Abstract
Objective:
The primary goal of our retrospective case-control study was to evaluate the ability of cardiopulmonary exercise testing to screen for underlying exercise-induced pulmonary hypertension (EIPH) in symptomatic patients who had a negative stress test and elevated right ventricular systolic pressure on echocardiogram. We also evaluated long-acting nitrates and ranolazine as medication challenges.
Setting:
Performed at a single, tertiary-care medical center in the United States.
Participants:
Based on the inclusion and exclusion criteria, 81 patients were included in this study. The primary outcome of the study was to measure mean pulmonary arterial pressure at rest and exertion, as well as Wasserman curves. We also recorded patient demographics and risk factors, left ventricular ejection fraction, and mean oxygen consumption. Additionally, patients were monitored symptomatically after receiving long-acting nitrates and ranolazine.
Results:
A total of 61 patients had resting pulmonary arterial hypertension, and 27 had EIPH. The EIPH group had a significantly higher mean age of 71.67 years. Wasserman curves calculated from the cardiopulmonary exercise testing data revealed 3 subgroups of EIPH patients: cardiac restriction, chronotropic incompetence, and combination of both patterns. The EIPH group showed significant improvement in symptoms after receiving long-acting nitrate therapy.
Conclusions:
Many patients with symptoms of angina, dyspnea, and/or fatigue on exertion with negative cardiac stress testing may have underlying pulmonary arterial hypertension, including EIPH. Therefore, these patients require adequate treatment and follow-up to prevent worsening of symptoms and pathology.
Keywords: cardiopulmonary exercise test, exercise-induced pulmonary hypertension, mean pulmonary arterial pressure, pulmonary arterial hypertension
ARTICLE SUMMARY
Strengths and Limitations of This Study
The invasive right heart catheterization was performed on supine patients; thus, large autonomic differences were not accounted for.
Because the patients were supine, the resting mean pulmonary arterial pressure was affected by the increased venous return and central venous pressure.
The Wasserman curves were interpreted based on the opinions of expert clinicians, instead of prespecified criteria.
INTRODUCTION
Exercise-induced pulmonary hypertension (EIPH) is often an unidentified cause of exercise intolerance and exertional dyspnea. It is speculated to be an early, mild phase of pulmonary arterial hypertension (PH).1-3 At the recent 6th World Symposium in 2018, the World Health Organization (WHO) altered the definition of PH from mean pulmonary arterial pressure (mPAP) of at least 25 mmHg to an mPAP of > 20 mmHg. The reasoning behind this was because it now represents the upper limit of normal mPAP in the general population, and individuals with an mPAP between 21 and 24 mmHg are at risk for poor outcomes.4,5 Historically, EIPH has been defined as an mPAP of > 30 mmHg during exercise2,6; however, this value has not been updated because of a lack of consensus of data. In certain patients, EIPH is associated with worse clinical outcomes, such as in those with decreased left ventricular ejection fraction (LVEF),7,8 mitral regurgitation,9,10 and aortic stenosis.11 Recent studies have shown that EIPH and normal LVEF have an incidence of up to 34%.3,12 Nevertheless, EIPH has remained controversial and poorly understood by both practicing clinicians and researchers. EIPH may remain undiagnosed in a significant proportion of patients because the conclusive diagnosis is ultimately dependent on an invasive testing methodology, namely, exercise right heart catheterization (RHC).13 Among such patients, cardiopulmonary exercise testing (CPET) may serve as a noninvasive screening methodology that further elucidates the presence of underlying EIPH. Furthermore, CPET can help rule out subclinical noncardiac comorbidities as the etiology, such as chronic obstructive pulmonary disease.
The primary goal of our retrospective study was to evaluate the ability of CPET to screen for underlying EIPH in patients with symptoms of dyspnea, effort intolerance, and/or exertional fatigue who had negative stress testing, but also elevated right ventricular systolic pressure (RVSP) on echocardiogram. In addition, we examined the treatment outcome after challenge with either long-acting nitrate (isosorbide mononitrate) or ranolazine. The decision to use ranolazine in this setting was based on clinician experience. The overall objective of our study was to provide a foundation for future prospectively designed studies that will seek to further validate CPET as a screening tool in patients suspected of having EIPH and potentially evaluate interventional approaches for such patients.
METHODS
Study Population
This study was a retrospective analysis of participants who underwent CPET at a single, tertiary-care medical center in the United States from January 2015 to January 2016. It involved patients who were referred to our institute for CPET evaluation because of angina, dyspnea, and/or fatigue on exertion to confirm or to exclude pulmonary hypertension PH by RHC. All participants eventually underwent RHC and CPET. Demographic variables and medical history were collected based on patient self-report and electronic medical record review. This history included age, sex, body mass index, smoking status, hypertension status, and dyslipidemia status. Patients were excluded from analysis if they did not undergo RHC with CPET or had hypertrophic cardiomyopathy, LVEF < 55%, and/or severe valvular disease. Additional exclusion criteria included age younger than 18 years, left ventricular diastolic or systolic dysfunction (assessed by echocardiography), impaired renal function (glomerular filtration rate < 60 mL/min), significant restrictive (total lung capacity < 80% of predicted) or obstructive (forced expiratory volume in 1 second < 70% of predicted) lung disease, acute right heart pressure, and/or volume overload. All procedures followed the institutional and ethical guidelines, and written informed consent was obtained from every patient.
In accordance with the Health Insurance Portability and Accountability Act, all patient records were deidentified.14 This study was exempt from review by the Institutional Review Board because it did not involve any human subjects.15
CPET Methodology
All CPET was performed at our facility’s pulmonary function laboratory, which meets applicable American Heart Association standards.16 CPET was performed before RHC using a cycle ergometer. All patients underwent hemodynamic measurement by performing arm movements (similar to jumping jacks) for 3 minutes with 2.5-pound weights fastened to their wrists. An arm ergometer was not available at our community facility. The mPAP was measured through RHC performed both before initiating and once concluding the arm exercise period. Figure 1 depicts the patient flow. To perform the arm exercise test, patients were required to undergo RHC without sedation. Any patient who requested sedation was unable to perform the exercise; thus, these patients were labeled as “no exercise” for this analysis. CPET was performed using a standardized protocol.17 Briefly, work rate was continuously increased by 5 to 15 W/min to a maximum tolerated level on an electromagnetically braked cycle ergometer (ViaSprint 150 p; Ergoline, Windhagen, Germany). Blood gas analysis was performed at rest and during maximal exercise. Heart rate was monitored continuously, and blood pressure was taken noninvasively every 2 min. Furthermore, oxygen (O2) uptake (VO2), minute ventilation (Ve), and carbon dioxide output (VCO2) were measured breath by breath through an adult facemask (Vmax Spectra 229 D, Sensormedics, Yorba Linda, CA). Maximum oxygen consumption (VO2 max) is the maximum oxygen an individual can utilize during maximal exercise (measured as milliliters of oxygen used in 1 min per kilogram of body weight). Peak VO2, O2 pulse, alveolar-arterial O2 difference, and functional dead space ventilation were calculated per their well-known equations.17 Peak VO2 was defined as the value of averaged data during the final 15 seconds of exercise. The anaerobic threshold was chosen at the peak VO2 at which the ventilatory equivalent for O2 (Ve/VO2) increased and the ventilatory equivalent for CO2 (Ve/VCO2) decreased or remained constant. The Ve/VCO2 slope was determined as the linear regression slope of Ve and VCO2 from the start of exercise until the respiratory compensation point (the time point at which ventilation is stimulated by acidemia and the end-tidal carbon dioxide begins to decrease).
Figure 1.
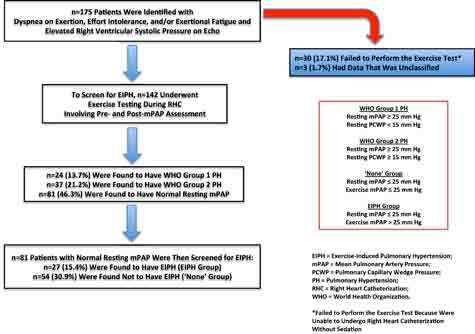
Patient flow diagram.
Right Heart Catheterization
All participants in the study underwent RHC and received no medications on the morning of the procedure. A True Size Thermodilution S-tip catheter (Edwards Lifesciences, Irvine, CA) was inserted via the right femoral vein. Hemodynamic measurements were performed in the supine position, which included heart rate, wedge position pressure, pulmonary arterial pressure (PAP), and right atrium pressure. O2 saturation was measured in mixed venous blood samples. Cardiac index (CI) was obtained using the thermodilution method (COM-2 Cardiac Output Computer; Edwards Lifesciences). CI and pulmonary vascular resistance (PVR) were calculated using standard formulas (CI = cardiac output/body surface area, and PVR = [mean PAP–wedge position pressure]/cardiac output).
Wrist Weight–Fastened Stress Testing
During the preparatory phase of the RHC, all patients were educated on how to perform the arm movements with the wrist weights fastened during the stress test, which was similar to jumping jacks. After hemodynamic measurements at rest, patients were asked to wear 2.5-pounds wrist weights on each arm and perform arm movements for 3 min in the supine body position. The mPAP was measured through RHC performed both before initiating and once concluding the arm exercise period. During the arm exercise test, EIPH was defined as mPAP ≥ 25 mmHg.
Medication Challenge
A long-acting nitrate, isosorbide mononitrate (Imdur 30 mg daily; Merck, Darmstadt, Germany), and ranolazine were challenged in both EIPH and not EIPH (N-EIPH) groups. An oral form of a long-acting nitrate has shown the ability to potentiate the pulmonary-selective vasodilatory effect of phosphodiesterase 5 inhibitors.18 Additionally, ranolazine was associated with improved functional class, decreased right ventricular size, improved right ventricular function, and improved exercise time.19
Statistical Analysis
Descriptive statistical analysis was performed using means and standard deviations for describing patient characteristics. Means, medians, interquartile ranges, and frequencies are reported for RHC, LVEF, and CPET findings, wherever appropriate. Also, continuous variables were compared using t-tests, and baseline categorical variables were compared using χ2 tests. To help examine risk factors that might have been associated with EIPH, each of these groups’ characteristics were compared by logistic regression analysis. Wasserman curves displaying heart rate and stroke volume with exercise from the CPET data for EPIH patients were also obtained.20 The proportion of patients in each group whose symptoms were resolved by treatment with long-acting nitrate was compared by χ2 analysis.
Patient and Public Involvement
No participants were involved in this retrospective study.
RESULTS
Of the 175 patients referred to our clinic, 145 underwent RHC for confirmatory investigation of PH. The other 30 patients were excluded because they failed to perform the test. On retrospective chart review, data from 3 patients were not available. A total of 81 patients were found to have normal resting mPAP; therefore, they were included in our data analysis. These included 29 men (36%) and 52 women (64%; Table 1). Before exercising, all patients had similar baseline symptoms and echocardiograms with normal LVEFs and elevated RVSP. Of these patients, 27 (33%) constituted the EIPH group. The remaining 54 (67%) were characterized as either none or N-EIPH.
Table 1.
Summary of variables for patients with normal resting mean pulmonary arterial pressure
| Variable | EIPH group | N-EIPH group | |
|---|---|---|---|
| Mean age (standard deviation), years | 71.67 (11.89) | 58.89 (12.69) | |
| Mean body mass index (standard deviation) | 30.70 (5.72) | 30.23 (6.91) | |
| Sex (No.) | Male | 10 | 19 |
| Female | 17 | 35 | |
| Hypertension (No.) | Yes | 23 | 38 |
| No | 16 | 4 | |
| Diabetes (No.) | Yes | 6 | 14 |
| No | 21 | 40 | |
| Dyslipidemia (No.) | Yes | 20 | 40 |
| No | 7 | 14 | |
| Smoking (No.) | Yes | 4 | 18 |
| No | 8 | 41 | |
| Active | 1 | 0 | |
| Nonsmoker | 4 | 3 | |
| Echocardiogram with diastolic dysfunction (No.) | 18 | 29 | |
EIPH = exercise induced pulmonary hypertension; N-EIPH = not EIPH.
EIPH patients were older, with a mean age at presentation of 71.67 years (SD ± 11.89 years) compared with 58.89 years (± 12.69 years) for the N-EIPH group; (p < 0.01), Table 1. In total, 61 (75%) patients had hypertension, 60 (74%) had dyslipidemia, 47 (58%) had diastolic dysfunction, 20 (24%) had diabetes, and 14 (17%) were smokers. There were no statistically significant differences between the groups with respect to these comorbidities.
The resting mPAP in all patients with EIPH was below the resting mPAP threshold of 25 mmHg, according to WHO’s definition of PH groups 1 and 2. Whereas mPAP during exercise for all 3 PH patient subgroups increased to > 25 mmHg (Figure 2), patients without any PH had an increase in mPAP that was significantly lower. Resting LVEF was similar among all groups (Figure 3) and was without significant differences during CPET across any groups in regard to VO2 max or mPAP. The mean LVEF for the EIPH group was between 53 and 57% (Figure 3), and the mean VO2 max was < 20 mL/kg/min (Figure 4). Patients with EIPH had an exercise capacity similar to those in WHO groups 1 and 2. The CPET data show that the mean VO2 max for EIPH patients was not significantly different from patients with resting PH (Figure 4). Patients with WHO group 1 PH had a broad distribution of VO2 max findings compared with patients in the EIPH and WHO group 2 study subgroups; however, this was not considered to be clinically significant. Patients with abnormal exercise hemodynamics had a lower VO2 max mean of 13 mL/kg/min compared with those with normal exercise hemodynamics, with a mean of 16.2 mL/kg/min.
Figure 2.
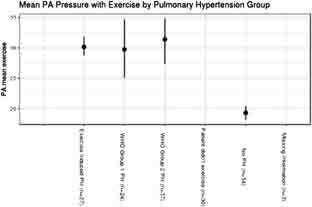
Comparison of mean pulmonary arterial pressure (mPAP) during exercise for all groups.
Figure 3.
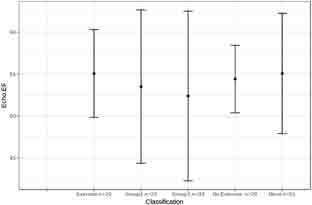
Comparison of resting left ventricular ejection fraction (LVEF) for all groups. -Excluded 1 patient in the exercise group, 1 in World Health Organization (WHO) group 1, 4 in WHO group 2, 1 in the no-exercise group, and 3 in the none group because they failed to produce an ejection fraction (EF) estimate on their echocardiogram.
Figure 4.
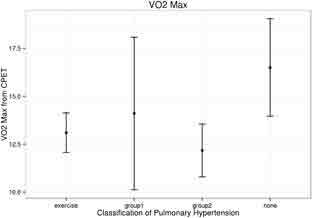
Comparison of maximum oxygen consumption (VO2 max) on cardiopulmonary exercise test (CPET) for those in exercise-induced pulmonary hypertension (EIPH), World Health Organization (WHO) Group 1, and WHO Group 2 pulmonary hypertension.
Risk factors that may have been associated with EIPH were examined. Logistic regression analysis was used to compare the mPAPs of patients with EIPH and those without EIPH versus age, sex, body mass index, smoking status, hypertension status, and dyslipidemia status. A moderate association was observed between increasing age and exercise mPAP among EIPH patients. For every 1-year increase in age, the odds of developing EIPH also increased by 1.08 (Figure 5). No other association was observed between EIPH and any baseline variable.
Figure 5.
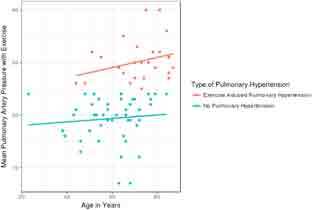
Association of increasing age with increasing ean pulmonary arterial pressure (mPAP).
Three particular patterns of Wasserman curves were identified from CPET data among the EIPH subgroups. The first group revealed a cardiac restricted pattern, where the stroke volume curve remained flat, even as the heart rate increased with exercise. The second group reported one of chronotropic incompetence, in which the heart rate did not increase appropriately with exercise. Finally, the third group illustrated a combination with flat heart rate and stroke volume curves throughout the duration of exercise.
After the diagnosis of EIPH, 17 of the patients were challenged with long-acting nitrate, isosorbide mononitrate. Of these patients, 76.5% reported symptomatic resolution, such as decreased dyspnea and improved routine activities. Additionally, 14 patients were similarly challenged in the N-EIPH group, and 42.9% reported symptomatic improvement. The nitrate response rate difference between EIPH and N-EIPH groups was statistically significant (p = 0.027). Moreover, 10 patients in the EIPH group were challenged with ranolazine, and 4 (40%) responded. In the N-EIPH group, 13 of 23 patients (56.5%) responded. The difference from ranolazine was not found to be statistically significant. In the EIPH group, 5 patients were challenged with both nitrate and ranolazine. Of these patients, 1 improved only after the ranolazine challenge, 2 improved only after nitrate, 1 improved with either, and 1 had no improvement with either. Eight patients in the N-EIPH group were challenged with both nitrate and ranolazine. Of these, 2 improved only after ranolazine, 3 improved only after nitrate, and 3 had no improvement after either. Furthermore, 21 (77.8%) patients from the EIPH group were on beta-blocker therapy before exercise challenge, compared with 31 (57.4%) patients in the N-EIPH group. Beta-blocker therapy was not used as a challenge medication and was held during the exercise challenge and CPET. Finally, before the exercise challenge, 3 (11.1%) EIPH patients were not on antiplatelet therapy, compared with 13 (24.0%) N-EIPH patients.
DISCUSSION
EIPH is often considered an interim phase between normal healthy individuals and resting stable PH. Whether it may also represent a distinct clinical entity apart from being a milder version of WHO-defined PH remains to be clarified. In our study, there was no clinically significant difference between the impairment in EIPH compared with stable resting PH. This indicates that EIPH should be diagnosed and treated as soon as possible, similarly to WHO-defined PH. EIPH appears to be most similar to WHO group 2 PH, and it may be the earlier manifestation of the condition.2 In our study, the CPET data showed that the mean VO2 max of EIPH was not significantly different from patients with stable resting PH. We also found that the mean age of patients with EIPH was significantly older than the mean age of patients with no PH (p < 0.01). In fact, the likelihood of developing EIPH was calculated to increase with each 1-year increase in age. This might be explained by the increasing underlying cardiac pathologies associated with aging. This is alongside the increased prevalence of preserved LVEF heart failure that is observed with increase in age.21
Rationale for Using an Exercise mPAP Cutoff of 25 mmHg
As of 2018, PH now includes patients with an mPAP > 20 mmHg. Before this change, the definitions were as follows: PH is an mPAP > 25 mmHg at rest and EIPH is an mPAP > 30 mmHg. The current guidelines do not delineate a particular criterion for defining EIPH because of the failure in reaching a consensus.22 It is difficult to define an upper limit of normal at exercise because it depends on cardiac output, pulmonary capillary wedge pressure, and PVR, all of which are affected by workload.23 Research has been recommended to delineate at which levels exercise-induced mPAP and PVR confer prognostic and therapeutic implications.24 We opted to use a cutoff of 25 mmHg to define and diagnose EIPH based on the updated guidelines for PH and because these patients had unexplained symptoms. This cutoff would allow us to capture more patients from a limited cohort to study who may require treatment to prevent sequelae. On review of our data, a cutoff of 30 mmHg would reduce the sample size to 14; however, no significant change would occur in results.
Patterns Identified on CPET Wasserman Curves
In our EIPH patients, we observed a flattened stroke volume response curve when heart rate increased throughout both halves of exercise capacities. This indicated a significant cardiac restriction limiting maximal cardiac output. Similarly, chronotropic incompetence was observed when heart rate failed to respond to increased cardiac demand. In some instances, we found a combined appearance of stroke volume response failure and chronotropic incompetence to cardiac demand. EIPH patients had a VO2 max that was almost as poor as patients with resting PH. Such patients would require adequate treatment and follow-up to prevent worsening of symptoms and pathology. Most EIPH patients had diastolic dysfunction on echocardiography. Underlying pathogenesis may involve microvascular angina leading to diastolic dysfunction, which worsens with age. Long-acting nitrates have been shown to reduce microvascular angina; thus, they alleviate symptoms in these patients. The CPET data showed that many EPIH patients were cardiac restricted or chronotropic incompetent, despite being off beta-blocking agents during the tests. Therefore, we demonstrated the valuable role of CPET in assessing dyspnea. All patients with elevated RVSP on echocardiogram should undergo RHC. Ultimately, no RHC is complete without exercise, which can unmask EIPH in undiagnosed patients.
EIPH in Patients with Preserved LVEF
EIPH is a common finding in patients with unexplained exertional dyspnea or anginal symptoms with preserved LVEF. Such patients are regularly referred for exercise echocardiography evaluation. In a study by Lim et al25, up to one-third of patients with preserved LVEF had EIPH. Furthermore, age, resting early mitral inflow velocity/mitral annular early diastolic velocity ratio, and resting systolic PAP of these patients were found to be independently associated with EIPH. In light of these findings, EIPH should be considered as a cause of unexplained exercise intolerance in symptomatic patients with preserved LVEF.
Use of CPET as a Testing Strategy
Despite its dominance over other testing methodologies, CPET has limitations, which include variability in existing protocols, use of sedatives, and exercise techniques. It also requires experienced interpreters to decipher fluctuations in measure readings caused by respiratory swing and upright or supine posturing during exercise, and it has challenges in identifying the zero level in catheter transducers.26-28 Protocol standardization may alleviate these concerns. In our study, the Wasserman curves, VO2 max, and LVEF were obtained by examining noninvasive CPET testing data. Whether using invasive CPET would have provided different results is questionable. We suggest that CPET be performed first, and if at least 1 of the 3 Wasserman curves is found to be abnormal, then exercise RHC is indicated. This may ensure that patients with resting mPAPs < 25 mmHg, including those with EIPH, are referred for invasive RHC testing. Additionally, it would exclude patients with resting mPAPs < 25 mmHg due to noncardiac causes from invasive testing. Without a screening tool, all patients would require invasive RHC on referral to the pulmonology vascular clinic. CPET testing in asymptomatic EIPH patients demonstrating similar Wasserman curve patterns would suggest a role for CPET as a potential screening tool for earlier detection.
Role of Long-Acting Nitrates in the Treatment of EIPH
Treatment of established PH at rest is dependent on its etiology. In our study, EIPH patients demonstrated a significant response to the long-acting nitrate, isosorbide mononitrate, with 86.67% having their symptoms resolved. Based on this response, further studies evaluating the effectiveness of long-acting nitrate for alleviating symptoms of preserved LVEF heart failure are indicated. Among our patients, epicardial coronary disease was excluded by either stress nuclear examination or left heart catheterization. However, neither of these techniques is particularly sensitive to diagnosing microvascular angina.29 Moreover, we postulate that microvascular angina is the driving pathophysiology of EIPH. Nitrates increase vasodilation in the pulmonary circulation, thereby increasing perfusion, adjusting the V/Q mismatch, and alleviating dyspnea on exertion and effort intolerance among EIPH patients.
Rationale for Avoiding Beta Blockers in the Management of EIPH
The use of beta blockers in these patients may not be beneficial. Beta blockers inherently slow the heart rate, which allows for increased ventricular filling and ejection fraction. However, these physiologic changes also lead to decreased cardiac output, resulting in increased mPAP, symptoms of exercise intolerance. Beta blockers might worsen chronotropic incompetence, subsequently exacerbating EIPH symptoms. Essentially, they could inhibit the only available mechanism for increasing the cardiac output during exercise in the patient with EIPH. This is supported by the observation of CPET data patterns that demonstrate EIPH-induced chronotropic incompetence leading to failure of adequate heart rate response during exercise. The same result would be seen in the Wasserman curve combined effect. Further prospectively designed clinical studies may further elucidate this clinical query for this patient population.
Limitations of This Study
Our retrospective study had limitations, such as the arm movement performed during the invasive RHC; exercising in a supine manner may not account for the large autonomic differences present before and after exercising. The influence on resting mPAP from increased venous return and central venous pressure in the supine position are also evident. Interpretation of CPET Wasserman curves was obtained by expert clinical opinion rather than by prespecified criteria, which would be preferred in a prospectively designed study.
CONCLUSIONS
Patients with symptoms of angina, dyspnea, and/or fatigue on exertion with negative left heart cardiac stress testing may have elevated right heart pressures attributable to resting PH. EIPH, a type of PH, is not a benign condition, but has the potential to develop into adverse clinical sequalae if left unaddressed. Despite the limitations from our retrospective analysis, our findings highlight the unmet burden of EIPH. It also demonstrates the potential role of CPET as a screening tool to detect characteristic Wasserman curve patterns in patients suspected of having any form of resting PH or EIPH. CPET also helps delineate patients who likely do not have underlying resting PH or EIPH and would benefit from avoiding confirmatory invasive exercise RHC testing. We propose that prospectively designed randomized clinical studies be performed to better clarify the role of CPET in early forms of resting PH or EIPH and asymptomatic patients.
Footnotes
Disclosure Statement: The author(s) have no conflicts of interest to disclose.
Funding: No funding was sought in writing this manuscript.
Author Contributions: Zulfiqar Qutrio Baloch, MD, wrote the original manuscript. Shabber Agha Abbas, MD, wrote the original manuscript. Rohan Madhu Prasad, DO, edited the original manuscript and added further references. Amin M Elamin, MD, FACP, provided a major contribution role, including supervision, data analysis, and revision of the final draft. Abbas Ali, MD, FACC, provided a supervisory role and revised the final draft.
Abbreviations: BMI, Body mass index; CI, Cardiac index; CPET, Cardiopulmonary exercise test; EIPH, Exercise-induced pulmonary hypertension; LVEF, Left ventricular ejection fraction; mPAP, Mean pulmonary arterial pressure; N-EIPH, Not EIPH; PAP, Pulmonary arterial pressure; PCWP, Wedge position pressure; PH, Pulmonary arterial hypertension; PVR, Pulmonary vascular resistance; RHC, Right heart catheterization; RVSP, Right ventricular systolic pressure; VO2 max, Maximum oxygen consumption. A measurement of the maximum amount of oxygen that an individual can utilize during intense, or maximal exercise. It is measured as milliliters of oxygen used in 1 minute per kilogram of body weight; WHO, World Health Organization
References
- 1.Proudman SM, Stevens WM, Sahhar J, Celermajer D. Pulmonary arterial hypertension in systemic sclerosis: The need for early detection and treatment. Intern Med J 2007 Jul;37(7):485-94. DOI: 10.1111/j.1445-5994.2007.01370.x, PMID:17547726 [DOI] [PubMed] [Google Scholar]
- 2.Tolle JJ, Waxman AB, Van Horn TL, Pappagianopoulos PP, Systrom DM. Exercise-induced pulmonary arterial hypertension. Circulation 2008 Nov;118(21):2183-9. DOI: 10.1161/CIRCULATIONAHA.108.787101, PMID:18981305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ha JW, Choi D, Park S, et al. Determinants of exercise-induced pulmonary hypertension in patients with normal left ventricular ejection fraction. Heart 2009 Mar;95(6):490-4. DOI: 10.1136/hrt.2007.139295, PMID:18653569 [DOI] [PubMed] [Google Scholar]
- 4.Condon DF, Nickel NP, Anderson R, Mirza S, de Jesus Perez VA. The 6th World Symposium on Pulmonary Hypertension: what’s old is new. F1000Res 2019 Jun;8:F1000 Faculty Rev-888. DOI: 10.12688/f1000research.18811.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas CA, Anderson RJ, Condon DF, de Jesus Perez VA. Diagnosis and management of pulmonary hypertension in the modern era: Insights from the 6th World Symposium. Pulm Ther 2020 Jun;6(1):9-22. DOI: 10.1007/s41030-019-00105-5, PMID:32048239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bossone E, Naeije R. Exercise-induced pulmonary hypertension. Heart Fail Clin 2012 Jul;8(3):485-95. DOI: 10.1016/j.hfc.2012.04.007, PMID:22748908 [DOI] [PubMed] [Google Scholar]
- 7.Maréchaux S, Pinçon C, Le Tourneau T, et al. Cardiac correlates of exercise induced pulmonary hypertension in patients with chronic heart failure due to left ventricular systolic dysfunction. Echocardiography 2008 Apr;25(4):386-93. DOI: 10.1111/j.1540-8175.2007.00616.x, PMID:18177381 [DOI] [PubMed] [Google Scholar]
- 8.Tumminello G, Lancellotti P,Lempereur M, D'Orio V, Pierard LA. Determinants of pulmonary artery hypertension at rest and during exercise in patients with heart failure. Eur Heart J 2007 Mar;28(5):569-74. DOI: 10.1093/eurheartj/ehl561, PMID:17314112 [DOI] [PubMed] [Google Scholar]
- 9.Lancellotti P, Magne J, Dulgheru R, Ancion A, Martinez C, Piérard LA. Clinical significance of exercise pulmonary hypertension in secondary mitral regurgitation. Am J Cardiol 2015 May;115(10):1454-61. DOI: 10.1016/j.amjcard.2015.02.028, PMID:25784516 [DOI] [PubMed] [Google Scholar]
- 10.Magne J, Lancellotti P, Piérard LA. Exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. Circulation 2010 Jul;122(1):33-41. DOI: 10.1161/CIRCULATIONAHA.110.938241, PMID:20566950 [DOI] [PubMed] [Google Scholar]
- 11.Lancellotti P, Magne J, Donal E, et al. Determinants and prognostic significance of exercise pulmonary hypertension in asymptomatic severe aortic stenosis. Circulation 2012 Aug;126(7):851-9. DOI: 10.1161/CIRCULATIONAHA.111.088427, PMID:22832784 [DOI] [PubMed] [Google Scholar]
- 12.Shim CY, Kim SA, Choi D, et al. Clinical outcomes of exercise-induced pulmonary hypertension in subjects with preserved left ventricular ejection fraction: Implication of an increase in left ventricular filling pressure during exercise. Heart 2011 Sep;97(17):1417–24. DOI: 10.1136/hrt.2010.220467, PMID:21653218 [DOI] [PubMed] [Google Scholar]
- 13.Rosenkranz S, Preston IR. Right heart catheterisation: Best practice and pitfalls in pulmonary hypertension. Eur Respir Rev 2015 Dec;24(138):642-52. DOI: 10.1183/16000617.0062-2015, PMID:26621978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United States Department of Health & Human Services . Medical privacy—national standards to protect the privacy of personal health information. Accessed December 29, 2020. www.hhs.gov/ocr/hipaa. [Google Scholar]
- 15.United States Department of Health & Human Services . Policy for protection of human research subjects. Accessed May 3, 2021. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html. [Google Scholar]
- 16.Balady, GJ, Arena, R, Sietsema, K, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation 2010 Jul;122(2):191-225. DOI: 10.1161/CIR.0b013e3181e52e69, PMID:20585013 [DOI] [PubMed] [Google Scholar]
- 17.Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Postgrad Med J 2007 Nov;83(985):675-82. DOI: 10.1136/hrt.2007.121558, PMID:17989266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stehlik J, Movsesian MA. Combined use of PDE5 inhibitors and nitrates in the treatment of pulmonary arterial hypertension in patients with heart failure. J Card Fail 2009 Feb;15(1):31-4. DOI: 10.1016/j.cardfail.2008.09.005, PMID:19181291 [DOI] [PubMed] [Google Scholar]
- 19.Khan SS, Cuttica MJ, Beussink-Nelson L, et al. Effects of ranolazine on exercise capacity, right ventricular indices, and hemodynamic characteristics in pulmonary arterial hypertension: A pilot study. Pulm Circ 2015 Sep;5(3):547-56. DOI: 10.1086/682427, PMID:26401256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasserman K, Hansen J, Sue D, et al. Principles of exercise testing and interpretation. 4th edition.Baltimore, MD: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 21.Dhingra A, Garg A, Kaur S, et al. Epidemiology of heart failure with preserved ejection fraction. Curr Heart Fail Rep 2014 Dec;11(4):354-65. DOI: 10.1007/s11897-014-0223-7, PMID:25224319 [DOI] [PubMed] [Google Scholar]
- 22.Galiè N, Simonneau G. The Fifth World Symposium on Pulmonary Hypertension. J Am Coll Cardiol 2013 Dec;62(25 Suppl):D1-3. DOI: 10.1016/j.jacc.2013.10.030, PMID:24355633 [DOI] [PubMed] [Google Scholar]
- 23.Naeije R, Vonk Noordegraaf A, Kovacs G. Exercise-induced pulmonary hypertension: At last! Eur Respir J 2015 Sep;46(3):583-6. DOI: 10.1183/09031936.00061015, PMID:26324684 [DOI] [PubMed] [Google Scholar]
- 24.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013 Dec;62(25 Suppl):D42-50. DOI: 10.1016/j.jacc.2013.10.032, PMID:24355641 [DOI] [PubMed] [Google Scholar]
- 25.Lim AY, Kim C, Park SJ, Choi JO, Lee SC, Park SW. Clinical characteristics and determinants of exercise-induced pulmonary hypertension in patients with preserved left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging 2017 Mar;18(3):276-83. DOI: 10.1093/ehjci/jew199, PMID:27679601 [DOI] [PubMed] [Google Scholar]
- 26.Boerrigter BG, Waxman AB, Westerhof N, Vonk-Noordegraaf A, Systrom DM. Measuring central pulmonary pressures during exercise in COPD: How to cope with respiratory effects. Eur Respir J 2014 May;43(5):1316-25. DOI: 10.1183/09031936.00016913, PMID:24177003 [DOI] [PubMed] [Google Scholar]
- 27.Kovacs G, Avian A, Pienn M, Naeije R, Olschewski H. Reading pulmonary vascular pressure tracings. How to handle the problems of zero leveling and respiratory swings. Am J Respir Crit Care Med 2014 Aug;190(3):252-7. DOI: 10.1164/rccm.201402-0269PP, PMID:24869464 [DOI] [PubMed] [Google Scholar]
- 28.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010 Sep;3(5):588-95. DOI: 10.1161/CIRCHEARTFAILURE.109.930701, PMID:20543134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanza GA, Crea F. Primary coronary microvascular dysfunction: Clinical presentation, pathophysiology, and management. Circulation 2010 Jun;121(21):2317-25. DOI: 10.1161/CIRCULATIONAHA.109.900191, PMID:20516386 [DOI] [PubMed] [Google Scholar]


