Abstract
Objective:
Myocardial infarction and ischemic stroke are leading causes of cardiovascular (CV) morbidity and mortality in ANCA-associated vasculitis (AAV), especially for the 20% with end-stage renal disease (ESRD). We assessed the impact of renal transplantation on the risk of myocardial infarction and stroke among patients with ESRD due to AAV.
Methods:
We identified patients from the United States Renal Data System with ESRD due to AAV between 2000 and 2016. We examined the association between renal transplantation and the risk of non-fatal and fatal myocardial infarction or ischemic stroke among waitlisted patients using Medicare claims and death data through 2017. We used time-varying Cox proportional hazards models with age as the time scale to estimate hazard ratios (HR) and 95% confidence intervals (CIs) for myocardial infarction and ischemic stroke events among patients who received a renal transplant compared to those who remained on the waitlist.
Results:
Of 1029 waitlisted patients, 593 (58%) were transplanted over a mean of 5.7 years. There were 17 events (4.6/1,000 person-years) in the transplanted group and 40 events (13.7/1,000 person-years) in the group that remained waitlisted. A renal transplant was associated with a 78% lower risk of myocardial infarction or ischemic stroke (HR=0.22, 95% CI 0.11 to 0.47). These findings persisted across sex and age groups and when censoring patients after living donor transplantation.
Conclusions:
Among AAV patients with ESRD, renal transplantation can substantially reduce the risk of myocardial infarction and ischemic stroke. Improving access to transplantation for this population may further improve outcomes.
Introduction
Glomerulonephritis is a common manifestation of ANCA-associated vasculitis (AAV) and leads to end-stage renal disease (ESRD) in up to 20% of patients (1). In the United States, there has been an increase in the rate of ESRD attributed to AAV over time (2), perhaps in the context of improved survival from AAV (3). Both AAV and ESRD patients are at a 2-fold and 3-fold increased risk, respectively, for cardiovascular disease (CV) events, including myocardial infarction (MI) and stroke (4–6). Indeed, among patients with ESRD, those with glomerulonephritis as the etiology of ESRD have the highest risk of CV events except when compared to those with diabetes mellitus (7).
In addition to chronic kidney disease, a confluence of unique factors put AAV patients with ESRD at a particularly high risk for MI and stroke. In part, patients with AAV are at increased risk of CV events because of glucocorticoid exposure, which contributes to dyslipidemia, lipodystrophy, hyperglycemia, and hypertension. Additionally, disease-mediated factors such as systemic inflammation, direct endothelial injury, and microvascular dysfunction further contribute to CV risk. Despite this excess burden, little is known about strategies that may reduce the risk of CV events in this high risk group.
We previously reported that renal transplantation is associated with a significant reduction in mortality, including cardiovascular-specific death, among patients with ESRD due to AAV (8). Additionally, prior studies of patients with ESRD attributed to diabetes mellitus in the 1990s found that renal transplantation was associated with a reduction in the risk of acute coronary syndrome (7, 9). To our knowledge, no studies have evaluated the impact of renal transplantation on the risk of non-fatal and fatal MI and stroke among patients with ESRD attributed to AAV, and there is no contemporary data on these outcomes in the general ESRD population. We evaluated the impact of renal transplantation on the risk of MI and stroke among patients waitlisted for a renal transplant in the United States.
Patients and methods
Data source and study population
The United States Renal Data System (USRDS) is a national registry of ESRD patients, representing an estimated 94% of patients who receive dialysis or kidney transplantation. Patients who refuse replacement therapy, die prior to enrollment, or receive transient dialysis for acute renal failure may not be enrolled. Nephrologists are required by law to submit a Medical Evidence Report, which includes the cause of ESRD according to ICD-9 codes, within 45 days of a patient starting a new ESRD treatment. Further information may be found in the USRDS Annual Data Report (10).
We identified all incident cases of ESRD attributed to AAV by ICD-9 code 446.4 recorded by the nephrologist on the Medical Evidence Report. Among those who received care within the Mass General Brigham healthcare system (N=90), we linked their electronic health record (EHR) data to their USRDS record. We then reviewed their EHR data, including physician notes, biopsy report, and laboratory results to evaluate whether the patient had a physician diagnosis of AAV. We confirmed that the ICD-9 code of 446.4 (granulomatosis with polyangiitis) on the Medical Evidence Report has a positive predictive value (PPV) of 94% for identifying AAV cases. Among the cases classified as AAV (N=85), 99% had a positive ANCA test. There are no specific ICD-9 codes for MPA, but using this linked data, we determined that ICD-9 code 446.4 includes patients characterized as GPA and MPA (68% and 32% of cases, respectively) as well as those with PR3- and MPO-ANCA+ disease (39% and 55% of cases, respectively), which is thought to be a more valid approach to classifying AAV (11).
We included AAV patients who were diagnosed with ESRD and waitlisted for a renal transplant between 2000–2016. We limited the study period to after 2000 because of the availability from USRDS of hospitalization data after this date. Data availability ended on 12/31/2017. We restricted our analysis to those patients waitlisted for a renal transplant to minimize confounding by indication given that patients waitlisted for a renal transplant are generally younger, healthier, have a higher socioeconomic status, and higher level of social support than those who are not waitlisted (12).
We excluded patients who were transplanted without being waitlisted or without receiving hemo- or peritoneal dialysis in order to minimize confounding by indication since these patients may have unique features (e.g., comorbidity burden, socioeconomic status) associated with better outcomes compared to those who are waitlisted, an approached used in our prior studies (8, 13, 14). We also excluded patients with an MI or ischemic stroke prior to the date that they were waitlisted for a renal transplant to identify incident cardiovascular events. Patients were followed from their first day on the waitlist for a renal transplant until the earliest of: a fatal or non-fatal cardiovascular event of interest, removal from the waitlist, end of Medicare coverage, end of follow-up (December 31, 2017), or non-CV death.
Cardiovascular outcomes
The primary outcome of interest was non-fatal or fatal CV events, specifically ischemic stroke or myocardial infarction. Non-fatal stroke or myocardial infarction were identified using Centers for Medicaid and Medicare Services (CMS) linked hospitalization data using previously validated ICD-9 codes for the identification of stroke or myocardial infarction in administrative claims data (15, 16). Fatal ischemic stroke or myocardial infarction were identified using vital status data (including primary cause of death) routinely collected as part of the USRDS documentation process through the mandatory completion of the CMS ESRD Death Notification form (CMS-2746). Fatal MIs were defined as cases in which the primary cause of death was acute myocardial infarction and fatal strokes were defined as cases in which stroke was the cause (excluding codes that included hemorrhage). Secondary outcomes included the risk of non-fatal or fatal MI and non-fatal or fatal stroke, analyzed separately.
Covariates
Details were obtained from the USRDS regarding relevant baseline covariates, including demographics (age, sex, race), body mass index, initial ESRD treatment, waitlisting date, calculated panel reactive antibodies (CPRA, a measure associated with the risk of transplant rejection, graft survival, and transplant likelihood), transplant status, type of transplant (living or deceased), date of renal transplant, and vital status. The Charlson comorbidity index (CCI) was used to estimate the burden of comorbidities that were identified using linked Part A and B CMS claims data (17). Hospitalization frequency in the six months prior to waitlisting was tabulated using hospitalization CMS data.
Statistical analysis
We determined incidence rates and 95% confidence intervals (CIs) of CV events per 1,000 person-years. Follow-up began at the date that a patient was waitlisted for their first renal transplant. In order to avoid immortal time bias, all transplanted patients contributed person-time to the waitlisted group until they received a transplant, after which they contributed time to the transplanted arm. We used a time-dependent Cox proportional hazards model with age as the time scale and renal transplantation as the time-varying exposure; we confirmed that the proportional hazards assumption was met. We accounted for the competing risk of death using the methods described by Fine and Gray (18, 19). We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) of the time to the first outcome. Multivariable models were adjusted for sex, race, first ESRD treatment method, CPRA, and time-varying CCI. Additional subgroup analyses evaluated the risk of cardiovascular events among those who received a renal transplant compared to those who did not receive a renal transplant during the study period according to age at ESRD onset and sex. The USRDS does not permit reporting of any cell sizes with less than 11 individuals in order to maintain privacy and confidentiality. Owing to this limitation and the small cell sizes in the subgroup analyses, we do not report the number of events or incidence rates, but only the resulting hazard ratios. In a sensitivity analysis, we censored patients who received a living donor transplant at the time of transplantation. We also calculated E-values to assess the potential effect of unmeasured confounders (20). All p values were two-sided with a significance threshold of <0.05. Statistical analyses were performed using SAS 9.4.
Data use
The data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government. This study was exempted from the Mass General Brigham Institutional Review Board because it only used deidentified data.
Role of the funding source
The National Institutes of Health had no role in the design or conduct of the study or the reporting of results.
Institutional Review Board approval
This study was exempted from the MGB Institutional Review Board.
Results
During the study period, 1029 patients were diagnosed with ESRD due to AAV and waitlisted for a renal transplant; 593 (58%) received a renal transplant between January 1st, 2000 and December 31st, 2016 (Table 1). Those who received a transplant were younger at the onset of ESRD than those who remained on the waitlist (46.4 years vs 52.6 years). Sex (43% vs 42% female, respectively), race (86% vs 80% white, respectively), ethnicity (14% vs 17% Hispanic, respectively), and initial dialysis modality (82% vs 88% hemodialysis, respectively) were similar among those who received a transplant compared to those who remained on the waitlist. The calculated panel reactive antibodies (0.14 [±0.30] vs 0.17 [±0.31], respectively), comorbidity score (4.2 [±3.2] vs 4.0 [±3.1], respectively), hospitalization frequency (106 vs 86, respectively), and time on dialysis prior to waitlisting (1.1 [±1.5] vs 1.4 [±2.2] years, respectively) were similar in those transplanted and those who remained on the waitlist. The mean time spent on the waitlist prior to transplantation was a mean (SD) 1.6 [±1.6] years among those who received a transplant.
Table 1.
Characteristics of Transplanted Subjects and Comparators with End-Stage Renal Disease Due to Granulomatosis with Polyangiitis
| Baseline Characteristics | Transplanted | Not Transplanted |
|---|---|---|
| N | 593 | 436 |
| Age at ESRD* (years, mean, SD) | 46.4, 17.4 | 52.6, 15.0 |
| <40 (%) | 32.0 | 18.1 |
| 40–49 (%) | 17.2 | 15.1 |
| 50–59 (%) | 20.4 | 26.2 |
| >=60 (%) | 30.4 | 40.6 |
| Female (%) | 42.7 | 41.7 |
| BMI (kg/m2, mean, SD) | 27.0, 6.4 | 27.7 |
| Race (%) | ||
| White | 85.5 | 79.6 |
| African American | 5.4 | 9.6 |
| Asian | 1.9 | 2.3 |
| Other | 7.3 | 8.5 |
| Hispanic (%) | 13.7 | 16.5 |
| OPTN Region (%) | ||
| 1 (CT, ME, MA, NH, RI, Eastern VT) | 4.9 | 4.6 |
| 2 (DE, DC, MD, NJ, PA, WV, Northern VA) | 9.8 | 10.6 |
| 3 (AL, AR, FL, GA, LA, MS) | 10.8 | 11.9 |
| 4 (OK, TX) | 7.9 | 9.9 |
| 5 (AZ, CA, NV, NM, UT) | 14.8 | 16.1 |
| 6 (AK, HI, ID, MT, OR, WA) | 6.6 | 5.1 |
| 7 (IL, MN, ND, SD, WI) | 11.5 | 9.9 |
| 8 (CO, IA, KA, MO, NE, WY | 9.1 | 6.9 |
| 9 (NY, Western VT) | 3.7 | 5.7 |
| 10 (IN, MI, OH) | 9.8 | 5.5 |
| 11 (KY, NC, SC, TN, VA) | 11.1 | 14.0 |
| Prior Organ Transplant (%) | <11 | <11 |
| Panel Reactive Antibodies (PRA) (mean, SD) | 0.14, 0.30 | 0.17, 0.31 |
| Comorbidities at Waitlisting | ||
| Charlson Comorbidity Index (mean, SD) | 4.2, 3.2 | 4.0, 3.1 |
| Hypertension (%) | 81.8 | 70.4 |
| Diabetes (with or without complications) (%) | 35.2 | 30.3 |
| Dyslipidemia (%) | 56.0 | 43.4 |
| Congestive heart failure (%) | 24.3 | 33.7 |
| Chronic obstructive pulmonary disease (%) | 34.2 | 32.1 |
| Asthma (%) | 23.8 | 23.2 |
| Malignancy (%) | 12.1 | 11.2 |
| No of Hosp in the Six Months Prior to Waitlisting (median, IQR) | 0 [0,0] | 0 [0,0] |
| Time on dialysis prior to waitlisting (mean, SD) | 1.1, 1.5 | 1.4, 2.2 |
| Time from waitlisting to transplantation (mean, SD) | 1.6, 1.6 | — |
| First Modality (%) | ||
| Hemodialysis | 82.3 | 87.6 |
| Peritoneal Dialysis | 17.7 | 12.4 |
| Transplant Type (%) | ||
| Living | 85.3 | 67.7 |
| Deceased | 77.8 | 32.3 |
Over 6,657 person-years of follow-up, there were 57 fatal or non-fatal myocardial infarction or ischemic stroke events (Table 2). Specifically, there were 17 myocardial infarctions and 40 ischemic strokes. Among the 593 patients who received a renal transplant, there were 17 events such that the incidence rate (95% CI) was 4.6 (95% CI: 2.4 to 6.7) per 1,000 person-years. In contrast, there were 40 events among those not transplanted during the study period (waitlisted), such that the incidence rate (95% CI) was 13.7 (95% CI: 9.4 to 17.9) per 1,000 person-years.
Table 2.
Risk of Myocardial Infarction or Ischemic Stroke According to Transplant Status Among Patients with End-Stage Renal Disease Due to Granulomatosis with Polyangiitis
| Total Follow-Up (Person Years) | Events, n | Incident rate* (95% CI) |
Unadjusted HR (95% CI) |
Fully-Adjusted HR† (95% CI) |
|
|---|---|---|---|---|---|
| Overall | 6657 | 57 | 8.6 (6.3, 10.8) | ||
| Transplanted | 3734 | 17 | 13.7 (9.4, 17.9) | 0.21 (0.10, 0.43) | 0.22 (0.11, 0.47) |
| Not Transplanted | 2922 | 40 | 4.6 (2.4, 6.7) | 1.0 | 1.0 |
HR, hazard ratio; ESRD, end-stage renal disease
Per 1,000 Person Years;
Additionally adjusted for sex, race, first ESRD treatment method, any comorbidity, and comorbidity score. Age was used as the time-scale in the Cox proportional hazards model.
In a fully-adjusted time-dependent cox model, patients who received a renal transplant had a 78% lower risk of a myocardial infarction or ischemic stroke compared to patients who did not receive a renal transplant (Figure 1, HR: 0.22, 95% CI: 0.11 to 0.47). These findings were similar among those younger than 50 years of age (HR: 0.22, 95% CI: 0.06 to 0.80) and older than 50 years of age (HR: 0.25, 95% CI: 0.10 to 0.63). These findings were also similar among women (HR: 0.17, 95% CI: 0.05 to 0.57) and men (HR: 0.32, 95% CI: 0.12 to 0.82). When myocardial infarction and ischemic stroke events were analyzed separately, the association between transplantation and reduced risk of MI (HR: 0.08, 95% CI 0.01 to 0.63) and stroke (HR: 0.27, 95% CI 0.12 to 0.61) persisted.
Figure 1.
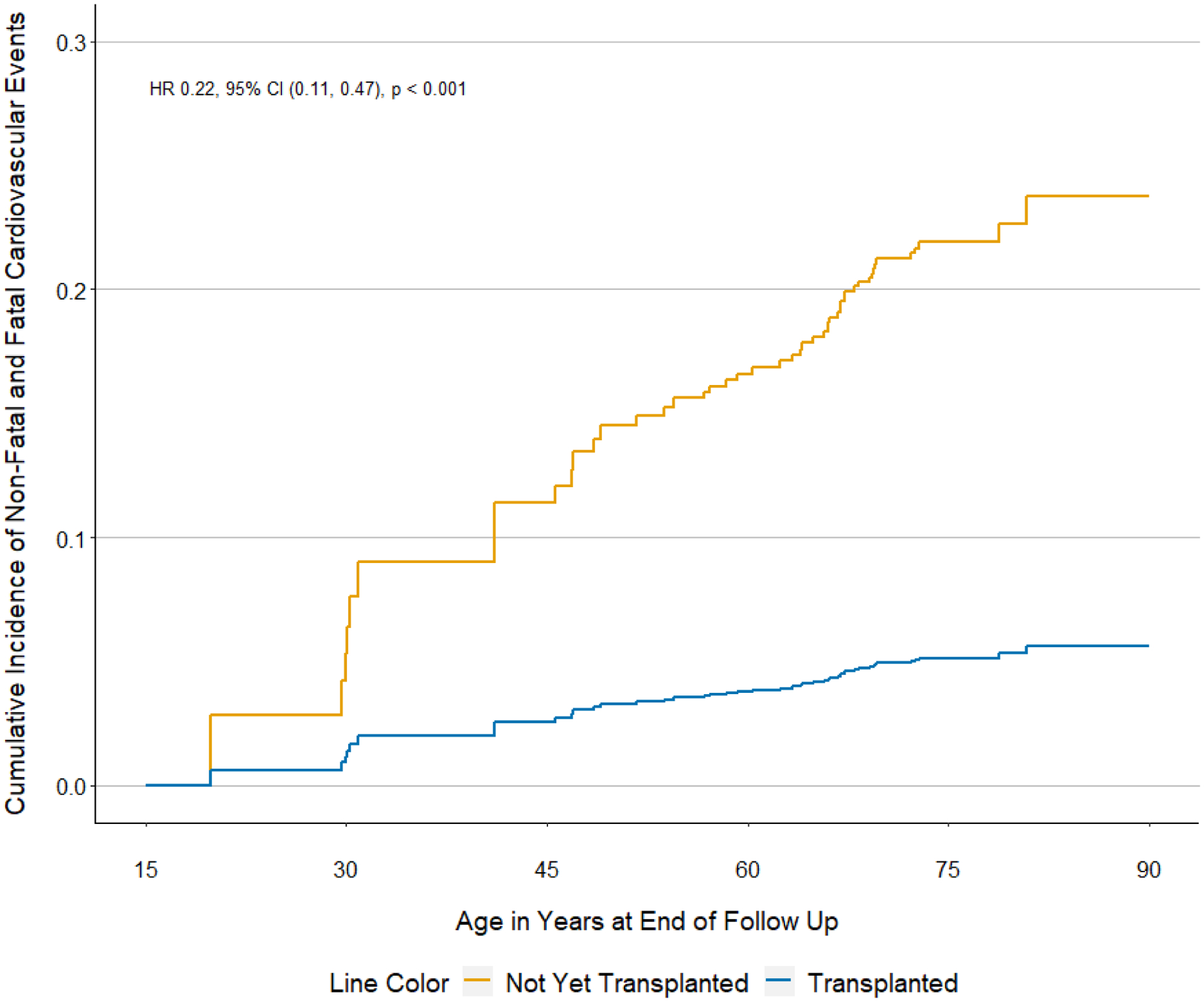
Cumulative incidence of non-fatal and fatal cardiovascular events according to transplant status among waitlisted patients with end-stage renal disease due to granulomatosis with polyangiitis
Our findings remained unchanged when patients who received a living donor transplant were censored (HR: 0.19, 95% CI 0.07 to 0.48). To assess the robustness of the association to unmeasured confounding, we determined that the observed HR of 0.22 for our primary outcome could be explained away by an unmeasured confounder that was associated with both the exposure (renal transplant) and the outcome (myocardial infarction or ischemic stroke) by an HR of at least 8.56 above and beyond the measured confounders but not by a weaker confounding. The corresponding CI could be moved to include the null by an unmeasured confounder that was associated with both transplant and all-cause mortality by an HR of at least 3.68.
Discussion
Using a large cohort of nearly all patients in the United States with ESRD due to AAV who are waitlisted, we found that renal transplantation is associated with a 78% lower risk of myocardial infarction or ischemic stroke when compared to patients who remain on the waitlist. Our findings remained consistent when myocardial infarction and ischemic stroke were analyzed as separate outcomes, persisted regardless of age or sex, and were robust in sensitivity analysis assessing the potential impact of unmeasured confounding. Given the significant burden of non-fatal and fatal myocardial infarction and ischemic stroke in patients with AAV (4) and in those with ESRD (5), our observations highlight the importance of referring patients with ESRD attributable to AAV for evaluation for renal transplantation and addressing modifiable CV risk factors for those on the waitlist. The benefits of renal transplantation observed in this study highlight the substantial impact that CKD and dialysis have on CV risk in this population which often has many other potential disease- and treatment-specific factors driving CV risk.
We previously established the survival benefit of renal transplantation in AAV and found that some of this benefit was driven by a reduction in CV-specific death, which includes death from arrhythmia, heart failure, valvular disease, and atherosclerotic events (8). The present study expands on those finding by demonstrating that the risks for non-fatal and fatal myocardial infarction and ischemic stroke in patients with ESRD attributed to AAV are markedly reduced following renal transplantation. AAV is associated with an increased risk of ischemic cardiovascular events compared with the general population (3), likely because of accelerated atherosclerosis in the context of glucocorticoid exposure, systemic inflammation, and direct endothelial injury. These factors compound those associated with ESRD that also drive an increased risk of cardiovascular disease, including uremic toxins, electrolyte disequilibrium, neurohumoral alterations, and systemic inflammation related to the dialysis procedure (5). Indeed, patients with ESRD due to glomerulonephritis are at higher risk for acute myocardial infarction than patients with ESRD from other causes (except diabetes mellitus) (7).
Our observations highlight that ESRD is an important factor contributing to atherosclerotic cardiovascular disease in patients with ESRD due to AAV and that at least some of its impact can be reversed following transplantation (5). There are surprisingly few studies evaluating the impact of renal transplantation on these outcomes using methods to address potential confounding and susceptibility to immortal time and other biases (21). A similar risk reduction has been reported in patients with ESRD due to diabetes mellitus in the 1990s, where renal transplantation was associated with a 62% reduction in the risk of acute coronary syndrome (9). A 55% reduction in the long-term risk of acute myocardial infarction was also observed in another USRDS study that included all-patients with ESRD from all causes through 2002 (7). In this study, we expanded upon these findings in a contemporary cohort and the population of patients with ESRD and AAV. Given the ongoing risk of MI and stroke before and after renal transplantation, regular assessment of and counseling for modifiable CV risk factors is critical in this population (5).
Ischemic strokes were more common in our study population than myocardial infarction. Similar observations have been made in patients with lupus nephritis and ESRD on the transplant waitlist (22). Our observations may reflect the previously reported excess risk of stroke in all-comers with ESRD compared to the general population, as well as the additional risk attributable to AAV-specific features like systemic inflammation, hypertension, and endothelial injury (23). Additional studies are needed to better understand the characteristics of stroke in patients with AAV. Some patients with AAV do have stroke as a manifestation of their AAV (i.e., central nervous system vasculitis), but this is typically rare, and the rates observed in this study are certainly higher than what one might attribute to AAV.
These findings highlight the critical need of rheumatologists, nephrologists, and other specialists who care for patients with ESRD attributed to AAV to refer these patients to renal transplant centers for evaluation. It is important to note that patients with ESRD in the United States can be waitlisted for a renal transplant when their estimated glomerular filtration rate (eGFR) is < 20 mL/min/1.73 m2 regardless of whether or not they are receiving dialysis, so referring patients whose eGFR is < 30 mL/min/1.73 m2 allows sufficient time for recipient evaluation. The CV benefits we observed with transplantation need to be balanced with the need for absent clinical disease activity of AAV prior to transplantation. Hence, at least six months, but preferably one year, of clinical remission is usually recommended prior to preceding with transplantation (24). Notably, a stable positive ANCA titer in a patient with stable clinical remission is not a contraindication to transplantation.
Our study has a number of strengths largely related to the data source, which includes nearly all patients in the United States with ESRD and their outcomes, including transplantation, comorbidities, hospitalizations, and death, as collected from UNOS, CMS, and nephrologists. However, our study also has certain limitations. First, this is an observational study and there is the possibility of unmeasured confounding. However, we addressed this by limiting our study population to patients waitlisted for renal transplantation, excluding those who received a transplant without receiving dialysis first, incorporating CPRA and comorbidity scores as covariates, performing a sensitivity analysis where we censored patients who received a living donor transplant, and estimating the E-value. Second, the number of observed events in patients who received a transplant was small, limiting our ability to report the results of additional subgroup analyses. Third, our outcome (myocardial infarction or ischemic stroke) was assessed using previously validated billing codes associated with hospitalization and death certificate data, but these approaches may lack sensitivity for some events of interest. However, we would not expect this misclassification to occur differentially between those who received a transplant and those who remained on the waitlist. Fourth, the USRDS does not include details of AAV disease or treatment history limiting our ability to account for these potential confounders. As such, we do not have details regarding AAV disease flare as a reason for a patient not receiving a transplant but did account for removal from the waitlist in our analyses.
In conclusion, we found that renal transplantation significantly reduces the risk of non-fatal and fatal myocardial infarction and ischemic stroke in ESRD attributed to AAV. These findings highlight the important roles that chronic kidney disease and hemodialysis play in the risk of cardiovascular disease in patients with AAV and ESRD who have many factors contributing to an excess risk of myocardial infarction and stroke. Given that renal transplants are a limited resource, additional studies are needed to identify other strategies to reduce the risk of myocardial infarction and stroke among patients with AAV and ESRD and to improve access to transplantation for those on the waitlist.
Funding/Disclosures
ZSW is supported by NIH/NIAMS [K23AR073334 and L30 AR070520]. ZSW reports research support from Bristol-Myers Squibb and Principia and consulting fees from Viela Bio and Medpace. AJ is supported by the Rheumatology Research Foundation Scientist Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moiseev S, Novikov P, Jayne D, Mukhin N. End-stage renal disease in ANCA-associated vasculitis. Nephrol Dial Transplant. 2017;32(2):248–53. doi: 10.1093/ndt/gfw046. [DOI] [PubMed] [Google Scholar]
- 2.Wallace ZS, Zhang Y, Lu N, Stone JH, Choi HK. Improving Mortality in End-Stage Renal Disease Due to Granulomatosis With Polyangiitis (Wegener’s) From 1995 to 2014: Data From the United States Renal Data System. Arthritis Care Res (Hoboken). 2018;70(10):1495–500. doi: 10.1002/acr.23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan JA, Dehghan N, Chen W, Xie H, Esdaile JM, Avina-Zubieta JA. Mortality in ANCA-associated vasculitis: a meta-analysis of observational studies. Ann Rheum Dis. 2017;76(9):1566–74. doi: 10.1136/annrheumdis-2016-210942. [DOI] [PubMed] [Google Scholar]
- 4.Houben E, Penne EL, Voskuyl AE, van der Heijden JW, Otten RHJ, Boers M, et al. Cardiovascular events in anti-neutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis of observational studies. Rheumatology (Oxford). 2018;57(3):555–62. doi: 10.1093/rheumatology/kex338. [DOI] [PubMed] [Google Scholar]
- 5.Bhatti NK, Karimi Galougahi K, Paz Y, Nazif T, Moses JW, Leon MB, et al. Diagnosis and Management of Cardiovascular Disease in Advanced and End-Stage Renal Disease. J Am Heart Assoc. 2016;5(8). doi: 10.1161/JAHA.116.003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 7.Kasiske BL, Maclean JR, Snyder JJ. Acute myocardial infarction and kidney transplantation. J Am Soc Nephrol. 2006;17(3):900–7. doi: 10.1681/asn.2005090984. [DOI] [PubMed] [Google Scholar]
- 8.Wallace ZS, Wallwork R, Zhang Y, Lu N, Cortazar FB, Niles JL, et al. Improved survival with renal transplantation for end-stage renal disease due to granulomatosis with polyangiitis: data from the United States Renal Data System. Ann Rheum Dis. 2018;77(9):1333–8. doi: 10.1136/annrheumdis-2018-213452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hypolite IO, Bucci J, Hshieh P, Cruess D, Agodoa LY, Yuan CM, et al. Acute coronary syndromes after renal transplantation in patients with end-stage renal disease resulting from diabetes. Am J Transplant. 2002;2(3):274–81. doi: 10.1034/j.1600-6143.2002.20313.x. [DOI] [PubMed] [Google Scholar]
- 10.United States Renal Data System. 2019 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2019. [Google Scholar]
- 11.Hilhorst M, van Paassen P, Tervaert JW, Limburg Renal R. Proteinase 3-ANCA Vasculitis versus Myeloperoxidase-ANCA Vasculitis. J Am Soc Nephrol. 2015;26(10):2314–27. doi: 10.1681/ASN.2014090903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 13.Jorge A, Wallace ZS, Lu N, Zhang Y, Choi HK. Renal Transplantation and Survival Among Patients With Lupus Nephritis: A Cohort Study. Ann Intern Med. 2019;170(4):240–7. doi: 10.7326/m18-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jorge A, Fu X, Cook C, Lu N, Zhang Y, Choi HK, et al. Kidney Transplantation and Cardiovascular Events Among Patients with End-Stage Renal Disease due to Lupus Nephritis: A Nationwide Cohort Study. Arthritis Care Res (Hoboken). 2021. doi: 10.1002/acr.24725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. American heart journal. 2004;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Murray AM, Seliger S, Lakshminarayan K, Herzog CA, Solid CA. Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol. 2013;24(7):1166–73. doi: 10.1681/asn.2012080841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133(6):601–9. doi: 10.1161/circulationaha.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–74. doi: 10.7326/m16-2607. [DOI] [PubMed] [Google Scholar]
- 21.Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11(10):2093–109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 22.Wallace ZS, Jorge A, Fu X, Zhang Y, Choi H. Reduced Risk of Cardiovascular Diseases Events with Renal Transplantation in Granulomatosis with Polyangiitis in the United States: Data from the US Renal Data System [abstract]. Arthritis Rheumatol. 2020;72 (suppl 10). https://acrabstracts.org/abstract/reduced-risk-of-cardiovascular-diseases-events-with-renal-transplantation-in-granulomatosis-with-polyangiitis-in-the-united-states-data-from-the-us-renal-data-system/. Accessed February 2, 2021. [Google Scholar]
- 23.Masson P, Kelly PJ, Craig JC, Lindley RI, Webster AC. Risk of Stroke in Patients with ESRD. Clin J Am Soc Nephrol. 2015;10(9):1585–92. doi: 10.2215/cjn.12001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck L, Bomback AS, Choi MJ, Holzman LB, Langford C, Mariani LH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am J Kidney Dis. 2013;62(3):403–41. doi: 10.1053/j.ajkd.2013.06.002. [DOI] [PubMed] [Google Scholar]


