Abstract
Spatial abilities contribute to life and occupational competencies, and certain spatial skills differ, on average, between males and females, typically favoring males when differences occur. Factors contributing to spatial skills could include prenatal as well as experiential/cultural influences, with biological and social influences likely interacting and difficult to disentangle. This meta-analysis examined the potential influence of prenatal androgen exposure on spatial skill by examining studies of patients with congenital adrenal hyperplasia (CAH). CAH involves elevated adrenal androgens prenatally, with overall androgen concentrations higher for females with CAH vs. same-sex controls but with little overall difference between males with CAH versus controls. We hypothesized that, if androgens contribute prenatally to neurobehavioral development in humans as in many other species, females with CAH would show spatial enhancement versus control females, but with no definitive hypothesis for males. Meta-analysis of 12 studies examining overall spatial skill and three spatial subcategories failed to support enhanced spatial performance for females with CAH; males with CAH showed lower spatial ability compared to control males, at least for the category of overall spatial skill. Although statistical logic precludes accepting the null hypothesis for females, the meta-analysis failed to support the idea that prenatal exposure to androgens explains spatial gender/sex differences in humans. Alternative explanations for average gender/sex differences in some spatial tasks could include androgen exposure at other times, such as mini-puberty, or different social factors experienced by males and females. We also discuss possible explanations for the different outcomes seen in females vs. males with CAH.
Keywords: Spatial ability, visuospatial ability, androgen, congenital adrenal hyperplasia (CAH), gender differences, sex differences
INTRODUCTION
Spatial (or visuospatial) abilities contribute to numerous every day behaviors such as wayfinding (Pazzaglia & Moè, 2013) and are thought to be central to occupations such as architecture, dentistry, medicine, design, engineering, and mechanics, among others (Halpern et al., 2007; Halpern & Collaer, 2005). Enhancement of spatial skill has been suggested as a way to promote mathematical performance (Gilligan, Flouri, & Farran, 2017; Nuttall, Casey, & Pezaris, 2005) as well as success and retention of individuals in science, technology, engineering, and mathematical (STEM) careers (Sorby, Veurink, & Streiner, 2018; Stieff & Uttal, 2015; Uttal et al., 2013). A subset of spatial abilities also shows average differences between males and females. When differences occur, they typically favor males, on average (Voyer, Voyer, & Bryden, 1995; but see Voyer, Voyer, & Saint-Aubin, 2017 for evidence of a difference in object location memory favoring females). Depending on the type of spatial task, the magnitude of a male advantage, if any, ranges widely, from negligible (d = .13 to .19 for spatial visualization) to large (as high as d = .94 for certain scoring approaches to 3D mental rotation) (Linn & Petersen, 1985; Voyer et al., 1995). Opinions diverge as to whether these differences are practically meaningful (Hyde, 2005; Voyer et al., 1995); however, given the relevance of spatial skills to numerous competencies, the origins of such differences are of interest.
A great deal of research has investigated why males and females differ behaviorally, including cognitively. Current thinking suggests that many behavioral gender/sex differences1 reflect a combination of inborn and experiential factors operating jointly in bi- or multi-directional ways (Hines, 2004). For example, inborn factors may shape certain behavioral starting points, but subsequently, behaviors are influenced by socialization from parents, peers, and the larger society, as well as self-socialization as children develop gender schemas and attempt to match their behavior to societal expectations (Halpern et al., 2007; Hines et al., 2016a; Maccoby & Jacklin, 1974; Martin & Ruble, 2010; Ruble et al., 2007; Wong & Hines, 2015).
Potential inborn factors might include hormonal influences such as prenatal exposure to androgens. During prenatal development, testosterone and other androgens influence somatic development, such as directing genital formation into a male-typical form. They also exert neurobehavioral effects, influencing development of the brain as well as subsequent behaviors in a wide range of mammals. Results of true experiments manipulating androgens in nonhuman species indicate that early androgens contribute organizationally to later sex differences in reproductive behaviors (Arnold, 2009), juvenile play (Meaney, 1988), and spatial cognition, including aspects of maze performance (Isgor & Sengelaub, 2003; Roof, 1993; Williams, Barnett, & Meck, 1990).
In humans, consistent evidence suggests that androgen exposure during early life also influences gender/sex-typed toy and activity interests, sexual orientation, gender identity, and some, though not all, gender/sex-related personality characteristics (Berenbaum & Hines, 1992; Hines, 2011; Hines, 2015; Hines, Golombok, Rust, Johnston, & Golding, 2002; Nordenstrom, Servin, Bohlin, Larsson, & Wedell, 2002). However, evidence for an influence of early androgen exposure on human cognition, particularly spatial abilities, has been less consistent (Hines, Constantinescu, & Spencer, 2015). True experimental evidence for such an effect cannot be obtained in humans due to ethical constraints; however, certain medical disorders mimic aspects of experiments involving androgen manipulations. One such disorder is classical congenital adrenal hyperplasia (CAH).
CAH is a family of disorders involving abnormal steroidogenesis by the adrenal glands, with deficient synthesis of cortisol and sometimes mineralocorticoids but elevated synthesis of adrenal androgens (El-Maouche, Arlt, & Merke, 2017; Witchel, 2017). The disorder occurs in approximately 1:14,000 to 18,000 births and usually results from a mutation of the gene encoding for the 21-hydroxylase (21-OH) enzyme (Speiser et al., 2018). Cortisol is normally regulated such that when its production is deficient, as in CAH, its precursors become elevated and are ultimately shunted into the intact pathway for adrenal androgens causing elevated androgen synthesis beginning prenatally (El-Maouche et al., 2017). Both males and females with CAH experience elevated adrenal androgens, but overall androgen exposure is more markedly atypical for females because the ovaries, unlike the testes, normally produce negligible concentrations of androgens and other steroids prenatally (Carr, 1998; Miller & Auchus, 2011). The high concentrations of androgens experienced prenatally by females with CAH virilize the external genitalia to varying degrees. Androgen concentrations of males with CAH appear to be in the normal to high normal range prenatally (Pang et al., 1980; Pang, Levine, Chow, Faiman, & New, 1979). Classical CAH due to 21-OH deficiency occurs in salt wasting (SW) and simple virilising (SV) forms (El-Maouche et al., 2017). Mineralocorticoids, such as aldosterone, are additionally deficient in the SW form of CAH leading to life-threatening crises involving severe dehydration, if untreated (White & Speiser, 2000). Diagnosis and treatment terminate the overproduction of androgens and the deficiencies of cortisol and aldosterone (if any) and help prevent electrolyte crises, also called SW episodes.
To understand whether androgen exposure prenatally may contribute to neurobehavioral development in humans, patients with CAH, particularly females, have been studied in comparison to controls for a number of behaviors that show average gender/sex differences in typical populations. Thus, research with CAH patients may help elucidate whether prenatal exposure to androgen contributes to gender/sex differences in spatial performance. In addition, research on spatial performance in individuals with CAH could provide a greater understanding of the clinical and practical consequences of diagnosis with CAH.
Despite the value of studying patients with CAH, large samples are difficult to obtain due to the rarity of the disorder. Challenges in recruiting patients often lead to studies with small samples, with some group sizes in published studies as low as four or five. Because of the statistical dangers of significance testing in and extrapolating results from very small samples, applying meta-analysis to studies of individuals with CAH can help clarify reliable patterns of findings in this population. Meta-analysis weighs results in terms of their statistical reliability, effectively aggregating numerous studies to compute a more stable estimate of the effect.
One meta-analysis of spatial ability in CAH concluded that spatial enhancement exists in females with CAH, and it was suggested that early-life androgens may have direct effects on underlying neural contributors to spatial abilities or to interests that might facilitate them (Puts, McDaniel, Jordan, & Breedlove, 2008). Conversely, that meta-analysis found impairment of spatial skill in males with CAH. New studies of spatial performance in individuals with CAH have become available since then, suggesting the need for an update. Further, our approach differs from that of the 2008 meta-analysis by analyzing skills according to defined spatial task subcategories. The 2008 study hoped to focus on three-dimensional mental rotation skills but ultimately also included two-dimensional rotational measures, as well as non-rotational measures. An additional goal of the current meta-analysis was to increase the size and, thus, the repress entativeness of the results by maximizing available samples. The 2008 meta-analysis di d not always include data from all participants in relevant studies. For example, for one study only a small subset of participants who were matched to same-sex family members were included (Baker & Ehrhardt, 1974) and in another, only a subset matched for intelligence (Helleday, Bartfai, Ritzen, & Forsman, 1994), although similar matching did not exist in other studies included in that meta-analysis.
Spatial abilities are heterogeneous in nature, tapping different constellations of skills (Voyer et al., 1995). Meta-analyses of spatial abilities, and particularly gender/sex differences in them, tend to divide tasks into three subcategories: mental rotation, spatial perception, and spatial visualization (Linn & Petersen, 1985). Voyer et al. (1995) adopted a test-by-test approach but also recognized these as reasonable spatial subcategories. Mental rotation is the ability to quickly and accurately imagine an object from a different perspective in two- or three-dimensional space; spatial perception involves assessing spatial relationships regardless of distracting information, often in reference to a body or gravitational reference; and spatial visualization involves evaluating and transforming complex visual material using multistep analytical spatial processes (Linn & Petersen, 1985; Voyer et al., 1995). Males outperform females, on average, for mental rotation (average category effect size, d = .56 to .73) and spatial perception (d = .44) tasks, with less consistent and very small or arguably negligible gender/sex differences obtained for spatial visualization (d = .13 to .19) (Linn & Petersen, 1985; Voyer et al., 1995). Because effects of sex steroid hormones are most likely to be observed on variables that show gender/sex differences (Hines et al., 2015), our meta-analysis focused on measures with well-characterized gender/sex differences, such as those studied in the meta-analysis of Voyer et al. (1995) or tasks with clear links to one of the three spatial subcategories.
If androgens in typical males contribute prenatally to their average advantage on the subset of spatial tasks that tend to show gender/sex differences, we hypothesized that females with CAH would perform better than typical females in overall spatial abilities. Further, we hypothesized that any performance advantage for females with CAH would manifest largely on mental rotation and spatial perception tasks, because these show substantial gender/sex differences, with negligible, or no, enhancement on spatial visualization measures. Considering potential outcomes in males, much of the evidence from prior studies has reported no significant differences in gender/sex-related behavior for males with CAH compared to controls, although the prior meta-analysis found reduced spatial performance in males with CAH. Therefore, we will evaluate spatial performance in males with and without CAH with no specific hypothesis.
METHOD
Eligibility Criteria and Study Selection
Eligible studies included (1) patients with classical CAH and healthy control participants, and (2) one or more spatial dependent measures fitting one of the spatial subcategories indicated earlier (see Table 1; Linn & Petersen, 1985; Voyer et al., 1995). Data were not analyzed for SW and SV subgroups separately because an insufficient number of studies report data in this fashion and because further subdivision would decrease already small sample sizes. We attempted to use the largest samples available within each study, avoiding reliance on more restricted groupings based on subset matching or intelligence cut offs. Additionally, we aimed to include all spatial dependent measures that fit into any one of the spatial subcategories.
Table 1.
Studies and tasks contributing to each category of spatial ability
| Study | Mental Rotation | Spatial Perception | Spatial Visualization | Intelligence Measure |
|---|---|---|---|---|
| Baker and Ehrhardt (1974) | *PMA-Spatial Relations | Wechsler FSIQ | ||
| Berenbaum et al. (2012) | V&K Mental Rotations | Water Level | Advanced Vocabulary | |
| PMA-Spatial Relations | ||||
| Dittmann et al. (1993) | Block Design | Shortened Wechsler IQ, German version | ||
| Hampson and Rovet (2015) | V&K Mental Rotations | DAT-Space Relations | Wechsler FSIQ | |
| Card Rotations | ||||
| Hampson et al. (1998) | *PMA-Spatial Relations | Wechsler FSIQ | ||
| Helleday et al. (1994) | *Figure Rotation | Rod-and-Frame | SRB3 (Block Design) Gottschaldt Hidden Figures | SRB 1–3 (Synonyms, Reasoning, Block Design) |
| Hines et al. (2003)/ Collaer et al. (2016) | *V&K Mental Rotations *PMA-Spatial Relations |
Water Level Rod-and-Frame JLAP | Vocabulary subtest of the Wechsler IQ scale | |
| Inozemtseva et al. (2008) | Lines Orientation (embedded figures-type test) | Wechsler IQ Scale for Mexican Children | ||
| Malouf et al. (2006) | *Cube Comparisons | NART Estimated FSIQ | ||
| McGuire et al. (1975) | *Block Design | Prorated Wechsler FSIQ | ||
| Resnick et al. (1986) | *V&K Mental Rotations Card Rotations | Paper Folding Paper Form Board Hidden Patterns | Ravens Progressive Matrices-Modified |
Note. PMA = Primary Mental Abilities battery; V & K = Vandenberg and Kuse; JLAP = Judgment of Line Angle and Position; DAT = Differential Aptitude Tests; FSIQ = Full Scale IQ; NART = National Adult Reading Test-Revised. Consult original sources for references to and details on the intelligence measures. Tasks with an asterisk represent ones included in both the current meta-analysis as well as Puts et al. (2008).
Relevant studies were sought in PsycINFO, PubMed, and ERIC using the search terms “congenital adrenal hyperplasia or CAH or adrenogenital syndrome” AND “spatial or visuospatial or cognit*”. In ProQuest, a more general database, these same terms were used to search keywords, titles and abstracts, and included searches of conference papers and proceedings, dissertations/theses, and scholarly journals. In each database, the titles of identified documents were read, and obviously irrelevant ones were eliminated. This produced a collection of 87 articles after removing duplicates. We read each abstract, retaining 66 potential articles for full text consideration. With one exception (Somajni et al., 2011, an article that we were unable to obtain via libraries at multiple institutions or interlibrary loan), each article was acquired in full text and examined for eligibility. In addition, 29 researchers in the field were contacted via email to explore the potential availability of unpublished spatial data for inclusion. Ten replied, but none had unpublished data to add. Ultimately, 12 articles representing 11 samples were identified.
Procedure
Spatial effect sizes in the form of standardized mean difference or Cohen’s d (Cohen, 1988) were computed comparing patients with CAH to same-sex controls for each sex and each spatial task. We adopted Cohen’s (1988) interpretations of effect size magnitudes (.20 small, .50 moderate, .80 large). Data were coded such that a positive effect size indicated better performance by patients with CAH vs. same-sex controls, and negative effects indicated better performance by controls. We also computed an effect size for intelligence scores for each of the studies in the meta-analysis. As with the spatial effect sizes, positive values indicated higher intelligence scores for CAH patients vs. controls, and negative values indicated higher scores for controls.
For accuracy, two individuals independently extracted task means, SDs, and sample sizes from manuscripts and computed d and variance values. Any discrepancies were resolved by identifying their source and correcting them. Standardized mean differences were adjusted using a small sample correction factor (J) to produce Hedges’ g (Borenstein, Hedges, Higgins, & Rothstein, 2009) that formed the computational basis of the meta-analysis.
Four meta-analyses were conducted (overall spatial ability, mental rotation, spatial perception, and spatial visualization) and results for each sex are presented separately. When a sample had multiple tasks in the same subcategory (e.g., two mental rotation tasks; see Table 1), the in-category effect sizes were averaged (Lipsey & Wilson, 2001). Additionally, for overall spatial performance, effect sizes were averaged across the set of all spatial tasks within a study. Ultimately, each contributing sample was represented by only one effect size per sex in any meta-analysis. See Table 2 for further information on measures, ages, and sample sizes. The analysis of overall spatial performance included effect sizes from 11 samples of females and 7 samples of males. The mental rotation category included 8 samples of females and 6 of males; spatial perception included 3 samples of females and 2 of males; and spatial visualization included 6 samples of females and 3 of males (see Table 3). Considering all possible spatial effect sizes, this meta-analysis reflects findings from a maximum of 207 females with CAH, 153 female controls, 86 males with CAH, and 98 male controls although these numbers are lower for individual spatial subcategories. For comparison, the earlier meta-analysis reported inclusion of 128 females with CAH, 108 female controls, 61 males with CAH, and 64 male controls (Puts et al., 2008). However, they appear to have included 98 distinct female controls rather than 108 because they apparently counted the same ten family controls twice from one study (Malouf, Migeon, Carson, Petrucci, & Wisniewski, 2006).
Table 2.
Studies, measure sources, ages, and sample sizes
| Study | Spatial measures | Age range (years) | Female CAH N | Female Control N | Male CAH N | Male Control N |
|---|---|---|---|---|---|---|
| Baker and Ehrhardt (1974); Baker (1993) for demographics | PMA-Spatial Relationsa | 4–29 | 13 | 11 | 8 | 14 |
| Berenbaum et al. (2012) | V&K Mental Rotationsb PMA-Spatial Relationsa
Water levelc |
16–30 | 19 | 13 | 12 | 14 |
| Dittmann et al. (1993) | Block Designd | 16–41s | 17 | 10 | - | - |
| Hampson and Rovet (2015) | V&K Mental Rotationsb
Card Rotationse DAT-Space Relationsf |
12–30t | 22 | 13 | 8 | 6 |
| Hampson et al. (1998) | PMA-Spatial Relationsg (suitable for 8–12 yr olds) | 8–12 | 7 | 6 | 5 | 4 |
| Helleday et al. (1994) | Figure Rotationh Rod-and-Framei SRB3 (Block Design)j Gottschaldt Hidden Figuresk |
17–34 | 22 | 22 | - | - |
| Hines et al.(2003)/Collaer et al. (2016) | V&K Mental Rotations-Revl PMA-Spatial Relationsa Water Levelm Rod-and-Framen JLAPo |
12–45 | 40 | 29 | 29 | 30 |
| Inozemtseva et al. (2008) | Lines Orientation (embedded figure task)p | 7–16 | 11 | 11 | - | - |
| Malouf et al. (2006) | Cube Comparisonse | 20–73 | 24 | 10 | - | - |
| McGuire et al. (1975); McGuire and Omenn (1975) | Block Designq | 5–32 | 15 | 15 | 16 | 16 |
| Resnick et al. (1986); Resnick (1982) | V&K Mental Rotationsb Card Rotationse
Paper Foldinge Paper Form Boardr Hidden Patternse |
11–31 | 17 | 13 | 8 | 14 |
Note. PMA = Primary Mental Abilities battery; V & K = Vandenberg and Kuse; JLAP = Judgment of Line Angle and Position; DAT = Differential Aptitude Tests.
Citations provided by authors, unless noted otherwise (see Supplementary Material for spatial test references as provided by original studies):
Thurstone (1963); despite minor name differences in original articles, PMA “Spatial” (Baker & Ehrhardt, 1974) and “Space” (Berenbaum et al., 2012) are presumed or confirmed to be the PMA-Spatial Relations test.
Vandenberg and Kuse (1978).
Harris (undated) personal communication, see Berenbaum et al. (2012).
Subtest of the German-language Wechsler Adult Intelligence Scale, Wechsler (1955).
Ekstrom et al. (1976).
Bennett et al. (1982).
Cited as Thurstone & Thurstone (1963), but the same as Thurstone (1963), personal communication with author.
Dureman et al., (1971).
Bergman et al. (1985).
Lezak (1983).
Kolb & Whishaw (1985).
Peters et al. (1995).
Liben (1990).
Oltman (1968), Witkin et al. (1962).
Collaer & Nelson (2002).
Matute et al. (2007).
Subtest of the Wechsler Intelligence Scale for Children or Wechsler Adult Intelligence Scale; Wechsler (1958, 1965).
No citation given, but presumably an edition or modification of the Minnesota Paper Form Board Test (Quasha & Likert, 1937).
Ages for the full sample started at 11 years, but the relevant spatial means and SDs were given only for participants aged 16 and older.
Participants aged 11.5 to 21 years were recruited and three females above 21 years also volunteered, with ages available for two of the three (22 and 30 years).
Table 3.
Summary statistics from the spatial meta-analyses
| Spatial Category | Sex | RE Effect Size (g) | [95% Cl] | Test of significance, z (p) | N of Samples | Homogeneity (Q)Ɨ | Evid. of Pub. Bias? |
|---|---|---|---|---|---|---|---|
| Overall Spatial | F | .005 | [−.219, .228] | 0.04 (.967) | 11 | 11.14 | Yes |
| Mental Rotation | F | .166 | [−.108, .440] | 1.19 (.235) | 8 | 8.75 | Yes |
| Spatial Perception | F | −.336 | [−.827, .156] | −1.34 (.181) | 3 | 4.26 | —a |
| Spatial Visualiz. | F | −.054 | [−.345, .237] | −0.36 (.718) | 6 | 4.09 | Yes |
| Overall Spatial | M | −.354 | [−.643, −.066] | −2.41 (.016)* | 7 | 3.97 | Yes |
| Mental Rotation | M | −.271 | [−.668, .126] | −1.34 (.181) | 6 | 7.04 | No |
| Spatial Perception | M | −.369 | [−.790, .052] | −1.72 (.086) | 2 | 0.01 | —a |
| Spatial Visualiz. | M | −.422 | [−.900, .056] | −1.73 (.084) | 3 | 0.35 | —a |
Note. F = female, M = male, RE = random effects, CI = confidence interval, Q = homogeneity statistic, Evid. of Pub. Bias? = Evidence of publication bias?, Visualiz. = Visualization.
Publication bias was not investigated for meta-analyses with as few as 2 or 3 contributing studies
p < .05
all ps > .12
We used Comprehensive Meta-Analysis (CMA) software, version 2 (Borenstein, Hedges, Higgins, & Rothstein, 2005) to compute the meta-analysis. As suggested by Lipsey and Wilson (2001) for meta-analyses with small samples and small numbers of studies, we adopted random rather than fixed effects models. Sensitivity analyses and investigations of publication bias were also conducted. We considered spatial categories with three or fewer studies to be insufficient for such investigations although we know of no definitive rules for this. In reality, because no spatial category in our meta-analysis included four or five studies, publication bias and sensitivity analyses were always based on at least six contributing studies (see Table 3). Sensitivity analyses explored the robustness of results when eliminating individual studies. Publication bias was evaluated by examining funnel plots for asymmetry; however, because this is somewhat subjective (Duval & Tweedie, 2000b), we also used more formalized techniques: the Begg and Mazumdar (1994) rank order correlation and Duval and Tweedie’s trim and fill method (Borenstein et al., 2009; Duval & Tweedie, 2000a), fitting data with a random effects model. When asymmetry is found, Duval and Tweedie’s approach also estimates an adjusted effect size and a confidence interval estimated to eliminate the bias.
Any potential role of group differences in intelligence in the results of the meta-analyses was explored using meta-regression. The dependent measure of the regression was the effect size for spatial performance while the predictor was the effect size for the standardized mean difference in intelligence (g for intelligence) between patients and controls for each sex from each study.
RESULTS
Overall Spatial Performance
Males and Females
Analysis of overall spatial performance for all patients compared to all controls (considering both male and female subgroups) produced a g of −.130, 95% confidence interval (CI) [−.307, .047], z = −1.44, p = .149. This indicates a lack of significant difference for overall spatial skill between patients and controls. However, the pattern of effect sizes was heterogeneous, differing significantly for males and females, Q(1) = 3.66, p = .056 (using the suggested p value of 0.10 for heterogeneity tests; Lau, Ioannidis, & Schmid, 1997). We also had different hypotheses regarding spatial task performance for males and females. Thus, for this and all subsequent spatial categories, the performance of males and females was analyzed separately.
Females
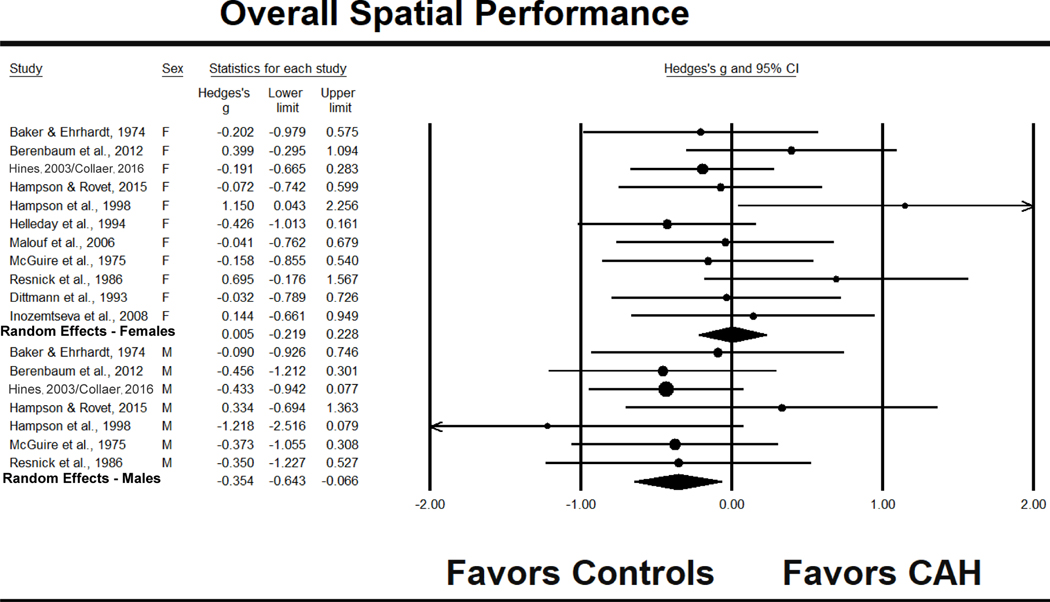
The overall spatial effect size, g, for females was .005, 95% CI [−.219, .228] (see Fig. 1). Table 3 provides additional information on z and p values, homogeneity, and publication bias. There was insufficient evidence to suggest that females with CAH differed significantly from control females in terms of overall spatial performance. There was no statistically significant heterogeneity between effect sizes (see Table 3), perhaps suggesting the use of a fixed effects model. As discussed in the Method, however, we report results from a random effects model for this and other analyses as recommended for small studies (Lipsey & Wilson, 2001). These findings were robust as shown by the “omit one study” analyses. Omitting each study individually produced effect sizes from −.049 to .056, with no outcome showing a statistically significant difference between females with CAH and control females.
Figure 1.
Effect sizes (g) for overall spatial performance comparing patients with CAH to same-sex controls. F = females, M = males.
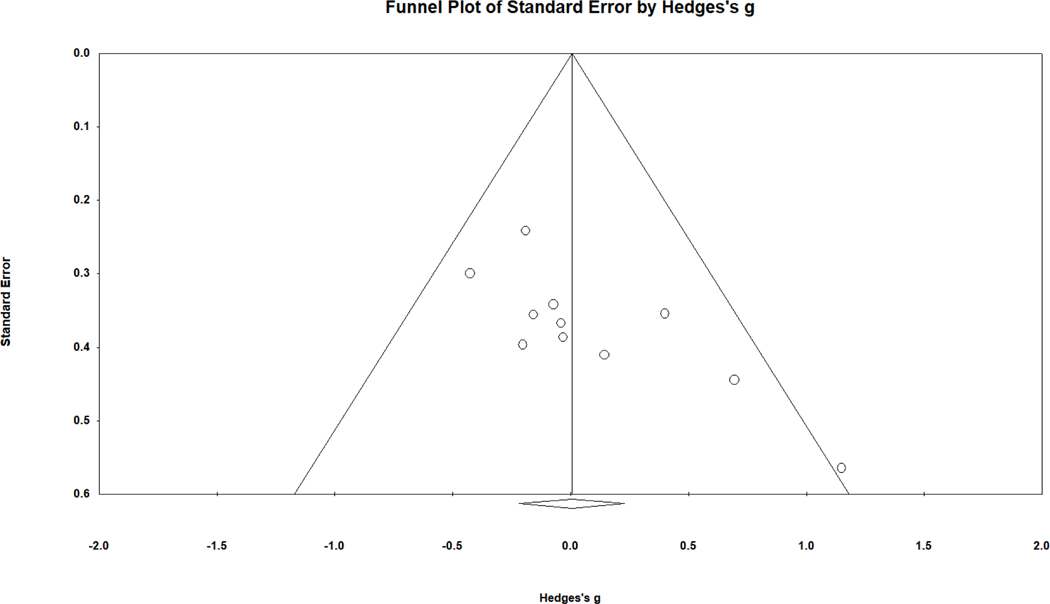
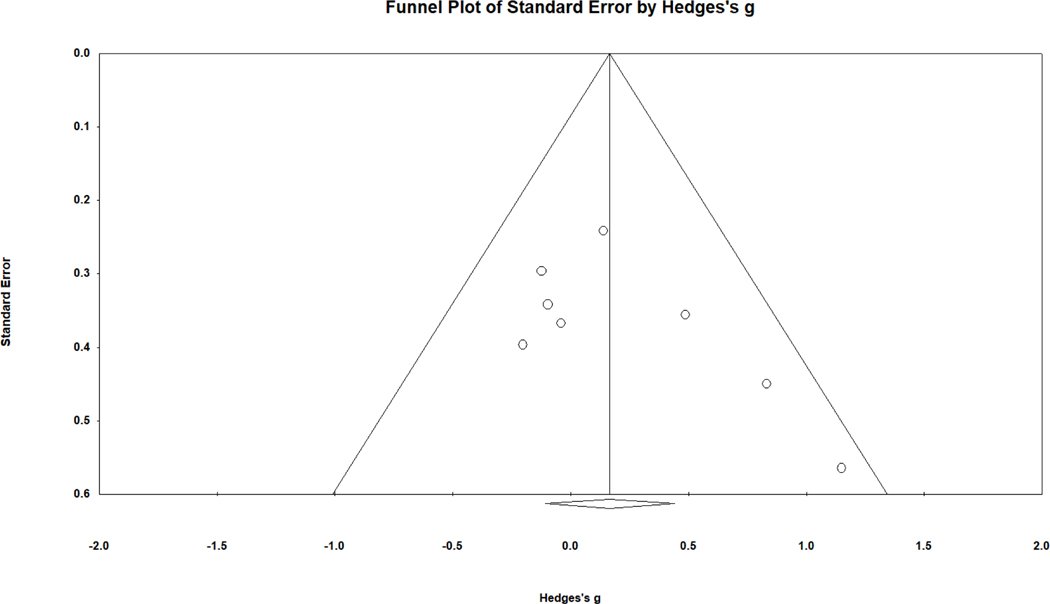
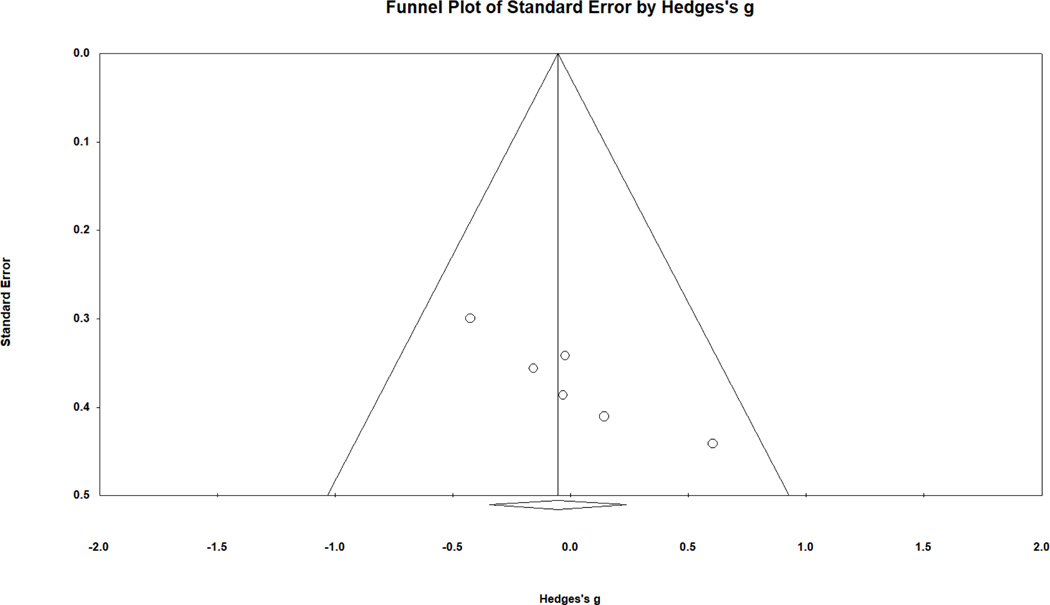
To explore the possibility of publication bias, a funnel plot was reviewed. A funnel plot demonstrates the relationship between study precision (roughly the inverse of standard error or sample size) and its obtained effect size. This funnel plot (see Fig. 2) indicates asymmetry, with the smallest samples (located near the bottom of the funnel plot and having the largest standard error) reporting the largest effect sizes favoring females with CAH. Removal of the most asymmetric study shifted the overall effect size to a negligible negative value, g = −.049, 95% CI [−.263, .165], again with no statistically significant difference from zero. In further support of the notion of asymmetrical publication patterns, the Begg and Mazumdar rank order correlation between sampling variance (higher for smaller samples) and effect size was .58, p = .006 (one-tailed as recommended). In other words, smaller samples produced larger effect sizes favoring females with CAH, an outcome suggestive of publication bias. The trim and fill technique did not identify asymmetry or suggest an adjusted effect size.
Figure 2.
Funnel plot of standard error in relation to effect size (g) for overall spatial performance comparing females with CAH to female controls. Higher precision (lower standard error) is observed for studies closer to the top of the graph.
Males
The overall spatial performance of males with CAH versus control males was g = −.354, 95% CI [−.643, −.066] (see Fig. 1). Thus, males with CAH showed a small, but statistically significant, reduction in overall spatial skill compared to male controls. Heterogeneity between effect sizes was not statistically significant. Omitting individual studies in a sequential fashion produced g values ranging from −.413 to −.309, leading to the same conclusion with one exception. Without the Hines et al. (2003)/Collaer et al. (2016) sample, the finding of lowered overall spatial skill in males with CAH only approached a conventional level of significance, z = −1.78, p = .076.
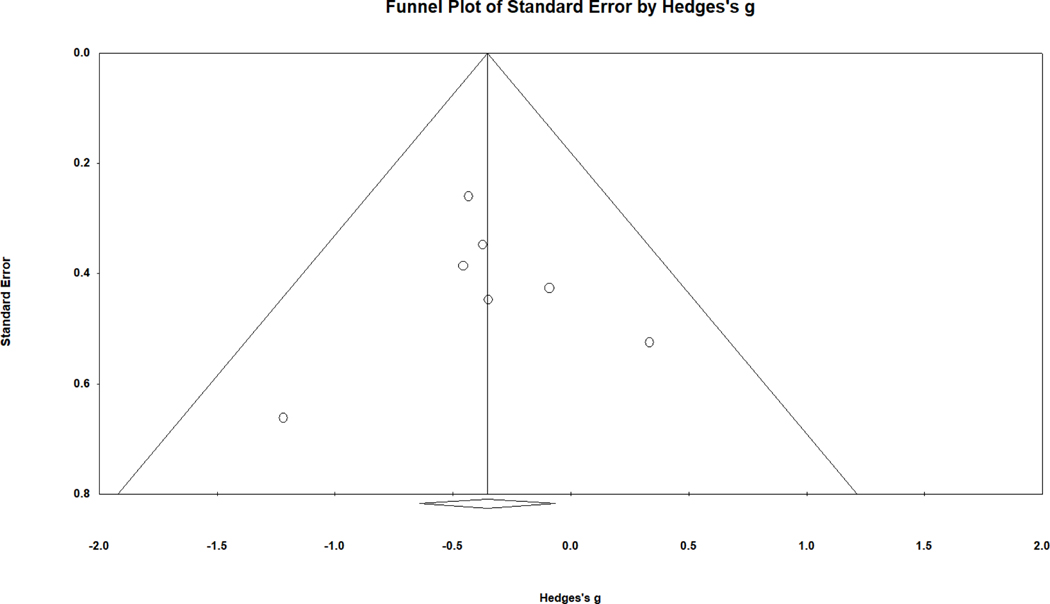
The funnel plot for males (see Fig. 3) also showed asymmetry, with the smallest samples reporting the largest effect sizes, but in this case favoring control males over males with CAH. The Begg and Mazumdar rank order correlation was not significant, but the trim and fill approach indicated asymmetry and produced an adjusted point estimate of g = −.309, 95% CI [−.591, −.028], again showing significantly lower performance by males with CAH. The fail-safe N value (Borenstein et al., 2009), three in this case, indicates the number of additional studies with null results (i.e., showing no difference between the male groups, g = 0) that would be needed to reduce the overall effect size to an insignificant level. Thus, this suggests that three studies with effect sizes near zero (compared to the seven studies of the meta-analysis) would be needed to negate this finding.
Figure 3.
Funnel plot of standard error in relation to effect size (g) for overall spatial performance comparing males with CAH to male controls. Higher precision (lower standard error) is observed for studies closer to the top of the graph.
Mental Rotation
Females
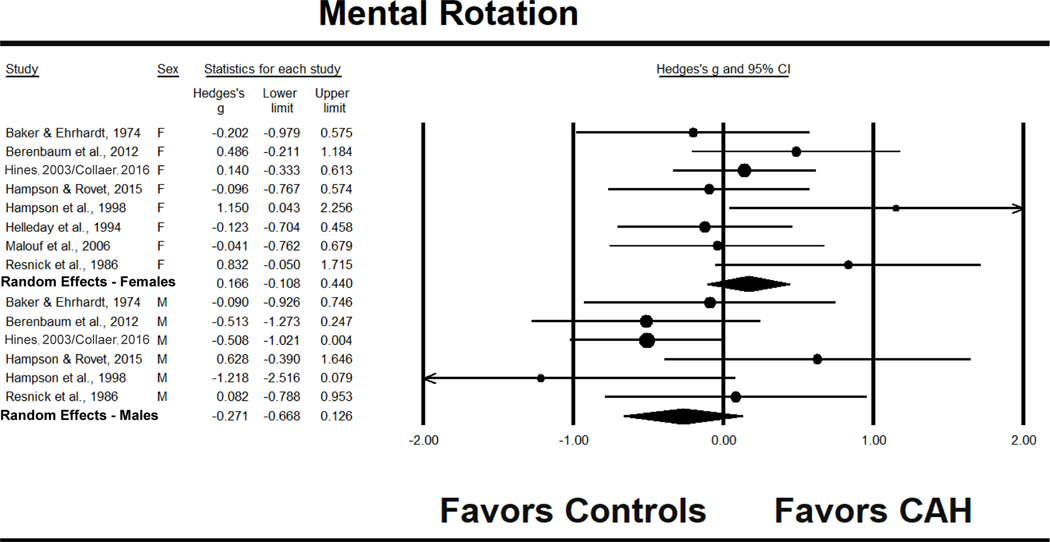
For the eight studies contributing effect sizes for mental rotation in females, g = .166, 95% CI [−.108, .440]. There was no statistically significant difference between females with CAH and control females and no significant heterogeneity (see Fig. 4 and Table 3). When omitting studies individually, effect sizes for the remainder ranged from .099 to .225, producing no outcome where females with CAH differed significantly from control females. The funnel plot indicated asymmetry (see Fig. 5). Application of the trim and fill procedure indicated bias, and adjustments correcting for the asymmetry produced a lower adjusted effect size, shifting from g = .166 to .112, 95% CI [−.190, .414]. The Begg and Mazumdar rank correlation exploring whether smaller studies produced larger effect sizes approached the conventional criterion of significance, p = .087, one-tailed.
Figure 4.
Effect sizes (g) for mental rotation performance comparing patients with CAH to same-sex controls. F = females, M = males.
Figure 5.
Funnel plot of standard error in relation to effect size (g) for mental rotation performance comparing females with CAH to female controls. Higher precision (lower standard error) is observed for studies closer to the top of the graph.
Males
The six studies investigating mental rotation in males produced a g = −.271, 95% CI [−.668, .126], with no statistically significant difference between males with CAH and control males, and no evidence of significant heterogeneity (see Fig. 4). Analyses omitting each of the six studies individually led to the same conclusion in five cases, with g values ranging from −.402 to −.172. However, after removal of Hampson and Rovet (2015), the remaining studies suggested a significant lowering of mental rotation performance in males with CAH, g = −.402, 95% CI [−.737,−.066], z = −2.35, p = .019. The funnel plot for these studies (see Fig. 6) was not obviously asymmetric. In support of this, the trim and fill analysis did not suggest asymmetry or an adjusted effect size, and the Begg and Mazumdar rank order correlation failed to reach statistical significance.
Figure 6.
Funnel plot of standard error in relation to effect size (g) for mental rotation performance comparing males with CAH to male controls. Higher precision (lower standard error) is observed for studies closer to the top of the graph.
Spatial Perception
Few studies assessed spatial perception performance in CAH: three studies included females with and without CAH and two included males. Given the small number of contributing studies, the “omit one study” and publication bias analyses were considered to not be appropriate.
Females and Males
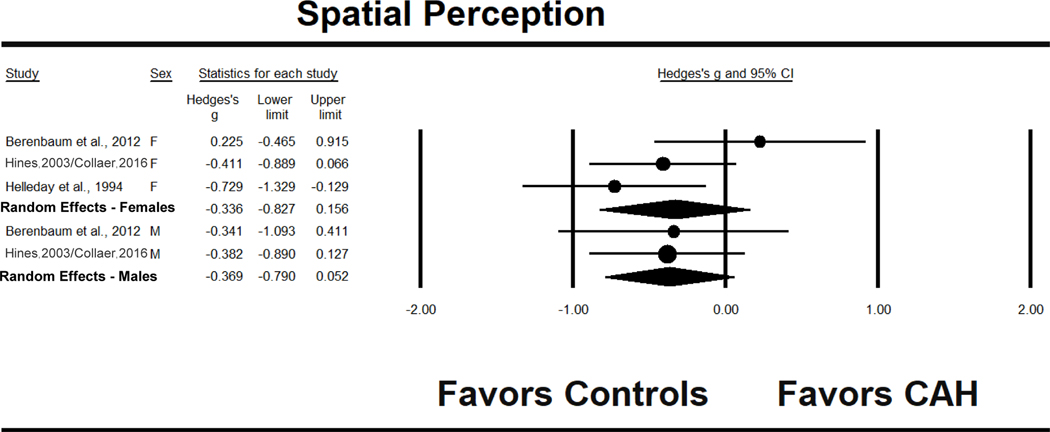
The spatial perception effect size for females was g = −.336, 95% CI [−.827, .156] and for males, g = −.369, 95% CI [−.790, .052] (see Fig. 7). Thus, both males and females with CAH showed effect sizes in a negative direction, but neither group differed significantly from same-sex controls. However, the results for males with CAH approached, but did not reach, the conventional threshold for significance (see Table 3). Heterogeneity results were not significant for females or males.
Figure 7.
Effect sizes (g) for spatial perception performance comparing patients with CAH to same-sex controls. F = females, M = males.
Spatial Visualization
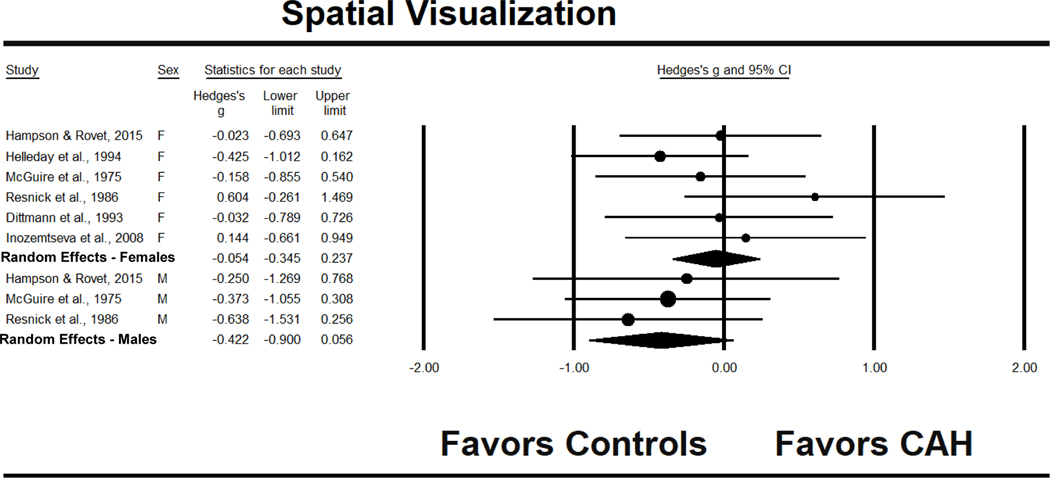
Females
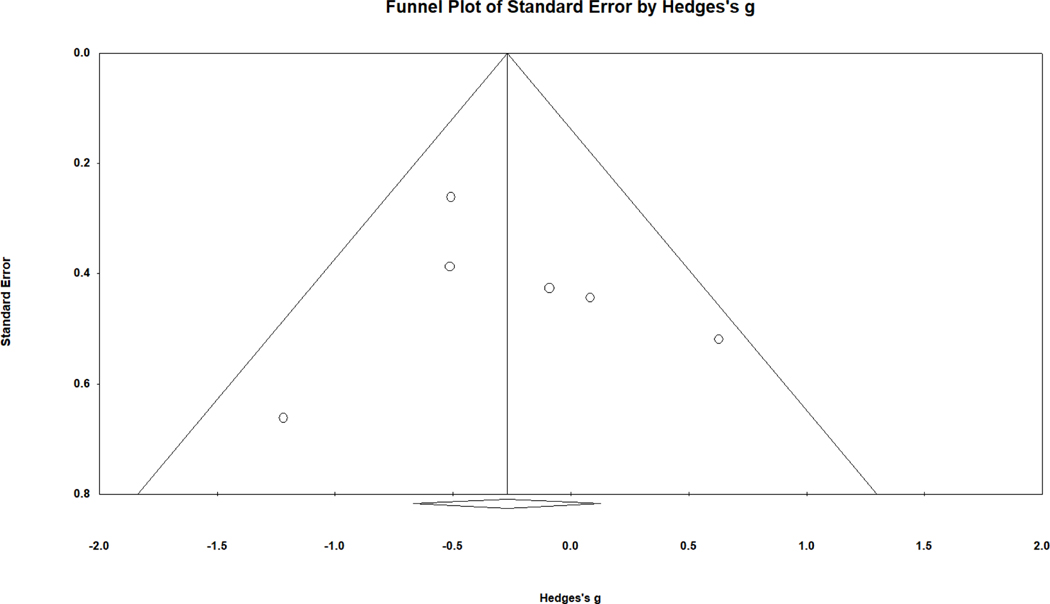
The effect size for females with CAH compared to control females, based on six studies, was g = −.054, 95% CI [−.345, .237], without significant heterogeneity (see Fig. 8). Omit one study analyses produced effect sizes ranging from g = −.138 to .067 and were consistent in showing no statistically significant differences between females with CAH and control females. To explore the possibility of publication bias, Figure 9 presents the funnel plot. Studies with larger samples tended to produce effect sizes that were near zero or negative, while smaller samples produced effect sizes that were larger and more positive. In support of this conclusion, the Begg and Mazumdar rank correlation was significant at .667, p = .030, and the trim and fill procedure also indicated asymmetry, producing an adjusted effect size of g = −.182, 95% CI [−.475, .111], a lower value, but again, without a statistically significant difference from zero.
Figure 8.
Effect sizes (g) for spatial visualization performance comparing patients with CAH to same-sex controls. F = females, M = males.
Figure 9.
Funnel plot of standard error in relation to effect size (g) for spatial visualization performance comparing females with CAH to female controls. Higher precision (lower standard error) is observed for studies closer to the top of the graph.
Males
The spatial visualization effect size for males based on three studies was g = −.422, 95% CI [−.900, .056], without significant heterogeneity. These results approached, but did not reach, the conventional threshold for significance (see Table 3). Given the small number of available studies, the omit one study and publication bias analyses were not considered to be appropriate for males.
Relationship of Task Performance to Intelligence
An early report on individuals with CAH reported enhanced general intelligence (Money & Lewis, 1966). Subsequent research suggested, however, that the apparent enhancement resulted from comparing individuals with CAH to outdated population norms (Wenzel et al., 1978). Most studies comparing individuals with CAH to controls, either unaffected relatives or matched controls, have not found group differences on measures of general intelligence (Berenbaum, Bryk, & Duck., 2010; Browne et al., 2015; Dittmann et al., 1990; Hampson, Rovet, & Altmann, 1998; Hines et al., 2003; Hirvikoski et al., 2007; Kelso, Nicholls, Warne, & Zacharin, 2000; Malouf et al., 2006; McGuire & Omenn, 1975; Merke et al., 2003; Nass & Baker, 1991; Resnick, Berenbaum, Gottesman, & Bouchard, 1986), but there are occasional reports of reduced intellectual performance in individuals with CAH (Hamed, Metwalley, & Farghaly, 2018; Helleday et al., 1994; Johannsen et al., 2006). In addition, one of the studies included in this meta-analysis (Hampson & Rovet, 2015) excluded some participants with CAH from statistical analyses, based on “questionable” neurological status, the criteria for which included reduced scores on a measure of general intelligence. Therefore, we investigated whether our results regarding spatial abilities might reflect variability in general intelligence.
To explore this question, we conducted meta-regressions on the same dependent measures of the meta-analysis (overall spatial performance and the three spatial subcategories) using the effect size for intelligence as the predictor when at least five samples were available to provide intelligence scores. Consequently, meta-regressions were conducted for (1) overall spatial ability and each of the three spatial subcategories when including both the male and female subgroups, (2) mental rotation for the female and male subgroups separately, and (3) spatial visualization for the female subgroup only.
When the subgroups for both males and females were entered simultaneously into meta-regressions, the effect size for intelligence never significantly related to the effect size for spatial ability (overall spatial skill, mental rotation, spatial perception, or spatial visualization, with p values for the models ranging from .13 to .74). Conducting meta-regressions for the sexes separately (assuming at least five samples per sex), again the effect size for intelligence never significantly predicted the effect size for spatial performance on any dependent measure (all p values > .20) for females (overall spatial skill, mental rotation, or spatial visualization) or males (overall spatial skill or mental rotation). Studies in the remaining subcategories (spatial perception for females (3 studies), spatial perception for males (2 studies), and spatial visualization for males (3 studies) were considered insufficient for conducting meta-regression. Rules of thumb recommend at least 10 samples, as opposed to five, per predictor variable; however, Borenstein et al. (2009) noted that “no hard and fast rules” exist regarding correct ratios. To summarize, across our studies, intelligence comparisons between patients and controls did not predict differences in spatial skill.
DISCUSSION
We did not find support for the hypothesis that females with CAH who experience elevated exposure to androgens prenatally excel at spatial performance in comparison to control females. Although the logic of statistical testing does not allow us to prove a null hypothesis of no difference, none of the four spatial effect sizes for females (overall spatial, mental rotation, spatial perception, or spatial visualization) differed significantly from zero. Thus, our conclusion contrasts with that of Puts et al. (2008). The relative robustness of our findings in females are supported by sensitivity analyses. Elimination of a study never altered the conclusion of no significant difference between females with CAH and unaffected controls, suggesting that the findings do not hinge on one atypical study.
We further hypothesized that, if androgens prenatally promote spatial skill, females with CAH might show a pattern of greater enhancement on the types of spatial tasks at which typical males most consistently outperform typical females, specifically mental rotation and spatial perception, with smaller (or no) enhancement for spatial visualization (Linn & Petersen, 1985; Voyer et al., 1995). Such a pattern did not emerge from the data. Although the highest relative performance of females with CAH was in mental rotation (g = .166 without adjustment for publication bias, g = .112 after adjustment), the lowest was in spatial perception (−.336), albeit with a small number of contributing studies. For comparison, the spatial visualization subcategory produced an effect size that was near zero or approached a small negative value with trim and fill adjustment (g = −.054 without adjustment for publication bias, g = −.182 after adjustment). Thus, against the hypothesis, this subcategory lay between the other two subcategories in effect size. Critically, however, none of these effect sizes differed significantly from zero, and the magnitudes provide little support for the notion of a graded spectrum of spatial effects that parallel differences between typical males and females.
Our results failing to support enhancement of spatial skills by androgens during prenatal development diverge from findings in some other behavioral domains that show increased male-typical characteristics for females with CAH. These have been observed, for example, in childhood play, sexual orientation, gender identity and some personality traits (Berenbaum & Hines, 1992; Hines, 2011; Hines et al., 2002; Nordenstrom et al., 2002). Of note, however, the gender/sex difference for spatial abilities is smaller in magnitude than for these other domains (Hines et al., 2015; Voyer et al., 1995), potentially reducing the power for detecting effects.
Males with CAH performed worse than control males in overall spatial performance and showed trends towards lowered performance, approaching conventional levels of significance, for spatial perception and spatial visualization. The number of contributing studies for males was lower than for females in each spatial category, perhaps limiting power for detecting differences. For males, only the overall spatial measure and mental rotation subcategory had sufficient studies to justify sensitivity and publication bias analyses, and the findings for males were less robust than for females in the sense that eliminating individual studies was sometimes sufficient to change outcomes in terms of statistical significance. Thus, our findings for males with CAH are less consistent than our findings for females, but they suggest a possible reduction in spatial abilities broadly, in agreement with Puts et al. (2008).
Publication Bias/Asymmetry Findings
Evidence of publication bias occurred consistently for females. All three of the categories with sufficient studies to explore publication bias (overall spatial skill, mental rotation, and spatial visualization) showed asymmetrical funnel plots for females, and each of those also produced formalized evidence of asymmetry based either on the Begg and Mazumdar rank order correlation or Duval and Tweedie’s trim and fill technique. The pattern of asymmetry indicated that smaller studies tended to produce larger effect sizes in favor of superior spatial performance in females with CAH. In agreement with this, when effect sizes were adjusted using the trim- and-fill method, the correction always moved the effect size to the left, meaning to smaller positive or more negative effects. Thus, eliminating publication bias presumably would only buttress confidence in the lack of significant spatial enhancement for females with CAH.
In males, evidence of publication bias/asymmetry was less consistent; however, fewer spatial categories (overall spatial skill and mental rotation) included sufficient studies, and smaller data sets were available for these investigations. One of these (overall spatial skill) showed asymmetry in the funnel plot as well as using a formalized procedure, but asymmetry was not obvious for the subcategory of mental rotation in males. As a caution, tests for publication bias tend to be underpowered, especially when analyzing small numbers of studies (under ten) as here; therefore, a lack of a significance cannot be assumed to guarantee a lack of bias or asymmetry (Ioannidis & Trikalinos, 2007).
Sources of asymmetry in funnel plots or presumed publication bias stem, in part, from the fact that not all studies and results are equally likely to be published (Duval & Tweedie, 2000a). For example, researchers may not report studies or variables that are not statistically significant, and editorial policy may discourage publication of such work (Hedges, 1992). Further, the degree of bias introduced is sensitive to sample size among other factors, and bias can be very large when samples are small (Hedges, 1992), a common situation in studies of CAH. Additional contributors to bias and effect size distortion in this and many other fields arise from the selective reporting of outcomes and “significance chasing” (Ioannidis & Trikalinos, 2007). Going forward, these issues might be addressed by pre-registering studies or storing data in open archives independent of publication outcomes (Ioannidis & Trikalinos, 2007; Voyer & Jansen, 2017).
Comparison to Prior Meta-Analysis
Compared to Puts et al. (2008), our results differed for females but were similar for males. For females, the Puts et al. (2008) effect size was 0.47 (also a random effects model) significantly favoring females with CAH over female controls. This is a sizeable value, approaching a moderate effect size of 0.50 (Cohen, 1988). However, their 95% confidence interval for the estimate ran from .01 to .92. In other words, according to Cohen’s (1988) designations, the effect size for CAH females in the prior meta-analysis might be as small as essentially zero or could extend up to a value exceeding the 0.80 criterion for a large effect–a range so wide that its descriptiveness could be considered of limited usefulness. By using the fuller samples compared to Puts et al., as well as new studies, our standard errors were smaller and the precision of the estimate has risen, with the CI for overall spatial ability in females cut roughly in half, −.219 to .228. For males, both the current and former meta-analyses found lowered spatial skills in CAH. However, the current effect size for males was smaller in magnitude and narrower in CI (g = −.354 [−.643, −.066]) compared to the earlier meta-analysis (g = −.60 [−1.15 −.05]; Puts et al., 2008). Another comparison relates to the specific tasks included. Puts et al. stated a desire to focus on tasks of 3D mental rotations, but given constraints of task availability, often included measures of 2D rotation or measures without strong rotational demands, such as the Block Design test, or no rotational component, the Healy Pictorial Completion task. The current meta-analysis provides an overall spatial measure for each sex in addition to well-defined subcategory results.
Patterns for Females vs. Males with CAH
Why do males with CAH appear to show significant impairment compared to same-sex controls but females with and without CAH do not differ? One possibility is that females benefited from earlier diagnosis due to ambiguous external genitalia and received earlier treatment leading to better control starting potentially shortly after birth, while males were typically less likely to be identified early, at least before widespread neonatal screening for CAH (White & Speiser, 2000). Consequently, males may have been more likely to experience electrolyte/SW crises, and these crises could produce some degree of neural impairment (White & Speiser, 2000). Females can also experience these crises but, before the availability of neonatal screening for CAH, they tended to experience fewer than males because of their earlier treatment. The onset of neonatal screening for CAH has varied by country and by state within the U.S. (El-Maouche et al., 2017). Given the publication dates of the studies in this meta-analysis, participant ages, and the relatively long time required to complete CAH studies, presumably most samples in this meta-analysis would predate widespread neonatal screening protocols, potentially suggesting more health or neural consequences for these males.
Second, androgens have been proposed to influence neurobehavioral development at several developmentally-sensitive time points: prenatally, during an early postnatal androgen surge, and at puberty. The early postnatal androgen surge, sometimes called mini-puberty (Hines et al., 2016b), occurs during the first six months of infancy, a time of intense cerebral development (Dubois et al., 2014). Androgen concentrations peak roughly 1 to 3 months after birth in males (Kuiri-Hänninen, Sankilampi, & Dunkel, 2014; Lamminmäki et al., 2012) and have been found to relate to aspects of gender/sex-typed behavioral development, including infant mental rotation (Constantinescu, Moore, Johnson, & Hines, 2018), childhood play (Lamminmäki et al., 2012; Pasterski et al., 2015), language (Kung, Browne, Constantinescu, Noorderhaven, & Hines, 2016; Schaadt, Hesse, & Friederici, 2015), and social preferences (Alexander, 2014). Males with CAH appear to lack the postnatal androgen surge that occurs in typically-developing males (Pang et al., 1979). Consequently, if spatial abilities in males are sensitive to androgens during the early postnatal surge (Constantinescu et al., 2018; Hier & Crowley, 1982), the absence of this exposure may explain the relative impairment observed in males with CAH. In contrast, neither typically-developing females nor females with CAH would experience a postnatal androgen surge, perhaps explaining the lack of significant difference between the female groups.
Third, the relationship between early androgens and spatial ability may be curvilinear such that an optimal concentration of early androgen exposure exists, with lower or higher exposures associated with poorer development of abilities (Puts et al., 2008). Limited support for this idea exists in nonhuman species; early administration of testosterone may improve spatial skill in intact females but impair skills in intact males (Roof, 1993; Roof & Havens, 1992). According to this reasoning, the finding that males with CAH have androgen concentrations on the high end of the normal range (Pang et al., 1979, 1980) could suggest that their early androgen exposure exceeds some optimal level.
Limitations
Three of the eight meta-analyses (four meta-analyses in each sex) had a small number of contributing studies (spatial perception for both males and females, and spatial visualization for males). Thus, these particular findings need to be interpreted cautiously. Further, some prior studies suggest different patterns of outcomes for patients with the SW vs. SV forms of CAH. In one study, a subset of patients with the SW form outperformed those with the SV form on mental rotation (Hampson & Rovet, 2015), while other studies suggest higher spatial skill on certain tasks in patients with the SV form (Helleday et al., 1994) or no difference (Malouf et al., 2006). Our inability to analyze results separately for the SW and SV patients means that, if differences in spatial ability exist between these subtypes, our results will fail to capture them. In addition, despite seeking to include all possible studies, the meta-analysis was still based on a reasonably small number.
A general limitation of research on individuals with CAH is that they are not randomly assigned to groups as in experimental research. In addition, the CAH condition may cause other problems that could affect cognitive performance. For instance, one of the studies included in our meta-analysis excluded data for participants who were considered to be neurologically questionable (Hampson & Rovet, 2015). In contrast, we included data for these participants in our meta-analysis. Similarly, different biases might motivate individuals with and without CAH to participate in research, and these biases could relate to intelligence. Therefore, we used meta-regression to evaluate the possibility that differences in intelligence explained our outcomes. Results of the meta-regressions did not support intelligence differences as an explanation of our spatial findings for either females or males.
Our results do not provide support for the idea that prenatal androgen exposure is responsible for average differences in selected spatial skills between males and females. In addition, although we cannot rule out effects of androgenic hormones at other times, for example, during early postnatal life, much research suggests that social factors, such as training, foster spatial abilities (Sorby et al., 2018; Uttal et al., 2013; Wright, Thompson, Ganis, Newcombe, & Kosslyn, 2008). If promoting spatial skills to encourage participation in STEM fields is a valuable goal, society may want to focus on changes in educational practices to increase participation in STEM. In addition, it would be useful to have additional information on factors that influence spatial skills in males with CAH.
Supplementary Material
ACKNOWLEDGMENTS
Marcia Collaer received support from the Middlebury College Research Leave Program; the A. Barton Hepburn Endowed Professorship; and an Overseas Fellowship from Churchill College, University of Cambridge. Melissa Hines received research support from the United States Public Health Service (HD081720). We thank Elise Park, Middlebury College, for her work extracting data and computing summary statistics for the meta-analysis.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of Interest: The authors declare that they have no conflict of interest. Ethical Approval: This article entails secondary, meta-analysis of fully anonymized and aggregated data that have been previously published. Therefore, no new data on human or nonhuman participants were collected for the present work.
Debate exists concerning whether to use the term “sex” or “gender,” with some arguing that “sex” should be reserved for biological distinctions and “gender” for social or cultural ones. Others contend that the distinction is irrelevant due to the intertwining of these factors. For a variety of reasons, including our inability to know the origins of most characteristics, we will typically use the terms “gender/sex” or “gender/sex difference” (see Hyde, Bigler, Joel, Tate, & van Anders, 2019).
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- Alexander GM (2014). Postnatal testosterone concentrations and male social development. Frontiers in Endocrinology, 5, 15. doi: 10.3389/fendo.2014.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP (2009). The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Hormones and Behavior, 55, 570–578. doi: 10.1016/j.yhbeh.2009.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SKW (1993). An exploratory family study of IQ and specific cognitive abilities in classical congenital adrenal hyperplasia:Examined by family, genetic, and salt wasting subgroups (Doctoral Dissertation). Columbia University, New York. [Google Scholar]
- Baker SW, & Ehrhardt AA (1974). Prenatal androgen, intelligence and cognitive sex differences. In Friedman RC, Richart RM, & Vande Wiele RL (Eds.), Sex differences in behavior (pp. 53–76). New York: Wiley. [Google Scholar]
- Begg CB, & Mazumdar M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, 50, 1088–1101. [PubMed] [Google Scholar]
- Berenbaum SA, Bryk KL, & Beltz AM (2012). Early androgen effects on spatial and mechanical abilities: Evidence from congenital adrenal hyperplasia. Behavioral Neuroscience, 126, 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum SA, Bryk KK, & Duck SC (2010). Normal intelligence in female and male patients with congenital adrenal hyperplasia. International Journal of Pediatric Endocrinology, 853103. doi: 10.1155/2010/853103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum SA, & Hines M. (1992). Early androgens are related to childhood sex-typed toy preferences. Psychological Science, 3, 203–206. [Google Scholar]
- Borenstein M, Hedges L, Higgins J, & Rothstein H. (2005). Comprehensive meta-analysis, version 2. Englewood, NJ: Biostat. www.meta-analysis.com [Google Scholar]
- Borenstein M, Hedges L, Higgins J, & Rothstein H. (2009). Introduction to meta-analysis. Chichester, UK: Wiley. [Google Scholar]
- Browne WV, Hindmarsh PC, Pasterski V, Hughes IA, Acerini CL, Spencer D, . . . Hines M. (2015). Working memory performance is reduced in children with congenital adrenal hyperplasia. Hormones and Behavior, 67, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr BR (1998). Disorders of the ovaries and female reproductive tract. In Wilson JD, Foster DW, Kronenberg HM, & Larsen PR (Eds.), Williams textbook of endocrinology (9th ed., pp. 751–817). Philadelphia: Saunders. [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Collaer ML, Hindmarsh PC, Pasterski V, Fane BA, & Hines M. (2016). Reduced short term memory in congenital adrenal hyperplasia (CAH) and its relationship to spatial and quantitative performance. Psychoneuroendocrinology, 64, 164–173. doi: 10.1016/j.psyneuen.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu M, Moore DS, Johnson SP, & Hines M. (2018). Early contributions to infants’ mental rotation abilities. Developmental Science, 21, e12613. doi: 10.1111/desc.12613 [DOI] [PubMed] [Google Scholar]
- Dittmann RW, Kappes MH, & Kappes ME (1993). Cognitive functioning in female patients with 21-hydroxylase deficiency. European Child & Adolescent Psychiatry, 2, 34–43. doi: 10.1007/BF02098828 [DOI] [PubMed] [Google Scholar]
- Dittmann RW, Kappes MH, Kappes ME, Börger D, Stegner H, Willig RH, & Wallis H. (1990). Congenital adrenal hyperplasia I: Gender-related behavior and attitudes in female patients and sisters. Psychoneuroendocrinology, 15, 401–420. [DOI] [PubMed] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Huppi PS, & Hertz-Pannier L. (2014). The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience, 276, 48–71. doi: 10.1016/j.neuroscience.2013.12.044 [DOI] [PubMed] [Google Scholar]
- Duval S, & Tweedie R. (2000a). A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. Journal of the American Statistical Association, 95, 89–98. doi: 10.1080/01621459.2000.10473905 [DOI] [Google Scholar]
- Duval S, & Tweedie R. (2000b). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56, 455–463. [DOI] [PubMed] [Google Scholar]
- El-Maouche D, Arlt W, & Merke DP (2017). Congenital adrenal hyperplasia. Lancet, 390(10108), 2194–2210. doi: 10.1016/s0140-6736(17)31431-9 [DOI] [PubMed] [Google Scholar]
- Gilligan KA, Flouri E, & Farran EK (2017). The contribution of spatial ability to mathematics achievement in middle childhood. Journal of Experimental Child Psychology, 163, 107–125. doi: 10.1016/j.jecp.2017.04.016 [DOI] [PubMed] [Google Scholar]
- Halpern DF, Benbow CP, Geary DC, Gur RC, Hyde JS, & Gernsbacher MA (2007). The science of sex differences in science and mathematics. Psychological Science in the Public Interest, 8, 1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern DF, & Collaer ML (2005). Sex differences in visuospatial abilities: More than meets the eye. In Shah P. & Akira M. (Eds.), The Cambridge handbook of visuospatial thinking (pp. 170–212). New York: Cambridge University Press. [Google Scholar]
- Hamed SA, Metwalley KA, & Farghaly HS (2018). Cognitive function in children with classic congenital adrenal hyperplasia. European Journal of Pediatrics, 177(11), 1633–1640. doi: 10.1007/s00431-018-3226-7 [DOI] [PubMed] [Google Scholar]
- Hampson E, & Rovet JF (2015). Spatial function in adolescents and young adults with congenital adrenal hyperplasia: Clinical phenotype and implications for the androgen hypothesis. Psychoneuroendocrinology, 54, 60–70. doi: 10.1016/j.psyneuen.2015.01.022 [DOI] [PubMed] [Google Scholar]
- Hampson E, Rovet JF, & Altmann D. (1998). Spatial reasoning in children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Developmental Neuropsychology, 14, 299–320. [Google Scholar]
- Healy W. (1914). A pictorial completion test. Psychological Review, 21, 189–203. doi: 10.1037/h0075712 [DOI] [Google Scholar]
- Hedges LV (1992). Modeling publication selection effects in meta-analysis. Statistical Science, 7, 246–255. [Google Scholar]
- Helleday J, Bartfai A, Ritzen EM, & Forsman M. (1994). General intelligence and cognitive profile in women with congenital adrenal hyperplasia (CAH). Psychoneuroendocrinology, 19, 343–356. [DOI] [PubMed] [Google Scholar]
- Hier DB, & Crowley WF Jr. (1982). Spatial ability in androgen-deficient men. New England Journal of Medicine, 306, 1202–1205. [DOI] [PubMed] [Google Scholar]
- Hines M. (2004). Brain gender. Oxford: Oxford University Press. [Google Scholar]
- Hines M. (2011). Gender development and the human brain. Annual Review of Neuroscience, 34, 67–86. [DOI] [PubMed] [Google Scholar]
- Hines M. (2015). Gendered development. In Lamb ME & Lerner RM (Eds.), Handbook of child psychology and developmental science: Socioemotional processes, Vol. 3, 7th ed. (pp. 842–887). Hoboken, NJ: John Wiley & Sons Inc. [Google Scholar]
- Hines M, Constantinescu M, & Spencer D. (2015). Early androgen exposure and human gender development. Biology of Sex Differences, 6, 3. doi: 10.1186/s13293-015-0022-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Fane BA, Pasterski VL, Mathews GA, Conway GS, & Brook C. (2003). Spatial abilities following prenatal androgen abnormality: Targeting and mental rotations performance in individuals with congenital adrenal hyperplasia. Psychoneuroendocrinology, 28, 1010–1026. [DOI] [PubMed] [Google Scholar]
- Hines M, Golombok S, Rust J, Johnston KJ, & Golding J. (2002). Testosterone during pregnancy and gender role behavior of preschool children: A longitudinal, population study. Child Development, 73, 1678–1687. doi: 10.1111/1467-8624.00498 [DOI] [PubMed] [Google Scholar]
- Hines M, Pasterski V, Spencer D, Neufeld S, Patalay P, Hindmarsh PC, . . . Acerini CL (2016a). Prenatal androgen exposure alters girls’ responses to information indicating gender-appropriate behaviour. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 371(1688), 20150125. doi: 10.1098/rstb.2015.0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Spencer D, Kung KT, Browne WV, Constantinescu M, & Noorderhaven RM (2016b). The early postnatal period, mini-puberty, provides a window on the role of testosterone in human neurobehavioural development. Current Opinion in Neurobiology, 38, 69–73. doi: 10.1016/j.conb.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Hirvikoski T, Nordenström A, Lindholm T, Lindblad F, Ritzén EM, Wedell A, & Lajic S. (2007). Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. Journal of Clinical Endocrinology and Metabolism, 92, 542–548. doi: 10.1210/jc.2006-1340 [DOI] [PubMed] [Google Scholar]
- Hyde JS (2005). The gender similarities hypothesis. American Psychologist, 60(6), 581–592. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Bigler RS, Joel D, Tate CC, & van Anders SM (2019). The future of sex and gender in psychology: Five challenges to the gender binary. American Psychologist, 74, 171–193. doi: 10.1037/amp0000307 [DOI] [PubMed] [Google Scholar]
- Inozemtseva O, Matute E, & Juárez J. (2008). Learning disabilities spectrum and sexual dimorphic abilities in girls with congenital adrenal hyperplasia. Journal of Child Neurology, 23, 862–869. doi: 10.1177/0883073808315618 [DOI] [PubMed] [Google Scholar]
- Ioannidis JPA, & Trikalinos TA (2007). The appropriateness of asymmetry tests for publication bias in meta-analyses: A large survey. Canadian Medical Association Journal, 176, 1091–1096. doi: 10.1503/cmaj.060410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, & Sengelaub DR (2003). Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. Journal of Neurobiology, 55, 179–190. doi: 10.1002/neu.10200 [DOI] [PubMed] [Google Scholar]
- Johannsen TH, Ripa CPL, Reinisch JM, Schwartz M, Mortensen EL, & Main KM (2006). Impaired cognitive function in women with congenital adrenal hyperplasia. Journal of Clinical Endocrinology & Metabolism, 91, 1376–1381. doi: 10.1210/jc.2005-1959 [DOI] [PubMed] [Google Scholar]
- Kelso WM, Nicholls MER, Warne GL, & Zacharin M. (2000). Cerebral lateralization and cognitive functioning in patients with congenital adrenal hyperplasia. Neuropsychology, 14, 370–378. [DOI] [PubMed] [Google Scholar]
- Kuiri-Hänninen T, Sankilampi U, & Dunkel L. (2014). Activation of the hypothalamic-pituitary-gonadal axis in infancy: Minipuberty. Hormone Research in Paediatrics, 82, 73–80. doi: 10.1159/000362414 [DOI] [PubMed] [Google Scholar]
- Kung KTF, Browne WV, Constantinescu M, Noorderhaven RM, & Hines M. (2016). Early postnatal testosterone predicts sex-related differences in early expressive vocabulary. Psychoneuroendocrinology, 68, 111–116. doi: 10.1016/j.psyneuen.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Lamminmäki A, Hines M, Kuiri-Hänninen T, Kilpeläinen L, Dunkel L, & Sankilampi U. (2012). Testosterone measured in infancy predicts subsequent sex-typed behavior in boys and in girls. Hormones and Behavior, 61, 611–616. [DOI] [PubMed] [Google Scholar]
- Lau J, Ioannidis JP, & Schmid CH (1997). Quantitative synthesis in systematic reviews. Annals of Internal Medicine, 127, 820–826. [DOI] [PubMed] [Google Scholar]
- Linn MC, & Petersen AC (1985). Emergence and characterization of sex differences in spatial ability: A meta-analysis. Child Development, 56, 1479–1498. [PubMed] [Google Scholar]
- Lipsey MW, & Wilson DB (2001). Practical meta-analysis (Vol. 49). Thousand Oaks, CA: Sage. [Google Scholar]
- Maccoby EE, & Jacklin CN (1974). The psychology of sex differences. Stanford, CA: Stanford University Press. [Google Scholar]
- Malouf MA, Migeon CJ, Carson KA, Petrucci L, & Wisniewski AB (2006). Cognitive outcome in adult women affected by congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hormone Research, 65, 142–150. doi: 10.1159/000091793 [DOI] [PubMed] [Google Scholar]
- Martin CL, & Ruble DN (2010). Patterns of gender development. Annual Review of Psychology, 61, 353–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire LS, & Omenn GS (1975). Congenital adrenal hyperplasia. I. Family studies of IQ. Behavior Genetics, 5, 165–173. [DOI] [PubMed] [Google Scholar]
- McGuire LS, Ryan KO, & Omenn GS (1975). Congenital adrenal hyperplasia. II. Cognitive and behavioral studies. Behavior Genetics, 5, 175–188. [DOI] [PubMed] [Google Scholar]
- Meaney MJ (1988). The sexual differentiation of social play. Trends in Neurosciences, 11, 54–58. doi: 10.1016/0166-2236(88)90164-6 [DOI] [PubMed] [Google Scholar]
- Merke DP, Fields JD, Keil MF, Vaituzis AC, Chrousos GP, & Giedd JN (2003). Children with classic congenital adrenal hyperplasia have decreased amygdala volume: Potential prenatal and postnatal hormonal effects. Journal of Clinical Endocrinology and Metabolism, 88, 1760–1765. doi: 10.1210/jc.2002-021730 [DOI] [PubMed] [Google Scholar]
- Miller WL, & Auchus RJ (2011). The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine Reviews, 32, 81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money J, & Lewis V. (1966). IQ, genetics and accelerated growth: Adrenogenital syndrome. Bulletin of the Johns Hopkins Hospital, 118, 365–373. [Google Scholar]
- Nass R, & Baker S. (1991). Learning disabilities in children with congenital adrenal hyperplasia. Journal of Child Neurology, 6, 306–312. [DOI] [PubMed] [Google Scholar]
- Nordenstrom A, Servin A, Bohlin G, Larsson A, & Wedell A. (2002). Sex-typed toy play behavior correlates with the degree of prenatal androgen exposure assessed by CYP21 genotype in girls with congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism, 87, 5119–5124. doi: 10.1210/jc.2001-011531 [DOI] [PubMed] [Google Scholar]
- Nuttall RL, Casey MB, & Pezaris E. (2005). Spatial ability as a mediator of gender differences on mathematics tests. In Gallagher AM & Kaufman JC (Eds.), Gender differences in mathematics: An integrative psychological approach (pp. 121–142). Cambridge: Cambridge University Press. [Google Scholar]
- Pang S, Levine LS, Cederqvist LL, Fuentes M, Riccardi VM, Holcombe JH, . . . New MI (1980). Amniotic fluid concentrations of steroids in fetuses with congenital adrenal hyperplasia due to 21-hydroxylase deficiency and in anencephalic fetuses. Journal of Clinical Endocrinology and Metabolism, 51, 223–229. [DOI] [PubMed] [Google Scholar]
- Pang S, Levine LS, Chow DM, Faiman C, & New MI (1979). Serum androgen concentrations in neonates and young infants with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clinical Endocrinology, 11, 373–384. [DOI] [PubMed] [Google Scholar]
- Pasterski V, Acerini CL, Dunger DB, Ong KK, Hughes IA, Thankamony A, & Hines M. (2015). Postnatal penile growth concurrent with mini-puberty predicts later sex-typed play behavior: Evidence for neurobehavioral effects of the postnatal androgen surge in typically developing boys. Hormones and Behavior, 69, 98–105. doi: 10.1016/j.yhbeh.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Pazzaglia F, & Moè A. (2013). Cognitive styles and mental rotation ability in map learning. Cognitive Processing, 14, 391–399. doi: 10.1007/s10339-013-0572-2 [DOI] [PubMed] [Google Scholar]
- Perlman SM (1973). Cognitive abilities of children with hormone abnormalities: Screening by psychoeducational tests. Journal of Learning Disabilities, 6, 21–29. [Google Scholar]
- Puts DA, McDaniel MA, Jordan CL, & Breedlove SM (2008). Spatial ability and prenatal androgens: Meta-analyses of congenital adrenal hyperplasia and digit ratio (2D:4D) studies. Archives of Sexual Behavior, 37, 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM (1982). Psychological functioning in individuals with congenital adrenal hyperplasia: Early hormonal influences on cognition and personality (Doctoral dissertation). University of Minnesota, Minneapolis. [Google Scholar]
- Resnick SM, Berenbaum SA, Gottesman II, & Bouchard TJ Jr. (1986). Early hormonal influences on cognitive functioning in congenital adrenal hyperplasia. Developmental Psychology, 22, 191–198. [Google Scholar]
- Roof RL (1993). Neonatal exogenous testosterone modifies sex difference in radial arm and Morris water maze performance in prepubescent and adult rats. Behavioural Brain Research, 53(1–2), 1–10. [DOI] [PubMed] [Google Scholar]
- Roof RL, & Havens MD (1992). Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Research, 572, 310–313. [DOI] [PubMed] [Google Scholar]
- Ruble DN, Taylor LJ, Cyphers L, Greulich FK, Lurye LE, & Shrout PE (2007). The role of gender constancy in early gender development. Child Development, 78, 1121–1136. doi: 10.1111/j.1467-8624.2007.01056.x [DOI] [PubMed] [Google Scholar]
- Schaadt G, Hesse V, & Friederici AD (2015). Sex hormones in early infancy seem to predict aspects of later language development. Brain and Language, 141, 70–76. doi: 10.1016/j.bandl.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Somajni F, Sovera V, Albizzati A, Russo G, Peroni P, Seragni G, & Lenti C. (2011). Neuropsychological assessment in prepubertal patients with congenital adrenal hyperplasia: preliminary study. Minerva pediatrica, 63(1), 1–9. [PubMed] [Google Scholar]
- Sorby S, Veurink N, & Streiner S. (2018). Does spatial skills instruction improve STEM outcomes? The answer is ‘yes’. Learning and Individual Differences, 67, 209–222. doi: 10.1016/j.lindif.2018.09.001 [DOI] [Google Scholar]
- Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, . . . White PC (2018). Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism, 103, 4043–4088. doi: 10.1210/jc.2018-01865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieff M, & Uttal D. (2015). How much can spatial training improve STEM achievement? Educational Psychology Review, 27, 607–615. doi: 10.1007/s10648-015-9304-8 [DOI] [Google Scholar]
- Uttal DH, Meadow NG, Tipton E, Hand LL, Alden AR, Warren C, & Newcombe NS (2013). The malleability of spatial skills: A meta-analysis of training studies. Psychological Bulletin, 139, 352–402. doi: 10.1037/a0028446 [DOI] [PubMed] [Google Scholar]
- Voyer D, & Jansen P. (2017). Motor expertise and performance in spatial tasks: A meta-analysis. Human Movement Science, 54, 110–124. doi: 10.1016/j.humov.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, & Bryden MP (1995). Magnitude of sex differences in spatial abilities: A meta-analysis and consideration of critical variables. Psychological Bulletin, 117, 250–270. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, & Saint-Aubin J. (2017). Sex differences in visual-spatial working memory: A meta-analysis. Psychomics Bulletin and Review, 24, 307–334. doi: 10.3758/s13423-016-1085-7 [DOI] [PubMed] [Google Scholar]
- Wenzel U, Schneider M, Zachmann M, Knorr-Mürset G, Weber A, & Prader A. (1978). Intelligence of patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency, their parents and unaffected siblings. Helvetica Paediatrica Acta, 33, 11–16. [PubMed] [Google Scholar]
- White PC, & Speiser PW (2000). Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocrine Reviews, 21, 245–291. doi: 10.1210/er.21.3.245 [DOI] [PubMed] [Google Scholar]
- Williams CL, Barnett AM, & Meck WH (1990). Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behavioral Neuroscience, 104, 84–97. [DOI] [PubMed] [Google Scholar]
- Witchel SF (2017). Congenital adrenal hyperplasia. Journal of Pediatric and Adolescent Gynecology, 30, 520–534. doi: 10.1016/j.jpag.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WI, & Hines M. (2015). Preferences for pink and blue: The development of color preferences as a distinct gender-typed behavior in toddlers. Archives of Sexual Behavior, 44, 1243–1254. doi: 10.1007/s10508-015-0489-1 [DOI] [PubMed] [Google Scholar]
- Wright R, Thompson WL, Ganis G, Newcombe NS, & Kosslyn SM (2008). Training generalized spatial skills. Psychonomics Bulletin and Review, 15, 763–771. doi: 10.3758/PBR.15.4.763 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.