Abstract
Various stocks of honey bees (Apis mellifera L. (Hymenoptera: Apidae)) employ multiple mechanisms to control varroa mite (Varroa destructor Anderson & Trueman (Mesostigmata: Varroidae)) infestations. Identification of trait-associated genes and markers can improve efficiency of selective breeding. Dopamine receptors show promise in this regard in their association with numerous traits in honey bees, high plasticity, and indicated association with varroa resistance through QTL analysis. We assessed the relationship between exposure to mite-infested brood and gene expression of the honey bee dopamine receptors, Amdop1, Amdop2, and Amdop3, in bees and stocks with known levels of varroa resistance, in Spring 2016 (VSH vs Italian) and Summer 2019 (Pol-line vs Italian). Relative mRNA expression levels varied both by honey bee stock and before/after exposure to varroa-infested brood, in 7-, 10-, and 14-day-old bees. However, the trials revealed contrasting patterns in expression of the three dopamine receptors. In 2016, downregulation was evident in VSH bees, but varied by days post-emergence and by gene. The 2019 trial showed upregulation post-exposure in both stocks, and at all ages, for Amdop1, Amdop2, and Amdop3, with the exception of 14 d Italian bees for Amdop2 and Amdop3. Stock comparison in 2019 showed upregulation of all three dopamine-like receptors in post-exposure bees of all ages. Season and associated differences in mite loads may have contributed to the differences observed across trials. Differential expression of all three dopamine receptors suggests a role for the dopaminergic system in varroa resistance and suggests that further characterization of these receptors for breeding potential is warranted.
Keywords: bee, varroa, dopamine, mite, resistance
Dopamine is involved in behavior, memory, cognition, and learning and acts as a catecholamine neurotransmitter and neuromodulator in all animals (Blenau and Erber 1998). In honey bees, Apis mellifera L. (Hymenoptera: Apidae), dopamine and the three associated dopamine receptors (both D1 and D2 classes) have been indicated in aversive learning (Vergoz et al. 2007, 2009; Jarriault et al. 2018), appetitive learning (Klappenbach et al. 2013), grooming, and motor function (Mustard et al. 2010), and are known to be heavily influenced by queen mandibular pheromone (Beggs et al. 2009, Sasaki and Harada 2020). Many of these traits have potential roles in the mechanisms driving honey bee resistance to the varroa mite, Varroa destructor (Anderson & Trueman), the primary pest of honey bees. This parasite has nearly a global distribution and is considered to be the single greatest health threat to honey bees (Genersch 2010, Guzmán-Novoa et al. 2010).
Various stocks of honey bees employ multiple mechanisms to control varroa mite infestations. The varroa-sensitive hygiene (VSH) trait is one such mechanism and bees/colonies exhibiting VSH consistently have low mite infestation levels. This highly heritable trait is complex, involving three primary behaviors that ultimately result in removal of mite-infested brood and in the suppression of mite reproduction (Harris 2007; Danka et al. 2008, 2011). While the behavioral components of the trait have been well-defined, the genetic mechanisms driving those behaviors have been difficult to discern. In honey bees bred for the VSH, the D2-dopamine receptor Amdop3 showed strong QTL association with the uncapping and removal components of the trait (Tsuruda et al. 2012). A sensory-related function of Amdop3 mRNA is strongly suggested by localization in cells around the optic and antennal lobes of the honey bee brain (Beggs et al. 2005, Beggs and Mercer 2009). The link to olfaction and motor activity and known association of Amdop3 with the VSH trait suggest that both the D1 and D2 dopamine receptors are promising candidate genes for improving resistance in honey bees to the varroa mite.
We conducted a two-phase study to assess the association of the three honey bee dopamine receptors with colony-level resistance to varroa mites. Specifically, bees sampled from colonies of known Varroa resistance were used to test the relationship between exposure to mite-infested brood and gene expression of Amdop1, Amdop2, and Amdop3. Ultimately, genes that correlate to the VSH trait could be developed for use in marker-assisted selection.
Materials and Methods
Sample Collection
Colony-Level VSH Versus Non-VSH
Honey bees were collected from colonies of known phenotype relative to VSH activity and mite resistance (high VSH/low mites and low VSH/high mites). At the time of testing in April 2016, the VSH colony had not been treated for Varroa since being established 2 yr earlier and had 0.3% of brood infested. The colony that showed no mite resistance had an Italian × Carniolan queen; it was untreated for 6 mo and had 27% of brood infested.
In total, 300 young worker bees from each colony were collected within 24 h after they emerged from individually caged brood combs (held in an incubator at 35°C and 50% RH) and marked with enamel paint on the thoracic dorsum to distinguish colony source. All bees then were placed together in a foster colony of Italian bees that was unrelated to the bees being tested. Mite infestation in brood of the foster colony was 1.3%.
Marked worker bees were collected at 7, 10, and 14 d post-emergence, the age at which the likelihood of performing the VSH behaviors is highest. On each sampling day, bees from each phenotype (VSH/NON) were collected for analysis of gene expression as follows: Thirty bees per phenotype were collected prior to colony manipulation each sampling day. A comb of mite-infested brood obtained from an unrelated source was then placed into the foster colony. Mite infestation in the introduced brood was 7.5%, 10.0%, and 12.5% at the three sampling times, respectively. Bees of the two phenotypes were then collected 10–45 min after mite-infested brood was added. Bees that were actively opening brood cells were noted. Each bee that was collected (pre- and post-exposure) was flash frozen in liquid nitrogen and then stored individually at −80°C until RNA analysis.
Pol-line Versus Italian
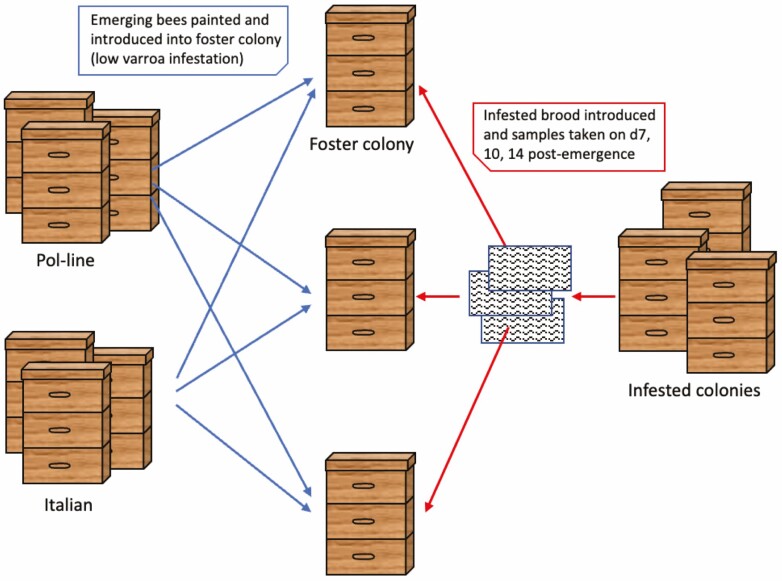
The procedure described above was repeated in July 2019 with 3 colonies each of Pol-line and Italian honey bees (Fig. 1). Pol-line bees are a stock bred via instrumental insemination by the USDA-ARS Honey Bee Breeding, Genetics & Physiology Laboratory in Baton Rouge, LA for high VSH activity, low mite reproduction, and other production traits (Danka et al. 2016). The Italian bees originated from a commercially available stock. Mite infestation of the source colonies was 1.3% ± 0.02 and 15.2 ± 0.06 for Pol-line and Italian stocks, respectively. Each of the 3 foster colonies used had 0 mites at the time of bee introduction. Mite infestation of the introduced brood frames was 14.3% ± 0.04, 22.5% ± 0.10, and 22.5% ± 0.07.
Fig. 1.
Depiction of colony-based phenotyping assay.
RNA Extraction, cDNA Synthesis, and Expression
For all samples, total RNA was extracted from whole heads of worker honey bees using the Maxwell RSC kit for tissues on the Maxwell RSC Instrument for nucleic acid purification, as per manufacturer instructions. Whole heads were used to encapsulate neuronal and physical mechanisms associated with olfactory response. All RNA were quantified on a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Rockland, DE) and 1 μg of total RNA was used for cDNA synthesis. cDNA was synthesized using the SuperScript II RT kit (Life Technologies, Inc.) as per manufacturer’s instructions on a Veriti Thermalcycler (Life Technologies, Inc.). The cDNA was then quantified by UV absorption on the NanoDrop 1000 spectrophotometer and all samples were diluted to 40 ng/μl. In total, 80 ng of cDNA per sample was used in expression analyses. In total, 48 RNA 2019 samples were deemed unusable due to extraction processing errors. These included samples from both stocks.
Expression of target mRNA was measured with real-time PCR on a StepOne Plus (ThermoFisher Scientific/Applied Biosystems, Grand Island, NY) thermalcycler for all VSH/NON trial samples and on a QuantStudio3 (ThermoFisher Scientific/Applied Biosystems) thermalcycler for all Pol-line/Italian samples. All real-time PCR amplifications were performed in duplicate. Each amplification reaction mixture (11.5 μl) contained 10 ng of cDNA; 1× Fast SYBR Green Master Mix (ThermoFisher Scientific/Applied Biosystems); and 10 nM of each primer. Primer sequences were designed with Beacon Designer 8.0 (Premier BioSoft, Inc, Palo Alto, CA) software. Primer sequences and GenBank accession numbers are listed in Table 1. The amplification profile was: 95°C held for 20 s, followed by 45 cycles of 95°C for 3 s, and 60°C for 30 s. Amplification products were normalized with two reference genes, B151178 (Le Conte et al. 2011) and elongation factor 1 alpha (ef1α) in colony-phenotyped bees (Vergoz et al. 2009), and deionized water was used as no-template negative control. Ten-fold serial dilutions of one sample, run in duplicate, were used to determine amplification efficiency for each set of reactions.
Table 1.
Primer sequences, qPCR efficiencies, and GenBank accession numbers of all genes tested
| Sequence name | GenBank ID | Primer sequence | qPCR efficiency (%) | |
|---|---|---|---|---|
| Forward | Reverse | |||
| Amdop1 | GB50192 | TGAACGATCTCCTCGGCTAT | ACCCAACGACCGTATCTGAG | 96.21 |
| Amdop2 | NM_001014983 | GGATCAACAGCGGAATGAAT | GCGAATCTTTGACTCGGTTT | 105.35 |
| Amdop3 | GB14561 | CGTTGCAAACTGTCACCAAT | GACGTCCATTGCGATGTAAA | 96.39 |
| Ef1α | XM_012490647 | TGCAACCTACTAAGCCGATG | GACCTTGCCCTGGGTATCTT | 95.76 |
| B151178 | CTCATCAGTTGTTGGTTCTCCTC | TCGTTTGGCTCTTCAGTCTTGT | 95.50 |
Data Analysis
Ct values for all samples were normalized to the reference genes and then relative mRNA expression of each gene was compared using the 2−ΔΔCt calculation (Livak and Schmittgen 2001). For each timepoint, the sample with the lowest value was set to 1 and then used for calibration and calculation of the fold change values. The data were analyzed using mixed analysis ANOVA in SAS 9.2 (SAS Institute, Inc.), by bee age (days post-emergence) to determine if differences existed between stocks and exposure to infested brood (pre/post). When statistical differences were found, pairwise comparisons of stock and exposure(pre/post) per day were conducted using two-tailed t-tests, setting P = 0.05. All data are reported as mean ± SEM.
Results
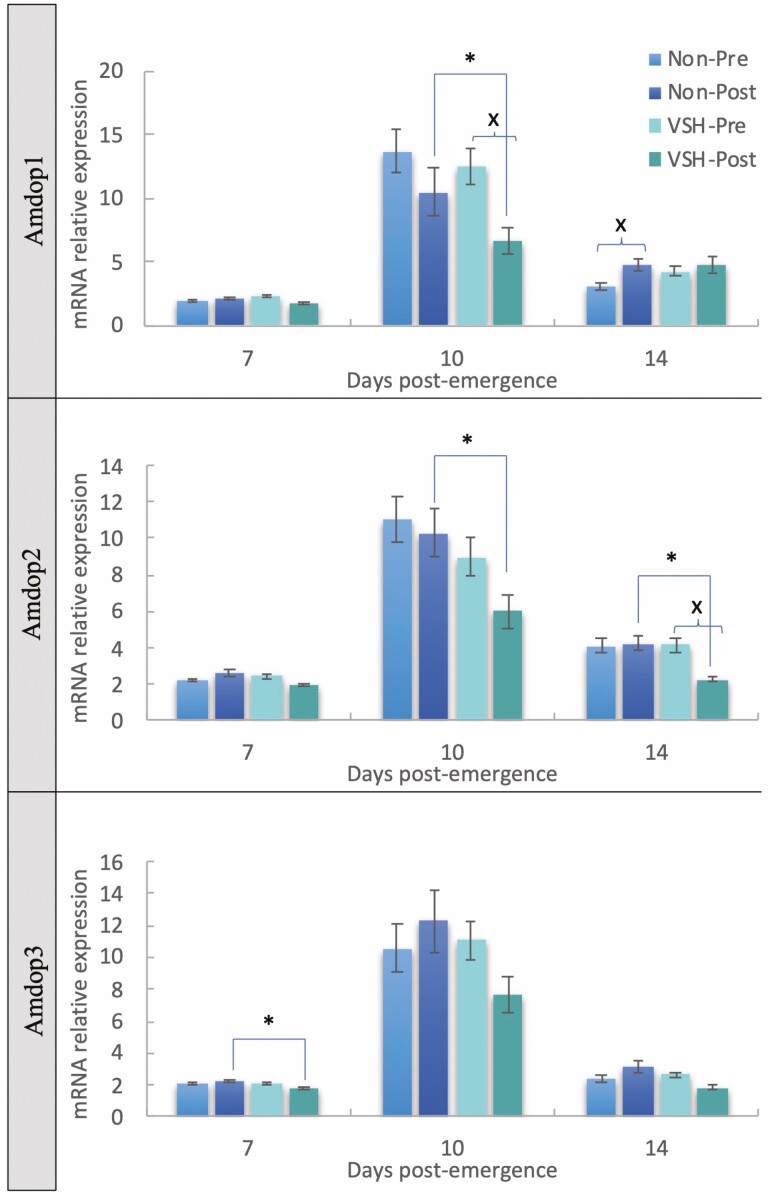
Relative mRNA expression levels for three dopamine receptors (Amdop1, Amdop2, and Amdop3) varied both by honey bee stock and before/after exposure to varroa-infested brood, in young bees that were 7, 10, and 14 d post-emergence (Table 2). In the 2016 sampling trial, upregulation was evident in non-VSH compared to VSH bees, post-exposure, for those 10 d for Amdop1, 10 d and 14 d for Amdop2, and 7 d for Amdop3 (Fig. 2). Exposure to infested brood reduced relative expression of Amdop1 in 10 d VSH bees, but was upregulated in NON-VSH bees on day 14. Amdop2 was downregulated post-exposure in VSH bees only and no exposure effects on Amdop3 expression were evident for VSH or NON-VSH bees of any age (Fig. 2).
Table 2.
Results of t-test analysis of stock and exposure effects on mRNA expression of 3 dopamine receptor genes in response to exposure of worker bees to varroa-infested brood
| Stocks | Gene | Effect | Day 7 | Day 10 | Day 14 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | t | P | DF | t | P | DF | t | P | |||
| VSH/NON | Amdop1 | Stock | 102 | 0.39 | 0.7004 | 83 | 1.66 | 0.1004 | 102 | −1.51 | 0.1345 |
| Exposure | 102 | −3.35 | 0.0011 | 83 | −2.95 | 0.0042 | 102 | 2.16 | 0.0334 | ||
| VSH/NON | Amdop2 | Stock | 102 | 1.64 | 0.1044 | 83 | 2.77 | 0.0068 | 102 | 2.67 | 0.0088 |
| Exposure | 102 | −0.41 | 0.6806 | 83 | −1.63 | 0.1059 | 102 | −2.62 | 0.0101 | ||
| VSH/NON | Amdop3 | Stock | 102 | 2.89 | 0.0047 | 83 | 1.38 | 0.1713 | 102 | 2.09 | 0.0940 |
| Exposure | 102 | −0.82 | 0.4118 | 83 | −0.57 | 0.5712 | 102 | −0.19 | 0.8488 | ||
| Pol-line/Ital | Amdop1 | Stock | 98 | −8.3 | <0.0001 | 106 | −4.43 | <0.0001 | 86 | −2.04 | 0.0447 |
| Exposure | 98 | 18.89 | <0.0001 | 106 | 7.03 | <0.0001 | 86 | 5.42 | <0.0001 | ||
| Pol-line/Ital | Amdop2 | Stock | 98 | −7.11 | <0.0001 | 106 | −7.07 | <0.0001 | 86 | −2.31 | 0.0232 |
| Exposure | 98 | 15.89 | <0.0001 | 106 | 11.23 | <0.0001 | 86 | 5.04 | <0.0001 | ||
| Pol-line/Ital | Amdop3 | Stock | 98 | −7.59 | <0.0001 | 106 | −7.42 | <0.0001 | 86 | −3.31 | 0.0014 |
| Exposure | 98 | 16.21 | <0.0001 | 106 | 11.89 | <0.0001 | 86 | 4.97 | <0.0001 |
Real-time PCR results were normalized to EF1α and B151178 reference genes and then calibrated to sample with the highest dCt value within each sampling day for each gene. Results in bold are signicant at P < 0.05.
Fig. 2.
Mean relative expression values (2−ΔΔCt, fold change) ± SEM for three honey bee dopamine receptors associated with varroa-sensitive hygiene (VSH) in worker honey bees collected from colonies of known phenotype, both pre- and post-exposure to varroa-infested brood, in bees 7, 10, and 14 d old. ‘*’ denotes a significant difference between resistance type (VSH/NON) in bees based on exposure status. ‘X’ denotes a significant difference between pre and post-exposure samples, within each resistant phenotype group. P < 0.05 based on t-test analysis per bee age and per gene of interest.
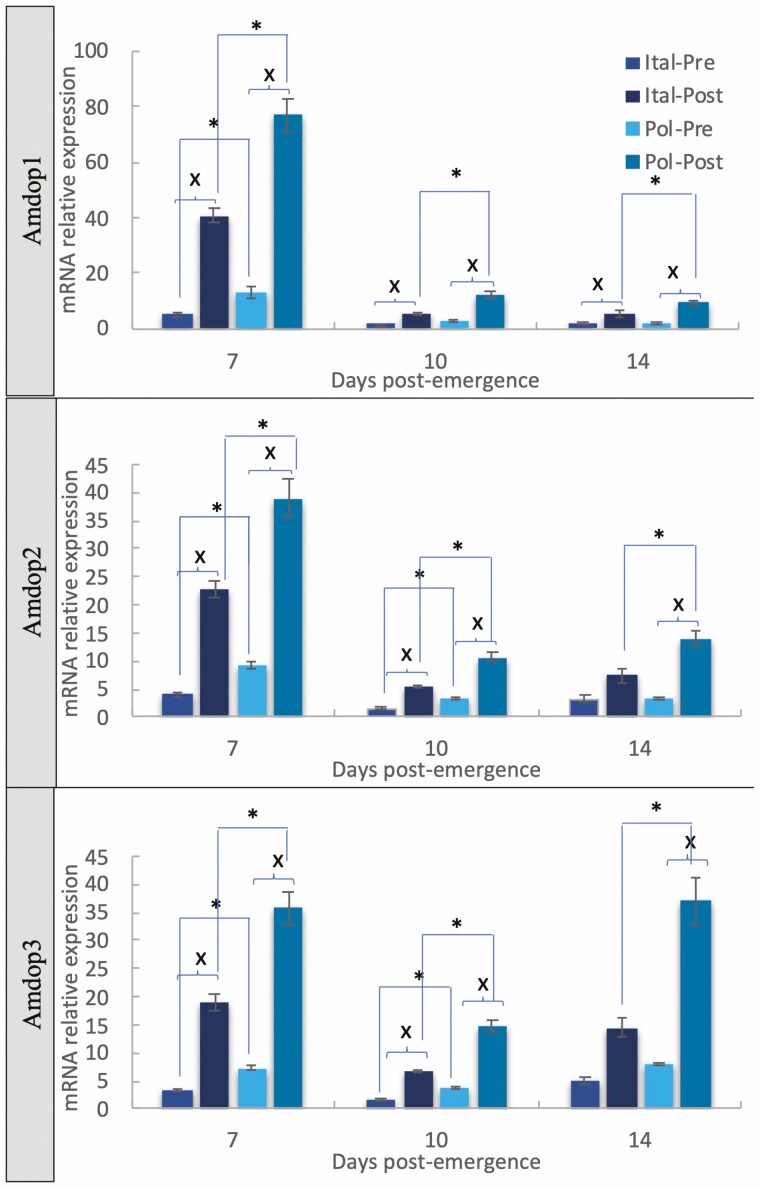
The comparison between Pol-line and Italian honey bees showed stronger differences among both stock and exposure groups (Table 2, Fig. 3). Upregulation post-exposure to infested brood compared to pre-exposed bees was evident for both stocks, at all ages, for Amdop1, Amdop2, and Amdop3, with the exception of 14 d Italian bees for Amdop2 and Amdop3. Stock comparison showed upregulation of all three dopamine-like receptors in post-exposure sampled bees of all ages (Fig. 3).
Fig. 3.
Mean relative expression values (2−ΔΔCt, fold change) ± SEM for three honey bee dopamine receptors associated with varroa resistance in worker honey bees collected from colonies representing stocks of known phenotype, both pre- and post-exposure to varroa-infested brood, in bees 7, 10, and 14 d old. ‘*’ denotes a significant difference between resistance type (Pol-line/Italian) in bees based on exposure status. ‘X’ denotes a significant difference between pre- and post-exposure samples, within each stock. P < 0.05 based on t-test analysis per bee age and per gene of interest.
Discussion
Colony-level phenotype for varroa resistance was used to assess if exposure to varroa-infested honey bee brood invoked differential expression of three dopamine receptors in worker bees. Comparisons based on colony of origin phenotype (based on measured VSH activity and stock characteristics) showed contrasting patterns between the two rounds of testing. The initial trial testing VSH versus NON-VSH bees showed minimal differences among groups and downregulation of each gene in bees that had been selected for high VSH activity. In contrast, upregulation was evident in Pol-line bees compared to Italian bees across ages and post-exposure to infested brood, for all three dopamine receptors. The second trial occurred during mid-summer, when varroa infestation levels are high, as was reflected by mite levels in the introduced infested brood comb. Foster colony infestation levels in both trials were comparable. In addition, Pol-line stock originated from bees bred for the VSH trait. However, this stock’s trait profile has been broadened to incorporate other beneficial production traits, as well as highly efficient VSH activity (Danka et al. 2016). These factors likely contributed to the differential response between the two trials.
Dopamine receptor differential expression, both up- and downregulated, in response to varroa-infested brood suggests a role for the dopaminergic system in varroa resistance. Dopamine regulation has many roles in honey bee physiology, the most relevant of which to varroa resistance are motor function, grooming, olfactory learning, and memory (Mustard et al. 2003, 2010; Agarwal et al. 2011; Jarriault et al. 2018; Sasaki and Harada 2020). Visual characterization of the dopaminergic neurons in the honey bee brain demonstrated perfusion throughout, confirming that olfactory, gustatory, and visual learning centers were all heavily innervated and showed variation with age and stimulation source (Tedjakumala et al. 2017). These centers are involved in aversive learning in honey bees and the likelihood of their involvement in the complex behavioral process of detecting and removing varroa-infested pupae is high. This coupled with the high plasticity of dopamine receptors and their strong response to varroa-infested brood shown here, and known association of Amdop3 with the VSH–QTL (Tsuruda et al. 2012) is suggestive of their potential use in breeding for improved resistance to the varroa mite. Further characterization of the honey bee dopamine D1 and D2 dopamine receptors is warranted and could offer the potential for valuable selective markers for improving varroa resistance breeding efforts in honey bees.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgments
We thank D. Dodge, V. Rainey, and D. Winfrey for assistance in colony management and sample collection. We thank G. Dodds for assistance in maintenance and propagation of the selected lines. We thank T. Adams for assistance in sample processing. Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the USA Department of Agriculture and does not imply approval to the exclusion of other products that may be suitable.
Author Contributions
L.B. designed the study, collected samples, and wrote the manuscript. L.B. collected and analyzed samples, contributed to development of the experimental design, and edited the manuscript. All authors have approved the final version of the manuscript.
References Cited
- Agarwal, M., Giannoni Guzman M., Morales-Matos C., Del Valle Diaz R. A., Abramson C. I., . et al. 2011. Dopamine and octopamine influence avoidance learning of honey bees in a place preference assay. PLoS One. 6: e25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs, K. T., Hamilton I. S., Kurshan P. T., Mustard J. A., and Mercer A. R.. . 2005. Characterization of a D2-like dopamine receptor (Amdop3) in honey bee, Apis mellifera. Insect. Biochem. Mol. Biol. 35: 873–882. [DOI] [PubMed] [Google Scholar]
- Beggs, K. T., and Mercer A. R.. . 2009. Dopamine receptor activation by honey bee queen pheromone. Curr. Biol. 19: 1206–1209. [DOI] [PubMed] [Google Scholar]
- Blenau, W., and Erber J.. . 1998. Behavioural pharmacology of dopamine, serotonin and putative aminergic ligands in the mushroom bodies of the honeybee (Apis mellifera). Behav. Brain Res. 96: 115–124. [DOI] [PubMed] [Google Scholar]
- Danka, R., Harris J., Ward K., and Ward R.. . 2008. Status of bees with the trait of varroa-sensitive hygiene (VSH) for varroa resistance. Am. Bee J. 148: 51–54. [Google Scholar]
- Danka, R. G., Harris J. W., and Villa J. D.. . 2011. Expression of Varroa sensitive hygiene (VSH) in commercial VSH honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 104: 745–749. [DOI] [PubMed] [Google Scholar]
- Danka, R. G., J. W. Harris, and G. E. Dodds. 2016. Selection of VSH-derived “pol-line” honey bees and evaluation of their Varroa-resistance characteristics. Apidologie 47: 483–490. [Google Scholar]
- Genersch, E. 2010. Honey bee pathology: current threats to honey bees and beekeeping. Appl. Micro. Biotech. 87: 87–97. [DOI] [PubMed] [Google Scholar]
- Guzmán-Novoa, E., Eccles L., Calvete Y., Mcgowan J., Kelly P. G., . et al. 2010. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie. 41: 443–450. [Google Scholar]
- Harris, J. W. 2007. Bees with varroa sensitive hygiene preferentially remove mite infested pupae aged ≤5 days post capping. J. Apic. Res./Bee World. 46: 134–139. [Google Scholar]
- Jarriault, D., Fuller J., Hyland B. I., and Mercer A. R.. . 2018. Dopamine release in mushroom bodies of the honey bee (Apis mellifera L.) in response to aversive stimulation. Sci. Rep. 8:16277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klappenbach, M., Kaczer L., and Locatelli F.. . 2013. Dopamine interferes with appetitive long-term memory formation in honey bees. Neurobiol. Learn. Mem. 106: 230–237. [DOI] [PubMed] [Google Scholar]
- Le Conte, Y., Alaux C., Martin J. F., Harbo J. R., Harris J. W., . et al. 2011. Social immunity in honeybees (Apis mellifera): transcriptome analysis of varroa-hygienic behaviour. Insect Molec. Biol. 20: 399–408. [DOI] [PubMed] [Google Scholar]
- Livak, K. J., and Schmittgen T. D.. . 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Mustard, J. A., Blenau W., Hamilton I. S., Ward V. K., Ebert P. R., . et al. 2003. Analysis of two D1-like dopamine receptors from the honey bee Apis mellifera reveals agonist-independent activity. Mol. Brain Res. 113: 67–77. [DOI] [PubMed] [Google Scholar]
- Mustard, J. A., Pham P. M., and Smith B. H.. . 2010. Modulation of motor behavior by dopamine and the D1-like dopamine receptor Amdop2 in the honey bee. J. Insect Physiol. 56: 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, K., and Harada M.. . 2020. Dopamine production in the brain is associated with caste-specific morphology and behavior in an artificial intermediate honey bee caste. PLoS One. 15: e0244140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedjakumala, S. R., Rouquette J., Boizeau M. L., Mesce K. A., Hotier L., Massou I., and Giurfa M.. . 2017. A tyrosine-hydroxylase characterization of dopaminergic neurons in the honey bee brain. Front. Syst. Neurosci. 11: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruda, J. M., Harris J. W., Bourgeois L., Danka R. G., and Hunt G. J.. . 2012. High-resolution linkage analyses to identify genes that influence Varroa Sensitive Hygiene behavior in honey bees. PLoS One. 7: e48276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergoz, V., McQuillan H. J., Geddes L. H., Pullar K., Nicholson B. J., . et al. 2009. Peripheral modulation of worker bee responses to queen mandibular pheromone. Proc. Nat. Acad. Sci. 106: 20930–20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergoz, V., Roussel E., Sandoz J.C., and Giurfa M.. . 2007. Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PLoS One. 2: e288. [DOI] [PMC free article] [PubMed] [Google Scholar]