Abstract
Introduction:
Type 2 diabetes mellitus has reached epidemic levels in the U.S. and worldwide. Ocular complications from this disease include diabetic retinopathy and keratopathy, both of which can lead to significant vision loss. While frequently underappreciated, diabetic keratopathy is associated with painful ocular surface disorders, including corneal erosions and delayed wound healing. Recent work in our laboratory has focused on the role of the insulin-like growth factor (IGF) system in diabetic corneal disease.
Methods:
Here we review recent findings on the presence of IGF-1, insulin, and the insulin-like binding protein (IGFBP-3) in human tear fluid and evaluate their potential use as biomarkers in diabetes. We further examine clinical evidence using in vivo confocal microscopy as an important imaging biomarker in diabetes and discuss associations between tear film changes in diabetes and corneal nerve loss.
Results:
IGFBP-3 was the only tear film marker significantly associated with nerve loss in Type 2 diabetes, whereas tear levels of IGF-1 were associated with aging. Interestingly, tear levels of IGFBP-3 were not directly related to serum levels of HbA1c, suggesting that hyperglycemia alone is not driving increased secretion of this protein.
Conclusions:
Overwhelming evidence supports the use of in vivo confocal microscopy as a tool to evaluate corneal nerve and epithelial changes induced by diabetes in research settings. The newly identified relationship between morphological changes in the corneal subbasal nerve plexus in diabetes and the increase in tear levels of IGFBP-3 suggest that this protein may represent an innovative new biomarker to assess risk for ocular and non-ocular complications in Type 2 diabetes mellitus.
Keywords: Cornea, subbasal nerve plexus, tear film, in vivo confocal microscopy, diabetes
Introduction
Diabetes is a worldwide epidemic and a leading cause of blindness in adults between 20 and 74 years of age.[1] Two of the most prominent ocular complications from diabetes are diabetic retinopathy and keratopathy, the latter of which is frequently underappreciated and under studied.[2] Diabetic keratopathy alone is a complication that occurs in 70% of diabetic patients.[3–5] In the cornea, complications from diabetes can range from mild to severe. Mild to moderate corneal complications include superficial punctate keratitis from disruption of the tight epithelial barrier, recurrent corneal erosions arising from a reduction in cellular adhesion and abnormal basement membrane composition, and aqueous deficient dry eye.[4, 6–12] Diabetes-induced aqueous deficient dry eye stems from hyperglycemic damage to the lacrimal gland and a corresponding loss of the subbasal nerve plexus.[13–16] This decrease in corneal innervation disrupts normal communication within the lacrimal functional unit, driving the loss of aqueous secretion from the lacrimal gland and altered tear dynamics.[16, 17] In severe cases, substantial loss of the subbasal nerve plexus depletes the corneal epithelium of necessary trophic factors, resulting in corneal epithelial thinning, persistent epithelial defects, neurotrophic keratitis, and loss of vision.[18–21] Due to both the loss of neurotrophic support and epigenetic changes induced by chronic inflammation and hyperglycemia, restoring normal homeostasis to the wounded corneal epithelium is often difficult. This is due to changes at the cellular and molecular level, as described in our recent review.[22]
The Insulin-like growth factor (IGF) family
The insulin-like growth factor (IGF) family has important functions in mammalian growth, development, and aging.[23–25] The IGF family consists of peptide and hormone ligands that include IGF-1, IGF-2, and insulin. There are also six known IGF-binding proteins (IGFBPs) and three transmembrane receptors.[26] These include IGF type 1 receptor (IGF-1R), IGF type 2 receptor (IGF-2R), and insulin receptor (INSR). Ligand-mediated receptor activation activates multiple cellular processes including metabolism, proliferation, survival, migration, and invasion.[23] In contrast to the other receptors, IGF-2R functions primarily to sequester the IGF-II ligand and attenuate IGF-1R activity.[23] Similarly, IGFBPs function is to sequester both IGF-I and IGF-II, thereby preventing receptor binding.
Due to the high level of homology between IGF-1R and INSR, they can readily hybridize with each other to form an IGF and insulin hybrid receptor (Hybrid-R). Expression and localization of Hybrid-R has been well-characterized in corneal epithelial cells.[27, 28] Formation of Hybrid-R is believed to be stochastic in nature and thought to be driven by expression levels of IGF-1R and INSR. In non-ocular tissues, increased expression of Hybrid-R has been documented in diabetes.[29, 30] Moreover, ligand specificity of Hybrid-R may account for insulin insensitivity in these tissues, since Hybrid-R shows a greater affinity towards IGF-1 than insulin.[31, 32] While IGF-1R, INSR, and Hybrid-R have been identified in the corneal epithelium, the exact functions of these receptors are still under investigation.[31, 33–35]
Of the six insulin-like growth factor binding proteins (IGFBPs), IGFBP-3 is the most abundant and widely studied in human serum.[36] IGFBP-3 is a glycosylated, phosphorylated N-linked secretory protein and exerts it’s primary functions by binding to the canonical ligand, IGF-1, to extend the half-life of IGF-1 in circulation and prevent activation of IGF-1R.[36] Given the well-established role of IGF-1R in regulating proliferation, it is not surprising that IGFBP-3 functions as an anti-proliferative factor in the corneal epithelium.[37] Over the past decade, novel roles for IGFBP-3 have evolved to include vital roles in apoptosis, autophagy, and insulin resistance, indicating that IGFBP-3 is a pleiotropic protein with cell and tissue specific effects.[38–41] In this review, we will summarize what is known about the presence of IGF-1, insulin, and IGFBP-3 in normal human tear fluid, how they may be altered in corneal disease, and their potential to serve as biomarkers in diabetes. In addition, we will evaluate what is known about these markers with respect to diabetic corneal nerve damage.
IGF-1
In skin, IGF-1 is secreted by dermal fibroblasts and functions to activate dermal keratinocytes to induce proliferation.[42, 43] In the cornea, Ko and colleagues were the first to report secretion of IGF-1 by rabbit corneal epithelial cells.[44] In our hands, we have been unable to detect IGF-1 protein secretion by telomerase-immortalized human corneal epithelial cells. In contrast to the corneal epithelium, we have detected low amounts of IGF-1 in tear fluid.[45, 46] In clinical studies, tear levels of IGF-1 did not appear to vary with sex or in patients with diabetes, although larger studies are necessary to confirm these findings.[45, 46] Tear IGF-1 levels however, are inversely related to age.[46] We postulate that this may be reflective of age-related changes in the lacrimal gland.
In a more recent study, we examined the effect of dry eye on tear levels of IGF-1.[45] We found that tear levels of IGF-1 were decreased to almost non-detectable levels in patients with shorter tear film break up times. Tear levels of IGF-1 were similarly reduced in patients with reduced Schirmer’s measurements. From these data, we concluded that the extremely low levels of IGF-1 present in tear fluid of dry eye patients are due to reduced secretion from the lacrimal gland and that IGF-1 is not suitable for use as a biomarker in corneal disease.[46]
Insulin
The corneal epithelium has been reported to be an insulin insensitive tissue, meaning that insulin is not required for glucose uptake.[34, 47] This is due to the presence of a constitutively active glucose uptake transporter, GLUT-1.[48] Despite this, insulin remains a key hormone that supports proliferation and growth of the corneal epithelium. Rocha and colleagues were the first to confirm the presence of insulin in human tear fluid. [34] Using a radioimmunoassay, they found a mean insulin concentration of 0.404 ng/ml in tear fluid and went on to show that insulin is not made, but stored and released by the lacrimal gland.[49] Continuous secretion of insulin from the lacrimal gland is thought to be essential for homeostasis of the corneal epithelium.[33, 50] Given the short half-life of insulin and the rapid turnover of human tears, the residence time of insulin in tear fluid is unknown. Moreover, while hyperinsulinemia frequently occurs in metabolic syndrome and Type 2 diabetes, it is unknown whether tear levels of insulin become similarly elevated. Thus, no current data exists to support that tear levels of insulin may be a suitable biomarker for ocular complications from diabetes.
IGFBP-3
IGFBP-3 is present in the human corneal and limbal epithelium.[51] Unlike IGF-1 and insulin, IGFBP-3 is secreted by corneal epithelial cells in culture and is present in human tear fluid.[46, 51] Our initial studies identifying IGFBP-3 in tear fluid compared tear levels of IGFBP-3 between healthy adults to those with a diagnosis of Type 1 or Type 2 diabetes.[46] Importantly, we found a three-fold increase in tear levels of IGFBP-3 in diabetes that appeared to be irrespective of Type 1 versus Type 2 disease. This paralleled our in vitro data that showed a similar three-fold increase in secreted IGFBP-3 by corneal epithelial cells in response to culture in high glucose medium.[46] Further, during culture IGFBP-3 secretion was not induced by treatment with mannitol, suggesting that glucose and not hyperosmolarity drove the increase in secreted IGFBP-3. The significance of an increase in IGFBP-3, as seen in diabetes, is the shift in the IGFBP-3 to IGF-1 ratio. When tested in a cell culture model, this shift led to an inhibition of IGF-1 activity and blunted activation of IGF-1R. The limitations of this early work included a small sample size in the clinical study, markers for disease control were obtained through medical chart review, and the mixed cohort of Type 1 and Type 2 diabetics.[46]
Diabetes-induced loss of the corneal subbasal nerve plexus
The subbasal nerve plexus is an intricate network consisting of primarily sensory nerves, also known as nerve leashes, that run throughout the basal layer of the corneal epithelium, just anterior to Bowman’s layer.[52, 53] The unmyelinated nerve fibers that comprise the plexus enter the cornea from the periphery to form an Archimedean spiral with the central vortex positioned in the inferior central cornea (Fig. 1A&B). From the subbasal nerve plexus, terminal nerve fibers branch and run anteriorly toward the apical epithelial surface. In addition to these perpendicular nerve fibers, terminal fibers cross within the wing and superficial cell layers, traversing for some distance, before either turning upward to the apical epithelium or re-anastomosing with other terminal fibers (Fig 1C).
Figure 1:
Imaging of the subbasal nerve plexus and terminal epithelial nerves. (A) Maximum intensity projection of neuronal tubulin (green) staining of the subbasal nerve plexus in the mouse cornea. Scale: 70 μm. Image taken from Cai et al. Am J Pathol 2014.[68] (B) Widefield mapping of the human subbasal nerve plexus using in vivo confocal microscopy. Scale bar: 400 μm. Image taken from Patel et al. Invest Ophthalmol Vis Sci 2005.[80] (C) Maximum intensity projection showing terminal epithelial nerves branching off the subbasal nerve plexus and running throughout the corneal epithelium. Neuronal tubulin staining in green showing corneal nerves and propidium iodide stained corneal epithelial cell nuclei shown in red. Scale: 10 μm. Image taken from Cai et al. Am J Pathol 2014.[68]
Clinical studies:
Due to the optically transparent nature of the cornea, in vivo confocal microscopy has been widely used to study the normal morphology of the subbasal nerve plexus and to assess changes in the subbasal nerve plexus associated with systemic and ocular disease.[54–58] In vivo confocal microscopy is a relatively rapid and non-invasive technique that only requires topical anesthesia prior to applanation of the cornea. Different confocal microscopes have been implemented over the years with advantages and disadvantages attributed to instrument design. Most devices initially all used white light and their specific details have been reviewed elsewhere.[59] The most recent design, the Heidelberg Retinal Tomograph with the Rostock Corneal Module (Heidelberg Engineering, GmBH, Dossenheim, Germany) employs the use of a long wavelength red laser that permits the acquisition of sequential high-resolution scans with excellent lateral and axial resolution compared to previous models.[60]
In vivo confocal microscopy was first used to evaluate loss of the human corneal subbasal nerve plexus in diabetes in 2000.[61] In their seminal study, Rosenberg and colleagues reported a significant decrease in nerve density in the subbasal nerve plexus in patients with diabetes compared to healthy, non-diabetic controls. They further went on to show that patients with severe neuropathy demonstrated both corneal epithelial thinning and a loss of corneal sensitivity. In contrast to this, in patients with either mild or no corneal neuropathy, there was no detectable loss in corneal sensitivity and there was an increase in thickness of the corneal epithelium, presumably from swelling secondary to an abnormal corneal endothelium.
Since that time, the number of human and animal studies using in vivo confocal microscopy to detect corneal nerve fiber loss in diabetes have increased and been extensively reviewed.[19, 62–64] In these studies, parameters for quantifying changes in the subbasal nerve plexus have expanded beyond nerve fiber density to include nerve fiber length, nerve fiber branching, nerve beading, and tortuosity. Stromal nerve architecture can also be simultaneously evaluated and diabetes-related alterations in the morphology of stromal nerves have been reported.[61] In addition, it has been suggested that changes at the center of the vortex or whorl pattern in the subbasal nerve plexus are the earliest and most sensitive measure for corneal nerve fiber damage.[65] Together, these studies have shown that the measurable changes in corneal nerves visible by in vivo confocal microscopy are associated with the development of peripheral diabetic neuropathy. These data are further supported by studies showing that clinical improvement in risk factors for diabetic peripheral neuropathy, including pancreas transplantation, are associated with an improvement in the measurable parameters for the subbasal nerve plexus. [66, 67]
Limitations to the use of confocal microscopy as a routine clinical test exist. These include the cost associated with the machine that precludes widespread use in clinical practice, the absence of any standardization in image acquisition, and the time intensive nature of image analysis. Depending on the clinic, image analysis may involve manual image assembly and assessment of corneal nerve morphology to more automated methodology. Thus, while current research data supports the use of the in vivo confocal microscope as a tool for tracking changes in peripheral corneal nerves in disease, continued research is needed to develop easily implemented acquisition and image analysis programs to facilitate data collection and established normative cutoff indices that can be widely applied in clinical practice.
Basic investigations:
Our laboratory and others have reported loss of the corneal subbasal nerve plexus in rodent models of diabetes.[68–70] Davidson et al. (2014) studied corneal nerve changes in Type 2 diabetic rats using high fat fed rodents exposed to a low dose of streptozotocin, a drug known to known to kill pancreatic beta cells and induce type 1 diabetes.[69] Using this model, they found a decrease in corneal nerve length in high fat fed/obese rodents and in diabetics. Further investigation into the pattern of nerve loss revealed a 50% decrease in corneal epithelial nerve fiber length in the corneal whorl. The amount of nerve loss decreased in the mid-periphery and no nerve loss was detected out in the peripheral cornea.
Wang and colleagues found similar changes in type 2 Goto-kakizaki rats with the most extensive nerve damage located within the central cornea.[70] In addition, these diabetic mice demonstrated delayed wound healing and reinnervation compared to healthy controls, a finding consistent in humans. In our own lab using 3-dimensional volumetric imaging of the mouse cornea, we were able to segment and quantify nerve fiber length and density in the subbasal nerve plexus independently of the terminal epithelial nerve fibers that branch and run perpendicularly to the apical corneal surface (Fig. 2, Supplementary videos 2 & 3).[58, 71, 72] In our Type 1 diabetes mouse model that used high dose streptozotocin, we found that after six weeks of disease, there was a significant loss in nerve fiber length in the subbasal nerve plexus, but not the terminal epithelial nerve fibers.[68] At a later disease stage we did detect a decrease in the terminal epithelial nerve fibers. This decrease was evident in both diabetic and control mice, suggesting an age-related, not disease related effect.[68] Consistent with this finding, work by Davidson and colleagues also saw a decrease in terminal epithelial nerve fiber length with age.[69]
Figure 2:
Loss of the corneal subbasal nerve plexus in diabetes. (A-B) Maximal intensity projection of neuronal tubulin stained nerve fibers in the (A) normal, and (B) diabetic mouse cornea. (C-D) 3-D surface renderings of nerve fibers in the (C) normal, and (D) diabetic cornea. Fibers in the subbasal nerve plexus are shown in red, terminal epithelial nerves in blue. Note the distinct loss of red fibers in diabetes. Scale bars: 10 μm. Images taken from Cai et al. Am J Pathol 2014.[68]
Our observations led to the conclusion that subbasal nerve plexus fibers without associated terminal epithelial nerves are lost before subbasal plexus nerve fibers with terminal epithelial nerves, suggesting that significant damage to the subbasal nerve plexus is required before terminal epithelial nerves are affected. Further studies are needed to explore this finding. However, since the terminal epithelial nerve fibers are sensory in nature, this would explain why confocal studies showing early loss of the subbasal nerve plexus do not correlate with a reduction in corneal sensitivity.[73] It is important to note that our findings of a reduction in length of fibers in the subbasal nerve plexus, but not in terminal epithelial nerves, differ than those from other rodent models of diabetes. We speculate that this is due to sampling differences when imaging 3-dimenstional whole tissue in situ as opposed to the use of 2-dimensional thinly sectioned images.[68, 70] Interestingly, our reported decrease in the terminal epithelial nerve fibers that occur in response to aging in the mouse parallel the decrease in human tear levels of IGF-1 during aging. Since IGF-1 is a known neurotrophic factor, the loss of IGF-1 may contribute to the reported age-related decrease in the terminal nerve fibers.[74]
Biomarkers for disease severity in diabetes
Hemoglobin A1c is a well-established clinical marker for monitoring disease control in diabetes. In vivo confocal microscopic studies of the subbasal nerve plexus have shown that patients with higher levels of HbA1c are associated with greater nerve loss.[75] Not unsurprisingly, this indicates that sugar control is tightly related to the onset of corneal neuropathy. More recently, we re-evaluated tear levels of IGFBP-3 in an age and sex-matched cohort of Type 2 diabetics, controlling for severity of disease, presence of corneal neuropathy, and the confounding effects of dry eye (Fig. 3).[76] In this study, we recruited 40 age- and sex-matched patients, with 18 patients in the Type 2 diabetes group, and 22 in the healthy, non-diabetic control group. Tears were collected using microcapillary tubes and in vivo confocal microscopy was performed to quantify changes in the subbasal nerve plexus. Standard dry eye testing was used to exclude any patients with moderate to severe dry eye that may have impacted tear film and corneal nerve measurements. These included measurements of tear production using Schirmers testing, non-invasive tear breakup time, corneal staining, and completion of the Ocular Surface Disease Index questionnaire. Phlebotomy was also performed to measure serum level of HbA1c.
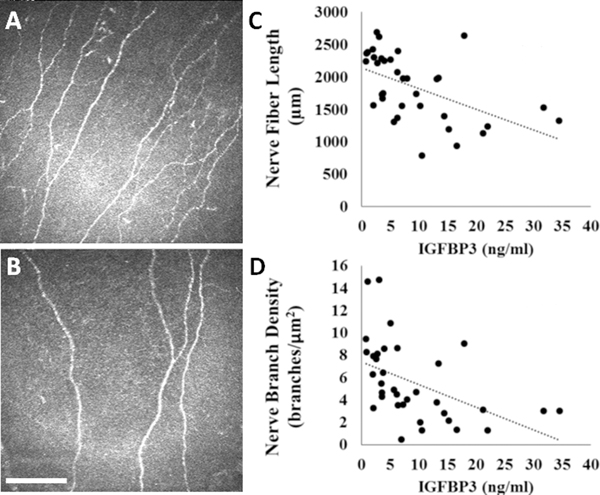
Figure 3:
Loss of the subbasal nerve plexus in diabetes is associated with an increase in tear levels of IGFBP-3. (A-B) In vivo confocal microscopy of the subbasal nerve plexus in (A) healthy, non-diabetic controls, and (B) diabetics. Scale bar: 400 μm. (C) Nerve fiber length was inversely correlated with tear levels of IGFBP-3 (R=−0.522, P=0.001). (D) Nerve branch density was inversely correlated with tear levels of IGFBP-3 (R=−0.481, P=0.003). Images taken from Stuard et al. Invest Ophthalmol Vis Sci 2017.[76]
Similar to our initial study, there was an approximate three-fold increase in tear levels of IGFBP-3 in Type 2 diabetics compared to non-diabetic controls.[46, 76] In contrast to this, tear levels of IGFBP-3 were not correlated with measured levels of HbA1c. Instead, we found that the increase in tear levels of IGFBP-3 were inversely correlated with nerve fiber length and nerve branch density, the two nerve parameters assessed in this study. Of high clinical relevance, this correlation between nerve changes and IGFBP-3 was stronger than the relationship between nerve changes and HbA1c, which is currently the gold standard for monitoring glycemic control in diabetes. These data suggest that tear levels of IGFBP-3 are not solely related to blood sugar, but reflect a total measure of stress to the corneal epithelium in diabetes. It is important to note however, that unlike Type 1 disease, complications in Type 2 diabetes are also not solely related to blood sugar control.[77] While optimal control is essential, other factors contribute to the underlying pathobiology. Future studies need to evaluate the effects of fluctuations with insulin administration or daily glucose changes on tear levels of IGFBP-3 in diabetic patients.
It is unknown whether the increase in tear levels of IGFBP-3 play a key role in the development of corneal complications in diabetes. Likewise, it remains unknown whether the full length binding protein is present in human tears or whether a specific cleavage fragment is present. IGFBP-3 is subject to cleavage by a high number of proteases and this may alter the bioavailability of this protein. It is also plausible that increased IGFBP-3 in human tears may not be due to increased secretion by stressed corneal epithelial cells but is simply a result of cellular damage and release from a dysfunctional system. Further studies are needed to tease out these questions.
Conclusions
In vivo confocal microscopy is a powerful tool for quantifying early peripheral nerve damage in diabetes in laboratory settings.[78, 79] Since changes in the corneal subbasal nerve plexus occurs prior to the onset of diabetic retinopathy, the ability to identify these early changes is critical to preventing permanent vision loss. Limitations of this technique do exist. These include the time involved in image processing and analysis, lack of full automation, and the absence of standardization. These have precluded successful integration into the clinical setting and the use of corneal nerve loss as a standard, imaging biomarker in diabetes.
The identification of potential tear film derived biomarkers, such as IGFBP-3, that correlate with corneal nerve damage, may represent a new, non-invasive measure for early corneal changes and function as a key marker to identify patients at risk for ocular and non-ocular complications. Large-scale studies are needed to validate whether this interesting pleiotropic binding protein may serve as a unique biomarker for complications in Type 2 diabetes mellitus.
Supplementary Material
A maximum intensity projection showing the detailed branching and anastomosing patterns of the terminal epithelial nerves in the corneal epithelium. Neuronal tubulin staining shown in green.
3-D surface rendering of the subbasal nerve plexus (red) and terminal epithelial nerves (blue) in the normal, non-diabetic, mouse cornea. Scale bar: 10 μm.
3-D surface rendering of the subbasal nerve plexus (red) and terminal epithelial nerves (blue) in a streptozotocin Type 1 diabetic mouse cornea. Scale bar: 15 μm.
Acknowledgments
Support: NEI grants R01 EY024546 (DMR), R01 EY029258 (DMR), T35 EY026510 (DMR), Fight for Sight Postdoctoral Fellowship (RT), UTSW Dean’s Research Scholar Fellowship (WLS), and an unrestricted grant from Research to Prevent Blindness, New York, NY.
References
- 1.Cheung N, Mitchell P, and Wong TY, Diabetic retinopathy. Lancet, 2010. 376(9735): p. 124–36. [DOI] [PubMed] [Google Scholar]
- 2.Vieira-Potter VJ, Karamichos D, and Lee DJ, Ocular Complications of Diabetes and Therapeutic Approaches. Biomed Res Int, 2016. 2016: p. 3801570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz RO, et al. , Diabetic keratopathy. Trans Am Ophthalmol Soc, 1981. 79: p. 180–99. [PMC free article] [PubMed] [Google Scholar]
- 4.Ljubimov AV, Diabetic complications in the cornea. Vision Res, 2017. 139: p. 138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skarbez K, et al. , Comprehensive Review of the Effects of Diabetes on Ocular Health. Expert Rev Ophthalmol, 2010. 5(4): p. 557–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bussan KA and Robertson DM, Contact lens wear and the diabetic corneal epithelium: a happy or disastrous marriage? J Diabetes Complications, 2019. 33(1): p. 75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikbova G, et al. , Diabetic corneal neuropathy: clinical perspectives. Clin Ophthalmol, 2018. 12: p. 981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bikbova G, et al. , Corneal changes in diabetes mellitus. Curr Diabetes Rev, 2012. 8(4): p. 294–302. [DOI] [PubMed] [Google Scholar]
- 9.Tabatabay CA, et al. , Reduced number of hemidesmosomes in the corneal epithelium of diabetics with proliferative vitreoretinopathy. Graefe’s Arch Clin Exp Ophthalmol, 1988. 226: p. 389–392. [DOI] [PubMed] [Google Scholar]
- 10.Azar DT, et al. , Altered epithelial-basement membrane interactions in diabetic corneas. Arch Ophthalmol, 1992. 110(4): p. 537–540. [DOI] [PubMed] [Google Scholar]
- 11.Azar DT, et al. , Decreased penetration of anchoring fibrils into the diabetic stroma. Arch Ophthalmol, 1989. 107(10): p. 1520–1523. [DOI] [PubMed] [Google Scholar]
- 12.Gekka M, et al. , Corneal epithelial barrier function in diabetic patients. Cornea, 2004. 23(1): p. 35–37. [DOI] [PubMed] [Google Scholar]
- 13.Zou X, et al. , Prevalence and clinical characteristics of dry eye disease in community-based type 2 diabetic patients: the Beixinjing eye study. BMC Ophthalmol, 2018. 18(1): p. 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goebbels M, Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol, 2000. 84: p. 19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf, 2007. 5(2): p. 179–93. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, et al. , Dry Eye Syndrome in Patients with Diabetes Mellitus: Prevalence, Etiology, and Clinical Characteristics. J Ophthalmol, 2016. 2016: p. 8201053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern ME, et al. , The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res, 2004. 78(3): p. 409–16. [DOI] [PubMed] [Google Scholar]
- 18.Lockwood A, Hope-Ross M, and Chell P, Neurotrophic keratopathy and diabetes mellitus. Eye, 2006. 20: p. 837–839. [DOI] [PubMed] [Google Scholar]
- 19.Markoulli M, et al. , The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul Surf, 2018. 16(1): p. 45–57. [DOI] [PubMed] [Google Scholar]
- 20.Chang P-Y, et al. , Decreased density of corneal basal epithelium and subbasal corneal nerve bundle changes in patients with diabetic retinopathy. Am J Ophthalmol, 2006. 142: p. 488–490. [DOI] [PubMed] [Google Scholar]
- 21.Sacchetti M. and Lambiase A, Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol, 2014. 8: p. 571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones L, et al. , TFOS DEWS II Management and Therapy Report. Ocul Surf, 2017. 15(3): p. 575–628. [DOI] [PubMed] [Google Scholar]
- 23.Bowers LW, et al. , The Role of the Insulin/IGF System in Cancer: Lessons Learned from Clinical Trials and the Energy Balance-Cancer Link. 2015. 6(77). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laron Z, Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol Pathol, 2001. 54(5): p. 311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chitnis MM, et al. , The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res, 2008. 14(20): p. 6364–70. [DOI] [PubMed] [Google Scholar]
- 26.Clemmons DR, IGF binding proteins and their functions. Mol Reprod Dev, 1993. 35(4): p. 368–74; discussion 374–5. [DOI] [PubMed] [Google Scholar]
- 27.Moxham CP, Duronio V, and Jacobs S, Insulin-like growth factor I receptor beta-subunit heterogeneity. Evidence for hybrid tetramers composed of insulin-like growth factor I and insulin receptor heterodimers. J Biol Chem, 1989. 264(22): p. 13238–44. [PubMed] [Google Scholar]
- 28.Soos MA, et al. , Receptors for insulin and insulin-like growth factor-I can form hybrid dimers. Characterisation of hybrid receptors in transfected cells. Biochem J, 1990. 270(2): p. 383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Federici M, et al. , Increased abundance of insulin/IGF-I hybrid receptors in adipose tissue from NIDDM patients. Mol Cell Endocrinol, 1997. 135(1): p. 41–7. [DOI] [PubMed] [Google Scholar]
- 30.Federici M, et al. , Increased expression of insulin/insulin-like growth factor-I hybrid receptors in skeletal muscle of noninsulin-dependent diabetes mellitus subjects. J Clin Invest, 1996. 98(12): p. 2887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y-C, Zhu M, and Robertson DM, Novel Nuclear Localization and Potential Function of Insulin-Like Growth Factor-1 Receptor/Insulin Receptor Hybrid in Corneal Epithelial Cells. PLoS One, 2012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandini G, et al. , Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem, 2002. 277(42): p. 39684–95. [DOI] [PubMed] [Google Scholar]
- 33.Titone R, Zhu M, and Robertson DM, Insulin mediates de novo nuclear accumulation of the IGF-1/insulin Hybrid Receptor in corneal epithelial cells. Sci Rep, 2018. 8(1): p. 4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocha EM, et al. , Identification of insulin in the tear film and insulin receptor and IGF-1R on the human ocular surface. Invest Ophthalmol Vis Sci, 2002. 43: p. 963–967. [PubMed] [Google Scholar]
- 35.Robertson DM, Zhu M, and Wu YC, Cellular distribution of the IGF-1R in corneal epithelial cells. Exp Eye Res, 2012. 94(1): p. 179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baxter RC, Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endo Metab, 2000. 278: p. E967–E976. [DOI] [PubMed] [Google Scholar]
- 37.Titone R, Zhu M, and Robertson DM, Mutual regulation between IGF-1R and IGFBP-3 in human corneal epithelial cells. 2019. 234(2): p. 1426–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan SSY, et al. , Insulin-like growth factor binding protein-3 leads to insulin resistance in adipocytes. J Clini Endocrinol Metab, 2005. 90(12): p. 6588–6595. [DOI] [PubMed] [Google Scholar]
- 39.Butt AJ and Williams AC, IGFBP-3 and apoptosis - a license to kill? Apoptosis, 2001. 6(3): p. 199–205. [DOI] [PubMed] [Google Scholar]
- 40.Muzumdar RH, et al. , Central and opposing effects of IGF-1 and IGF-binding protein-3 on systemic insulin action. Diabetes, 2006. 55(10): p. 2788–2796. [DOI] [PubMed] [Google Scholar]
- 41.Yoo E-G, et al. , Insulin-like growth factor-binding protein-3 mediates high glucose-induced apoptosis by increasing oxidative stress in proximal tubular epithelial cells. Endocrinology, 2011. 152: p. 3135–3142. [DOI] [PubMed] [Google Scholar]
- 42.Lewis DA, et al. , The IGF-1/IGF-1R signaling axis in the skin: a new role for the dermis in aging-associated skin cancer. Oncogene, 2010. 29(10): p. 1475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudman SM, et al. , The role of IGF-I in human skin and its appendages: morphogen as well as mitogen? J Invest Dermatol, 1997. 109(6): p. 770–7. [DOI] [PubMed] [Google Scholar]
- 44.Ko JA, Yanai R, and Nishida T, IGF-1 released by corneal epithelial cells induces up-regulation of N-cadherin in corneal fibroblasts. J Cell Physiol, 2009. 221(1): p. 254–61. [DOI] [PubMed] [Google Scholar]
- 45.Patel R, Zhu M, and Robertson DM, Shifting the IGF-axis: An age-related decline in human tear IGF-1 correlates with clinical signs of dry eye. Growth Horm IGF Res, 2018. 40: p. 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y-C, et al. , Elevated IGFBP3 levels in diabetic tears: a negative regulator of IGF-1 signaling in the corneal epithelium. Ocul Surf, 2012. 10(2): p. 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friend J, et al. , Insulin sensitivity and sorbitol production of the normal rabbit corneal epithelium in vitro. Invest Ophthalmol Vis Sci, 1980. 19(8): p. 913–9. [PubMed] [Google Scholar]
- 48.Kuipers DP, et al. , Differential regulation of GLUT1 activity in human corneal limbal epithelial cells and fibroblasts. Biochimie, 2013. 95(2): p. 258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cunha DA, et al. , Insulin secretion by rat lacrimal glands: effects of systemic and local variables. Am J Physiol Endocrinol Metab, 2005. 289(5): p. E768–75. [DOI] [PubMed] [Google Scholar]
- 50.Zagon IS, et al. , Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch Ophthalmol, 2007. 125(8): p. 1082–8. [DOI] [PubMed] [Google Scholar]
- 51.Robertson DM, et al. , Insulin-like growth factor binding protein-3 expression in the human corneal epithelium. Exp Eye Res, 2007. 85(4): p. 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marfurt CF, et al. , Anatomy of the human corneal innervation. Exp Eye Res, 2010. 90(4): p. 478–92. [DOI] [PubMed] [Google Scholar]
- 53.Muller LJ, et al. , Architecture of human corneal nerves. Invest Ophthalmol Vis Sci, 1997. 38(5): p. 985–94. [PubMed] [Google Scholar]
- 54.Patel DV and McGhee CN, In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol, 2009. 93(7): p. 853–60. [DOI] [PubMed] [Google Scholar]
- 55.Papanas N. and Ziegler D, Corneal confocal microscopy: a new technique for early detection of diabetic neuropathy. Curr Diab Rep, 2013. 13(4): p. 488–99. [DOI] [PubMed] [Google Scholar]
- 56.Cruzat A, Pavan-Langston D, and Hamrah P, In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol, 2010. 25(5–6): p. 171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allgeier S, et al. , Image reconstruction of the subbasal nerve plexus with in vivo confocal microscopy. Invest Ophthalmol Vis Sci, 2011. 52(9): p. 5022–5028. [DOI] [PubMed] [Google Scholar]
- 58.Cruzat A, Qazi Y, and Hamrah P, In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul Surf, 2017. 15(1): p. 15–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petroll WM and Robertson DM, In Vivo Confocal Microscopy of the Cornea: New Developments in Image Acquisition, Reconstruction, and Analysis Using the HRT-Rostock Corneal Module. Ocul Surf, 2015. 13(3): p. 187–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petroll WM, et al. , Quantitative 3-dimensional corneal imaging in vivo using a modified HRT-RCM confocal microscope. Cornea, 2013. 32(4): p. e36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenberg ME, et al. , Corneal structure and senstivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci, 2000. 2000(41): p. 10. [PubMed] [Google Scholar]
- 62.Papanas N. and Ziegler D, Corneal confocal microscopy: Recent progress in the evaluation of diabetic neuropathy. J Diabetes Investig, 2015. 6(4): p. 381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tavakoli M, Petropoulos IN, and Malik RA, Corneal confocal microscopy to assess diabetic neuropathy: an eye on the foot. J Diabetes Sci Technol, 2013. 7(5): p. 1179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang MS, et al. , Corneal confocal microscopy for assessment of diabetic peripheral neuropathy: a meta-analysis. Br J Ophthalmol, 2016. 100(1): p. 9–14. [DOI] [PubMed] [Google Scholar]
- 65.Petropoulos IN, et al. , The Inferior Whorl For Detecting Diabetic Peripheral Neuropathy Using Corneal Confocal Microscopy. Invest Ophthalmol Vis Sci, 2015. 56(4): p. 2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mehra S, et al. , Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care, 2007. 30(10): p. 2608–12. [DOI] [PubMed] [Google Scholar]
- 67.Tavakoli M, et al. , Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes, 2013. 62(1): p. 254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cai D, et al. , The impact of type 1 diabetes mellitus on corneal epithelial nerve morphology and the corneal epithelium. Am J Pathol, 2014. 184(10): p. 2662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davidson EP, et al. , Differences and similarities in development of corneal nerve damage and peripheral neuropathy and in diet-induced obesity and type 2 diabetic rats. Invest Ophthalmol Vis Sci, 2014. 55(3): p. 1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang F, et al. , Reduced innervation and delayed re-innervation after epithelial wounding in type 2 diabetic Goto-Kakizaki rates. Am J Pathol, 2012. 181(6): p. 2058–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He J. and Bazan HE, Mapping the nerve architecture of diabetic human corneas. Ophthalmology, 2012. 119(5): p. 956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kovács I, et al. , Abnormal activity of corneal cold thermoreceptors underlies the unpleasant sensations in dry eye disease. Pain, 2016. 157(2): p. 399–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenberg ME, et al. , Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci, 2000. 41: p. 2915–2921. [PubMed] [Google Scholar]
- 74.Zheng WH, et al. , Insulin-like growth factor-1 (IGF-1): a neuroprotective trophic factor acting via the Akt kinase pathway. J Neural Transm Suppl, 2000(60): p. 261–72. [DOI] [PubMed] [Google Scholar]
- 75.Wu T, et al. , Variables associated with corneal confocal microscopy parameters in healthy volunteers: implications for diabetic neuropathy screening. Diabet Med, 2012. 29(9): p. e297–303. [DOI] [PubMed] [Google Scholar]
- 76.Stuard WL, Titone R, and Robertson DM, Tear levels of insulin-like growth factor binding protein 3 correlate with subbasal nerve plexus changes in patients with type 2 diabetes mellitus. Invest Ophthalmol Vis Sci, 2017. 58(14): p. 6105–6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hansen T, Type 2 diabetes mellitus--a multifactorial disease. Ann Univ Mariae Curie Sklodowska Med, 2002. 57(1): p. 544–9. [PubMed] [Google Scholar]
- 78.Ziegler D, et al. , Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes, 2014. 63(7): p. 2454–63. [DOI] [PubMed] [Google Scholar]
- 79.Maddaloni E. and Sabatino F, In vivo corneal confocal microscopy in diabetes: Where we are and where we can get. World J Diabetes, 2016. 7(17): p. 406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patel DV and McGhee CNJ, Mapping of the Normal Human Corneal Sub-Basal Nerve Plexus by In Vivo Laser Scanning Confocal Microscopy. Investigative Ophthalmology & Visual Science, 2005. 46(12): p. 4485–4488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A maximum intensity projection showing the detailed branching and anastomosing patterns of the terminal epithelial nerves in the corneal epithelium. Neuronal tubulin staining shown in green.
3-D surface rendering of the subbasal nerve plexus (red) and terminal epithelial nerves (blue) in the normal, non-diabetic, mouse cornea. Scale bar: 10 μm.
3-D surface rendering of the subbasal nerve plexus (red) and terminal epithelial nerves (blue) in a streptozotocin Type 1 diabetic mouse cornea. Scale bar: 15 μm.