Significance
Persistent viral infection remains a major source of global morbidity and mortality. Studies using a persistent clone of lymphocytic choriomeningitis virus (LCMV) revealed that in addition to optimal functions and interactions of T and B cells, production of cytokines is essential in promoting long-term control of infection. Here we report that B cells are an indispensable source of IL-27 during persistent LCMV infection. B cell–derived IL-27 promotes viral control via supporting accumulations of virus-specific CD8 and CD4 T cells. During later stages of infection, B cell–derived IL-27 promotes production of IFN-γ and IL-21 by virus-specific CD4 T and Tfh cells, respectively. Our study unveils the critical role of a B cell–secreted cytokine in controlling a persistent infection.
Keywords: IL-27, viral infection, B cells, CD4 T cells, antibody
Abstract
Recent studies have identified a critical role for B cell–produced cytokines in regulating both humoral and cellular immunity. Here, we show that B cells are an essential source of interleukin-27 (IL-27) during persistent lymphocytic choriomeningitis virus (LCMV) clone 13 (Cl-13) infection. By using conditional knockout mouse models with specific IL-27p28 deletion in B cells, we observed that B cell–derived IL-27 promotes survival of virus-specific CD4 T cells and supports functions of T follicular helper (Tfh) cells. Mechanistically, B cell–derived IL-27 promotes CD4 T cell function, antibody class switch, and the ability to control persistent LCMV infection. Deletion of IL-27ra in T cells demonstrated that T cell–intrinsic IL-27R signaling is essential for viral control, optimal CD4 T cell responses, and antibody class switch during persistent LCMV infection. Collectively, our findings identify a cellular mechanism whereby B cell–derived IL-27 drives antiviral immunity and antibody responses through IL-27 signaling on T cells to promote control of LCMV Cl-13 infection.
Persistent viral infections, such as HIV, hepatitis B virus, and hepatitis C virus, cause devastating disease burden around the globe. Viral persistence leads to sustained viremia, prolonged immune activation, disorganized microarchitecture of secondary lymphoid organs, reduced numbers and compromised functions of virus-specific T and B cells, as well as aberrant production of virus-specific antibodies (1–5). Infected hosts are confronted with long-term health challenges which can ultimately result in medical diseases and in some cases lead to death. Research efforts using persistent lymphocytic choriomeningitis virus (LCMV) clone 13 (Cl-13) as a model of persistent viral infection in mice primarily led to our mechanistic understanding of how interactions between different immune cell populations contribute to virus clearance. There are three major stages of LCMV Cl-13 infection: initial infection and establishment of virus persistence, maintenance of persistence, and finally immune control and eventual purging of the virus from the host. Initially upon infection, LCMV Cl-13 induces higher production of type 1 interferon (IFN-I) and expression of immunoregulatory molecules such as interleukin-10 (IL-10) and programmed cell death ligand 1 (PD-L1), which restrains T cell functions and sets up virus persistence. Blockade of IFN-I, PD-1/PD-L1, and IL-10 were shown to improve functions of virus-specific T cells to kill infected cells and produce effector cytokines such as IFN-γ, tumor necrosis factor-α (TNF-α), and IL-2 (6–12). During the later stages of persistent LCMV infection, virus-specific CD4 T cells display differentiation bias toward T follicular helper (Tfh) cells and produce the immune stimulatory cytokine IL-21 to provide help to both CD8 T cells and B cells. IL-21 signaling leads to improved CD8 T cell functions and generation of LCMV-specific neutralizing antibodies that drives viral control (13–17). A recent study identified IL-27 as an essential immune regulator required for the control of persistent LCMV infection (18). Ablation of IL-27ra led to increased viral replication and reduction of both virus-specific CD4 T cells and LCMV-specific immunoglobulin G (IgG) antibodies at later stages of infection (18, 19).
IL-27 is a pleiotropic cytokine composed of IL-27p28 and Epstein–Barr virus inducible protein-3 (EBI3) subunits. It signals through a heterodimeric receptor complex consisting of WSX-1 (IL-27ra) and gp130 to activate both STAT1 and STAT3 transcription factors (20). Although dendritic cells (DCs) and macrophages were identified as major producers of IL-27 required for maintenance of early antiviral immunity, the cellular source of IL-27 required for long-term control of persistent LCMV infection is unknown (19). Previous studies demonstrated that B cells secrete cytokines to provide signals to other cells to modulate both humoral and cellular immunity (21–25). Intriguingly, in addition to IL-6, IL-10, IL-12, and IL-35, B cells were shown to independently secrete EBI3 and IL-27p28 upon in vitro activation (23, 26). Taken together, these studies are highly suggestive that B cells may play a key role in IL-27 production during persistent LCMV infection. In the present study, we identified B cells as an essential source of IL-27 that promotes survival and functions of virus-specific CD8 and CD4 T cells. B cell–derived IL-27 induces IFN-γ and IL-21 production by virus-specific CD4 T cells and Tfh cells at later stages of LCMV infection that contribute to virus clearance. Our findings reveal the central role of B cells in the regulation of T cell functions and describes IL-27–dependent cellular interactions between T and B cells necessary for control of persistent LCMV infection.
Results and Discussion
Various B Cell Populations Express IL-27 during Persistent LCMV Infection.
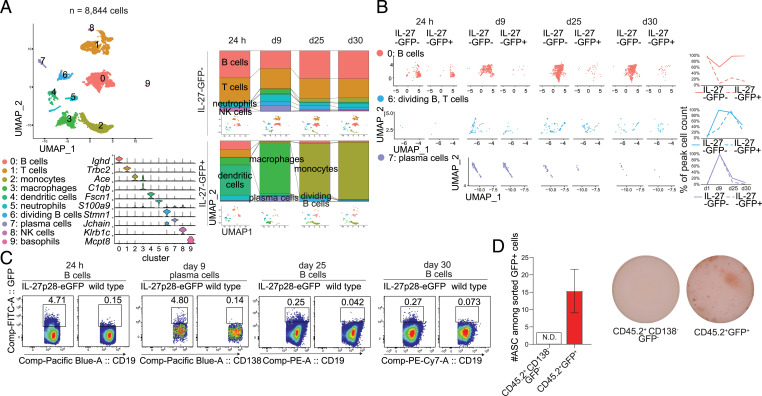
Following persistent virus infection, IL-27 is necessary to purge the virus from animals (18, 27). However, the specific cellular sources of IL-27 required for viral clearance are currently unknown. To generate a complete picture of the cellular and temporal production of IL-27 following LCMV Cl-13 infection, we characterized the landscape of IL-27p28–expressing and IL-27p28–nonexpressing cells using IL-27p28-eGFP (enhanced green fluorescent protein) reporter mice by sequencing transcriptomes of single sorted IL-27p28-GFP+ and IL-27p28-GFP− splenocytes at days 1, 9, 25, and 30 postinfection (p.i.). The cells were reliably separated into clusters associated with known cell types (Fig. 1A and SI Appendix, Fig. S1A). As expected, myeloid cells were the dominant source of IL-27p28: Early IL-27p28+ cells displayed transcriptomic signatures of DCs whereas macrophages and monocytes predominated at later times following infection (Fig. 1A and SI Appendix, Fig. S1B). DCs and myeloid cells have previously been identified as major producers of IL-27 required for early control of persistent LCMV infection (19). Given that chronic infection persists for several weeks and B cells were shown to secrete IL-27 upon in vitro activation, we were particularly interested in the possibility that IL-27 could be produced at later stages of infection and potentially by cellular sources other than DCs and myeloid cells. From our single-cell data, we observed that B cells and plasma cells comprised a minor but significant fraction of IL-27p28-GFP+ cells at all times following infection (Fig. 1A). The combined percentage of B cells and plasma cells of total IL-27p28-GFP+ splenocytes ranged from 14.4% on day 1 to 10.5, 9.5, and 3.8% on days 9, 25, and 30 p.i., respectively. At day 1 p.i., IL-27p28-GFP+ B cells were predominantly from the Ighd+ naïve-like cluster but later B cell IL-27p28 was associated with mitotic B cells and plasma cells (Fig. 1 A and B). Interestingly, IL-27p28-GFP+ B cells in cluster 0 expressed lower levels of genes associated with naïve B cells and higher levels of genes associated with germinal center B cells compared with IL-27p28-GFP− B cells in the same cluster (SI Appendix, Fig. S1 C and D. Further, whereas the percentage of IL-27p28-GFP+ cells of nondividing B cells peaked on day 1 p.i., the highest percentage of IL-27p28-GFP–producing cells among dividing B cells occurred on day 25 p.i. (Fig. 1B). These observations are consistent with the association of IL-27p28 expression with activated B cells. We next verified that B cells derived from IL-27p28-eGFP mice were able to express IL-27p28 upon in vitro activation by TLR4 and CD40 as previously described (SI Appendix, Fig. S1E) (23). In addition, we observed that IL-27p28 production in B cells was markedly enhanced in the presence of IL-21, which was accompanied by sustained elevated levels of EBI3 (SI Appendix, Fig. S1 E and F).
Fig. 1.
B cell populations express IL-27. (A, B, and D) IL-27p28-eGFP mice were infected with 2 × 106 PFU LCMV Cl-13 intravenously. (C) IL-27p28-eGFP mice and WT C57BL/6 mice were infected with 2 × 106 PFU LCMV Cl-13. (A) Analysis of single-cell RNA-seq of splenocytes at days 1, 9, 25, and 30 p.i. (A, Left) Dimensionality-reduced uniform manifold approximation and projection (UMAP) plot and expression of cluster marker genes. (A, Right) Cluster distribution of IL-27p28-GFP+ and IL-27p28-GFP− cells at each time point; the identity of each cluster is indicated by color. (B) Time-resolved distribution of cells within each cluster; the graph indicates relative abundances of IL-27p28-GFP+ (denoted IL-27-GFP+) and IL-27p28-GFP− (denoted IL-27-GFP−) cells in each cluster scaled to peak relative abundance within each experimental group. (C) GFP expression on B cells (1, 25, and 30 d p.i.) or plasma cells (9 d p.i.) was determined by flow cytometry, gated on splenic B cells or plasma cells in IL-27p28-eGFP reporter mice and WT mice (the latter as a negative control for background fluorescence). (D) Frequencies of antibody-secreting cells (ASCs) in CD45.2+GFP+ and CD45.2+GFP−CD138− populations were determined using IgG B cell ELISpot at day 9 p.i. Data in C and D are representative of two experimental replicates and the error bar represents mean ± SD from nine mice per group. FITC, fluorescein isothiocyanate; N.D., not detected.
To confirm our observation from single-cell RNA sequencing (RNA-seq), we performed flow cytometry analyses of fluorescence signals in splenocytes derived from IL-27p28-eGFP mice at 24 h and days 9, 25, and 30 p.i. B cells from wild-type (WT) mice were examined concomitantly as negative controls to confirm the specificity of the GFP reporter signal. Consistently, total B cells and plasma B cells from IL-27p28-eGFP reporter mice were significant producers of IL-27p28 at 24 h and day 9 p.i., respectively, whereas WT B cells showed negligible GFP fluorescence (Fig. 1C and SI Appendix, Fig. S1G). By performing IgG B cell enzyme-linked immunosorbent spot (ELISpot) assays of GFP+ cells derived from IL-27p28-eGFP mice at day 9 p.i., we observed that some IL-27 producers were capable of secreting antibodies (Fig. 1D), suggesting that plasma cells are significant sources of IL-27 during LCMV Cl-13 infection. Taken together, these data demonstrate that, in addition to DCs and macrophages, B cell populations and plasma cells produce IL-27 during persistent LCMV infection.
B Cell–Derived IL-27 Is Required to Control Persistent LCMV Infection.
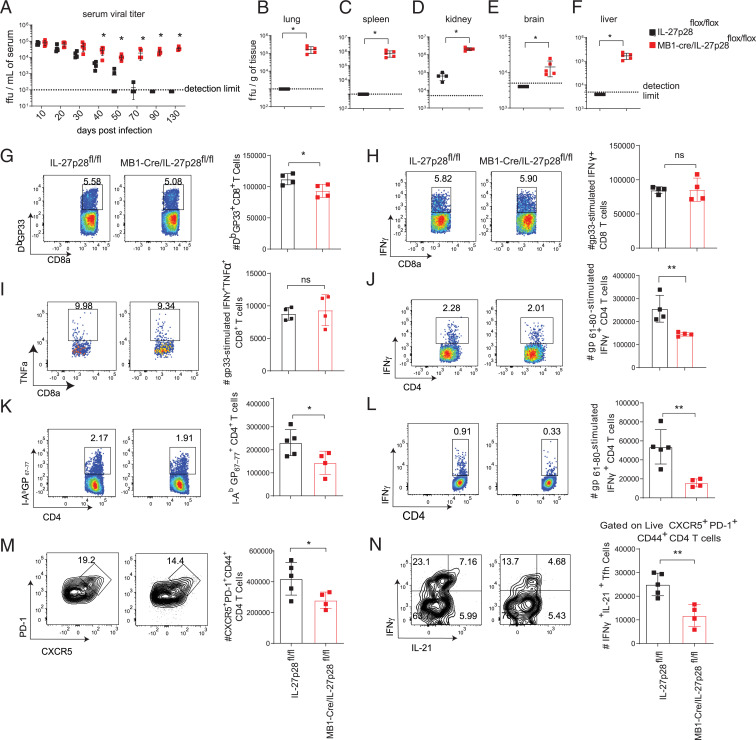
Given that B cells are a significant cellular source of IL-27, we asked if B cell–derived IL-27 promotes long-term control of viral persistence. To explore the impact of B cell–specific deletion of IL-27p28 during chronic LCMV infection, we interbred IL-27p28f/f mice to MB1-Cre mice and infected them with LCMV Cl-13. Deletion efficiency and specificity were subsequently determined by assessing the expression of IL-27p28 in CD138+ plasma B cells using flow cytometry and assessing the relative expression of Il27 messenger RNA (mRNA) using qPCR of sorted CD138+ plasma cells, B cells, DCs, and macrophages from MB1-Cre+/−/IL-27p28f/f and IL-27p28f/f mice at day 9 p.i. We observed a significant reduction of relative Il27 mRNA expression in B cells and plasma B cells but not in DCs and macrophages derived from MB1-Cre+/−/IL-27p28f/f mice compared with IL-27p28f/f mice (SI Appendix, Fig. S2 A–E). Our results verified that the MB1-Cre deletion strategy is specific to B cell populations. MB1-Cre+/−/IL-27p28f/f mice were unable to control viral loads in serum at various times p.i. and in multiple tissues including lung, spleen, kidney, brain, and liver at day 130 p.i. (Fig. 2 A–F). Analysis of the antiviral T cell responses at day 9 p.i. revealed a modest but significant reduction in GP33 tetramer–positive CD8 T cells (Fig. 2G); however, no defect in cytokine production was observed in virus-specific CD8 T cells (Fig. 2 H and I). Interestingly, we observed a significant reduction in the numbers of virus-specific IFN-γ–producing CD4 T cells at day 9 p.i. (Fig. 2J). Deleterious effects of MB1-Cre deletion during persistent LCMV infection were ruled out as MB1-Cre+/− mice did not display defects in T cells and were able to control persistent LCMV infection as efficiently as WT mice (SI Appendix, Fig. S2 F–I).
Fig. 2.
IL-27–producing B cells promote control of persistent LCMV infection. IL-27p28flox/flox (WT) and MB1-Cre/IL-27p28flox/flox (B cell–specific IL-27p28 KO) mice were infected with 2 × 106 PFU LCMV Cl-13. (A) Serum viral loads were determined throughout infection. (B–F) At day 130 p.i., viral loads were measured in (B) lung, (C) spleen, (D) kidney, (E) brain, and (F) liver. (G–J) At day 9 p.i., splenocytes were analyzed by flow cytometry to determine the total number of (G) H2-Db GP33–41 virus-specific CD8 T cells, (H) IFN-γ–producing GP33–41 virus-specific CD8 T cells, (I) polyfunctional IFN-γ– and TNF-α–producing GP33–41 virus-specific CD8 T cells, and (J) IFN-γ–producing GP61–80 virus-specific CD4 T cells. (K–N) At day 40 p.i., splenocytes were analyzed by flow cytometry to determine the number of (K) I-Ab GP67–77+ CD4 T cells, (L) IFN-γ+ CD4 T cells after GP61–80 peptide stimulation, (M) Tfh cells, and (N) IL-21+IFN-γ+ Tfh cells after PMA and ionomycin stimulation. Data are representative of three experimental replicates and error bars represent mean ± SD from four or five mice per group. Statistical analyses of experimental groups were performed using the Mann–Whitney U test (A–F) or Student’s two-tailed t test (G–N); not significant (ns), P > 0.05; *P ≤ 0.05, **P ≤ 0.01.
Since LCMV Cl-13 infection persists for several weeks and is associated with dysfunctional exhausted CD8 T cells and differentiation bias of CD4 T cells toward Tfh cells (17), we wondered whether B cell–derived IL-27 affects T cell responses at later stages of infection. At day 40 p.i., we observed that LCMV-infected MB1-Cre+/−/IL-27p28f/f displayed fewer virus-specific I-Ab GP67–77 tetramer+ CD4 T cells and IFN-γ–producing CD4 T cells compared with IL-27p28f/f littermate controls, suggesting that B cell–derived IL-27 is essential for the maintenance of both the expansion and function of virus-specific CD4 T cells during chronic LCMV infection (Fig. 2 K and L). In addition to regulating survival and functions of virus-specific CD4 T cells, IL-27 has been shown to play an important role in supporting Tfh functions during ovalbumin priming (28). Recent findings also indicated that Tfh cells are essential for the generation of LCMV-specific antibodies that drive clearance of persistent LCMV infection (7, 16). Therefore, we investigated the impact of B cell–derived IL-27 on the numbers and function of Tfh cells during the chronic phase of LCMV infection. At day 40 p.i., MB1-Cre+/−/IL-27p28f/f displayed fewer Tfh cells compared with IL-27p28f/f littermate controls (Fig. 2M). We stimulated splenocytes derived from B cell–specific IL-27p28 knockout (KO) mice and littermate controls ex vivo with either phorbol myristate acetate (PMA) and ionomycin or LCMV GP61–80 peptide and observed KO Tfh cells produced markedly fewer IFN-γ+IL-21+ compared with WT cells (Fig. 2N and SI Appendix, Fig. S2J). Moreover, mice lacking IL-27p28 in B cells displayed similar levels of LCMV-specific IgG1 but reduced IgG2a/2c and neutralizing antibodies (SI Appendix, Fig. S2 K–M), which have been previously demonstrated to promote control of Cl-13 infection (16, 29, 30). These results jointly suggest that B cell–derived IL-27 is required for the maintenance of Tfh function, antibody production, and clearance of a persistent virus but also that IL-27 produced by other cells is insufficient to compensate for loss of the B cell–derived IL-27 pool.
T Cell–Intrinsic IL-27 Signaling Is Required to Control Persistent LCMV Infection.
To address mechanistically how B cell–derived IL-27 facilitates host defense against persistent viral infection, we next investigated the cellular populations that require IL-27 signaling to promote viral control. We first investigated the possibility that B cell–intrinsic IL-27R signaling may be required to control the virus. To test this, IL-27raf/f mice were crossed with MB1-Cre mice (MB1-Cre+/−/IL-27raf/f) to generate conditional KO mice with B cell–specific IL-27ra deletion before infecting them with LCMV Cl-13. We assessed serum viral titers from day 9 until day 120 p.i. and observed that mice deficient in IL-27ra specifically in B cells were able to control Cl-13 infection similar to littermate controls (SI Appendix, Fig. S3 A–C), suggesting that B cell–intrinsic IL-27R signaling is not required for the control of persistent LCMV infection. Besides controlling virus as effectively as IL-27raf/f (WT) controls, mice lacking IL-27ra in B cells displayed normal LCMV-specific IgG2a/2c production (SI Appendix, Fig. S3D). In addition, B cell–specific deletion of IL-27ra did not alter numbers of germinal center (GC) B cells, virus-specific CD4 T cells, and virus-specific CD8 T cells at day 9 p.i. (SI Appendix, Fig. S1 E–G).
Since CD8 T cells are essential effectors that drive the antiviral immune response during chronic viral infection, we next tested whether IL-27 signaling in effector CD8 T cells was required for protection against persistent LCMV infection. We generated conditional KO mice where IL-27ra was deleted in granzyme B–expressing cells (including effector CD8 T cells and natural killer cells) by crossing IL-27raf/f mice to granzyme B Cre mice (GZMB-Cre+/−/IL-27raf/f). Following LCMV Cl-13 infection, GZMB-Cre+/−/IL-27raf/f controlled viral loads as efficiently as GZMB-Cre− littermate controls (SI Appendix, Fig. S3 H–J). Moreover, we did not observe defects in antiviral T cell responses nor was there a difference in virus-specific IgG2a/2c production (SI Appendix, Fig. S3K). Numbers of virus-specific CD8 T cells, IFN-γ–producing CD8 T cells, and IFN-γ–producing CD4 T cells were not affected by the lack of IL-27ra in granzyme B–expressing cells (SI Appendix, Fig. S3 L–N). Together, these results indicate that IL-27R signaling in granzyme B–expressing cells is not required for control of persistent LCMV infection.
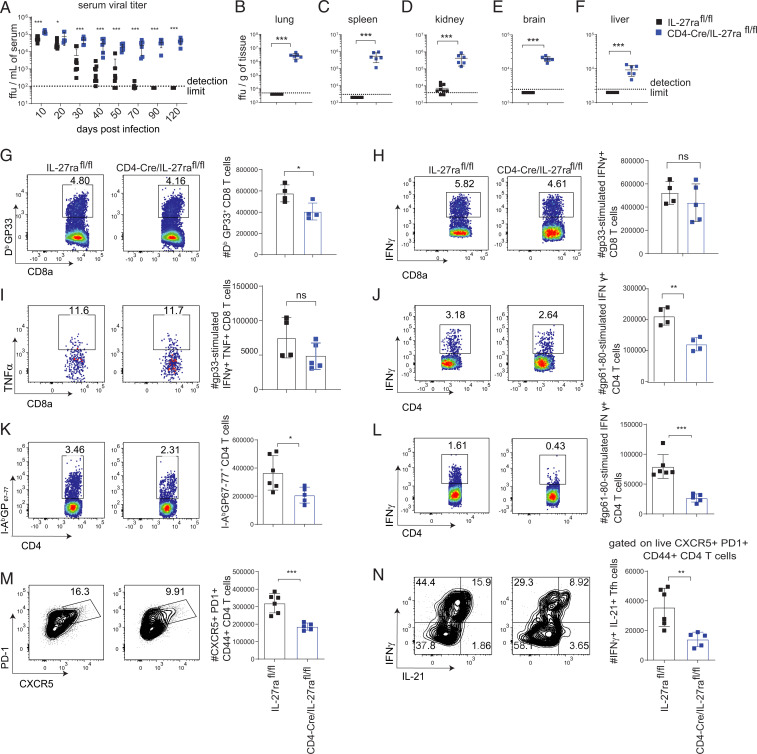
Since our findings indicated that both B cell– and effector CD8 T cell–specific IL-27R signaling are not required to control LCMV viremia, we further tested whether IL-27R signaling in all T cells was required for protection against persistent LCMV infection. By crossing IL-27raf/f mice with CD4-Cre mice (CD4-Cre+/−/IL-27raf/f), we generated conditional KO mice with IL-27ra deletion in both CD4 and CD8 T cells. Deletion efficiency and specificity were verified by flow cytometry analyses of splenocytes at days 9 and 40 p.i. Our findings revealed that IL-27ra expression was specifically reduced in CD8 and CD4 T cells in CD4-Cre/IL-27raflox/flox mice at both time points when B cells, DCs, and macrophages displayed WT levels of IL-27Ra (SI Appendix, Fig. S3 O and P). Notably, CD4-Cre+/−/IL-27raf/f mice were unable to clear LCMV Cl-13 from serum at various times p.i. and lung, spleen, kidney, brain, and liver at 120 d p.i. (Fig. 3 A–F). Analysis of antiviral T cell responses revealed a small but significant reduction in GP33 tetramer–positive CD8 T cells (Fig. 3G); however, no significant reductions in cytokine production were observed in GP33-specific CD8 T cells (Fig. 3 H and I). Analysis of antiviral CD4 T cells revealed significant reductions in IFN-γ–producing CD4 T cells at day 9 p.i. (Fig. 3J). In accordance with MB1-Cre/IL-27p28f/f mice, CD4-Cre/IL-27raf/f mice displayed reduced virus-specific I-Ab GP67–77+ CD4 T cells, IFN-γ–producing CD4 T cells, Tfh cells, and IFN-γ+ IL-21+ Tfh cells at day 40 p.i. (Fig. 3 K–N and SI Appendix, Fig. S3Q). Taken together, these data suggest that IL-27p28 is produced by B cells and signals on CD4 T cells to promote expansion and functional responses of virus-specific CD4 T cells and Tfh cell differentiation and function at later times post-LCMV infection that result in control of persistent LCMV infection. In line with the observed defects in Tfh responses, mice lacking IL-27ra in T cells also displayed reduced LCMV-specific IgG2a/2c but not IgG1 titers (SI Appendix, Fig. S3 R and S).
Fig. 3.
T cell–intrinsic IL-27 signaling mediates control of persistent LCMV infection. IL-27raflox/flox (WT) and CD4-Cre/IL-27raflox/flox mice (T cell–specific IL-27ra KO) were infected with 2 × 106 PFU LCMV Cl-13. (A) Serum viral loads were determined throughout infection. (B–F) At day 120 p.i., viral loads were measured in (B) lung, (C) spleen, (D) kidney, (E) brain, and (F) liver. (G–J) At day 9 p.i., splenocytes were analyzed by flow cytometry to determine the total number of (G) H2-Db GP33–41 virus-specific CD8 T cells, (H) IFN-γ–producing GP33–41 LCMV-specific CD8 T cells, (I) polyfunctional IFN-γ– and TNF-α–producing GP33–41 LCMV-specific CD8 T cells, and (J) IFN-γ–producing GP61–80 LCMV-specific CD4 T cells. (K–N) At day 40 p.i., splenocytes were analyzed by flow cytometry to determine the number of (K) I-Ab GP67–77+ CD4 T cells, (L) IFN-γ+–producing CD4 T cells after GP67–80 peptide stimulation, (M) Tfh cells, and (N) IL-21+IFN-γ+ Tfh cells after PMA and ionomycin stimulation. Data are representative of three experimental replicates and error bars represent mean ± SD from 4 to 10 mice per group. Statistical analyses of experimental groups were performed using the Mann–Whitney U test (A–F) or Student’s two-tailed t test (G–N); not significant (ns), P > 0.05; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
IFN-I Blockade Requires IL-27 Signaling for Enhanced Control of Persistent LCMV Infection.
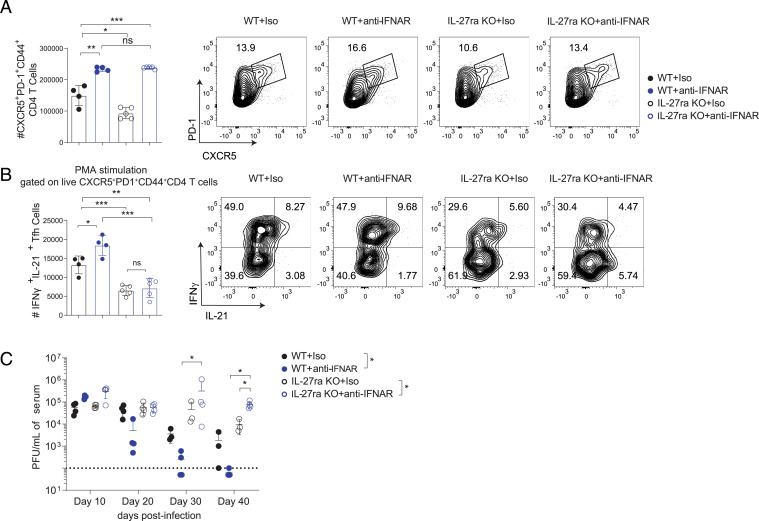
Our group recently showed that blockade of IFN-I signaling led to increased numbers of Tfh cells in both WT and IL-27ra KO mice during persistent LCMV infection (6–8, 31), and thus we further tested whether anti-IFNAR1 immunotherapy is able to improve functions of CD4 T cells and promote viral clearance. We first assessed Tfh numbers and function following anti-IFNAR1 antibody treatment at day 40 p.i. IL-27ra KO mice treated with anti-IFNAR1 displayed similar numbers of Tfh cells to WT controls that received anti-IFNAR1 antibody (Fig. 4A). However, we observed significantly fewer IFN-γ+IL-21+ Tfh cells in IL-27ra KO mice even after anti-IFNAR1 blockade (Fig. 4B and SI Appendix, Fig. S4). Consequently, unlike anti-IFNAR1 blockade in WT mice which leads to hastened control of Cl-13 infection (Fig. 4C) (6, 8), anti-IFNAR1–treated IL-27ra KO mice were unable to control the virus and even displayed elevated serum Cl-13 titers compared with isotype-treated animals at day 40 p.i. (Fig. 4C). Taken together, these findings emphasize the importance of IL-27 for maintaining and enhancing the function of Tfh cells during later stages of LCMV infection.
Fig. 4.
IL-27R signaling is required for anti-IFNAR immunotherapy to promote IL-21 production by Tfh cells and viral clearance. C57BL/6 WT and IL-27ra KO mice were treated with isotype control or anti-IFNAR1 antibody 1 d prior to LCMV Cl-13. (A and B) At day 40 p.i., spleens were analyzed by flow cytometry to determine the number of (A) Tfh cells and (B) IL-21+IFN-γ+ Tfh cells after PMA and ionomycin stimulation. (C) Serum viral loads were measured at different time points. Data are representative of three experimental replicates and error bars represent mean ± SD from four or five mice per group. For A and B, statistical comparisons of experimental groups were performed using Student’s two-tailed t test; for C, experimental groups were compared by two-way ANOVA (group-level) and Mann–Whitney U test (specific time points); not significant (ns), P > 0.05; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Persistent LCMV infection is maintained for several months while the presence and optimal functions of both virus-specific T and B cells are required to purge the infection. The role of Tfh cells in driving production of LCMV-specific antibodies required for viral clearance has recently been established (16). IL-27 is an immunostimulatory cytokine that drives expansion of virus-specific stem-like CXCR5+Tim3− CD8 T cells, accumulation of virus-specific CD4 T cells, and promotion of IgG2a/2c class switch (18, 31, 32). Given that IL-27 can influence multiple cell types to drive viral clearance, it is crucial to understand how cell-specific IL-27 signaling mediates control of persistent viral infection.
In this study, we identified B cells as an essential source of IL-27 and provide insights into how B cell–derived IL-27 drives antiviral immunity and antibody response by regulating virus-specific CD4 T cells and Tfh cell functions during late stages of persistent viral infection. During persistent LCMV infection, B cell–derived IL-27 promotes the accumulation and function of both virus-specific CD4 T cells and Tfh cells. Importantly, our data suggest that IL-27R signaling on Tfh cells drives IFN-γ and IL-21 production at later stages of chronic viral infection, which in turn results in generation of antiviral antibodies that keep the viral titers in check during the phase of antiviral CD8 T cell exhaustion. Intriguingly, although myeloid cells comprise the majority of IL-27p28+ splenocytes during Cl-13 infection, they are unable to compensate for the loss of B cell–derived IL-27, suggesting a possible requirement for IL-27 specifically in areas where B cell–T cell interactions occur. Given that IL-27p28 can be secreted without EBI3 and modulate immune responses (26), we cannot definitively conclude that IL-27 (p28 + EBI3) is the active cytokine required. However, we find that activated B cells also produce significant levels of EBI3 (SI Appendix, Fig. S1F), suggesting that IL-27 is the active cytokine in our system.
Given there are multiple sources of IL-27 during persistent LCMV infection, we speculate that the spatial-temporal production of IL-27 serves distinct nonredundant roles. Early IL-27 during persistent LCMV infection, which is produced mainly by DCs and myeloid cells, was shown to signal on both innate and adaptive immune cells to mediate early viral containment as IL-27ra KO mice showed higher viral titer than WT mice at day 9 p.i. (19). In contrast, B cell–derived IL-27 did not play a role in early viral containment (Fig. 2A), but instead we observed reduction of Tfh and Tfh-derived IL-21 and IFN-γ in the absence of B cell–derived IL-27 at later times p.i. (Fig. 2N and SI Appendix, Fig. S2J). Given that stable cognate interactions between T and B cells are required for Tfh to drive differentiation of GC B cells into plasma and memory B cells (33, 34), our finding suggests that while cognate Tfh provides IL-21 to support B cell differentiation, local B cell–produced IL-27 maintains Tfh functions.
Overall, we demonstrate the ability of B cells to produce IL-27p28, which orchestrates a variety of important T cell functions that lead to optimal antiviral antibody responses during persistent LCMV infection. Given these results, we hypothesize that B cell–derived IL-27 may play a key role in regulating immunity in other diseases where T cells are important for pathogenesis. Intriguingly, multiple recent studies have shown that B cells in human tumors’ tertiary lymphoid structures are associated with a better response to immunotherapy (35–37). Taken together, our study adds to the list of essential cytokines produced by B cells (38) and further underscores the importance of studying B cells and their cytokine production in promoting control of persistent viral infections and possibly cancer.
Materials and Methods
Mice.
C57BL/6 WT, conditional KO, and germline KO mice were used for this study. IL-27p28-eGFP mice were generated by Ross Kedl, University of Colorado Anschutz, Aurora, CO, and generously gifted to us by Ross Kedl and Zhenming Xu, University of Texas at San Antonio, San Antonio, TX (39). IL-27p28flox/flox were kindly provided by Li-Fan Lu, University of California San Diego, La Jolla, CA (40). The generation and characterization of IL-27raflox/flox was described previously (41). CD45.1+ GP66–77 T cell receptor tg (SMARTA), Ebi3−/−, WSX-1−/−, and Cre-expressing lines were purchased from The Jackson Laboratory. To obtain Il27ra−/− SMARTA CD45.1+ mice, WSX-1−/− mice were crossed to CD45.1+ SMARTA mice. To generate conditional deletion of IL-27p28flox/flox and IL-27raflox/flox, mice were crossed to Cre-expressing lines obtained from The Jackson Laboratory: Mb1-Cre to target B cells, granzyme B-Cre to target activated T cells, and CD4-Cre to target T cells. All mice were bred and maintained under specific pathogen-free conditions.
In Vivo LCMV Infection.
Seven- to 12-wk-old mice were injected intravenously with 2 × 106 focus-forming units (FFUs) of LCMV Cl-13. Viral titers were assessed by focus assays using Vero cell monolayers as previously described (7, 42). All experiments were conducted in accordance with guidelines and approval of the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Antibody Treatments.
For blockade of IFN-I, mice were treated with 1 mg of anti-IFNAR1 antibody intraperitoneally (clone MAR1-5A3; Leinco Technologies) or a mouse IgG1 isotype control (clone MOPC21; Leinco Technologies) 1 d prior to infection.
B Cell Isolation, Stimulation, and Cytokine Production Measurement.
Splenic B cells were purified from naïve mice using an EasySep Mouse B Cell Isolation Kit (Stemcell Technologies). Isolated B cells were cultured at 1 × 105 cells per well in a 96-well plate in complete RPMI containing lipopolysaccharide (3 mg/mL; Invivogen) for 24 h before being stimulated with agonistic anti-CD40 antibody (5 mg/mL; BioLegend) and IL-21 (50 ng/mL; R&D Systems). After 24 h poststimulation, B cells were harvested, stained with surface markers and viability dye (eBioscience), and analyzed using flow cytometry.
Cell Staining for Flow Cytometry.
For surface staining, antibodies for cell-surface markers were added to single-cell suspensions prepared from spleens at dilution 1:200 in phosphate-buffered saline (PBS) supplemented with 2% fetal bovine serum (FBS) and 1 mM ethylenediaminetetraacetate, followed by incubation for 30 min at 4 °C. Staining of CXCR5 of Tfh was performed as previously described (7). Staining of CXCR5+CD8+ T cells was performed as previously described (31). Tetramer staining was performed as previously described using major histocompatibility complex (MHC) tetramers provided by the NIH (8). Live/Dead Fixable Dead Cell Stain (Invitrogen) was used to identify live cells. Cells were fixed with 4% paraformaldehyde. Flow cytometry analysis was performed using a BD LSR II (Becton Dickinson) and data were analyzed using FlowJo (Tree Star).
Ex Vivo Cell Stimulation and Intracellular Cytokine Staining.
Splenocytes were stimulated with MHC class I–restricted LCMV-GP33–41 (2 μg/mL) or MHC class II–restricted LCMV-GP61–80 peptide (5 μg/mL) for 1 h in the absence of brefeldin A and then 5 h in the presence of brefeldin A (4 μg/mL; Sigma-Aldrich). Cells were fixed and permeabilized with 2% saponin, and intracellular staining was performed with antibodies to IFN-γ (XMG1.2), TNF-α (MP6-XT22), and IL-2 (JES6-5H4). For polyclonal stimulation, single-cell suspensions were stimulated with eBioscience Cell Stimulation Mixture according to the manufacturer’s protocol. Intracellular IL-21 staining was performed as previously described (18).
LCMV-Specific IgG Enzyme-Linked Immunosorbent Assay.
Serum antibody enzyme-linked immunosorbent assays were performed as previously described (7). Microplates were coated with baby hamster kidney (BHK) cell lysates infected with LCMV Cl-13. Serial dilutions of serum were carried out and antibody was detected by using purified biotin-conjugated anti-mouse IgG, IgG1, or IgG2a (1030-08, 1070-08, 1080-08; Southern Biotech) antibodies. A CLARIOstar Plus microplate reader was used to quantify the results.
LCMV Cl-13 Neutralization Assay.
LCMV neutralization in vitro was determined as previously described (42). Sera were prediluted 10-fold and inactivated at 56 °C for 30 min. Serial 2-fold dilutions of sera were incubated with 40 FFU LCMV Cl-13 at 37 °C for 60 min before being transferred to a 96-well plate containing Vero cells. Neutralization capacities were determined at 16 h postincubation as the relative reduction of infectious foci.
ELISpot Assay.
Nitrocellulose Millititer Multiscreen plates were coated with BHK cell lysates infected with LCMV Cl-13. Nonspecific binding was blocked with RPMI supplemented with 10% FBS and 1% penicillin and streptomycin. Serial 3-fold dilutions of IL-27p28-eGFP+ cells were carried out and plates were incubated overnight in a 37 °C incubator supplemented with 5% CO2. Plates were washed with PBS with 0.1% Tween (PBST) and then incubated with biotinylated anti-mouse IgGγ (Jackson ImmunoResearch) overnight at 4 °C. The plates were washed with PBST and incubated with horseradish peroxidase–conjugated avidin-D (Vector Laboratories) followed by incubation with a 3-amino-9-ethylcarbazole substrate. The plates were subsequently washed and air-dried in the dark and then spots were counted.
qPCR.
Total RNA extraction from sorted cells was performed using an RNeasy Plus Mini Kit (Qiagen; 74134). Isolated RNA was reverse-transcribed into complementary DNA (cDNA) using a QuantiTect Reverse Transcription Kit (Qiagen; 205311). cDNA quantification was performed using Fast SYBR Green Master Mix (Applied Biosystems; 4385612). The relative RNA levels were normalized to Gapdh. The following primers were used at 300 nM:
Il27p28 forward (F), 5′-CTGTTGCTGCTACCCTTGCTT-3′ and reverse (R), 5′-CACTCCTGGCAATCGAGATTC-3′
Gapdh F, 5′-TCCCACTCTTCCACCTTCGA-3′ and R, 5′-AGTTGGGATAGGGCCTCTCTT-3′.
Quantification and Statistical Analysis.
Data were analyzed using GraphPad Prism 9.0 software. R version 4.1.0 was used for RNA-seq analysis. ANOVA, Tukey’s posttest, Student’s t test, and Mann–Whitney U test were used to assess differences between experimental groups as indicated in the respective figure legends. Statistical significance is displayed as *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001.
Single-Cell RNA-Seq and Analysis.
Splenocytes from IL-27p28-eGFP reporter mice infected with Cl-13 for 1, 9, 25, and 30 d were harvested and live cells were sorted into IL-27p28-GFP+ and IL-27p28-GFP− fractions by flow cytometry, stained using TotalSeq-B cell hashing antibodies (BioLegend), and subjected to 3′ single-cell transcriptome library preparation (v3.1; 10X Genomics). Libraries were sequenced on a NextSeq 500 (Illumina) to an average depth of 8,504 reads per cell. Cell Ranger version 4.0.0 was used for cell demultiplexing, reads-per-gene counting, and hashing barcode counting. Seurat version 4.0.3 was used for cell filtering, sample demultiplexing, clustering, differential expression analysis, dimensionality reduction, and plotting (43). R version 4.1.0 was used for Seurat analysis and additional transcriptome analyses (44). Packages ggplot2 and ComplexHeatmap were used for additional plotting. To analyze the expression profile of differentially expressed genes in B cell subsets, bulk RNA-seq data from ImmGen (GSE109125) were used. Sequencing data have been deposited in the Gene Expression Omnibus (GEO) under accession no. GSE186898.
Supplementary Material
Acknowledgments
We thank The Scripps Research Institute Flow Cytometry and Genomics cores for experimental assistance and Dr. Ross Kedl (University of Colorado Anschutz) and Dr. Li-Fan Lu (University of California San Diego) for kindly sharing mouse strains. This work was supported by NIH Grants R01AI164744 and R01AI118862 (to J.R.T.). J.Z. is a recipient of a Cancer Research Institute/Irvington postdoctoral fellowship.
Footnotes
Reviewers: J.S., Mayo Clinic; and A.Z., University of Alabama at Birmingham.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2116741119/-/DCSupplemental.
Data Availability
The single-cell RNA-seq data reported in this article have been deposited in the GEO (accession no. GSE186898) (45).
References
- 1.Wherry E. J., T cell exhaustion. Nat. Immunol. 12, 492–499 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Zajac A. J., et al. , Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallimore A., Dumrese T., Hengartner H., Zinkernagel R. M., Rammensee H.-G., Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J. Exp. Med. 187, 1647–1657 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks D. G., Teyton L., Oldstone M. B., McGavern D. B., Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J. Virol. 79, 10514–10527 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergthaler A., et al. , Impaired antibody response causes persistence of prototypic T cell-contained virus. PLoS Biol. 7, e1000080 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson E. B., et al. , Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340, 202–207 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Z., et al. , IFNAR1 signaling in NK cells promotes persistent virus infection. Sci. Adv. 7, eabb8087 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teijaro J. R., et al. , Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340, 207–211 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber D. L., et al. , Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Brooks D. G., et al. , Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12, 1301–1309 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng C. T., et al. , Blockade of interferon beta, but not interferon alpha, signaling controls persistent viral infection. Cell Host Microbe 17, 653–661 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osokine I., et al. , Type I interferon suppresses de novo virus-specific CD4 Th1 immunity during an established persistent viral infection. Proc. Natl. Acad. Sci. U.S.A. 111, 7409–7414 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsaesser H., Sauer K., Brooks D. G., IL-21 is required to control chronic viral infection. Science 324, 1569–1572 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi J. S., Du M., Zajac A. J., A vital role for interleukin-21 in the control of a chronic viral infection. Science 324, 1572–1576 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fröhlich A., et al. , IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324, 1576–1580 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Greczmiel U., et al. , Sustained T follicular helper cell response is essential for control of chronic viral infection. Sci. Immunol. 2, eaam8686 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Fahey L. M., et al. , Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J. Exp. Med. 208, 987–999 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harker J. A., Dolgoter A., Zuniga E. I., Cell-intrinsic IL-27 and gp130 cytokine receptor signaling regulates virus-specific CD4+ T cell responses and viral control during chronic infection. Immunity 39, 548–559 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harker J. A., et al. , Interleukin-27R signaling mediates early viral containment and impacts innate and adaptive immunity after chronic lymphocytic choriomeningitis virus infection. J. Virol. 92, e02196-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirahara K., et al. , Asymmetric action of STAT transcription factors drives transcriptional outputs and cytokine specificity. Immunity 42, 877–889 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schultze J. L., et al. , Human non-germinal center B cell interleukin (IL)-12 production is primarily regulated by T cell signals CD40 ligand, interferon gamma, and IL-10: Role of B cells in the maintenance of T cell responses. J. Exp. Med. 189, 1–12 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arkatkar T., et al. , B cell–derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. J. Exp. Med. 214, 3207–3217 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen P., et al. , IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507, 366–370 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R.-X., et al. , Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med. 20, 633–641 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fillatreau S., Sweenie C. H., McGeachy M. J., Gray D., Anderton S. M., B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3, 944–950 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Stumhofer J. S., et al. , A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat. Immunol. 11, 1119–1126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harker J. A., Wong K. A., Dolgoter A., Zuniga E. I., Cell-intrinsic gp130 signaling on CD4+ T cells shapes long-lasting antiviral immunity. J. Immunol. 195, 1071–1081 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batten M., et al. , IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J. Exp. Med. 207, 2895–2906 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldridge J. R., McGraw T. S., Paoletti A., Buchmeier M. J., Antibody prevents the establishment of persistent arenavirus infection in synergy with endogenous T cells. J. Virol. 71, 755–758 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnett B. E., et al. , Cutting edge: B cell-intrinsic T-bet expression is required to control chronic viral infection. J. Immunol. 197, 1017–1022 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Z., et al. , IL-27 promotes the expansion of self-renewing CD8+ T cells in persistent viral infection. J. Exp. Med. 216, 1791–1808 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Q., et al. , Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature 407, 916–920 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Victora G. D., Nussenzweig M. C., Germinal centers. Annu. Rev. Immunol. 30, 429–457 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Ma C. S., Deenick E. K., Batten M., Tangye S. G., The origins, function, and regulation of T follicular helper cells. J. Exp. Med. 209, 1241–1253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cabrita R., et al. , Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Helmink B. A., et al. , B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petitprez F., et al. , B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Lund F. E., Cytokine-producing B lymphocytes—Key regulators of immunity. Curr. Opin. Immunol. 20, 332–338 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kilgore A. M., et al. , IL-27p28 production by XCR1+ dendritic cells and monocytes effectively predicts adjuvant-elicited CD8+ T cell responses. Immunohorizons 2, 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang S., et al. , High susceptibility to liver injury in IL-27 p28 conditional knockout mice involves intrinsic interferon-γ dysregulation of CD4+ T cells. Hepatology 57, 1620–1631 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Do J., et al. , Treg-specific IL-27Rα deletion uncovers a key role for IL-27 in Treg function to control autoimmunity. Proc. Natl. Acad. Sci. U.S.A. 114, 10190–10195 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Battegay M., et al. , Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods 33, 191–198 (1991). [DOI] [PubMed] [Google Scholar]
- 43.Hao Y., et al. , Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.R Development Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2021).
- 45.J. Zak, I. Pratumchai, J. R. Teijaro, IL-27 production by splenic immune cells throughout persistent LCMV infection. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE186898. Deposited 1 November 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The single-cell RNA-seq data reported in this article have been deposited in the GEO (accession no. GSE186898) (45).