Significance
Sweet taste neurons in both Drosophila and mice are often thought to be hardwired to promote appetitive responses and signal the presence of reward. Here, exploiting Drosophila females’ robust rejection of sucrose substrates over plain ones during egg-laying in one specific context, we discovered that Drosophila sweet neurons can be divided into at least two anatomically and functionally distinct groups that confer positive and negative values, respectively, to options during egg-laying. This discovery reveals one design feature of the Drosophila sweet taste system that allows sweetness/sugars to be valued differently according to context and animals’ behavioral goal (i.e., feeding versus egg-laying), pointing to a level of flexibility and sophistication that is not seen in the system’s mammalian counterparts.
Keywords: sweet neurons, egg-laying, labeled-line coding, functional division, Drosophila
Abstract
Sucrose is an attractive feeding substance and a positive reinforcer for Drosophila. But Drosophila females have been shown to robustly reject a sucrose-containing option for egg-laying when given a choice between a plain and a sucrose-containing option in specific contexts. How the sweet taste system of Drosophila promotes context-dependent devaluation of an egg-laying option that contains sucrose, an otherwise highly appetitive tastant, is unknown. Here, we report that devaluation of sweetness/sucrose for egg-laying is executed by a sensory pathway recruited specifically by the sweet neurons on the legs of Drosophila. First, silencing just the leg sweet neurons caused acceptance of the sucrose option in a sucrose versus plain decision, whereas expressing the channelrhodopsin CsChrimson in them caused rejection of a plain option that was “baited” with light over another that was not. Analogous bidirectional manipulations of other sweet neurons did not produce these effects. Second, circuit tracing revealed that the leg sweet neurons receive different presynaptic neuromodulations compared to some other sweet neurons and were the only ones with postsynaptic partners that projected prominently to the superior lateral protocerebrum (SLP) in the brain. Third, silencing one specific SLP-projecting postsynaptic partner of the leg sweet neurons reduced sucrose rejection, whereas expressing CsChrimson in it promoted rejection of a light-baited option during egg-laying. These results uncover that the Drosophila sweet taste system exhibits a functional division that is value-based and task-specific, challenging the conventional view that the system adheres to a simple labeled-line coding scheme.
The taste systems of many animal species are known to possess a dedicated “channel” for detecting sugars, a class of chemicals that is highly nutritious. For example, mice have been shown to encode gustatory receptors that specifically sense sugars, and the taste neurons that express these sugar receptors on their tongues generally do not express receptors that sense chemicals of another taste modality (e.g., bitterness) (1–3). Furthermore, activation of these sugar-sensing taste neurons by artificial means has been shown to be able to drive appetitive sugar-induced innate responses (e.g., licking) and act as a positive reinforcer for learning (3–5). In some recent studies, these properties of the sweet taste neurons have been found to be present in some of their central nervous system (CNS) targets (e.g., taste-sensitive neurons in the insular cortex), too (6, 7). Thus, one school of thought is that taste coding for sweetness in mice may follow the simple “labeled-line” rule: sweet taste neurons, and potentially some of their central targets, are hardwired to detect sugars specifically and drive sugar-induced reinforcing neural signals and appetitive behaviors (1–7).
Drosophila melanogaster also possess sugar-detecting taste neurons. Pioneering early studies have shown that sugar-sensing taste neurons in flies are molecularly, anatomically, and functionally distinct from taste neurons that sense bitterness; sweet-sensing and bitter-sensing taste neurons express different gustatory receptors, project axons to different areas in the brain, and are required to promote different (appetitive versus aversive) behaviors (8–12). Moreover, the activation of sweet neurons by artificial means can drive appetitive behaviors and act as a positive reinforcer for learning (10, 13, 14), while artificial activation of bitter-sensing neurons can induce rejection behaviors and be used as a punishment for learning (10, 13, 15). Interestingly, while these results suggest that Drosophila sweet neurons and their mammalian counterparts have some shared properties, subsequent studies suggest that significant differences exist between them, too. First, the Drosophila genome appears to encode many more sweet receptors than mouse genome does (12, 16–23). Second, Drosophila sweet neurons appear to be able to detect some chemicals that belong to another taste modality [e.g., acetic acid (AA)] (24–27). Third, Drosophila sweet neurons can be found on several body parts (e.g., proboscis and legs) (8, 12, 18, 20, 23, 28–30). Interestingly, sweet neurons on different body parts of Drosophila do not promote identical behavioral outputs (8, 20, 23, 24, 28, 29). For example, labellar sweet neurons and esophageal sweet neurons on the proboscis have been shown to promote proboscis extension reflex (PER) and ingestion, respectively, whereas leg sweet neurons have been shown to promote PER and slowing down of locomotion (8, 12, 28, 29). Collectively, these results suggest that in contrast to the apparent homogeneity of sweet neurons in some mammals, a functional division exists among Drosophila sweet neurons, although the different behavioral responses promoted by different Drosophila sweet neurons generally appear appetitive in nature.
In this work, we report yet another striking feature of Drosophila sweet neurons that sets them apart from their mammalian counterparts, namely a functional division that is value-based and task-specific. We discovered this by taking advantage of a context-dependent but highly robust sugar rejection behavior exhibited by egg-laying females (31–34). Previous studies have shown that when selecting for egg-laying site in a small enclosure (dimension ∼16 × 10 × 18 mm), Drosophila readily accept a sucrose-containing agarose for egg-laying when it is the sole option but strongly reject it when a plain option is also available (31, 32). Importantly, silencing their sweet neurons causes the females to no longer reject the sucrose option when choosing between the sucrose versus plain options (31, 32). Thus, in addition to promoting appetitive behaviors and acting as a positive reinforcer, activation of sweet neurons on an egg-laying option can also decrease the value of such an option (thereby causing its rejection over an option that does not activate sweet neurons). These observations not only suggest the existence of an apparent “antiappetitive” role of Drosophila sweet neurons when the task of animals is to select for egg-laying sites but also raise a key question as to whether such counterintuitive, value-decreasing property of sweetness detection during egg-laying may be 1) solely an emergent property of specific neurons in the brain that respond similarly to all peripheral sweet neurons but are sensitive to animals’ behavioral goal and context or 2) carried out by specific sweet neurons at the periphery and then transmitted into the brain via a unique neural pathway activated by these neurons. To disambiguate between these possibilities, we genetically targeted different subsets of sweet neurons to assess their circuit properties as well as their behavioral roles as the animals decided in either a regular or a virtual sweet versus plain decision during egg-laying, taking advantage of a high-throughput closed-loop optogenetic stimulation platform we developed recently. Our collective results support the second scenario and suggest that the value-decreasing property of sweetness/sucrose is conveyed specifically by the sweet neurons on the legs of Drosophila—and not by other sweet neurons—and the unique postsynaptic target(s) of the leg sweet neurons that send long-range projections to the superior lateral protocerebrum (SLP) in the brain. These results reveal a previously unappreciated functional and anatomical division of the Drosophila sweet taste neurons that is both task-specific and value-based, pointing to a level of complexity and sophistication that seems unmatched by their mammalian counterparts so far.
Results
Sweet Neurons at Different Locations Contribute Differentially to Devaluing the Sweet Option for Egg-laying.
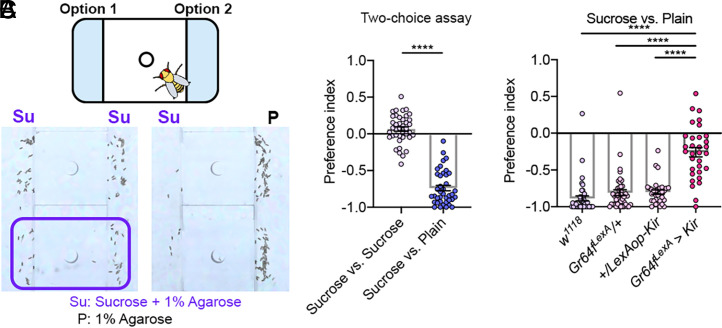
We first reconfirmed that Drosophila showed context-dependent sucrose rejection for egg-laying. Indeed, when tested in our high-throughput apparatus, wild-type (w1118) females accepted the sucrose-containing agarose when given two sucrose-containing agaroses but rejected the sucrose option when the other option was sucrose-free (Fig. 1 A and B) (31, 32). In addition, when we used either the Gr64f-GAL4 or the Gr64fLexA drivers (18, 35, 36) to silence virtually all their peripheral sweet neurons, the affected females no longer rejected the sweet agarose in the same sweet versus plain task (Figs. 1C and 2A). Thus, sweet neurons play a critical role in devaluing an agarose that contains sucrose in our decision task, thereby promoting its rejection over a plain agarose.
Fig. 1.
Drosophila rely on sweet neurons to reject the sucrose option in a sucrose versus plain task. (A) (Top) Arena for examining egg-laying preference of a single fly. The two substrates are separated by a plastic divider in the middle. (Bottom) Representative images showing preferences of two WT (w1118) females in the sucrose versus sucrose task and two in the sucrose versus plain task. Su: 1% agarose with 150 mM sucrose; P: 1% agarose. The rectangle denotes the approximate area for one arena (SI Appendix, Fig. S1G). Note that throughout this work, [sucrose] in the sweet substrate is 150 mM. (B) Egg-laying preference index (PI) of WT females in the sucrose versus sucrose and the sucrose versus plain tasks. Note that throughout the work, egg-laying PI in an option1 versus option2 task is calculated as follows: (no. of eggs on option1 − no. of eggs on option2)/(total no. of eggs). We calculated the PI only if the fly had laid ≥10 eggs. Unpaired t test with Welch’s correction; n = 40 and 39. (C) Egg-laying PI of females with their sweet neurons inhibited. Welch’s ANOVA test with Dunnett’s posttest; n = 31 to 44. Note that throughout this work, we use the following abbreviations: ns, P ≥ 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; and ω, mean is significantly different (P < 0.05) from zero in one-sample t test.
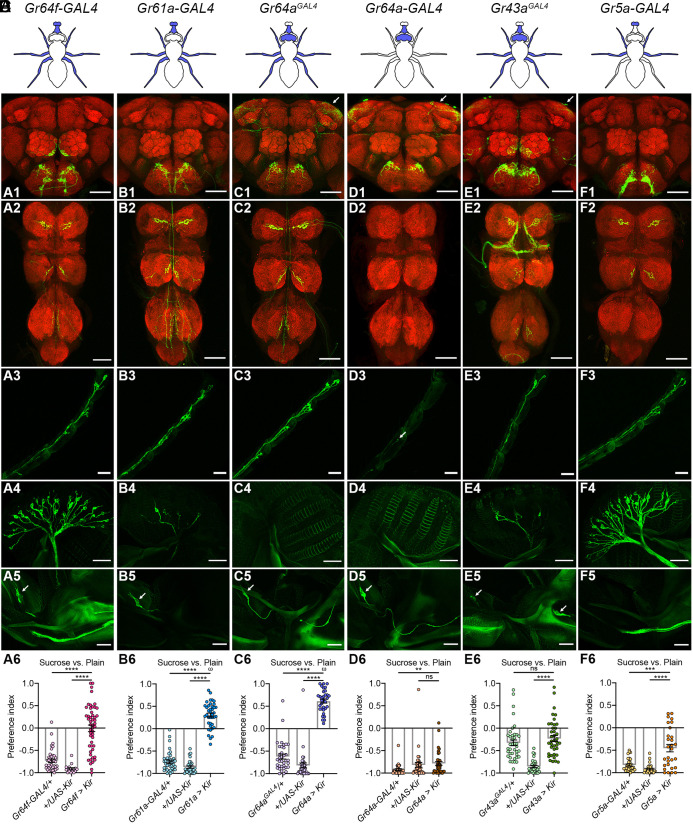
Fig. 2.
Inhibiting different subsets of sweet neurons differentially impacts sucrose rejection. (A–F) properties of different sweet Gr-GAL4s. (Top) Schematics indicating the cell body locations of sweet neurons (blue) labeled by different sweet Gr-GAL4s. (Middle) Expression of different sweet Gr-GAL4s. Top to bottom: in the brain (1), VNC (2), foreleg (3), labellum (4), and esophagus (5). (Bottom) Egg-laying PIs of females with different Gr-GAL4–labeled neurons silenced. Welch’s ANOVA test with Dunnett’s posttest, n = 16 to 50 (scale bars, 60 μm).
Previous studies have shown that cell bodies of sweet neurons can be found at different locations in a fly’s body such as its proboscis (i.e., the labellum and the esophagus), legs, and brain (8, 18, 20, 28, 30). To begin to assess whether sweet neurons at different locations contribute similarly to devaluing the sweet agarose for egg-laying, we collected several GAL4 drivers, each of which labeled a different combination of sweet neurons (Fig. 2; SI Appendix, Fig. S1 A–F). We then attempted to deduce the roles of different sweet neurons by correlating the expression patterns of these drivers with the phenotypes that they produced.
We found that inactivation of sweet neurons labeled by either Gr61a-GAL4 (36) or Gr64aGAL4 (18, 35) caused females to switch from rejecting to preferring the sweet agarose in the sweet versus plain task (Fig. 2 B and C; SI Appendix, Fig. S1G). Because these two GAL4s labeled the same sweet neurons on the legs and the LSO (labral sense organ, on the esophagus) but differed in their labeling of neurons at other locations (Fig. 2 B and C; SI Appendix, Fig. S1 B and C), these results suggest that sweet neurons on the legs, the LSO, or possibly both might be more critical for promoting the rejection of the sweet agarose. However, the importance of the LSO sweet neurons was called into question as inactivating these neurons by using either Gr43aGAL4 (20, 37) or Gr64a-GAL4 (36) (a driver that differs from the “knocked-in” Gr64aGAL4)—both of which labeled LSO neurons—did not significantly impact sweet rejection (Fig. 2 D and E). Lastly, we found that the labellar sweet neurons may not promote sweet rejection either, as while inactivating the Gr5a-GAL4–expressing neurons reduced sweet rejection, it did so to a lesser degree than inactivating the Gr64aGAL4 neurons (Fig. 2 C and F). Gr5a-GAL4 labeled many labellar sweet neurons (16) but fewer sweet neurons on the legs (and essentially no other sweet neurons) (Fig. 2F; SI Appendix, Fig. S1F). Thus, this result is more consistent with the view that the weaker rejection exhibited by the Gr5a-GAL4 > Kir2.1 animals may be because fewer leg sweet neurons of theirs were inactivated.
Taken together, these results suggest that sweet neurons at different locations do not contribute equally to promoting rejection of the sweet agarose in the sweet versus plain decision and that those on the legs likely play more significantly a role than the rest.
Sweet Neurons on the Legs Are Solely Necessary for Devaluing the Sweet Option for Egg-Laying.
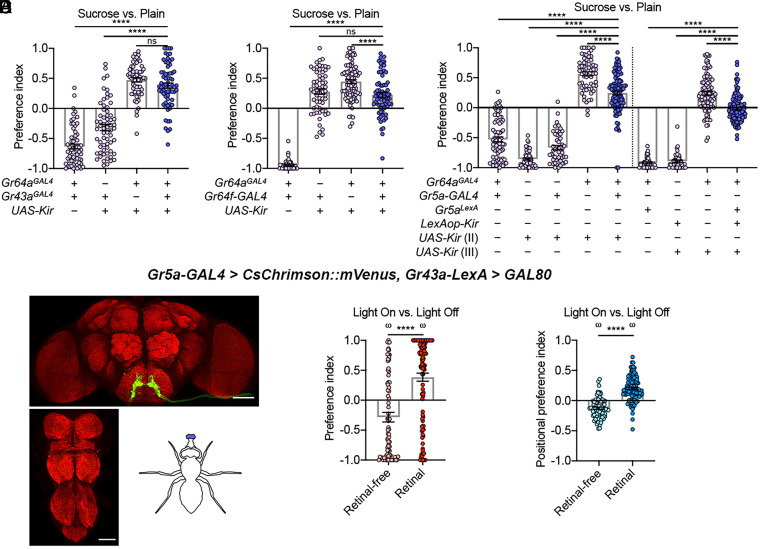
To confirm that the sweet neurons on the legs are essential for promoting sweet rejection in the sweet versus plain task, we next used three intersectional approaches to manipulate different subsets of the neurons labeled by Gr64aGAL4. Gr64aGAL4 labeled the leg sweet neurons strongly and a few sweet neurons on the LSO and in the brain and, importantly, produced a very strong lack-of-sweet-rejection phenotype when used to inactivate neurons (Fig. 2C; SI Appendix, Fig. S1 C and G). First, we sought corroboration that the inability of Gr64aGAL4 > Kir2.1 animals to reject sweet option was due to inactivation of their leg sweet neurons. To that end, we found a transgene combination, Gr43a-LexA>GAL80, that can block GAL4-dependent expression in the sweet neurons on the legs (and on the LSO), but not on the labellum (SI Appendix, Fig. S2 A and C; Fig. 6D). Introducing this combination into the Gr64aGAL4 > Kir2.1 animals reverted them from not rejecting the sweet agarose to clearly rejecting it again in the sweet versus plain decision (SI Appendix, Fig. S2B). Moreover, the same combination also reverted the Gr64f-GAL4 > Kir2.1 and the Gr5a-GAL4 > Kir2.1 animals from not rejecting to clearly rejecting the sweet option, too (SI Appendix, Fig. S2D). These results support that the leg, but not labellar, sweet neurons are essential for devaluing the sweet agarose.
Fig. 6.
Labellar sweet neurons promote sucrose acceptance during egg-laying. (A) Egg-laying PI of flies with their Gr64aGAL4-expressing neurons, Gr43aGAL4-expressing neurons, or both silenced in the sucrose versus plain task. Welch’s ANOVA test with Dunnett’s posttest; n = 60 to 67. (B) Egg-laying PI of flies with their Gr64aGAL4-expressing neurons, Gr64f-GAL4–expressing neurons, or both silenced in the sucrose versus plain task. Welch’s ANOVA test with Dunnett’s posttest; n = 42 to 92. (C) Egg-laying PI of flies with their Gr64aGAL4-expressing neurons, Gr5a-GAL4-expressing neurons, or both silenced in the sucrose versus plain task. Welch’s ANOVA test with Dunnett’s posttest; n = 40 to 130. (D) Gr5a-GAL4–dependent expression in the presence of Gr43a-LexA>GAL80. Note that GAL4-dependent expression in the legs is blocked. (E and F) Egg-laying and positional PIs of flies with CsChrimson expressed in only their labellar Gr5a-GAL4–expressing neurons in the light-on versus light-off task. Unpaired t test with Welch’s correction and one-sample t test against zero; n = 75 and 106 in E and n = 84 and 107 in F (scale bars, 60 μm).
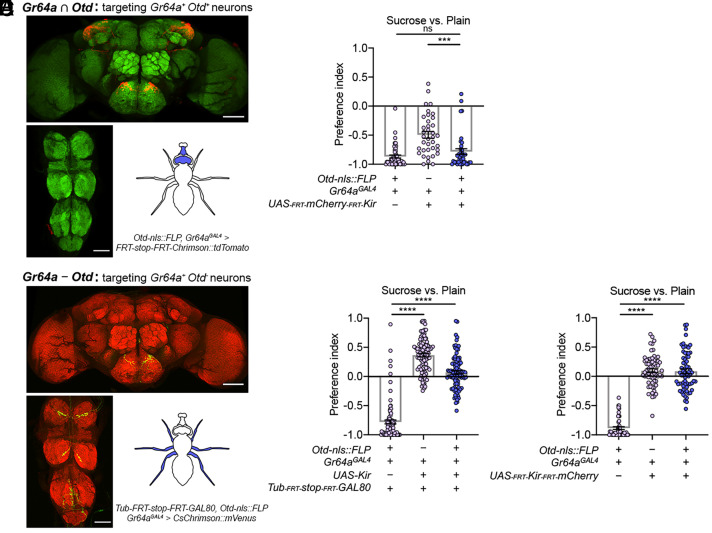
In our second approach, we directly ruled out a significant contribution from the LSO and the brain-intrinsic sweet neurons labeled by Gr64aGAL4. We created flies that contained the following transgenes: Otd-nls::FLP, UAS-FRT-mCherry-FRT-Kir2.1, and Gr64aGAL4. Because Otd-nls::FLP (38) is expressed in only the brain-intrinsic neurons and a few subsets of sensory neurons that project into the brain (e.g., LSO sweet neurons) but not in any sensory neurons on the legs, this transgene combination allowed Kir2.1 to be expressed in only the Gr64aGAL4-expressing sweet neurons in the brain and LSO (Fig. 3A). These animals clearly rejected the sweet agarose in the sweet versus plain task (Fig. 3B), in stark contrast to the lack of sweet rejection exhibited by the Gr64aGAL4 > Kir2.1 animals (Fig. 2C; SI Appendix, Fig. S1G), suggesting that neither the brain-intrinsic nor the LSO sweet neurons labeled by Gr64aGAL4 are essential for devaluing the sweet agarose.
Fig. 3.
Sweet neurons on the legs are solely necessary for sucrose rejection. (A) Images showing our intersection scheme restricted GAL4-dependent expression to Gr64aGAL4-expressing neurons in the brain and LSO only. (Lower right) Schematic highlighting the cell body locations of the intersected neurons. (B) Egg-laying PI of females whose Gr64aGAL4-labeled neurons in brain and LSO were selectively silenced. Welch’s ANOVA test with Dunnett’s posttest; n = 37 to 49. (C) Images showing our subtraction scheme restricted GAL4-dependent expression to Gr64aGAL4-expressing neurons on the legs only. (Lower right) Schematic highlighting the cell body locations of the neurons spared from the subtraction. (D and E) Egg-laying PI of females whose Gr64aGAL4-expressing sweet neurons on the legs were selectively silenced. Welch’s ANOVA test with Dunnett’s posttest; n = 50 to 96 (scale bars, 60 μm).
In our third approach, we restricted Kir2.1 to be expressed in only the Gr64aGAL4-expressing sweet neurons on the legs by putting Otd-nls::FLP together with Tub-FRT-stop-FRT-GAL80, UAS-Kir2.1, and Gr64aGAL4 (Fig. 3C). These animals no longer rejected the sweet agarose for egg-laying (Fig. 3D), directly demonstrating the requirement of the leg sweet neurons in promoting sweet rejection in the sweet versus plain decision task. We obtained a similar result when we used a different transgene combination to inhibit just the Gr64aGAL4-expressing neurons on the legs (Fig. 3E).
Thus, results from these intersectional approaches strongly suggest that sweet neurons on the legs, but not in other locations, are solely necessary for females to devalue a sucrose-containing agarose in our sweet versus plain task.
A Closed-Loop Platform to Examine the Impact of Artificial Stimulation of Sweet Neurons During Egg-Laying.
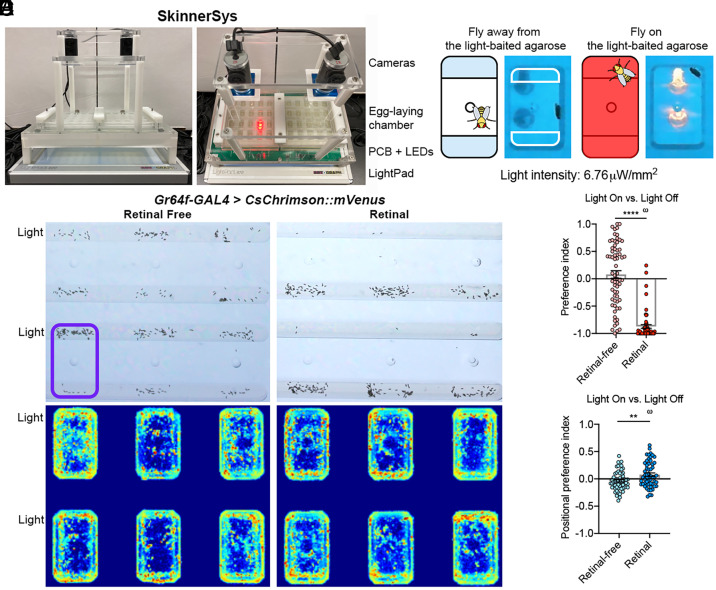
Next, we wished to determine whether artificial activation of the leg sweet neurons on a plain agarose is sufficient to decrease its value for egg-laying. One approach is to express the channelrhodopsin CsChrimson (39) in the leg sweet neurons and assess how the flies would choose between two plain agaroses, one of which is “baited” with light. We designed and built SkinnerSys, a high-throughput platform that can illuminate flies in closed loop during egg-laying (Fig. 4 A and B; Movies S1 and S2). Briefly, SkinnerSys consists of three major components: 1) SkinnerTrax, code we previously developed for real-time tracking and delivering light to multiple individual animals in closed loop (15, 40); 2) an apparatus that contains 40 individual two-choice arenas; and 3) a custom printed circuit board (PCB) that allows LED illumination of different arenas to be independently controlled (Fig. 4A). Thus, SkinnerSys can assay in a highly parallel manner how individual females respond to activation of specific neurons of interest as they explore different options for egg-laying. Importantly, because SkinnerSys can deliver light pulses to animals according to their position in real time, we can control optogenetic activation of neurons of interest with desired spatial precision (Fig. 4B; Movies S1 and S2).
Fig. 4.
A closed-loop platform for stimulating neurons in a two-choice egg-laying task. (A) SkinnerSys, a high-throughput platform for tracking and illuminating flies in closed loop. See Movies S1 and S2 for “live action.” (B) SkinnerSys-administered light-on versus light-off task. In this task, only the top agarose is “baited with light”: Light is off when the fly is away from it (Left), but light is on when the fly is contacting it (Right). The agarose strips are separated by a plastic divider. (C) Representative images of egg-laying and positional preferences of retinal-fed and retinal-free Gr64f-GAL4>CsChrimson flies in the light-on versus light-off task. The purple rectangle denotes the approximate area for a single arena. Note that for all the representative images collected by SkinnerSys in this work, egg-laying pictures and positional heatmaps are matched. Warmer colors in the heatmaps indicate more time spent in the corresponding locations. (D) Egg-laying PI for light of Gr64f-GAL4>CsChrimson flies in the light-on versus light-off task. Unpaired t test with Welch’s correction and one-sample t test against zero; n = 67 and 59. (E) Positional PI of Gr64f-GAL4>CsChrimson flies in the light-on versus light-off task. Unpaired t test with Welch’s correction and one-sample t test against zero; n = 58 and 56. Positional PI for light is calculated as follows: (time spent on light-on side − time spent on light-off side)/(time spent on both), with the arena midline split into two sides.
As a proof-of-concept experiment, we first used Gr64f-GAL4 to express CsChrimson in all peripheral sweet neurons and examined how the females would choose when given a plain agarose on which light is consistently off versus a plain agarose on which light is turned on only when the fly is on the agarose (Fig. 4B). We found that Gr64f-GAL4 > CsChrimson animals not fed with retinal showed no clear biases for either option (Fig. 4 C and D). In contrast, retinal-fed animals robustly rejected the light-on agarose over the light-off agarose (Fig. 4 C and D). This is a demonstration that artificial activation of sweet neurons on a plain option was sufficient to drive its rejection for egg-laying over another plain option on which such activation did not occur. Positional heatmaps revealed that retinal-fed Gr64f-GAL4 > CsChrimson females had a slight but statistically significant positional preference for the light-on substrate (Fig. 4 C and E), suggesting that while activation of all sweet neurons on an option can decrease its value for egg-laying, it still confers a mild appetitive quality to flies when they are not laying eggs.
Activation of the Leg Sweet Neurons Is Sufficient to Decrease the Value of a Plain Option for Egg-Laying.
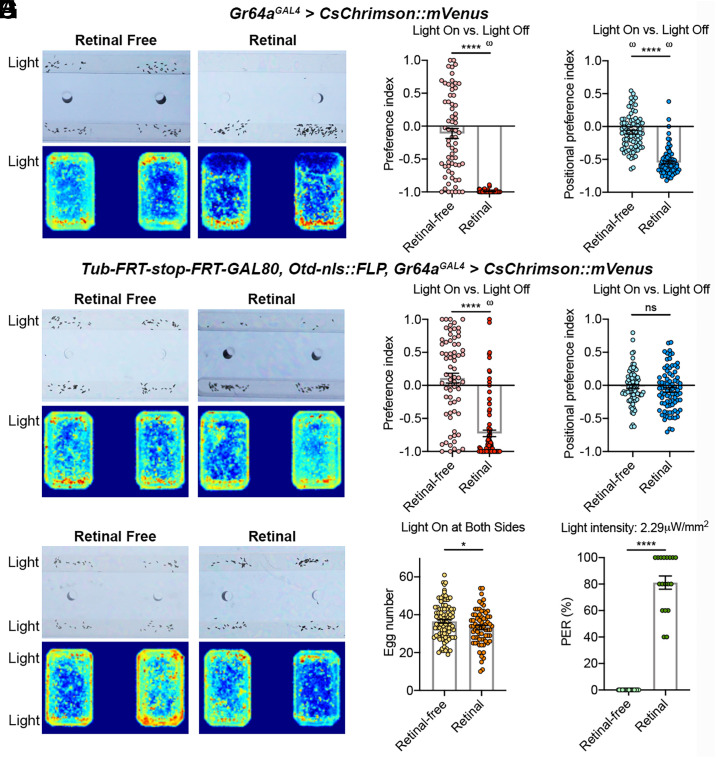
To test whether optogenetic activation of the leg sweet neurons on a plain option can cause the animal to devalue it for egg-laying, we first stimulated Gr64aGAL4-expressing neurons in closed loop. As described earlier, Gr64aGAL4 labeled sweet neurons on the legs strongly, as well some LSO and brain-intrinsic sweet neurons, but no labellar sweet neurons (Fig. 2C; SI Appendix, Fig. S1C). Similar to Gr64f-GAL4 > CsChrimson females, retinal-fed Gr64aGAL4 > CsChrimson females strongly rejected the light-baited plain agarose in the light-on versus light-off task (Fig. 5 A and B). Curiously, however, positional heatmaps showed that these animals preferred to spend time away from the light-on agarose, too (Fig. 5 A and C).
Fig. 5.
Artificial activation of the leg sweet neurons on a plain option decreases its value for egg-laying. (A) Representative egg-laying preferences and positional heatmaps of Gr64aGAL4>CsChrimson flies in the light-on versus light-off task. (B and C) Egg-laying and positional PIs of Gr64aGAL4>CsChrimson flies in the light-on versus light-off task. Unpaired t test with Welch’s correction and one-sample t test against zero; n = 67 and 69 in B and n = 80 and 79 in C. (D) Representative egg-laying preferences and positional heatmaps of flies that expressed CsChrimson in only their Gr64aGAL4-labeled leg neurons in the light-on versus light-off task. (E and F) Egg-laying and positional PIs of flies described in D in the light-on versus light-off task. Unpaired t test with Welch’s correction and one-sample t test against zero; n = 70 and 88 in E and n = 83 and 84 in F. (G) Representative egg-laying preferences and positional heatmaps of flies described in D in the light-on versus light-on task. (H) Number of eggs laid by flies described in D in the light-on versus light-on task. Unpaired t test with Welch’s correction; n = 93 and 71. (I) Light-induced PER of flies described in D. Unpaired t test with Welch’s correction; n = 20 and 18.
We next restricted CsChrimson to be expressed in only the leg sweet neurons by combining the following transgenes: Gr64aGAL4, UAS-CsChrimson, Otd-nls::FLP, and Tub-FRT-stop-FRT-GAL80 (Fig. 3C). When fed with retinal, these flies rejected the light-on option over the light-off one for egg-laying (Fig. 5 D and E), demonstrating the sufficiency of optogenetic activation of just the leg sweet neurons in promoting rejection of an option on which such activation occurs over an option on which it does not. Interestingly, these animals no longer avoided spending time on the illuminated option (Fig. 5 D and F). Further, males, virgins, and non–egg-laying mated females of the same genotype all showed similar positional indifference between the light-on versus the light-off options (SI Appendix, Fig. S3), so we observed devaluing of the light-baited option only in egg-laying females and only for egg-laying.
Next, we examined how females with CsChrimson expressed in only their leg sweet neurons behaved when both options were baited with light (Fig. 5 G and H). This experiment is important because optogenetic activation of the leg sweet neurons may simply shut down egg-laying as opposed to devaluing an option. We found that females readily laid eggs on both options in the light-on versus light-on task, even though the number of eggs laid by the retinal-fed flies was slightly lower (Fig. 5 G and H). This result suggests that activation of the leg sweet neurons does not inhibit egg-laying per se; instead, it causes the option on which activation occurs to be devalued. This result mirrors the observation that whereas WT females strongly rejected the sweet option for egg-laying in the sweet versus plain task, they readily accepted the sweet option in the sweet versus sweet task (Fig. 1 A and B). Lastly, we found that light induced PER from these animals effectively (Fig. 5I), consistent with previous findings that activation of the leg sweet neurons is capable of promoting an appetitive feeding response (8, 29).
Some of the Labellar Sweet Neurons Have a Role in Increasing the Value of the Sweet Agarose for Egg-Laying.
We have so far focused on assigning the “value-decreasing function” of sweet neurons during egg-laying to those on the legs. Here, we address the peculiar results that whereas inhibiting neurons using different pan–sweet-neuron drivers caused females to become indifferent between the sweet and plain options (Figs. 1C and 2A), inhibiting neurons using either Gr64aGAL4 or Gr61a-GAL4 caused preference for the sweet option over the plain one (Fig. 2 B and C; SI Appendix, Fig. S1G). These findings raise the intriguing possibility that some of the sweet neurons that are not labeled by these two GAL4 drivers (e.g., labellar and some pharyngeal sweet neurons) may act to increase the value of a sweet option over a plain one when activated; however, their impact on the sweet versus plain decision may normally be suppressed or obscured by the dominant value-decreasing function of the leg sweet neurons.
To test this idea, we first asked whether some of the pharyngeal neurons may be responsible for promoting the sweet preference exhibited by the Gr64aGAL4 > Kir2.1 flies. We simultaneously silenced Gr64aGAL4- and Gr43aGAL4-expressing neurons and found that these “double inhibition” animals still exhibited a preference for the sweet option (Fig. 6A). Because Gr43aGAL4 labeled virtually all pharyngeal sweet neurons (20, 37) (Fig. 2E), this result rules out a significant role of these neurons in promoting the peculiar sweet preference we observed. Next, we simultaneously silenced Gr64aGAL4- and Gr64f-GAL4–expressing neurons and found that the sweet preference of these animals reduced significantly (Fig. 6B), suggesting that the labellar sweet neurons may play a role in promoting sweet preference as they were clearly labeled by Gr64f-GAL4 (Fig. 2A). Indeed, simultaneously inhibiting Gr64aGAL4- and Gr5a-GAL4–expressing neurons or simultaneously inhibiting Gr64aGAL4- and Gr5aLexA-expressing neurons significantly reduced the sweet preference exhibited by the Gr64aGAL4 > Kir2.1 animals (Fig. 6C), too. Like Gr5a-GAL4, Gr5aLexA also labeled many labellar sweet neurons (18, 35).
Next, we asked whether animals may prefer a plain agarose on which their labellar sweet neurons are optogenetically activated. To restrict CsChrimson to be present only in their labellar sweet neurons, we again introduced Gr43a-LexA>GAL80, a transgene combination that suppressed GAL4-dependent expression in virtually all leg sweet neurons but spared the labellar ones, into Gr5a-GAL4 > CsChrimson animals (Fig. 6D). Interestingly, retinal-fed animals that carried all these transgenes indeed showed a statistically significant preference to lay eggs, as well as to spend time, on the illuminated option over the unilluminated one (Fig. 6 E and F).
Collectively, these results support the idea that activation of some of the labellar sweet neurons on an option increases its value for egg-laying, but such value-increasing function has little behavioral impact when the leg sweet neurons are activated on the same option. This result is consistent with the recent findings that some of the labellar sweet neurons can be activated by AA and promote egg-laying preference for AA in an AA versus plain task (24).
The Leg Sweet Neurons Receive Different Presynaptic Modulations from the Labellar Sweet Neurons.
Having found that the leg sweet neurons were uniquely critical for sweet rejection during egg-laying, we next explored whether information collected by the leg neurons might be processed differently from that collected by the rest of the sweet neurons. We first assess specific presynaptic modulations received by sweet neurons by using GFP Reconstitution Across Synaptic Partners (GRASP), a tool that can detect whether two groups of neurons have direct contacts as well as the directionality of such contacts (41, 42). Previous studies have reported that axons of sweet neurons labeled by Gr5a-LexA—a driver that labels both the labellar and the leg sweet neurons (29)—receive direct presynaptic inputs from the dopaminergic (DA), octopaminergic (OA), and GABAergic neurons in the subesophageal zone (SEZ) and that these inputs can modify sugar-activated feeding responses (43–46). We replicated these experiments by using Gr64f-GAL4 to label virtually all peripheral sweet neurons and indeed detected GRASP signals between axons of Gr64f-GAL4–expressing neurons and processes from the DA, OA, and GABAergic neurons in the SEZ (SI Appendix, Fig. S4).
In contrast, axons of the leg sweet neurons (labeled by Gr64aGAL4) did not appear to directly contact either the DA or the OA neurons (SI Appendix, Fig. S5), although they did form bidirectional contacts with the GABAergic neurons in the ventral nerve cords (VNCs) and possibly also the SEZ (SI Appendix, Fig. S5). These results suggest that the DA and the OA systems do not directly modulate the output of—nor do they receive any direct input from—the leg sweet neurons, revealing one difference in how information collected by the leg versus the labellar sweet neurons is processed at the first stage of information relay.
The Leg Sweet Neurons Have a Direct Postsynaptic Target That Is Not Shared by the Rest of the Sweet Neurons.
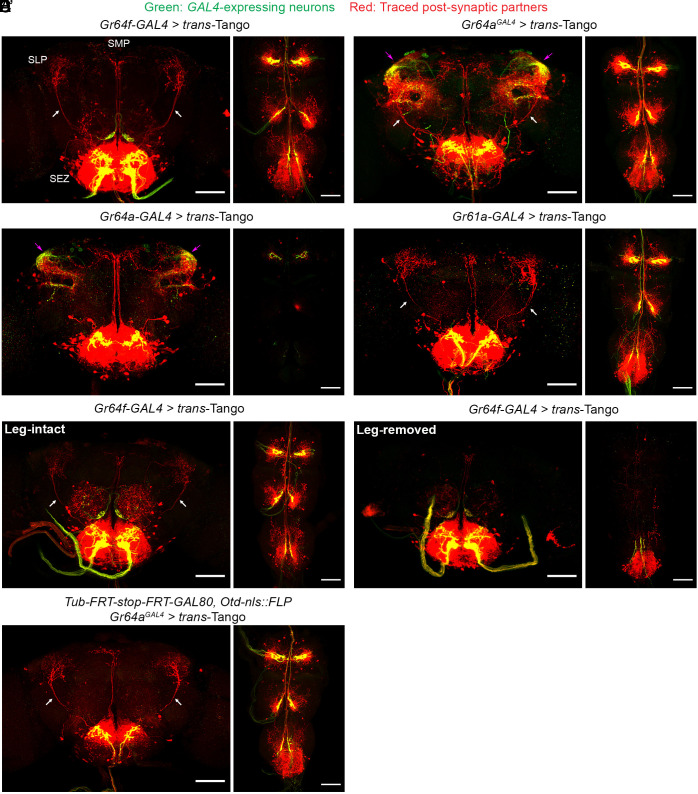
To examine whether information collected by the leg sweet neurons and the rest of the sweet neurons might be routed differently into higher brain areas, we next used trans-Tango to assess their postsynaptic targets. trans-Tango is a circuit-tracing technique that can label the direct postsynaptic targets of specific GAL4-expressing neurons of interest (47). In fact, the developers of trans-Tango were among the first to show that the Gr64f-GAL4–expressing sweet neurons have numerous direct postsynaptic partners in the brain (47). We replicated this experiment and found that trans-Tango tracing of Gr64f-GAL4 indeed labeled neurons that elaborated processes in the SEZ as well as neurons that projected to the superior medial protocerebrum (SMP) and the SLP in the brain (Fig. 7A).
Fig. 7.
Postsynaptic partners of the leg sweet neurons have a unique long-range projection in the brain. (A–D) Representative images showing sweet neurons labeled by different GAL4s (green) and their traced postsynaptic partners (red): (A) Gr64f-GAL4–expressing neurons and their partners, (B) Gr64aGAL4-expressing neurons and partners, (C) Gr64a-GAL4–expressing neurons and partners, (D) Gr61a-GAL4–expressing neurons and partners. Pink arrows: processes of brain-intrinsic sweet neurons not labeled by Gr64f-GAL4. White arrows: SLP-targeting projections. (E and E′) Representative images showing Gr64f-GAL4–expressing neurons and their traced postsynaptic partners in intact (E) and leg-amputated flies (E′). (F) Representative images showing specifically the Gr64aGAL4-expressing leg neurons and their partners (scale bars, 60 μm).
We then compared trans-Tango–traced targets of the Gr64aGAL4- versus Gr64a-GAL4–expressing neurons (Fig. 7 B and C). We chose these two GAL4s for comparison because while they labeled the same brain-intrinsic and LSO sweet neurons, only Gr64aGAL4 labeled the leg sweet neurons (Fig. 2 C and D; SI Appendix, Figs. S1 C–D and S6A). These GAL4s produced quite similar trans-Tango patterns (and both had targets that were not seen when we trans-Tango traced Gr64f-GAL4, as Gr64f-GAL4 did not label brain-intrinsic sweet neurons). However, one major difference was evident: whereas the long-range SLP projection was present among the targets of Gr64aGAL4-expressing neurons, it was absent among the targets of Gr64a-GAL4–expressing neurons (Fig. 7 B and C). This result suggests that the SLP projection may be unique to the postsynaptic targets of the leg sweet neurons. Consistent with this idea, the SLP projection was also present when we traced the targets of Gr61a-GAL4–expressing sweet neurons (Fig. 7D); like Gr64aGAL4, Gr61a-GAL4 also labeled many leg sweet neurons (Fig. 2B; SI Appendix, Fig. S1B).
To assess whether the SLP projection might indeed be unique to the postsynaptic partners of the leg sweet neurons, we trans-Tango traced targets of Gr64f-GAL4–expressing neurons in intact versus leg-amputated animals (Fig. 7 E and E′). A previous study has shown that leg amputation can cause axons of leg taste neurons to degenerate (8), and we thus reasoned it may also prevent the targets of leg taste neurons from being traced. Indeed, we found that axons of the leg sweet neurons were barely visible in the leg-amputated Gr64f-GAL4 > trans-Tango animals (Fig. 7E′), and, importantly, the SLP projection traced by trans-Tango was eliminated, while the SMP projection remained intact (Fig. 7E′). We observed a similar absence of the SLP projection when we amputated the legs of Gr5a-GAL4 > trans-Tango flies (SI Appendix, Fig. S6B). These results suggest that among all sweet neurons, only the leg neurons have postsynaptic partners that send prominent projections to the SLP.
To rule out that the trans-Tango–traced SLP projection may be more sensitive to leg amputation for nonspecific reasons, we attempted tracing by restricting GAL4 activity to only the leg sweet neurons (Fig. 7F) and confirmed that postsynaptic partners of the leg sweet neurons sent a clear long-range projection to SLP (Fig. 7F), as well as to the SMP and the SEZ, two areas that were targeted by postsynaptic partners of some other sweet neurons (Fig. 7F).
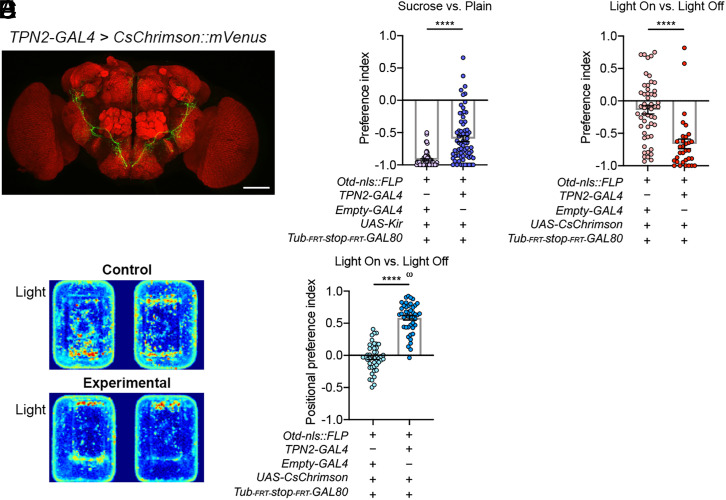
In our last set of experiments, we addressed the potential functional significance of the SLP-projecting targets of the leg sweet neurons. A group of projection neurons known as TPN2 has recently been shown—which we confirmed (SI Appendix, Fig. S7 A–C and Movie S3)—to be a direct postsynaptic target of the leg sweet neurons (13). Cell bodies and dendrites of TPN2 are located in the VNCs, but their long axons ascend and target the SLP in the brain prominently (Fig. 8A; SI Appendix, Fig. S7A) (13). To assess the role of these SLP-projecting TPN2 neurons in egg-laying decisions, we selectively 1) inhibited them and assessed how the animals chose in the sucrose versus plain task and 2) expressed CsChrimson in them and assessed how the animals chose in the light-on versus light-off task. We found that females with reduced activities of TPN2 showed reduced rejection of sucrose in the sucrose versus plain task, while retinal-fed animals with CsChrimson expressed in their TPN2 clearly rejected the light-baited agarose for egg-laying, despite having a positional preference for it, in the light-on versus light-off task (Fig. 8 B–E). [Curiously, while virgins and non–egg-laying mated females showed positional preference for light, too, males did not (SI Appendix, Fig. S7D).] Thus, the egg-laying decision phenotypes of TPN2 parallel that of bidirectional manipulations of the leg sweet neurons.
Fig. 8.
One of the SLP-projecting partners of the leg sweet neurons also promotes sucrose rejection. (A) Processes of TPN2-GAL4–expressing neurons in the brain (SI Appendix, Fig. S8). (B) Egg-laying PI of flies with their TPN2-GAL4–expressing neurons silenced in the sucrose versus plain task. Unpaired t test with Welch’s correction; n = 61 and 70. (C) Egg-laying PI of flies that expressed CsChrimson in their TPN2-GAL4–expressing neurons in the light-on versus light-off task. Both experimental flies and the controls were retinal-fed. Unpaired t test with Welch’s correction; n = 52 and 32. (D) Representative heatmaps of the flies described in C in the light-on versus light-off task. (E) Positional PI of the flies described in C in the light-on versus. light-off task. Unpaired t test with Welch’s correction and one-sample t test against zero; n for each = 44 (scale bars, 60 μm).
Collectively, our results support the view that information collected by the leg sweet neurons is processed differently from that by the rest of the sweet neurons and that the SLP is a candidate area for housing neurons that process option values during egg-laying site selection.
Discussion
In this work, we present results suggesting that sweet neurons on different body parts of Drosophila do not contribute equally to determining how a sweet option should be valued during egg-laying. First, whereas inactivating their leg sweet neurons abolished females’ plain preference in the sweet versus plain decision, inactivating the rest of the sweet neurons did not. Second, optogenetic activation of just the leg sweet neurons on a plain agarose was sufficient to cause its rejection over another plain agarose on which such activation was withheld. In contrast, selective activation of just the labellar sweet neurons promoted preference for the plain agarose on which it occurred. Third, bidirectional manipulations of the activities of TPN2—a postsynaptic partner unique to the leg sweet neurons—produced egg-laying decision phenotypes that were qualitatively similar to those of the leg sweet neurons. These findings suggest strongly that the Drosophila sweet taste system possesses a functional and anatomical division that is valued-based and task-specific.
How do our findings add to our understanding of the Drosophila sweet taste system in general? First, our findings suggest that sweetness is not always appetitive; rather, it is a cue that can bidirectionally modify the value of an egg-laying option. Second, our findings suggest that sweet neurons on the legs can drive diverse outputs depending on the task the animals are tending to; they can produce context-independent acceptance during feeding (PER) in both sexes and context-dependent rejection during egg-laying. Lastly, we suspect our understanding of the full behavioral functions of the Drosophila sweet taste system may have been limited because the field has been focusing on a limited set of behavioral tests. For example, our findings suggest that some of the sweet neurons can even promote genuine avoidance, albeit in an artificial setting (Fig. 5 A–C), hinting at the potential existence of another function of sweet taste neurons that has yet to be explored.
Why develop a circuit that allows detection of sweetness on an option to modify its value for egg-laying as opposed to promoting its reflexive acceptance? We have several speculations. First, eggs are precious; thus, it should be more advantageous for females to control their deposition through a decision process as opposed to a reflex. Second, converting different sensory cues (e.g., sweetness and firmness) associated with an option into value-modifying signals may allow options of disparate properties to be compared directly via a so-called “common currency.” Third, having sweetness engage a value-modifying circuit may allow animals more flexibility in adjusting the value of a sweet option according to decision contexts. For example, while flies prefer the plain option in certain two-choice contexts (31, 32, 48), they prefer the sweet option when laying eggs in a significantly larger enclosure (49). Perhaps larger enclosures promote sweet preference either by enhancing the output of the value-increasing pathway potentially mediated by the labellar sweet neurons or by dampening the value-decreasing one mediated by the leg sweet neurons.
Finally, where might the sweet versus plain egg-laying decision be made in the Drosophila brain? While egg-laying preference has been frequently used for studying the function of different sensory systems (24, 50–61), the central circuit that assigns, retains, and compares values of egg-laying options has yet to be elucidated. Two recent studies suggest that the descending egg-laying command neurons (oviDNs) must be an integral component of the decision circuit as they not only are capable of triggering egg-laying when directly activated but also express a [Ca2+] signal that tracks the relative value of an egg-laying option (33, 48). On the other hand, while successful rejection of the otherwise acceptable sweet option in the sweet versus plain task requires that the animals hold in memory their recent encounters with the preferred plain option, our previous studies have ruled out a critical role of the learning-and-memory center mushroom bodies in this decision (32). Our current results put forward the SLP as a candidate for housing the neurons that signal values to the decision-maker (Figs. 7 and 8). Given that dendrites of oviDNs arborize in the SMP (33), scrutinizing the electron microscopy (EM) connectome (62) for neurons that relay information from the SLP to SMP may help uncover some of the components that convert sweetness into a value-modifying signal during egg-laying.
Methods
In this work, we used various fly strains we obtained from colleagues and the Bloomington Stock Center, several published methods, and a closed-loop stimulation setup (SkinnerSys) we developed to assess the behavioral roles of neurons of interest during egg-laying site selection. Origins of the fly strains and more detailed descriptions of the published methods as well as SkinnerSys can be found in the SI Appendix. The code we used for closed-loop stimulation and the chamber and PCB design files for SkinnerSys can be found at https://github.com/ulrichstern/SkinnerTrax.
Supplementary Material
Acknowledgments
We thank Dr. Hubert Amrein, Dr. David Anderson, Dr. Anupama Dahanukar, Dr. Kristin Scott, and the Bloomington Stock Center for providing us with flies; Dr. Hiro Matsunami and Dr. Fan Wang for helpful discussions; and Dr. Barry Dickson and Dr. Kaiyu Wang for suggestions and critical reading of the manuscript. This work is supported by the Holland-Trice Scholar Award and the Invertebrate Neuroscience Fund.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2110158119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Chandrashekar J., Hoon M. A., Ryba N. J., Zuker C. S., The receptors and cells for mammalian taste. Nature 444, 288–294 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Liman E. R., Zhang Y. V., Montell C., Peripheral coding of taste. Neuron 81, 984–1000 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarmolinsky D. A., Zuker C. S., Ryba N. J., Common sense about taste: From mammals to insects. Cell 139, 234–244 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao G. Q., et al. , The receptors for mammalian sweet and umami taste. Cell 115, 255–266 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Mueller K. L., et al. , The receptors and coding logic for bitter taste. Nature 434, 225–229 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Barretto R. P., et al. , The neural representation of taste quality at the periphery. Nature 517, 373–376 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X., Gabitto M., Peng Y., Ryba N. J., Zuker C. S., A gustotopic map of taste qualities in the mammalian brain. Science 333, 1262–1266 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z., Singhvi A., Kong P., Scott K., Taste representations in the Drosophila brain. Cell 117, 981–991 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Thorne N., Chromey C., Bray S., Amrein H., Taste perception and coding in Drosophila. Curr. Biol. 14, 1065–1079 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Marella S., et al. , Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron 49, 285–295 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Moon S. J., Köttgen M., Jiao Y., Xu H., Montell C., A taste receptor required for the caffeine response in vivo. Curr. Biol. 16, 1812–1817 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Dahanukar A., Lei Y. T., Kwon J. Y., Carlson J. R., Two Gr genes underlie sugar reception in Drosophila. Neuron 56, 503–516 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H., Kirkhart C., Scott K., Long-range projection neurons in the taste circuit of Drosophila. eLife 6, e23386 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon M. D., Scott K., Motor control in a Drosophila taste circuit. Neuron 61, 373–384 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stern U., Yang C. H., SkinnerTrax: High-throughput behavior-dependent optogenetic stimulation of Drosophila. bioRxiv [Preprint] (2017). 10.1101/080614. Accessed 24 December 2021. [DOI]
- 16.Chyb S., Dahanukar A., Wickens A., Carlson J. R., Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc. Natl. Acad. Sci. U.S.A. 100 (suppl. 2), 14526–14530 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahanukar A., Foster K., van der Goes van Naters W. M., Carlson J. R., A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat. Neurosci. 4, 1182–1186 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Fujii S., et al. , Drosophila sugar receptors in sweet taste perception, olfaction, and internal nutrient sensing. Curr. Biol. 25, 621–627 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiao Y., Moon S. J., Wang X., Ren Q., Montell C., Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr. Biol. 18, 1797–1801 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyamoto T., Slone J., Song X., Amrein H., A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell 151, 1113–1125 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slone J., Daniels J., Amrein H., Sugar receptors in Drosophila. Curr. Biol. 17, 1809–1816 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto T., Chen Y., Slone J., Amrein H., Identification of a Drosophila glucose receptor using Ca2+ imaging of single chemosensory neurons. PLoS One 8, e56304 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joseph R. M., Sun J. S., Tam E., Carlson J. R., A receptor and neuron that activate a circuit limiting sucrose consumption. eLife 6, e24992 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H. L., Stern U., Yang C. H., Molecular control limiting sensitivity of sweet taste neurons in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 116, 20158–20168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaeger A. H., et al. , A complex peripheral code for salt taste in Drosophila. eLife 7, e37167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tauber J. M., et al. , A subset of sweet-sensing neurons identified by IR56d are necessary and sufficient for fatty acid taste. PLoS Genet. 13, e1007059 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn J. E., Chen Y., Amrein H., Molecular basis of fatty acid taste in Drosophila. eLife 6, e30115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeDue E. E., Chen Y. C., Jung A. Y., Dahanukar A., Gordon M. D., Pharyngeal sense organs drive robust sugar consumption in Drosophila. Nat. Commun. 6, 6667 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thoma V., et al. , Functional dissociation in sweet taste receptor neurons between and within taste organs of Drosophila. Nat. Commun. 7, 10678 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman E. G., Dahanukar A., Molecular neurobiology of Drosophila taste. Curr. Opin. Neurobiol. 34, 140–148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang C. H., Belawat P., Hafen E., Jan L. Y., Jan Y. N., Drosophila egg-laying site selection as a system to study simple decision-making processes. Science 319, 1679–1683 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang C. H., He R., Stern U., Behavioral and circuit basis of sucrose rejection by Drosophila females in a simple decision-making task. J. Neurosci. 35, 1396–1410 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang F., et al. , Neural circuitry linking mating and egg laying in Drosophila females. Nature 579, 101–105 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cury K. M., Prud’homme B., Gompel N., A short guide to insect oviposition: When, where and how to lay an egg. J. Neurogenet. 33, 75–89 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Yavuz A., Jagge C., Slone J., Amrein H., A genetic tool kit for cellular and behavioral analyses of insect sugar receptors. Fly (Austin) 8, 189–196 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon J. Y., Dahanukar A., Weiss L. A., Carlson J. R., Molecular and cellular organization of the taste system in the Drosophila larva. J. Neurosci. 31, 15300–15309 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto T., Amrein H., Diverse roles for the Drosophila fructose sensor Gr43a. Fly (Austin) 8, 19–25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asahina K., et al. , Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell 156, 221–235 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klapoetke N. C., et al. , Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stern U., et al. , Learning a spatial task by trial and error in Drosophila. Curr. Biol. 29, 2517–2525.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Feinberg E. H., et al. , GFP reconstitution across synaptic partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron 57, 353–363 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Macpherson L. J., et al. , Dynamic labelling of neural connections in multiple colours by trans-synaptic fluorescence complementation. Nat. Commun. 6, 10024 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu B., Chui V., Mann K., Gordon M. D., Presynaptic gain control drives sweet and bitter taste integration in Drosophila. Curr. Biol. 24, 1978–1984 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Marella S., Mann K., Scott K., Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron 73, 941–950 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inagaki H. K., et al. , Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell 148, 583–595 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Youn H., Kirkhart C., Chia J., Scott K., A subset of octopaminergic neurons that promotes feeding initiation in Drosophila melanogaster. PLoS One 13, e0198362 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talay M., et al. , Transsynaptic mapping of second-order taste neurons in flies by trans-Tango. Neuron 96, 783–795.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vijayan V., et al. , A rise-to-threshold signal for a relative value deliberation. bioRxiv [Preprint] (2021). 10.1101/2021.09.23.461548. Accessed 24 December 2021. [DOI]
- 49.Schwartz N. U., Zhong L., Bellemer A., Tracey W. D., Egg laying decisions in Drosophila are consistent with foraging costs of larval progeny. PLoS One 7, e37910 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joseph R. M., Devineni A. V., King I. F., Heberlein U., Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 106, 11352–11357 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joseph R. M., Heberlein U., Tissue-specific activation of a single gustatory receptor produces opposing behavioral responses in Drosophila. Genetics 192, 521–532 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu S. F., Ja Y. L., Zhang Y. J., Yang C. H., Sweet neurons inhibit texture discrimination by signaling TMC-expressing mechanosensitive neurons in Drosophila. eLife 8, e46165 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guntur A. R., et al. , H2O2-sensitive isoforms of Drosophila melanogaster TRPA1 act in bitter-sensing gustatory neurons to promote avoidance of UV during egg-laying. Genetics 205, 749–759 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu E. Y., Guntur A. R., He R., Stern U., Yang C. H., Egg-laying demand induces aversion of UV light in Drosophila females. Curr. Biol. 24, 2797–2804 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gou B., Liu Y., Guntur A. R., Stern U., Yang C. H., Mechanosensitive neurons on the internal reproductive tract contribute to egg-laying-induced acetic acid attraction in Drosophila. Cell Rep. 9, 522–530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dweck H. K., et al. , Olfactory preference for egg laying on citrus substrates in Drosophila. Curr. Biol. 23, 2472–2480 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Zhang L., et al. , Parallel mechanosensory pathways direct oviposition decision-making in Drosophila. Curr. Biol. 30, 3075–3088.e4 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Hussain A., et al. , Ionotropic chemosensory receptors mediate the taste and smell of polyamines. PLoS Biol. 14, e1002454 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y., Amrein H., Ionotropic receptors mediate drosophila oviposition preference through sour gustatory receptor neurons. Curr. Biol. 27, 2741–2750.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stensmyr M. C., et al. , A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 151, 1345–1357 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Mansourian S., et al. , Fecal-derived phenol induces egg-laying aversion in Drosophila. Curr. Biol. 26, 2762–2769 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Scheffer L. K., et al. , A connectome and analysis of the adult Drosophila central brain. eLife 9, e57443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.