Abstract
Bone tissue engineering has emerged as a significant research area that provides promising novel tools for the preparation of biomimetic hydrogels applied in bone-related diseases (e.g., bone defects, cartilage damage, osteoarthritis, etc.). Herein, thermal sensitive polymers (e.g., PNIPAAm, Soluplus, etc.) were introduced into main chains to fabricate biomimetic hydrogels with injectability and compatibility for those bone defect need minimally invasive surgery. Mineral ions (e.g., calcium, copper, zinc, and magnesium), as an indispensable role in maintaining the balance of the organism, were linked with polymer chains to form functional hydrogels for accelerating bone regeneration. In the chemically triggered hydrogel section, advanced hydrogels crosslinked by different molecular agents (e.g., genipin, dopamine, caffeic acid, and tannic acid) possess many advantages, including extensive selectivity, rapid gel-forming capacity and tunable mechanical property. Additionally, photo crosslinking hydrogel with rapid response and mild condition can be triggered by different photoinitiators (e.g., I2959, LAP, eosin Y, riboflavin, etc.) under specific wavelength of light. Moreover, enzyme triggered hydrogels were also utilized in the tissue regeneration due to its rapid gel-forming capacity and excellent biocompatibility. Particularly, some key factors that can determine the therapy effect for bone tissue engineering were also mentioned. Finally, brief summaries and remaining issues on how to properly design clinical-oriented hydrogels were provided in this review.
Keywords: Different crosslinking conditions, Biomimetic hydrogel, Bone tissue engineering
Graphical abstract
Biomimetic hydrogels based on different crosslinking conditions, possess three-dimensional (3D) network with high aqueous content and functional properties, enabling their wide applications in bone tissue engineering. Recent advances in the design, preparation and application of light trigger hydrogels, thermal response hydrogels, small molecule crosslinking hydrogels, ionic crosslinking hydrogels and enzyme triggered hydrogels in the field of bone tissue engineering are reviewed.
Highlights
-
•
Diverse fabrication methods of advanced hydrogels were overviewed.
-
•
The process, advantages and drawbacks of advanced hydrogels were discussed.
-
•
Some key points in design of hydrogels for clinical use were summarized in this review.
-
•
The challenges, future directions and transformation potentials of hydrogels in bone tissue engineering were mentioned.
1. Introduction
Bone-related diseases caused by trauma, infection, or age-related diseases, including fracture, bone defect, and osteoarthritis, still significantly affect the life quality of patients [1]. In the bone microenvironment, various cells took part in bone reparation under well-orchestrated regulation in vivo. There are commonly four phases during fracture healing or injuries: hematoma phase, hematoma organization phase, callus phase, and remodeling phase. During the first phase, immune cells like neutrophils and monocytes are recruited and create hematoma via high-level inflammation, the inflammatory factor IL-1 and TNF-α took critical parts in this period. The level of inflammation and immune response falls rapidly in the second phase, under the regulation of anti-inflammatory factors IL-10 and IL-4. Activated TGF-β signaling recruits MSCs and accelerate chondrogenic differentiation, along with fast angiogenesis at the beginning of callus phase. Next, epithelial cells digest the newborn cartilage and induce osteogenesis, with a relatively fast bone turn-over rate. During the remodeling phase, the unordered woven bone is degraded by osteoclasts and relative cell factors such as MMPs, and is replaced by hollow, strong, and well-organized lamellar bone [2,3].
As early as 2000BC, a plate of hammered gold was used on skull of human fossils in Peru [4]. Animal bone graft was observed on the Ishtkunui fossil to fill the skull defect. Over the last few decades, surgeons and scientists have made incredible progress in bone reconstruction techniques. During the first generation, scaffold-based tissue engineering products enabled surgeons with variform fillers for all shapes of bone defects. With the deepening of bone regeneration mechanism, researchers created degradable materials which regulated adjacent microenvironments by releasing pharmacological assets. Nearest bioactive materials are designing to stimulate regeneration ability of host organs or cells [5]. Fortunately, biomaterials (e.g., mimic cement [6], polymer scaffold [7] and hydrogel [8], etc.) acted as bionic extracellular matrix have been widely utilized in the field of bone tissue engineering and have gained excellent therapy effects in recent decades.
During the regeneration process of natural bone, the extracellular matrix (ECM) plays a vital role in undertaking the signaling and substance exchange between the nascent tissue and the original tissue [9]. Similarly, hydrogels possess aqueous-swellable polymers with a 3D network structure formed by various crosslinking reactions, which are suitable for cell growth and the transfer of bioactive molecules [10]. Consequently, hydrogel materials have natural advantages compared with other bioactive materials in tissue regeneration engineering. Concretely, hydrogels enable proliferation, adhesion, and differentiation properties of stem cells, especially BMSCs, during bone reparation [11]. According to Ovijit et al., cell spreading, proliferation, and osteogenic differentiation of BMSCs were accelerated inside of hydrogels with tunable stress relaxation. On the other side, pharmacological assets carried by degradable hydrogels could regulate cell activities in situ. For instance, bisphosphonate released by an injectable hydrogel that controlled tooth relapse movement and osteoclasts activity after administration [12].
Various triggered conditions, including physical and chemical, have been employed to prepare advanced hydrogels for enhancing bone regeneration capacity in recent years [13,14]. In physically triggered gels, light or temperature stimulate the occurrence of crosslinking within the hydrogels. While in chemically triggered gels, covalent bonds or coordinate bonds between polymer chains create a stable hydrogel network via molecule or ionic crosslinking agents, respectively [15].
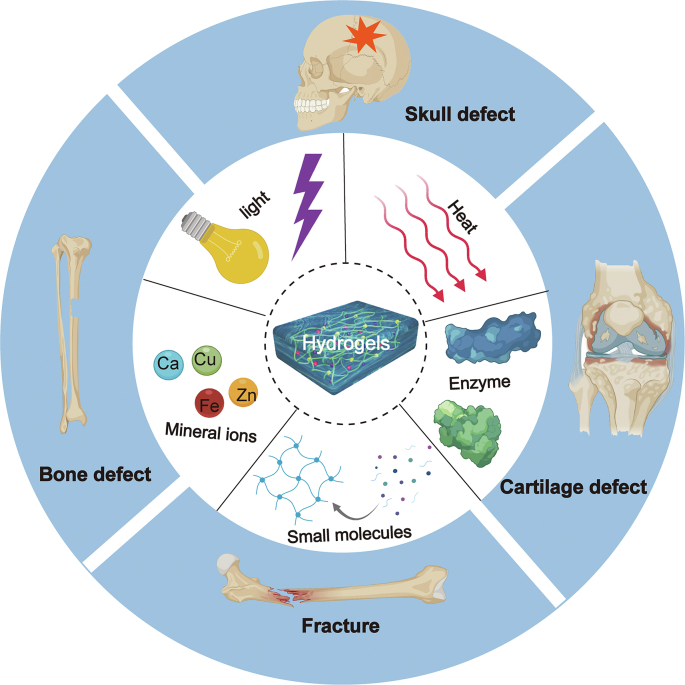
In this review, different trigger conditions for fabrications of various bionic hydrogels in bone tissue engineering have been discussed (Fig. 1). Among of them, hydrogels prepared under physically triggered conditions have unique advantages for clinical application, such as mild gel-forming temperature and low toxic crosslinking reaction. Reasonably, such prepared hydrogels have potential applications for bone fractures as well as bone defects with small holes. In addition, chemically triggered hydrogels can be utilized in those hard and large bone defects via mono, dual or multiple covalent crosslinking hydrogels. Moreover, some key factors in bone-related hydrogels design were also discussed. This review presents a brief overview of novel gels based on physically and chemically triggered methods for cell growth, drug storage, and bone regeneration.
Fig. 1.
Various bionic hydrogels fabricated by different triggered conditions (e.g., Heat, Mineral ions, Small molecules, Light, Enzyme, etc.) for bone tissue engineering. Created with BioRender.com.
2. Hydrogels crosslinked by the physical conditions
Physically crosslinked hydrogels are formed due to the physical crosslinking interactions, which includin, ionic interactions [104], and temperature [18]. There has been growing attention in designing physically crosslinked hydrogels for bone tissue engineering owing to the gelation process often occurs under the mild condition and absence of chemical crosslinking agents.
2.1. Temperature-triggered hydrogels
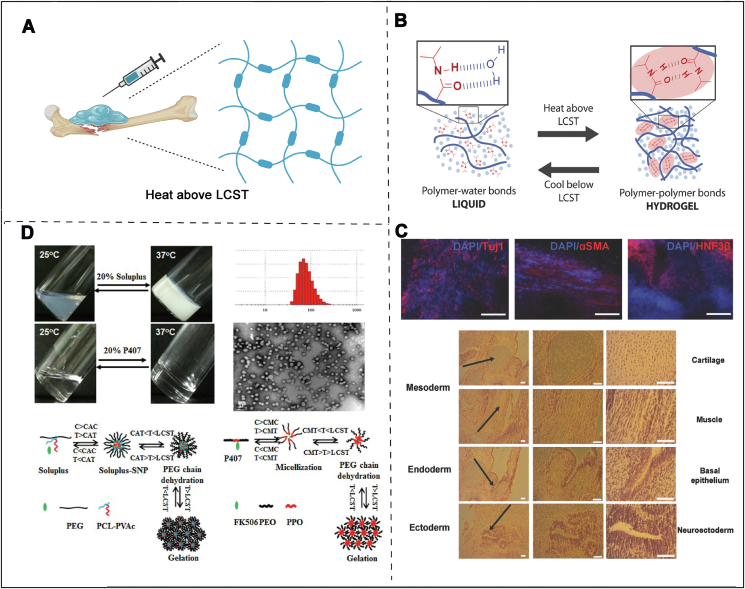
Bio-oriented hydrogels with temperature-triggered characteristics offer opportunities to create ‘friendly’ materials which may respond to thermal-stimuli with some change in their shapes or inner structure in vivo [19]. Over the last twenty years, smart hydrogels have been fabricated to respond various stimuli such as ionic or temperature [20]. Among of them, strategies triggered by changing temperature are especially appropriate for bone tissue engineering, because of its unique sol to gel property following temperature changes (Fig. 2A) [21]. Common polymers used in the biomedicine field have little thermosensitive behaviors, which are usually prepared via covalent crosslinking of the thermo-responsive chains into the polymers. Hence, to fabricate thermosensitive hydrogels, some frequently-used thermo-responsive polymers including poly (lactic co-glycolic acid)-PEG [22], poly(N,N-diethyl acrylamide) [23], PNIPAAm [24], and soluplus [25] have been introduced.
Fig. 2.
A) Thermal sensitive hydrogels used in bone defect model. Created with BioRender.com B) The sol to gel phase transition under different temperatures. Used with permission [26]. Copyright 2018, Wiley-VCH GmbH. C) The in vivo treatment effect of thermal sensitive hydrogel via H&E staining. Reproduced with permission [26]. Copyright 2018, Wiley-VCH GmbH. D) Schematic diagram of FK506 loaded Soluplus-SNPs hydrogels and Kolliphor P407 hydrogels. Used with permission [27]. Copyright 2017, Elsevier.
As a typical representative of thermal respond polymer, PNIPAAm has become the most popular material due to its sensitive phase translation capacity when the temperature goes to 32 °C (lower critical solution temperature) [28]. Based on this fact, linear PNIPAAm presents an unstable state at room temperature. Therefore, binding PNIPAAm with the other polymer chains can improve its stability and mechanical property [29]. To fabricate a PNIPAAm-based hydrogel with enhanced mechanical property, Ekerdt et al. have designed a PEG-PNIPAAm hydrogel as a 3D cell culture system for enhancing cell adhesion and growth (Fig. 2B) [30]. Compared to the 2D culture platform, this 3D hydrogel system critically improved cell proliferation and significantly promoted cell-directed differentiation. More importantly, the introduction of PNIPAAm endowed the hydrogel with thermoresponsive capacity, which allowed facile gel shaping and simultaneous cell encapsulation in the irregular tissue defects (Fig. 2C). Although the temperature-sensitive hydrogels have obvious superiorities and are promising in bone regeneration, this original hydrogel presents limited tunable mechanical property and rheological behavior. Hence, to overcome these drawbacks, lots of strategies have been developed, for example, hyaluronic acids (HA) were incorporated into the thermo-responsive hydrogel system to enhance the mechanical property of the hydrogel matrix [26]. In this design, HA was selected as the major hydrophilic chain of hydrogel for its abundant groups (hydroxyl and carboxyl groups). Additionally, HA is an important component of the extracellular matrix (ECM) and has a series of positive effects on cell behavior in vivo. HA-PNIPAAm polymers can be generated from thiol-terminated PNIPAAm modified with pendant vinyl groups of HA-VS. Ultimately, the mechanical behaviors of the gels could be mediated to suit specific stem cell differentiation, for instance, stiffer hydrogels have a better promoting effect on osteogenic differentiation of BMSCs. In a word, the ability to control the physical and chemical characteristics of HA-PNIPAAm hydrogels provides a promising therapeutic platform for different bone-relevant diseases.
Soluplus®, poly (N-vinyl caprolactam)-poly (vinyl acetate)-poly (ethylene glycol) graft copolymer, is a novel drug excipient utilized frequently in the manufacture of pharmaceutical preparations (e.g. micelles [31], solid dispersions [32]). These years, soluplus has also been widely utilized in intelligent responsive hydrogels for tissue engineering. As we all know, some bioactive molecules which can treat bone-related diseases, are hard to achieve ideal drug treatment effect in vivo due to poor water-soluble property.
To avoid the dissoluble property and sudden release behavior, Wu and his co-workers fabricated a micelle linked hydrogel composed of soluplus and tacrolimus for treating rheumatoid arthritis (RA) (Fig. 2D) [27]. In this crafty design, soluplus played a vital role in regulating drug release rate and mechanical property. In detail, soluplus in sol state was self-assembled to form micelles and loaded with tacrolimus in the hydrophobic layer. While under the body temperature, hydrogels, attributed by stronger hydrophobic interactions, were formed to create a local drug storage with stable mechanical property. In rat models, tacrolimus-loaded soluplus hydrogels possessed better therapeutic efficacy against RA compared with reported poloxamer 407 delivery systems. In brief, this tacrolimus-loaded soluplus hydrogel with sensitive temperature response is a promising drug delivery platform for RA therapy.
2.2. Ionic crosslinking agents
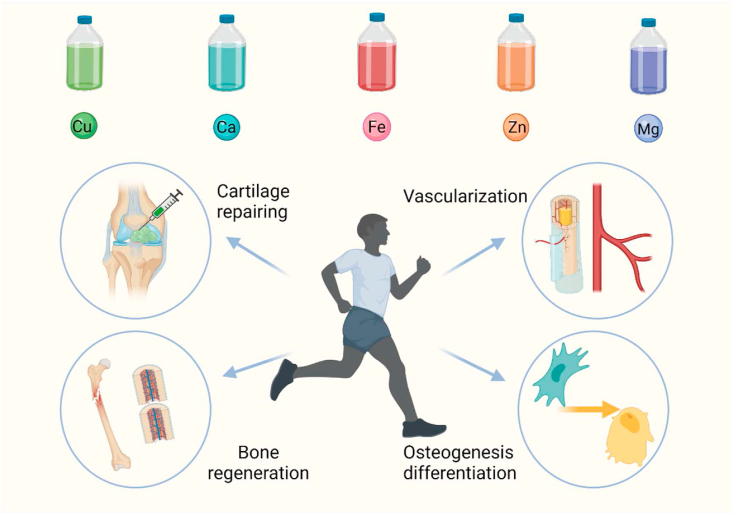
Daily uptake of four major nutrients (vitamins, minerals, amino acids, and essential fatty acids) is the basic life maintenance activities. Hence, minerals, as the basic nutrients, are often important to maintain normal body function. The mineral ions such as iron, copper, zinc, and calcium, play key roles in regulating or maintaining cellular behavior and organism balance (Fig. 3) [33,34]. For example, irons, an essential component of cells, have hematopoietic functions, which are involved in hemoglobin production and the synthesis of cytochrome and various enzymes to promote body growth. In addition, irons also play vital roles in transporting nutrients in the blood. Based on these theoretical bases, irons are often introduced into bionic materials to accelerate vascularization in the process of bone regeneration, neurorepair and blood remodeling. Copper is another important element in body, which has significant influence on lots of physiological activities. Furthermore, copper is a strong antioxidant that can remove free radicals from tissues and protect the cells [35]. Notably, copper acts a vital role in the maintenance of bone quantity and accelerating wound repairing. Therefore, ionic-based hydrogels have gained wide application in tissue engineering.
Fig. 3.
Mineral ions, including copper, calcium, iron, zinc, and magnesian, play an irreplaceable roles in regulating cellular behavior and accelerating bone tissue regeneration. Created with BioRender.com.
Recent studies also proved that Mg2+ with an appropriate concentration (50–200 ppm) can observably promote osteogenic differentiation and considerable new bone regeneration in bone defects [36,37]. Based on the above facts, Pan et al. designed a macro-porous structured GelMA hydrogel with the incorporation of MgO nanoparticles (NPs) via thiol-ene click reactions [38]. The experimental results showed that such prepared hydrogels had excellent mechanical property and uniform porous structure. In vivo experiments also indicated that the hydrogels with macro-porous structure not only provided an ECM microenvironment for in situ osteogenesis, but also released Mg2+ ions to enhance the osteogenic differentiation of BMSCs for accelerating bone tissue regeneration. The hydroxyapatite/MgO nanocrystals with high mechanical property and osteoinductive capacity were also applied in bone reconstruction. Chen et al. designed a 3D hydrogel scaffold incorporated with hydroxyapatite/MgO nanocrystal which enhanced bone tissue repair in diabetes mellitus rats due to the controlled release of Mg2+ [39]. In vitro studies showed that this 3D hydrogel scaffold promoted cellular migration, proliferation, and osteogenic differentiation of BMSCs by inducing osteogenic gene expression. Besides, the in vivo diabetic bone defects were completely repaired after the implantation of hydroxyapatite/MgO nanocrystal hybrid hydrogels 8 weeks later.
In order to prepare hydrogels that can be applicated in the field of soft tissue, Mahapatra et al. developed a strategy to prepare hydrogels with superior toughness via dual networks crosslinking in the presence of positively charged quaternary poly (ethylene imine) (Q-PEI) and micelles formed by Pluronic F127 diacrylate [40]. Ca2+ and Cu2+ ions were, meanwhile, introduced into the system to form the coordination bond, which effectively improved the mechanical strength and tensile properties of the hydrogels. So far, hydrogels with a double ion crosslinked network and super extensibility have been successfully designed. The strength tests showed that hydrogels with the participation of Ca2+ ions stretched to 108 times of the original length, and the maximum toughness is 177 MJ m−3. A series of performance tests have proved that the prepared hydrogel is a super flexible soft material with excellent mechanical strength and has perspective applications in various fields.
Morais et al. prepared hydrogels with bone substitute based on the development of alginate [41]. Alginate and calcium crosslinked hydrogels (Alg), alginate and chitosan double-network hydrogels (Alg/Ch) were designed respectively, and alginate and hyaluronic acid hydrogels (Alg/HA) were used as carriers for bone substitute biomimetic material, glass reinforced hydroxyapatite (GR-HAP). The weight change study showed that three types of hydrogels could expand and degrade within 72 h under the conditions of pH 7.4 or 4, among of them, Alg/HA hydrogels have the fastest degradation rate. In summary, the three types of hydrogels prepared are potential bone substitute carriers combined with GR-HAP particles.
Strategy such as introducing the copper contained nanoparticles into the hydrogel network structure is a commonly tactic. For example, a new platform of composite injectable hydrogel for local drug delivery of bioactive ions has been designed to ensure the prolonged-release behavior of bioactive substances [42]. This hybrid hydrogel showed rapid gelation and broad injectability under physiological conditions, while the addition of copper ions in hydrogel system could maintained its network structure for several days at local. Hence, the herein-designed hydrogels exhibited great potential as a local drug storehouse for treating delayed bone repairing or wound healing. Except for osteogenic capacity, the function of copper on chondrogenic differentiation and its potential in biomaterials have been rarely investigated. Specifically, Xu and his co-workers developed a copper-engaged biomaterial to promote chondrogenic. In vitro studies exhibited that Cu can promote MSCs morphology change, glycosaminoglycan (GAG) production, and the expression of chondrogenic genes [43]. The in vivo Col-2 expression emerged in four weeks after operation and the stronger expression of Col-2 was detected in the lacunae and around the chondrocytes in 8 weeks. Photothermal therapy combined with copper-contained hydrogels is another critical strategy to synergistically promote bone repairing.
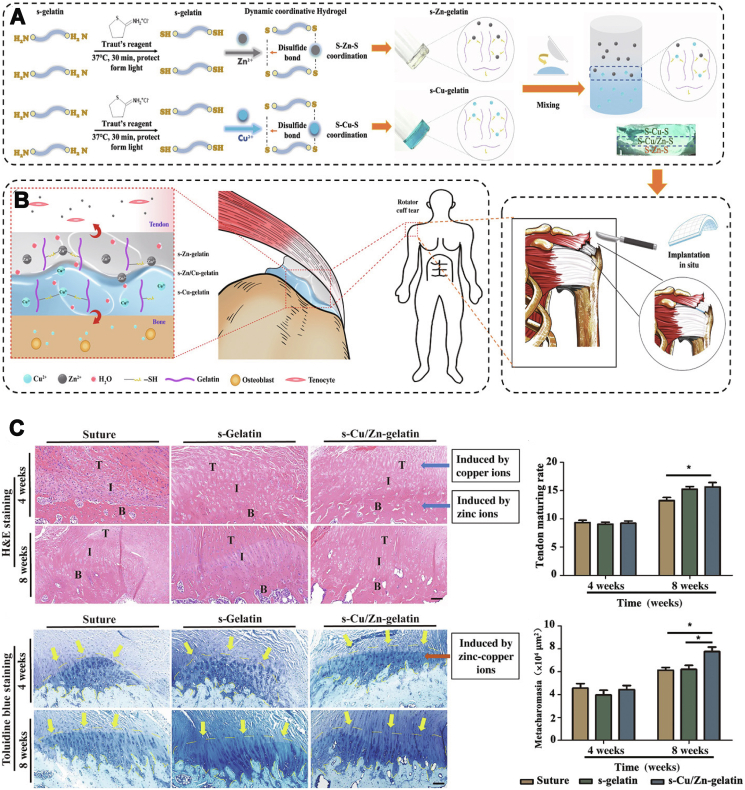
Yang et al. prepared a copper-doped double crosslinked injectable hybrid hydrogel for bone tissue repair while providing photothermal therapy [45]. Micro-CT and tissue staining showed that the introduction of copper into the functional hydrogels apparently enhanced bone regeneration, collagen formation in bone tissue, and the expression of corresponding osteogenic substances. Since fracture repair in clinical practice is often accompanied with the risk of infection, Lu et al. reported that copper-containing hydrogels can exhibit significant antimicrobial activity due to the released copper ions which promoted cell adhesion and osteogenesis in vivo [46]. To gain healing at the surface of tendon-to-bone, Yang et al. fabricated a gradient bimetallic ion-based hydrogel based on metal ions (zinc and copper ions) and gelatin polymers [44]. In this ECM-mimicking system, zinc and copper ions not only acted as ion crosslinkers, but also offered obviously antibacterial abilities and induced regenerative activity in vitro (Fig. 4A). The in vivo study of rat rotator cuff tear models demonstrated that this gradient hybrid hydrogel is capable of promoting tenogenesis and osteogenesis. Furthermore, the copper/zinc gradient layer induced considerable collagen and fibrocartilage alignment and regrowth at the interface between tendon and bone tissues (Fig. 4B and C). In a word, novel gradient ion hydrogels ensure feasibility and offer opportunities for the regeneration of gradient or interfacial tissues with physiological complexity. In conclusion, many strategies containing copper ions have gained admirable results. However, most of the current studies only select small animal models and more large animal studies should be encouraged to more accurately mimic human structures.
Fig. 4.
Representative case of ionic hydrogels in bone tissue regeneration. A) The preparation process of the gradient bimetallic ion-based hydrogels and the operation diagram. B) Schematic diagram of the gradient ionic hydrogels for synchronous regeneration in the tendon-to-bone insertion. C) H&E staining and toluidine blue staining of different experimental groups (Suture, s-Gelatin, s-Cu/Zn-Gelatin.) at four weeks and eight weeks. Used with permission [44]. Copyright 2021, AAAS. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
As far, the most significant feature of the ionic crosslinking method is its self-healing capacity as a result of which gel network can be destroyed at high shearing force and repair once the shearing force is removed. However, poor mechanical property seriously limits the application of hydrogels in bone and other hard tissues owing to the physical crosslinking method of ionic interaction.
3. Hydrogels crosslinked by chemical conditions
Up to now, different chemical crosslinking methods have been reported to form covalent bonds among modified polymer chains in hydrogel systems and they involve small molecule crosslink, photo induced crosslink and enzymatic induced crosslink. Compared with physical crosslinking methods, chemical crosslinking methods usually present enhanced mechanical properties and stability.
3.1. Small molecule crosslinking agents
Traditionally, the incorporation of specific small crosslinking agents including glutaraldehyde, genipin, dopamine, and tannic acid (TA) is regarded as an effective way to simultaneously tailor functionality and mechanical properties of the gels. Among them, the application of glutaraldehyde is restricted due to its toxic side effect on cell and tissue [47]. Therefore, as the ideal substitutes, genipin, dopamine, caffeic acid and tannic acid have grasped much interest and are frequently introduced into polymer networks to enhance the performance of biomaterials.
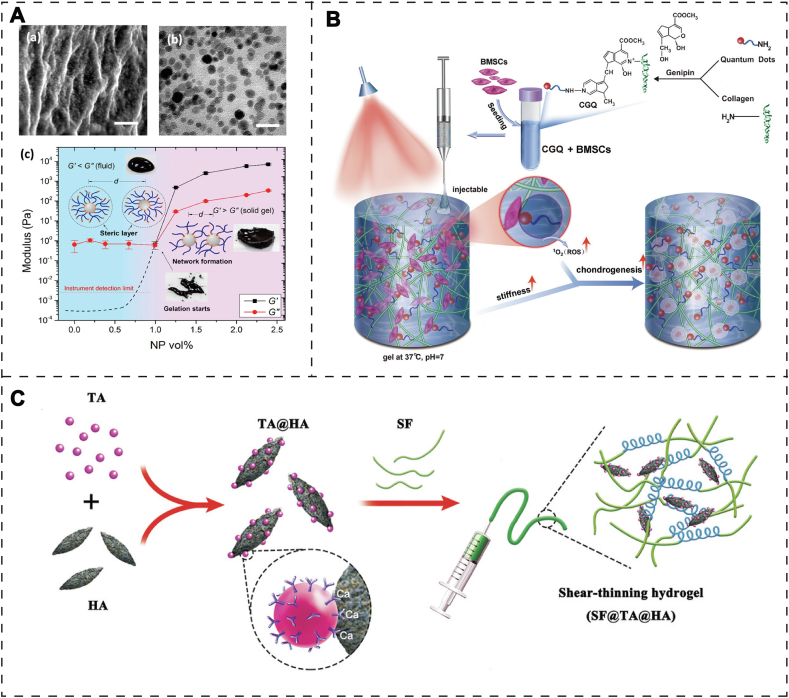
Inspired by the adhesion mechanism of mussel organisms, catechoolic compounds (e.g., dopamine, caffeic acid) with catechol functional groups have been frequently utilized in regeneration medicine due to its robust surface adhesion. Li et al. designed a self-healing hydrogel formed by the coordination bonds between Fe3O4 NPs and dopamine-modified PEG [48]. Briefly, Fe3+ ions and phenolic hydroxyl groups can form dynamic covalent bonds in PEG polymer, which maintained hydrogel network with tunable mechanical properties via changing the concentration of Fe3O4 NPs. More importantly, due to the metal coordination bonds are structurally reversible, this hydrogel also possessed self-healing capacity (Fig. 5A). This strategy provides a wide-spectrum hydrogel model with controlled mechanical property and self-healing capacity for apply in those soft (skin wound) or hard (bone defect) tissues.
Fig. 5.
A) (a) SEM image of Fe3O4 nanoparticle-cross-linked hydrogels (scale bar: 200 nm); (b) TEM image of ultramicro-cut of a NP gel. (c) Study on the rheological properties of the hydrogels. Used with permission [48]. Copyright 2015, American Chemical Society. B) The preparation process and implementation of collagen-genipin-quantum dot (CGQ) composite hydrogels in cartilage defects. Used with permission [51]. Copyright 2020, Wiley-VCH GmbH. C) Fabrication process of SF@TA@HA hydrogel. Used with permission [52]. Copyright 2020, Wiley-VCH GmbH.
Similarly, caffeic acid is another catechoolic compound frequently used in the modification of polymers. However, caffeic acid, as a plant-derived catechol derivative, possesses lots of advantages compared with dopamine, including accessibility, low-cost and desirable esthetics, and it presents inner biological characteristics such as antibacterial, anti-inflammatory, and antioxidant effects [49]. Inspired by natural bone structure, a nanoclay-organic hydrogel bone sealant (NoBS) was firstly designed via catechol chemistry interaction to accelerate bone regeneration [50]. Nanoclays chosen as a nanosized crosslinking agent, were incorporated into the system of caffeic acid-modified chitosan. Then, phenolic hydroxyl groups of caffeic acids are capable of forming the network, alone or with encapsulated nanoclays in the gel system, resulting in a multifunctional and self-healing hydrogel that can be arbitrary injected into an arbitrary bone defect. Significantly, the nanoclay-crosslinked hydrogels showed well osteoinductivity through regulating the Wnt/β-catenin pathway. Furthermore, smoothened agonists were loaded in nanoclays to enhance the effect of osteogenic differentiation via activating the Hedgehog pathway. Finally, a safer and faster bone regeneration exhibited in NoBS group when compared with other groups in critical-sized bone defect models. Overall, this design of engineered nanocomposite hydrogels provides a new idea for bone defect repairing.
Genipin is a natural compound extracted from the fruit of Gardenia jasminoides, which has been used as a high-performance cross-linking agent for connecting the polymers with amino-group. The reason for being a popular small molecule is that genipin can guarantee favorable compatibility [53], maintain injectability at room temperature [54] and obtain the anti-inflammatory effect [55].
For example, the natural biomolecular materials containing chitosan, l-arginine, and hydroxyapatite nanocrystals were used to fabricate scaffolds that simulate natural bones via 3D printed technology [56]. Firstly, genipin was used as a crosslinker to enhance the mechanical performance of the 3D scaffold. Subsequently, the amine of chitosan and l-Arginine attack C3 carbon of genipin, and the dihydrophosphate ring openings form crosslinking. With the increase of the concentration of genipin in the system, mechanical properties were enhanced (under 1%). In order to explore the biocompatibility of the 3D printed scaffold, osteosarcoma cells MG-63 were co-cultured with the scaffold for 1, 2, 3, and 7 days, and the surface of the scaffold provides a friendly microenvironment. Although this genipin-linked hydrogel presented good biocompatibility and potential osteogenic capacity, its application has been restricted to the level of cell culture. Lu et al. fabricated a collagen-based injectable hydrogel, which was formed by crosslinking with carbon dot nanoparticles (CD NPs) via introducing genipin as a small molecular linker [57]. In this system, the introduced CD NPs could not only act as a physical crosslinking agent to enhance the mechanical stress, but also enable the hydrogels to generate ROS under 808 nm NIR via the mechanism of photodynamic therapy. Hence, this composite hydrogel showed increased stiffness, facile injectability, and controllable degradation rate. According to the result of mechanical properties, CGN hydrogel exhibited tougher stress than collagen hydrogel. Additionally, the CGN hydrogel also presented a 21-fold higher compression modulus over collagen hydrogel. The chondrogenic differentiation ability of BMSCs was accelerated by this composite hydrogel in vitro. More importantly, this novel hydrogel was capable of improving cartilage repair in cartilage defect via regulating the TGF-β/SMAD signaling pathway. Cadmium selenide quantum dots (CdSe QDs), similar to CD NPs with nontoxic characteristic, can be used as crosslinkers in hydrogel and bring about multifunctional properties [58]. Zheng and his co-workers followed the previous design ideas and fabricated a hybrid hydrogel that consists of collagen type I acting as a basic scaffold for containing CdSe QDs and BMSCs [51]. In this system, CdSe QDs were chosen as a biocompatible crosslinker to be covalently crosslinked with collagen I to obtain injectability and enhanced mechanical property. Meanwhile, CdSe QDs also acted as photosensitizers and generated ROS for photodynamic therapy (PDT) (Fig. 5B). A series of studies proved that the enhanced mechanical stress and increased ROS production in this injectable hydrogel system can improve in vitro chondrogenic differentiation and accelerate in vivo cartilage regeneration.
Compared to dopamine and caffeic acid, tannic acid (TA) possesses more pyrogallol and catechol groups per molecule, which is derived from green tea, grapes, wine, and others. Abundant phenolic compounds in TA have strong adhesive ability with organic or inorganic surface, which characteristic has been developed as a potential crosslinking agent for engineering functional hydrogels. Besides, because of the large number of catechol groups present in the structure, its free radical clearance capability can be utilized in materials for anti-cancer, and anti-inflammatory therapy.
Bai et al. selected tannic acid (TA), belonging to phenolic glue molecules, showing good affinity to combine with silk fibroin (SF), and combined with hydroxyapatite (HA) in the form of coordination bonds (Fig. 5C) [52]. Finally, inorganic-organic hybrid hydrogels with high toughness and strong adhesion were successfully prepared (SF@TA @HA). The osteogenic differentiation ability of this hybrid hydrogel was detected by in vitro osteogenesis test. After 14 days of culture, the activity of alkaline phosphatase (ALP) in the SF@TA@HA group was significantly enhanced compared with the pure SF control group, indicating that MSCs were more likely to differentiate into osteoblasts, suggesting that this advanced hydrogel had the ability to induce osteogenic differentiation. Then, the hydrogels were implanted into the bone defect of mice, 8 weeks later, more newly formed bone tissue was observed in the original defect area of the hydrogel group compared with the control group through micro-CT and quantitative analysis of bone mass. In vivo tests showed that the hydrogels could achieve adequate fixation and timely mechanical repair, and guide bone regeneration in the critical size defect of the femur in rats. In the other case, considering some polymers such as poloxamer without strong tissue adhesiveness, Wang et al. fabricated a multifunctional hydrogel consisting of poloxamer 407 (PX), bioactive nanoparticles, and tannic acid (TA). The optimal combination (PXNT) was screened according to in vitro and in vivo bioactivity levels [59]. Adding TA into PXNT showed excellent tissue adhesive property. In vivo experiment of rat models, the dura mater around the laminectomy site of rats treated with PX, PL, and PLN were compressed respectively, and the dural sclerosis scar was serious. However, the formation of scar tissue was limited and the epidural adhesion was not serious in PXNT group. Meanwhile, MRI images showed that only a thin edge enhancement could be seen around the dural sac in PXNT group, suggesting scar granulation tissue, but there was no direct adhesion of dura. In the rabbit lumbar laminectomy experiment to prevent epidural adhesion, the laminectomy site was rechecked three weeks after operation. Because of the lack of scar tissue, it exhibited that the dura treated with PXNT was easy to be exposed. Moreover, MR images and T1 and T2 weighted axial images showed that the tight adhesion between dense scar and dura was significantly reduced after PXNT treatment in the model group. The above models indicated that in situ therapy with PXNT hydrogels can availably prevent some side effect after lumbar laminectomy.
A variety of chemical crosslinking agents has been applied to prepare novel hydrogels with stable and tunable properties, including drug release behavior, mechanical property, degradation, and gelation time. It is worth noting that some small molecules should be restricted to use due to their potential cytotoxicity in vivo.
3.2. Photo-triggered hydrogels
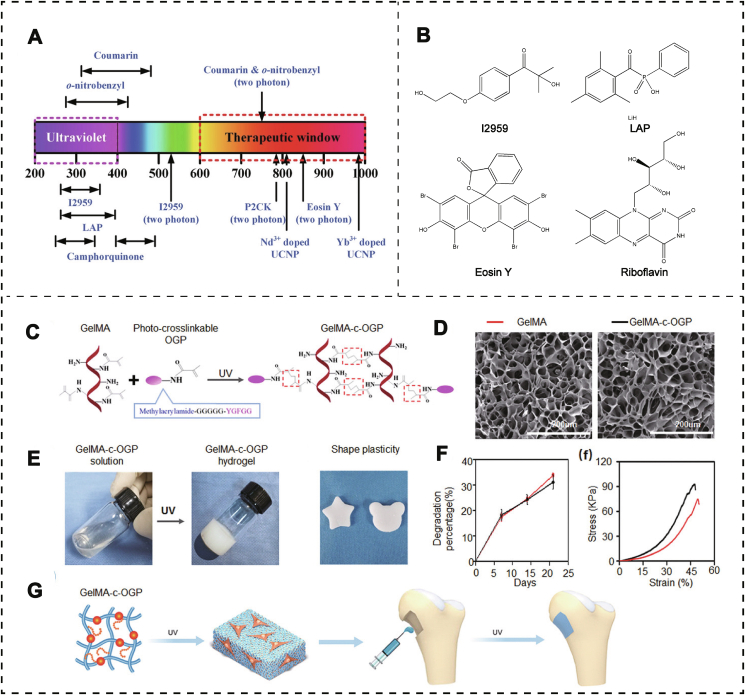
Photo-triggered hydrogels are one of the most promising hydrogels in bone tissue engineering; it can be triggered by light at specific wavelength (ultraviolet (UV) or visible light) (Fig. 6A). and induce the formation of three-dimensional network and morphological changes of gel precursor [60]. Owing to the in situ gelation and facile preparation, this triggered method meets various requirements of bone-related disease. Lots of polymers based on natural and synthetic sources are modified with photo-respond crosslinked groups. For instance, PEG and PVA with good cytocompatibility have been often chosen as matrix materials for photo-crosslinked hydrogels. Similarly, nature-derived polymers such as collagen, hyaluronic acid, and gelatin have also been modified to fabricate this type of hydrogel. Remarkably, the polymerization of hydrogels does not respond to light irradiation in the absence of photoinitiators. Hence, photoinitiators play a key factor in the process of photo-crosslinking gels, which determine both the irradiation wavelength and gel formation quality (Fig. 6B). In general, photoinitiators are divided into two types based on different reaction mechanisms. Type I, also called “cleavage” type, can generate initiating radicals upon intramolecular bond cleavage under UV light. It is usually composed of only one component such as I2959 (2-Hydroxy-[4'-(2-Hydroxyethoxy) Phenyl] -2-Methyl Propanone; 2-Hydroxy-1-(4'-(2-hydroxyethoxy phenyl)-2-methyl propanone), I651 (2,2-dimethoxy-2-phenylacetophenone, DMPA), I184 (1-hydroxycyclohexyl phenyl ketone) and LAP (lithium acylphosphinate). Hydrogen abstraction type is regarded as type II, which needs lower excitation energy than type I. The commonly used types II are eosin Y and riboflavin. Eosin Y is one of the most popular type II initiators and usually couples with amines or thiols to initiate radical polymerization. Riboflavin, another type II initiator, possesses good biocompatibility and has been widely used to prepare hydrogels under visible light [61].
Fig. 6.
Photo crosslinked hydrogels. A) Absorption wavelength distribution of different photosensitizers. Used with permission [60]. Copyright 2020, Wiley-VCH GmbH. B) Structural formula of some representative photosensitizers, including I2959, LAP, Eosin Y, and Riboflavin. C) The chemical molecular structure of the methacrylated OGP polypeptide and gelatin (GelMA), and the structure of GelMA-c-OGP with ultraviolet (UV) light. D) Porous structure topography diagram of GelMA and GelMA-c-OGP via SEM. E) Gel formation process of GelMA-c-OGP hydrogels. F) Degradation percentage (%) and stress properties (KPa) of GelMA and GelMA-c-OGP hydrogels. G) Photograph of GelMA-c-OGP solution and GelMA-c-OGP hydrogel formation after UV light and the pipeline of GelMA-c-OGP applied for bone regeneration. Used with permission [62]. Copyright 2020, Wiley-VCH GmbH.
Gelatin, the most abundant protein in the ECM of mammals, is approved by FDA for application in the biomedicine field. It is very suitable for cell culture and tissue repair because of its high side chain reactivity, excellent biocompatibility, and superior hydrophilicity. However, aiming as a photo-respond polymer, gelatin should be modified by photo-crosslinking groups including methacryloyl, acrylamide as well as norborene. Among them, Gelatin methacryloyl (GelMA) is the most studied and used photo-crosslinking polymer. GelMA has low osteogenic activity itself, hence, bioactive ingredients, such as ions, drugs or other materials, should be introduced to endow GelMa with ideal bone regeneration capacity.
Osteogenic growth peptide (OGP) is a bioactive compound, which can improve the growth and differentiation of osteoclast in vitro and can enhance bone repairing in vivo [63]. To improve osteogenic capacity of GelMA-based hydrogel, Qiao et al. prepared a novel GelMA hydrogel through covalent connection between the OGP and the GelMA under UV light (Fig. 6C–F) [62]. This co-photo-cross-linked hydrogel not only prolonged the therapy time of OGP, but also offered a stable microenvironment for regulating cell behavior. In rat distal femur defects model, this OGP crosslinked GelMA hydrogel exhibited an accelerating new bone regeneration rate and stronger repair capacity in vivo (Fig. 6G). All above studies indicated that OGP crosslinked GelMA hydrogel is a promising biomaterial for improving cell adhesion, growth, accelerating the expression of the osteogenic genes, and enhancing new tissue regeneration in bone defects. The strategy to incorporate nanoparticles is another way to improve bioactivity of GelMA. Phosphate also plays a key role in bone reconstruction [64]. However, some drawbacks cannot be overlooked including uncontrolled phosphate ionic release behavior and apparent cytotoxic (e.g., red phosphate, white phosphate) [65].
Recently, Huang and his co-workers have fabricated a GelMA hydrogel with encapsulated black phosphorus nanosheets (BPNs) for enhanced bone regeneration [66]. BPNs, another main allotrope of phosphorus, have steady physicochemical property and well biocompatibility. The introduced BPNs in system markedly improved mechanical property of GelMA hydrogels. In addition, sustained phosphorus release from BPNs was beneficial to long-term bone regeneration. The in vitro results indicated that BPNs-hydrogels present photo-responsively released phosphate and enhanced mineralization ability. Moreover, the ability for osteogenic differentiation of hDPSCs was enhanced through the BMP-RUNX2 pathway. Besides, the GelMA hydrogels with BPNs also promoted in vivo bone repair. Overall, related experiments indicated that the GelMA hydrogels observably improve bone regeneration in defect model when adding BPNs as extra phosphorus. Except for gelatin, one of the main components of joint fluid, HA-based macromers can also be selected to prepare photo-responsive hydrogels based on its biodegradability, easily modified and amphiphilic properties. Wang et al. designed an HA-based injectable hydrogel with biocompatibility and certain mechanical strength through dual-crosslinking reaction [67]. Its first crosslinked point was produced by adding the photoinitiator (LAP) into pre drive fluid of furan-modified HA under a 365 nm UV light, then, covalent bonds were generated between two furan groups. Next, the second crosslinked point was produced by maleimide-modified PEG and furan-modified HA based on the Diels-Alder reaction. Finally, this injectable hydrogel possesses enough mechanical stress to create a durable scaffold for bone tissue engineering.
Various type II initiators (e.g., eosin Y, riboflavin) have been reported to prepare the functional hydrogel for biomedical field. In eosin Y-triggered reaction, N-Vinylpyrrolidone (NVP) has been selected as polymer monomer to form the hydrogels under visible light, which is against the inhibitory effect of physiological oxygen on the radical initiation process [68]. As a natural ECM material, collagen is an ideal base polymer for bone restoration, with a three helical structure. However, shortcomings of weak stability and prolonged crosslinking time prevent its application used as bionic material. Combined with 3D bioprinting technology, a Col-I-based hydrogel was fabricated by riboflavin photo-crosslinking method [69]. The result of the cytotoxicity assay showed that cytoactive decreased from 95% to 75% because of adding the visible-light activated agent. When the concentration of riboflavin changed from 0.5 mM to 0.2 mM, its cell viability was significantly improved. Accordingly, a lower concentration of riboflavin brought about lower-modulus levels of hydrogels. Hence, it is critical to keep a balance between mechanical stiffness and cell viability via control riboflavin concentration.
The advantage of the photo-crosslinking method is its gel-forming in situ and accurate spatiotemporal control. The mechanical stiffness and, as a result, biochemical properties of hydrogel can be easily altered by regulating the distance, wavelength, power and exposure time of the light used [70,71]. However, cell-loaden type and its biocompatible characteristic are limited by drawbacks including toxic photoinitiators and unwanted free radical reactions, which will damage the friendly inner environment of hydrogel and subsequently limit their wide use in bone tissue engineering [72].
3.3. Enzyme catalyzed agents
Through traditional chemical crosslinking methods, such as photo crosslinking, covalent crosslinking, the fabricated the hydrogels present excellent stiffness and rapid gelation time. However, the potential cytotoxicity of residual reagents and chemical crosslinking agents remains a tricky problem. Currently, enzymatic crosslinking hydrogels have been paid great attention to improve material properties, including fast gelation time and excellent biocompatibility [73].
So far, there are many kinds of enzymatic crosslinking approaches used to prepare biomimetic hydrogels for repairing bone tissue and cartilage tissue. For instance, horse radish peroxidase (HRP) triggered crosslinking reaction was used to form covalent bonds between tyramine-modified polymers in the presence of hydrogen peroxide (H2O2). Zhang and his co-workers developed a bi-component (collagen type I-tyramine (Col-TA) and hyaluronic acid-tyramine (HA-TA)) hydrogel based on HRP triggered the crosslinking reaction [74]. Remarkably, its physiochemical properties, such as gelation time, mechanical strength, water solubility, and biocompatibility, were well improved, which expanded the application of Col-I in the field of tissue engineering. The engaged TGF-β1 released from hydrogel greatly promoted the chondrogenic differentiation of BMSCs in vitro. Furthermore, in vivo experiments showed that this enzyme-catalyzed hydrogel can achieve a good cartilage repair effect according to the result of histological and immunohistochemical analysis. Similarly, another enzyme-catalyzed hydrogel was formed by HA and chondroitin sulfate (CS) in the presence of H2O2 and HRP [75]. BMP-2 and BMSCs were simultaneously packaged into the hydrogel system to build a biomimetic microenvironment for osteogenic differentiation. Hence, the in vitro and in vivo experiments demonstrated that the obtained hydrogel not only supplied a suitable microenvironment for osteogenic differentiation of BMSCs, but also promoted bone tissue repair in the rat femur.
MMP Lys-peptide grafted CS precursor (LYS-CS) and transglutaminase crosslinked PEG (TG-PEG) hydrogel was selected as hybrid materials to form a CS-PEG hybrid hydrogel via another crosslinking method based on the FXIIIa-mediated crosslinking mechanism [76]. With the CS-PEG hybrid hydrogels degradated by chondroitinase, the encapsulated BMP-2 released from system to promote osteogenic differentiation. Additionally, this composite system with different amount of CS can be employed to control growth factor binding and release behavior for generate ECM, which can further fill the bone defect.
In summary, such enzyme-catalyzed gel-forming strategies can be as a promising platform for bone tissue engineering due to its fast gel-forming time, tunable stiffness and good biocompatibility.
4. Key points of hydrogel design in clinical
To satisfy the demand of biocompatibility and biodegradability in clinical applications, polymers as the source of hydrogels used in bone tissue engineering can be derived from macromolecules within the organisms, such as nucleic acid substances [77], macromolecular proteins [78], and glycosaminoglycans [79]. In addition, many non-natural polymers, including PVA [80], PEG [81], and PCL [82], can also be selected as the potential materials to prepare functional hydrogels for treating various bone-relevant diseases. Although these polymers are biocompatible and without any toxic effect on cell proliferation, they cannot significantly stimulate migration, growth, and differentiation of cells. Hence, novel designs based on those common polymers should be proposed for bone tissue engineering.
Bone tissues are constituted by collagen fibers and mineralized HAP at the nanometric scale, and the hierarchical structure comprises of cortical bone and cancellous bone. Cortical bone shows a porosity of 5–10%, while cancellous bone has a porosity of 50–90% Cortical bone endures most of the physical stress due to the dense structure and high compressive strength [83,84]. The spongy-like structure of cancellous bone facilitates the ingrowth of blood vessels, transportation of nutrition and migration of cells. Cartilage extracellular matrix (ECM) is a dense connective tissue with a pore size of only a few nanometers, which evidently prevents cell infiltration and vascularization [[85], [86], [87]], the occurrence of osteoarthritis is highly relevant to the damaged structure and upregulated permeation in cartilage. Hence, porous structure is critical for cellular penetration, distribution, and revascularization. Thus, the pore size of prepared hydrogels is another important factor that can guide the inner cells into hydrogel network. Pore size with 2–50 nm is defined as small pore hydrogels, which possess the largest surface area to load high dose drug for good therapy effect [88]. Middle pore size (10 μm) can accelerate the exchange speed of mineral ions (calcium, magnesium, and zinc), hydroxyapatite formation, and the introduction of bone-forming proteins [89]. Hydrogel scaffolds of large pore size with size over 100 μm can apparently promote the cell migration and adhesion [90]. Therefore, hydrogel structures with different pore sizes can stimulate different cell behavior. In a word, the suitable pore size should be controlled to make high-performance hydrogels for bone repairing. However, hydrogels with different pore sizes still have their limitations due to the lack of hierarchical structure and topological morphology. To address this problem, three-dimensional printing and other microfabrication techniques have been widely developed to obtain precise fabrication of hydrogels with tunable morphology, tailored pore size/porosity, customized architecture, and matched mechanical strength for bone tissue engineering [84,87,[91], [92], [93]].
Depending on the mechanical conduction of signaling pathways, mechanical property of hydrogels can be apperceived by stem cells, which are capable of regulating cell adhesion, determining the types of cells, and stimulating the cells to produce specific cytokines [94]. Thus, a suitable mechanical property, that matches the aimed bone defect, can enhance the bone repairing quality. While most of the bioactive hydrogels have weak mechanical strength, which limited their applications in bone tissue engineering. Researchers have made lots of efforts in preparing stiff and tough hydrogels with tunable mechanics. Various ways have been accepted to adjust the mechanical property of hydrogels, including multi-crosslinking methods via intermolecular bonds (e.g., covalent bonds [95], ionic bonds [96], Van der Waals' force [97], etc.), incorporating the nanomaterials into the gel system (e.g., nanoparticles [98], nanosheets [99], quantum dot [100], etc.) and mixing hydrogels with other support materials (e.g., 3D printed scaffolds, electrostatic spinning scaffolds etc.). It is worth mentioning that Tiller et al. obtained ultra-stiff and tough hydrogels via enzyme-induced mineralization, which was a big breakthrough in developing hybrid hydrogels with tunable mechanics [101]. Hence, we deem that more attentions should be paid on the innate structure of natural bone which ultimately determines its ultra-tough mechanics and provides new eyes for the design of artificial biomimetic materials for bone regeneration.
As an emerging area, drug delivery system (DDS) has obtained increasing attention in the field of material science. Hydrogels, especially intelligent hydrogels, can be stimulated by the external response conditions (e.g., light, heat, magnetism, and pH, etc.) for on-demand drug release behavior. An ideal drug-loaded hydrogel system is supposed to have high drug-loading capacity, durable stability and support, controlled drug release, and stimulus response properties [102,103]. In term of bone tissue therapy, in situ hydrogels can not only act as a site for cell adhesion and growth, but also serve as drug storage for promoting tissue regeneration by sustained and controlled drug delivery.
In brief, great progress has been made in over mentioned points (e.g., material source, porous size, mechanical strength, and drug release behavior) for bone tissue engineering. In the future, comprehensive strategies should be introduced to design novel hydrogels for facilitating cell growth, regulating cell behavior, precise controlling drug release, and accelerating bone tissue repair.
5. Outlook and future
The powerful self-repairing capacity of natural bone tissue has been driving researchers to explore high-performance hydrogels through reasonable approaches. In the past two decades, artificial ECM-mimicking hydrogels were fabricated via different trigger approaches, such as physical conditions and chemical conditions, and they showed admirable treatment in bone-related diseases. For instance, a novel hydrogel, prepared by light trigger in mild condition, may be suit to the defect site in urgent need of treatment. In addition, a tough hydrogel with dual crosslinking network perfectly matches with those hard bone tissue. Moreover, thermal responded hydrogels obtain injectable capacity, which may be help those bone defects need to minimally invasive surgery. So far, based on different trigger conditions, a variety of smart approaches have been explored and applied to prepare novel hydrogels with a correct way for using in bone tissue engineering. 1) Exploiting new polymers to design and fabricate the biodegradable and biocompatible hydrogels to solve the biocompatibility issues. 2) Some thermal sensitive chains can be linked in polymers to form injectable hydrogels, which can be used as microfracture surgery to treat those small hole defects. 3) According to the clinical requirements, the drug loaded systems are designed into different administration types to achieve satisfied therapy effects for corresponding bone diseases.
Despite these advances, several points must be paid more attention to design the next generation hydrogel for bone tissue engineering. First, we may focus on the balance between complexity and simplicity in bone-related hydrogels design. On the one hand, to meet the requirements of the multiple-stage structure of the natural bone, properly complex hydrogel systems could be constructed for mimicking the microenvironment of bone defect. On the other hand, advanced hydrogels with simple fabrication processes and structures are beneficial to mass production-scale, especially aimed at commercial transformation applications. Second, inspired by the rapid development of immunotherapy, new generation of hydrogels can be designed to regulate immune cell behavior to accelerate the hydrogel-bone tissue connection. For instance, conscious regulation of macrophage types (M0 to M2) is helpful to maximize the function of hydrogels and promotes bone tissue regeneration. Third, considering complicity of bone tissue and uncertainty of defect location, computational material science and computational biology should be introduced to establish huge databases for cell behaviors, hydrogel material characteristics, and cell-material interactions from existing scientific researches.
In the nearly future, there are still lots of blind spots of hydrogel design that should be solved to appease the clinical needs. Many types of hydrogels have been investigated in animal models; however, very few hydrogel products based on the human model are further put into effect. From the perspective of materials, some future concerns are as follows: 1) Hydrogel materials are usually implanted into subcutaneous to evaluate the corresponding osteogenic index, which cannot present the real microenvironment of bone defect. 2) The uncertainty of the degradation rate in vivo and the interaction between vehicles and bioactive moleculars will determine the release behavior of drug, thereby affecting the ultimate therapy effect.3) The storage of hydrogels is also a tricky thing, which is the one of blocks in clinical application. Water-contained hydrogels are more easily damaged during storage and transport, which can eventually result in drug leakage. 4) Although lots of bioactive hydrogel systems for bone tissue regeneration have been made, a low-cost, general purpose and easily handled method aimed to commercial hydrogels has not yet been constructed. Herein, it is expected that, a deeper understanding of physical and chemical methods with respect to reasonable design of the hydrogels is key to exploiting regenerative bone-related materials. More importantly, researching the basic mechanisms underlying material-biological interactions can bring about inspiration for engineering advanced biomimetic hydrogels to deepen the integration of the material and the organism.
Authorship contribution statement
Xu Xue, Yan Hu and Sicheng Wang contributed equally to this work. Xu Xue, Yan Hu and Sicheng Wang drafted and wrote the manuscript of this review. Xiao Chen, Yingying Jiang and Jiacan Su guided and revised the manuscript of this review.
Declaration of competing interest
The authors declare no conflict of interest in this review.
Acknowledgements
The authors acknowledge the financial support from National Key R&D Program of China (2018YFC2001500); National Natural Science Foundation of China (91749204, 82172098, 81771491, 81871099, 81972254); Shanghai Rising-Star Program (21QA1412000).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Xiao Chen, Email: sirchenxiao@126.com.
Yingying Jiang, Email: YJiang8@shu.edu.cn.
Jiacan Su, Email: jiacansu@smmu.edu.cn.
References
- 1.Stolzing A., Jones E., McGonagle D., Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech. Ageing Dev. 2008;129(3):163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Loeffler J., Duda G.N., Sass F.A., Dienelt A. The metabolic microenvironment steers bone tissue regeneration. Trends Endocrinol. Metabol. 2018;29(2):99–110. doi: 10.1016/j.tem.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Salhotra A., Shah H.N., Levi B., Longaker M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020;21(11):696–711. doi: 10.1038/s41580-020-00279-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker F.G. Repairing holes in the head: a history of cranioplasty. Neurosurgery. 1997;41(4) doi: 10.1097/00006123-199710000-00067. 999-999. [DOI] [PubMed] [Google Scholar]
- 5.Henkel J., Woodruff M.A., Epari D.R., Steck R., Glatt V., Dickinson I.C., Choong P.F., Schuetz M.A., Hutmacher D.W. Bone regeneration based on tissue engineering conceptions - a 21st century perspective. Bone Res. 2013;1(3):216–248. doi: 10.4248/BR201303002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schröter L., Kaiser F., Stein S., Gbureck U., Ignatius A. Biological and mechanical performance and degradation characteristics of calcium phosphate cements in large animals and humans. Acta Biomater. 2020;117:1–20. doi: 10.1016/j.actbio.2020.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Kim H.D., Amirthalingam S., Kim S.L., Lee S.S., Rangasamy J., Hwang N.S. Biomimetic materials and fabrication approaches for bone tissue engineering. Adv Healthc Mater. 2017;6(23) doi: 10.1002/adhm.201700612. [DOI] [PubMed] [Google Scholar]
- 8.Liu M., Zeng X., Ma C., Yi H., Ali Z., Mou X.B., Li S., Deng Y., He N.Y. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5 doi: 10.1038/boneres.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang F., Yu Y., Liu S., Ming L., Zhang Y., Zhou Z., Zhao J., Jin Y. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact Mater. 2021;6(3):666–683. doi: 10.1016/j.bioactmat.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui Z.K., Kim S., Baljon J.J., Wu B.M., Aghaloo T., Lee M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019;10(1):3523. doi: 10.1038/s41467-019-11511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhuri O., Gu L., Klumpers D., Darnell M., Bencherif S.A., Weaver J.C., Huebsch N., Lee H.P., Lippens E., Duda G.N., Mooney D.J. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016;15(3):326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Utari T.R., Ana I.D., Pudyani P.S., Asmara W. The intrasulcular application effect of bisphosphonate hydrogel toward osteoclast activity and relapse movement. Saudi Dent J. 2021;33(5):292–298. doi: 10.1016/j.sdentj.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennink W.E., van Nostrum C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002;54(1):13–36. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- 14.Hu W., Wang Z., Xiao Y., Zhang S., Wang J. Advances in crosslinking strategies of biomedical hydrogels. Biomater Sci. 2019;7(3):843–855. doi: 10.1039/c8bm01246f. [DOI] [PubMed] [Google Scholar]
- 15.Voorhaar L., Hoogenboom R. Supramolecular polymer networks: hydrogels and bulk materials. Chem. Soc. Rev. 2016;45(14):4013–4031. doi: 10.1039/c6cs00130k. [DOI] [PubMed] [Google Scholar]
- 18.Nonoyama T., Lee Y.W., Ota K., Fujioka K., Hong W., Gong J.P. Instant thermal switching from soft hydrogel to rigid plastics inspired by thermophile proteins. Adv. Mater. 2020;32(4) doi: 10.1002/adma.201905878. [DOI] [PubMed] [Google Scholar]
- 19.Xian S.J., Webber M.J. Temperature-responsive supramolecular hydrogels. J. Mater. Chem. B. 2020;8(40):9197–9211. doi: 10.1039/d0tb01814g. [DOI] [PubMed] [Google Scholar]
- 20.Webber M.J. Engineering responsive supramolecular biomaterials: toward smart therapeutics. Bioeng Transl Med. 2016;1(3):252–266. doi: 10.1002/btm2.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu S., Ni P., Wang B., Chu B., Zheng L., Luo F., Luo J., Qian Z. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials. 2012;33(19):4801–4809. doi: 10.1016/j.biomaterials.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 22.Li X., Chen L., Lin H., Cao L., Cheng J., Dong J., Yu L., Ding J. Efficacy of poly(D,L-Lactic acid-co-glycolic acid)-poly(ethylene glycol)-poly(D,L-Lactic acid-co-glycolic acid) thermogel as a barrier to prevent spinal epidural fibrosis in a postlaminectomy rat model. Clin Spine Surg. 2017;30(3):E283–e290. doi: 10.1097/BSD.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 23.Hanyková L., Krakovský I., Šestáková E., Šťastná J., Labuta J. Poly(N,N'-Diethylacrylamide)-Based thermoresponsive hydrogels with double network structure. Polymers. 2020;12(11) doi: 10.3390/polym12112502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekerdt B.L., Fuentes C.M., Lei Y., Adil M.M., Ramasubramanian A., Segalman R.A., Schaffer D.V. Thermoreversible hyaluronic acid-PNIPAAm hydrogel systems for 3D stem cell culture. Adv Healthc Mater. 2018;7(12) doi: 10.1002/adhm.201800225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu H.M., Wang K.Y., Wang H.N., Chen F., Huang W.C., Chen Y.Q., Chen J.L., Tao J., Wen X.G., Xiong S.B. Novel self-assembled tacrolimus nanoparticles cross-linking thermosensitive hydrogels for local rheumatoid arthritis therapy. Colloids Surf., B. 2017;149:97–104. doi: 10.1016/j.colsurfb.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Ekerdt B.L., Fuentes C.M., Lei Y.G., Adil M.M., Ramasubramanian A., Segalman R.A., Schaffer D.V. Thermoreversible hyaluronic acid-PNIPAAm hydrogel systems for 3D stem cell culture. Adv Healthc Mater. 2018;7(12) doi: 10.1002/adhm.201800225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H., Wang K., Wang H., Chen F., Huang W., Chen Y., Chen J., Tao J., Wen X., Xiong S. Novel self-assembled tacrolimus nanoparticles cross-linking thermosensitive hydrogels for local rheumatoid arthritis therapy. Colloids Surf. B Biointerfaces. 2017;149:97–104. doi: 10.1016/j.colsurfb.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Hu X.M., Cheng W.M., Shao Z.L., Xin L. Synthesis and characterization of temperature-sensitive hydrogels. E-Polymers. 2015;15(5):353–360. [Google Scholar]
- 29.Li J., Wang B., Lin J., Cheng D., Lu Y. Multifunctional surface modification of mulberry silk fabric via PNIPAAm/chitosan/PEO nanofibers coating and cross-linking technology. Coatings. 2018;8(2) [Google Scholar]
- 30.Adil M.M., Vazin T., Ananthanarayanan B., Rodrigues G.M.C., Rao A.T., Kulkarni R.U., Miller E.W., Kumar S., Schaffer D.V. Engineered hydrogels increase the post-transplantation survival of encapsulated hESC-derived midbrain dopaminergic neurons. Biomaterials. 2017;136:1–11. doi: 10.1016/j.biomaterials.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Jin I.S., Jo M.J., Park C.W., Chung Y.B., Kim J.S., Shin D.H. Physicochemical, pharmacokinetic, and toxicity evaluation of soluplus (R) polymeric micelles encapsulating fenbendazole. Pharmaceutics. 2020;12(10) doi: 10.3390/pharmaceutics12101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nair A.R., Lakshman Y.D., Anand V.S.K., Sree K.S.N., Bhat K., Dengale S.J. Overview of extensively employed polymeric carriers in solid dispersion technology. AAPS PharmSciTech. 2020;21(8) doi: 10.1208/s12249-020-01849-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z., Jia B., Yang H., Han Y., Wu Q., Dai K., Zheng Y. Biodegradable ZnLiCa ternary alloys for critical-sized bone defect regeneration at load-bearing sites: in vitro and in vivo studies. Bioact Mater. 2021;6(11):3999–4013. doi: 10.1016/j.bioactmat.2021.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z., Jia B., Yang H., Han Y., Wu Q., Dai K., Zheng Y. Zn0.8Li0.1Sr-a biodegradable metal with high mechanical strength comparable to pure Ti for the treatment of osteoporotic bone fractures: in vitro and in vivo studies. Biomaterials. 2021;275:120905. doi: 10.1016/j.biomaterials.2021.120905. [DOI] [PubMed] [Google Scholar]
- 35.Tomaszewska E., Dobrowolski P., Kwiecien M., Winiarska-Mieczan A., Tomczyk A., Muszynski S. The influence of the dietary Cu-Glycine complex on the histomorphology of cancellous bone, articular cartilage, and growth plate as well as bone mechanical and geometric parameters is dose dependent. Biol. Trace Elem. Res. 2017;178(1):54–63. doi: 10.1007/s12011-016-0894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Z., Wu J., Qiao W., Zhao Y., Wong K.H.M., Chu P.K., Bian L., Wu S., Zheng Y., Cheung K.M.C., Leung F., Yeung K.W.K. Precisely controlled delivery of magnesium ions thru sponge-like monodisperse PLGA/nano-MgO-alginate core-shell microsphere device to enable in-situ bone regeneration. Biomaterials. 2018;174:1–16. doi: 10.1016/j.biomaterials.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Obata A., Ogasawara T., Kasuga T. Combinatorial effects of inorganic ions on adhesion and proliferation of osteoblast-like cells. J. Biomed. Mater. Res. 2019;107(5):1042–1051. doi: 10.1002/jbm.a.36623. [DOI] [PubMed] [Google Scholar]
- 38.Pan H.T., Gao H.C., Li Q.T., Lin Z.F., Feng Q., Yu C.X., Zhang X.H., Dong H., Chen D.F., Cao X.D. Engineered macroporous hydrogel scaffolds via pickering emulsions stabilized by MgO nanoparticles promote bone regeneration. J. Mater. Chem. B. 2020;8(28):6100–6114. doi: 10.1039/d0tb00901f. [DOI] [PubMed] [Google Scholar]
- 39.Chen R., Chen H.B., Xue P.P., Yang W.G., Luo L.Z., Tong M.Q., Zhong B., Xu H.L., Zhao Y.Z., Yuan J.D. HA/MgO nanocrystal-based hybrid hydrogel with high mechanical strength and osteoinductive potential for bone reconstruction in diabetic rats. J. Mater. Chem. B. 2021;9(4):1107–1122. doi: 10.1039/d0tb02553d. [DOI] [PubMed] [Google Scholar]
- 40.Das Mahapatra R., Imani K.B.C., Yoon J. Integration of macro-cross-linker and metal coordination: a super stretchable hydrogel with high toughness. ACS Appl. Mater. Interfaces. 2020;12(36):40786–40793. doi: 10.1021/acsami.0c11167. [DOI] [PubMed] [Google Scholar]
- 41.Morais D.S., Rodrigues M.A., Silva T.I., Lopes M.A., Santos M., Santos J.D., Botelho C.M. Development and characterization of novel alginate-based hydrogels as vehicles for bone substitutes. Carbohydr. Polym. 2013;95(1):134–142. doi: 10.1016/j.carbpol.2013.02.067. [DOI] [PubMed] [Google Scholar]
- 42.Pontremoli C., Boffito M., Fiorilli S., Laurano R., Torchio A., Bari A., Tonda-Turo C., Ciardelli G., Vitale-Brovarone C. Hybrid injectable platforms for the in situ delivery of therapeutic ions from mesoporous glasses. Chem. Eng. J. 2018;340:103–113. [Google Scholar]
- 43.Xu C.K., Chen J.R., Li L.H., Pu X.B., Chu X., Wang X.L., Li M., Lu Y., Zheng X.F. Promotion of chondrogenic differentiation of mesenchymal stem cells by copper: implications for new cartilage repair biomaterials. Mat Sci Eng C-Mater. 2018;93:106–114. doi: 10.1016/j.msec.2018.07.074. [DOI] [PubMed] [Google Scholar]
- 44.Yang R., Li G., Zhuang C., Yu P., Ye T., Zhang Y., Shang P., Huang J., Cai M., Wang L., Cui W., Deng L. Gradient bimetallic ion-based hydrogels for tissue microstructure reconstruction of tendon-to-bone insertion. Sci Adv. 2021;7(26) doi: 10.1126/sciadv.abg3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Z., Zhao F.J., Zhang W., Yang Z.Y., Luo M., Liu L., Cao X.D., Chen D.F., Chen X.F. Degradable photothermal bioactive glass composite hydrogel for the sequential treatment of tumor-related bone defects: from anti-tumor to repairing bone defects. Chem. Eng. J. 2021;419 [Google Scholar]
- 46.Lu Y., Li L.H., Zhu Y., Wang X.L., Li M., Lin Z.F., Hu X.M., Zhang Y., Yin Q.S., Xia H., Mao C.B. Multifunctional copper-containing carboxymethyl chitosan/alginate scaffolds for eradicating clinical bacterial infection and promoting bone formation. Acs Appl Mater Inter. 2018;10(1):127–138. doi: 10.1021/acsami.7b13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oryan A., Kamali A., Moshiri A., Baharvand H., Daemi H. Chemical crosslinking of biopolymeric scaffolds: current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018;107(Pt A):678–688. doi: 10.1016/j.ijbiomac.2017.08.184. [DOI] [PubMed] [Google Scholar]
- 48.Li Q.C., Barret D.G., Messersmith P.B., Holten-Andersen N. Controlling hydrogel mechanics via bio-inspired polymer-nanoparticle bond dynamics. ACS Nano. 2016;10(1):1317–1324. doi: 10.1021/acsnano.5b06692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryu J.H., Messersmith P.B., Lee H. Polydopamine surface chemistry: a decade of discovery. ACS Appl. Mater. Interfaces. 2018;10(9):7523–7540. doi: 10.1021/acsami.7b19865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee C.S., Hwang H.S., Kim S., Fan J.B., Aghaloo T., Lee M. Inspired by nature: facile design of nanoclay-organic hydrogel bone sealant with multifunctional properties for robust bone regeneration. Adv. Funct. Mater. 2020;30(43) doi: 10.1002/adfm.202003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng L., Liu S.J., Cheng X.J., Qin Z.N., Lu Z.H., Zhang K., Zhao J.M. Intensified stiffness and photodynamic provocation in a collagen-based composite hydrogel drive chondrogenesis. Adv. Sci. 2020;7(6) doi: 10.1002/advs.202000588. vol 6, 1900099, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bai S.M., Zhang X.L., Lv X.L., Zhang M.Y., Huang X.W., Shi Y., Lu C.H., Song J.B., Yang H.H. Bioinspired mineral-organic bone adhesives for stable fracture fixation and accelerated bone regeneration. Adv. Funct. Mater. 2020;30(5) [Google Scholar]
- 53.Sung H.W., Huang R.N., Huang L.L., Tsai C.C. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J. Biomater. Sci. Polym. Ed. 1999;10(1):63–78. doi: 10.1163/156856299x00289. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z., Liu H., Luo W., Cai T., Li Z., Liu Y., Gao W., Wan Q., Wang X., Wang J., Wang Y., Yang X. Regeneration of skeletal system with genipin crosslinked biomaterials. J. Tissue Eng. 2020;11 doi: 10.1177/2041731420974861. 2041731420974861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen L., Li M., Yang Z., Tao W., Wang P., Tian X., Li X., Wang W. Gardenia jasminoides Ellis: ethnopharmacology, phytochemistry, and pharmacological and industrial applications of an important traditional Chinese medicine. J. Ethnopharmacol. 2020;257:112829. doi: 10.1016/j.jep.2020.112829. [DOI] [PubMed] [Google Scholar]
- 56.Zafeiris K., Brasinika D., Karatza A., Koumoulos E., Karoussis I.K., Kyriakidou K., Charitidis C.A. Additive manufacturing of hydroxyapatite-chitosan-genipin composite scaffolds for bone tissue engineering applications. Mat Sci Eng C-Mater. 2021;119 doi: 10.1016/j.msec.2020.111639. [DOI] [PubMed] [Google Scholar]
- 57.Lu Z., Liu S., Le Y., Qin Z., He M., Xu F., Zhu Y., Zhao J., Mao C., Zheng L. An injectable collagen-genipin-carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomaterials. 2019;218:119190. doi: 10.1016/j.biomaterials.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 58.Rahman M.M., Karim M.R., Alharbi H.F., Aldokhayel B., Uzzaman T., Zahir H. Cadmium selenide quantum dots for solar cell applications: a review. Chem. Asian J. 2021;16(8):902–921. doi: 10.1002/asia.202001369. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., Li L.L., Ma Y.C., Tang Y., Zhao Y., Li Z.M., Pu W.D., Huang B., Wen X., Cao X.J., Chen J.F., Chen W., Zhou Y., Zhang J.X. Multifunctional supramolecular hydrogel for prevention of epidural adhesion after laminectomy. ACS Nano. 2020;14(7):8202–8219. doi: 10.1021/acsnano.0c01658. [DOI] [PubMed] [Google Scholar]
- 60.Zhu H.Y., Yang H.Q., Ma Y.F., Lu T.J., Xu F., Genin G.M., Lin M. Spatiotemporally controlled photoresponsive hydrogels: design and predictive modeling from processing through application. Adv. Funct. Mater. 2020;30(32) doi: 10.1002/adfm.202000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piluso S., Gomez D.F., Dokter I., Texeira L.M., Li Y., Leijten J., van Weeren R., Vermonden T., Karperien M., Malda J. Rapid and cytocompatible cell-laden silk hydrogel formation via riboflavin-mediated crosslinking. J. Mater. Chem. B. 2020;8(41):9566–9575. doi: 10.1039/d0tb01731k. [DOI] [PubMed] [Google Scholar]
- 62.Qiao Y.S., Liu X.Z., Zhou X.C., Zhang H.B., Zhang W., Xiao W., Pan G.Q., Cui W.G., Santos H.A., Shi Q. Gelatin templated polypeptide Co-Cross-Linked hydrogel for bone regeneration. Adv Healthc Mater. 2020;9(1) doi: 10.1002/adhm.201901239. [DOI] [PubMed] [Google Scholar]
- 63.Gabarin N., Gavish H., Muhlrad A., Chen Y.C., Namdar-Attar M., Nissenson R.A., Chorev M., Bab I. Mitogenic G(i) protein-MAP kinase signaling cascade in MC3T3-E1 osteogenic cells: activation by C-terminal pentapeptide of osteogenic growth peptide [OGP(10-14)] and attenuation of activation by cAMP. J. Cell. Biochem. 2001;81(4):594–603. doi: 10.1002/jcb.1083. [DOI] [PubMed] [Google Scholar]
- 64.Fukumoto S. The role of bone in phosphate metabolism. Mol. Cell. Endocrinol. 2009;310(1–2):63–70. doi: 10.1016/j.mce.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 65.Brown R.B. Stress, inflammation, depression, and dementia associated with phosphate toxicity. Mol. Biol. Rep. 2020;47(12):9921–9929. doi: 10.1007/s11033-020-06005-1. [DOI] [PubMed] [Google Scholar]
- 66.Huang K.Q., Wu J., Gu Z.P. Black phosphorus hydrogel scaffolds enhance bone regeneration via a sustained supply of calcium-free phosphorus. Acs Appl Mater Inter. 2019;11(3):2908–2916. doi: 10.1021/acsami.8b21179. [DOI] [PubMed] [Google Scholar]
- 67.Wang G., Zhu J.H., Chen X.F., Dong H., Li Q.T., Zeng L., Cao X.D. Alginate based antimicrobial hydrogels formed by integrating Diels-Alder "click chemistry" and the thiol-ene reaction. RSC Adv. 2018;8(20):11036–11042. doi: 10.1039/c8ra00668g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee M., Rizzo R., Surman F., Zenobi-Wong M. Guiding lights: tissue bioprinting using photoactivated materials. Chem. Rev. 2020;120(19):10670–10747. doi: 10.1021/acs.chemrev.0c00077. [DOI] [PubMed] [Google Scholar]
- 69.Unagolla J.M., Jayasuriya A.C. Hydrogel-based 3D bioprinting: a comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl Mater Today. 2020;18 doi: 10.1016/j.apmt.2019.100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kirillova A., Yeazel T.R., Asheghali D., Petersen S.R., Dort S., Gall K., Becker M.L. Fabrication of biomedical scaffolds using biodegradable polymers. Chem. Rev. 2021;121(18):11238–11304. doi: 10.1021/acs.chemrev.0c01200. [DOI] [PubMed] [Google Scholar]
- 71.Zhang K., Feng Q., Fang Z., Gu L., Bian L. Structurally dynamic hydrogels for biomedical applications: pursuing a fine balance between macroscopic stability and microscopic dynamics. Chem. Rev. 2021;121(18):11149–11193. doi: 10.1021/acs.chemrev.1c00071. [DOI] [PubMed] [Google Scholar]
- 72.Balakrishnan B., Banerjee R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem. Rev. 2011;111(8):4453–4474. doi: 10.1021/cr100123h. [DOI] [PubMed] [Google Scholar]
- 73.Akhtar M.F., Hanif M., Ranjha N.M.… Methods of synthesis of hydrogels. A review, Saudi Pharm J. 2016;24(5):554–559. doi: 10.1016/j.jsps.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y.J., Cao Y., Zhao H.B., Zhang L.W., Ni T.Y., Liu Y.S., An Z., Liu M., Pei R.J. An injectable BMSC-laden enzyme-catalyzed crosslinking collagen-hyaluronic acid hydrogel for cartilage repair and regeneration. J. Mater. Chem. B. 2020;8(19):4237–4244. doi: 10.1039/d0tb00291g. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y.J., Chen H., Zhang T.T., Zan Y., Ni T.Y., Cao Y., Wang J.E., Liu M., Pei R.J. Injectable hydrogels from enzyme-catalyzed crosslinking as BMSCs-laden scaffold for bone repair and regeneration. Mat Sci Eng C-Mater. 2019;96:841–849. doi: 10.1016/j.msec.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 76.Anjum F., Lienemann P.S., Metzger S., Biernaskie J., Kallos M.S., Ehrbar M. Enzyme responsive GAG-based natural-synthetic hybrid hydrogel for tunable growth factor delivery and stem cell differentiation. Biomaterials. 2016;87:104–117. doi: 10.1016/j.biomaterials.2016.01.050. [DOI] [PubMed] [Google Scholar]
- 77.Khajouei S., Ravan H., Ebrahimi A. Developing a colorimetric nucleic acid-responsive DNA hydrogel using DNA proximity circuit and catalytic hairpin assembly. Anal. Chim. Acta. 2020;1137:1–10. doi: 10.1016/j.aca.2020.08.059. [DOI] [PubMed] [Google Scholar]
- 78.Nabavi M.H., Salehi M., Ehterami A., Bastami F., Semyari H., Tehranchi M., Nabavi M.A., Semyari H. A collagen-based hydrogel containing tacrolimus for bone tissue engineering. Drug Deliv Transl Res. 2020;10(1):108–121. doi: 10.1007/s13346-019-00666-7. [DOI] [PubMed] [Google Scholar]
- 79.Neves M.I., Araújo M., Moroni L., da Silva R.M.P., Barrias C.C. Glycosaminoglycan-inspired biomaterials for the development of bioactive hydrogel networks. Molecules. 2020;25(4) doi: 10.3390/molecules25040978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y., Xue Y., Wang J., Zhu Y., Zhu Y., Zhang X., Liao J., Li X., Wu X., Qin Y.X., Chen W. A composite hydrogel with high mechanical strength, fluorescence, and degradable behavior for bone tissue engineering. Polymers. 2019;11(7) doi: 10.3390/polym11071112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ni P., Ding Q., Fan M., Liao J., Qian Z., Luo J., Li X., Luo F., Yang Z., Wei Y. Injectable thermosensitive PEG-PCL-PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects. Biomaterials. 2014;35(1):236–248. doi: 10.1016/j.biomaterials.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 82.Chen M., Feng Z., Guo W., Yang D., Gao S., Li Y., Shen S., Yuan Z., Huang B., Zhang Y., Wang M., Li X., Hao L., Peng J., Liu S., Zhou Y., Guo Q. PCL-MECM-Based hydrogel hybrid scaffolds and meniscal fibrochondrocytes promote whole meniscus regeneration in a rabbit meniscectomy model. ACS Appl. Mater. Interfaces. 2019;11(44):41626–41639. doi: 10.1021/acsami.9b13611. [DOI] [PubMed] [Google Scholar]
- 83.Dalby M.J., Gadegaard N., Tare R., Andar A., Riehle M.O., Herzyk P., Wilkinson C.D.W., Oreffo R.O.C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007;6(12):997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 84.Wang C., Huang W., Zhou Y., He L., He Z., Chen Z., He X., Tian S., Liao J., Lu B., Wei Y., Wang M. 3D printing of bone tissue engineering scaffolds. Bioact Mater. 2020;5(1):82–91. doi: 10.1016/j.bioactmat.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng N.-C., Estes B.T., Awad H.A., Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng. 2008;15(2):231–241. doi: 10.1089/ten.tea.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rowland C.R., Colucci L.A., Guilak F. Fabrication of anatomically-shaped cartilage constructs using decellularized cartilage-derived matrix scaffolds. Biomaterials. 2016;91:57–72. doi: 10.1016/j.biomaterials.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen W., Xu Y., Li Y., Jia L., Mo X., Jiang G., Zhou G. 3D printing electrospinning fiber-reinforced decellularized extracellular matrix for cartilage regeneration. Chem. Eng. J. 2020;382 [Google Scholar]
- 88.Huang B., Yuan Y., Ding S., Li J., Ren J., Feng B., Li T., Gu Y., Liu C. Nanostructured hydroxyapatite surfaces-mediated adsorption alters recognition of BMP receptor IA and bioactivity of bone morphogenetic protein-2. Acta Biomater. 2015;27:275–285. doi: 10.1016/j.actbio.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 89.Zhang J., Zhou H., Yang K., Yuan Y., Liu C. RhBMP-2-loaded calcium silicate/calcium phosphate cement scaffold with hierarchically porous structure for enhanced bone tissue regeneration. Biomaterials. 2013;34(37):9381–9392. doi: 10.1016/j.biomaterials.2013.08.059. [DOI] [PubMed] [Google Scholar]
- 90.Li Y., Xiao Y., Liu C. The horizon of materiobiology: a perspective on material-guided cell behaviors and tissue engineering. Chem. Rev. 2017;117(5):4376–4421. doi: 10.1021/acs.chemrev.6b00654. [DOI] [PubMed] [Google Scholar]
- 91.Wang C., Lai J., Li K., Zhu S., Lu B., Liu J., Tang Y., Wei Y. Cryogenic 3D printing of dual-delivery scaffolds for improved bone regeneration with enhanced vascularization. Bioact Mater. 2021;6(1):137–145. doi: 10.1016/j.bioactmat.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matai I., Kaur G., Seyedsalehi A., McClinton A., Laurencin C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. doi: 10.1016/j.biomaterials.2019.119536. [DOI] [PubMed] [Google Scholar]
- 93.Gauvin R., Chen Y.C., Lee J.W., Soman P., Zorlutuna P., Nichol J.W., Bae H., Chen S., Khademhosseini A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials. 2012;33(15):3824–3834. doi: 10.1016/j.biomaterials.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dubin-Thaler B.J., Giannone G., Dobereiner H.G., Sheetz M.P. Nanometer analysis of cell spreading on matrix-coated surfaces reveals two distinct cell states and STEPs. Biophys. J. 2004;86(3):1794–1806. doi: 10.1016/S0006-3495(04)74246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu M., Qin M., Zhang X., Zhang X., Li J., Hu Y., Chen W., Huang D. Porous PVA/SA/HA hydrogels fabricated by dual-crosslinking method for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2020;31(6):816–831. doi: 10.1080/09205063.2020.1720155. [DOI] [PubMed] [Google Scholar]
- 96.Zhao Y., Li M., Liu B., Xiang J., Cui Z., Qu X., Qiu D., Tian Y., Yang Z. Ultra-tough injectable cytocompatible hydrogel for 3D cell culture and cartilage repair. J. Mater. Chem. B. 2018;6(9):1351–1358. doi: 10.1039/c7tb03177g. [DOI] [PubMed] [Google Scholar]
- 97.Chao Y., Ge Y., Chen Z., Cui X., Zhao C., Wang C., Wallace G.G. One-pot hydrothermal synthesis of solution-processable MoS(2)/PEDOT:PSS composites for high-performance supercapacitors. ACS Appl. Mater. Interfaces. 2021;13(6):7285–7296. doi: 10.1021/acsami.0c21439. [DOI] [PubMed] [Google Scholar]
- 98.Jiang Y., Krishnan N., Heo J., Fang R.H., Zhang L. Nanoparticle-hydrogel superstructures for biomedical applications. J. Contr. Release. 2020;324:505–521. doi: 10.1016/j.jconrel.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zohreband Z., Adeli M., Zebardasti A. Self-healable and flexible supramolecular gelatin/MoS(2) hydrogels with molecular recognition properties. Int. J. Biol. Macromol. 2021;182:2048–2055. doi: 10.1016/j.ijbiomac.2021.05.106. [DOI] [PubMed] [Google Scholar]
- 100.Rajabnejadkeleshteri A., Basiri H., Mohseni S.S., Farokhi M., Mehrizi A.A., Moztarzadeh F. Preparation of microfluidic-based pectin microparticles loaded carbon dots conjugated with BMP-2 embedded in gelatin-elastin-hyaluronic acid hydrogel scaffold for bone tissue engineering application. Int. J. Biol. Macromol. 2021;184:29–41. doi: 10.1016/j.ijbiomac.2021.05.148. [DOI] [PubMed] [Google Scholar]
- 101.Rauner N., Meuris M., Zoric M., Tiller J.C. Enzymatic mineralization generates ultrastiff and tough hydrogels with tunable mechanics. Nature. 2017;543(7645):407–+. doi: 10.1038/nature21392. [DOI] [PubMed] [Google Scholar]
- 102.Sun Z.Y., Song C.J., Wang C., Hu Y.Q., Wu J.H. Hydrogel-based controlled drug delivery for cancer treatment: a review. Mol. Pharm. 2020;17(2):373–391. doi: 10.1021/acs.molpharmaceut.9b01020. [DOI] [PubMed] [Google Scholar]
- 103.Elkhoury K., Russell C.S., Sanchez-Gonzalez L., Mostafavi A., Williams T.J., Kahn C., Peppas N.A., Arab-Tehrany E., Tamayol A. Soft-nanoparticle functionalization of natural hydrogels for tissue engineering applications. Adv Healthc Mater. 2019;8(18) doi: 10.1002/adhm.201900506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chan Wing P, Kung Fu-Chen, Kuo Yu-Lin, Yang Ming-Chen, Thomas Lai Wen-Fu. Alginate/Poly(γ-glutamic Acid) Base Biocompatible Gel for Bone Tissue Engineering. Bio Med Res Int. 2015;2015:185841. doi: 10.1155/2015/185841. [DOI] [PMC free article] [PubMed] [Google Scholar]