Abstract
Background
: Torque teno virus (TTV) DNA load in plasma directly associates with the net state of immunosuppression and inflammation in different clinical settings, including transplantation and chronic inflammatory diseases.
Objectives
: We investigated whether plasma TTV DNA load may predict the occurrence of certain infectious events and overall mortality in critically ill COVID-19 patients.
Patients and Methods
: 50 patients (median age, 65.5 years) were recruited. TTV DNA load was quantitated in serial plasma specimens by real-time PCR. Serum levels of interleukin-6, C-reactive protein, ferritin, lactate dehydrogenase, Gamma-Glutamyl Transferase (GGT), alanine transaminase (ALT) and aspartate transaminase (AST) and absolute lymphocyte counts (ALC) in paired specimens were available. Nosocomial bloodstream infections and ventilator-associated pneumonia and overall mortality were the clinical outcomes.
Results
: TTV DNA was detected in 38 patients (76%). A weak inverse correlation (Rho=-0.28; P = 0.004) was observed between TTV DNA loads and ALC. No direct correlation was found between TTV DNA load and serum levels of any of the above biomarkers. Patients with detectable TTV DNA had an increased risk of subsequently developing infectious events (HR 9.28; 95% CI, 1.29–69.5; P = 0.03). A trend (P = 0.05) towards higher TTV DNA area under a curve between days 7 and 17 after ICU admission (AUC7–17) was observed in patients who died, as compared to survivors.
Conclusion
: Our findings suggested that plasma TTV DNA load monitoring may be helpful for predicting the occurrence of severe nosocomial infections and mortality in critically ill COVID-19 patients.
Keywords: Torque teno virus (TTV), TTV DNAemia, COVID-19, Intensive care unit, Mortality
JCV-d-21–00,857. R3
1. Background
Anelloviridae are small (30–50 nm) non-enveloped single-stranded DNA (2.2–3.9 Kb) of negative sense (ss-) viruses that comprise three genera, Alphatorquevirus, which includes torque teno virus (TTV), a prototypic member of the anelloviruses, Betatorquevirus and Gammatorquevirus [1]. TTV, which has not been consistently linked to any human disease, establishes a chronic-persistent infection presumably in multiple body sites [2], representing a major component of the human virome [3]. TTV DNAemia is commonly documented in healthy infected individuals and remains relatively stable in magnitude over years [4]; in contrast, the level of TTV DNAemia fluctuates widely in transplant recipients with a clear linkage to the net state of immunosuppression; in fact, peripheral blood TTV DNA load consistently correlates directly with the intensity of host immunosuppression, and as such it may predict the occurrence of allograft rejection (low viral loads) or infectious events (high viral loads), notably in the solid organ transplantation setting-SOT-[5], [6], [7], [8], [9], [10]. Outside the immunosuppressed population, high plasma and respiratory tract TTV DNA loads have been reported in individuals with chronic lung disorders of inflammatory nature, such as asthma, chronic bronchiectasis or chronic obstructive lung disease, as compared to healthy individuals [11], [12], [13]; while the ability of TTV to stimulate inflammatory responses, as shown in vitro models [14], may account for this latter observations, it may also be that pro-inflammatory environments trigger TTV replication. In this context, lymphopenia and excessive generation of proinflammatory cytokines are hallmarks of severe COVID-19, the latter being pathogenetically involved in the development and aggravation of acute respiratory distress syndrome (ARDS), and tissue damage, which eventually result in multi-organ failure and death [15], [16], [17]. Since a certain degree of immunosuppression and an hyperinflammatory state co-exist in severe COVID-19 patients, and both conditions may impact on TTV replication, in this exploratory study we aimed at characterizing the kinetics of plasma TTV DNA load in critically ill COVID-19 patients, how does it compare to that of absolute lymphocyte counts (ALC) and serum levels of inflammatory and tissue damage parameters and assessing whether it may behave as a surrogate biomarker of poor clinical outcomes.
TTV is hepatotropic and has been associated historically with acute and chronic liver disease, although formal proof lending support to that assumption is lacking [18]. In turn, acute liver injury develops frequently in COVID-19 and it is associated with poor outcome [19]. On the basis of the above, we also sought to determine whether TTV DNA loads were associated with serum levels of transaminases.
2. Study design
2.1. Patients and specimens
In this prospective single-center observational study, 50 critically ill COVID-19 patients (34 males and 16 females; median age, 65.5 years; range, 21–79) were recruited between 16 October 2020 and 20 February 2021 (Table 1 ). All cases were due to the Wuhan-Hu-1 D614G variant, as determined by whole-genome sequencing (not shown). Sequential plasma specimens were scheduled to be collected at least once a week since ICU admission until release or death. The only patient inclusion criterium was the availability of at least two specimens for the analyses detailed below. Plasma were obtained by centrifugation of whole blood EDTA tubes, cryopreserved at −80 °C and retrieved for TTV DNAemia analyses. Medical history and laboratory data were prospectively recorded. The current study was approved by the Ethics Committee of Hospital Clínico Universitario INCLIVA (May 2020).
Table 1.
Clinical characteristics of the study population.
| Variables | no. (%) |
|---|---|
| Sex | |
| Male | 34 (68) |
| Female | 16 (32) |
| Type of comorbidity | |
| Diabetes mellitus | 11 (22) |
| Asthma/ Chronic lung disease | 8 (16) |
| Hypertension | 22 (44) |
| Dyslipidemia/ Obesity | 26 (52) |
| Cancer | 2 (1) |
| Immunosuppression | 2 (1) |
| Number of comorbidities | |
| One | 17 (34) |
| Two or more | 21 (42) |
| None | 12 (24) |
| On mechanical ventilation | 50 (100) |
| Acute renal failure | 15 (30) |
| Acute Physiology And Chronic Health Evaluation II | |
| <10 | 9 (18) |
| 10–15 | 17 (34) |
| >15 | 24 (48) |
| Antiviral or anti-inflammatory treatment | |
| Remdesivir | 11 (22) |
| Corticosteroids | 50 (100) |
| Tocilizumab | 19 (38) |
| Adrenalin | 24 (48) |
2.2. TTV DNA quantitation
TTV DNA load in plasma was quantified with a TaqMan real-time PCR assay kit which amplifies a highly conserved segment of the untranslated region of the viral genome, as previously reported [20, 21]. Specimens with undetectable TTV DNA loads were assigned a value of 0 for analysis purposes. All samples from each patient were assayed simultaneously in singlets.
2.3. Diagnosis of SARS-CoV-2 infection
Real-time PCR assays were used for detection of SARS-CoV-2 RNA in upper or lower respiratory tract specimens, as previously described [22].
2.4. Laboratory measurements
Clinical laboratory tests included serum levels of ferritin, d-Dimer (D-D), C reactive protein (CRP), interleukin-6 (IL-6), lactate dehydrogenase (LDH), Gamma-Glutamyl Transferase (GGT), alanine transaminase (ALT), aspartate transaminase (AST) and ALC in blood specimens paired to plasma samples used for TTV DNA quantitation.
2.5. Definitions
Bacteremia, fungemia and ventilation associated pneumonia (VAP) were defined as previously reported [23, 24, respectively]. According to the CDC (Centers for Disease Control and prevention) [25], critical illness was defined by the presence of respiratory failure, septic shock, and/or multiple organ dysfunction.
2.6. Statistical methods
Frequency comparisons for categorical variables were carried out using Fisher's exact test. Differences between medians were compared using the Mann-Whitney U test. Correlations between variables of interest were assessed using the Spearman's rank test. The TTV DNA load area under a curve (AUC) was calculated with the STATGRAPHIC Centurion XVII statistics package (Statpoint Technologies, Inc., Warrenton, VA, USA) and required 2 or more specimens/patient. Two-sided P-values < 0.05 were considered significant. Univariate Cox regression models were built to identify risk factors for defined clinical events. Statistical analyses were performed using SPSS version 25.0 (SPSS, Chicago, IL, USA).
3. Results
3.1. Patient clinical features
Patients were admitted to ICU at a median of 9 days (range, 2–21) after the onset of COVID-19 symptoms. All patients presented with pneumonia and were treated with ceftaroline-fosamil plus azithromycin or ceftriaxone plus azithromycin either prior to or at the time of ICU admission. No patient received prophylactic treatment with antifungals. All patients eventually needed mechanical ventilation. Of interest, all patients received corticosteroid treatment while at ICU and no patient had a canonical immunosuppressive condition. Median time of ICU stay was 20 days (range, 5–67).
3.2. Plasma TTV DNA kinetics
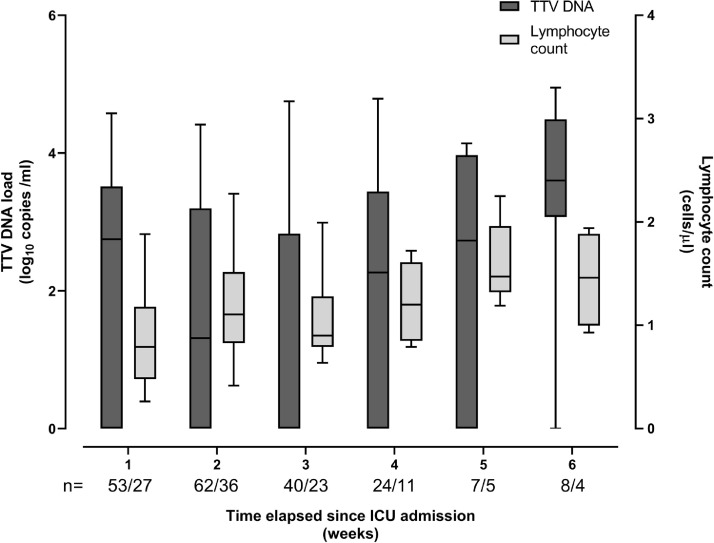
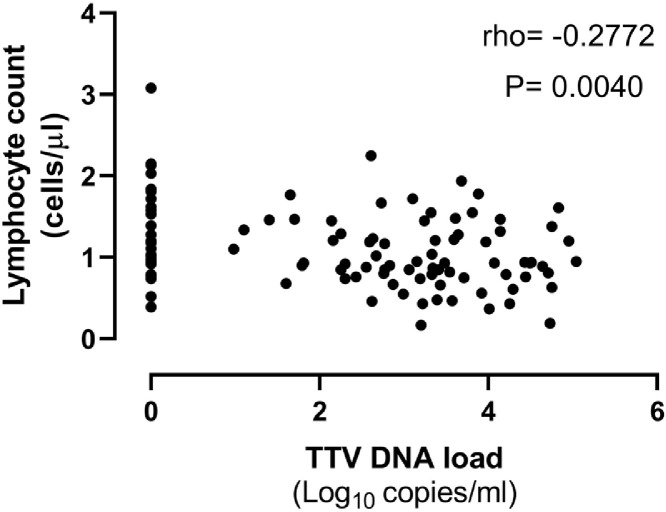
A total of 194 plasma specimens from 50 patients were processed for TTV DNA quantitation by real-time PCR. A median of 4 specimens/patient (range, 2–8) were available for the analyses. TTV DNA could be detected in one or more specimens (n = 111; 57.2%) from 38 patients (76%). A total of 30 patients had detectable TTV DNA at baseline, a median of 4 days (range, 1–7 days) after ICU admission. TTV DNAemia was documented in the remaining 8 patients during ICU stay. Fig. 1 depicts the kinetics TTV DNAemia since ICU admission. Overall, median TTV DNA load at baseline in the 38 patients who had one or more positive PCR result during ICU stay was 2.8 log10 copies/ml (range, 0–4.7). Plasma TTV DNA load tended to decrease over time reaching though level by week 3, to increase later on to slightly higher level than that measured at baseline by week ≥6. TTV DNA peak load was achieved beyond week 4 with a maximum value of 4.75 log10 copies/ml. The dynamics of ALC is also shown in Fig. 1. A significant drop (P = 0.01) of ALC was noticed by week 2 after ICU admission. Afterwards, ALC steadily increased to reach higher levels than those present at baseline (by weeks 5/≥6). We observed no consistent relationship between the kinetics of TTV DNA load and that of peripheral blood lymphocytes; yet, a significant (P = 0.004), but week inverse correlation (Rho=−0.28) was observed between these two parameters (Fig. 2 ).
Fig. 1.
Whisker plots depicting plasma TTV DNA loads and absolute lymphocyte counts in critically ill COVID-19 patients. The number of specimens available for analyses at different timepoints following intensive care unit admission is shown.
Fig. 2.
Correlation between plasma TTV DNA load and absolute lymphocyte counts in critically ill COVID-19 patients. Rho and P values are shown.
3.3. Plasma TTV DNA load and serum levels of COVID-19 severity biomarkers
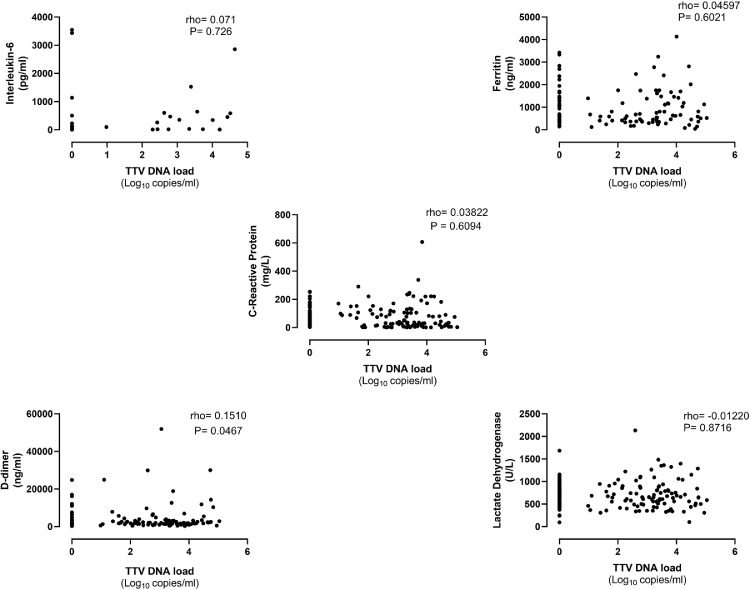
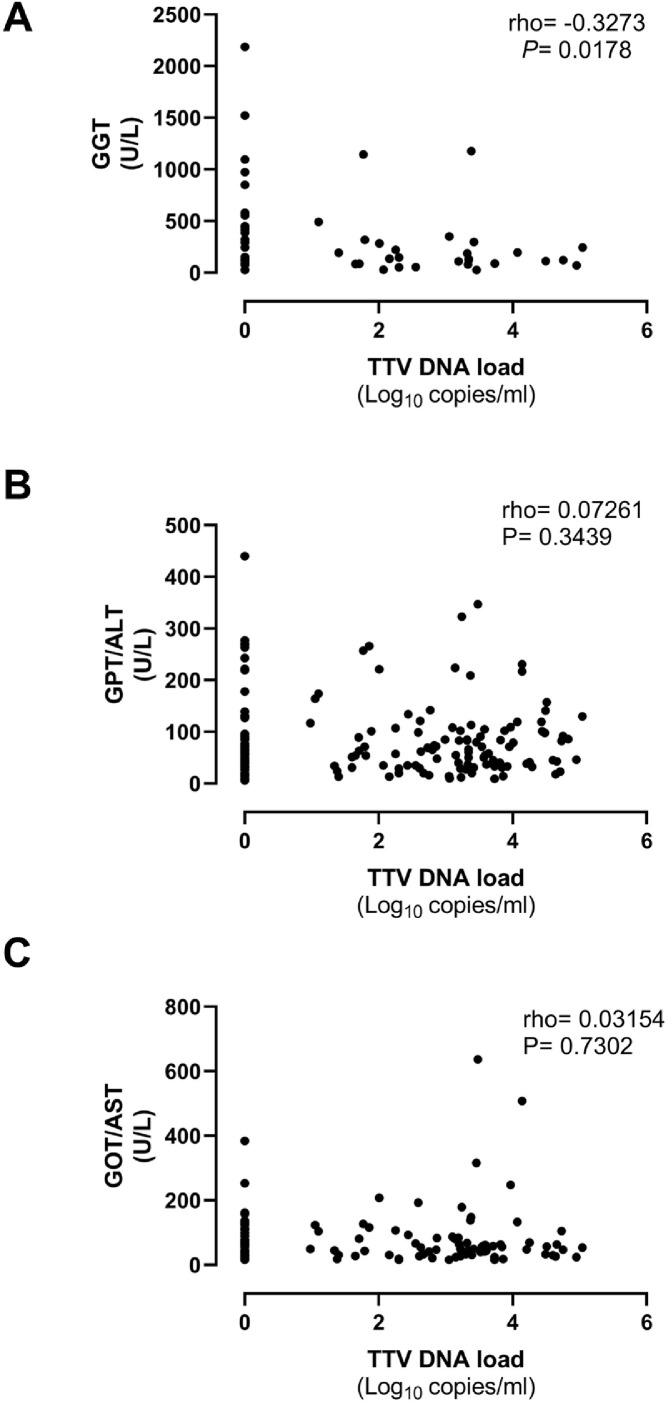
We next investigated whether plasma TTV DNA loads did correlate with levels of biomarkers of inflammation (IL-6, ferritin, and CRP), pro-coagulative activity (Dimer-D) and tissue damage (LDH) in paired sera. The data are shown in Fig. 3 . We also assessed the potential association between TTV DNA loads and surrogate biomarkers of liver function (GGT, ALT, and AST) (Fig. 4 ). We found no direct correlation between TTV DNA load and serum levels of any of these biomarkers.
Fig. 3.
Correlation between plasma TTV DNA load and levels of interleukin-6, ferritin, Dimer-D and lactate dehydrogenase in paired sera in critically ill COVID-19 patients. Rho and P values are shown.
Fig. 4.
Correlation between plasma TTV DNA load and levels of Gamma-Glutamyl Transferase (GGT), alanine transaminase (ALT) and aspartate transaminase (AST) in paired sera in critically ill COVID-19 patients. Rho and P values are shown.
3.4. TTV DNA load and clinical events
We next sought to investigate whether plasma TTV DNA load monitoring may allow prediction of the occurrence of certain nosocomial infectious events (bloodstream infections and VAP) and overall mortality. Twenty-eight patients had one or more infectious events during ICU stay (Table 2 ). Bloodstream infections and VAP were diagnosed at a median 19 days (range, 4–62) and 10 days (range, 3–48), respectively, following ICU admission. As shown in Table 3 , overall, patients with detectable TTV DNA in plasma (in one or more specimens) during ICU stay (n = 38) had a higher rate of infectious events as compared to those who systematically tested negative for TTV DNA over the study period (P = 0.013). Moreover, a trend (P = 0.16) towards higher TTV DNA loads at baseline (<7 days since ICU admission) was noticed among patients who subsequently developed infectious events as compared to those who did not (median, 2.5 log10 copies/ml; range, 0–4.75, vs. median, 0 log10 copies/ml; range, 0–4.25). Cox models adjusted to age, sex, and APACHE II indicated that qualitative detection of TTV DNA within the first week of ICU stay and TTV DNA loads ≥3.3 log10 copies/ml were associated with an increased risk of subsequent infectious events (HR 9.28; 95% CI, 1.29–69.5; P = 0.03 and HR, 2.8; CI 95%, 0.9–5.2; P = 0.08, respectively).
Table 2.
Causative agents of bacteremia/fungemia and ventilator-associated pneumonia in critically ill COVID-19 patients.
| Parameter | no. (%) |
|---|---|
| Clinical condition | |
| Ventilator-associated pneumonia | 14 (28) |
| Bacteremia/fungemia | 9 (18) |
| Ventilator-associated pneumonia and bacteremia/fungemia | 5 (10) |
| Causative agent | |
| Ventilator-associated pneumonia | |
| Klebsiella spp. | 9 (18) |
| Pseudomonas aeruginosa | 5 (10) |
| Staphylococcus aureus | 2 (4) |
| Enterobacter spp. + Aspergillus spp. | 1 (2) |
| Enterococcus faecalis | 1 (2) |
| No identification of the causative agent | 1 (2) |
| Bacteremia/Fungemia | |
| Enterococcus faecalis | 5 (10) |
| Candida spp. | 4 (8) |
| Pseudomonas aeruginosa | 1 (2) |
| Enterobacter cloacae | 1 (2) |
| Klebsiella aerogenes | 1 (2) |
Table 3.
TTV DNA detection and load in plasma from critically ill COVID-19 patients stratified by occurrence of clinical events and death.
| Clinical outcome | TTV DNA-based parameter | |||
|---|---|---|---|---|
| Detection of TTV DNA in plasma during ICU stay. No. of patients (%) | Non-detection of TTV DNA in plasma during ICU stay. No. of patients (%) | TTV DNA load ≥3.3 log10 copies/ml within week 1 after ICU admission. No. of samples (%)a | TTV DNA load <3.3 log10 copies/ml within week 1 after ICU admission. No. of samples (%)a | |
| Infectious eventsb | 25 (89.2) | 3 (10.7) | 10 (45.4) | 12 (54.5) |
| No infectious eventsb | 13 (59.0) | 9 (40.9) | 4 (22.2) | 14 (77.7) |
| Death | 15 | 6 | 7 (43.7) | 9 (56.2) |
| Survival | 23 | 6 | 7 (29.1) | 17 (70.8) |
ICU, intensive care unit; TTV, Torque teno virus.
A total of 13 patients had 2 plasma specimens drawn within the first week after ICU admission).
Bloodstream infections (bacteremia/fungemia), ventilator-associated pneumonia or both.
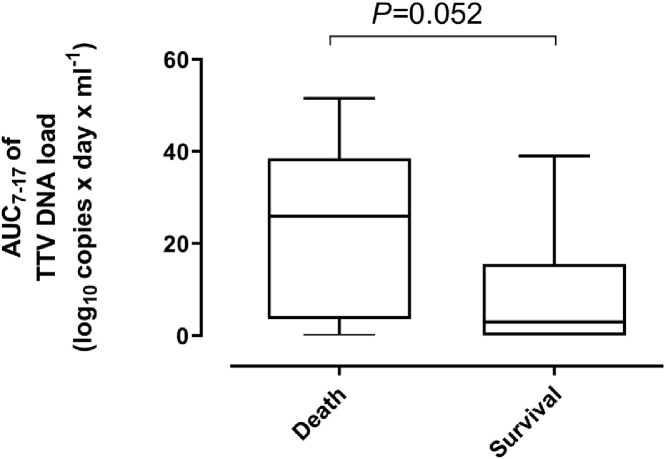
A total of 21 out of 50 patients died. Death occurred at a median of 32 days (range, 19–59) after ICU admission. Mortality was comparable (P = 0.52) across patients with or without detectable plasma TTV DNA (Table 3). Moreover, TTV DNA load within 1 week after ICU admission was also similar (P = 0.33) among patients who died or survived (median, 1.74 log10 copies/ml; range, 0–4.75 vs. median, 2.33 log10 copies/ml; range, 0–4.14). Since deaths occurred after day 19 after ICU admission, we were able to calculate the TTV DNA load AUCs prior to that timepoint in patients with 2 or more consecutive PCR positive results and investigate whether this parameter was associated with subsequent overall mortality. Specifically, in order to avoid any bias due to different sampling times, we calculated AUCs from a median of day 7 (range, days 4–10) until a median of day 17 (range, 14 to 19) since ICU admission (AUC7–17) in 27 patients, of whom 11 died and 16 survived. A shown in Fig. 5 , a clear trend (P = 0.05) towards higher TTV DNA AUC0–14 was observed in patients who died (median, log10 25.8 copies × days × mL−1; range 0–51.5) compared with survivors (median, log10 2.95 copies × days × mL−1; range 0–39.9).
Fig. 5.
Whisker plots depicting plasma TTV DNA load area under a curve (AUC) between day 7 to day 17 (medians) after ICU admission (AUC7–17), reported as copies x day x mL-1) in critically ill COVID-19 patients who either died or survived. The P value is shown.
4. Discussion
In this exploratory, proof-of-principle study we hypothesized that TTV DNA monitoring in plasma may serve the purpose of identifying critically ill COVID-19 patients at highest risk of developing infectious events leading to poor prognosis, such as bloodstream infections and VAP [26,27], and overall mortality, both conditions pathogenetically linked to either a deficient, or dysbalanced SARS-CoV-2-driven immune responses, or both. TTV DNA load in plasma inversely correlate with peripheral ALC by 1 to 3 months after kidney transplantation [9] and at late times (≥ day 100) following allogeneic hematopoietic stem cell transplantation-allo-HSCT- [28]. We investigated whether this was the case in critically ill COVID-19 patients. ALC described a trajectory typically reported for this population group [29]; plasma TTV DNA load trajectory showed no apparent relationship with that of ALC; yet, a significant, but rather week inverse correlation between these two parameters was noticed.
Both, the in vitro pro-inflammatory nature of TTV [14] and the fact that TTV DNA load in the blood compartment is increased in patients with chronic inflammatory diseases as compared to healthy controls [11], [12], [13] led us to investigate whether a correlation between serum levels of surrogate biomarkers of inflammation or tissue damage, such as IL-6, CRP, d-D and LDH and plasma TTV DNA load could be documented in critically ill COVID-19 patients. Nevertheless, we observed no direct correlation at all between plasma TTV DNA loads and serum levels of the above biomarkers in paired specimens. In this respect, it is of relevance to mention that data on IL-6 levels were only available for 27 specimens from 23 patients and that 9 of these patients were under tocilizumab treatment at the time of sampling. Despite this, the lack of correlation between TTV DNA loads and serum levels of CRP and ferritin, both reliable markers of an hyperimflammatory state in patients with COVID-19 [15] argues against TTV DNA load being a good surrogate marker of inflammation in clinical setting.
Although controversial, TTV has been linked to acute and chronic liver disease in both immunocompetent and immunosuppressed individuals [18]. Since acute liver injury commonly presents severe COVID-19 [19], we investigated whether TTV DNA loads were associated with serum levels of transaminases. No direct correlation between these parameters was found, this arguing against a potential role of TTV in producing liver damage in critically ill COVID-19 patients.
Superinfection events occur frequently in critically ill COVID-19 patients and notably worsen their prognosis [26,27]. Monitoring of TTV DNAemia has been shown to be a useful tool to anticipate the occurrente of a variety of infectious events in SOT and allo-HSCT settings [[8], [9], [10], [30], [31], [32], [33]]. Our data suggested that this might also be the case in critically ill COVID-19 patients regarding nosocomial bloodstream infections and VAP. The potential relevance of this finding stems on two assumptions that are debatable: (i) most if not all adults are chronically infected by one or more TTV species that may access the blood compartment with no apparent clinical consequences [2]; (ii) the lack of TTV DNAemia detection, even in healthy individuals, may be due to analytical drawbacks (namely, insufficient sensitivity of current PCR assays), the existence of sanctuaries of viral persistence in organ and tissues other than peripheral blood [2,4,34], or both; natural or iatrogenic immunosuppression may decrease immune surveillance of TTV in virus-persistence sites allowing TTV DNAemia to become detectable [2]. Moreover, an increased risk of subsequent infections was observed for patients displaying detectable TTV DNA in plasma or exhibiting TTV DNA load ≥3.3 log10 copies/ml within the first week since ICU admission. If our observations were confirmed in more robust studies, monitoring of TTV DNA load in plasma early after ICU admission may trigger the administration of antimicrobial phrophylaxis targeting opportunistic bacterial and fungal agents involved in severe infections.
High TTV DNAemia levels have been associated with increased mortality risk in different settings, including the elderly population [35,36], septic patients at ICU recruited prior to the SARS-CoV-2 pandemic [37] and allogeneic hematopietic stem cell transplant recipients [33]. Here, a trend towards an association with increased mortality rate was observed for TTV DNA AUC7–17. This observation, however, must be interpreted with caution due to the scarce number of death events in the cohort.
The current study is merely exploratory and does not allow to draw robust conclusions; even so, our findings suggested that the magnitude of plasma TTV DNA load in critically ill COVID-19 patients may reflect their state of immunocompetence, as in other clinical settings, and point to a use of viral load monitoring as an ancillary tool for predicting the occurrence of severe nosocomial infections in critically ill COVID-19 patients and perhaps mortality. Whether it may anticipate the development of reactivation of persistent viruses such as Cytomegalovirus or Herpes simplex viruses, which may impact on patient survival, could not be addressed in the current study. The potential clinical relevance of our observations warrants further studies involving larger and if possible multicenter cohorts.
Funding
This work received no private or public funds.
Author contributions
LF, EA, EG, and IT: Methodology and validation of data. NC, JF and MLB: Medical care of ICU patients. DN: Conceptualization, supervision, writing the original draft. All authors reviewed the original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to all personnel who work at Clinic University Hospital, in particular to those at Microbiology laboratory and the Intensive Care Unit for their commitment in the fight against COVID-19. Eliseo Albert and Estela Giménez hold a Juan Rodés Contract from the Health Institute Carlos III (JR20/00011 and JR18/00053, respectively). Ignacio Torres holds a Río Hortega Contract (CM20/00090) the Health Institute Carlos III.
References
- 1.Family—Anelloviridae . In: Virus Taxonomy. King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Elsevier; San Diego: 2012. pp. 331–3411. [Google Scholar]
- 2.Focosi D., Antonelli G., Pistello M., Maggi F. Torquetenovirus: the human virome from bench to bedside. Clin. Microbiol. Infect. 2016;22:589–593. doi: 10.1016/j.cmi.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Liang G., Bushman F.D. The human virome: assembly, composition and host interactions. Nat. Rev. Microbiol. 2021;19:514–527. doi: 10.1038/s41579-021-00536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Focosi D., Spezia P.G., Macera L., Salvadori S., Navarro D., Lanza M., et al. Assessment of prevalence and load of torquetenovirus viraemia in a large cohort of healthy blood donors. Clin. Microbiol. Infect. 2020;26:1406–1410. doi: 10.1016/j.cmi.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 5.De Vlaminck I., Khush K., Strehl C., Kohli B., Luikart H., Neff N.F., et al. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell. 2013;155:1178–1187. doi: 10.1016/j.cell.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiemann M., Puchhammer-Stöckl E., Eskandary F., Kohlbeck P., Rasoul-Rockenschaub S., Heilos A., et al. Torque teno virus load-inverse association with antibody-mediated rejection after kidney transplantation. Transplantation. 2017;101:360–367. doi: 10.1097/TP.0000000000001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Focosi D., Macera L., Pistello M., Maggi F. Torque teno virus viremia correlates with intensity of maintenance immunosuppression in adult orthotopic liver transplant. J. Infect. Dis. 2014;210:667–668. doi: 10.1093/infdis/jiu209. [DOI] [PubMed] [Google Scholar]
- 8.Strassl R., Schiemann M., Doberer K., Görzer I., Puchhammer-Stöckl E., Eskandary F., et al. Quantification of torque teno virus viremia as a prospective biomarker for infectious disease in kidney allograft recipients. J. Infect. Dis. 2018;218:1191–1199. doi: 10.1093/infdis/jiy306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Ruiz M., Albert E., Gimenez E., Ruiz-Merlo T., Parra P., López-Medrano F., et al. Monitoring of alphatorquevirus DNA levels for the prediction of immunosuppression-related complications after kidney transplantation. Am. J. Transplant. 2019;19:1139–1149. doi: 10.1111/ajt.15145. [DOI] [PubMed] [Google Scholar]
- 10.Gorzer I., Haloschan M., Jaksch P., Klepetko W. Puchhammer-Stockl E. Plasma DNA levels of torque teno virus and immunosuppression after lung transplantation. J. Heart Lung Transplant. 2014;33:320–323. doi: 10.1016/j.healun.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Pifferi M., Maggi F., Andreoli E., Lanini L., Marco E.D., Fornai C., et al. Associations between nasal torquetenovirus load and spirometric indices in children with asthma. J. Infect. Dis. 2005;192:1141–1148. doi: 10.1086/444389. [DOI] [PubMed] [Google Scholar]
- 12.Freer G., Maggi F., Pifferi M., Di Cicco M.E., Peroni D.G., Pistello M. The virome and its major component, anellovirus, a convoluted system molding human immune defenses and possibly affecting the development of asthma and respiratory diseases in childhood. Front. Microbiol. 2018;9:686. doi: 10.3389/fmicb.2018.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Y., Xue Q., Jiao W., Wu J., Yu Y., Zhao L., et al. Associations between sputum torque teno virus load and lung function and disease severity in patients with chronic obstructive pulmonary disease. Front. Med. (Lausanne) 2021;8 doi: 10.3389/fmed.2021.618757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocchi J., Ricci V., Albani M., Lanini L., Andreoli E., Macera L., et al. Torquetenovirus DNA drives proinflammatory cytokines production and secretion by immune cells via toll-like receptor 9. Virology. 2009;394:235–242. doi: 10.1016/j.virol.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 15.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N. Engl. J. Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 16.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leisman D.E., Ronner L., Pinotti R., aylor M.D., Sinha P., Calfee C.S., et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reshetnyak V.I., Maev I.V., Burmistrov A.I., Chekmazov I.A., Karlovich T.I. Torque teno virus in liver diseases: on the way towards unity of view. World J. Gastroenterol. 2020;26:1691–1707. doi: 10.3748/wjg.v26.i15.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma A., Jaiswal P., Kerakhan Y., Saravanan L., Murtaza Z., Zergham A., et al. Liver disease and outcomes among COVID-19 hospitalized patients - A systematic review and meta-analysis. Ann. Hepatol. 2021;21 doi: 10.1016/j.aohep.2020.10.001. Mar-Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albert E., Solano C., Pascual T., Torres I., Macera L., Focosi D., et al. Dynamics of torque teno virus plasma DNAemia in allogeneic stem cell transplant recipients. J. Clin. Virol. 2017;94:22–28. doi: 10.1016/j.jcv.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Albert E., Solano C., Giménez E., Focosi D., Pérez A., Macera L., et al. The kinetics of torque teno virus plasma DNA load shortly after engraftment predicts the risk of high-level CMV DNAemia in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2018;53:180–187. doi: 10.1038/bmt.2017.235. [DOI] [PubMed] [Google Scholar]
- 22.Olea B., Albert E., Torres I., Gozalbo-Rovira R., Carbonell N., Ferreres J., et al. Lower respiratory tract and plasma SARS-CoV-2 RNA load in critically ill adult COVID-19 patients: relationship with biomarkers of disease severity. J. Infect. 2021;(21) doi: 10.1016/j.jinf.2021.05.036. S0163-445300279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.IDSA guidelines. https://www.idsociety.org/practice-guideline/practice-guideline 2010.
- 24.Martin-Loeches I., Rodriguez A.H., Torres A. New guidelines for hospital-acquired pneumonia/ventilator-associated pneumonia: USA vs. Eur. Curr. Opin. Crit. Care. 2018;24:347–352. doi: 10.1097/MCC.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 25.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed [06/2021]. [PubMed]
- 26.Musuuza J.S., Watson L., Parmasad V., Putman-Buehler N., Christensen L., Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0251170. e0251170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Povoa P., Martin-Loeches I., Nseir S. Secondary pneumonias in critically ill patients with COVID-19: risk factors and outcomes. Curr. Opin. Crit. Care. 2021 Jul 27 doi: 10.1097/MCC.0000000000000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert E., Solano C., Giménez E., Focosi D., Pérez A., Macera L., et al. Kinetics of Alphatorquevirus plasma DNAemia at late times after allogeneic hematopoietic stem cell transplantation. Med. Microbiol. Immunol. 2019;208:253–258. doi: 10.1007/s00430-019-00586-w. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y., Tan W., Chen H., Zhu Y., Wan L., Jiang K., et al. Dynamic changes in lymphocyte subsets and parallel cytokine levels in patients with severe and critical COVID-19. BMC Infect. Dis. 2021;21:79. doi: 10.1186/s12879-021-05792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maggi F., Focosi D., Statzu M., Bianco G., Costa C., Macera L., et al. Early post-transplant torquetenovirus viremia predicts cytomegalovirus reactivations in solid. Organ Transplant Recipients Sci. Rep. 2018;8:15490. doi: 10.1038/s41598-018-33909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albert E., Solano C., Giménez E., Focosi D., Pérez A., Macera L., et al. The kinetics of torque teno virus plasma DNA load shortly after engraftment predicts the risk of high-level CMV DNAemia in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2018;53:180–188. doi: 10.1038/bmt.2017.235. [DOI] [PubMed] [Google Scholar]
- 32.Fernández-Ruiz M., Albert E., Giménez E., Rodríguez-Goncer I., Andrés A., Navarro D., et al. Early kinetics of torque teno virus DNA load and BK polyomavirus viremia after kidney transplantation. Transpl Infec.t Dis. 2020;22 doi: 10.1111/tid.13240. e13240. [DOI] [PubMed] [Google Scholar]
- 33.Pradier A., Masouridi-Levrat S., Bosshard C., Dantin C., Vu D.L., Zanella M.C., et al. Torque teno virus as a potential biomarker for complications and survival after allogeneic hematopoietic stem cell transplantation. Front. Immunol. 2020;11:998. doi: 10.3389/fimmu.2020.00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bueno F., Albert E., Piñana J.L., Pérez A., Úbeda C., Gómez M.D., et al. Kinetics of Torque Teno virus DNA in stools may predict occurrence of acute intestinal graft versus host disease early after allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2021;23 doi: 10.1111/tid.13507. e13507. [DOI] [PubMed] [Google Scholar]
- 35.Giacconi R., Maggi F., Macera L., Pistello M., Provinciali M., Giannecchini S., et al. Torquetenovirus (TTV) load is associated with mortality in Italian elderly subjects. Exp. Gerontol. 2018;112:103–111. doi: 10.1016/j.exger.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Giacconi R., Maggi F., Macera L., Spezia P.G., Pistello M., Provinciali M., et al. Prevalence and loads of torquetenovirus in the European MARK-AGE study population. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75:1838–1845. doi: 10.1093/gerona/glz293. [DOI] [PubMed] [Google Scholar]
- 37.Walton A.H., Muenzer J.T., Rasche D., Boomer J.S., Sato B., Brownstein B.H., et al. Reactivation of multiple viruses in patients with sepsis. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0098819. e98819. [DOI] [PMC free article] [PubMed] [Google Scholar]