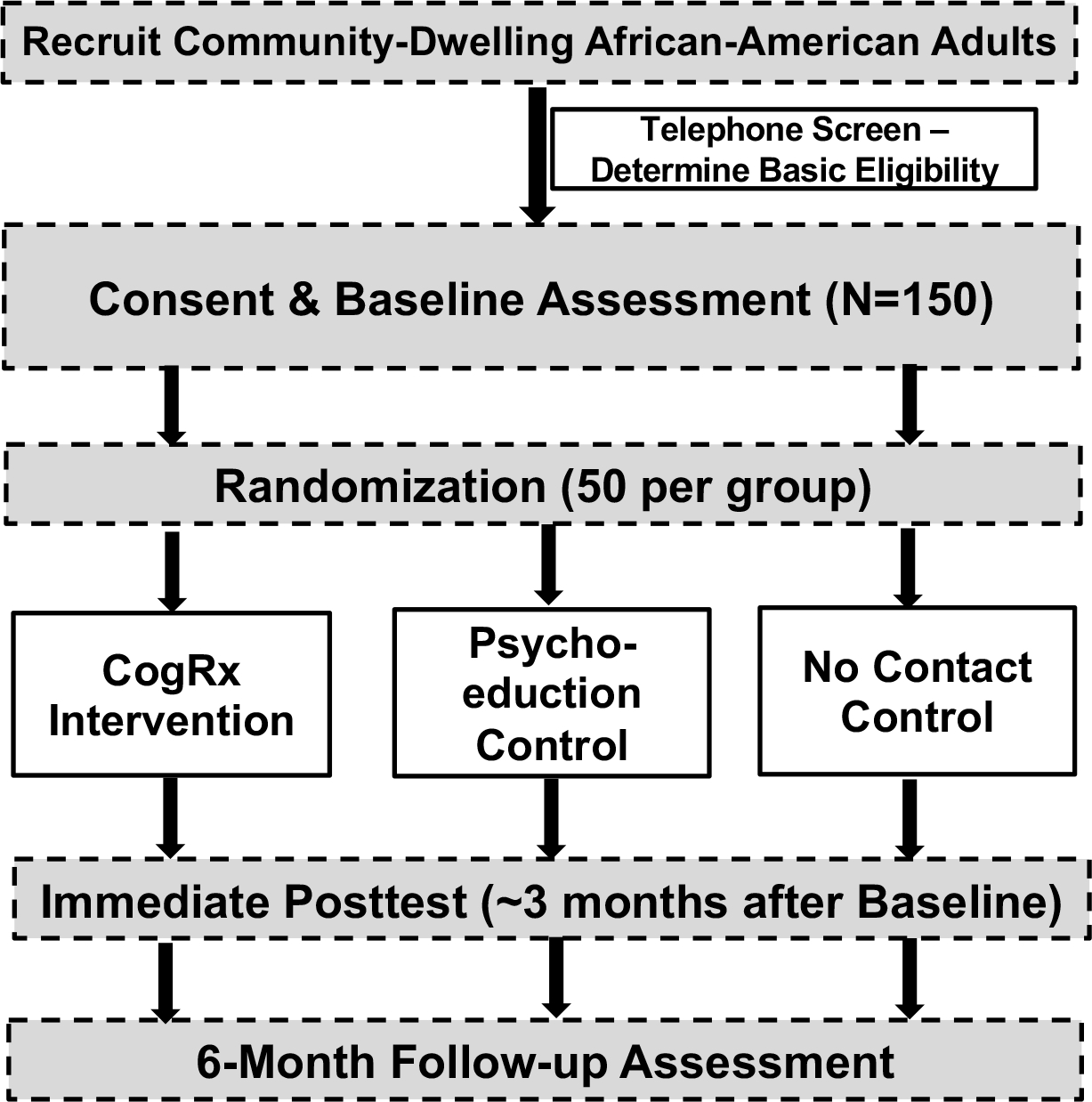
Study Design
Study Aims
The overall aim of this study is to explore the feasibility and preliminary efficacy of individualized CogRxs in improving engagement in healthy behaviors and other outcomes and to gain feedback on future implementation of the program in middle-aged AAs. Specific Aim 1, focusing on proximal outcomes, seeks to determine whether the CogRx condition is superior to psychoeducation alone in improving engagement in healthy lifestyle behaviors at 3 months and whether these changes remain at 6 months. We also examine whether the CogRx and psychoeducation only conditions will experience improved dementia knowledge compared to the no-contact control at 3 months and whether this knowledge is retained at 6 months. Aim 1 will also examine within the CogRx condition, the association between adherence and self-efficacy data gathered via text messaging and improvements in lifestyle behaviors. Specific Aim 2, focusing on distal outcomes, will compare the three conditions on a brief battery of cognitive and psychological measures (eg, depressive symptoms, self-rated successful aging) after 3 months and 6 months. This study will also examine if these effects on distal outcomes will be mediated by gains in the aforementioned primary outcomes (ie, health behaviors, dementia knowledge, and adherence and self-efficacy [in the intervention group only]). Finally, the exploratory aim employs qualitative interviews with CogRx participants to determine feedback for future implementation of this program, including barriers and facilitators to engagement/adherence, and likelihood of continuing the program. As mentioned below, our study will also have data to examine the impact of COVID-19 on engagement in health behaviors as well as COVID-19 literacy.
Participants and Procedure
This study is funded by the National Institute on Aging, was approved by the UAB Institutional Review Board (protocol # 300003029), and is being conducted in accordance with the Declaration of Helsinki. The ClinicalTrials.gov number is NCT03864536. Community-dwelling middle-aged AA adults aged 45–65 are being recruited for this study, with flyers in the community and an advertisement in the online listing of University studies. After obtaining verbal consent to screen, a telephone screen determines the following self-reported eligibility criteria: age 45 to 65, no neurological (including dementia diagnosis) or severe psychiatric (eg, schizophrenia or bipolar disorder) disorders, no insomnia (to eliminate those with clinical sleep deficiencies that may not be amendable to behavioral intervention), must be ambulatory (in order to reduce confounding effect of this factor on baseline physical activity and to allow for physical activity Rx), and must have a working cell phone with unlimited texting. The Telephone Interview for Cognitive Status (TICS)32 is also administered to exclude those with cognitive impairment in the moderate to severe range (score ≤23). The rationale for including those with normal cognitive functioning and mild impairment, but not more severe impairment is threefold: 1) this allows for examination of both prophylactic and rehabilitative effects on outcomes, as well as the ability to examine adherence in those who may already have some impairment; 2) by including community-dwelling middle-aged adults with normal functioning or subtle impairment, we reduce the confounding effect of using a clinical sample (ie, mild cognitive impairment [MCI] or AD), yielding more generalizable results; and 3) in this middle-aged sample of AAs, it is expected that a large percent will have some subtle impairment attributable to multiple factors; thus, including only those with normal cognitive functioning would not be feasible for this 2-year study.
Eligible participants then provide written informed consent and complete a baseline assessment including an assessment of strengths and deficiencies in CogRx domains (Tables 1 & 2). After baseline testing it must be determined that participants have deficiencies in at least two of five CogRx domains in order to be further randomized to one of three conditions: no-contact control, psychoeducation only, and psychoeducation + CogRx. The excel-based randomization algorithm matches conditions on demographics (age, education, gender). If participants are not deficient in at least two of five CogRx domains, the baseline visit completes participation and they are not further randomized in the study. The intervention groups return for a one-time visit (described below). All participants return for 3- and 6-month follow-ups. Research assistants conduct the assessments while a dedicated interventionist administers the intervention protocol. Participants are compensated $50 each for the 4-hour baseline, and 3-month and 6-month posttests. Intervention groups receive $50 for the intervention visit. Additional compensation of $20 is provided to CogRx participants who respond to a majority of their adherence text-messages (described below).
Table 1.
Study Measures
| Domain | Measure | Time | |
|---|---|---|---|
| Sociodemographics & Health | Demographicsa,M: age, education, gender, income; Wide Range Achievement Test 4th Edition33 (quality of education)a,IP; self-reported medical comorbidities and medicationsa,P; Alcohol, Smoking and Substance Involvement Screening Test (ASSIST)-Lite34 (substance use)P; Rapid Estimate of Adult Literacy in Medicine (REALM)a,IP and Vital SignsIP (health literacy); Childhood Trauma Questionnaire35 (adverse childhood experiences)a,M | 20 min | |
| Psychological, Mental Health, Successful Aging |
Resilience: Connor Davidson Resilience Scale36,M Attitudes About Aging: Philadelphia Geriatric Morale Scale37,M Personal Mastery: Personal Mastery Scale38,M Coping: Proactive Coping Scale39,M |
Self-rated Successful Aging: Montross Measure40,M Quality of Life: Medical Outcomes Study Short Form 1241,M Mood: Center for Epidemiological Studies – Depression Scale42,IP Locus of Control: Personality in Intellectual Aging Contexts43,M |
30 min |
| Cognitive Function |
Delayed Recall: Hopkins Verbal Learning Test (HVLT)44,P, Brief Visuospatial Memory Test (BVMT)45,IP Learning: HVLTP, BVMTIP Verbal: Controlled Oral Word Association Test46,P, Category Fluency47,P |
Executive Function: Trails B48,IP, Stroop Color Word Trial49,IP Processing Speed: Trails A48,IP, Stroop Color Trial49,IP Attention/Working Memory: Paced Auditory Serial Addition Test50,IP, Letter Number Sequencing51,P Motor: Grooved Pegboard52,IP |
1 hr |
| CogRx Domain Deficiencies P | See Table 2 | 30 min | |
| Dementia Knowledge | Dementia Knowledge Assessment Scale53, 54,d,M, Dementia Risk/Prevention Knowledge Questionnaired,IP | 10 min | |
| Barriers & Facilitators | Qualitative interview on barriers and facilitators to CogRx engagementc,d,P | 5 min | |
| Intervention Feedback | Quantitative and qualitative items pertaining to likes and dislikes about the study and self-reported improvements and engagement in CogRx domainsb,d,P | 10 min | |
| COVID-19 | Influence of COVID-19 on Lifestyle Behaviors Surveya,d,P, Coronavirus Impact Scale 55,a,d,M, COVID-19 Social Impact Questionnaire 56,a,d,M, Knowledge and Perceptions of COVID-19 57,a,d,IP |
20 min | |
Notes. Measures given at baseline and 3 and 6-month follow-ups, except where noted.
= baseline only
= CogRx group only at 3 and 6 month follow-ups
= Administered at baseline to all participants and only CogRx group at 3-month follow-up
=measure explained in detail in text of manuscript; As described in the text, the COVID-19 hybrid data collection protocol resulted in some measures being
=mailed
=phone
=in-person.
Table 2.
Determination of Deficiency in CogRx
| Domain | Assessment of Deficiency | Intervention |
|---|---|---|
| Physical Activity | International Physical Activity Questionnaire 58 to determine CDC criteria CDC, 59: At least 150 min of moderate aerobic activity OR 75 min of vigorous aerobic activity per week (or an equivalent mix). Deficient: not meeting either criteria | • At least 150 min of moderate aerobic activity OR 75 min of vigorous aerobic activity per week (or an equivalent mix) • Participant choses specific activities (e.g., walking, jogging, bicycling) |
| Diet | Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet Questionnaire. 60, 61 Deficient: score of 5 or lower (lowest tertile) |
MIND diet: Encourage: Green leafy vegetable, berries, nuts, olive oil, whole grains, fish & poultry, beans, wine. Limit: Butter & margarine, red meat, fast fried food, pastries & sweets, cheese |
| Social Activity | Social Network Index (SNI) 62. Deficient: score lower than 6 (indicative of social network diversity) | • Encourage greater frequency of contact with satisfying friends/family (can be face-to-face, via phone) • Encourage socially active volunteer work 63, 64 |
| Sleep | Pittsburg Sleep Quality Index (PSQI) to determine sleep quantity and quality. Deficient: less than 7 hours actual sleep per night reported and Overall Sleep Quality rating of “Fairly Bad” or “Very Bad”65 | Aim for at least 7 hours of sleep per night and encourage healthy sleep habits: limit screen time before bed, limit alcohol & caffeine before bed, go to bed at reasonable hour, use sound machine, relaxation/breathing techniques for resting the mind 66 |
| Cognitive Activity | Leisure Activity Questionnaire. 67 Deficient: score lower than 12 (indicative of “activity days”) |
Encourage cognitive activity during leisure time: reading, writing, musical instruments, playing games, taking classes |
In light of COVID-19, several modifications have been made to the study protocol in an attempt to both ensure the safety of study staff and participants, as well as to capture the influence of the pandemic on study measures and outcomes. Specifically, the need has emerged to limit in-person data collection to only those measures that are absolutely essential to be done in person, while administering the remaining measures via phone and mail. Much consideration has been given to maintaining data integrity and consistency with this hybrid protocol. This is further described in the Study Progress and Modifications section below. Further, several additional measures have been added to the battery regarding COVID-19 (described below).
Measures
A comprehensive battery is administered at the baseline, 3-month, and 6-month follow-ups (Table 1). Specifically, this battery assesses cognitive functioning, as well as a broad range of psychosocial domains. Several study-developed questionnaires are also included, such as a Barriers and Facilitators Survey, Exit Survey, and an Influence of COVID-19 on Lifestyle Behaviors Survey. A validated measure of dementia knowledge is included along with a study-developed measure of dementia risk/prevention knowledge, which includes several items from existing measures,68, 69 as well as new items. This validated battery of widely-used cognitive and psychosocial measures are listed in Table 1. Measures developed specifically for this study, as well as dementia knowledge and COVID-19 measures are described further below.
Barriers and Facilitators Survey
The Barriers and Facilitators Survey is a study-developed qualitative interview that covers the five CogRx domains. This survey is administered to all participants at the baseline visit and then only to participants in the CogRx intervention group at the 3-month posttest. For each of the CogRx domains, participants are asked about limitations (eg, “What prevents or limits you from being able to engage in physical activity?”) and motivations in that specific area (eg, “What helps or motivates you to engage in physical activity?”) to better understand factors that influence lifestyle behaviors. Participants are recorded with a digital audio recorder and responses are then transcribed verbatim for later qualitative analysis.
Dementia Knowledge Assessment Scale
The Dementia Knowledge Assessment Scale (DKAS)54 is used to measure understanding of dementia at baseline as well as examine any improvements in knowledge at follow-up visits. The DKAS is a 27-item measure comprised of statements about dementia (eg, “Difficulty eating and drinking generally occurs in the later stages of dementia.”) that span four dimensions (ie, causes and characteristics, communication and engagement, care needs, and risks and health promotion). Responses range from strongly agree to strongly disagree, as well as I don’t know which is encouraged over simply guessing. Specifically, this scale will be used to determine whether the CogRx and psychoeducation conditions improve on dementia knowledge compared to the no-contact control, and thus is administered at baseline and 3 and 6 months.
Dementia Risk/Prevention Knowledge Questionnaire
The Dementia Risk/Prevention Knowledge questionnaire is an 11-item study-developed measure that combines items from existing measures68, 69 with new items developed for this study. This measure includes questions assessing knowledge of protective factors (eg, “What do you think a person can do to help reduce their risk of Alzheimer’s disease and other forms of dementia?”), as well as items regarding confidence and beliefs about reducing personal risk of dementia (eg, “What is your confidence level that you could take action now to reduce your risk of Alzheimer’s disease and other kinds of dementia?”). It also determines exposure to someone with dementia (“Have you had personal contact with a person with Alzheimer’s Disease or other kind of dementia?”). Items include multiple choice, Likert-scale, visual analogue, and open-ended responses. This measure is administered at baseline and 3 and 6 months.
Exit Survey
The Exit and Engagement Survey is a study-developed questionnaire administered only to the CogRx condition at 3-month and 6-month follow-up visits. The 3-month survey gathers qualitative feedback on what participants liked and disliked about the intervention, as well as quantitative data on self-reported improvements, difficulty sticking to one’s goals, and likelihood of adherence to healthy lifestyle habits following the intervention. The 6-month survey asks specifically about the immediate 3 month timeframe since the intervention ended, and queries on how well participants believed they continued meeting their goals after the intervention ended as well as qualitative items on things that motivated them or limited them from continuing with their goals.
Influence of COVID-19 on Lifestyle Behaviors Survey
The Influence of COVID-19 on Lifestyle Behaviors was developed in direct response to the pandemic and subsequent modifications to the study protocol. Since this study targets five areas that may be affected by social distancing restrictions, it is crucial to understand the continued effects that COVID-19 has on lifestyle habits. This questionnaire is administered to all participants at the baseline visit to measure changes since the emergence of the coronavirus on a scale of 1 (not at all) to 5 (extremely) in each CogRx domain (eg, “How much has COVID-19 affected your ability to follow a healthy diet?”) along with two open-ended questions at the end for further feedback.
Coronavirus Impact Scale
The Coronavirus Impact Scale55 measures the extent that COVID-19 has impacted several lifestyle areas (eg, daily routine, food access, experiences of stress-related symptoms) ranging from 0 (no change) to 3 (severe change). The scale asks participants about personal diagnosis as well as about family members or friends that have been diagnosed with COVID-19 and the extent of their symptoms (ranging from mild to the most severe symptoms resulting in death). This questionnaire is administered to all participants at the baseline visit
COVID-19 Social Impact Questionnaire
The COVID-19 Social Impact Questionnaire56 covers similar yet distinct areas from the Coronavirus Impact Scale. The questionnaire includes items about personal COVID-19 diagnosis and self-reported symptoms, new actions taken in response to the pandemic (eg, avoiding in-person contact), frequency of communication with others, changes in sleep pattern, and access to medical care. The last three questions include a loneliness scale (eg, How often do you feel left out?”) to assess feelings of social isolation. This questionnaire is administered to all participants at the baseline visit.
Knowledge and Perceptions of COVID-19 Survey
The Knowledge and Perceptions of COVID-19 questionnaire57 spans across multiple categories: 1) perceived risk (eg, “How many people in the US do you think will die from the new coronavirus by the end of the year?”), 2) transmission (eg, “What is the main way in which people are currently getting infected with the new coronavirus?”), 3) signs and symptoms (eg, “What are common signs or symptoms of an infection with the new coronavirus?”), and 4) beliefs about actions taken by the public (eg, “Which of the following actions help prevent catching an infection with the new coronavirus?”) and the government (eg, “At this point in the coronavirus pandemic, do you think the government should implement the following measures to prevent spreading of the virus?”). This questionnaire is administered to all participants at the baseline visit and will allow us to assess general knowledge and beliefs related to COVID-19 as understanding of the pandemic continues to evolve throughout the course of the study.
Intervention
The intervention (both CogRx and psychoeducation control) is administered by a race-concordant staff member. The CogRx and psychoeducation control sessions are each one time sessions conducted in a one-on-one format with the participant and the staff member. The psychoeducation program is approximately 1 hour and consists of a ~30/45 minute PowerPoint presentation conducted by the staff member followed by an opportunity for participants to ask questions. In accordance with the Health Belief Model, the topics include dementia severity (statistics on prevalence and prognosis of the disease), susceptibly (increased risk in AAs, including genetics, family history, and vascular risk factors), and finally risk/protective factors (general info on the five CogRx domains plus vascular control and depression management). This concludes the visit for the psychoeducation only condition.
After this presentation, the CogRx condition develops their tailored intervention plan. The research assistant explains whether they had “optimal” or “suboptimal” levels in each of the five domains using published guidelines and/or evidenced-based criteria59, 61, 65, 67, 70 (Table 2). For domains that are optimal, participants are encouraged to maintain their level of engagement. For domains that are suboptimal, corresponding science-based strategies to bolster those areas are explained using a tailored informational presentation (Figure 3 shows materials provided to participants). Participants then engage in a structured goal-setting process to identify specific goals for each domain. Participants with deficits in all five domains prioritize three to avoid over burdening the participant. They are instructed to choose domains that are the most important to them and that are the most feasible. The goal-setting process is conducted using the Bangor Goal Setting Interview (BGSI)20 approach which is grounded in Social Cognitive Theory of Behavior Change29 and the concept of motivational interviewing. Specifically these steps include: 1) identifying areas to work on, 2) setting goals, and 3) assessing goal attainment (at follow-up). Goals are identified in accordance with SMART71 principles (specific, measurable, achievable, realistic, and timely) (eg, “I will go for a 30-minute walk once per day”) (Figure 4 shows materials provided to participants). The Rx in each domain is relatively general and standard with some choices within each (eg, type of physical activity). Participants are also queried on barriers to these goals, and ways to overcome such barriers when possible. Participants are asked to integrate activities in their daily lives and work on their goals over the following 3-month period.
FIGURE 3.

Intervention Material: Introduction to the 5 CogRx Domains with Examples
FIGURE 4.

Intervention Material: Introduction to SMART Goals and Examples
Text-Messaging Protocol
At the intervention visit, text messaging procedures are set up and explained to participants in the CogRx condition. Specifically, motivational reminders and adherence and self-efficacy questions are sent automatically via text-messages to participants’ personal cell phones using the SurveySignal program. Participants receive one automated daily text-based reminder in the morning at 8 am (ie, “Don’t forget to do your healthy lifestyle activities today!”) as well as one query on their self-efficacy perceptions (ie, “I feel confident that I have the power to achieve my CogRx goals.”) to which they respond with a simple number (1=not true at all to 10=very true). Then in the evening at 8 pm they receive up to three text messages (depending on how many CogRx domains they are assigned, which may be 2 or 3) with questions regarding adherence for that day (eg, “Overall, how well would you say you met your diet goals today?”) to which they respond with a simple number (1=not very well to 10=very well). These responses will be used to create an adherence and self-efficacy score for each participant, which will be an average of their response each day of the 3-month intervention period. The text messages are monitored by a research assistant to track responsiveness and check for any technical issues (eg, automated texts not sent at correct time). If a participant has not responded to any text messages in two days, a research assistant calls them to see if they are having any issues and reminds them to respond to all texts in a timely manner. To incentivize responsiveness, if participants respond to at least 75% of text messages by the end of the 3-month intervention period they receive an additional $20 in compensation.
Bi-Weekly Check-ins
In addition to the text-based adherence gauging protocol, the research assistant also calls participants biweekly to assess progress and motivate engagement throughout the duration of the 3-months. The biweekly telephone interview is brief and queries participants only on their activities from the day prior. Participants are interviewed on varying days of the week over the 3-month period to allow for more diverse data collection.
Results
Study Progress and Modifications
Though planned analyses (described below) will be conducted when the study concludes in 2022, study modifications and current enrollment numbers are provided here. Enrollment began in January 2020 however due to a research shutdown due to COVID-19, no new participants were enrolled from March 2020 to August 2020. Thus far, the study has screened 95 people and enrolled 46 participants, of which 39 participants were randomized (14 control, 12 psychoeducation, 13 intervention). Thirty-three participants have completed 3 month follow-up, 22 have completed 6 month follow-up, and 0 have been lost to attrition. Modifications to the study when research resumption began in September 2020 included moving to a hybrid data collection model. Specifically, study assessments and the intervention transitioned to a hybrid of in-person, phone, and mail assessment modality to limit in-person contact. A footnote in Table 1 details the format for each data collection measure. The intervention session for psychoeducation (for both psychoeducation control group and CogRx group) remained as an in person format, while the additional CogRx program (i.e., feedback and goal setting) was conducted via phone. Another change is that whereas the original protocol proposed to complete full baseline assessments to determine eligibility, now in order to minimize non-essential contact with participants, participants complete the cognitive prescription domain measures via phone, after determining basic eligibility criteria. Therefore, only eligible participants complete a full baseline assessment. Finally, a change in recruitment has been made, such that the study now also recruits via social media (i.e., targeted Facebook ads). Of the 95 total people who have been screened, 60 found out about the study via the study ad, and 26 of those were actually enrolled.
Planned Analyses
A statistical significance will be established using alpha of 0.05 and the significance will be adjusted for multiple testing using the false discovery rate technique.72 Descriptive statistics and visualization tools will be used to explore all the outcome variables. Next, the distributions of each outcome will be analyzed and if needed transformations will be applied. Missing data will be handled using mixed effects models which deliver unbiased estimates when the data are missing conditionally at random. Otherwise, multiple imputation techniques with sensitivity analyses will be employed.73 The G* Power program74 was used for sample size analysis which indicated that a total sample size of 150 (50 per group) provides an 80% power to detect a moderate effect size75 (Cohen’s d = 0.64), at a corrected significance level of 0.01. We plan to enroll a sample size of N = 165 to account for a conservative estimated attrition rate of 10%, resulting in a N = 150 final sample.
For Specific Aim 1/Proximal Outcomes, to determine whether the CogRx condition is superior to psychoeducation alone in improving engagement in healthy lifestyle behaviors and dementia knowledge, longitudinal analyses using mixed-effects modeling approach will be employed.76 Specifically, the dependent variables we will examine are lifestyle behaviors (ie, the total scores for the individual surveys for each of the CogRx domains, as well as binary variables indicating whether or not the participant is in the risk range (Table 2) and dementia knowledge (ie, total scores for these measures). As a sensitivity analysis, generalized estimating equations will be used to estimate linear models with the repeated measurements. Strength of relationships among variables will be interpreted using effect sizes such as standardized regression coefficients (for continuous predictors) and standardized mean differences (for categorical predictors). Regression (mediation analysis) will be used to examine whether adherence and self-efficacy data gathered via text messaging and improvements in dementia knowledge are associated with gains in lifestyle behaviors.
For Specific Aim 2/Distal Outcomes, to compare the three conditions on a brief battery of cognitive and psychosocial measures, a similar approach as Aim 2 will be used, with longitudinal analyses using mixed-effects modeling approach.76 Specifically, the dependent variables are cognitive functioning and psychosocial measures (Table 1). For cognitive functioning z-score composites within each domain will be created as well as a global cognition composite. Regression (mediation analysis) will be used to examine whether gains in these outcomes are mediated by Aim 1 gains in lifestyle behaviors.
For the Exploratory/Qualitative Feedback Aim, to conduct qualitative interviews with CogRx participants to determine feedback for future implementation of this program, including barriers and facilitators to engagement/adherence and likelihood of continuing the program, interview data will first be transcribed and imported into NVivo qualitative analytic software. Content analysis will be used to examine themes.
Discussion
Despite the increased risk for AD and other dementias in AAs, the significant evidence on the role health behaviors may play in this risk, and evidence of less AD knowledge in AAs, virtually no studies have targeted this population in behavioral modification dementia risk reduction interventions. The implications of this study are important for healthcare professionals who are ideally positioned to educate and improve brain health literacy for their patients at risk of developing dementia. Identifying culturally relevant, feasible and ecologically-valid lifespan intervention approaches for this population are paramount. The current study addresses aforementioned gaps with existing multi-domain lifestyle modification approaches, including examining a broader range of domains. Indeed, studies suggest that a broad spectrum of activities provide more benefit than any one specific activity17 and that up to half of AD cases may be attributable to modifiable factors.9 Another limitation of exisiting multi-domain lifestyle interventions that the current study addresses is measurement of adherence with text-messaging. Indeed, studies support the acceptability of using mobile technology in AAs as both reminders/motivators of health behaviors and to assess adherence and other real-time queries.77, 78 This innovative study provides the first attempt to test the feasibility and preliminary efficacy of dementia psychoeducation combined with tailored “Cognitive Prescriptions”79 compared to psychoeducation only and no-contact control groups in middle-aged AAs in the Deep South. The psychoeducation targets general dementia knowledge, concerns, and beliefs by presenting information about dementia severity (eg, prevalence, prognosis) and susceptibility (eg, increased risk in AA) as well as risk and protective factors. The CogRx condition also receives tailored feedback on their profile of risk/protective factors (ie, physical activity, cognitive activity, social activity, diet, sleep) as well as developing intervention goals. These malleable domains may be especially viable behavioral targets for intervention and may have secondary positive reciprocal outcomes (eg, physical activity and sleep). This approach is grounded in Social Cognitive Theory29 and the Health Belief Model,30 with the premise that in order to change behavior, perceived susceptibility, severity, benefits, and barriers must be addressed.
Results from this study will fill many gaps in the literature. First, this study will determine whether a brief dementia psychoeducational program is effective in improving dementia knowledge in middle-aged AA adults, and whether this learned knowledge is durable up to 6 months. Next, this study will elucidate whether psychoeducation plus individualized behavioral plans are more effective in improving engagement in healthy lifestyle behaviors than psychoeducation alone. The text messaging adherence and self-efficacy data will also allow for determining whether these variables are associated with pre-post gains in the CogRx intervention group. This study will also allow for examining if these individualized plans tailored to one’s deficient areas are effective not only in improving engagement in the respective domains, but also whether there are downstream positive effects on domains not directly targeted in one’s intervention plan. While this pilot feasibility study only follows up participants for 6 months and given that participants will not have severe cognitive impairment at baseline, our results will nonetheless shed light on whether this intervention has any positive effects at 3 and 6 months on cognitive functioning and psychosocial outcomes. Importantly, the qualitative feedback provided will allow for determination of areas of the intervention that warrant modification in future work, as well as common barriers and facilitators that AAs experience in engaging in healthy lifestyle behaviors that will inform future iterations of this program. Importantly per barriers, our study will examine qualitative and quantitative data on how COVID-19 has affected participants’ lives, including specifically engagement in healthy lifestyle behaviors. We will also have a rich dataset of additional COVID-19 variables, including COVID-19 knowledge/literacy. These measures are particularly germane in the current study sample, as AAs are heavily burdened by the US COVID-19 pandemic.80–82 In addition to adding in COVID-19 specific measures, we have also modified our protocol by implementing a COVID-19 hybrid data collection plan that minimizes social contact by only conducting in-person assessments for measures that are absolutely essential. We have also added in social media as a recruitment approach, which has proven very successful. Therefore, the study team has overcome the obstacles of conducting a clinical trial during a pandemic. Indeed, we have had an excellent retention rate.
This study is not without limitations, in terms of study design and COVID-related obstacles previously mentioned. First, the sample size is small, though this is appropriate for this pilot study that is focused on generating feasibility, acceptability, and effect size data. Second, this study is limited by subjective measurement of the CogRx domains, including of behavior change over time. Third, and related to the prior point, is the lack of objective biomarker data that may shed light on any mechanisms whereby these domains may impact cognition. Fourth, the text messaging protocol is not personalized, which may be more beneficial in promoting behavior change.
If positive results emerge in the current study, future studies of this approach could address several important topics. First, future work should include objective assessment of improvement in CogRx domains (eg, actigraphy) and mechanisms whereby these changes affect cognition (eg, imaging, biomarkers) over longer follow-up periods to examine preventing/delaying MCI and AD vs. improving existing impairment. Second, future work may include individualized text-based reminders (eg, preferences for time of day, content) and also further stratify intervention groups into those that receive motivational reminders versus those who do not receive them, which will allow for a better understanding on the efficacy in such reminders in promoting behavior change. Third, examination of the CogRx approach in other at-risk populations (eg, HIV, traumatic brain injury) is a worthy avenue for future work. Finally, future implementation science research will provide important implications for translating this approach into community and clinical settings.
The clinical implications of this work are significant. Results may provide support for increasing dissemination of psychoeducation on dementia prevention health behaviors, which may be provided in diverse healthcare settings by interdisciplinary clinicians. Similarly, if the CogRx tailored plans are effective at improving engagement in health behaviors, this may support the deployment of a similar approach in clinical settings. For example, brief patient work-ups on multi-domain risk factor profiles will not only provide a basis for cognitive prescriptions, but the process of providing feedback to patients on deficient areas and engaging in goal setting may promote adherence. Further, many patients may not otherwise know that they are deficient in certain areas and this process would shed light on their brain health behaviors.
Acknowledgements
This work was supported by the National Institutes of Health [R21AG059954]. We would like to thank the staff who are assisting with this study and to the research volunteers who have made this work possible.
Funding:
This work was supported by the National Institutes of Health [R21AG059954].
Footnotes
Competing Interests: The Authors declare that there is no conflict of interest.
References
- 1.Hebert LE, Beckett LA, Scherr PA, Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. Oct-Dec 2001;15(4):169–73. [DOI] [PubMed] [Google Scholar]
- 2.2021 Alzheimer’s disease facts and figures. Alzheimers Dement. Mar 2021;17(3):327–406. doi: 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- 3.Potter GG, Plassman BL, Burke JR, et al. Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimers Dement. Nov 2009;5(6):445–53. doi: 10.1016/j.jalz.2009.04.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. Jan 9 2001;56(1):49–56. [DOI] [PubMed] [Google Scholar]
- 5.Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. Aug 30 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack CR Jr., Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. May 2009;132(Pt 5):1355–65. doi: 10.1093/brain/awp062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiman EM, Quiroz YT, Fleisher AS, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. Dec 2012;11(12):1048–56. doi: 10.1016/S1474-4422(12)70228-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. Apr 2013;12(4):357–67. doi: 10.1016/S1474-4422(13)70044-9 [DOI] [PubMed] [Google Scholar]
- 9.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. Sep 2011;10(9):819–28. doi: 10.1016/S1474-4422(11)70072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Academies of Sciences E, and Medicine, Division HaM, Policy BoHS, et al. Preventing Cognitive Decline and Dementia: A Way Forward. 2017; [PubMed]
- 11.Yaffe K, Falvey C, Harris TB, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. Dec 19 2013;347:f7051. doi: 10.1136/bmj.f7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noble JM, Manly JJ, Schupf N, Tang MX, Luchsinger JA. Type 2 diabetes and ethnic disparities in cognitive impairment. Ethn Dis. Winter 2012;22(1):38–44. [PMC free article] [PubMed] [Google Scholar]
- 13.Link BG, Phelan JC. Understanding sociodemographic differences in health--the role of fundamental social causes. Am J Public Health. Apr 1996;86(4):471–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. Sep 2008;18(3):223–54. doi: 10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- 15.Graham G Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev. 2015;11(3):238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saffer H, Dave D, Grossman M, Leung LA. Racial, Ethnic, and Gender Differences in Physical Activity. J Hum Cap. Winter 2013;7(4):378–410. doi: 10.1086/671200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karp A, Paillard-Borg S, Wang HX, Silverstein M, Winblad B, Fratiglioni L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord. 2006;21(2):65–73. doi: 10.1159/000089919 [DOI] [PubMed] [Google Scholar]
- 18.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. Jun 6 2015;385(9984):2255–63. doi: 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 19.Bredesen DE. Reversal of cognitive decline: a novel therapeutic program. Aging (Albany NY). Sep 2014;6(9):707–17. doi: 10.18632/aging.100690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clare L, Hindle JV, Jones IR, et al. The AgeWell study of behavior change to promote health and wellbeing in later life: study protocol for a randomized controlled trial. Trials. Jul 24 2012;13:115. doi: 10.1186/1745-6215-13-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dannhauser TM, Cleverley M, Whitfield TJ, Fletcher BC, Stevens T, Walker Z. A complex multimodal activity intervention to reduce the risk of dementia in mild cognitive impairment--ThinkingFit: pilot and feasibility study for a randomized controlled trial. BMC Psychiatry. May 05 2014;14:129. doi: 10.1186/1471-244X-14-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kivipelto M, Solomon A, Ahtiluoto S, et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimers Dement. Nov 2013;9(6):657–65. doi: 10.1016/j.jalz.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 23.Vellas B, Carrie I, Gillette-Guyonnet S, et al. Mapt Study: A Multidomain Approach for Preventing Alzheimer’s Disease: Design and Baseline Data. J Prev Alzheimers Dis. Jun 2014;1(1):13–22. [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts JS, Connell CM, Cisewski D, Hipps YG, Demissie S, Green RC. Differences between African Americans and whites in their perceptions of Alzheimer disease. Alzheimer Dis Assoc Disord. Jan-Mar 2003;17(1):19–26. [DOI] [PubMed] [Google Scholar]
- 25.Ayalon L, Arean PA. Knowledge of Alzheimer’s disease in four ethnic groups of older adults. Int J Geriatr Psychiatry. Jan 2004;19(1):51–7. doi: 10.1002/gps.1037 [DOI] [PubMed] [Google Scholar]
- 26.Howell JC, Soyinka O, Parker M, et al. Knowledge and Attitudes in Alzheimer’s Disease in a Cohort of Older African Americans and Caucasians. Am J Alzheimers Dis Other Demen. Jun 2016;31(4):361–7. doi: 10.1177/1533317515619037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connell CM, Scott Roberts J, McLaughlin SJ, Akinleye D. Racial differences in knowledge and beliefs about Alzheimer disease. Alzheimer Dis Assoc Disord. Apr-Jun 2009;23(2):110–6. doi: 10.1097/WAD.0b013e318192e94d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rovner BW, Casten RJ, Hegel MT, Leiby B. Preventing Cognitive Decline in Black Individuals With Mild Cognitive Impairment: A Randomized Clinical Trial. JAMA Neurol. Dec 1 2018;75(12):1487–1493. doi: 10.1001/jamaneurol.2018.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bandura A Health promotion by social cognitive means. Health Educ Behav. Apr 2004;31(2):143–64. doi: 10.1177/1090198104263660 [DOI] [PubMed] [Google Scholar]
- 30.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the Health Belief Model. Health Educ Q. Summer 1988;15(2):175–83. [DOI] [PubMed] [Google Scholar]
- 31.Joseph RP, Ainsworth BE, Mathis L, Hooker SP, Keller C. Utility of Social Cognitive Theory in Intervention Design for Promoting Physical Activity among African-American Women: A Qualitative Study. Am J Health Behav. Sep 1 2017;41(5):518–533. doi: 10.5993/AJHB.41.5.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandt J SM, Folstein MF. The Telephone Interview for Cognitive Status. Neuropsychiatry, Neuropsychology and Behavioral Neurology. 1988;1(2):111–117. [Google Scholar]
- 33.Wilkinson GS, Robertson GJ. Wide Range Achievement Test Professional manual - Fourth Edition. 2006
- 34.Ali R, Meena S, Eastwood B, Richards I, Marsden J. Ultra-rapid screening for substance-use disorders: the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST-Lite). Drug Alcohol Depend. 2013;132(1–2):352–61. doi:doi: 10.1016/j.drugalcdep.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Pennebaker JW, Susman JR. Disclosure of traumas and psychosomatic processes. Social Science & Medicine. 1988;26(3):327–332. doi: 10.1016/0277-9536(88)90397-8 [DOI] [PubMed] [Google Scholar]
- 36.Campbell-Sills L, Stein MB. Psychometric analysis and refinement of the Connor-davidson Resilience Scale (CD-RISC): Validation of a 10-item measure of resilience. J Trauma Stress. Dec 2007;20(6):1019–28. doi: 10.1002/jts.20271 [DOI] [PubMed] [Google Scholar]
- 37.Lawton MP. The Philadelphia Geriatric Center Morale Scale: a revision. J Gerontol. Jan 1975;30(1):85–9. [DOI] [PubMed] [Google Scholar]
- 38.Pearlin LI, Lieberman MA, Menaghan EG, Mullan JT. The stress process. J Health Soc Behav. Dec 1981;22(4):337–56. [PubMed] [Google Scholar]
- 39.Greenglass ER, Marques S, deRidder M, Behl S. Positive coping and mastery in a rehabilitation setting. Int J Rehabil Res. Dec 2005;28(4):331–9. [DOI] [PubMed] [Google Scholar]
- 40.Montross LP, Depp C, Daly J, et al. Correlates of self-rated successful aging among community-dwelling older adults. Am J Geriatr Psychiatry. Jan 2006;14(1):43–51. doi: 10.1097/01.JGP.0000192489.43179.31 [DOI] [PubMed] [Google Scholar]
- 41.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. Jun 1992;30(6):473–83. [PubMed] [Google Scholar]
- 42.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. Jun 1997;12(2):277–87. [DOI] [PubMed] [Google Scholar]
- 43.Lachman ME, Baltes P, Nesselroade JR, Willis SL. Examination of personality-ability relationships in the elderly: The role of the contextual (interface) assessment mode. Journal of Research in Personality. 1982;16:485–501. [Google Scholar]
- 44.Rasmusson DX, Bylsma FW, Brandt J. Stability of performance on the Hopkins Verbal Learning Test. Arch Clin Neuropsychol. Jan 1995;10(1):21–6. [PubMed] [Google Scholar]
- 45.Benedict R Brief Visuospatial Memory Test-revised. Psychological Assessment Resources, Inc.; 1997. [Google Scholar]
- 46.Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–38. [PubMed] [Google Scholar]
- 47.Rosen WG. Verbal fluency in aging and dementia. Journal of Clinical Neuropsychology. 1980/10/01 1980;2(2):135–146. doi: 10.1080/01688638008403788 [DOI] [Google Scholar]
- 48.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. Oct 1955;19(5):393–4. [DOI] [PubMed] [Google Scholar]
- 49.Trenerry MR, Crosson B, DeBoe J, & Leber WR. Stroop neuropsychological screening test manual. 1989
- 50.Diehr MC, Heaton RK, Miller W, Grant I. The Paced Auditory Serial Addition Task (PASAT): norms for age, education, and ethnicity. Assessment. Dec 1998;5(4):375–87. doi: 10.1177/107319119800500407 [DOI] [PubMed] [Google Scholar]
- 51.Taylor MJ, Heaton RK. Sensitivity and specificity of WAIS-III/WMS-III demographically corrected factor scores in neuropsychological assessment. J Int Neuropsychol Soc. Nov 2001;7(7):867–74. [PubMed] [Google Scholar]
- 52.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests 3rd ed. Oxford University Press; 2006. [Google Scholar]
- 53.Carpenter BD, Balsis S, Otilingam PG, Hanson PK, Gatz M. The Alzheimer’s Disease Knowledge Scale: development and psychometric properties. Gerontologist. Apr 2009;49(2):236–47. doi: 10.1093/geront/gnp023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Annear MJ, Toye CM, Eccleston CE, et al. Dementia Knowledge Assessment Scale: Development and Preliminary Psychometric Properties. Journal of the American Geriatrics Society. 2015;63(11):2375–2381. doi:doi: 10.1111/jgs.13707 [DOI] [PubMed] [Google Scholar]
- 55.Stoddard L, Kaufman J. Coronavirus Impact Scale. 2020;
- 56.Cawthon P, Orwoll E, Ensrud K, et al. Assessing the impact of the covid-19 pandemic and accompanying mitigation efforts on older adults. J Gerontol A Biol Sci Med Sci. 2020:glaa099. doi: 10.1093/gerona/glaa099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knowledge Geldsetzer P. and Perceptions of COVID-19 Among the General Public in the United States and the United Kingdom: A Cross-sectional Online Survey. Ann Intern Med. 2020;173(2):157–160. doi: 10.7326/M20-0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. Aug 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 59.Prevention. CfDCa. 2008 Physical Activity Guidelines for Americans. 2017. https://www.cdc.gov/physicalactivity/basics/index.htm
- 60.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. Sep 2015;11(9):1015–22. doi: 10.1016/j.jalz.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. Sep 2015;11(9):1007–14. doi: 10.1016/j.jalz.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM, Jr. Social ties and susceptibility to the common cold. JAMA. 1997;277(24):1940–4. [PubMed] [Google Scholar]
- 63.Anderson ND, Damianakis T, Kroger E, et al. The benefits associated with volunteering among seniors: a critical review and recommendations for future research. Psychol Bull. Nov 2014;140(6):1505–33. doi: 10.1037/a0037610 [DOI] [PubMed] [Google Scholar]
- 64.Griep Y, Hanson LM, Vantilborgh T, Janssens L, Jones SK, Hyde M. Can volunteering in later life reduce the risk of dementia? A 5-year longitudinal study among volunteering and non-volunteering retired seniors. PLoS One. 2017;12(3):e0173885. doi: 10.1371/journal.pone.0173885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sabia S, Fayosse A, Dumurgier J, et al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. Apr 20 2021;12(1):2289. doi: 10.1038/s41467-021-22354-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vance DE, Heaton K, Eaves Y, Fazeli PL. Sleep and cognition on everyday functioning in older adults: implications for nursing practice and research. J Neurosci Nurs. Oct 2011;43(5):261–71; quiz 272–3. doi: 10.1097/JNN.0b013e318227efb2 [DOI] [PubMed] [Google Scholar]
- 67.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. Jun 19 2003;348(25):2508–16. doi: 10.1056/NEJMoa022252 [DOI] [PubMed] [Google Scholar]
- 68.Smith BJ, Ali S, Quach H. Public knowledge and beliefs about dementia risk reduction: a national survey of Australians. BMC Public Health. 2014/06/28 2014;14(1):661. doi: 10.1186/1471-2458-14-661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marcum ZA, Hohl SD, Gray SL, Barthold D, Crane PK, Larson EB. Brain Health and Dementia Prevention: A Mixed-method Analysis. Am J Health Behav. 2019;43(2):300–310. doi:doi: 10.5993/AJHB.43.2.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. Apr 15 2000;355(9212):1315–9. doi: 10.1016/S0140-6736(00)02113-9 [DOI] [PubMed] [Google Scholar]
- 71.Bovend’Eerdt TJ, Botell RE, Wade DT. Writing SMART rehabilitation goals and achieving goal attainment scaling: a practical guide. Clin Rehabil. Apr 2009;23(4):352–61. doi: 10.1177/0269215508101741 [DOI] [PubMed] [Google Scholar]
- 72.Benjamini Y, Yekutieli D. False Discovery Rate–Adjusted Multiple Confidence Intervals for Selected Parameters. Journal of the American Statistical Association. 2005/03/01 2005;100(469):71–81. doi: 10.1198/016214504000001907 [DOI] [Google Scholar]
- 73.van Buren S Flexible imputation of missing data. CRC Press; 2012. [Google Scholar]
- 74.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. May 2007;39(2):175–91. [DOI] [PubMed] [Google Scholar]
- 75.Cohen J Statistical power analysis for the behavioral sciences. 2nd ed. ed. Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 76.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. 2nd ed. SAS Institute Inc.; 2006. [Google Scholar]
- 77.Bowen PG, Clay OJ, Lee LT, Browning W, Schoenberger YM, Martin MY. Texting Older Sisters to Step: The TOSS Study. West J Nurs Res. May 1 2018:193945918770784. doi: 10.1177/0193945918770784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim BH, Glanz K. Text messaging to motivate walking in older African Americans: a randomized controlled trial. Am J Prev Med. Jan 2013;44(1):71–5. doi: 10.1016/j.amepre.2012.09.050 [DOI] [PubMed] [Google Scholar]
- 79.Vance DE, Eagerton G, Harnish B, McKie P, Fazeli PL. Cognitive prescriptions. J Gerontol Nurs. Apr 2011;37(4):22–9; quiz 30–1. doi: 10.3928/00989134-20101202-03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laurencin CT, McClinton A. The COVID-19 Pandemic: a Call to Action to Identify and Address Racial and Ethnic Disparities. Journal of Racial and Ethnic Health Disparities. 2020/06/01 2020;7(3):398–402. doi: 10.1007/s40615-020-00756-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferdinand KC, Nasser SA. African-American COVID-19 Mortality: A Sentinel Event. Journal of the American College of Cardiology. 2020/06/02/ 2020;75(21):2746–2748. doi: 10.1016/j.jacc.2020.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fouad MN, Ruffin J, Vickers SM. COVID-19 Is Disproportionately High in African Americans. This Will Come as No Surprise…. Am J Med. 2020:S0002-9343(20)30411-3. doi: 10.1016/j.amjmed.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]


