Abstract
Introduction:
High-flow nasal cannula (HFNC) oxygen therapy is being used in pediatric wards at increasing rates, including community hospitals that do not have a pediatric intensive care unit (PICU). This study describes the use of HFNC in a pediatric ward at a community hospital, evaluating safety, flow limits, and outcomes for children transferred to a PICU.
Methods:
A descriptive, single center retrospective cohort study of consecutive subjects from birth to 24 months of age treated with HFNC for bronchiolitis in our pediatric ward from January 2016 to May 2019. We report demographic and clinical characteristics of the patients. The outcomes of interest include episodes of aspiration, pneumothorax, intubation, cardiorespiratory arrest, and transfers to the PICU.
Results:
There were 157 hospitalizations. One hundred twenty-three children (78.3%) were weaned off HFNC and discharged to home. Flow rates of up to 3 L/kg/min (average, 1.22 L/kg/min; range 0.28-3.08 L/kg/min) were tolerated. Of the 34 children transferred to the PICU, 29 were continued on HFNC, 1 required continuous positive airway pressure, and 4 were intubated. The median time from initiation of HFNC to transfer was 13 hours (interquartile range 6.0-23.0). There were no documented episodes of aspiration, pneumothorax, cardiorespiratory arrest, or death.
Conclusion:
HFNC could be safely administered in a community hospital pediatric ward without PICU expertise and capability. Most patients who deteriorate on HFNC do so within the first 24 hours when close monitoring is needed. For children transferred to a PICU, the vast majority did not require more invasive forms of respiratory support.
Keywords: bronchiolitis, high-flow nasal cannula, pediatric, pediatric intensive care unit, respiratory therapy, safety
INTRODUCTION
High-flow nasal cannula (HFNC) oxygen therapy is being increasingly used in the treatment of bronchiolitis in general pediatric wards outside of a pediatric intensive care unit (PICU).1 It has been shown to decrease cost, the overall length of stay, and PICU admissions.2-6 Despite this evidence, some have questioned its role in the treatment of children with bronchiolitis, questioning the efficacy7-9 and potential for overuse.10 The safety of HFNC has been primarily evaluated in children’s hospitals and academic centers that have PICUs on-site,2,5,11-14 flow limits of 1-2 L/kg/min have been shown as safe and efficacious in those settings.15,16 There are some studies completed in a community hospital setting that have reported on safety and efficacy.2,17,18 Overall though, less is known about the use of HFNC in community hospitals without PICUs.
In the community hospital without a PICU, the providers in the pediatric ward do not have the benefit of having PICU-trained physicians, nursing, and respiratory therapy staff on hand to assist with care and provide ongoing education. In addition, there is a time delay involved in the transfer process that can lead to adverse outcomes. Thus, there is theoretically more risk in the use of HFNC in a community hospital setting. In this study, we examine the use of HFNC in a community hospital pediatric ward and describe the outcomes of patients discharged to home and those transferred to an outside PICU.
METHODS
Study Design and Setting
We conducted a descriptive retrospective cohort study of consecutive subjects from birth to 24 months treated with HFNC for bronchiolitis in a pediatric ward at Kaiser Permanente Orange County from January 1, 2016, to May 1, 2019. This medical center is primarily an adult hospital that has 264 total beds, including a neonatal intensive care unit, a newborn nursery, and a pediatric ward. There is no PICU. Our pediatric ward has 17 licensed pediatric beds and 6 adolescent beds.
Our hospital is part of Kaiser Permanente Southern California, an integrated health delivery system that provides health care to over 4 million members. There are 14 medical centers in total. Three of the medical centers located in surroundings counties have PICUs. The PICUs are 17, 28, and 35 miles from our facility. We have 3 internal critical care transport teams for pediatrics, comprised of a pediatric hospitalist, registered nurse, and respiratory therapist. HFNC can be used on the transports. It is ground transport only.
At our facility, we use the Vapotherm device to deliver HFNC. We started using HFNC in the last quarter of 2015. During the study period, the decision to initiate HFNC was at the discretion of the treating physician. The general guideline given to our providers to initiate HFNC in infants with bronchiolitis was to trial the patient on a simple nasal cannula (up to 3 L flow depending on age and size) and if the work of breathing was still labored, to consult with a respiratory therapist and consider initiation of HFNC. At the time of this study, there were no specific parameters regarding respiratory rate, work of breathing, or oxygen saturation. Our protocol for use of HFNC during the study period was as follows: ≤ 1 year of age could receive a maximum flow of 8 L; ≥ 1 year of age was a maximum flow of 20 L. The maximum FIO2 provided needed to stay at ≤ 50%. Any flow or oxygen need above these parameters for greater than 6 hours (time is given for stabilization) would then need to be considered for transfer to a PICU at one of our other Kaiser facilities.
Subject Selection and Data Collection
The subjects were identified by age (0-24 months), treatment in the pediatric ward, and the order code for HFNC, which is specific and required to start a child on HFNC: order code 232126: Administer oxygen by high flow/NRB/Hood/Venti/Tent, RT. The principal investigator then reviewed the encounters to identify those that had a clinical diagnosis of bronchiolitis based on the American Academy of Pediatrics Clinical Practice Guideline recommendations.19 This methodology was used instead of International Classification of Diseases (ICD) coding due to the variability of ICD coding and the belief that using the order code would lead to a more precise and inclusive study group. Once children were identified, the electronic medical record, which contains both inpatient and outpatient data, was manually reviewed and data were recorded on a standardized information sheet. The primary chart review was completed by a research intern (Alan M Castro, BS, author). Each chart was also reviewed by the principal investigator (Patrick J Van Winkle, MD).
Subjects were excluded from the study for the following reasons: the presence of congenital heart disease diagnosed by echocardiogram and with ongoing care by a cardiologist, presence of tracheostomy, or hypotonia. Children that received HFNC for reasons other than bronchiolitis were also excluded.
The following patient characteristics were included: age, sex, race, weight on admission, weight and gestational age at birth, history of asthma, and treatment in the neonatal intensive care unit. Time on HFNC was determined from documentation in the respiratory therapy flow sheet. The time fed while on HFNC was calculated as the time overlap between documented feeding in the nursing flow sheet and documented time. Safety was determined by evaluating episodes of aspiration, pneumothorax, cardiopulmonary arrest, or death. To identify these events, as well as the diagnoses listed above in the exclusion criteria, the problem list, principal, and secondary diagnoses in the discharge summary were reviewed.
Statistical Analysis
Demographics and clinical characteristics were stratified by discharge disposition: medians with interquartile range (Q1-Q3) for continuous variables and number of observations with percentage for categorical variables were presented. Differences were assessed using the Wilcoxon test, χ2 test, or Fisher exact test as appropriate. All analyses were conducted using SAS version 9.4, Cary, NC.
This study was approved by the Kaiser Permanente Southern California Institutional Review Board according to the declaration of Helsinki and federal regulations.
RESULTS
Demographic and Clinical Characteristics
One hundred fifty-seven encounters were included in the final analysis of children with bronchiolitis treated with HFNC. There were 149 unique children, with 7 children having 2 hospitalizations and 1 child having 3 hospitalizations during the study period. Thirty-four (21.7%) were transferred to a PICU, the remaining 123 (78.3%) were discharged to home. There were no episodes of pneumothorax, cardiopulmonary arrest, or death. Four children were readmitted within 30 days of discharge. One from the PICU was readmitted for bronchiolitis from a different virus. Three were readmitted from the pediatric ward, 1 for bronchiolitis from a different virus, 1 for feeding difficulties, and 1 for asthma. Characteristics of the study group are given in Table 1.
Table 1.
Demographics and clinical characteristics of children treated with high-flow nasal cannula in a community hospital stratified by discharge disposition, home versus pediatric intensive care unit
| Discharge to home (n = 123) | Transfer to PICU (n = 34) | p-value | |
|---|---|---|---|
| Age in months, median (Q1, Q3) | 9.8 (4.3, 17.0) | 7.0 (2.7, 15.1) | 0.32 |
| Gender, n (%) | 0.49 | ||
| Female | 44 (35.8%) | 10 (29.4%) | |
| Male | 79 (64.2%) | 24 (70.6%) | |
| Race/ethnicity, n (%) | 0.33 | ||
| White | 51 (41.5%) | 9 (26.5%) | |
| Black | 4 (3.3%) | 1 (2.9%) | |
| Hispanic | 41 (33.3%) | 18 (52.9%) | |
| Asian/Pacific Islander | 21 (17.1%) | 5 (14.7%) | |
| Other | 6 (4.9%) | 1 (2.9%) | |
| Weight in kilograms on admission, median (Q1, Q3) | 8.7 (6.6, 10.5) | 7.9 (6.2, 9.6) | 0.21 |
| Weight in kilograms at birth, median (Q1, Q3) | 3.2 (2.6, 3.6) | 3.1 (2.6, 3.5) | 0.55 |
| Gestational age at birth, median (Q1, Q3) | 39.1 (36.9, 39.7) | 37.6 (36.6, 39.0) | 0.01 |
| History of asthma or past albuterol use | 20 (16.3%) | 8 (23.5%) | 0.33 |
| History of treatment in NICU or prior hospitalization | 24 (19.5%) | 10 (29.4%) | 0.21 |
| Year treated, n (%) | 0.03 | ||
| 2016-2017 | 46 (37.4%) | 6 (17.6%) | |
| 2018-2019 | 77 (62.6%) | 28 (82.3%) | |
| Virus isolateda | |||
| Respiratory syncytial virus | 36 (29.3%) | 23 (67.6%) | < 0.001 |
| Enterovirus/rhinovirus | 40 (32.5%) | 10 (29.4%) | 0.84 |
| Adenovirus | 1 (0.8%) | 1 (2.9%) | 0.39 |
| Human metapneumovirus | 12 (9.8%) | 3 (8.8%) | > 0.99 |
| Influenza virus | 3 (2.4%) | 1 (2.9%) | > 0.99 |
| Parainfluenza virus | 6 (4.9%) | 0 (0%) | 0.34 |
| Coronavirus | 5 (4.1%) | 0 (0%) | 0.59 |
| Respiratory viral panel positive | 99 (80.5%) | 33 (97.1%) | 0.02 |
Note that some children had > 1 virus isolated, thus the number of viruses isolated is greater than the number of encounters.
NICU = neonatal intensive care unit; PICU = pediatric intensive care unit.
HFNC Use in the Pediatric Ward
There was a significant difference between the maximum flow rate (average; range) used in the pediatric ward for children discharged to home (1.22 L/kg/min; 0.28-3.08 L/kg/min) versus those transferred to the PICU (1.57 L/kg/min; 0.85-2.55 L/kg/min) (P < 0.001). There was however substantial overlap between these flow rates as can be seen by the range of maximum flows (Figure 1). The maximum FIO2 used between the 2 groups was also significantly different with an FIO2 median (Q1-Q3) of 40% (30.0-45.0) for those children discharged to home and 50% (40.0-60.0) for those transferred to the PICU (P < 0.001). For the 34 children transferred to the PICU, the median time from initiation of HFNC in the pediatric ward to transfer was 13 hours (interquartile range 6.0-23.0 hours) (Figure 2).
Figure 1.
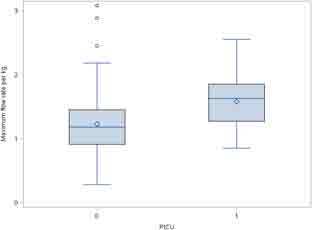
Comparison of maximum flow rate per kilogram for high-flow nasal cannula between children discharged to home and those transferred to a pediatric intensive care unit. PICU = pediatric intensive care unit.
Figure 2.
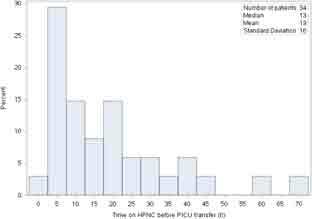
Time in hours on high-flow nasal cannula in the pediatric ward prior to transfer to the pediatric intensive care unit. HFNC = high-flow nasal cannula; PICU = pediatric intensive care unit.
Feeding on HFNC
One hundred fifty-three (94.7%) children had a diet order at some point during their time on HFNC. Children discharged to home were fed a significantly greater percentage of time while on HFNC, with the median percentage (Q1, Q3) of the time fed in hours equal to 46.8% (8.2-65.7) for PICU transfers and 94.9% (79.3-100.0) for those discharged to home (P < 0.001). No children had a nasogastric tube placed for feeds. Three of the children had gastrostomy tubes already in place that were used for feeding. There were no documented episodes of aspiration.
PICU Outcomes
Thirty-four (21.7%) of the children were transferred from our pediatric ward to a PICU at 1 of our other medical centers. Five (14.7%) required a higher level of oxygen delivery in the PICU, 1 received continuous positive airway pressure (CPAP), and 4 were intubated. For the children intubated, 2 had significant comorbidities, 3 were respiratory syncytial virus positive, and 3 were intubated within 2 hours of arrival in the PICU. The HFNC parameters and summary of outcomes in the PICU are presented in Table 2.
Table 2.
Characteristics of pediatric intensive care unit admissions for children transferred after receiving high-flow nasal cannula in a community hospital pediatric ward
| N = 34 | |
| Number of hours treated with HFNC, median (Q1, Q3) | 45.0 (14.0, 64.0) |
| Maximum flow rate per kilogram for HFNC, median (Q1, Q3) | 1.7 (1.5, 2.0) |
| Maximum FIO2 for HFNC, median (Q1, Q3) | 100.0 (50.0, 100.0) |
| Children needing a higher level of oxygen delivery for respiratory illness | 5 (14.7) |
| Intubated, n (%) | 4 (11.8) |
| CPAP, n (%) | 1 (3.0) |
| Cardiorespiratory arrest or death, n (%) | 0 (0) |
CPAP = continuous positive airway pressure; HFNC = high-flow nasal cannula.
DISCUSSION
HFNC could be safely administered to children comparable to our cohort 0-24 months of age with bronchiolitis in a community hospital pediatric ward without a PICU on site. There were no significant adverse events in the study group, including pneumothorax, need for intubation in the pediatric ward, cardiorespiratory arrest, or death. The majority of studies showing the safety of HFNC use outside of the PICU setting have been done in hospitals, such as children’s hospitals or academic centers, that have a PICU on site.5,11-14 While there are studies that have shown the safety of HFNC use in a community hospital setting,2,17,18 our study adds to these by elaborating on the demographic and clinical factors associated with a community hospital population and the care provided on the floor before transfer.
Our population was relatively healthy, with the majority born at term with normal birth weights and no prior hospitalizations after birth. The median length of stay for children discharged to home was just over 3 days. The median number of hours on HFNC was 39, which mirrors past studies showing time on HFNC between 1-2- and one to two days.7,12 Over 80% of children had a positive respiratory viral panel with approximately 30% of those positive being respiratory syncytial virus.
While on HFNC in the pediatric ward, the vast majority of children were fed without an incidence of aspiration noted. Past studies have also shown that it is safe to feed while on HFNC.5,12,20 In our study, we evaluated the overlap of documented feeding during HFNC use and found that children discharged to home were fed for a significantly greater number of hours than those transferred to a PICU, although those transferred were also fed almost half of the time they were on HFNC. Children did not have nasogastric tubes placed but for the 3 children that had gastrostomy tubes, the gastrostomy tubes were used. Overall, we show that on flows of 1-3 L/kg/min children were fed without incidences of aspiration.
Our study indicates that flow rates used in academic and pediatric tertiary care centers can be used in pediatric wards at community hospitals. For our 157 encounters, the median maximum flow rate was 1.2 L/kg/min for those discharged to home and 1.6 L/kg/min for those transferred to the PICU. This flow rate between 1 and 2 L/kg/min has been shown to have a positive effect on the work of breathing and has been suggested as a useful range for HFNC.15,16 Studies completed at children’s hospitals or academic centers have indicated a variety of weight and non-weight-based flow rates up to 2 L/kg/min are safe.2,4,5 There was a study done in a community hospital setting that reported maximum flow rates. In the study, 61 children aged 1-23 months treated in the emergency department and then in the pediatric ward were evaluated with flow rates starting at 1-2 L/kg/min with a range from 0.6 to 3.3 L/kg/min17 These maximum flow limits are comparable to the rates found in our study. Our study adds to the evidence that flow rates between 1 and 2 L/kg/min are safe in a pediatric ward at a community hospital. In addition, with the broad range of 0.3-3.1 L/kg/min, our study also indicates that flows higher than 2 L/kg/min are tolerated in a pediatric ward, although due to the retrospective nature of this study and limited sample size, further work would need to be done to evaluate this.
For children transferred to the PICU, the majority were transferred within 24 hours, which mirrors a past report.12 Additional studies have shown that the clinical benefit of HFNC delivered at appropriate flows should be evident within 60-90 minutes.5,6,17,18,20 Thus, these observations indicate a need for heightened monitoring for children on HFNC within the first 24 hours, with an emphasis placed on obtaining proper flow rates and more strict monitoring in the first 2 hours. For children that are past this initial 24 hours on HFNC at appropriate flow rates, the monitoring can potentially be relaxed.
Our study indicates that children can be transferred from a community hospital on HFNC to an outside PICU with good outcomes. Twenty-one percent of our children were transferred with no incidences of pneumothorax, cardiorespiratory arrest, or death registered up to PICU discharge. This percentage transferred is in line with past studies showing a transfer rate between 8% and 41%.2,12,17,21 Of the 34 children transferred, only 5 (14.7%) required a higher level of oxygen delivery in the PICU, 1 received CPAP, and 4 were intubated. This ability to maintain patients on HFNC in the PICU was partially based on the fact that we set up relatively strict parameters for HFNC use on the pediatric ward in terms of flow and FIO2. This was done so that patients would be transferred earlier as opposed to later in their disease progression. The goal was to not intubate patients being transferred. Our rates of intubation and CPAP in the PICU are comparable to a study that reported an 11% intubation rate and a 13% CPAP rate21 and a second study that reported a 5% intubation rate and a 1% rate of cardiopulmonary arrest.11
Our transport teams can use HFNC during transport, and thus the care with HFNC is not interrupted. This ability to continue HFNC without the need to intubate for transport or without an interruption of oxygen support may help to stabilize children during transport. This idea is supported by a past study that noted a decreased need for mechanical ventilation during transport with HFNC use in pediatric critical care transports.22 This continuation of HFNC could also partially account for the fact that 29 of the 34 children transferred were able to continue HFNC oxygen therapy and did not require a higher level of oxygen delivery in the PICU.
Our study has limitations. First, because this was an observational study, there was no control group to assess for outcomes of children not treated on HFNC, and all findings are associations and not causal. Second, we were not able to objectively assess the severity of illness on presentation, at the initiation of HFNC or at the time of PICU transfer. Third, we have a relatively small sample size in a single institution. Fourth, inclusion criteria were based on an order placed for HFNC, and while this should be accurate, it is assumed that some encounters were missed if this order was not placed correctly. Fifth, while we do have a guideline for the use of HFNC and parameters for PICU transfers, these decisions are at the discretion of the treating physician and thus not entirely uniform. Sixth, we are a large managed health care system with an integrated medical record, the generalizability of these results to other care settings is unclear.
CONCLUSION
HFNC was safely administered in a community hospital pediatric ward for both children that were discharged to home and those that were transferred to an outside PICU. HFNC flow rates of 1-2 L/kg/min shown to be safe in pediatric wards in children’s hospitals can also be used in this setting. Most patients who deteriorated on HFNC did so in the first 24 hours; thus, close monitoring is needed during this time. For children transferred to a PICU, the vast majority were managed on HFNC without an escalation to more invasive forms of respiratory support.
Supplementary Information
aSupplemental Material is available at: www.thepermanentejournal.org/files/2021/20.261supp.pdf
Footnotes
Disclosure Statement: The authors have no conflicts of interest to disclose.
Funding: There were no sources of financial support for this work.
Authors’ Contributions: Patrick J Van Winkle, MD, participated in all aspects of the preparation, including study design, data collection, data analysis, manuscript preparation, and review of the manuscript. Alan M Castro, BS, participated in data collection, data analysis, manuscript preparation, and review of the manuscript. Shareemae A Salvador-Lloyd, RN, participated in study design, data collection, data analysis, manuscript preparation, and review of the manuscript. Janet M GilbertLambert, RRT, participated in study design, data collection, data analysis, manuscript preparation, and review of the manuscript. Qiaoling Chen, MS, participated in study design, data collection, data analysis, manuscript preparation, and review of the manuscript.
Abbreviations: CPAP, continuous positive airway pressure; HFNC, high-flow nasal cannula; ICD, International Classification of Diseases; PICU, pediatric intensive care unit
References
- 1.Panciatici M, Fabre C, Tardieu S, et al. Use of high-flow nasal cannula in infants with viral bronchiolitis outside pediatric intensive care units. Eur J Pediatr 2019 Oct;178(10):1479-84. DOI: 10.1007/s00431-019-03434-4, PMID:31372745 [DOI] [PubMed] [Google Scholar]
- 2.Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med 2018 Mar;378(12):1121-31. DOI: 10.1056/NEJMoa1714855, PMID:29562151 [DOI] [PubMed] [Google Scholar]
- 3.Collins C, Chan T, Roberts JS, Haaland WL, Wright DR. High-flow nasal cannula in bronchiolitis: Modeling the economic effects of a ward-based protocol. Hosp Pediatr 2017 Aug;7(8):451-9. DOI: 10.1542/hpeds.2016-0167 [DOI] [PubMed] [Google Scholar]
- 4.Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): An open, phase 4, randomised controlled trial. Lancet 2017 Mar;389(10072):930-9. DOI: 10.1016/S0140-6736(17)30061-2 [DOI] [PubMed] [Google Scholar]
- 5.Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: Pilot study. J Paediatr Child Health 2014 May;50(5):373-8. DOI: 10.1111/jpc.12509, PMID:24612137 [DOI] [PubMed] [Google Scholar]
- 6.Haq I, Gopalakaje S, Fenton AC, McKean MC, J O'Brien C, Brodlie M. The evidence for high flow nasal cannula devices in infants. Paediatr Respir Rev 2014 Jun;15(2):124-34. DOI: 10.1016/j.prrv.2013.12.002, PMID:24472697 [DOI] [PubMed] [Google Scholar]
- 7.Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr 2017 Apr;7(4):197-203. DOI: 10.1542/hpeds.2016-0195, PMID:28292850 [DOI] [PubMed] [Google Scholar]
- 8.Piper L, Stalets EL, Statile AM. Clinical progress note: High flow nasal cannula therapy for bronchiolitis outside the ICU in infants. J Hosp Med 2020 Jan;15(01):49-50. DOI: 10.12788/jhm.3328 [DOI] [PubMed] [Google Scholar]
- 9.Beggs S, Wong ZH, Kaul S, Ogden KJ, Walters JA. High-flow nasal cannula therapy for infants with bronchiolitis. Cochrane Database Syst Rev 2014 Jan;(1):CD009609. DOI: 10.1002/14651858.CD009609.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: Should we go with the flow? Arch Dis Child 2018 Mar. 103(3):303. DOI: 10.1136/archdischild-2017-313950 [DOI] [PubMed] [Google Scholar]
- 11.Betters KA, Gillespie SE, Miller J, Kotzbauer D, Hebbar KB. High flow nasal cannula use outside of the ICU; factors associated with failure. Pediatr Pulmonol 2017 Jun;52(6):806-12. DOI: 10.1002/ppul.23626, PMID:27870384 [DOI] [PubMed] [Google Scholar]
- 12.Dadlez NM, Esteban-Cruciani N, Khan A, et al. Safety of high-flow nasal cannula outside the ICU for previously healthy children with bronchiolitis. Respir Care 2019 Nov;64(11):1410-5. DOI: 10.4187/respcare.06352, PMID:30914486 [DOI] [PubMed] [Google Scholar]
- 13.Daverio M, Da Dalt L, Panozzo M, Frigo AC, Bressan S. A two-tiered high-flow nasal cannula approach to bronchiolitis was associated with low admission rate to intensive care and no adverse outcomes. Acta Paediatr 2019 Nov;108(11):2056-62. DOI: 10.1111/apa.14869, PMID:31102551 [DOI] [PubMed] [Google Scholar]
- 14.Sachs N, Rom E, Schonfeld T, Gavish R, Berger I, Krause I. Short-term high-flow nasal cannula for moderate to severe bronchiolitis is effective in a general pediatric ward. Clin Pediatr (Phila) 2019 Dec;58(14):1522-7. DOI: 10.1177/0009922819877881, PMID:31556700 [DOI] [PubMed] [Google Scholar]
- 15.Yurtseven A, Turan C, Erseven E, Saz EU. Comparison of heated humidified high-flow nasal cannula flow rates (1-L·kg·min-1 vs 2-L·kg·min -1 ) in the management of acute bronchiolitis. Pediatr Pulmonol 2019 Jun;54(6):894-900. DOI: 10.1002/ppul.24318, PMID:30887731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr 2017 Oct;189:66-e3.PMID:28669609. DOI: 10.1016/j.jpeds.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 17.Davison M, Watson M, Wockner L, Kinnear F. Paediatric high-flow nasal cannula therapy in children with bronchiolitis: A retrospective safety and efficacy study in a non-tertiary environment. Emerg Med Australas 2017 Apr;29(2):198-203. DOI: 10.1111/1742-6723.12741, PMID:28332328 [DOI] [PubMed] [Google Scholar]
- 18.Heikkilä P., Sokuri P., Mecklin M., et al. . Using high-flow nasal cannulas for infants with bronchiolitis admitted to paediatric wards is safe and feasible. Acta Paediatr 2018 Nov;107(11):1971-6. DOI: 10.1111/apa.14421 [DOI] [PubMed] [Google Scholar]
- 19.Ralston SL, Lieberthal AS, Meissner HC, et al. . Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014 Nov;134(5):e1474-502. DOI: 10.1542/peds.2014-2742 [DOI] [PubMed] [Google Scholar]
- 20.Kalburgi S, Halley T, Kolaitis IN, Hood K, Mittal V. A review of heated high-flow nasal cannula in pediatrics-from critical care to ward use. Curr Treat Options Peds 2018 Jun;4(2):319-29. DOI: 10.1007/s40746-018-0128-x [DOI] [Google Scholar]
- 21.Kallappa C, Hufton M, Millen G, Ninan TK. Use of high flow nasal cannula oxygen (HFNCO) in infants with bronchiolitis on a paediatric ward: A 3-year experience. Arch Dis Child 2014 Aug;99(8):790-1. DOI: 10.1136/archdischild-2014-306637, PMID:24938537 [DOI] [PubMed] [Google Scholar]
- 22.Schlapbach LJ, Schaefer J, Brady AM, Mayfield S, Schibler A. High-flow nasal cannula (HFNC) support in interhospital transport of critically ill children. Intensive Care Med 2014 Apr;40(4):592-9. DOI: 10.1007/s00134-014-3226-7, PMID:24531340 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
aSupplemental Material is available at: www.thepermanentejournal.org/files/2021/20.261supp.pdf


