Abstract
Patients with metastatic uterine leiomyosarcoma (uLMS) have poor prognosis due to limited treatment options, especially when disease progresses on doxorubicin and gemcitabine-docetaxel regimens. Here we report a patient whose metastatic uLMS contains a BRCA2 deep deletion as well as TP53 and PTEN deep deletion. The patient responded rapidly to olaparib, a poly (ADP-ribose) polymerase inhibitor, after progressing on gemcitabine-docetaxel, doxorubicin, and temozolomide regimens. This case report shall be helpful to the treatment of other patients with metastatic uLMS that harbors a BRCA2 mutation or deletion.
Keywords: BRCA2, Olaparib, PARP inhibitor, uterine leiomyosarcoma
INTRODUCTION
Uterine leiomyosarcoma (uLMS) is the most common histologic subtype of uterine sarcoma, accounting for more than 60% of cases. The majority of cases are high grade with an aggressive course. The risk of relapse is high after surgical resection of early-stage disease. Treatment options are limited after disease progression on doxorubicin and gemcitabine/docetaxel regimens. Other agents, such as trabectedin, temozolomide, and pazopanib, have response rates below 10%.1-3 We report a case of metastatic uLMS with somatic deep deletion of BRCA2 that responds rapidly to the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib after progressing on doxorubicin, gemcitabine, and docetaxel and oral temozolomide.
CASE PRESENTATION
A 54-year-old Black woman presented with pelvic pain in May 2019 and was found to have a solid heterogeneous pelvic mass with areas of fluid and hemorrhagic density. The mass measured approximately 20.9 × 22.1 × 12.4 cm on a computer-aided topography. A positron emission topography (PET) scan showed the mass to be hypermetabolic and arising from the uterus with regions of hypoattenuation and photopenia indicating cystic/necrotic changes as well as fluorodeoxyglucose-avid lytic expansile right anterior acetabular and superior ramus lesion (Figure 1). A biopsy of the mass revealed uterine leiomyosarcoma. StrataNGS (Ann Arbor, Michigan) molecular profiling of the tumor showed deep deletion of BRCA2, PTEN, and TP53 genes. The StrataNGS result also showed microsatellite stable, tumor mutation burden of 8, low programmed death-ligand 1 expression, and low immune signature profile. She was evaluated by a genetic counselor, and germline testing of single-gene BRCA2 analysis was performed, which revealed no pathologic BRCA2 mutation but revealed a variant of unknown significance in the BRCA2 gene (specifically p.P3292L). There was no significant family history of cancer. The patient was evaluated by both gynecologic oncology and medical oncology, and the decision was made to initiate neoadjuvant chemotherapy followed by surgical resection. However, after 2 cycles of chemotherapy with gemcitabine and docetaxel, the disease progressed, and the patient experienced worsening pain. The patient had complete resection of the mass, with total hysterectomy and bilateral salpingo-oophorectomies as well as resection of the lytic bony lesions, in August 2019. The patient received 4 cycles of doxorubicin single agent for adjuvant therapy after the surgical resection. However, in April 2020, 3 months after she discontinued doxorubicin, she developed rapid progression with numerous pulmonary metastasis. She was given 2 cycles of oral temozolomide with disease progression. In June 2020, she started olaparib 300 milligram, twice a day. PET scan performed 6 weeks after the initiation of olaparib showed major partial response (Figure 2A and B). She continues to tolerate olaparib well without significant side effects. Her cough and shortness of breath improved dramatically a few days after she started olaparib. Eight months after she initiated olaparib, she continues to experience quality of life improvement, walking 5-7 miles a day without shortness of breath. The timeline of her diagnostic and therapeutic events is summarized in Table 1.
PATIENT PERSCPECTIVE
Olaparib was offered to me after three different chemotherapy regimens had failed. My biggest symptoms were shortness of breath and pain in the areas of my body where tumors were growing. The PET scan confirmed that my symptoms were related to the aggressive growth of the tumors.
I started taking Olaparib and the side effects I experienced were fatigue, dizziness, tingling, metallic taste in my mouth, trouble with memory and attention as well as aching joints. I found that the tingling and aching joints got better within weeks, as did the fatigue. I still experience some dizziness and some trouble with focus and distraction. The biggest change was that the shortness of breath started to go away and I went from not being able to walk more than 1/4 mile to averaging between 3‐7 miles most days of the week, including some uphill climbs. Notably, from being pushed in a wheelchair a year ago to being able to walk up four floors to the oncology department of Kaiser was a huge accomplishment for me.
Figure 1.
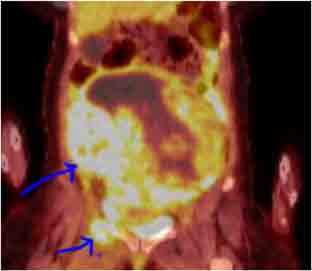
Metastatic uLMS at the diagnosis. Blue arrows point to the very large fluorodeoxyglucose-avid large tumor mass in the abdomen/pelvis with tumor necrosis and the bony metastasis to the acetabulum and superior ramus.
Figure 2.
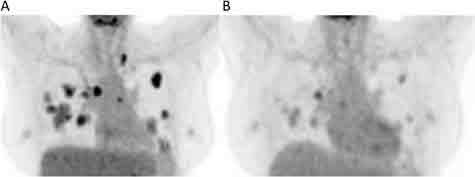
Representative image of metastatic uLMS to the lungs (A) prior to and (B) after treatment with olaparib. Note the fluorodeoxyglucose-avid lesions in the bilateral lungs are dramatically reduced after olaparib treatment.
Table 1.
Timeline of the major diagnostic and therapeutic events
| Timeline | Events | |
|---|---|---|
| May 2019 | Patient presents with symptoms | |
| July 2019 | PET scan showed a large uterus mass and bony metastasis | |
| July 2019 | Biopsy revealed uLMS | |
| July 2019 | Gemcitabine-docetaxel initiated | |
| August 2019 | Complete resection of the uLMS and bony metastasis | |
| September 2019 | Doxorubicin initiated | |
| April 2020 | PET scan showed progression | |
| April 2020 | Temozolomide initiated | |
| June 2020 | Olaparib initiated | |
| July 2020 | PET scan showed major response | |
DISCUSSION
BRCA1 and BRCA2 play key roles in double-strand DNA repair by homologous recombination, whereas PARP is important in single-strand DNA repair by base excision. Inhibition of PARP in cancer cells that are deficient in BRCA1 or BRCA2 leads to synthetic lethality as cancer cells lose the ability to repair both single-strand and double-strand DNA breaks.4-6 Several PARP inhibitors, including olaparib, have shown significant efficacy in metastatic ovarian, breast, prostate, and pancreatic cancers with germline or somatic BRCA1/2 mutation.7-9 BRCA1/2 mutation is uncommon in soft tissue sarcomas (< 1%). However, Seligson et al10 reported a BRCA1/2 mutation in 6 out of 61 cases of uLMS through reviewing the Cancer Genome Atlas and data from Ohio State University. Three of 6 cases with BRCA2 mutation treated with olaparib had stable disease, and 1 had a partial response.
Our patient experienced rapid symptomatic improvement and imaging response after initiating olaparib. Interestingly, in addition to the deep deletion of BRCA2, her tumor harbors deep deletion of 2 other important tumor suppressor genes: TP53 and PTEN. There is in vitro evidence that TP53 as well as PTEN deficiency increases the sensitivity of cancer cell lines to PARP inhibition.11,12 For example, mantle cell lymphoma cells that are deficient in both ATM and p53 are more sensitive to olaparib than cells that are deficient in ATM alone,12 and endometrioid endometrial cancer cells that are deficient of PTEN are more sensitive to PARP inhibition due to the inability of the cancer cells to mount a homologous recombination repair response.11,13 Whether or not the deep deletion of TP53 and/or PTEN sensitized this patient’s metastatic uLMS to olaparib is not certain. Further studies in this area shall result in additional insight. Our case report adds additional evidence that PARP inhibition may be of benefit for patients with metastatic uLMS with a somatic BRCA2 deletion and highlights the importance of obtaining NGS in the treatment of patients with metastatic cancer.
Footnotes
Disclosure Statement: The author(s) have no conflicts of interest to disclose.
Authors’ Contributions: Minggui Pan, Kristen Ganjoo, and Amer Karam participated in conceiving the study and writing the manuscript.
Abbreviations: NGS = next-generation sequencing; PARP = poly (ADP-ribose) polymerase; uLMS = uterine leiomyosarcoma
References
- 1.Hensley ML, Blessing JA, Mannel R, Rose PG. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II trial. Gynecol Oncol 2008 Jun;109(3):329-34. DOI: 10.1016/j.ygyno.2008.03.010, PMID:18534250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tap WD. GeDDiS: Insight into frontline therapy in soft tissue sarcoma. Lancet Oncol 2017 Oct;18(10):1297-9. DOI: 10.1016/S1470-2045(17)30672-1, PMID:28882535 [DOI] [PubMed] [Google Scholar]
- 3.Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016 Mar;34(8):786-93. DOI: 10.1200/JCO.2015.62.4734, PMID:26371143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005 Apr;434(7035):917-21. DOI: 10.1038/nature03445, PMID:15829967 [DOI] [PubMed] [Google Scholar]
- 5.Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature 2012 Jan;481(7381):287-94. DOI: 10.1038/nature10760, PMID:22258607 [DOI] [PubMed] [Google Scholar]
- 6.Dedes KJ, Wilkerson PM, Wetterskog D, Weigelt B, Ashworth A, Reis-Filho JS. Synthetic lethality of PARP inhibition in cancers lacking BRCA1 and BRCA2 mutations. Cell Cycle 2011 Apr;10(8):1192-9. DOI: 10.4161/cc.10.8.15273, PMID:21487248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 2020 May;382(22):2091-102. DOI: 10.1056/NEJMoa1911440, PMID:32343890 [DOI] [PubMed] [Google Scholar]
- 8.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian Ovarian Cancer Study Group. J Clin Oncol 2012 Jul;30(21):2654-63. DOI: 10.1200/JCO.2011.39.8545, PMID:22711857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018 Dec;379(26):2495-505. DOI: 10.1056/NEJMoa1810858, PMID:30345884 [DOI] [PubMed] [Google Scholar]
- 10.Seligson ND, Kautto EA, Passen EN, et al. BRCA1/2 functional loss defines a targetable subset in leiomyosarcoma. Oncologist 2019 Jul;24(7):973-9. DOI: 10.1634/theoncologist.2018-0448, PMID:30541756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dedes KJ, Wetterskog D, Mendes-Pereira AM, et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med 2010 Oct;2(53):53ra75. DOI: 10.1126/scitranslmed.3001538, PMID:20944090 [DOI] [PubMed] [Google Scholar]
- 12.Williamson CT, Kubota E, Hamill JD, et al. Enhanced cytotoxicity of PARP inhibition in mantle cell lymphoma harbouring mutations in both ATM and p53. EMBO Mol Med 2012 Jun;4(6):515-27. DOI: 10.1002/emmm.201200229, PMID:22416035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forster MD, Dedes KJ, Sandhu S, et al. Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nat Rev Clin Oncol 2011 May;8(5):302-6. DOI: 10.1038/nrclinonc.2011.42, PMID:21468130 [DOI] [PubMed] [Google Scholar]


