Abstract
There is a persistent bias toward higher prevalence and increased severity of coronavirus disease 2019 (COVID-19) in males. Underlying mechanisms accounting for this sex difference remain incompletely understood. Interferon responses have been implicated as a modulator of COVID-19 disease in adults and play a key role in the placental antiviral response. Moreover, the interferon response has been shown to alter Fc receptor expression and therefore may affect placental antibody transfer. Here, we examined the intersection of maternal-fetal antibody transfer, viral-induced placental interferon responses, and fetal sex in pregnant women infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Placental Fc receptor abundance, interferon-stimulated gene (ISG) expression, and SARS-CoV-2 antibody transfer were interrogated in 68 human pregnancies. Sexually dimorphic expression of placental Fc receptors, ISGs and proteins, and interleukin-10 was observed after maternal SARS-CoV-2 infection, with up-regulation of these features in placental tissue of pregnant individuals with male fetuses. Reduced maternal SARS-CoV-2–specific antibody titers and impaired placental antibody transfer were also observed in pregnancies with a male fetus. These results demonstrate fetal sex-specific maternal and placental adaptive and innate immune responses to SARS-CoV-2.
INTRODUCTION
Mortality and morbidity risk during the perinatal period and infancy is higher in males than in females (1–4). The underlying susceptibility of males may relate to evolutionary differences that occur throughout pregnancy and in the perinatal period, but the precise mechanistic differences that lead to this differential female survival benefit are not completely understood. Consistent with perinatal male vulnerability in general, male infants and children fare worse in the setting of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, with higher rates of severe disease in infants and of SARS-CoV-2–associated multisystem inflammatory syndrome (MIS-C) in male children (5–10). The biological basis for the observed relative vulnerability of the male immune system to SARS-CoV-2 in pediatric populations is likely multifactorial (11, 12). Emerging data point to a retrograde impact of infant sex on maternal immunity (13, 14), with specific differences in innate immune signaling across fetal sex, which may contribute to a differential dialogue between female and male fetuses and their mothers. This differential dialogue may critically affect immunity across the dyad, pointing to one potential explanation for sex differences in perinatal vulnerability to infectious disease: differential transplacental antibody transfer from the mother may provide female and male infants with different degrees of immunity.
Newborn antiviral immunity relies heavily on the placental transfer of maternal immunoglobulin G (IgG) to the fetal circulation (15–17). Public health strategies to protect newborns from potentially devastating respiratory infections such as pertussis and influenza capitalize on the ability of the placenta to transfer vaccine-induced maternal IgG to the fetal circulation (18, 19). Although the neonatal Fc receptor (FcRn) was classically identified as the primary receptor responsible for transferring maternal IgG to fetal circulation (20–22), recent findings have also demonstrated critical roles for the Fc-γ receptors (FCγRs) I, II and III in facilitating maternal IgG transfer (15, 16, 23–25). FCγRI expression is regulated by type I and II interferons (IFNs), and emerging data have clearly demonstrated perturbed placental transfer in the setting of other coinfections, including HIV (26) and malaria (27). Whether differences in inflammatory responses to SARS-CoV-2 infection could influence placental antibody transfer is unknown. In addition, little is known regarding sex differences in neonatal immune profiles and in maternal-fetal antibody transfer. Recent work has demonstrated reduced transplacental transfer of SARS-CoV-2–specific antibodies relative to influenza and pertussis antibodies (28, 29) and associated alterations in expression and localization of specific Fc receptors in the placenta (29), but sex differences in neonatal antibody-mediated immunity to SARS-CoV-2 and in placental receptors involved in antibody transfer have not yet been characterized.
Type I, type II, and type III IFNs are induced after innate recognition of viruses (30). Upon binding to their receptors, they induce expression of downstream effectors, IFN-stimulated genes (ISGs), which inhibit viral infection by a number of different mechanisms (31). However, viruses have evolved to evade these IFN responses, and IFN responses can also be drivers of inflammatory pathology. Type I IFN signaling correlates strongly with pathogenicity and fatality in both SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV) infections (32–34). Dysregulated type I IFN signaling is also associated with severe disease and drives pathogenicity in SARS-CoV-2 infection in both humans and murine models (33, 35–41). Sex differences in adult peripheral blood and pulmonary IFN signaling have been observed in both SARS-CoV-1 and SARS-CoV-2 infection (42–44), but there is a dearth of information about sex differences in fetal and pediatric populations. Type I and type III IFN responses at the maternal-fetal interface play a crucial role in limiting viral infection but may also be drivers of abnormal development (45–47). Less is known about the role of type II IFN signaling (initiated by IFN-γ) in the placenta and in SARS-CoV-2 infection (48–53). Sex differences have been noted in the placental immune response to bacterial infection (54, 55) and to other prenatal alterations such as maternal stress and maternal high-fat diet (12, 56, 57). Placental expression of ISGs in maternal SARS-CoV-2 infection, the role of sex differences, and potential impact on placental function have not yet been examined.
Given known sex differences in immune responses to SARS-CoV-2 infection (42), the observed sex differences in disease prevalence and severity in the pediatric population including infants (6–9), and the placenta’s critical role as an immune organ mediating antiviral responses and antibody transfer at the maternal-fetal interface (47), we sought to examine sex differences in the placental immune response to SARS-CoV-2 and how this may affect placental expression of receptors associated with antibody transfer. In a cohort of 38 participants infected with SARS-CoV-2 during pregnancy (19 male and 19 female fetuses) and a comparator group of 30 contemporaneously enrolled pregnant women testing negative for SARS-CoV-2 (15 male and 15 female fetuses), notable differences were noted in placental transfer of antibodies to male and female infants, as well as sexually dimorphic placental Fc receptor expression and ISG and IFN-stimulated protein expression in the setting of maternal SARS-CoV-2 infection. These data point to unexpected sex-driven bilateral communication across the maternal-fetal interface, associated with sex differences in SARS-CoV-2–specific antibody transfer that may provide insight into sex-biased differences in susceptibility to infectious diseases in male infants.
RESULTS
Demographic and clinical characteristics of study participants
Maternal demographic and clinical characteristics of study participants for placental analyses grouped by offspring sex are depicted in Table 1, and those providing matched maternal and umbilical cord blood are depicted in table S1. Of the 68 participants, 34 were pregnant with females and 34 with males. There were no differences between groups with respect to maternal age, parity, obesity, diabetes, hypertension, or gestational age at delivery, among other characteristics examined. Women with SARS-CoV-2 infection during pregnancy were more likely to be Hispanic compared to negative controls, concordant with our prior report of ethnic disparities in coronavirus disease 2019 (COVID-19) vulnerability in our hospital catchment area (58). Of the 38 women with SARS-CoV-2 infection during pregnancy, there were no differences between male and female fetuses with respect to gestational age at diagnosis of SARS-CoV-2 infection, days from positive SARS-CoV-2 test to delivery, or severity of COVID-19 illness. Although both male and female fetuses of mothers with SARS-CoV-2 had lower birthweights than their sex-matched control counterparts, clinically, this difference was not meaningful as all neonatal birthweights were in the normal range. No neonates born to mothers with SARS-CoV-2 infection during pregnancy were infected with SARS-CoV-2.
Table 1.
Demographic and clinical characteristics of study cohort by fetal sex and maternal SARS-CoV-2 status.
| All (68) | Female | Male | P † | |||
|---|---|---|---|---|---|---|
| SARS-CoV-2 negative (15) | SARS-CoV-2 positive (19)* | SARS-CoV-2 negative (15) | SARS-CoV-2 positive (19)* | |||
| Maternal age, years | 33 [28–38] | 33 [30–36] | 34 [30–40] | 30 [27–37] | 31 [26–36] | 0.34 |
| Parity, n | 1 [0–2] | 1 [0–2] | 1 [0–2] | 1 [0–2] | 1 [0–2] | 0.34 |
| Race, n (%) | 0.19 | |||||
| White | 40 (59) | 11 (73) | 8 (42) | 12 (80) | 9 (59) | |
| Black | 5 (7) | 1 (7) | 3 (16) | 0 (0) | 1 (7) | |
| Asian | 1 (1) | 0 (0) | 0 (0) | 1 (7) | 0 (0) | |
| Other | 12 (18) | 3 (20) | 4 (21) | 1 (7) | 4 (8) | |
| Not reported | 10 (15) | 0 (0) | 4 (21) | 1 (7) | 5 (15) | |
| Ethnicity, n (%) | 0.01 | |||||
| Hispanic | 32 (47) | 4 (27) | 11 (58) | 2 (13) | 15 (79) | |
| Non-Hispanic | 33 (48) | 10 (67) | 7 (37) | 12 (80) | 4 (21) | |
| Not reported | 3 (4) | 1 (7) | 1 (5) | 1 (7) | 0 (0) | |
| Chronic hypertension, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | N/A |
| DM/GDM, n (%) | 6 (9) | 1 (7) | 3 (16) | 1 (7) | 1 (5) | 0.67 |
| BMI ≥ 30 kg/m2, n (%) | 20 (29) | 2 (13) | 7 (37) | 3 (20) | 8 (42) | 0.21 |
| Prepregnancy BMI, kg/m2 | 27.4 [21.5–30.8] | 25.8 [21.6–29.4] | 28.5 [26.5–32.7] | 21.5 [20.3–29.8] | 29.5 [22.3–32.0] | 0.06 |
| GA at delivery, weeks | 39.2 [38.6–40.3] | 39.3 [39.0–39.4] | 39.3 [38.4–40.1] | 39.1 [39.0–41.1] | 39.0 [35.3–40.3] | 0.09 |
| Any labor, n (%) | 52 (76) | 8 (53) | 16 (84) | 11 (73) | 17 (89) | 0.07 |
| Neonatal birthweight, g | 3283 [3005–3590] | 3400 [3060–3590] | 3255 [2920–3340] | 3435 [3260–3730] | 3115 [2615–3590] | 0.06 |
| GA at positive SARS-CoV-2 test, weeks | 38.7 [35.6–39.6] | N/A | 36.3 [32.4–39.4] | N/A | 36.3 [32.4–39.5] | 0.90 |
| COVID-19 disease severity at diagnosis‡, n | 0.82 | |||||
| Asymptomatic | 16 (42) | N/A | 8 (42) | N/A | 8 (42) | |
| Mild/moderate | 19 (50) | N/A | 9 (47) | N/A | 10 (53) | |
| Severe/critical | 3 (8) | N/A | 2 (11) | N/A | 1 (5) | |
| Time between SARS-CoV-2 symptom onset and delivery, days | 36.5 [18–57] | N/A | 44 [28–64] | N/A | 32 [8–51] | 0.18 |
DM/GDM, diabetes mellitus/gestational diabetes mellitus; BMI, body mass index; GA, gestational age; N/A, not applicable.
SARS-CoV-2 status determined by nasopharyngeal RT-PCR at time of sample collection. If a participant was SARS-CoV-2 positive at any time in pregnancy, then she was included in “SARS-CoV-2 positive” category.
Significant differences between groups were determined using chi-square test for categorical variables, and Kruskal-Wallis test for continuous variables presented as median [interquartile range].
Disease severity classifications are based on published National Institutes of Health criteria.
Sex differences were observed in placental transfer of SARS-CoV-2–specific antibodies and antibody function
Reduced transplacental antibody transfer has been observed in the context of maternal infections such as HIV (26) and malaria (27), and our group and others have previously described deficits in transplacental antibody transfer of SARS-CoV-2–specific antibodies (28, 29, 59, 60). Given the known vulnerability of male infants to more severe respiratory disease (11), we sought to determine whether the sex of the fetus would affect transplacental transfer of maternal SARS-CoV-2–specific antibodies. We comprehensively profiled SARS-CoV-2 antibodies targeting the spike protein (S), the receptor binding domain (RBD), the spike S1 subunit (S1), the spike S2 subunit (S2), and the nucleocapsid protein (N) including titer, function, and placental receptor interactions in maternal-cord plasma pairs of SARS-CoV-2–exposed and SARS-CoV-2–unexposed (SARS-CoV-2–negative) pregnancies, using a previously described systems serology approach (29, 61). Maternal IgG titers against SARS-CoV-2–specific antigens, an important driver of maternal-fetal immune transfer (62, 63), were significantly lower in mothers carrying a male fetus (Fig. 1A and fig. S1; P < 0.05). Rather than the expected transplacental transfer observed for other pathogens [cord-to-maternal ratio of 1.1 to 1.5 or greater (24, 64, 65)], IgG titers against all examined SARS-CoV-2 antigens were significantly reduced in cord relative to maternal plasma across IgG subtypes in pregnancies with a male fetus compared with those with a female fetus (Fig. 1, B to F, and fig. S2; P < 0.05, P < 0.01, or P < 0.001). In contrast, significant reduction in cord versus maternal titers was only observed in the case of N protein–specific IgG2 in pregnancies with a female fetus (fig. S2, P < 0.05). There were significantly reduced cord:maternal transfer ratios for SARS-CoV-2–specific antibodies in male versus female fetuses (Fig. 1G; P < 0.05) and reduced transfer of SARS-CoV-2–specific antibodies capable of binding to FcRn, FCγRIIA/B, and FCγRIIIA/B predominantly in males (Fig. 1H and fig. S2). Although transfer ratios for SARS-CoV-2–specific antibodies were higher for female fetuses than those for males, they were still lower than the expected 1.1 to 1.5 ratios often observed for other pathogens (29, 64, 65). SARS-CoV-2–specific functional antibodies were also transferred less efficiently, with a marked male-specific decrease in antibody-dependent complement deposition (ADCD)–inducing antibodies and antibodies that induce natural killer (NK) cell chemokine secretion [macrophage inflammatory protein-1β (MIP-1β); Fig. 1, I and J].
Fig. 1. SARS-CoV-2–positive mothers with male fetuses demonstrate reduced maternal titers and placental transfer of SARS-CoV-2–specific antibodies compared to those with female fetuses.
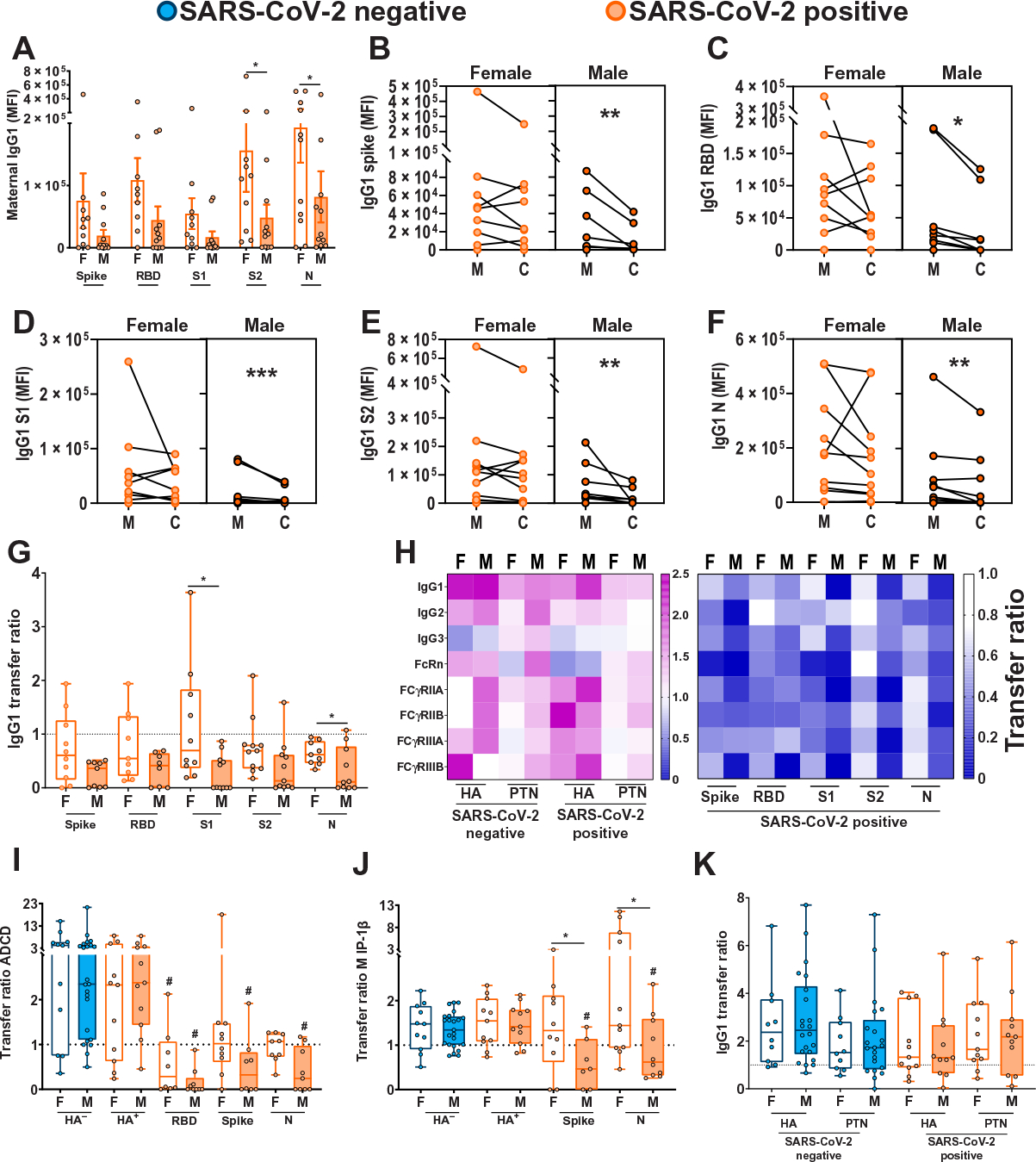
(A) Bar graphs depict spike protein–, RBD-, S1-, S2-, and N protein–specific maternal IgG1 titers (n = 11 per group). F, female fetus; M, male fetus. Differences across groups were assessed by two-way ANOVA followed by Bonferroni’s post hoc analyses. There was a main effect of fetal sex on maternal IgG1 titers. MFI, median fluorescence intensity. *P < 0.05. (B to F) Dot plots showing relative spike protein– (B), RBD- (C), S1- (D), S2- (E), and N protein–specific (F) maternal blood (M) and cord blood (C) titers of IgG1 (n = 11 per group). Female neonates of SARS-CoV-2–positive mothers are shown in light orange. Males born to SARS-CoV-2–positive mothers are shown in dark orange. All values reflect phosphate-buffered saline (PBS) background correction. Group differences were assessed by Wilcoxon matched-pairs signed rank test. *P < 0.05, **P < 0.01, and ***P < 0.001. (G) Box and whisker plots show the placental transfer ratios (cord:maternal ratios) for IgG1 against the SARS-CoV-2 antigens spike protein, RBD, S1, S2, and N. SARS-CoV-2–positive maternal status is shown in orange [female (F), open bars; male (M), shaded bars]. All values are PBS background corrected (n = 11 per group). Differences across groups were assessed by Kruskal-Wallis test followed by Dunn’s post hoc analyses. Dotted line denotes a transfer ratio of 1 or 100%. *P < 0.05. (H) Heatmap depicting the median PBS background–corrected cord:maternal transfer ratio of HA, PTN, spike protein, RBD, S1, S2, and N protein across all antibody subclasses and Fc receptor binding profiles in both SARS-CoV-2–negative and SARS-CoV-2–positive maternal:neonate dyads. (I and J) Box and whisker plots depicting transfer ratios (cord:maternal) for HA-, RBD-, spike protein–, or N protein–specific antibodies (n = 11 to 23 per group) mediating antibody-dependent complement deposition [ADCD (I)] and macrophage inflammatory protein-1β (MIP-1β) expression (J). SARS-CoV-2–negative maternal status is shown in blue (female, open bars; male, shaded bars), and SARS-CoV-2–positive maternal status is shown in orange (female, open bars; male, shaded bars). HA− indicates presence of antibodies specific to HA antigen in SARS-CoV-2–negative pregnancies, HA+ indicates presence of antibodies specific to HA antigen in SARS-CoV-2–positive pregnancies. Differences across groups were assessed by Kruskal-Wallis test followed by Dunn’s post hoc analyses. For each SARS-CoV-2 antigen, post hoc analyses were performed against the HA-specific activity using the matched SARS-CoV-2–positive dyad. #P < 0.01 compared to HA transfer ratio for SARS-CoV-2–positive samples of that sex, and *P < 0.01 compared to indicated group. (K) The box and whisker plots show the transfer ratios (cord:maternal ratio) for IgG1 against HA and PTN in maternal:neonate dyads from either SARS-CoV-2–negative or SARS-CoV-2–positive pregnancies (n = 11 to 23 per group). SARS-CoV-2 negative are shown in blue (female, open bars; male, shaded bars), and SARS-CoV-2 positive are shown in orange (female, open bars; male, shaded bars). Differences across groups were assessed by Kruskal-Wallis test followed by Dunn’s post hoc analyses. In (I) to (K), dotted line denotes transfer ratio of 1 or 100%. *P < 0.05. For box and whisker plots in (A), (G), and (I) to (K), the box extends from the 25th to 75th percentile, the whiskers depict minimum and maximum, and horizontal line depicts the median.
In contrast to SARS-CoV-2–specific antibodies, efficient transfer of pertussis antigen pertactin (PTN) and the influenza hemagglutinin (HA) glycoprotein-specific titers (Fig. 1K and fig. S3) and functions (Fig. 1, I and J) was observed across pregnancies with both female and male fetuses. Timing from maternal vaccination to sample collection for titer determination is depicted in table S2. In addition to influenza and pertussis antibodies, which reflect maternal vaccination during pregnancy, we assessed the transfer of antibodies against endemic, chronic, and other childhood vaccinatable pathogens including measles, mumps, rubella, varicella zoster virus (VZV), common coronaviruses NL63 and OC43, cytomegalovirus (CMV), and Epstein-Barr virus (EBV) and found no sex differences in maternal IgG1 titers for these pathogens (fig. S4). We did, however, identify increased transplacental transfer of anti-measles, anti-mumps, anti-VZV IgG1, and anti-NL63 IgG1 in females but not males exposed to maternal SARS-CoV-2, relative to sex-matched controls (fig. S4A). Although significantly lower maternal titers of SARS-CoV-2–specific IgG1 were observed in the setting of a male fetus (Fig. 1A; P < 0.05), this effect was specific to anti–SARS-CoV-2 antibodies, with no impact of fetal sex on maternal anti-HA, PTN, tetanus, VZV, CMV, measles, mumps, rubella, NL63, OC43, or EBV titers (fig. S4, B and C). Similar results were observed for maternal IgG2, IgG3, and IgM (fig. S5). Overall, these results suggest a reduced maternal SARS-CoV-2–specific humoral immune response in pregnancies with male fetuses, consistent with prior studies demonstrating suppressed maternal proinflammatory responses in mothers with a male fetus relative to a female fetus (13, 14) and the known direct correlation between proinflammatory response and increased antibody production in COVID-19 infection (66, 67).
Sexually dimorphic placental Fc receptor expression was observed in response to maternal SARS-CoV-2 infection
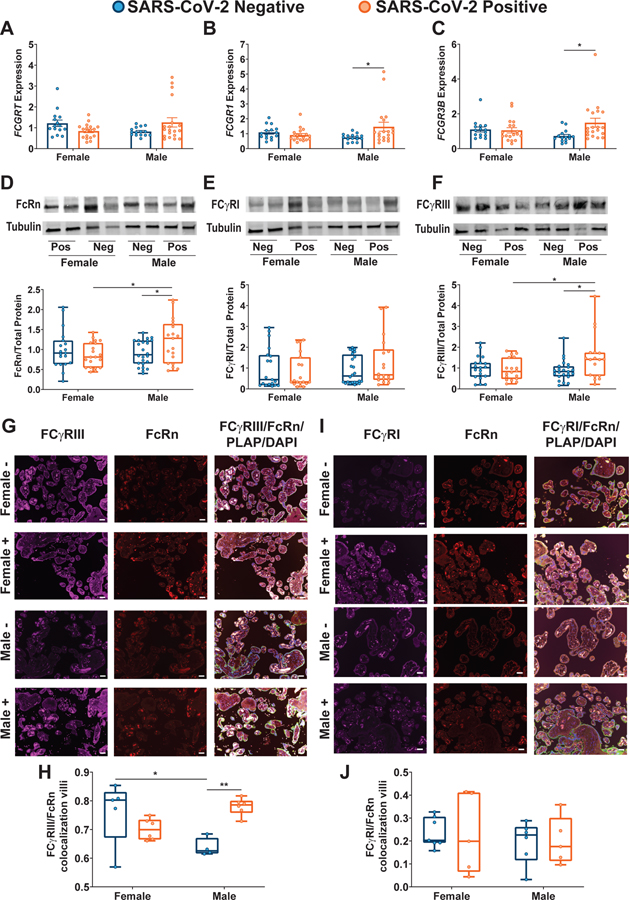
The transfer of maternal antibodies across the placenta is mediated by Fc receptors (15, 16). FcRn was considered the classical receptor mediating transplacental transfer of IgG (20–22), although other Fc receptors (FcγRI, FcγRII, and FcγRIII) are increasingly recognized as being expressed in the placenta and likely to play a role in transfer (15, 16, 23–25). We observed sexually dimorphic expression of FCGRT, FCGR1, and FCGR3A/B genes in the setting of maternal SARS-CoV-2 infection (Fig. 2, A to C, fig. S6, A to C, and table S3), driven by increased expression in male SARS-CoV-2–exposed placentas. There were no fetal sex– or maternal SARS-CoV-2 infection–mediated differences in placental expression of FCGR2A/B (fig. S6, B and C, and table S3). These findings point to sexual dimorphism in Fc receptor expression with up-regulation in male placentas. To distinguish whether increased transcript expression of Fc receptors was compensatory in the setting of diminished protein expression or whether protein expression mirrored gene expression, we performed immunoblot analyses of FcRn, FCγRIII, FCγRII, and FCγRI. Protein expression was consistent with gene expression for FcRn, FCγRII, and FCγRIII (Fig. 2, D to F, fig. S6D, and table S3). There was no impact of maternal SARS-CoV-2 infection or fetal sex on protein expression of FCγRI (Fig. 2E and table S3). In addition to Fc receptor quantity, it has been demonstrated that colocalization of other Fcγ receptors with FcRn typically augments efficiency of placental antibody transfer (16, 29). Immunohistochemical analyses of placental villi revealed sexual dimorphism in placental Fc receptor colocalization, with a significant increase in colocalization of FCγRIII and FcRn in male placentas only (Fig. 2, G and H, and table S3; P < 0.01). No fetal sex or maternal SARS-CoV-2 exposure differences in colocalization with FcRn were observed for FCγRI (Fig. 2, I and J, and table S3) or FCγRII (fig. S6, E to G, and table S3). The gene and protein expression results suggest that maternal SARS-CoV-2 infection has a sexually dimorphic impact on placental Fcγ receptor expression, driven by an increase in overall expression and increased FCγRIII/FcRn colocalization in male placentas. Increased placental Fc receptor expression was not sufficient to restore normal placental transfer of humoral immunity to male fetuses.
Fig. 2. Sexually dimorphic regulation of placental Fc receptor gene and protein expression is observed in the setting of maternal SARS-CoV-2 infection.
(A to C) RT-qPCR analyses of male or female placental expression of FCGRT (A), FCGR1 (B), and FCGR3B (C) in placental biopsies from SARS-CoV-2–negative (blue) or SARS-CoV-2–positive (orange) pregnancies (n = 15 to 18 per group). Expression shown is relative to reference genes YWHAZ and TOP1. Bar plots in (A) to (C) indicate means ± SEM. (D to F) Representative immunoblots and quantification of fetal female or fetal male expression of FcRn (D), FCγRI (E), and FCγRIII (F) in placental biopsies from SARS-CoV-2–negative (blue) or SARS-CoV-2–positive (orange) pregnancies (n = 19 per group). Neg and Pos on Western blot designates SARS-CoV-2–negative and SARS-CoV-2–positive pregnancies, respectively. For box and whisker plots in (D) to (F), the box extends from the 25th to 75th percentile, the whiskers depict minimum and maximum, and horizontal line depicts the median. (G) Placental tissue sections from SARS-CoV-2–positive and SARS-CoV-2–negative mothers were stained for FCγRIII (purple), FcRn (red), and placental alkaline phosphatase (PLAP; green), a trophoblast marker, and 4′,6-diamidino-2-phenylindole (DAPI, blue). (H) Box and whisker plots showing FCγRIII/FcRn colocalization in placental villi (n = 4 to 6 per group). (I) Placental tissue sections from SARS-CoV-2–positive and SARS-CoV-2–negative mothers were stained for FCγRI (purple), FcRn (red), and PLAP (green), a trophoblast marker, and DAPI (blue). (J) Box and whisker plots showing FCγRI/FcRn colocalization in placental villi (n = 5 to 7 per group). Scale bars, 100 μm (G and I). For box and whisker plots in (H) and (J), the box extends from the 25th to 75th percentile, the whiskers depict minimum and maximum, and horizontal line depicts the median. Differences across groups were assessed by two-way ANOVA followed by Bonferroni’s post hoc analyses. *P < 0.05 and **P < 0.01.
Spike protein–specific Fc-glycan profiles coupled with placental Fc receptor expression patterns drive reduced placental antibody transfer in male pregnancies
Given that antibody glycosylation has been demonstrated to be a key driver of reduced SARS-CoV-2–specific antibody transfer in maternal-cord dyads (68), we next profiled glycosylation of bulk and spike protein–specific antibodies in SARS-CoV-2–positive pregnancies in the context of a male versus a female fetus. We identified significant differences between bulk and spike protein–specific glycosylation profiles, more pronounced in the context of a male fetus (Fig. 3, A and B; P < 0.05). Spike protein–specific Fc-glycan profiles in SARS-CoV-2–positive mothers were marked by enhanced fucosylation (F) and digalactosylation (G2), and reduced bisecting-n-acetyl-glucosamine (b-GlcNAc, B) and agalactosylation (G0) on spike protein–specific antibodies in pregnancies with both male and female fetuses (Fig. 3, A and B, and fig. S7). Given that fucosylated antibodies are not transferred efficiently through FCγRIII (69–71), the increased expression of FCγRIII and increased colocalization of FCγRIII and FcRn in placentas of SARS-CoV-2–positive male pregnancies may have contributed to impaired placental transfer of SARS-CoV-2–specific antibodies. Because of the attenuated transfer of fucosylated antibodies resulting from up-regulation of FCγRIII, males preferentially transferred afucosylated antibodies (such as G0 and B), which were relatively scarce among the spike protein–specific antibodies. In contrast, pregnancies with a female fetus had relatively higher maternal titers of SARS-CoV-2–specific antibodies and decreased FCγRIII placental expression, allowing for more efficient transplacental antibody transfer of the available Fc-glycan profile on spike protein–specific antibodies. Bulk antibodies (including HA, PTN, MMR, VZV, common coronaviruses, CMV, and EBV) had a different glycan profile from spike protein–specific antibodies, with bulk antibodies overall enriched for G0 and B (Fig. 3, A and B). Sex-specific differences were noted in the glycosylation profile of antibodies transferred from mother to neonate (Fig. 3C). Pregnancies with a male fetus demonstrated significantly increased transfer of agalactosylated (G0), sialyated (S), and bisected GlcNAc (B) spike protein–specific antibodies relative to bulk (Fig. 3C; P < 0.05). Although both male and female spike protein–specific transfer of fucosylated (F) antibodies was decreased relative to bulk transfer, only males had impaired transfer of galactosylated (G1) antibodies (Fig. 3C). A possible etiology for the fundamental Fc-glycan differences between spike protein–specific and bulk antibodies within SARS-CoV-2–exposed dyads is the production of SARS-CoV-2–specific antibodies during a de novo infection in pregnancy. Inflammation has been demonstrated to alter the glycosylation profile of antibodies produced during that episode (72) and may be a driver of the differences noted in glycosylation profile of bulk compared with spike protein–specific antibodies.
Fig. 3. Sex differences in placental transfer are driven by differences in bulk versus spike protein–specific Fc-glycan transfer.
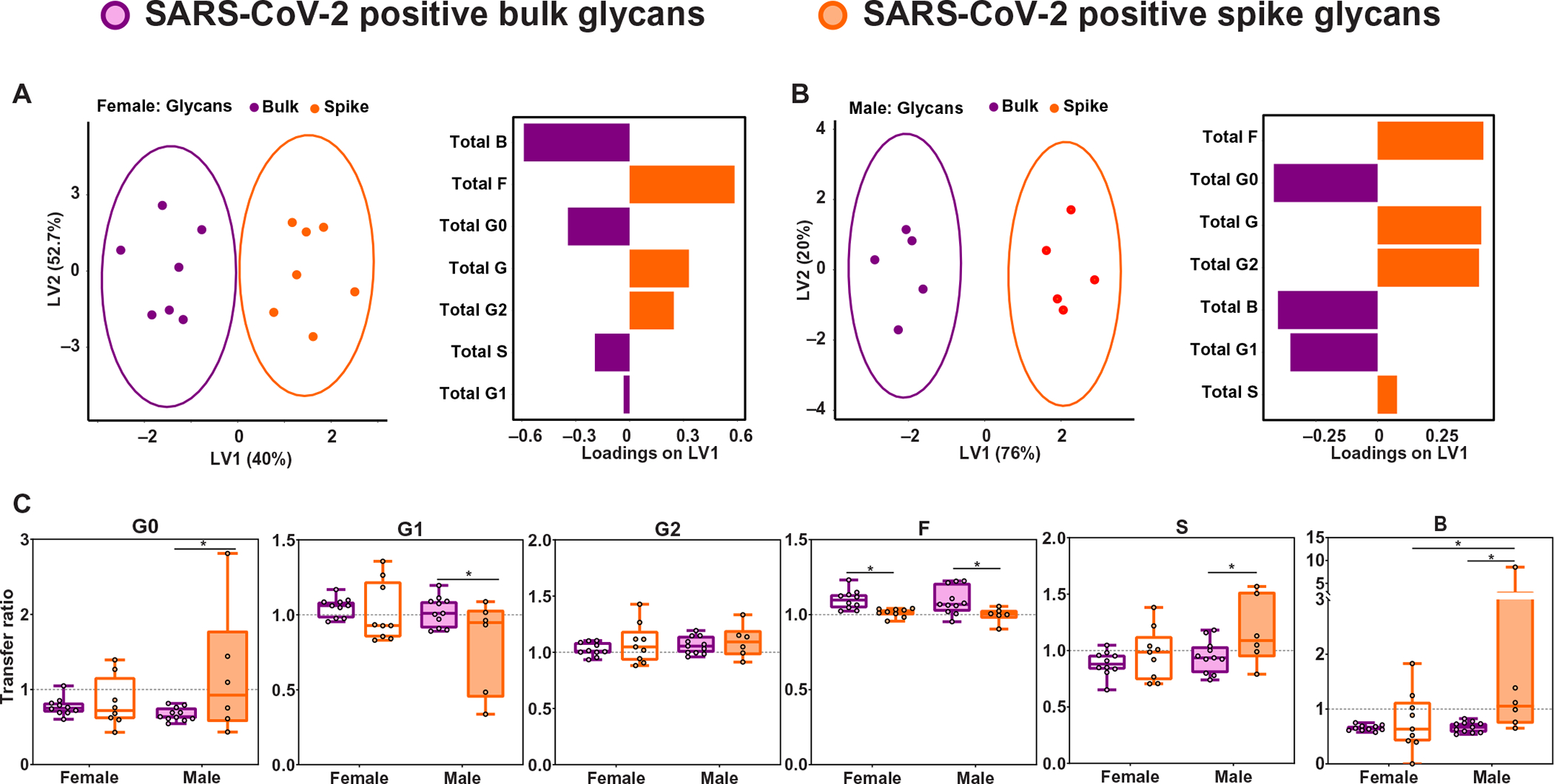
(A and B) A multilevel orthogonal partial least squares discriminant analysis (O-PLSDA) was built using bulk (purple) and spike protein–specific (orange) antibody glycan data from SARS-CoV-2–positive mothers pregnant with a female fetus (A) or male fetus (B) for which matched data were available. Each maternal sample is depicted as a dot on the scores plot (left). The glycan feature loadings on the first latent variable (LV1) are depicted by the bar graph (right). The model performance was assessed using leave-one-out cross-validation, with the average accuracy scores reported to be 98 and 100% predictive accuracy for the female and male O-PLSDA models, respectively. (C) Box and whisker plots showing the transfer ratios (cord:maternal ratios) for bulk and spike protein–specific Fc-glycans. Glycoforms depicted are agalactosylated (G0), monogalactosylated (G1), digalactyosylated (G2), fucosylated (F), sialylated (S), and bisected n-acetyl-glucosamine (GlcNAc, B). Bulk glycans are shown in purple, and spike protein–specific glycans are shown in orange (n = 8 to 11 per group). Pregnancies with female neonates are shown as open bars, whereas pregnancies with male neonates are shown as shaded bars. For box and whisker plots in (C), box extends from the 25th to 75th percentile, the whiskers depict minimum and maximum, and horizontal line depicts the median. Differences across groups were assessed by two-way ANOVA followed by FDR multiple comparisons correction post hoc analyses. *P < 0.05.
Sexually dimorphic placental expression of ISGs were observed in response to maternal SARS-CoV-2 infection
Given the key role ISGs are known to play in the placental antiviral response (46, 47, 73) and placental barrier function (74), and previous reports of sex differences in expression of IFN-stimulated proteins in the plasma of patients with COVID-19 (42), we examined whether maternal SARS-CoV-2 infection was associated with sex-specific alterations in placental type I, II, and III IFN pathways (fig. S8A). There was a sexually dimorphic expression pattern of the classical ISGs IFI6, CXCL10, and OAS1 driven by increased expression in male SARS-CoV-2–exposed placentas compared with SARS-CoV-2–negative controls (Fig. 4, A to C, and table S4). We next examined expression of CCL2/MCP-1, a type I IFN–stimulated cytokine implicated in monocyte chemotaxis (75) and up-regulated in lung samples from patients with COVID-19 (39, 40), as well as MX1, an antiviral response gene induced by type I/III IFNs and up-regulated during SARS-CoV-2 infection (76). Maternal SARS-CoV-2 exposure was associated with increased expression of CCL2 and MX1 in the placenta, with these differences primarily driven by increases in male placentas (Fig. 4, D and E, and table S4). Although maternal SARS-CoV-2 infection did not affect expression of placental TNF, IL6, or CCL7 compared with expression in placentas from women negative for SARS-CoV-2 (fig. S8, B to D, and table S4), there was a sexually dimorphic effect of maternal SARS-CoV-2 infection on the expression of anti-inflammatory factor IL10 (77), driven by significantly increased expression in SARS-CoV-2–exposed male placentas (Fig. 4F and table S4; P < 0.01). No changes in expression of the reference genes YWHAZ or TOP1 were observed across fetal sex or maternal SARS-CoV-2 status groups (fig. S9 and table S4).
Fig. 4. Male-specific up-regulation of ISGs in placentas exposed to maternal SARS-CoV-2 infection.
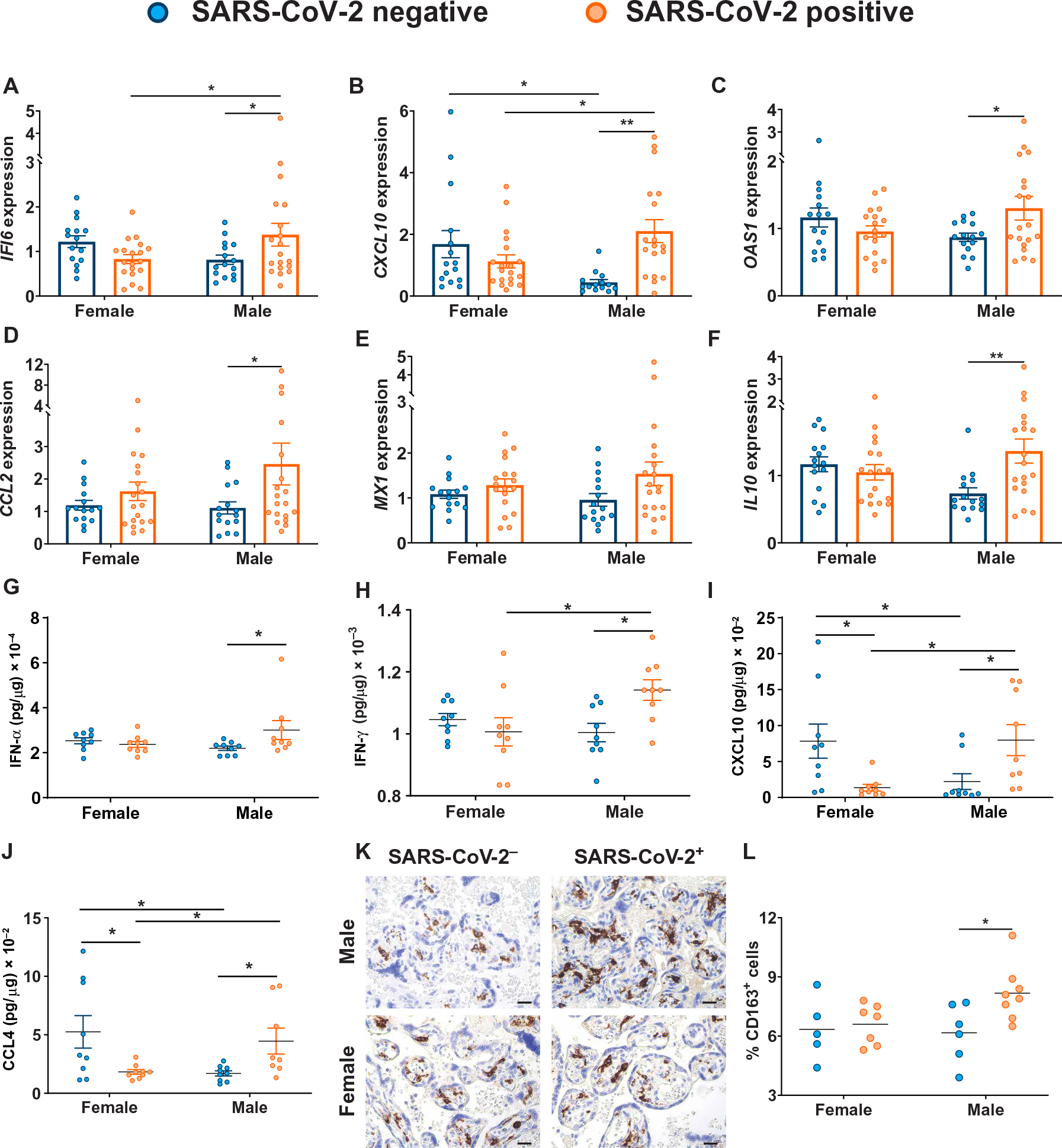
(A to F) RT-qPCR analyses of male or female placental expression of IFI6 (A), CXCL10 (B), OAS1 (C), CCL2 (D), MX1 (E), and IL10 (F) in placental biopsies from SARS-CoV-2–negative (blue) or SARS-CoV-2–positive (orange) pregnancies (n = 15 to 18 per group). Expression shown is relative to reference genes YWHAZ and TOP1. Bar plots in (A) to (F) indicate means ± SEM. (G to J) Placental homogenate protein concentrations of IFN-α (G), IFN-γ (H), CXCL10 (I), and CCL4 (J) were quantified by Luminex multiplex assay (n = 9 per group). Data are presented as means ± SEM. (K and L) Representative immunohistochemistry images (K) and quantification (L) of CD163-positive cells (brown) in placental sections from SARS-CoV-2–negative (blue) or SARS-CoV-2–positive (orange) pregnancies are shown (n = 5 to 8 per group). Scale bars, 100 μm (K). Horizontal bars in (L) indicate mean. Differences across groups were assessed by two-way ANOVA followed by Bonferroni’s post hoc analyses. *P < 0.05 and **P < 0.01
To confirm that the sexually dimorphic ISG response was translated at the protein level, we next assessed protein expression within the type I, II, and III IFN–stimulated pathways using a multiplex immunoassay. There was sexually dimorphic expression of placental IFN-α and IFN-γ, driven by increased IFN expression in male SARS-CoV-2–exposed placentas compared with SARS-CoV-2–negative controls (Fig. 4, G to H). Consistent with our gene expression profiling, we confirmed sexually dimorphic response of IFN-stimulated proteins CXCL10, CCL3, and CCL4 to maternal SARS-CoV-2 infection, although protein expression of CCL2 was not different, in contrast to what was observed at the gene level (Fig. 4, I and J, fig. S10, and table S4). Expression of some ISGs and proteins, in particular CXCL10 and CCL4, was also significantly lower in male compared with female control placentas in the absence of SARS-CoV-2 infection (Fig. 4, B, I, and J; P < 0.05). Protein analyses confirmed the lack of sex-specific alterations in placental expression of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-12p70, IL-13, IL-17A, IL-8, IL-1α, IL-1β, IL-4, CD62E, or CD62P in the setting of maternal SARS-CoV-2 infection (fig. S10 and table S4).
We also observed a male-specific increase in density of CD163+ Hofbauer cells, placental resident fetal macrophages (78), in response to maternal SARS-CoV-2 exposure (Fig. 4, K and L, and table S4). Although placental Hofbauer cell hyperplasia has been described in maternal SARS-CoV-2 infection (79, 80), sex differences have not yet been examined and may reflect a sex-specific fetal placental immune response to maternal SARS-CoV-2 infection. Together, these results suggest a sex-specific and, in some cases, sexually dimorphic response of placental ISGs to maternal SARS-CoV-2 infection, with up-regulation of ISGs in male placentas exposed to maternal SARS-CoV-2 infection.
Modeling the relative impact of placental gene expression, antibody features, and disease timing and severity
Although we observed sexually dimorphic expression of individual placental genes and sex-biased transplacental antibody transfer in response to maternal SARS-CoV-2 infection, it was still unclear whether placental gene expression influenced the transfer of humoral immunity in a sex-specific manner. To investigate the relationship between fetal sex, placental gene expression, and antibody transfer, we examined whether S1-specific IgG1 transplacental transfer could be predicted by differential inflammatory, Fc receptor, and IFN-stimulated placental gene expression in male and female orthogonal partial least squares discriminant analysis (O-PLSDA) models. S1-specific IgG1 transfer was modeled because the most robust sex difference was noted in the transfer of this antibody (Fig. 1G). Given that transfer of SARS-CoV-2 specific antibodies in fetuses of both sexes was less than the expected cord:maternal ratio of greater than 1.1 to 1.5, there was no truly high or efficient transfer. Ratio thresholds for distinguishing higher and lower transfer were therefore determined on the basis of the natural distribution of the data. For female neonates, this ratio was 0.85, and for males, this ratio was 0.5 (reflective of more impaired transfer in males). To investigate whether multivariate analysis could identify gene signatures specific to relatively high or low transfer, we performed O-PLSDA separately on the female and male neonates (Fig. 5, A to D). To avoid overfitting the O-PLSDA model, features were reduced to the “optimal” set of nonredundant features using the least absolute shrinkage and selection operator (LASSO) feature selection (81). In both male and female pregnancies, high placental expression of FCGR3B was associated with lower IgG1 S1 transfer efficiency (Fig. 5, A to D). Higher placental IL10 expression was associated with higher IgG1 S1 transfer in female pregnancies, but lower transfer efficiency in male pregnancies (Fig. 5, B and D). In male pregnancies, high expression of OAS1 was associated with higher transfer (Fig. 5D). Overall, these O-PLSDA models support a systemic sexually dimorphic response that triggers distinct inflammatory cascades likely critical for driving different titers of antibody production in mothers.
Fig. 5. Placental gene expression affects transplacental antibody transfer.
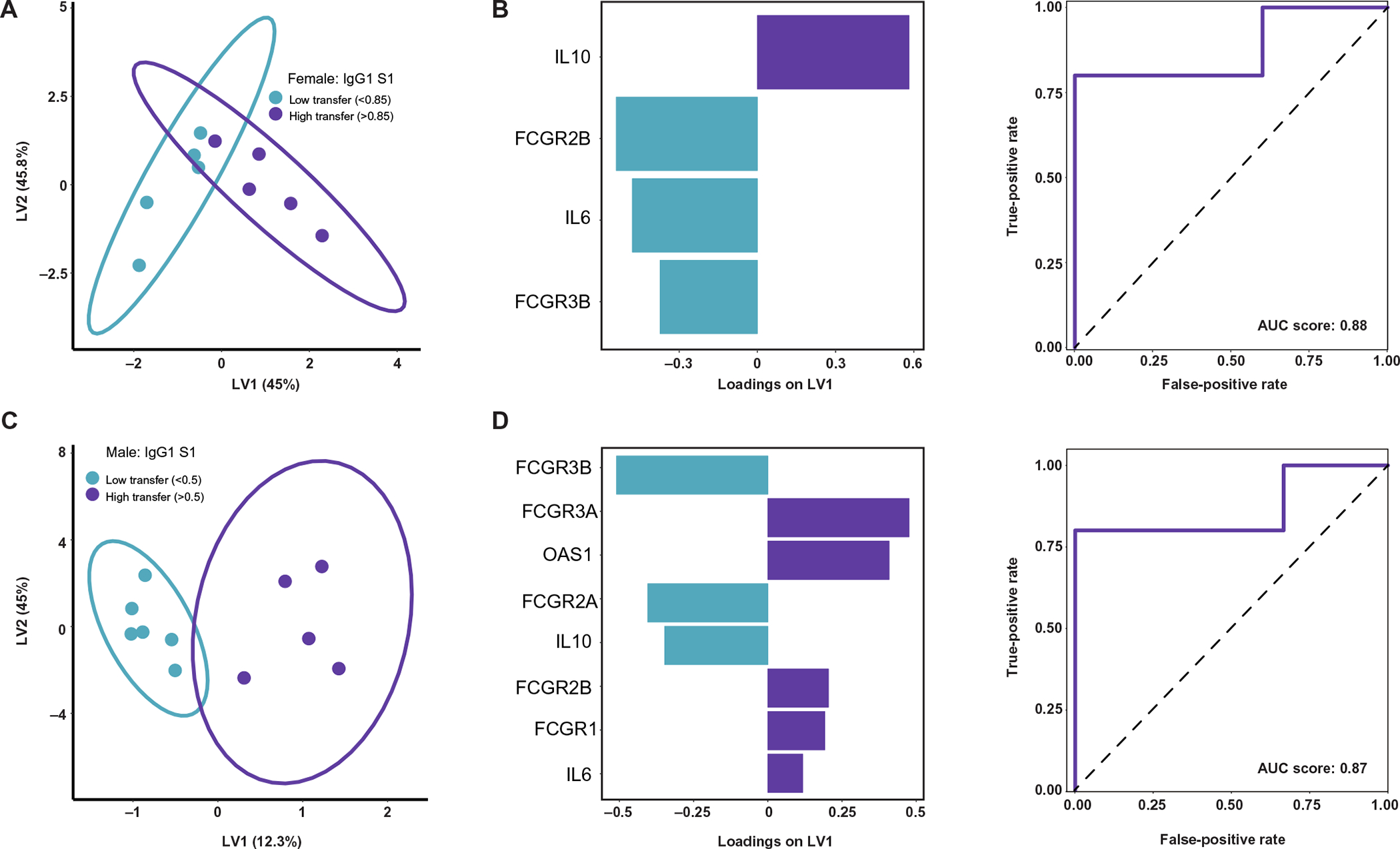
(A) O-PLSDA model classifying relatively low (ratio < 0.85) versus high (ratio > 0.85) IgG1 S1 transfer to female neonates based on placental gene expression. The model was built on gene expression features after LASSO feature selection. Each dot represents one mother-cord pair. (B) Gene expression feature loadings on the LV1 for the female model in (A). Bar color corresponds with which transfer classification (low versus high) is characterized by enriched expression of that gene, with blue bars enriched in low transfer and purple bars enriched in high transfer. The receiver operating characteristic (ROC) curve (solid purple line) for this model quantifies the accuracy in classifying IgG1 S1 transfer to female neonates after leave-one-out cross-validation. The area under the curve (AUC) score is reported. The black dashed line represents the expected performance of a random model (AUC score = 0.5). (C) O-PLSDA model classifying relatively low (ratio < 0.5) versus high (ratio > 0.5) IgG1 S1 transfer to male neonates based on placental gene expression. The model was built on gene expression features after LASSO feature selection. (D) Gene expression feature loadings on the LV1 for the male model in (C). Bar color corresponds with which transfer classification (low versus high) is characterized by enriched expression of that gene, with blue bars enriched in low transfer and purple bars enriched in high transfer, respectively. The ROC curve (solid purple line) for this model quantifies the accuracy in classifying IgG1 S1 transfer to male neonates after leave-one-out cross-validation. The AUC score is reported with black dashed line representing the expected performance of a random model (AUC score = 0.5).
We also examined the sex-specific impacts of other disease and birth-related factors on placental ISG and Fc receptor expression and placental antibody transfer. Using linear regression and O-PLSDA models, we did not observe an impact of time from infection, disease severity, or maternal labor status on male or female placental expression of any ISGs, proinflammatory cytokines, Fc receptors, or IgG transfer (fig. S11). In addition, although both male and female neonates born to SARS-CoV-2–positive mothers had reduced birthweight compared with sex-matched controls, birthweights were in the normal range for both groups (Table 1) and birthweight did not affect IgG antibody transfer in linear regression models (fig. S11). There were also no associations between antibody transfer and days from infection or gestational age at delivery for either male or female neonates (fig. S11). In summary, disease severity, time from infection, maternal labor status, neonatal birthweight, and gestational age at delivery do not appear to confound our findings of decreased transplacental antibody transfer in the context of a male fetus and sexually dimorphic placental gene expression in the context of maternal SARS-CoV-2 infection.
DISCUSSION
Our results demonstrate the impact of fetal sex on the maternal and placental immune response to SARS-CoV-2 and the potential consequences for neonatal antibody-mediated immunity. We show that maternal SARS-CoV-2 infection is associated with reduced maternal SARS-CoV-2–specific IgG titers in the setting of a male fetus. SARS-CoV-2–specific placental antibody transfer to the male fetus was reduced despite up-regulation of placental Fc receptors in SARS-CoV-2–exposed male placentas; males were unable to overcome the reduced maternal titers and the highly fucosylated glycan profile of the spike protein–specific antibodies. Mirroring Fc receptor expression, placental expression of ISGs and proteins was also sexually dimorphic, with notable up-regulation noted in male placentas in the setting of maternal SARS-CoV-2 infection. Collectively, these findings provide evidence of maternal-placental-fetal immune cross-talk in the setting of maternal viral infection, with fetal sex playing a key role in modifying maternal humoral responses and placental innate and adaptive immune responses.
Epidemiologic data point to a persistent male bias in the development and severity of COVID-19 disease in adults, children, and infants (6, 8, 9, 82, 83). Male COVID-19 patients are three times more likely to require admission to intensive care units and have higher odds of death than females (84). This male-biased vulnerability to maternal SARS-CoV-2 infection mirrors the male-biased risk of mortality and morbidity across the perinatal period (1–3). Our findings of sexually dimorphic placental innate immune responses to infection, coupled with sex differences in transfer of maternal humoral immunity, may provide insight into increased vulnerability of male infants to morbidity and mortality.
Although the impact of fetal sex is not consistently evaluated in studies of placental function (85), sex-specific alterations in the placental transcriptome have been described in both normal and pathologic pregnancies (86–89). Sex differences in the placental immune response to prenatal infections and other immune stressors have been described in human and animal models (54, 90–93) but have not been examined in SARS-CoV-2 infection. Here, we report that maternal SARS-CoV-2 infection induces a sexually dimorphic placental antiviral innate immune response, with up-regulation of ISGs in male, but not female, placentas. Male-specific stimulation of placental ISGs after SARS-CoV-2 exposure is consistent with the heightened male immune responses reported in SARS-CoV-2–infected adult and pediatric cohorts (5, 6, 8–10, 42, 94). Although we did not see evidence of maternal viremia or placental, cord blood, or neonatal SARS-CoV-2 infection (28, 95), and the majority of maternal infections represent mild or moderate disease, there is still evidence of altered placental gene expression and an antiviral response in the placentas of male pregnancies. This indicates that even a mild maternal infection in the absence of placental or fetal infection has the potential to affect placental function and fetal development.
Because of their immature immune system, newborns rely on the passive transplacental transfer of maternal antibodies for initial protection against infectious pathogens (15, 18, 96). Although previous reports in adults have noted sex differences in the production of SARS-CoV-2–specific antibodies (42, 97), sex-biased maternal production and transplacental transfer of SARS-CoV-2–specific antibodies have not been well described. We previously reported impaired placental transfer of maternal SARS-CoV-2–specific antibodies in the setting of maternal COVID-19 (28, 29). Although there are known sex differences in adult antibody production in response to SARS-CoV-2 infection (42, 94), little is known about sex differences in maternal titers or transplacental antibody transfer (98, 99) in the setting of maternal SARS-CoV-2 infection. Our finding of decreased maternal antibody titers against all measured SARS-CoV-2–specific antigens (S, S1, S2, RBD, and N) when the fetus was male versus female was a difference not observed for influenza- or pertussis-specific antibodies. Reduced maternal SARS-CoV-2–specific IgG titer in male pregnancies was undoubtedly a driver of the reduced transplacental transfer noted in male fetuses (15). This finding of impaired placental transfer of SARS-CoV-2–specific antibodies, more pronounced in males, is consistent with the male-specific reduction of placental transfer of maternal IgG reported in a nonhuman primate model of maternal stress (98). Reduced maternal antibody titers in the setting of a male fetus were likely attributable to suppressed maternal proinflammatory responses in the setting of a male fetus, which have been described in prior studies and may function to improve tolerance of the fetal allograft (13, 14). The direct correlation between proinflammatory response and increased antibody production noted in COVID-19 infection (66, 67) suggests that blunted maternal inflammatory responses in the setting of a male fetus may limit maternal antibody production in the setting of acute infection. Whether the male-biased impairment in placental SARS-CoV-2–specific antibody transfer renders male infants more vulnerable to early-life SARS-CoV-2 infection remains unclear, as the amount of antibody necessary for protection against SARS-CoV-2 infection is unknown and there are few sex-disaggregated reports of neonatal (100, 101) or infant infection (8).
Although the up-regulation of Fc receptor expression in male placentas may represent a compensatory placental response driven by reduced maternal antibody titer and transplacental transfer of SARS-CoV-2 antibodies (22, 102, 103), this response was likely reinforced by the increased IFN signaling in males versus females. IFN-stimulated signaling may affect placental antibody transfer via alteration in Fc receptor expression and function (104–106); for example, type I IFN signaling is known to up-regulate Fcγ receptor expression on monocytes (107). Hofbauer cells, tissue-resident macrophages of the placenta, express FcγRI, FcγRII, and FcγRIII (23). The male-specific Hofbauer cell hyperplasia in placentas exposed to maternal SARS-CoV-2 infection could therefore also be contributing to increased placental FcγRI and FcγRIII expression in males.
Although the low maternal antibody titers in male pregnancies may have driven a compensatory up-regulation of Fc receptors in the male placenta, the up-regulation of FcγRIII and colocalization of FcγRIII with FcRn in the male placenta likely impeded placental transfer of SARS-CoV-2–specific antibodies, given their distinct Fc-glycan profile. Our Fc-glycan analysis demonstrated that SARS-CoV-2–specific antibodies were highly fucosylated in both male and female pregnancies, a posttranslational modification that lowers antibody affinity for FcγRIII (69–71). The male-specific placental increase in FcγRIII expression and colocalization of FcγRIII with FcRn might therefore present an additional impediment to transferring the already-low maternal titers of SARS-CoV-2–specific antibodies to the fetus. Males instead preferentially transferred bisected (afucosylated) and agalactosylated (G0), afucosylated spike protein–specific antibodies, as afucosylated antibodies are more easily transferred by FcγRIII. Given the inflammatory nature of G0 and B antibodies (72, 108, 109), their preferential transfer might promote a more inflammatory immune response in male fetuses.
Innate immune sensing of SARS-CoV-2 involves the activation of type I and type III IFNs and up-regulation of ISGs in target cells (110). Given the relative paucity of SARS-CoV-2 placental infection (28) in comparison to other pandemic infections such as Zika virus (ZIKV) (111), the increased ISG production and up-regulated IL10 expression in exposed male placentas may be a protective mechanism to prevent direct placental infection and pathology. High IFN concentrations during pregnancy have proven protective against placental herpes simplex virus infection (112), and type III IFNs impair ZIKV transplacental transmission (113). Induction of ISGs is likely not universally protective, however. Whereas type III IFNs primarily serve a barrier defense role, type I and type II IFNs can serve a more classical immune activating or inflammatory role (31, 45). Animal models of viral infection in pregnancy implicate type I and type II IFNs and ISGs in impaired placental development and fetal growth restriction (46, 50, 51, 114), conditions that can have both short- and long-term impact on fetal and offspring health. We demonstrated increased expression of IFN-γ, initiator of type II IFN signaling, in male SARS-CoV-2–exposed placentas. IFN-γ and the type II IFN response have been implicated in placental spiral artery remodeling and may mediate fetal growth restriction and fetal demise in malarial and Toxoplasma gondii infection in pregnancy (50, 51, 115, 116). A transcriptomic analysis of SARS-CoV-2 response genes demonstrated that IFN-γ was an upstream regulator of host viral response in the setting of SARS-CoV-2 infection (117), with higher IFN-γ abundance associated with increased risk for SARS-CoV-2 viral entry (52) and increased mortality in moderate and severe COVID-19 illness (53). Thus, it remains unclear whether the male-specific up-regulation of ISGs described here is potentially beneficial (protection from viral infection) versus harmful (increased placental inflammation, increased risk for fetal growth restriction, or poor placental function). It was noteworthy that female placentas from SARS-CoV-2–negative control pregnancies generally had higher expression of ISGs and proteins than did male SARS-CoV-2–negative placentas. The potential for a baseline female “antiviral placental advantage” is consistent with the established increased vulnerability of the male fetus to in utero insults, including viral and bacterial infection (92, 118), and observed sex differences in baseline innate immunity described in nonplacental cells and tissues (12, 119). These findings highlight the necessity of future studies assessing baseline differences in male and female placental immune responses. The long-term consequences of SARS-CoV-2–associated placental induction of type I, II, and III IFN responses for fetal development and in utero programming of later-life metabolic and neurodevelopmental outcomes remains to be determined.
A limitation of our study is the infection of participants primarily in the third trimester, because these samples were collected during the initial wave of the SARS-CoV-2 pandemic in Boston. Whether maternal SARS-CoV-2 infection in the first and second trimester alters ISG and Fc receptor expression, and how such altered expression might durably affect placental immune function, is a question that remains to be answered in future studies. It remains unclear whether the reduced SARS-CoV-2–specific maternal antibody titers, highly fucosylated glycan profile of spike protein–specific antibodies, and attenuated male-specific transplacental antibody transfer are unique to SARS-CoV-2 biology or whether these phenomena instead reflect a common response to de novo infection during pregnancy. Future studies should assess the effect of fetal sex on maternal SARS-CoV-2 antibody titers and transplacental transfer in women infected before pregnancy and the effect of fetal sex on maternal antibody responses to other de novo infections during pregnancy. In addition, although we found no association between disease severity and placental gene expression or antibody transfer, such examinations were limited by the relatively small number of women with severe or critical illness. Although our results demonstrate male-specific up-regulation of type I and II IFNs (IFN-α and IFN-γ) and ISGs and proteins downstream of type I–III signaling cascades, this study did not assess protein expression of type III IFN-λ. Last, although our regression models did not find time from infection to delivery to be a substantial contributor to the antibody transfer ratios, we cannot entirely rule out any contribution of timing of maternal infection to the reduced antibody transfer noted in males. However, our robust sexually dimorphic gene and protein expression results, with up-regulation of both placental ISGs and Fc receptors in males, demonstrate placental factors are a stronger driver of antibody transfer than any time-from-infection effect.
In conclusion, our comprehensive evaluation of the impact of fetal sex on placental gene expression and transplacental antibody transfer in maternal SARS-CoV-2 infection provides insight into sexually dimorphic or sex-specific placental innate and adaptive immune responses to maternal SARS-CoV-2 infection. The increased impact of maternal SARS-CoV-2 infection on male placental and neonatal immunity highlights the importance of evaluating fetal sex in future studies of placental pathology and infant outcomes in SARS-CoV-2, as well as the critical importance of disaggregating sex data in follow-up studies of offspring neurodevelopmental and metabolic outcomes. These findings may have broader implications for understanding placental immune response, male vulnerability, and passive transfer of maternal antibody in other viral infections. Studies investigating SARS-CoV-2 vaccine safety and efficacy in pregnant women should also evaluate placental immune response and antibody transfer effects, in addition to neonatal infection rates, and report these data in a sex-disaggregated fashion (120).
MATERIALS AND METHODS
Study design
The primary objective of this study was to assess maternal-fetal antibody transfer, viral-induced placental IFN responses, and the impact of fetal sex in pregnant humans infected with SARS-CoV-2. No sample size calculation was performed as expected effect sizes were not known. Upon enrollment, collected samples were assigned ordinal deidentified sample identification numbers not specific to disease status (SARS-CoV-2 positive versus negative). Pregnant women at two tertiary care centers in Boston, Massachusetts, were approached for enrollment in a COVID-19 pregnancy biorepository study starting 2 April 2020 (Massachusetts General Hospital and Brigham and Women’s Hospital; Mass General Brigham IRB approval no. 2020P000804). All participants provided written informed consent (virtual). Pregnant women were eligible for inclusion if they were (i) 18 years or older, (ii) able to provide informed consent or had a health care proxy to do so, and (iii) diagnosed with, or at risk for, SARS-CoV-2 infection. Because of wide community spread in Massachusetts during the study period (121), all pregnant women presenting for hospital care were deemed “at risk” for SARS-CoV-2 infection. Universal COVID-19 screening of all patients admitted to the labor and delivery units by nasopharyngeal reverse transcription polymerase chain reaction (RT-PCR) testing was performed (initiated 16 April 2020). Maternal SARS-CoV-2 positivity was defined by a positive clinical nasopharyngeal RT-PCR test result. The number of replicates is detailed in the figure legends for individual experiments.
Maternal and cord blood collection and processing
Sample collection protocols have been described in a previous publication (122). Briefly, blood from pregnant women was collected at delivery by venipuncture into EDTA tubes. Umbilical cord blood was collected immediately after delivery. The umbilical cord was wiped clean, and blood was drawn directly from the vein using a syringe and transferred to EDTA vacutainer tubes. Blood was centrifuged at 1000g for 10 min at room temperature. Plasma was aliquoted into cryogenic vials and stored at −80°C.
Placental sampling and processing
Fetal side biopsies (0.5 cm3 per biopsy) were collected immediately after delivery. Biopsies were taken from two separate locations at least 4 cm from the cord insertion avoiding surface vasculature, edges, and areas of gross pathology. Fetal membranes were dissected from the fetal and maternal surfaces and not included in the sample. Biopsies were then dissected in half, serially washed in Dulbecco’s phosphate-buffered saline, submerged in 5 volumes of RNAlater (Invitrogen), and stored at least 24 hours at 4°C. Excess RNAlater was removed by blotting on Kimwipes, and biopsies were subdivided into 50-mg segments, placed into cryovials, flash frozen in liquid nitrogen, and stored at −80°C. For histopathological examination, placentas were fixed in formalin, weighed, examined grossly, and routine sections were taken for histopathologic diagnoses per the Amsterdam Consensus Criteria recommendations. Additional details on placental sectioning and staining are provided in the Supplementary Materials and in Methods. Placental homogenates were used to determine concentrations of the immune proteins using the Human Inflammation 20-Plex ProcartaPlex Panel (Thermo Fisher Scientific) according to the manufacturer’s instructions. Immune proteins included IFN-α, IFN-γ, IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-12p70, IL-13, IL-17A, TNF-α, CXCL10, CCL2, CCL3, CCL4, CD62E, and CD62P, and protein was quantified using ProcartaPlex Analysis software (Thermo Fisher Scientific).
Statistical analyses
All multivariate analyses were performed using R (version 4.0.0). Multivariate analyses included reverse transcription quantitative PCR (RT-qPCR) and Luminex antibody measurements. Missing data measurements were imputed using the k-nearest neighbor algorithm. Features were then centered and scaled to unit variance. O-PLSDA was performed to classify data on the basis of relative antibody transfer. Before building the O-PLSDA models, LASSO feature regularization and variable selection was performed, resulting in a reduced set of features that were consistently included in at least 80 of the 100 LASSO models. O-PLSDA models were built using the R “ropls” Bioconductor package (orthI = 1 and PredI = 1). Models were validated by evaluating the mean accuracy score after 100 trials of fivefold coefficient of variation (CV). Accuracy scores for analogous models with permutated labels and random features were also reported for comparison.
Statistical tests used and statistical significance are reported in the figure legends. Statistical analyses were performed using GraphPad Prism 9 and R (version 4.0.0). Significant differences in participant characteristics were evaluated using Kruskal-Wallis tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables. Gene expression measurements, immunoblot measurements, and immunohistochemistry staining comparing maternal SARS-CoV-2 exposure status and fetal sex were evaluated by two-way analysis of variance (two-way ANOVA) with Bonferroni’s post hoc analysis. Kruskal-Wallis test followed by Dunn’s post hoc test was used to determine significant differences between transfer ratios. Wilcoxon matched-pairs signed rank test was performed to determine significant differences between paired maternal-cord blood samples. All linear regression models were built in R (version 4.0.0) using the “stats” package. False discovery rate (FDR) multiple comparisons correction was performed for fig. S6 using a corrected P value of 0.05 as the cutoff for significance.
Supplementary Material
Funding:
This work was supported by the National Institutes of Health, including NICHD (R01HD100022 and 3R01HD100022–02S2 to A.G.E., K12HD103096–01 to L.L.S., and 1R01HD094937 to D.J.S.), NIAID (1R21AI145071 to D.J.S. and 3R37AI080289–11S1, R01AI146785, U19AI42790–01, U19AI135995–02, U19AI42790–01, and 1U01CA260476–01 to G.A.; UM-1 AI069412–15S1 to J.Z.L.), NIMH (F32MH116604 to E.A.B. and U54MH118919 supporting A.G.E.) and NHLBI (5K08HL143183 to L.M.Y. and K08HL146963–02 and 3K08HL146963–02S1 to K.J.G.). This work was supported, in part, by March of Dimes grant no. 6-FY20–223 (to A.G.E.). This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under grant no. 1745302 to K.M.P. Additional support was provided by the Massachusetts General Hospital Department of Surgery, the Robert and Donna E. Landreth Family Fund, the Department of Pediatrics at Massachusetts General Hospital, The Regione Campania Italy (CUP G58D20000240002–SURF 20004BP000000011 to A.F.), the Gates Foundation, the Ragon Institute of MGH and MIT, and the Massachusetts General Hospital and Brigham and Women’s Hospital Departments of Obstetrics and Gynecology.
Competing interests: K.J.G. has consulted for Illumina, BillionToOne, and Aetion outside the submitted work. A.F. reported serving as a cofounder and owning stock in Alba Therapeutics and serving on scientific advisory boards for NextCure and Viome outside the submitted work. G.A. reported serving as a founder of Systems Seromyx. J.Z.L. reported serving as a consultant for Abbvie and Jan Biotech. D.P. reported owning stock in Gilead Sciences, BioNano Genomics, Biogen, Bluebird Bio, ImmunoGen, Pfizer, and Bristol-Myers Squibb. D.J.R. reported receiving author royalties from UpToDate and Cambridge University Press outside the submitted work. Any opinion, findings, and conclusions or recommendation expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. All others declare that they have no other competing interest.
Footnotes
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Aghai ZH, Goudar SS, Patel A, Saleem S, Dhaded SM, Kavi A, Lalakia P, Naqvi F, Hibberd PL, McClure EM, Nolen TL, Iyer P, Goldenberg RL, Derman RJ, Gender variations in neonatal and early infant mortality in India and Pakistan: A secondary analysis from the Global Network Maternal Newborn Health Registry. Reprod. Health 17, 178 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner N, Danesh K, Moran K, The evolution of infant mortality inequality in the United States, 1960–2016. Sci. Adv. 6, eaba5908 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pongou R, Why is infant mortality higher in boys than in girls? A new hypothesis based on preconception environment and evidence from a large sample of twins. Demography 50, 421–444 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD; National Institute of Child Health and Human Development Neonatal Research Network, Intensive care for extreme Prematurity — Moving beyond gestational age. N. Engl. J. Med. 358, 1672–1681 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arditi M, Bahar I, Multisystem inflammatory syndrome in children in the United States. N. Engl. J. Med. 383, 1794 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Godfred-Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J, Roguski K, Wallace B, Prezzato E, Koumans EH, Lee EH, Geevarughese A, Lash MK, Reilly KH, Pulver WP, Thomas D, Feder KA, Hsu KK, Plipat N, Richardson G, Reid H, Lim S, Schmitz A, Pierce T, Hrapcak S, Datta D, Morris SB, Clarke K, Belay E; California MIS-C Response Team, COVID-19–associated multisystem inflammatory syndrome in children — United States, March–July 2020. MMWR Morb. Mortal. Wkly Rep. 69, 1074–1080 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, Gabrovska N, Velizarova S, Prunk P, Osterman V, Krivec U, Vecchio AL, Shingadia D, Soriano-Arandes A, Melendo S, Lanari M, Pierantoni L, Wagner N, L’Huillier AG, Heininger U, Ritz N, Bandi S, Krajcar N, Roglić S, Santos M, Christiaens C, Creuven M, Buonsenso D, Welch SB, Bogyi M, Brinkmann F, Tebruegge M; ptbnet COVID-19 Study Group, COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc. Health 4, 653–661 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim L, Whitaker M, O’Halloran A, Kambhampati A, Chai SJ, Reingold A, Armistead I, Kawasaki B, Meek J, Yousey-Hindes K, Anderson EJ, Openo KP, Weigel A, Ryan P, Monroe ML, Fox K, Kim S, Lynfield R, Bye E, Shrum Davis S, Smelser C, Barney G, Spina NL, Bennett NM, Felsen CB, Billing LM, Shiltz J, Sutton M, West N, Talbot HK, Schaffner W, Risk I, Price A, Brammer L, Fry AM, Hall AJ, Langley GE, Garg S; COVID-NET Surveillance Team, Hospitalization rates and characteristics of children aged & <18 years hospitalized with laboratory-confirmed COVID-19 — COVID-NET, 14 States, March 1–July 25, 2020. MMWR Morb. Mortal. Wkly Rep 69, 1081–1088 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yonker LM, Neilan AM, Bartsch Y, Patel AB, Regan J, Arya P, Gootkind E, Park G, Hardcastle M, John AS, Appleman L, Chiu ML, Fialkowski A, De la Flor D, Lima R, Bordt EA, Yockey LJ, D’Avino P, Fischinger S, Shui JE, Lerou PH, Bonventre JV, Yu XG, Ryan ET, Bassett IV, Irimia D, Edlow AG, Alter G, Li JZ, Fasano A, Pediatric Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Clinical presentation, infectivity, and immune responses. J. Pediatr. 227, 45–52.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma X, Liu S, Chen L, Zhuang L, Zhang J, Xin Y, The clinical characteristics of pediatric inpatients with SARS-CoV-2 infection: A meta-analysis and systematic review. J. Med. Virol. 93, 234–240 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muenchhoff M, Goulder PJR, Sex differences in pediatric infectious diseases. J Infect Dis 209 (Suppl. 3), S120–S126 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein SL, Flanagan KL, Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Taylor BD, Ness RB, Klebanoff MA, Tang G, Roberts JM, Hougaard DM, Skogstrand K, Haggerty CL, The impact of female fetal sex on preeclampsia and the maternal immune milieu. Pregnancy Hypertens 12, 53–57 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell AM, Palettas M, Christian LM, Fetal sex is associated with maternal stimulated cytokine production, but not serum cytokine levels, in human pregnancy. Brain Behav. Immun. 60, 32–37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jennewein MF, Abu-Raya B, Jiang Y, Alter G, Marchant A, Transfer of maternal immunity and programming of the newborn immune system. Semin. Immunopathol. 39, 605–613 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Jennewein MF, Goldfarb I, Dolatshahi S, Cosgrove C, Noelette FJ, Krykbaeva M, Das J, Sarkar A, Gorman MJ, Fischinger S, Boudreau CM, Brown J, Cooperrider JH, Aneja J, Suscovich TJ, Graham BS, Lauer GM, Goetghebuer T, Marchant A, Lauffenburger D, Kim AY, Riley LE, Alter G, Fc glycan-mediated regulation of placental antibody transfer. Cell 178, 202–215.e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Moraes-Pinto MI, Verhoeff F, Chimsuku L, Milligan PJ, Wesumperuma L, Broadhead RL, Brabin BJ, Johnson PM, Hart CA, Placental antibody transfer: Influence of maternal HIV infection and placental malaria. Arch. Dis. Child. Fetal Neonatal Ed. 79, F202–F205 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albrecht M, Arck PC, Vertically transferred immunity in neonates: Mothers, mechanisms and mediators. Front. Immunol. 11, 555 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention, Vaccinating Pregnant Women (2019); https://www.cdc.gov/vitalsigns/maternal-vaccines/index.html.
- 20.Clements T, Rice TF, Vamvakas G, Barnett S, Barnes M, Donaldson B, Jones CE, Kampmann B, Holder B, Update on transplacental transfer of IgG subclasses: Impact of maternal and fetal factors. Front. Immunol. 11, 1920 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roopenian DC, Akilesh S, FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7, 715–725 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Lozano NA, Lozano A, Marini V, Saranz RJ, Blumberg RS, Baker K, Agresta MF, Ponzio MF, Expression of FcRn receptor in placental tissue and its relationship with IgG levels in term and preterm newborns. Am. J. Reprod. Immunol. 80, e12972 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez DR, Fouda GG, Peng X, Ackerman ME, Permar SR, Noncanonical placental Fc receptors: What is their role in modulating transplacental transfer of maternal IgG? PLOS Pathog. 14, e1007161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez DR, Fong Y, Li SH, Yang F, Jennewein MF, Weiner JA, Harrell EA, Mangold JF, Goswami R, Seage III GR, Alter G, Ackerman ME, Peng X, Fouda GG, Permar SR, Fc characteristics mediate selective placental transfer of IgG in HIV-infected women. Cell 178, 190–201.e11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simister NE, Story CM, Human placental Fc receptors and the transmission of antibodies from mother to fetus. J. Reprod. Immunol. 37, 1–23 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Cumberland P, Shulman CE, Maple PAC, Bulmer JN, Dorman EK, Kawuondo K, Marsh K, Cutts FT, Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis 196, 550–557 (2007). [DOI] [PubMed] [Google Scholar]
- 27.McLean ARD, Stanisic D, McGready R, Chotivanich K, Clapham C, Baiwog F, Pimanpanarak M, Siba P, Mueller I, King CL, Nosten F, Beeson JG, Rogerson S, Simpson JA, Fowkes FJI, P. falciparum infection and maternofetal antibody transfer in malaria-endemic settings of varying transmission. PLOS ONE 12, e0186577 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edlow AG, Li JZ, Collier A-RY, Atyeo C, James KE, Boatin AA, Gray KJ, Bordt EA, Shook LL, Yonker LM, Fasano A, Diouf K, Croul N, Devane S, Yockey LJ, Lima R, Shui J, Matute JD, Lerou PH, Akinwunmi BO, Schmidt A, Feldman J, Hauser BM, Caradonna TM, De la Flor D, D’Avino P, Regan J, Corry H, Coxen K, Fajnzylber J, Pepin D, Seaman MS, Barouch DH, Walker BD, Yu XG, Kaimal AJ, Roberts DJ, Alter G, Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw. Open 3, e2030455 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atyeo C, Pullen KM, Bordt EA, Fischinger S, Burke J, Michell A, Slein MD, Loos C, Shook LL, Boatin AA, Yockey LJ, Pepin D, Meinsohn M-C, Nguyen NMP, Chauvin M, Roberts D, Goldfarb IT, Matute JD, James KE, Yonker LM, Bebell LM, Kaimal AJ, Gray KJ, Lauffenburger D, Edlow AG, Alter G, Compromised SARS-CoV-2-specific placental antibody transfer. Cell 184, 628–642.e10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Busnadiego I, Fernbach S, Pohl MO, Karakus U, Huber M, Trkola A, Stertz S, Hale BG, Antiviral activity of type I, II, and III interferons counterbalances ACE2 inducibility and restricts SARS-CoV-2. MBio 11, e01928–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazear HM, Schoggins JW, Diamond MS, Shared and distinct functions of type I and type III interferons. Immunity 50, 907–923 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cameron MJ, Ran L, Xu L, Danesh A, Bermejo-Martin JF, Cameron CM, Muller MP, Gold WL, Richardson SE, Poutanen SM, Willey BM, DeVries ME, Fang Y, Seneviratne C, Bosinger SE, Persad D, Wilkinson P, Greller LD, Somogyi R, Humar A, Keshavjee S, Louie M, Loeb MB, Brunton J, McGeer AJ; Canadian SARS Research Network, Kelvin DJ, Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 81, 8692–8706 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S, Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 19, 181–193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Zhao J, Gale MJ Jr., Baric RS, Enjuanes L, Gallagher T, McCray PB Jr., Perlman S, Rapid generation of a mouse model for Middle East respiratory syndrome. Proc. Natl. Acad. Sci. U.S.A. 111, 4970–4975 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanco-Melo D, Nilsson-Payant BE, Liu W-C, Uhl S, Hoagland D, Møller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, tenOever BR, Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181, 1036–1045.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Israelow B, Song E, Mao T, Lu P, Meir A, Liu F, Alfajaro MM, Wei J, Dong H, Homer RJ, Ring A, Wilen CB, Iwasaki A, Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J. Exp. Med. 217, e20201241 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JS, Park S, Jeong HW, Ahn JY, Choi SJ, Lee H, Choi B, Nam SK, Sa M, Kwon J-S, Jeong SJ, Lee HK, Park SH, Park S-H, Choi JY, Kim S-H, Jung I, Shin E-C, Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci. Immunol. 5, eabd1554 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, Ellingson MK, Mao T, Oh JE, Israelow B, Takahashi T, Tokuyama M, Lu P, Venkataraman A, Park A, Mohanty S, Wang H, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ, Muenker MC, Fournier JB, Campbell M, Odio CD, Casanovas-Massana A; Yale IMPACT Team, Herbst R, Shaw AC, Medzhitov R, Schulz WL, Grubaugh ND, Dela Cruz C, Farhadian S, Ko AI, Omer SB, Iwasaki A, Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584, 463–469 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian D-S, Dysregulation of immune response in patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 71, 762–768 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, Guo D, Hu W, Yang J, Tang Z, Wu H, Lin Y, Zhang M, Zhang Q, Shi M, Liu Y, Zhou Y, Lan K, Chen Y, Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 9, 761–770 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Z, Ren L, Zhang L, Zhong J, Xiao Y, Jia Z, Guo L, Yang J, Wang C, Jiang S, Yang D, Zhang G, Li H, Chen F, Xu Y, Chen M, Gao Z, Yang J, Dong J, Liu B, Zhang X, Wang W, He K, Jin Q, Li M, Wang J, heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe 27, 883–890.e2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, Silva J, Mao T, Oh JE, Tokuyama M, Lu P, Venkataraman A, Park A, Liu F, Meir A, Sun J, Wang EY, Casanovas-Massana A, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ; Yale IMPACT Research Team, Shaw A, Fournier JB, Odio CD, Farhadian S, Dela Cruz C, Grubaugh ND, Schulz WL, Ring AM, Ko AI, Omer SB, Iwasaki A, Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 588, 315–320 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S, Sex-based differences in susceptibility to Severe Acute Respiratory Syndrome Coronavirus infection. J. Immunol. 198, 4046–4053 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gadi N, Wu SC, Spihlman AP, Moulton VR, What’s sex got to do with COVID-19? Gender-based differences in the host immune response to coronaviruses. Front. Immunol. 11, 2147 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wells AI, Coyne CB, Type III interferons in antiviral defenses at barrier surfaces. Trends Immunol. 39, 848–858 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yockey LJ, Jurado KA, Arora N, Millet A, Rakib T, Milano KM, Hastings AK, Fikrig E, Kong Y, Horvath TL, Weatherbee S, Kliman HJ, Coyne CB, Iwasaki A, Type I interferons instigate fetal demise after Zika virus infection. Sci. Immunol 3, eaao1680 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yockey LJ, Lucas C, Iwasaki A, Contributions of maternal and fetal antiviral immunity in congenital disease. Science 368, 608–612 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Platanias LC, Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Sadler AJ, Williams BRG, Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 8, 559–568 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yockey LJ, Iwasaki A, Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 49, 397–412 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy SP, Tayade C, Ashkar AA, Hatta K, Zhang J, Croy BA, Interferon gamma in successful pregnancies. Biol. Reprod. 80, 848–859 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heuberger J, Trimpert J, Vladimirova D, Goosmann C, Lin M, Schmuck R, Mollenkopf H-J, Brinkmann V, Tacke F, Osterrieder N, Sigal M, Epithelial response to IFN-γ promotes SARS-CoV-2 infection. EMBO Mol. Med. 13, e13191 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gadotti AC, de Castro Deus M, Telles JP, Wind R, Goes M, Garcia Charello Ossoski R, de Padua AM, de Noronha L, Moreno-Amaral A, Baena CP, Tuon FF, IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 289, 198171 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braun AE, Carpentier PA, Babineau BA, Narayan AR, Kielhold ML, Moon HM, Shankar A, Su J, Saravanapandian V, Haditsch U, Palmer TD, “Females are not just ‘Protected’ Males”: Sex-specific vulnerabilities in placenta and brain after prenatal immune disruption. eNeuro 6, ENEURO.0358–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trus I, Udenze D, Cox B, Berube N, Nordquist RE, van der Staay FJ, Huang Y, Kobinger G, Safronetz D, Gerdts V, Karniychuk U, Subclinical in utero Zika virus infection is associated with interferon alpha sequelae and sex-specific molecular brain pathology in asymptomatic porcine offspring. PLOS Pathog. 15, e1008038 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clifton VL, Review: Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta 31 (Suppl), S33–S39 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Leon-Garcia SM, Roeder HA, Nelson KK, Liao X, Pizzo DP, Laurent LC, Parast MM, LaCoursiere DY, Maternal obesity and sex-specific differences in placental pathology. Placenta 38, 33–40 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Goldfarb IT, Clapp MA, Soffer MD, Shook LL, Rushfirth K, Edlow AG, Boatin AA, Kaimal AJ, Barth WH Jr., Bryant AS, Prevalence and Severity of Coronavirus Disease 2019 (COVID-19) illness in symptomatic pregnant and postpartum women stratified by hispanic ethnicity. Obstet. Gynecol. 136, 300–302 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joseph NT, Dude CM, Verkerke HP, Irby LS, Dunlop AL, Patel RM, Easley KA, Smith AK, Stowell SR, Jamieson DJ, Velu V, Badell ML, Maternal antibody response, neutralizing potency, and placental antibody transfer after Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection. Obstet. Gynecol. 138, 189–197 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song D, Prahl M, Gaw SL, Narasimhan SR, Rai DS, Huang A, Flores CV, Lin CY, Jigmeddagva U, Wu A, Warrier L, Levan J, Nguyen CBT, Callaway P, Farrington L, Acevedo GR, Gonzalez VJ, Vaaben A, Nguyen P, Atmosfera E, Marleau C, Anderson C, Misra S, Stemmle M, Cortes M, McAuley J, Metz N, Patel R, Nudelman M, Abraham S, Byrne J, Jegatheesan P, Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: Prospective cohort study. BMJ Open 11, e053036 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atyeo C, DeRiso EA, Davis C, Bordt EA, DeGuzman RM, Shook LL, Yonker LM, Fasano A, Akinwunmi B, Lauffenburger DA, Elovitz MA, Gray KJ, Edlow AG, Alter G, COVID-19 mRNA vaccines drive differential Fc-functional profiles in pregnant, lactating, and non-pregnant women. Sci. Transl. Med. 13, abi8631 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M, IgG placental transfer in healthy and pathological pregnancies. J. Immunol. Res. 2012, 985646 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kubiak JM, Murphy EA, Yee J, Cagino K, Friedlander RL, Glynn SM, Matthews KC, Jurkiewicz M, Sukhu AC, Zhao Z, Prabhu M, Riley L, Yang YJ, Severe acute respiratory syndrome coronavirus 2 serology levels in pregnant women and their neonates. Am. J. Obstet. Gynecol. 225, 73.e1–73.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Munoz FM, Bond NH, Maccato M, Pinell P, Hammill HA, Swamy GK, Walter EB, Jackson LA, Englund JA, Edwards MS, Healy CM, Petrie CR, Ferreira J, Goll JB, Baker CJ, Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: A randomized clinical trial. JAMA 311, 1760–1769 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonçalves G, Cutts FT, Hills M, Rebelo-Andrade H, Trigo FA, Barros H, Transplacental transfer of measles and total IgG. Epidemiol. Infect. 122, 273–279 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woodruff MC, Ramonell RP, Nguyen DC, Cashman KS, Saini AS, Haddad NS, Ley AM, Kyu S, Howell JC, Ozturk T, Lee S, Suryadevara N, Case JB, Bugrovsky R, Chen W, Estrada J, Morrison-Porter A, Derrico A, Anam FA, Sharma M, Wu HM, Le SN, Jenks SA, Tipton CM, Staitieh B, Daiss JL, Ghosn E, Diamond MS, Carnahan RH, Crowe JE Jr., Hu WT, Lee FE-H, Sanz I, Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 21, 1506–1516 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia-Beltran WF, Lam EC, Astudillo MG, Yang D, Miller TE, Feldman J, Hauser BM, Caradonna TM, Clayton KL, Nitido AD, Murali MR, Alter G, Charles RC, Dighe A, Branda JA, Lennerz JK, Lingwood D, Schmidt AG, Iafrate AJ, Balazs AB, COVID-19-neutralizing antibodies predict disease severity and survival. Cell 184, 476–488.e1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahan AE, Jennewein MF, Suscovich T, Dionne K, Tedesco J, Chung AW, Streeck H, Pau M, Schuitemaker H, Francis D, Fast P, Laufer D, Walker BD, Baden L, Barouch DH, Alter G, Antigen-specific antibody glycosylation is regulated via vaccination. PLOS Pathog. 12, e1005456 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chung S, Quarmby V, Gao X, Ying Y, Lin L, Reed C, Fong C, Lau W, Qiu ZJ, Shen A, Vanderlaan M, Song A, Quantitative evaluation of fucose reducing effects in a humanized antibody on Fcγ receptor binding and antibody-dependent cell-mediated cytotoxicity activities. MAbs 4, 326–340 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Falconer DJ, Subedi GP, Marcella AM, Barb AW, Antibody fucosylation lowers the FcγRIIIa/CD16a affinity by limiting the conformations sampled by the N162-Glycan. ACS Chem. Biol. 13, 2179–2189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV, Wang L-X, Modulating IgG effector function by Fc glycan engineering. Proc. Natl. Acad. Sci. U.S.A. 114, 3485–3490 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alter G, Ottenhoff THM, Joosten SA, Antibody glycosylation in inflammation, disease and vaccination. Semin. Immunol. 39, 102–110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Casazza RL, Lazear HM, Miner JJ, Protective and pathogenic effects of interferon signaling during pregnancy. Viral Immunol. 33, 3–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ETDA Jr., Cherry S, Sadovsky Y, Coyne CB, Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 19, 705–712 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lehmann MH, Torres-Domínguez LE, Price PJR, Brandmüller C, Kirschning CJ, Sutter G, CCL2 expression is mediated by type I IFN receptor and recruits NK and T cells to the lung during MVA infection. J. Leukoc. Biol. 99, 1057–1064 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Bizzotto J, Sanchis P, Abbate M, Lage-Vickers S, Lavignolle R, Toro A, Olszevicki S, Sabater A, Cascardo F, Vazquez E, Cotignola J, Gueron G, SARS-CoV-2 infection boosts MX1 antiviral effector in COVID-19 patients. iScience 23, 101585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L, SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 54, 62–75 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zulu MZ, Martinez FO, Gordon S, Gray CM, The elusive role of placental macrophages: The hofbauer cell. J. Innate Immun. 11, 447–456 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hecht JL, Quade B, Deshpande V, Mino-Kenudson M, Ting DT, Desai N, Dygulska B, Heyman T, Salafia C, Shen D, Bates SV, Roberts DJ, SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: A series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 33, 2092–2103 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Facchetti F, Bugatti M, Drera E, Tripodo C, Sartori E, Cancila V, Papaccio M, Castellani R, Casola S, Boniotti MB, Cavadini P, Lavazza A, SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine 59, 102951 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fonti V, Belitser E, Feature selection using lasso. VU Amsterdam Res. Paper Business Anal. 30, 1–25 (2017). [Google Scholar]
- 82.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI, Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 369, m1966 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abate BB, Kassie AM, Kassaw MW, Aragie TG, Masresha SA, Sex difference in coronavirus disease (COVID-19): A systematic review and meta-analysis. BMJ Open 10, e040129 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, Rosser EC, Webb K, Deakin CT, Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 11, 6317 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walker N, Filis P, Soffientini U, Bellingham M, O’Shaughnessy PJ, Fowler PA, Placental transporter localization and expression in the Human: The importance of species, sex, and gestational age differences†. Biol. Reprod. 96, 733–742 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buckberry S, Bianco-Miotto T, Roberts CT, Imprinted and X-linked non-coding RNAs as potential regulators of human placental function. Epigenetics 9, 81–89 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buckberry S, Bianco-Miotto T, Bent SJ, Dekker GA, Roberts CT, Integrative transcriptome meta-analysis reveals widespread sex-biased gene expression at the human fetal-maternal interface. Mol. Hum. Reprod. 20, 810–819 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stuart TJ, O’Neill K, Condon D, Sasson I, Sen P, Xia Y, Simmons RA, Diet-induced obesity alters the maternal metabolome and early placenta transcriptome and decreases placenta vascularity in the mouse. Biol. Reprod. 98, 795–809 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun T, Gonzalez TL, Deng N, DiPentino R, Clark EL, Lee B, Tang J, Wang Y, Stripp BR, Yao C, Tseng H-R, Karumanchi SA, Koeppel AF, Turner SD, Farber CR, Rich SS, Wang ET, Williams J, Pisarska MD, Sexually dimorphic crosstalk at the maternal-fetal interface. J. Clin. Endocrinol. Metab. 105, e4831–e4847 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Allard M-J, Giraud A, Segura M, Sebire G, Sex-specific maternofetal innate immune responses triggered by group B Streptococci. Sci. Rep. 9, 8587 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bronson SL, Bale TL, Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 155, 2635–2646 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goldenberg RL, Andrews WW, Faye-Petersen OM, Goepfert AR, Cliver SP, Hauth JC, The Alabama preterm birth study: Intrauterine infection and placental histologic findings in preterm births of males and females less than 32 weeks. Am. J. Obstet. Gynecol. 195, 1533–1537 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Barke TL, Money KM, Du L, Serezani A, Gannon M, Mirnics K, Aronoff DM, Sex modifies placental gene expression in response to metabolic and inflammatory stress. Placenta 78, 1–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL, Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 20, 442–447 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shook LL, Bordt EA, Meinsohn M-C, Pepin D, De Guzman RM, Brigida S, Yockey LJ, James KE, Sullivan MW, Bebell LM, Roberts DJ, Kaimal AJ, Li JZ, Schust D, Gray KJ, Edlow AG, Placental expression of ACE2 and TMPRSS2 in maternal SARS-CoV-2 infection: Are placental defenses mediated by fetal sex? J Infect Dis 2021, jiab335 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dowling DJ, Levy O, Ontogeny of early life immunity. Trends Immunol. 35, 299–310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bunders MJ, Altfeld M, Implications of sex differences in immunity for SARS-CoV-2 pathogenesis and design of therapeutic interventions. Immunity 53, 487–495 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coe CL, Crispen HR, Social stress in pregnant squirrel monkeys (Saimiri boliviensis peruviensis) differentially affects placental transfer of maternal antibody to male and female infants. Health Psychol. 19, 554–559 (2000). [PubMed] [Google Scholar]
- 99.Maertens K, Tran TMP, Hens N, Van Damme P, Leuridan E, Effect of prepregnancy pertussis vaccination in young infants. J Infect Dis 215, 1855–1861 (2017). [DOI] [PubMed] [Google Scholar]
- 100.Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shao J, Zhou W, Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 174, 722–725 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yuan J, Qian H, Cao S, Dong B, Yan X, Luo S, Zhou M, Zhou S, Ning B, Zhao L, Is there possibility of vertical transmission of COVID-19: A systematic review. Transl. Pediatr. 10, 423–434 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rahman A, Tiwari A, Narula J, Hickling T, Importance of feedback and feedforward loops to adaptive immune response modeling. CPT Pharmacometrics Syst. Pharmacol. 7, 621–628 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Webb NE, Bernshtein B, Alter G, Tissues: The unexplored frontier of antibody mediated immunity. Curr. Opin. Virol. 47, 52–67 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao C, Gao Y, Yu N, Li T, Zhang Y, Zhang H, Lu G, Gao Y, Guo X, Unidirectional transport of IgG by neonatal Fc receptor in human thyrocytes varies across different IgG subclasses. Mol. Cell. Endocrinol. 477, 103–111 (2018). [DOI] [PubMed] [Google Scholar]
- 105.Fairchild KD, Hudson RG, Douglas SD, McKenzie SE, Polin RA, Effect of gamma interferon on expression of Fc gamma receptors in monocytes of newborn infants and adults. Clin. Diagn. Lab. Immunol. 3, 464–469 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pearse RN, Feinman R, Shuai K, Darnell JE Jr., Ravetch JV, Interferon gamma-induced transcription of the high-affinity Fc receptor for IgG requires assembly of a complex that includes the 91-kDa subunit of transcription factor ISGF3. Proc. Natl. Acad. Sci. U.S.A. 90, 4314–4318 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Y, Lee PY, Kellner ES, Paulus M, Switanek J, Xu Y, Zhuang H, Sobel ES, Segal MS, Satoh M, Reeves WH, Monocyte surface expression of Fcγ receptor RI (CD64), a biomarker reflecting type-I interferon levels in systemic lupus erythematosus. Arthritis Res. Ther. 12, R90 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wuhrer M, Selman MHJ, McDonnell LA, Kümpfel T, Derfuss T, Khademi M, Olsson T, Hohlfeld R, Meinl E, Krumbholz M, Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J. Neuroinflammation 12, 235 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Plomp R, Ruhaak LR, Uh H-W, Reiding KR, Selman M, Houwing-Duistermaat JJ, Slagboom PE, Beekman M, Wuhrer M, Subclass-specific IgG glycosylation is associated with markers of inflammation and metabolic health. Sci. Rep. 7, 12325 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, Levantovsky R, Malle L, Moreira A, Park MD, Pia L, Risson E, Saffern M, Salomé B, Selvan ME, Spindler MP, Tan J, van der Heide V, Gregory JK, Alexandropoulos K, Bhardwaj N, Brown BD, Greenbaum B, Gümüş ZH, Homann D, Horowitz A, Kamphorst AO, de Lafaille MAC, Mehandru S, Merad M, Samstein RM; Sinai Immunology Review Project, Immunology of COVID-19: Current state of the science. Immunity 52, 910–941 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ades AE, Soriano-Arandes A, Alarcon A, Bonfante F, Thorne C, Peckham CS, Giaquinto C, Vertical transmission of Zika virus and its outcomes: A Bayesian synthesis of prospective studies. Lancet Infect. Dis. 21, 537–545 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zdravkovic M, Knudsen HJ, Liu X, Hager H, Zachar V, Aboagye-Mathiesen G, Ebbesen P, High interferon alpha levels in placenta, maternal, and cord blood suggest a protective effect against intrauterine herpes simplex virus infection. J. Med. Virol. 51, 210–213 (1997). [DOI] [PubMed] [Google Scholar]
- 113.Jagger BW, Miner JJ, Cao B, Arora N, Smith AM, Kovacs A, Mysorekar IU, Coyne CB, Diamond MS, Gestational stage and IFN-λ signaling regulate ZIKV infection in utero. Cell Host Microbe 22, 366–376.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baines KJ, Rampersaud AM, Hillier DM, Jeyarajah MJ, Grafham GK, Eastabrook G, Lacefield JC, Renaud SJ, Antiviral inflammation during early pregnancy reduces placental and fetal growth trajectories. J. Immunol. 204, 694–706 (2020). [DOI] [PubMed] [Google Scholar]
- 115.Senegas A, Villard O, Neuville A, Marcellin L, Pfaff AW, Steinmetz T, Mousli M, Klein JP, Candolfi E, Toxoplasma gondii-induced foetal resorption in mice involves interferon-gamma-induced apoptosis and spiral artery dilation at the maternofoetal interface. Int. J. Parasitol. 39, 481–487 (2009). [DOI] [PubMed] [Google Scholar]
- 116.Niikura M, Inoue S-I, Mineo S, Asahi H, Kobayashi F, IFNGR1 signaling is associated with adverse pregnancy outcomes during infection with malaria parasites. PLOS ONE 12, e0185392 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sajuthi SP, DeFord P, Li Y, Jackson ND, Montgomery MT, Everman JL, Rios CL, Pruesse E, Nolin JD, Plender EG, Wechsler ME, Mak ACY, Eng C, Salazar S, Medina V, Wohlford EM, Huntsman S, Nickerson DA, Germer S, Zody MC, Abecasis G, Kang HM, Rice KM, Kumar R, Oh S, Rodriguez-Santana J, Burchard EG, Seibold MA, Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat. Commun. 11, 5139 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ghidini A, Salafia CM, Gender differences of placental dysfunction in severe prematurity. BJOG 112, 140–144 (2005). [DOI] [PubMed] [Google Scholar]
- 119.DiPietro JA, Voegtline KM, The gestational foundation of sex differences in development and vulnerability. Neuroscience 342, 4–20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bischof E, Wolfe J, Klein SL, Clinical trials for COVID-19 should include sex as a variable. J. Clin. Invest. 130, 3350–3352 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.COVID-19 Response Reporting; https://www.mass.gov/info-details/covid-19-response-reporting.
- 122.Shook LL, Shui JE, Boatin AA, Devane S, Croul N, Yonker LM, Matute JD, Lima RS, Schwinn M, Cvrk D, Gardner L, Azevedo R, Stanton S, Bordt EA, Yockey LJ, Fasano A, Li JZ, Yu XG, Kaimal AJ, Lerou PH, Edlow AG, Rapid establishment of a COVID-19 perinatal biorepository: Early lessons from the first 100 women enrolled. BMC Med. Res. Methodol. 20, 215 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.