Key summary points
Aim
COVID-19 vaccine efficacy is high in older people, but declines after 4–6 months.
Findings
Booster doses are effective for restoring COVID-19 vaccine efficacy in older people.
Message
COVID-19 vaccine boosters generate higher protection than primary vaccination.
Keywords: COVID-19, SARS-COV-2, Vaccination, Booster, Older people
Abstract
Purpose
We provide here an updated analysis on efficacy of COVID-19 vaccine booster doses in older people (i.e., aged ≥ 80 years) based on ongoing Italian nationwide COVID-19 vaccination campaign.
Methods
Data were obtained from the COVID-19 national integrated surveillance program, made available and regularly updated by the Italian National Institute of Health.
Results
Compared to those who completed the COVID-19 vaccination cycle for ≥ 5 months (n = 2,385,897), those receiving booster doses (n = 1,549,747) had 75% lower risk of SARS-CoV-2 infection, 82–83% lower risk of COVID-19 hospitalization and ICU admission, and 81% lower risk of death. Administration of COVID-19 vaccine boosters generated also greater protection (between 63 and 87% higher) against all these same endpoints compared to early completing (i.e., < 5 months; n = 335,458) a primary COVID-19 vaccination cycle.
Conclusions
The administration of COVID-19 vaccine booster doses is advisable for reducing the risk of morbidity and mortality in older people.
Introduction
It is now virtually undeniable that the ongoing coronavirus disease 2019 (COVID-19) pandemic is one of the most dramatic events that has challenged humanity since the former Spanish flu pandemic outbreak, in 1918–1919 [1]. One of the most important aspects of this infectious respiratory disease, sustained by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is that older, fragile and comorbid people represent the most vulnerable part of the population, whereby the vast majority of COVID-19-related hospitalizations, intensive care unit (ICU) admissions and even deaths involve people aged 65 years or older [2, 3]. Several lines of evidence now attest that SARS-CoV-2 has a devastating impact on older adults, by increasing several folds their risk of unfavourable outcomes such as the need of hospitalization, respiratory support, intensive care, and ultimately magnifying their risk of death [4]. Last but not least, increased frailty and loss of function are commonplace in older people after recovering from COVID-19, thus generating dramatic consequences on healthcare, society and economy, since these older patients may need closer follow-up, as well as reinforced functional and psychologic support [5, 6].
The clinical evidence accumulated so far would hence suggest that older people should be especially protected from SARS-CoV-2 infection. Nonetheless, physical measures such as wearing face mask, social distancing, hand hygiene and so forth are only partially effective for preventing viral infection and COVID-19-related complications [7], so that widespread administration of COVID-19 vaccines is now regarded as the most effective preventive strategy in older persons [8], as already proven for other infectious diseases such as influenza, meningococcal and pneumococcal diseases, hepatitis, herpes zoster, diphtheria and tetanus [9].
Although the efficacy of COVID-19 vaccines against the risk of developing symptomatic disease is noteworthy, even higher than that of other vaccines such as those against Influenza (i.e., 80–90% vs. 50–60% efficacy) [10], waned post-vaccination immunity has been widely reported between 4 and 6 months after completing a primary COVID-19 vaccination cycle, with the risk of developing severe COVID-19 illness becoming nearly twofold higher after such period in people aged 60 years or older [11]. One of the potential solutions to contrast such decline of post-vaccine immunity encompasses the administration of additional vaccine doses (i.e., the so-called “boosters”), with the aim of restoring immunity to levels similar to, or even greater than, those achieved immediately after completing a primary COVID-19 vaccination. This article is hence aimed at providing an analysis on efficacy of COVID-19 vaccine booster doses in older people based on data retrieved from the ongoing nationwide Italian COVID-19 vaccination campaign.
Methods
This analysis was based on the official statistics of the COVID-19 national integrated surveillance program, which is made available and regularly updated by the Italian National Institute of Health (Istituto Superiore di Sanità, ISS; Last available update, December 17, 2021) [12]. This official bulletin includes complete nationwide epidemiologic information concerning the cumulative burden of COVID-19 cases, hospitalizations, ICU admissions and deaths, together with data on advancement of the nationwide COVID-19 vaccination campaign, which started on December 27, 2020. Vaccine administration in Italy was essentially based on two mRNA-based formulations (Pfizer/BioNTech Comirnaty and Moderna Spikevax) and two adenovirus-based vaccines (AstraZeneca Vaxzevria and Janssen Ad26.COV2-S). Starting from October 2021, an additional vaccination campaign has begun, with administration of vaccine booster doses (i.e., Pfizer/BioNTech Comirnaty or Moderna Spikevax) to older (i.e., > 60 years) and fragile individuals.
The official information available in the ISS bulletin was downloaded within an Excel Worksheet (Microsoft Excel; Microsoft, Redmond, WA, US) and statistical analysis (calculation of the odds ratio [OR] and 95% confidence interval [95%CI]) was carried out using MedCalc (Version 20.015; MedCalc Software Ltd., Ostend, Belgium). Statistical significance was set at p < 0.05. The study was performed in accordance with the Declaration of Helsinki, under all relevant terms of the local legislation. The research was based on public ISS data [12], so that Ethical Committee approval was unnecessary.
Results
The total number of people aged 80 years or older who were unvaccinated, who completed the primary COVID-19 vaccination cycle < 5 or ≥ 5 months and those who received a COVID-19 vaccine booster dose were 216,424, 335,458, 2,385,897 and 1,549,747, respectively. Vaccine coverage was as follows: Pfizer/BioNTech Comirnaty 70.1%, Moderna Spikevax 16.9%, AstraZeneca Vaxzevria 11.6% and Janssen Ad26.COV2-S 1.4%, respectively. As shown in Table 1 and Fig. 1, the overall COVID-19 vaccine efficacy within 5 months from completing a primary vaccination cycle was considerably high in this older population, associated with 80% lower risk of SARS-CoV-2 infection, 86% lower risk of COVID-19 hospitalization and ICU admission and 88% lower risk of death compared to unvaccinated people. Nonetheless, this protection tended to slightly wane after 5 months since the efficacy against the risk of SARS-CoV-2 infection, COVID-19 hospitalization, ICU admission and death was 70%, 83%, 86% and 88%, respectively. Compared to those who had completed the COVID-19 vaccination cycle for more than 5 months those who received vaccine booster doses had 75% lower risk of SARS-CoV-2 infections, and between 81 and 83% lower risk of COVID-19 hospitalization, ICU admission and death, respectively. The administration of COVID-19 vaccine boosters generated also greater protection (i.e., between 63 and 87% higher) against all these same endpoints compared to people who early completed (i.e., < 5 months) a primary COVID-19 vaccination cycle.
Table 1.
Efficacy of primary COVID-19 vaccine cycle and vaccine boosters from the ongoing Italian nationwide COVID-19 vaccination campaign
| Unvaccinated | Vaccinated (≥ 5 months) | Vaccinated (< 5 months) | |
|---|---|---|---|
| OR (95%CI) total SARS-CoV-2 infections | |||
| Vaccinated (≥ 5 months) | 0.30 (0.2900.31; p < 0.001) | – | – |
| Vaccinated (< 5 months) | 0.20 (0.19–0.21; p < 0.001) | 0.66 (0.63–0.69; p < 0.001) | – |
| Vaccinated (Booster) | 0.07 (0.06–0.08; p < 0.001) | 0.25 (0.23–0.27; p < 0.001) | 0.37 (0.35–0.40; p < 0.001) |
| OR (95%CI) COVID-19-related hospitalizations | |||
| Vaccinated (≥ 5 months) | 0.17 (0.16–0.19; p < 0.0019 | – | – |
| Vaccinated (< 5 months) | 0.14 (0.13–0.15; p < 0.001) | 0.80 (0.71–0.90; p < 0.001) | – |
| Vaccinated (Booster) | 0.03 (0.02–0.04; p < 0.001) | 0.17 (0.14–0.21; p < 0.001) | 0.21 (0.17–0.26; p < 0.001) |
| OR (95%CI) COVID-19 related ICU admissions | |||
| Vaccinated (≥ 5 months) | 0.10 (0.07–0.16; p < 0.001) | – | – |
| Vaccinated (< 5 months) | 0.14 (0.10–0.21; p < 0.001) | 1.41 (0.82–2.43; p = 0.210) | – |
| Vaccinated (Booster) | 0.02 (0.01–0.04; p < 0.001) | 0.18 (0.06–0.53; p < 0.001) | 0.13 (0.04–0.37; p < 0.001) |
| OR (95%CI) COVID-19 related deaths | |||
| Vaccinated (≥ 5 months) | 0.12 (0.10–0.14; p < 0.001) | – | – |
| Vaccinated (< 5 months) | 0.12 (0.10–0.14; p < 0.001) | 0.99 (0.81–1.22; p = 0.958) | – |
| Vaccinated (Booster) | 0.02 (0.01–0.03; p < 0.001) | 0.19 (0.13–0.27; p < 0.001) | 0.19 (0.13–0.27; p < 0.001) |
Statistically significant values (p < 0.05) are reported in bold
OR odds ratio, 95%CI 95% confidence interval, COVID-19 coronavirus disease 2019, ICU intensive care unit, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2
Fig. 1.
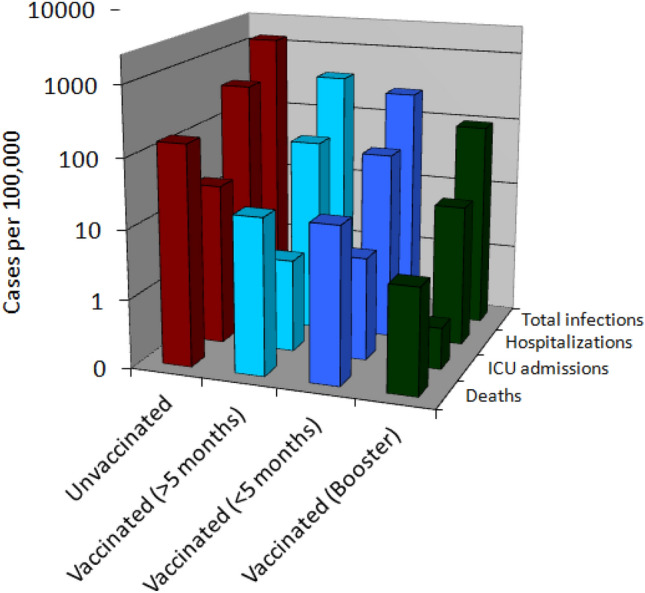
Cases per 100,000 of SARS-CoV-2 infection, COVID-19-related hospitalizations, intensive care unit admissions and deaths reported from the ongoing nationwide COVID-19 vaccination campaign in older Italian people (i.e., aged > 80 years). SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, COVID-19 coronavirus disease 2019; ICU, intensive care unit
Discussion
The results of our investigation based on data garnered from the ongoing nationwide COVID-19 vaccination campaign in Italy are in keeping with those reported in Israel [13, 14], and confirm that the efficacy of COVID-19 vaccines tends to decrease over time, especially against the risk of developing SARS-CoV-2 infection and COVID-19-related hospitalization. However, the administration of vaccine booster doses not only was found effective for restoring high protection against all unfavourable COVID-19 outcomes in older people (i.e., ≥ 97% efficacy compared to unvaccinated people), but was also associated with a considerably decreased vulnerability to SARS-CoV-2 infections and complications (i.e., between 63 and 87% lower) compared to what is commonly observed shortly after (i.e., within 5 months) completing a primary COVID-19 vaccination cycle. Unfortunately, no specific information could be garnered from the official ISS bulletin on the outcome of different vaccines as well as on the frail status of the population, two pivotal aspects in influencing vaccination efficacy, as shown in a recent study in residents of long-term care facilities in Israel [15].
A plausible biological explanation supports these findings, wherein the serum levels of anti-SARS-CoV-2 neutralizing antibodies tends to gradually decline over time, and such reduction seems even amplified in older people, as we have recently demonstrated [16]. The efficacy of COVID-19 vaccines largely depends on anti-SARS-CoV-2 neutralizing antibodies titer, irrespective of the type of vaccine [17], and COVID-19 vaccine boosters are especially effective to increase the serum levels of these antibodies by over twofold in older people (i.e., aged 65–85 years) compared to younger subjects [18]. It is hence advisable that COVID-19 vaccine booster doses shall be urgently made available to older people, who seem to display a more rapid decline of post-vaccine immunity and protection, and are also much more vulnerable to SARS-CoV-2 complications.
Declarations
Conflict of interest
The authors have no conflicts of interests.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lippi G, Sanchis-Gomar F, Henry BM. Coronavirus disease 2019 (COVID-19): the portrait of a perfect storm. Ann Transl Med. 2020;8:497. doi: 10.21037/atm.2020.03.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorrell JM. Losing a generation: the impact of COVID-19 on older Americans. J Psychosoc Nurs Ment Health Serv. 2021;59:9–12. doi: 10.3928/02793695-20210315-03. [DOI] [PubMed] [Google Scholar]
- 3.Lippi G, Sanchis-Gomar F, Henry BM. COVID-19: unravelling the clinical progression of nature's virtually perfect biological weapon. Ann Transl Med. 2020;8:693. doi: 10.21037/atm-20-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lozano-Montoya I, Quezada-Feijoo M, Jaramillo-Hidalgo J, Garmendia-Prieto B, Lisette-Carrillo P, Gómez-Pavón FJ. Mortality risk factors in a Spanish cohort of oldest-old patients hospitalized with COVID-19 in an acute geriatric unit: the OCTA-COVID study. Eur Geriatr Med. 2021;12:1169–1180. doi: 10.1007/s41999-021-00541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrillo-Garcia P, Garmendia-Prieto B, Cristofori G, Montoya IL, Hidalgo JJ, Feijoo MQ, Cortés JJB, Gómez-Pavón J. Health status in survivors older than 70 years after hospitalization with COVID-19: observational follow-up study at 3 months. Eur Geriatr Med. 2021;12:1091–1094. doi: 10.1007/s41999-021-00516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinohara T, Saida K, Tanaka S, Murayama A, Higuchi D. Did the number of older adults with frailty increase during the COVID-19 pandemic? A prospective cohort study in Japan. Eur Geriatr Med. 2021;12:1085–1089. doi: 10.1007/s41999-021-00523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadjadi M, Mörschel KS, Petticrew M. Social distancing measures: barriers to their implementation and how they can be overcome—a systematic review. Eur J Public Health. 2021 doi: 10.1093/eurpub/ckab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham AL, McIntyre P, Subbarao K, Booy R, Levin MJ. Vaccines for older adults. BMJ. 2021;372:n188. doi: 10.1136/bmj.n188. [DOI] [PubMed] [Google Scholar]
- 9.Doherty TM, Connolly MP, Del Giudice G, Flamaing J, Goronzy JJ, Grubeck-Loebenstein B, Lambert PH, Maggi S, McElhaney JE, Nagai H, Schaffner W, Schmidt-Ott R, Walsh E, Di Pasquale A. Vaccination programs for older adults in an era of demographic change. Eur Geriatr Med. 2018;9:289–300. doi: 10.1007/s41999-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monto AS. The future of SARS-CoV-2 vaccination—lessons from influenza. N Engl J Med. 2021;385:1825–1827. doi: 10.1056/NEJMp2113403. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, Milo R, Alroy-Preis S, Ash N, Huppert A. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Istituto Superiore di Sanità. Epidemia COVID-19—Aggiornamento Nazionale 15 Dicembre 2021. https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_15-dicembre-2021.pdf. Accessed 17 Dec 2021
- 13.Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, Mizrahi B, Alroy-Preis S, Ash N, Milo R, Huppert A. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, Reis BY, Balicer RD. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muhsen K, Maimon N, Mizrahi A, Varticovschi B, Bodenheimer O, Gelbshtein U, Grotto I, Cohen D, Dagan R. Effects of BNT162b2 Covid-19 vaccine booster in long-term care facilities in Israel. N Engl J Med. 2021 doi: 10.1056/NEJMc2117385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvagno GL, Henry BM, Pighi L, De Nitto S, Gianfilippi G, Lippi G. The pronounced decline of anti-SARS-CoV-2 spike trimeric IgG and RBD IgG in baseline seronegative individuals six months after BNT162b2 vaccination is consistent with the need for vaccine boosters. Clin Chem Lab Med. 2021;60(2):e29–e31. doi: 10.1515/cclm-2021-1184. [DOI] [PubMed] [Google Scholar]
- 17.Cromer D, Steain M, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Kent SJ, Triccas JA, Khoury DS, Davenport MP. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2021 doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falsey AR, Frenck RW, Jr, Walsh EE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Bailey R, Swanson KA, Xu X, Koury K, Kalina W, Cooper D, Zou J, Xie X, Xia H, Türeci Ö, Lagkadinou E, Tompkins KR, Shi PY, Jansen KU, Şahin U, Dormitzer PR, Gruber WC. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385:1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]


