Highlights
-
•
This study evaluated baseline routine biochemical parameters in COVID-19 patients admitted to the ICU so as to identify risk predictors of disease severity and poor outcomes.
-
•
Lactate dehydrogenase (LDH) and N-terminal pro B-type natriuretic peptide (NT-proBNP) were found to be independent risk factors of poor prognosis among COVID-19 patients admitted in the ICU.
-
•
More detailed investigations on the predictors of COVID-19 severity and mortality in the ICU are required.
KEYWORDS: COVID-19, ICU, Biochemical parameters, Prognostic, Biomarkers
Abbreviations: ACE, angiotensin-converting enzyme; ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; AUC, area under the curve; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CRP, C-reactive protein; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; HbA1c, haemoglobin A1c; hs-TnT, high-sensitivity troponin T; ICU, intensive care unit; IL, interleukin; KDIGO, Kidney Disease Improving Global Outcomes; IQR, interquartile range; K+, potassium; LDH, lactate dehydrogenase; Na+, sodium; NHLS, National Health Laboratory Service; NT-proBNP, N-terminal pro B-type natriuretic peptide; PCR, polymerase chain reaction; PCT, procalcitonin; ROC, receiver operating characteristic; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TnT, troponin T
Abstract
Background
Data on biochemical markers and their association with mortality rates in patients with severe coronavirus disease 2019 (COVID-19) admitted to intensive care units (ICUs) in sub-Saharan Africa are scarce. An evaluation of baseline routine biochemical parameters was performed in COVID-19 patients admitted to the ICU, in order to identify prognostic biomarkers.
Methods
Demographic, clinical, and laboratory data were collected prospectively from patients with PCR-confirmed COVID-19 admitted to the adult ICU of a tertiary hospital in Cape Town, South Africa, between October 2020 and February 2021. Robust Poisson regression methods and the receiver operating characteristic (ROC) curve were used to explore the association of biochemical parameters with severity and mortality.
Results
A total of 82 patients (median age 53.8 years, interquartile range 46.4–59.7 years) were enrolled, of whom 55 (67%) were female and 27 (33%) were male. The median duration of ICU stay was 10 days (interquartile range 5–14 days); 54/82 patients died (66% case fatality rate). Baseline lactate dehydrogenase (LDH) (adjusted relative risk 1.002, 95% confidence interval 1.0004–1.004; P = 0.016) and N-terminal pro B-type natriuretic peptide (NT-proBNP) (adjusted relative risk 1.0004, 95% confidence interval 1.0001–1.0007; P = 0.014) were both found to be independent risk factors of a poor prognosis, with optimal cut-off values of 449.5 U/l (sensitivity 100%, specificity 43%) and 551 pg/ml (sensitivity 49%, specificity 86%), respectively.
Conclusions
LDH and NT-proBNP appear to be promising predictors of a poor prognosis in COVID-19 patients in the ICU. Studies with a larger sample size are required to confirm the validity of this combination of biomarkers.
1. Introduction
As of July 28, 2021, the coronavirus disease 2019 (COVID-19) pandemic had caused over 195 million infections and over 4.1 million deaths globally (Johns Hopkins University, 2021). South Africa was the worst affected country in Africa, with up to 2.3 million confirmed cases and 70 338 deaths (Johns Hopkins University, 2021). A range of COVID-19 manifestations have been observed, ranging from asymptomatic, to mild, moderate, severe, and fulminant (Wu et al., 2020). The more severe clinical presentations have been associated with a high viral load, increasing age, and the presence of comorbidities (Jin et al., 2020), resulting in higher risks of acute respiratory distress syndrome (ARDS), acute renal, hepatic, or cardiac injury, and death (Huang et al., 2020). Several reports from Asia, Europe, and the USA have indicated that deranged biochemical, inflammatory, and immunological parameters may be associated with a poor prognosis (Mehta et al., 2020; Thompson et al., 2020; Ferrari et al., 2020; Song et al., 2020; Karakoyun et al., 2021). Data on biochemical markers and the association with mortality observed in patients with severe COVID-19 disease admitted to intensive care units (ICUs) in sub-Saharan Africa are scarce.
The aim of this study was to evaluate baseline routine biochemical findings in patients admitted to the ICU of a tertiary hospital in the Western Cape of South Africa during the second wave and to correlate these to the severity of the disease.
2. Methods
2.1. Study population
This cohort study took place at Tygerberg Hospital, a 1380-bed tertiary hospital in Cape Town, South Africa. The hospital provides tertiary services to approximately 3.5 million people from the Western Cape Province.
The study population comprised 82 consecutive patients with a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PCR test, admitted to the adult ICU during the second wave between October 29, 2020 and February 10, 2021. The biochemical parameters assessed were those routinely collected in the ICU. Patients were triaged by the intensivists according to disease severity and the likely prognosis, in accordance with provincial guidelines (Critical Care Society of Southern Africa, 2020), and admission was dictated by bed availability.
2.2. Data collection
Due to the infection control risk, data were captured prospectively using photographs of written clinical notes at the bedside, which were securely stored electronically. The clinical data were entered remotely by a data-capturer into a Redcap database. The laboratory results were imported from the National Health Laboratory Service (NHLS) Laboratory Information System (TrakCare Lab Enterprise) into the database. Data were quality checked by the data entry supervisor to ensure that the data entered were of good quality and reliable.
2.3. Ethics
Patient confidentiality was ensured by labelling data with a unique episode number. The study was approved by the Health Research Ethics Committee of Stellenbosch University (approval number N20/04/002_COVID-19). The research project was conducted according to the ethical principles of the Declaration of Helsinki.
2.4. Laboratory analyses
Serum samples were collected on ICU admission for all study participants. The samples were analysed in the NHLS Chemical Pathology Laboratory on a Roche cobas 6000 analyser (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's recommendations. The NHLS is the South African national state laboratory, providing laboratory services to over 80% of the population. The levels of the following parameters were determined: sodium (Na+) and potassium (K+) using indirect ion-selective electrode potentiometry, creatinine enzymatically, urea using a kinetic assay with urease, alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) enzymatically, bilirubin using the colorimetric diazo method, C-reactive protein (CRP) immunoturbidimetrically, high-sensitivity troponin T (hs-TnT), N-terminal pro-brain natriuretic peptide (NT-proBNP), procalcitonin (PCT), and ferritin using an electrochemiluminescent immunoassay, and glycated haemoglobin (HbA1c) using a turbidimetric inhibition immunoassay. The estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula without correction for race, a calculation recommended by the Kidney Disease Improving Global Outcomes (KDIGO) report (Levey et al., 2020). The NHLS Chemical Pathology Laboratory is accredited by the South African National Accreditation Services (SANAS), a regulatory body responsible for laboratory conformity to ISO15189 assessments in South Africa. Result quality is validated with internal quality control and the laboratory participates in an external quality control scheme.
2.5. Outcomes and predictor variables
The following data were collected: sociodemographic (age, sex), pre-existing comorbidities associated with severe COVID-19 outcome (hypertension, diabetes mellitus, and hyperlipidaemia), and routinely collected biochemistry. The primary outcome was the proportion of patients who died (non-survivors) after admission to the ICU. Time to death or discharge and length of stay in the ICU were assessed.
2.6. Statistical analysis
Continuous variables were expressed as the mean and standard deviation for normally distributed data and as the median with interquartile range (IQR) for non-normally distributed data. Categorical variables were expressed using frequencies and percentages. Robust Poisson regression was used to assess the significant association between demographic, laboratory results, and survival. Factors associated with death at a P-value <0.15 in the unadjusted univariable robust Poisson regression were included in a multivariable model to identify independent factors associated with death. Due to the high prevalence of mortality, around 66%, the logistic regression overestimated the effect measure with large standard errors, resulting in wide confidence intervals; therefore robust Poisson regression was used. Adjusted incidence rate ratios and their 95% confidence intervals (CI) were used as a measure of association. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic performance of various biochemical analytes to discriminate between patients with severe disease who survived and died. Factors with a P-value <0.05 were considered significantly associated with mortality. All statistical analyses were performed using Stata version 16 (StataCorp, College Station, TX, USA) and R version 4.0.2 (R Core Team) with R Studio version 1.3 (R Studio Team) statistical software.
3. Results
3.1. Patient demographics
The study cohort comprised 82 patients with a median age of 53.8 years (IQR 46.4–59.7 years) who were admitted between October 2020 and February 2021. Table 1 shows the demographic characteristics of the study patients. There were 55 (67%) female patients and 27 (33%) male patients, and underlying comorbidities considered in this analysis were hypertension (n = 39, 57%), diabetes mellitus (n = 34, 50%), and hyperlipidaemia (n = 5, 7%).
Table 1.
Demographic characteristics of COVID-19 patients admitted to the ICU
| Characteristic | Total (%) | Survivors(n = 28) | Non-survivors(n = 54) | P-value |
|---|---|---|---|---|
| Age (years), median (IQR) | 53.8 (46.4–59.7) | 49.6 (42.0–60.2) | 55.3 (47.2–58.1) | 0.310 |
| Sex | 0.550 | |||
| Female | 55 (67%) | 20 (71%) | 35 (65%) | |
| Male | 27 (33%) | 8 (29%) | 19 (35%) | |
| Smoker | 13 (25%) | 3 (17%) | 10 (27%) | 0.670 |
| Duration of ICU stay | 10 (5–14) | 9.5 (5.5–14) | 10 (5–13) | 0.880 |
| Hypertension | 39 (57%) | 12 (55%) | 27 (59%) | 0.750 |
| Diabetes mellitus | 34 (50%) | 12 (55%) | 22 (48%) | 0.600 |
| Hyperlipidaemia | 5 (7%) | 1 (5%) | 4 (9%) | 0.540 |
ICU, intensive care unit; IQR, interquartile range.
The median duration of stay in the ICU was 10 days (IQR 5–14 days). Of the 82 patients with outcome data available at database censure, 54 (66%) died in the ICU. Table 1 provides descriptive data, showing that there was no statistically significant difference between survivors and non-survivors admitted to the ICU with regard to demographic characteristics, age, sex, and comorbidities.
3.2. Baseline biochemical parameters
The comparison of baseline biochemical parameters in survivors and non-survivors is shown in Table 2. Electrolyte results, namely Na+ and K+, showed no significant differences between the two groups (P = 0.37 and P = 0.51, respectively). Regarding markers of renal function, there was no significant difference in urea or creatinine level between the two groups (P = 0.18 and P = 0.097, respectively); however, the eGFR was decreased in the non-survivor group (P = 0.046). There was no statistically significant difference in HbA1c between survivors and non-survivors (P = 0.33).
Table 2.
Baseline biochemical characteristics of COVID-19 patients admitted to the ICU
| Analytes | Total | Reference intervals | TotalMedian (IQR) | Survivors(n = 28)Median (IQR) | Non-survivors(n = 54)Median (IQR) | P-value |
|---|---|---|---|---|---|---|
| Na+ | 82 | 135–145 mmol/l | 137.5 (135–140) | 137 (135–139.5) | 138 (135–141) | 0.370 |
| K+ | 82 | 3.5–5.1 mmol/l | 4.4 (3.8–4.7) | 4.2 (3.8–4.7) | 4.4 (3.9–4.7) | 0.510 |
| Urea | 82 | 2.1–7.1 mmol/l | 6.3 (4.9–8.3) | 5.95 (4.4–7.9) | 6.5 (5.1–8.8) | 0.180 |
| Creatinine | 82 | 49–90 μmol/l | 73.5 (64–98) | 71 (63–78) | 75.5 (66–107) | 0.097 |
| eGFR | 82 | >60 ml/min | 83 (71–99) | 88 (75.5–104.5) | 80 (70–94) | 0.046 |
| HbA1c | 77 | <6.5% | 7.6 (6.3–8.7) | 7.8 (6.3–11.6) | 7.5 (6.3–8.4) | 0.330 |
| Ca2+ | 58 | 2.12–2.59 mmol/l | 2.07 (2.02–2.17) | 2.1 (2.03–2.19) | 2.06 (2.01–2.16) | 0.450 |
| Mg2+ | 53 | 0.63–1.05 mmol/l | 0.94 (0.88–1.07) | 0.89 (0.81–0.94) | 0.98 (0.89–1.08) | 0.019 |
| Phosphate | 53 | 0.78–1.42 mmol/l | 1.15 (0.94–1.53) | 1.16 (1.09–1.35) | 1.14 (0.93–1.55) | 0.940 |
| TBil | 73 | 5–21 μmol/l | 7 (5–9) | 7 (5–9) | 7 (5–9) | 0.650 |
| ALT | 74 | <41 U/l | 38.5 (23–50) | 40 (23–65) | 37 (22–49) | 0.810 |
| LDH | 42 | 100–190 U/l | 722 (579–900) | 606 (346–846) | 772 (633–957.5) | 0.014 |
| TnT | 68 | <100 ng/l | 13.5 (6–28) | 6 (5–16) | 15 (9–37) | 0.006 |
| NT-proBNP | 67 | <125 pg/ml | 220 (102–845) | 116 (63–259) | 309 (125–1390) | 0.004 |
| CRP | 81 | <10 mg/l | 147 (96–221) | 106.5 (69.5–186.5) | 162 (110–235) | 0.014 |
| PCT | 70 | <0.5 ng/ml | 0.36 (0.13–1.12) | 0.16 (0.08–0.36) | 0.57 (0.19–1.68) | 0.003 |
| Ferritin | 61 | 13–150 μg/l | 891 (440–1400) | 937 (344–1400) | 770 (440–1496) | 0.880 |
ICU, intensive care unit; IQR, interquartile range; Na+, sodium; K+, potassium; eGFR, estimated glomerular filtration rate; HbA1c, haemoglobin A1c; Ca2+, calcium; Mg2+, magnesium; TBil, total bilirubin; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; TnT, troponin T; NT-proBNP, N-terminal B-type natriuretic peptide; CRP, C-reactive protein; PCT, procalcitonin.
With regard to markers of liver function, there was no significant difference in bilirubin or ALT between the two groups (P = 0.65 and P = 0.81, respectively), but the non-specific marker LDH was significantly increased in non-survivors (P = 0.014). Markers of cardiac function showed significantly increased levels of TnT and NT-proBNP in non-survivors (P = 0.006 and P = 0.004, respectively). Markers of inflammation showed significantly higher levels of CRP and PCT in non-survivors (P = 0.014 and P = 0.003, respectively). Although ferritin levels were increased, there was no statistically significant difference between survivors and non-survivors (P = 0.88).
3.3. Association of socio-demographic and biochemical parameters with survival
Table 3 shows the association between socio-demographic and biochemical parameters and survival in the study cohort. In the unadjusted univariate analysis, the relative risk (RR) was statistically significant for urea (1.03, 95% CI 1.01–1.06; P = 0.020), creatinine (1.01, 95% CI 1.01–1.07; P = 0.003), eGFR (0.99, 95% CI 0.98–0.99; P = 0.005), LDH (1.01, 95% CI 1.01–1.04; P = 0.001), TnT (1.02, 95% CI 1.01–1.03; P = 0.012), NT-proBNP (1.01, 95% CI 1.01–1.03; P = 0.001), CRP (1.02, 95% CI 1.03–1.04, P = 0.021), and PCT (1.21, 95% CI 1.10–1.35; P < 0.001). However, LDH and NT-proBNP also showed a significant association with a poor prognosis in the adjusted univariate analysis: adjusted RR 1.002 (95% CI 1.0004–1.004; P = 0.016) and adjusted RR 1.0004 (95% CI 1.0001–1.0007; P = 0.014), respectively.
Table 3.
Association of socio-demographic and biochemical parameters with mortality status among patients admitted to the COVID-19 ICU
| Characteristic | Unadjusted RR(95% CI) | P-value | Adjusted RR(95% CI) | P-value |
|---|---|---|---|---|
| Age | 1.00 (0.99–1.03) | 0.308 | ||
| Sex: Male | 1.19 (0.86–1.64) | 0.298 | ||
| Duration of ICU stay | 0.99 (0.97–1.01) | 0.424 | ||
| Hypertension | 1.06 (0.75–1.48) | 0.753 | ||
| Asthma | 0.73 (0.18–2.99) | 0.665 | ||
| Diabetes mellitus | 0.92 (0.66–1.28) | 0.608 | ||
| Hyperlipidaemia | 1.20 (0.75–1.93) | 0.452 | ||
| Na+ | 1.01 (0.97–1.04) | 0.722 | ||
| K+ | 1.10 (0.90–1.35) | 0.370 | ||
| Urea | 1.03 (1.01–1.06) | 0.020 | 0.86 (0.74–1.01) | 0.073 |
| Creatinine | 1.01 (1.01–1.07) | 0.003 | 0.99 (0.98–1.01) | 0.530 |
| eGFR | 0.99 (0.98–0.99) | 0.005 | 0.99 (0.97–1.01) | 0.453 |
| HbA1c | 0.95 (0.87–1.03) | 0.206 | ||
| Ca2+ | 0.95 (0.27–3.39) | 0.941 | ||
| Mg2+ | 1.02 (0.44–2.39) | 0.956 | ||
| Phosphate | 1.13 (0.95–1.35) | 0.172 | ||
| TBil | 1.01 (1.00–1.03) | 0.667 | ||
| ALT | 1.00 (0.99–1.00) | 0.631 | ||
| LDH | 1.01 (1.01–1.04) | 0.001 | 1.002 (1.0004–1.004) | 0.016 |
| TnT | 1.02 (1.01–1.03) | 0.012 | 0.99 (0.98–1.004) | 0.199 |
| NT-proBNP | 1.01 (1.01–1.03) | 0.001 | 1.0004 (1.0001–1.0007) | 0.014 |
| CRP | 1.02 (1.03–1.04) | 0.021 | 1.001 (0.999–1.003) | 0.251 |
| PCT | 1.21 (1.10–1.35) | <0.001 | 1.14 (0.96–1.36) | 0.135 |
| Ferritin | 1.00 (0.99–1.00) | 0.893 |
ICU, intensive care unit; RR, relative risk; CI, confidence interval; Na+, sodium; K+, potassium; eGFR, estimated glomerular filtration rate; HbA1c, haemoglobin A1c; Ca2+, calcium; Mg2+, magnesium; TBil, total bilirubin; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; TnT, troponin T; NT-proBNP, N-terminal B-type natriuretic peptide; CRP, C-reactive protein; PCT, procalcitonin.
3.4. Cut-offs and ROC
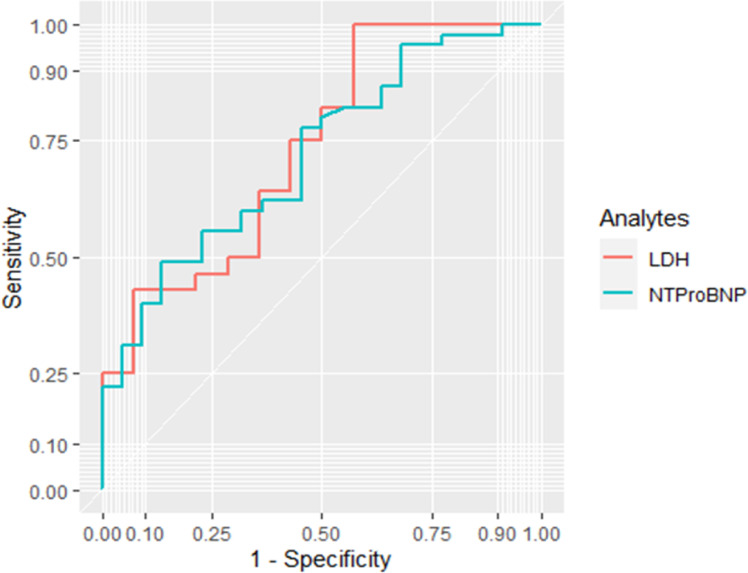
As the adjusted RR was significant for LDH and NT-proBNP, the optimal cut-offs to predict non-survival were determined, and the performance of these two parameters was tested using ROC curves. The proposed optimum cut-off points that could predict COVID-19 severity and mortality in the ICU were ≥449.5 U/l for LDH (100% sensitivity, 43% specificity, area under the curve (AUC) 0.73) and ≥551 pg/ml for NT-proBNP (49% sensitivity, 86% specificity, AUC 0.72) (Table 4). However, the performance of both was suboptimal to allow the use of either one as a predictive marker on its own (see Figure 1).
Table 4.
Optimal cut-off, sensitivity, specificity, and AUC for LDH and NT-proBNP
| Analyte | Direction | Optimal cut-off point | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| LDH | >= | 449.5 U/L | 1 | 0.43 | 0.73 |
| NT-proBNP | >= | 551 pg/mL | 0.49 | 0.86 | 0.72 |
AUC, area under the receiver operating characteristic curve; LDH, lactate dehydrogenase; NT-proBNP, N-terminal pro B-type natriuretic peptide.
Figure 1.
ROC curve of the predictor effect of combined LDH (lactate dehydrogenase) and NT-proBNP (N-terminal pro B-type natriuretic peptide) for a poor prognosis.
4. Discussion
Although numerous studies globally have evaluated biochemical abnormalities in the first and second waves of COVID-19 (Mehta et al., 2020; Thompson et al., 2020; Ferrari et al., 2020; Song et al., 2020; Karakoyun et al., 2021), there is a paucity of data from Africa. South Africa experienced the second wave in late 2020 and this study examined patients admitted to the ICU during that period.
There were several notable findings from this study, including the observation that in non-survivors the eGFR was significantly decreased while the LDH levels were significantly increased. Furthermore, surrogate markers of cardiac dysfunction, namely TnT and NT-proBNP, and inflammatory markers, namely CRP and PCT, were significantly elevated among non-survivors. However, only LDH and NT-proBNP were significant predictors of disease severity.
Other biochemical parameters such as serum Na+ and K+ (Chen et al., 2020; Lippi et al., 2020a; Wang et al., 2020), CRP (Bonetti et al., 2020; Luo et al., 2020; Ponti et al., 2020; Tan et al., 2020; Yamada et al., 2020; Poggiali et al., 2020; Boufrioua et al., 2021), PCT (Ponti et al., 2020; Lippi and Plebani, 2020; Hesse et al., 2021), pro-inflammatory cytokines, albumin, and ferritin, which are biomarkers that have been linked with multi-organ failure among patients with severe COVID-19 admitted to the ICU (Mehta et al., 2020; Thompson et al., 2020; Zeng et al., 2020), were not significantly associated with COVID-19 after adjustment for confounders. However, several markers of organ dysfunction have been described as prognostic markers in COVID-19. These include markers of hepatocyte injury (bilirubin, ALT, and the non-specific enzyme, LDH) (Bonetti et al., 2020; Poggiali et al., 2020; Kermali et al., 2020), renal function (urea, creatinine, and eGFR) (Henry and Lippi, 2020), and cardiac function (hs-TnT and NT-proBNP) (Lippi et al., 2020b). Biochemical markers associated with severity include raised LDH, CRP, cardiac troponins, and NT-proBNP.
It appears that there is only one study from South Africa on biomarkers in the first wave. A study by Hesse et al. investigated laboratory findings of all patients with COVID-19 during a 4-month period (11.7% positive), and found that disease severity was associated with raised inflammatory markers, coagulation markers, liver, cardiac markers, and urea (Hesse et al., 2021). Two studies compared the first and second waves in South Africa. Jassat et al. only compared clinical outcomes and described more hospitalizations in patients who were older with fewer comorbidities and a 20% increased risk of in-hospital mortality (Jassat et al., 2021). Maslo et al. compared clinical and laboratory data in patients hospitalized in one hospital during both waves and found that patients admitted during the second wave were also older with fewer comorbidities and had significantly higher D-dimer and interleukin (IL)-6 levels (Maslo et al., 2021). As these findings are thought to be due to increased transmissibility of the variants of concern, other laboratory biomarkers may be useful to predict disease severity.
In the present study cohort, routine biochemical tests were examined, which included electrolytes, markers of renal, hepatic, and cardiac dysfunction, and markers of inflammation, and these were correlated with survival.
As hepatocytes express angiotensin-converting enzyme 2 (ACE2) receptors, liver damage may be due to direct infection (Chai et al., 2020), but also to hepatotoxic drugs, the systemic inflammatory response, hypoxia, or multi-organ failure (Feng et al., 2020). In the present study cohort, no significant difference in bilirubin or ALT levels was found; however, LDH was significantly raised in non-survivors. This is in agreement with the results of previous studies, and high LDH was one of the first markers of disease severity described (Poggiali et al., 2020; Kermali et al., 2020; Ferrari et al., 2020). LDH is not liver-specific and this increase in LDH is thought to be due to lung damage induced by SARS-CoV-2 (Han et al., 2020). It must be noted that even though levels were significantly higher in the non-survivors in the study cohort, both groups had levels well above the higher reference limit, in concordance with the observation by Kermali et al. (Kermali et al., 2020).
The risk of cardiac dysfunction is increased in COVID-19 and possible causes include direct cytopathic injury due to the presence of ACE2 receptors on cardiac myocytes, cytokine-mediated damage, ischaemia, or exacerbation of pre-existing cardiac disease (Akhmerov and Marbán, 2020; Khan et al., 2020). Like previous studies, significantly higher TnT and NT-proBNP levels were found in non-survivors (Lippi et al., 2020b; Gao et al., 2020). The American College of Cardiology stated that increased TnT and NT-proBNP levels do not necessarily suggest acute coronary syndrome and must be interpreted in the correct clinical context, taking the clinical picture of the patient into consideration (American College of Cardiology, 2020).
The cytokine storm in COVID-19 is associated with worse outcomes and increased levels of pro-inflammatory cytokines such as IL-6 (Mehta et al., 2020; Song et al., 2020). This affects acute phase reactants, leading to increased CRP and ferritin and decreased albumin levels (Mehta et al., 2020). Most laboratories, including the NHLS Chemical Pathology Laboratory, do not routinely analyse IL-6. However, CRP is synthesized in the liver under the influence of IL-6 and therefore reflects IL-6 levels (Boras et al., 2014; Sproston and Ashworth, 2018). Significantly increased CRP levels were found in non-survivors, which is in agreement with the published literature (Bonetti et al., 2020; Luo et al., 2020; Ponti et al., 2020; Tan et al., 2020; Yamada et al., 2020; Poggiali et al., 2020; Boufrioua et al., 2021). Unfortunately, albumin was not measured routinely in the study patients and therefore numbers were too few to analyse.
PCT is normally produced in the C-cells of the thyroid and levels are undetectable in health. However, during infection (especially bacterial), levels rise due to extra-thyroidal synthesis (Karzai et al., 1997). Increased PCT levels were found in non-survivors in the present study, which is also consistent with other studies (Ponti et al., 2020; Lippi and Plebani, 2020; Hesse et al., 2021). Although previous studies have found increased ferritin (Cheng et al., 2020) or no difference in ferritin levels (Wu et al., 2020) between COVID-19 survivors and non-survivors, this study found increased levels in survivors. However, this difference was not statistically significant and the numbers were small.
When analysing the unadjusted RR in the study cohort, urea, creatinine, eGFR, LDH, TnT, NT-proBNP, CRP, and PCT were significantly associated with the risk of non-survival. However, regarding the adjusted RR, only LDH and NT-proBNP were significantly associated with increased risk. The optimal cut-off values of these two analytes to predict severity were then determined. However, the performance of both was suboptimal to allow for the use of either one as a predictive marker on its own.
Chronic kidney disease is associated with worse outcomes in COVID-19 (Henry and Lippi, 2020). In this study, slightly higher urea and creatinine with lower eGFR levels were found in non-survivors, but these differences were neither statistically nor clinically significant. Other studies have reported higher urea levels to be associated with severity (Bonetti et al., 2020; Boufrioua et al., 2021).
No significant difference in Na+ or K+ levels was found between survivors and non-survivors. Few studies have evaluated electrolyte disturbances in COVID-19 (Chen et al., 2020; Lippi et al., 2020a; Wang et al., 2020) and many have reported hypokalaemia to be associated with worse outcomes and exacerbation of ARDS and cardiac injury. As the SARS-CoV-2 virus enters the cells using the ACE2 receptor and thereby influences the renin–angiotensin system, renal loss of K+ is thought to be the cause of electrolyte disorders in these patients (Chen et al., 2020; Lippi et al., 2020a). Electrolytes may be also influenced by other clinical manifestations of these patients, such as gastrointestinal loss due to diarrhoea and vomiting and multi-organ failure (Chen et al., 2020).
The study also found that non-survivors were slightly older and more likely to be smokers, and that the proportion of male patients who died was slightly higher than the proportion of female patients who died, although none of these results were statistically significant. Comorbidities such as hypertension and diabetes mellitus have been shown to be predictors of non-survival (Savoia et al., 2021; Prattichizzo et al., 2021). The association of hypertension with a severe outcome has been postulated to be because of the action of SARS-CoV-2 on the renin–angiotensin system (Savoia et al., 2021). Diabetes mellitus with high HbA1c is associated with worse SARS-CoV-2 outcomes (Prattichizzo et al., 2021). However, in the current study cohort, although 57% of the participants were hypertensive, no significant difference in the frequency of hypertension or diabetes mellitus was found between survivors and non-survivors.
It must however be noted that the median HbA1c levels were raised at >7% in both groups. A level of >6.5% is diagnostic of diabetes. The study results agree with those of a recent study conducted in South Africa (Hesse et al., 2021).
This study has some limitations. The population was small and only baseline ICU admission laboratory data were analysed. A larger sample population may have increased the statistical significance of the markers. Ideally, an analysis of the trend over their ICU stay would have been performed, but the numbers were too small on subsequent days. This study also has certain strengths, namely, all patients were admitted to the same ICU and had samples analysed on admission in the same laboratory, ensuring harmonization of the pre-analytical and analytical phases of testing. Further larger studies are needed to evaluate these biomarkers to determine their use in developing a risk score, as earlier identification of patients at high risk of a poor prognosis and the institution of early interventions may be effective in reducing COVID-19 mortality among patients admitted to the ICU.
In conclusion, this study identified LDH and NT-proBNP as biochemical markers possibly associated with poor COVID-19 outcomes in patients admitted to the ICU . To increase the predictive probability, there is a need to combine biomarker assessments and not only rely on a single parameter estimate; for example combining LDH and NT-proBNP increases the sensitivity of predicting mortality rather than relying on a single biomarker.
Declaration of Competing Interest
No conflict of interest declared.
Acknowledgments
Acknowledgements
Prof. Sir Alimuddin Zumla is a co-Principal Investigator of the PANDORA-ID-NET, the Pan-African Network for Rapid Research, Response, Relief and Preparedness for Infectious Disease Epidemics, supported by the European & Developing Countries Clinical Trials Partnership (EDCTP). He is in receipt of a UK National Institutes of Health Research, Senior Investigator Award, and is a Mahathir Foundation Science Award laureate.
Author contributions
All authors contributed to the ideation, the collection, analyses, and interpretation of the data, and writing of the manuscript.
Research funding
This work was conducted under the COVID-19 Africa Rapid Grant Fund supported under the auspices of the Science Granting Councils Initiative in Sub-Saharan Africa (SGCI) and administered by South Africa's National Research Foundation (NRF) in collaboration with Canada's International Development Research Centre (IDRC), the Swedish International Development Cooperation Agency (Sida), South Africa's Department of Science and Innovation (DSI), the Fonds de Recherche du Québec (FRQ), the United Kingdom's Department of International Development (DFID), United Kingdom Research and Innovation (UKRI) through the Newton Fund, and the SGCI participating councils across 15 countries in sub-Saharan Africa.
Informed consent statement
The investigators obtained ethical approval and a waiver of consent from the Health Research Ethics Committee of the Faculty of Medicine and Health Sciences, Stellenbosch University, and the Research Ethics Committee of Tygerberg Hospital (approval number N20/04/002_COVID-19).
References
- Akhmerov A, Marbán E. COVID-19, and the heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Cardiology. Troponin and BNP Use in COVID-19, 2020. https://www.acc.org/latest-in-cardiology/articles/2020/03/18/15/25/troponin-and-bnp-use-in-covid19 (Accessed 15 June 2021)
- Bonetti G, Manelli F, Patroni A, Bettinardi A, Borrelli G, Fiordalisi G, et al. Laboratory predictors of death from coronavirus disease 2019 (COVID-19) around Valcamonica, Italy. Clin Chem Lab Med. 2020;58:1100–1105. doi: 10.1515/cclm-2020-0459. [DOI] [PubMed] [Google Scholar]
- Boras E, Slevin M, Alexander MY, Aljohi A, Gilmore W, Ashworth J, et al. Monomeric C-reactive protein, and Notch-3 co-operatively increase angiogenesis through PI3K signalling pathway. Cytokine. 2014;69:165–179. doi: 10.1016/j.cyto.2014.05.027. [DOI] [PubMed] [Google Scholar]
- Boufrioua E, Awati E, Ouahidi I, Elomari L, Ouassif H, et al. Comparative Analysis of Biochemical Indexes of Severe and Non-Severe Patients Infected with COVID-19. Arch Med. 2021;13:17. [Google Scholar]
- Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 2020.02.03.931766. [Google Scholar]
- Chen D, Li X, Song Q, Hu C, Su F, Dai J, et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Li H, Li L, Liu C, Ya S, Chen H, Li Y. Ferritin in the coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Lab Anal. 2020;34:e23618. doi: 10.1002/jcla.23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critical Care Society of Southern Africa. Allocation of scarce critical care resources during the COVID-19 pandemic health emergency in South Africa. 2020. https://criticalcare.org.za/wp-content/uploads/2020/04/Allocation-of-Scarce-Critical-Care-Resources-During-the-COVID-19-Public-Health-Emergency-in-South-Africa.pdf (Accessed 20 July 2021) [PubMed]
- Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, et al. COVID-19, and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transpl Hepatol. 2020;8:18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Motta Z, Strollo M, Banfi G, Locatelli M. Routine blood tests as a potential diagnostic tool for COVID-19. Clin Chem Lab Med. 2020;58:1095–1099. doi: 10.1515/cclm-2020-0398. [DOI] [PubMed] [Google Scholar]
- Gao L., Jiang D., Wen XS., Cheng XC., Sun M., He B., et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res. 2020;21(1):83. doi: 10.1186/s12931-020-01352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Zhang H, Mu S, Wei W, Jin C, Tong C, et al. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: a retrospective and observational study. Aging (Albany NY) 2020;12:11245. doi: 10.18632/aging.103372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52:1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse R, van der Westhuizen DJ, George JA. COVID-19-Related Laboratory Analyte Changes and the Relationship Between SARS-CoV-2 and HIV, TB, and HbA1c in South Africa. Adv Exp Med Biol. 2021;1321:183–197. doi: 10.1007/978-3-030-59261-5_16. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassat W, Mudara C, Ozougwu L, Tempia S, Blumberg L, Davies MA, et al. Increased mortality among individuals hospitalised with COVID-19 during the second wave in South Africa. medRix. 2021 2021.03.09.21253184. [Google Scholar]
- Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins University & Medicine. Coronavirus Resource Center: Global map. https://coronavirus.jhu.edu/map.html [Accessed 28 July 2021].
- Karzai W., Oberhoffer M., Meier-Hellmann A., Reinhart K. Procalcitonin – a new indicator of the systemic response to severe infections. Infection. 1997;25:329–334. doi: 10.1007/BF01740811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakoyun I, Colak A, Turken M, Altin Z, Arslan FD, Iyilikci V, et al. Diagnostic utility of C-reactive protein to albumin ratio as an early warning sign in hospitalized severe COVID-19 patients. Int Immunopharmacol. 2021;91 doi: 10.1016/j.intimp.2020.107285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis on COVID-19 – a systematic review. Life Sci. 2020;254 doi: 10.1016/j.lfs.2020.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IH, Zahra SA, Zaim S, Harky A. At the heart of COVID-19. J Card Surg. 2020;35:1287–1294. doi: 10.1111/jocs.14596. [DOI] [PubMed] [Google Scholar]
- Levey AS, Eckardt KU, Dorman NM, Christiansen SL, Cheung M, Jadoul M, et al. Nomenclature for kidney function and disease—executive summary and glossary from a kidney disease: Improving Global Outcomes (KDIGO) consensus conference. Eur Heart J. 2020;41:4592–4598. doi: 10.1093/eurheartj/ehaa650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, South AM, Henry BM. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19) Ann Clin Biochem. 2020;57:262–265. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 19 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XM, Zhou W, Yan XJ, Guo TG, Wang BC, et al. Prognostic value of C-reactive protein in patients with COVID-19. Clin Infect Dis. 2020;71:2174–2179. doi: 10.1093/cid/ciaa641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslo C, Messina A, Laubscher A, Toubkin M, Sitharam L, Feldman C, et al. COVID-19: a comparative study of severity of patients hospitalized during the first and the second wave in South Africa, 2021. https://www.medrxiv.org/content/10.1101/2021.05.11.21257033v1.full.pdf (Accessed 20 June 2021)
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggiali E, Zaino D, Immovilli P, Rovero L, Losi G, Dacrema A, et al. Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in COVID-19 patients. Clin Chim Acta. 2020;509:135–138. doi: 10.1016/j.cca.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57:389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Li W, Xie J, Ho Y, You C. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prattichizzo F, de Candia P, Nicolucci A, Ceriello A. Elevated HbA1c levels in pre-Covid-19 infection increases the risk of mortality: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2021;20:e3476. doi: 10.1002/dmrr.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C, Huang Y, Shi F, Tan K, Ma Q, Chen Y, et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol. 2020;92:856–862. doi: 10.1002/jmv.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S, Bohn MK, Mancini N, Loh TP, Wang CB, Grimmler M, et al. IFCC interim guidelines on biochemical/hematological monitoring of COVID-19 patients. Clin Chem Lab Med. 2020;58:2009–2016. doi: 10.1515/cclm-2020-1414. [DOI] [PubMed] [Google Scholar]
- Savoia C, Volpe M, Kreutz R. Hypertension, a Moving Target in COVID-19: Current Views and Perspectives. Circ Res. 2021;128:1062–1079. doi: 10.1161/CIRCRESAHA.121.318054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immonol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Li R, Wang J, Jiang Q, Gao C, Yang J, et al. Correlation analysis between disease severity and clinical and biochemical characteristics of 143 cases of COVID-19 in Wuhan, China: a descriptive study. BMC Infect Dis. 2020;20:519. doi: 10.1186/s12879-020-05242-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Chen X, Cai Y, Xia J, Zhiou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Wakabayashi M, Yamaji T, Chopra N, Mikami T, Miyashita H, et al. Value of leucocytosis and elevated C-reactive protein in predicting severe coronavirus 2019 (COVID-19): a systematic review and meta-analysis. Clin Chim Acta. 2020;509:235–243. doi: 10.1016/j.cca.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Huang Y, Guo Y, Yin M, Chen X, Xiao L, et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]